Abstract
Aims/Introduction
Nucleotide‐binding oligomerization domain‐like receptor family pyrin domain containing 3 (NLRP3) inflammasomes produce IL‐18 upon being activated by various stimuli via the P2 receptors. Previously, we showed that serum and urine IL‐18 levels are positively associated with albuminuria in patients with type 2 diabetes, indicating the involvement of inflammasome activation in the pathogenesis of diabetic kidney disease (DKD). In the present study, we investigated whether the administration of suramin, a nonselective antagonist of the P2 receptors, protects diabetic KK.Cg‐A y /TaJcl (KK‐Ay) mice against DKD progression.
Materials and Methods
Suramin or saline was administered i.p. to KK‐Ay and C57BL/6J mice once every 2 weeks for a period of 8 weeks. Mouse mesangial cells (MMCs) were stimulated with ATP in the presence or absence of suramin.
Results
Suramin treatment significantly suppressed the increase in the urinary albumin‐to‐creatinine ratio, glomerular hypertrophy, mesangial matrix expansion, and glomerular fibrosis in KK‐Ay mice. Suramin also suppressed the upregulation of NLRP3 inflammasome‐related genes and proteins in the renal cortex of KK‐Ay mice. P2X4 and P2X7 receptors were significantly upregulated in the isolated glomeruli of KK‐Ay mice and mainly distributed in the glomerular mesangial cells of KK‐Ay mice. Although neither ATP nor suramin affected NLRP3 expression in MMCs, suramin inhibited ATP‐induced NLRP3 complex formation and the downstream expression of caspase‐1 and IL‐18 in MMCs.
Conclusions
These results suggest that the NLRP3 inflammasome is activated in a diabetic kidney and that inhibition of the NLRP3 inflammasome with suramin protects against the progression of early stage DKD.
Keywords: Diabetic kidney disease, Inflammasomes, Suramin
Suramin, a nonselective antagonist of the P2 receptors, suppresses NLRP3 inflammasome activation and exerts renoprotective effects in diabetic KK‐Ay mice. Suramin protects against DKD development via the inhibition of P2X receptors, and can be expected to be an important drug for the treatment of DKD.
INTRODUCTION
The prevalence of diabetic kidney disease (DKD) is rising rapidly owing to the increasing prevalence of diabetes worldwide 1 , 2 , 3 . Furthermore, DKD contributes to the development of cardiovascular diseases and has led to increased mortality rates 4 . Therefore, understanding the mechanisms underlying the pathogenesis of DKD and discovering new therapeutic targets will have important implications for the treatment of patients with diabetes 5 .
It is now well recognized that inflammatory processes play critical roles in the pathogenesis of DKD 5 , 6 , 7 , 8 , 9 . Accumulating data also suggest that inflammasomes, multiprotein complexes, play a role in the pathogenesis of renal inflammation 10 . Pattern recognition receptors, such as nucleotide‐binding oligomerization domain‐like receptors (NLRs), recognize danger‐associated molecular patterns (DAMPs) to stimulate inflammasome assembly 11 . Inflammasome assembly in turn results in caspase‐1 activation and the subsequent secretion of IL‐1β and IL‐18 into the extracellular space 11 . We previously showed that serum and urine IL‐18 levels are positively associated with albuminuria in patients with type 2 diabetes, indicating the involvement of inflammasome activation in the pathogenesis of DKD 12 . Among the different types of inflammasomes, the NLR family pyrin domain containing 3 (NLRP3) inflammasome has been associated with DKD 13 , 14 . NLRP3 knockout (KO) and caspase‐1 KO mice showed renoprotective effects against a diabetes‐induced increase in albuminuria and renal morphological changes 13 . Additionally, a locus‐ and gene‐based genome‐wide association study meta‐analysis has associated a single‐nucleotide polymorphism in the NLRP3 gene with DKD 15 .
Extracellular ATP can act as a DAMP to activate the NRLP3 inflammasome via P2 receptors (P2X and P2Y receptors) 16 . P2X receptors are ligand‐gated ion channels with seven known subtypes (P2X1–7) in mammals. On the other hand, P2Y receptors include eight subtypes (P2Y1, 2, 4, 6, 11–14) 17 . Using mass spectrometry imaging, we demonstrated that ATP is increased in the glomeruli of diabetic mice, which may be explained by the activation of the glycolytic pathway 18 , and indicates that inhibition of the P2 receptors could be a potential therapeutic approach for the treatment of DKD.
Suramin acts as a nonselective antagonist of P2X and P2Y receptors by inhibiting Na‐K‐ATPase at an intracellular site, conceivably by interfering with ATP binding 19 . Suramin has been used to treat African trypanosomiasis for almost a century 20 . A study reported that the injection of suramin (10 mg/kg) suppresses the progression of renal fibrosis and prevents the decline in creatinine clearance in db/db mice 21 . However, clinical trials in which very high doses (e.g., blood levels of 150–270 μmol/L) of suramin were used to treat patients with cancer have led to concerns about the potential toxicity of the drug 22 . Accordingly, we hypothesized that the lower dose suramin may be more beneficial as a potential therapy for DKD without adverse effects.
Here, we evaluated whether low concentrations of suramin could play a role in preventing the progression of DKD in KK‐Ay mice (a mouse model for type 2 diabetes and obesity) by suppressing the NLRP3 inflammasome.
MATERIALS AND METHODS
Animals
Male non‐diabetic C57BL/6J (BL6) and diabetic KK.Cg‐A y /TaJcl (KK‐Ay) mice (4 weeks old; CLEA Japan, Tokyo, Japan) were randomly assigned to one of the five groups (n = 12, respectively): (1) BL6 mice treated with saline (BL6); (2) BL6 mice treated with a moderate‐dose of suramin (BL6(S)); (3) KK‐Ay mice treated with saline (KK‐Ay); (4) KK‐Ay mice treated with a low‐dose of suramin (KK‐Ay(LS)); and (5) KK‐Ay mice treated with a moderate‐dose of suramin (KK‐Ay(S)). Low (0.01 mg/kg) and moderate (1 mg/kg) doses of suramin (Sigma‐Aldrich, St. Louis, MO, USA) were injected i.p., once every 2 weeks for a period of 8 weeks. All mice were killed at 12 weeks of age and their kidneys were harvested. For the detailed protocols, refer to Appendix S1.
Quantification of glomerular size and mesangial matrix area
Periodic acid‐Schiff (PAS)‐stained sections were analyzed after modifications to the procedure described previously 18 . For the detailed protocols, refer to Appendix S1.
Immunoperoxidase staining
Immunoperoxidase staining was performed following modification to a protocol described previously 9 , 23 . For the detailed protocol and antibodies, refer to Appendix S1.
Immunofluorescence staining
Immunofluorescence staining was performed using the protocol described previously 9 . For the detailed protocol and antibodies, refer to Appendix S1.
Double immunofluorescence staining
Nuclei were stained with DAPI and the sections were observed under a fluorescence microscope (BZ‐8000; Keyence, Osaka, Japan). For the detailed antibodies, refer to Appendix S1.
RNA extraction and quantitative real‐time RT‐PCR (qPCR)
Total RNA was extracted from each sample (renal cortex, glomeruli, and cells), and qPCR was performed using StepOnePlus (Thermo Fisher Scientific, Waltham, MA, USA). For the detailed protocol and primer sequences used for qPCR, refer to Appendix S1 and Table S1.
Immunoblotting
Proteins extracted from the renal cortex or cellular lysates were separated on 4–12% or 12% Bis‐Tris gels and transferred onto PVDF membranes. For the detailed protocol and antibodies, refer to Appendix S1.
Cell culture
Mouse mesangial cells (MMCs; SV40 MES 13; ATCC, Manassas, VA, USA) were cultured in DMEM (5.5 mmoL/L d‐glucose) supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin–streptomycin–glutamine, in a 5% CO2 incubator at 37°C.
The effect of ATP in MMCs
After reaching 50% confluence, the cell culture medium of MMCs was replaced with DMEM supplemented with 0.5% (vol/vol) FBS, and incubated for 24 h. The MMCs were then exposed to the following conditions: (1) vehicle alone (control), (2) 1 μM suramin (control (S)), (3) 5 mM ATP (ATP), (4) 5 mM ATP and 0.1 μM suramin (ATP(LS)), or (5) 5 mM ATP and 1 μM suramin (ATP(S)). The medium was changed every 12 h. After 24 h of incubation, the MMCs were collected and subjected to qPCR, western blotting, or blue native polyacrylamide gel electrophoresis (BN‐PAGE).
BN‐PAGE and two‐dimensional polyacrylamide gel electrophoresis (2D‐PAGE)
BN‐PAGE was performed using a NativePAGE Bis‐Tris Gel System (Thermo Fisher Scientific), according to the manufacturer's instructions. We also performed 2D‐PAGE (BN‐PAGE as the first dimension and SDS‐PAGE as the second dimension) using a 4–12% Bis‐Tris ZOOM Gel (Thermo Fisher Scientific). For the detailed protocol and antibodies, refer to Appendix S1.
siRNA transfection of MMCs
Transfection of MMCs with P2X4 and P2X7 siRNA (Horizon Discovery, Cambridge, UK) was performed using a Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific) according to the manufacturer's instructions. After the transfection, the MMCs were treated with 5 mM ATP and 1 μM suramin, or vehicle as described earlier. The MMCs were collected and subjected to qPCR and western blotting. For the detailed protocol, refer to Appendix S1.
Statistical analysis
All values are expressed as mean ± SEM. Differences between groups were examined for statistical significance using one‐way anova followed by Tukey's test. Statistical significance was set at P < 0.05.
RESULTS
Suramin suppresses albuminuria in KK‐Ay mice without affecting major metabolic parameters
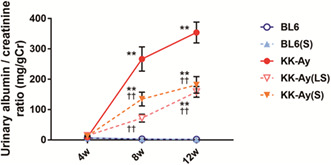
As shown in Table 1, body weight, blood glucose, HbA1c, serum creatinine, systolic blood pressure, and kidney weight (per tibial length) were significantly higher and creatinine clearance was significantly lower in KK‐Ay mice than in BL6 mice (12 weeks old); however, there was no difference between the KK‐Ay and suramin‐treated diabetic KK‐Ay mice. The urinary albumin‐to‐creatinine ratio (UACR) increased progressively in KK‐Ay mice during the study (Figure 1a). Notably, the administration of not only a moderate but also a low‐dose of suramin to KK‐Ay mice caused a significant decrease in the UACR (Figure 1a).
Table 1.
Metabolic parameters of C57BL/6J (BL6) and KK.Cg‐A y /TaJcl (KK‐Ay) mice at 4 and 12 weeks of age
| Variable | BL6 mice | KK‐Ay mice | |||
|---|---|---|---|---|---|
| BL6 | BL6(S) | KK‐Ay | KK‐Ay(LS) | KK‐Ay(S) | |
| At 4 weeks of age | |||||
| Body weight (g) | 15.7 ± 0.3 | 16.3 ± 0.3 | 18.6 ± 0.2** | 18.7 ± 0.4** | 17.9 ± 0.6** |
| Blood glucose (mg/dL) | 149.8 ± 4.7 | 145.7 ± 3.3 | 209.9 ± 15.4** | 224.9 ± 9.2** | 231.3 ± 9.7** |
| HbA1c (mmol/mol) | 13.0 ± 0.2 | 16.3 ± 0.5 | 13.3 ± 0.4 | 14.3 ± 0.6 | 13.2 ± 0.3 |
| HbA1c (%) | 3.3 ± 0.0 | 3.6 ± 0.0 | 3.4 ± 0.0 | 3.5 ± 0.1 | 3.4 ± 0.0 |
| Systolic blood pressure (mmHg) | 85.6 ± 1.0 | 81.8 ± 0.9 | 83.5 ± 2.1 | 89.7 ± 2.5 | 89.5 ± 1.1 |
| At 12 weeks of age | |||||
| Body weight (g) | 28.6 ± 0.5 | 27.8 ± 0.6 | 43.6 ± 0.6** | 45.5 ± 1.0** | 44.4 ± 0.9** |
| Blood glucose (mg/dL) | 187.6 ± 14.9 | 183.6 ± 17.5 | 569.3 ± 11.5** | 572.3 ± 8.2** | 532.3 ± 23.0** |
| HbA1c (mmol/mol) | 22.0 ± 1.4 | 24.9 ± 0.6 | 61.1 ± 1.7** | 54.6 ± 2.7** | 61.9 ± 2.0** |
| HbA1c (%) | 4.2 ± 0.1 | 4.4 ± 0.1 | 7.7 ± 0.2** | 7.2 ± 0.2** | 7.8 ± 0.2** |
| Serum creatinine (mg/dL) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0** | 0.4 ± 0.1* | 0.4 ± 0.0* |
| Creatinine clearance (mL/min/kg) | 7.3 ± 1.8 | 6.9 ± 1.8 | 2.2 ± 0.3* | 3.5 ± 0.6 | 2.7 ± 0.3* |
| Systolic blood pressure (mmHg) | 100.5 ± 1.4 | 97.9 ± 1.6 | 109.9 ± 1.5* | 108.7 ± 2.8 | 112.2 ± 2.5* |
| Kidney weight/tibia (mg/mm) | 8.9 ± 0.2 | 8.6 ± 0.4 | 18.0 ± 0.8** | 19.4 ± 0.8** | 17.2 ± 0.7** |
| Food intake (g/day) | 3.1 ± 0.1 | 2.8 ± 0.1 | 5.6 ± 0.3** | 6.0 ± 0.1** | 6.4 ± 0.3**,† |
Data are mean ± SEM. n = 6–12, systolic blood pressure was measured 5 times from each mouse. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05 vs KK‐Ay group.
BL6(S), BL6 mice treated with a moderate‐dose of suramin group; BL6, BL6 mice treated with saline group; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin group; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin group; KK‐Ay, KK‐Ay mice treated with saline group.
Figure 1.
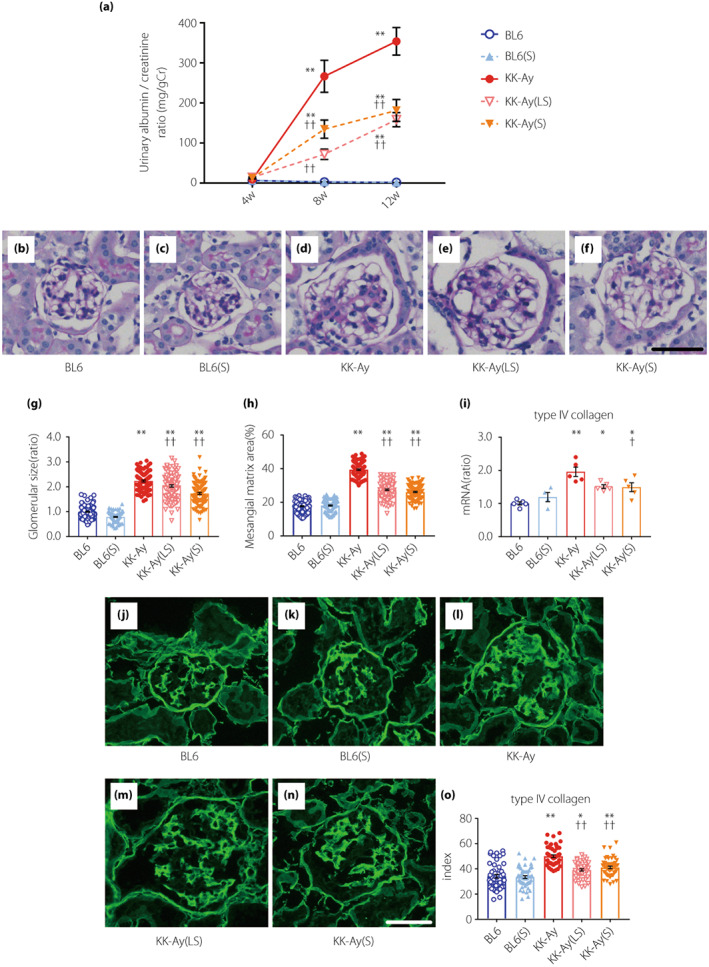
Suramin suppresses diabetes‐induced albuminuria, mesangial matrix expansion, and type IV collagen accumulation. (a) Time course analysis of urinary albumin‐to‐creatinine ratio (n = 12). (b–f) PAS staining of glomeruli of BL6 (b), BL6(S) (c), KK‐Ay (d), KK‐Ay(LS) (e), and KK‐Ay(S) (f) mice. (g, h) Quantification of the glomerular size (tuft area) (g) and mesangial matrix area (h), as calculated by the PAS‐positive area in the tuft area of 20 randomly selected glomeruli from each mouse (n = 5–6). Scale bar, 50 μm; original magnification, 200×. (i) Quantification of type IV collagen mRNA expression in the renal cortex by real‐time RT‐PCR (n = 4–5). (j–n) Histological expression of type IV collagen in the glomeruli of BL6 (j), BL6(S) (k), KK‐Ay (l), KK‐Ay(LS) (m), and KK‐Ay(S) (n) mice. (o) Type IV collagen expression was estimated using the fluorescence intensities of 10 randomly selected glomeruli from each mouse (n = 5). Scale bar, 50 μm; original magnification, 200×. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05, †† P < 0.001 vs KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline; PAS, Periodic acid‐Schiff.
Kidney morphology and inhibition of kidney fibrosis by suramin
The glomeruli in PAS‐stained sections are shown in Figure 1b–f. Glomerular hypertrophy was observed in KK‐Ay mice but not in BL6 mice. Treatment with low‐ and moderate‐doses of suramin caused a significant improvement in the glomerular size (Figure 1b–g), and significantly attenuated mesangial matrix expansion in KK‐Ay mice (Figure 1b–f,h). mRNA expression and index for type IV collagen were examined to evaluate whether suramin could prevent extracellular matrix (ECM) accumulation in the renal cortex and glomeruli (Figure 1i–o). We observed that type IV collagen was upregulated in the renal cortex of KK‐Ay mice, and downregulated by the administration of a moderate‐dose of suramin (Figure 1i). Moreover, the type IV collagen index was significantly higher in the KK‐Ay group than in the BL6 group, and significantly decreased by treatment with low‐ and moderate‐doses of suramin (Figure 1o). These findings suggest that suramin treatment suppresses ECM accumulation in the glomeruli of KK‐Ay mice.
Suramin suppresses the NLRP3 inflammasome
To study the effect of suramin on the NLRP3 inflammasome in the renal cortex, we performed qPCR and western blotting. NLRP3 mRNA expression increased significantly in KK‐Ay mice (Figure 2a). NLRP3 protein expression was significantly increased in KK‐Ay mice and administration of low‐ and moderate‐doses of suramin caused a significant decrease in the NLRP3 protein expression (Figure 2c,d). IL‐18 mRNA expression increased significantly in KK‐Ay mice and decreased significantly by treatment with a moderate‐dose of suramin (Figure 2b). Moreover, caspase‐1 activation was monitored via immunohistochemical staining (Figure 2e–j). The average number of caspase‐1 (p10)‐positive cells in the glomeruli of KK‐Ay(LS) and KK‐Ay(S) mice were significantly lower than that in KK‐Ay mice (Figure 2j).
Figure 2.
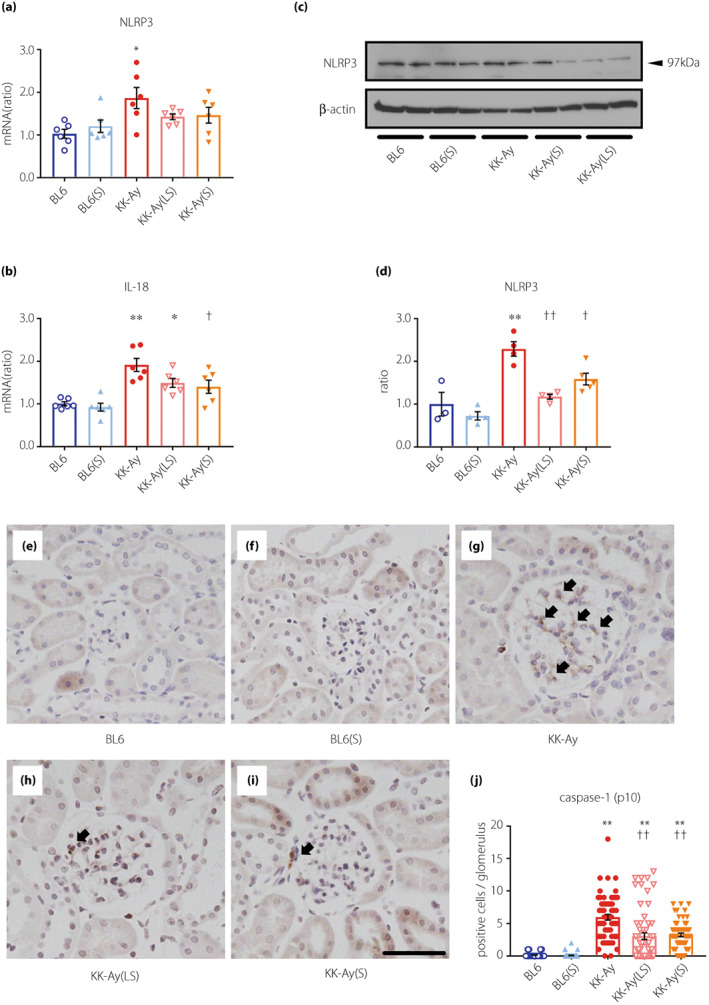
Suramin treatment suppresses the upregulation of inflammasome‐associated genes and proteins in KK‐Ay mice. (a, b) Quantification of NLRP3 (a) and IL‐18 (b) mRNA expression in the renal cortex by real‐time RT‐PCR (n = 6). (c, d) Western blotting images (c) and quantified data (d) for the NLRP3 protein expression in renal cortex (n = 3–5). (e–i) Histological analysis of caspase‐1 (p10) in the glomeruli of BL6 (e), BL6(S) (f), KK‐Ay (g), KK‐Ay(LS), (h) and KK‐Ay(S) (i) mice. (j) Quantification of caspase‐1 (p10) positive cells in the glomerulus of 20 randomly selected glomeruli from each mouse (n = 3). Scale bar, 50 μm; original magnification, 200×. Arrows indicate caspase‐1 (p10)‐positive cells. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05, †† P < 0.001 vs KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline; NLRP3, nucleotide‐binding oligomerization domain‐like receptor family pyrin domain containing 3.
Suramin attenuates renal inflammation in KK‐Ay mice
To study the anti‐inflammatory properties of suramin, we assessed the effect of suramin on diabetes‐induced expression of pro‐inflammatory genes and infiltration of macrophages. Renal cortical expression of C‐C motif chemokine 2 was significantly increased in KK‐Ay mice and decreased by a low‐dose of suramin (Figure 3a). In the renal cortex of KK‐Ay mice, upregulation of C‐X‐C motif chemokine ligand 10 was almost completely blocked by low‐ and moderate‐doses of suramin (Figure 3b). However, suramin had no significant effect on the renal cortical expression of toll‐like receptor 2 (Figure 3c), toll‐like receptor 4 (Figure 3d), and intercellular adhesion molecule 1 (Figure 3e) in KK‐Ay mice. CD68 mRNA expression (marker for macrophages) showed significant upregulation in the renal cortex of KK‐Ay mice. Importantly, low‐ and moderate‐doses of suramin suppressed CD68 mRNA expression (Figure 3f). Additionally, immunohistochemical staining for F4/80 (another marker of murine macrophages) showed a similar suppression pattern by suramin treatment. To elaborate, the increase in the average number of glomerular F4/80 positive cells in KK‐Ay mice was significantly suppressed by the administration of low‐ and moderate‐doses of suramin (Figure 3g–l).
Figure 3.
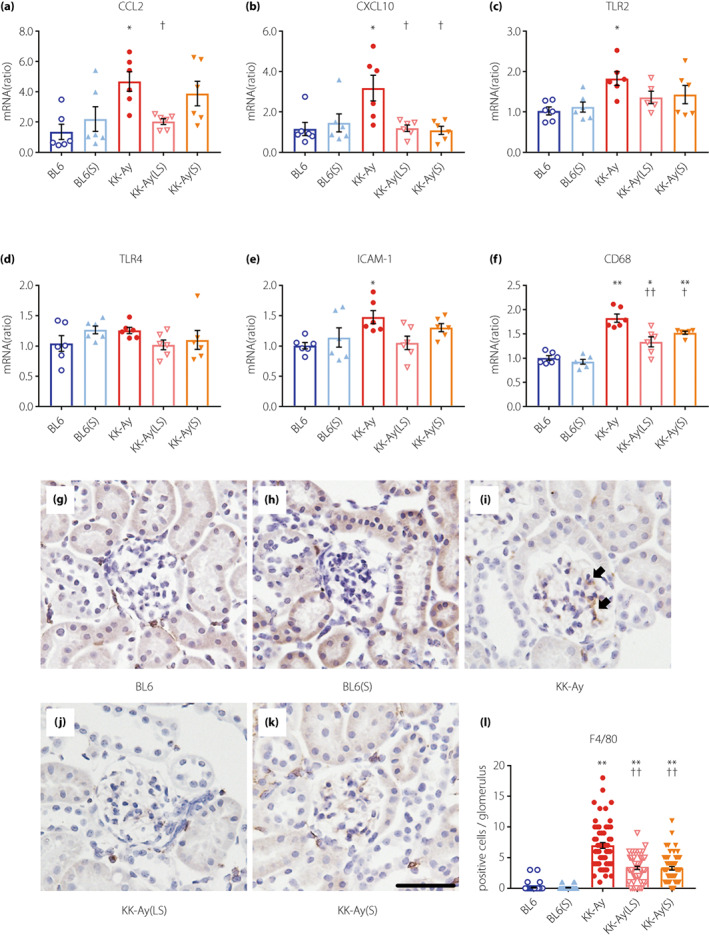
Suramin treatment attenuates renal inflammation in KK‐Ay mice. (a–f) Quantification of CCL2 (a), CXCL10 (b), TLR2 (c), TLR4 (d), ICAM‐1 (e), and CD68 (f) mRNA expression in the renal cortex by real‐time RT‐PCR (n = 5–6). (g–k) Histological analysis of F4/80 in the glomeruli of BL6 (g), BL6(S) (h), KK‐Ay (i), KK‐Ay(LS) (j) and KK‐Ay(S) (k) mice. (l) Quantification of F4/80 positive cells in the glomerulus of 20 randomly selected glomeruli from each mouse (n = 3). Scale bar, 50 μm; original magnification, 200×. Arrows indicate F4/80‐positive cells. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05, †† P < 0.001 vs KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; CCL2, C‐C motif chemokine 2; CXCL10, C‐X‐C motif chemokine ligand 10; ICAM‐1, intercellular adhesion molecule 1; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline; TLR2, toll‐like receptor 2; TLR4, toll‐like receptor 4.
Effect of suramin on P2X and P2Y expression in the renal cortex
To examine whether suramin exerts its beneficial effects by antagonizing the P2 receptors, we evaluated the mRNA expression of P2X and P2Y receptors. Among the P2X receptor family, renal cortical expression of P2X1, P2X4, P2X5, P2X6, and P2X7 was significantly higher in KK‐Ay mice than in BL6 mice (Figure 4a–g). We observed a significant upregulation in the expression of P2X4 and P2X7 receptors in KK‐Ay mice, which was significantly downregulated by low‐ and moderate‐doses of suramin (Figure 4d,g). Among the P2Y receptor family, there was no difference between the suramin‐treated and untreated KK‐Ay mice (Figure 4h–n). Suramin did not affect the expression of ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1) or ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1), which hydrolyze extracellular ATP (Figure 4o,p).
Figure 4.
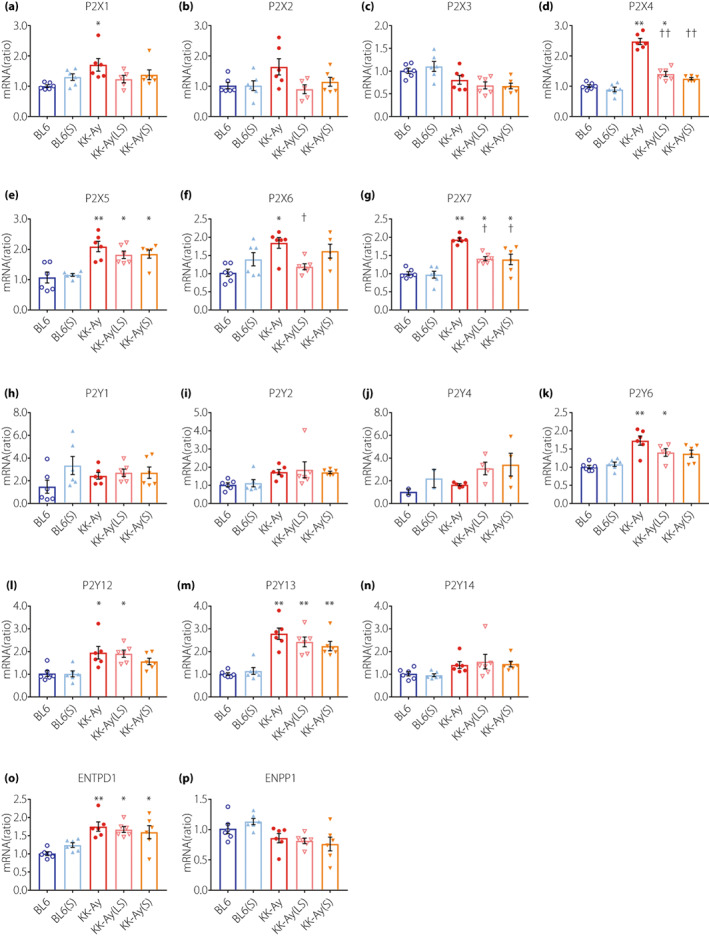
Changes in the mRNA expression of the P2X and P2Y receptors in renal cortex by diabetes and suramin treatment. (a–p) Quantification of P2X1 (a), P2X2 (b), P2X3 (c), P2X4 (d), P2X5 (e), P2X6 (f), P2X7 (g), P2Y1 (h), P2Y2 (i), P2Y4 (j), P2Y6 (k), P2Y12 (l), P2Y13 (m), P2Y14 (n), ENTPD1 (o), and ENPP1 (p) mRNA expression in the renal cortex by real‐time RT‐PCR. n = 6, 1–4 out of 6 samples were below detection limit. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05, †† P < 0.001 vs. KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; ENPP1, ectonucleotide pyrophosphatase/phosphodiesterase 1; ENTPD1, ectonucleoside triphosphate diphosphohydrolase 1; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline.
Distribution of the P2X4 and P2X7 receptors in the glomeruli
To determine the localization of P2X4 and P2X7 receptors in the glomeruli of KK‐Ay mice, we performed double immunofluorescence staining for CD90 (a marker for mesangial cells), P2X4, and P2X7 (Figure 5a–h). P2X4 and P2X7 were mainly distributed in the glomerular mesangial cells of KK‐Ay mice. Furthermore, the glomerular expression of P2X4 and P2X7 was significantly upregulated in the glomeruli from KK‐Ay mice and completely downregulated in glomeruli from KK‐Ay(LS) and KK‐Ay(S) mice (Figure 5i,j).
Figure 5.
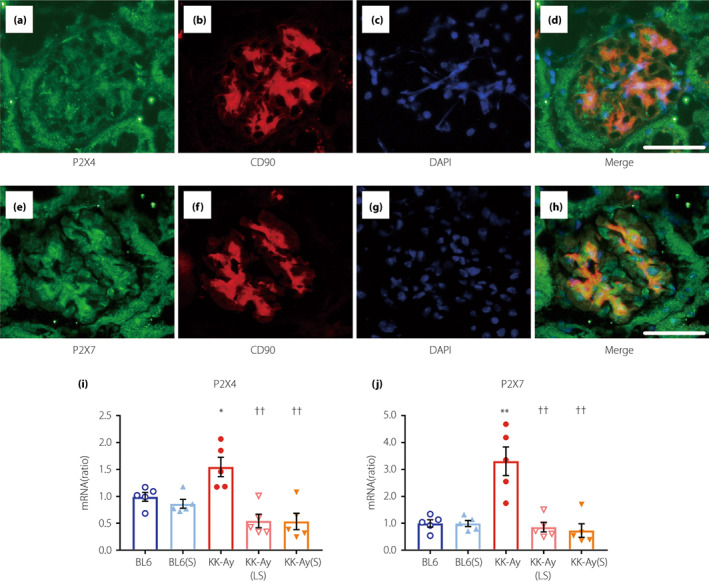
P2X4 and P2X7 are localized in the mesangial cells of the glomerulus. (a–d) Double immunofluorescence staining for P2X4 and CD90 (a marker of mouse mesangial cells). (e–h) Double immunofluorescence staining of P2X7 and CD90. Scale bars, 50 μm; original magnification: objective lens, 20×; zoom, 2×. (i, j) Quantification of P2X4 (i) and P2X7 (j) mRNA expression in the isolated glomeruli by real‐time RT‐PCR (n = 5). Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. †† P < 0.001 vs KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline.
Suramin prevents the decrease of the slit diaphragm proteins in the glomeruli
To investigate the effect of suramin on glomerular filtration barrier, we performed immunofluorescence staining of podocin (Figure 6a–f) and nephrin (Figure 6g–l). The podocin index was significantly decreased in KK‐Ay mice, and attenuated by low‐ and moderate‐doses of suramin (Figure 6f). Moreover, the decrease of nephrin index in KK‐Ay mice was also significantly attenuated by moderate‐dose suramin treatment (Figure 6l). These results suggest that the slit diaphragm proteins (podocin and nephrin) were decreased in the diabetic mice, and attenuated by suramin treatment.
Figure 6.
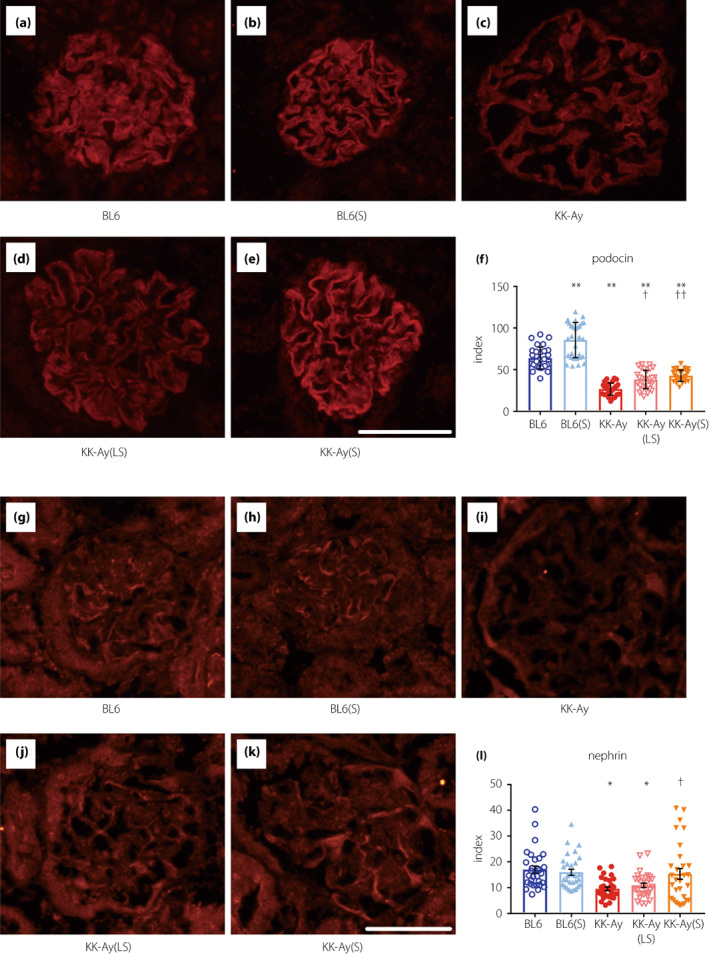
Effect of suramin on immunofluorescence staining for glomerular podocin and nephrin. (a–e) Histological expression of podocin in the glomeruli of BL6 (a), BL6(S) (b), KK‐Ay (c), KK‐Ay(LS) (d), and KK‐Ay(S) (e) mice. (f) Podocin expression was estimated using the fluorescence intensities of 10 randomly selected glomeruli from each mouse (n = 3). (g–k) Histological expression of nephrin in the glomeruli of BL6 (g), BL6(S) (h), KK‐Ay (i), KK‐Ay(LS) (j), and KK‐Ay(S) (k) mice. (l) Nephrin expression was estimated using the fluorescence intensities of 10 randomly selected glomeruli from each mouse (n = 3). Scale bar, 50 μm; original magnification, 200×. Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs BL6 group. † P < 0.05, †† P < 0.001 vs KK‐Ay group. BL6(S), BL6 mice treated with a moderate‐dose of suramin; BL6, BL6 mice treated with saline; KK‐Ay(LS), KK‐Ay mice treated with a low‐dose of suramin; KK‐Ay(S), KK‐Ay mice treated with a moderate‐dose of suramin; KK‐Ay, KK‐Ay mice treated with saline.
Suramin inhibits ATP‐induced NRLP3 complex formation
Since suramin treatment resulted in remarkable changes in the expression of P2X4 and P2X7 receptors in KK‐Ay mice, and because both the receptors were predominantly localized in glomerular mesangial cells, we investigated whether suramin suppressed ATP‐induced NLRP3 inflammasome activation in MMCs. The mRNA expression of P2X4 and P2X7 was significantly increased by ATP stimulation (Figure 7a,b); however, there was no effect of ATP on P2X4 and P2X7 protein expression (Figure 7c–f). Unlike in the renal cortex or glomeruli of KK‐Ay mice, both the mRNA and protein expression of P2X4 and P2X7 in MMCs did not differ between the suramin‐treated and untreated groups (Figure 7a–f). This indicated that suramin does not directly regulate the expression of P2X4 and P2X7 receptors in MMCs. Conversely, the expression of active caspase‐1 and mature IL‐18 was significantly increased by ATP stimulation and decreased by suramin treatment (Figure 7i–l), suggesting that suramin suppresses the events downstream of NLRP3 inflammasome activation. Unexpectedly, there was no significant change in NLRP3 expression among the groups (Figure 7g,h). Therefore, we performed BN‐PAGE to evaluate the formation of NLRP3 complexes. As shown in Figure 8a, large protein complexes were detected at positions >1,000 kilodalton (kDa). The density of these protein bands increased by ATP stimulation and decreased by suramin treatment. We performed 2D‐PAGE (first dimension being BN‐PAGE and second dimension being SDS‐PAGE) to verify the components of large protein complexes. We detected NLRP3 protein spots (>100 kDa) in the second dimension for the ATP‐stimulated group, which were >1,000 kDa in size in the first dimension (Figure 8b). This observation suggested that the large protein complexes consisted of NRLP3. On the other hand, the NLRP3 protein spots were weakly detected in control and ATP(S) groups (Figure 8b). These results indicate that suramin suppresses ATP‐induced formation of the NLRP3 inflammasome complex, possibly by antagonizing the P2 receptors in MMCs.
Figure 7.
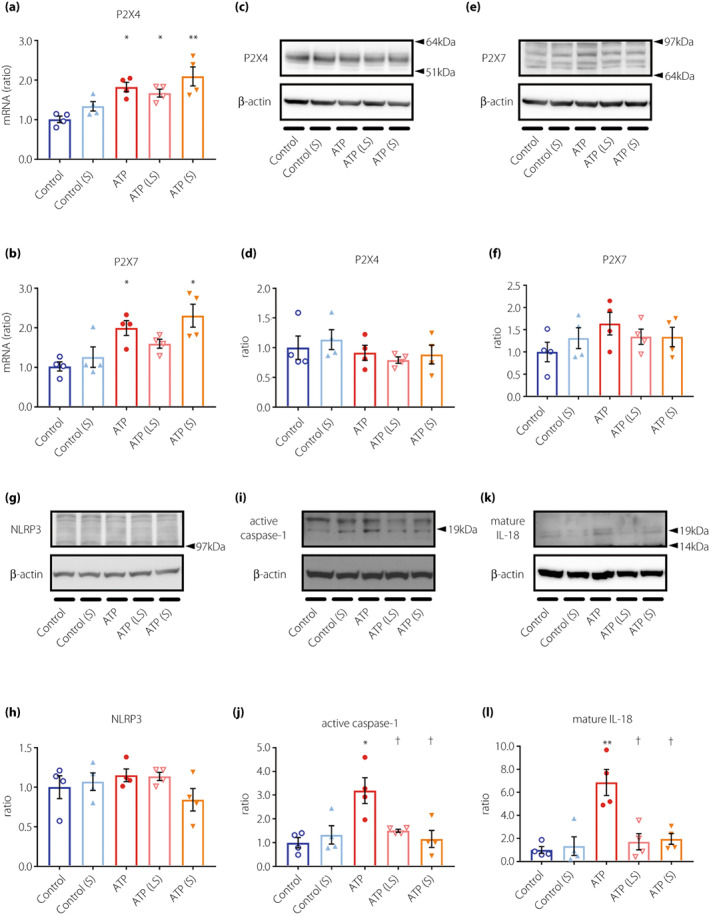
Suramin treatment suppresses the upregulation of active caspase‐1 and mature IL‐18 in ATP‐stimulated MMCs. (a, b) Quantification of P2X4 (a) and P2X7 (b) mRNA expression in MMCs by real‐time RT‐PCR (n = 4). (c) Analysis of P2X4, (e) P2X7, (g) NLRP3, (i) caspase‐1, and (k) IL‐18 protein expression in MMCs by western blotting analysis. Quantified data for (d) P2X4, (f) P2X7, (h) NLRP3, (j) caspase‐1, and (l) IL‐18 expression (n = 4). Data are presented as mean ± SEM. *P < 0.05, **P < 0.001 vs control group. † P < 0.05 vs ATP group. Control, MMCs + vehicle alone; Control(S), MMCs +1 μM suramin; ATP, MMCs +5 mM ATP; ATP(LS), MMCs +5 mM ATP and 0.1 μM suramin; ATP(S), MMCs +5 mM ATP and 1 μM suramin. MMCs, mouse mesangial cells; NLRP3, nucleotide‐binding oligomerization domain‐like receptor family pyrin domain containing 3.
Figure 8.
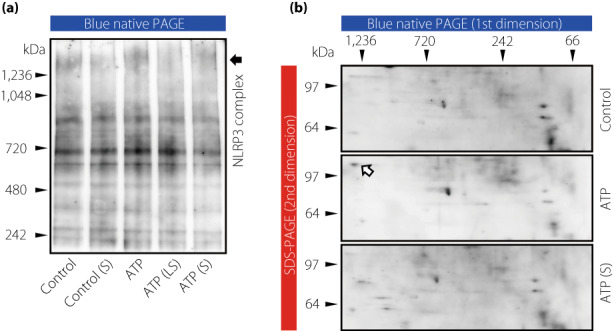
ATP‐induced NLRP3 inflammasome complex formation is suppressed by suramin treatment in MMCs. (a) Detection of the NLRP3 protein complex in MMCs by BN‐PAGE. (b) Detection of NLRP3 protein expression in control, ATP, and ATP(S) treated MMCs by 2D‐PAGE. Arrows indicate the NLRP3 protein spot in the second dimension of the ATP group. Control, MMCs + vehicle alone; Control(S), MMCs +1 μM suramin; ATP, MMCs +5 mM ATP; ATP(LS), MMCs +5 mM ATP and 0.1 μM suramin; ATP(S), MMCs +5 mM ATP and 1 μM suramin. 2D‐PAGE, two‐dimensional polyacrylamide gel electrophoresis; BN‐PAGE, blue native polyacrylamide gel electrophoresis; MMCs, mouse mesangial cells; NLRP3, nucleotide‐binding oligomerization domain‐like receptor family pyrin domain containing 3.
To examine which was the more dominant P2X receptor subtype in the action of suramin, we transfected MMCs with P2X4 and/or P2X7 siRNA. Transfection with siRNAs targeting P2X4 or P2X7 significantly downregulated the expression level of P2X4 and P2X7 (>70% and >65% respectively; Figure S1a,b). However, in both ATP and suramin treated MMCs, no additional effect on active caspase‐1 protein expression was observed by the knockdown of P2X4 or P2X7 (Figure S1c,d). These results suggest that suramin may have a potential to antagonize both P2X4 and P2X7 receptors to a similar degree.
DISCUSSION
Here, we report that suramin exerted renoprotective effects by suppressing NLRP3 inflammasome‐related genes and proteins in the renal cortex of KK‐Ay mice, which was independent of HbA1c, body weight, or systolic blood pressure. Moderate‐dose suramin is superior in nephrin protein expression in the glomeruli, and mRNA expressions of type IV collagen and IL‐18 in the renal cortex; however, even low‐dose suramin also exerts sufficient renoprotective effects.
We previously showed that serum and urinary IL‐18 levels associate positively with the UACR in patients with type 2 diabetes and DKD 6 , 24 . Another recent report showed a significant correlation between plasma IL‐18 levels and DKD severity 25 . Here, the expression of inflammasome‐related genes and proteins, such as IL‐18, increased significantly in the renal cortex of KK‐Ay mice and ATP‐stimulated MMCs, and decreased with suramin treatment. Suramin also suppressed UACR in KK‐Ay mice. We speculated that suramin improved UACR via the same mechanism as that responsible for the suppression of IL‐18 levels i.e., by inhibiting activation of the NLRP3 inflammasome.
P2 receptors have been known to play an essential role in vascular tone regulation. A recent study showed that the afferent arteriole exhibits a sustained vasoconstriction in response to ATP 26 . Other study has reported that the P2X antagonist reduces the afferent and efferent resistances, and increases the single‐nephron glomerular filtration rate in rats that received angiotensin II 27 . Here, we showed that suramin improved UACR, but did not affect creatinine clearance in KK‐Ay mice, suggesting that suramin does not alter renal hemodynamics in KK‐Ay mice. These differences in renal hemodynamics may be attributed to the difference in animal models.
Pathological damage of the glomerular filtration barrier, such as the slit diaphragm, is known to be associated with glomerular proteinuria 28 and db/db mice have shown to exhibit pathological damage of the glomerular filtration barrier 29 . Here, we showed that the decrease of podocin and nephrin protein expression was attenuated by suramin, suggesting that suramin has the potential to suppress diabetes‐induced injury and dysfunction of the slit diaphragm, which may lead to the suppression of albuminuria by suramin administration.
Recent studies have shown that suramin treatment attenuates the development of peritoneal fibrosis in a rat model of peritoneal fibrosis, and renal fibrosis in a mouse model of obstructive nephropathy 30 , 31 . This report suggested that suramin attenuated peritoneal fibrosis by either inhibiting the activation of the TGF‐β1‐smad3 signaling pathway or preventing the phosphorylation of signal transducer and activator of transcription 3 and extracellular signal‐regulated kinase 1/2 30 . Our experiments aimed to investigate ECM accumulation in early stage DKD and showed that suramin suppressed mesangial matrix accumulation and type IV collagen deposition in the glomeruli.
ENTPD1 and ENPP1 metabolize extracellular ATP. Polymorphisms in ENTPD1 and ENPP1 have been associated with type 2 diabetes and DKD 32 , 33 . We observed no difference in renal ENTPD1 or ENPP1 gene expression between suramin treated and untreated KK‐Ay mice, suggesting that suramin did not affect ENTPD1‐ or ENPP1‐dependent ATP metabolism.
A number of studies have suggested that the P2X and P2Y receptors can be therapeutic targets for various pathological conditions 34 . Selective P2X receptor antagonists, such as A438079 and oxidized ATP (oATP) for P2X7 35 , 36 , have been shown to exert renoprotective effects. Serum creatinine and blood urea nitrogen (BUN) levels were reported to improve after A438079 treatment in an ischemia/reperfusion (I/R)‐induced acute kidney injury (AKI) mouse model 37 . Mesangial ECM production was significantly suppressed by oATP in rat mesangial cells that were incubated under high‐glucose conditions 38 . On the other hand, suramin is known to act as a nonselective antagonist of P2X and P2Y receptors 36 . Suramin has been used clinically to treat human African trypanosomiasis, with five i.v. injections (20 mg/kg) administered every 3–7 days over a period of 4 weeks 39 . A recent study showed that suramin treatment caused a significant reduction in serum creatinine and BUN levels in mice with renal I/R‐induced AKI 40 . Another report showed that suramin suppresses the progression of renal fibrosis and prevents the decline in creatinine clearance in db/db mice 21 . However, suramin may cause side effects, such as nausea, vomiting, and urticaria; and less often, renal damage when injected at very high doses 41 . Recently, a randomized clinical trial (Phase I/II) reported that a single i.v. injection of suramin (20 mg/kg) improved symptoms in 5–14 years‐old children with the autism spectrum disorder while averting serious adverse events 22 . Here, we showed that the renoprotective effects of suramin can be achieved even when a relatively low dose (0.01 and 1 mg/kg) of the drug is administered to KK‐Ay mice, indicating that suramin can be used for the treatment of DKD without any adverse reactions.
We observed that expression of P2X4 and P2X7 increased significantly in the renal cortex of KK‐Ay mice and decreased with suramin treatment. Moreover, no additional effect on active caspase‐1 was observed by the knockdown of P2X4 or P2X7 in both ATP and suramin treated MMCs. Our results indicate that P2X4 and P2X7 are associated with diabetes and DKD, and that multiple P2X receptors may participate in the pathogenesis of DKD. Recent investigations using selective antagonists of P2X receptors and P2X receptor‐KO mice (especially P2X7 receptor‐KO mice) clearly show that the blockade of individual P2X receptors can facilitate the improvement of DKD symptoms 42 , 43 . However, our results suggest that inhibition by nonselective P2X receptor antagonists, such as suramin, may be a more beneficial strategy to treat DKD.
There were some limitations in this study. First, because this study was in animal and cell studies, it is unclear whether suramin is effective for patients with DKD or not. In addition, adverse effects of suramin in patients with DKD are difficult to evaluate. Therefore, clinical trials using suramin are needed. Second, the current results suggest that suramin exerts renoprotective effects via the suppression of NLRP3 inflammasome in consequence of inhibiting inflammation in whole kidney. However, detailed underlying mechanisms by which suramin inhibits glomerular filtration barrier damage has not been completely clarified.
In conclusion, we report that suramin suppresses NLRP3 inflammasome activation and exerts renoprotective effects in KK‐Ay mice without lowering the blood glucose levels. This study provides the first evidence that repeated long‐term administration of a low‐dose of suramin protects against DKD development via the inhibition of P2X receptors. Suramin can be expected to be an important drug for the treatment of DKD.
DISCLOSURE
SM reports honoraria for speaking from Daiichi Sankyo, Mitsubishi Tanabe, and Taisho. JW receives speaker honoraria from Astra Zeneca, Daiichi Sankyo, Novo Nordisk Pharma, Tanabe Mitsubishi, and receives grant support from Astellas, Baxter, Bayer, Chugai, Dainippon Sumitomo, Kyowa Kirin, Novo Nordisk Pharma, Ono, Otsuka, Tanabe Mitsubishi, and Teijin. KS received speaker fees from MSD, Eli Lilly Japan, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Mitsubishi Tanabe, AstraZeneca, Ono, Sumitomo Dainippon Pharma, Kyowa Hakko Kirin, and research support from Takeda, MSD, Kyowa Hakko Kirin, and Mitsubishi Tanabe. All the other authors declare no conflict of interest.
Approval of the research protocol: N/A.
Informed consent: N/A.
Registry and the registration no. of the study/trial: N/A.
Animal studies: All animal experiments were conducted following the national guidelines and the relevant national laws on the protection of animals.
Supporting information
Appendix S1 | Materials and methods.
Figure S1 | No additional effect on active caspase‐1 is observed by the knockdown of P2X4 or P2X7 in both ATP and suramin treated MMCs.
Table S1 | Real time RT‐PCR probe and primer designs (Takara Bio).
ACKNOWLEDGMENTS
Part of this study was supported by a JSPS Grant‐in‐Aid for Scientific Research (KAKENHI) Grant Number (JP17K09698 and JP20K08635 to KS and JP18K08211 and JP22K08353 to MS) and a research grant obtained through Eli Lilly Japan K.K.
REFERENCES
- 1. Roglic G, Unwin N, Bennett PH, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005; 28: 2130–2135. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 3. Federation TID . IDF Diabetes Atlas, 10th edn, 2021. Available from: https://www.diabetesatlas.org/en/resources/ Accessed April 06, 2022. [Google Scholar]
- 4. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom prospective diabetes study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 5. Yozai K, Shikata K, Sasaki M, et al. Methotrexate prevents renal injury in experimental diabetic rats via anti‐inflammatory actions. J Am Soc Nephrol 2005; 16: 3326–3338. [DOI] [PubMed] [Google Scholar]
- 6. Shikata K, Makino H. Microinflammation in the pathogenesis of diabetic nephropathy. J Diabetes Investig 2013; 4: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakamura A, Shikata K, Nakatou T, et al. Combination therapy with an angiotensin‐converting‐enzyme inhibitor and an angiotensin II receptor antagonist ameliorates microinflammation and oxidative stress in patients with diabetic nephropathy. J Diabetes Investig 2013; 4: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyamoto S, Shikata K, Miyasaka K, et al. Cholecystokinin plays a novel protective role in diabetic kidney through anti‐inflammatory actions on macrophage: anti‐inflammatory effect of cholecystokinin. Diabetes 2012; 61: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kodera R, Shikata K, Kataoka HU, et al. Glucagon‐like peptide‐1 receptor agonist ameliorates renal injury through its anti‐inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965–978. [DOI] [PubMed] [Google Scholar]
- 10. Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol 2011; 22: 1007–1018. [DOI] [PubMed] [Google Scholar]
- 11. Wada J, Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 2016; 12: 13–26. [DOI] [PubMed] [Google Scholar]
- 12. Nakamura A, Shikata K, Hiramatsu M, et al. Serum interleukin‐18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 2005; 28: 2890–2895. [DOI] [PubMed] [Google Scholar]
- 13. Shahzad K, Bock F, Dong W, et al. Nlrp3‐inflammasome activation in non‐myeloid‐derived cells aggravates diabetic nephropathy. Kidney Int 2015; 87: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol 2019; 15: 501–520. [DOI] [PubMed] [Google Scholar]
- 15. Saeed M. Locus and gene‐based GWAS meta‐analysis identifies new diabetic nephropathy genes. Immunogenetics 2018; 70: 347–353. [DOI] [PubMed] [Google Scholar]
- 16. Lee HM, Kim JJ, Kim HJ, et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013; 62: 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giuliani AL, Sarti AC, Di Virgilio F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol Lett 2019; 205: 16–24. [DOI] [PubMed] [Google Scholar]
- 18. Miyamoto S, Hsu CC, Hamm G, et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine 2016; 7: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dunn PM, Blakeley AG. Suramin: a reversible P2‐purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol 1988; 93: 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williamson J, Scott‐Finnigan TJ. Trypanocidal activity of antitumor antibiotics and other metabolic inhibitors. Antimicrob Agents Chemother 1978; 13: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Korrapati MC, Howell LA, Shaner BE, et al. Suramin: a potential therapy for diabetic nephropathy. PLoS One 2013; 8: e73655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naviaux RK, Curtis B, Li K, et al. Low‐dose suramin in autism spectrum disorder: a small, phase I/II, randomized clinical trial. Ann Clin Transl Neurol 2017; 4: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J, Miyamoto S, You YH, et al. AMP‐activated protein kinase (AMPK) activation inhibits nuclear translocation of Smad4 in mesangial cells and diabetic kidneys. Am J Physiol Renal Physiol 2015; 308: F1167–F1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kajitani N, Shikata K, Nakamura A, et al. Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2010; 88: 171–176. [DOI] [PubMed] [Google Scholar]
- 25. Wong CK, Ho AW, Tong PC, et al. Aberrant activation profile of cytokines and mitogen‐activated protein kinases in type 2 diabetic patients with nephropathy. Clin Exp Immunol 2007; 149: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishiyama A, Rahman M, Inscho EW. Role of interstitial ATP and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertens Res 2004; 27: 791–804. [DOI] [PubMed] [Google Scholar]
- 27. Bautista‐Perez R, Perez‐Mendez O, Cano‐Martinez A, et al. The role of P2X7 purinergic receptors in the renal inflammation associated with angiotensin II‐induced hypertension. Int J Mol Sci 2020; 21: 4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawachi H, Fukusumi Y. New insight into podocyte slit diaphragm, a therapeutic target of proteinuria. Clin Exp Nephrol 2020; 24: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Gu C, Lawrence DA, et al. A plasminogen activator inhibitor type 1 mutant retards diabetic nephropathy in db/db mice by protecting podocytes. Exp Physiol 2014; 99: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xiong C, Liu N, Shao X, et al. Delayed administration of suramin attenuates peritoneal fibrosis in rats. BMC Nephrol 2019; 20: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu N, Tolbert E, Ponnusamy M, et al. Delayed administration of suramin attenuates the progression of renal fibrosis in obstructive nephropathy. J Pharmacol Exp Ther 2011; 338: 758–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedman DJ, Talbert ME, Bowden DW, et al. Functional ENTPD1 polymorphisms in African Americans with diabetes and end‐stage renal disease. Diabetes 2009; 58: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sortica DA, Buffon MP, Souza BM, et al. Association between the ENPP1 K121Q polymorphism and risk of diabetic kidney disease: a systematic review and meta‐analysis. PLoS One 2015; 10: e0118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol 2017; 8: 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dell'Antonio G, Quattrini A, Cin ED, et al. Relief of inflammatory pain in rats by local use of the selective P2X7 ATP receptor inhibitor, oxidized ATP. Arthritis Rheum 2002; 46: 3378–3385. [DOI] [PubMed] [Google Scholar]
- 36. Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology 2016; 104: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan Y, Bai J, Zhou X, et al. P2X7 receptor inhibition protects against ischemic acute kidney injury in mice. Am J Physiol Cell Physiol 2015; 308: C463–C472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solini A, Iacobini C, Ricci C, et al. Purinergic modulation of mesangial extracellular matrix production: role in diabetic and other glomerular diseases. Kidney Int 2005; 67: 875–885. [DOI] [PubMed] [Google Scholar]
- 39. Babokhov P, Sanyaolu AO, Oyibo WA, et al. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathog Glob Health 2013; 107: 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhuang S, Lu B, Daubert RA, et al. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int 2009; 75: 304–311. [DOI] [PubMed] [Google Scholar]
- 41. Etchegorry MG, Helenport JP, Pecoul B, et al. Availability and affordability of treatment for human African trypanosomiasis. Trop Med Int Health 2001; 6: 957–959. [DOI] [PubMed] [Google Scholar]
- 42. Solini A, Menini S, Rossi C, et al. The purinergic 2X7 receptor participates in renal inflammation and injury induced by high‐fat diet: possible role of NLRP3 inflammasome activation. J Pathol 2013; 231: 342–353. [DOI] [PubMed] [Google Scholar]
- 43. Menzies RI, Booth JWR, Mullins JJ, et al. Hyperglycemia‐induced renal P2X7 receptor activation enhances diabetes‐related injury. EBioMedicine 2017; 19: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 | Materials and methods.
Figure S1 | No additional effect on active caspase‐1 is observed by the knockdown of P2X4 or P2X7 in both ATP and suramin treated MMCs.
Table S1 | Real time RT‐PCR probe and primer designs (Takara Bio).


