Abstract
Background
Information regarding the frequency of L waves and their prognostic relevance in dogs with secondary atrial fibrillation (AF) is limited.
Hypothesis/Objectives
To determine whether L waves occur and ascertain their prognostic role, as well as the role of other clinical and echocardiographic variables in dogs with AF.
Animals
Fifty‐five dogs with AF associated with myxomatous mitral valve disease or dilated cardiomyopathy.
Methods
Retrospective, multicenter observational study. In addition to L waves analysis, other clinical and echocardiographic variables, including type of antiarrhythmic treatment, were evaluated. A survival analysis was performed to test for predictors of cardiac death and all‐cause mortality using Cox proportional hazards regression models.
Results
L waves were evident in 33/55 dogs (60%, 95% confidence interval [CI] = 47%‐72%) but their presence did not influence outcome. Increased left ventricular end‐systolic diameter normalized for body weight (LVSDn) was a significant predictor of both cardiac death (hazard ratio [HR] = 4.41, 95% CI = 1.18‐16.54; P = .03) and all‐cause mortality (HR = 9.39, 95% CI = 2.49‐35.32; P < .001). Heart rate assessed during echocardiography (Echo‐HR) represented an additional significant predictor of cardiac death (HR = 1.01, 95% CI = 1.00‐1.01; P = .04) and all‐cause mortality (HR = 1.01, 95% CI = 1.00‐1.01; P = .04).
Conclusions and Clinical Importance
L waves occurred frequently in dogs with AF, but held no prognostic relevance. Conversely, LVSDn and Echo‐HR represented independent predictors of negative outcome in these animals.
Keywords: canine, cardiac arrhythmias, dilated cardiomyopathy, myxomatous mitral valve disease, survival analysis, transthoracic echocardiography
Abbreviations
- AF
atrial fibrillation
- Ao
aortic root
- CHF
congestive heart failure
- CI
confidence interval
- DCM
dilated cardiomyopathy
- Echo‐HR
heart rate obtained during echocardiographic examination
- FS
fractional shortening
- HR
hazard ratio
- LA
left atrial
- LV
left ventricular
- LVDD
left ventricular end‐diastolic diameter
- LVDDn
left ventricular end‐diastolic diameter normalized for body weight
- LVSD
left ventricular end‐systolic diameter
- LVSDn
left ventricular end‐systolic diameter normalized for body weight
- MMVD
myxomatous mitral valve disease
- MST
median survival time
- OT
other treatment
- TTE
transthoracic echocardiography
1. INTRODUCTION
Atrial fibrillation (AF) is a common cardiac rhythm disturbance and the most common supraventricular tachyarrhythmia in dogs. 1 , 2 , 3 , 4 It is characterized by rapid, uncoordinated atrial electrical activity, which compromises the atrial contribution to ventricular filling, leading to decreased cardiac output, increased atrial filling pressures, and development or worsening of clinical signs of heart failure. 5 , 6 In dogs, AF usually is associated with atrial dilatation caused by underlying structural heart diseases (so‐called “secondary” AF), 6 , 7 , 8 with the most common being myxomatous mitral valve disease (MMVD) in small‐to‐medium sized breeds of dogs and dilated cardiomyopathy (DCM) in large‐to‐giant breeds. 9 , 10 , 11 , 12 , 13 , 14 Development of AF is associated with decreased survival time in dogs with MMVD and DCM, primarily because of congestive heart failure (CHF) and sudden cardiac death. 10 , 13 , 15 , 16 , 17 In light of the high prevalence and clinical impact of secondary AF, knowledge of factors associated with a negative prognosis is crucial for proper management of affected dogs. Regrettably, the available veterinary literature on this topic is scant and mainly limited to retrospective studies investigating the negative effects on survival of high heart rate assessed using 24‐hour Holter monitoring or comparing the effect of 2 antiarrhythmic protocols (i.e., digoxin and diltiazem vs monotherapy with diltiazem). 10 , 11 Although Holter monitoring is best suited to evaluate the actual ventricular rate in dogs with AF, it is time consuming and requires specialized equipment and trained operators. In addition to Holter monitoring, transthoracic echocardiography (TTE) is a useful diagnostic tool in dogs with secondary AF. Interestingly, despite the abundance of studies listing TTE among diagnostic procedures performed in dogs with AF, 5 , 7 , 8 , 12 , 13 , 14 , 15 , 16 , 17 no previous study has specifically addressed the prognostic utility of echocardiographic parameters in dogs with AF. In humans, several echocardiographic parameters have demonstrated prognostic value in patients with AF, primarily those related to left‐sided cardiac chambers dimension and systolic as well as diastolic function. 18 , 19 Included echocardiographic factors associated with decreased survival time are detection of mitral L waves, mid‐diastolic filling waves suggestive of advanced diastolic dysfunction, increased filling pressures, and a distended noncompliant left atrium (LA). 20 , 21 , 22 Currently, L waves represent a neglected echocardiographic parameter in animals with only a single study reporting their detection in dogs with MMVD without cardiac arrhythmias. 23
Therefore, our aims were 2‐fold: to evaluate whether L waves occur in dogs with AF secondary to MMVD and DCM and to investigate the ability of selected clinical and echocardiographic variables, including different antiarrhythmic treatment and presence of L waves, to predict survival in this population of dogs. We hypothesized that L waves would be identifiable in dogs with AF secondary to MMVD and DCM, and that cardiac evaluation including TTE would provide relevant prognostic information in these dogs.
2. MATERIAL AND METHODS
2.1. Animals
For this multicenter observational retrospective study, data were collected from the internal databases of 2 veterinary teaching hospitals. The study was approved by the management committee of the veterinary teaching hospital of the University of Padova and all owners gave informed written consent for all investigations. Dogs were recruited to the study from those undergoing cardiac diagnostic evaluation and final diagnosis of AF secondary to MMVD and DCM between May 2012 and November 2021. Diagnosis of MMVD was based on clinical and echocardiographic findings, including a left apical systolic murmur and thickened or prolapsing mitral valve leaflets or both on 2‐dimensional echocardiography associated with mitral valve regurgitation on color flow Doppler interrogation. 24 , 25 Disease severity was classified according to the guidelines of the American College of Veterinary Internal Medicine into stages B1, B2, C, and D. 25 For an echocardiographic diagnosis of DCM, breed‐specific cut‐off values were used when available. 26 , 27 Specifically, in Doberman pinschers, diagnosis of DCM was based on left ventricular end‐diastolic volume normalized to body surface area >95 mL/m2, end‐systolic volumes normalized to body surface area >55 mL/m2, or both obtained using the Simpson's method of disks. 27 , 28 In Boxers, DCM was defined as left ventricular (LV) fractional shortening (FS%) <21%, LV end‐diastolic diameter (LVDD) >4.8 cm, and left ventricular end‐systolic diameter (LVSD) >3.3 cm. 29 In breeds lacking specific diagnostic cut‐off values for DCM, diagnosis was made when LVDD and LVSD exceeded the upper 97.5 percentile breed‐specific reference interval and FS was <20%. 26 If breed‐specific reference intervals were not available, breed‐independent reference intervals 30 were used as recommended. 26 In dogs with DCM, disease severity was classified according to the recently proposed classification scheme into stages B1, B2, C, and D. 27 The presence of AF was based on at least 1 of the following methods: 6 or 12‐lead surface ECG of least 1‐minute duration, or good quality 1‐lead ECG during the echocardiographic examination. In particular, the echocardiographic diagnosis of AF was based on the combined presence of the following findings: irregularly irregular cardiac rhythm with narrow QRS complexes, lack of recognizable P waves, and absence of A wave on mitral inflow on Doppler analysis. For dogs with more visits available during the period of observation, data obtained during the first visit documenting AF were analyzed.
Dogs with equivocal cardiac diagnosis or other concomitant congenital or acquired cardiac disease were excluded from the study. The absence of a diagnosis of AF, presence of paroxysmal AF, and evidence of lone atrial fibrillation (ie, atrial fibrillation not associated with underlying structural heart disease) also were considered exclusion criteria (so that only dogs with chronic, secondary AF associated with MMVD or DCM were included). Conversely, the presence of concomitant systemic diseases and previous use of cardiovascular drugs did not represent exclusion criteria. Concerning systemic disease, dogs with hypothyroidism were included in the DCM group if they were on treatment with levothyroxine and the condition was considered stable at the time of inclusion. Concerning cardiovascular drugs, before inclusion, these were prescribed according to referring veterinarians' opinion. After inclusion, we optimized treatments according to patient needs and scientific evidence. In particular, pimobendan was prescribed to all dogs with stage B2 MMVD satisfying the EPIC study's criteria as well as dogs with stage B2 DCM. All dogs with stage C MMVD as well as those with stage C DCM received at least a combination of furosemide and pimobendan, often associated with additional drugs such as angiotensin‐converting enzyme inhibitors or spironolactone or both. 25 , 31 The antiarrhythmic protocols were optimized for each dog according to our experience and the underlying cardiac and systemic condition because of the lack of specific guidelines for medical management of arrhythmias in dogs.
2.2. Echocardiographic examination
At each Institution, an experienced operator performed TTE using echocardiographic units (iE33 and CX50 ultrasound systems, Philips Healthcare) equipped with dedicated multifrequency phased array transducers (S5‐1 and S3‐8 MHz) and continuous ECG. All echocardiographic examinations were obtained on conscious dogs placed in right and left lateral recumbency using gentle manual restraint and without chemical restraint. 32 Images and video clips of echocardiographic investigations were stored in dedicated workstations and measurements subsequently were carried out by 2 experienced investigators. Specifically, LVDD and LVSD were measured from M‐mode short‐axis echocardiographic images at the level of the chordae tendinae and FS then was calculated according to the standard formula. Left ventricular diameters also were normalized for body weight by allometric scaling in diastole (LVDDn) and systole (LVSDn). 33 In Doberman pinschers, LV chamber volumes also were determined using Simpson's method of disks in the right parasternal long‐axis 4‐chamber view. 27 , 28 Left atrial diameter and aortic root (Ao) diameter were measured in early diastole from echocardiographic short axis images obtained at the heart base level and the LA/Ao ratio then was calculated. 34 The LA antero‐posterior diameter also was calculated from the right parasternal long‐axis view by measuring the widest distance, parallel to the mitral valve annulus, from the inner wall of the middle of the interatrial septum to the inner wall of the posterior free wall at end‐systole. 35 Pulsed‐wave Doppler analysis of transmitral blood flow was obtained from the left apical 4‐chamber view by positioning the sample volume at the tip of the mitral leaflets, and the peak velocity of the early diastolic wave was measured. Moreover, particular attention was paid to identify L waves, defined as distinct positive mid‐diastolic flow velocities recorded after the E waves, leading to a biphasic pattern in dogs with AF (i.e., E wave followed by an L wave). 20 , 36 , 37 , 38 Although low velocity flow could be recorded during diastasis, an L wave was defined when forward mid‐diastolic flow after the E wave had a peak velocity >0.2 m/s (Figure 1). 20 , 36 , 37 , 38 All echocardiographic images for identification of L waves were obtained or reviewed or both by a board‐certified veterinary cardiologist (G.R.). Multiple views were used to evaluate transtricuspid blood flow, and the maximal value of any tricuspid regurgitation (TR) velocity was measured using continuous‐wave Doppler. In the absence of right ventricular outflow obstruction, the presence of pulmonary hypertension was considered when TR maximal velocity was ≥3.4 m/s (corresponding to a systolic pressure gradient ≥46 mm Hg using the modified Bernoulli equation). 39 All measurements were replicated on 5 consecutive beats, and the mean values then were calculated. 40 The heart rate (Echo‐HR) provided by the continuous single‐lead ECG performed during each echocardiographic examination was annotated. For the purpose of our study, only the Echo‐HR annotated during the acquisition of mitral inflow was considered. This variable was obtained by calculating the mean of the coupling intervals recorded over at least 5 consecutive QRS complexes.
FIGURE 1.
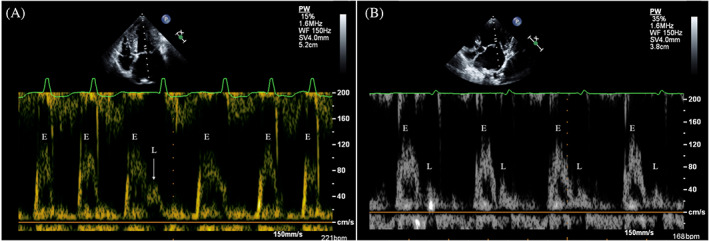
Pulsed wave Doppler mitral inflow showing E waves (E) and L waves (L) in 2 dogs with atrial fibrillation. (A) In this dog with high ventricular rate, only 1 L wave is recognizable during the longer diastolic phase (arrow); in the following cardiac cycle the E and L waves are partially fused. (B) In this dog with lower ventricular rate, L waves are always visible after E waves. Note that all L waves appears as well‐defined positive mid‐diastolic flow velocity with a peak velocity >0.2 m/s
2.3. Statistical analyses
Data were analyzed using different commercially available software packages (SAS version 9.3, SAS Institute, Inc, Cary, North Carolina; MedCalc version 12.6.1.0, MedCalc Software, Ostend, Belgium).
Demographic and clinical characteristics included: sex, breed, age, body weight, type of cardiac disease, class of CHF, type of CHF, cardiac treatment at inclusion, presence of concurrent diseases and ongoing treatment at the time of inclusion, and antiarrhythmic treatment after inclusion. For sex, breed, cardiac disease, and class of CHF, the following binary categories were considered, respectively: male or female, purebred or crossbred, MMVD or DCM, and compensated or decompensated CHF. For type of CHF, the following tertiary categories were considered: left‐sided CHF, right‐sided CHF, and bilateral CHF. For cardiac treatment at inclusion, concurrent diseases, and ongoing treatment, binary categories (yes or no) were used. For antiarrhythmic treatment after inclusion, the following tertiary categories were considered: digoxin, digoxin and diltiazem, and other treatment (OT). The following continuous Echo‐ECG and echocardiographic variables were considered: Echo‐HR, LA diameter, LA/Ao, LVDDn, LVSDn, FS, peak velocity of the early transmitral diastolic wave, and TR maximal velocity. For the echocardiographic categorical variables L‐wave and TR, the binary categories (yes or no) were used.
After assessment of normal distribution of data using the Shapiro‐Wilk's test, continuous data were reported as mean and SD. Categorical variables were reported as number and percentage. Comparison of clinical and echocardiographic variables between dogs with and without an L wave was conducted using the Student's t‐test and the z‐test for continuous and categorical variables, respectively.
Survival data were derived from the electronic databases of each institution or through telephone interviews with the referring veterinarians or dogs' owners. Dogs were classified as still alive, dead from cardiac‐related causes, dead from all‐cause mortality, or lost to follow‐up when no further data were available after presentation. Death from cardiac‐related causes was defined as sudden cardiac death, CHF refractory to medical treatment, euthanasia because of worsening of the cardiac condition, or death within 4 hours from the diagnosis of pulmonary edema. Time in days from inclusion (i.e., diagnosis of AF) to death (survival time) or to the telephone interview for dogs still alive (follow‐up time) was annotated. Right‐censoring was applied for dogs still alive at the end of the observational period. Kaplan‐Meier survival analysis was employed to evaluate the effect of presence of an L wave and type of antiarrhythmic treatment (ie, digoxin, digoxin and diltiazem, or OT) on median survival time (MST) before cardiac death.
Univariate and multivariable regression with Cox models was performed to determine whether a significant relationship existed between clinical and echocardiographic variables and survival. The endpoints were cardiac death and all‐cause mortality. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated considering 1‐unit change or reference level for continuous or categorical variables, respectively. Variables with P ≤ .1 in univariate analysis were included in the final multivariable Cox proportional hazards regression models with stepwise selection method. A value of P < .05 was considered significant for all analyses.
3. RESULTS
3.1. Study population
Fifty‐five dogs, 44 males (80%) and 11 females (20%), met the inclusion criteria and follow‐up data were available for 49 (89.1%) of them. These dogs belonged to 21 different breeds, with mixed breed being the most frequently presented group (23/55, 41.8%), followed by Doberman Pinscher and German Shepherd (4/55, 7.3% each), Corso (3/55, 5.5%), and Dogue de Bordeaux, Golden retriever, English Setter, and Italian Spinone (2/55, 3.6% each). Their mean age was 10.6 ± 2.9 years and body weight was 28.9 ± 17.8 kg. Forty dogs (72.7%) and 15 dogs (27.3%) were diagnosed with MMVD and DCM, respectively. Five dogs (9.1%) had compensated heart disease, whereas 50 dogs (90.9%) had CHF. Among the latter, 3 dogs (6.0%), 25 dogs (50.0%), and 22 dogs (44.0%) had right‐sided CHF, left‐sided CHF, and bilateral CHF, respectively. Thirty‐five dogs (63.6%) were receiving ≥1 drugs for cardiovascular treatment at inclusion (Appendix S1). Nineteen dogs (34.5%) had concurrent noncardiovascular diseases including neoplasia (9 cases), inflammatory diseases and chronic kidney disease (2 cases each), and degenerative arthropathy, esophageal foreign body, prostatic hyperplasia, medically‐controlled hypothyroidism, medically‐controlled leishmaniosis, and urinary tract infection (1 case each). On pulsed‐wave Doppler examination of mitral inflow, 33 dogs (60%, 95% CI = 47%‐72%) had a recognizable L wave. Table 1 summarizes the descriptive statistics for clinical, electrocardiographic, and echocardiographic variables of all dogs and shows the comparison of variables between dogs with and without an L wave. No difference was found for any of the considered variables between the 2 groups of dogs.
TABLE 1.
Descriptive data obtained from 55 dogs with atrial fibrillation divided in two groups according to presence or absence of an L wave on pulsed wave Doppler interrogation of mitral diastolic blood flow
| Variable | Total cohort (n = 55) | L wave present (n = 33) | L wave absent (n = 22) | P value |
|---|---|---|---|---|
| Sex, M/F (%) | 44/11 (80/20) | 27/6 (72/18) | 17/5 (77/23) | .95 |
| Age (year) | 11 ± 3 | 11 ± 3 | 10 ± 3 | .17 |
| Body weight (kg) | 29 ± 18 | 26 ± 17 | 32 ± 20 | .22 |
| Cardiac disease, MMVD/DCM (%) | 40/15 (73/27) | 25/8 (76/24) | 15/7 (68/32) | .77 |
| ACVIM stage, B2/C + D (%) | 5/50 (9/91) | 3/30 (9/91) | 2/20 (9/91) | .99 |
| L‐CHF (%) | 25 (50) | 14 (47) | 11 (55) | .77 |
| R‐CHF (%) | 3 (6) | 1 (3) | 2 (10) | NA |
| B‐CHF (%) | 22 (44) | 15 (50) | 7 (35) | .45 |
| Previous treatment, yes/no (%) | 35/20 (64/36) | 22/11 (67/33) | 13/9 (59/41) | .78 |
| Concurrent disease, yes/no (%) | 19/36 (34/66) | 11/22 (33/67) | 8/22 (36/64) | .99 |
| Heart rate (bpm) | 198 ± 44 | 198 ± 45 | 197 ± 44 | .91 |
| Left atrial diameter (cm) | 5.42 ± 0.94 | 5.43 ± 0.99 | 5.41 ± 0.88 | .94 |
| LA/Ao | 2.56 ± 0.61 | 2.55 ± 0.61 | 2.56 ± 0.64 | .96 |
| LVDDn | 2.04 ± 0.31 | 2.04 ± 0.30 | 2.04 ± 0.34 | .98 |
| LVSDn | 1.34 ± 0.28 | 1.37 ± 0.24 | 1.30 ± 0.33 | .35 |
| FS (%) | 29.9 ± 13.1 | 27.8 ± 10.7 | 33.1 ± 15.8 | .15 |
| Mitral E Vmax (cm/s) | 140.0 ± 35.2 | 141.4 ± 35.0 | 137.9 ± 36.3 | .72 |
| TR, yes/no (%) | 49/6 (89/11) | 31/2 (94/6) | 18/4 (82/18) | .33 |
| TR Vmax (m/s) | 2.77 ± 0.46 | 2.79 ± 0.48 | 2.75 ± 0.44 | .80 |
| Outcome | ||||
| Dead/alive + lost FU (%) | 47/8 (85/15) | 30/3 (91/9) | 17/5 (77/23) | .31 |
| Cardiac death (%) | 38 (81) | 23 (77) | 15 (88) | .56 |
Abbreviations: ACVIM, American College of Veterinary Internal Medicine; B‐CHF, bilateral congestive heart failure; B2, dogs from MMVD group with cardiac enlargement or dogs from DCM group showing left ventricular systolic dysfunction with or without concurrent left ventricular chamber enlargement in diastole; C, dogs with MMVD or DCM and past or present clinical signs of CHF D, dogs with MMVD or DCM and clinical signs of CHF refractory to standard treatment; DCM, dilated cardiomyopathy; F, female; FS, fractional shortening; LA/Ao, left atrial diameter to aortic diameter; L‐CHF, left‐sided CHF; Lost FU, lost to follow‐up; LVDDn, left ventricular diastolic diameter normalized to body weight; LVSDn, left ventricular systolic diameter normalized to body weight; Mitral E Vmax, mitral valve maximal E wave velocity; M, male; MMVD, myxomatous mitral valve disease; NA, not applicable; R‐CHF, right‐sided CHF; TR, tricuspid regurgitation; TR Vmax, tricuspid regurgitation maximal velocity.
After inclusion, 15 dogs (27.3%), 26 dogs (47.3%), and 14 dogs (25.4%) received digoxin, digoxin and diltiazem, and OT, respectively (Appendix S2). The OT group included either dogs without any antiarrhythmic treatment (i.e., antiarrhythmic treatment not instituted because of low ventricular penetrance associated with AF) or dogs for which complete data on antiarrhythmic treatment were not available (7 cases), and dogs only receiving diltiazem (4 cases), amiodarone, digoxin and sotalol, or diltiazem and amiodarone (1 case each).
Complete follow‐up data were available for 49 dogs (89.1%). Among them, 38 (77.5%) and 9 dogs (18.4%) experienced cardiac death and death from noncardiac causes, respectively, whereas 2 dogs (4.1%) were still alive at the end of the study. Among cases of cardiac death, sudden death represented the most common cause (27 dogs, 71.1%), followed by euthanasia because of worsening of the cardiac condition (10 dogs, 26.3%), and CHF refractory to medical treatment (1 dog, 2.6%). The overall median follow‐up time was 219 days (range, 1‐994 days). No significant difference was found in MST between dogs with (326 days, 95% CI interval = 134‐421 days) and without L waves (97 days, 95% CI = 12‐359 days; log‐rank test P = .07; Figure 2). Similarly, no difference was found in MST among antiarrhythmic treatment groups: 212 days (95% CI = 14‐287 days), 359 days (95% CI = 87‐421 days), and 326 days (95% CI = 13‐326 days; log‐rank test P = .74) for dogs receiving digoxin, digoxin and diltiazem, and OT, respectively (Figure 3).
FIGURE 2.
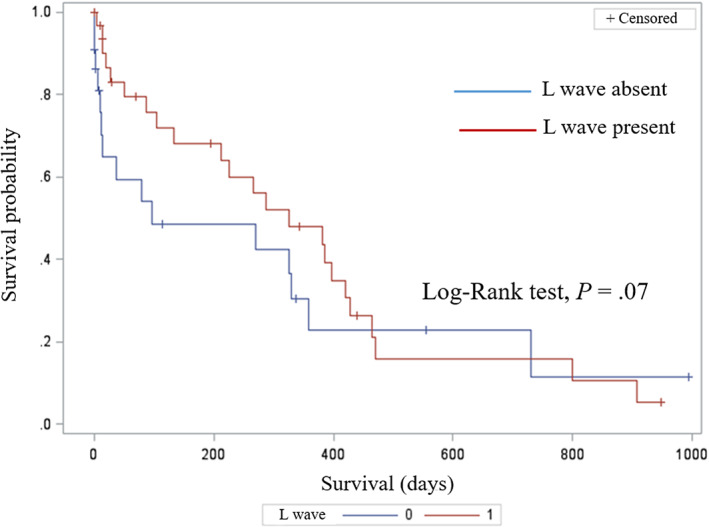
Kaplan‐Meier survival curve for cardiac‐related death in 55 dogs with atrial fibrillation divided based on presence (red line) or absence (blue line) of an L wave on pulsed‐wave Doppler interrogation of mitral inflow. Dogs still alive at the end of the observational period and dogs that died for noncardiac related events were right‐censored
FIGURE 3.
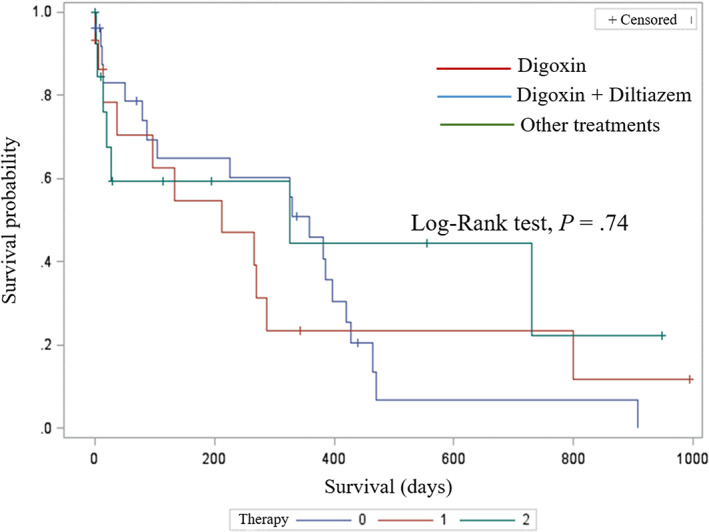
Kaplan‐Meier survival curve for cardiac‐related death in 55 dogs with atrial fibrillation divided based on antiarrhythmic treatment: digoxin (red line), digoxin and diltiazem (blue line), other treatments (green line). Dogs still alive at the end of the observational period and dogs that died for noncardiac related events were right‐censored
3.2. Univariate and multivariable regression analysis
Univariate logistic regression showed that LVDDn (P = .02), LVSDn (P = .01), and Echo‐HR (P = .02) were associated with cardiac death. Furthermore, LVDDn (P = .01), LVSDn (P = .01), and Echo‐HR (P = .02) as well as age (P = .10) and decompensated CHF (P = .08) were associated with all‐cause mortality in univariate analysis (Table 2).
TABLE 2.
Results of univariate logistic regression analysis showing the association between clinical and echocardiographic variables and the risk of cardiac death and all‐cause mortality in 55 dogs with atrial fibrillation
| Variable | Cardiac death | All‐cause mortality |
|---|---|---|
| P value | P value | |
| Sex (M) | .57 | .68 |
| Age (year) | .58 | .10 |
| Body weight (kg) | .22 | .23 |
| Heart rate (bpm) | .02 | .02 |
| Cardiac disease (MMVD) | .89 | .98 |
| ACVIM stage (C + D) | .99 | .08 |
| Previous treatment | .72 | .57 |
| Concurrent diseases (Yes) | .58 | .86 |
| Left atrial diameter (cm) | .75 | .98 |
| LA/Ao | .25 | .30 |
| LVDDn | .02 | .01 |
| LVSDn | .01 | .01 |
| FS (%) | .92 | .65 |
| Mitral E Vmax (cm/s) | .30 | .27 |
| TR (Yes) | .99 | .67 |
| TR Vmax (m/s) | .41 | .37 |
| L Wave (Yes) | .38 | .84 |
Note: Variables with P ≤ .1 used for final multivariable models in bold characters.
Abbreviations: ACVIM, American College of Veterinary Internal Medicine; C, dogs with myxomatous mitral valve disease (MMVD) or dilated cardiomyopathy (DCM) and past or present clinical signs of congestive heart failure; D, dogs with MMVD or DCM and clinical signs of congestive heart failure refractory to standard treatment; FS, Fractional shortening; LA/Ao, left atrial diameter to aortic diameter; LVDDn, left ventricular diastolic diameter normalized to body weight; LVSDn, left ventricular systolic diameter normalized to body weight; M, male; Mitral E Vmax, mitral valve maximal E wave velocity; TR, tricuspid regurgitation; TR Vmax, tricuspid regurgitation maximal velocity.
Final multivariable models confirmed that increased LVSDn (HR = 4.41, 95% CI = 1.18‐16.54; P = .03) and Echo‐HR (HR = 1.01, 95% CI = 1.00‐1.01; P = .04) were significant predictors of cardiac death. Moreover, LVSDn (HR = 9.39, 95% CI = 2.49‐35.32; P < .001), Echo‐HR (HR = 1.01, 95% CI = 1.00‐1.01; P = .04), and age (HR = 1.17, 95% CI = 1.04‐1.32; P = .01) were significant predictors of all‐cause mortality (Table 3).
TABLE 3.
Results of multivariable logistic regression analyses for the risks for cardiac death (Model 1) and all‐cause mortality (Model 2) in 55 dogs with atrial fibrillation
| Predictors | Hazard ratio | Lower 95% CI | Upper 95% CI | P value |
|---|---|---|---|---|
| Risk of cardiac death | ||||
| Heart rate (bpm) | 1.01 | 1.00 | 1.01 | .04 |
| LVSDn | 4.41 | 1.18 | 16.54 | .03 |
| Risk of all‐cause mortality | ||||
| Age (year) | 1.17 | 1.04 | 1.32 | .01 |
| Heart rate (bpm) | 1.01 | 1.00 | 1.01 | .04 |
| LVSDn | 9.39 | 2.49 | 35.32 | <.001 |
Abbreviations: CI, confidence interval; LVSDn, left ventricular systolic diameter normalized to body weight.
4. DISCUSSION
Our aims were to investigate the presence and prognostic role of L waves, as well as that of selected clinical and echocardiographic variables in dogs with AF. For the first time in this species, we found that L waves often are detectable in dogs with AF, but their presence was not influenced by demographic, clinical, and echocardiographic variables. The presence of L waves did not influence the outcome in dogs with AF, but increased LVSDn and Echo‐HR were negative prognostic factors for cardiac death and, in addition to age, all‐cause mortality in these animals.
The mitral L waves represent forward mid‐diastolic flow recordable during diastasis using different echocardiographic techniques. 20 , 41 , 42 , 43 Because L waves coincide temporally with diastasis, their genesis has been attributed to blood flow moving from pulmonic veins, through the LA and into the LV after early rapid filling. 41 Blood flow during diastasis is intrinsically related to the transmitral pressure gradient and diastolic time. Accordingly, these 2 parameters can determine whether L waves are detectable or not. 37 , 41 In humans, L waves become evident in pathologic conditions characterized by decreased LV diastolic active relaxation, increased LV stiffness, increased filling pressures or some combination of these, such as LV hypertrophy, 38 , 43 , 44 severe mitral valve disease, 45 heart diseases characterized by severe LV systolic dysfunction, 22 , 43 and AF. 20 , 36 , 45 In these patients, detection of L waves has been associated with increased LA pressure, pseudonormal filling state, and higher probability of future hospitalization for heart failure and cardiac death. 20 , 21 , 38 , 43 , 44 In our study, L waves were identified in 60% of dogs with AF. Although this prevalence is similar to that described in dogs with advanced MMVD with sinus rhythm (59%), 23 it was higher than that reported in human patients with LV hypertrophy and sinus rhythm (32.4%), 38 and those affected by AF (34%). 20 Another difference between our findings and those from the human medical literature concerns the relationship between L waves and demographic, clinical, and echocardiographic findings. In our study, no difference was found when comparing selected variables from dogs with and without L waves. Conversely, human patients with L waves were older, predominantly female, and typically with higher transmitral filling pressures and larger LA volumes compared to those without L waves. 20 , 21 , 22 , 44 These discrepancies are likely because of species‐related differences as well as different structural cardiac diseases and their effect on diastolic function. Hence, L waves have been studied mainly in humans with LV hypertrophy and ischemic heart disease that generally cause more severe diastolic dysfunction compared to MMVD and DCM in dogs. 44 , 46 , 47 , 48
Despite the observed high prevalence of L waves, we could not identify a significant prognostic role in dogs with AF. Hence, neither was significantly different MST found between dogs with and without L waves nor was the presence of L waves associated with cardiac death or all‐cause mortality in Cox proportional hazards models. Some studies have determined that the presence of an L wave in human patients with AF was independently associated with an increase in adverse cardiac events, all‐cause mortality or both, but information on the underlying cardiac diseases was lacking. 20 , 21 The reasons for the discrepancy between these results and those of our study are not immediately clear and, again, could be a result of species‐related differences as well as different structural cardiac diseases and their effects on diastolic function. Although the number of dogs in our study that reached the end‐point was high for either cardiac death or all‐cause mortality, type II statistical error cannot be excluded because the sample size was relatively small.
Increased LVSDn and Echo‐HR were significantly associated with either cardiac death or all‐cause mortality in dogs in our study after multivariable evaluation. Furthermore, increased age also had a negative prognostic role for the end‐point all‐cause mortality. In particular, for each 1 unit increase of LVSDn there was 4‐fold and 9‐fold increased risk of cardiac death and all‐cause mortality, respectively, and each 1 beat per minute increase in Echo‐HR was associated with a 1% increase in both cardiac death and all‐cause mortality, whereas for each 1 year increase in age there was 17% increase in all‐cause mortality. An increase of LVSD has already been identified as a negative prognostic indicator in dogs with sinus rhythm and MMVD 49 as well as in those with DCM, 31 , 50 suggesting that this parameter has remarkable clinical consequences even before the onset of AF in dogs with cardiac diseases. Moreover, LVSDn is a moderately accurate predictor of the development of AF in dogs with MMVD, 14 indicating that it also plays a relevant role in the shift from sinus rhythm to AF. Our study identified the negative prognostic role of increased LVSDn in dogs with AF, confirming that this echocardiographic variable also acts as a reliable prognostic indicator in the advanced stages of the natural history of MMVD and DCM. In humans, an increased LVSD is independently associated with increased mortality in patients with mitral regurgitation under medical management and after mitral surgery. 51 Moreover, it is associated with rehospitalization and cardiovascular death in patients with heart failure and decreased LV ejection fraction, including those affected by DCM. 52 , 53 Additionally, this echocardiographic parameter acts as an early sign of systolic dysfunction, which often is associated with increased LV wall stress, LV end‐diastolic pressure as well as LA pressure and LA wall tension in humans. 54 , 55 These factors may help in understanding why dogs with increased LVSDn experience worse outcome compared to those with normal LVSDn. Regarding the link between AF and LV systolic compromise and worse prognosis, several pathophysiological mechanisms should be considered. First, during AF, lack of atrial systole decreases the ventricular preload, leading to decreased cardiac output. Second, the decreased LV filling indirectly affects contractility according to the Frank‐Starling mechanism with further compromise in cardiac output. Third, rapid sustained ventricular rate may lead to tachycardia‐induced cardiomyopathy, which may additionally contribute to LV maladaptive remodeling and dysfunction. 6 , 56 Concerning the negative prognostic role of increased Echo‐HR, this finding is consistent with previous studies investigating the negative effects of a high mean ventricular response assessed by 24‐hour Holter monitoring on survival in dogs with AF. 10 , 11 The method used in our study to determine the ventricular rate differs from that employed in previous investigations in dogs and Holter monitoring allows a more accurate evaluation of the ventricular response during secondary AF, a condition usually associated with high sympathetic tone. Regardless of the method of assessment, our results confirm that increased heart rate is harmful and inevitably associated with decreased MST in dogs with AF. Age represented an additional negative prognostic indicator for the end‐point all‐cause mortality. This finding overall agrees with previous data from dogs with MMVD, DCM, and AF, 57 , 58 , 59 and may be explained not only by higher susceptibility to adverse outcomes associated with extracardiac diseases in the elderly, but theoretically also to a higher susceptibility to negative effects of AF in adult‐to‐old dogs. Indeed, in humans, not only prevalence, but also morbidity of elderly patients suffering from AF are higher compared to those of younger individuals; moreover, these former patients tend to exhibit increased and longer hospitalization times because of cardiovascular complications. 60
Among echocardiographic variables not associated with a negative prognosis in our study, LA measurement deserves some consideration. Echocardiographic features of LA remodeling and dysfunction previously have been identified as important negative prognostic factors both in dogs with MMVD 49 , 57 , 59 and DCM. 50 Furthermore, increased LA size has been identified as a significant predisposing factor for AF development in dogs. 7 , 14 Nevertheless, none of the LA echocardiographic parameters investigated in our study reached statistical significance in the survival analysis. Hypothetically, this result could have been a consequence of the relatively limited number of measured LA variables. For example, we did not include LA volumetric measurement in our analysis, which had high accuracy in detecting LA enlargement and was an important negative prognostic factor in dogs with MMVD. 61 , 62 Similarly, advanced echocardiographic techniques, namely tissue Doppler imaging and speckle tracking echocardiography, were not employed in the dogs of our study. These echocardiographic modalities have been used to evaluate the LA function in dogs with MMVD associated with sinus rhythm 61 , 63 , 64 , 65 and in dogs with tachyarrhythmias, 66 and have been shown to be useful in predicting the onset of AF in this species. 12 , 67 In humans, LA analysis by tissue Doppler imaging and speckle tracking echocardiography has important prognostic relevance in patients with AF. 68 , 69 Although the prognostic role of these techniques remains to be established in dogs with AF, it cannot be excluded that the use of these modalities in our study could have expanded the list of prognostic factors. On the other hand, it is possible that LA enlargement, regardless of the methods employed for its measurement, is an inciting factor for AF development in dogs but, once AF has occurred, its clinical relevance may become secondary.
Concerning prognostic implications related to antiarrhythmic treatment, we could not identify any significant effect on survival of the considered treatment groups. In particular, no significant difference was found in the MST of dogs receiving digoxin and diltiazem compared to those treated with digoxin alone. The combination treatment digoxin and diltiazem represented the most frequently applied treatment in dogs in our study, followed by monotherapy with digoxin alone, whereas a third group of dogs received miscellaneous treatments. A previous retrospective study determined that administration of combination treatment digoxin and diltiazem significantly decreased median heart rate and significantly prolonged MST compared to monotherapy with diltiazem in medium to large‐sized dogs with MMVD and AF. 10 Furthermore, digoxin and diltiazem combination treatment provided better ventricular rate control than digoxin or diltiazem alone in dogs with AF and advanced heart disease. 70 Differences in study population and treatment comparison may explain the different results on MST of dogs in our study compared to those previously reported. 10 Evidence‐based prospective studies on antiarrhythmic treatment are lacking in the veterinary literature, and there is a need for well‐designed clinical studies comparing the effects of different treatments on survival in dogs with AF.
Our study had some limitations. First, we explored the presence of L waves at a single time point, which may represent a limitation because L waves may increase in size, disappear, and reappear over time in the same patient according to several dynamic variables, primarily the heart rate. 37 However, we identified L waves in the majority of dogs in our study, with a percentage similar to that of a previous study in dogs. Second, the choice of studying dogs with MMVD and DCM together may have biased potential statistically significant results that could be identified in studies designed to investigate the role of AF in each structural cardiac disease of dogs studied individually. Third, the method we used to assess heart rate based on 1‐lead recording during echocardiographic examination can overestimate the actual mean ventricular response in dogs with AF. 71 However, our study was not aimed at precisely determining the daily ventricular heart rate in dogs with AF, but rather to assess the prognostic role of several parameters that can be obtained during echocardiographic examination. Finally, the retrospective design precluded further classification of AF (i.e., as persistent, long‐standing, or permanent) as well as the standardization of therapeutic interventions.
In conclusion, we showed that L waves represent a common finding in dogs with MMVD and DCM complicated by AF, but their presence is not associated with a worse prognosis. Conversely, LVSDn and Echo‐HR represent 2 important negative prognostic factors for either cardiac death or all‐cause mortality in dogs with secondary AF. Advanced age also has a negative effect on all‐cause mortality in the same animals.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This was a retrospective study performed on nonexperimental animals that exclusively underwent selected noninvasive diagnostic procedures made necessary by their underlying heart disease (eg, echocardiography, electrocardiography). The study was approved by the Management Committee of the Veterinary Teaching Hospital of the University of Padua. All owners of dogs enrolled in the study gave informed written consent for all of the investigations.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1 Supporting information
Appendix S2 Supporting information
ACKNOWLEDGMENT
No funding was received for this study. Presented in part as an abstract at the 32th Congress of the European College of Veterinary Internal Medicine — Companion Animals, Gothenburg, Sweden, 1st‐3rd September 2022.
Romito G, Darida S, Valente C, et al. Prevalence and prognostic role of L wave and selected clinical and echocardiographic variables in dogs with atrial fibrillation. J Vet Intern Med. 2023;37(1):47‐57. doi: 10.1111/jvim.16584
REFERENCES
- 1. Buchanan JW. Spontaneous arrhythmias and conduction disturbances in domestic animals. Ann N Y Acad Sci. 1965;127:224‐238. [DOI] [PubMed] [Google Scholar]
- 2. Noszczyk‐Nowak A, Michałek M, Kałuża E, Cepiel A, Pasławska U. Prevalence of arrhythmias in dogs examined between 2008 and 2014. J Vet Res. 2017;61:103‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romito G, Guglielmini C, Poser H, Baron Toaldo M. Lorenz plot analysis in dogs with sinus rhythm and tachyarrhythmias. Animals (Basel). 2021;11:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hellemans A, Schittekatte M, Covents M, Smets P. Diagnosis and management of arrhythmias in dogs: a cross‐sectional online survey among Flemish veterinary practitioners. Vet Rec Open. 2022;9:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ward J, Ware W, Viall A. Association between atrial fibrillation and right‐sided manifestations of congestive heart failure in dogs with degenerative mitral valve disease or dilated cardiomyopathy. J Vet Cardiol. 2019;21:18‐27. [DOI] [PubMed] [Google Scholar]
- 6. Pedro B, Fontes‐Sousa AP, Gelzer AR. Canine atrial fibrillation: pathophysiology, epidemiology and classification. Vet J. 2020;265:105548. [DOI] [PubMed] [Google Scholar]
- 7. Guglielmini C, Chetboul V, Pietra M, et al. Influence of left atrial enlargement and body weight on the development of atrial fibrillation: retrospective study on 205 dogs. Vet J. 2000;160:235‐241. [DOI] [PubMed] [Google Scholar]
- 8. Menaut P, Bélanger MC, Beauchamp G, Ponzio NM, Moïse NS. Atrial fibrillation in dogs with and without structural or functional cardiac disease: a retrospective study of 109 cases. J Vet Cardiol. 2005;7:75‐83. [DOI] [PubMed] [Google Scholar]
- 9. Tidholm A, Jönsson L. A retrospective study of canine dilated cardiomyopathy (189 cases). J Am Anim Hosp Assoc. 1997;33:544‐550. [DOI] [PubMed] [Google Scholar]
- 10. Jung SW, Sun W, Griffiths LG, Kittleson MD. Atrial fibrillation as a prognostic indicator in medium to large‐sized dogs with myxomatous mitral valvular degeneration and congestive heart failure. J Vet Intern Med. 2016;30:51‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pedro B, Dukes‐McEwan J, Oyama MA, Kraus MS, Gelzer AR. Retrospective evaluation of the effect of heart rate on survival in dogs with atrial fibrillation. J Vet Intern Med. 2018;32:86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baron Toaldo M, Mazzoldi C, Romito G, et al. Echocardiographic predictors of first onset of atrial fibrillation in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2020;34:1787‐1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friederich J, Seuß AC, Wess G. The role of atrial fibrillation as a prognostic factor in Doberman pinschers with dilated cardiomyopathy and congestive heart failure. Vet J. 2020;264:105535. [DOI] [PubMed] [Google Scholar]
- 14. Guglielmini C, Goncalves Sousa M, Baron Toaldo M, et al. Prevalence and risk factors for atrial fibrillation in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2020;34:2223‐2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calvert CA, Pickus CW, Jacobs GJ, Brown J. Signalment, survival, and prognostic factors in Doberman pinschers with end‐stage cardiomyopathy. J Vet Intern Med. 1997;11:323‐326. [DOI] [PubMed] [Google Scholar]
- 16. Vollmar C, Vollmar A, Keene BW, Fox PR, Reese S, Kohn B. Dilated cardiomyopathy in 151 Irish wolfhounds: characteristic clinical findings, life expectancy and causes of death. Vet J. 2019;245:15‐21. [DOI] [PubMed] [Google Scholar]
- 17. Borgeat K, Pack M, Harris J, et al. Prevalence of sudden cardiac death in dogs with atrial fibrillation. J Vet Intern Med. 2021;35:2588‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papadopoulos CH, Oikonomidis D, Lazaris E, Nihoyannopoulos P. Echocardiography and cardiac arrhythmias. Hellenic J Cardiol. 2018;59:140‐149. [DOI] [PubMed] [Google Scholar]
- 19. Abe H, Kosugi S, Ozaki T, et al. Impact of echocardiographic congestion grade in HFpEF with and without atrial fibrillation. JACC Asia. 2022;2:73‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakai H, Takeuchi M, Nishikage T, Nagakura T, Otani S. The mitral L wave: a marker of advanced diastolic dysfunction in patients with atrial fibrillation. Circ J. 2007;71:1244‐1249. [DOI] [PubMed] [Google Scholar]
- 21. Su HM, Lin TH, Hsu PC, et al. Incremental prognostic value of identifying mitral L wave in patients with atrial fibrillation. Int J Cardiol. 2013;168:4501‐4503. [DOI] [PubMed] [Google Scholar]
- 22. Masai K, Mano T, Goda A, et al. Correlates and prognostic values of appearance of L wave in heart failure patients with preserved vs. reduced ejection fraction. Circ J. 2018;82:2311‐2316. [DOI] [PubMed] [Google Scholar]
- 23. Morgan KRS, Monteith G, Raheb S, Colpitts M, Fonfara S. Echocardiographic parameters for the assessment of congestive heart failure in dogs with myxomatous mitral valve disease and moderate to severe mitral regurgitation. Vet J. 2020;263:105518. [DOI] [PubMed] [Google Scholar]
- 24. Chetboul V, Tissier R. Echocardiographic assessment of canine degenerative mitral valve disease. J Vet Cardiol. 2012;14:127‐148. [DOI] [PubMed] [Google Scholar]
- 25. Keene BW, Atkins C, Bonagura J, et al. ACVIM consensus guidelines for diagnosis and treatment of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2019;33:1127‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bonagura JD, Visser LC. Echocardiographic assessment of dilated cardiomyopathy in dogs. J Vet Cardiol. 2022;40:15‐50. [DOI] [PubMed] [Google Scholar]
- 27. Wess G. Screening for dilated cardiomyopathy in dogs. J Vet Cardiol. 2022;40:51‐68. [DOI] [PubMed] [Google Scholar]
- 28. Wess G, Domenech O, Dukes‐McEwan J, Häggström J, Gordon S. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman pinschers. J Vet Cardiol. 2017;19:405‐415. [DOI] [PubMed] [Google Scholar]
- 29. Meurs KM, Stern JA, Sisson DD, et al. Association of dilated cardiomyopathy with the striatin mutation genotype in boxer dogs. J Vet Intern Med. 2013;27:1437‐1440. [DOI] [PubMed] [Google Scholar]
- 30. Wess G, Bauer A, Kopp A. Echocardiographic reference intervals for volumetric measurements of the left ventricle using the Simpson's method of disc in 1331 dogs. J Vet Intern Med. 2021;35:724‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Summerfield NJ, Boswood A, O'Grady MR, et al. Efficacy of pimobendan in the prevention of congestive heart failure or sudden death in Doberman pinschers with preclinical dilated cardiomyopathy (the PROTECT study). J Vet Intern Med. 2012;26:1337‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 33. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med. 2004;18:311‐321. [DOI] [PubMed] [Google Scholar]
- 34. Rishniw M. Erb HN evaluation of four 2‐dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med. 2000;14:429‐435. [DOI] [PubMed] [Google Scholar]
- 35. Marchesotti F, Vezzosi T, Tognetti R, et al. Left atrial anteroposterior diameter in dogs: reference interval, allometric scaling, and agreement with the left atrial‐to‐aortic root ratio. J Vet Med Sci. 2019;81:1655‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ari H, Ari S, Akkaya M, et al. A novel echocardiographic predictor of atrial fibrillation recurrence: L‐wave. Echocardiography. 2013;30:1180‐1186. [DOI] [PubMed] [Google Scholar]
- 37. Morisawa D, Ohno Y, Ohta Y, et al. Serial changes of L wave according to heart rates in a heart failure patient with persistent atrial fibrillation. J Cardiol Cases. 2019;20:213‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saito C, Minami Y, Arai K, et al. Prognostic significance of the mitral L‐wave in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2020;130:130‐136. [DOI] [PubMed] [Google Scholar]
- 39. Reinero C, Visser LC, Kellihan HB, et al. ACVIM consensus statement guidelines for the diagnosis, classification, treatment, and monitoring of pulmonary hypertension in dogs. J Vet Intern Med. 2020;34:549‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233‐270. [DOI] [PubMed] [Google Scholar]
- 41. Keren G, Meisner JS, Sherez J, Yellin EL, Laniado S. Interrelationship of mid‐diastolic mitral valve motion, pulmonary venous flow, and transmitral flow. Circulation. 1986;74:36‐44. [DOI] [PubMed] [Google Scholar]
- 42. Kerut EK. The mitral L‐wave: a relatively common but ignored useful finding. Echocardiography. 2008;25:548‐550. [DOI] [PubMed] [Google Scholar]
- 43. Ha JW, Ahn JA, Moon JY, et al. Triphasic mitral inflow velocity with mid‐diastolic flow: the presence of mid‐diastolic mitral annular velocity indicates advanced diastolic dysfunction. Eur J Echocardiogr. 2006;7:16‐21. [DOI] [PubMed] [Google Scholar]
- 44. Lam CSP, Han L, Ha JW, Oh JK, Ling LH. The mitral L wave: a marker of pseudonormal filling and predictor of heart failure in patients with left ventricular hypertrophy. J Am Soc Echocardiogr. 2005;18:336‐341. [DOI] [PubMed] [Google Scholar]
- 45. Bhasin D, Arora GK, Gupta A, Isser HS, Bansal S. L‐wave in severe mitral stenosis. Acta Cardiol. 2021;76:678‐679. [DOI] [PubMed] [Google Scholar]
- 46. Ohara T, Little WC. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr Opin Cardiol. 2010;25:613‐621. [DOI] [PubMed] [Google Scholar]
- 47. Camacho RR, Sousa MG, Franco RP, et al. Diastolic function is impaired in dogs with myxomatous mitral valve disease. Ars Veterinaria. 2016;32:16‐23. [Google Scholar]
- 48. Pilz PM, Ward JE, Chang WT, et al. Large and small animal models of heart failure with reduced ejection fraction. Circ Res. 2022;130:1888‐1905. [DOI] [PubMed] [Google Scholar]
- 49. Häggström J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124‐1135. [DOI] [PubMed] [Google Scholar]
- 50. Martin MW, Stafford Johnson MJ, Strehlau G, et al. Canine dilated cardiomyopathy: a retrospective study of prognostic findings in 367 clinical cases. J Small Anim Pract. 2010;51:428‐436. [DOI] [PubMed] [Google Scholar]
- 51. Tribouilloy C, Grigioni F, Avierinos JF, et al. Survival implication of left ventricular end‐systolic diameter in mitral regurgitation due to flail leaflets a long‐term follow‐up multicenter study. J Am Coll Cardiol. 2009;54:1961‐1968. [DOI] [PubMed] [Google Scholar]
- 52. Miura K, Matsumori A, Nasermoaddeli A, et al. Prognosis and prognostic factors in patients with idiopathic dilated cardiomyopathy in Japan. Circ J. 2008;72:343‐348. [DOI] [PubMed] [Google Scholar]
- 53. Ito K, Li S, Homma S, et al. Left ventricular dimensions and cardiovascular outcomes in systolic heart failure: the WARCEF trial. ESC Heart Fail. 2021;8:4997‐5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wakami K, Ohte N, Asada K, et al. Correlation between left ventricular end‐diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr. 2009;22:847‐851. [DOI] [PubMed] [Google Scholar]
- 55. Liao YC, Liao JN, Lo LW, et al. Left atrial size and left ventricular end‐systolic dimension predict the progression of paroxysmal atrial fibrillation after catheter ablation. J Cardiovasc Electrophysiol. 2017;28:23‐30. [DOI] [PubMed] [Google Scholar]
- 56. Manolis AA, Manolis TA, Melita H, et al. Atrial fibrillation‐induced tachycardiomyopathy and heart failure: an underappreciated and elusive condition. Heart Fail Rev. 2022;27:2119‐2135. doi: 10.1007/s10741-022-10221-1 [DOI] [PubMed] [Google Scholar]
- 57. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120‐128. [DOI] [PubMed] [Google Scholar]
- 58. Savarese A, Probo M, Locatelli C, et al. Iron status in dogs with myxomatous mitral valve disease. Pol J Vet Sci. 2018;21:507‐515. [DOI] [PubMed] [Google Scholar]
- 59. Vollmar C, Vollmar A, Keene B, Fox PR, Reese S, Kohn B. Irish wolfhounds with subclinical atrial fibrillation: progression of disease and causes of death. J Vet Cardiol. 2019;24:48‐57. [DOI] [PubMed] [Google Scholar]
- 60. Karamichalakis N, Letsas KP, Vlachos K, et al. Managing atrial fibrillation in the very elderly patient: challenges and solutions. Vasc Health Risk Manag. 2015;11:555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baron Toaldo M, Romito G, Guglielmini C, et al. Prognostic value of echocardiographic indices of left atrial morphology and function in dogs with myxomatous mitral valve disease. J Vet Intern Med. 2018;32:914‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tidholm A, Häggström J. Prognostic value of selected one‐, two‐ and three‐dimensional and Doppler echocardiographic methods to assess severity in dogs with myxomatous mitral valve disease. J Vet Cardiol. 2022;39:89‐101. [DOI] [PubMed] [Google Scholar]
- 63. Nakamura K, Kawamoto S, Osuga T, et al. Left atrial strain at different stages of myxomatous mitral valve disease in dogs. J Vet Intern Med. 2017;31:316‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baron Toaldo M, Romito G, Guglielmini C, et al. Assessment of left atrial deformation and function by 2‐dimensional speckle tracking echocardiography in healthy dogs and dogs with myxomatous mitral valve disease. J Vet Intern Med. 2017;31:641‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Caivano C, Rishniw M, Birettoni F, et al. Left atrial deformation and phasic function determined by two‐dimensional speckle‐tracking echocardiography in dogs with myxomatous mitral valve disease. J Vet Cardiol. 2018;20:102‐114. [DOI] [PubMed] [Google Scholar]
- 66. Baron Toaldo M, Romito G, Cipone M, Diana A, Tursi M. Electrocardiographic, echocardiographic, and left atrial strain imaging features of a dog with atrial flutter and third‐degree atrioventricular block. J Vet Cardiol. 2017;19:462‐468. [DOI] [PubMed] [Google Scholar]
- 67. Neves J, Pedro B, Christley R, Dukes‐McEwan J. Usefulness of pulsed‐wave tissue Doppler imaging at the mitral annulus for prediction of new‐onset atrial fibrillation in dogs. J Vet Cardiol. 2018;20:425‐437. [DOI] [PubMed] [Google Scholar]
- 68. Jain V, Ghosh R, Gupta M, et al. Contemporary narrative review on left atrial strain mechanics in echocardiography: cardiomyopathy, valvular heart disease and beyond. Cardiovasc Diagn Ther. 2021;11:924‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pastore MC, De Carli G, Mandoli GE, et al. The prognostic role of speckle tracking echocardiography in clinical practice: evidence and reference values from the literature. Heart Fail Rev. 2021;26:1371‐1381. [DOI] [PubMed] [Google Scholar]
- 70. Gelzer AR, Kraus MS, Rishniw M, et al. Combination therapy with digoxin and diltiazem controls ventricular rate in chronic atrial fibrillation in dogs better than digoxin or diltiazem monotherapy: a randomized crossover study in 18 dogs. J Vet Intern Med. 2009;23:499‐508. [DOI] [PubMed] [Google Scholar]
- 71. Gelzer AR, Kraus MS, Rishniw M. Evaluation of in‐hospital electrocardiography versus 24‐hour Holter for rate control in dogs with atrial fibrillation. J Small Anim Pract. 2015;56:456‐462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Appendix S2 Supporting information


