Abstract
Sensitization of central pain and inflammatory pathways play essential roles in migraine, a primary neurobiological headache disorder. Since hypoxia-inducible factor-1α (HIF-1α) is implicated in neuroprotection and inflammation inhibition, herein we investigated the role of HIF-1α in migraine. A chronic migraine model was established in mice by repeated injection of nitroglycerin (10 mg/kg, i.p.) every other day for 5 total injections. In the prevention and acute experiments, roxadustat, a HIF-1α stabilizer, was orally administered starting before or after nitroglycerin injection, respectively. Pressure application measurement, and tail flick and light-aversive behaviour tests were performed to determine the pressure pain threshold, thermal nociceptive sensitivity and migraine-related light sensitivity. At the end of experiments, mouse serum samples and brain tissues were collected for analyses. We showed that roxadustat administration significantly attenuated nitroglycerin-induced basal hypersensitivity and acute hyperalgesia by improving central sensitization. Roxadustat administration also decreased inflammatory cytokine levels in serum and trigeminal nucleus caudalis (TNC) through NF-κB pathway. Consistent with the in vivo results showing that roxadustat inhibited microglia activation, roxadustat (2, 10, and 20 μM) dose-dependently reduced ROS generation and inflammation in LPS-stimulated BV-2 cells, a mouse microglia cell line, by inhibiting HIF-1α/NF-κB pathway. Taken together, this study demonstrates that roxadustat administration ameliorates migraine-like behaviours and inhibits central pain sensitization in nitroglycerin-injected mice, which is mainly mediated by HIF-1α/NF-κB/inflammation pathway, suggesting the potential of HIF-1α activators as therapeutics for migraine.
Keywords: migraine, HIF-1α, roxadustat, central sensitization, neuroinflammation, ROS
Introduction
Migraine is a common primary disease encompassing episodic and chronic migraine that potently affects the social life of patients. Episodic migraine can develop to the chronic form. Gene, environment, age, and hormone differences are risk factors for the progression and occurrence of migraine [1–3]. At present, the main clinical drugs for acute migraine treatment are nonsteroidal anti-inflammatory drugs, triptans and ergotamine [2]. Calcium antagonists, β-adrenergic receptor antagonists and selective serotonin-reuptake inhibitors are mostly used for the preventive treatment of migraine [4]. However, these drugs are not effective for all people with migraines, as they have some limitations and may cause severe side effects. Nonsteroidal anti-inflammatory drugs are usually restricted to no more than 2–3 days per week and may lead to addiction and cognitive impairment [5]. Triptans are prohibited for patients with cardiovascular disease [6]. The use of ergotamine is associated with adverse vascular events [7]. Calcitonin gene-related peptide (CGRP) is a neuropeptide released from trigeminal nerve fibers and plays a critical role in migraine pathophysiology [8]. Studies have shown that blocking CGRP or CGRP receptor seems to be effective and safe in migraine patients. Rimegepant, which is administered in an orally disintegrating tablet formulation, is a highly selective CGRP antagonist that was approved by the FDA in 2020 for the acute treatment of migraine [9]. However, blocking CGRP may have risks in patients with comorbidities such as cardiovascular disease [10]. Moreover, four therapeutic monoclonal antibodies targeting CGRP or CGRP receptor have been approved by the FDA in recent years for migraine treatment. However, monoclonal antibodies also have some limitations, such as a high price and the potential for side effects, such as red rash, itching, and pain [11]. Therefore, there is an urgent need to develop new drugs for migraine treatment.
To date, the detailed mechanism of migraine is unclear. A large number of studies have shown that central sensitization, especially in the trigeminal nucleus caudalis (TNC), plays a critical role in migraine and can increase the frequency and maintain the occurrence of chronic migraine by amplifying pain signals and causing enhanced pain and disability [12, 13]. Cutaneous allodynia (CA) is a clinical manifestation of central sensitization, characterized by hypersensitivity to pain triggered by mechanical pressure, cold, heat, or other stimuli [14, 15]. CA affects more than half of migraine patients and is related to the severity of the migraine [16]. Pain hypersensitivity also occurs during migraine attacks [17]. Phosphorylated extracellular regulated protein kinase 1/2 (p-ERK1/2) and c-Fos are highly expressed in the TNC after innocuous stimulation and are often used as markers of central sensitization [18].
Based on accumulating evidence, oxidative stress and neuroinflammation both play important roles in the pathogenesis of migraine. Previous studies have shown that most of the common triggers of migraine can generate oxidative stress [19]. Lower activities of superoxide dismutase and glutathione peroxidase and reduced antioxidant capacity have been found in migraine patients [20]. The process of migraine is complex and controversial, and the neuroinflammatory cascade between neurons, astrocytes, and microglia is closely related to pain sensitivity of migraine. In addition to oxidative stress, neuroinflammation can also be elevated by common triggers. It has been reported that inflammatory response mediated by interleukin 1β (IL-1β) in TNC contributes to the process of central sensitization in a nitroglycerin (NTG)-induced chronic migraine mouse model [13]. Moreover, IL-18-mediated microglia-astrocyte interactions and activation of toll-like receptor 4/nuclear factor-kappa B (NF-κB) also promote migraine-related allodynia [21]. As a master regulator of proinflammatory cytokines, NF-κB activation results in the enhanced transcription and secretion of IL-1β, IL-6, and tumour necrosis factor α (TNF-α) [22]. It should be noted that multiple inflammatory cytokines, including IL-1β, IL-6, TNF-α, monocyte chemotactic protein-1 (MCP-1), and intercellular adhesion molecule-1, are significantly increased in serum of people with migraines, indicating that migraine is accompanied by systemic inflammation [23–26].
Transcription factor hypoxia-inducible factor-1α (HIF-1α) plays an important protective role in hypoxia and inflammatory pathways [27]. Activation of HIF-1α protects against doxorubicin-induced cardiotoxicity by increasing levels of the antiapoptotic gene Bcl-2 and antioxidant enzyme superoxide dismutase 2 to reduce apoptosis and oxidative stress pathways [28]. In addition, cisplatin-induced acute kidney injury can be attenuated by roxadustat through enhanced HIF-1α levels, which is related to the protection against renal tubular cell apoptosis and the inhibition of proinflammatory factors expression [29]. Moreover, there is evidence that HIF-1α stability has neuroprotective effects, which are mainly mediated by enhancing vascular endothelial growth factor production within neuronal cells [30]. Studies have shown that in a neuropathic pain rat model, HIF-1α stabilization or activation attenuates neuropathic pain and promotes functional recovery by enhancing the expression of HIF-1α downstream target gene BNIP3, reducing inflammatory cytokines, and activating vascular endothelial growth factor and nerve growth factor [31, 32]. Therefore, we speculated that HIF-1α may be a therapeutic target for migraine. As a new HIF prolyl hydroxylase inhibitor, roxadustat can stabilize HIF-1α to inhibit its degradation and further activate the transcription of downstream target genes. Roxadustat is approved for anaemia treatment in patients with chronic kidney disease (CKD) because it induces erythropoietin production and iron metabolism [33]. A recent study showed that roxadustat can reverse depressive-like behaviours in rats by upregulating the expression of HIF-1α and erythropoietin [34]. Moreover, roxadustat can promote the functional recovery and neuroprotection of experimental spinal cord injury by stabilizing HIF-1α [35]. In addition, the anti-inflammatory effect of roxadustat has been widely reported [36, 37]. A previous study proved that roxadustat can cross the blood-brain barrier to some extent and induce the expression of HIF-1α in brain tissue of mice [38]. Therefore, to gain insight into the anti-migraine pharmacological effects and mechanisms of HIF-1α, we constructed an NTG-induced chronic migraine mouse model and treated mice with roxadustat. In this study, we employed two treatment strategies, including acute and preventive treatment. Moreover, we used BV-2 cells, a mouse microglia cell line for mechanisms exploration.
Materials and methods
Reagents
Roxadustat was purchased from MedChemExpress (Jiangsu, China). Nitroglycerin was purchased from Beijing Yimin Pharmaceutical Co., Ltd. (Beijing, China). Lipopolysaccharide (LPS) and thiazolyl blue tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, USA). HIF-1α siRNA was purchased from Guangzhou RiboBio Co., Ltd. (Guangdong, China). PMSF was purchased from Beyotime Biotechnology (Shanghai, China).
Rabbit anti-IL-1β, NF-κB, ERK1/2, and c-Fos polyclonal and β-actin monoclonal antibodies were purchased from ABclonal Inc. (Hubei, China). Rabbit anti-p-ERK1/2, IκBα, phosphorylated IκBα (p-IκBα) and mouse anti-TNF-α and α-Tubulin monoclonal antibodies were purchased from Proteintech Inc. (Chicago, USA). Rabbit anti-HIF-1α, phosphorylated-NF-κB (p-NF-κB), and IL-6 monoclonal antibodies were purchased from Affinity Inc. (Wuhan, China).
Cell culture
BV-2 cells, a murine microglial cell line (ATCC, Manassas, USA), were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Biological Industries, Kibbutz Beit Haemek, Israel) supplemented with 10% fetal bovine serum (FBS, AusGeneX, Brisbane, AUS) and 1% penicillin/streptomycin (HyClone, Logan, USA) in a humidified incubator with 5% CO2 at 37 °C.
Determination of cell viability
Cell viability was determined by MTT assay. Briefly, after treatment, 0.5 mg/mL MTT solution (100 μL/well) was added to BV-2 cells in each well, and the cells were incubated for 4 h at 37 °C. After being washed with 1× PBS, the cells were incubated in DMSO (150 μL/well) for 20 min, and the absorbance at 550 nm was measured by a microplate reader (BioTek Instruments, Winooski, USA).
Determination of cellular reactive oxygen species (ROS)
Cellular ROS levels were determined by the fluorescent dye 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA). After treatment, DCFH-DA (30 or 1000 μL/well for 96- or 6-well plates, respectively) was added to BV-2 cells for 20 min in a cell incubator. Then, the dye was removed, and the cells were washed twice with PBS. The fluorescence intensity at 488 nm (excitation) and 525 nm (emission) in the samples in the 96-well plates was determined using a microplate reader (BioTek Instruments, Winooski, USA), while samples in 6-well plates were photographed with a fluorescence microscope (Leica, Wetzlar, Germany).
Knockdown of gene expression by siRNA transfection
BV-2 cells were transfected with HIF-1α siRNA (siHIF-1α, 40 nM/well) or the corresponding negative control siRNA (siCtrl) using LipofectamineTM RNAiMAX in serum-free medium according to the manufacturer’s instruction. After incubation in transfection medium for 24 h, BV-2 cells were subjected to further treatment.
Animals and drug administration
C57BL/6J mice (male, ~8 weeks old) were purchased from GemPharmatech Co., Ltd. (Jiangsu, China). All animal experimental procedures were approved by the Ethics Committee of Hefei University of Technology (HFUT20210226001) and were performed in accordance with the animal care guidelines of the National Institutes of Health.
The migraine mouse model was constructed based on previous reports [39]. In both prevention and acute treatment experiments, mice were randomly divided into 3 groups (8 mice/group): normal saline control group (NS), NTG-induced migraine group (NTG), and NTG plus roxadustat treatment group (NTG + Rox). Mice received the following treatments: the NS group received intraperitoneal (i.p.) injection of normal saline, and the NTG group received i.p. injection of NTG (10 mg/kg bodyweight) every other day for a total of 5 injections; the NTG + Rox group received i.p. injection of NTG and were fed normal chow containing roxadustat (12.8 mg/100 g food) for the prevention experiment or treated by the intragastric (i.g.) administration of roxadustat (12.8 mg/kg body weight once a day) for the acute treatment experiment. In the prevention experiment, mice received roxadustat treatment 2 days before the NTG injection, behaviour tests were conducted before the NTG injection, and mice were sacrificed on the second day of the last NTG injection. In the acute treatment experiment, mice received roxadustat treatment 30 min after the NTG injection, underwent behaviour tests 2 h after the NTG injection, and were sacrificed after the behaviour tests. At the end of experiments, serum samples and brain tissues were collected.
Sensory sensitivity tests
The pressure pain threshold was measured by a pressure application measurement (PAM) test using an electronic tenderness instrument (YLS-3, Yiyan Technology, Jinan, China) [40, 41]. Mouse was put into a fixed cylinder with an audio amplification device, and the tail was placed between the indenter and a flat support. Mouse tail was slowly pressed until the mouse screamed, and the pressure value (g) was recorded. According to a previous study and based on animal ethics standards, we used 500 g as the pressure cut-off in our experiment [42]. The pressure values were recorded three times in each test, and the average value was defined as the pressure pain threshold.
The tail-flick test was applied to determine the thermal nociceptive threshold using an electronic tenderness instrument (YLS-12A, Yiyan Technology, Jinan, China) [40]. Mouse tail was placed on a platform with an infrared spot to heat the platform. The time of tail flick was recorded automatically. If the latency was less than two seconds, the test was performed again. The latency of the thermal pain reaction was recorded three times in each test, and the average value was considered as the thermal nociceptive threshold.
Before NTG injection, one test was conducted and considered as a baseline threshold (basal responses). Two hours after NTG injection, another test was performed and defined as the acute responses.
Light-aversive behaviour test
A light/dark box with two equally sized chambers was used to quantify migraine-related light sensitivity [43, 44]. The experimental box was made of opaque white wood. The light chamber is white and equipped with lighting equipment and a camera system, while the dark chamber is wrapped with black paper and covered with a lid. There is a square gate connecting these two chambers. Before the test, mouse was put into the box for 1 min to adapt to the experimental environment. Time and motion trajectory in the light chamber were recorded by a video analysis system during a 5 min test session. In the preventive experiment, the light-aversive behaviour test was performed before NTG injection, while in the acute treatment experiment, it was performed 1 h after NTG injection to detect photophobia in the acute phase.
Determination of protein expression by Western blot and mRNA expression by quantitative real-time PCR (qRT-PCR)
After treatment, BV-2 cells or TNC tissues were lysed in RIPA lysis buffer containing PMSF and an inhibitor cocktail. Protein expression was determined by Western blot [45]. The band density was analysed by ImageJ and normalized to α-Tubulin or HSP90 in the corresponding samples.
Total RNA was extracted from BV-2 cells or TNC tissues using total RNApure reagent (Zoman Biotechnology, Beijing, China). mRNA expression was determined using SYBR® PremixEx TaqTM II and the indicated primers in a Bio-Rad CFX System. Primers used in the experiment are shown in Table 1. mRNA expression was normalized to β-actin mRNA expression in the corresponding samples.
Table 1.
Sequences of the primers for the qRT-PCR assay.
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| m-β-actin | ATGGAGGGGAATACAGCCC | TTCTTTGCAGCTCCTTCGTT |
| m-HIF-1α | GGCAGCGATGACACAGAAAC | TGGGACTGTTAGGCTCAGGT |
| m-IL-1β | GACCTTCCAGGATGAGGACA | AGCTCATATGGGTCCGACAG |
| m-IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| m-TNF-α | CGTCGTAGCAAACCACCAAG | TTGAAGAGAACCTGGGAGTAGACA |
m: Mus musculus, HIF-1α hypoxia inducible factor-1α, IL-1β/6 interleukin 1β or 6, TNF-α tumour necrosis factor-α.
Immunofluorescence staining
At the end of experiment, mice were anaesthetized by i.p. injection of 2% sodium pentobarbital, followed by perfusion with cold PBS. Brain tissue was quickly removed and frozen on dry ice, and the whole brain was fixed with 4% paraformaldehyde at 4 °C overnight. Tissues were transferred to 20% and 30% sucrose solutions until they sank. To determine the expression of c-Fos, p-ERK1/2, HIF-1α, p-NF-κB, Iba-1, NeuN, and IL-1β in TNC, the sections within the bregma at −7.47 mm to −8.24 mm were collected for immunofluorescence staining [46]. Briefly, tissues were embedded in OCT and sectioned at 10 μm thickness. Tissue sections were incubated with the indicated primary antibodies overnight at 4 °C and then with fluorescence-conjugated secondary antibodies for 2 h at room temperature. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI) (Beyotime, China) at room temperature for 10 min. Images were obtained by laser scanning confocal microscopy (Leica, Wetzlar, Germany). The mean fluorescence intensity (MFI) was assessed by Photoshop software and expressed as fold change relative to the NS group.
Antibody array and Gene Ontology (GO) analysis
Mouse serum from the therapeutic experiment group was collected and analysed using the G-Series Mouse Cytokine Array 1 (GSM-CYT-1, RayBiotech, Guangzhou, China) according to the manufacturer’s instructions. The original data were normalized by RayBiotech software. The moderated t-statistics based on linear models for microarray data (limma) database from R/Bioconductor was used to analyse proteins with significant differences (P < 0.05). GO analysis was performed on the obtained protein data using the clusterProfiler database (http://bioconductor.org/), which is based on biological process, molecular function, and cellular component. Fisher’s exact test was used for statistical analysis.
Statistical analysis
All values are presented as the mean ± standard deviation (S.D.). GraphPad Prism 7.0 software was used for statistical analysis. Two-way ANOVA was used to evaluate the differences between two groups in the in vivo study, and one-way analysis of variance (ANOVA) followed by a post hoc Bartlett’s test was used in the other study. Differences were considered significant at P < 0.05.
Results
Roxadustat protects against LPS-induced inflammation in BV-2 cells
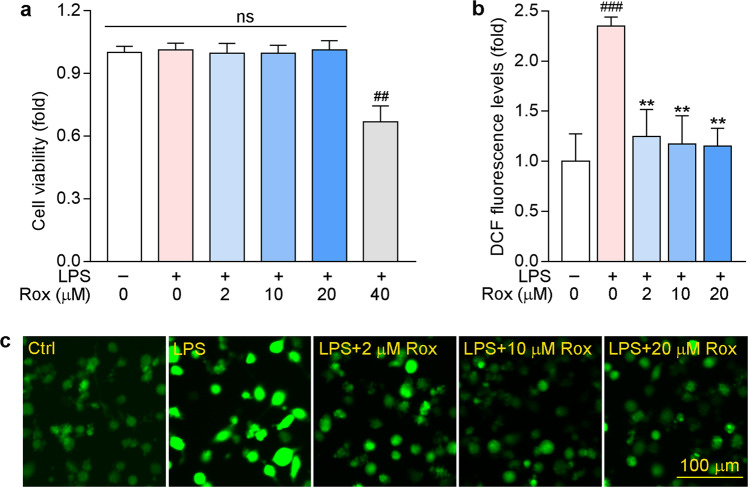
Studies have shown that microglia play a critical role in the induction and maintenance of neuropathic pain by releasing proinflammatory cytokines and chemokines [47]. In this study, we first used BV-2 cells, a murine microglial cell line, to explore the anti-inflammatory and antioxidative stress roles of roxadustat in vitro. To determine whether roxadustat can cause cytotoxicity, we initially pre-treated BV-2 cells with roxadustat for 1 h, followed by co-treatment with LPS for another 24 h. As shown in Fig. 1a, cell viability was not affected by roxadustat treatment at low concentrations but was decreased by 40 μM roxadustat. These results indicated that the safe dose of roxadustat in BV-2 cells was 20 μM or less. Then, we found that LPS increased ROS accumulation in BV-2 cells, which was substantially blocked by roxadustat treatment (Fig. 1b, c).
Fig. 1. Roxadustat reduces LPS-induced intracellular ROS levels in BV-2 cells.
a BV-2 cells were pre-treated with roxadustat at indicated concentrations for 1 h, then co-treated with 1 μg/mL LPS for another 24 h, followed by determination of cell viability by MTT assay. b, c BV-2 cells were pre-treated with indicated concentrations of roxadustat for 1 h, then co-treated with 1 μg/mL LPS for another 12 h. ROS levels were detected using DCFH-DA. ##P < 0.01; ###P < 0.001 vs. Control group (Ctrl); **P < 0.01 vs. LPS treated group; ns: not significantly different (n = 5), Rox: roxadustat.
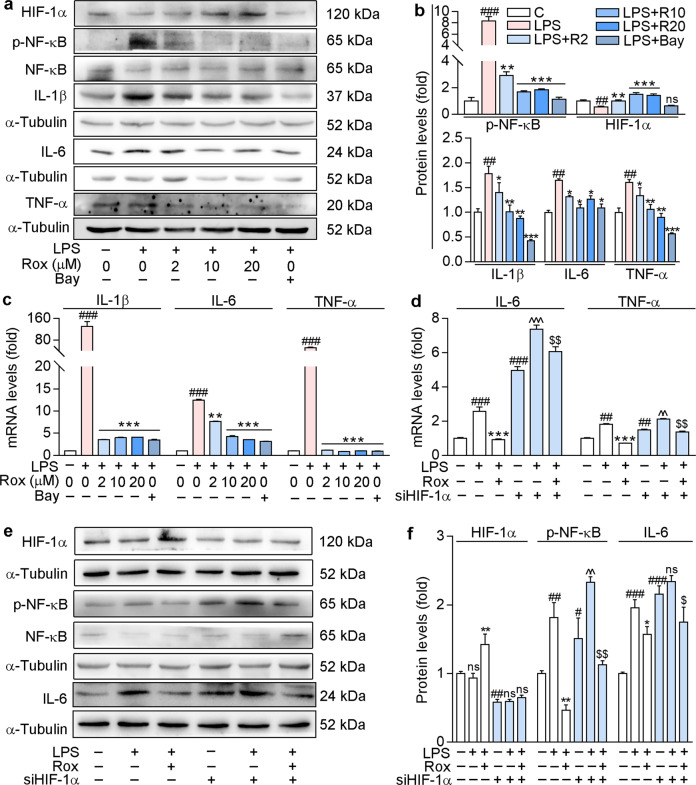
ROS accumulation can lead to an increase in proinflammatory cytokines. Indeed, our results showed that LPS increased the protein levels of proinflammatory factors IL-1β, IL-6, TNF-α, and p-NF-κB, the active form of NF-κB (Fig. 2a, b). However, the increases in both the proinflammatory cytokines and nuclear transcription factor were reversed by roxadustat or Bay 11-7082 (an NF-κB inhibitor). Similarly, LPS-induced IL-1β, IL-6, and TNF-α mRNA levels were also attenuated by roxadustat or Bay 11-7082 (Fig. 2c). Moreover, our results indicated that 20 μM roxadustat showed anti-inflammatory effects similar to those of the NF-κB inhibitor. Furthermore, as shown in Fig. 2a, b, roxadustat enhanced LPS-mediated reduction in HIF-1α expression, but BAY 11-7082 did not, indicating that NF-κB may be downstream of HIF-1α. These results suggest that roxadustat may inhibit cellular inflammation by regulating HIF-1α/NF-κB pathway.
Fig. 2. Roxadustat protects against LPS-induced inflammation in BV-2 cells.
a–c BV-2 cells were pre-treated with BAY (5 μM) or roxadustat at the indicated concentrations for 1 h, then co-treated with 1 μg/mL LPS for another 12 h. Protein expression of HIF-1α, NF-κB, p-NF-κB, IL-6, IL-1β and TNF-α was determined by Western blot with quantitative analysis of band intensity (a and b); mRNA levels of IL-1β, IL-6 and TNF-α were determined by qRT-PCR (c). d–f BV-2 cells were transfected with HIF-1α siRNA or the corresponding negative control siRNA for 24 h, then pre-treated with 10 μM roxadustat for 1 h, followed by 1 μg/mL LPS co-treatment for another 12 h. mRNA levels of IL-6 and TNF-α were determined by qRT-PCR (d); protein expression of HIF-1α, NF-κB, p-NF-κB and IL-6 was determined by Western blot with quantitative analysis of band intensity (e and f). #P < 0.05; ##P < 0.01; ###P < 0.001 vs. Control group (Ctrl). *P < 0.05; **P < 0.01; ***P < 0.001 vs. LPS treated group; ^P < 0.05; ^^P < 0.01; ^^^P < 0.001 vs. siHIF-1α group; $P < 0.05, $$P < 0.01 vs. LPS treated siHIF-1α group, ns: not significantly different (n = 3). Bay: Bay 11-7082; Rox: roxadustat; R2/10/20: roxadustat 2 or 10 or 20 μM.
To explore the mechanisms by which roxadustat regulates the inflammatory response, we transfected BV-2 cells with HIF-1α siRNA. The results in Fig. 2d–f demonstrate that silencing HIF-1α dramatically enhanced the mRNA expression of inflammatory factors IL-6 and TNF-α and the protein expression of IL-6 in BV-2 cells in both the presence and absence of LPS. Similar results were observed for p-NF-κB levels (Fig. 2e, f). Roxadustat had little effect on LPS-induced inflammatory cytokines and p-NF-κB levels in HIF-1α knockdown cells compared to control cells (Fig. 2d–f). Hence, the above results suggest that HIF-1α may be a key regulator that inhibits the inflammatory response. Taken together, these results indicate that roxadustat reduces ROS production and cellular inflammation, at least in part, through the HIF-1α/NF-κB pathway.
Roxadustat prevents NTG-induced migraine in C57BL/6J mice
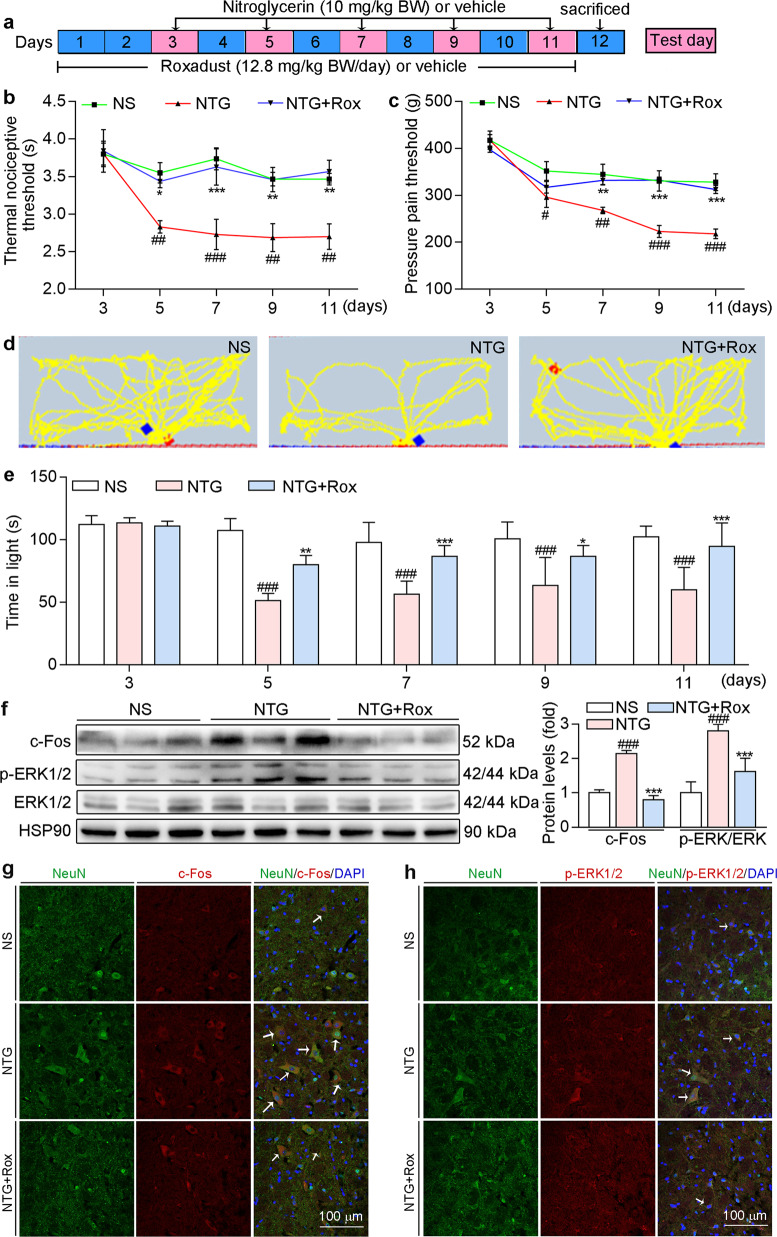
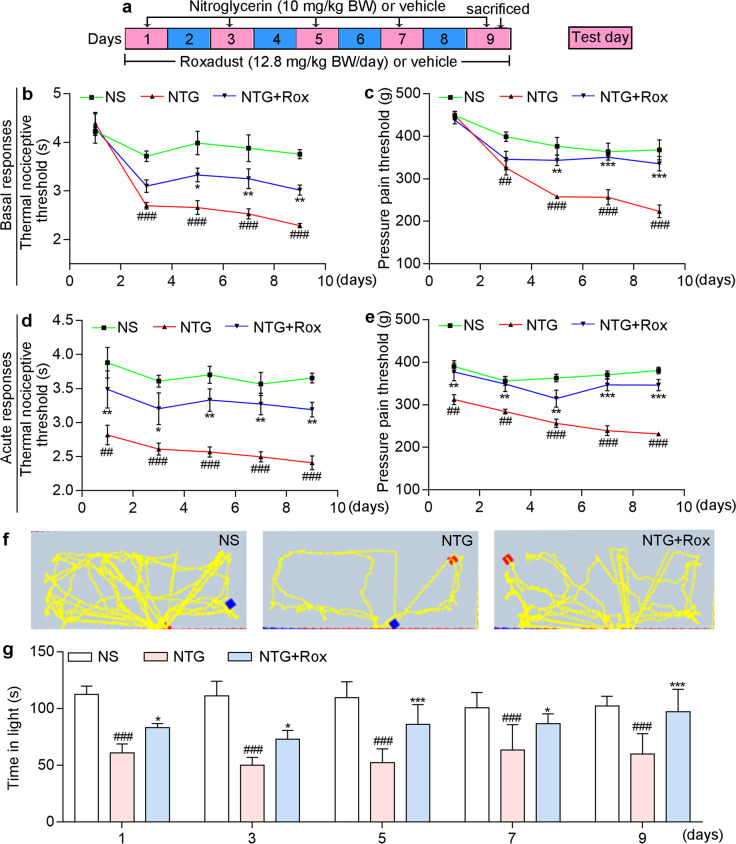
NTG can cause hyperalgesia and is widely used to construct migraine mouse model [39]. To explore the protective effects of roxadustat in vivo, we pre-fed mice normal chow containing roxadustat for 2 days before NTG injection (Fig. 3a). The PAM and tail-flick tests were performed on Days 3, 5, 7, 9, and 11. As shown in Fig. 3b, c, there was no significant difference between groups in the baseline threshold before NTG administration (the first day of NTG injection). Compared with those in the NS group, mice in the NTG group showed a significant decrease in both the pressure pain threshold and thermal nociceptive threshold of basal responses after NTG injection (Fig. 3b, c). However, roxadustat treatment significantly restored NTG-induced basal hypersensitivity, indicating the protective effects of HIF-1α activation on NTG-induced migraine. In addition, migraine can also cause light aversion. Light-aversive behaviour test was performed to evaluate the photophobic behaviour of mice. In general, mice in each group showed a strong tendency to stay in the dark environment. Compared with those in the NS group, mice in the NTG group showed less activity and a shorter residence time in the light chamber, suggesting the occurrence of migraine-related photophobia in the model group (Fig. 3d, e). In contrast, mice in the preventive group treated with roxadustat moved more frequently and showed a strong tendency to stay in the light chamber, indicating that roxadustat improved NTG-induced light sensitivity.
Fig. 3. Preventative treatment with roxadustat improves NTG-induced migraine in C57BL/6J mice.
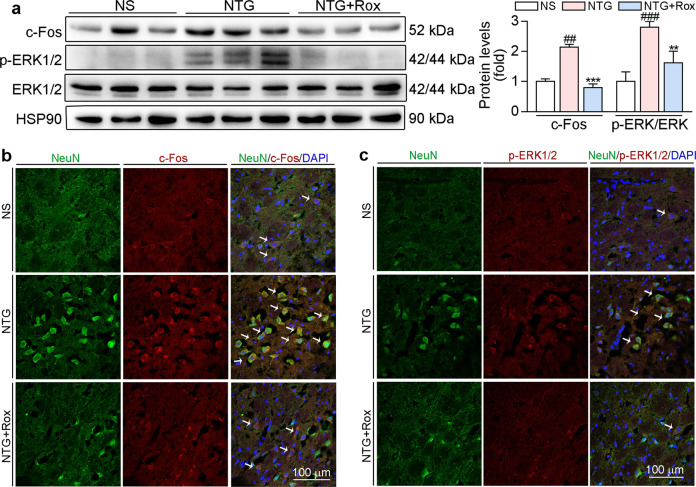
a In the prevention experiment, mice were fed with normal chow or chow containing roxadustat (12.8 mg/100 g food) for 11 days. On day 3, 5, 7, 9 and 11, mice received i.p. injection of normal saline or NTG (10 mg/kg bodyweight). Before NTG injection, mouse was conducted tail-flick and PAM test for determination the threshold of thermal nociceptive (b) and pressure pain (c), respectively. Before NTG injection, mouse was conducted light-aversive behaviour test for determination the motion trajectory (d) and the time (e) of mouse in the light chamber. After treatment, mice were sacrificed, followed by collection of brain tissues. Protein expression of c-Fos and p-ERK1/2 in TNC was determined by Western blot with quantitative analysis of band intensity (f); TNC frozen sections were conducted co-immunofluorescence staining with c-Fos (red, g) or p-ERK1/2 (red, h) and NeuN (neuronal marker, green) antibodies. White arrow indicates expression of c-Fos or p-ERK1/2 in neuron cells of TNC. #P < 0.05; ##P < 0.01; ###P < 0.001 vs. NS group; *P < 0.05; **P < 0.01; ***P < 0.001 vs. NTG group (n = 5); Rox: roxadustat.
There is increasing evidence that c-Fos and p-ERK1/2 are reliable molecular markers of central sensitization [18]. Our results showed that the repeated administration of NTG increased p-ERK1/2 and c-Fos protein levels in TNC (Fig. 3f). Moreover, co-immunofluorescence staining indicated that p-ERK1/2 and c-Fos protein levels were higher in the NTG group than in the NS group and these proteins were localized in neuron cells of TNC (Fig. 3g, h). Compared with the NTG group, the preventive group treated with roxadustat showed a significant decrease in the protein levels of c-Fos and p-ERK1/2 in TNC (Fig. 3f–h), suggesting that roxadustat can ameliorate NTG-induced central sensitization.
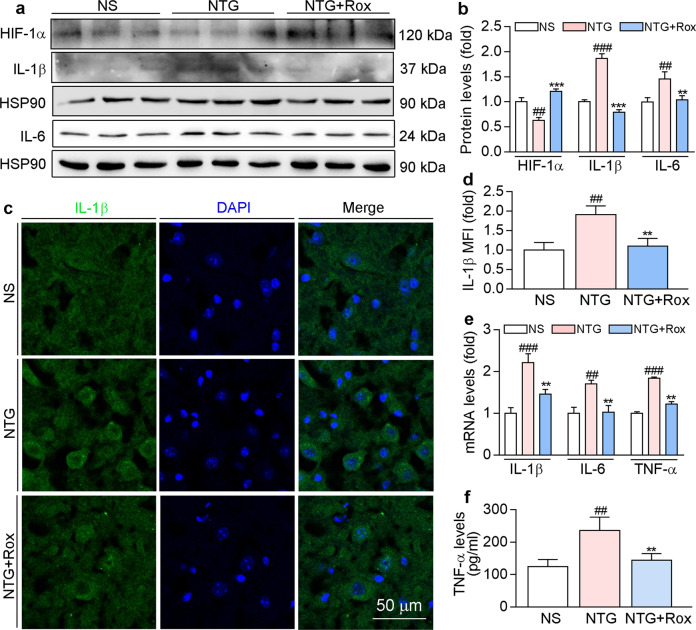
Our in vitro results showed that roxadustat can stabilize HIF-1α and improve LPS-induced inflammation in BV-2 cells. Consistently, preventive treatment with roxadustat enhanced HIF-1α levels that were reduced by NTG (Fig. 4a, b). Moreover, roxadustat improved the neuroinflammation induced by the repeated administration of NTG, as evidenced by the decreased protein levels of proinflammatory factors IL-6 and IL-1β (Fig. 4a–d) and mRNA levels of IL-1β, IL-6, and TNF-α in TNC (Fig. 4e). In addition, the ELISA results showed that serum TNF-α levels were increased in the NTG group but reduced by roxadustat treatment (Fig. 4f). Taken together, the above results suggest that roxadustat attenuates central sensitization by regulating neuroinflammation in TNC.
Fig. 4. Preventative treatment with roxadustat ameliorates NTG-induced neuroinflammation.
TNC tissues collected from mice used in Fig. 3 were conducted the following assays. a and b Protein expression of HIF-1α, IL-1β and IL-6 was determined by Western blot with quantitative analysis of band intensity. c and d TNC sections were used to determine IL-1β protein expression by immunofluorescence staining with quantitation of MFI. e mRNA levels of IL-1β, IL-6, and TNF-α were determined by qRT-PCR. f Serum TNF-α levels were determined using a mouse TNF-α ELISA kit according to the manufacturer’s instructions. ##P < 0.01; ###P < 0.001 vs. NS group; **P < 0.01; ***P < 0.001 vs. NTG group (n = 3); Rox: roxadustat.
HIF-1α activation exerts treatment effects on NTG-induced migraine-like behaviours
Migraine is a periodic disease that encompasses preventive and acute stages in which treatment can be administered. To elucidate the ability of roxadustat to improve symptoms in the acute stage, mice in the acute treatment group received i.g. administration of roxadustat daily after NTG injection (Fig. 5a). The results of behavioural experiments highlighted that acute treatment with roxadustat significantly attenuated NTG-induced basal hypersensitivity, acute hyperalgesia, and light sensitivity (Fig. 5b–g), indicating that HIF-1α activation may be a therapeutic strategy for migraine. Compared to NS treatment, NTG administration significantly induced hyperalgesia. However, acute treatment with roxadustat improved the thermal nociceptive threshold and pressure pain threshold of basal responses from the fifth day (Fig. 5b, c), and acute responses and photophobia from the first administration of NTG (Fig. 5d–g).
Fig. 5. Acute treatment with roxadustat improves NTG-induced migraine in C57BL/6 J mice.
a In the acute treatment experiment, mice received i.p. injection of normal saline or NTG (10 mg/kg bodyweight) on day 1, 3, 5, 7 and 9. After 30 min of NTG injection, mice were given i.g. administration of normal saline or roxadustat (12.8 mg/kg bodyweight). Mouse was conducted tail flick (b and d) and PAM test (c and e). The basal responses (b and c) were recorded before NTG injection, while the acute responses (d and e) were recorded 2 h after NTG injection. After NTG injection, mouse was conducted light-aversive behaviour test for determination of motion trajectory (f) and the time (g) of mouse in the light chamber. ##P < 0.01; ###P < 0.001 vs. NS group; *P < 0.05; **P < 0.01; ***P < 0.001 vs. NTG group (n = 5); Rox: roxadustat.
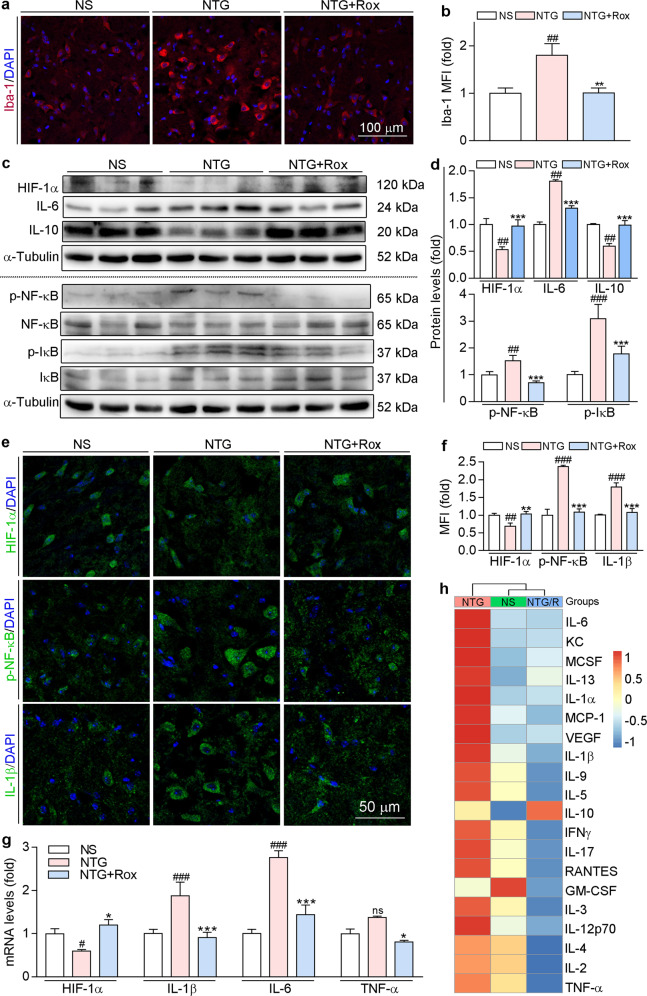
Consistent with the results in Fig. 3, we found that acute treatment with roxadustat decreased the markers of central sensitization, p-ERK1/2 and c-Fos, in TNC (Fig. 6). The results of co-immunofluorescence staining further confirmed that roxadustat treatment in the acute stage decreased p-ERK1/2 and c-Fos levels in TNC neurons (Fig. 6b, c). Activated microglia can secrete a number of proinflammatory cytokines, which are the main contributors to central sensitization [13]. To explore the mechanisms of roxadustat in migraine treatment, we examined whether roxadustat can reduce the activation of microglia in TNC by conducting immunofluorescence staining using an Iba-1 (a microglia marker) antibody. As shown in Fig. 7a, b, NTG stimulation induced microglia activation, which was reduced by roxadustat treatment. In addition, we measured proinflammatory and anti-inflammatory cytokines in TNC. Our results showed that roxadustat reversed the NTG-mediated increase in IL-6 and decrease in IL-10 protein expression (Fig. 7c, d). Moreover, the main regulator of inflammation, p-NF-κB (the active form of NF-κB), was reduced by roxadustat (Fig. 7c, d). IκBα, a member of the IκB family, is relevant for the transcriptional activity of NF-κB. We found that NTG enhanced the levels of p-IκB (the active form of IκB), which was attenuated by roxadustat (Fig. 7c, d). Consistent with the findings in BV-2 cells, roxadustat treatment stabilized HIF-1α in mouse TNC (Fig. 7c–g). The immunofluorescence staining results in Fig. 7e, f further confirmed that inflammation was downregulated by roxadustat. In addition, we found that mRNA levels of IL-1β, IL-6, and TNF-α were enhanced in TNC of the NTG group and were greatly attenuated by roxadustat (Fig. 7g).
Fig. 6. Acute treatment with roxadustat improves NTG-induced central sensitization in TNC.
TNC tissues collected from mice used in Fig. 5 were conducted the following assays. a Protein expression of c-Fos, p-ERK1/2 and ERK1/2 in TNC was determined by Western blot with quantitative analysis of band intensity. TNC frozen sections were conducted co-immunofluorescence staining with c-Fos (red, b) or p-ERK1/2 (red, c) and NeuN (neuronal marker, green) antibodies. White arrow indicates expression of c-Fos or p-ERK1/2 in neuron cells of TNC. ##P < 0.01; ###P < 0.001 vs. NS group; **P < 0.01; ***P < 0.001 vs. NTG group (n = 3); Rox: roxadustat.
Fig. 7. Acute treatment with roxadustat ameliorates NTG-induced neuroinflammation and systemic inflammation.
Brain tissues and serum collected from mice used in Fig. 5 were conducted the following assays. a and b TNC frozen sections were used to determine Iba-1 protein expression by immunofluorescence staining with quantitation of MFI. c and d Protein expression levels of HIF-1α, IL-6, IL-10, NF-κB, p-NF-κB, IκB, and p-IκB in TNC were determined by Western blot with quantitative analysis of band intensity. e and f TNC frozen sections were used to determine HIF-1α, p-NF-κB, and IL-1β protein expression by immunofluorescence staining with quantitation of MFI. g mRNA levels of HIF-1α, IL-1β, IL-6, and TNF-α in TNC were determined by qRT-PCR. h Serum proinflammatory cytokines were analyzed by RayBio® Label-Based Mouse Antibody Arrays according to the manufacturer’s instructions. #P < 0.05; ##P < 0.01; ###P < 0.001 vs. NS group; *P < 0.05; **P < 0.01; ***P < 0.001 vs. NTG group (n = 3); ns: not significantly different, Rox: roxadustat.
There is clinical evidence that the levels of serum inflammatory biomarkers are increased in patients during migraine attacks [25, 26]. Hence, to determine whether roxadustat can regulate systemic inflammation in a migraine mouse model, we detected serum proinflammatory factors using RayBio® Label-Based Mouse Antibody Arrays. According to the GO analysis results, there was an acute inflammatory response in the serum of NTG-injected mice (Fig. S1). The expression of proinflammatory cytokines, especially IL-6, keratinocyte chemoattractant (KC), MCP-1, and IL-1β, was increased in the NTG group compared with the NS group. In contrast, the elevated inflammatory cytokines were improved by roxadustat (Fig. 7h). Overall, these results indicate that roxadustat administration can attenuate central sensitization by regulating microglia activation and neuroinflammation in TNC and systemic inflammation through HIF-1α/NF-κB/inflammation pathway.
Discussion
In the present study, to gain insight into the pharmacological effects of HIF-1α activation on anti-migraine activity, roxadustat was employed in an NTG-induced migraine mouse model. The clinical strategy for migraine is generally divided into preventive and acute treatment [5]. Therefore, we treated mice before NTG or after NTG administration in this study. In both the prevention and acute treatment groups, roxadustat improved the pressure pain and thermal nociceptive thresholds and ameliorated photophobia (Figs. 3 and 5). Additionally, the levels of central sensitization markers, p-ERK1/2, and c-Fos, in TNC were inhibited by roxadustat (Figs. 3 and 6). In addition, we found that roxadustat had anti-inflammatory and antioxidative stress properties both in vivo and in vitro, evidenced by decreased inflammatory cytokines in mouse TNC, serum and BV-2 cells (Figs. 2, 4, and 7). Furthermore, siRNA experiments demonstrated that the ability of roxadustat to reduce inflammation was dependent on the HIF-1α/NF-κB pathway (Fig. 2). Roxadustat is approved in many countries and regions, such as the UK, EU, China, Japan, South Korea and Chile, for the treatment of anaemia in CKD patients [48]. Therefore, our study may not only provide an effective strategy for migraine treatment but also broaden the indications of roxadustat in other diseases.
The occurrence and development of migraine are accompanied by the central sensitization of trigeminal pain pathway [13]. Allodynia, an abnormal sensory state that is usually caused by harmless stimuli, is the main manifestation of central sensitization in most patients with migraine [49]. In our study, the PAM and tail flick tests showed that roxadustat treatment significantly restored NTG-induced acute and basal responses (Figs. 3b, c, and 5b–e). Photophobia is one of the most common symptoms of migraine [50]. The light-aversive behaviour test showed that roxadustat treatment significantly improved NTG-induced photophobia (Figs. 3d, e and 5f, g). p-ERK1/2 and c-Fos have been widely used as markers of nociceptive neuronal activation and central sensitization. Inhibition of c-Fos protein expression by the intrathecal injection of c-Fos antisense oligodeoxynucleotides can decrease the thermal hyperalgesia induced by complete Freund’s adjuvant in rats [51]. ERK is a member of the mitogen-activated protein kinase (MAPK) family. ERK activation in nociceptive spinal neurons may maintain persistent inflammatory pain hypersensitivity by regulating the transcription of prodynorphin and neurokinin-1 [52]. Under strong noxious stimulation, p-ERK1/2 levels were found to increase, while MEK inhibitors reduced p-ERK1/2 levels to attenuate formalin-induced pain behaviours [53]. In our study, we found that roxadustat treatment significantly decreased the protein levels of c-Fos and p-ERK1/2 in TNC (Figs. 3f–h and 6), suggesting that roxadustat ameliorates NTG-induced central sensitization by regulating c-Fos and p-ERK1/2 pathways.
Although the causes of migraine are still unclear, inflammation has been demonstrated to be a universal trigger [54]. The process of migraine is accompanied by systemic inflammation. Interestingly, the inflammatory response is altered with disease progression [55]. In our study, the RayBio® Label-Based Mouse Antibody Array results showed that NTG injection enhanced the systemic inflammatory response. Most notably, the expression of IL-6, KC, MCP-1 and IL-1β was increased in mice of the NTG group. However, the elevated inflammatory cytokines levels were improved by roxadustat (Fig. 7h). Central sensitization, especially in the TNC, plays a critical role in migraine. Glial cells are activated in the TNC of patients with migraine and can promote central sensitization by releasing proinflammatory mediators. Inflammatory factors can further promote and maintain chronic pain [56]. In an NTG-induced migraine mouse model, microglia were activated to mediate IL-1β release and promote central sensitization [13]. IL-6 participates in migraine-related pain behaviour by enhancing the sensitization of dural afferents [57]. Therefore, inhibiting inflammation is a therapeutic strategy for migraine treatment. Nonsteroidal anti-inflammatory drugs are used in clinical treatment of migraine [2]. In addition to serum, TNC also showed significantly increased proinflammatory factors levels in the NTG group. However, the enhanced levels of proinflammatory factors in TNC and serum were noticeably reduced by roxadustat treatment (Figs. 4 and 7). NF-κB is a master regulator of inflammation [58]. Rhynchophylline was found to attenuate NTG-induced migraine in a rat model by suppressing MAPK/NF-κB signalling [59]. In our study, NTG-induced p-NF-κB and p-IκB levels were reduced by roxadustat (Fig. 7c–f). In addition, our results showed that roxadustat improved NTG-induced microglia activation (Fig. 7a, b). Consistent with the in vivo results, roxadustat treatment decreased LPS-induced ROS production and inflammatory cytokines expression (Figs. 1 and 2). Taken together, these results suggest that roxadustat can improve neuroinflammation through NF-κB pathway.
Activation of HIF-1α can improve inflammation, which contributes to neuroprotective effects in nerve cells [30, 60]. In our study, we found that roxadustat treatment stabilized HIF-1α both in vivo and in vitro. In addition, the levels of inflammatory factors were enhanced after HIF-1α expression was inhibited by siRNA in BV-2 cells. Moreover, the inhibitory effect of roxadustat on inflammatory cytokines was restored in HIF-1α siRNA transfected BV-2 cells (Fig. 2d–f). Similarly, roxadustat had little effect on LPS-induced p-NF-κB levels in HIF-1α knockdown cells (Fig. 2e, f). Based on the above results, we suggest that the anti-inflammatory effect of roxadustat is mediated by HIF-1α/NF-κB pathway.
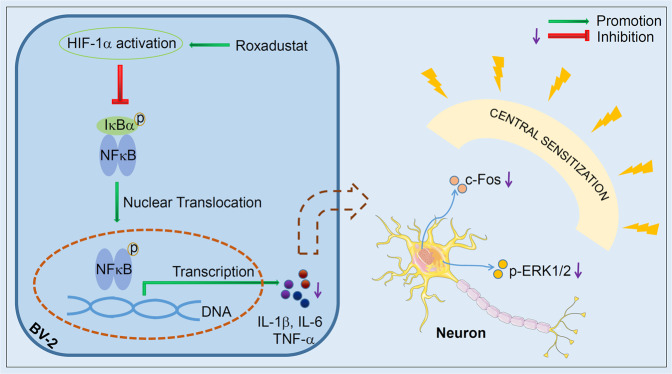
In conclusion, we demonstrated that the HIF-1α stabilizer roxadustat can protect against NTG-induced migraine in both the prevention and acute treatment strategies. In BV-2 cells, we found that LPS-induced cellular ROS production and inflammatory cytokines expression were inhibited by the stabilization of HIF-1α. The in vivo study showed that roxadustat treatment improved TNC activation by reducing markers of central sensitization and inhibiting inflammation in TNC and serum. Clearly, these data indicate that the anti-inflammatory effect of roxadustat is regulated by HIF-1α/NF-κB pathway (Fig. 8). Taken together, our results suggest that HIF-1α may be an effective therapeutic target for migraine and that roxadustat may be a candidate drug for migraineur.
Fig. 8. Schematic diagram for the role of roxadustat in NTG-induced migraine.
Roxadustat ameliorated NTG-induced migraine by activating HIF-1α, which is mainly mediated by reducing NF-κB/inflammation pathway to attenuate central sensitization markers, p-ERK1/2, and c-Fos. HIF-1α activators might be potential drugs for treatment of migraine.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) Grants 81973316 to JHH, 82173807 to YJD; Tianjin Municipal Science and Technology Commission of China Grant 20JCZDJC00710 and the Fundamental Research Funds for the Central Universities (Nankai University) 63211045 to HJH; the Fund from Tianjin Municipal Health Commission Grant ZC200093 the Open Fund of Tianjin Central Hospital of Gynecology Obstetrics/Tianjin Key Laboratory of human development and reproductive regulation to XDF.
Supporting information
Author contributions
XXY and JHH conceived and designed the study. DGY and YYG, ZQY, XRW, XSM, and TFZ, performed, analyzed, and interpreted studies data. DGY and XXY wrote the paper. XXY, JHH, YJD, YLC, CZL, ZLX, LS and XDF contributed reagents/materials/analysis tools and revised the paper. All authors discussed the data and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Dai-gang Yang, Yong-yao Gao
Contributor Information
Ji-hong Han, Email: jihonghan2008@nankai.edu.cn.
Xiao-xiao Yang, Email: yangxiaoxiao@hfut.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-00941-3.
References
- 1.Al-Hassany L, Haas J, Piccininni M, Kurth T, Maassen Van Den Brink A, Rohmann JL. Giving researchers a headache—sex and gender differences in migraine. Front Neurol. 2020;11:549038. doi: 10.3389/fneur.2020.549038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper W, Doty EG, Hochstetler H, Hake A, Martin V. The current state of acute treatment for migraine in adults in the United States. Postgrad Med. 2020;132:581–9. doi: 10.1080/00325481.2020.1767402. [DOI] [PubMed] [Google Scholar]
- 3.Hranilovich JA, Kaiser EA, Pace A, Barber M, Ziplow J. Headache in transgender and gender-diverse patients: a narrative review. Headache. 2021;61:1040–50. doi: 10.1111/head.14171. [DOI] [PubMed] [Google Scholar]
- 4.Silberstein SD. Migraine. Lancet. 2004;363:381–91. doi: 10.1016/S0140-6736(04)15440-8. [DOI] [PubMed] [Google Scholar]
- 5.Goadsby PJ, Lipton RB, Ferrari MD. Migraine-current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 6.Welch KM, Mathew NT, Stone P, Rosamond W, Saiers J, Gutterman D. Tolerability of sumatriptan: clinical trials and post-marketing experience. Cephalalgia. 2000;20:687–95. doi: 10.1046/j.1468-2982.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 7.Tfelt-Hansen P, Saxena PR, Dahlof C, Pascual J, Lainez M, Henry P, et al. Ergotamine in the acute treatment of migraine: a review and European consensus. Brain. 2000;123:9–18. doi: 10.1093/brain/123.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Al-Hassany L, Goadsby PJ, Danser AHJ, MaassenVanDenBrink A. Calcitonin gene-related peptide-targeting drugs for migraine: how pharmacology might inform treatment decisions. Lancet Neurol. 2022;21:284–94. doi: 10.1016/S1474-4422(21)00409-9. [DOI] [PubMed] [Google Scholar]
- 9.Scott LJ. Rimegepant: first approval. Drugs. 2020;80:741–6. doi: 10.1007/s40265-020-01301-3. [DOI] [PubMed] [Google Scholar]
- 10.Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltrán E, et al. Blocking CGRP in migraine patients—a review of pros and cons. J Headache Pain. 2017;18:96. doi: 10.1186/s10194-017-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Yu C, Wang L, Zhang F, Wang K, Huang J, et al. Development and validation of a reporter gene assay for bioactivity determination of anti-CGRP monoclonal antibodies. Anal Biochem. 2021;634:114291. doi: 10.1016/j.ab.2021.114291. [DOI] [PubMed] [Google Scholar]
- 12.Gaul C, Messlinger K, Holle-Lee D, Neeb L. Pathophysiology of headaches. Dtsch Med Wochenschr. 2017;142:402–8. doi: 10.1055/s-0042-111694. [DOI] [PubMed] [Google Scholar]
- 13.He W, Long T, Pan Q, Zhang S, Zhang Y, Zhang D, et al. Microglial NLRP3 inflammasome activation mediates IL-1beta release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model. J Neuroinflammation. 2019;16:78. doi: 10.1186/s12974-019-1459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen N, Zhang J, Wang P, Guo J, Zhou M, He L. Functional alterations of pain processing pathway in migraine patients with cutaneous allodynia. Pain Med. 2015;16:1211–20. doi: 10.1111/pme.12690. [DOI] [PubMed] [Google Scholar]
- 15.Lovati C, Giani L, Castoldi D, Mariotti D’Alessandro C, DeAngeli F, Capiluppi E, et al. Osmophobia in allodynic migraineurs: cause or consequence of central sensitization? Neurol Sci. 2015;36(Suppl 1):145–7. doi: 10.1007/s10072-015-2141-1. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–58. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Tommaso M, Guido M, Libro G, Losito L, Sciruicchio V, Monetti C, et al. Abnormal brain processing of cutaneous pain in migraine patients during the attack. Neurosci Lett. 2002;333:29–32. doi: 10.1016/S0304-3940(02)00967-9. [DOI] [PubMed] [Google Scholar]
- 18.Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–7. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borkum JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache. 2016;56:12–35. doi: 10.1111/head.12725. [DOI] [PubMed] [Google Scholar]
- 20.Goschorska M, Gutowska I, Baranowska-Bosiacka I, Barczak K, Chlubek D. The use of antioxidants in the treatment of migraine. Antioxidants. 2020;9:116. [DOI] [PMC free article] [PubMed]
- 21.Gong Q, Lin Y, Lu Z, Xiao Z. Microglia-astrocyte cross talk through IL-18/IL-18R signaling modulates migraine-like behavior in experimental models of migraine. Neuroscience. 2020;451:207–15. doi: 10.1016/j.neuroscience.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Erdener SE, Kaya Z, Dalkara T. Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine. J Headache Pain. 2021;22:138. doi: 10.1186/s10194-021-01353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata E, Fujii N, Hosomichi K, Mitsunaga S, Suzuki Y, Mashimo Y, et al. Possible association between dysfunction of vitamin D binding protein (GC Globulin) and migraine attacks. PLoS One. 2014;9:e105319. doi: 10.1371/journal.pone.0105319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martami F, Razeghi Jahromi S, Togha M, Ghorbani Z, Seifishahpar M, Saidpour A. The serum level of inflammatory markers in chronic and episodic migraine: a case-control study. Neurol Sci. 2018;39:1741–9. doi: 10.1007/s10072-018-3493-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, He Q, Ren Z, Li F, Chen W, Lin X, et al. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci. 2015;36:535–40. doi: 10.1007/s10072-014-2010-3. [DOI] [PubMed] [Google Scholar]
- 26.Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, et al. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. Eur Rev Med Pharmacol Sci. 2011;15:1111–6. [PubMed] [Google Scholar]
- 27.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Long G, Chen H, Wu M, Li Y, Gao L, Huang S, et al. Antianemia drug roxadustat (FG-4592) protects against doxorubicin-induced cardiotoxicity by targeting antiapoptotic and antioxidative pathways. Front Pharmacol. 2020;11:1191. doi: 10.3389/fphar.2020.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Yu X, Zhang Y, Ding G, Zhu C, Huang S, et al. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin Sci (Lond) 2018;132:825–38. doi: 10.1042/CS20171625. [DOI] [PubMed] [Google Scholar]
- 30.Kakio S, Funakoshi-Tago M, Kobata K, Tamura H. Coffee induces vascular endothelial growth factor (VEGF) expression in human neuroblastama SH-SY5Y cells. Nutr Neurosci. 2017;20:336–42. doi: 10.1080/1028415X.2015.1133106. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Huo X, Chen H, Li B, Liu J, Ma W, et al. Hydrogen-rich saline activated autophagy via HIF-1α pathways in neuropathic pain model. Biomed Res Int. 2018;2018:4670834. doi: 10.1155/2018/4670834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α) J Comp Neurol. 2012;520:2903–16. doi: 10.1002/cne.23072. [DOI] [PubMed] [Google Scholar]
- 33.Li ZL, Tu Y, Liu BC. Treatment of renal anemia with roxadustat: advantages and achievement. Kidney Dis. 2020;6:65–73. doi: 10.1159/000504850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Zhao M, Cheng X, Zhao T, Feng Z, Zhao Y, et al. FG-4592 improves depressive-like behaviors through HIF-1-mediated neurogenesis and synapse plasticity in rats. Neurotherapeutics. 2020;17:664–75. doi: 10.1007/s13311-019-00807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu K, Zhou K, Wang Y, Zhou Y, Tian N, Wu Y, et al. Stabilization of HIF-1α by FG-4592 promotes functional recovery and neural protection in experimental spinal cord injury. Brain Res. 2016;1632:19–26. doi: 10.1016/j.brainres.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Miao AF, Liang JX, Yao L, Han JL, Zhou LJ. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against renal ischemia/reperfusion injury by inhibiting inflammation. Ren Fail. 2021;43:803–10. doi: 10.1080/0886022X.2021.1915801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han F, Wu G, Han S, Li Z, Jia Y, Bai L, et al. Hypoxia-inducible factor prolyl-hydroxylase inhibitor roxadustat (FG-4592) alleviates sepsis-induced acute lung injury. Respir Physiol Neurobiol. 2020;281:103506. doi: 10.1016/j.resp.2020.103506. [DOI] [PubMed] [Google Scholar]
- 38.Hoppe G, Yoon S, Gopalan B, Savage AR, Brown R, Case K, et al. Comparative systems pharmacology of HIF stabilization in the prevention of retinopathy of prematurity. Proc Natl Acad Sci USA. 2016;113:E2516–2525. doi: 10.1073/pnas.1523005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–74. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh L, Kaur A, Garg S, Bhatti R. Skimmetin/osthole mitigates pain-depression dyad via inhibiting inflammatory and oxidative stress-mediated neurotransmitter dysregulation. Metab Brain Dis. 2021;36:111–21. doi: 10.1007/s11011-020-00604-4. [DOI] [PubMed] [Google Scholar]
- 42.Minett MS, Eijkelkamp N, Wood JN. Significant determinants of mouse pain behaviour. PLoS One. 2014;9:e104458. doi: 10.1371/journal.pone.0104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y, Liu S, Shu H, Xing Y, Tao F. AMPA receptor GluA1 Ser831 phosphorylation is critical for nitroglycerin-induced migraine-like pain. Neuropharmacology. 2018;133:462–9. doi: 10.1016/j.neuropharm.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casili G, Lanza M, Filippone A, Campolo M, Paterniti I, Cuzzocrea S, et al. Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J Neuroinflammation. 2020;17:59. doi: 10.1186/s12974-020-01736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma J, Zhao D, Wang X, Ma C, Feng K, Zhang S, et al. LongShengZhi capsule reduces established atherosclerotic lesions in apoE-deficient mice by ameliorating hepatic lipid metabolism and inhibiting inflammation. J Cardiovasc Pharmacol. 2019;73:105–17. doi: 10.1097/FJC.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 46.Pinskiy V, Tolpygo AS, Jones J, Weber K, Franciotti N, Mitra PP. A low-cost technique to cryo-protect and freeze rodent brains, precisely aligned to stereotaxic coordinates for whole-brain cryosectioning. J Neurosci Methods. 2013;218:206–13. doi: 10.1016/j.jneumeth.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henry DH, Glaspy J, Harrup R, Mittelman M, Zhou A, Carraway HE, et al. Roxadustat for the treatment of anemia in patients with lower-risk myelodysplastic syndrome: Open-label, dose-selection, lead-in stage of a phase 3 study. Am J Hematol. 2022;97:174–84. doi: 10.1002/ajh.26397. [DOI] [PubMed] [Google Scholar]
- 49.Goadsby PJ. Migraine, allodynia, sensitisation and all of that. Eur Neurol. 2005;53(Suppl 1):10–6. doi: 10.1159/000085060. [DOI] [PubMed] [Google Scholar]
- 50.Wilkins AJ, Haigh SM, Mahroo OA, Plant GT. Photophobia in migraine: A symptom cluster? Cephalalgia. 2021;41:1240–8. doi: 10.1177/03331024211014633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugiyo S, Yonehara N, Appenteng K, Nokubi T, Shigenaga Y, Takemura M. Effects of intrathecal c-fos antisense oligodeoxynucleotide on adjuvant-induced thermal hyperalgesia. Exp Brain Res. 2001;140:198–205. doi: 10.1007/s002210100809. [DOI] [PubMed] [Google Scholar]
- 52.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–85. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–9. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 54.Ramachandran R. Neurogenic inflammation and its role in migraine. Semin Immunopathol. 2018;40:301–14. doi: 10.1007/s00281-018-0676-y. [DOI] [PubMed] [Google Scholar]
- 55.Ceylan M, Bayraktutan OF, Becel S, Atis O, Yalcin A, Kotan D. Serum levels of pentraxin-3 and other inflammatory biomarkers in migraine: Association with migraine characteristics. Cephalalgia. 2016;36:518–25. doi: 10.1177/0333102415598757. [DOI] [PubMed] [Google Scholar]
- 56.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–48. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan J, Melemedjian OK, Price TJ, Dussor G. Sensitization of dural afferents underlies migraine-related behavior following meningeal application of interleukin-6 (IL-6) Mol Pain. 2012;8:6. doi: 10.1186/1744-8069-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao G, Man YH, Li AR, Guo Y, Dai Y, Wang P, et al. NO up-regulates migraine-related CGRP via activation of an Akt/GSK-3beta/NF-kappaB signaling cascade in trigeminal ganglion neurons. Aging. 2020;12:6370–84. doi: 10.18632/aging.103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai T, Chen L, Chen X, He J, Lv P, Ge H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-small ka, CyrillicB signaling. Mol Cell Biochem. 2019;461:205–12. doi: 10.1007/s11010-019-03603-x. [DOI] [PubMed] [Google Scholar]
- 60.Liu XL, Lu J, Xing J. Stabilization of HIF-1alpha modulates VEGF and caspase-3 in the hippocampus of rats following transient global ischemia induced by asphyxial cardiac arrest. Life Sci. 2016;151:243–9. doi: 10.1016/j.lfs.2016.03.005. [DOI] [PubMed] [Google Scholar]