Abstract
Aim
Both the clinical manifestation and molecular characteristics of colorectal cancer (CRC) vary according to the anatomical site. We explored the risk factors for four groups of colorectal neoplasms (CRN) at different anatomical sites.
Methods
We extracted data from the database of Tianjin Colorectal Cancer Screening Program from 2010 to 2020. According to the CRN anatomical sites, patients were divided into four groups: the proximal colon group, the distal colon group, the rectum group, and the multiple colorectal sites. Binary logistic regression analysis was used to explore the differences in risk factors of CRN at different anatomical sites.
Results
The numbers of patients with CRN in the proximal colon, distal colon, rectum, and multiple colorectal sites were 4023, 6920, 3657, and 7938, respectively. Male sex was associated with a higher risk from the proximal colon to the rectum. Advanced age and obesity were also significantly associated with overall colorectal CRN risk, but there were some differences between men and women. Smoking was associated with CRN risk only in the distal colon and rectum in both men and women. Frequent alcohol consumption and family history of CRC in first-degree relatives (FDRs) were associated with the risk of multisite colorectal CRN only in males.
Conclusions
We observed differences in advanced age, obesity, smoking, alcohol consumption, and family history of colorectal cancer at different anatomical sites of colorectal neoplasms. These factors vary by gender.
Keywords: Colorectal neoplasms, Different anatomical sites, Risk factors, Colorectal adenoma, Colorectal cancer
Introduction
Colorectal cancer is the third most common cancer in the world and the second most common cancer in the world [1]. According to the latest statistics in 2022, colorectal cancer is the second most common cancer and the fifth leading cause of cancer death in China [2]. Adenoma is considered a precancerous lesion[3]. Most colorectal cancers are transformed from preexisting adenomas over many years. The adenoma-carcinoma sequence may involve the activation of multiple oncogenes by mutation and the inactivation of multiple tumor suppressor genes [4], a progressive progression from normal tissue to abnormal tissue to cancer [5]. Therefore, early screening for colorectal cancer can prevent and diagnose colorectal cancer. High-risk adenomas can be detected and removed by colonoscopy, thereby reducing the incidence and mortality of colorectal cancer [6].
Embryologic development from the proximal and distal colon differs in origin, with the portion from the cecum to the proximal two-thirds of the transverse colon originating in the midgut and the portion from the distal transverse colon to the rectum originating in the hindgut [7]. In 1990, Bufill proposed that proximal colon cancer and distal colon cancer were two distinct tumors and proved this from a molecular genetic point of view [8]. Later, some scholars explored the risk factors for colorectal cancer in different locations [9, 10]. However, there are few studies to analyze the risk factors of colorectal neoplasms (CRN) (colorectal adenoma and cancer) at different sites. Approximately 5% of adenomas become malignant within 5 to 10 years [4], so adenoma is also a dangerous disease. Our screening program was designed to detect CRN in a timely manner, so as to intervene early in the transformation of adenoma to cancer or make preventive recommendations. Therefore we will comprehensively study the difference in risk factors between colorectal adenoma and colorectal cancer in different sites.
In this study, we divided colorectal neoplasms into the proximal colon group, distal colon group, rectal group, and multiple colorectal site groups (whole colon or colorectal) according to their anatomical sites. Exploring the risk factors of colorectal neoplasms in different anatomical sites is of great significance for colorectal cancer screening, which can guide people to prevent the occurrence of colorectal neoplasms by changing their lifestyle, and ultimately plays a role in preventing colorectal cancer and reducing the incidence and mortality of colorectal cancer.
Materials and methods
Data source
Since 2010, the Tianjin municipal government has organized and commissioned the Tianjin Union Medical Center and other community hospitals to conduct colorectal cancer screening. Participants completed a questionnaire for colorectal cancer risk factors or underwent a stool immunochemical test, and those who were positive for either were subjected to colonoscopy. The population in this study was part of the colorectal Cancer Screening Program in Tianjin. All participants signed an informed consent form. This study was approved by the Ethics Committee of Tianjin Union Medical Center. This trial was conducted following the Declaration of Helsinki guidelines.
Colonoscopy procedures and definition of CRN
All colonoscopies are performed by experienced physicians at the hospital who have undergone formal training, rigorous examination, and certification. Good bowel preparation was performed for each colonoscopy, and a clear photographic record was maintained for adequate examination time. All pathological findings were compared and reported by a physician specializing in pathology against the latest guidelines. Colonic pathologic findings included colorectal cancer, advanced adenomas (≥ 1.0 cm in diameter or with villous components or pathological findings of high-grade intraepithelial neoplasia), non-advanced adenoma, ulcerative colitis, Crohn’s disease, benign tumor, and non-adenomatous polyps. CRN include carcinoma, advanced adenoma, and non-advanced adenoma.
Select study participants
We first screened participants who were positive on the high-risk questionnaire or fecal immunochemical test. Those who meet any or more of the following criteria are considered to be at high risk, and those at high risk are advised to undergo colonoscopy to confirm the diagnosis: (1) first-degree relatives with colorectal cancer; (2) personal history of malignant tumors or intestinal polyps; (3) patients with two or more of the following: (a) chronic constipation, (b) chronic diarrhea, (c) mucinous stool or bloody stool, (d) history of adverse life events, (e) history of chronic appendicitis or appendectomy, and (f) history of chronic cholecystitis or cholecystectomy; and (4) positive fecal occult blood test.
Participants with successful colonoscopy and complete pathological reports were included, and benign tumors, ulcerative colitis, Crohn’s disease, and non-adenomatous polyps were excluded. Participants with incomplete questionnaires were also excluded.
Classification of anatomical sites of CRN
According to the site of CRN, we divided the participants into four groups: proximal colon group, distal colon group, rectum group, and multisite colorectal site group. The proximal colon group included the cecum, ascending colon, colonic liver curvature, and transverse colon. The distal colon group included the splenic curvature, descending colon, and sigmoid colon. The rectum group included the rectosigmoid junction. Whole colon or multiple colorectal groups were set to explore which exposure factors promote the development of adenomas in multiple anatomical sites within the colorectum.
Assessment of exposure factors
We collated data from the questionnaire, including sex, age, lesion site, pathology, smoking status, frequency of alcohol consumption, body mass index (BMI), and history of colorectal cancer in first-degree relatives (FDRs). Age was divided into four groups (40–50, 51–60, 61–70, and older than 70). Smoking status was divided into two groups (never, present, or past). Drinking frequency was divided into two groups (never or occasionally, weekly, or daily). According to the criteria of the Asian population recommended by the WHO, the critical point of BMI for overweight and obesity is defined as 23 kg/m2 and 27.5 kg/m2, respectively [11].
Statistical analysis
All statistical analyses were performed using SPSS software version 26.00. The number and proportion of patients with CRN features at each site were calculated. The independent risk factors of colorectal adenoma in different anatomical sites were analyzed by dichotomous logistic regression. Univariate analysis and multivariate analysis were performed, and P < 0.05 was statistically significant by two-sided test. The odds ratio (OR) and 95% confidence interval (95% CI) of each variable were calculated.
Results
Characteristics of participants
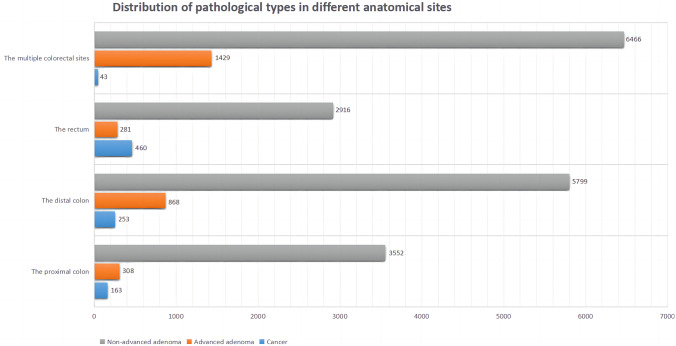
A total of 41,352 participants were enrolled in this study, of whom 22,538 (54.5%) were diagnosed with CRN, including 919 (2.2%) with CRC, 18,733 (45.3%) with non-advanced adenoma, and 2866 (6.9%) with advanced adenoma. Among all patients diagnosed with CRN, 9945 (44.1%) were females and 12,593 (55.9%) were males. There were 4023 cases (17.8%) in the proximal colon group, 6920 cases (30.7%) in the distal colon group, 3657 cases (16.2%) in the rectum group, and 7938 cases (35.2%) in the whole colon or colorectal group. Table 1 shows the basic characteristics of all participants. Figure 1 shows the distribution of pathological types of CRN in different sites. The percentages of non-advanced adenoma, advanced adenoma, and cancer in each group were as follows: proximal colon group (88.3%, 7.7%, 4.1%), distal colon group (83.8%, 12.5%, 3.7%), rectum group (79.7%, 7.7%, 12.6%), and multi-site colorectal group (81.5%, 18.0%, 0.5%).
Table 1.
Characteristics of the 41,352 participants
| Variables | Participants without CRN | Participants with CRN | |||
|---|---|---|---|---|---|
| The proximal colon | The distal colon | The rectum | The multiple colorectal sites | ||
| (n = 18,814) | (n = 4023) | (n = 6920) | (n = 3657) | (n = 7938) | |
| Sex (%) | |||||
| Female | 11,669 (62.0) | 2038 (50.7) | 3120 (45.1) | 1800 (49.2) | 2987 (37.6) |
| Male | 7145 (38.0) | 1985 (49.3) | 3800 (54.9) | 1857 (50.8) | 4951 (62.4) |
| Age (years) (%) | |||||
| 40–50 | 1943 (10.3) | 230 (5.7) | 436 (6.3) | 236 (6.5) | 276 (3.5) |
| 51–60 | 5813 (30.9) | 1170 (29.1) | 2123 (30.7) | 904 (24.7) | 1506 (19.0) |
| 61–70 | 8400 (44.6) | 1975 (49.1) | 3372 (48.7) | 1818 (49..7) | 4247 (53.5) |
| Greater than 70 | 2658 (14.1) | 648 (16.1) | 989 (14.3) | 699 (19.1) | 1909 (24.0) |
| BMI (kg/m2) (%) | |||||
| Less than 23 | 6013 (32.0) | 1087 (27.0) | 1899 (27.4) | 1041 (28.5) | 1904 (24.0) |
| 23–27.5 | 9714 (51.6) | 2177 (54.1) | 3672 (53.1) | 1970 (53.9) | 4260 (53.7) |
| Greater than 27.5 | 3087 (16.4) | 759 (18.9) | 1349 (19.5) | 646 (17.7) | 1774 (22.3) |
| Smoking status (%) | |||||
| Never | 15,541 (82.6) | 3189 (79.3) | 4960 (71.7) | 2736 (74.8) | 5347 (67.4) |
| Present or past | 3273 (17.4) | 834 (20.7) | 1960 (28.3) | 921 (25.2) | 2591 (32.6) |
| Alcohol intake (%) | |||||
| Never or occasional | 17,424 (92.6) | 3628 (90.2) | 6063 (87.6) | 3248 (88.8) | 6670 (84.0) |
| Weekly or daily | 1390 (7.4) | 395 (9.8) | 857 (12.4) | 409 (11.2) | 1268 (16.0) |
| Family history of CRC (%) | |||||
| No | 17,331 (92.1) | 3668 (91.2) | 6370 (92.1) | 3368 (92.1) | 7270 (91.6) |
| Yes | 1483 (7.9) | 355 (8.8) | 550 (7.9) | 289 (7.9) | 668 (8.4) |
| Regular exercise (%) | |||||
| Yes | 7885 (41.8) | 1795 (44.6) | 2993 (43.3) | 1660 (45.4) | 3880 (48.9) |
| No | 10,949 (58.2) | 2228 (55.4) | 3927 (56.7) | 1997 (54.6) | 4058 (51.1) |
| History of chronic diarrhea (%) | |||||
| No | 15,065 (80.1) | 3338 (83.0) | 5769 (83.4) | 2955 (80.8) | 6610 (83.3) |
| Yes | 3749 (19.9) | 685( 17.0) | 1151 (16.6) | 702 (19.2) | 1328 (16.7) |
| History of chronic constipation (%) | |||||
| No | 14,855 (79.0) | 3266 (81.2) | 5718 (82.6) | 3004 (82.1) | 6578 (82.9) |
| Yes | 3959 (21.0) | 757 (18.8) | 1202 (17.4) | 653 (17.9) | 1360 (17.0) |
| History of mucous and bloody stool (%) | |||||
| No | 16,199 (86.1) | 3514 (87.3) | 6068 (87.7) | 3110 (85.0) | 6983 (88.0) |
| Yes | 2615 (13.9) | 509 (12.7) | 852 (12.3) | 547 (15.0) | 955 (12.0) |
Family history of colorectal cancer was defined only in first-degree relatives
*CRN colorectal neoplasms, CRC colorectal cancer, BMI body mass index
Fig. 1.
Distribution of carcinoma, advanced adenoma, and non-advanced adenoma in different groups
Univariate analysis and multivariate analysis
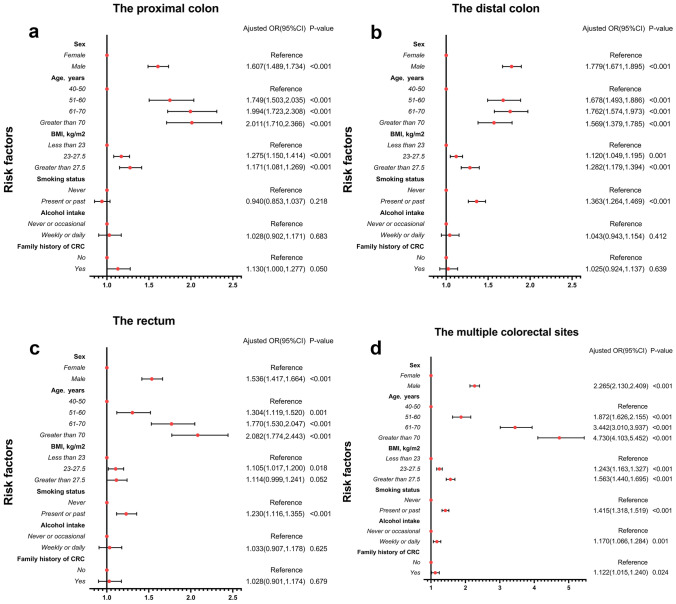
Table 2 shows the results of univariate logistic regression analysis. Male sex, advanced age, overweight or obesity, smoking in the past or present, and drinking multiple times per week were risk factors significantly associated with CRN in the proximal colon, distal colon, rectum, and multiple sites of colorectum. A history of colorectal cancer in a first-degree relative was only a risk factor for proximal colon CRN. Figure 2 shows the results of multivariate logistic regression analysis, and Table 3 shows the differences between men and women after stratification:
Table 2.
Univariate logistic regression analysis was used to analyze the risk factors of CRN at different anatomical sites
| Risk factors | The proximal colon | The distal colon | The rectum | The multiple colorectal sites | |||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P value | Unadjusted OR (95% CI) | P value | Unadjusted OR (95% CI) | P value | Unadjusted OR (95% CI) | P value | ||
| Sex | |||||||||
| Female | Reference | Reference | Reference | Reference | |||||
| Male | 1.591 (1.485, 1.7032) | < 0.001 | 1.989 (1.881, 2.103) | < 0.001 | 1.685 (1.569, 1.809) | < 0.001 | 2.707 (2.564, 2.858) | < 0.001 | |
| Age (years) | |||||||||
| 40–50 | Reference | Reference | Reference | Reference | |||||
| 51–60 | 1.700 (1.463, 1.976) | < 0.001 | 1.628 (1.451, 1.826) | < 0.001 | 1.280 (1.100, 1.491) | 0.001 | 1.824 (1.588, 2.094) | < 0.001 | |
| 61–70 | 1.986 (1.718, 2.297) | < 0.001 | 1.789 (1.601, 2.000) | < 0.001 | 1.782 (1.542, 2.058) | < 0.001 | 3.559 (3.121, 4.059) | < 0.001 | |
| Greater than 70 | 2.060 (1.753, 2.420) | < 0.001 | 1.658 (1.460, 1.883) | < 0.001 | 2.165 (1.847, 2.538) | < 0.001 | 5.056 (4.399, 5.811) | < 0.001 | |
| BMI (kg/m2) | |||||||||
| Less than 23 | Reference | Reference | Reference | Reference | |||||
| 23–27.5 | 1.240 (1.145, 1.342) | < 0.001 | 1.197 (1.123, 1.276) | < 0.001 | 1.171 (1.080, 1.271) | < 0.001 | 1.385 (1.301, 1.475) | < 0.001 | |
| Greater than 27.5 | 1.360 (1.228, 1.507) | < 0.001 | 1.384 (1.275, 1.502) | < 0.001 | 1.209 (1.086, 1.346) | 0.001 | 1.815 (1.679, 1.962) | < 0.001 | |
| Smoking status | |||||||||
| Never | Reference | Reference | Reference | Reference | |||||
| Present or past | 1.242 (1.141, 1.352) | < 0.001 | 1.8776 (1.759, 2.001) | < 0.001 | 1.598 (1.470, 1.738) | < 0.001 | 2.301 (2.166, 2.444) | < 0.001 | |
| Alcohol intake | |||||||||
| Never or occasional | Reference | Reference | Reference | Reference | |||||
| Weekly or daily | 1.365 (1.214, 1.535) | < 0.001 | 1.772 (1.619, 1.939) | < 0.001 | 1.578 (1.405, 1.773) | < 0.001 | 2.383 (2.197, 2.585) | < 0.001 | |
| Family history of CRC | |||||||||
| No | Reference | Reference | Reference | Reference | |||||
| Yes | 1.131 (1.002, 1.277) | 0.046 | 1.009 (0.911, 1.117) | 0.863 | 1.003 (0.879, 1.144) | 0.967 | 1.074 (0.976, 1.181) | 0.143 | |
Family history of colorectal cancer was defined only in first-degree relatives
*CRN colorectal neoplasms, CRC colorectal cancer, BMI body mass index, OR odds ratio, CI confidence interval
Fig. 2.
Forest plots used to show odds ratios for CRN risk factors in different groups. a The proximal colon subgroup. b The distal colon subgroup. c The rectum subgroup. d The multiple colorectal sites subgroup. *CRC colorectal cancer, BMI body mass index, OR odds ratio, CI confidence interval. Family history of colorectal cancer was defined only in first-degree relatives
Table 3.
Risk factors for CRN at different anatomical sites stratified by sex
| Risk factors | The proximal colon | The distal colon | The rectum | The multiple colorectal sites | ||||
|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Age (years) | ||||||||
| Female | ||||||||
| 40–50 | Reference | Reference | Reference | Reference | ||||
| 51–60 | 1.822 (1.455, 2.280) | < 0.001 | 1.646 (1.380, 1.965) | < 0.001 | 1.099 (0.883, 1.368) | 0.397 | 1.700 (1.347, 2.146) | < 0.001 |
| 61–70 | 2.111 (1.695, 2.629) | < 0.001 | 1.806 (1.520, 2.147) | < 0.001 | 1.684 (1.368, 2.074) | < 0.001 | 3.431 (2.745, 4.289) | < 0.001 |
| Greater than 70 | 2.466 (1.938, 3.139) | < 0.001 | 1.764 (1.450, 2.145) | < 0.001 | 2.051 (1.629, 2.584) | < 0.001 | 5.404 (4.277, 6.828) | < 0.001 |
| Male | ||||||||
| 40–50 | Reference | Reference | Reference | Reference | ||||
| 51–60 | 1.720 (1.399, 2.115) | < 0.001 | 1.730 (1.479, 2.023) | < 0.001 | 1.549 (1.250, 1.920) | < 0.001 | 2.032 (1.701, 2.428) | < 0.001 |
| 61–70 | 1.913 (1.570, 2.331) | < 0.001 | 1.713 (1.474, 1.992) | < 0.001 | 1.818 (1.482, 2.228) | < 0.001 | 3.435 (2.903, 4.065) | < 0.001 |
| Greater than 70 | 1.658 (1.328, 2.068) | < 0.001 | 1.402 (1.181, 1.664) | < 0.001 | 2.068 (1.656, 2.583) | < 0.001 | 4.184 (3.497, 5.005) | < 0.001 |
| BMI (kg/m2) | ||||||||
| Female | ||||||||
| Less than 23 | Reference | Reference | Reference | Reference | ||||
| 23–27.5 | 1.112 (0.999, 1.238) | 0.052 | 1.133 (1.035, 1.241) | 0.007 | 1.125 (1.004, 1.260) | 0.042 | 1.306 (1.185, 1.439) | < 0.001 |
| Greater than 27.5 | 1.207 (1.049, 1.387) | 0.008 | 1.303 (1.160, 1.465) | < 0.001 | 1.161 (1.001, 1.348) | 0.049 | 1.599 (1.419, 1.801) | < 0.001 |
| Male | ||||||||
| Less than 23 | Reference | Reference | Reference | Reference | ||||
| 23–27.5 | 1.249 (1.106, 1.410) | < 0.001 | 1.104 (1.005, 1.213) | 0.040 | 1.079 (0.956, 1.218) | 0.217 | 1.187 (1.085, 1.299) | < 0.001 |
| Greater than 27.5 | 1.356 (1.162, 1.582) | < 0.001 | 1.253 (1.111, 1.414) | < 0.001 | 1.058 (0.903, 1.241) | 0.485 | 1.514 (1.353, 1.694) | < 0.001 |
| Smoking status | ||||||||
| Female | ||||||||
| Never | Reference | Reference | Reference | Reference | ||||
| Present or past | 1.078 (0.880, 1.320) | 0.467 | 1.707 (1.470, 1.983) | < 0.001 | 1.483 (1.227, 1.794) | < 0.001 | 1.803 (1.558, 2.087) | < 0.001 |
| Male | ||||||||
| Never | Reference | Reference | Reference | Reference | ||||
| Present or past | 0.897 (0.803, 1.002) | 0.053 | 1.262 (1.158, 1.375) | < 0.001 | 1.147 (1.026, 1.283) | 0.016 | 1.298 (1.198, 1.407) | < 0.001 |
| Alcohol intake | ||||||||
| Female | ||||||||
| Never or occasional | Reference | Reference | Reference | Reference | ||||
| Weekly or daily | 1.490 (0.863, 2.572) | 0.152 | 1.083 (0.659, 1.779) | 0.753 | 0.958 (0.499, 1.839) | 0.897 | 1.460 (0.935, 2.28) | 0.096 |
| Male | ||||||||
| Never or occasional | Reference | Reference | Reference | Reference | ||||
| Weekly or daily | 1.031 (0.900, 1.181) | 0.656 | 1.079 (0.972, 1.198) | 0.155 | 1.075 (0.938, 1.230) | 0.298 | 1.202 (1.092, 1.323) | < 0.001 |
| Family history of CRC | ||||||||
| Female | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.121 (0.953, 1.319) | 0.168 | 1.015 (0.880, 1.170) | 0.839 | 1.126 (0.947, 1.339) | 0.178 | 1.068 (0.924, 1.236) | 0.373 |
| Male | ||||||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 1.147 (0.953, 1.381) | 0.147 | 1.049 (0.901, 1.221) | 0.541 | 0.918 (0.747, 1.128) | 0.415 | 1.182 (1.028, 1.359) | 0.019 |
Family history of colorectal cancer was defined only in first-degree relatives
*CRN colorectal neoplasms, CRC colorectal cancer, BMI body mass index, OR odds ratio, CI confidence interval
Sex and age
Male sex was an independent risk factor for CRN from the proximal colon to the rectum. Males had the strongest correlation with CRN risk in the distal colon (OR = 1.779, 95% CI = 1.671, 1.895). The risk of CRN increased with age in both the proximal colon and rectum. For all sexes, age over 70 conferred the greatest risk in the proximal colon. The risk of CRN was greatest in the distal colon at 61–70 years of age (OR = 1.762, 95% CI = 1.574, 1.973). For women, age over 70 years (OR = 2.466, 95% CI = 1.938, 3.139) was most strongly associated with CRN risk in the proximal colon. However, for men, age over 70 years (OR = 2.068, 95% CI = 1.656, 2.583) was most strongly associated with rectal CRN risk. In the colon, older age was associated with a higher risk of CRN in women than in men. However, advanced age in the rectum confers a greater risk to men.
BMI
Obesity (BMI greater than 27.5 kg/m2) was associated with CRN risk from the proximal colon to the rectum. For women, obesity (OR = 1.303, 95% CI = 1.160, 1.465) conferred the greatest risk in the distal colon. For men, however, obesity (OR = 1.356, 95% CI = 1.162, 1.582) conferred the greatest risk in the proximal colon. There is no significant correlation between obesity and the increased risk of rectal CRN in men.
Unhealthy lifestyle and family history of CRC (FDRs)
Multivariate analysis showed that smoking was only associated with CRN in the distal colon (OR = 1.363, 95% CI = 1.264, 1.469) and rectum (OR = 1.230, 95% CI = 1.116, 1.355). And the risk is greater in the distal colon. This is true for all sexes. In the distal colon, for women, the OR for smoking is 1.707 (95% CI = 1.470, 1.983); for men, the OR for smoking is 1.262 (95% CI = 1.158, 1.375). Among CRN patients with single intestinal segment, we found that multiple alcohol consumption per week and family history of CRC were not significantly associated with CRN occurrence, but were only associated with CRN at multiple sites. Further analysis in men and women revealed that these two factors were surprisingly only associated with the incidence of colorectal multisite CRN in men. Among men with colorectal multisite CRN, the OR for frequent drinking is 1.202 (95% CI = 1.092, 1.323), and the OR for family history of CRC (FDRs) is 1.182 (95% CI = 1.028, 1.359).
Notably, older age, obesity, and smoking were more associated with multiple-site CRN than with a single bowel segment in both men and women. But this group also had the largest proportion of non-advanced adenomas and advanced adenomas.
Discussion
Male sex and advanced age were significantly associated with CRN risk in any part of the colorectal region: males had the strongest association with CRN in the distal colon. Age over 70 years was most associated with the risk of CRN in the proximal colon among women and rectal CRN among men. Men are more likely to develop CRN than women, possibly because men lack the protective effect of estrogen [12]. Moreover, it has been suggested that androgens may be involved in the formation of colorectal tumors by regulating the proliferation of intestinal epithelial cells [13]. By comparison, we found that only when CRN occurred in the proximal colon did the proportion of women exceed that of men, which is consistent with previous findings that women are at higher risk for CRC in the proximal colon [14], especially in the ascending colon [9]. In many studies, advanced age is a risk factor for colorectal adenoma and colorectal cancer [9, 12, 15, 16]. This may be related to the adenoma-carcinoma sequence of colorectal tumors [5, 17]; when normal cells accumulate multiple genetic mutations over many years, they become cancerous [18, 19]. Therefore, early detection of high-risk adenomas is also one of the goals of our screening program, and the progression of colorectal neoplasms can be cut off by interfering with the adenoma-carcinoma sequence of CRC [20].
Obesity has repeatedly been shown to be a risk factor for colorectal adenoma, colorectal cancer, and recurrence after adenoma resection [19, 21]. In Asian populations, 23 kg/m2 and 27.5 kg/m2 are the latest recommended cut-off values for overweight and obesity, respectively, which are both risk factors for CRN from the proximal colon to the rectum, when not stratified by sex. However, there was no significant association between obesity and rectal CRN risk in men. For multiple colorectal CRN, high BMI was significantly more associated with CRN risk than single intestinal segment, which may indicate that obesity is a risk factor without anatomical heterogeneity, and obesity may increase the risk of CRN in both the colon and rectum indiscriminately. Racial and ethnic differences may influence obesity-related differences in CRN risk across colorectal sites [21, 22].
Smoking and MSI-H, CIMP (CpG island methylator phenotype), and a mutated BRAF gene are related [23], and the frequency of these three genetic changes from the rectum to the ascending colon increases gradually [24], which may be the molecular basis for the different associations between smoking and CRN risk at different sites. We found that smoking was a risk factor for CRN in the distal colon, rectum, and multiple colorectal sites, but not in the proximal colon. However, it was previously reported that smoking was most strongly associated with the risk of CRC in the proximal colon (primarily the transverse colon) and rectum [9, 25]. Some studies have suggested that smoking may have a greater effect on non-advanced adenomas, and our study included a large proportion of patients with colorectal adenomas, which may have contributed to the difference in results [26]. Smoking is more associated with CRN risk in women, which has been confirmed in other studies[27]. But we do not come to the same conclusion. Although the odds ratio for smoking was higher in women than in men, it is not sufficient to conclude that smoking is more harmful to women, because men are at higher risk for CRN, which may also contribute to the difference in this data. Although smoking mainly causes malignant tumors of the respiratory system, it also increases the risk of developing tumors of the digestive and urinary systems [28]. Smoking generates a variety of carcinogenic compounds through the digestive system, circulatory system, and intestinal contact; causes intestinal flora metabolic disorders; and induces the generation of adenoma and progression to CRC [29]. Quitting smoking can limit the progression of CIMP [22], which has guiding significance for the prevention of CRC.
Alcohol also increases the risk of CRC by causing DNA damage and dysregulation of cellular REDOX reactions through the production of acetaldehyde and acetate by metabolism in the body [30]. We found that although frequent alcohol consumption was associated with CRN risk at all sites in univariate analysis, it was only an independent risk factor in males for colorectal multisite CRN in multivariate analysis. Previous studies are consistent with our results that alcohol consumption only poses a risk for men [31]. Alcohol consumption has been shown to be a risk factor for colorectal adenoma [32], but the association with colorectal cancer is weaker [33]. In our study, the proportions of non-advanced and advanced adenomas in the colorectal multisite group were 34.5% and 49.5%, respectively. Therefore, at this point, our results may mainly show the risk of colorectal adenomas.
We found that a history of colorectal cancer in a first-degree relative was an independent risk factor for CRN in the proximal colon and multisite colorectal sites. For all sexes, it was only a marginal risk factor for the proximal colon. However, when stratified by sex, it was only associated with the risk of multisite CRN in males. Many studies have shown that people with a family history of colorectal cancer are more likely to develop CRN [34–37]. The American College of Gastroenterology guidelines for colorectal cancer screening define those with colorectal cancer in first-degree relatives as at high risk and recommend colonoscopy starting at age 40 [6]. Other researchers have reached similar conclusions: A family history of colorectal cancer is most strongly associated with the risk of proximal colon cancer or adenoma [38, 39]. Family history of CRC may be associated with most molecular subtypes, and some types of colorectal cancer contain multiple molecular subtypes, for example, LINE-1 (long interspersed nucleotide element 1) methylation and CIMP-low of MSI-high cancer. These molecular isoforms will change along the gut [24, 40]. Moreover, MSI-high tumors are more likely to occur in the proximal colon [41], which may underlie the anatomical heterogeneity of CRC family history.
There are some limitations in our study. First, the classification of anatomical sites is not sufficiently refined, and some potential variations may be obscured by broad divisions. Second, there may be recall bias in the contents of the questionnaire. Third, there are many bad living habits, and we only considered some of them. Fourth, some participants were excluded because of incomplete pathology reports or questionnaire content, which may have interfered with the results.
In conclusion, we explored risk factors for CRN based on anatomical sites. Men are an immutable risk factor. Advanced age increases the risk of CRN, but the sites with the strongest correlation differ by sex. Obesity was associated with CRN risk in both men and women, but was not significantly associated with an increased risk of rectal CRN in men. Smoking is more harmful to women. Alcohol consumption and family history of colorectal cancer contributed less to CRN than other factors. Recognition of these differences will guide the development of population-specific prevention and treatment strategies in colorectal cancer screening programs.
Author contribution
All authors participated in discussions about the article and approved the final version. Main manuscript: Huaqing Wang, Zhen Yuan, Shuyuan Wang, Wenwen Pang, and Chunze Zhang. Study design: Huaqing Wang, Zhen Yuan, Shuyuan Wang, Wenwen Pang, Qinghuai Zhang, Xipeng Zhang, and Chunze Zhang. Data collection: Huaqing Wang, Zhen Yuan, Shuyuan Wang, Wenwen Pang, Wanting Wang, Xinyu Liu, Ben Yi, Qiurong Han, Yao Yao, Qinghuai Zhang, Xipeng Zhang, and Chunze Zhang. Statistical analysis: Huaqing Wang, Zhen Yuan, Shuyuan Wang, Wenwen Pang, Chunze Zhang, Wanting Wang, Xinyu Liu, Ben Yi, Qiurong Han, and Yao Yao. Figures and tables: Huaqing Wang, Zhen Yuan, Shuyuan Wang, Wenwen Pang, and Chunze Zhang. Funding acquisition: Qinghuai Zhang, Xipeng Zhang, and Chunze Zhang.
Funding
This research was supported by the Key R&D Projects in the Tianjin Science and Technology Pillar Program (Grant number 19YFZCSY00420), Natural Science Foundation of Tianjin (21JCZDJC00060, 21JCYBJC00180 and 21JCYBJC00340), Tianjin Key Medical Discipline (Specialty) Construction Project (Grant number TJYXZDXK-044A), and Tianjin Hospital Association Hospital Management Research Project (Grant number 2019ZZ07).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was approved by the Ethics Committee of Tianjin Union Medical Center. This trial was conducted following the Declaration of Helsinki guidelines.
Consent to participate
Written informed consent was obtained from all participants before entering the study.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huaqing Wang, Zhen Yuan, Shuyuan Wang, and Wenwen Pang contributed equally to this article.
Contributor Information
Xipeng Zhang, Email: xipengzhangtj@163.com.
Chunze Zhang, Email: chunze.zhang@nankai.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conteduca V, Sansonno D, Russi S, Dammacco F. Precancerous colorectal lesions (Review) Int J Oncol. 2013;43(4):973–984. doi: 10.3892/ijo.2013.2041. [DOI] [PubMed] [Google Scholar]
- 4.Midgley R, Kerr D. Colorectal cancer. The Lancet. 1999;353(9150):391–399. doi: 10.1016/S0140-6736(98)07127-X. [DOI] [PubMed] [Google Scholar]
- 5.Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89(7):845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 6.Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. 2021;116(3):458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 7.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review. Eur J Surg Oncol. 2015;41(3):300–308. doi: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Lo CH, He X, Hang D, Wang M, Wu K, Chan AT, Ogino S, Giovannucci EL, Song M (2020) Risk factor profiles differ for cancers of different regions of the colorectum. Gastroenterology 159(1):241–256 e13 [DOI] [PMC free article] [PubMed]
- 10.Huang L, Wang X, Gong W, Huang Y, Jiang B. The comparison of the clinical manifestations and risk factors of colorectal cancer and adenomas: results from a colonoscopy-based study in southern Chinese. Int J Colorectal Dis. 2010;25(11):1343–1351. doi: 10.1007/s00384-010-1030-6. [DOI] [PubMed] [Google Scholar]
- 11.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 12.Park SH, Hong KI, Park HC, Kim YS, Bok GH, Kim KH, Shin DS, Han JY, Kim YK, Choi YJ, Eun SH, Lim BH, Kwack KK, Korean Society of Digestive Endoscopy Polyp Study W (2021) Colon polyp detection in primary health care institutions of Korea: detection rate and issues with following the guidelines. Korean J Gastroenterol 78(6):328–336 [DOI] [PMC free article] [PubMed]
- 13.Yu X, Li S, Xu Y, Zhang Y, Ma W, Liang C, Lu H, Ji Y, Liu C, Chen D, Li J. Androgen maintains intestinal homeostasis by inhibiting bmp signaling via intestinal stromal cells. Stem Cell Reports. 2020;15(4):912–925. doi: 10.1016/j.stemcr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SE, Paik HY, Yoon H, Lee JE, Kim N, Sung MK. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21(17):5167–5175. doi: 10.3748/wjg.v21.i17.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Je IJ, de Wit K, van der Vlugt M, Bastiaansen BA, Fockens P, Dekker E. Prevalence, distribution and risk of sessile serrated adenomas/polyps at a center with a high adenoma detection rate and experienced pathologists. Endoscopy. 2016;48(8):740–746. doi: 10.1055/s-0042-105436. [DOI] [PubMed] [Google Scholar]
- 16.Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu LC, Potter JD, Newcomb PA. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177(7):625–637. doi: 10.1093/aje/kws282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–2270. doi: 10.1002/cncr.2820360944. [DOI] [PubMed] [Google Scholar]
- 18.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond JH. Interference with the adenoma-carcinoma sequence. Eur J Cancer. 1995;31(7–8):1115–1117. doi: 10.1016/0959-8049(95)00213-3. [DOI] [PubMed] [Google Scholar]
- 21.Schlesinger S, Aleksandrova K, Abar L, Vieria AR, Vingeliene S, Polemiti E, Stevens CAT, Greenwood DC, Chan DSM, Aune D, Norat T. Adult weight gain and colorectal adenomas-a systematic review and meta-analysis. Ann Oncol. 2017;28(6):1217–1229. doi: 10.1093/annonc/mdx080. [DOI] [PubMed] [Google Scholar]
- 22.Laake I, Thune I, Selmer R, Tretli S, Slattery ML, Veierod MB. A prospective study of body mass index, weight change, and risk of cancer in the proximal and distal colon. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1511–1522. doi: 10.1158/1055-9965.EPI-09-0813. [DOI] [PubMed] [Google Scholar]
- 23.Nishihara R, Morikawa T, Kuchiba A, Lochhead P, Yamauchi M, Liao X, Imamura Y, Nosho K, Shima K, Kawachi I, Qian ZR, Fuchs CS, Chan AT, Giovannucci E, Ogino S. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178(1):84–100. doi: 10.1093/aje/kws431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6):847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy N, Ward HA, Jenab M, Rothwell, JA Boutron-Ruault MC, Carbonnel F, Kvaskoff M, Kaaks R, Kuhn T, Boeing H, Aleksandrova K, Weiderpass E, Skeie G, Borch KB, Tjonneland A, Kyro C, Overvad K, Dahm CC, Jakszyn P, Sanchez MJ, Gil L, Huerta JM, Barricarte A, Quiros JR, Khaw KT, Wareham N, Bradbury KE, Trichopoulou A, La Vecchia C, Karakatsani A, Palli D, Grioni S, Tumino R, Fasanelli F, Panico S, Bueno-de-Mesquita B, Peeters PH, Gylling B, Myte R, Jirstrom K, Berntsson J, Xue X, Riboli E, Cross AJ, Gunter MJ (2019) Heterogeneity of colorectal cancer risk factors by anatomical subsite in 10 European countries: a multinational cohort study. Clin Gastroenterol Hepatol 17(7):1323–1331 e6 [DOI] [PMC free article] [PubMed]
- 26.Tiemersma EW, Bunschoten A, Kok FJ, Glatt H, de Boer SY, Kampman E. Effect of SULT1A1 and NAT2 genetic polymorphism on the association between cigarette smoking and colorectal adenomas. Int J Cancer. 2004;108(1):97–103. doi: 10.1002/ijc.11533. [DOI] [PubMed] [Google Scholar]
- 27.Gram IT, Park SY, Wilkens LR, Haiman CA, Le Marchand L. Smoking-related risks of colorectal cancer by anatomical subsite and sex. Am J Epidemiol. 2020;189(6):543–553. doi: 10.1093/aje/kwaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3(10):733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 29.Bai X, Wei H, Liu W, Coker OO, Gou H, Liu C, Zhao L, Li C, Zhou Y, Wang G, Kang W, Ng EK, Yu J (2022) Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut [DOI] [PMC free article] [PubMed]
- 30.Johnson CH, Golla JP, Dioletis E, Singh S, Ishii M, Charkoftaki G, Thompson DC, Vasiliou V (2021) Molecular mechanisms of alcohol-induced colorectal carcinogenesis. Cancers (Basel) 13(17) [DOI] [PMC free article] [PubMed]
- 31.Kontou N, Psaltopoulou T, Soupos N, Polychronopoulos E, Xinopoulos D, Linos A, Panagiotakos D. Alcohol consumption and colorectal cancer in a Mediterranean population: a case-control study. Dis Colon Rectum. 2012;55(6):703–710. doi: 10.1097/DCR.0b013e31824e612a. [DOI] [PubMed] [Google Scholar]
- 32.Longnecker MP, Chen M-J, Probst-Hensch NM, Harper JM, Lee ER, Frankl HD, Haile RW. Alcohol and smoking in relation to the prevalence of adenomatous colorectal polyps detected at sigmoidoscopy. Epidemiology. 1996;7(3):275–280. doi: 10.1097/00001648-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Erhardt JG, Kreichgauer HP, Meisner C, Bode JC, Bode C. Alcohol, cigarette smoking, dietary factors and the risk of colorectal adenomas and hyperplastic polyps–a case control study. Eur J Nutr. 2002;41(1):35–43. doi: 10.1007/s003940200004. [DOI] [PubMed] [Google Scholar]
- 34.Song M, Emilsson L, Roelstraete B, Ludvigsson JF. Risk of colorectal cancer in first degree relatives of patients with colorectal polyps: nationwide case-control study in Sweden. BMJ. 2021;373:n877. doi: 10.1136/bmj.n877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quintero E, Carrillo M, Leoz ML, Cubiella J, Gargallo C, Lanas A, Bujanda L, Gimeno-Garcia AZ, Hernandez-Guerra M, Nicolas-Perez D, Alonso-Abreu I, Morillas JD, Balaguer F, Muriel A, de Oncology Group of the Asociacion Espanola de G (2016) Risk of advanced neoplasia in first-degree relatives with colorectal cancer: a large multicenter cross-sectional study. PLoS Med 13(5):e1002008 [DOI] [PMC free article] [PubMed]
- 36.Lindgren G, Liljegren A, Jaramillo E, Rubio C, Lindblom A. Adenoma prevalence and cancer risk in familial non-polyposis colorectal cancer. Gut. 2002;50(2):228–234. doi: 10.1136/gut.50.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng SC, Lau JY, Chan FK, Suen BY, Leung WK, Tse YK, Ng SS, Lee JF, To KF, Wu JC, Sung JJ. Increased risk of advanced neoplasms among asymptomatic siblings of patients with colorectal cancer. Gastroenterology. 2013;144(3):544–550. doi: 10.1053/j.gastro.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Samadder NJ, Valentine JF, Guthery S, Singh H, Bernstein CN, Leighton JA, Wan Y, Wong J, Boucher K, Pappas L, Rowe K, Burt RW, Curtin K, Smith KR (2019) Family history associates with increased risk of colorectal cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 17(9):1807–1813 e1 [DOI] [PubMed]
- 39.Armelao F, Paternolli C, Franceschini G, Franch R, Orlandi PG, Miori G, Avancini I, Togni M, Rossi M, Meggio A, Tasini E, Manfrini R, Giacomin D, Fasoli R, Faitini K, Mastromauro M, Costa S, Ridolfi F, Rosi P, de Pretis G (2011) Colonoscopic findings in first-degree relatives of patients with colorectal cancer: a population-based screening program. Gastrointest Endosc 73(3):527–534 e2 [DOI] [PubMed]
- 40.Ogino S, Nishihara R, Lochhead P, Imamura Y, Kuchiba A, Morikawa T, Yamauchi M, Liao X, Qian ZR, Sun R, Sato K, Kirkner GJ, Wang M, Spiegelman D, Meyerhardt JA, Schernhammer ES, Chan AT, Giovannucci E, Fuchs CS. Prospective study of family history and colorectal cancer risk by tumor LINE-1 methylation level. J Natl Cancer Inst. 2013;105(2):130–140. doi: 10.1093/jnci/djs482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bapat B, Lindor NM, Baron J, Siegmund K, Li L, Zheng Y, Haile R, Gallinger S, Jass JR, Young JP, Cotterchio M, Jenkins M, Grove J, Casey G, Thibodeau SN, Bishop DT, Hopper JL, Ahnen D, Newcomb PA, Le Marchand L, Potter JD, Seminara DR, Family CC. The association of tumor microsatellite instability phenotype with family history of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(3):967–975. doi: 10.1158/1055-9965.EPI-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.