Abstract
The role of the alternative sigma factor ςB in Staphylococcus epidermidis was investigated by the construction, complementation, and characterization of a sigB deletion mutant. Electrophoretic analyses confirmed a profound influence of ςB on the expression of exoproteins and cytoplasmic proteins. Detailed investigation revealed reduced lipase and enhanced protease activity in the ςB mutant. Furthermore, no significant influence of ςB on heterologous biofilm formation or on the activity of the global regulator agr was detected.
In several bacteria, various alternative sigma factors modulate gene expression in response to environmental and metabolic signals (12). Based on the information available for the Staphylococcus aureus genome, staphylococci appear to possess only one alternative sigma factor, ςB. Therefore, ςB is presumed to play a crucial role in global regulation. Expression of virulence factors in S. aureus has been shown to depend on ςB (17) and on at least two global regulatory systems: agr (accessory gene regulator) (20) and sar (staphylococcal accessory regulator) (4). Since sar is responsible for agr activation and ςB influences SarA expression (5), ςB represents the superior global regulator in the S. aureus regulatory network controlling exoprotein expression.
Staphylococcus epidermidis ranks among the most important nosocomial pathogens, mainly because of its ability to colonize indwelling medical devices by forming a biofilm (9, 10, 29). In addition, many antibiotics lose their effectiveness against S. epidermidis in the biofilm environment because of the impenetrable slime capsule (6, 28). A further factor contributing to the severe threat of S. epidermidis to public health is the occurrence of multiresistant and vancomycin-resistant strains (24). Virtually nothing is known about the regulation of virulence factors in S. epidermidis. An agr deletion mutant has recently been constructed and characterized by our group (30), but no other global regulator has been studied so far.
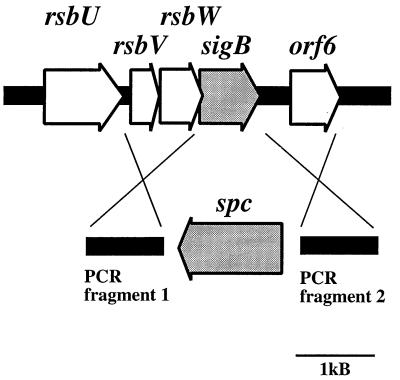
A region of the chromosome of S. epidermidis Tü3298 comprising four open reading frames with strong sequence similarities to the S. aureus sigB operon was sequenced by direct chromosomal sequencing (GenBank accession number AF359562), starting with primer S1 (CACGAAGATTTAGTTCAAGTTGGTATGGTGG), derived from the S. aureus sequence. The striking similarity in both sequence and overall genetic organization suggested that this region is the sigB operon of S. epidermidis (Fig. 1).
FIG. 1.
Physical map of the sigB operon of S. epidermidis and construction of a sigB deletion mutant using plasmid pBTΔsigB. Arrows depict open reading frames and indicate their orientation and size. The sigB gene was replaced with the spectinomycin resistance gene (spc) as shown in the lower part of the figure. The spc gene and two PCR-amplified regions flanking sigB were cloned into plasmid pBT2, yielding integration vector pBTΔsigB. The crosses indicate the sites of homologous recombination.
In order to analyze its function in S. epidermidis, sigB was replaced by a spectinomycin resistance gene (spc) from Tn554 (19) by homologous recombination (Fig. 1). Since the rsb genes are tightly clustered and rsbW and sigB overlap, we decided not to replace the first bases of sigB, leaving the anti-sigma factor rsbW intact. Fragments of about 870 bp upstream and downstream of sigB were amplified using primer pairs SigEcoRI and SigBamHI (GAT TAAAG TGAAT TCATG TAGGG TATAGG and CAGGTGATGGATCCCTAGCTGAT TTCGAC) for fragment 1 and SigSphI and SigHindIII (GCTGCATGCCAGTAAACGAGT TGTTAAC and GAGGAAAAGCTTAGTCCCTGATTAAAAACATC) for fragment 2. The fragments were cloned into the polylinker region of plasmid pBT2 (2), flanking the spectinomycin resistance gene. The resulting plasmid, pBTΔsigB, was introduced into S. epidermidis Tü3298 by electroporation (1). Allelic replacement of the wild-type sigB gene by spc was carried out as previously described (2). The correct insertion of the antibiotic resistance marker in the resulting strain, S. epidermidis TüΔsigB, was confirmed by Southern blot analysis and chromosomal sequencing.
To investigate the influence of ςB on protein expression, protein samples from stationary phase (16 h) cultures were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein profiles of the ςB mutant S. epidermidis TüΔsigB and the wild-type strain S. epidermidis Tü3298 were compared with the complemented mutant S. epidermidis TüΔsigB(pTXsigB) and S. epidermidis Tü(pTXsigB), which overexpresses sigB. To generate plasmid pTXsigB, the sigB gene of S. epidermidis Tü3298 was amplified by PCR with primers SigUBamHI (GATTAAGGATCCAAAAAAAGAGCAGGTGCG) and SigUMluI (CTCTGTTAACAACGCGTTTACTGTCTTGCAGC) and cloned in plasmid pTX15 (23, 31). S. epidermidis TüΔsigB(pTX16) and S. epidermidis Tü(pTX16) served as control strains to show that complementation and overexpression effects are not caused by the pTX plasmid. Plasmid pTX16 (23, 31) is used as a negative control in pTX15 expression studies, because cloning in vector pTX15 deletes a lipase gene which had also been deleted in pTX16.
Strains were cultivated in B-medium (1% peptone [Difco], 0.5% yeast extract [Gibco-BRL], 0.5% NaCl, 0.1% K2HPO4, 0.1% glucose). Vectors pTX15 and pTX16 allow xylose-inducible and glucose-repressible gene expression (23, 31), and therefore strains containing these plasmids were grown in modified B-medium containing 0.5% xylose and lacking glucose. Cultures were adjusted to the same cell density and grown for 16 h. Exoprotein samples were precipitated with 1/9 volume of trichloracetic acid, and cytoplasmic and membrane fractions were prepared as described previously (22). The growth rates of all the strains tested were the same.
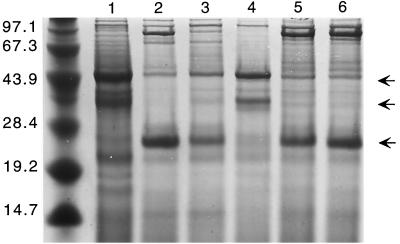
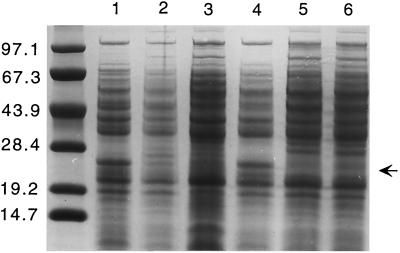
Deletion of sigB in S. epidermidis resulted in a pleiotropic alteration of the protein pattern of exoproteins (Fig. 2) and cytoplasmic proteins (Fig. 3), while the composition of membrane proteins was not altered significantly (data not shown). The most striking differences in the ςB -mutant samples containing exoproteins were the absence of an approximately 27-kDa protein and clearly more pronounced 44-kDa and 38-kDa proteins. The profiles of cytoplasmic proteins showed a distinct change in the pattern, especially in the range from about 25 to 30 kDa. Most remarkable in this respect is a protein of about 27 kDa, which is present in significantly higher amounts in the mutant strain. Comparison of protein profiles show that the ςB -minus phenotype was complemented by plasmid pTXsigB; in contrast, the wild-type strain bearing the same plasmid did not show a protein pattern different from that of the wild type. Our studies suggest a crucial role for ςB in S. epidermidis gene regulation. Since the most striking differences in the protein patterns were seen in the exoprotein fractions, we decided to analyze exoprotein expression in more detail.
FIG. 2.
Influence of S. epidermidis ςB on production of exoproteins. Protein profiles of 16-h cultures of the following strains are shown: S. epidermidis sigB deletion mutant S. epidermidis Tü3298ΔsigB (lane 1), S. epidermidis wild-type Tü3298 (lane 2), the complemented mutant S. epidermidis TüΔsigB(pTXsigB) (lane 3), the control strain S. epidermidis TüΔsigB(pTX16) (lane 4), the ςB-overexpressing strain S. epidermidis Tü(pTXsigB) (lane 5), and its corresponding control strain S. epidermidis Tü(pTX16) (lane 6). The proteins were separated on SDS–10% polyacrylamide gels and stained with Coomassie brilliant blue G250. The arrows mark the positions of 27-kDa, 38-kDa, and 44-kDa proteins present or absent in the different strains. The molecular masses (in kilodaltons) of size standard proteins are shown on the left.
FIG. 3.
Influence of S. epidermidis ςB on production of cytoplasmic proteins. Protein profiles of 16-h cultures of the following strains are shown: S. epidermidis sigB deletion mutant S. epidermidis Tü3298ΔsigB (lane 1), S. epidermidis wild-type Tü3298 (lane 2), the complemented mutant S. epidermidis TüΔsigB(pTXsigB) (lane 3), the control strain S. epidermidis TüΔsigB(pTX16) (lane 4), the ςB-overexpressing strain S. epidermidis Tü(pTXsigB) (lane 5), and its corresponding control strain S. epidermidis Tü(pTX16) (lane 6). The proteins were separated on SDS–10% polyacrylamide gels and stained with Coomassie brilliant blue G250. The arrow indicates the position of a 27-kDa protein present or absent in the different strains. The molecular masses (in kilodaltons) of size standard proteins are shown on the left.
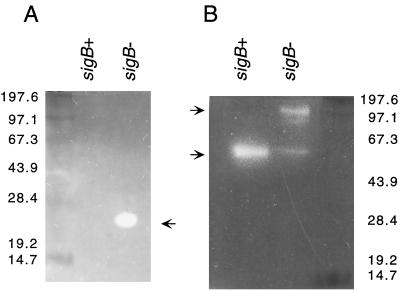
Lipases and proteases are known to be virulence factors in S. aureus (8, 11) and may also contribute to the pathogenicity of S. epidermidis. Therefore, lipase and protease activity in the supernatant of the S. epidermidis wild-type and ΔsigB mutant strains was analyzed by zymography (Fig. 4). Exoprotein samples were separated on nondenaturing SDS-polyacrylamide gels and incubated on agarose test plates containing 1% Tween (for lipase detection) or 1% casein according to the method of Hammersten (Amersham Pharmacia Biotech, Freiburg, Germany) for protease detection as described previously (30). Staphylococcal lipases are organized as preproenzymes and are secreted in the prolipase form (11, 27). Lipase test plates revealed a single lipolytic band at about 50 kDa in the exoprotein sample from the S. epidermidis wild-type strain, which was less pronounced in samples from the ςB mutant strain. A second lipolytic band of about 100 kDa was also present in samples from the mutant strain; the size corresponds to that of the proform of staphylococcal lipase (11, 27). Thus, the processing of this lipase shows pronounced ςB dependence. In contrast, lipase production was enhanced in three different S. aureus ςB mutants compared to their isogenic wild-type strains (17). These data indicate that the involvement of ςB in lipase gene expression may vary among staphylococcal species. Protease test plates of the ςB mutant strain showed a distinct proteolytic band at about 25 kDa which was not detectable in the wild-type strain. For strain S. epidermidis Tü3298, an extracellular protease with a corresponding molecular mass has not been reported so far. The results indicate that the expression or secretion of this novel protease is repressed by ςB.
FIG. 4.
Zymographic analysis of protease (A) and lipase (B) activity. Exoprotein samples of strains S. epidermidis Tü3298 (wild-type; sigB+) and S. epidermidis TüΔsigB (sigB deletion mutant; sigB−) were separated on SDS–10% polyacrylamide gels. Zymographic analysis of the gels was carried out on protease or lipase agarose test plates. Arrows indicate the positions of proteolytic or lipolytic bands. The molecular masses (in kilodaltons) of size standard proteins are shown on the left (A) and right (B).
In S. aureus and Bacillus subtilis, ςB modulates expression of several stress and starvation proteins. We carried out heat shock (54°C, 10 min) and salt stress (2 M) studies in B-medium. The wild-type strain and the ςB mutant had similar growth patterns after stress exposure (data not shown). In B. subtilis, the regulatory factor RsbU is essential for full ςB activity under stress conditions but is not required for activation of ςB during the stationary phase (13). The occurrence of natural rsbU mutants of S. aureus harboring an 11-bp deletion has been reported (3, 16, 17), but based on sequence comparisons, rsbU appears to be intact in S. epidermidis Tü3298. Since the signal transduction pathway influencing RsbU expression in bacilli and in staphylococci is not known, we speculate that S. epidermidis Tü3298 might contain a mutation in this pathway, leading to the nonresponsiveness of ςB expression to environmental stress.
In S. epidermidis, biofilm formation and cell aggregation are dependent on the production of the polysaccharide intercellular adhesin (PIA) (18), which is synthesized by the gene products of the ica gene locus. Strain S. epidermidis Tü3298 used in this study lacks the ica genes. This was proven by PCR, Southern blot analysis, and direct chromosomal sequencing (data not shown). Plasmid pCN27 containing the S. epidermidis RP62A ica genes has been shown to lead to the formation of cell clusters and PIA expression in the heterologous host Staphylococcus carnosus (14).
To investigate the impact of ςB in biofilm formation, plasmid pCN27 was introduced into S. epidermidis Tü3298 and S. epidermidis TüΔsigB. Light microscopy analysis of 16-h cultures of the two strains showed formation of large cell clusters but did not reveal any significant differences in cell aggregation between the two strains. The same result was obtained with cultures that had been exposed to heat or salt stress before growth (data not shown). External stress has been demonstrated to induce biofilm formation in S. aureus (25) and S. epidermidis (26), and the response was shown to depend on functional RsbU (15). The nonresponsiveness of cell aggregation in strain S. epidermidis Tü3298 to the presence or absence of ςB might therefore be explained by a nonfunctional RsbU-mediated signal transduction pathway. Furthermore, as S. epidermidis Tü3298 lacks the ica genes, regulatory elements needed for the control of ica gene expression might also be missing.
To understand a putative regulatory cascade of global regulators in S. epidermidis, we elucidated the role of ςB in agr expression. Staphylococcal delta toxin is encoded within the gene coding for RNAIII, the effector molecule of the agr regulation system. Therefore, quantitative analysis of delta toxin allows the measurement of agr activity in different strains. The delta toxin concentration was directly detected by analytical high-pressure liquid chromatography is the supernatant of S. epidermidis Tü3298 and S. epidermidis Tü3298ΔsigB, as described previously (21). Delta toxin production was measured in five independent stationary-phase cultures of each strain. The amount of delta toxin in the two strains was not significantly different (wild-type strain, 7.6 μg/ml; ςB deletion mutant, 7.0 μg/ml), indicating no direct influence of ςB on agr activity.
Given that ςB regulates sar activity, as has been shown for S. aureus, we cannot rule out that at least some of the alterations reported in this study are caused by reduced transcription of the sar regulatory locus. To understand the regulation of virulence genes in S. epidermidis, it will be important to distinguish the Sar and ςB target genes and to test the virulence of a ςB mutant in an animal model.
Acknowledgments
We thank Reinhold Brückner for helpful discussions, Vera Augsburger and Detlinde Futter-Bryniok for technical assistance, Ulrike Pfitzner for photography, and Karen A. Brune for editing the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (60371/3-1).
REFERENCES
- 1.Augustin J, Götz F. Transformation of Staphylococcus epidermidis and other staphylococcal species with plasmid DNA by electroporation. FEMS Microbiol Lett. 1990;54:203–207. doi: 10.1016/0378-1097(90)90283-v. [DOI] [PubMed] [Google Scholar]
- 2.Brückner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. doi: 10.1111/j.1574-6968.1997.tb10387.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan P F, Foster S J, Ingham E, Clements M O. The Staphylococcus aureus alternative sigma factor ςB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deora R, Tseng T, Misra T K. Alternative transcription factor ςSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol. 1997;179:6355–6359. doi: 10.1128/jb.179.20.6355-6359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber B F, Kaplan M H, Clogston A G. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990;161:37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Gertz S, Engelmann S, Schmid R, Ziebandt A K, Tischer K, Scharf C, Hacker J, Hecker M. Characterization of the ςB regulon in Staphylococcus aureus. J Bacteriol. 2000;182:6983–6991. doi: 10.1128/jb.182.24.6983-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goguen J D, Hoe N P, Subrahmanyam Y V. Proteases and bacterial virulence: a view from the trenches. Infect Agents Dis. 1995;4:47–54. [PubMed] [Google Scholar]
- 9.Götz F, Heilmann C, Cramton S E. Molecular basis of catheter associated infections by staphylococci. London, England: Kluwer Academic Plenum Publishers; 2000. [DOI] [PubMed] [Google Scholar]
- 10.Götz F, Peters G. Colonization of medical devices by coagulase-negative staphylococci. 3rd ed. Washington, D.C.: ASM Press; 2000. [Google Scholar]
- 11.Götz F, Verheij H M, Rosenstein R. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem Phys Lipids. 1998;93:15–25. doi: 10.1016/s0009-3084(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 12.Hecker M, Völker U. General stress response of Bacillus subtilis and other bacteria. Adv Microb Physiol. 2001;44:35–91. doi: 10.1016/s0065-2911(01)44011-2. [DOI] [PubMed] [Google Scholar]
- 13.Hecker M, Völker U. Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the sigmaB regulon. Mol Microbiol. 1998;29:1129–1136. doi: 10.1046/j.1365-2958.1998.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 15.Knobloch J K, Bartscht K, Sabottke A, Rohde H, Feucht H H, Mack D. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J Bacteriol. 2001;183:2624–2633. doi: 10.1128/JB.183.8.2624-2633.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullik I, Giachino P. The alternative sigma factor sigmaB in Staphylococcus aureus regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 17.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy E, Huwyler L, de Freire Bastos M D C. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 1985;4:3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeb S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto M, Götz F. Analysis of quorum sensing activity in staphylococci by RP-HPLC of staphylococcal delta-toxin. Biotechniques. 2000;28:1088. doi: 10.2144/00286bm07. , 1090, 1092, 1096. [DOI] [PubMed] [Google Scholar]
- 22.Peschel A, Götz F. Analysis of the Staphylococcus epidermidis genes epiF, -E, and -G involved in epidermin immunity. J Bacteriol. 1996;178:531–536. doi: 10.1128/jb.178.2.531-536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peschel A, Ottenwälder B, Götz F. Inducible production and cellular location of the epidermin biosynthetic enzyme EpiB using an improved staphylococcal expression system. FEMS Microbiol Lett. 1996;137:279–284. doi: 10.1111/j.1574-6968.1996.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 24.Raad I, Alrahwan A, Rolston K. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin Infect Dis. 1998;26:1182–1187. doi: 10.1086/520285. [DOI] [PubMed] [Google Scholar]
- 25.Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. Alternative transcription factor ς(B) is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol. 2000;182:6824–6826. doi: 10.1128/jb.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:3357–3363. doi: 10.1128/aac.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenstein R, Götz F. Staphylococcal lipases: biochemical and molecular characterization. Biochimie. 2000;82:1005–1014. doi: 10.1016/s0300-9084(00)01180-9. [DOI] [PubMed] [Google Scholar]
- 28.Slack M P, Nichols W W. Antibiotic penetration through bacterial capsules and exopolysaccharides. J Antimicrob Chemother. 1982;10:368–372. doi: 10.1093/jac/10.5.368. [DOI] [PubMed] [Google Scholar]
- 29.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18:843–846. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 30.Vuong C, Götz F, Otto M. Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis. Infect Immun. 2000;68:1048–1053. doi: 10.1128/iai.68.3.1048-1053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wieland K P, Wieland B, Götz F. A promoter-screening plasmid and xylose-inducible, glucose-repressible expression vectors for Staphylococcus carnosus. Gene. 1995;158:91–96. doi: 10.1016/0378-1119(95)00137-u. [DOI] [PubMed] [Google Scholar]