Abstract
Background
In Belgian Malinois, a KCNJ10 variant causes progressive spinocerebellar degeneration.
Hypothesis/Objectives
Describe the clinical, diagnostic, pathological and genetic features of spinocerebellar degeneration in the Bouvier des Ardennes breed.
Animals
Five affected Bouvier des Ardennes puppies with spinocerebellar ataxia (SCA), 8 healthy related dogs, and 63 healthy unrelated Bouvier des Ardennes.
Methods
Sequential case study.
Results
Clinical signs started at 6 weeks of age in 1 puppy with severe signs of cerebellar disease, and at 7 to 10 weeks of age in the 4 remaining puppies with milder signs of spinocerebellar disease. The first puppy displayed severe intention tremors and rapidly progressive generalized hypermetric ataxia, whereas the 4 others developed a milder progressive SCA. Euthanasia after progression to nonambulatory status was performed by 8 weeks of age in the first puppy, and before 11 months of age in the 4 remaining puppies. Histopathology revealed cerebellar spongy degeneration and a focal symmetrical demyelinating myelopathy. All cases were homozygous for KCNJ10 XM_545752.6:c.986T>C(p.(Leu329Pro)), which is pathogenic for SCA with (or without) myokymia, seizures or both (SAMS) and spongy degeneration and cerebellar ataxia (SDCA) 1 in Belgian Malinois dogs. All sampled parents were heterozygous and none of the healthy dogs were homozygous for this recessive variant. This variant has an allele frequency of 15% in the 63 healthy dogs studied.
Conclusions and Clinical Importance
Inherited spinocerebellar degeneration also affects the Bouvier des Ardennes breed and is caused by a KCNJ10 variant. It can present with a spectrum of severity grades, ranging from severe cerebellar to milder spinocerebellar signs.
Keywords: demyelinating myelopathy, hypermetric ataxia, intention tremors, SAMS, SDCA, spongy degeneration
Abbreviations
- ATP1B2
ATPase Na+/K+ transporting beta‐2 polypeptide
- BEAR
brainstem auditory evoked responses
- CAPN1
calpain 1
- DAB
diaminobenzidine
- EDTA
ethylenediaminetetraacetic acid
- GFAP
glial fibrillary acidic protein
- GRM1
glutamate receptor metabotropic 1
- HE
hematoxylin eosin
- IHC
immunohistochemistry
- ITPR1
inositol 1,4,5‐triphosphate receptor, type 1
- JRT
Jack Russell Terrier
- KCNIP4
potassium channel‐interacting protein 4
- KCNJ10
potassium inwardly rectifying channel subfamily J member 10
- LFB
luxol fast blue
- MRI
magnetic resonance imaging
- NF200
total neurofilaments
- OMIA
Online Mendelian Inheritance in Animals
- OMIM
Online Mendelian Inheritance in Man
- PBS
phosphate‐buffer saline
- PRT
Parson Russell Terrier
- SAMS
spinocerebellar ataxia with (or without) myokymia, seizures or both
- SCA
spinocerebellar ataxia
- SCN8A
sodium voltage gated channel, alpha subunit 8
- SDCA
spongy degeneration and cerebellar ataxia
- SLC12A6
solute carrier family 12 (potassium/chloride transporter), member 6
1. INTRODUCTION
Spinocerebellar ataxia (SCA) with myokymia, seizures or both (SAMS) is a form of hereditary ataxia reported in several breeds, including the Jack Russell Terrier (JRT), 1 Parson Russell Terrier (PRT), 2 Smooth Haired and Toy Fox Terriers, 3 , 4 Patterdale Terrier 5 and Belgian Malinois Shepherd (also termed “SeSAME/EAST homologue” in this breed). 6 This syndrome is typically caused by variants in the KCNJ10 gene, which encodes for a voltage‐gated potassium channel that is highly expressed in the brain, spinal cord, inner ear and kidneys. 7 Dogs affected with SAMS can present with SCA only, or alternatively have combinations of SCA, myokymia, neuromyotonia and epileptic seizures. 2 , 4 , 5 , 6 Three different canine KCNJ10 variants have been described so far, the c.627C>G variant, 2 , 4 causal for SAMS in the JRT, PRT, Smooth Haired and Toy Fox Terriers as well as the Patterdale Terrier 5 breeds, the g.22141027insC variant, 8 also causal for SAMS in the JRT and PRT breeds, and the c.986T>C variant in the Belgian Malinois breed. 6 In the Belgian Malinois, this genetic variant causes SAMS 6 as well as spongy degeneration and cerebellar ataxia (SDCA) 1, 9 , 10 which likely represent different severity grades of the same disease. In this report, we describe the clinical, diagnostic, pathological and genetic features of 5 related Bouvier des Ardennes puppies with inherited SCA.
2. MATERIAL AND METHODS
2.1. Clinical examination
This case series was designed as a sequential hospital‐based case study with follow‐up. Two 7‐week old male intact Bouviers des Ardennes siblings were presented at the Small Animal Department of the Faculty of Veterinary Medicine of Ghent University for an uncoordinated gait since the age of 6 weeks (dog 1) and 7 weeks (dog 2). Two related Bouviers des Ardennes siblings (a male and a female) were presented at the Small Animal Clinic of the Faculty of Veterinary Medicine of the University of Liège (dogs 3 and 4) at 4 and 6 months of age respectively. A clinical and neurological examination was performed in all 4 dogs. A complete bloodwork including electrolytes, Toxoplasma gondii and Neospora caninum serologies was performed in dogs 1 and 4, and brainstem auditory evoked responses (BAER) were recorded in dogs 1 and 2. The BAER test was performed under sedation with a commercially available electrophysiological unit (Natus Synergy UltraPro, Acertys Healthcare NV, Aartselaar, Belgium). A 1,5 T magnetic resonance imaging (MRI) of the caudal cranial fossa, cervical and cranial thoracic spinal cord was performed in dog 3 (Siemens Magnetom, Siemens Healthcare). A video from 1 more related 5‐month old Bouvier des Ardennes female puppy was assessed (dog 5).
2.2. Pathological examination
A full necropsy was performed in dogs 1 and 2, immediately following euthanasia. Samples from the brain, spinal cord, femoral nerve roots and nerves as well as skeletal muscle from the quadriceps were collected, formalin‐fixed paraffin‐embedded and stained with hematoxylin‐eosin (HE) as well as luxol fast blue (LFB) and Nissl stain. Immunohistochemistry (IHC) was performed using neuronal (total neurofilaments, NF200) and glial (glial fibrillary acidic protein [GFAP] for astrocytes and Iba1 for microglia) markers. Technical details are available in Video S1. Sections of 5 μm thicknesses were mounted on capillary glass slides, deparaffinized and rinsed with water. Primary antibodies used are summarized in Table 1. Sections were pretreated in 10 mM citrate buffer pH 6.0 for 20 minutes at 96°C, cooled 30 minutes at room temperature and rinsed in phosphate‐buffer saline (PBS). Previously, sections were treated 35 minutes with 3% peroxidase to block endogenous peroxydase activity. Nonspecific binding was blocked by normal goat or rabbit serum 30% diluted with PBS for 1 hour. Samples were incubated with primary antibodies 40 minutes at room temperature. Sections were therefore rinsed with PBS and incubated during 40 minutes with a labeled polymer according to the manufacturer's instructions (mouse kit K4007 or rabbit kit K011, Dakocytomation). For Iba1, the incubation was performed using a secondary goat anti‐rabbit antibody and then with a standard ABC Peroxidase staining kit (Thermo Scientific, kit number 32020) diluted with PBS for 1 hour at room temperature. Staining was completed by a 10‐minute incubation with 3,3′‐diaminobenzidine (DAB) and counterstained in hematoxylin for 3 seconds. The positive control used was normal canine brain tissue including gray matter and white matter. In all experiments, negative controls were done by omitting the primary antibody.
TABLE 1.
Overview of described canine variants potentially involved in the phenotype of the affected Bouviers des Ardennes
| OMIA ID 12 | Gene ID | Gene symbol | Variant | Reference | OMIM gene ID 13 |
|---|---|---|---|---|---|
| 002110‐9615 | 489479 | ATP1B2 a | NC_006587.3(XM_546597.6):c.130_131insLT796559.1:g.50‐276 | Mauri et al, 2017 14 | 182331 |
| 001820‐9615 | 483745 | CAPN1 a | NC_006600.3(XM_540866.5):c.344G>A | Forman et al, 2013 15 | 114 220 |
| 000078‐9615 | 484024 | GRM1 b | NC_006583.3(XM_005615490.3):c.2331_2332ins62bp | Zeng et al, 2011 16 | 604473 |
| 002097‐9615 | 476548 | ITPR1 b | NC_006602.3:g.12880740CTT[7_651] | Forman et al, 2015 17 | 147265 |
| 002240‐9615 | 479129 | KCNIP4 b | NC_006585.3(XM_005618660.3):c.436T>C | Jenkins et al, 2020 18 | 608182 |
| 002089‐9615 | 488635 | KCNJ10 a | NC_006620.3(XM_545752.6):c.627C>G | Gilliam et al, 2014 2 | 602208 |
| 002089‐9615 | 488635 | KCNJ10 a | NC_006620.3:g. 22141027_22141028insC | Gast et al, 2016 8 | 602208 |
| 002089‐9615 | 488635 | KCNJ10 a | NC_006620.3(XM_545752.6):c.986T>C | Van Poucke et al, 2017 6 | 602208 |
| 002194‐9615 | 477604 | SCN8A a | NC_006609.3(XM_022411522.1):c.4898G>T | Letko et al, 2019 19 | 600702 |
| 002279‐9615 | 478239 | SLC12A6 a | NC_006612.3(XM_014109414.2):c.178_181delinsCATCTCACTCAT | Van Poucke et al, 2019 20 | 604878 |
Known canine variants that were hypothesized to possibly account for the different phenotypes between the cases.
Additional known canine variants that could cause the earlier onset of abnormal gait observed in some of the cases.
2.3. Genetic analysis
An EDTA blood sample were obtained from the 5 affected puppies as well as 8 healthy closely related dogs (Figure 1), and from 63 healthy Belgian Bouviers des Ardennes with owner consent. Given the small size of the Bouvier des Ardennes sample in Belgium, these 63 dogs are almost all related to some extent to the affected families. Genotyping assays for variants known to be able to cause hereditary ataxia, or affect the earlier onset and increased severity of the clinical signs as observed in some of the cases, were performed as described in their respective papers (Table 1). The complete KCNJ10 coding sequence was also screened for new onset‐effecting variants in the affected dogs via Sanger sequencing as described previously. 6
FIGURE 1.
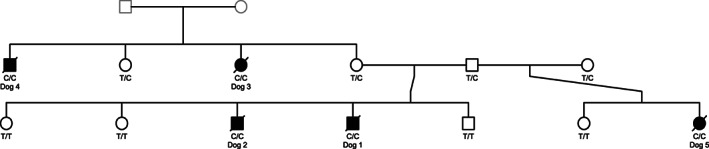
Pedigree information of the affected Bouvier des Ardennes family, drawn with the kinship2 package in RStudio. 11 Squares represent males, circles females, black icons sampled dogs, gray icons nonsampled dogs, shaded icons cases, nonshaded icons healthy dogs, strikethrough icons deceased dogs. The KCNJ10 XM_545752.6:c.986T>C genotype is added for all sampled dogs
3. RESULTS
3.1. Clinical features
Abnormalities were not detected on general clinical examination in all 4 examined puppies. Neurological examination at 7 weeks of age revealed a severe generalized borderline ambulatory hypermetric ataxia with pronounced truncal sway, mildly decreased proprioception in both pelvic limbs, severe intention tremors, bilateral absent patellar reflexes and menace responses in dog 1, suggestive for cerebellar as well as L4‐L6 spinal cord segment or femoral nerve roots or nerves dysfunction. Dog 2 showed a wide‐based stance as well as bilateral absent patellar reflexes and menace responses but only minimal ataxia (Video S1). Dog 1 was humanely euthanized a few days later because of the rapid progression and severity of his clinical signs. By 4 months of age, the clinical signs of dog 2 had progressed to a clear hypermetric and dysmetric generalized ataxia worse on the hind limbs, truncal sway and mild intention tremors (Video S2), which were similar to the clinical signs of dogs 3 (4 months of age) and 4 (6 months of age) at presentation, and on the video of dog 5 (5 months of age). These clinical signs were suggestive of spinocerebellar as well as L4‐L6 spinal cord segment or femoral nerve roots or nerves dysfunction. The owners of dog 4 reported a 20‐minute episode of rigidity and tremors in lateral recumbency with preserved consciousness which caused the dog to develop a life threatening hyperthermia of 42°C. Dogs 2 to 5 progressed to nonambulatory status because of their pronounced ataxia (Video S3) between 7 and 11 months of age, and were therefore euthanized.
CBC and serum biochemistry including electrolytes (Na2+, K+, Cl−, Ca2+) did not reveal any abnormalities, and N. caninum and T. gondii serologies were negative in dogs 1 and 4. BAER recordings revealed loss of waves III/VI and V in dogs 1 and 2 (Figure 2). The MRI in dog 3 did not reveal any abnormalities at the level of the cerebellum nor the cervical spinal cord.
FIGURE 2.
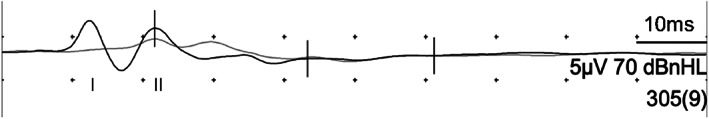
BAER recordings from dog 1 showing disappearance of waves III/VI and V
3.2. Pathological features
Necropsy was performed on dog 1 and 2 at respectively 8 weeks and 7 months of age. No macroscopic abnormalities were found. On histopathology, cerebellar spongy degeneration as well as a bilateral symmetrical demyelinating myelopathy targeting the dorsolateral spinocerebellar tracts was found in both dogs. Middle to small, different sized vacuoles apparently empty or containing cellular debris, were mainly found in the granular layer of the cerebellum, intracellularly located in Purkinje cells and in the neuronal bodies of the cerebellar nuclei. Purkinje cells were decreased in number and the remaining ones had a degenerating appearance. Associated gliosis was present and confirmed by IHC in all affected areas. A few differences were noticed between these 2 dogs. In dog 1 (Figure 3), microgliosis was moderate and restricted to cerebellar spongiotic areas. Spinal spinocerebellar tracts were also spongiotic, losing myelin and axonal components. The femoral ventral nerve roots showed a proliferative astrocytosis associated with spongiotic changes.
FIGURE 3.
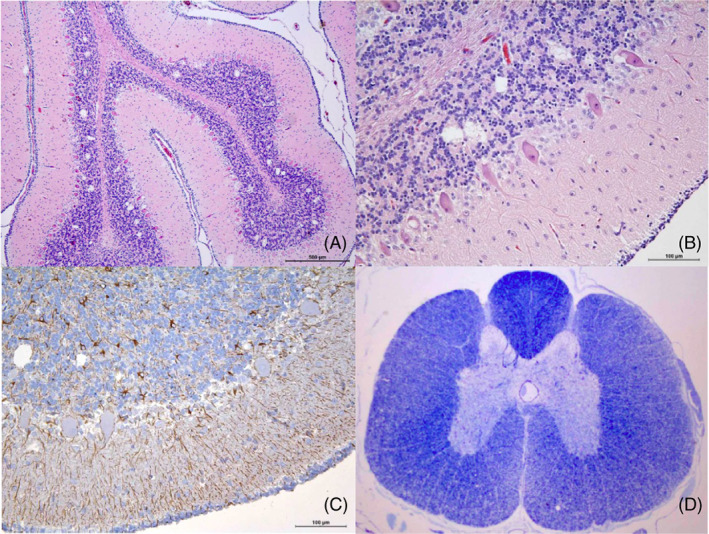
Histopathology of dog 1 at 8 weeks of age: (A and B) HE stain of the cerebellar cortex, vacuoles are restricted mainly to granular layer or intraneuronal in Purkinje cells; (C) GFAP immunopositives reactive astrocytes in the cerebellar cortex; (D) LFB stain of the spinal cord shows pale areas in the lateral and ventral funiculi
In dog 2 (Figure 4), Purkinje cells were diminished in number showing mild intracellular vacuoles. Degeneration of some neuronal bodies in the cerebellar nuclei, as well as spheroids in the cerebellar cortical white matter were evident. Cerebellar peduncles, pyramids and pyramidal tracts, trapezoid body and associated tracts showed marked spongiotic changes. All funiculi were affected showing a marked spongiotic pattern mainly affecting the spinocerebellar, cuneate, and corticospinal tracts. In these areas, myelin vacuolation and loss and a markedly diminished number of axons were detected. A strong and diffuse reactive microgliosis with microglial nodule formation was also associated with all spinal spongiotic areas. The femoral ventral nerve roots showed moderate to mild astrocytosis associated to spongiotic areas.
FIGURE 4.
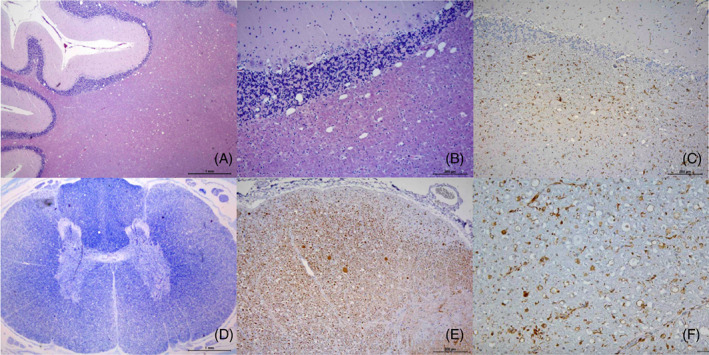
Histopathology of dog 2 at 7 months of age: (A and B) HE stain shows spongiosis in the cortical white matter, and infiltrating the cortical cerebellar granular layer. (C) IHC for Iba1 shows reactive microglial cells associated to spongiotic cerebellar area; (D) LFB stain of the spinal cord shows marked pallor of lateral and ventral funiculi. (E and F) IHC of the dorsal spinocerebellar tract of the cervical spinal cord. Absence or decreased immune positivity for NF200 (E) evidence loose of axonal component, some spheroids are also present. Marked immune positivity for Iba1 (F) in that area corresponding to reactive microgliosis is also evident
3.3. Genetics
All 5 affected dogs were homozygous for the KCNJ10 XM_545752.6:c.986T>C missense variant, previously reported to be pathogenic for SAMS and SDCA1 in the Belgian Malinois dog breed. 6 , 10 All sampled parents were heterozygous and none of the healthy dogs were homozygous for this recessive variant (Figure 1 and Table 2). This variant has an estimated allele frequency of 15% in the Belgian Bouviers des Ardennes study sample (Table 2).
TABLE 2.
Overview of the KCNJ10 XM_545752.6:c.986T>C genotypes in the 5 affected dogs, 8 healthy family members and 63 other healthy Belgian Bouviers des Ardennes, as well as the calculated C‐allele frequencies
| c.986T>C genotype | T/T | T/C | C/C | Total | C‐allele frequency |
|---|---|---|---|---|---|
| Cases | 0 | 0 | 5 | 5 | 100% |
| Healthy (family members) | 4 | 4 | 0 | 8 | 25% |
| Healthy (other) | 44 | 19 | 0 | 63 | 15% |
No additional variant was found in the KCNJ10 coding sequence of any of the 5 affected cases. No other tested variant known to be able to cause the observed spinocerebellar degeneration, or affect the earlier onset and increased severity of the clinical signs as observed in some of the cases (Table 1), was present in any of the 5 cases.
4. DISCUSSION
Two clinical presentations were seen in our 5 affected dogs. Dog 1 had very severe early‐onset and fast progressing cerebellar signs, which much resembles SDCA1 in Belgian Malinois dogs. SDCA1 is characterized by severe generalized cerebellar (hypermetric) ataxia, stumbling and falling as well as severe intention tremors, with an onset at 4 to 8 weeks of age and euthanasia of all puppies by 13 to 17 weeks of age. Also the histopathological findings of dog 1 are similar to the ones described in SDCA1, where the main lesions are localized in the cerebellum and include vacuolization of the cerebellar nuclei, Purkinje cells, granular cell layer and white matter, whereas much less severe or no lesions are found in the spinal cord. 9 , 10
Dogs 2 to 5 presented with much milder signs at onset, and showed a much slower progression, more resembling SAMS (also termed “SeSAME/EAST homologue” in this breed) which was also described in the Belgian Malinois breed. This disease is characterized by generalized (hypermetric) SCA with an onset at 6 to 8 weeks of age, a consistent feature in these patients is the absence of patellar reflexes. In some cases, the SCA is associated with a variable combination of myokymia, neuromyotonia and epileptic seizures. No myokymia nor seizures were seen in our Bouvier des Ardennes patients, even though the description of the episode of rigidity and hyperthermia with preserved consciousness in dog 4 could have been compatible with an episode of neuromyotonia. In the Belgian Malinois dog, progression of the ataxia results in nonambulatory status and euthanasia before 6 months of age in most cases, which is a bit earlier than in our patients (7‐11 months of age). The predominant histopathological lesion found in dogs with SAMS is a bilateral myelopathy with a predominant axonopathy and myelin vacuolization, most severe in the corticospinal tracts, whereas similar but less severe lesions are found in the cerebellar white matter, 6 which resembles the histopathological findings of dog 2.
SDCA1 and SAMS are believed to be part of a continuous spectrum of severity grade of one same disease, as demonstrated genetically by the fact that they are caused by the same KCNJ10 c.986T>C (p.(Leu329Pro)) variant in the Belgian Malinois breed. The KCNJ10 c.986T>C variant was considered a strong genetic candidate after recognition of these different severity phenotypes in our Bouvier des Ardennes patients. Phenotypic variability has been reported in dogs and humans with KCNJ10 variants. 1 , 6 , 9 , 10 , 21 , 22 , 23 In humans, the presence of residual channel function or compensatory mechanisms in certain organs have been shown to contribute to this phenotypic heterogeneity within KCNJ10 variants. 24 It was therefore hypothesized that this different phenotypic severity could be influenced by a combination of the KCNJ10 variant with another variant. Testing of several other variants known to cause hereditary ataxia and resequencing the complete KCNJ10 coding sequence was therefore conducted in our 5 affected dogs, but no additional variant was found.
The Bouvier des Ardennes breed was well established in Belgium in the 19th century. The breed almost became extinct at the beginning of the 20th century wherefore the current, limited Bouvier des Ardennes population (estimated to be only few hundreds individuals in the world [unpublished data, Club Belge du Bouvier des Flandres et des Ardennes]) originates from just a few individuals that were discovered in Belgian farms in 1985. 25 , 26 Investigating the breeding schemes revealed that some Bouvier des Flandres and Belgian Malinois dogs were used to strengthen the Bouvier des Ardennes blood lines in the early 21th century, which explains why a variant reported in the Belgian Malinois dog breed 6 , 10 was found in the Bouvier des Ardennes breed. This bottleneck effect has caused the current Belgian Bouvier des Ardennes population to be quite closely related, which probably accounts for the high allele frequency (around the 15%) of this KCNJ10 variant in the current study. 27 This study is an interesting example of how a certain variant's presence in a particular breed can uncover some details about its ancestry.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval was granted by the local ethical (Faculty of Veterinary Medicine, Ghent University, Belgium) and deontological (Federal Public Service Health, Food Chain Safety and Environment, Brussels, Belgium) committees (EC2017/86) for the use of all left‐over samples in this study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1. Technical details about the different antibodies used for the IHC of dogs 1 and 2.
Video S1. Dogs 1 and 2 at 8 weeks of age: a severe generalized borderline ambulatory hypermetric ataxia with pronounced truncal is evident in dog 1, whereas only minimal ataxia is noticeable in dog 2.
Video S2. Dog 2 at 4 months of age: the clinical signs have progressed into a wide based stance, truncal sway and clear hypermetric and dysmetric generalized ataxia, worse on the hind limbs.
Video S3. Dog 2 at 7 months of age: the clinical signs have now progressed to profound ataxia and truncal sway, resulting in nonambulatory status.
ACKNOWLEDGMENT
No funding was received for this study.
Stee K, Van Poucke M, Pumarola M, et al. Spinocerebellar ataxia in the Bouvier des Ardennes breed is caused by a KCNJ10 missense variant. J Vet Intern Med. 2023;37(1):216‐222. doi: 10.1111/jvim.16594
Kimberley Stee and Mario Van Poucke contributed equally to this manuscript.
DATA AVAILABILITY STATEMENT
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
REFERENCES
- 1. Bhatti SF, Vanhaesebrouck AE, Van Soens I, et al. Myokymia and neuromyotonia in 37 Jack Russell terriers. Vet J. 2011;189:284‐288. [DOI] [PubMed] [Google Scholar]
- 2. Gilliam D, O'Brien DP, Coates JR, et al. A homozygous KCNJ10 mutation in Jack Russell terrier and related breeds with spinocerebellar ataxia with myokymia, seizures or both. J Vet Intern Med. 2014;28:871‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rohdin C, Lüdtke L, Wohlstein P, et al. New aspects of hereditary ataxia in smooth‐haired fox terriers. Vet Rec. 2010;166:557‐560. [DOI] [PubMed] [Google Scholar]
- 4. Rohdin C, Gilliam D, O'Leary CA, et al. A KCNJ10 mutation previously identified in the Russell group of terriers also occurs in smooth‐haired fox terriers with hereditary ataxia an in related breeds. Acta Vet Scand. 2015;57:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher C, Liebel F. Neuromyotonia, myokymia and spinocerebellar ataxia in two Patterdale terrier littermates. Vet Rec. 2022; 10(4):e439. [Google Scholar]
- 6. Van Poucke M, Stee K, Bhatti SFM, et al. The novel homozygous KCNJ10 c.986T>C (p.(Leu329Pro)) variant is pathogenic for the SeSAME/EAST homologue in Malinois dogs. Eur J Hum Genet. 2017;25:222‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salal‐Rabanal M, Kucheryavykh LY, Skatchkov SN, et al. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10). J Biol Chem. 2010;285:36040‐36048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gast AC, Metzger J, Tipold A, Distl O. Genome‐wide association study for hereditary ataxia in the Parson Russell Terrier and DNA‐testing for ataxia‐associated mutations in the Parson and Jack Russell Terrier. BMC Vet Res. 2016;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kleiter M, Högler S, Kneissl S, Url A, Leschnik M. Spongy degeneration with cerebellar ataxia in Malinois puppies: a hereditary autosomal recessive disorder? J Vet Intern Med. 2011;25:490‐496. [DOI] [PubMed] [Google Scholar]
- 10. Mauri N, Kleiter M, Leschnik M, et al. A missense variant in KCNJ10 in Belgian shepherd dogs affected by spongy degeneration with cerebellar ataxia (SDCA1). G3. 2017;7:663‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinnwell JP, Therneau TM, Schaid DJ. The kinship2 R package for pedigree data. Hum Hered. 2014;78:91‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. http://omia.angis.org.au/. Accessed March 2022.
- 13. https://omim.org/. Accessed March 2022.
- 14. Mauri N, Kleiter M, Dietschi E, et al. A SINE insertion in ATP1B2 in Belgian shepherd dogs affected by spongy degeneration with cerebellar ataxia (SDCA2). G3. 2017;7:2729‐2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forman OP, De Risio L, Mellersh CS. Missense mutation in CAPN1 is associated with spinocerebellar ataxia in the Parson Russell Terrier dog breed. PLoS One. 2013;8:e64627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng R, Farias FHG, Johnson GS, et al. A truncated retrotransposon disrupts the GRM1 coding sequence in Coton de Tulear dogs with Bandera's neonatal ataxia. J Vet Intern Med. 2011;25:267‐272. [DOI] [PubMed] [Google Scholar]
- 17. Forman OP, De Risio L, Matiasek K, et al. Spinocerebellar ataxia in the Italian Spinone dog is associated with an intronic GAA repeat expansion in ITPR1. Mamm Genome. 2015;26:108‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jenkins CA, Kalmar L, Matiasek K, et al. Characterisation of canine KCNIP4: a novel gene for cerebellar ataxia identified by whole‐genome sequencing two affected Norwegian Buhund dogs. PLoS Genet. 2020;16:e1008527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Letko A, Dietschi E, Nieburg M, et al. A missense variant in SCN8A in alpine dachsbracke dogs affected by spinocerebellar ataxia. Genes. 2019;10:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Poucke M, Stee K, Sonck L, et al. Truncating SLC12A6 variants cause different clinical phenotypes in humans and dogs. Eur J Hum Genet. 2019;27:1561‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. New Engl J Med. 2009;360:1960‐1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scholl UI, Choi M, Liu T, et al. Seizures, sensorineural deafness, ataxia, mental retardation and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842‐5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cross JH, Arora R, Heckemann RA, et al. Neurological features of epilepsy, ataxia, sensorineural deafness, tubulopathy syndrome. Dev Med Child Neurol. 2013;55:846‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morin M, Forst AL, Pérez‐Torre P, et al. Novel mutations in the KCNJ10 gene associated to a distinctive ataxia, sensorineural hearing loss and spasticity clinical phenotype. Neurogenetics. 2020;21:135‐143. [DOI] [PubMed] [Google Scholar]
- 25. https://www.ukcdogs.com/bouvier-des-ardennes. Accessed March 2022.
- 26. http://www.fci.be/Nomenclature/Standards/171g01-en.pdf. Accessed March 2022.
- 27. Wijnrocx K, François L, Stinckens A, Janssens S, Buys N. Half of 23 Belgian dog breeds has a compromised genetic diversity, as revealed by genealogical and molecular data analysis. J Anim Breed Genet. 2016;133:375‐383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Technical details about the different antibodies used for the IHC of dogs 1 and 2.
Video S1. Dogs 1 and 2 at 8 weeks of age: a severe generalized borderline ambulatory hypermetric ataxia with pronounced truncal is evident in dog 1, whereas only minimal ataxia is noticeable in dog 2.
Video S2. Dog 2 at 4 months of age: the clinical signs have progressed into a wide based stance, truncal sway and clear hypermetric and dysmetric generalized ataxia, worse on the hind limbs.
Video S3. Dog 2 at 7 months of age: the clinical signs have now progressed to profound ataxia and truncal sway, resulting in nonambulatory status.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.


