Abstract
Nowadays, Artificial Intelligence (AI) and machine learning (ML) have successfully provided automated solutions to numerous real-world problems. Healthcare is one of the most important research areas for ML researchers, with the aim of developing automated disease prediction systems. One of the disease detection problems that AI and ML researchers have focused on is dementia detection using ML methods. Numerous automated diagnostic systems based on ML techniques for early prediction of dementia have been proposed in the literature. Few systematic literature reviews (SLR) have been conducted for dementia prediction based on ML techniques in the past. However, these SLR focused on a single type of data modality for the detection of dementia. Hence, the purpose of this study is to conduct a comprehensive evaluation of ML-based automated diagnostic systems considering different types of data modalities such as images, clinical-features, and voice data. We collected the research articles from 2011 to 2022 using the keywords dementia, machine learning, feature selection, data modalities, and automated diagnostic systems. The selected articles were critically analyzed and discussed. It was observed that image data driven ML models yields promising results in terms of dementia prediction compared to other data modalities, i.e., clinical feature-based data and voice data. Furthermore, this SLR highlighted the limitations of the previously proposed automated methods for dementia and presented future directions to overcome these limitations.
Keywords: Dementia prediction, Feature selection, Machine learning, Deep learning
Introduction
Over a period of time, the advancements made in the field of medical science helped to increase the lifespan in the modern world [1]. This increased life expectancy raised the prevalence of neurocognitive disorders, affecting a significant part of the older population as well as global economies. In 2010, it was estimated that $604 billion have been spent on dementia patients in the USA alone[2]. The number of dementia patients is rapidly increasing worldwide, and statistical projections suggest that 135 million people might be affected by dementia by 2050 [3]. There are several risk factors that contribute to the development of dementia, including aging, head injury, and lifestyle. While age is the most prominent risk factor for dementia; figures suggest that a person at the age of 65 years old has 1–2% risk of developing dementia disease. By the age of 85 years old, this risk can reach to 30% [4].
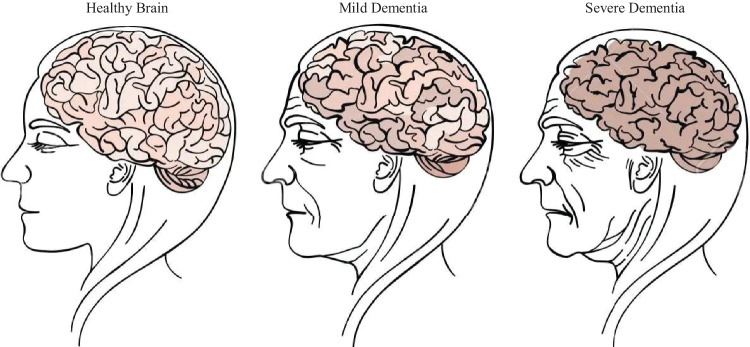
Dementia is a mental disorder that is characterized by a progressive deterioration of cognitive functions that can affect daily life activities such as memory, problem solving, visual perception, and the ability to focus on a particular task. Usually, older adults are most vulnerable to dementia, and people take it as an inevitable consequence of aging, which is perhaps the wrong perception. Dementia is not a part of the normal ageing process; however, it should be considered a serious form of cognitive decline that affects your daily life. Actually, the primary cause for the development of dementia is the several diseases and injuries that affect the human brain [5]. Dementia is ranked on the seventh place in the leading causes of deaths in the world [6]. Furthermore, it is the major cause of disability and dependency among older people globally [6]. A change in the person’s ordinary mental functioning and obvious signs of high cognitive deterioration are required for a diagnosis of dementia [7]. Figure 1 presents the progression of dementia with age.
Fig. 1.
Progression of dementia disease with ageing
Types of dementia
Dementia is not a single disease, but, it is used as a generic term for several different cognitive disorders. Figure 2 provides the overview of different types of dementia along with the percentage of particular dementia type occurrence in the patients [8]. To have a better idea about dementia, we have studied common types of dementia for better problem awareness.
Fig. 2.
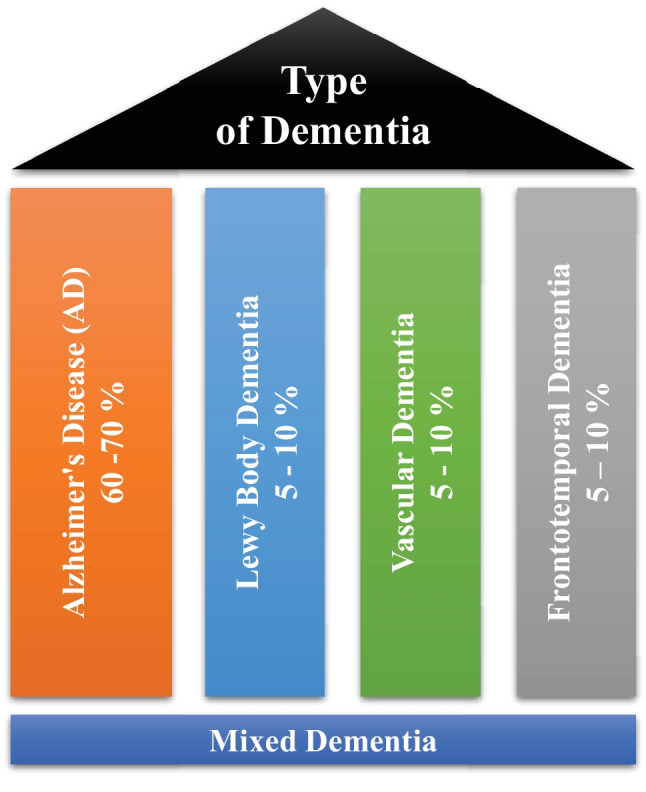
Types of dementia disease
Alzheimer’s disease
Alzheimer’s disease (AD) is thought to develop when abnormal amounts of amyloid beta (A) build up in the brain, either extracellularly as amyloid plaques, tau proteins or intracellularly as neurofibrillary tangles, affecting neuronal function, connectivity and leading to progressive brain function loss [9]. This diminished ability to eliminate proteins with ageing is regulated by brain cholesterol [10] and is linked to other neurodegenerative illnesses [11]. Except for 1–2% of cases where deterministic genetic anomalies have been discovered, the aetiology of the majority of Alzheimer’s patients remains unexplained [12]. The amyloid beta (A) hypothesis and the cholinergic hypothesis are two competing theories presented to explain the underlying cause of AD [13].
Vascular dementia
Vascular dementia (VaD) is a subtype of dementia caused by problems with the brain’s blood flow, generally in the form of a series of minor strokes, which results in a slow decline of cognitive capacity [14]. The VaD refers to a disorder characterized by a complicated mix of cerebrovascular illnesses that result in structural changes in the brain, as a result of strokes and lesions, which lead to cognitive impairment. A chronological relationship between stroke and cognitive impairments is necessary to make the diagnosis [15]. Ischemic or hemorrhagic infarctions in several brain areas, such as the anterior cerebral artery region, the parietal lobes, or the cingulate gyrus, are associated with VaD. In rare cases, infarcts in the hippocampus or thalamus might cause dementia [16]. A stroke increases the risk of dementia by 70%, whereas a recent stroke increases the risk by almost 120% [17]. Brain vascular lesions can also be caused by diffuse cerebrovascular disease, such as small vessel disease [18]. Risk factors for VaD include age, hypertension, smoking, hypercholesterolemia, diabetes mellitus, cardiovascular disease, and cerebrovascular sickness; geographic origin, genetic proclivity, and past strokes are also risk factors [19]. Cerebral amyloid angiopathy, which develops when beta amyloid accumulates in the brain, can occasionally lead to vascular dementia.
Lewy body dementia
Lewy body dementia (LBD) is a subtype of dementia characterized by abnormal deposits of the protein alpha-synuclein in the brain. These deposits, known as Lewy bodies, affect brain chemistry, causing problems with thinking, movement, behavior, and mood. Lewy body dementia is one of the most common causes of dementia [20]. Progressive loss of mental functions, visual hallucinations, as well as changes in alertness and concentration are prevalent in persons with LBD. Other adverse effects include tight muscles, delayed movement, difficulty walking, and tremors, all of which are also signs and symptoms of Parkinson’s disease [21]. LBD might be difficult to identify. Early LBD symptoms are commonly confused with those of other brain diseases or mental problems. Lewy body dementia can occur alone or in conjunction with other brain disorders [22]. It is a progressive disorder, which means that symptoms emerge gradually and worsen with time. A timespan of five to eight years is averaged, although it can last anywhere from two to twenty years for certain people [23]. The rate at which symptoms arise varies greatly from person to person, depending on overall health, age, and the severity of symptoms.
Frontotemporal dementia
Frontotemporal Dementia (FTD) is a subtype of dementia characterized by nerve cell loss in the frontal and temporal lobes of the brain [24]. As a result, the lobes contract. FTD can have an impact on behavior, attitude, language, and movement. This is one of the most common dementias in people under the age of 65. FTD most commonly affects persons between the ages of 40 and 65; however, it may also afflict young adults and older individuals [25]. The lobes decrease, and behavior, attitude, language, and mobility can all be affected by FTD. FTD affects both men and women equally. Dissociation from family, extreme oniomania, obscene speech, screaming, and the inability to regulate emotions, behavior, personality, and temperament are examples of social display patterns caused by FTD [26]. The symptoms of FTD appeared several years prior to visiting a neurologist [27].
Mixed Dementia (MD)
Mixed dementia occurs, when more than one kind of dementia coexists in a patient, and it is estimated to happen in around 10% of all dementia cases [6]. AD and VaD dementia are the two subtypes that are most common in MD [28]. This case is usually associated with factors such as old age, high blood pressure, and brain blood vessel damage [29]. Because one dementia subtype often predominates, MD is difficult to identify. As a result, the individuals affected by MD are rarely treated and miss out on potentially life-changing medicines. MD can cause symptoms to begin earlier than the actual diagnosis of the disease and spread swiftly to affect the most areas of the brain [30].
Method
Recently, numerous automated methods have been developed based on machine learning for early the prediction of different diseases [31–48]. This systematic literature review (SLR) presented hereby, investigates machine learning-based automated diagnostic systems that are designed and developed by scientists to predict dementia and its subtypes, such as AD, VaD, LBD, FTD and MD. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) criteria to conduct this SLR [49, 50]. A comprehensive search was conducted to retrieve the research articles that contain ML approaches to predict the development of dementia and its subtypes using three different types of data modalities (images, clinical-variables, voice).
Aim of the study
SLRs are done to synthesize current evidence, to identify gaps in the literature, and to provide the groundwork for future studies [51]. Previous, SLRs studies have been done on automated diagnostic systems for dementia prediction based on ML approaches, which focused on a single sort of data modality. These SLR investigations did not emphasize the limits of previously published automated approaches for dementia prediction. The SLR presented herein assesses the previously proposed automated diagnostic systems based on deep learning (DL) and ML algorithms for the prediction of dementia and its common subtypes (e.g. AD, VaD, FTD, MD). The aim of this SLR is to analyse and evaluate the performance of automated diagnostic systems for dementia prediction using different data modalities. The main question is decomposed in the following sub-research questions:
What types of ML and DL techniques have been used by researchers to diagnose dementia?
Examine the methods of feature extraction or selection used by the researchers.
Analyze the different performance evaluation measures that are adopted by the researcher to validate the effectiveness of the proposed diagnostic system for demetnia.
Analyze the performance of ML models on various data types.
Identification of weaknesses in previously proposed ML models for dementia prediction.
Article selection
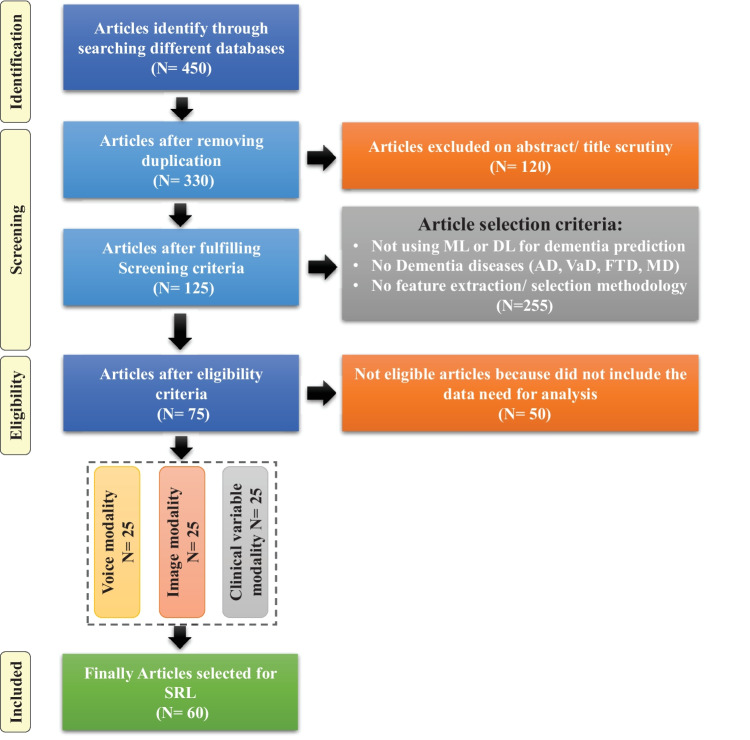
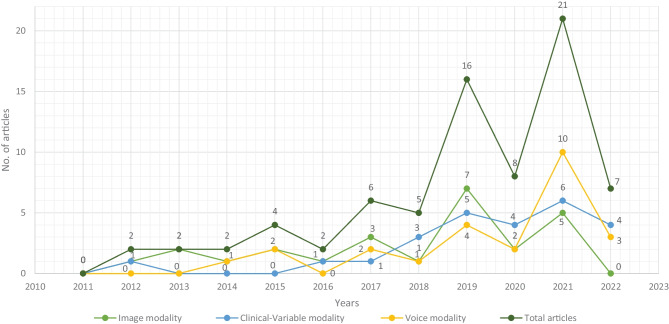
For this SLR study, the research articles were selected based on keywords such as ML, DL, dementia and its subtypes (AD, VaD, FTD, and MD). For the collection of research articles, we conducted an electronic search from different online databases such as ScienceDirect, PubMed, IEEE Xplore Digital Library, Springer, Hindawi, and PLOs, which helped to gather 450 research studies on the specific topic. After reviewing the title and abstract in each study, 120 publications were found to be ineligible for processing, while 330 articles were selected for further processing. Following the deduplication of data, 125 full-text publications were retrieved for further processing after the screening phase of the article selection, with 205 of them being eliminated due to not satisfying the article selection criteria of the screening phase. Finally, 50 research articles were eliminated due to not fulfilling the eligibility criteria for article selection. The final set of selected papers consisted of 75 research papers, among these final selected articles, each of the data modalities (image, clinical-variables, voice) contained 25 papers. After rerunning the database searches in May 2022, no further suitable research article was found for the selection. Figure 3 presents the workflow for article selection, which includes the four PRISMA guidelines-recommended steps such as identification, screening, eligibility, and inclusion [49, 50]. In recent years, ML scientists have shown a strong interest in designing and developing ML-based automated diagnostic systems for dementia prediction. Therefore, the number of research articles in this research area has been increased and it can be depicted from Fig. 4 where research articles are published years wise with regarding data modality. The publications utilized in this study were selected based on the following criteria:
Studies that present automated diagnostic systems for dementia and its common subtypes (AD,VaD, FTD, MD).
Studies published between 2011 and 2022.
Studies employing ML approaches for dementia diagnosis.
Studies which have utilized several data modalities.
Studies published in the English language.
Fig. 3.
Flow diagram of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses)
Fig. 4.
Selected research articles which are published from 2011 to 2022 regarding data modality
Machine learning for dementia
Over the years, the increasing use and availability of medical equipment has resulted in a massive collection of electronic health records (EHR) that might be utilized to identify dementia using developing technologies such as ML and DL [52]. These EHRs are one of the most widely available and used clinical datasets. They are a crucial component of contemporary healthcare delivery, providing rapid access to accurate, up-to-date, comprehensive patient information while also assisting with precise diagnosis and coordinated, efficient care [53]. Laboratory tests, vital signs, drugs, and other therapies, as well as comorbidities, can be used to identify the people at risk of dementia using the EHRs’ data [54]. In some situations, patients may also be subjected to costly and invasive treatments such as neuroimaging scans i.e., magnetic resonance imaging (MRI) and position emission tomography (PET)) and cerebrospinal fluid (CSF) collection for biomarker testing [55–57]. These tests’ findings may also be found in the EHR. According to researchers, such longitudinal clinical EHR data can be used to track the advancement of AD dementia over time [58]. Recently, several automated diagnostic systems for different diseases, such as Parkinson’s disease [59], hepatitis [47], carcinoma [41], and heart failure [60–62] prediction have been designed by employing ML and DL techniques. Inspired by this fact, the unmet demand for dementia knowledge, along with the availability of relevant huge datasets, has motivated scientists to investigate the utility of artificial intelligence (AI), which is gaining a prominent role in the area of healthcare innovation [63]. ML, a subset of AI, can model the relationship between input quantities and clinical outcomes, identify hidden patterns in enormous volumes of data, and draw conclusions or make decisions that help with more accurate clinical decision-making [51]. However, computational hypotheses generated by ML models must still be confirmed by subject matter experts in order to achieve enough precision for clinical decision-making [64].
In this SLR, we have included studies that have used ML predictive models (supervised and unsupervised) for dementia prediction and excluded studies that have used statistical methods for cohort summarization and hypothesis testing (e.g., odds ratio, chi-square distribution, Kruskal-Wallis test, and Kappa-Cohen test). Furthermore, we have referenced the data modality-based study [65] for this literature review, where we have categorized the three data modality types such as image, clinical-variable and voice. Thus, we have studied each modality-based automated diagnostic system for dementia prediction that has been proposed in the past using ML and DL.
Datasets
This section explains the datasets that were used in the selected research papers for experiments and performance evaluation of the proposed automated diagnostic systems designed by the researchers using ML algorithms for dementia and its subtypes. A total of 61 datasets were studied from the selected research articles. These datasets are compiled from a wide range of organizations and hospitals throughout the world. Only a few datasets are openly available to the public, while others are compiled by researchers from various hospitals and healthcare institutes. We have only included datasets that have been used to diagnose AD, VaD, FTD, MD, and LBD using ML and DL techniques. On the basis of data modality, we have categorised the dataset into three types: images, clinical_variables and voice datasets. The datasets differ in terms of the number of variables (features) and samples. As a result, we examined each modality of the dataset one by one.
Image modality based datasets
There are several image datasets based on brain imaging, such as magnetic resonance imaging (MRI), collected by the researchers for the diagnosis of dementia. From the Table 1, it can be depicted that Open Access Series of Imaging Studies (OASIS) and Alzheimer’s Disease Neuroimaging Initiative (ADNI) datasets are mostly used by the researchers for the experimental purpose. OASIS aims to make neuroimaging datasets available to the scientific community for free. By gathering and openly disseminating this multimodal dataset produced by the Knight ADRC and its related researchers, they had used different samples and variables of the datasets in their research work. ADNI researchers acquire, validate, and use data such as MRI, PET imaging, genetics, cognitive assessments, CSF, and blood biomarkers as disease predictors. The ADNI website contains research information and data from the North American ADNI project, which includes Alzheimer’s disease patients, people with mild cognitive impairment, and older controls. Table 1 provides us with the following information: dataset_id, dataset name, number of samples in the particular dataset, variables in the dataset, and finally, the type of dementia.
Table 1.
Summary of image-modality based datasets
| Dataset_ID | Dataset | Samples | Variables | Dementia_Type |
|---|---|---|---|---|
| 01 | NA | 373 | 5 | AD |
| 02 | ADNI | 300 | 4 | AD |
| 03 | ALZ201 | 72 | 4 | AD |
| 04 | ADNI | 138 | 6 | AD |
| 05 | NINDS-AIREN | 93 | 62 | VaD, AD |
| 06 | OASIS | 373 | 8 | AD |
| 07 | OASIS-1 | 216 | 10 | AD |
| 08 | OASIS-2 | 373 | 12 | AD |
| 09 | ADNI | 273 | 8 | AD |
| 10 | OASIS | 420 | 5 | AD |
| 11 | ADNI | 750 | 10 | AD |
| 12 | NA | 373 | 15 | AD |
| 13 | ADNI | 506 | 7 | AD |
| 14 | OASIS | 436 | NA | Dementia |
| 15 | ADNI | 750 | 10 | AD |
| 16 | OASIS | 373 | 8 | AD |
Clinical-variables modality based datasets
Throughout the course of time, the growing usage and availability of medical devices have resulted in an overwhelming collection of clinical EHR data. Furthermore, the patient’s medical history consists of medical tests and clinical records that can be used for the prediction of diseases. Thus, the importance of clinical data emerges as a vital tool for proactive management of disease. The dataset based on clinical variables for dementia consists of medical tests that are used by doctors to check the dementia status in patients, such as the Mini Mental Status Exam (MMSE), the Montreal Cognitive Assessment (MoCA), the Telephone Interview for Cognitive Status (TICS), and the Brief Interview for Mental Status (BIMS). Clinical-variables based datasets consist of information about these medical tests along with patient personal information, i.e., age, sex, and marital status. Hereby, Table 2 provides the information regarding clinical-variables modality-based datasets that are used by the researchers for the design and development of automated diagnostic systems for dementia patients based on ML. Table 2 presents the dataset_id, dataset name, number of samples in the particular dataset, variables in the dataset, and finally the type of dementia.
Table 2.
Summary of clinical-variable modality based datasets
| Dataset_ID | Dataset | Samples | Variables | Dementia_Type |
|---|---|---|---|---|
| 17 | HAICDDS | 1354 | 45 | MD |
| 18 | Adnimerge | 1851 | 49 | MD |
| 19 | EMIF-AD cohort | 500 | 4 | AD |
| 20 | OASIS | 373 | 10 | Dementia |
| 21 | ADRD | 44945 | 50 | Dementia |
| 22 | multi-sensor | 17 | 13 | Dementia |
| 23 | Bremen-Ost | 158 | 7 | MCI, AD |
| 24 | microRNA | 39 | 25 | AD |
| 25 | Raman spectral | 37 | 360 | AD |
| 26 | Cheng Kung | 81 | 10 | AD |
| 27 | NA | 169 | 14 | Dementia |
| 28 | LASI-DAD | 2528 | 30 | Dementia |
| 29 | NACC | 15307 | 258 | Dementia |
| 30 | NCT | 138 | 15 | Dementia |
| 31 | Lebanon | 1002 | 20 | Dementia |
| 32 | FDG-PET | 329 | 32 | AD |
| 33 | OASIS | 450 | NA | AD |
| 34 | LOHAS | 123 | 40 | Dementia |
| 35 | GIST | 60 | 6 | MCI |
| 36 | ADRD | 1038 | 35 | Dementia |
| 37 | HRS | 9979 | 52 | Dementia |
| 38 | MCSA | 3265 | 36 | MD |
| 39 | MNCD | 84 | 38 | AD,FTD |
Voice modality based datasets
Speech analysis is a useful technique for clinical linguists in detecting various types of neurodegenerative disorders affecting the language processing areas. Individuals suffering from Parkinson’s disease (PD, deterioration of voice quality, unstable pitch), Alzheimer’s disease (AD, monotonous pitch), and the non-fluent form of Primary Progressive Aphasia (PPA-NF, hesitant, non-fluent speech) may experience difficulties with prosody, fluency, and voice quality. Besides imaging and clinical-variables data, the researchers employed voice recording data to identify dementia using ML and DL algorithms. The data collection process for voice data varies from dataset to dataset, for example, in a few datasets, patients were requested to answer a prepared set of questions (interview) in a specific time interval. In a few datasets, selected neuropsychological tests were carried out, the description of each neuropsychological test was played and was followed by an answering window. Table 3 presents the dataset_id, dataset name, number of samples in the particular dataset, variables in the dataset, and finally the subtype of dementia.
Table 3.
Summary of voice modality based datasets
| Dataset_ID | Dataset | Samples | Variables | Dementia_Type |
|---|---|---|---|---|
| 40 | ADReSS | 156 | NA | AD |
| 41 | Aishell | 120 | NA | AD |
| 42 | TICS-J | 123 | 36 | Dementia |
| 43 | NA | 16 | 192 | Dementia |
| 44 | VBSD | 36 | 254 | AD |
| 45 | PAR | 48 | 20 | AD |
| 46 | ADReSS | 156 | NA | AD |
| 47 | NBD | 09 | 03 | Dementia |
| 48 | FHS | 81 | 27 | Dementia |
| 49 | Cheng Kung | 98 | 8 | AD |
| 50 | Aphasia | 72 | 232 | Dementia |
| 51 | EHC-Istanbul | 51 | 30 | AD |
| 52 | ADReSS | 156 | NA | AD |
| 53 | DementiaBank’s | 1273 | 108 | AD |
| 54 | NA | 09 | 03 | Dementia |
| 55 | Carolina Collection | 21 | 3 | AD |
| 56 | FHS | 5449 | NA | Dementia |
| 57 | PROMPT | 120 | 82 | Dementia |
| 58 | ADReSS | 144 | 11 | AD |
| 59 | DementiaBank’s | 497 | 108 | AD |
| 60 | AZTIAHO | 70 | NA | AD |
| 61 | Pitt Corpus | 550 | 17 | AD |
Data sharing challenges
In this digital era, public health decision-making has grown progressively complicated, and the utilization of data has become critical [66]. Data are employed at the local level to observe public health and target interventions; at the national scale for resource allocation, prioritization, and planning; and at the global scale for disease burden estimates, progress in health and development measurement, and the containment of evolving global health threats [67, 68]. Van Panhuis et al. have adequately described the challenges to exchanging health data [69]. Based on our initial analysis, we built on this taxonomy to identify the hurdles related to data sharing in global public health, and we have highlighted how they may apply to each typology as given below.
Lack of complete data, lost data, restrictive as well as conflicting data formats, a lack of metadata and standards, a lack of interoperability of datasets (e.g., structure or “language”), and a lack of appropriate analytic solutions are examples of technical barriers encountered by health information management systems.
Individuals and organizations face motivational challenges when it comes to sharing data. These impediments include a lack of incentives, opportunity costs, apprehension about criticism, and disagreements over data usage and access.
The potential and present costs of sharing data are both economic hurdles.
Political obstacles are those that are built into the norms of local health governance and often emerge as regulations and guidelines. They can also entail trust and ownership difficulties.
Legal issues that arise as a result of data collection, analysis, and usage include questions regarding who owns or controls the data, transparency, informed permission, security, privacy, copyright, human rights, damage, and stigma.
Ethical constraints include a lack of perceived reciprocity (i.e., the other side will not disclose data) and proportionality (i.e., deciding not to share data based on an assessment of the risks and benefits). An overall concern is that frameworks, rules, and regulations have not kept up with technological changes that are transforming how data is collected, analyzed, shared, and used.
ML based diagnostic models for dementia: Image modality
In recent years, researchers have designed many ML and DL algorithms for the detection of dementia and its subtypes using MRI images of the brain. For example, Dashtipour et al. [70] proposed a ML based method for the prediction of Alzheimer’s disease. In their proposed model, they used DL techniques to extract the features from brain images, and for classification purposes, they deployed SVM and bidirectional long short-term memory (BiLSTM). Through their proposed model, they had reported the classification accuracy of 91.28%. Moreover, for early detection of the AD, a DL based approach was proposed by Helaly et al. In their proposed work, they employed convolutional neural networks (CNN). The Alzheimer’s disease spectrum is divided into four phases. Furthermore, different binary medical image classifications were used for each two-pair class of Alzheimer’s disease stages. Two approaches were used to categorize medical images and diagnose Alzheimer’s disease. The first technique employs basic CNN architectures based on 2D and 3D convolution to cope with 2D and 3D structural brain images from the ADNI dataset. They had achieved highly promising accuracies for 2D and 3D multi-class AD stage classification of 93.61% and 95.17%, respectively. The VGG19 pre-trained model had been fine-tuned and obtained an accuracy of 97% for multi-class AD stage classification [71]. Vandenberghe et al. had proposed a method for binary classification of 18F-flutemetramol PET using ML techniques for AD and mild cognitive impairment (MCI). They had tested whether support vector machines (SVM), a supervised ML technique, can duplicate the assignments made by blindfolded visual readers, as well as which image components had the highest diagnostic value according to SVM and how 18F-fluoromethylamol-based SVM classification compares to structural MRI-based SVM classification in the same cases. Their F-flutemetamol based classifier was able to replicate the assignments obtained by visual read with 100% accuracy [72]. Odusami et al. proposed a novel method for the detection of early-stage dementia from functional brain changes in MRI using a fine-tuned ResNet-18 network. Their research work presents a DL based technique for predicting MCI, early MCI, late MCI, and Alzheimer’s disease (AD). The ADNI fMRI dataset was used for analysis and consisted of 138 participants. On EMCI vs. AD, LMCI vs. AD, and MCI vs. AD, the fine-tuned ResNet18 network obtained classification accuracy of 99.99%, 99.95%, and 99.95%, respectively [73]. Zheng et al. had presented a ML based framework for differential diagnosis between VaD and AD using structural MRI features. The least absolute shrinkage and selection operator (LASSO) was then used to build a feature set that was fed into SVM for classification. To ensure unbiased evaluation of model performance, a comparative analysis of classification models was conducted using different ML algorithms to discover which one had better performance in the differential diagnosis between VaD and AD. The diagnostic performance of the classification models was evaluated using quantitative parameters derived from the receiver operating characteristic curve (ROC). The experimental finding had shown that the SVM with RBF performed well for the differential diagnosis of VaD and AD, with sensitivity (SEN), specificity (SPE), and accuracy (ACC) values of 82.65%, 87.17%, and 84.35%, respectively (AUC = 86.10–95%, CI = 0.820–0.902) [74]. Basheer et al. [75] had presented an innovative technique by making improvements in capsule network design for the best prediction outcomes. The study used the OASIS dataset with dimensions (373 X 15) to categorize the labels as demented or non-demented. To make the model swifter and more accurate, several optimization functions were performed on the variables, as well as the feature selection procedure. The claims were confirmed by demonstrating the correlation accuracy at various iterations and layers with an allowable accuracy of 92.39%. L. K. Leong and A. A. Abdullah had proposed a method for the prediction of AD based on ML techniques with the Boruta algorithm as a feature selection method. According to the Boruta algorithm, Random Forest Grid Search Cross Validation (RF GSCV) outperformed other 12 ML models, including conventional and fine-tuned models, with 94.39% accuracy, 88.24% sensitivity, 100.00% specificity, and 94.44% AUC even for the small OASIS-2 longitudinal MRI dataset [76]. Battineni et al. had presented a SVM based ML model for the prediction of dementia. Their proposed model had achieved an accuracy and precision of 68.75% and 64.18% using the OASIS-2 dataset [77]. Mathotaarachchi et al. had analyzed the amyloid imaging using ML approaches for the detection of dementia. To overcome the inherent unfavorable and imbalance proportions between persons with stable and progressing moderate cognitive impairment in a short observation period. The innovative method had achieved 84.00% accuracy and an AUC of 91.00% for the ROC [78]. Aruna and Chitra had presented a ML approach for the identification of dementia from MRI images, where they had deployed Independent Component Analysis (ICA) to extract the features from the images, and for classification purposes, SVM with different kernels is used. Through their proposed method, they had obtained an accuracy of 90.24% [79] (Fig. 5).
Fig. 5.
Accuracy comparison of different ML models based on image modality
Supervised ML techniques and CNNs were examined by Herzog and Magoulas. They had achieved the accuracy of 92.5% and 75.0% for NC vs EMCI, 93.0% and 90.5% for NC vs. AD, respectively [80]. Battineni et al. had comprehensive applied ML model on MRI to predict Alzheimer’s disease (AD) in older subjects, and they had proposed two ML models for AD detection. In the first trial, manual feature selection was utilized for model training, and ANN produced the highest AUC of 81.20% by ROC. The NB had earned the greatest AUC of 94.20% by ROC in the second trial, which included wrapping approaches for the automated feature selection procedure [81]. Ma et al. had conducted a study where they compared feature-engineered and non-feature-engineered ML methods for blinded clinical evaluation for dementia of Alzheimer’s type classification using FDG-PET. The highest accuracy of 84.20% was obtained through CNN’s [82]. Bidani et al. had presented a novel approach in the field of DL that combines both the deep convolutional neural network (DCNN) model and the transfer learning model to detect and classify dementia. When the features were retrieved, the dementia detection and classification strategy from brain MRI images using the DCNN model provided an improved classification accuracy of 81.94%. The transfer learning model, on the other hand, had achieved an accuracy of 68.13% [83].
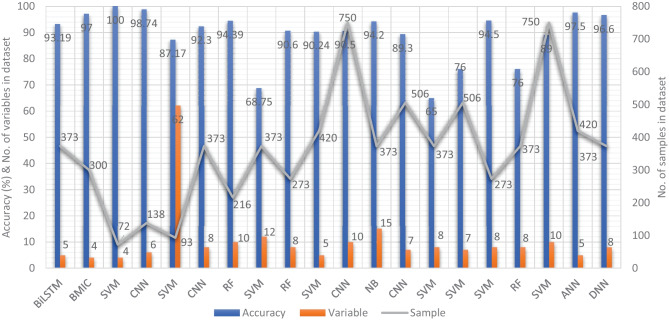
Moscoso et al. had designed a predictive model for the prediction of Alzheimer’s disease using MRI images. Their proposed model had obtained the highest accuracy of 84.00% [84]. Khan and Zubair had presented an improved multi-modal based ML approach for the prognosis of AD. Their proposed model had a five-stage ML pipeline, where each stage was further categorized into different sub-levels. Their proposed model had reported the highest accuracy of 86.84% using RF [85]. Mohammed et al. had evaluated the two CNN models (AlexNet and ResNet-50) and hybrid DL/ML approaches (AlexNet+SVM and ResNet-50+SVM) for AD diagnosis using the OASIS dataset. They had found that RF algorithm had attained an overall accuracy of 94%, as well as precision, recall, and F1 scores of 93%, 98%, and 96%, respectively [86]. Salvatore et al. had developed a ML method for early AD diagnosis using magnetic resonance imaging indicators. In their proposed ML model, they used PCA for extracting features from the images and SVM for the classification of dementia. They had achieved a classification accuracy of 76% using a 20-fold cross validation scheme [87]. Katako et al. had identified the AD related FDGPET pattern that is also found in LBD and Parkinson’s disease dementia using ML approaches. They studied different ML algorithms, but SVM with an iterative single data algorithm produced the best performance, i.e., sensitivity 84.00%, specificity 95.00% through 10-fold cross-validation [88]. Gray et al. had presented a system in which RF proximities were utilized to learn a low-dimensional manifold from labelled training data and then infer the clinical labels of test data that translated to this space. Their proposed model, voxel-based (FDG-PET), obtained an accuracy of 87.9% using ten-fold cross-validation [89]. Table 4 provides the overall performance evaluation of the ML models that were presented by the researchers for the prediction of dementia and its subtypes by using image data as a modality.
Table 4.
Performance evaluation of image-modality based ML models for dementia
| S.No | Authors | Year | D_ID | Feature | Model | Metrics | Acc.% | Spec.% | Sens.% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dashtipour et al. [70] | 2021 | 01 | CNN | BiLSTM | k-fold | 93.19 | 93.00 | 93.00 |
| 2 | Helaly et al. [71] | 2021 | 02 | CNN | BMIC | MCC | 97.00 | 98.00 | 94.00 |
| 3 | Vandenberghe et al. [72] | 2012 | 03 | Voxels | SVM | MCC | 100 | 92.00 | 85.00 |
| 4 | Odusami et al. [73] | 2021 | 04 | ReLU | CNN | MCC | 98.74 | 100 | 97.24 |
| 5 | Zheng et al. [74] | 2019 | 05 | LASSO | SVM | ROC | 87.17 | 87.17 | 82.65 |
| 6 | Basheer et al. [75] | 2019 | 06 | KPCA | CNN | MCC | 92.30 | NA | NA |
| 7 | Leong et al. [76] | 2019 | 07 | Boruta | RF | MCC | 94.39 | 100 | 84.24 |
| 8 | Battineni et al. [77] | 2019 | 08 | RBF | SVM | MCC | 68.75 | NA | NA |
| 9 | Mathotaarachchi et al. [78] | 2017 | 9 | RUSRF | F1-score | 90.60 | 86.50 | 70.80 | |
| 10 | Aruna and Chitra [79] | 2015 | 10 | ICA | SVM | MCC | 90.24 | NA | NA |
| 11 | Herzog et al. [80] | 2021 | 11 | std, RMS | CNN | MCC | 90.50 | 90.00 | 93.00 |
| 12 | Battineni et al. [81] | 2020 | 12 | ANN | NB | ROC | 94.20 | 89.56 | 89.92 |
| 13 | Ma et al. [82] | 2021 | 13 | FPDS | CNN | MCC | 89.30 | 67.00 | 84.50 |
| 14 | Bidani et al. [83] | 2019 | 14 | K-means | DNN | MCC | 81.94 | NA | NA |
| 15 | Moscoso et al. [84] | 2019 | 15 | NA | ML | AUC | 84.00 | 71.00 | 78.00 |
| 16 | De Bruijne [90] | 2016 | 16 | DNN | SVM | ROC | 65.00 | 88.00 | 80.00 |
| 17 | Mohammed et al. [86] | 2020 | 16 | IFS | ML | ROC | 90.00 | 93.00 | 98.00 |
| 18 | Salvatore et al. [87] | 2015 | 13 | PCA | SVM | k-fold | 76.00 | NA | NA |
| 19 | Katako et al. [88] | 2018 | 09 | ISDA | SVM | AUC | 94.50 | 95.00 | 84.00 |
| 20 | Moscoso et al. [89] | 2019 | 16 | FSL FAST | RF | MCC | 76.00 | 89.80 | 88.90 |
| 21 | Tong et al. [91] | 2014 | 11 | mi-Graph | SVM | k-Fold | 89.00 | 92.60 | 84.90 |
| 22 | Akhila et al. [92] | 2017 | 10 | SFTA | ANN | MCC | 97.50 | 97.50 | 97.50 |
| 23 | Chen and Pham [93] | 2013 | 16 | mi-Graph | Markov | k-Fold | 78.30 | 76.00 | 79.00 |
| 24 | Patil and Yardi [94] | 2013 | NA | DCT | ANN | MCC | 100 | NA | NA |
| 25 | Gulhare et al. [95] | 2017 | 06 | niblack | DNN | MCC | 96.60 | NA | NA |
D_ID: is a reference number of Dataset ID
ML based diagnostic models for dementia: Clinical-variable modality
Aside from image-based ML techniques for dementia prediction, several research studies have utilized clinical-variable data with ML algorithms to predict dementia and its subtypes. For instance, Chiu et al. had designed a screening instrument to detect MCI and dementia using ML techniques. They had developed a questionnaire to assist neurologists and neuropsychologists in the screening of MCI and dementia. The contribution of 45 items that matched the patient’s replies to questions was ranked using feature selection through information gain (IG). Among the 45 items, 12 were ranked the highest in feature selection. The ROC analysis showed that AUC in test group was 94.00% [96]. Stamate et al. had developed a framework for the prediction of MCI and dementia. Their proposed framework was based on the ReliefF approach paired with statistical permutation tests for feature selection, model training, tweaking, and testing using ML algorithms such as RF, SVM, Gaussian Processes, Stochastic Gradient Boosting, and eXtreme Gradient Boosting. The stability of model performances was studied using computationally expensive Monte Carlo simulations, and the results of their proposed framework were given as for dementia detection, the accuracy was 88.00%, sensitivity was 93.00%, and the specificity was 94.00%, whereas moderate cognitive impairment had a sensitivity of 86.00% and a specificity of 90% [97]. Stamate et al. developed a system for detecting dementia subtypes (AD) in blood utilizing DL and other supervised ML approaches such as RF and extreme gradient boosting. The AUC for the proposed DL method was 85% (0.80–0.89), for XGBoost it was 88% (0.86–0.89), and for RF it was 85% (0.83–0.87). In comparison, CSF measurements of amyloid, p-tau, and t-tau (together with age and gender) gave AUC values of 78%, 83%, and 87%, respectively, by using the XGBoost [98]. Bansal1 et al. had performed the comparative analysis of the different ML methods for the detection of dementia using clinical-variables. In their experiments, they exploited the performance of four ML models, such as J48, NB, RF, and multilayer perceptrons. From the results of experiments, they had concluded that j48 outperformed the rest of the ML models for the detection of dementia [99]. Nori et al. had experimented the lasso algorithm on a big dataset of patient and identify the 50 variables by ML model with an AUC of 69.30% [100]. Alam et al. [101]used signal processing on wearable sensor data streams (e.g., electrodermal activity (EDA), photoplethysmogram (PPG), and accelerometer (ACC)) and machine learning techniques to measure cognitive deficits and their relationship with functional health deterioration.
Gurevich et al. had used SVM and neuropsychological test for the classification of AD from other causes of cognitive impairment. The highest classification accuracy they had achieved through their proposed method was 89.00% [102]. Karaglani et al. had proposed a ML based automated diagnosis system for AD by using blood-based biosignatures. In their proposed method, they used mRNA-based statistically equivalent signatures for feature ranking and a RF model for classification. Their proposed automated diagnosis system had reported the accuracy of 84.60% using RF [103]. Ryzhikova et al. had analyzed cerebrospinal fluid for the diagnosis of AD by using ML algorithms. For classification purposes, artificial neural networks (ANN) and SVM discriminant analysis (SVM-DA) statistical methods were applied, with the best findings allowing for the distinguishing of AD and HC participants with 84.00% sensitivity and specificity. The proposed classification models have a high discriminative power, implying that the technique has a lot of potential for AD diagnosis [104]. Cho and Chen had designed a double layer dementia diagnosis system based on ML where fuzzy cognitive maps (FCMs) and probability neural networks (PNNs) were used to provide initial diagnoses at the base layer, and Bayesian networks (BNs) were used to provide final diagnoses at the top layer. Diagnosis results, “proposed treatment,” and “no treatment required” might be used to provide medical institutions with self-testing or secondary dementia diagnosis. The highest accuracy reported by their proposed system was 83.00% [105]. Facal et al. had studied the role of cognitive reserve in the conversion from MCI to dementia using ML. Nine ML classification algorithms were tried in their study, and seven relevant performance parameters were generated to assess the prediction accuracy for converted and non-converted individuals. The use of ML algorithms on socio-demographic, basic health, and CR proxy data allowed for the prediction of dementia conversion. The Gradient Boosting Classifier (ACC = 0.93; F1 = 0.86 and Cohen’s kappa = 0.82) and RF Classifier (ACC = 92%; F1 = 0.79 and Cohen’s kappa = 0.71) performed the best [106]. Jin et al. had proposed automatic classification of dementia from learning of clinical consensus diagnosis in India using ML techniques. All viable ML models exhibited remarkable discriminative skills (AUC >90%) as well as comparable accuracy and specificity (both around 95%). The SVM model beat other ML models by obtaining the highest sensitivity (0.81), F1 score (0.72), kappa (.70, showing strong agreement), and accuracy (second highest) (0.65). As a consequence, the SVM was chosen as the best model in their research work [107]. James et al. had evaluated the performance of ML algorithms for predicting the progression of dementia in memory clinic patients. According to their findings, ML algorithms outperformed humans in predicting incident all-cause dementia within two years. Using all 258 variables, the gradient-boosted trees approach had an overall accuracy of 92% , sensitivity of 0.45, specificity of 0.97, and an AUC of 0.92. Analysis of variable significance had indicated that just 6 variables were necessary for ML algorithms to attain an accuracy of 91% and an AUC of at least 89.00% [108]. Bougea et al. had investigated the effectiveness of logistic regression (LR), K-nearest neighbours (K-NNs), SVM, the Naive Bayes classifier, and the Ensemble Model to correctly predict PDD or DLB. The K-NN classification model exhibited an overall accuracy of 91.2% based on 15 top clinical and cognitive scores, with 96.42% sensitivity and 81% specificity in distinguishing between DLB and PDD. Based on the 15 best characteristics, the binomial logistic regression classification model had attained an accuracy of 87.5%, with 93.93% sensitivity and 87% specificity. Based on the 15 best characteristics, the SVM classification model had achieved an accuracy of 84.6% of overall instances, 90.62% sensitivity, and 78.58% specificity. A model based on NB classification obtained an accuracy of 82.05%, sensitivity of 93.10%, and a specificity of 74.41%. Finally, an ensemble model, which was constructed by combining the separate ones, attained 89.74% accuracy, 93.75% sensitivity, and 85.73% specificity [109] (Fig. 6).
Fig. 6.
Accuracy comparison of different ML models based on clinical-variable modality
Salem et al. had presented a regression-based ML model for the prediction of dementia. In their proposed method, they had investigated ML approaches for unbalanced learning. In their suggested supervised ML approach, they started by intentionally oversampling the minority class and undersampling the majority class, in order to reduce the bias of the ML model to be trained on the dataset. Furthermore, they had deployed cost-sensitive strategies to penalize the ML models when an instance was misclassified in the minority class. According to their findings, the balanced RF was the most resilient probabilistic model (with just 20 features/variables) with an F1 score of 0.82, a G-Mean of 0.88, and an AUC of 0.88 using ROC. With a F1-score of 0.74 and an AUC of 0.80 by ROC, the calibrated-weighted SVM was their top classification model for the same number of features [110]. Gutierrez et al. had designed an automated diagnosis system for the detection of AD and FTD by using feature engineering and genetic algorithms. Their proposed system had obtained the accuracy of 84% [111]. Mirzaei and Adeli had analyzed the state-of-the-art ML techniques used for the detection and classification of AD [112]. Hsiu et al. had studied ML algorithms for early identification of cognitive impairment. Their proposed model had obtained the accuracy of 70.32% by threefold cross-validation scheme [113]. Several classification models were constructed using various ML and feature selection methodologies to automate MCI detection using gait biomarkers. They had demonstrated, however, that dual-task walking differentiated between MCI and CN individuals. The ML model used for MCI pre-screening based on inertial sensor-derived gait biomarkers achieved 71.67% accuracy and 83.33% sensitivity, respectively, as reported by Shahzad et al. [114]. Hane et al. investigated the use of deidentified clinical notes acquired from multiple hospital systems over a 10-year period to enhance retrospective ML models predicting the risk of developing AD. The AUC improved from 85.00% to 94.00% by utilizing clinical notes, and the positive predictive value (PPV) rose from 45.07% (25,245/56,018) to 68.32% (14,153/20,717) in the model at the beginning of disease [115]. Table 5 provides the overall performance evaluation of the ML models that were presented by the researchers for the prediction of dementia and its subtypes by using clinical-variable data as a modality.
Table 5.
Performance evaluation of Clinical-Variable based ML models for dementia
| S.No | Authors | Year | D_ID | Feature | Model | Metrics | Acc.% | Spec.% | Sens.% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chiu et al. [96] | 2019 | 17 | IG | DT | ROC | 96.00 | 93.00 | 92.00 |
| 2 | Stamate et al. [97] | 2018 | 18 | SVM | RF | k-fold | 88.00 | 94.00 | 93.00 |
| 3 | Visser et al. [98] | 2019 | 19 | XGBoost | RF | k-fold | 85.00 | NA | NA |
| 4 | Bansal et al. [99] | 2018 | 20 | WEKA | J48 | k-fold | 98.66 | 95.00 | 81.00 |
| 5 | Nori et al. [100] | 2019 | 21 | Lasso | RF | AUC | 69.30 | 98.70 | 16.40 |
| 6 | Alam et al. [101] | 2016 | 22 | high-pass filter | RF | k-fold | 91.50 | 91.80 | 95.70 |
| 7 | Gurevich et al. [102] | 2017 | 23 | PCA | SVC | MCC | 89.00 | 94.00 | 74.00 |
| 8 | Karaglani et al. [103] | 2020 | 24 | mRNA | RF | ROC | 84.60 | NA | NA |
| 9 | Ryzhikova et al. [104] | 2021 | 25 | GA | SVM-DA | k-fold | 90.30 | 84.00 | 84.00 |
| 10 | Cho and Chen [105] | 2012 | 26 | FCM | PNNs | NA | 82.00 | NA | NA |
| 11 | Facal et al. [106] | 2019 | 27 | ANN | RF | k-fold | 92.00 | 88.00 | 88.00 |
| 12 | Jin et al. [107] | 2021 | 28 | NA | SVM | MCC | 96.00 | 95.00 | 81.00 |
| 13 | James et al. [108] | 2021 | 29 | NA | GBT | k-fold | 92.00 | 97.00 | 45.00 |
| 14 | Bougea et al. [109] | 2021 | 30 | NA | K-NN | AUC | 91.00 | 85.73 | 93.75 |
| 15 | Salem et al. [110] | 2021 | 31 | NA | RF | ROC | 88.00 | NA | NA |
| 16 | Garcia-Gutierrez et al. [111] | 2022 | 32 | GA | DT | F1-score | 88.00 | NA | NA |
| 17 | Mirzaei and Adeli [112] | 2022 | 33 | RF | CNN | k-fold | 95.74 | NA | NA |
| 18 | Hsiu et al. [113] | 2022 | 34 | LDA | KNN | k-fold | 70.32 | 72.00 | 68.00 |
| 19 | Shahzad et al. [114] | 2022 | 35 | EFA | SVM | k-fold | 71.67 | NA | 83.33 |
| 20 | Hane et al. [115] | 2020 | 36 | NA | ML | AUC | 94.00 | 98.00 | 45.00 |
| 21 | Aschwanden et al. [116] | 2020 | 37 | NA | RF | AUC | 64.00 | NA | 67.00 |
| 22 | Ryu et al. [117] | 2020 | 20 | HPO | XGBoost | K-fold | 85.61 | 81.40 | 77.27 |
| 23 | de Langavant et al. [118] | 2018 | 37 | PCA | k-mean | AUC | 91.00 | 93.30 | 93.60 |
| 24 | Fouladvand et al. [119] | 2019 | 38 | RNN | RF | F1-score | 82.00 | 44.30 | 13.00 |
| 25 | Balea-Fernandez et al. [120] | 2021 | 39 | GA | RF | AUC | 80.00 | 71.00 | 100 |
D_ID: is a reference number of Dataset ID
ML based diagnostic models for dementia: Voice modality
Similar to the image and clinical-variable modalities, researchers had also developed automated diagnostic systems based on voice data for the prediction of dementia. Hereby, we have reviewed the research work done by the scientists in detail. For example, Chlasta and Wolk had worked on the computer-based automated screening of dementia patients by spontaneous speech analysis using DL and ML techniques. In their work, they used neural networks to extract the features from the voice data; the extracted features were then fed into a linear SVM for classification purposes. Their SVM model had obtained the accuracy of 59.1% while CNN based ML model had reported the accuracy of 63.6% [121]. Chien et al. had presented an ML model for the assessment of AD using speech data. Their suggested model included a feature sequence that was used to extract the features from the raw audio data, as well as a recurrent neural network (RNN) for classification. Their proposed ML model had reported an accuracy of 83.80% based on the ROC curve [122]. Shimoda et al. had designed an ML model that identified the risk of dementia based on the voice feature in telephone conversations. Extreme gradient boosting (XGBoost), RF, and LR based ML models were used, with each audio file serving as one observation. The predictive performance of the constructed ML models was tested by characterizing the ROC curve and determining the AUC, sensitivity, and specificity [123]. Nishikawa et al. had developed an ensemble discriminating system based on a classifier with statistical acoustic characteristics and a neural network of transformer models, with an F1-score of 90.70% [124]. Liu et al. had introduced a new technique for recognizing Alzheimer’s disease that used spectrogram features derived from speech data, which aided families in comprehending the illness development of patients at an earlier stage, allowing them to take preventive measures. They used ML techniques to diagnose AD using speech data collected from older adults who displayed the attributes described in the speech. Their proposed method had obtained the maximum accuracy of 84.40% based on LogisticRegressionCV [125]. Searle et al. had created a ML model to assess spontaneous speech, which might potentially give an efficient diagnostic tool for earlier AD detection. Their suggested model was a fundamental Term Frequency-Inverse Document Frequency (TF-IDF) vectorizer as input into an SVM model, and the top performing models were a pre-trained transformer-based model ’DistilBERT’ when used as an embedding layer into simple linear models. The proposed model had obtained the highest accuracy of 82.00% [126]. Zhu et al. had suggested an ML model that employed the speech pause as an effective biomarker in dementia detection, with the purpose of reducing the detection, model’s confidence levels by adding perturbation to the speech pauses of the testing samples. They next investigated the impact of the perturbation in training data on the detection model using an adversarial training technique. The proposed model had achieved an accuracy of 84.00% [127]. Ossewaarde et al. had proposed ML model based on SVM for the classification of spontaneous speech of individuals with dementia based on automatic prosody analysis. Their findings suggest that the classifier can distinguish some dementia types (PPA-NF, AD), but not others (PD) [128]. Xue et al. had developed an ML model based on DL for the detection of dementia by using voice recordings. In their ML model, long short-term memory (LSTM) network and the convolutional neural network (CNN) utilized audio recordings to categorize whether the recording contained a participant with either NC or only DE and to discriminate between recordings belonging to those with DE and those without DE (i.e., NDE (NC+MCI)) [129]. Weiner et al. had presented two pipelines of feature extraction for dementia detection: the manual pipeline used manual transcriptions, while the fully automatic pipeline used transcriptions created by automatic speech recognition (ASR). The acoustic and linguistic features that they had extracted need no language specific tools other than the ASR system. Using these two different feature extraction pipelines, they had automatically detect dementia [130] (Fig. 7).
Fig. 7.
Accuracy comparison of different ML models based on voice modality
Furthermore, Sadeghian et al. had presented the empirical evidence that a combination of acoustic features from speech, linguistic features were extracted from an automatically determined transcription of the speech including punctuation, and results of a mini mental state exam (MMSE) had achieved strong discrimination between subjects with a probable AD versus matched normal controls [131]. Khodabakhsh et al. had evaluated the linguistic and prosodic characteristics in Turkish conversational language for the identification of AD. Their research suggested that prosodic characteristics outperformed linguistic features by a wide margin. Three of the prosodic features had helped to achieve a classification accuracy of more than 80%, However, their feature fusion experiments did not improve classification performance any more [132]. Edwards et al. had analyzed the text data at both the word level and phoneme level, which leads to the best-performing system in combination with audio features. Thus, the proposed system was both multi-modal (audio and text) and multi-scale (word and phoneme levels). Experiments with larger neural language models had not resulted in improvement, given the small amount of text data available [133]. Kumar et al. had identified speech features relevant in predicting AD based on ML. They had deployed neural network for the classification and obtained the accuracy of 92.05% [134]. Ossewaarde et al. had built ML model based on SVM for the classification from spontaneous speech of individuals with dementia by using automatic prosody [128]. Luz et al. had developed an ML approach for analyzing patient speech in dialogue for dementia identification. They had designed a prediction model, and the suggested strategy leveraged additive logistic regression (ML boosting method) on content-free data gathered through dialogical interaction. Their proposed model obtained the accuracy of 86.50% [135]. Sysed et al. had designed a multimodal system that identified linguistic and paralinguistic traits of dementia using an automated screening tool. Their proposed system had used bag-of-deep-feature for feature selection and ensemble model for classification [136]. Moreover, Sarawgi et al. had used multimodal inductive transfer learning for AD detection and severity. Their proposed system further achieved state-of-the-art AD classification accuracy of 88.0% when evaluated on the full benchmark DementiaBank Pitt database. Table 6 provides the overall performance evaluation of the ML models that were presented by the researchers for the prediction of dementia and its subtypes by using voice-modality data.
Table 6.
Performance evaluation of voice-modality based ML models for dementia
| S.No | Authors | Year | D_ID | Feature | Model | Metrics | Acc.% | Spec.% | Sens.% |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chlasta and Wołk [121] | 2021 | 40 | VGGish | CNN | F1-score | 63.60 | NA | NA |
| 2 | Chien et al. [122] | 2021 | 41 | CRNN | RNN | ROC | 83.80 | 83.80 | 75.60 |
| 3 | Shimoda et al. [123] | 2021 | 42 | PRAAT | RF | AUC | 88.20 | 80.00 | 96.40 |
| 4 | Nishikawa et al. [124] | 2022 | 43 | lightGBM | ViT_b16 | F1-score | 90.70 | NA | 87.5 |
| 5 | Liu et al. [125] | 2019 | 44 | Spectrogram | LR | k-fold | 84.40 | 91.30 | 87.50 |
| 6 | Searle et al. [126] | 2022 | 45 | CRF | SVM | F1-score | 82.00 | NA | NA |
| 7 | Zhu et al. [127] | 2021 | 46 | Wav2vec | BERT | ROC | 83.00 | NA | NA |
| 8 | Ossewaarde et al. [128] | 2019 | 47 | VAD | SVM | ROC | 93.00 | NA | NA |
| 9 | Xue et al. [129] | 2021 | 48 | RNN | CNN | AUC | 80.50 | 75.00 | 87.30 |
| 10 | Weiner [130] | 2017 | 49 | ASR | DNN | Acc | 90.24 | NA | NA |
| 11 | Sadeghian et al. [131] | 2017 | 50 | VAD | NN | Acc | 91.70 | NA | NA |
| 12 | Khodabakhsh et al. [132] | 2015 | 51 | VAD | NN | Acc | 80.00 | NA | NA |
| 13 | Edwards et al. [133] | 2020 | 52 | LDA | RF | F1-score | 92.30 | NA | NA |
| 14 | Kumar et al. [134] | 2021 | 53 | SVC | NN | MCC | 92.05 | NA | NA |
| 15 | Ossewaarde et al. [128] | 2019 | 54 | VAD | SVM | ROC | 84.00 | NA | NA |
| 16 | Luz et al. [135] | 2018 | 55 | VGO | SVM | ROC | 86.50 | 88.00 | 81.20 |
| 17 | Xue et al. [129] | 2021 | 56 | LSTM | CNN | AUC | 80.50 | NA | NA |
| 18 | Orsulic-Jeras et al. [137] | 2021 | 57 | PCA | SVM | k-fold | 76.00 | NA | NA |
| 19 | Syed et al. [136] | 2021 | 58 | Fusion | SVC | Acc | 95.37 | 94.44 | 96.30 |
| 20 | Sarawgi et al. [138] | 2020 | 59 | PCA | Ensemble | F1-score | 88.00 | NA | NA |
| 21 | Calza et al. [139] | 2021 | NA | SSVAD v1.0 | RF | F1-score | 75.00 | NA | NA |
| 22 | Haider et al. [140] | 2019 | 60 | eGeMAPS | LDA | F1-score | 78.70 | NA | NA |
| 23 | López-de-Ipiña et al. [141] | 2015 | 61 | SSF + EF | SVM | k-fold | 86.04 | NA | NA |
| 24 | Orimaye et al. [142] | 2014 | 60 | IG | SVM | F1-score | 74.00 | 75.00 | 73.00 |
| 25 | Santander-Cruz et al. [143] | 2022 | 61 | BERT | SVM | k-fold | 77.00 | 80.00 | 80.00 |
D_ID: is a reference number of Dataset ID
Discussion
In this SLR, we examined the research work that employed ML and DL algorithms to analyze clinical data in order to identify variables that might help predict dementia. We studied 75 research articles that were published in the last 10 years that used image, clinical-variable, and voice data to predict dementia and its subtypes. Nowadays, the healthcare industry creates a vast quantity of data on patients’ health; this data is used by researchers to enhance individual health by utilizing developing technologies such as ML and DL. As a result, researchers can not only distinguish dementia patients from healthy people with high accuracy, but also forecast the disease progression of MCI patients.
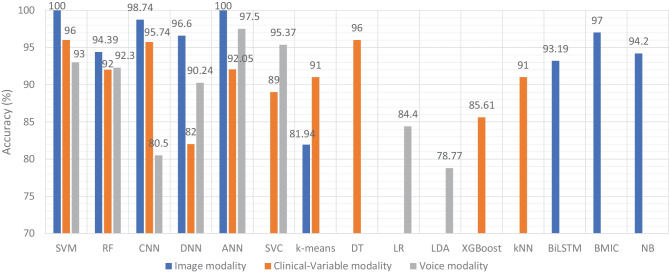
Therefore, researchers have expressed a strong interest in designing and developing automated diagnostic systems based on ML and DL techniques. As seen in Fig. 4., there has been an exponential increase in the number of such research publications that use ML algorithms for dementia prediction and detection in the previous four years. We investigated the selected papers using significant performance assessment criteria for ML and DL approaches such as data attributes, computational methodologies, and study emphasis. In this SLR, we have uncovered research gaps in the present literature as well as anticipated future research opportunities. Additionally, in Fig. 8 model comparison, we examined the performance of multiple ML algorithms for dementia prediction based on three types of data modalities: image, clinical-variable, and voice. The accuracy gained by image-based ML algorithms is higher when compared to clinical-variable and voice modalities, as shown in Fig. 8 model comparison. Moreover, the researchers’ suggested SVM, RF, and ANN-based ML techniques outperformed the rest of the ML algorithms in terms of performance. According to Fig. 8 model comparison, voice modality-based ML models show worse accuracy when compared to image and clinical-variable modality data. As a result, there is still a performance gap for researchers to close in order to improve the performance of ML algorithms for the prediction of dementia using voice data. Hence, researchers have shown a strong interest in the creation of automated diagnosis systems for dementia prediction utilizing speech data and ML algorithms, as illustrated in Fig. 4.
Fig. 8.
Accuracy comparison of ML models based on data modality
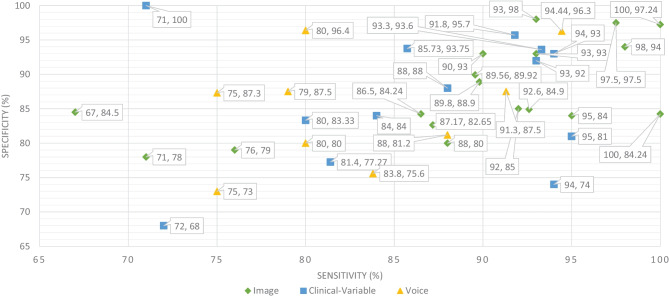
The ML and DL models are likely prone to problems such as poor quality of data, poor selection of ML model, Bias Variance tradeoff and training too complex models. Thus, scientists have developed various evaluation metrics (i.e., ROC, AUC, MCC, F1-score, K-fold) and methods to avoid these problems. The data is a crucial element in ML because ML models work only with numeric data; therefore, poor data quality results in lower performance of ML models. Moreover, imbalance classes in the dataset also cause the bias results from the ML models. Thus, this problem can be overcome by oversampling or undersampling the training data. There are different techniques that are used by the AI engineers for oversampling, such as random oversampling and the synthetic minority oversampling technique (SMOTE). To evaluate the bias researchers’ work, use sensitivity and specificity as an evaluation metric to measure the bias of the ML model. Higher values of sensitivity and specificity means model is free from the biasness while having either one parameter value higher and other one is lower means there is biasness exist. Thus, we have also studied the sensitivity and specificity, along with the accuracy, of the previously proposed ML models for dementia prediction. Figure 9 Comparison provides a brief description of the sensitivity and specificity of the ML models for the detection of dementia based on different data modalities. From Fig. 9, we can observe that ML models have higher values for sensitivity and specificity when using image data as compared to clinical-variable and voice modality data. In comparison to accuracy from Fig. 8 to sensitivity and specificity from Fig. 9, we have noted that the results obtained from image based modality are more reliable and precise using ML and DL algorithms in spite of clinical and voice modality.
Fig. 9.
Sensitivity and specificity comparison of ML based on modality
Furthermore, the correlation between sensitivity and specificity would help us understand the efficacy of the ML models, which are designed for automated disease prediction. The mathematical terms “sensitivity” and “specificity” indicate the accuracy of a test that reports the presence or absence of a disease. Individuals who meet the requirement are labelled “positive,” while those who do not are considered “negative”. The chance of a positive test, conditioned on being actually positive, is referred to as sensitivity (the true positive rate), while specificity (true negative rate) is the likelihood of a negative test if it is actually negative. Sensitivity and specificity are inversely proportional, which means that as sensitivity rises, specificity falls, and vice versa. Mathematically, sensitivity and specificity are given as:
| 1 |
| 2 |
On the other hand, accuracy is a ratio of number of correct assessments / number of all assessments. The proportion of genuine positive outcomes (both true positive and true negative) in the selected population is represented by the numerical value of accuracy. The test result is accurate 99% of the time, whether positive or negative. For the most part, this is right. However, it is worth noting that the equation of accuracy means that even if both sensitivity and specificity are high, say 99%, this does not imply that the test’s accuracy is also high. In addition to sensitivity and specificity, accuracy is determined by the prevalence of the illness in the target population. A diagnosis for a rare ailment in the target group may have high sensitivity and specificity but low accuracy. However, for a balanced dataset, ML models with higher sensitivity and specificity result in higher accuracy. Hence, accuracy must be interpreted carefully. The mathematical formula for accuracy is given as:
| 3 |
where, TP stands for the number of true positives, FP stands for the number of false positives, TN stands for the true negative, and FN stands for the false negative.
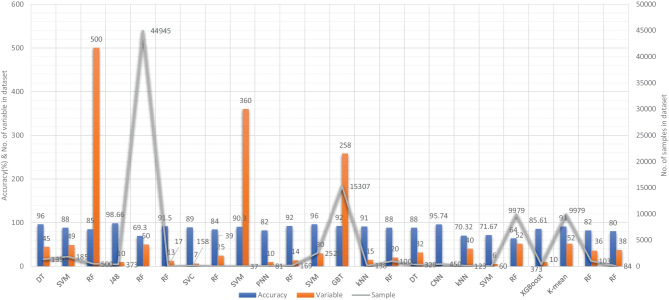
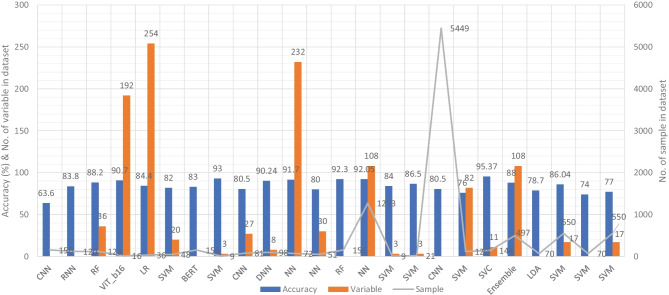
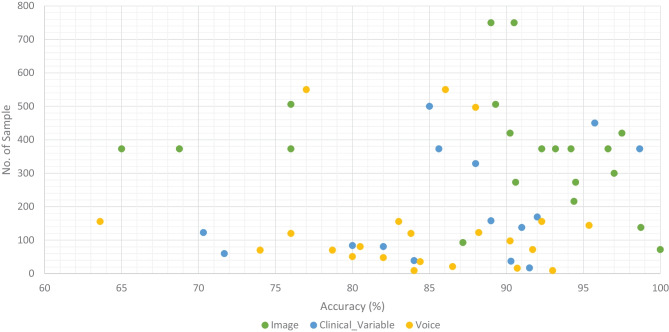
We classified all datasets that were used by researchers to test the performance of their proposed ML models for the prediction of dementia (AD, VaD, MCI, and FTD) into three types: image, clinical-variable, and voice. A total of 61 datasets were examined in terms of the number of samples and variables in the datasets. In image modality datasets from the Table 1, it can be observed that the ADNI dataset has a significant number of samples, which is 750, while the NINDS-AIREN dataset has more variables as compared to the rest of the datasets in the image modality data. Moreover, from the Table 2 of clinical-variable modality datasets, it can be noticed that the ADRD dataset has the highest number of samples (44945) as compared to the rest of the dataset, while the Raman spectral dataset has the highest number of variables (366). In the last, Table 3 of voice data modality elaborated the dataset of voice modality where FHS dataset has highest number of samples of 5449 while VBSD dataset had highest variables of 254 as compared to rest of the datasets in voice modality. The type of data and the size of the dataset are two important factors that have a significant influence on the performance of ML models. Thus, we have also studied this factor by comparing the accuracy along with the number of samples in the dataset with respect to data modalities. From Fig. 10, it can be observed that the majority of the ML models that used image data have higher accuracy along with a higher number of samples in the dataset. There are few ML models that show poor performance when the number of samples in the dataset is large. While, clinical-variable and voice modalities show prominent performance when the number of samples in the dataset is small.
Fig. 10.
Accuracy comparison of ML models along with number of sample in the dataset based on data modality
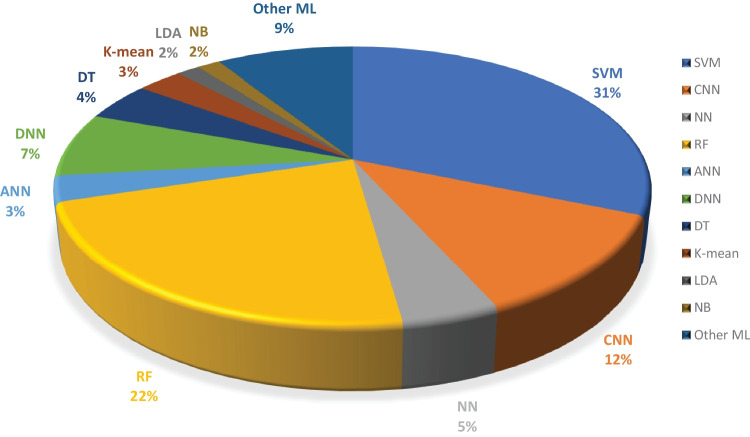
Moreover, we examined the effectiveness of ML classifiers utilized by the researchers in their proposed automated diagnostic systems for dementia prediction and classification. According to the selected studies of this SLR, SVM is the most commonly used ML classifier by researchers for the classification of patients and normal subjects using three data modalities (i.e., image, clinical-variable, voice), RF is the second most commonly used ML classifier by researchers, and CNN is the third most commonly used ML classifier by researchers. It can be observed from the Fig. 11. SVMs are the most powerful tools for the binary classification task, along with RF. From Fig. 8, we can see that SVM also obtained the highest average accuracy based on three types of data modalities. Hence, this factor also encourages the scientists to employ SVM as a binary classifier for dementia prediction or other disease prediction systems. From Fig. 11, we can observe the percentage of other ML classifiers that were used by the researchers in selected research articles for the automated diagnosis of dementia.
Fig. 11.
Overall percentage of ML models used in the selected research articles regardless of data modality
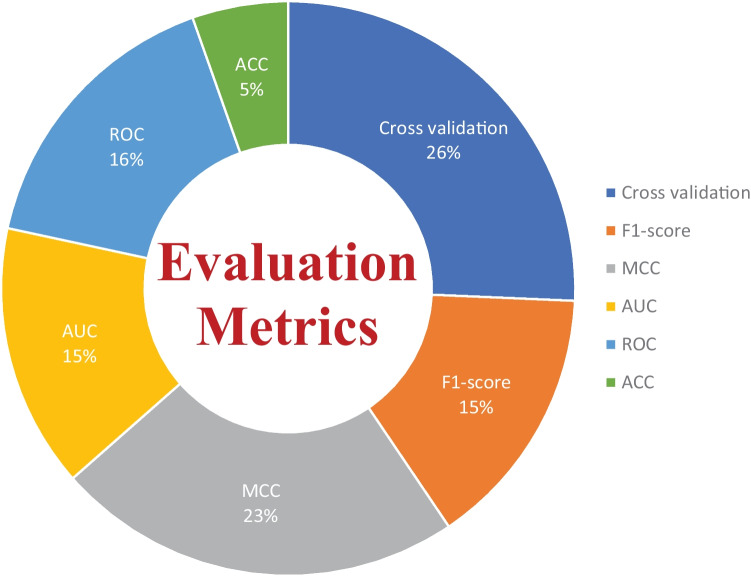
There are several evaluation metrics that are used for the performance assessment of ML models, such as F1score, AUC, ROC, Matthew’s correlation coefficient (MCC), cross-validation, K-fold, specificity, sensitivity, and accuracy. Each evaluation metric has its own pros and cons. Thus, the selection of appropriate evaluation metrics for the assessment of the ML model is essential to understanding its efficiency and performance. For instance, when data plays a vital role in ML models for decision-making and a dataset has unbalanced classes, it may be possible that results from the ML predictive model might be biassed due to the unbalanced nature of the data in the dataset. Thus, here evaluation metrics help to eliminate the factor of biasness in the results, i.e., the k-fold. The F1-score evolution metric is suitable for the classification of multiple classes in the dataset. while ROC tells us how well the ML model can differentiate binary classes. As a result, AUC and ROC reveal how effectively the probabilities from the positive classes are separated from the probabilities from the negative classes. From Fig. 12, it can be depicted that cross validation is mostly used in the studies that were selected for this SLR to evaluate the performance of proposed ML models. MCC is the second most used evaluation metric, while ROC is in third place. The proposition of other evaluation metrics used by the researchers to validate the efficiency of their proposed ML models can be observed from Fig. 12.
Fig. 12.
Overall percentage of evaluation metrics of ML models used by the researchers in the selected research articles
Limitations in the previously proposed ML models
ML algorithms have been effectively applied to a broad range of real-world challenges, including banking, cybersecurity, transportation, and robots. They do, however, have fundamental limitations that make them inappropriate for every problem. In the clinical domain, researchers have concentrated on the supervised learning approach, developing various automated diagnostics for AD, MCI, and dementia prediction using supervised machine algorithms. From the Figs. 8 and 11, It can be noticed that supervised ML classifiers are mostly used by the researchers in the selected past research articles. Because supervised machine learning approaches have various limitations, automated diagnostic methods for dementia prediction based on supervised techniques suffer from some, if not all, of these constraints. In this part, we have examined the drawbacks of supervised ML-based techniques for dementia prediction, which are as follows:
The model overfitting problem affects the performance of ML models. As previously indicated, several researchers have used the k-fold cross-validation approach to evaluate the efficacy of their constructed diagnostic system. However, because of data leaks, it may result in highly biassed findings.
To deal with problem of imbalance classes in the dataset, Researchers and scientists had devised several techniques to eliminate the problem of imbalance classes such as random oversampling example (ROSE), synthetic minority over-sampling technique (SMOTE) and random over sampling (ROS) etc. Unfortunately, in the selected study, the researchers had not considered this factor to deal with the problem of imbalanced classes in the dataset that cause problems of bias.
Supervised ML models require training on a dataset; nevertheless, training on a large quantity of significant data is a hard and time-consuming job, especially for slow learning algorithms like kNN.
For training and testing of the ML models, researchers had used different data partitioning methods, which resulted in inconsistent comparisons of accuracy and other evaluation metrics among the proposed ML models for dementia prediction. Thus, standard data partition schemes should be adopted (holdout) for the comparison of ML models developed by the researcher for dementia prediction.
Another challenge with ML-based automated diagnostic systems for dementia is the time complexity of the proposed ML algorithms. The time complexity means the overall time require to complete all the computational tasks by the ML model for making a prediction. The ML model can forecast results only after it has been trained on the training data, which takes time to analyze. Furthermore, ML models include a large number of parameters that must be manually modified in the case of supervised learning. As a result, it takes a significant amount of effort and time to fine-tune the hyperparameters of the ML model in order to get higher performance.
DL technology has demonstrated cutting-edge performances for the prediction of various diseases in the recent years. However, DL technology needs a massive quantity of data for model training, which is a time-consuming and tough task. Due to the complexity of data models, training is quite costly. Furthermore, DL necessitates the use of pricey GPUs and hundreds of workstations, which are not effective in terms of economics.
Future research directions
In recent years, several ML models have been presented for the prediction of AD and MCI; nevertheless, there are still certain areas that need to be explored by academics and experts. In this section, we have discussed different research areas and the future prospects of ML algorithms for dementia detection. We infer from this study that the following major parameters have a role in the efficient identification of dementia and its forms.
Data is extremely important in the case of ML-based automated detection of dementia, especially when DL models are considered. Many of the publicly available datasets, however, are modest in size. But future research should concentrate on gathering a huge number of samples for the datasets. In this SLR, we studied ML-based automated diagnostic systems for dementia prediction using three different kinds of data modalities (image, clinical_variable, voice). From Fig. 10, it can be observed that only the image modality based ML model obtained the higher accuracy along with the large size of the dataset, while the voice modality based ML model obtained the higher accuracy on a small dataset. Thus, for the researchers, there is still room available for designing and developing the automated prediction of dementia and its sub-types by using voice data. Therefore, the interest of researchers have been tremendously raised for the development of automated diagnostic systems for dementia prediction using voice data modality and this trend can be confirmed from the Fig. 4. There is still a lot room available for the improvement in design and construction of automated diagnostic systems for the dementia using clinical-variable data modality for the researchers. Because, the ML model was developed in the past using clinical-variable data, it displays mix performance by using clinical_variable modality, i.e., when the number of samples is lower in the dataset, the ML shows lower accuracy. Thus. In the future, we need to increase the number of samples in the dataset so that we have larger datasets for experimental purposes and the designed ML model can be effectively evaluated.
In selected studies of this SLR, the majority of ML algorithms belong to the supervised category of learning. While few researchers used an unsupervised ML approach for the prediction of dementia and its subtypes, Altough, unspervsied learning approaches suffer from the limitation such as less accuracy, more expensive in term of computational etc. Therefore, it will encourage scientists and researchers to design and construct new techniques and methods using supervised ML algorithms that are more precise and accurate for the prediction of dementia and its subtypes. Moreover, in this SLR, we have analysed the various ML models based on three data modalities (image, clinical-variable, and voice), and we have comprehensively compared previously proposed ML-based systems in terms of various evaluation metrics, but with different data modalities, it would be suggested that multimodal processing techniques based on ML would provide more reliable and efficient results. Hence, in the future, researchers should exploit multimodal approaches based on ML for a better prediction of dementia and its subtypes.
Conclusion
In contrast to earlier SLR studies that examined numerous ML techniques proposed for the automated diagnosis of dementia and its subtypes (AD, VaD, FTD, and MCI) using one type of data modality, this study reviewed ML methods for dementia considering different types of data modalities such as image data, clinical variables, and voice data. The research articles published from 2011 to 2022 were gathered using different databases. It was pointed out that ML approaches based on image data modality has shown better performance compared with ML methods trained on clinical variables based data and voice data modality. Furthermore, this study critically evaluated the previously proposed methods and highlighted limitations in these methods. To overcome these limitations, this study presented future research directions in the domain of automated dementia prediction using ML approaches. We hope that this SLR will be helpful for AI and ML researchers and medical practitioners who are working in the domain of automated diagnostic systems for dementia prediction.
Acknowledgements
The first author’s learning process was supported by the National E-Infrastructure for Aging Research (NEAR), Sweden. NEAR is working on improving the health condition of older adults in Sweden.
Author Contribution
Conceptualization by Peter Anderber, Data curation by Liaqat ALi, Formal analysis by Ana Luiza Dallora, Write up and Methodology by Ashir Javeed, Proofread by Arif Ali, Supervised by Johan Sanmartin Berglund. If any of the sections are not relevant to your manuscript.
Funding
Open access funding provided by Blekinge Institute of Technology. This research received no external funding.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethics Approval
This study was carried out in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee at Blekinge Institute of Technology (BTH).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
The original online version of this article was revised: The author name "Liaqata Ali" was incorrect in the original publication of this article. The name should be "Liaqat Ali"
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ashir Javeed and Ana Luiza Dallora contributed equally to this work.
Change history
9/14/2024
A Correction to this paper has been published: 10.1007/s10916-024-02109-4
References
- 1.Menéndez, G.: La revolución de la longevidad: cambio tecnológico, envejecimiento poblacional y transformación cultural. Revista de Ciencias Sociales 30(41), 159–178 (2017)
- 2.Prince, M.J., Wimo, A., Guerchet, M.M., Ali, G.C., Wu, Y.-T., Prina, M.: World alzheimer report 2015-the global impact of dementia: An analysis of prevalence, incidence, cost and trends (2015)
- 3.Vrijsen, J., Matulessij, T., Joxhorst, T., de Rooij, S.E., Smidt, N.: Knowledge, health beliefs and attitudes towards dementia and dementia risk reduction among the dutch general population: a cross-sectional study. BMC public health 21(1), 1–11 (2021) [DOI] [PMC free article] [PubMed]
- 4.Widiger, T.A., Costa, P.T., Association, A.P., et al: Personality Disorders and the Five-factor Model of Personality. JSTOR, (2013)
- 5.Lo, R.Y.: The borderland between normal aging and dementia. Tzu-Chi Medical Journal 29(2), 65 (2017) [DOI] [PMC free article] [PubMed]
- 6.WHO: Dementia. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/dementia
- 7.Budson, A.E., Solomon, P.R.: Memory Loss E-book: A Practical Guide for Clinicians. Elsevier Health Sciences, (2011)
- 8.friendly wyoming, D.: Types of dementia. Dementia friendly wyoming. https://www.dfwsheridan.org/types-dementia
-
9.Tackenberg, C., Kulic, L., Nitsch, R.M.: Familial alzheimer’s disease mutations at position 22 of the amyloid
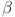 -peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system. PloS one 15(9), 0239584 (2020) [DOI] [PMC free article] [PubMed]
-peptide sequence differentially affect synaptic loss, tau phosphorylation and neuronal cell death in an ex vivo system. PloS one 15(9), 0239584 (2020) [DOI] [PMC free article] [PubMed] - 10.Wang, H., Kulas, J.A., Wang, C., Holtzman, D.M., Ferris, H.A., Hansen, S.B.: Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proceedings of the National Academy of Sciences 118(33) (2021) [DOI] [PMC free article] [PubMed]
- 11.Vilchez, D., Saez, I., Dillin, A.: The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nature communications 5(1), 1–13 (2014) [DOI] [PubMed]
- 12.Breijyeh, Z., Karaman, R.: Comprehensive review on alzheimer’s disease: Causes and treatment. Molecules 25(24), 5789 (2020) [DOI] [PMC free article] [PubMed]
- 13.Long, J.M., Holtzman, D.M.: Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179(2), 312–339 (2019) [DOI] [PMC free article] [PubMed]
- 14.Román, G.C.: Vascular dementia may be the most common form of dementia in the elderly. Journal of the neurological sciences 203, 7–10 (2002) [DOI] [PubMed]
- 15.Gold, G., Bouras, C., Canuto, A., Bergallo, M.F., Herrmann, F.R., Hof, P.R., Mayor, P.-A., Michel, J.-P., Giannakopoulos, P.: Clinicopathological validation study of four sets of clinical criteria for vascular dementia. American Journal of Psychiatry 159(1), 82–87 (2002) [DOI] [PubMed]
- 16.Lina, R.: Atrial fibrillation in aging; methodological aspects and the relation to dementia and cerebral vascular disease (2022)
- 17.Kuźma, E., Lourida, I., Moore, S.F., Levine, D.A., Ukoumunne, O.C., Llewellyn, D.J.: Stroke and dementia risk: a systematic review and meta-analysis. Alzheimer’s & Dementia 14(11), 1416–1426 (2018) [DOI] [PMC free article] [PubMed]
- 18.Tay, J., Morris, R.G., Tuladhar, A.M., Husain, M., de Leeuw, F.-E., Markus, H.S.: Apathy, but not depression, predicts all-cause dementia in cerebral small vessel disease. Journal of Neurology, Neurosurgery & Psychiatry 91(9), 953–959 (2020) [DOI] [PMC free article] [PubMed]
- 19.Morton, R.E., St. John, P.D., Tyas, S.L.: Migraine and the risk of all-cause dementia, alzheimer’s disease, and vascular dementia: A prospective cohort study in community-dwelling older adults. International journal of geriatric psychiatry 34(11), 1667–1676 (2019) [DOI] [PubMed]
- 20.Sanford, A.M.: Lewy body dementia. Clinics in geriatric medicine 34(4), 603–615 (2018) [DOI] [PubMed]
- 21.Taylor, J.-P., McKeith, I.G., Burn, D.J., Boeve, B.F., Weintraub, D., Bamford, C., Allan, L.M., Thomas, A.J., T O’Brien, J.: New evidence on the management of lewy body dementia. The Lancet Neurology 19(2), 157–169 (2020) [DOI] [PMC free article] [PubMed]
- 22.McKEITH, I.G., Galasko, D., Wilcock, G.K., Byrne, E.J.: Lewy body dementia–diagnosis and treatment. The British Journal of Psychiatry 167(6), 709–717 (1995) [DOI] [PubMed]
- 23.Surendranathan, A., Kane, J.P., Bentley, A., Barker, S.A., Taylor, J.-P., Thomas, A.J., Allan, L.M., McNally, R.J., James, P.W., McKeith, I.G., et al.: Clinical diagnosis of lewy body dementia. BJPsych open 6(4) (2020) [DOI] [PMC free article] [PubMed]
- 24.Finger, E.C.: Frontotemporal dementias. Continuum: Lifelong Learning in Neurology 22(2 Dementia), 464 (2016) [DOI] [PMC free article] [PubMed]
- 25.Rabinovici, G.D., Miller, B.L.: Frontotemporal lobar degeneration. CNS drugs 24(5), 375–398 (2010) [DOI] [PMC free article] [PubMed]
- 26.Organization, W.H., et al.: The ICD-10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines; 2004. Geneva: WHO (2021)
- 27.Ghetti, B., Buratti, E., Boeve, B., Rademakers, R.: Frontotemporal Dementias vol. 320. Springer, (2021)
- 28.Custodio, N., Montesinos, R., Lira, D., Herrera-Pérez, E., Bardales, Y., Valeriano-Lorenzo, L.: Mixed dementia: A review of the evidence. Dementia & neuropsychologia 11, 364–370 (2017) [DOI] [PMC free article] [PubMed]
- 29.Garcia-Ptacek, S., Kåreholt, I., Cermakova, P., Rizzuto, D., Religa, D., Eriksdotter, M.: Causes of death according to death certificates in individuals with dementia: a cohort from the swedish dementia registry. Journal of the American Geriatrics Society 64(11), 137–142 (2016) [DOI] [PubMed]
- 30.Arvanitakis, Z., Shah, R.C., Bennett, D.A.: Diagnosis and management of dementia. Jama 322(16), 1589–1599 (2019) [DOI] [PMC free article] [PubMed]
- 31.Ullah, H., Bin Heyat, M.B., AlSalman, H., Khan, H.M., Akhtar, F., Gumaei, A., Mehdi, A., Muaad, A.Y., Islam, M.S., Ali, A., et al.: An effective and lightweight deep electrocardiography arrhythmia recognition model using novel special and native structural regularization techniques on cardiac signal. Journal of Healthcare Engineering 2022 (2022) [DOI] [PMC free article] [PubMed] [Retracted]
- 32.Javeed, A., Ali, L., Mohammed Seid, A., Ali, A., Khan, D., Imrana, Y.: A clinical decision support system (cdss) for unbiased prediction of caesarean section based on features extraction and optimized classification. Computational Intelligence and Neuroscience 2022 (2022) [DOI] [PMC free article] [PubMed] [Retracted]
-
33.Imrana, Y., Xiang, Y., Ali, L., Abdul-Rauf, Z., Hu, Y.-C., Kadry, S., Lim, S.:
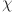 2-bidlstm: A feature driven intrusion detection system based on
2-bidlstm: A feature driven intrusion detection system based on 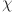 2 statistical model and bidirectional lstm. Sensors 22(5), 2018 (2022) [DOI] [PMC free article] [PubMed]
2 statistical model and bidirectional lstm. Sensors 22(5), 2018 (2022) [DOI] [PMC free article] [PubMed] - 34.Ali, L., Zhu, C., Zhao, H., Zhang, Z., Liu, Y.: An integrated system for unbiased parkinson’s disease detection from handwritten drawings. In: Advances in Intelligent Systems and Computing, pp. 3–13. Springer, (2022)
- 35.Ali, L., Niamat, A., Khan, J.A., Golilarz, N.A., Xingzhong, X., Noor, A., Nour, R., Bukhari, S.A.C.: An optimized stacked support vector machines based expert system for the effective prediction of heart failure. IEEE Access 7, 54007–54014 (2019)
- 36.Ali, L., Zhu, C., Zhou, M., Liu, Y.: Early diagnosis of parkinson’s disease from multiple voice recordings by simultaneous sample and feature selection. Expert Systems with Applications 137, 22–28 (2019)
- 37.Ali, L., Khan, S.U., Golilarz, N.A., Yakubu, I., Qasim, I., Noor, A., Nour, R.: A feature-driven decision support system for heart failure prediction based on statistical model and gaussian naive bayes. Computational and Mathematical Methods in Medicine 2019 (2019) [DOI] [PMC free article] [PubMed]
- 38.Mehbodniya, A., Khan, I.R., Chakraborty, S., Karthik, M., Mehta, K., Ali, L., Nuagah, S.J.: Data mining in employee healthcare detection using intelligence techniques for industry development. Journal of Healthcare Engineering 2022 (2022) [DOI] [PMC free article] [PubMed] [Retracted]
- 39.Ali, L., Khan, S.U., Arshad, M., Ali, S., Anwar, M.: A multi-model framework for evaluating type of speech samples having complementary information about parkinson’s disease. In: 2019 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), pp. 1–5 (2019). IEEE
- 40.Ahmad, F.S., Ali, L., Khattak, H.A., Hameed, T., Wajahat, I., Kadry, S., Bukhari, S.A.C., et al: A hybrid machine learning framework to predict mortality in paralytic ileus patients using electronic health records (ehrs). Journal of Ambient Intelligence and Humanized Computing 12(3), 3283–3293 (2021)
- 41.Ali, L., Wajahat, I., Golilarz, N.A., Keshtkar, F., Bukhari, S.A.C.: Lda–ga–svm: improved hepatocellular carcinoma prediction through dimensionality reduction and genetically optimized support vector machine. Neural Computing and Applications 33(7), 2783–2792 (2021)
- 42.Ali, L., Zhu, C., Zhang, Z., Liu, Y.: Automated detection of parkinson’s disease based on multiple types of sustained phonations using linear discriminant analysis and genetically optimized neural network. IEEE journal of translational engineering in health and medicine 7, 1–10 (2019) [DOI] [PMC free article] [PubMed]
- 43.Ali, L., He, Z., Cao, W., Rauf, H.T., Imrana, Y., Heyat, M.B.B.: Mmdd-ensemble: A multimodal data–driven ensemble approach for parkinson’s disease detection. Frontiers in Neuroscience 15 (2021) [DOI] [PMC free article] [PubMed]
- 44.Rehman, A., Khan, A., Ali, M.A., Khan, M.U., Khan, S.U., Ali, L.: Performance analysis of pca, sparse pca, kernel pca and incremental pca algorithms for heart failure prediction. In: 2020 International Conference on Electrical, Communication, and Computer Engineering (ICECCE), pp. 1–5 (2020). IEEE
- 45.Ahmed, F.S., Ali, L., Joseph, B.A., Ikram, A., Mustafa, R.U., Bukhari, S.A.C.: A statistically rigorous deep neural network approach to predict mortality in trauma patients admitted to the intensive care unit. Journal of Trauma and Acute Care Surgery 89(4), 736–742 (2020) [DOI] [PubMed]
- 46.Imrana, Y., Xiang, Y., Ali, L., Abdul-Rauf, Z.: A bidirectional lstm deep learning approach for intrusion detection. Expert Systems with Applications 185, 115524 (2021)
- 47.Akbar, W., Wu, W.-p., Saleem, S., Farhan, M., Saleem, M.A., Javeed, A., Ali, L.: Development of hepatitis disease detection system by exploiting sparsity in linear support vector machine to improve strength of adaboost ensemble model. Mobile Information Systems 2020 (2020)
- 48.Ali, L., Bukhari, S.: An approach based on mutually informed neural networks to optimize the generalization capabilities of decision support systems developed for heart failure prediction. Irbm 42(5), 345–352 (2021)
- 49.McInnes, M.D., Moher, D., Thombs, B.D., McGrath, T.A., Bossuyt, P.M., Clifford, T., Cohen, J.F., Deeks, J.J., Gatsonis, C., Hooft, L., et al: Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the prisma-dta statement. Jama 319(4), 388–396 (2018) [DOI] [PubMed]
- 50.Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P., Clarke, M., Devereaux, P.J., Kleijnen, J., Moher, D.: The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology 62(10), 1–34 (2009) [DOI] [PubMed]
- 51.Martí-Juan, G., Sanroma-Guell, G., Piella, G.: A survey on machine and statistical learning for longitudinal analysis of neuroimaging data in alzheimer’s disease. Computer methods and programs in biomedicine 189, 105348 (2020) [DOI] [PubMed]
- 52.McGinnis, J.M., Olsen, L., Goolsby, W.A., Grossmann, C., et al: Clinical Data as the Basic Staple of Health Learning: Creating and Protecting a Public Good: Workshop Summary. National Academies Press, (2011) [PubMed]
- 53.Vaughn, V.M., Linder, J.A.: Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Publishing Group Ltd (2018) [DOI] [PubMed]
- 54.Doody, R., Stevens, J., Beck, C., Dubinsky, R., Kaye, J., Gwyther, L., Mohs, R., Thal, L., Whitehouse, P., DeKosky, S., et al: Practice parameter: Management of dementia (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology. Neurology 56(9), 1154–1166 (2001) [DOI] [PubMed]
- 55.Chi, C.-L., Zeng, W., Oh, W., Borson, S., Lenskaia, T., Shen, X., Tonellato, P.J.: Personalized long-term prediction of cognitive function: Using sequential assessments to improve model performance. Journal of biomedical informatics 76, 78–86 (2017) [DOI] [PubMed]
- 56.Johnson, K.A., Minoshima, S., Bohnen, N.I., Donohoe, K.J., Foster, N.L., Herscovitch, P., Karlawish, J.H., Rowe, C.C., Carrillo, M.C., Hartley, D.M., et al: Appropriate use criteria for amyloid pet: a report of the amyloid imaging task force, the society of nuclear medicine and molecular imaging, and the alzheimer’s association. Alzheimer’s & Dementia 9(1), 1–16 (2013) [DOI] [PMC free article] [PubMed]
- 57.Shaw, L.M., Arias, J., Blennow, K., Galasko, D., Molinuevo, J.L., Salloway, S., Schindler, S., Carrillo, M.C., Hendrix, J.A., Ross, A., et al: Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of alzheimer’s disease. Alzheimer’s & Dementia 14(11), 1505–1521 (2018) [DOI] [PMC free article] [PubMed]
- 58.Mills, K.L., Tamnes, C.K.: Methods and considerations for longitudinal structural brain imaging analysis across development. Developmental cognitive neuroscience 9, 172–190 (2014) [DOI] [PMC free article] [PubMed]
- 59.Ali, L., Zhu, C., Golilarz, N.A., Javeed, A., Zhou, M., Liu, Y.: Reliable parkinson’s disease detection by analyzing handwritten drawings: construction of an unbiased cascaded learning system based on feature selection and adaptive boosting model. Ieee Access 7, 116480–116489 (2019)
- 60.Javeed, A., Rizvi, S.S., Zhou, S., Riaz, R., Khan, S.U., Kwon, S.J.: Heart risk failure prediction using a novel feature selection method for feature refinement and neural network for classification. Mobile Information Systems 2020 (2020)
- 61.Javeed, A., Zhou, S., Yongjian, L., Qasim, I., Noor, A., Nour, R.: An intelligent learning system based on random search algorithm and optimized random forest model for improved heart disease detection. IEEE Access 7, 180235–180243 (2019)
- 62.Ali, L., Rahman, A., Khan, A., Zhou, M., Javeed, A., Khan, J.A.: An automated diagnostic system for heart disease prediction based on chi2 statistical model and optimally configured deep neural network. IEEE Access 7, 34938–34945 (2019)
- 63.Maddox, T.M., Rumsfeld, J.S., Payne, P.R.: Questions for artificial intelligence in health care. Jama 321(1), 31–32 (2019) [DOI] [PubMed]
- 64.Chen, P.-H.C., Liu, Y., Peng, L.: How to develop machine learning models for healthcare. Nature materials 18(5), 410–414 (2019) [DOI] [PubMed]
- 65.Javeed, A., Khan, S.U., Ali, L., Ali, S., Imrana, Y., Rahman, A.: Machine learning-based automated diagnostic systems developed for heart failure prediction using different types of data modalities: A systematic review and future directions. Computational and Mathematical Methods in Medicine 2022 (2022) [DOI] [PMC free article] [PubMed] [Retracted]
- 66.Thacker, S.B., Qualters, J.R., Lee, L.M., for Disease Control, C., Prevention, et al: Public health surveillance in the united states: evolution and challenges. MMWR Suppl 61(3), 3–9 (2012) [PubMed]
- 67.Boerma, J.T., Stansfield, S.K.: Health statistics now: are we making the right investments? The Lancet 369(9563), 779–786 (2007) [DOI] [PubMed]
- 68.Walport, M., Brest, P.: Sharing research data to improve public health. The Lancet 377(9765), 537–539 (2011) [DOI] [PubMed]
- 69.Van Panhuis, W.G., Paul, P., Emerson, C., Grefenstette, J., Wilder, R., Herbst, A.J., Heymann, D., Burke, D.S.: A systematic review of barriers to data sharing in public health. BMC public health 14(1), 1–9 (2014) [DOI] [PMC free article] [PubMed]
- 70.Dashtipour, K., Taylor, W., Ansari, S., Zahid, A., Gogate, M., Ahmad, J., Assaleh, K., Arshad, K., Imran, M.A., Abbai, Q.: Detecting alzheimer’s disease using machine learning methods. In: EAI (2021)
- 71.Helaly, H.A., Badawy, M., Haikal, A.Y.: Deep learning approach for early detection of alzheimer’s disease. Cognitive Computation, 1–17 (2021) [DOI] [PMC free article] [PubMed]
- 72.Vandenberghe, R., Nelissen, N., Salmon, E., Ivanoiu, A., Hasselbalch, S., Andersen, A., Korner, A., Minthon, L., Brooks, D.J., Van Laere, K., et al: Binary classification of 18f-flutemetamol pet using machine learning: comparison with visual reads and structural mri. Neuroimage 64, 517–525 (2013) [DOI] [PubMed]
- 73.Odusami, M., Maskeliūnas, R., Damaševičius, R., Krilavičius, T.: Analysis of features of alzheimer’s disease: Detection of early stage from functional brain changes in magnetic resonance images using a finetuned resnet18 network. Diagnostics 11(6), 1071 (2021) [DOI] [PMC free article] [PubMed]
- 74.Zheng, Y., Guo, H., Zhang, L., Wu, J., Li, Q., Lv, F.: Machine learning-based framework for differential diagnosis between vascular dementia and alzheimer’s disease using structural mri features. Frontiers in Neurology, 1097 (2019) [DOI] [PMC free article] [PubMed]
- 75.Basheer, S., Bhatia, S., Sakri, S.B.: Computational modeling of dementia prediction using deep neural network: Analysis on oasis dataset. IEEE Access 9, 42449–42462 (2021)
- 76.Leong, L.K., Abdullah, A.A.: Prediction of alzheimer’s disease (ad) using machine learning techniques with boruta algorithm as feature selection method. In: Journal of Physics: Conference Series, vol. 1372, p. 012065 (2019). IOP Publishing
- 77.Battineni, G., Chintalapudi, N., Amenta, F.: Machine learning in medicine: Performance calculation of dementia prediction by support vector machines (svm). Informatics in Medicine Unlocked 16, 100200 (2019)
- 78.Mathotaarachchi, S., Pascoal, T.A., Shin, M., Benedet, A.L., Kang, M.S., Beaudry, T., Fonov, V.S., Gauthier, S., Rosa-Neto, P., Initiative, A.D.N., et al: Identifying incipient dementia individuals using machine learning and amyloid imaging. Neurobiology of aging 59, 80–90 (2017) [DOI] [PubMed]
- 79.Aruna, S., Chitra, S.: Machine learning approach for identifying dementia from mri images. International Journal of Computer and Information Engineering 9(3), 881–888 (2016)
- 80.Herzog, N.J., Magoulas, G.D.: Brain asymmetry detection and machine learning classification for diagnosis of early dementia. Sensors 21(3), 778 (2021) [DOI] [PMC free article] [PubMed]
- 81.Battineni, G., Chintalapudi, N., Amenta, F., Traini, E.: A comprehensive machine-learning model applied to magnetic resonance imaging (mri) to predict alzheimer’s disease (ad) in older subjects. Journal of Clinical Medicine 9(7), 2146 (2020) [DOI] [PMC free article] [PubMed]
- 82.Ma, D., Yee, E., Stocks, J.K., Jenkins, L.M., Popuri, K., Chausse, G., Wang, L., Probst, S., Beg, M.F.: Blinded clinical evaluation for dementia of alzheimer’s type classification using fdg-pet: A comparison between feature-engineered and non-feature-engineered machine learning methods. Journal of Alzheimer’s Disease 80(2), 715–726 (2021) [DOI] [PMC free article] [PubMed]
- 83.Bidani, A., Gouider, M.S., Travieso-González, C.M.: Dementia detection and classification from mri images using deep neural networks and transfer learning. In: International Work-Conference on Artificial Neural Networks, pp. 925–933 (2019). Springer
- 84.Moscoso, A., Silva-Rodríguez, J., Aldrey, J.M., Cortés, J., Fernández-Ferreiro, A., Gómez-Lado, N., Ruibal, Á., Aguiar, P., Initiative, A.D.N., et al: Prediction of alzheimer’s disease dementia with mri beyond the short-term: Implications for the design of predictive models. NeuroImage: Clinical 23, 101837 (2019) [DOI] [PMC free article] [PubMed]
- 85.Khan, A., Zubair, S.: An improved multi-modal based machine learning approach for the prognosis of alzheimer’s disease. Journal of King Saud University-Computer and Information Sciences (2020)
- 86.Mohammed, B.A., Senan, E.M., Rassem, T.H., Makbol, N.M., Alanazi, A.A., Al-Mekhlafi, Z.G., Almurayziq, T.S., Ghaleb, F.A.: Multi-method analysis of medical records and mri images for early diagnosis of dementia and alzheimer’s disease based on deep learning and hybrid methods. Electronics 10(22), 2860 (2021)
- 87.Salvatore, C., Cerasa, A., Battista, P., Gilardi, M.C., Quattrone, A., Castiglioni, I.: Magnetic resonance imaging biomarkers for the early diagnosis of alzheimer’s disease: a machine learning approach. Frontiers in neuroscience 9, 307 (2015) [DOI] [PMC free article] [PubMed]
- 88.Katako, A., Shelton, P., Goertzen, A.L., Levin, D., Bybel, B., Aljuaid, M., Yoon, H.J., Kang, D.Y., Kim, S.M., Lee, C.S., et al: Machine learning identified an alzheimer’s disease-related fdg-pet pattern which is also expressed in lewy body dementia and parkinson’s disease dementia. Scientific reports 8(1), 1–13 (2018) [DOI] [PMC free article] [PubMed]
- 89.Gray, K.R., Aljabar, P., Heckemann, R.A., Hammers, A., Rueckert, D.: Random forest-based manifold learning for classification of imaging data in dementia. In: International Workshop on Machine Learning in Medical Imaging, pp. 159–166 (2011). Springer
- 90.De Bruijne, M.: Machine learning approaches in medical image analysis: From detection to diagnosis. Elsevier (2016) [DOI] [PubMed]
- 91.Tong, T., Wolz, R., Gao, Q., Hajnal, J.V., Rueckert, D.: Multiple instance learning for classification of dementia in brain mri. In: International Conference on Medical Image Computing and Computer-Assisted Intervention, pp. 599–606 (2013). Springer [DOI] [PubMed]
- 92.Akhila, J., Markose, C., Aneesh, R.: Feature extraction and classification of dementia with neural network. In: 2017 International Conference on Intelligent Computing, Instrumentation and Control Technologies (ICICICT), pp. 1446–1450 (2017). IEEE
- 93.Chen, Y., Pham, T.D.: Development of a brain mri-based hidden markov model for dementia recognition. Biomedical engineering online 12(1), 1–16 (2013) [DOI] [PMC free article] [PubMed]
- 94.Patil, M., Yardi, A.: Ann based dementia diagnosis using dct for brain mr image compression. In: 2013 International Conference on Communication and Signal Processing, pp. 451–454 (2013). IEEE
- 95.Gulhare, K.K., Shukla, S., Sharma, L.: Deep neural network classification method to alzheimer’s disease detection. International Journals of Advanced Research in Computer Science and Software Engineering 7(6), 1–4 (2017)
- 96.Chiu, P.-Y., Tang, H., Wei, C.-Y., Zhang, C., Hung, G.-U., Zhou, W.: Nmd-12: A new machine-learning derived screening instrument to detect mild cognitive impairment and dementia. PloS one 14(3), 0213430 (2019) [DOI] [PMC free article] [PubMed]
- 97.Stamate, D., Alghamdi, W., Ogg, J., Hoile, R., Murtagh, F.: A machine learning framework for predicting dementia and mild cognitive impairment. In: 2018 17th IEEE International Conference on Machine Learning and Applications (ICMLA), pp. 671–678 (2018). IEEE
- 98.Visser, P.J., Lovestone, S., Legido-Quigley, C.: A metabolite-based machine learning approach to diagnose alzheimer-type dementia in blood: Results from the european medical information framework for alzheimer disease biomarker discovery cohort (2019) [DOI] [PMC free article] [PubMed]
- 99.Bansal, D., Chhikara, R., Khanna, K., Gupta, P.: Comparative analysis of various machine learning algorithms for detecting dementia. Procedia computer science 132, 1497–1502 (2018)
- 100.Nori, V.S., Hane, C.A., Martin, D.C., Kravetz, A.D., Sanghavi, D.M.: Identifying incident dementia by applying machine learning to a very large administrative claims dataset. PLoS One 14(7), 0203246 (2019) [DOI] [PMC free article] [PubMed]
- 101.Alam, M.A.U., Roy, N., Holmes, S., Gangopadhyay, A., Galik, E.: Automated functional and behavioral health assessment of older adults with dementia. In: 2016 IEEE First International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE), pp. 140–149 (2016). IEEE
- 102.Gurevich, P., Stuke, H., Kastrup, A., Stuke, H., Hildebrandt, H.: Neuropsychological testing and machine learning distinguish alzheimer’s disease from other causes for cognitive impairment. Frontiers in aging neuroscience 9, 114 (2017) [DOI] [PMC free article] [PubMed]
- 103.Karaglani, M., Gourlia, K., Tsamardinos, I., Chatzaki, E.: Accurate blood-based diagnostic biosignatures for alzheimer’s disease via automated machine learning. Journal of clinical medicine 9(9), 3016 (2020) [DOI] [PMC free article] [PubMed]
- 104.Ryzhikova, E., Ralbovsky, N.M., Sikirzhytski, V., Kazakov, O., Halamkova, L., Quinn, J., Zimmerman, E.A., Lednev, I.K.: Raman spectroscopy and machine learning for biomedical applications: Alzheimer’s disease diagnosis based on the analysis of cerebrospinal fluid. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 248, 119188 (2021) [DOI] [PubMed]
- 105.Cho, P.-C., Chen, W.-H.: A double layer dementia diagnosis system using machine learning techniques. In: International Conference on Engineering Applications of Neural Networks, pp. 402–412 (2012). Springer
- 106.Facal, D., Valladares-Rodriguez, S., Lojo-Seoane, C., Pereiro, A.X., Anido-Rifon, L., Juncos-Rabadán, O.: Machine learning approaches to studying the role of cognitive reserve in conversion from mild cognitive impairment to dementia. International journal of geriatric psychiatry 34(7), 941–949 (2019) [DOI] [PubMed]
- 107.Jin, H., Chien, S., Meijer, E., Khobragade, P., Lee, J., et al: Learning from clinical consensus diagnosis in india to facilitate automatic classification of dementia: Machine learning study. JMIR Mental Health 8(5), 27113 (2021) [DOI] [PMC free article] [PubMed]
- 108.James, C., Ranson, J.M., Everson, R., Llewellyn, D.J.: Performance of machine learning algorithms for predicting progression to dementia in memory clinic patients. JAMA network open 4(12), 2136553–2136553 (2021) [DOI] [PMC free article] [PubMed]
- 109.Bougea, A., Efthymiopoulou, E., Spanou, I., Zikos, P.: A novel machine learning algorithm predicts dementia with lewy bodies versus parkinson’s disease dementia based on clinical and neuropsychological scores. Journal of geriatric psychiatry and neurology, 0891988721993556 (2021) [DOI] [PubMed]
- 110.Salem, F.A., Chaaya, M., Ghannam, H., Al Feel, R.E., El Asmar, K.: Regression based machine learning model for dementia diagnosis in a community setting. Alzheimer’s & Dementia 17, 053839 (2021)
- 111.Garcia-Gutierrez, F., Delgado-Alvarez, A., Delgado-Alonso, C., Díaz-Álvarez, J., Pytel, V., Valles-Salgado, M., Gil, M.J., Hernández-Lorenzo, L., Matías-Guiu, J., Ayala, J.L., et al.: Diagnosis of alzheimer’s disease and behavioural variant frontotemporal dementia with machine learning-aided neuropsychological assessment using feature engineering and genetic algorithms. International journal of geriatric psychiatry 37(2) (2022) [DOI] [PubMed]
- 112.Mirzaei, G., Adeli, H.: Machine learning techniques for diagnosis of alzheimer disease, mild cognitive disorder, and other types of dementia. Biomedical Signal Processing and Control 72, 103293 (2022)
- 113.Hsiu, H., Lin, S.-K., Weng, W.-L., Hung, C.-M., Chang, C.-K., Lee, C.-C., Chen, C.-T.: Discrimination of the cognitive function of community subjects using the arterial pulse spectrum and machine-learning analysis. Sensors 22(3), 806 (2022) [DOI] [PMC free article] [PubMed]
- 114.Shahzad, A., Dadlani, A., Lee, H., Kim, K.: Automated prescreening of mild cognitive impairment using shank-mounted inertial sensors based gait biomarkers. IEEE Access (2022)
- 115.Hane, C.A., Nori, V.S., Crown, W.H., Sanghavi, D.M., Bleicher, P.: Predicting onset of dementia using clinical notes and machine learning: case-control study. JMIR medical informatics 8(6), 17819 (2020) [DOI] [PMC free article] [PubMed]
- 116.Aschwanden, D., Aichele, S., Ghisletta, P., Terracciano, A., Kliegel, M., Sutin, A.R., Brown, J., Allemand, M.: Predicting cognitive impairment and dementia: A machine learning approach. Journal of Alzheimer’s Disease 75(3), 717–728 (2020) [DOI] [PMC free article] [PubMed]
- 117.Ryu, S.-E., Shin, D.-H., Chung, K.: Prediction model of dementia risk based on xgboost using derived variable extraction and hyper parameter optimization. IEEE Access 8, 177708–177720 (2020)
- 118.de Langavant, L.C., Bayen, E., Yaffe, K., et al: Unsupervised machine learning to identify high likelihood of dementia in population-based surveys: development and validation study. Journal of medical Internet research 20(7), 10493 (2018) [DOI] [PMC free article] [PubMed]
- 119.Fouladvand, S., Mielke, M.M., Vassilaki, M., Sauver, J.S., Petersen, R.C., Sohn, S.: Deep learning prediction of mild cognitive impairment using electronic health records. In: 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), pp. 799–806 (2019). IEEE [DOI] [PMC free article] [PubMed]
- 120.Balea-Fernandez, F.J., Martinez-Vega, B., Ortega, S., Fabelo, H., Leon, R., Callico, G.M., Bibao-Sieyro, C.: Analysis of risk factors in dementia through machine learning. Journal of Alzheimer’s Disease 79(2), 845–861 (2021) [DOI] [PubMed]
- 121.Chlasta, K., Wołk, K.: Towards computer-based automated screening of dementia through spontaneous speech. Frontiers in Psychology, 4091 (2021) [DOI] [PMC free article] [PubMed]
- 122.Chien, Y.-W., Hong, S.-Y., Cheah, W.-T., Yao, L.-H., Chang, Y.-L., Fu, L.-C.: An automatic assessment system for alzheimer’s disease based on speech using feature sequence generator and recurrent neural network. Scientific Reports 9(1), 1–10 (2019) [DOI] [PMC free article] [PubMed]
- 123.Shimoda, A., Li, Y., Hayashi, H., Kondo, N.: Dementia risks identified by vocal features via telephone conversations: A novel machine learning prediction model. PloS one 16(7), 0253988 (2021) [DOI] [PMC free article] [PubMed]
- 124.Nishikawa, K., Akihiro, K., Hirakawa, R., Kawano, H., Nakatoh, Y.: Machine learning model for discrimination of mild dementia patients using acoustic features. Cognitive Robotics (2021)
- 125.Liu, L., Zhao, S., Chen, H., Wang, A.: A new machine learning method for identifying alzheimer’s disease. Simulation Modelling Practice and Theory 99, 102023 (2020)
- 126.Searle, T., Ibrahim, Z., Dobson, R.: Comparing natural language processing techniques for alzheimer’s dementia prediction in spontaneous speech. arXiv preprint arXiv:2006.07358 (2020)
- 127.Zhu, Y., Tran, B., Liang, X., Batsis, J.A., Roth, R.M.: Towards interpretability of speech pause in dementia detection using adversarial learning. arXiv preprint arXiv:2111.07454 (2021) [DOI] [PMC free article] [PubMed]
- 128.Ossewaarde, R., Jonkers, R., Jalvingh, F., Bastiaanse, R.: Classification of spontaneous speech of individuals with dementia based on automatic prosody analysis using support vector machines (svm). In: The Thirty-Second International Flairs Conference (2019)
- 129.Xue, C., Karjadi, C., Paschalidis, I.C., Au, R., Kolachalama, V.B.: Detection of dementia on voice recordings using deep learning: a framingham heart study. Alzheimer’s research & therapy 13(1), 1–15 (2021) [DOI] [PMC free article] [PubMed]
- 130.Weiner, J., Engelbart, M., Schultz, T.: Manual and automatic transcriptions in dementia detection from speech. In: INTERSPEECH, pp. 3117–3121 (2017)
- 131.Sadeghian, R., Schaffer, J.D., Zahorian, S.A.: Speech processing approach for diagnosing dementia in an early stage (2017)
- 132.Khodabakhsh, A., Yesil, F., Guner, E., Demiroglu, C.: Evaluation of linguistic and prosodic features for detection of alzheimer’s disease in turkish conversational speech. EURASIP Journal on Audio, Speech, and Music Processing 2015(1), 1–15 (2015)
- 133.Edwards, E., Dognin, C., Bollepalli, B., Singh, M.K., Analytics, V.: Multiscale system for alzheimer’s dementia recognition through spontaneous speech. In: INTERSPEECH, pp. 2197–2201 (2020)
- 134.Kumar, Y., Maheshwari, P., Joshi, S., Baths, V.: Ml-based analysis to identify speech features relevant in predicting alzheimer’s disease. arXiv preprint arXiv:2110.13023 (2021)
- 135.Luz, S., de la Fuente, S., Albert, P.: A method for analysis of patient speech in dialogue for dementia detection. arXiv preprint arXiv:1811.09919 (2018)
- 136.Syed, Z.S., Syed, M.S.S., Lech, M., Pirogova, E.: Automated recognition of alzheimer’s dementia using bag-of-deep-features and model ensembling. IEEE Access 9, 88377–88390 (2021)
- 137.Orsulic-Jeras, S., Sanders, B., Powers, S., Ejaz, F., Cordell, A., Wilk, C.: Developing a reminiscence therapy platform-lifebio memorytm-with a novel machine-learning-based application that transfers speech to text and generates life stories for nursing home residents with dementia. Alzheimer’s & Dementia 17, 052281 (2021)
- 138.Sarawgi, U., Zulfikar, W., Soliman, N., Maes, P.: Multimodal inductive transfer learning for detection of alzheimer’s dementia and its severity. arXiv preprint arXiv:2009.00700 (2020)
- 139.Calzà, L., Gagliardi, G., Favretti, R.R., Tamburini, F.: Linguistic features and automatic classifiers for identifying mild cognitive impairment and dementia. Computer Speech & Language 65, 101113 (2021)
- 140.Haider, F., De La Fuente, S., Luz, S.: An assessment of paralinguistic acoustic features for detection of alzheimer’s dementia in spontaneous speech. IEEE Journal of Selected Topics in Signal Processing 14(2), 272–281 (2019)
- 141.Lopez-de-Ipiña, K., Alonso, J.B., Solé-Casals, J., Barroso, N., Henriquez, P., Faundez-Zanuy, M., Travieso, C.M., Ecay-Torres, M., Martinez-Lage, P., Eguiraun, H.: On automatic diagnosis of alzheimer’s disease based on spontaneous speech analysis and emotional temperature. Cognitive Computation 7(1), 44–55 (2015)
- 142.Orimaye, S.O., Wong, J.S.-M., Golden, K.J.: Learning predictive linguistic features for alzheimer’s disease and related dementias using verbal utterances. In: Proceedings of the Workshop on Computational Linguistics and Clinical Psychology: From Linguistic Signal to Clinical Reality, pp. 78–87 (2014)
- 143.Santander-Cruz, Y., Salazar-Colores, S., Paredes-García, W.J., Guendulain-Arenas, H., Tovar-Arriaga, S.: Semantic feature extraction using sbert for dementia detection. Brain Sciences 12(2), 270 (2022) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.