Mature B cells control lymphotoxin-beta receptor signaling in mesenchymal stem cells to promote emergency myelopoiesis.
Abstract
Systemic inflammation halts lymphopoiesis and prioritizes myeloid cell production. How blood cell production switches from homeostasis to emergency myelopoiesis is incompletely understood. Here, we show that lymphotoxin-β receptor (LTβR) signaling in combination with TNF and IL-1 receptor signaling in bone marrow mesenchymal stem cells (MSCs) down-regulates Il7 expression to shut down lymphopoiesis during systemic inflammation. LTβR signaling in MSCs also promoted CCL2 production during systemic inflammation. Pharmacological or genetic blocking of LTβR signaling in MSCs partially enabled lymphopoiesis and reduced monocyte numbers in the spleen during systemic inflammation, which correlated with reduced survival during systemic bacterial and viral infections. Interestingly, lymphotoxin-α1β2 delivered by B-lineage cells, and specifically by mature B cells, contributed to promote Il7 down-regulation and reduce MSC lymphopoietic activity. Our studies revealed an unexpected role of LTβR signaling in MSCs and identified recirculating mature B cells as an important regulator of emergency myelopoiesis.
Introduction
Blood cell production is a tightly regulated process that is critical for organismal homeostasis. Myeloid, erythroid, and lymphoid lineages are continuously produced at defined rates under homeostasis, but during infection and/or systemic inflammation, the production of short-lived innate immune cells of myeloid lineage is prioritized, whereas lymphopoiesis is temporarily shut down, a process described as emergency myelopoiesis (Ueda et al., 2004, 2005; Manz & Boettcher, 2014). This response is essential for survival during infections as myeloid cells are required for pathogen clearance and for instructing adaptive immunity. A large body of work has shown that pattern recognition receptors (PRRs) expressed on hematopoietic stem and progenitor cells have an important role in skewing hematopoietic differentiation toward myeloid lineages (Boettcher & Manz, 2017; Capitano, 2019; Schultze et al, 2019). However, the mechanisms controlling the shutdown of lymphopoiesis remain poorly understood.
Mesenchymal stem cells (MSCs) and endothelial cells in the bone marrow form a specialized niche for hematopoietic stem cell (HSC) maintenance and differentiation into lymphoid lineages because of their expression of critical stem cell maintenance and lymphopoietic cytokines, namely, KITL and IL-7 (Mendez-Ferrer et al, 2010; Ding et al, 2012; Cordeiro Gomes et al, 2016; Fistonich et al, 2018; Comazzetto et al, 2019). Recent single-cell RNA-sequencing studies revealed that in addition to stem cell maintenance and lymphopoietic cytokines, individual MSCs also express additional lymphoid and myeloid cell lineage–instructive cytokines (Baryawno et al, 2019; Tikhonova et al, 2019), suggesting that myeloid cell production could also be controlled by the MSC niche. In support of this model, myeloid progenitors have been shown to localize in proximity to MSCs under homeostasis and during inflammation/infection (Herault et al, 2017; Zhang et al, 2021). This model of centralized control of hematopoietic stem and progenitor cell differentiation at the bone marrow MSC niche (Miao et al, 2020) led us to hypothesize that MSCs are able to sense and respond to systemic cues such as inflammatory cytokines to coordinate HSC differentiation for an appropriate hematopoietic response.
Results
Systemic inflammation turns off lymphopoietic niche activity by blocking IL-7 production
To better understand the effects of systemic inflammation on HSC differentiation, we treated mice with CFA i.p. for several days and quantified hematopoietic stem and progenitor cell subsets within the bone marrow by flow cytometry. The total bone marrow cellularity reduced within the first 12 h and remained lower than that during homeostasis until day 3 (Fig 1A). HSCs and multipotent progenitor cells (MPPs) were numerically reduced at 12 and 24 h after CFA administration, but their numbers increased between days 1 and 5 (Fig 1B). In contrast, common lymphoid progenitor and megakaryocyte progenitor numbers remained unchanged, whereas common myeloid progenitors (CMPs) and granulocyte and monocyte progenitors (GMPs) declined until day 3, with GMPs recovering on day 5. Within the lymphoid lineages, proB-cell numbers dropped sharply 12 h post-CFA administration and remained low through day 5. PreB- and immature B-cell numbers changed more gradually, declining progressively from 12 h through day 5 (Fig 1C). NK cells and NK progenitors were also significantly reduced within 12 h of systemic inflammation, and numbers remained lower than homeostatic levels until day 5 (Fig 1D). Neutrophils and monocytes were also reduced at 12 h, but both cell populations expanded to reach numbers higher than baseline by day 5 post-CFA (Fig 1E).
Figure 1. Effects of systemic inflammation on hematopoiesis.
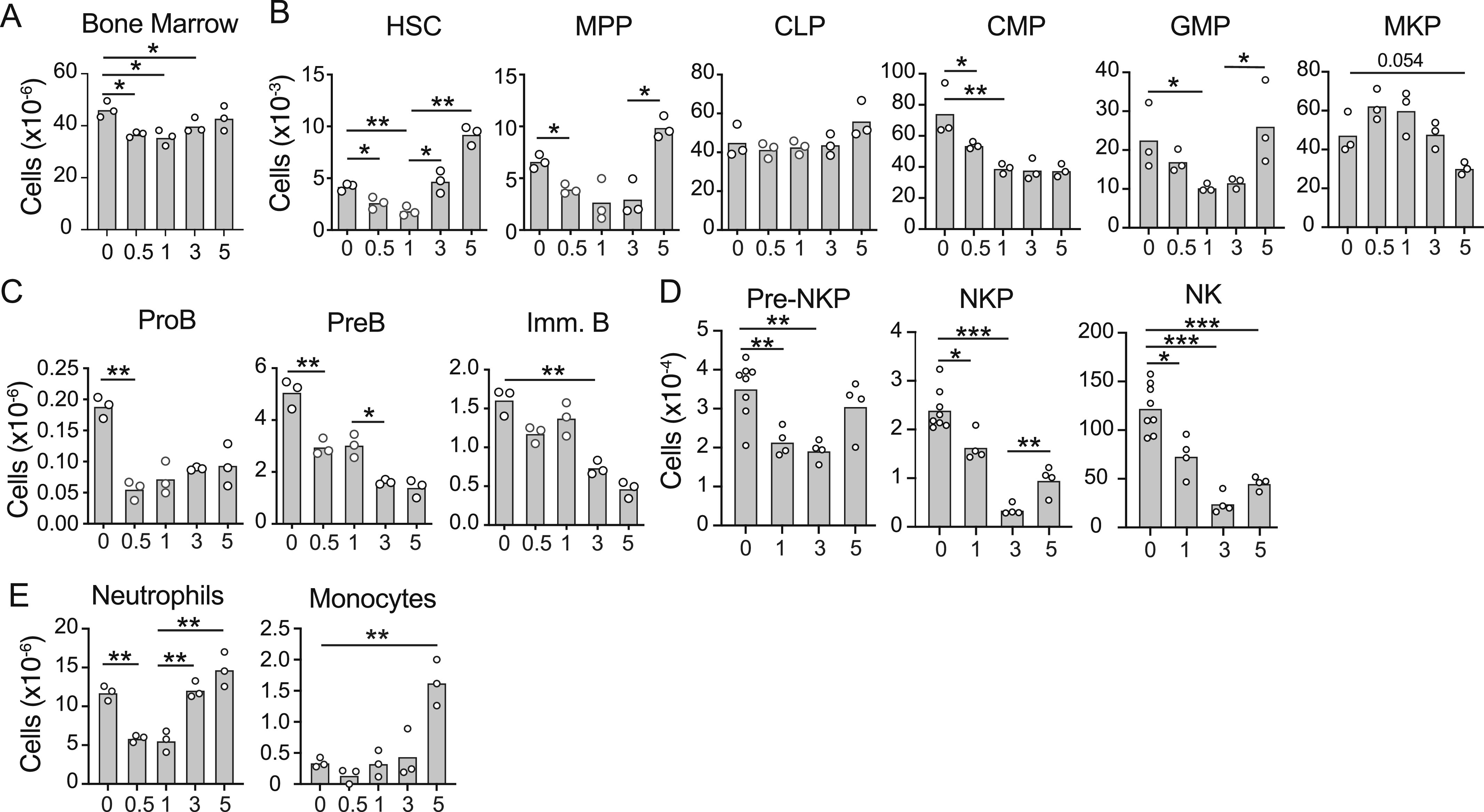
(A) Total cell number in bone marrow from femur and tibia. (B) HSC, MPP, CLP, CMP, GMP, and MKP numbers in bone marrow. (C) ProB-, PreB-, and immature B-cell numbers in bone marrow. (D) pre-NKP, NKP, and NK cell numbers. (E) Bone marrow neutrophil and monocyte numbers in femur and tibia. Mice were administered CFA i.p. on day 0 and analyzed at the indicated time points (days, x-axis). Bars indicate the mean, and circles depict individual mice. *P < 0.05 and **P < 0.005 by a t test. Data are representative of three independent experiments.
Because the shutdown in lymphopoiesis was most evident at the IL-7–dependent proB-cell stage, we analyzed the effect of CFA administration on IL7 expression in bone marrow MSCs using an Il7-GFP reporter mouse strain (Miller et al, 2013). Indeed, CFA induced a profound down-regulation of Il7-GFP in leptin receptor (Lepr)+ MSCs 5 d after administration (Fig 2A and B). The number of Lepr+ MSCs remained unchanged (Fig 2C), suggesting that IL-7 is reduced during systemic inflammation. Consistent with this possibility, IL-7 receptor abundance, which is controlled by an IL-7 receptor–mediated feedforward loop (Ochiai et al, 2012; Clark et al, 2014), declined in proB and preB cells during systemic inflammation (Fig S1A). To test whether reduced IL-7 availability during systemic inflammation caused the shutdown in lymphopoiesis, we treated mice with recombinant IL-7 complexed with a neutralizing anti-IL-7 (aIL-7, clone M25) monoclonal antibody, which increases the half-life of recombinant IL-7 in vivo (Boyman et al, 2008). In vivo IL-7/aIL-7 treatment raised the number of proB and preB cells significantly under non-inflammatory conditions (Fig S1B). During CFA-induced systemic inflammation, IL-7/aIL-7 treatment restored proB- and preB-cell numbers in the bone marrow but did not affect immature and mature B cells, which are not dependent on IL-7 (Fig 2D). Interestingly, IL-7/aIL-7 treatment also blunted the expansion of bone marrow myeloid populations (Fig 2E). Altogether, these studies suggest that inflammation-induced lymphopenia is at least partly controlled by reduced IL-7 production in bone marrow MSCs.
Figure 2. Inflammation-induced Il7 down-regulation and impact on emergency myelopoiesis.
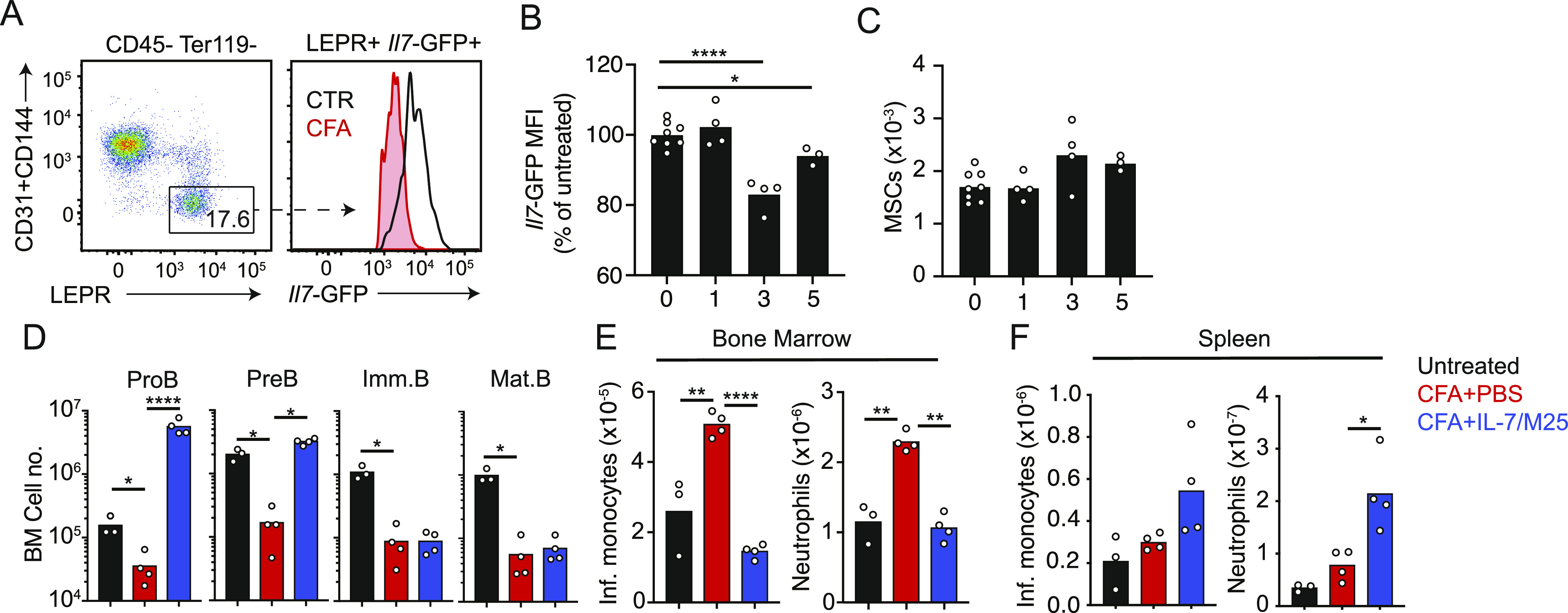
(A) Il7-GFP expression on gated bone marrow MSCs in mice injected i.p. with CFA (red) or saline (blue) for 5 d. (B) Il7-GFP Geo. mean in gated Lepr+ MSCs. (C) Number of Lepr+ MSCs in bone marrow. (D, E, F) Mice immunized with CFA i.p. and pretreated with the IL-7/aIL-7 cytokine/antibody complex (1.5/15 μg, respectively, clone M25) i.v. (D) Developing B-cell subsets in bone marrow. (E, F) Myeloid cell subsets in bone marrow (E) and spleen (F). Bars represent the average, and circles depict individual mice. *P < 0.05; **P < 0.005; ***P < 0.0005; and ****P < 0.00005 by an unpaired t test. Data in all panels are representative of two experiments.
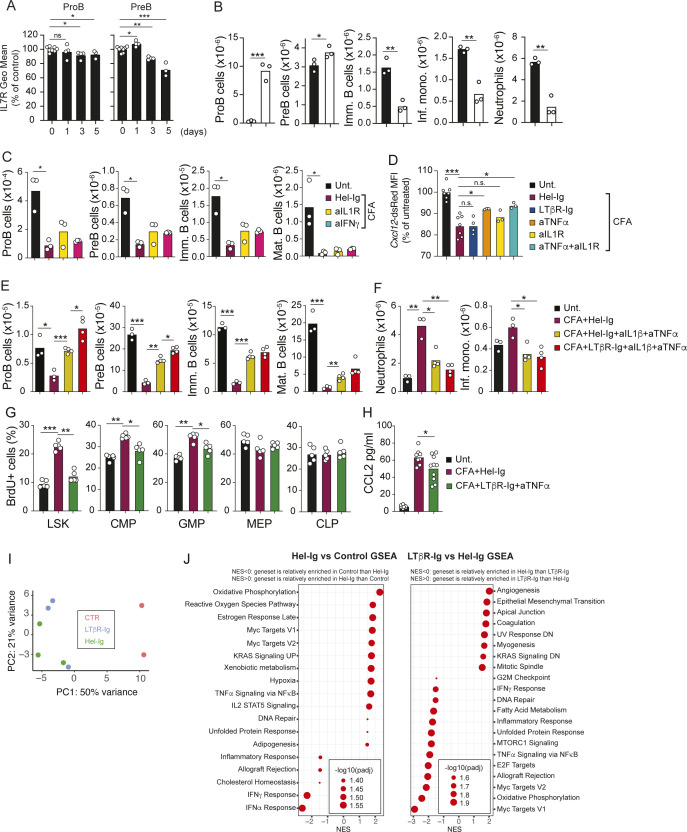
Figure S1. Effects of cytokine and LTβR blocking on emergency myelopoiesis.
(A) IL-7 receptor expression in proB and preB cells during CFA-induced systemic inflammation. (B) Numbers of developing B-cell subsets in the bone marrow of mice treated with IL-7/aIL-7 for 3 d. (C) Numbers of proB, preB, immature B, and mature B cells in bone marrow of mice injected with CFA (i.p.) and cytokine blocking antibodies over 5 d. (D) Cxcl12-dsRed Geo. mean expression in bone marrow MSCs of mice injected with CFA (i.p.) and cytokine blocking antibodies over 5 d. (E, F) Numbers of proB, preB, immature B, and mature B cells in bone marrow (E) and of neutrophils and inflammatory monocytes in the spleen (F) of mice administered CFA ip and treated with cytokine blocking antibodies. (G) Frequency of BrdU+ cells within the indicated hematopoietic progenitor population, as measured by flow cytometry, of CFA-challenged mice and mice treated with control or LTβR-Ig+ anti-TNF-α blocking reagents. BrdU administration was 1 h before euthanasia. (H) CCL2 protein measurements in bone marrow interstitial fluids recovered from CFA-challenged mice and mice treated with control or LTβR-Ig + anti-TNF-α blocking reagents. Data in all panels are representative of two individual experiments. Bars represent the average, and circles depict individual mice. *P < 0.05; **P < 0.005; and ***P < 0.0005 by an unpaired t test. (I) Principal component analysis distribution plot from RNA sequencing of MSCs in control mice, and in mice challenged with CFA for 2 d treated with Hel-Ig or with LTβR-Ig. (J) GSEA-MSigDB Hallmark pathway analyses from differentially expressed genes. Data are representative of one experiment.
LTβR signaling and inflammatory cytokine receptor signaling control Il7 transcription in MSCs
Prior studies have shown critical roles of inflammatory cytokines in driving emergency myelopoiesis (Cain et al, 2009; Manz & Boettcher, 2014). To identify the signals and receptors on MSCs responsible for controlling IL7 transcription in response to inflammation, we performed RNA-sequencing of Lepr+ bone marrow MSCs. Transcriptomic analyses showed that Lepr+ MSCs express multiple inflammatory cytokine receptors, including lymphotoxin-β receptor (LTβR; Fig 3A), the latter being a TNF superfamily receptor that is required for peripheral lymphoid organ development, and for maturation of stromal cell subsets in secondary lymphoid organs (Ruddle, 2014; Cheng et al, 2019). MSCs also expressed low to undetectable amounts of PRRs (Fig 3A), but a prior study showed no measurable roles of PRRs in MSCs during emergency myelopoiesis (Boettcher et al, 2014). To test whether inflammatory cytokines and/or LTβR control IL7 expression in bone marrow MSCs, we challenged Il7GFP/+ mice with CFA for 5 d and blocked LTβR and/or inflammatory cytokines using a soluble LTβR-Ig decoy and cytokine blocking antibodies. Interestingly, inflammation-induced IL7 down-regulation was partially blocked with LTβR-Ig, anti-IL-1β, or anti-TNF-α, and was completely blocked with LTβR-Ig + anti-TNF-α (Fig 3B). Although IFN-γ has been shown to play important roles in emergency myelopoiesis (Baldridge et al., 2010; de Bruin et al., 2012; Kaufmann et al, 2018), blocking IFN-γ did not prevent IL7 down-regulation (Fig 3B), nor did it recover B-cell progenitor numbers in vivo (Fig S1C). The complete block of IL7 down-regulation with LTβR-Ig and anti-TNF-α treatment led to significantly increased IL7–dependent progenitor B-cell production, which resulted in increased numbers of immature B cells (Fig 3C). Nevertheless, proB- and preB-cell numbers were still reduced when compared to baseline (Fig 3C), likely because of down-regulation of CXCL12 (Fig S1D). Systemic inflammation and infection down-regulate CXCL12 transcripts and protein levels in vivo (Ueda et al., 2004, 2005; Saw et al, 2019), whereas reduced CXCR4 function (CXCL12 receptor) in B-cell progenitors impairs B lymphopoiesis (Beck et al, 2014; Cordeiro Gomes et al, 2016; Mandal et al, 2019). Furthermore, IL-1 has been reported to directly inhibit the differentiation of MPPs to the B lineage (Kennedy & Knight, 2015) and triple blocking of LTβR, IL-1R, and TNFR signaling resulted in a small but significant increase in B-lineage cell production when compared to LTβR and TNFR double blocking (Fig S1E). Likewise, combined blocking of IL-1β and TNF-α also completely restored proB-cell numbers in the BM (Fig S1E), whereas LTβR and TNFR double blocking only partially restored proB-cell numbers (Fig 3C). These results show that redundancy between IL-1R, TNFR, and LTβR signaling promotes IL7 down-regulation.
Figure 3. LTβR signaling and TNF receptor signaling control IL-7 production and B lymphopoiesis.
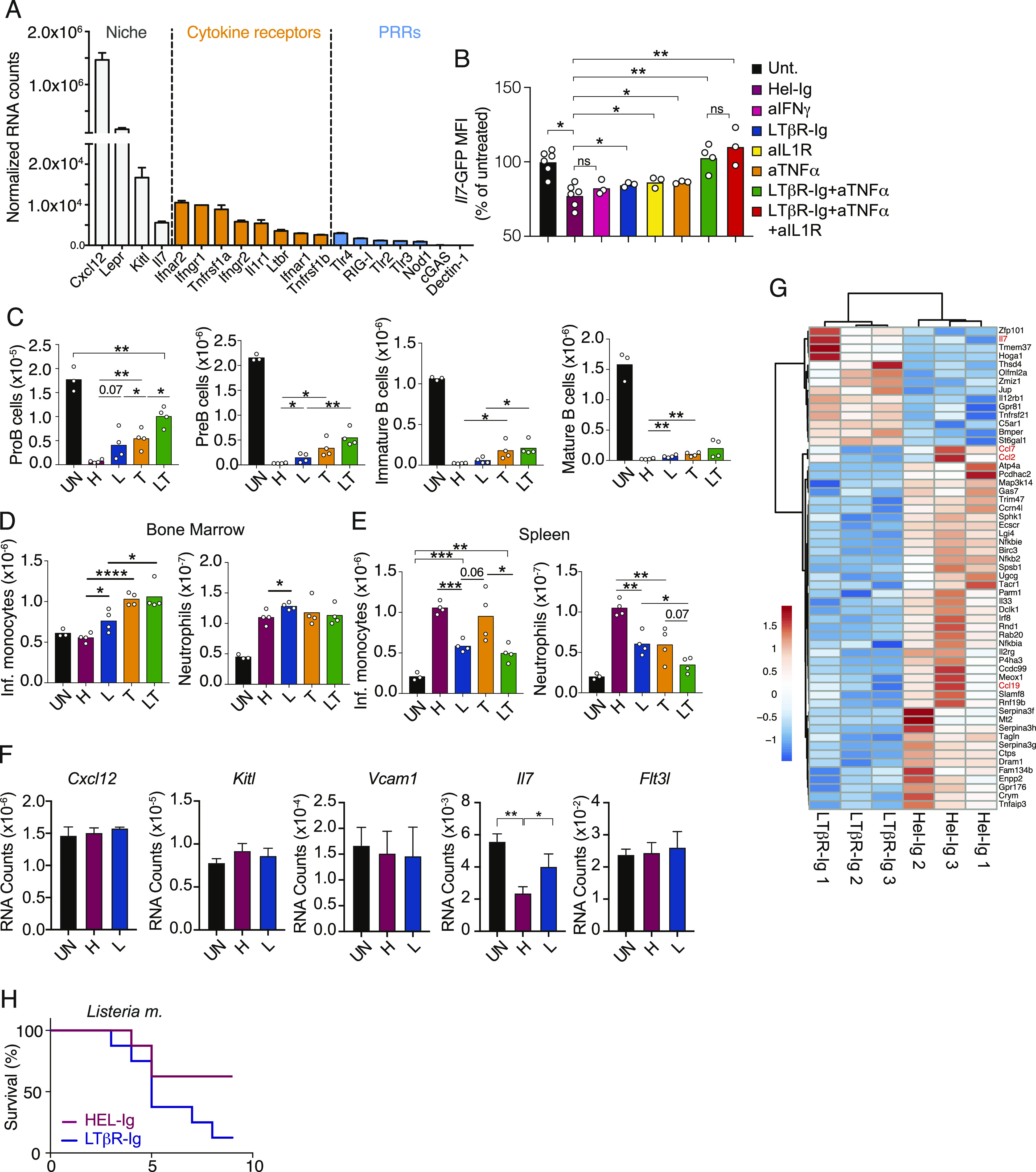
(A) Normalized RNA counts of cytokine and pattern recognition receptors in bone marrow MSCs; data obtained from MSC RNA sequencing. (B) Il7-GFP expression in gated bone marrow MSCs relative to untreated mice (expressed as percent of untreated). (C) Numbers of proB, preB, immature B, and mature B cells in bone marrow. (D, E) Numbers of inflammatory monocytes and neutrophils in bone marrow (D) and spleen (E). In panels (B, C, D, E), data were obtained from untreated control (UN, black) or CFA-immunized mice treated with HEL-Ig (H, wine red), LTβR-Ig (L, blue), anti-TNF-α (T, orange), and LTβR-Ig + anti-TNF-α (LT, green) for 5 d. (F) CFA-induced changes in lymphopoietic factors expressed by MSCs. (G) Differentially expressed genes in MSCs isolated from mice injected with CFA for 24 h and treated with LTβR-Ig (L) or HEL-Ig (H, FDR-adjusted q-value < 0.05). (H) Survival after i.v. infection with Listeria monocytogenes (50,000 CFU) after pretreatment with either HEL-Ig or LTβR-Ig (n = 8 per group). Data in panels (A, B, C, D, E) are representative of two to six experiments. Data in panels (F, G) were from one experiment. Bars represent the average, circles depict individual mice, and error bars indicate the SEM. *P < 0.05; **P < 0.005; ***P < 0.0005; and ****P < 0.00005 by an unpaired t test.
Monocytes and neutrophils were significantly increased in CFA-inflamed mice but were modestly increased in the bone marrow of LTβR-Ig–treated when compared to Hel-Ig–treated mice (Fig 3D). In contrast, both myeloid cell subsets were significantly reduced in the spleen of mice treated with LTβR-Ig, and even further reduced in mice treated with combined LTβR-Ig and anti-TNF-α antibodies (Fig 3E). The concomitant accumulation in bone marrow and reduction in the periphery suggested that myeloid cell egress from bone marrow was impaired by blocking LTβR or TNFR signaling. LTβR and TNFR signaling blockade also reduced the rate of myeloid progenitor proliferation in vivo, whereas BrdU incorporation in common lymphoid progenitors and in megakaryocyte/erythroid progenitors (MEPs) was unaffected in vivo (Fig S1G). In line with the reported role of IL-1R in supporting inflammation-induced emergency myelopoiesis (Ueda et al, 2009) and LTβR signaling in the homeostasis of neutrophils in the spleen (Shou et al, 2021), blocking of IL-1R in addition to LTβR and TNFR led to a further reduction in inflammatory monocytes and neutrophil numbers in the spleen (Fig S1F).
As the role of LTβR signaling in bone marrow MSCs in the control of emergency myelopoiesis was unexpected, we decided to focus our attention on this signaling axis. To understand which MSC-expressed genes are controlled by LTβR signaling, we examined global transcriptomic changes in MSCs isolated from CFA-injected mice that were also pretreated with LTβR-Ig or with control IgG1 (anti-hen egg lysozyme, HEL-Ig) for 1 d. Systemic inflammation segregated MSCs from untreated samples on principal component (PC)-1, but LTβR-Ig treatment did not separate samples on PC1 or PC2 (Fig S1I). CFA significantly changed the expression of 576 genes, of which 56 were directly controlled by LTβR signaling (Table S1). Gene set enrichment analyses revealed up-regulation of genes associated with metabolic changes (e.g., oxidative phosphorylation, reactive oxygen species, and Myc-regulated genes) and with increased inflammatory cytokine receptor signaling (e.g., NF-κB and STAT5 signaling) in MSCs isolated from CFA-injected and Hel-Ig mice. LTβR blocking led to increased expression of genes associated with mesenchymal cell differentiation and angiogenesis, and reduced expression of genes associated with inflammation (Fig S1J). Importantly, among factors important for lymphopoiesis, such as CXCL12, Kit and FLT3 ligands, VCAM1, and IL7, CFA-induced inflammation only promoted IL7 down-regulation, which was partially prevented by LTβR blocking (Fig 3F). Besides IL7, LTβR signaling presumably in MSCs also controlled CCL2 expression (Figs 3G and S1H and Table S1), consistent with prior studies (Cuff et al, 1999). CCL2 is a chemokine that promotes monocyte egress from bone marrow by binding to its receptor CCR2 (Serbina & Pamer, 2006; Shi et al, 2011). CCL7, another ligand for CCR2, was also altered by LTβR blocking during systemic inflammation (Fig 3G). In contrast, LTβR-Ig did not affect CXCL12 transcription (Figs 3F and S1D and Table S1). Because CCR2 signaling promotes CXCR4 desensitization in monocytes (Jung et al, 2015), these data could explain the apparent defect in myeloid cell egress from bone marrow during systemic inflammation with LTβR and TNFR blocking (Fig 3D and E). Although the LTβR-dependent fold changes observed in MSC gene expression, and in lymphoid and myeloid cell production, were relatively small (<threefold), these were nevertheless physiologically important given that mice became more susceptible to systemic infection with Listeria monocytogenes (Fig 3H), which is consistent with a prominent role of monocytes in defense against systemic L. monocytogenes infection (Serbina et al, 2008).
Table S1 LTβR-regulated genes in Lepr+ MSPCs during CFA-induced systemic inflammation. (5MB, xlsx)
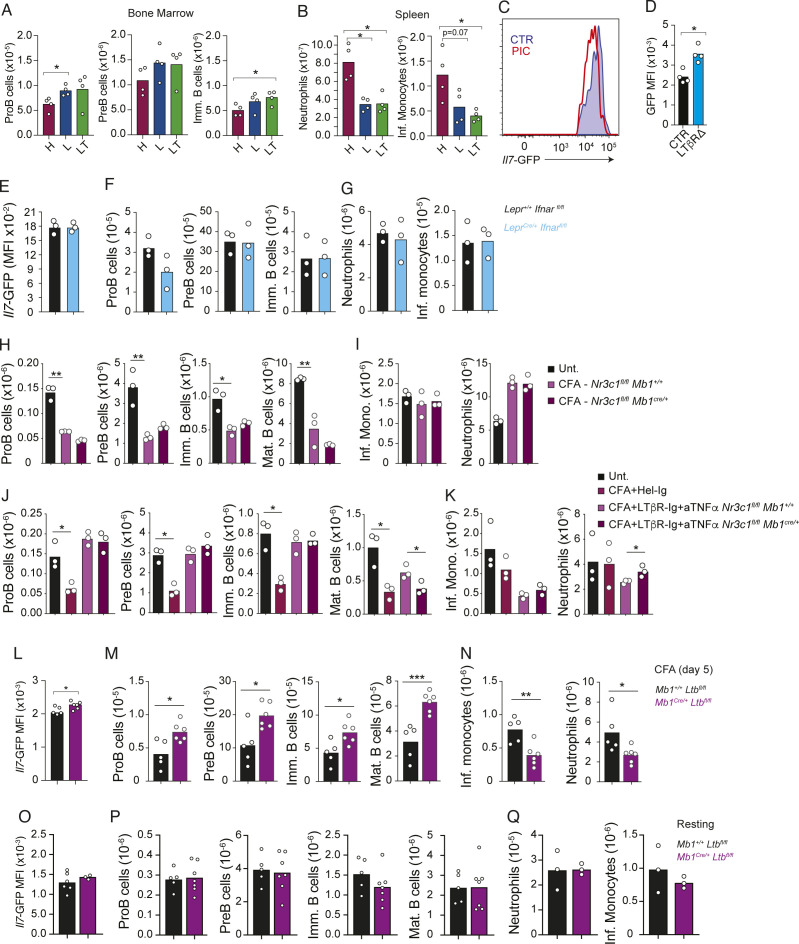
To determine whether cell-intrinsic LTβR signaling in MSCs is required for emergency myelopoiesis, we challenged mice conditionally deficient in Ltbr in bone marrow MSCs (LTβR cKO) with CFA. Lepr-cre–mediated deletion abrogated LTβR with about 80% efficiency in bone marrow MSCs (Fig 4A), which was sufficient for preventing inflammation-induced IL7 down-regulation (Fig 4B). This resulted in increased B-cell progenitor production during systemic inflammation (Fig 4C). Although monocyte and neutrophil numbers in the bone marrow were similar, splenic monocyte numbers were significantly reduced (Fig 4D and E). The difference in bone marrow monocyte numbers between systemic LTβR-Ig treatment and LTβR cKO mice during inflammation may be due to the fact that endothelial cells in bone marrow, which express CCL2 and contribute to monocyte egress (Shi et al, 2011), also express LTβR (Tikhonova et al, 2019).
Figure 4. LTβR signaling in bone marrow MSCs controls emergency myelopoiesis.
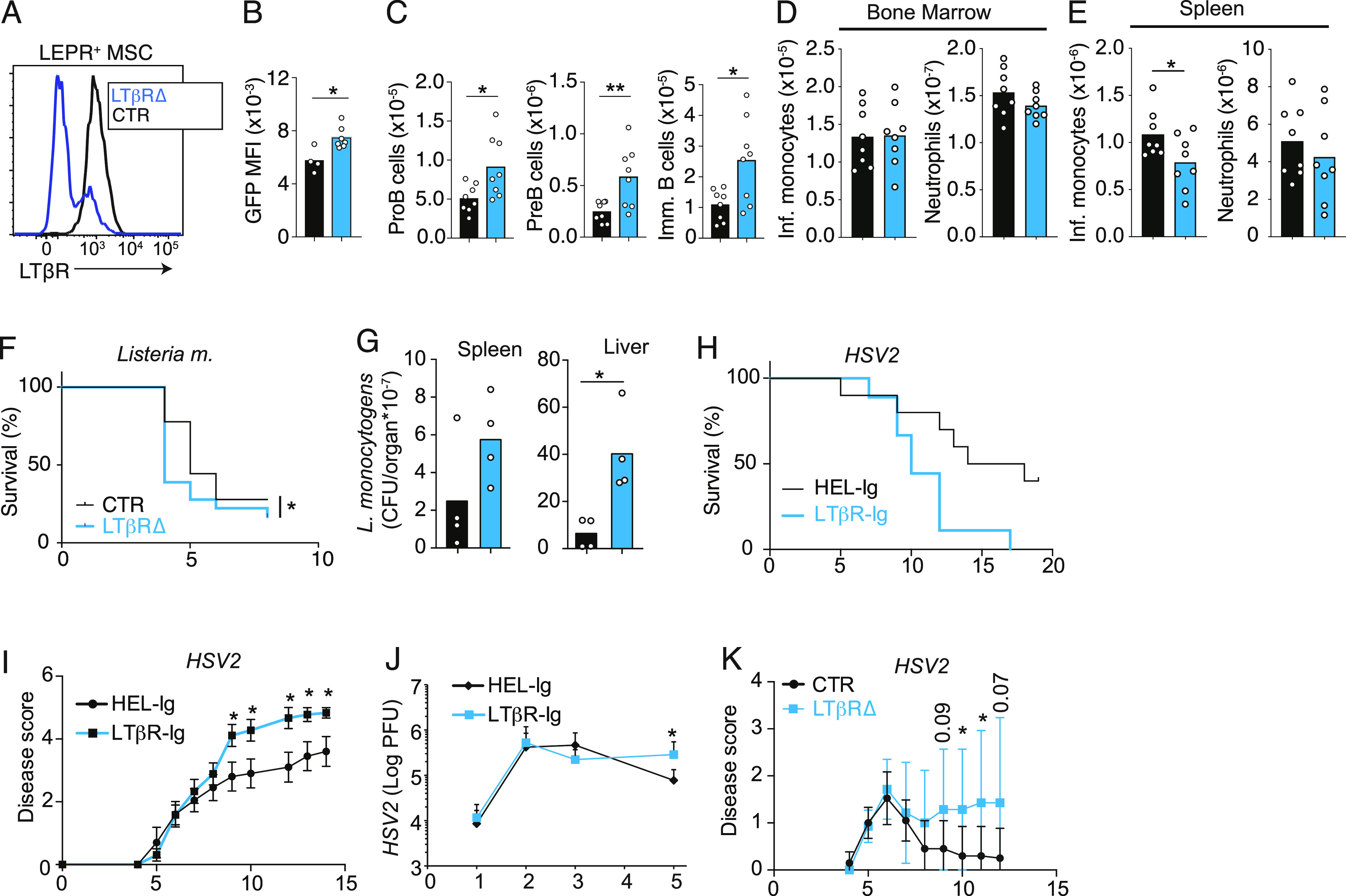
(A) Histogram of LTβR expression on gated bone marrow Lepr+ MSCs from Lepr+/+; Ltbrfl/fl (CTR, black) and LeprCre/+; Ltbrfl/fl (blue, LTβR∆) mice. (B, C, D, E) Il7 expression in MSCs, (C) developing B-cell subsets in bone marrow, and (D, E) inflammatory monocytes and neutrophils in bone marrow (D) and in the spleen (E) of CTR (black) and of LTβR∆ (blue) mice immunized with CFA i.p. for 5 d. Bars represent the average, circles depict individual mice, and error bars indicate the SEM. (F) Survival of CTR (black, n = 9) and of LTβR∆ (blue, n = 11) mice after i.v. infection with Listeria monocytogenes (370,000 CFU). (G) Listeria monocytogenes CFUs in spleen and liver of CTR (black) and of LTβR∆ (blue) mice at day 3 post-infection. (H, I, J) Survival (H), disease score (I), and HSV-2 viral titers (J) in vaginal wash of mice infected with 1,000 PFU after pretreatment with either HEL-Ig (n = 8) or LTβR-Ig (n = 6). (K) Disease score of CTR (black) and of LTβR∆ (blue) mice infected with HSV2 (1,000 PFU) intravaginally. In panels (G, H, I, J), x-axis shows time post-infection (days). *P < 0.05 and **P < 0.005 by an unpaired t test. Data in panels (A, B, C, D, E, F) are representative of two to four experiments. Data in panels (G, H, I, J) were from one experiment. Bars represent the average, circles depict individual mice, and error bars indicate the SEM.
Conditional Ltbr deficiency in MSCs did not alter the rate of hematopoietic progenitor, monocyte, and neutrophil production, as measured by BrdU incorporation over a 2-h period (Fig S2A). However, there was a trend toward reduced numbers of newly generated BrdU+ monocytes reaching the spleen over a period of 48-h of BrdU exposure (Fig S2B), and a significant reduction in the total number of monocytes in the spleen (Fig S2C). During homeostasis, LTβR cKO mice had a higher number of proB cells in the bone marrow, consistent with our prior studies (Zehentmeier et al, 2022), but similar numbers of monocytes and neutrophils in bone marrow and spleen (Fig S2E–G). The number of MSCs was unchanged (Fig S2D). Although Ltbr deficiency in MSCs led to smaller than twofold changes in B-cell and myeloid cell numbers during inflammation, these changes were physiologically relevant given that LTβR cKO mice were significantly more susceptible to systemic infection with L. monocytogenes by day 5 (Fig 4F). Consistent with this, proB-cell numbers were increased in the bone marrow of LTβR cKO mice infected with Listeria on day 2 (Fig S2H–J), and splenic monocytes were significantly reduced on day 3 (Fig S2K–M). Importantly, LTβR cKO animals had significantly higher pathogen burden compared with WT mice (Fig 4G), suggesting that the impairment in emergency myelopoiesis compromised pathogen clearance. However, we cannot rule out the possibility that alterations in splenic niches of LTβR cKO mice may have contributed to the reduction in splenic monocytes given the fact that a few stromal cells are targeted by Lepr-cre (Zehentmeier et al, 2022). To test whether the LTβR axis was important in systemic infectious inflammation generally, we also examined models of acute viral challenges. As in the CFA model of bacterial inflammation, challenge with polyinosinic:polycytidylic acid (polyI:C), a mimetic of viral RNA, also reduced IL7 expression and B-cell production, and promoted emergency myelopoiesis in an LTβR (and TNF-α)-dependent manner (Fig S3A–D). These findings are consistent with early studies showing a requirement for TNF-α and lymphotoxin-α in B-cell progenitor apoptosis induced by systemic infections with influenza A virus (Sedger et al, 2002). In contrast, conditional Ifnar1 deficiency in MSCs did not impact IL7 expression nor B-cell and myeloid cell development during polyI:C-induced inflammation (Fig S3E–G). Like in the Listeria model, mice treated with LTβR-Ig, or mice conditionally deficient in Ltbr in bone marrow MSCs, were significantly more susceptible to infection of HSV2 (Fig 4H–K), a pathogen that is also controlled by monocyte-mediated immunity (Iijima et al, 2011).
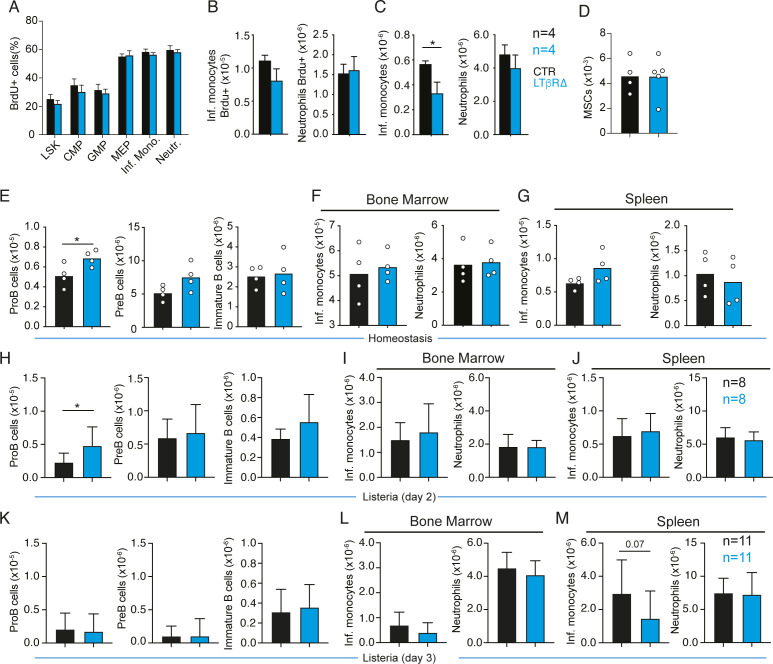
Figure S2. LTβR signaling and TNF receptor signaling control Il7 down-regulation and promote emergency myelopoiesis in response to Listeria.
(A, B, C) Effects of CFA-induced systemic inflammation in hematopoietic progenitor and myeloid cell proliferation. (A) BrdU incorporation in LSKs, CMPs, GMPs, MEPs, monocytes, and neutrophils on day 3 after CFA (i.p.) of control (black) and LTβR cKO (blue) mice. BrdU injection was 2 h before euthanasia. (B) Number of BrdU+ monocytes and neutrophils in the spleen. Mice were kept under BrdU exposure for 48 h. (C) Total number of monocytes and neutrophils in the spleen. (E, F, G) Total number of developing B cells and myeloid cells in control (black) and LTβR cKO (blue) mice under homeostasis. (D) Number of Lepr+ MSCs in bone marrow. (E) B-cell subsets in bone marrow. (F) Monocytes and neutrophils in bone marrow. (G) Monocytes and neutrophils in the spleen. (H, I, J) Total number of developing B cells and myeloid cells in control (black) and LTβR cKO (blue) mice analyzed 2 d after infection with Listeria. (H) B-cell subsets in bone marrow. (I) Monocytes and neutrophils in bone marrow. (J) Monocytes and neutrophils in the spleen. (K, L, M) Total number of developing B cells and myeloid cells in control (black) and LTβR cKO (blue) mice analyzed 3 d after infection with Listeria. (K) B-cell subsets in bone marrow. (L) Monocytes and neutrophils in bone marrow. (M) Monocytes and neutrophils in the spleen. Bars represent the average, and circles depict individual mice. *P < 0.05 by an unpaired t test.
Figure S3. LTβR signaling and TNF receptor signaling control Il7 down-regulation and promote emergency myelopoiesis in response to the viral mimetic polyI:C, and effects of glucocorticoid receptor deficiency in B-lineage cells in emergency myelopoiesis.
(A, B) Number of developing B-cell subsets in bone marrow (A) and myeloid cell subsets in the spleen (B) of mice immunized with polyI:C i.v. (1.4 mg/Kg/d) for 4 d pretreated with Hel-Ig, LTβR-Ig, or anti-LTβR-Ig + anti-TNF-α. (C, D) Il7-GFP expression in saline versus polyI:C-immunized control or LTβR∆ mice (day 5). (E, F, G) Impact of Ifnar deletion in MSCs in emergency myelopoiesis in response to viral mimetic and susceptibility to HSV2 viral infection. (E) Il7-GFP Geo. mean in Lepr+ MSCs. (F) Number of developing B cells in bone marrow. (G) Monocytes and neutrophils in the spleen. (H, I) Number of developing B-cell subsets in bone marrow (H) and myeloid cell subsets in the spleen (I) of control and Nr3c1fl/fl Mb1cre/+ mice immunized with CFA for 5 d. (J, K) Number of developing B-cell subsets in bone marrow (J) and myeloid cell subsets in the spleen (K) of control and Nr3c1fl/fl Mb1cre/+ mice immunized with CFA for 5 d and pretreated with HEL-Ig or with LTβR-Ig + anti-TNF-α. (L, M, N) Effects of CFA-induced systemic inflammation in mice conditionally deficient in Ltb in B-lineage cells. (L) Il7-GFP Geo. mean in Lepr+ MSCs in bone marrow. (M) Developing B-cell numbers. (N) Neutrophil and monocyte numbers in the spleen. Mice were Mb1+/+; Ltbfl/fl (CTR, black) and Mb1cre/+; Ltbfl/fl and challenged with CFA (ip) for 5 d. (O, P, Q) Numbers of developing B cells, monocytes, and neutrophils in mice conditionally deficient in Ltb in B-lineage cells under homeostasis. (O) Il7-GFP Geo. mean in Lepr+ MSCs in bone marrow. (P) Developing B-cell numbers. (Q) Neutrophil and monocyte numbers in the spleen. Bars represent the average, and circles depict individual mice. *P < 0.05; **P < 0.005; and ***P < 0.0005 by an unpaired t test.
Systemic inflammatory cytokines, such as IL-1 and TNF-α, act on the hypothalamic–pituitary–adrenal axis to increase glucocorticoid (GC) production, which in turn exerts anti-inflammatory effects (Cain & Cidlowski, 2017). GCs also act directly on B and T lymphocytes by activating the mitochondrial pathway of cellular apoptosis (Cain et al, 2020; Taves & Ashwell, 2021). However, conditional deletion of the GC receptor NR3C1 in B-lineage cells had no effect on CFA-induced lymphopoiesis shutdown and emergency myelopoiesis (Fig S3H and I). Furthermore, lymphopoiesis and emergency myelopoiesis were indistinguishable in Nr3c1 cKO and control littermate mice treated with LTβR and TNF-α blocking reagents (Fig S3J and K), ruling out a significant role of GCs in emergency myelopoiesis.
Lymphotoxin-α1β2 from recirculating mature B cells promotes emergency myelopoiesis
Finally, we focused on determining the source of LTβR ligands. In the first 24 h after systemic infection, although myeloid cells and developing B cells are significantly reduced (Fig 1), mature B cells accumulate in the bone marrow and are reduced in the spleen (Fig 5A), consistent with prior observations (Moreau et al, 2015). Thus, we hypothesized that recirculating mature B cells might play a role in driving IL7 down-regulation given the fact that naïve B cells express lymphotoxin-α1β2 heterotrimers (a membrane-bound ligand for LTβR) and organize the stromal compartments of secondary lymphoid organ through interactions with stromal cell–expressed LTβR (Fu & Chaplin, 1999; Cyster, 2005). Furthermore, naïve B cells transiting through the bone marrow express measurable amounts of LTβR ligands even during systemic inflammation (Fig 5B), which led us to consider the possibility that these cells deliver lymphotoxin-α1β2 signals to LTβR on bone marrow MSCs during the early hours of systemic inflammation. Importantly, MSCs expressed significantly higher IL7 transcripts during systemic inflammation when naïve B cells were conditionally deficient in Ltb using Cd21-cre (Fig 5C). Similar results were obtained when B-lineage cells were conditionally deficient in Ltb (Fig S3L–N). In contrast, no changes in Il7-GFP, developing B-cell subsets, or myeloid cells were noted in the bone marrow and spleen of mice conditionally deficient in Ltb in B-lineage cells during homeostasis (Fig S3O–Q). Increased IL7 was consistent with increased B-cell production in bone marrow, which correlated with significantly reduced monocytes in the spleen (Fig 5D and E), similar to that seen in LTβR cKO (Fig 4D). These data revealed an unexpected and previously unknown role of naïve recirculating B cells in turning off B-lymphopoiesis through IL7 down-regulation in bone marrow MSCs during systemic inflammation. It also suggests that by engaging LTβR on MSCs, mature B cells contribute to promoting monocyte egress from bone marrow and fine-tuning emergency myelopoiesis. Hematopoietic stem and progenitor cells, and myeloid cells, in the bone marrow also produce LTβR ligands (Zehentmeier et al, 2022). Although it is possible that other hematopoietic cells deliver LTβR ligands to MSCs, our results suggest that such contribution may be minimal.
Figure 5. Circulatory mature B cells provide lymphotoxin-α1β2 to MSCs and promote emergency myelopoiesis.
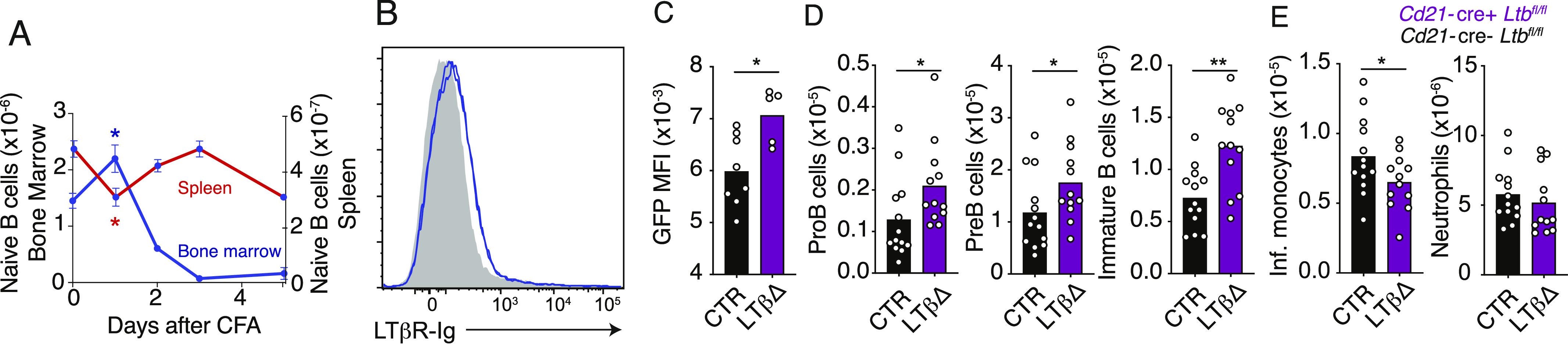
(A) Changes in mature B-cell number in bone marrow (blue) and spleen (red) of mice injected with CFA (i.p.) over 5 d. (B) LTβR ligand expression in mature B cells in bone marrow of mice immunized with CFA for 24 h (Ltb−/−, gray-filled histogram; Ltb+/+, blue histogram). (C, D, E) Il7-GFP expression Geo. mean in MSCs (C); developing B-cell subsets in bone marrow (D); and inflammatory monocytes and neutrophils in the spleen (E) of control (black) or Cd21-cre Ltbfl/fl mice (purple) injected with CFA i.p. for 5 d. Bars represent the average, and error bars indicate the SEM. *P < 0.05 and **P < 0.005 by an unpaired t test. Data in all panels are representative of two to four experiments.
Discussion
In this study, we showed that LTβR signaling, IL-1R signaling, and TNF-α receptor signaling in bone marrow MSCs promote lymphopoiesis shutdown by turning off IL7 production. Mature recirculating B cells accumulate in the bone marrow for a period of 12–24 h during systemic inflammation where they deliver LTα1β2 to LTβR expressed on MSCs. Besides shunting IL7 production, LTβR signaling also modulates the expression of CCL2, a chemokine expressed by MSCs that engages CCR2 and promotes monocyte egress from bone marrow (Serbina & Pamer, 2006; Shi et al, 2011).
The reciprocal production of lymphocytes and myeloid cells during systemic infection suggests that expansion of myelopoiesis cannot occur, whereas homeostatic numbers of lymphoid cells are still being produced. By turning off IL7 transcripts and promoting myeloid cell egress, LTβR, in combination with TNFR and IL-1R signaling in MSCs, acts as a molecular switch between homeostatic hematopoiesis and a temporary state of lymphopenia that is required for emergency myelopoiesis to proceed. Why lymphopoiesis needs to be turned off temporarily to allow myeloid cell expansion remains unknown. We speculate that sickness behaviors, and particularly anorexia induced by systemic infection (a metabolic adaptation that is essential for organismal tolerance to systemic inflammation (Wang et al, 2016)), impose metabolic constraints in the bone marrow microenvironment possibly because of reduced cytokines (e.g., SCF) or nutrient availability (e.g., glucose, amino acids) that may enforce an upper limit to the rate of myeloid cell production. It is possible that some blood cancers, such as Philadelphia chromosome acute lymphoblastic leukemia and acute myeloid leukemias, which are known to induce IL7 down-regulation in bone marrow MSCs (Fistonich et al, 2018; Baryawno et al, 2019; Zehentmeier & Pereira, 2019) exploit molecular mechanisms, like LTβR, to prevent normal blood cell development and in this way reduce competition for limiting nutrients in the bone marrow interstitium.
Materials and Methods
Mice
C57BL/6 mice were purchased from the Jackson Laboratories or the National Cancer Institute. Lepr-cre, Mb1cre/+, Nr3c1fl/fl, Ifnarfl/fl, and Cd21-cre mice were purchased from the Jackson Laboratories. Il7GFP/+ mice were from our internal colony. Ltbfl/fl (Tumanov et al, 2002) and Ltbrfl/fl (Wang et al, 2010) mice were bred at Yale Animal Resources Center. Adult males (8–13 wk) were used for L. monocytogenes infections, and adult females (8–12 wk) were used for HSV-2 infections. Male and female adult mice (8–12 wk) were used for all other experiments. All mice were maintained under specific pathogen-free conditions at the Yale Animal Resources Center and were used according to the protocol approved by the Yale University Institutional Animal Care and Use Committee.
Flow cytometry
BM stromal cells were isolated as previously described (Cordeiro Gomes et al, 2016). Briefly, long bones were flushed with HBSS supplemented with 2% of heat-inactivated fetal bovine serum, penicillin/streptomycin, L-glutamine, Hepes, and 200 U/ml collagenase IV (Worthington Biochemical Corporation), and digested for 30 min at 37°C. Cell clumps were dissociated by gentle pipetting. Cells were filtered through a 100-μm nylon mesh and washed with HBSS/2% FBS. All centrifugation steps were done at 300g for 5 min, and all stains were done on ice. LEPR stains were done for 1 h and all other stains for 20 min. BM MSCs were identified as CD45− Ter119− CD31− CD144− LEPR+ cells. For analysis of hematopoietic populations, long bones were flushed with DMEM supplemented with 1.5% fetal bovine serum, penicillin/streptomycin, L-glutamine, and Hepes and spleens were mashed through a 70-μm nylon mesh. Red blood cells were lysed with ammonium chloride buffer. Hematopoietic cell populations were identified as follows: ProB: CD19+ CD93+ IgM− cKit+; PreB: CD19+ CD93+ IgM− cKit−; immature B: CD19+ IgM+ CD93+; mature B: CD19+ IgM+ CD93−; developing neutrophils: CD115− Gr1+ CD11bhi CXCR4hi; mature neutrophils: CD115− Gr1hi CD11blo; immature monocytes: CD115+ Gr1+ CXCR4hi; mature inflammatory monocytes: CD115+ Gr1+ CXCR4lo; splenic mature neutrophils: CD115− Gr1hi CD11blo; splenic inflammatory monocytes: CD115+ Gr1+; CMP: lineage− cKit+ SCA-1− CD34+ CD16/32lo; GMP: lineage− cKit+ SCA-1− CD34+ CD16/32hi; MEP: lineage− cKit+ SCA-1− CD34− CD16/32−; and erythrocytes: Ter119+ CD71− or CD71+. The lineage cocktail was as follows: CD19, B220, CD3e, CD4, Gr1, NK1.1, Ter119, and CD11b.
Systemic inflammation and cytokine/cytokine receptor blocking in vivo
CFA-induced inflammation was induced by i.p. injection of 200–400 μl of a 1:1 emulsion of DPBS and CFA (Sigma-Aldrich). Mice were analyzed at the stated time points after CFA injection. PolyI:C treatments were performed intravenously at 1.4 mg/Kg/d. LTβR signaling was blocked with 200 μg of LTβR-Ig consisting of the LTβR ectodomain fused with the Fc domain of a mouse IgG1 antibody specific for hen egg lysozyme (Hel-Ig). Control experiments were performed by treatment with 200 μg HEL-Ig. Inflammatory cytokines TNF-α, IFN-γ, and IL-1β were blocked with anti-TNF (#BE0058; Bio-X-Cell), anti-INF-γ (#BE0055; Bio-X-Cell), or anti-IL-1β (#BE0246; Bio-X-Cell) antibodies at 100 μg/mouse injected intravenously via the tail vein or retro-orbital sinus immediately before CFA injection. For LTβR-Ig treatment at steady state, mice were injected with 100 μg LTβR-Ig i.v. once a week for 3 wk and analyzed 3 wk after the first injection. In vivo BrdU incorporation was performed by BrdU injection i.v. (1 mg/mouse). Flow cytometry detection of incorporated BrdU was performed with FITC or APC BrdU Flow Kit (BD Biosciences) by following the manufacturer’s protocol.
Infection models
Adult male mice between 8 and 13 wk were used for the infections. Mice were infected via retro-orbital injection. C57BL/6 mice were infected with 50,000 CFU and Lepr-cre; Ltbrfl/fl mice with 370,000 CFU L. Monocytogenes. CFU titers were determined by plating titrated amounts of spleen and liver homogenate on brain heart infusion plates. Briefly, spleen and liver were harvested 3 d after infection and weighed. Tissues were then mashed through a 70-μm strainer, and titrated dilutions were generated in 1% Triton X-100. The tissue homogenates were then plated on brain heart infusion plates and grown overnight at 37°C. Adult female mice were infected by vaginal inoculation with 1,000 PFU HSV-2. Mice were scored for disease progression every day beginning 4 d after inoculation, as previously described (Iijima et al, 2011). Vaginal swabs were taken from infected mice at day 5 to determine HSV2 PFUs, as described (Iijima et al, 2011).
MSC sorting and RNA-sequencing
Long bones were flushed with HBSS supplemented with 2% of heat-inactivated fetal bovine serum, penicillin/streptomycin, L-glutamine, Hepes, and 200 U/ml collagenase IV (Worthington Biochemical Corporation), and digested for 30 min at 37°C. Cell clumps were dissociated by gentle pipetting. Cells were filtered through a 100-μm nylon mesh and washed with HBSS/2% FBS. Hematopoietic cells were depleted by staining with biotin-conjugated CD45 and Ter119 antibodies, and using Dynabeads Biotin Binder (#11047; Invitrogen). After the depletion of hematopoietic cells, the remaining cells were stained with antibodies against CD31, CD144, and PDGFRβ. BM MSCs were identified as CD45− Ter119− CD31− CD144− PDGFRβ+ cells. Sorting was performed using a BD FACSAria II. Cells were sorted directly into 350 μl RLT Plus buffer (QIAGEN), and RNA was extracted using the RNeasy Plus Micro Kit (#74034; QIAGEN). RNA sequencing was performed by the Yale Center for Genome Analysis using the Illumina HiSeq 2000 system, with paired-end 2 × 76 bp read length. The sequencing reads were aligned onto the Mus musculus GRCm38/mm10 reference genome using HISAT2 v2.2.1 software. The mapped reads were converted into the count matrix with default parameters using HTSeq v0.8.0 software, followed by the variance stabilizing transformation offered by DESeq2. Differentially expressed genes were identified using the same software, DESeq2, based on a negative binomial generalized linear model, and visualized in hierarchically clustered heatmaps using the pheatmap v1.0.12 in R package. Gene set enrichment analyses were conducted using the preranked gene set enrichment algorithm, fgsea v1.20.0, with default parameters on the MSigDB Hallmark gene set. Gene sets were considered significantly enriched when the adjusted P-value is less than 0.05.
Data Availability
Accession number to any data relating to the article was deposited in NCBI (GSE218505).
Supplementary Material
Acknowledgements
We thank Mathias Skadow, Harding H Luan, Neeha Kothapali, and Josh Yan for technical assistance. We thank Shuang Yu and Ruslan Medzhitov for help with Listeria infections. LTβR-Ig and HEL-Ig were generously provided by Linda Burkly and Mia Rushe (Biogen Inc., Ma.). These studies were funded by the NIH (RO1AI113040 awarded to JP Pereira); X Feng was funded by the NIH (T32 DK007356). S Zehentmeier was funded by a fellowship from the German Research Foundation (DFG) ZE1060/1-1. AV Tumanov was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) grant (RP220470). J Choi was supported by the National Research Foundation of Korea (NRF) grant funded by the South Korean government (2020R1F1A1076705).
Author Contributions
VY Lim: conceptualization, data curation, formal analysis, and writing—review and editing.
X Feng: data curation, formal analysis, and writing—review and editing.
R Miao: data curation and formal analysis.
S Zehentmeier: data curation and formal analysis.
N Ewing-Crystal: data curation and formal analysis.
M Lee: formal analysis.
AV Tumanov: resources and writing—review and editing.
JE Oh: data curation, formal analysis, and writing—review and editing.
A Iwasaki: resources and writing—review and editing.
A Wang: conceptualization and writing—review and editing.
J Choi: conceptualization, formal analysis, supervision, methodology, and writing—review and editing.
JP Pereira: conceptualization, data curation, formal analysis, supervision, funding acquisition, project administration, and writing—original draft.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA (2010) Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 465: 793–797. 10.1038/nature09135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, Kokkaliaris KD, Mercier F, Tabaka M, Hofree M, et al. (2019) A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell 177: 1915–1932.e16. 10.1016/j.cell.2019.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck TC, Gomes AC, Cyster JG, Pereira JP (2014) CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J Exp Med 211: 2567–2581. 10.1084/jem.20140457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher S, Manz MG (2017) Regulation of inflammation- and infection-driven hematopoiesis. Trends Immunol 38: 345–357. 10.1016/j.it.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Boettcher S, Gerosa RC, Radpour R, Bauer J, Ampenberger F, Heikenwalder M, Kopf M, Manz MG (2014) Endothelial cells translate pathogen signals into G-CSF-driven emergency granulopoiesis. Blood 124: 1393–1403. 10.1182/blood-2014-04-570762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD (2008) IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol 180: 7265–7275. 10.4049/jimmunol.180.11.7265 [DOI] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17: 233–247. 10.1038/nri.2017.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain D, Kondo M, Chen H, Kelsoe G (2009) Effects of acute and chronic inflammation on B-cell development and differentiation. J Invest Dermatol 129: 266–277. 10.1038/jid.2008.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DW, Bortner CD, Diaz-Jimenez D, Petrillo MG, Gruver-Yates A, Cidlowski JA (2020) Murine glucocorticoid receptors orchestrate B cell migration selectively between bone marrow and blood. J Immunol 205: 619–629. 10.4049/jimmunol.1901135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitano ML (2019) Toll-like receptor signaling in hematopoietic stem and progenitor cells. Curr Opin Hematol 26: 207–213. 10.1097/moh.0000000000000511 [DOI] [PubMed] [Google Scholar]
- Cheng HW, Onder L, Novkovic M, Soneson C, Lutge M, Pikor N, Scandella E, Robinson MD, Miyazaki JI, Tersteegen A, et al. (2019) Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp. Nat Commun 10: 1739. 10.1038/s41467-019-09728-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MR, Mandal M, Ochiai K, Singh H (2014) Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol 14: 69–80. 10.1038/nri3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comazzetto S, Murphy MM, Berto S, Jeffery E, Zhao Z, Morrison SJ (2019) Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin Receptor+ niche cells in the bone marrow. Cell Stem Cell 24: 477–486.e6. 10.1016/j.stem.2018.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, Tani-Ichi S, Schlenner S, Richie E, Rodewald HR, et al. (2016) Hematopoietic stem cell niches produce lineage-instructive signals to control multipotent progenitor differentiation. Immunity 45: 1219–1231. 10.1016/j.immuni.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff CA, Sacca R, Ruddle NH (1999) Differential induction of adhesion molecule and chemokine expression by LTα3 and LTαβ in inflammation elucidates potential mechanisms of mesenteric and peripheral lymph node development. J Immunol 162: 5965–5972. 10.4049/jimmunol.162.10.5965 [DOI] [PubMed] [Google Scholar]
- Cyster JG (2005) Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 23: 127–159. 10.1146/annurev.immunol.23.021704.115628 [DOI] [PubMed] [Google Scholar]
- de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA (2012) IFNγ induces monopoiesis and inhibits neutrophil development during inflammation. Blood 119: 1543–1554. 10.1182/blood-2011-07-367706 [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ (2012) Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462. 10.1038/nature10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fistonich C, Zehentmeier S, Bednarski JJ, Miao R, Schjerven H, Sleckman BP, Pereira JP (2018) Cell circuits between B cell progenitors and IL-7(+) mesenchymal progenitor cells control B cell development. J Exp Med 215: 2586–2599. 10.1084/jem.20180778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YX, Chaplin DD (1999) Development and maturation of secondary lymphoid tissues. Annu Rev Immunol 17: 399–433. 10.1146/annurev.immunol.17.1.399 [DOI] [PubMed] [Google Scholar]
- Herault A, Binnewies M, Leong S, Calero-Nieto FJ, Zhang SY, Kang YA, Wang X, Pietras EM, Chu SH, Barry-Holson K, et al. (2017) Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature 544: 53–58. 10.1038/nature21693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Mattei LM, Iwasaki A (2011) Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A 108: 284–289. 10.1073/pnas.1005201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Mithal DS, Park JE, Miller RJ (2015) Localized CCR2 activation in the bone marrow niche mobilizes monocytes by desensitizing CXCR4. PLoS One 10: e0128387. 10.1371/journal.pone.0128387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E, Sanz J, Dunn JL, Khan N, Mendonca LE, Pacis A, Tzelepis F, Pernet E, Dumaine A, Grenier JC, et al. (2018) BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172: 176–190.e19. 10.1016/j.cell.2017.12.031 [DOI] [PubMed] [Google Scholar]
- Kennedy DE, Knight KL (2015) Inhibition of B lymphopoiesis by adipocytes and IL-1-producing myeloid-derived suppressor cells. J Immunol 195: 2666–2674. 10.4049/jimmunol.1500957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Okoreeh MK, Kennedy DE, Maienschein-Cline M, Ai J, McLean KC, Kaverina N, Veselits M, Aifantis I, Gounari F, et al. (2019) CXCR4 signaling directs Igk recombination and the molecular mechanisms of late B lymphopoiesis. Nat Immunol 20: 1393–1403. 10.1038/s41590-019-0468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG, Boettcher S (2014) Emergency granulopoiesis. Nat Rev Immunol 14: 302–314. 10.1038/nri3660 [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466: 829–834. 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R, Lim VY, Kothapalli N, Ma Y, Fossati J, Zehentmeier S, Sun R, Pereira JP (2020) Hematopoietic stem cell niches and signals controlling immune cell development and maintenance of immunological memory. Front Immunol 11: 600127. 10.3389/fimmu.2020.600127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Hartigan-O’Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, Matloubian M, Coughlin SR, McDonald DM, McCune JM (2013) IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int Immunol 25: 471–483. 10.1093/intimm/dxt012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JM, Berger A, Nelles ME, Mielnik M, Furlonger C, Cen SY, Besla R, Robbins CS, Paige CJ (2015) Inflammation rapidly reorganizes mouse bone marrow B cells and their environment in conjunction with early IgM responses. Blood 126: 1184–1192. 10.1182/blood-2015-03-635805 [DOI] [PubMed] [Google Scholar]
- Ochiai K, Maienschein-Cline M, Mandal M, Triggs JR, Bertolino E, Sciammas R, Dinner AR, Clark MR, Singh H (2012) A self-reinforcing regulatory network triggered by limiting IL-7 activates pre-BCR signaling and differentiation. Nat Immunol 13: 300–307. 10.1038/ni.2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddle NH (2014) Lymphotoxin and TNF: How it all began-a tribute to the travelers. Cytokine Growth Factor Rev 25: 83–89. 10.1016/j.cytogfr.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw S, Weiss A, Khokha R, Waterhouse PD (2019) Metalloproteases: On the watch in the hematopoietic niche. Trends Immunol 40: 1053–1070. 10.1016/j.it.2019.09.006 [DOI] [PubMed] [Google Scholar]
- Schultze JL, Mass E, Schlitzer A (2019) Emerging principles in myelopoiesis at homeostasis and during infection and inflammation. Immunity 50: 288–301. 10.1016/j.immuni.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Sedger LM, Hou S, Osvath SR, Glaccum MB, Peschon JJ, van Rooijen N, Hyland L (2002) Bone marrow B cell apoptosis during in vivo Influenza virus infection requires TNF-α and lymphotoxin-α. J Immunol 169: 6193–6201. 10.4049/jimmunol.169.11.6193 [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317. 10.1038/ni1309 [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG (2008) Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452. 10.1146/annurev.immunol.26.021607.090326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG (2011) Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34: 590–601. 10.1016/j.immuni.2011.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou Y, Koroleva E, Spencer CM, Shein SA, Korchagina AA, Yusoof KA, Parthasarathy R, Leadbetter EA, Akopian AN, Munoz AR, et al. (2021) Redefining the role of lymphotoxin beta receptor in the maintenance of lymphoid organs and immune cell homeostasis in adulthood. Front Immunol 12: 712632. 10.3389/fimmu.2021.712632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taves MD, Ashwell JD (2021) Glucocorticoids in T cell development, differentiation and function. Nat Rev Immunol 21: 233–243. 10.1038/s41577-020-00464-0 [DOI] [PubMed] [Google Scholar]
- Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Dominguez A, Pinho S, Akhmetzyanova I, Gao J, Witkowski M, et al. (2019) The bone marrow microenvironment at single-cell resolution. Nature 569: 222–228. 10.1038/s41586-019-1104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumanov AV, Kuprash DV, Lagarkova MA, Grivennikov SI, Abe K, Shakhov AN, Drutskaya LN, Stewart CL, Chervonsky AV, Nedospasov SA (2002) Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 17: 239–250. 10.1016/s1074-7613(02)00397-7 [DOI] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G (2004) Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med 199: 47–58. 10.1084/jem.20031104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G (2005) Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med 201: 1771–1780. 10.1084/jem.20041419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Cain DW, Kuraoka M, Kondo M, Kelsoe G (2009) IL-1R type I-dependent hemopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J Immunol 182: 6477–6484. 10.4049/jimmunol.0803961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Koroleva EP, Kruglov AA, Kuprash DV, Nedospasov SA, Fu YX, Tumanov AV (2010) Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity 32: 403–413. 10.1016/j.immuni.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R (2016) Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166: 1512–1525.e12. 10.1016/j.cell.2016.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehentmeier S, Pereira JP (2019) Cell circuits and niches controlling B cell development. Immunol Rev 289: 142–157. 10.1111/imr.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehentmeier S, Lim VY, Ma Y, Fossati J, Ito T, Jiang Y, Tumanov AV, Lee HJ, Dillinger L, Kim J, et al. (2022) Dysregulated stem cell niches and altered lymphocyte recirculation cause B and T cell lymphopenia in WHIM syndrome. Sci Immunol 7: eabo3170. 10.1126/sciimmunol.abo3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu Q, Johnson CB, Pham G, Kinder JM, Olsson A, Slaughter A, May M, Weinhaus B, D’Alessandro A, et al. (2021) In situ mapping identifies distinct vascular niches for myelopoiesis. Nature 590: 457–462. 10.1038/s41586-021-03201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 LTβR-regulated genes in Lepr+ MSPCs during CFA-induced systemic inflammation. (5MB, xlsx)
Data Availability Statement
Accession number to any data relating to the article was deposited in NCBI (GSE218505).