Abstract
Background.
Air pollution and noise exposures individually associate with major adverse cardiovascular events (MACE) via a mechanism involving arterial inflammation (ArtI); however, their combined impact on ArtI and MACE remains unknown. We tested whether dual (vs. one or neither) exposure associates with greater ArtI and MACE risk and whether MACE risk is mediated via ArtI.
Methods.
Individuals (N = 474) without active cancer or known cardiovascular disease with clinical 18F-FDG-PET/CT imaging were followed for 5 years for MACE. ArtI was measured. Average air pollution (particulate matter ≤ 2.5 μm, PM2.5) and transportation noise exposure were determined at individual residences. Higher exposures were defined as noise > 55 dBA (World Health Organization cutoff) and PM2.5 ≥ sample median.
Results.
At baseline, 46%, 46%, and 8% were exposed to high levels of neither, one, or both pollutants; 39 experienced MACE over a median 4.1 years. Exposure to an increasing number of pollutants associated with higher ArtI (standardized β [95% CI: .195 [.052, .339], P = .008) and MACE (HR [95% CI]: 2.897 [1.818–4.615], P < .001). In path analysis, ArtI partially mediated the relationship between pollutant exposures and MACE (P < .05).
Conclusion.
Air pollution and transportation noise exposures contribute incrementally to ArtI and MACE. The mechanism linking dual exposure to MACE involves ArtI. (J Nucl Cardiol 2022)
Keywords: Air pollution, transportation noise pollution, arterial inflammation, 18F-FDG-PET/CT
INTRODUCTION
There is growing evidence that environmental factors such as noise and air pollution are important in the development of chronic non-communicable diseases.1,2 Exposure to either pollutant independently increases cardiovascular disease (CVD) risk.3-6 Although significant progress has been made in clarifying the individual pathobiological mechanisms by which each of these pollutants contribute to CVD,7-13 it is not known if there are shared pathways.4,14-16 There are reasons to believe that there could be common mechanisms despite the distinct routes by which these pollutants enter the body and initiate pathologic changes. Air pollution enters through the lungs and triggers oxidative stress that leads to downstream leukopoietic tissues and systemic inflammation that result in endothelial dysfunction, pro-thrombotic activity, and atherosclerotic inflammation (ArtI).5,15,16 Exposure to unhealthy noise levels triggers stress-associated neural activity and promotes downstream systemic inflammation, endothelial dysfunction, and ArtI due to neurohormonal activation.4,13,14 Thus, several downstream processes (e.g., ArtI and systemic inflammation) are relevant to both types of pollution exposure that often coexist.4 Although the individual impacts of each pollutant on ArtI and major adverse cardiovascular events (MACE) have been shown,4,16 their joint contribution to ArtI and MACE has been challenging to disentangle.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging provides a well-validated imaging marker of ArtI.17 Importantly, whole body 18F-FDG-PET/CT imaging also allows simultaneous assessment of the metabolic activities of stress-associated neural tissues (e.g., amygdalar activity or AmygA) and leukopoietic tissues (e.g., bone marrow) to derive insights about the interplay between these organs.4,16,18 Since heightened ArtI results from exposure to either pollutant, ArtI may provide a useful measurement at the node of integration for the adverse cardiovascular effects of both pollutants.4,16-18
Accordingly, we evaluated a cohort of individuals who underwent clinically indicated 18F-FDG-PET/CT imaging to test whether combined chronic exposure to both higher levels of air and transportation noise pollution (vs. one or neither pollutant) independently associates with higher ArtI. Furthermore, we evaluated whether combined exposure associated with greater MACE risk after multivariable adjustments for potential confounders and whether ArtI was an important participant in the mechanism underlying this relationship.
METHODS
Study sample
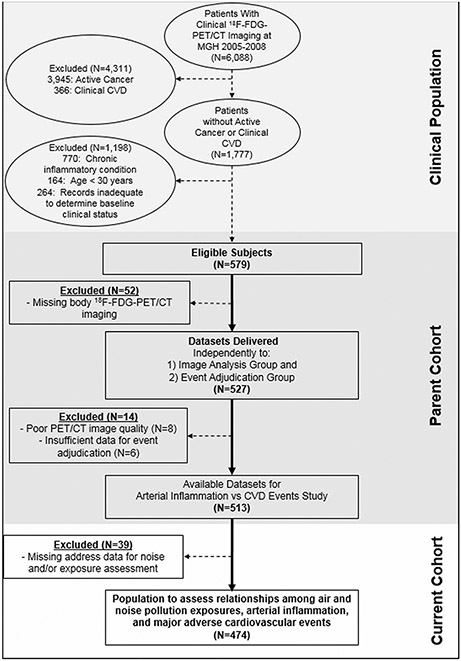
The retrospective study sample (N = 474) was derived from 6088 patients who underwent clinically indicated 18F-FDG-PET/CT imaging from 2005 to 2008, predominantly for cancer surveillance or screening, at Massachusetts General Hospital (Boston, MA, USA, Figure 1). The final cohort was identified from the sample of 1,777 patients without active cancer (no prior cancer or remission for ≥ one year before imaging and throughout follow-up) or known CVD (assessed by medical record review).18 Additional pre-defined inclusion criteria are provided (Supplement). All individuals provided 18F-FDG-PET/CT imaging that allowed assessment of ArtI. A subgroup (N = 265) provided brain images that allowed measurement of AmygA. A separate group (N = 424) provided imaging that allowed measurement of bone marrow activity. The Mass General Brigham Institutional Review Board approved the study protocol. Informed consent was not required.
Figure 1.
Study subject selection.
18F-FDG-PET/CT imaging protocol
18F-FDG-PET/CT was performed on an integrated scanner (e.g., Biograph 64, Siemens Healthcare, Erlangen, Germany). 18F-FDG was injected intravenously (after fasting overnight), and imaging was performed approximately 60 minutes thereafter. A low-dose, ungated CT was obtained for attenuation correction.
Assessment of tissue activities and coronary calcification
Analyses of 18F-FDG-PET/CT images were performed while blinded to all clinical data. To assess ArtI, the 18F-FDG signal in the ascending aorta was measured with CT guidance at 3 mm intervals from 1 cm above the aortic annulus to the aortic arch. Maximum tracer uptake intensity was measured in each slice as a standardized uptake value (SUV). The mean SUV for all slices was corrected for background blood activity from the superior vena cava, yielding a target-to-background ratio.18 Leukopoietic activity (as bone marrow activity) was measured using validated methods.18 AmygA was defined as the ratio of the mean bilateral amygdalar SUV corrected for mean temporal lobe SUV.18 Coronary artery calcification (CAC) was measured using the attenuation correction CT. Further description is provided (Supplement).
Assessment of air pollution and transportation noise exposure
Individual home addresses were derived from medical records. Annual air pollution exposure at each address was quantified as the mean concentration of particulate matter with a diameter ≤ 2.5 μm (PM2.5) for the year 2017 using the United States (US) Environmental Protection Agency Air Quality System Data Mart.15,19,20 Because air pollution exposure associates with cardiovascular outcomes at levels well below National Ambient Air Quality Standards of < 12 mg/m3, higher air pollution exposure was defined as exposure to PM2.5 concentrations that were ≥ median for the study sample (approximately 9 μg/m3).15,21,22 Average 24-hour transportation noise exposure was determined at each individual’s home using the US Department of Transportation’s Road and Aviation Noise Map, which provides a combined model of traffic and aircraft noise using 2014 data.23 Noise exposure was provided in 5 dBA increments, and high exposure was defined as >55 dBA, a threshold established by the World Health Organization (WHO) that associates with adverse health consequences.24 Additional details are provided (Supplement).
Assessment of clinical, socioeconomic, geographic, and demographic covariables
CVD risk factors (i.e., hypertension, hyperlipidemia, smoking, and diabetes), medical history, and healthcare access factors (i.e., baseline health insurance and in-state residence) were assessed from the medical records.4 Using zip code level data from the US Census Bureau’s 2015 American Community Survey 5-year estimates, median income was derived.25 Straight-line distance between individual home residences and the closest major roadway (i.e., a primary or secondary road according to the US Census Bureau designated 2016 Master Address File/Topologically Integrated Geographic Encoding and Referencing Feature Class Code) was assessed.26,27 Urban/rural status was evaluated using Rural-Urban Commuting Area Codes (RUCAs, version 2.0) and each subject’s zip code at the census tract level.28,29
Assessment of MACE
Two cardiologists, blinded to imaging and pollutant exposure data, reviewed clinical records to identify MACE within five years of index imaging.4,16,18 Qualifying events were cardiovascular death, unstable angina, myocardial infarction, cerebrovascular accident, heart failure, and coronary or peripheral artery revascularization.30,31 Further details are provided (Supplement).
Statistical analyses
Analyses were performed using SPSS (Version 26, IBM Corporation, Armonk, NY, USA). Continuous variables were presented as mean and standard deviation (SD) when normally distributed, or as median and interquartile range (IQR) when not. Correlations between continuous variables were evaluated using Pearson and Spearman coefficients. Independent sample t-tests were performed to evaluate differences in groups for continuous variables, and Chi-squared tests were used to assess differences in binary variables. Individuals were grouped by number of heightened pollutant exposures as: (1) neither, (2) one, or (3) both pollutants. They were also grouped by type of heightened pollutant exposures: (1) <median air pollution and ≤55 dBA, (2) ≥median air pollution and ≤55 dBA, (3) <median air pollution and >55 dBA, and (4) ≥median air pollution and >55 dBA. Relationships between the number of heightened pollutant exposures and tissue measurements were assessed with linear regression, as β and 95% confidence intervals (CIs). Cox proportional-hazard models and Kaplan–Meier survival were used to evaluate hazard ratios (HRs) for MACE and MACE-free survival, respectively, within five years of index imaging. Cox models were also performed for each separate pollutant using continuous measurements. Patients were censored by the first date of MACE, death, or last available follow-up within five years. We evaluated for multiplicative interactions between noise and air pollution exposure with ArtI and MACE. Covariables were selected a priori, and all multivariable models included age and sex. Additional models adjusted further for CVD risk factors (i.e., hypertension, hyperlipidemia, diabetes, current smoking), baseline CAC score, statin use, healthcare access factors (i.e., health insurance, in-state residence), malignancy and treatment history, median neighborhood income, and geographic area factors (i.e., distance from nearest roadway, urban/rural status) in other analyses. For survival models with more than two covariables, backwards selection was implemented. Mediation analysis was evaluated using the SPSS PROCESS Macro version 3.4 Model 4 to evaluate the prespecified path of greater number of pollutant exposures → higher ArtI → increased MACE risk using a logistic regression framework to approximate direct and indirect effects with 5000 bootstrap samples. For the indirect pathway, an exact P-value was not available; thus, a 95% CI that did not include zero was considered significant. For all other analyses, a two-sided P-value <.05 indicated significance. Additional details are provided (Supplement).
RESULTS
Baseline characteristics
Among 474 subjects, the median age was 55 (IQR 44-66) years and 42.8% were male. Totals of 218 (46%), 217 (46%), and 39 (8%) individuals were chronically exposed to increased levels of neither, one, or both pollutants, respectively. Those exposed to both pollutants (vs. one or neither) were less likely to have prior cancer (P = .008) or cancer treatment (P = .012); however, they were more likely to have hypertension (P = .004). Additional details are provided (Table 1).
Table 1.
Baseline characteristics.
| Variables | Overall (N = 474) | Exposure to one or neither pollutant (N = 435) |
Exposure to both pollutants (N = 39) |
P-value |
|---|---|---|---|---|
| Demographics | ||||
| Median age, years (IQR) | 55 (44-66) | 54 (44-66) | 59 (46-67) | .055 |
| Male sex | 203 (42.8%) | 189 (43.4%) | 14 (35.9%) | .361 |
| White race | 428 (90.3%) | 394 (90.6%) | 34 (87.2%) | .913 |
| Cardiovascular risk factors | ||||
| Current smoker | 50 (10.5%) | 47 (10.8%) | 3 (7.7%) | .536 |
| Hypertension | 166 (35.0%) | 144 (33.1%) | 22 (56.4%) | .004 |
| Diabetes mellitus | 40 (8.4%) | 35 (8.0%) | 5 (12.8%) | .307 |
| Hyperlipidemia | 134 (28.3%) | 123 (26.9%) | 14 (35.9%) | .274 |
| Mean total cholesterol (SD), mg/dL | 191.76 (42.53) | 193.27 (42.60) | 179.35 (40.63) | .115 |
| Mean LDL (SD), mg/dL | 111.11 (37.22) | 112.34 (37.61) | 101.27 (32.91) | .153 |
| Statin therapy | 95 (20.0%) | 87 (20.0%) | 8 (20.5%) | .944 |
| Median Framingham risk score (IQR) | 3.00 (1.00-8.00) | 3.00 (1.00-7.50) | 4.00 (1.75-12.00) | .685 |
| Median body mass index (IQR), kg/m2 | 26.43 (23.43-30.99) | 26.43 (23.41-30.90) | 26.06 (23.48-32.17) | .860 |
| Coronary artery calcium score | ||||
| 0-10 | 304 (64.1%) | 282 (64.8%) | 22 (56.4%) | .675 |
| 11-99 | 56 (11.8%) | 52 (12.0%) | 4 (10.3%) | |
| ≥100 | 55 (11.6%) | 50 (11.5%) | 5 (12.8%) | |
| Malignancy History | ||||
| Previous cancer | 399 (84.2%) | 372 (85.5%) | 27 (69.2%) | .008 |
| Previous chemotherapy/radiation | 358 (75.5%) | 335 (77.0%) | 23 (59.0%) | .012 |
| Socioeconomic and Environmental Factors | ||||
| Median average transportation noise | 35-40 (<35-50) | 35-40 (<35-50) | 55-60 (55-60) | <.001 |
| exposure, dBA (IQR) | ||||
| Transportation noise exposure >55 dBA (WHO cutoff) | 62 (13.1%) | 23 (5.3%) | 39 (100%) | <.001 |
| Median mean air pollution, particulate matter ≤ 2.5 μm, μg/m3 (IQR) | 5.80 (4.60-7.60) | 5.30 (4.60-6.90) | 8.80 (6.90-8.80) | <.001 |
| Air pollution exposure ≥ population median | 233 (49.2%) | 194 (40.9%) | 39 (100%) | <.001 |
| Straight-line distance between residence and nearest primary or secondary roads (IQR), miles | .30 (.14, .63) | .29 (.14, .63) | .22 (.06, .31) | .264 |
| Urban status | 466 (98.3) | 427 (98.2) | 39 (100) | .500 |
| Median income (IQR), $ | 80,148 (61,775-100,301) | 80,866 (61,919-100,347) | 76,596 (50,865-94,518) | .521 |
| Healthcare Access factors | ||||
| Health insurance | 413 (87.1%) | 379 (87.1%) | 34 (87.2%) | .992 |
| In-state residence | 419 (88.4%) | 381 (87.6%) | 38 (97.4%) | .066 |
| Variables | No MACE (N = 435) |
MACE (N = 39) |
Cox P-value |
|---|---|---|---|
| Demographics | |||
| Median age, years (IQR) | 53 (44-64) | 68 (61-78) | <.001 |
| Male sex | 187 (43.0%) | 16 (41.0%) | .880 |
| White race | 393 (90.3%) | 35 (89.7%) | .924 |
| Cardiovascular risk factors | |||
| Current smoker | 39 (9.0%) | 11 (28.2%) | .001 |
| Hypertension | 142 (32.6%) | 24 (61.5%) | .001 |
| Diabetes mellitus | 32 (7.4%) | 8 (20.5%) | .011 |
| Hyperlipidemia | 118 (27.1%) | 16 (41.0%) | .070 |
| Mean total cholesterol (SD), mg/dL | 192.38 (43.35) | 188.25 (37.90) | .610 |
| Mean LDL (SD), mg/dL | 111.46 (37.85) | 109.19 (33.92) | .829 |
| Statin therapy | 79 (18.2%) | 16 (41%) | .001 |
| Median Framingham risk score (IQR) | 2.00 (1.00-7.00) | 8.50 (3.25-13.75) | <.001 |
| Median body mass index (IQR), kg/m2 | 26.34 (23.37-31.09 | 27.00 (24.65-31.06) | .463 |
| Coronary artery calcium score | |||
| 0-10 | 291 (66.9%) | 13 (33.3%) | .001 |
| 11-99 | 48 (11.0%) | 8 (20.5%) | |
| ≥100 | 47 (10.8%) | 8 (20.5%) | |
| Malignancy History | |||
| Previous cancer | 376 (86.4%) | 23 (59.0%) | <.001 |
| Previous chemotherapy/radiation | 340 (78.2%) | 18 (46.2%) | <.001 |
| Socioeconomic and Environmental Factors | |||
| Median average transportation noise exposure, dBA (IQR) | 35-40 (<35-50) | 50-55 (35-60) | <.001 |
| Transportation noise exposure >55 dBA (WHO cutoff) | 48 (11.0%) | 14 (35.9%) | <.001 |
| Median mean air pollution, particulate matter ≤ 2.5 μm, μg/m3 (IQR) | 5.23 (4.83-6.00) | 6.00 (4.88-7.10) | .001 |
| Air pollution exposure ≥ population median | 205 (47.1%) | 28 (71.7%) | .007 |
| Straight-line distance between residence and nearest primary or secondary roads (IQR), miles | .29 (.14, .63) | .21 (.07, .34) | .058 |
| Urban status | 427 (98.2) | 39 (100) | .500 |
| Median income (IQR), $ | 80,532 (63,026-101,467) | 76,596 (50,865-87,381) | .012 |
| Healthcare Access factors | |||
| Health insurance | 388 (89.2%) | 25 (64.1%) | <.001 |
| In-state residence | 382 (87.8%) | 37 (94.8%) | .215 |
Data are N (%) unless specified. A total of 239 patients provided data for Framingham risk score.
Relationship between heightened pollutant exposures and baseline characteristics
Air pollution exposure (continuous, μg/m3) and transportation noise exposure (per 5 dBA increase) were significantly correlated (R = .344, P < .001, Supplemental Tables 1A and 1B). Higher air pollution exposure additionally associated with lower median income (R = −.143, P = .002). Further, individuals exposed to higher levels of air pollution were more likely to have health insurance (P = .015). Transportation noise exposure similarly correlated with neighborhood median income (R = −.129, P = .002). Higher noise exposure also associated with distance from the nearest roadway (R = −.273, P = .003), hypertension (P = .007), urban residence (P = .024), and in-state residence (P < .001)
Relationship between pollutant exposures and tissue activities
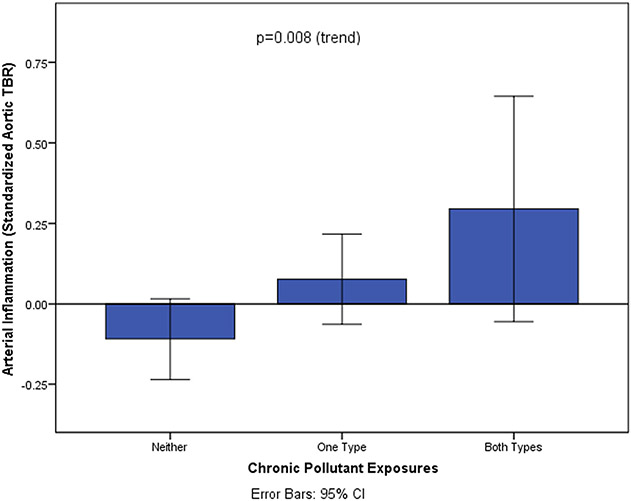
The number of pollutants to which individuals were exposed (i.e., 0 vs. 1 vs. 2) incrementally associated with ArtI in univariable (standardized β [95% CI] = .195 [.052, .339], p (trend) = .008) and multivariable models (Figures 2 and 3, Table 2). This relationship remained significant after multivariable adjustment (.247 [.103, .392], p (trend) = .001). There was no multiplicative interaction between air and noise pollution exposure with ArtI (P = .540). Further, the number of pollutant exposures did not significantly associate with increased amygdalar (.177 [−.016, .370], p (trend)=.072) or bone marrow (.143 [−.011, .298], p (trend)=.068) activities (Supplemental Figures 1A and 1B).
Figure 2.
Relationship between number of heightened pollutant exposures and arterial inflammation. Error bars represent 95% CI.
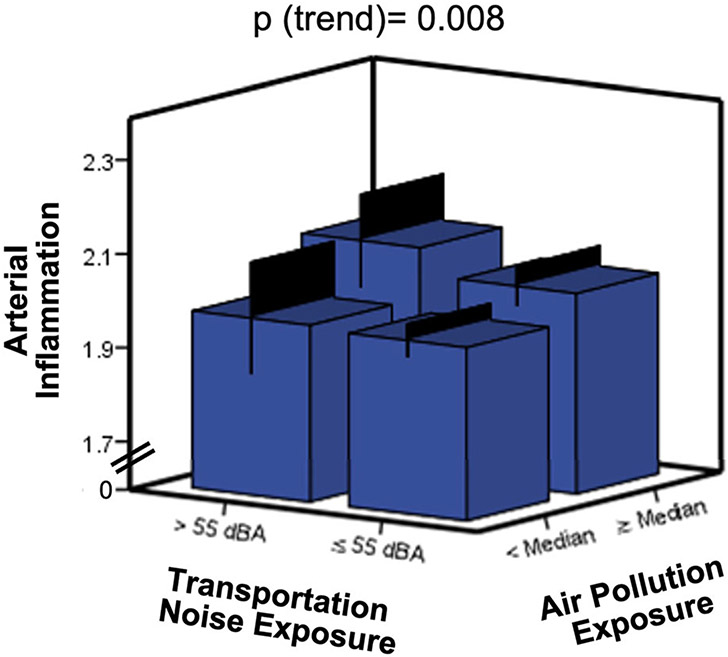
Figure 3.
Relationships between types of heightened pollutant exposures and arterial inflammation. Error bars represent 95% CI, and the unadjusted P-value for the relationship between the number of pollutant exposures and arterial inflammation is shown.
Table 2.
Relationships between the number of pollutant exposures (neither, one, and both) and arterial inflammation
| Covariables | Arterial inflammation |
|
|---|---|---|
| Standardized β [95% CI] | P-value for trend | |
| Univariable | .195 [.052, .339] | .008 |
| Age and sex | .191 [.053, .329] | .007 |
| CVD risk factors | .179 [.042, .316] | .011 |
| Malignancy and treatment | .191 [.053, .330] | .007 |
| Median neighborhood income | .187 [.050, .324] | .008 |
| Geographic area status | .260 [.114, .407] | .001 |
| Healthcare access factors | .191 [.052, .330] | .007 |
| Statin treatment | .186 [.048, .324] | .008 |
| Fully adjusted (all factors above) ^ | .247 [.103, .392] | .001 |
CVD risk factors = diabetes, current smoking, hypertension, and hyperlipidemia
Malignancy and treatment = history of cancer and chemotherapy/radiation
Geographic area status = straight-line distance between residence and the nearest primary or secondary roads and urban/rural status
Healthcare access = baseline health insurance and in-state residence
All multivariable models included the adjustments specified in each row addition to age and sex and are not serially additive
Risk for MACE by number of pollutant exposures
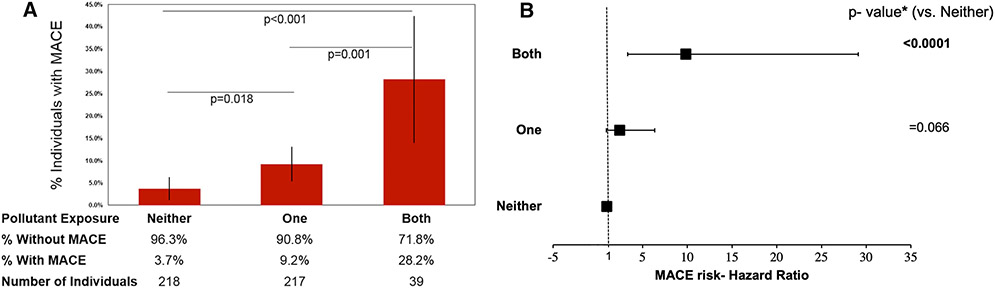
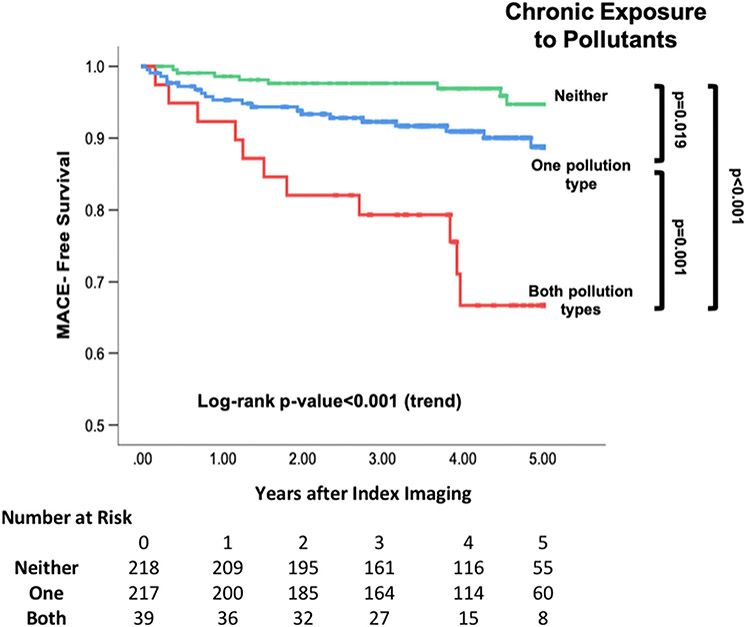
Thirty-nine subjects experienced MACE over a median 4.1 (IQR 3.0–5.0) years. Air pollution exposure as a continuous variable in μg/m3 (HR [95% CI] = 1.258 [1.053–1.502], P = .011) and transportation noise exposure (per 5 dBA) associated with MACE (1.358 [1.157–1.595], P < .001) in models adjusted for age and sex. Histograms showing MACE by number and type of pollutant exposures are shown (Figure 4, Supplemental Figure 2) in addition to a three-dimensional plot of MACE by type of pollutant exposure (Supplemental Figure 3). CVD risk factors and neighborhood income associated with MACE (Table 1). Exposure to a greater number of pollutants (i.e., 0 vs. 1 vs. 2) associated with greater MACE risk in univariable (2.897 [1.818–4.615], p (trend) < .001) and multivariable models (Table 3, Figures 4 and 5). Notably, individuals exposed to both pollutants had a >10-fold increased risk of MACE (relative to pollutant unexposed) in fully adjusted models (11.844 [3.154–44.475], P < .001). A similar relationship was seen when MACE risk was assessed by type of pollutant exposure (Supplemental Figure 2). Further, among subgroups with lower presumed CVD risk (Supplemental Table 2), the number of pollutants remained associated with MACE risk through multivariable adjustments. There was no multiplicative interaction between heightened air and noise pollution exposure in Cox regression with MACE (P = .738).
Figure 4.
(A) Histogram showing the distribution of MACE by number of pollutant exposures. Error bars represent 95% CI, and P-values are unadjusted. (B) Point estimates of hazard ratios are represented by black squares with 95% CI depicted by horizontal lines. * P-values are fully adjusted with backwards selection.
Table 3.
Risk for MACE by number of heightened pollutant exposures
| Covariables | Pollutant exposures |
|||||
|---|---|---|---|---|---|---|
| Air and noise vs. air or noise vs. neither |
Air and noise vs. air or noise |
Air and noise vs. neither |
||||
| HR [95% CI] |
P- value |
HR [95% CI] |
P- value |
HR [95% CI] |
P- value |
|
| Univariable | 2.897 [1.818-4.615] | <.001 | 3.189 [1.527-6.659] | .002 | 8.364 [3.362-20.811] | <.001 |
| Age and sex | 2.567 [1.604-4.106] | <.001 | 2.804 [1.333-5.900] | .007 | 6.630 [2.618-16.786] | <.001 |
| CVD risk factors^ | 2.580 [1.574-4.230] | <.001 | 3.413 [1.605-7.258] | .001 | 6.305 [2.493-15.946] | <.001 |
| Malignancy and treatment^ | 2.339 [1.468-3.728] | <.001 | 2.264 [1.064-4.819] | .034 | 6.513 [2.588-16.396] | <.001 |
| Median neighborhood income^ | 2.415 [1.516-3.846] | <.001 | 2.797 [1.337-5.853] | .006 | 6.513 [2.588-16.396] | <.001 |
| Geographic area factors^ | 3.082 [1.829-5.191] | <.001 | 2.903 [1.258-6.700] | .013 | 9.635 [3.340-27.800] | <.001 |
| Healthcare access factors^ | 2.385 [1.468-3.874] | <.001 | 2.577 [1.214-5.473] | .014 | 5.758 [2.212-14.986] | <.001 |
| Coronary artery calcium score^ | 2.336 [1.357-4.023] | .002 | 3.413 [1.413-8.244] | .006 | 6.979 [2.469-19.726] | <.001 |
| Statin treatment^ | 2.526 [1.580-4.037] | <.001 | 2.797 [1.337-5.853] | .006 | 6.379 [2.528-16.279] | <.001 |
| Fully adjusted (all factors above) ^ | 2.469 [1.289-4.729] | .015 | 4.493 [1.443-13.984] | .009 | 11.844 [3.154-44.475] | <.001 |
All multivariable models (aside from fully adjusted model) included the adjustments specified in each row addition to age and sex and are not serially additive
Backwards selection implemented
Figure 5.
Kaplan-Meier MACE-free survival plot by number of pollutant exposures. Log-rank P-values are provided.
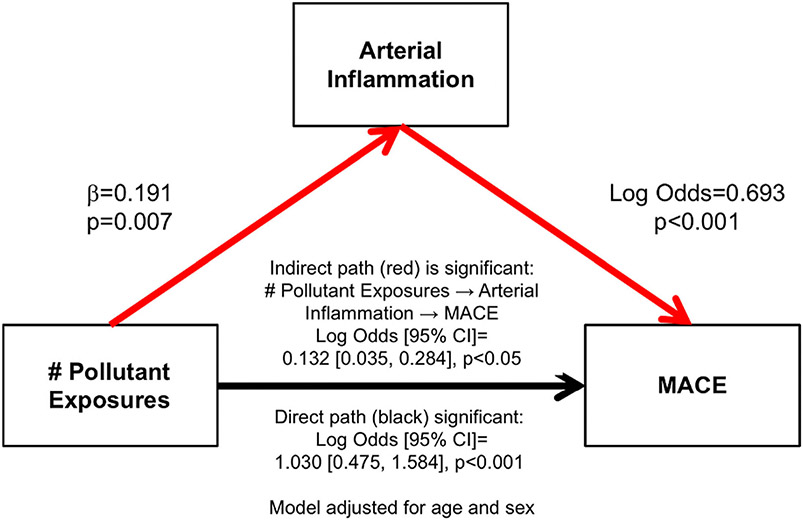
Role of heightened arterial inflammation in the link between pollutant exposures and MACE
To evaluate the role of ArtI in linking heightened pollutant exposures to MACE, the single mediator path of greater number of pollutant exposures to higher ArtI to increased MACE risk was assessed. This path was significant (indirect path: log odds [95% CI]: .132 [.035, .284], P < .05, adjusted for age and sex, Figure 6) and accounted for 11.4% of the total effect. Importantly, the direct path (i.e., the path linking pollutant exposures to MACE after removing the role of ArtI) also remained significant (1.030 [.475, 1.584], P < .001).
Figure 6.
Single mediator model adjusted for age and sex for the role of increased arterial inflammation in the relationship between the number of pollutant exposures and MACE.
DISCUSSION
Chronic air pollution and transportation noise exposure have each consistently been shown to independently associate with atherosclerotic CVD.32,33 Recent work has shown that each pollutant increases arterial inflammation and that increased arterial inflammation participates in the separate mechanisms linking each exposure to CVD events; however, data regarding the impact of combined pollutant exposure on arterial inflammation and adverse CVD events are limited.4,14,16,33,34 Furthermore, whether heightened arterial inflammation partially mediates the relationship between combined pollution exposure and adverse CVD events is unknown. Herein, we addressed these knowledge gaps. Through a retrospective 18F-FDG-PET/CT imaging study, we observed that combined exposure to both noise and air pollution associates with incrementally increased arterial inflammation and MACE risk after robust adjustments for potential confounders. Moreover, the relationship between number of pollutant exposures and MACE is in part mediated by increased arterial inflammation.
Mechanistic insights
Air and noise pollution both lead to pathophysiologic adaptations such as sympathetic and hypothalamic-pituitary-adrenal axis activation, inflammation, endothelial dysfunction, and altered biochemical profiles that eventually result in increased cardiovascular risk factors and CVD.13,14 Although many of these mechanisms are common between air and noise pollution, the distinct nature of these exposures has prompted the hypothesis that combined exposure to both pollutants may be synergistic, or at least additive. Given the frequency of chronic exposure to both pollutants, a greater understanding of the pathobiological consequences of the co-exposures is needed.35-37
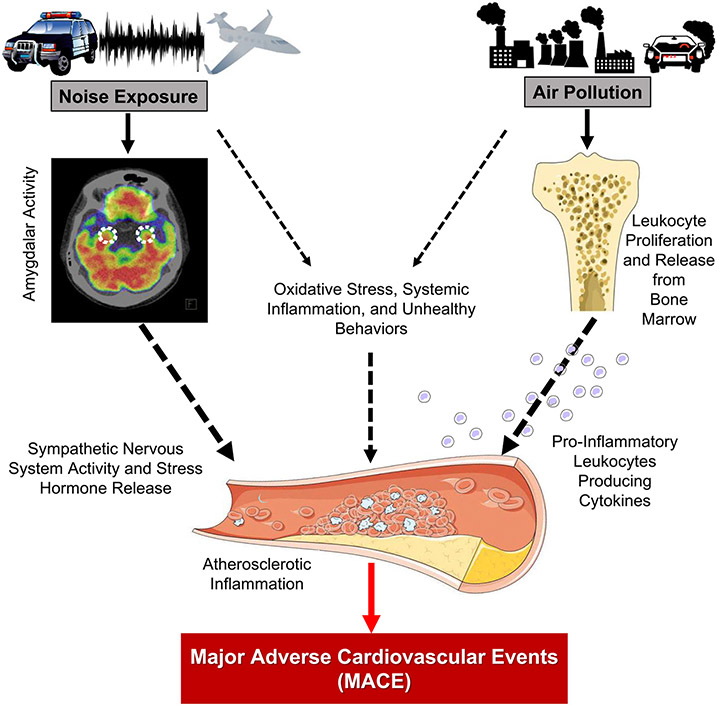
The entry portals through which these two pollutants initiate their respective pathologic cascades appear to be distinct. Air pollution enters the body through the lungs and subsequently increases bone marrow (i.e., leukopoietic) activity. This in turn augments systemic inflammation and oxidative stress and potentiates downstream CVD.5,15,16 Chronic noise exposure initiates a pathobiological cascade that begins with activation of brain centers involved in stress perception, such as the amygdala. This triggers a chronic stress reaction that increases vascular inflammation.4,38 Notably, ArtI is a potent marker for CVD risk that is a downstream consequence of both air pollution and noise exposure when studied separately (via their respective impacts on leukopoietic activity and stress-associated neural centers).4,16,17 The current study demonstrates that ArtI serves as a node of confluence for both pollutant exposures, in spite of their different routes for entering the body (Figure 7). Moreover, the study provides novel insights by demonstrating that the link between combined pollution exposure and heightened MACE risk is partially mediated via a mechanism that includes increased ArtI. Additional contributions to this mechanistic link likely include adverse health behaviors (e.g., diet, exercise), impaired vascular reactivity, oxidative stress, and greater risk for typical cardiovascular risk factors among others.14,15,37
Figure 7.
The hypothesized biological mechanism linking combined air and transportation noise pollution to major adverse cardiovascular events through heightened atherosclerotic inflammation. Black dotted lines refer to paths highlighted in previous studies, while the solid red line depicts the common path highlighted by the current study’s findings.
Heightened risk for MACE with combined pollutant exposure
While it is clearly demonstrated that air and noise pollution both increase CVD risk independently of one another, studies to date have yielded inconsistent results regarding whether combined exposure leads to a higher risk of CVD.3,5,6,10,39 Several studies have shown an increased risk for individual adverse CVD events (e.g., myocardial infarction, cerebrovascular accident, heart failure) with combined exposure to both air (as nitrogen dioxide) and noise pollution. However, others have failed to show such a relationship.7-9,11 Such conflicting findings may have resulted from inconsistent measurement of important confounders, including socioeconomic status, geographic status, and healthcare access. Additionally, not all prior studies have measured the component of air pollution most associated with CVD (i.e., PM2.5). The current study overcomes many of these relative limitations. Notably, the current results show that combined exposure to air (as PM2.5) and noise pollution substantially increases the risk for MACE (vs. 0 or 1 pollutant exposures) in a graded and incremental fashion, even after adjustment for confounders including CVD risk factors, prior cancer or cancer treatment, baseline atherosclerosis, healthcare access factors, and neighborhood income. As such, individuals with combined exposure to higher levels of environmental pollutants may merit additional attention to limit the impact of pollution-associated CVD.
Future directions
These findings underscore the need to attend to the increasing levels of environmental pollutants to limit their impact on health. In addition to efforts to reduce exposure on both population and individual scales, these results suggest the possibility of therapeutically targeting ArtI with drugs (e.g., statins) to biologically reduce the impact of combined pollutant exposure on CVD in those who cannot avoid it. These results support a hypothesized model linking increased pollution to MACE via arterial inflammation and underscore the need for an experimental study with controlled pollutant exposures and a prospective study with direct measurement of exposures and confounders to confirm these findings.
Limitations
The current study has several limitations. The sample was derived from a retrospective cohort of predominantly white individuals who underwent clinically indicated 18F-FDG-PET/CT imaging at a single academic medical center and, therefore, may not reflect the entire population exposed to pollutants. Air and noise pollution exposure were modeled using each individual’s home address. Exposure misclassification thus is a potential concern, since pollutant concentrations were not measured at other locations where exposure may have occurred (e.g., work) and may not reflect a comprehensive picture of individual exposures. The possibility that some individuals may have relocated during the follow-up period was not assessed. Further, relatively small numbers of individuals had exposure to heightened noise in isolation (N = 23) or to both pollutants in combination (N = 39), and there was a low number of adverse events (N = 39) in the study sample. Additionally, while we assessed exposure to PM2.5 (the principle component air pollution that is known to primarily contribute to CVD risk),16 other components of pollution that may also contribute to CVD were not evaluated. Air pollution data was evaluated from 2017, as this was the earliest year that provided the most inclusive data for our population, while noise pollution was evaluated from 2014, the only year data was available from the measurement tool for noise at the time of the study. As a result, temporal differences in pollutant profiles exist. Nevertheless, the relationship between air pollution and ArtI and MACE has previously been shown to be consistent across other date ranges of air pollution measurement.16 Further, health behavior data and additional serological measures of inflammation and oxidative stress were not available for this retrospective cohort. Lastly, despite the relationships observed, causality cannot be determined from this retrospective study. Despite these relative limitations, many of which would theoretically weaken the observed associations, we found substantial gradients between the number of pollution exposures and measured outcomes after multivariable adjustments.
CONCLUSIONS
Combined exposure to both air and transportation noise pollution incrementally increases arterial inflammation and CVD event risk compared to exposure to one or neither pollutant. Further, combined pollutant exposure may synergistically potentiate CVD via a biological pathway involving increased arterial inflammation. Prospective studies are required to confirm these relationships and determine the impact of therapies targeting atherosclerotic inflammation on pollution-associated CVD.
NEW KNOWLEDGE GAINED
Combined exposure to air and transportation noise pollution significantly increases ArtI and MACE risk compared to exposure to neither or one pollutant in isolation.
Increased MACE risk consequent to combined pollutant exposure is partially mediated by a pathway that involves heightened ArtI.
Our study identifies a potential pathologic link that could be targeted by therapies to attenuate pollution-associated CVD risk
Supplementary Material
Funding
This work is supported in part by the following US National Institutes of Health grants: #KL2TR002542 (MTO), #K23HL151909 (MTO), #P01HL131478 (ZAF and AT), #R01HL137913 (AT), #R01HL152957 (AT), #R01HL149516 (AT), #R56AR077187 (AT), #R33HL141047 (AT), #R01ES017290 (SR), and #R35ES031702 (SR).
Abbreviations
- MACE
Major adverse cardiovascular events
- ArtI
Atherosclerotic inflammation
- 18F-FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- AmygA
Amygdalar activity
- SUV
Standardized uptake value
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s12350-022-03003-7.
Disclosure
Michael T. Osborne, MD received consulting fees from WCG Intrinsic Imaging for unrelated work, and Ahmed Tawakol, MD received grants from Actelion and Genentech (grants to institution), Actelion and Esperion (minor consulting fees). The remaining authors have no relevant disclosures.
References
- 1.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fritschi L, Brown AL, Kim R, Schwela DH, Kephalopoulos SE. Burden of Disease from Environmental Noise: Quantification of healthy life years lost in Europe: World Health Organization, Regional Office for Europe, Bonn, and European Commission Joint Research Centre; 2011. [Google Scholar]
- 3.Gan WQ, Davies HW, Koehoorn M, Brauer M. Association of long-term exposure to community noise and traffic-related air pollution with coronary heart disease mortality. Am J Epidemiol 2012;175:898–906. [DOI] [PubMed] [Google Scholar]
- 4.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, et al. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur Heart J 2020;41:772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. [DOI] [PubMed] [Google Scholar]
- 6.Kempen EV, Casas M, Pershagen G, Foraster M. WHO environmental noise guidelines for the European region: A systematic review on environmental noise and cardiovascular and metabolic effects: A summary. Int J Environ Res Public Health. 2018;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roswall N, Raaschou-Nielsen O, Ketzel M, Gammelmark A, Overvad K, Olsen A, et al. Long-term residential road traffic noise and NO2 exposure in relation to risk of incident myocardial infarction: A Danish cohort study. Environ Res 2017;156:80–6. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen M, Luhdorf P, Ketzel M, Andersen ZJ, Tjonneland A, Overvad K, et al. Combined effects of road traffic noise and ambient air pollution in relation to risk for stroke? Environ Res 2014;133:49–55. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen M, Wendelboe Nielsen O, Sajadieh A, Ketzel M, Tjonneland A, Overvad K, et al. Long-term exposure to road traffic noise and nitrogen dioxide and risk of heart failure: A cohort study. Environ Health Perspect 2017;125:097021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetreault LF, Perron S, Smargiassi A. Cardiovascular health, traffic-related air pollution and noise: Are associations mutually confounded? A systematic review. Int J Public Health 2013;58:649–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang WT, Wang VS, Chang LT, Chuang KJ, Chuang HC, Liu CS, et al. Road traffic noise, air pollutants, and the prevalence of cardiovascular disease in Taichung. Taiwan. Int J Environ Res Public Health. 2018;15:1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen M, Pershagen G. Transportation noise linked to cardiovascular disease independent from air pollution. Eur Heart J 2019;40:604–6. [DOI] [PubMed] [Google Scholar]
- 13.Osborne MT, Naddaf N, Abohashem S, Radfar A, Ghoneem A, Dar T, et al. A neurobiological link between transportation noise exposure and metabolic disease in humans. Psychoneuroendocrinology 2021;131:105–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, et al. Environmental stressors and cardio-metabolic disease: Part II-mechanistic insights. Eur Heart J 2017;38:557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–70. [DOI] [PubMed] [Google Scholar]
- 16.Abohashem S, Osborne MT, Dar T, Naddaf N, Abbasi T, Ghoneem A, et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur Heart J 2021;42:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging 2013;6:1250–9. [DOI] [PubMed] [Google Scholar]
- 18.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, et al. Relation between resting amygdalar activity and cardiovascular events: A longitudinal and cohort study. Lancet 2017;389:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riggs DW, Zafar N, Krishnasamy S, Yeager R, Rai SN, Bhatnagar A, et al. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ Res 2020;180:108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Environmental Protection Agency, 2017. AQS Data Mart. https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html/ (accessed 15 December 2019). 2017.
- 21.Cai Y, Hodgson S, Blangiardo M, Gulliver J, Morley D, Fecht D, et al. Road traffic noise, air pollution and incident cardiovascular disease: A joint analysis of the HUNT, EPIC-Oxford and UK Biobank cohorts. Environ Int 2018;114:191–201. [DOI] [PubMed] [Google Scholar]
- 22.Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, et al. Risk of nonaccidental and cardiovascular mortality in relation to long-term exposure to low concentrations of fine particulate matter: A Canadian national-level cohort study. Environ Health Perspect 2012;120:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.United States Department of Transportation and Bureau of Transportation Statistics. National transportation noise map road and aviation noise in the United States. https://maps.bts.dot.gov/arcgis/apps/webappviewer/index.html?id=a303ff5924c9474790464cc0e9d5c9fb/ (accessed 15 December 2019). 2014.
- 24.European Environmental Agency. Noise in Europe 2014. EEA Report. 2014;10:1–68. [Google Scholar]
- 25.United States Census Bureau. 2015 American Community Survey’s 5-year estimates. https://factfinder.census.goc/faces/nav/jsf/pages/index.xhtml/ (accessed 15 December 2019). 2015.
- 26.Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multiethnic Study of Atherosclerosis (MESA). Environ Health Perspect 2008;116:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Census Bureau. MAF/TIGER Feature Class Code. https://www.census.gov/library/reference/code-lists/mt-feature-class-codes.html/ (accessed 1 May 2020). 2016.
- 28.Danaher BG, Hart LG, McKay HG, Severson HH. Measuring participant rurality in Web-based interventions. BMC Public Health 2007;7:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.University of Washington School of Medicine: Washington W, Alaska, Montana and Idaho. Rural Urban Commuting Area Codes (version 2.0). http://depts.washington.edu/uwmca/ruca-download.php/ (accessed 1 May 2020). 2006.
- 30.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, et al. ACCF/AHA focused update incorporated into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011;2011:e215–367. [DOI] [PubMed] [Google Scholar]
- 31.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–76. [DOI] [PubMed] [Google Scholar]
- 32.Al-Kindi S, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol 2020;17:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bevan GH, Al-Kindi S, Brook RD, Munzel T, Rajagopalan S. Ambient air pollution and atherosclerosis: Insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. 2020:ATVBAHA120315219. [DOI] [PubMed] [Google Scholar]
- 34.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook J, et al. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 2017;38:550–6. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen M, Hjortebjerg D, Eriksen KT, Ketzel M, Tjonneland A, Overvad K, et al. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: A cross-sectional study. Environ Int 2015;85:238–43. [DOI] [PubMed] [Google Scholar]
- 36.Fuks KB, Weinmayr G, Basagana X, Gruzieva O, Hampel R, Oftedal B, et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J 2017;38:983–90. [DOI] [PubMed] [Google Scholar]
- 37.Cai Y, Hansell AL, Blangiardo M, Burton PR, BioShaRe, de Hoogh K, et al. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and lifelines cohorts. Eur Heart J. 2017;38:2290–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, et al. Stress-associated neurobiological pathway linking socioeconomic disparities to cardiovascular disease. J Am Coll Cardiol 2019;73:3243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: A nationwide cohort study in Switzerland. Eur Heart J 2019;40:598–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.