Abstract
In vitro and in vivo adhesive properties of flagella and recombinant flagellin FliC and flagellar cap FliD proteins of Clostridium difficile were analyzed. FliC, FliD, and crude flagella adhered in vitro to axenic mouse cecal mucus. Radiolabeled cultured cells bound to a high degree to FliD and weakly to flagella deposited on a membrane. The tissue association in the mouse cecum of a nonflagellated strain was 10-fold lower than that of a flagellated strain belonging to the same serogroup, confirming the role of flagella in adherence.
Clostridium difficile is now well established as the main cause of nosocomial infections such as pseudomembranous colitis, antibiotic-associated diarrhea, and antibiotic-associated colitis (3, 6, 15, 24), especially in elderly and immunocompromised patients (2, 6). Toxigenic C. difficile strains produce two virulence factors, toxins A and B (26). The proposed accessory virulence factors include (i) capsule, an antiphagocytic factor (10); (ii) fimbriae (7); (iii) hydrolytic enzymes, which are potentially involved in mucus degradation and penetration (5, 30, 34, 35); and (iv) adhesins mediating adherence to mucosa (14, 18, 20, 21, 39, 40).
Flagella have been implicated in internalization of Campylobacter jejuni and Legionella pneumophila (12, 17) and in cell adherence and colonization by C. jejuni (27), Helicobacter pylori (13), and Aeromonas caviae (31). Motility mediated by flagella is responsible for the invasiveness of Salmonella enterica serovar Typhi (25) and Borrelia burgdorferi (33) and the pathogenicity of Vibrio cholerae (32). The flagellin FliC is the major structural component of the flagellar filament, and assembly of a flagellum requires other proteins called hook-associated proteins (HAP1, HAP2, and HAP3). The fliD gene encodes structural component HAP2 of the flagellar cap at the distal end of the filament (4, 19). In a previous study we characterized the fliC and fliD genes of C. difficile, which encode the 39-kDa flagellin protein (36, 37) and the 56-kDa flagellar cap protein (38), respectively. The aim of this work was to study the potential role of C. difficile flagella in adherence and colonization.
In vitro adherence of recombinant FliC, FliD, and crude flagella to mucus.
Inasmuch as during the colonization process C. difficile is likely to encounter a layer of mucus first in the intestine, the properties of adhesion of FliC, FliD, and crude flagella to cecal axenic mouse mucus were investigated. Thirteen-week-old C3H axenic mice, obtained from l'Institut National de Recherche Agronomique (Jouy-en-Josas, France) and from our breeding program, were maintained in sterile isolators (Isoconcept, Orléans, France) and received standard nutrients sterilized by irradiation. Mucus was obtained from excised ceca that were opened lengthwise after the contents were removed by gentle shaking twice in phosphate-buffered saline (PBS) (10 mM phosphate buffer, 150 mM NaCl; pH 7.2). The mucus was gently scraped off and suspended in 10 ml of PBS containing 0.02% (wt/vol) sodium azide by stirring for 16 h at 4°C. Cellular debris was removed by centrifugation at 20,000 × g for 15 min at 4°C, and the mucus was dried by freeze-drying. The recombinant proteins and flagella were purified as previously described (11, 36, 38).
The adherence of the FliC and FliD proteins and crude flagella to mucus in vitro was examined by dot blotting. Ten-microgram portions of axenic mouse cecal mucus and porcine stomach mucus (Sigma) or 5-μg portions of proteins (purified FliC and FliD, crude flagella as a positive control, glutathione S-transferase [GST] as a negative control) were applied to three Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore) with a Minifold I dot blotter (Schleicher and Schuell). The dot blotter allowed us to deposit equal quantities of mucus or proteins in 4-mm-diameter dots on the membranes. Each membrane was dried at 37°C for 15 min and then incubated overnight at 4°C in sodium carbonate buffer (pH 9.6) containing 20 μg of FliC per ml, 20 μg of FliD per ml, or 20 μg of crude flagella per ml. After two washes with PBS for 10 min, the membranes were incubated for 1 h at room temperature in TBS buffer (20 mM Tris-HCl, 0.5 M NaCl; pH 7.5) containing 0.1% (vol/vol) Tween 20 and 10% (wt/vol) skim milk and then for 1 h at 37°C with polyclonal rabbit antibodies (1:2,000 dilution) raised against FliC or FliD. The membranes were washed three times (10 min each) with TBS buffer containing 0.1% (vol/vol) Tween 20, and bound antibodies were detected with goat anti-rabbit IgG alkaline phosphatase conjugate (1:2,500 dilution; Sigma) by using Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma) as the substrates.
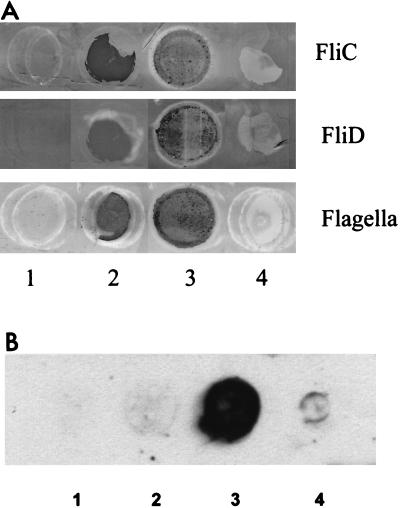
As shown in Fig. 1A, crude flagella, as well as recombinant FliC and FliD proteins, could bind specifically to mucus isolated from axenic mice, suggesting that there is a receptor for flagella in murine mucus. In contrast, no binding to porcine stomach mucus was observed. These results suggest that C. difficile flagella play a role in attachment to mucus, the first barrier encountered during colonization. Attachment to mucus could facilitate establishment of infection in the gut. FliD of Pseudomonas aeruginosa is responsible for tracheobronchial mucin adhesion in patients afflicted with cystic fibrosis (1) and is involved in colonization of the mouse gastric mucosa by H. pylori (22) and the invasiveness of Proteus mirabilis (28).
FIG. 1.
(A) Adherence of recombinant FliC and FliD and crude flagella to mucus. The dots on the PVDF membrane contained 5 μg of GST (negative control; a nonadhesive protein; Amersham Pharmacia Biotech) (lane 1), 5 μg of purified FliC, FliD, or flagella (positive controls) (lane 2), 10 μg of murine cecal mucus (lane 4), and 10 μg of porcine stomach mucus (lane 4). After incubation with the proteins, they were revealed by using antibodies raised against FliC and FliD. Lanes 1 and 4, negative reactions; lanes 2 and 3, positive reactions. (B) Adherence of radiolabeled Vero cells to recombinant FliC and FliD and crude flagella. Proteins were dot blotted on a PVDF membrane, incubated with labeled cells, and revealed by autoradiography. Lane 1, GST protein (negative control); lane 2, FliC; lane 3, FliD; lane 4, crude flagella. Lanes 1 and 2, negative reactions; lane 3, positive reaction; lane 4, weakly positive reaction. All experiments were performed in duplicate, and the results were confirmed in two independent assays.
In vitro adherence of radiolabeled cultured cells to flagellar proteins.
In order to circumvent problems in assays of cell adhesion of flagellated bacteria due to the removal of flagella during manipulations and the multifactor nature of C. difficile adherence (18, 21, 40), we decided to perform direct cell adhesion experiments with radiolabeled target cells adhering to purified flagellum preparations and recombinant FliC and FliD proteins. Five micrograms of FliC, FliD, flagella, or GST was transferred to a PVDF membrane by dot blotting as described above. Vero cells (monkey kidney origin; Bio-Whittaker), cultured as previously described (20), were labeled with 1 mCi of l-[35S]methionine (NEN) in minimal essential medium (MEM) complemented with 10% (vol/vol) fetal calf serum (Life Technologies) for 6 h at 37°C under 5% CO2 and were resuspended in MEM (2 × 105 cells/cm2 of membrane). The cells were washed twice with PBS and were incubated twice for 30 min in MEM without methionine (Life Technologies) complemented with 10% (vol/vol) fetal calf serum (Life Technologies). After blotting of the proteins, the membrane was dried at 37°C for 15 min, incubated for 3 h at 37°C in PBS with 5% (wt/vol) skim milk, and washed three times with PBS. The membrane was incubated for 90 min at 37°C under 5% CO2 with labeled cells and washed three times with PBS containing 5% (wt/vol) skim milk, and protein-bound cells on the blotted membrane were detected by exposure of the membrane to Kodak Biomax photographic film (Sigma) for 16 h at −80°C.
As shown in Fig. 1B, no adhesion to FliC or GST was observed. In contrast, the radiolabeled cells adhered strongly to the recombinant FliD protein, as shown by the presence of a large spot on the membrane, in spite of the small diameter of the deposits applied. Weak binding to the crude flagellar preparation was also observed. These results suggest that the flagellar cap protein, unlike the flagellar subunit, could play a role in cell adherence. The low level of binding to whole flagella could be explained by the small amount of FliD protein present at the tips of flagella compared with the number of polymerized flagellin subunits forming the flagellar filaments (ratio, several thousand to one). When the binding experiment was carried out with proteins denatured by migration in a sodium dodecyl sulfate-polyacrylamide gel (23), no adhesion to the 39- and the 56-kDa proteins corresponding to the flagellin and the flagellar cap protein was observed (data not shown). The adhesion of radiolabeled Vero cells to the FliD protein in nondenaturing conditions shows that the native structure of the FliD protein is required for adherence. In some other bacterial species, such as Bacillus thuringiensis (41) and C. jejuni (17), the flagellar structure has been implicated in adhesion to cultured cell lines, whereas in H. pylori, Helicobacter mustelae, and Vibrio anguillarum, flagella are not involved in adherence (9, 29).
Role of flagella in C. difficile implantation in the mouse intestine.
Prior to implantation experiments, expression of the two flagellar proteins in the strains of C. difficile used (Table 1) was assessed by immunoblot analysis of flagellar preparations as previously described (36, 38). Antibodies raised against purified recombinant FliD (56 kDa) and FliC (39 kDa) recognized proteins with the same molecular masses in crude flagellar preparations of strains ATCC 43593 and ATCC 43598. The aflagellate strains EX560 and 6058 did not express the two flagellar proteins (Table 1).
TABLE 1.
C. difficile strains used in this study
| Strain | Serogroup | Toxin
|
Protein
|
Source | ||
|---|---|---|---|---|---|---|
| A | B | FliC | FliD | |||
| 79685 | S3 | + | + | + | + | Institut de Bactériologie, Strasbourg, France |
| ATCC 43593 | B | − | − | + | + | Université Catholique de Louvain, Brussels, Belgium |
| EX560 | B | − | − | − | − | Université Catholique de Louvain, Brussels, Belgium |
| ATCC 43598 | F | − | + | + | + | Université Catholique de Louvain, Brussels, Belgium |
| 6058 | F | − | + | − | − | Université Catholique de Louvain, Brussels, Belgium |
Functional studies of C. difficile proteins are currently hampered by the fact that construction of isogenic mutants through inactivation of potential virulence-associated genes by site-directed mutagenesis is not feasible due to a lack of appropriate genetic tools. Therefore, to examine the function of a particular protein in vivo, we must employ indirect methods. In vivo colonization experiments with axenic mice were performed by using flagellated and nonflagellated strains belonging to the same serogroup, since we can assume that strains belonging to the same serogroup have quite similar compositions in terms of surface proteins which are determinants of adhesion and serogroup specificity. Nontoxigenic (ToxA−) strains were chosen for these experiments because mice die within 48 h after inoculation with a toxigenic strain (ToxA+B+).
Mice were orally gavaged with 0.5 ml of anaerobically cultured C. difficile (cells grown for 24 h in tryptone glucose yeast extract infusion broth [Difco]). The numbers of bacteria present in suspensions were determined by using serial dilutions in saline buffer (0.8% [wt/vol] NaCl, 0.33% [wt/vol] Na2HPO4, 0.11% [wt/vol] KH2PO4; pH 7.2). Dilutions were seeded in duplicate and cultured in tubes containing 1.5% GAPTT agar (1% [wt/vol] yeast hydrolysate, 1.5% [wt/vol] Bacto Peptone, 1% [wt/vol] Bacto Tryptone, 0.1% [vol/vol] Tween 80; pH 6.5) at 37°C for 24 to 48 h. Viable C. difficile cells were enumerated. On days 1, 2, 6, and 7, a fecal sample was collected from each mouse at the anus and weighed. The feces were homogenized and diluted in LCY buffer (0.2% [wt/vol] acid casein hydrolysate, 0.5% [wt/vol] NaCl, 0.1% [wt/vol] KH2PO4, 0.2% [wt/vol] yeast extract; pH 7.0), and the cells were enumerated separately by using serial dilutions in an anaerobic chamber. In all colonization experiments, dilutions were seeded in duplicate and cultured in 1.5% GAPTT agar tubes as described above.
In the first experiment, to determine whether flagella play a role in intestinal implantation, flagellated strain ATCC 43593 (ToxA−B− Fla+) and nonflagellated strain EX560 (ToxA−B− Fla−), belonging to serogroup B (Table 1), were fed to axenic mice by using preparations containing 8.5 × 106 and 12.5 × 106 CFU/ml, respectively. On day 1, the two strains rapidly colonized mouse intestines in equal numbers (approximately 108 CFU/g of feces), and the numbers were close to 109 CFU/g of feces on day 7.
Since in a previous study Borriello et al. (8) suggested that C. difficile toxins can influence in vivo colonization, a second experiment was carried out with strains ATCC 43598 (ToxA−B+ Fla+) and 6058 (ToxA−B+ Fla−), belonging to serogroup F (Table 1). The initial bacterial suspensions fed to the mice contained 5 × 106 and 2.6 × 106 CFU/ml for strains ATCC 43598 and 6058, respectively. Colonization was rapid for both the flagellated strain and the nonflagellated strain and progressed in similar fashions in the mice. The bacterial concentrations remained constant at a level of 1010 CFU/g of feces up to the end of the experiment (data not shown).
The data suggest that flagella play no role in implantation in the intestines of axenic mice; nonflagellated strains colonized axenic mouse intestines at the same rate as flagellated strains. These results could be explained by the fact that axenic mice do not have an intestinal barrier flora and any strain fed orally becomes implanted at a high level in the gut. Our results seem to corroborate a possible enhancing role for toxins in gut implantation, perhaps due to increasing adhesion to epithelia through the cell-binding domain of toxins.
Tissue association of C. difficile strains in the mouse cecum.
In order to study the influence of flagella on adhesion to the ceca of axenic mice, the four C. difficile strains described above were used. Seven days after inoculation of the bacteria, the mice were sacrificed and placed in an anaerobic chamber. The entire cecum of each mouse was removed as described by Gomez-Trevino et al. (16), rinsed by gently shaking it eight times in a phosphate buffer (pH 7.2), and weighed. Each cecum was crushed with an Ultra-Turrax apparatus (T25; Janke&Kunkel, IKA-Labortechnik, Staufen, Germany) for 1 min at 13,500 rpm and diluted in LCY buffer in order to obtain a concentration of 10 mg/ml. Serial dilutions were seeded in duplicate and cultured in 1.5% GAPTT agar tubes as described above.
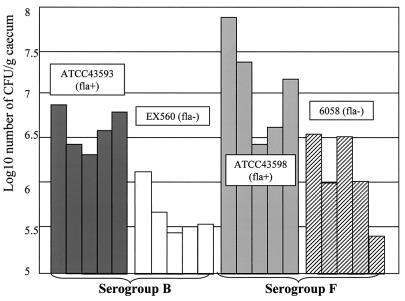
The results obtained for strains belonging to the same serogroup were compared (Fig. 2). The adherence of nonflagellated strain EX560 (ToxA−B−Fla−, serogroup B) to ceca was significantly less (10-fold less; P < 0.01, as assessed by Student's t test) than that of flagellated strain ATCC 43593 (ToxA−B− Fla+, serogroup B); the average numbers of bacteria per gram of cecum for these two strains were 4 × 105 and 3.9 × 106 CFU, respectively. A similar significant difference (P < 0.01) was observed for the adhesion to ceca of flagellated strain ATCC 43598 (ToxA−B+ Fla+, serogroup F) and nonflagellated strain 6058 (ToxA−B+ Fla−, serogroup F); the average numbers of bacteria per gram of cecum for these strains were 1.4 × 107 and 1.3 × 106 CFU, respectively (Fig. 2). Thus, flagella seem to be implicated in adherence to the mouse cecum in vivo. Toxin B appears to enhance attachment to the cecum since strains belonging to serogroup F adhere better than strains belonging to serogroup B.
FIG. 2.
Adherence of flagellated and nonflagellated C. difficile strains to axenic mouse cecum. The adherence of flagellated and nonflagellated strains belonging to the same serogroup is expressed as log10 number of bacteria per gram of cecum from five mice. Strains belonging to serogroup B are toxin A negative, whereas strains belonging to serogroup F are toxin A and B negative.
Taken together, our in vitro results suggest that both the FliC and FliD proteins are implicated in attachment of C. difficile to the mucus layer of the intestine. Flagellated strains appeared to have a better capacity to associate with the cecal wall in vivo; the FliD protein could play a role in this process. In contrast, no difference in the level of implantation was observed between the two groups, although toxin B seems to promote colonization and association with the cecum. Flagella and especially the flagellar FliD protein appear to be some of the multiple cell adhesins of this microorganism. We are initiating vaccination experiments in which FliD and other adhesins are immunogens.
Acknowledgments
We thank Sylvie Lambert-Bordes and Sandra Hoys for technical assistance in experiments performed with germfree mice. We are grateful to Béatrice Pedron and Nathalie Radegonde for their kind help. We thank M. Delmée, Université Catholique de Louvain, Brussels, Belgium, for kindly providing the four strains used in colonization experiments.
REFERENCES
- 1.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. The Pseudomonas aeruginosa flagellar cap protein, FliD, is responsible for mucin adhesion. Infect Immun. 1998;66:1000–1007. doi: 10.1128/iai.66.3.1000-1007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbut F, Mario N, Meyohas M C, Binet D, Frottier J, Petit J C. Investigation of a nosocomial outbreak of Clostridium difficile-associated diarrhoea among AIDS patients by random amplified polymorphic DNA (RAPD) assay. J Hosp Infect. 1994;26:181–189. doi: 10.1016/0195-6701(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett J G, Chang T W, Gurwiyh M, Gorbach S L, Onderdonk A M. Antibiotic associated pseudomembranous colitis due to toxin producing clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 4.Blair D F, Dutcher S K. Flagella in prokaryotes and lower eukaryotes. Curr Opin Genet Dev. 1992;2:756–767. doi: 10.1016/s0959-437x(05)80136-4. [DOI] [PubMed] [Google Scholar]
- 5.Bongaerts G P, Lyerly D M. Role of bacterial metabolism and physiology in the pathogenesis of Clostridium difficile disease. Microb Pathog. 1997;22:253–256. doi: 10.1006/mpat.1996.0119. [DOI] [PubMed] [Google Scholar]
- 6.Borriello S P, Larson H E. Antibiotic and pseudomembranous colitis. J Antimicrob Chemother. 1981;7(Suppl. A):53–65. doi: 10.1093/jac/7.suppl_a.53. [DOI] [PubMed] [Google Scholar]
- 7.Borriello S P, Davies H A, Barclay F E. Detection of fimbriae amongst strains of Clostridium difficile. FEMS Microbiol Lett. 1988;49:65–67. [Google Scholar]
- 8.Borriello S P, Welch A R, Barclay F E, Davies M A. Mucosal association by Clostridium difficile in the hamster gastrointestinal tract. J Med Microbiol. 1988;25:191–196. doi: 10.1099/00222615-25-3-191. [DOI] [PubMed] [Google Scholar]
- 9.Clyne M, Ocroinin T, Suerbaum S, Josenhans C, Drumm B. Adherence of isogenic flagellum-negative mutants of Helicobacter pylori and Helicobacter mustelae to human and ferret gastric epithelial cells. Infect Immun. 2000;68:4335–4339. doi: 10.1128/iai.68.7.4335-4339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies H A, Borriello S P. Detection of capsule in strains of Clostridium difficile of varying virulence and toxigenicity. Microb Pathog. 1990;9:141–146. doi: 10.1016/0882-4010(90)90088-8. [DOI] [PubMed] [Google Scholar]
- 11.Delmée M, Avesani V, Delferriere N, Burtonboy G. Characterization of flagella of Clostridium difficile and their role in serogrouping reactions. J Clin Microbiol. 1990;28:2210–2214. doi: 10.1128/jcm.28.10.2210-2214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich C, Heuner K, Brand B C, Hacker J, Steinert M. Flagellum of Legionella pneumophila positively affects the early phase of infection of eukaryotic host cells. Infect Immun. 2001;69:2116–2122. doi: 10.1128/IAI.69.4.2116-2122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton K A, Suerbaum S, Josenhams C, Krakowka S. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 1996;64:2445–2448. doi: 10.1128/iai.64.7.2445-2448.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eveillard M, Fourel V, Barc M C, Kerneis S, Coconnier M H, Karjalainen T, Bourlioux P, Servin A L. Identification and characterization of adhesive factors of Clostridium difficile involved in adhesion to human colonic enterocyte-like Caco-2 and mucus-secreting HT29 cells in culture. Mol Microbiol. 1993;7:371–381. doi: 10.1111/j.1365-2958.1993.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 15.George W L. Antimicrobial agent associated colitis and diarrhea: historical background and clinical aspects. Rev Infect Dis. 1984;6:208–213. doi: 10.1093/clinids/6.supplement_1.s208. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Trevino M, Boureau H, Karjalainen T, Bourlioux P. Clostridium difficile adherence to mucus: results of an in vivo and ex vivo assay. Microb Ecol Health Dis. 1996;9:329–334. [Google Scholar]
- 17.Grant C C, Konkel M E, Cieplak W J, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennequin C, Porcheray F, Waligora A-J, Collignon A, Bourlioux P, Karjalainen T. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology. 2001;147:87–96. doi: 10.1099/00221287-147-1-87. [DOI] [PubMed] [Google Scholar]
- 19.Homma M, Kutsukake K, Iino T, Yamaguchi S. Hook-associated proteins essential for flagellar filament formation in Salmonella typhimurium. J Bacteriol. 1984;157:100–108. doi: 10.1128/jb.157.1.100-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karjalainen T, Barc M C, Collignon A, Trolle S, Boureau H, Cotte-Laffitte J, Bourlioux P. Cloning of a genetic determinant from Clostridium difficile involved in adherence to tissue culture cells and mucus. Infect Immun. 1994;62:4347–4355. doi: 10.1128/iai.62.10.4347-4355.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karjalainen T, Waligora-Dupriet A-J, Cerquetti M, Spigaglia P, Mauri P, Mastrantonio P. Molecular and genomic analysis of two genes encoding surface-anchored proteins from Clostridium difficile. Infect Immun. 2001;69:3442–3446. doi: 10.1128/IAI.69.5.3442-3446.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J S, Chang J H, Chung S I, Yum J S. Molecular cloning and characterization of the Helicobacter pylori fliD gene, an essential factor in flagellar structure and motility. J Bacteriol. 1999;181:6969–6976. doi: 10.1128/jb.181.22.6969-6976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Larson H E, Honour P, Price A B, Borriello S P. Clostridium difficile and aetiology of pseudomembranous colitis. Lancet. 1978;i:1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- 25.Liu S L, Ezaki T, Miura H, Matsui K, Yabuuchi E. Intact motility as a Salmonella typhi invasion-related factor. Infect Immun. 1988;56:1967–1973. doi: 10.1128/iai.56.8.1967-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyerly D M, Saum K E, MacDonald D K, Wilkins T D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSweegan E, Walker R I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986;53:141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mobley H L, Belas R, Lockatell V, Chippendale G, Trifillis A L, Johnson D E, Warren J W. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1996;64:5332–5340. doi: 10.1128/iai.64.12.5332-5340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ormonde P, Horstedt P, O'Toole R, Milton D L. Role of motility in adherence to and invasion of a fish cell line by Vibrio anguillarum. J Bacteriol. 2000;182:2326–2328. doi: 10.1128/jb.182.8.2326-2328.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poilane I, Karjalainen T, Barc M-C, Bourlioux P, Collignon A. Protease activity of Clostridium difficile strains. Can J Microbiol. 1998;44:157–161. [PubMed] [Google Scholar]
- 31.Rabaan A A, Gryllos I, Tomas J M, Shaw J G. Motility and the polar flagellum are required for Aeromonas caviae adherence to HEp-2 cells. Infect Immun. 2001;69:4257–4267. doi: 10.1128/IAI.69.7.4257-4267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson K. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect Immun. 1991;59:2727–2736. doi: 10.1128/iai.59.8.2727-2736.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadziene A, Thomas D D, Bundoc V G, Holt S C, Barbour A G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Investig. 1991;88:82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon S V, Borriello S P. Proteolytic activity of Clostridium difficile. J Med Microbiol. 1992;36:307–311. doi: 10.1099/00222615-36-5-307. [DOI] [PubMed] [Google Scholar]
- 35.Steffen E K, Hentges D J. Hydrolytic enzymes of anaerobic bacteria isolated from human infections. J Clin Microbiol. 1981;14:153–156. doi: 10.1128/jcm.14.2.153-156.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasteyre A, Barc M-C, Karjalainen T, Dodson P, Hyde S, Bourlioux P, Borriello S P. A Clostridium difficile gene encoding flagellin. Microbiology. 2000;146:957–966. doi: 10.1099/00221287-146-4-957. [DOI] [PubMed] [Google Scholar]
- 37.Tasteyre A, Karjalainen T, Avesani V, Delmée M, Collignon A, Bourlioux P, Barc M-C. Phenotypic and genotypic diversity of the flagellin gene (fliC) among Clostridium difficile isolates from different serogroups. J Clin Microbiol. 2000;38:3179–3186. doi: 10.1128/jcm.38.9.3179-3186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tasteyre A, Karjalainen T, Avesani V, Delmée M, Collignon A, Bourlioux P, Barc M-C. Molecular characterization of fliD gene encoding flagellar cap and its expression among Clostridium difficile isolates from different serogroups. J Clin Microbiol. 2001;39:1178–1183. doi: 10.1128/JCM.39.3.1178-1183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waligora A-J, Barc M-C, Bourlioux P, Collignon A, Karjalainen T. Clostridium difficile cell attachment is modified by environmental factors. Appl Environ Microbiol. 1999;65:4234–4238. doi: 10.1128/aem.65.9.4234-4238.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waligora A-J, Hennequin C, Mullany P, Collignon A, Bourlioux P, Karjalainen T. Characterization of a cell surface protein of Clostridium difficile with adhesive properties. Infect Immun. 2001;69:2144–2153. doi: 10.1128/IAI.69.4.2144-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M Y, Lovgren A, Landen R. Adhesion and cytotoxicity of Bacillus thuringiensis to cultured Spodoptera and Drosophila cells. J Invertebr Pathol. 1995;66:46–51. doi: 10.1006/jipa.1995.1059. [DOI] [PubMed] [Google Scholar]