Abstract
Objectives:
Lower daily methadone dose is negatively associated with retention in methadone maintenance treatment (MMT). Cannabis use during MMT is common, with many patients reporting its use for opioid withdrawal mitigation. We sought to test whether the association between lower MMT dose and treatment retention differs by concurrent high-frequency cannabis use in a community sample of people on MMT.
Methods:
We obtained data from participants initiating MMT in two community-recruited prospective cohorts of people who use drugs in Vancouver, Canada. We built multivariable Cox frailty models to estimate the relationships between MMT dose (<90 mg/day vs. ≥90 mg/day) and time to treatment discontinuation. We included an interaction term to test whether high-frequency (≥daily) cannabis use modified the measured effect of lower treatment dose on treatment retention.
Results:
Between December 2005 and December 2018, 829 (54.1%) participants initiated at least one MMT episode and were included in the analysis. Lower MMT dose was strongly positively associated with treatment discontinuation regardless of concurrent high-frequency cannabis use (interaction p>0.05). Structural factors including homelessness and incarceration were significantly and positively associated with treatment discontinuation.
Conclusions:
Although we previously found the magnitude and strength of the relationship between lower MMT dose and high-frequency unregulated opioid use to be tempered during high-frequency cannabis use periods, this effect measure modification does not appear to translate to time retained in treatment. Cannabis-based interventions to promote retention in MMT are unlikely to produce long-term benefit without addressing external factors that place MMT patients at increased risk of treatment discontinuation.
Keywords: Cannabis, cannabinoids, opioid use disorder, methadone, drug treatment
Introduction
Drug overdose continues to be a leading cause of premature death across Canada and the U.S.1 For people living with opioid use disorder (OUD), pharmacological management with an opioid agonist, such as methadone or buprenorphine/naloxone, is the most effective medication-based intervention against opioid overdose.2,3 Retention in evidence-based treatment is critical to preventing unregulated opioid use4 and subsequent overdose.5 Studies from diverse settings demonstrate that higher methadone doses are strongly positively correlated with retention in treatment6–9 and negatively correlated with continued use of unregulated opioids.10–12 Yet, it remains common for patients to be prescribed treatment doses that do not yield maximum treatment benefit.13
It is estimated that about half of patients on medication-based treatment of OUD use cannabis at least once during their treatment.14 In a recent survey of people who use cannabis and opioids, over 60% reported using cannabis to mitigate opioid withdrawal with perceived benefit—particularly in addressing anxiety, tremors, and sleep problems arising from withdrawal.15 Though mitigation of OUD symptoms with cannabis was recorded as early as the 1940s,16 there has been a renewed scientific interest in cannabinoids as potential treatment agents for OUD 17,18} and two recent small human experimental studies demonstrate improvements in severity of opioid withdrawal with the administration of THC19 and suppression of opioid cravings with the administration of CBD.20
Studies to date have produced inconsistent evidence of the impact of cannabis use during medication-based treatment for OUD, including MMT. For example, in a recent systematic review of studies documenting the relationship between cannabis use and treatment outcomes for patients in medication-based treatment of OUD, while most studies did not document a significant relationship between cannabis use and treatment outcomes, a small number found worse outcomes and a similarly small number found more favourable outcomes among patients who used cannabis.14 Furthermore, studies have been limited by several common features including cross-sectional design, lack of control for confounding, or low precision in cannabis use measurement (e.g., captured only at treatment baseline or quantified at a level too low to plausibly detect a biological effect).14 While laws governing medical or non-medical cannabis use have been reformed in Canada and over 30 U.S. states, evidence of cannabis use (e.g., through detection of tetrahydrocannabinol [THC] in urine) can still result in treatment restrictions such as denial of take-home doses in some treatment settings.21,22 In contrast, despite a need for more evidence of treatment benefit, a growing number of U.S. state medical cannabis programs are authorizing medical cannabis in the treatment of OUD.23,24
We recently tested the hypothesis that the well-established relationship between lower MMT doses and high-frequency unregulated opioid use during treatment differs based on concurrent cannabis use status and found evidence that the magnitude and strength of this relationship is tempered during periods of high-frequency cannabis use.25 However, whether a similar effect measure modification pattern exists for treatment retention—one of the most important clinical indicators of success in medication-based treatment of OUD—is not known. Building off our previous study, we sought to explore the cannabis-dependent relationship between methadone dose and treatment discontinuation in a large community-recruited sample of people who use unregulated drugs in Vancouver, Canada.
Methods
Study population and procedures
Data for this study were obtained from two open prospective cohorts of people who use unregulated drugs (PWUD) in Vancouver, Canada. The Vancouver Injection Drug Users Study (VIDUS) includes HIV at-risk people who inject drugs. The AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS) includes PWUD living with HIV. The studies began open recruitment in 1996 (VIDUS) and 2005 (ACCESS) through extensive street outreach in areas across Vancouver’s downtown core, with concentrated efforts in the Downtown Eastside (DTES), a low-income neighbourhood with an open unregulated drug market and widespread marginalization and criminalization of PWUD. Many health and social services for marginalized PWUD are concentrated within this neighbourhood, including low-barrier clinics and pharmacies providing medication-based treatment for OUD, primarily methadone.
Eligibility criteria for VIDUS include: (1) being HIV-negative and (2) having injected drugs at least once use in the 30 days before study enrolment. Eligibility criteria for ACCESS include: (1) being HIV-positive and (2) using an unregulated drug by injection or non-injection (other than cannabis, which was a controlled substance in Canada until October 2018) in the 30 days before study enrolment. HIV serostatus is confirmed through serology. Additionally, participants in both studies must: (3) be at least 18 years of age; (4) reside in the Greater Vancouver Regional District; and (5) provide written informed consent. Except for HIV-specific study assessments (ACCESS only), all study instruments and follow-up procedures described below are harmonized such that data can be pooled for statistical analyses and interpretation.
At study enrolment, participants in each cohort complete a structured interviewer-administered questionnaire eliciting information on socio-demographic factors, current substance use patterns, health and social service utilization, and social- and structural-level exposures (e.g., incarceration), physical and mental health, disability, other health-related concerns. Blood is collected for HIV antibody testing (VIDUS) or HIV clinical monitoring (ACCESS) and hepatitis C serology (both cohorts). Participants are scheduled for a follow-up interview every six months to allow for time-updated analyses of the information obtained at baseline. Participants receive a $40 (CAD) honorarium for participation at each study visit. Ethical approval for both studies was granted by the University of British Columbia/Providence Health Care Research Ethics Board.
Study sample
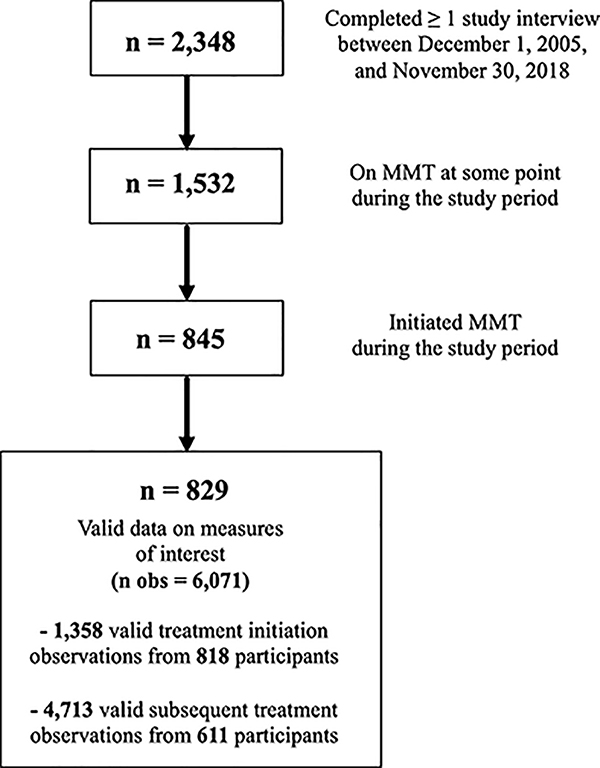
From December 1, 2005, to November 30, 2018, we asked participants about their current and past six-month enrolment in MMT for OUD at baseline and each six-month follow-up interview. To analyze the outcome of treatment retention, the sample was restricted to participants who initiated (or re-initiated) MMT during the study period (December 1, 2005, to November 30, 2018), defined as reporting past six-month MMT after at least one interview of reporting no past six-month MMT. Participants who reported being on MMT at study recruitment (baseline) were not eligible for analysis until they re-initiated a subsequent treatment episode during the study period (Figure 1).
Figure 1.
Flowchart illustrating the composition of the analytic samples
Measures
Outcome measure
The outcome of interest was time-to-discontinuation of treatment. Time zero was defined as the date of initiating or re-initiating MMT and was estimated at the beginning of the first six-month period in which the participant endorsed past six-month MMT enrolment. We estimated the time of treatment discontinuation from time-updated self-reported information about past six-month and current MMT enrolment at each interview. Participants who endorsed current and past six-month enrolment were considered retained on treatment for that six-month period; participants who did not endorse current or past six-month MMT enrolment were estimated to have discontinued treatment at the mid-point between their previous interview date and the beginning of that six-month period; and participants who did not endorse current MMT enrolment but endorsed past six-month enrolment were estimated to have discontinued treatment at the mid-point between the beginning of that six-month interview period and the current interview date. After a participant discontinued treatment, all their subsequent follow-up data was censored until (if) they re-initiated another treatment episode during the study period. Participants who were still enrolled in MMT at the end of the study period were right-censored. Participants were considered lost to regular follow-up if the time between two consecutive interviews exceeded 24 months. In this case, they were censored at the time of their last interview before being lost to regular follow-up and were considered re-eligible for analysis once they returned for a follow-up interview. If MMT enrolment was reported during this interview, it was considered a new treatment episode.
Primary independent variables
The main exposures of interest were daily methadone dose (primary independent variable) and cannabis use (hypothesized effect measure modifier). All participants who reported past six-month MMT enrolment were asked to report their current daily dose in mL. For participants who were estimated to have discontinued MMT at a date preceding their interview, the dose reported at the time of their previous interview during that treatment episode was used (participants who discontinued a new treatment episode before six months were handled separately—see statistical analysis). We dichotomized the daily dose at 90 mg such that patients reporting doses of <90 mg were characterized as being on a lower dose. This cut-point is supported by previous evidence showing more complete opioid blockade and improved treatment outcomes at doses of ≥100 mg/d8,9,26,27 and ensures consistency with our previous analysis.25
The relationship between methadone dose and treatment retention was hypothesized to differ by frequency of past six-month cannabis use such that the association would be tempered or rendered non-significant during periods of high-frequency cannabis use. At each interview, participants who endorsed cannabis use in the past six months were asked to estimate their average frequency of use over this time (none, about once/month, about 2–3 times/month, about once/week, 2–3 times/week, and about once/day). Consistent with our previous analysis,25 high-frequency cannabis use was defined as ≥daily vs. <daily.
Secondary variables
We included several secondary variables known or a priori hypothesized to confound the relationship between treatment dose and treatment retention. The following variables were considered for these analyses: (1) sociodemographic factors, including sex (male vs. female), current age (per year older), racial identity (White vs. non-White), legal employment (yes vs. no), homelessness (defined as living on the street with no fixed address, consistent with previous analyses,28 yes vs. no), and incarceration (yes vs. no); (2) substance use and health-related factors, including HIV serostatus (positive vs. negative), ≥daily alcohol use (yes vs. no), and ≥daily stimulant (crystal methamphetamine or crack/powder cocaine) use (yes vs. no); and (3) treatment-related factors, including engagement in MMT at study recruitment (yes vs. no), treatment episode number (corresponding to each additional continuous period of treatment from initiation to discontinuation/censorship [categorized into episodes 1, 2, ≥3], and engagement in other substance use treatment (e.g., counselling, residential treatment), and calendar year of treatment (≥2014 vs. <2014). This cut-off corresponds with province-wide changes to the province’s methadone formulation from a 1mg/mL pharmacy-compounded formulation to a 10 mg/mL commercially-available formulation. We hypothesized an increased likelihood of treatment discontinuation after the formula change given immediate patients reports that the new formulation failed to suppress opioid withdrawal for 24 hours,29 and later studies confirming that the switch had widespread unintended impacts on opioid relapse.30,31 Aside from HIV status, which is confirmed through serology, all variables are derived from self-reported data. All variables are time-varying (except sex and race) and refer to the previous six-month period at each study interview. Of note, opioid use was conceptualized as an intermediate factor in the relationship between MMT dose and retention and was not statistically treated as a confounder in these analyses.
Statistical analysis
Socio-demographic and health-related characteristics at the beginning of the first treatment episode (i.e., treatment baseline) were examined for all participants who initiated a treatment episode during the study. These observations were stratified by cannabis use status and group differences were tested using Pearson’s Chi-Square test (categorical variables) or Wilcoxon rank-sum test (numeric variables).
Because MMT dose was only reported by participants who were on MMT at the time of their interview, the effect of treatment dose could not be estimated for participants who discontinued MMT within the first six months of initiating a new treatment episode. As the first few months after treatment initiation represent a high-risk period in which stabilizing on treatment may be challenging due to withdrawal and craving, the dataset was split into observations to be analyzed separately for the potential relationship between cannabis use and ≤6 month discontinuation (analysis 1), and the cannabis-dependent relationship between dose and time-to-discontinuation after 6 months (analysis 2; Figure 1).
Given low variability in the measurable number of days until discontinuation or censorship within the first six months of treatment resulting from the study’s biannual interview protocol, short-term retention was modelled as a binary outcome (i.e., retained ≤six months; yes vs. no). To prevent underestimating ≤six-month discontinuation in cases where participants could not be scheduled for a subsequent interview at exactly six months, short-term retention was defined as ≤200 days to allow for an approximate three-week buffer period. Given the potential for multiple treatment episodes per person, we built generalized estimating equations (GEEs) with logit link to examine the relationship between high-frequency cannabis use and discontinuing treatment within six months of initiation, adjusting for the hypothesized confounders above.
To model the relationship between MMT dose (and its potential modification by high-frequency cannabis use) and time-to-treatment discontinuation after six months, we built bivariable and multivariable Cox gamma-frailty models. The frailty term represents an unobservable random variable corresponding to each person’s deviation from the baseline hazard function and accounts for the potential within-person correlation of recurrent treatment episode lengths. This approach is useful when participants can have recurring discontinuation events and it has been applied to previous observational research of MMT retention.6,32 First, we examined the crude bivariable relationships to the outcome for lower MMT dose and cannabis use separately. Then, we added an interaction term for dose and cannabis to explore effect measure modification. Following this, all hypothesized confounders outlined above were added to the model to estimate the adjusted association between methadone dose and time-to-treatment discontinuation within each stratum of cannabis use. In both steps, the significance of the interaction term was checked to explore possible effect measure modification.
All analyses were conducted in R (Version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria) using RStudio (Version 1.2.5033). All p-values are two-sided.
Results
Between December 1, 2005, and November 30, 2018, a total of 2348 participants were recruited into the studies and completed at least one study interview. Overall, 1532 (65.2%) participants endorsed past six-month MMT at least once, of whom 829 (55.1%) initiated at least one treatment episode during the study period and had complete data for all measures of interest (Figure 1). These individuals contributed 6,071 observations to the study, representing 1,390 distinct MMT episodes across 3,356 person-years. Participants spent a median of 37.9 cumulative months (Interquartile Range [IQR]: 11.8 – 48.6) in treatment. Most of them (n = 477; 57.5%) experienced only one treatment episode. Of the remaining 352 participants, most (n = 212, 60.2%) re-enrolled in MMT just once more during the study. Overall, 530 (63.9%) participants discontinued treatment a total of 872 times over 3,356 person-years for a crude treatment discontinuation incidence rate of 26.0 per 100 person-years (95% Confidence Interval [CI]: 24.3 – 27.7).
Of the 818 MMT participants who had complete data in the first interview after initiating MMT, 139 (17.0%) reported high-frequency cannabis use at the start of their first treatment episode. They were more likely to be male (69.8% vs. 56.3%, p=0.004) and employed (27.3% vs. 17.7%, p=0.012; Table 1). In total, 240 (29.3%) participants discontinued treatment at <six months in at least one treatment episode. As shown in Table 2, there was not a significant relationship observed between high-frequency cannabis use and retention in treatment at six months (Odds Ratio [OR] = 1.02, 95% CI: 0.71 – 1.47; Adjusted Odds Ratio [AOR] = 0.98, 95% CI: 0.66 – 1.45; both p>0.05).
Table 1.
Baseline characteristics of 818 PWUD who initiated an MMT episode between December 1, 2005, and November 30, 2018
| Characteristic | Overall n = 818 | Daily cannabis use1 |
p-value | |
|---|---|---|---|---|
| Yes n = 139; 17.0% | No n = 679; 83.0% | |||
|
| ||||
| Sociodemographic factors | ||||
|
| ||||
| Sex | ||||
| Male | 479 (58.6) | 97 (69.8) | 382 (56.3) | 0.004 |
| Female | 339 (41.4) | 42 (30.2) | 297 (43.7) | |
| Age | ||||
| Median (IQR) | 42.4 (35.0 – 49.2) | 43.4 (35.7 – 48.4) | 42.2 (34.9 – 49.5) | 1.000 |
| Racial identity | ||||
| White | 464 (56.8) | 82 (59.0) | 383 (56.4) | 0.641 |
| Non-white | 353 (43.2) | 57 (41.0) | 296 (43.6) | |
| Employment 1 | ||||
| Yes | 158 (19.3) | 38 (27.3) | 120 (17.7) | 0.012 |
| No | 660 (80.7) | 101 (72.7) | 559 (82.3) | |
| Homelessness 1 | ||||
| Yes | 274 (33.5) | 41 (29.5) | 233 (34.3) | 0.318 |
| No | 544 (66.5) | 98 (70.5) | 446 (65.7) | |
| Incarceration 1 | ||||
| Yes | 90 (11.0) | 16 (11.5) | 90 (13.3) | 0.675 |
| No | 712 (89.0) | 123 (88.5) | 589 (86.7) | |
|
| ||||
| Substance use, health, treatment factors | ||||
|
| ||||
| Daily alcohol use 1 | ||||
| Yes | 36 (4.4) | 8 (5.8) | 28 (4.1) | 0.530 |
| No | 782 (95.6) | 131 (94.2) | 651 (95.9) | |
| Daily stimulant use 1 | ||||
| Yes | 346 (42.3) | 60 (43.2) | 286 (42.1) | 0.894 |
| No | 472 (57.7) | 79 (56.8) | 393 (57.9) | |
| Daily opioid use 1 | ||||
| Yes | 329 (40.1) | 52 (38.5) | 277 (41.6) | 0.571 |
| No | 472 (59.9) | 83 (61.5) | 389 (58.4) | |
| HIV status | ||||
| HIV-positive | 273 (33.4) | 50 (36.0) | 223 (32.8) | 0.539 |
| HIV-negative | 545 (66.6) | 89 (64.0) | 456 (67.2) | |
| Other addiction treatment 1 | ||||
| Yes | 172 (21.0) | 33 (23.7) | 139 (20.5) | 0.455 |
| No | 646 (79.0) | 106 (76.3) | 540 (79.5) | |
| MMT dose 2,3 | ||||
| Lower (< 90 mg/d) | 436 (64.1) | 75 (64.1) | 361 (64.1) | 1.000 |
| Higher (≥ 90 mg/d) | 244 (35.9) | 42 (35.9) | 202 (35.9) | |
Note:
Refers to exposures in the previous six months
Daily MMT dose was reported at the time of interview
Cells for MMT dose do not add up to 818 as participants who discontinued treatment before their interview were ineligible for this question
IQR = Interquartile range
Table 2.
Bivariable and multivariable associations between all independent variables and ≤six-month retention among 818 PWUD initiating an MMT episode between December 1, 2005 and November 30, 2018
| Variable | MMT discontinuation at six months |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Adjusted Odds Ratio (95% CI) | p-value | |
|
| ||||
| Primary independent variable | ||||
|
| ||||
| Daily cannabis use 1 | ||||
| (Yes vs. no) | 1.02 (0.71 – 1.47) | 0.908 | 0.98 (0.66 – 1.45) | 0.920 |
|
| ||||
| Socio-demographic factors | ||||
|
| ||||
| Sex | ||||
| (Male vs. female) | 1.15 (0.87 – 1.52) | 0.318 | 1.28 (0.94 – 1.75) | 0.120 |
| Age | ||||
| (Per year increase) | 0.98 (0.97 – 1.00) | 0.035 | 0.98 (0.97 – 1.00) | 0.056 |
| Racial identity | ||||
| (White vs. non-white) | 0.78 (0.60 – 1.03) | 0.082 | 0.82 (0.61 – 1.10) | 0.180 |
| Employed 1 | ||||
| (Yes vs. no) | 1.23 (0.90 – 1.68) | 0.189 | 1.17 (0.83 – 1.64) | 0.359 |
| Homeless 1 | ||||
| (Yes vs. no) | 1.32 (0.99 – 1.76) | 0.056 | 1.17 (0.86 – 1.58) | 0.313 |
| Incarcerated 1 | ||||
| (Yes vs. no) | 1.47 (1.01 – 2.12) | 0.042 | 1.23 (0.84 – 1.82) | 0.290 |
|
| ||||
| Substance use and health factors | ||||
|
| ||||
| Daily alcohol use 1 | ||||
| (Yes vs. no) | 1.55 (0.93 – 2.59) | 0.091 | 1.42 (0.84 – 2.41) | 0.187 |
| Daily stimulant use 1 | ||||
| (Yes vs. no) | 0.95 (0.72 – 1.25) | 0.697 | 1.00 (0.74 – 1.35) | 0.994 |
| Daily opioid use 1,2 | ||||
| (Yes vs. no) | 2.61 (2.00 – 3.42) | <0.001 | -- | -- |
| HIV serostatus | ||||
| (Positive vs. negative) | 0.71 (0.53 – 0.97) | 0.029 | 0.74 (0.53 – 1.01) | 0.060 |
|
| ||||
| Treatment-related factors | ||||
|
| ||||
| Calendar year | ||||
| (≥2014 vs. <2014) | 2.11 (1.61 – 2.75) | <0.001 | 2.59 (1.92 – 3.49) | <0.001 |
| Other addiction treatment 1 | ||||
| (Yes vs. no) | 1.27 (0.92 – 1.74) | 0.212 | 1.23 (0.89 – 1.69) | 0.243 |
| Treatment episode number | ||||
| (2 vs. 1) | 0.98 (0.72 – 1.32) | 0.871 | 0.98 (0.72 – 1.32) | 0.184 |
| (≥3 vs. 1) | 1.00 (0.68 – 1.48) | 0.982 | 0.62 (0.39 – 0.97) | 0.035 |
| MMT at study recruitment | ||||
| (Yes vs. no) | 0.59 (0.43 – 0.81) | 0.001 | 0.57 (0.41 – 0.80) | 0.001 |
Note:
Refers to the six-month period preceding interview
Opioid use is conceptualized as an intermediate factor in the relationship between low MMT dose and treatment discontinuation
95% CI = 95% Confidence interval
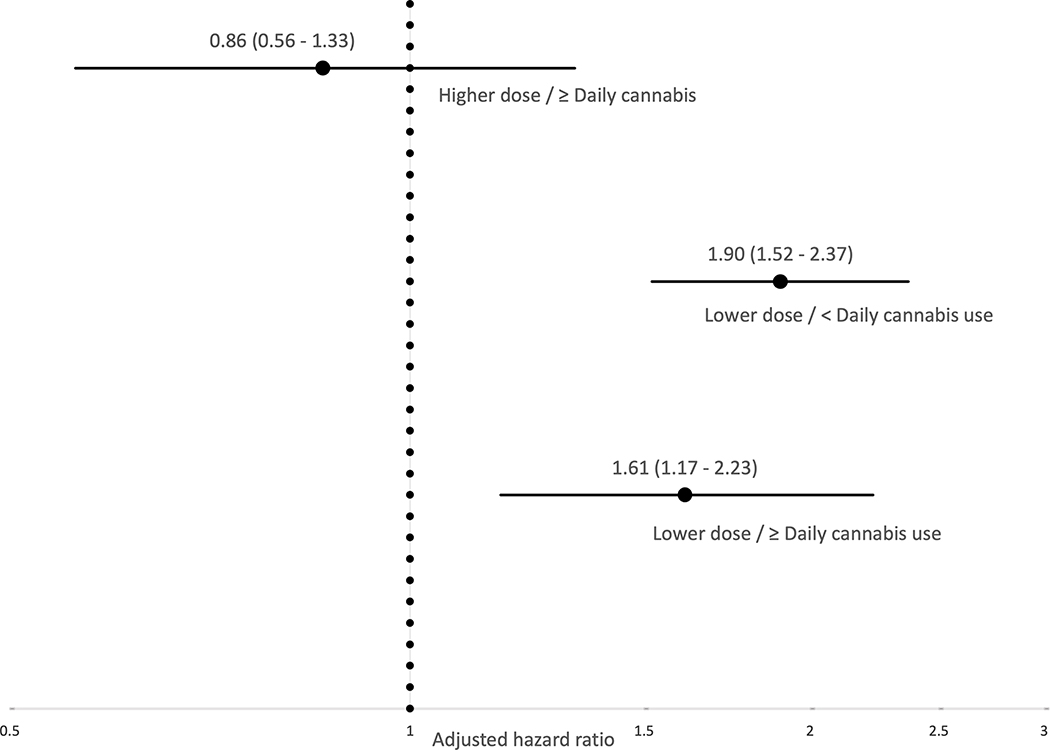
A further 611 (73.7%) participants remained in MMT for >six months and were included in the longer-term retention analysis (Figure 1). Table 3 shows the results of the bivariable and multivariable Cox frailty models. Before considering a potential interaction with cannabis, at the bivariable-level, lower MMT dose was significantly associated with treatment discontinuation (Hazard Ratio [HR] = 2.05, 95% CI: 1.66 – 2.53, p<0.001), while high-frequency cannabis use was not significantly associated with discontinuation (HR = 0.91, 95% CI: 0.71 – 1.18, p=0.496). The estimate for lower MMT dose was similar between cannabis frequency strata (HR = 2.10, 95% CI: 1.66 – 2.65, p<0.001 during no/low-frequency cannabis use; HR = 1.85, 95% CI: 1.13 – 3.03, p=0.014 during high-frequency cannabis use), consistent with a lack of effect measure modification, as confirmed through the interaction term (p=0.650). This finding held after accounting for hypothesized confounders (interaction p=0.692). The adjusted relative hazard of treatment discontinuation for each combination of cannabis and dose (reference: <daily cannabis, high dose) is depicted in Figure 2.
Table 3.
Bivariable and multivariable associations between all independent variables and time-to-discontinuation after six months among 611 PWUD on MMT between December 1, 2005 and November 30, 2018
| Variable | Time-to-MMT discontinuation |
|||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | Adjusted Hazard Ratio (95% CI) | p-value | |
|
| ||||
| Treatment dose1 (primary independent variable), pooled estimate | ||||
|
| ||||
| MMT dose | -- | -- | ||
| (<90 mg/d vs. ≥90 mg/d) | 2.05 (1.66 – 2.53) | <0.001 | ||
|
| ||||
| Cannabis use2 (hypothesized effect measure modifier), pooled estimate | ||||
|
| ||||
| Daily cannabis use | -- | -- | ||
| (Yes vs. no) | 0.91 (0.71 – 1.18) | 0.496 | ||
|
| ||||
| Treatment dose estimate 2 , stratified by cannabis use 2,3 | ||||
|
| ||||
| (Daily cannabis use = no): MMT dose | ||||
| (<90 mg/d vs. ≥90 mg/d) | 2.10 (1.66 – 2.65) | <0.001 | 1.90 (1.52 – 2.37) | <0.001 |
| (Daily cannabis use = yes): MMT dose | ||||
| (<90 mg/d vs. ≥90 mg/d) | 1.85 (1.13 – 3.03) | 0.014 | 1.87 (1.16 – 3.01) | 0.010 |
|
| ||||
| Socio-demographic factors | ||||
|
| ||||
| Sex | ||||
| (Male vs. female) | 1.14 (0.92 – 1.41) | 0.220 | 1.22 (1.00 – 1.49) | 0.055 |
| Age | ||||
| (Per year increase) | 0.98 (0.97 – 1.00) | 0.007 | 0.99 (0.98 – 1.00) | 0.079 |
| Racial identity | ||||
| (White vs. non-white) | 0.74 (0.60 – 0.91) | 0.004 | 0.77 (0.64 – 0.94) | 0.010 |
| Employed 2 | ||||
| (Yes vs. no) | 1.05 (0.82 – 1.33) | 0.702 | 1.02 (0.80 – 1.29) | 0.897 |
| Homeless 2 | ||||
| (Yes vs. no) | 1.83 (1.44 – 2.32) | <0.001 | 1.44 (1.13 – 1.83) | 0.003 |
| Incarcerated 2 | ||||
| (Yes vs. no) | 2.07 (1.49 – 2.89) | <0.001 | 1.54 (1.11 – 2.14) | 0.011 |
|
| ||||
| Substance use and health factors | ||||
|
| ||||
| Daily alcohol use 2 | ||||
| (Yes vs. no) | 1.21 (0.85 –1.73) | 0.283 | 1.11 (0.80 – 1.55) | 0.535 |
| Daily stimulant use 2 | ||||
| (Yes vs. no) | 1.23 (1.00 –1.52) | 0.050 | 1.20 (0.98 – 1.47) | 0.083 |
| Daily opioid use 2,4 | ||||
| (Yes vs. no) | 2.48 (2.03 – 3.03) | <0.001 | -- | -- |
| HIV status | ||||
| (Positive vs. negative) | 0.87 (0.70 – 1.09) | 0.218 | 0.94 (0.77 – 1.15) | 0.560 |
|
| ||||
| Treatment-related factors | ||||
|
| ||||
| Calendar year | ||||
| (≥2014 vs. <2014) | 1.25 (1.02 – 1.52) | 0.029 | 1.27 (1.03 – 1.57) | 0.027 |
| Other addiction treatment 2 | ||||
| (Yes vs. no) | 1.39 (1.09 – 1.76) | 0.007 | 1.26 (1.00 – 1.59) | 0.052 |
| Treatment episode number | ||||
| (2 vs. 1) | 1.34 (1.08 – 1.65) | 0.008 | 1.28 (1.03 – 1.60) | 0.030 |
| (≥3 vs. 1) | 1.50 (1.13 – 1.98) | 0.005 | 1.30 (0.96 – 1.76) | 0.090 |
| MMT at study recruitment | ||||
| (Yes vs. no) | 0.77 (0.61 – 0.96) | 0.018 | 0.84 (0.68 – 1.03) | 0.085 |
Note:
Daily MMT dose was reported at the time of the interview
Refers to the six-month period preceding interview
Interaction term p-value (unadjusted model) = 0.650; Interaction term p-value (adjusted model) = 0.962
Opioid use is conceptualized as an intermediate factor in the relationship between low MMT dose and treatment discontinuation
95% CI = 95% Confidence interval
Figure 2.
Adjusted hazard of treatment discontinuation within strata of treatment dose and cannabis use (relative to higher dose / < daily cannabis use) among 611 MMT initiates in Vancouver, Canada, December 2005 - December 2018
Note: Estimates are adjusted for sex, age, racial identity, employment, homelessness, incarceration, daily alcohol use, daily stimulant use, HIV serostatus, calendar year of treatment, treatment episode, and enrolment in other addiction treatment; Adjusted hazard ratios are shown on the log scale.
In the adjusted Cox frailty model, additional significant associations with treatment discontinuation were observed for non-white racial identity, homelessness, incarceration, later (≥2014) year of treatment, and second treatment attempt during the study (all p<0.05; Table 3).
Discussion
Building off our previous observational research showing a possible role of high-frequency cannabis use in reducing the estimated association between lower methadone treatment dose and high-frequency unregulated opioid use, we sought to investigate whether a similar pattern could be observed for methadone treatment retention.
First, we found that the odds of retention within six month of treatment initiation were not significantly different during periods of high-frequency cannabis use. This finding for short-term retention mimics those reported previously in other settings.33–35 Of note, however, this finding contrasts one previously observed among the current study population, whereby high-frequency (but not occasional) cannabis use was associated with increased odds of retention in opioid agonist treatment (MMT or buprenorphine-naloxone) six months later.36 As the authors confirmed a similar finding after restricting their sample to MMT patients only,37 the difference in findings may have resulted from the current study’s examination of six-month discontinuation only at the first study observation after treatment initiation.
Following this short-term retention analysis, using Cox frailty models testing for interaction between dose and cannabis frequency on treatment discontinuation, we found that the lower dose-associated risk of treatment discontinuation was elevated to a similar level regardless of concurrent cannabis use. This is despite evidence from our previous study that the relationship between lower methadone dose and high-frequency use of illicit opioids was relatively lower during periods of high-frequency cannabis use,25 as well as evidence from the current study that high-frequency unregulated opioid use is a strong risk factor for treatment discontinuation (see Tables 2 and 3).
The secondary findings of our study offer insight into possible reasons why our earlier finding for unregulated opioid use did not translate to increased retention. Consistent with an established body of research,9,38 we observed several indicators of social and structural marginalization to be strongly associated with treatment discontinuation, including non-white racial identity, homelessness, and incarceration It is possible that, even if our previous finding of an interaction between cannabis and dose on unregulated opioid use reflected an underlying therapeutic effect of cannabis on managing immediate treatment needs associated with sub-therapeutic dosing, any individual-level symptom mitigation may be negligible against the structural barriers within the broader social, physical, political, and economic environments that help shape treatment access, adherence, and retention for this population.39 Given the observational nature of these studies, it is again worth acknowledging the possibility that our previous finding was better explained by some latent factor related to treatment dose and opioid use that differed by cannabis use status (e.g., different pre-treatment opioid use intensity). Considering the current finding, however, this latent factor would need to be unrelated to treatment retention.
While our data clearly shows that long-term retention is more likely at higher treatment doses, for many patients, adjustments to treatment dose should be reviewed as part of an individualized and integrated treatment approach—one that examines and addresses other aspects of the patient’s day-to-day life that are currently obstructing their ability to retain in treatment, such as access to adequate housing or involvement in the criminal justice system. Insofar as cannabis use in this setting goes, our findings lead us to conclude that cannabis use should not be taken as a signal of treatment “non-noncompliance” or a marker of eventual treatment discontinuation.
The ability to exploit up to 13 years’ worth of multiple MMT episodes per participant from over 800 PWUD in a community setting with widespread low-barrier access to MMT is a major strength of this research. However, the observational nature of this study presents some limitations that should be considered when interpreting these findings. First, despite a diverse strategy for community recruitment, the cohorts cannot be generalized to all PWUD in Vancouver or elsewhere. Second, the six-month data collection structure prevented the ability to record important details regarding possible changes to methadone dose and exact timing of enrolment/discontinuation within each six-month period. Past six-month independent variable measures may relate to behaviours or exposures that occurred or persisted after the estimated treatment discontinuation when participants endorsed past six-month but not current MMT enrolment (5.7% of study observations). Aside from HIV serostatus, all information is obtained via self-report; although self-report of MMT dose, substance use, and associated risk behaviours among PWUD are generally valid and reliable.40,41 Finally, the study questionnaire did not elicit information about certain details of cannabis use, including cannabinoid composition, potency, modes of administration, quantity used per occasion, that could better illuminate the findings.
Conclusions
In the first study to investigate the role of cannabis as a hypothesized effect measure modifier between lower methadone dose and treatment discontinuation, findings suggest that high-frequency cannabis use is not significantly associated with reduced risk of treatment discontinuation at lower methadone doses. While our study suggests that cannabis-based interventions on their own may not provide long-term benefit to patients at a high risk of MMT discontinuation, we did not uncover any evidence to indicate that cannabis use poses an additional harm to patients on MMT. For clinicians and opioid treatment service providers, these findings, which reflect time-updated and high-intensity cannabis use patterns in patients with severe OUD, highlight the importance of repealing policies that penalize patients for using cannabis during treatment.
Acknowledgments
We would like to thank the study participants for their invaluable contributions to this research as well as past and present study interviewers, nurses, and administrative staff. This study was funded by the US National Institutes of Health (ACCESS: U01-DA021525; VIDUS: U01-DA038886). S.L. was supported through doctoral awards from the Canadian Institutes of Health Research (CIHR) and the Pierre Elliott Trudeau Foundation for this work. Outside of this work, Z.D.C. reports grants from National Center for Complementary and Integrative Health, Center for Medical Cannabis Research, and the California Bureau of Cannabis Control. M.E.S. is supported by a Michael Smith Foundation for Health Research (MSFHR)/St. Paul’s Hospital Foundation Scholar Award. K.H. is supported by a CIHR New Investigator Award (MSH-141971), a MSFHR Scholar Award, and the St. Paul’s Foundation. N.F. is supported by a MSFHR/St. Paul’s Hospital Foundation Scholar Award and the Philip Owen Professorship in Addiction Medicine. M.-J.M. is supported by the United States National Institutes of Health (U01-DA0251525).
Funding sources:
This study was funded by the US National Institutes of Health (ACCESS: U01-DA021525; VIDUS: U01-DA038886). Study funders had no role in the study design, data collection or analysis, interpretation of the findings, or manuscript writing/publishing.
Footnotes
Conflicts of interest: M.-J.M. is the Canopy Growth professor of cannabis science at the University of British Columbia (UBC), a position created from unstructured arms’ length gifts to the university from Canopy Growth Corporation, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. UBC has also received unstructured funding from NG Biomed, Ltd., an applicant to the Canadian federal government for a license to produce cannabis, to support his research. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. M.-J.M. and M.E.S. were awarded funds by the Canadian Institutes of Health Research to study the safety and feasibility of co-dispensing tetrahydrocannabinol with methadone; Tilray will provide the study drug. Over the past 12 months, Z.D.C. reports receiving honoraria from Canopy Growth Corporation. All other authors declare no conflicts of interest.
References
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013–2017. Morb Mortal Wkly Rep. 2018;67(51/52):1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krawczyk N, Mojtabai R, Stuart EA, et al. Opioid agonist treatment and fatal overdose risk in a state-wide US population receiving opioid use disorder services. Addiction. 2020;doi: 10.1111/add.14991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:Cd002209. doi: 10.1002/14651858.CD002209.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosyk B, MacNab YC, Sun H, et al. Proportional hazards frailty models for recurrent methadone maintenance treatment. Am J Epidemiol. 2009;170(6):783–92. doi: 10.1093/aje/kwp186 [DOI] [PubMed] [Google Scholar]
- 7.Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013;132(1–2):378–82. doi: 10.1016/j.drugalcdep.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peles E, Schreiber S, Sason A, Adelson M. Similarities and changes between 15- and 24-year survival and retention rates of patients in a large medical-affiliated methadone maintenance treatment (MMT) center. Drug Alcohol Depend. 2018;185:112–119. doi: 10.1016/j.drugalcdep.2017.11.034 [DOI] [PubMed] [Google Scholar]
- 9.Lo A, Kerr T, Hayashi K, et al. Factors associated with methadone maintenance therapy discontinuation among people who inject drugs. J Subst Abuse Treat. 2018;94:41–46. doi: 10.1016/j.jsat.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proctor SL, Copeland AL, Kopak AM, Hoffmann NG, Herschman PL, Polukhina N. Outcome predictors for patients receiving methadone maintenance treatment: Findings from a retrospective multi-site study. J Subst Use. 2016;21(6):601–613. [Google Scholar]
- 11.Swensen G, Ilett KF, Dusci LJ, et al. Patterns of drug use by participants in the Western Australian methadone program, 1984–1991. Med J Australia. 1993;159(6):373–376. [DOI] [PubMed] [Google Scholar]
- 12.Hser Y-I, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Aunno T, Pollack HA, Frimpong JA, Wutchiett D. Evidence-based treatment for opioid disorders: a 23-year national study of methadone dose levels. J Subst Abuse Treat. 2014;47(4):245–50. doi: 10.1016/j.jsat.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake S, St. Pierre M The relationship between cannabis use and patient outcomes in medication-based treatment of opioid use disorder: A systematic review. Clin Psychol Rev. 2020;82:101939. doi: 10.1016/j.cpr.2020.101939 [DOI] [PubMed] [Google Scholar]
- 15.Bergeria CL, Huhn AS, Dunn KE. The impact of naturalistic cannabis use on self-reported opioid withdrawal. J Subst Abuse Treat. 2020;113:108005. doi: 10.1016/j.jsat.2020.108005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grinspoon L Marihuana Reconsidered. Cambridge, MA: Harvard University Press; 1971. [Google Scholar]
- 17.Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–54. doi: 10.1016/j.neuroscience.2013.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiese B, Wilson-Poe AR. Emerging Evidence for Cannabis’ Role in Opioid Use Disorder. Cannabis Cannabinoid Res. 2018;3(1):179–189. doi: 10.1089/can.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisaga A, Sullivan MA, Glass A, et al. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015;154:38–45. doi: 10.1016/j.drugalcdep.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry. 2019;176(11):911–22. doi: 10.1176/appi.ajp.2019.18101191 [DOI] [PubMed] [Google Scholar]
- 21.McElrath K Medication-assisted treatment for opioid addiction in the United States: Critique and commentary. Subst Use Misuse. 2018;53(2):334–343. doi: 10.1080/10826084.2017.1342662 [DOI] [PubMed] [Google Scholar]
- 22.Frank D “That’s no longer tolerated”: Policing patients’ use of non-opioid substances in methadone maintenance treatment. J Psychoactive Drugs. 2021;53(1):10–17. doi: 10.1080/02791072.2020.1824046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.New York State Department of Health announces opioid replacement now a qualifying condition for medical marijuana. New York State. July 12, 2020. https://www.health.ny.gov/press/releases/2018/2018-07-12_opioid_replacement.htm [Google Scholar]
- 24.New Jersey to allow medical marijuana for opioid addiction treatment. Practical Pain Management. December 1, 2020. https://www.practicalpainmanagement.com/treatments/pharmacological/new-jersey-allow-medical-marijuana-opioid-addiction-treatment
- 25.Lake S, Kerr T, Buxton J, et al. The cannabis-dependent relationship between methadone treatment dose and illicit opioid use in a community-based cohort of people who use drugs. Cannabis Cannabinoid Res. 2021;Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappalainen L, Nolan S, Dobrer S, et al. Dose-response relationship between methadone dose and adherence to antiretroviral therapy among HIV-positive people who use illicit opioids. Addiction. 2015;110(8):1330–9. doi: 10.1111/add.12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donny EC, Walsh SL, Bigelow GE, Eissenberg T, Stitzer ML. High-dose methadone produces superior opioid blockade and comparable withdrawal suppression to lower doses in opioid-dependent humans. Psychopharmacology (Berl). 2002;161(2):202–12. doi: 10.1007/s00213-002-1027-0 [DOI] [PubMed] [Google Scholar]
- 28.Milloy MJ, Kerr T, Bangsberg DR, et al. Homelessness as a structural barrier to effective antiretroviral therapy among HIV-seropositive illicit drug users in a Canadian setting. AIDS Patient Care STDs. 2012;26(1):60–7. doi: 10.1089/apc.2011.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullins G (Host). (2019, February 27) Change Intolerance (No 2) [Audio podcast episode]. In Crackdown. https://crackdownpod.com/podcast/episode-2-change-intolerance/ [Google Scholar]
- 30.Socías ME, Wood E, McNeil R, et al. Unintended impacts of regulatory changes to British Columbia Methadone Maintenance Program on addiction and HIV-related outcomes: An interrupted time series analysis. Int J Drug Policy. 2017;45:1–8. doi: 10.1016/j.drugpo.2017.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer AM, Hu S, Amlani A, Moreheart S, Sampson O, Buxton JA. Patient perspectives of methadone formulation change in British Columbia, Canada: outcomes of a provincial survey. Subst Abuse Treat Prev Policy. 2016;11:3. doi: 10.1186/s13011-016-0048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cousins G, Boland F, Barry J, et al. J-shaped relationship between supervised methadone consumption and retention in methadone maintenance treatment (MMT) in primary care: National cohort study. Drug Alcohol Depend. 2017;173:126–131. doi: 10.1016/j.drugalcdep.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 33.Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98(3):269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scavone JL, Sterling RC, Weinstein SP, Van Bockstaele EJ. Impact of cannabis use during stabilization on methadone maintenance treatment. Am J Addict. 2013;22(4):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weizman T, Gelkopf M, Melamed Y, Adelson M, Bleich A. Cannabis abuse is not a risk factor for treatment outcome in methadone maintenance treatment: A 1-year prospective study in an Israeli clinic. Aust N Z J Psychiatry. 2004;38(1–2):42–46. [DOI] [PubMed] [Google Scholar]
- 36.Socías ME, Wood E, Lake S, et al. High-intensity cannabis use is associated with retention in opioid agonist treatment: A longitudinal analysis. Addiction. 2018;113(12):2250–58. doi: 10.1111/add.14398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Socias ME, Nosova E, Lake S, Hayashi K, Kerr T, Milloy M-J. A call for experimental research on the risks and benefits of cannabis in the context of treatment for opioid use disorder [Letter to the Editor]. CMAJ Open. July 16, 2020. http://cmajopen.ca/content/7/4/E665.short/reply#cmajo_el_1922 [Google Scholar]
- 38.Lundgren LM, Sullivan LM, Maina AW, Schilling RF. Client factors associated with length of stay in methadone treatment among heroin users who inject drugs: quantitative analysis of state-level substance abuse treatment utilization data. J Addict Med. 2007;1(1):26–32. doi: 10.1097/ADM.0b013e318044e8fe [DOI] [PubMed] [Google Scholar]
- 39.Rhodes T Risk environments and drug harms: A social science for harm reduction approach. Int J Drug Policy. 2009;20(3):193–201. doi: 10.1016/j.drugpo.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 40.Darke S Self-report among injecting drug users: A review. Drug Alcohol Depend. 1998;51(3):253–263. doi: 10.1016/S0376-8716(98)00028-3 [DOI] [PubMed] [Google Scholar]
- 41.Langendam MW, van Haastrecht HJ, van Ameijden EJ. The validity of drug users’ self-reports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int J Epidemiol. 1999;28(3):514–20. doi: 10.1093/ije/28.3.514 [DOI] [PubMed] [Google Scholar]