Summary
Background
The effectiveness of the second BNT162b2 (Pfizer–BioNTech) mRNA COVID-19 booster vaccine dose (ie, fourth inoculation) is well established, but its safety has yet to be fully understood. The absence of sufficient vaccine safety information is one of the key contributors to vaccine hesitancy. In this study, we aimed to evaluate the safety profile of the second BNT162b2 mRNA COVID-19 booster vaccine using data from a retrospective cohort and a prospective cohort.
Methods
To evaluate the safety profile of the second booster vaccine, we analysed its short-term effects and compared them to those of the first booster by using data from, first, a retrospective cohort of 250 000 random members of the second-largest health-care organisation in Israel (Maccabi Healthcare Services) and, second, a prospective cohort (the PerMed study) of 4698 participants from all across Israel. Individuals who were aged 18 years or older who received the second BNT162b2 mRNA COVID-19 vaccine booster during the vaccination campaign, from Dec 30, 2021, to July 22, 2022, were eligible for inclusion in the retrospective cohort analysis. To be included in the PerMed study, participants needed to be 18 years or older, members of Maccabi Healthcare Services at the time of enrolment, using their own smartphone, and be able to give informed consent by themselves. Participants from the prospective cohort received smartwatches, downloaded a dedicated mobile application, and granted access to their medical records. The smartwatches continuously monitored several physiological measures, including heart rate. For analysis of the prospective cohort data, we used the Kruskal-Wallis test to compare heart rate levels observed before and after vaccination. The mobile application collected daily self-reported questionnaires on local and systemic reactions. Medical records of the retrospective cohort were accessed to examine the occurrence of 25 potential adverse events, and we evaluated the risk differences between 42 days in the periods before and after vaccination in a pairwise method using non-parametric percentile bootstrap.
Findings
The retrospective cohort included 94 169 participants who received the first booster and 17 814 who received the second booster. Comparing the 42 days before and after vaccination, the second booster was not associated with any of the 25 adverse events investigated, including myocardial infarction (risk difference, 2·25 events per 10 000 individuals [95% CI –3·93 to 8·98]) and Bell's Palsy (–1·68 events [–5·61 to 2·25]). None of the individuals was diagnosed with myocarditis or pericarditis following vaccination with the second booster. The prospective cohort included 1785 participants who received the first booster and 699 who received the second booster. We found no significant differences after inoculation with the first booster compared with the second booster (heart rate: day 2 [p=0·3], day 6 [p=0·89]; extent of self-reported reactions [p=0·06]). We found a significant increase in mean heart rate relative to that observed during the week before vaccination (baseline) levels during the first 3 days following the second booster (p<0·0001), peaking on day 2 (mean difference of 1·61 bpm [1·07 to 2·16] compared with baseline). Mean heart rate values returned to baseline levels by day 6 (–0·055 bpm [–0·56 to 0·45] compared with baseline).
Interpretation
Both our retrospective and prospective analyses support the safety of the second booster, with our findings reflecting physicians' diagnoses, patients' objective physiological measures, and patients' subjective reactions. We believe this study provides safety assurances to the global population who are eligible to receive an additional COVID-19 booster inoculation. These assurances can help increase the number of high-risk individuals who opt to receive this booster vaccine and thereby prevent severe outcomes associated with COVID-19.
Funding
European Research Council (ERC).
Introduction
The rapid spread of the COVID-19 omicron variant and the sharp rise in omicron-associated hospitalisations led the Israeli Government to initiate a world-leading second mRNA booster vaccine campaign with either the Moderna or Pfizer–BioNTech mRNA vaccines, which was launched on Dec 30, 2021.1 Initially targeting individuals who are at high risk of complications associated with COVID-19, the campaign was expanded shortly after its launch to include all individuals aged 60 years and older.1 Several countries have since followed suit. The US Centers for Disease Control and Prevention (CDC), for example, issued a recommendation on March 29, 2022, that individuals who are aged 50 years or older and patients who are immunocompromised should receive a second mRNA booster dose.2
Research in context.
Evidence before this study
Safety is one of the key factors in deciding whether or not to get vaccinated. Yet, to the best of our knowledge, no extensive study has been conducted to evaluate the safety of the second booster dose of the COVID-19 BNT162b2 mRNA vaccine (or, indeed, of any mRNA vaccines). We searched Google Scholar, Pubmed, and preprint services (including medRxiv, bioRrxiv, and SSRN) for safety trials of the second booster published between Dec 30, 2021 (our study's launch) and Aug 10, 2022, with no language restrictions, using the terms “COVID-19 vaccine safety” OR “COVID-19 second booster vaccine dose” OR “COVID-19 fourth vaccine dose” OR “safety of the fourth COVID-19 BNT162b2 (Pfizer–BioNTech) mRNA dose”. We included only research regarding mRNA vaccines. We identified non-peer-reviewed research regarding the second booster's efficacy and safety among 112 health-care workers. The most frequent adverse event reported was pain at the injection site, and most of the reported adverse events were considered mild. Additionally, we found a peer-reviewed, randomised controlled trial involving 166 recipients of the fourth mRNA vaccine dose. Fatigue, headaches, malaise, and muscle aches were the most commonly reported reactions. Neither of these two reports covered a large enough cohort to present credible safety information that could quell concerns regarding vaccine side-effects and complications, nor do they amalgamate multiple sources of complementary information to provide credible evidence as to measured and subjectively experienced side-effects.
Added value of this study
To the best of our knowledge, this is the first comprehensive safety assessment of the second BNT162b2 mRNA COVID-19 booster vaccine. We analysed data from: (1) smartwatches and daily questionnaires on reactions to the vaccine in a prospective cohort of 4698 participants, of whom 1785 received the first booster and 699 received the second booster; and (2) the medical records from a retrospective cohort of 250 000 random members of the second-largest health-care organisation in Israel, of whom 94 169 received the first booster and 17 814 received the second booster. Our prospective cohort analysis provided evidence to show that the second booster induces significant changes in heart rate levels, with measures peaking on the second day after taking the vaccine and returning to each individual's baseline by the sixth day. No significant differences in heart rate levels and self-reported reactions were found between the second and first booster doses. Vaccination in the retrospective cohort was not associated with any of the 25 adverse events investigated, and no events of myocarditis or pericarditis were reported for the 17 814 cohort members following vaccination with the second booster dose.
Implications of all the available evidence
Both our prospective and retrospective analyses further support the safety profile of the second BNT162b2 mRNA COVID-19 booster in high-risk populations, with our findings reflecting patients' objective and subjective responses. Thus, we believe this study provides safety assurances to the global population who are eligible to receive an additional COVID-19 booster inoculation. These assurances can help increase the number of high-risk individuals who opt to receive this booster vaccine and thereby prevent severe outcomes associated with COVID-19.
Despite the second BNT162b2 (Pfizer–BioNTech) booster dose showing effectiveness in preventing the severe outcomes of COVID-193 with a promising safety profile,4, 5 there has been a notable global public reluctance to be vaccinated. In the USA, 2 months after the CDC recommendation, only 21·5% of eligible individuals had followed the suggestion to receive the second booster.6 Studies indicate that COVID-19 vaccine hesitancy is mainly motivated by safety concerns rather than efficacy considerations.7, 8 Raising confidence in the booster vaccines requires closing the knowledge gap on vaccine safety, a gap that has thus far been filled by non-scientific, somewhat speculative theories.9 The need to rectify this situation is highlighted by the revival of the Global Vaccine Data Network. In this largest-ever vaccine safety project, scientists from over 20 countries gather the data needed to investigate rare complications that are linked to COVID-19 vaccines to improve the prediction and treatment of, and potentially prevent, these side-effects.10
Although severe events after vaccination will likely lead to a medical consultation, which would be noted in the patient's medical records, milder reactions are generally underreported. One promising direction to close this gap and provide a more comprehensive assessment of the safety of vaccines is to conduct extensive, continuous, and detailed monitoring and surveillance of physiological changes in vaccinated individuals11, 12, 13, 14 Moreover, several studies have shown the excellent ability of wearable sensors from smartwatches to detect unnoticeable medical conditions. For example, as part of the Apple heart study,15 the ability to continuously monitor smartwatch sensors to detect atrial fibrillation based on an irregular heart pulse was shown. Specifically, studies showed that heart measures, including heart rate, heart rate variability (HRV), and resting heart rate, are powerful indicators of COVID-19 infection in the pre-symptomatic stage,16 and can be used to detect COVID-19 infection in real time.17 In the context of the primary COVID-19 vaccination series (first and second dose), studies using wearables found more apparent changes in heart measures following vaccination with the second dose compared with the first dose on the second day following vaccination, in individuals vaccinated with the Moderna vaccine versus the Pfizer–BioNTech vaccine, and among vaccinated individuals not previously infected with COVID-19 versus those who were vaccinated and infected.11, 12, 13 All of these heart measures were previously reported to correlate with subjective symptoms after the COVID-19 vaccine.18, 19 Nevertheless, the value of smartwatch measurements (eg, heart rate) for the identification of vaccine-associated adverse events has yet to be established.
In this study, we aimed to evaluate the safety profile of the second BNT162b2 mRNA COVID-19 booster vaccine. To do so, we examined the short-term effects (ie, the effects lasting up to 42 days) of the second BNT162b2 mRNA COVID-19 booster vaccine and compared it with the first booster by analysing data from both a prospective cohort of 4698 participants as part of the PerMed observational prospective study11, 12, 14 and a retrospective cohort of 250 000 random members of Maccabi Healthcare Services, the second-largest health-care provider in Israel.
Methods
Study design and participants
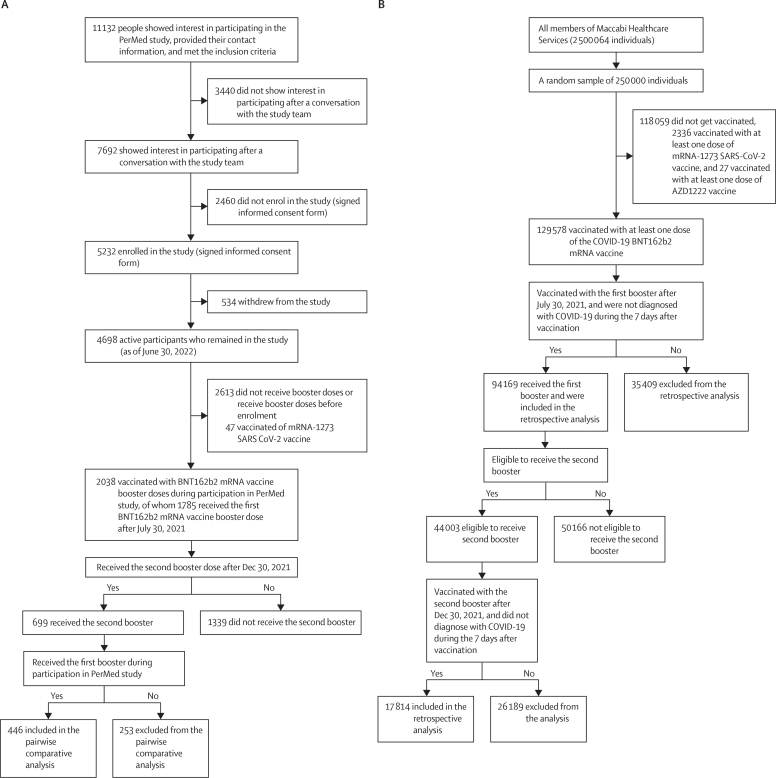
The ongoing PerMed prospective observational study included 4698 participants aged 18 years and older who were recruited between Nov 16, 2020, and June 30, 2022, from all across Israel.11, 14 (appendix pp 2–7, 12–13). Participant recruitment was conducted via advertisements on social media and word-of-mouth. Each participant signed an informed consent form after receiving a comprehensive explanation of the study by a professional survey company.
Maccabi Healthcare Services is the second-largest health-care provider in Israel, serving about 25% of the population (approximately 2·5 million members). Maccabi Healthcare Service's members are representative of the Israeli population and reflect all demographic, ethnic, and socioeconomic groups and levels.20 To be included in the PerMed study, participants needed to be 18 years or older, members of Maccabi Healthcare Services at the time of enrolment and for at least 2 years prior, using their own smartphone, and be able to give written informed consent by themselves. We analysed the data from participants that received the first or the second booster between the day of enrolment and June 30, 2022 (figure 1A). Participants were excluded if they knew that they would be outside of Israel for more than 3 months continuously at any point for the 2 years after enrolment. Participants were also excluded if they were students or employees of the principal investigator.
Figure 1.
Trial profile for prospective (A) and retrospective (B) cohort
All participants in the prospective study were advised, both orally and in writing, about the study and provided written informed consent to participate. The entire study (prospective and retrospective parts) was approved by Maccabi Healthcare Service's Helsinki institutional review board (protocol number 0122-20-MHS).
For the retrospective cohort, we analysed anonymised electronic medical records (from the year 2012 to 2022) of 250 000 randomly sampled members of Maccabi Healthcare Services. Individuals who were aged 18 years or older who received the second BNT162b2 mRNA COVID-19 vaccine booster during the vaccination campaign, from Dec 30, 2021, to July 22, 2022, were eligible for inclusion. People who were not members of Maccabi Healthcare Services during the entire study period were excluded. As the retrospective data was pseudonymised, the Helsinki institutional review board approved the use of the retrospective cohort data, without requiring specific consent from the members of Maccabi Healthcare Services (protocol number 0122-20-MHS).
Procedures
Upon enrolment to the prospective study, we collected information on participants' sex, age, and underlying medical conditions. The list of underlying medical conditions consisted of hypertension, diabetes, heart disease, chronic lung disease, immune suppression, cancer, renal failure, and whether participants' BMI was 30 or higher (BMI was computed as the weight in kilograms divided by the square of the height in metres). After enrolment, participants used the PerMed mobile application to complete a daily questionnaire.11, 12, 14 The questionnaire allowed participants to report on signs and symptoms (appendix pp 3–5) that were previously observed in BNT162b2 mRNA Covid-19 clinical trials,21 with an option to add other symptoms as free text.
Participants in the prospective cohort were equipped with Garmin Vivosmart 4 smart fitness trackers. Among other features, the smartwatch collects data for all-day heart rate, HRV-based stress,22 and daily resting heart rate.23 We focused on these measures because they provide continuous information on two major systems of the human body: the cardiovascular system and the nervous system. We also had access to other measures such as step counts, night blood oxygen saturation, sleep duration, and sleep-level classification, including light, deep, rapid eye movement (REM), and awake periods (appendix pp 2–3, 12–13).
The smartwatch's optical wrist heart rate monitor was designed to continuously measure the user's heart rate. The frequency at which the heart rate was measured varied and sometimes depended on the level of activity of the user: when the user started an activity, the optical heart rate monitor's measurement frequency increased. As HRV was not easily accessible through Garmin's application programming interface, we used Garmin's stress level instead, which is calculated based on HRV.22 Specifically, the device uses heart rate data to determine the interval between each heartbeat. The variable length of time between each heartbeat is regulated by the body's autonomic nervous system. Less variability between beats correlates with higher stress levels, whereas an increase in variability indicates less stress.24 When examining the data collected in our study, we identified a heart rate sample approximately every 15 s and an HRV sample every 180 s.
The data preprocessing and inclusion criteria are detailed in the appendix (pp 6–7). We developed a dedicated data collection platform that collects for each participant data from the smartphone sensors and daily questionnaires via the Permed application and the smartwatch sensors via the Garmin server (appendix p 12). These collected data are securely stored in Tel Aviv University facilities. The exact hour of each vaccination administration was recorded in the individual's medical record.
Participants completed a one-time enrolment questionnaire, received Garmin Vivosmart 4 smartwatches, and installed two applications on their mobile phones: (1) the PerMed application,11, 12, 14 which collects daily self-reported questionnaires; and (2) an application that passively records smartwatch data. Participants were asked to wear their smartwatches as much as possible. Using a dedicated dashboard to monitor compliance, a survey company ensured that participants' questionnaires were filled at least twice a week, that their smartwatches were charged and properly worn, and that any technical problems with the mobile applications or smartwatch were resolved (appendix pp 12–13).
We implemented several preventive measures to minimise participant attrition and discomfort to improve the quality, continuity, and reliability of the collected data. First, each day, participants who did not fill out their daily questionnaire by 1900h received a reminder notification through the PerMed application. Second, we developed a dedicated dashboard that allowed the survey company to identify participants who repeatedly neglected to complete the daily questionnaire or did not wear their smartwatch for extended periods of time. These participants were contacted by the survey company (either by text message or phone call) and were encouraged to better adhere to the study protocol. Third, to strengthen participants' engagement, a weekly summary report was generated for each participant, which was available inside the PerMed application. Similarly, a monthly newsletter with recent findings from published studies and useful tips regarding the smartwatch's capabilities was sent to the participants. At the end of the 2 year study, participants received all the obtained personal insights and could keep the smartwatch as a gift (appendix p 13).
In the retrospective portion of the study, we accessed data from the electronic medical records of 250 000 random members of Maccabi Healthcare Services. Records are automatically collected and updated monthly in the Maccabi Health Services databases from all clinics and medical facilities across the country. The data are coded, pseudonymised, viewed, stored, and processed within the Maccabi Healthcare Services research room. Maccabi Healthcare Services uses the International Classification of Diseases, Ninth Revision, and Clinical Modification. Procedures are coded using Current Procedural Terminology codes. We accessed each patient demographic information and information on potential chronic illnesses (table 1). We examined potential adverse events from a list diagnosed by clinicians in clinics or hospitals (appendix p 8–11).
Table 1.
Characteristics of the prospective and retrospective cohorts
|
Prospective cohort |
Retrospective cohort |
||||
|---|---|---|---|---|---|
| First booster (n=1785) | Second booster (n=699) | First and second booster (n=446) | First booster (n=94 169) | First and second booster* (n=17 814) | |
| Sex | |||||
| Male | 866 (48·5%) | 348 (48·8%) | 215 (48·2%) | 45 208 (48·0%) | 8879 (49·8%) |
| Female | 919 (51·5%) | 350 (50·2%) | 231 (51·8%) | 48 961 (52·0%) | 8935 (50·2%) |
| Unspecified | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Age group, years | |||||
| Median (IQR) | 52 (34–61) | 62 (53–68) | 64 (57–70) | 47 (31–61) | 69 (62–76) |
| 0–29 years | 275 (15·4%) | 26 (3·7%) | 11 (2·5%) | 21 711 (23·1%) | 285 (1·6%) |
| 30–39 years | 350 (19·6%) | 55 (7·9%) | 23 (5·2%) | 14 445 (15·3%) | 489 (2·8%) |
| 40–49 years | 201 (11·3%) | 61 (8·7%) | 22 (4·9%) | 15 514 (16·5%) | 799 (4·5%) |
| 50–59 years | 429 (24·0%) | 130 (18·6%) | 86 (19·3%) | 16 650 (17·7%) | 1850 (10·4%) |
| 60–69 years | 362 (20·3%) | 277 (39·6%) | 185 (41·5%) | 12 596 (13·4%) | 5770 (32·4%) |
| 70 years | 168 (9·4%) | 150 (21·5%) | 119 (26·7%) | 13 253 (14·1%) | 8621 (48·4%) |
| BMI† | |||||
| Median (IQR) | 26·04 (23·33–29·23) | 26·80 (24·42–29·98) | 26·96 (24·61–30·07) | 25·35 (22·20–29·06) | 27·27 (24·38–30·66) |
| <30·0 | 1381 (77·4%) | 510 (73·0%) | 318 (71·3%) | 71 347 (75·8%) | 12 476 (70·0%) |
| ≥30·0 | 363 (20·3%) | 170 (24·3%) | 110 (24·7%) | 18 650 (19·8%) | 5098 (28·6%) |
| Unspecified | 41 (2·3%) | 19 (2·7%) | 18 (4·0%) | 4172 (4·4%) | 240 (1·4%) |
| Comorbidities‡ | |||||
| Yes | 397 (22·2%) | 260 (37·2%) | 186 (41·7%) | 24 585 (26·1%) | 10 781 (60·5%) |
| No | 1342 (75·2%) | 412 (58·9%) | 238 (53·4%) | 69 584 (73·9%) | 7033 (39·5%) |
| Unspecified | 46 (2·6%) | 27 (3·9%) | 22 (4·9%) | 0 (0%) | 0 (0%) |
| Population sector | |||||
| General Jewish | 1570 (88·0%) | 637 (91·1%) | 411 (92·2%) | 82 954 (88·1%) | 16 613 (93·3%) |
| Ultra-Orthodox Jewish | 37 (2·07%) | 8 (1·15%) | 6 (1·35%) | 3797 (4·03%) | 412 (2·31%) |
| Religious (non-ultra-Orthodox) Jewish | 71 (4·0%) | 27 (3·9%) | 17 (3·8%) | 3355 (3·6%) | 488 (2·7%) |
| Arab | 18 (1·0%) | 1 (<1%) | 0 (0%) | 4046 (4·3%) | 300 (1·7%) |
| Unspecified | 89 (5·0%) | 26 (3·7%) | 12 (2·7%) | 17 (<1%) | 1 (<1%) |
| Time between first and second booster | |||||
| Median, days (IQR) | NA | 158 (153–174) | 157 (153–172) | NA | 157 (153–169) |
NA=not applicable.
All participants who received a second dose were documented in the medical records with the first booster as well.
The BMI is the weight in kilograms divided by the square of the height in metres.
Comorbidities were at least one of the following: hypertension, diabetes, heart disease, chronic lung disease, immune suppression, cancer, or renal failure.
Outcomes
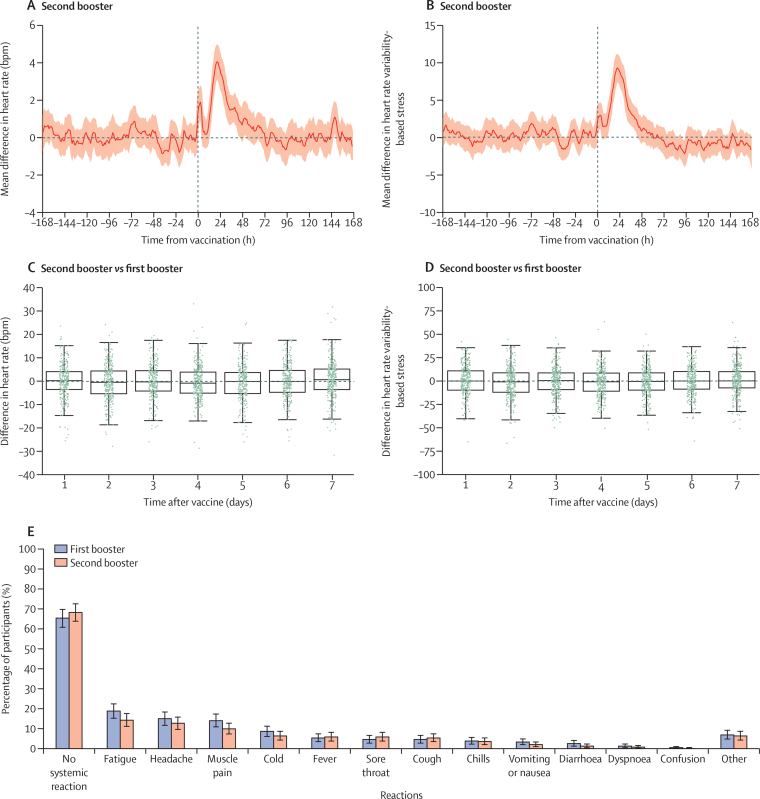
For the prospective study, we analysed heart rate, HRV-based stress, resting heart rate, and step counts from the smartwatches. For each measure, the prespecified outcomes are the differences between the mean value of each measure (on a daily and hourly basis) recorded 0–42 days after vaccination, compared with those recorded during the week before vaccination (baseline), keeping the same day of the week and the same hour during the day. As post-hoc validation measures, we also calculated the differences between the mean value of each measure 14 days before vaccination and those observed 7 days before vaccination. To compare the first and second boosters, we calculate for the difference between the daily changes in heart rate and HRV-based stress for each individual, recorded after the second and the first booster (figure 2C, D).
Figure 2.
Self-reported reactions and physiological reactions to the second booster dose compared with the first booster dose
Reactions to the second booster, as recorded by the smartwatches (A, B). The figures show the mean difference between the baseline period and period after vaccination for heart rate (n=570; A) and heart rate variability-based stress (n=567; B). Mean values are depicted as solid lines and 95% CIs are presented as shaded regions. Boxplots of the differences between the daily mean changes in the smartwatch indicators for heart rate (n=363; C) and heart rate variability-based stress (n=358; D) between the second and first booster. Each change is between the period after vaccination and the baseline periods. Each green dot represents a single participant. (E) A comparison of the reactions reported by participants between the first and second boosters (n=392). The bars represent the percentage of participants who reported a reaction. Error bars represent 95% CIs. For each panel, the sample size represents the number of participants that we had sufficient data points to conduct the analysis using the criteria presented in the methods section.
In analysing the daily questionnaires, we calculated the percentage of participants who reported each of the following new reactions in the 7 days after vaccination: fatigue, headache, muscle pain, cold, fever, sore throat, cough, chills, vomiting or nausea, diarrhoea, dyspnoea, confusion, loss of taste and smell, or shortness of breath. Participants could also report any other symptoms using free text. We define a reaction post-vaccination as a new reaction, if it had not been reported in the most recent questionnaire completed in the 7 days before vaccination.
For each individual in the retrospective cohort, we noted the existence of 25 potential adverse events during the 42-day period before and after each of the two booster vaccinations. This set of 25 adverse events was composed based on a previous large-scale study25 that examined the safety of the first and second (primary series) vaccine dose (appendix pp 8–11).
Statistical analysis
For the prospective cohort, we compared heart rate levels observed before and after vaccination. We focused on heart rate in the statistical analyses to refrain from multiple testing and potential type 1 errors. Heart rate is a vital sign that is often used to detect inflammations in general and heart inflammations26 and is recorded by a wide variety of smartwatches and sampled in high frequency in our database (every 15 s as opposed to resting heart rate, which was calculated on a daily basis).
First, we defined the baseline period as the difference in the daily mean heart rate between the 7-day period before vaccination and the 7-day period 2 weeks before vaccination (keeping the same day of the week). These daily differences follow the same mean and variance and are very weakly correlated (appendix pp 14–16). Then, using the Kruskal-Wallis test, we examined whether there were differences in the heart rate levels between the baseline period and those observed during the first 3 days after vaccination, which was consistent with clinical trials that indicated that most symptoms appear within 24–72 h after vaccination.21 We also tested whether these differences faded by the sixth day after vaccination, consistent with a previous study that modeled reactions with smartwatches after vaccinating with the primary series.13
For participants who received both the first and second boosters, we used a paired sample t-test to determine the statistical significance of the difference in the daily heart rate (measured on the second day and sixth day after vaccination) between the second booster and the first booster. Likewise, to determine the statistical significance of the difference between the extent of self-reported reactions to the second booster versus the first booster, we conducted a t-test for dependent samples. To refrain from type 1 errors, we used the Bonferroni correction across all statistical tests done for the prospective cohort study. Analysing the smartwatch measures, we excluded participants who did not have at least one overlapping period of recorded data (ie, the same day of the week and the same hour during the day) during their baseline and post-vaccination periods. Analysing the reaction from the daily questionnaires, we excluded participants who did not fill in at least one questionnaire during the baseline period and at least one questionnaire during the 7 days post-vaccination.
To calculate the changes in smartwatch measures after vaccination (appendix p 18), we did the following steps. First, for each participant and each hour, we calculated the difference between the mean value of the measure tested during that hour and that of the corresponding hour in the previous week (keeping the same day of the week and same hour during the day). If these data were not recorded (eg, if a participant did not wear the smartwatch in the same period before and after vaccination), we excluded the participant from this analysis. Then, we aggregated each hour's differences divided by all participants to calculate a mean difference and the associated 95% CI. We present this analysis starting a week before and after vaccination.
To associate the extent of new self-reported reactions that occurred after vaccination we first noted any pre-existing signs and symptoms reported in the most recent completed questionnaire during the 7 days before vaccination (baseline period). Next, we calculated the percentage of participants who reported new (ie, not pre-existing) systemic reactions in the 7-day period after vaccination. For example, if a participant reported a headache a day before vaccination and a day after vaccination, we did not count the symptom as a vaccination-associated reaction. For each reaction, we used a binomial distribution to calculate the 95% CI.
For the retrospective cohort, diagnoses with 25 potential adverse events were retrieved from the Maccabi Healthcare Service's database using their International Classification of Disease Ninth Revision codes (ICD-9).
For each participant, we first calculated the daily mean changes in heart rate between the post-vaccination period and the baseline period. We did this separately for the first and second boosters. Then, we calculated the difference between these two mean values for each participant and each of the 7 days after inoculation.
To assess the difference in the risk of exhibiting each of the potential adverse events between the periods before and after vaccination, we conducted a pairwise comparison for each individual. Consistent with a previous study,25 we chose a time interval of 42 days to evaluate the potential short-term effects of vaccination. Specifically, for each individual (i) and event (j), we set a Boolean variable to be
if event j was recorded in the individual's medical record at least once in the period after vaccination (ie, 0–42 days after vaccination), and to
otherwise. Likewise, we set a Boolean variable representing a baseline (or control) period to be
if the adverse event was recorded in the individual's medical records at least once in the period before vaccination (ie, 42–0 days before vaccination). For each individual, we then calculated the difference between the values after and before vaccination,
Hence, if
the event is potentially associated with the vaccine or a random event. If
it is potentially associated with a random event. The risk difference for event is the mean value of all the vaccinated individuals,
Note that if a specific event was reported in both the periods before and after vaccination,
and thus we assumed that the event was not associated with the vaccine. This approach is identical to the standard estimations of risk differences in exposed versus unexposed groups,27 but also accounts for the paired nature of the samples. If an individual is found to be positive for COVID-19 in the period after vaccination, we compared only the events recorded in the period between inoculation and a week before the COVID-19 test was conducted and match it with the same time interval in the baseline period (ie, before vaccination). To calculate the 95% CI for the difference without imposing any unknown distribution, we applied a non-parametric percentile bootstrap method with 10 000 repetitions, similarly to previous safety studies.25, 28 We repeated the same procedure to assess the risk difference between the period after vaccination for the first and second boosters.
Myocarditis and pericarditis are recognised as adverse events of special interest.26 Thus, we extended the analysis to examine whether these events are also associated with the primary series (ie, first and second doses) and the first booster in participants who are eligible to receive the second booster dose in Israel. This population includes individuals aged 60 years or older or individuals aged older than 18 years with particular comorbidities that have already received their first booster. We note that health-care workers and individuals who work with high-risk groups were also eligible to receive the second booster dose but were not explicitly tracked because their profession is not recorded in our data. For validation and to allow comparison with previous study that examined the safety of the primary vaccine series,25 we also conducted a post-hoc matching-process analysis, in which persons vaccinated with the second booster were matched in a 1:1 ratio to persons vaccinated with the first booster only (appendix pp 21–23).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
In the prospective cohort, 2038 participants received either the first or the second booster (figure 1). During the study period, 1785 participants received the first booster, 699 participants received the second booster, and 446 received the first and second booster (table 1). Among the recipients of the second booster, 350 (50·2%) participants were women and 348 (49·8%) participants were men (one participant did not specify their sex). Their age ranged from 21–87 years, with a median age of 62 years (table 1).
In the retrospective cohort of 250 000 individuals, 94 169 (37·7%) received the first booster and 44 003 (17·6%) were eligible for the second booster dose. Among the individuals who were eligible for the second booster, 17 814 (40·5%) received the second booster. Among the recipients of the second booster doses, 8935 (50·2%) were women and 8879 (49·8%) were men. Their age ranged from 18 years to 104 years, with a median age of 69 years. The average age and number of underlying medical conditions differed between the datasets because of the eligibility criteria (age, comorbidities) for the second booster dose (table 1).
In the prospective cohort, for 570 of the 699 participants who received the second booster dose, we had sufficient heart rate data, and for 567 participants we had sufficient HRV-based stress data to compare those measures from the 7-day period before the administration of the second booster dose (baseline period) with those in the 7 days after the vaccination. We identified a significant rise in heart rate (figure 2A) during the first 3 days following the administration of the second booster compared with baseline levels (Kruskal-Wallis test, statistic: 120·792; p<0·0001). This rise peaked on the second day following vaccination, with a mean difference compared with the baseline of 1·613 beats per minute (bpm; SD 0·276) for the heart rate. Heart rate levels returned to their baseline levels on the sixth day after inoculation (0·945; p=0·62). Similar trends were observed for other measurements, including HRV-based stress and resting heart rate (figure 2B, appendix p 18).
Similarly, for 648 (92·7%) of the 699 participants who received the second booster, we had sufficient questionnaire data to assess the extent of reactions reported following the second booster vaccine dose (appendix p 17). Specifically, 436 (67%; 95% CI 64–71) participants did not report any new signs or symptoms after receiving the second booster dose. The most frequently reported reactions were fatigue, headache, muscle pain, cold, and a sore throat. These reactions faded in nearly all participants within 3 days of receiving the second vaccine. We also found that participants who reported more severe reactions to the first booster tended to likewise report more severe reactions to the second booster (appendix p 24).
We also conducted a pairwise comparative analysis between the reactions of the two boosters for participants who received the two boosters during the study period (figure 2C–E). Our analysis showed no significant difference between the first and the second booster in terms of the peak (t-test, second day mean difference: –0·470 bpm, statistic: –1·027, p=0·30) and the sixth day (t-test, sixth day mean difference: 0·058 bpm, statistic: 0·135, p=0·89), suggesting that heart rate measures returned to baseline shortly after the inoculation with each of the vaccines. Similarly, an analysis of the self-reported reactions after vaccination showed that the extent of systematic reactions reported following the second booster dose was similar to those observed following the first booster dose (paired t-test, statistic: 1·883, p=0·06; figure 2E). For example, out of 392 participants (who had sufficient questionnaire data for the first and second boosters), 268 (68% [95% CI 64–73]) did not report any new signs or symptoms after receiving the second booster dose, compared with 257 (66% [61–70]) after the first booster dose. Moreover, the most frequently reported reactions (ie, fatigue, headache, muscle pain, fever, and cold) were similar after the first and second booster doses.
For the retrospective cohort, we compared the frequency of 25 potential adverse events 42 days before the first and second booster vaccination with those 42 days after vaccination. We observed no significant positive risk difference due to the second booster uptake for any of the adverse events tested (table 2). For example, the risk difference for Bell's Palsy was –1·68 (95% CI –5·61 to 2·25), 2·25 (–3·93 to 8·98) for myocardial infarction, and –2·25 (–4·49 to –0·56) for pericarditis. Furthermore, there were no events of myocarditis before or after vaccination. We also confirmed the safety of the second booster by conducting an alternative analysis similar to the one presented in a previous study (appendix pp 21–23).25
Table 2.
Adverse events associated with SARS-CoV-2 vaccination
| Number of individuals with an event before the second booster* | Number of individuals with an event after the second booster* | Risk difference after second booster (95% CI; number of events per 10 000 people)† | Number of individuals with event after the first booster* | Risk difference of second boosters versus first boosters (95% CI; number of events per 10 000 people)† | |
|---|---|---|---|---|---|
| Acute kidney injury | 8 | 11 | 1·68 (−3·37 to 6·74) | 5 | 3·93 (−0·56 to 8·42) |
| Anaemia | 203 | 193 | −5·61 (−27·51 to 16·28) | 191 | −2·25 (−25·82 to 21·89) |
| Appendicitis | 2 | 3 | 0·56 (−1·68 to 2·81) | 6 | −0·56 (−3·93 to 2·81) |
| Arrhythmia | 170 | 158 | −6·74 (−26·95 to 12·91) | 144 | 7·86 (−12·91 to 28·63) |
| Arthritis or arthropathy | 38 | 29 | −5·05 (−14·03 to 3·93) | 32 | −1·68 (−10·10 to 6·74) |
| Bell's palsy | 8 | 5 | −1·68 (−5·61 to 2·25) | 5 | −2·25 (−6·18 to 1·68) |
| Cerebrovascular accident | 95 | 91 | −2·25 (−17·40 to 12·91) | 80 | 10·10 (−5·05 to 25·26) |
| Deep vein thrombosis | 0 | 2 | 1·12 (0 to 2·81) | 2 | 0 (−2·81 to 2·81) |
| Herpes simplex virus infection | 12 | 15 | 1·68 (−3·93 to 7·29) | 11 | 2·25 (−3·37 to 7·86) |
| Herpes zoster infection | 20 | 33 | 7·30 (−0·56 to 15·16) | 24 | 2·81 (−5·61 to 11·23) |
| Intracranial haemorrhage | 4 | 9 | 2·81 (−1·12 to 6·74) | 2 | 2·25 (−2·25 to 7·30) |
| Lymphadenopathy | 26 | 20 | −3·37 (−10·67 to 4·50) | 30 | −7·30 (−15·72 to 0·56) |
| Lymphopenia | 0 | 0 | .. | 0 | .. |
| Myocardial infarction | 15 | 19 | 2·25 (−3·93 to 8·98) | 15 | 3·93 (−2·81 to 11·23) |
| Myocarditis | 0 | 0 | .. | 1 | −0·56 (−1·68 to 0) |
| Neutropenia | 11 | 12 | 0·56 (−4·50 to 5·61) | 8 | 2·81 (−2·25 to 7·86) |
| Other thrombosis | 4 | 3 | −0·56 (−3·37 to 2·25) | 1 | 0·56 (−1·68 to 2·81) |
| Paresthesia | 60 | 64 | 2·25 (−10·11 to 14·60) | 51 | 2·81 (−9·54 to 15·16) |
| Pericarditis | 4 | 0 | −2·25 (−4·49 to −0·56) | 2 | −2·25 (−4·49 to −0·56) |
| Pulmonary embolus | 8 | 6 | −1·12 (−5·05 to 2·81) | 4 | −0·56 (−3·93 to 2·81) |
| Seizures | 7 | 4 | −1·68 (−5·62 to 1·69) | 2 | 1·69 (−1·12 to 4·49) |
| Syncope | 38 | 41 | 1·68 (−7·86 to 11·23) | 35 | 9·54 (−0·56 to 20·21) |
| Thrombocytopenia | 17 | 9 | −4·49 (−10·10 to 1·12) | 9 | −1·12 (−6·18 to 3·93) |
| Uveitis | 3 | 3 | 0 (−2·81 to 2·81) | 3 | 0 (−2·81 to 2·81) |
| Vertigo | 137 | 153 | 8·98 (−9·54 to 27·51) | 133 | 3·93 (−16·84 to 24·14) |
Pairwise comparative analysis including 17 814 participants.
42-day period before or after the booster dose.
The risk difference and CIs were estimated for each individual in a paired fashion with the use of percentile bootstrap method with 10 000 repetitions.
We also observed a similar trend when comparing, in a pairwise method, the potential adverse events after the second booster dose to the ones observed following the first booster dose. For example, the risk difference for lymphadenopathy was –7·30 (95% CI –15·72 to 0·56), and for appendicitis –0·561 (–3·93 to 2·81). Additionally, we compared, in a pairwise method, the potential adverse events before and after vaccination with the first booster among recipients of the second booster. The only adverse event that was positively significant was lymphadenopathy after the first booster, with a risk difference of 8·98 (1·68 to 16·28; appendix p 19)
Myocarditis and pericarditis are recognised as adverse events of special interest. We found that none of the 17 814 recipients of the second booster dose were diagnosed with myocarditis or pericarditis following the second booster. For individuals who were eligible to receive the second booster, we also extended the analysis to examine whether these events were associated with the primary series (ie, first and second doses) or the first booster. Among the 44 003 eligible individuals, five individuals were diagnosed with myocarditis following inoculation with the primary series (risk difference 1·14 [95% CI 0·23 to 2·27]) and two after the first booster (risk difference –0·68 [–1·82 to 0·46]); 12 were diagnosed with pericarditis following the primary series (1·59 [–0·23 to 3·41]), and seven following the first booster (–0·23 [–2·05 to 1·59]; appendix p 20).
Discussion
Our prospective and retrospective analyses provide evidence to further support the safety of the second BNT162b2 mRNA booster in eligible populations, with our findings reflecting patients' objective and subjective responses. Our prospective cohort provided evidence to show that the second booster induces significant changes in heart rate levels, with measures peaking on day 2 and returning to each individual's baseline by day 6. These trends were not different from those observed in the same individuals following the first booster. The retrospective cohort of 17 814 Maccabi Healthcare Services members further suggested that none of the 25 events examined was associated with the second booster dose of the vaccine, and no events of myocarditis or pericarditis were observed following vaccination with the second booster (ie, fourth dose).
The clinical presentation of myocarditis after COVID-19 mRNA vaccination is one of the major contributors to vaccine hesitancy.29 In our retrospective cohort analysis, we found that none of the 17 814 individuals who received the second booster had a report of myocarditis in their electronic medical records within 42 days after vaccination. This absence of reported events following the second booster, and the presentation of two events after the first dose out of the 44 003 individuals eligible for vaccination with the second dose, should quell concerns on this matter. Our results are also in line with a large-scale database showing that the risk of myocarditis after the primary series is low, but with the highest frequency being in young men.25, 26
Although our prospective cohort's size is similar to other studies that used wearable devices, it has a rather large pairwise sample.11, 12, 13 In particular, most of the participants in our second booster analysis were aged over 50 years, in line with Israeli and CDC vaccine administration guidelines.30, 31 This age group is characterised by a higher rate of underlying medical conditions and might be more sensitive to changes caused by vaccine reactions. Thus, if guidelines are changed to include all individuals aged 18 years and older, our findings might not be generalisable.
In our analysis of the prospective cohort, we found an increase of 1·61 bpm in daily mean heart rate difference during the second day after vaccination. Previous studies have suggested that even a minor long-lasting increase in heart rate is associated with an increased risk of death.32, 33 However, in our study, the daily mean difference of heart rate values returned to baseline levels by the sixth day after vaccination, so we do not expect an increased risk by this temporal change.
Our study includes several limitations. First, the clinical importance of the continuous monitoring of heart measures, including heart rate, HRV, and resting heart rate, are yet to be fully established. Second, the Garmin smartwatches we used are not medical-grade wearable devices, nor are smartwatches representative of all wearable devices. Third, although some details are available on how the Garmin HRV-based stress measure is calculated,22, 34 the exact algorithm is proprietary and is not fully disclosed. Fourth, participants in the prospective cohort were recruited via advertisements on social media and word-of-mouth, making our cohort a convenient sampling. Fifth, the demanding study requirements to wear the smartwatch and fill the questionnaires at least twice a week for 2 years made participation in the study less appealing to particular populations. Indeed, our participants were slightly older than the Israeli population, so our analyses might not be generalisable to the entire Israeli or global population. However, the frequency and duration of the self-reported reactions were consistent with those of the clinical trial on the first and second doses of the BNT162b2 mRNA vaccine.21 Sixth, our retrospective study's sample size (17 814 participants) might not suffice to identify rare severe events. For example, a previous study found that vaccination with the primary course was weakly associated with herpes zoster infection, with 0·8–2·4 events per 10 000 people in the general population.25 Among the recipients of the second booster, we observed a risk difference of 7·3 events per 10 000 participants, but with a 95% CI range of –0·56 to 15·15. Of note, among the recipients of the second booster, 2029 (11·39%) participants were vaccinated against herpes zoster. Lastly, our analysis did not necessarily represent those who are eligible to receive the second dose but those who actually received the second booster. For example, individuals who had a severe adverse response to the first booster might opt not to receive the second booster and thus are not represented in our data.
Our main analysis of the retrospective cohort was different from that of a previous large-scale study that examined the short-term safety of the primary vaccine course in two aspects.25 First, our study was not a matching-process analysis, in which vaccinated individuals are matched to controls on the basis of general criteria. Instead, we compared each individual to their own baseline. This difference between analyses is particularly important given that people who are vaccinated have differential testing behaviours relative to those who are unvaccinated.35 Second, for each potential severe event tested, the previous study excluded individuals who had previously been diagnosed with that event. We did not disregard these individuals because it might be possible for the vaccine to trigger a previous condition or that these individuals are more prone to specific medical complications. We therefore believe that, for those diagnosed with an event before the baseline period and during the period after vaccination, it would be reasonable to conservatively attribute such an adverse event to the vaccine. Encouragingly, even when including these individuals and adopting this conservative assumption, our results support the safety of mRNA COVID-19 vaccines. Notably, to allow a comparison with the previous study, we conducted an alternative analysis, which also confirmed the safety of the second booster. In conclusion, our findings further highlight the short-term safety of the BNT162b2 second booster dose, as reflected in both our subjective and objective data.
Data sharing
Researchers who are interested in obtaining an aggregated version of the data sufficient to reproduce the results reported in this paper should contact the corresponding author. Statistical code will be available after acceptance.
Declaration of interests
All authors declare no competing interests.
Acknowledgments
Acknowledgments
This research was supported by the European Research Council (project number 949850) and the Israel Science Foundation (grant number 3409/19), within the Israel Precision Medicine Partnership programme. We thank Natalie Page for the insightful conversations about the study and the valuable editorial inputs. We thank the anonymous reviewers and the academic editor for their academic input.
Acknowledgments
Contributors
DY, MY, and ES were responsible for the conception and design of the study. DY, TP, SG, and ES were responsible for the collection and assembly of the data. DY, MY, and ES were responsible for verifying the data. DY, MY, and ES had access to the raw data. MY, MM, DY, and ES were responsible for analysing and interpreting the data. AP, MY, MM, DY, and ES were responsible for all statistical analyses. MY, DY, and ES were responsible for drafting the article. DY and ES were responsible for critically revising the article for important intellectual content. All authors were responsible for the final approval of the article. DY was responsible for the decision to submit the article for publication. DY and ES were responsible for obtaining funding.
Supplementary Material
References
- 1.Ministry of Health Fourth dose of the vaccine approved for people with a weakened immune system. 2021. https://www.gov.il/en/departments/news/30122021-05
- 2.Centres for Disease Control and Prevention CDC recommends additional boosters for certain individuals. 2022. https://www.cdc.gov/media/releases/2022/s0328-covid-19-boosters.html
- 3.Gazit S, Saciuk Y, Perez G, Peretz A, Pitzer VE, Patalon T. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113. doi: 10.1136/bmj-2022-071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero-Ibarguengoitia ME, González-Cantú A, Rivera-Salinas D, et al. Analysis of immunization, adverse events, and efficacy of a fourth dose of BNT162b2 vaccine. Vaccines (Basel) 2022;10:1139. doi: 10.3390/vaccines10071139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centres for Disease Control and Prevention COVID data tracker: vaccinations in the US. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-people-onedose-pop-5yr
- 7.Štěpánek L, Janošíková M, Nakládalová M, Štěpánek L, Boriková A, Vildová H. Motivation to COVID-19 vaccination and reasons for hesitancy in employees of a Czech tertiary care hospital: a cross-sectional survey. Vaccines. 2021;9:863. doi: 10.3390/vaccines9080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machingaidze S, Wiysonge CS. Understanding COVID-19 vaccine hesitancy. Nat Med. 2021;27:1338–1339. doi: 10.1038/s41591-021-01459-7. [DOI] [PubMed] [Google Scholar]
- 9.Pertwee E, Simas C, Larson HJ. An epidemic of uncertainty: rumors, conspiracy theories and vaccine hesitancy. Nat Med. 2022;28:456–459. doi: 10.1038/s41591-022-01728-z. [DOI] [PubMed] [Google Scholar]
- 10.Couzin-Frankel J. Global project gears up to study vaccine safety. Science. 2022;376:227–228. doi: 10.1126/science.abq4273. [DOI] [PubMed] [Google Scholar]
- 11.Mofaz M, Yechezkel M, Guan G, et al. Self-reported and physiologic reactions to third BNT162b2 mRNA COVID-19 (booster) vaccine dose. Emerg Infect Dis. 2022;28:1375. doi: 10.3201/eid2807.212330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gepner Y, Mofaz M, Oved S, et al. Utilizing wearable sensors for continuous and highly-sensitive monitoring of reactions to the BNT162b2 mRNA COVID-19 vaccine. Commun Med (Lond) 2022;2:27. doi: 10.1038/s43856-022-00090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quer G, Gadaleta M, Radin JM, et al. Inter-individual variation in objective measure of reactogenicity following COVID-19 vaccination via smartwatches and fitness bands. NPJ Digit Med. 2022;51:49. doi: 10.1038/s41746-022-00591-z. 2022; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oved S, Mofaz M, Lan A, et al. Differential effects of COVID-19 lockdowns on well-being: interaction between age, gender and chronotype. J R Soc Interface. 2021;18:20210078. doi: 10.1098/rsif.2021.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381:1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra T, Wang M, Metwally AA, et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng. 2020;4:1208–1220. doi: 10.1038/s41551-020-00640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alavi A, Bogu GK, Wang M, et al. Real-time alerting system for COVID-19 and other stress events using wearable data. Nat Med. 2021;28:175–184. doi: 10.1038/s41591-021-01593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-Based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 19.Mathioudakis AG, Ghrew M, Ustianowski A, et al. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: a vaccine recipient survey. Life (Basel, Switzerland) 2021;3:249. doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maccabi Our healthcare sysem. https://www.maccabi4u.co.il/1781-he/Maccabi.aspx
- 21.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Google Patents Procedure for detection of stress by segmentation and analyzing a heart beat signal. https://patents.google.com/patent/US20050256414A1/en
- 23.VÍVOSMART ® 4 Owner's Manual 2018. https://www8.garmin.com/manuals/webhelp/vivosmart4/EN-US/vivosmart_4_OM_EN-US.pdf
- 24.Garmin What is the stress level feature on my Garmin watch? https://support.garmin.com/en-US/?faq=WT9BmhjacO4ZpxbCc0EKn9
- 25.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong H-L, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399:2191–2199. doi: 10.1016/S0140-6736(22)00791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothman KJ. Epidemiology: an introduction. Oxford University Press; Oxford: 2012. [Google Scholar]
- 28.Sáez-Llorens X, Bandyopadhyay AS, Gast C, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials. Lancet. 2021;397:27–38. doi: 10.1016/S0140-6736(20)32540-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troiano G, Nardi A. Vaccine hesitancy in the era of COVID-19. Public Health. 2021;194:245–251. doi: 10.1016/j.puhe.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corono.Health. Vaccines. https://corona.health.gov.il/en/vaccine-for-covid/
- 31.Centres for Disease Control and Prevention CDC strengthens recommendations and expands eligibility for COVID-19 booster shots. https://www.cdc.gov/media/releases/2022/s0519-covid-booster-acip.html
- 32.Chen XJ, Barywani SB, Hansson PO, et al. Impact of changes in heart rate with age on all-cause death and cardiovascular events in 50-year-old men from the general population. Open Hear. 2019;6:e000856. doi: 10.1136/openhrt-2018-000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raisi-Estabragh Z, Cooper J, Judge R, et al. Age, sex and disease-specific associations between resting heart rate and cardiovascular mortality in the UK BIOBANK. PLoS One. 2020;15:e0233898. doi: 10.1371/journal.pone.0233898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firstbeat Technologies Ltd Stress and recovery analysis method based on 24-hour heart rate variability. 2014; : 1–13. https://assets.firstbeat.com/firstbeat/uploads/2015/11/Stress-and-recovery_white-paper_20145.pdf (accessed Aug 14)
- 35.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers who are interested in obtaining an aggregated version of the data sufficient to reproduce the results reported in this paper should contact the corresponding author. Statistical code will be available after acceptance.