Abstract
Background
Vector‐borne diseases are of increasing importance in Germany. Since 2015, autochthonous cases have been increasingly documented in Berlin/Brandenburg.
Objectives
Describe autochthonous Babesia canis infection in the Berlin/Brandenburg region.
Animals
Forty‐nine dogs with autochthonous B. canis infection.
Methods
Evaluation of history, clinical signs, laboratory abnormalities, treatment, and outcome.
Results
Dogs were presented between March and August (9) and September and January (40) in the years 2015‐2021. Historical and clinical findings were lethargy (100%), pale mucous membranes (63%), fever (50%), and pigmenturia (52%). Common clinicopathological findings were thrombocytopenia (100%), anemia (85%), intravascular hemolysis (52%), pancytopenia (41%), and systemic inflammatory response syndrome (SIRS; 37%). Babesia detection was based on blood smear evaluation (n = 40) and PCR targeting the 18S rRNA gene of piroplasms (n = 49). Sequencing indicated 99.47% to 100% identity to B. canis sequences from GenBank. All dogs were treated with imidocarb (2.4‐6.3 mg/kg; median, 5 mg/kg); 8 dogs received 1, 35 received 2, and 1 dog each received 3, 4, or 5 injections, respectively. Continued PCR‐positive results were detected in 7 dogs after the 1st, in 5 after the 2nd, in 2 after the 3rd, and in 1 28 days after the 4th injection. Four dogs were euthanized and 3 dogs died.
Conclusions and Clinical Importance
Autochthonous B. canis infections in Berlin/Brandenburg were associated with severe clinicopathological changes, SIRS, and multiorgan involvement. Testing by PCR during and after treatment is advisable to monitor treatment success. Screening of blood donors in high‐risk areas and year‐round tick protection is strongly recommended.
Keywords: canine babesiosis, complications, genotype, vector‐borne disease
Abbreviations
- aPTT
activated partial thromboplastin time
- CRP
canine C‐reactive protein
- cTnI
canine troponin I
- DIC
disseminated intravascular coagulation
- Hct
hematocrit
- PT
prothrombin time
- SIRS
systemic inflammatory response syndrome
1. INTRODUCTION
Canine babesiosis is a vector‐borne disease caused by Babesia canis, Babesia vogeli, Babesia gibsoni, and Babesia vulpes in Europe. 1 , 2 , 3 In Germany, babesiosis is recognized to be mainly an infection in dogs that have traveled to endemic regions or have been imported, but autochthonous cases caused by B. canis have been known for many years. Transmission occurs through the bite of an infected Dermacentor reticulatus tick. Dermacentor reticulatus has become increasingly widespread in Germany because of growing suburban wasteland, a high density of potential hosts, and climate changes. 4 , 5 , 6 Case reports describing autochthonous B. canis infections in dogs have been published for several German regions including Lower Rhine, Munich, Lower Saxony, Rhineland‐Palatinate, Saarland, and Baden‐Wuerttemberg. 7 , 8 , 9 , 10 , 11 , 12 Despite the frequent occurrence of D. reticulatus in Berlin/Brandenburg, only individual case reports have been described in the literature in this region until a few years ago. 4 Since 2015, however, autochthonous cases have been documented with increasing frequency. Herein, we report the clinical signs, diagnosis, treatment, and outcome of dogs with acute autochthonous babesiosis from the Berlin/Brandenburg region.
2. MATERIALS AND METHODS
2.1. Patients and inclusion criteria
Dogs diagnosed with B. canis infection between April 2015 and December 2021 were included. These dogs had either never left the region of Berlin/Brandenburg or had not left the region during the 6 weeks before diagnosis.
2.2. Diagnostic methods
Diagnosis was initiated by a screening blood smear (venous and capillary blood; LT‐SYS, Haema rapid staining, Laboratory and Technology, Berlin; Figure 1) and completed by PCR testing with subsequent genotyping. The Babesia spp. specific PCR used primers RLB‐F (5′‐GAGGTAGTGACAAGAAATAACAATA‐3′) and RLB‐R (5′‐TCTTCGATCCCCTAACTTTC‐3′) to amplify a 460 bp Babesia spp. 18S rRNA gene fragment. 13 The Master‐Mix was composed of 0.25 mM dNTPs, 0.5 μM of each primer, and 0.02 U/μL Phusion High‐Fidelity DNA Polymerase (ThermoFisher, Waltham, Massachusetts) in 20 μL 1× Phusion HF PCR buffer. Reactions further contained 2 μL template DNA (60‐280 ng/μL). After initial denaturation at 98°C for 30 s, 40 cycles of 98°C for 10 s, 65°C for 30 s, and 72°C for 30 s were conducted before final incubation at 72°C for 5 min. Positive control reactions with 200 copies of a plasmid DNA with the amplicon as insert as well as negative controls were always included in parallel with the samples. As a negative control, water was added to the master mix used for all samples instead of the DNA template. All PCR products were purified and cloned before sequences were generated by Sanger sequencing at LGC Genomics (Berlin). The sequences then were analyzed using BLASTn in GenBank. 14
FIGURE 1.
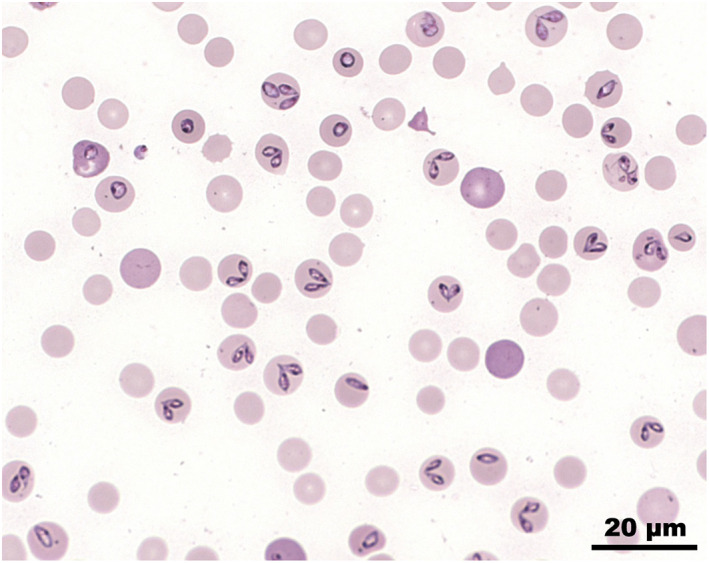
Blood smear with intracellular merozoites of Babesia canis (Giemsa)
2.3. Clinical variables
Anaplasma phagocytophilum infection was excluded in all dogs by PCR testing (Laboklin, Bad Kissingen, Germany). Dogs that had left the Berlin/Brandenburg region in the past were thoroughly tested for pathogens that are endemic to the respective regions. Such was the case in 3 dogs that were tested for Ehrlichia canis (EDTA blood PCR, serum immunofluorescence antigen test [IFAT]), Leishmania infantum (EDTA blood PCR, serum IFAT), Dirofilaria immitis (serum antigen enzyme‐linked immunosorbent assay, ELISA, Knott test) in addition (Laboklin, Bad Kissingen, Germany, Institute for Experimental Parasitology LMU Munich, Germany). Hematology (Sysmex XT2000i, Sysmex Deutschland GmbH, Norderstedt, Germany) and biochemistry (Konelab 60i, Thermo Electron GmbH, Dreieich, Germany) were performed in all dogs, as well as a manual differential blood count in most dogs. Thrombocytopenia was confirmed by manual counting (Thrombo Plus, Sarstedt, Nümbrecht, Germany). Coagulation times activated partial thromboplastin time (aPTT; C.K. Prest, Diagnostica Stago, S.A.S., Asnieres sur Seine, France) and prothrombin time (PT; ThromborelS, Siemens Healthcare GmbH, Erlangen, Germany) were measured in most dogs (Schnitger‐Gross coagulometer, Amelung, Lemgo, Germany). Disseminated intravascular coagulation (DIC) was suspected in the event of prolonged aPTT and PT combined with thrombocytopenia. In most cases, 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester lipase (DGGR lipase; Roche, Diagnostics GmbH, Mannheim, Germany), lactate (GEM Premier 3500, Werfen GmbH, Munich, Germany), C‐reactive protein (CRP; Gentian Canine CRP Reagent Kit, Gentian AS, Moss, Norway), and cardiac troponin I (cTnI; TnI Ultra, Siemens, Frimley, UK) were measured. Coombs' test and platelet‐bound antibody tests were performed at the School of Veterinary Medicine, Hannover, Germany (Immunology Unit). Radiographs of the thorax and abdomen (DigitalDiagnost, Philips, Hamburg, Germany) as well as sonographic examination (Logic S7, Scil animal care company GmbH, Viernheim, Germany) of the abdomen were performed. Systolic blood pressure was determined by Doppler method (Doppler Eickemeyer, Tuttlingen, Germany). In numerous dogs, an ECG (PC‐EKG 2000, Eickemeyer, Tuttlingen) and echocardiography (Vivid 7 Dimension, Scil animal care company GmbH, Viernheim) were performed. The presence of systemic inflammatory response syndrome (SIRS) was determined using SIRS criteria: hypothermia (rectal temperature < 37.8°C), fever (rectal temperature > 39.7°C), heart rate > 160/min, respiratory rate > 40/min, leukopenia (<4 × 109/L), leukocytosis (>12 × 109/L), and presence of >10% immature leukocytes. 15 Systemic inflammatory response syndrome was diagnosed if ≥2 of the above criteria were present.
Descriptive statistics were performed, including determination of median, range, minimum, and maximum (Microsoft Excel, Munich, Germany).
3. RESULTS
3.1. Patients
Acute babesiosis was diagnosed in 49 dogs between 2015 and 2021. Of these, 46 cases were patients of the Clinic for Small Animals, Freie Universität Berlin; 3 dogs were diagnosed in a veterinary practice in Brandenburg. Thirty‐five dogs had never left the region, and 14 had not left during the 6 weeks preceding initial presentation. Five dogs presented between April 2015 to May 2016 (retrospective evaluation) and 44 dogs from October 2019 to December 2021 (prospective evaluation). Most dogs (n = 40) developed clinical signs from September to January whereas a smaller number was presented between March and August (n = 9; Figure 2). One dog was presented twice, first in November 2019 and again 12 months later. Only the findings of the first presentation were included in the analysis. In 2 instances, 2 dogs each belonged to the same owner. The study population consisted of dogs of 23 different breeds and 11 mixed‐breed dogs. The age of the dogs ranged from 0.5 to 12 years (median, 7 years). More males (32; 9 of which were neutered) than females (17; 5 of which were spayed) were affected. Body weight ranged from 6 to 53.5 kg (median, 27.8 kg) of which 34 dogs (69%) weighed >20 kg. In 32 dogs (74%), no (n = 20) or inadequate (n = 12) ectoparasite prophylaxis was reported. In 6 cases (14%), tick prophylaxis was performed (according to the owner): 4 dogs were wearing a collar (containing flumethrin) and in 2 dogs fipronil had been administered as a spot‐on. In 5 other dogs (12%), nonregistered tick prophylaxis (collar from a pet store, coconut oil, herbal spot‐on, garlic, vinegar) had been used. In the remaining 6 dogs, no information was available on tick prophylaxis. In the days before presentation, 36 owners had found and removed ticks from their dogs.
FIGURE 2.
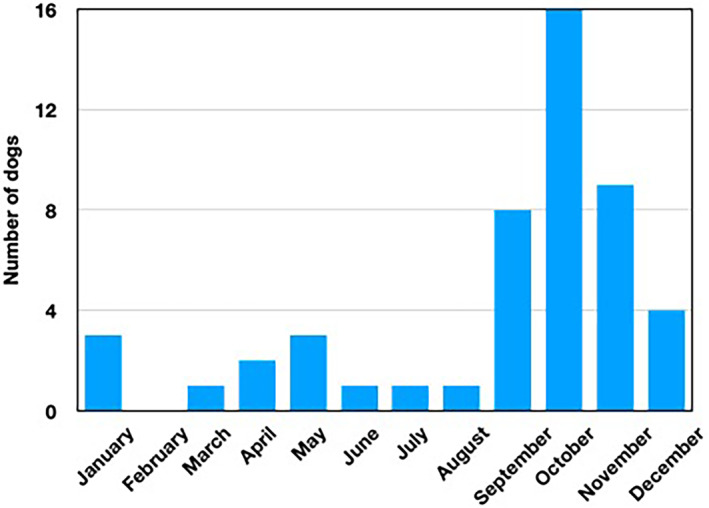
Calendar month of presentation of 49 dogs with autochthonous Babesia canis infection
Complete examination results and course of disease were available for 46 dogs presented at the Clinic for Small Animals, Freie Universität Berlin.
3.2. Diagnosis of B. canis infection
Babesia organisms were detected in the blood smears of 40 dogs (87%). Polymerase chain reaction testing for detection of the 18S rRNA gene of piroplasm was positive in all dogs, and sequencing showed 99.47 to 100% identity with other sequences of B. canis from GenBank (Accession number ON152329‐ON152375). 16 Seventeen different genotypes were identified, with all cases from 2015 attributable to a single genotype.
3.3. History and clinical presentation
Reasons for presentation included lethargy (100%), inappetence (54%), fever (50%), red urine (39%), vomiting (9%), polyuria and polydipsia (4%), and increased panting (4%). Common initial clinical findings were pale pink (50%), white (13%) or icteric (4%) mucous membranes, fever (37%), and red urine (52%). Systolic blood pressure ranged from 90 to 180 mm Hg (median, 119 mm Hg). Three dogs were hypotensive (<100 mm Hg). One dog that had B. canis infection twice developed uveitis on both occasions, which resolved after treatment. Another dog that was normoglycemic and had normal liver and kidney function was comatose 24 hours after admission. Neurologic examination identified miosis, temporary horizontal nystagmus, protrusion of the nictitating membrane and hypersalivation; spinal reflexes were normal.
3.4. Laboratory testings
Initial laboratory results are shown in Table 1. All dogs had thrombocytopenia, with platelet counts ranging from 0 to 139 × 109/L (median, 13 × 109/μL) confirmed by manual counting. Severe thrombocytopenia (<40 × 109/L) was seen in 39 dogs (85%), of which only 2 dogs exhibited detectable bleeding (epistaxis and hematuria, respectively). Twenty‐seven dogs (59%) had bicytopenia and 19 (41%) pancytopenia. Intravascular hemolysis (hemoglobinuria, hemoglobinemia) was present in 24 dogs. Hematocrit ranged from 15% to 54% (median, 33%) at presentation. Thirty‐nine dogs (85%) initially were anemic (regenerative in 13 dogs with reticulocytes >60 000/μL). However, severe anemia (Hct <20%) was present in only 3 cases. The Coombs' test was positive in 3 of 32 cases, and the Hct of these dogs was 25%, 30%, and 33%, respectively. Eleven of 33 dogs had platelet‐bound antibodies; the platelet counts of these dogs were 0 to 99 × 103/μL (median, 5 × 103/μL). The presence of DIC was suspected in 12 dogs (26%). Prolonged aPTT and PT were detected in 34 (87%) and 13 dogs (33%), respectively.
TABLE 1.
Clinicopathological findings in 46 dogs with autochthonous Babesia canis infection
| Parameter | Range (median) | Increased (n) | Decreased (n) | Reference value |
|---|---|---|---|---|
| Hematology | ||||
| Leukocytes (×109/L) | 1.37‐27.4 (5.25) | 6 | 26 | 5.6‐14 |
| Band neutrophils (×109/L) a (n = 12) | 0‐1.394 (0.18) | 2 | … | 0‐0.5 |
| Segmented neutrophils (×109/L) a (n = 12) | 1.45‐20 (3.2) | 1 | 5 | 3.0‐9.0 |
| Eosinophils (×109/L) a (n = 12) | 0‐0.145 (0) | 0 | … | 0‐0.6 |
| Lymphocytes (×109/L) a (n = 12) | 0.14‐6.11 (0.83) | 1 | 8 | 1‐3.6 |
| Monocytes (×109/L) a (n = 12) | 0.17‐5.26 (0.365) | 3 | … | 0‐0.5 |
| Hematocrit (%) | 15‐54 (33) | 0 | 41 | 42‐56 |
| Hemoglobin (g/dL) | 4.6‐18.0 (4.4) | 0 | 43 | 14.6‐19.8 |
| Erythrocytes (×106/μL) | 2.3‐8.3 (4.9) | 0 | 36 | 5.9‐8.3 |
| Reticulocytes (/μL) | 5100‐339 700 (38 800) | 13 | … | >60 000 regenerative |
| Platelets (×109/μL) | 0‐139 (13) | 0 | 46 | 165‐400 |
| Clinical chemistry | ||||
| Sodium (mmol/L) | 127‐150 (138) | 0 | 26 | 140‐150 |
| Potassium (mmol/L) | 3‐5.8 (3.8) | 2 | 14 | 3.6‐4.8 |
| Glucose (mmol/L) | 2.6‐11.1 (5.5) | 13 | 6 | 4.5‐6.2 |
| Creatinine (μmol/L) | 41‐1016 (105.5) | 25 | 2 |
54‐106 (<20 kg) 54‐123 (>20 kg) |
| Urea (mmol/L) | 2.8‐83.2 (14.1) | 29 | 2 | 3.5‐10 |
| ALT (U/L) | 14.8‐1092 (52.5) | 17 | … | −76 |
| AP (U/L) | 10‐669 (134) | 32 | … | −97 |
| AST (U/L) | 12.9‐1868 (94) | 36 | … | −41 |
| Bilirubin (μmol/L) | 1.8‐782 (10) | 39 | … | −5 |
| Calcium (mmol/L) | 2‐3.3 (2.5) | 0 | 20 | 2.5‐2.9 |
| Phosphorus (mmol/L) | 0.86‐3.89 (1.6) | 29 | 1 | 0.96‐1.4 |
| Protein (g/L) (n = 45) | 41‐94.8 (54.9) | 5 | 21 | 54‐66 |
| Albumin (g/L) (n = 45) | 16.3‐33.1 (24.8) | 0 | 34 | 28‐36 |
| Coagulation parameters | ||||
| aPTT (s) (n = 39) | 11.3‐31.4 (15.5) | 34 | 0 | 10‐13.1 |
| PT (s) (n = 39) | 9.2‐45.5 (22.7) | 13 | 1 | 16.5‐25 |
| Other variables | ||||
| cTnI (ng/mL) (n = 34) | 0.01‐20.2 (0.43) | 30 | … | −0.08 |
| Lipase U/L (n = 34) | 14‐3404 (86.5) | 2 b /11 c | … | −260 b /400 c |
| CRP (mg/L) (n = 40) | 17‐153.8 (62.1) | 40 | … | −10 |
| Lactate (mmol/L) (n = 24) | 0.9‐5 (2.1) | 5 | … | −2.5 |
Included in analysis only if a differential blood count was obtained on the day of presentation.
260‐400 U/L.
>400 U/L.
Common clinicopathological findings were increases of liver enzyme activities (ALT 37%; AP 70%), hyperbilirubinemia (85%), hyperphosphatemia (63%), hypoproteinemia (47%), hypoalbuminemia (76%), and increased serum creatinine (54%) or urea (63%) concentration. Azotemia was prerenal (high urine specific gravity, normalization of azotemia the next day) in 8 dogs and renal in 17 dogs. In 2 of 17 dogs with renal azotemia urea concentration and creatinine concentration were normal initially and increased on day 3. C‐reactive protein concentration was increased in all dogs. In 13 dogs (38%) an increased DGGR lipase concentration was found, and in 8 of these cases there was clinical suspicion of acute pancreatitis (vomiting, inappetence, painful cranial abdomen, sonographically swollen hypoechoic pancreas). At initial presentation, SIRS criteria were present in 16 (of 43) dogs (37%): leukopenia (13) and fever (9) were the most common findings. The cTnI concentration was increased in 30/34 dogs (88%) and ranged from 0.09 to 20 ng/mL (median, 0.5 ng/mL).
3.5. Cardiological examination
Cardiac examination was performed by a specialist in cardiology in 24 dogs. Eleven dogs (46%) had ECG abnormalities (ventricular premature beats [4], ST segment depression [3], atrioventricular block [2], ST segment elevation [1], ventricular tachycardia [1], atrial premature beats [1], negative T wave [1]). Echocardiographic changes were detected in only 2 cases: 1 dog had heterogeneous echogenicity of the myocardium, the other had hypertrophy of the left ventricular outer wall and ventricular septum with a roughened surface of the endocardium. These 2 dogs also exhibited arrhythmias. In dogs with abnormal findings on cardiac examination, cTnI concentration ranged from 0.08 to 11 ng/mL (median, 0.93 ng/mL).
3.6. Abdominal imaging
Abdominal radiography identified splenomegaly in all dogs and hepatomegaly in 5 dogs (11%). Abnormal abdominal sonographic findings were splenomegaly, which was diffusely inhomogeneous in 11 cases. Two dogs were splenectomized. Other abnormal ultrasonographic findings included small amounts of free fluid (12), sludge in the gallbladder (8), a painful and swollen pancreas (8), hepatomegaly (5), enlarged mesenterial lymph nodes (4), suspicion of splenic infarction (2), perirenal free fluid (2), and gallbladder wall edema (2).
3.7. Treatment and outcome
Forty‐five dogs (98%) were hospitalized over a period of 1 to 21 days (median, 4.5 days), 1 dog was treated as an outpatient. All dogs were treated with imidocarb (Carbesia, Intervet, Beaucouze, France) at a dosage of 2.4 to 6.3 mg/kg (median, 5.0 mg/kg). In dogs with azotemia, the dosage ranged from 2.4 to 6 mg/kg (median, 4 mg/kg). Eight dogs (17%) received a single injection (6 dogs died or were euthanized before the 2nd injection, 1 dog each did not return for reevaluation and continued treatment with the referring veterinarian), 35 (76%) received 2, and 1 dog each received 3, 4, and 5 injections. Injections were given at an interval of 12 to 85 days (median, 15 days). The interval between injections varied because dogs were presented irregularly or did not return for follow‐up appointments. Several adverse effects were documented in 7 dogs (15%): painful swelling at the injection site occurred 4 times, vomiting (2), mucopurulent bloody diarrhea (2), salivation (1), panting and restlessness (1).
A total of 94 PCR follow‐up controls were performed in 35 dogs. The day of sample collection for PCR testing after injection was determined by the veterinarian and ranged from 5 to 265 days (median, 15 days). The first negative Babesia PCR follow‐up test was detected after the first injection in 19 cases (5 to 18 days; median, 12 days; imidocarb dosage 3 to 6 mg/kg; median, 6 mg/kg) or after the second injection in 15 cases (6 to 28 days; median, 14 days; imidocarb dose 4 to 6 mg/kg; median, 5 mg/kg). In 9 dogs, positive Babesia PCR follow‐up tests were obtained (Table S1): in 7 dogs after the first (after 7 to 32 days; median, 13 days; imidocarb dosage: 3 to 6 mg/kg; median, 4.4 mg/kg), in 5 dogs after the second (after 8 to 10 days; median, 8 days; imidocarb dose: 3 to 5 mg/kg; median, 4.3 mg/kg), in 2 dogs after the third (after 11 and 22 days; imidocarb dosage 3.9 and 4.5 mg/kg) and in 1 dog 28 days after the fourth injection (imidocarb dosage 5 mg/kg; Table S1). This dog received another injection (5 mg/kg) and was negative thereafter.
Further supportive therapeutic measures, depending on clinical signs, included the administration of maropitant (1 mg/kg, q24h IV; Emex, CP Pharma, Burgdorf, Germany), omeprazole (1 mg/kg, q12h PO; Omeprazol‐ratiopharm, Ratiopharm GmbH, Ulm, Germany), or esomeprazole (1 mg/kg, q12h IV; Nexium, Grünenthal GmbH, Aachen, Germany) and metamizole (10 to 20 mg/kg, q8h IV or PO; Novacen, CP Pharma, Burgdorf, Germany; Novaminsulfon‐ratiopharm, Ratiopharm GmbH, Ulm, Germany). In 39 dogs, an antibiotic (doxycycline, n = 17; 5 mg/kg, q12h PO; Doxybactin, Dechra, Aulendorf, Germany) or amoxicillin‐clavulanic acid (n = 22; 12.5‐20 mg/kg, q12h IV or PO, Amoxclav Hexal, Holzkirchen; Synulox, Zoetis, Berlin, Germany) was administered initially. Dogs with arrhythmias were given magnesium (0.5 to 1 mmol/kg PO, Magnetrans forte, Stada Consumer Health Deutschland GmbH, Bad Vilbel, Germany). Atenolol (Atenolol‐ratiopharm, Ratiopharm GmbH, Ulm, Germany) was administered to treat hemodynamically relevant supraventricular arrhythmias.
Forty‐five dogs received a balanced crystalloid solution (Sterofundin ISO, Braun, Melsungen, Germany) during hospitalization. Thirteen dogs received dog erythrocyte antigen (DEA) 1‐compatible packed red blood cell transfusions (1 to 4 transfusions; median, 1 transfusion), 4 dogs with prolonged coagulation parameters received fresh frozen plasma (1 transfusion each), and 1 dog with severe thrombocytopenia and prolonged coagulation parameters received platelet‐rich plasma.
In 1 dog, secondary immune‐mediated hemolytic anemia developed. After an initial Hct increase (from 25% to 38%), a decrease in Hct (to 15%) occurred on day 11 with a negative Babesia PCR and a positive Coombs' test. After treatment with prednisolone (0.6 mg/kg/day PO; Prednisolon, CP Pharma, Burgdorf, Switzerland) the Hct normalized within 14 days. Five of 17 dogs with persistently increased serum creatinine concentrations died or were euthanized, respectively. In 8 dogs, renal function tests normalized within 8 to 35 days and in the remaining 4 dogs, serum creatinine concentrations increased on days 27 to 427 (144; 139; 124; 123 μmol/L).
Thirty‐nine dogs (85%) survived. Four dogs were euthanized after 2 to 18 days (financial reasons and acute renal failure [2], acute renal failure [1], hepatopathy and arrhythmias [1]). Three dogs died 2 to 8 days after initial presentation (cerebral babesiosis and coma [1], acute renal failure and oliguria [1], hematemesis and acute renal disease [1]). Three dogs that died (2) or were euthanized (1) fulfilled SIRS criteria at initial presentation.
4. DISCUSSION
Our study includes a large number of dogs with presumed autochthonous babesiosis in Germany. An increasing prevalence of the vector D. reticulatus in different German regions made increasing numbers of Babesia infections in dogs foreseeable. 6
The screening of a blood smear was diagnostic for infection with large piroplasms in 87% of cases. This finding supports the importance of this rapid and inexpensive diagnostic tool. All dogs in our study were infected with B. canis, which was confirmed by PCR and sequencing. In Europe, 3 genotypes (based on Bc28.1) of B. canis have been described to date. 17 The Bc28.1‐A type is found in Poland, Hungary, and Ukraine and is associated with moderate to low‐grade virulence. The Bc28.1‐B type is dominant in western and southwestern Europe and is considered to be moderately virulent. 17 The Bc28.1‐34 G type only has been detected in France. Dogs in our study were found to have 17 different genotypes that could not be assigned to any of those previously described. The occurrence of different genotypes possibly is attributable to the introduction of Babesia by dogs imported from different regions. In contrast, the species B. vogeli, which is a large Babesia, was not detected. Indeed, spread of this species would be unlikely because the vector Rhipicephalus sanguineus thus far is not endemic in Germany. Dermacentor reticulatus exhibits a bimodal activity pattern. 5 , 18 In contrast to Ixodes ricinus, D. reticulatus shows a first activity peak between March and May. The second activity peak usually occurs in August through November, but the tick previously has been found active over the winter months in the Berlin/Brandenburg region. 5 This finding may explain why the dogs in our study presented with clinical signs mainly between autumn and January and into spring. Dogs of all ages and breeds were affected, but almost 70% of the dogs described here weighed >20 kg. This observation might be a result of the higher incidence of tick infestation in large dogs. 18
Initially, 89% and, during the course of disease, 98% of the dogs experienced anemia (only 1 dog was not anemic). In addition to intravascular hemolysis (because of proliferation of Babesia and subsequent erythrolysis), extravascular hemolysis also may contribute to anemia. Increased phagocytosis of infected and noninfected erythrocytes may be triggered by oxidative and other damage in addition to opsonization of erythrocytes by antibodies. 19 , 20 In 9% of the dogs, the Coombs' test was positive at first presentation, and in another dog the test became positive during the course of disease. The immune‐mediated pathomechanism seems of minor importance for the development of anemia. Similar results were obtained in a study using flow cytometry for detection of erythrocyte membrane binding immunoglobulin M and immunoglobulin G in B. canis (n = 24) and B. vogeli (n = 6) positive dogs. 21 Although 4/6 dogs with B. vogeli were positive, no dog with B. canis had antierythrocyte antibodies.
Severe thrombocytopenia itself can lead to hemorrhage, and in dogs with babesiosis caused by B. canis or B. gibsoni, no correlation was found between the degree of parasitemia and the severity of anemia. 19 , 22 As in earlier case studies, thrombocytopenia was a very common finding. 20 , 23 , 24 , 25 Consumption of platelets, platelet sequestration as well as immune‐mediated destruction may be causative factors for thrombocytopenia. 26 An associative (secondary) immune‐mediated thrombocytopenia has been described in cases of babesiosis and other vector‐borne infections (e.g., granulocytic anaplasmosis which can resolve without glucocorticoid therapy). 27 , 28 , 29 Similar to other studies, 56% of the affected dogs were leukopenic. 23 , 24 , 25 Lymphopenia caused by hypercortisolism has been suggested as a possible pathomechanism in dogs with peracute B. rossi infection. 24 , 30 Sequestration of leukocytes in the spleen is also possible. 23 Although leukopenia was identified as a negative prognostic factor in 1 study, it was found in 60% of dogs with a mild course in another study. 23 , 24 Forty‐one percent of the patients had pancytopenia that also has been described in other vector‐borne diseases (e.g., ehrlichiosis, leishmaniasis, anaplasmosis). In the context of B. canis infection, the prevalence of pancytopenia has not been evaluated in most studies. However, leukopenia, anemia, and thrombocytopenia are commonly occurring laboratory abnormalities. 23 , 24 , 25 Different B. canis strains potentially may result in different severity of clinicopathological abnormalities.
Our findings indicate that B. canis infection frequently was associated with SIRS (37%) and multi‐organ involvement (48%), as described in previous case reports. 25 , 31 The presence of SIRS did not correlate with the severity of parasitemia but was a negative prognostic factor in some studies. 31 , 32 Thirty‐two percent of the dogs described here had renal abnormalities. Different pathomechanisms such as hypoxia, hypotension, vascular stasis caused by sludging of erythrocytes, and damage to the kidneys by hemoglobin have been considered as possible mechanisms. 26 Histological changes in the kidneys (e.g., vacuolar degeneration, necrosis, detachment of the tubular epithelium in the proximal tubule) are consistent with hypoxia‐induced injury. 33 Only rarely, hemoglobin casts or droplets have been identified in the tubular epithelium. In addition to tubular damage, glomerular injury with subsequent development of glomerulopathy also has been described. 34
Approximately two‐thirds of the dogs had increased liver enzyme activities. Hypoxia, hypotension, SIRS, and pancreatitis are possible pathomechanisms. The increases in AST activities, sometimes marked, also can be explained by intravascular hemolysis. 35
Pancreatitis, which occurred in 8 dogs (17%), also has been described in association with babesiosis and may be the result of hypoxia or hypotension. 36 , 37
Abnormalities were detected in 11/24 dogs in which a thorough cardiac evaluation was performed (45%). Anemia, hypotension, electrolyte abnormalities, metabolic acidosis, and azotemia may have contributed to cardiac complications. 26 The development of cardiac abnormalities has been investigated in 3 studies of B. rossi infections in South Africa and B. canis infections in Poland. 38 , 39 , 40 Electrocardiographic changes occurred in 33 to 41% of cases and arrhythmias were described in 12% to 30%. Increased cTnI concentrations, which also have been described in several other studies, were present in 88% of our cases (markedly increased in 8 cases [2.4‐20.2 ng/mL]) and are indicative of myocardial injury, which can result from both cardiac and extracardiac disease. 41 , 42 , 43 Increased cTnI concentration has been shown to be a negative prognostic factor in dogs with SIRS and in dogs with B. rossi infection in South Africa. 38 , 43 Factors influencing the troponin concentration in our cases could not be identified. In a previous study, increased cTnI concentrations in dogs without cardiac disease but with renal disorders were observed. Subclinical ischemic damage to cardiac myocytes, uremic pericarditis or myocarditis, and decreased clearance have been considered as possible pathomechanisms for increased troponin concentrations in dogs with renal disease. 44 Furthermore, falsely low troponin concentrations may occur in patients with hemolysis. 45
To the best of our knowledge, splenic infarction associated with B. canis infection, as suspected in 2 of our cases, has not been reported previously. Interestingly, splenic infarction is described in human patients as a rare complication of B. microti infection. 46 , 47 In addition to vascular obstruction caused by adhesion of infected erythrocytes to the endothelium, endothelial damage and rapid splenic enlargement associated with sequestration of Babesia‐laden erythrocytes and platelets are possible pathomechanisms. Splenic rupture also has been described in this context. 46
One dog in our study developed neurologic signs that could not be explained by metabolic causes (eg, hypoglycemia, liver and kidney failure). Cerebral babesiosis is a rare complication and is associated with high mortality. 23 , 48 , 49 , 50 Possible pathomechanisms include circulatory disturbances caused by accumulation of parasites and erythrocytes in the capillaries, endothelial damage, vasculitis, immune complex deposition in vessels, and hypoxia. 49
Uveitis was another rare complication observed in a dog in our study. The affected dog had B. canis infection twice, 1 year apart, and each time with uveitis. After imidocarb treatment, the dog became asymptomatic both times. To our knowledge, uveitis has not been reported previously in association with babesiosis.
The imidocarb dosage recommended by the manufacturer for treatment of babesiosis is 2.12 mg/kg, and the dosage for prevention is 4.25 mg/kg. The dosage reported in the veterinary literature is 5 to 6.6 mg/kg. 26 The differences in dosage recommendations are based on the fact that the treatment goal for dogs in endemic areas (where reinfection is very likely) is solely reduction in the number of babesia with subsequent formation of pre‐immunity. 51 Described adverse effects of imidocarb, which also were noted in our study, include pain and swelling at the injection site, cholinergic signs (e.g., salivation, vomiting, diarrhea), renal tubular necrosis, and hepatic necrosis. 33 , 52 , 53 Imidocarb should be used with caution in patients with impaired renal and hepatic function. 33 , 54 In our study, the frequency of adverse effects may be underrepresented (15%) because of the partially retrospective evaluation. In addition, treatment was administered by different veterinarians and adverse effects may not have been documented completely. Our data indicate, that a second imidocarb injection often is necessary, because 7 dogs still had positive PCR results 7 to 32 days after the first injection. In 5 cases, a positive PCR result even occurred 8 to 20 days after treatment despite administration of imidocarb twice. One dog was treated with 5 injections of imidocarb because its PCR results remained positive. Evaluation of treatment success by PCR before concluding and 2 months after completion of treatment is recommended. 1 Interestingly, 1 dog suffered from babesiosis twice, 1 year apart. This dog initially received 2 injections of imidocarb (4.8 and 4 mg/kg) 14 days apart. Because the dog was asymptomatic and PCR was negative 14 days after the first course of treatment, the second occurrence most likely represented a new infection, independent of the first infection. Tick prophylaxis was provided with flumethrin/imidacloprid. Chronic infection with B. canis however cannot be excluded in this case. 55 After experimental infection, protective immunity lasts between 5 and 8 months. 56
The mortality rate of B. canis infection in Europe ranges from 1.5% to 20%. Depending on genotype, the mortality rate in central Europe and northeastern Europe is 12% to 20% and in southwestern Europe is <5%. 17 Mortality also differs when compared with other Babesia species. Although infection with B. vogeli is associated with a mild course of disease, a high death rate is described for infection with B. rossi. 26 In our study population, complications (especially renal, cardiac, pancreatitis, and DIC) occurred in 19 cases (41%). The mortality rate was 15%. Three dogs died of babesiosis and 4 were euthanized. Systemic inflammatory response syndrome occurred in 3 of 7 of the dogs that died or were euthanized in our study, whereas 13 dogs with SIRS survived B. canis infection.
The majority (70%) of dogs received irregular, inadequate or no tick prevention. However, 6 dogs were treated with fipronil (2) or flumethrin/imidacloprid (4) according to information provided by the owners. Factors associated with the maintenance and distribution of the active ingredients (e.g., frequent contact with water or improper application such as irregular wearing of a collar or misapplication of spot on solutions) can result in variation in the sustained efficacy of topical products and cannot be evaluated retrospectively for the affected dogs. It was shown that a collar with flumethrin/imidacloprid is highly effective against infection with B. canis and efficacy can be expected after 2 days of wearing the collar. 57 , 58 Furthermore, indirect protection against transmission of B. canis (by D. reticulatus ticks) has been shown in a laboratory study at day 28 after treatment, thereby decreasing the risk of disease under the conditions of the study. 59
Fipronil is approved for Ixodes ricinus and Dermacentor variabilis and has a killing effect of 48 hours after infestation, but no repellent effect. 60 Experimental infection trials have shown that D. reticulatus transmit Babesia after at least 48 hours. A special feature of this tick species is that the male ticks also occasionally suck small amounts of blood. If the male tick has taken a blood meal and feeding is interrupted, transmission of Babesia can occur within 8 hours. 61 This finding emphasizes the important role of repellents. However, antiparasitics without a repellent effect (e.g., fluralaner, afoxolaner, lotilaner) also can be protective against the transmission of B. canis because of a rapid killing effect. 62 , 63 , 64
A limitation of our study is that a few dogs had adequate previous travel history, and infection outside the Berlin/Brandenburg region cannot be entirely excluded. Another limitation is that the dogs were under the care of different veterinarians in the clinic. Therefore, the timing of PCR re‐testing was highly variable which may have resulted in biased medians.
5. CONCLUSIONS
Our case series indicates that autochthonous babesiosis is of high clinical relevance in Berlin/Brandenburg. The increased occurrence of infections may be caused by the spread of the vector, import of dogs infected with B. canis, infection of the ticks with B. canis in the region or some combination of these factors. Severe clinical signs and laboratory abnormalities, SIRS, and multiorgan involvement often necessitated intensive medical treatment, including administration of blood products. Re‐testing for B. canis using PCR during and after treatment is advised. Screening of blood donors in high‐risk areas and year‐round tick protection with products having repellent or rapid‐killing effect in order to prevent or decrease the risk of transmission of B. canis are strongly recommended. 65 , 66
CONFLICT OF INTEREST DECLARATION
Christiane Weingart has held lectures for veterinary pharmaceutical and diagnostic companies. Barbara Kohn and Georg von Samson‐Himmelstjerna have acted as consultants for veterinary pharmaceutical and diagnostic companies and have previous and ongoing research collaborations with various companies. Christina S. Helm, Elisabeth Müller, Ingo Schäfer, Marianne Skrodzki, and Jürgen Krücken do not have a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1: Positive follow‐up PCR results in 9 dogs with Babesia canis infection after imidocarb treatment: Day of imidocarb injection, imidocarb dosage and result of PCR test after injection
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge Dres Scholz in Brandenburg for providing the information of 3 B. canis infected dogs.
Weingart C, Helm CS, Müller E, et al. Autochthonous Babesia canis infections in 49 dogs in Germany. J Vet Intern Med. 2023;37(1):140‐149. doi: 10.1111/jvim.16611
REFERENCES
- 1. Solano‐Gallego L, Sainz A, Roura X, Estrada‐Peña A, Miró G. A review of canine babesiosis: the European perspective. Parasit Vectors. 2016;9:336‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baneth G, Cardoso L, Brilhante‐Simões P, Schnittger L. Establishment of Babesia vulpes n. sp. (Apicomplexa: Babesiidae), a piroplasmid species pathogenic for domestic dogs. Parasit Vectors. 2019;12:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Birkenheuer AJ, Buch J, Beall MJ, Braff J, Chandrashekar R. Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Vet Parasitol reg Stud Rep. 2020;22:100471. [DOI] [PubMed] [Google Scholar]
- 4. Heile C, Heydorn AO, Schein E. Dermacentor reticulatus (Fabricius, 1794)—distribution, biology and vector for Babesia canis in Germany. Berl Munch Tierarztl Wochenschr. 2006;119:330‐334. [PubMed] [Google Scholar]
- 5. Kohn M, Krücken J, McKay‐Demeler J, Pachnicke S, Krieger K, von Samson‐Himmelstjerna G. Dermacentor recticulatus in Berlin/Brandenburg (Germany): activity patterns and associated pathogens. Ticks Tick Borne Dis. 2019;10:191‐206. [DOI] [PubMed] [Google Scholar]
- 6. Drehmann M, Springer A, Lindau A, et al. The spatial distribution of Dermacentor Ticks (Ixodidae) in Germany – evidence of a continuing spread of Dermacentor reticulatus . Front Vet Sci. 2020;7:578220. doi: 10.3389/fvets.2020.578220.eCollection [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dürbaum M, Daugschies A, Leopold‐Temmler B. Die Babesiose des Hundes durch Babesia canis (Piana und Gallo‐Valerio 1895). Prakt Tierarzt. 2000;82:699‐710. [Google Scholar]
- 8. Zahler M, Steffen T, Lutz S, et al. Babesia canis und Dermacentor reticulatus in Munich: a new endemic focus in Germany. Tierarztl Praxis. 2000;28:116‐120. [Google Scholar]
- 9. Jensen J, Nolte I. Autochthonous infection with Babesia canis in a dog from northern Germany. Tierarztl Prax. 2005;33:408‐412. [Google Scholar]
- 10. Kehl A, Hübner J, Müller E. An endemic case of babesiosis in a dog. Kleintiermedizin. 2005;9(10):258‐261. [Google Scholar]
- 11. Barutzki D, Reule M, Scheunemann R, et al. Die Babesiose des Hundes. Eine autochthone Erkrankung in Deutschland. Deutsches Tierärzteblatt. 2007;3(2007):284‐293. [Google Scholar]
- 12. Beelitz P, Schumacher S, Marholdt F, Pfister K, Silaghi C. Untersuchungen zur Prävalenz von Babesia canis canis in Auwaldzecken (Dermacentor reticulatus) im Saarland. Berl Munch Tierarztl Wochenschr. 2012;125:168‐171. [PubMed] [Google Scholar]
- 13. Gubbels JM, De Vos AP, Van der Weide M, et al. Simultaneous detection of bovine Theileria and Babesia species by reverse line blot hybridization. J Clin Microbiol. 1999;37:1782‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403‐410. [DOI] [PubMed] [Google Scholar]
- 15. Okano S, Yoshida M, Fukushima U, Higuchi S, Takase K, Hagio M. Usefulness of systemic inflammatory response syndrome criteria as an index for prognosis judgement. Vet Rec. 2002;150:245‐246. [DOI] [PubMed] [Google Scholar]
- 16. Helm SC, Weingart C, Ramünke S, et al. High genetic diversity of Babesia canis (Piana & Galli‐Valerio, 1895) in a recent local outbreak in Berlin/Brandenburg, Germany. Transbound Emerg Dis. 2022;69:e3336. doi: 10.1111/tbed.14617 [DOI] [PubMed] [Google Scholar]
- 17. Carcy B, Randazzo S, Depoix L, et al. Classification of Babesia canis strains in Europe based on polymorphism of the Bc28.1‐gene from the Babesia canis Bc28 multigene family. Vet Parasitol. 2015;211:111‐123. [DOI] [PubMed] [Google Scholar]
- 18. Beck S, Schreiber C, Schein E, et al. Tick infestation and prophylaxis of dogs in northeastern Germany: a prospective study. Ticks Tick Borne Dis. 2014;5:336‐342. [DOI] [PubMed] [Google Scholar]
- 19. Crnogaj M, Ceron JJ, Smit I, et al. Relation of antioxidant status at admission and disease severity and outcome in dogs naturally infected with Babesia canis canis . BMC Vet Res. 2017;13:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teodorowski O, Winiarczyk S, Tarhan D, et al. Antioxidant status, and blood zinc and copper concentrations in dogs with uncomplicated babesiosis due to Babesia canis infections. J Vet Res. 2021;65:169‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carli E, Tasca S, Trotta M, Furlanello T, Caldin M, Solano‐Gallego L. Detection of erythrocyte binding IgM and IgG by flow cytometry in sick dogs with Babesia canis canis or Babesia canis vogeli infection. Vet Parasitol. 2009;162:51‐57. [DOI] [PubMed] [Google Scholar]
- 22. Murase T, Maede Y. Increased erythrophagocytic activity of macrophages in dogs with Babesia gibsoni infection. Jpn J Vet Sci. 1990;52:321‐327. [DOI] [PubMed] [Google Scholar]
- 23. Mathe A, Voros K, Papp L, et al. Clinical manifestation of canine babesiosis in Hungary (63 cases). Acta Vet Hung. 2006;54:367‐385. [DOI] [PubMed] [Google Scholar]
- 24. Eichenberger RM, Riond B, Willi B, Hofmann‐Lehmann R, Deplazes P. Prognostic markers in acute Babesia canis infection. J Vet Intern Med. 2016;30:174‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strobl A, Künzel F, Tichy A, Leschnik M. Complications and risk factors regarding the outcomes of canine babesiosis in Central Europe – a retrospective analysis of 240 cases. Acta Vet Hung. 2020;68:160‐168. [DOI] [PubMed] [Google Scholar]
- 26. Solano‐Gallego L, Baneth G. Babesiosis in dogs and cats – expanding parasitological and clinical spectra. Vet Parasitol. 2011;181:48‐60. [DOI] [PubMed] [Google Scholar]
- 27. Wilkerson MJ, Shuman W, Swist S, Harkin K, Meinkoth J, Kocan AA. Platelet size, platelet surface associated IgG, and reticulated platelets in dogs with immune‐mediated thrombocytopenia. Vet Clin Pathol. 2001;30:141‐149. [DOI] [PubMed] [Google Scholar]
- 28. Goddard A, Leisewitz AL, Kristensen AT, Schoeman JP. Platelet activation and platelet‐leukocyte interaction in dogs naturally infected with Babesia rossi . Vet J. 2015;205:387‐392. [DOI] [PubMed] [Google Scholar]
- 29. Chirek A, Silaghi C, Pfister K, Kohn B. Granulocytic anaplasmosis in 63 dogs: clinical signs, laboratory results, therapy and course of disease. J Small Anim Pract. 2018;59:112‐120. [DOI] [PubMed] [Google Scholar]
- 30. Schoeman JP, Herrtage ME. Adrenal response to the low dose ACTH stimulation test and the cortisol‐to‐adrenocorticotrophic hormone ratio in canine babesiosis. Vet Parasitol. 2008;154:205‐213. [DOI] [PubMed] [Google Scholar]
- 31. Matijatko V, Kis I, Torti M. Systemic inflammatory response syndrome and multiple organ dysfunction syndrome in canine babesiosis. Vet Arhiv. 2010;80:611‐626. [Google Scholar]
- 32. Beletic A, Janjic F, Radakovic M, et al. Systemic inflammatory response syndrome in dogs with naturally infected with Babesia canis: association with the parasite load and host factors. Vet Parasitol. 2021;291:109366. [DOI] [PubMed] [Google Scholar]
- 33. Mathe A, Dobos‐Kovacs M, Vörös K. Histological and ultrastructural studies of renal lesions in Babesia canis infected dogs treated with imidocarb. Acta Vet Hung. 2007;55:511‐523. [DOI] [PubMed] [Google Scholar]
- 34. Kules J, Bilic P, Ljubic BB, et al. Glomerular and tubular kidney damage markers in canine babesiosis caused by Babesia canis . Ticks Tick Borne Dis. 2018;9:1508‐1517. [DOI] [PubMed] [Google Scholar]
- 35. Hall EJ, German AJ. Laboratory evaluation of hepatic disease. In: Villiers E, Ristic J, eds. BSAVA Manual of Canine and Feline Clinical Pathology. Gloucester: British Small Animal Veterinary Association; 2016:237‐261. [Google Scholar]
- 36. Köster LS, Steiner JM, Suchodolski JS, Schoeman JP. Serum canine pancreatic‐specific lipase concentrations in dogs with naturally occurring Babesia rossi infection. J S Afr Vet Assoc. 2015a;86:E1‐E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Köster LS, Lobetti RG, Kelly P. Canine babesiosis: a perspective on clinical complications, biomarkers, and treatment. Vet Med. 2015b;6:119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lobetti R, Dvir E, Pearson J. Cardiac troponins in canine babesiosis. J Vet Intern Med. 2002;16:63‐68. [DOI] [PubMed] [Google Scholar]
- 39. Dvir E, Lobetti RG, Jacobsen LS, et al. Electrocardiographic changes and cardiac pathology in canine babesiosis. J Vet Cardiol. 2004;6:15‐23. [DOI] [PubMed] [Google Scholar]
- 40. Bartnicki M, Lyp P, Debial P, et al. Cardiac disorders in dogs infected with Babesia canis . Polis J Vet Sci. 2017;20:573‐581. [DOI] [PubMed] [Google Scholar]
- 41. Lobetti R, Kirberger R, Keller N, Kettner F, Dvir E. NT‐ProBNP and cardiac troponin I in virulent canine babesiosis. Vet Parasitol. 2012;190:333‐339. [DOI] [PubMed] [Google Scholar]
- 42. Hamacher L, Dörfelt R, Müller M, Wess G. Serum cardiac Troponin I concentrations in dogs with systemic inflammatory response syndrome. J Vet Intern Med. 2015;29:164‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gommeren K, Desmas I, Garcia A, et al. Cardiovascular biomarkers in dogs with systemic inflammatory response syndrome. J Vet Emerg Crit Care. 2019;29:256‐263. [DOI] [PubMed] [Google Scholar]
- 44. Sharkey L, Berzina I, Ferasin L, et al. Evaluation of serum cardiac troponin I concentration in dogs with renal failure. J Am Vet Med Assoc. 2009;234:767‐770. [DOI] [PubMed] [Google Scholar]
- 45. Herman DS, Kavsak PA, Greene DN. Variability and error in cardiac troponin testing. An ACLPS critical review. Am J Clin Pathol. 2017;148:281‐295. [DOI] [PubMed] [Google Scholar]
- 46. Blackwood B, Binder W. Unusual complications from Babesia infection: splenic infarction and splenic rupture in two separate patients. J Emerg Med. 2018;55:e113‐e117. [DOI] [PubMed] [Google Scholar]
- 47. Sung LH, Sundaram AH, Glick AL, Chen DF, Shipton L. Babesiosis as a cause of atraumatic splenic injury: two case reports and a review of literature. J Gen Intern Med. 2021;36:3869‐3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van de Maele I, Savary‐Bataille K, Giehlen I, et al. An unusual form of canine babesiosis. Can Vet J. 2008;49:283‐286. [PMC free article] [PubMed] [Google Scholar]
- 49. Adaszek L, Gorna M, Klimiuk P, et al. A presumptive case of cerebral babesiosis in a dog in Poland caused by a virulent Babesia canis strain. Tierarztl Prax. 2012;40:367‐371. [PubMed] [Google Scholar]
- 50. Daste T, Lucas MN, Aumann M. Cerebral babesiosis and acute respiratory distress syndrome in a dog. J Vet Emerg Crit Care. 2013;23:615‐623. [DOI] [PubMed] [Google Scholar]
- 51. Birkenheuer AJ. Babesiosis. In: Sykes JE, ed. Canine and Feline Infectious Diseases. Vol 2014. St. Louis, MO: Elsevier; 2014:727‐737. [Google Scholar]
- 52. Abdullah AS, Sheikh‐Omar AR, Baggot JD, Zamri M. Adverse effects of imidocarb dipropionate in a dog. Vet Res Com. 1984;8:55‐59. [DOI] [PubMed] [Google Scholar]
- 53. Baneth G. Antiprotozoal treatment of canine babesiosis. Vet Parasitol. 2018;254:58‐63. [DOI] [PubMed] [Google Scholar]
- 54. Kock N, Kelly P. Massive hepatic necrosis associated with accidental imidocarb dipropionate toxicosis in a dog. J Comp Pathol. 1991;104:113‐116. [DOI] [PubMed] [Google Scholar]
- 55. Milanovic Z, Beletic A, Vekic J, et al. Evidence of acute phase reaction in asymptomatic dogs naturally infected with Babesia canis . Vet Parasitol. 2020;282:109140. [DOI] [PubMed] [Google Scholar]
- 56. Vercammen F, De Deken R, Maes L. Duration of protective immunity in experimental canine babesiosis after homologous and heterologous challenge. Vet Parasitol. 1997;68:51‐57. [DOI] [PubMed] [Google Scholar]
- 57. Fourie JJ, de Vos C, Crafford D, Pollmeier M, Schunack B. A study on the long‐term efficacy of Seresto® collars in preventing Babesia canis (Piana&Gall‐Valerio, 1895) transmission to dogs by infected Dermacentor reticulatus (fabricuis, 1794) ticks. Parasit Vectors. 2019;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stanneck D, Kruedewagen EM, Fourie JJ, Horak IG, Davis W, Krieger KJ. Efficacy of an imidacloprid/flumethrin collar against fleas, ticks, mites and lice on dogs. Parasit Vectors. 2012;5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fourie JJ, Stanneck D, Jongejan F. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs treated with an imidacloprid/flumethrin collar. Vet Parasitol. 2013;192:273. [DOI] [PubMed] [Google Scholar]
- 60. Beugnet F, Franc M. Insecticide and acaricide molecules and/or combinations to prevent pet infestation by ectoparasites. Trends Parasitol. 2012;28:257‐279. [DOI] [PubMed] [Google Scholar]
- 61. Varloud M, Liebenberg J, Fourie J. Early Babesia canis transmission in dogs within 24 h and 8 h of infestation with infected pre‐activated male Dermacentor reticulatus ticks. Parasit Vectors. 2018;11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Taenzler J, Liebenberg J, Roepke RKA, Heckeroth AR. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs after topical administration of fluralaner spot‐on solution. Parasit Vectors. 2016;9:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beugnet F, Halos L, Larsen D, Labuschagné M, Erasmus H, Fourie J. The ability of an oral formulation of afoxolaner to block the transmission of Babesia canis by Dermacentor reticulatus ticks to dogs. Parasit Vectors. 2014;7:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cavalleri D, Murphy M, Seewald W, Drake J, Nanchen S. Two randomized, controlled studies to assess the efficacy and safety of lotilaner (Credelio™) in preventing Dermacentor reticulatus transmission of Babesia canis to dogs. Parasit Vectors. 2017;10:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wardrop KJ, Birkenheuer A, Blais MC, et al. Update on canine and feline blood donor screening for blood‐borne pathogens. J Vet Intern Med. 2016;30:15‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Otranto D, Dantas‐Torres F, Fourie JJ, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for studies evaluating the efficacy of parasiticides in reducing the risk of vector‐borne pathogen transmission in dogs and cats. Vet Parasitol. 2021;290:109369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Positive follow‐up PCR results in 9 dogs with Babesia canis infection after imidocarb treatment: Day of imidocarb injection, imidocarb dosage and result of PCR test after injection


