Abstract
Background
A glycemic challenge test is used for the diagnosis of insulin dysregulation (ID) in horses and ponies. Different forms of the test exist where the administrative route and dose of glucose vary, which makes interpretation of results challenging.
Hypothesis/Objectives
To evaluate the palatability of, and blood glucose and insulin responses to, carbohydrate pellets fed as an oral glucose test (OGT), and to establish the diagnostic threshold for ID when using the pellets.
Animals
University and privately‐owned horses and ponies (n = 157) comprised of 31 breeds and both sexes.
Methods
Multicenter cohort study. A custom‐produced glycemic pellet was offered for free intake at 0.5 g/kg BW soluble carbohydrate and serum insulin and blood glucose concentrations measured before and after (60, 120, and 180 minutes) the pellets were offered. Pellet acceptance and intake time (those that finished within 10 minutes) were determined to assess palatability.
Results
The pellets were palatable to 132/157 animals, and ponies found the pellets more (P = .004) palatable than horses. The median intake time (4 [3‐6] minutes) was positively correlated with acceptance grade (r = .51; P < .0001). Consumption of the pellets elicited peak blood glucose (6.6 [5.8‐7.8] mmol/L) and serum insulin (40.5 [19‐99.8] μIU/mL) responses at 120 minutes. At 120 minutes the optimal cut‐off was 83 μIU/mL (95% CI: 70‐99 μIU/mL) for the IMMULITE 2000XPi assay.
Conclusions and Clinical Importance
The pellets were palatable and a suitable, novel carbohydrate source for the OGT.
Keywords: equine metabolic syndrome, horse, hyperinsulinemia, laminitis, oral glucose test, palatability, pony
Abbreviations
- BCS
body condition score
- BW
bodyweight
- CNS
cresty neck score
- EMS
equine metabolic syndrome
- ID
insulin dysregulation
- NSC
nonstructural carbohydrate
- OGT
oral glucose test
- OST
oral sugar test
- PPID
pituitary pars intermedia dysfunction
1. INTRODUCTION
Insulin dysregulation (ID), the key component of equine metabolic syndrome (EMS), increases a horse's risk of laminitis. 1 In animals with ID excessive postprandial insulin secretion and tissue resistance to insulin action (either or both can occur) cause transient or persistent hyperinsulinemia, 2 which can trigger lamellar damage. 3 An underlying genetic risk for disease coupled with extrinsic factors, such as consumption of diets high in nonstructural carbohydrates and a lack of exercise, likely underpins the relatively high incidence of ID and laminitis in equids worldwide. 4 , 5
The diagnosis of ID remains a challenge for multiple reasons. Phenotypically, horses and ponies with ID are often overweight with regional adiposity in key locations, such as around the nuchal ligament. 1 However, ID can also occur in lean animals limiting the diagnostic utility of assessment of body condition. The diagnosis of tissue insulin resistance requires the use of specific tests. 6 Further, resting insulin concentrations can be normal in animals with ID, which necessitates the use of a dynamic test that measures the insulin response to an oral glycemic challenge. 1 Unfortunately, the type and dose of glucose used in the test, as well as its route of administration, varies globally. The oral sugar test (OST) entails oral administration of a commercial corn syrup (Karo 7 ) that is not available worldwide. 8 An oral glucose test (OGT) that uses dextrose powder replaces this test where corn syrup cannot be sourced. The unreliable palatability of dextrose powder means that the dextrose is either dosed using a nasogastric tube or mixed with chaff and fed. 9 , 10 The result of this diversity in test protocols is that test‐specific cut‐off values for insulin concentration are required for diagnosing ID. 1 Further, multiple assays have been validated for the measurement of circulating insulin concentrations, each necessitating adjustment of the test cut‐off value for ID diagnosis. 11 The fact that ID requires monitoring compounds this problem, as most horses will require repeated testing to monitor disease and use of a consistent diagnostic approach for each patient is essential. Lastly, the lack of consistency in the assessment of ID is also problematic when trying to compare data from different regions. Thus, a widely available glucose product that is administered in a uniform manner to horses undergoing a glycemic challenge test is needed.
The aim of this study was to evaluate the palatability of, and blood glucose and insulin responses to, a custom produced carbohydrate pellet given as an oral glycemic challenge to horses and ponies for the diagnosis of ID. A second aim was to establish the diagnostic threshold for postprandial insulin concentrations consistent with ID when using the carbohydrate pellet in combination with commonly used immunoassays for quantification of equine insulin.
2. MATERIALS AND METHODS
2.1. Study design
This was a multicenter study undertaken concurrently (over a 12‐month period) in Australia Germany, Sweden, the United Kingdom and the United States. A mixture of university‐owned and privately‐owned horses and ponies (n = 157) of varying ages (not less than 2 years old), sex, breed and metabolic health were enrolled in the study by the investigators in each location. All eligible animals at each location were included so that the test could be undertaken in animals with a broad spectrum of metabolic health. All subjects needed to have resided with the respective owner for a minimum of 1 year, been up to date with routine vaccinations applicable to their home region and had been dewormed with an anthelmintic. Routine animal management practices at each location were not affected by the study. Donkeys, mules and pregnant or lactating mares were excluded from the study and subjects could not currently be receiving SGLT‐2 inhibitors, metformin or levothyroxine. The study was approved by the relevant Animal Care and Ethics Committee in each location and written consent was obtained from owners of privately‐owned subjects.
Carbohydrate pellets containing oligosaccharides based on glucose units, linseed flour, maize germ flour and highly refined plant oils and extracts were custom produced and transported in sealed containers to each study location for use in a glycemic challenge test administered by the different investigators adhering to the same protocol. The night before testing, overnight access to feed or pasture was withheld, subjects were housed individually, and provided with 0.2 to 0.4 kg/100 kg bodyweight (BW) hay or haylage. 1 Access to water was unrestricted. The next morning a 0‐hour timepoint blood sample was taken by jugular venipuncture to establish baseline insulin and glucose concentrations, and to enable measurement of ACTH for the diagnosis or confirmation of pituitary pars intermedia dysfunction (PPID; diagnosis made using a combination of clinical signs and geographically appropriate, seasonally‐adjusted cut‐off values). After sampling, the subjects were fed a pelleted dose of 0.5 g of soluble carbohydrate per kg BW. The pellets were offered for free intake over a 10 minutes period. After this time the feed bucket was removed, any refused portion estimated by visual inspection of the proportion remaining, and the refused pellets discarded. Subsequent blood samples were collected for measurement of insulin and glucose concentrations by jugular venipuncture 60 (except for the United Kingdom), 120, and 180 minutes after dosing.
The palatability of the carbohydrate pellet was determined by assessing the speed of consumption and amount consumed, and recording any signs indicative of an aversion to consumption. A simple 3‐point scale was used to grade the palatability of the pellet and is described in Table 1. Metabolic responses were only relevant in subjects that consumed greater than 90% of the pellets and were further investigated in an “acceptance” subset of animals graded as 1 or 2 on the acceptance scale (Figure 1; n = 132). Therefore, the blood insulin and glucose concentrations were analyzed in subsets of the cohort that were assigned a score of either 1 or 2 on the acceptance scale and had been tested for the variable being investigated.
TABLE 1.
A 3‐point grading scale was used to determine the palatability of a carbohydrate pellet offered to horses and ponies for a 10 min period (n = 157)
| Acceptance | Aversion | |
|---|---|---|
| Score 1 | Very good: test item was accepted without hesitation | None: no signs of aversion to the pellets |
| Score 2 | Good: test item was accepted with minor hesitation, but eaten within 10 min, up to 10% refusal | Moderate: slight to moderate aversive behavior when offered the pellets |
| Score 3 | Unacceptable: test item was not accepted within 10 min, >10% refusal | High: pronounced aversive behavior when offered the pellets (eg, head shaking, flehmen) |
FIGURE 1.
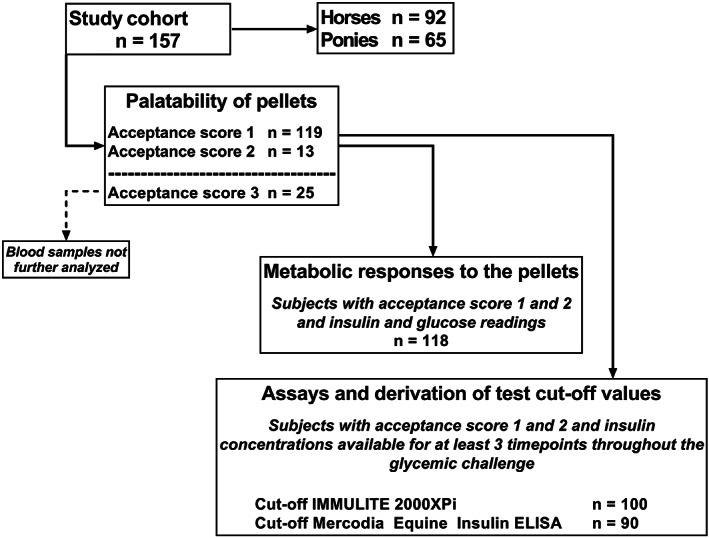
A flow chart describing subsets of the study cohort used for data analyses
2.2. Animals
A thorough history was recorded for each subject that included the recent diet (access over the past 4 weeks to forage/concentrate/pasture), housing (inside/outside/both), current or recent medications and relevant medical history (previous diagnoses of PPID, EMS or laminitis noting that not all animals had previously been assessed for these diseases). Age, breed, and sex were recorded. Where possible BW was measured using an electronic scale (n = 113) or, where no scale was available, calculated using a formula (n = 35; BW = heart girth2 (cm2) × body length (cm)/11 880 (cm3)) or estimated (n = 9). A body condition score (BCS; 1‐9) 12 and cresty neck score (CNS; 0‐5) 13 were assigned and the presence of supraorbital, rump and mammary or preputial fat deposits recorded. A thorough physical examination that included a detailed examination of the hooves was performed by an experienced veterinarian.
2.3. Sample processing
For the 0‐hour timepoint blood was divided equally between plastic K3EDTA and serum (clot activator) tubes (Greiner Bio‐One, Austria) for the measurement of ACTH and insulin respectively. The K3EDTA tubes were placed on wet ice or in a cooler immediately after collection, centrifuged (1500g for 10 minutes for all tubes) and the plasma transferred in 1 mL aliquots to cryovials and stored at −80°C. The clot activator tubes were left at ambient temperature for 30 to 60 minutes and then centrifuged (Australia, Germany and Sweden) or placed on ice and centrifuged within 5 hours (the United Kingdom and the United States), and the serum stored using the same process. Blood collected at the remaining 3 timepoints was used to obtain plasma and serum for insulin analyses.
2.4. Assays
A drop of fresh blood was used to measure blood glucose concentration with a hand‐held glucometer validated for use in horses (Australia, Germany, Sweden, and the United States: Accu‐Check [Roche, Switzerland] 10 or the United Kingdom: AlphaTRAK 2 [Zoetis, New Jersey]). 14 Plasma and serum samples were shipped frozen to a regional laboratory for initial analyses (Australia: VetPath Laboratories, Washington; Germany and Sweden: SYNLAB.vet GmbH, Leverkusen; UK: Liphook Equine Hospital Laboratory, Hampshire and USA: Animal Health Diagnostic Center, Cornell, New York). The analysis of ACTH was undertaken using IMMULITE 2000XPi (Siemens Healthcare, Germany) 15 in all locations except Germany where the IMMULITE 1000 (Siemens Healthcare, Germany) was used. The IMMULITE 2000XPi (n = 101) and the Mercodia Equine Insulin ELISA (Mercodia AB, Uppsala, Sweden; n = 103) were used to analyze insulin concentration. An interlaboratory comparison of the IMMULITE 2000XPi data was undertaken as part of the study to ensure that results obtained in the different regions could be compared. These assays have been validated previously. 1 , 11 , 16
2.5. Data analyses
The distribution of each dataset was checked for normality with a Shapiro‐Wilk test. Normally distributed continuous data are presented as mean ± SE and all other data are presented as median [IQR]. Significance was considered met at P < .05.
Continuous variables were compared with ANOVA (eg, age and BW) using Dunn's method for multiple pairwise comparisons. Categorical variables were compared with a Chi‐square test (eg, housing, diet and palatability grades) or ANOVA on ranks with Dunn's method (eg, BCS, CNS and intake time). The intake time was compared between horses and ponies using a Mann‐Whitney rank sum test. Area under the curve (AUC) was calculated using zero as the baseline with the trapezoidal method for the blood glucose and insulin (IMMULITE 2000XPi) responses to the pellets. These data were log‐transformed for a 2‐way ANOVA (with Holm‐Sidak method for pairwise comparisons) looking at the effect of type (ie, horse or pony) and clinical examination findings on glucose and insulin responses. Correlation of variables used Spearman's test except the correlation between the Mercodia Equine Insulin ELISA and IMMULITE 2000XPi insulin measurements which used Pearson's test and were also compared visually with a scatterplot. The agreement of sample classification in both datasets using the previously derived cut‐offs was described as percent and with Cohen's kappa statistic. 17 The limits of agreement between the 2 assays was estimated using Bland‐Altman analysis with a mixed model accounting for correlation within individuals. 18 These data were analyzed using SigmaPlot v.13 (Systat software, CA, USA) and R version 4.2.0.
Cut‐off determination was performed separately for the insulin concentrations obtained using the IMMULITE 2000XPi (Figure 1; n = 100) and the Mercodia Equine Insulin ELISA (n = 90) by determining the optimal number and composition of clusters of individuals with similar insulin time courses in 2 phases. First, clusters of samples were identified by consensus clustering using complete linkage hierarchical clustering on 1000 random subsets of the data. 19 A priori determination of the number of clusters was achieved by the majority rule of methods in the NbClust package. 20 Secondly, the cut‐off minimizing the absolute difference between sensitivity and specificity was determined in a bootstrap approach for every timepoint using the cutpointr R‐package. 21 Values below the limit of quantification were imputed by random draws from a truncated normal distribution (msm R‐package 22 ) whose parameters were estimated using regression on order statistics as provided by the NADA R‐package. 23 Missing 60 minutes samples (the United Kingdom) were imputed using the k‐nearest neighbor algorithm (as in the impute package 24 ).
3. RESULTS
3.1. Animals
The study cohort consisted of 58.6% horses (n = 92) and 41.4% ponies (n = 65) of 31 breeds (Supporting information item 1) and comprised 76 geldings, 8 stallions and 73 mares. They ranged in age from 4 to 40 years (14 ± 0.48 years) and were most frequently housed predominantly outside (45.5%) or indoors (38.5%), with a smaller number of subjects provided with an even mix of inside and outside time (16%). As expected, all subjects received some form of forage (hay > forage/pasture > silage/haylage) as their principal dietary component (Supporting information item 2). Some type of concentrate (eg, grain or pellet) was also provided as a secondary dietary component to 48.1% of subjects. These demographic data differed between the geographic locations (Table 2).
TABLE 2.
Demographic data (reported as median [IQR]) for the study cohort (n = 157) grouped according to their geographic location
| Overall | Germany | United Kingdom | Australia | United States | Sweden | P value | |
|---|---|---|---|---|---|---|---|
| Number | 157 | 55 | 33 | 25 | 24 | 20 | – |
| Type (%) | <.001 | ||||||
| Horse | 58.6 | 43.6 | 39.4 | 52 | 91.7 | 100 | |
| Pony | 41.4 | 56.4 | 60.6 | 48 | 8.3 | 0 | |
| Age (y) | 14 [10‐18] | 16 [12‐20] a | 17 [9–20] | 11 [9.5‐15] a | 13.5 [5.25‐18] | 13 [10.3‐16.8] | .02 |
| Sex (%) | <.001 | ||||||
| Gelding | 48.4 | 47.3 | 39.4 | 88 | 45.8 | 20 | |
| Stallion | 5.1 | 10.9 | 0 | 0 | 4.2 | 5 | |
| Mare | 46.5 | 41.8 | 60.6 | 12 | 50 | 75 | |
| Housing (%) | # (n = 23) | <.001 | |||||
| Outside | 45.5 | 23.6 | 66.7 | 100 | 47.9 | 0 | |
| Inside | 38.5 | 47.3 | 33.3 | 0 | 13 | 100 | |
| Both | 16 | 29.1 | 0 | 0 | 39.1 | 0 | |
| Diet (%) | <.001 | ||||||
| Forage only | 51.9 | 40 | 100 | 40 | 20.8 | 0 | |
| Forage plus concentrate | 48.1 | 60 | 0 | 60 | 79.2 | 100 |
Note: #, missing data.
Groups that differ statistically within variable.
The mean BW of the cohort was 461 ± 12.6 kg and BW differed significantly by geographical region (Figure 2). For the cohort the BCS (6 5 , 6 , 7 ) and CNS (2 1 , 2 , 3 ) were positively correlated (r = .61; P < .0001) and indicated a generally overweight cohort, where 61.8% were either overweight or obese (ie, ≥ 6/9). However, the degree of obesity varied significantly between the locations (Figure 2). Regional fat deposits were present in 46.5% (n = 73) of the cohort. For subjects with regional adiposity 48% had a fat deposit in only 1 region, while 35.6% had fat deposits in 2 areas and the remaining 16.4% had 3 areas of fatty deposits.
FIGURE 2.
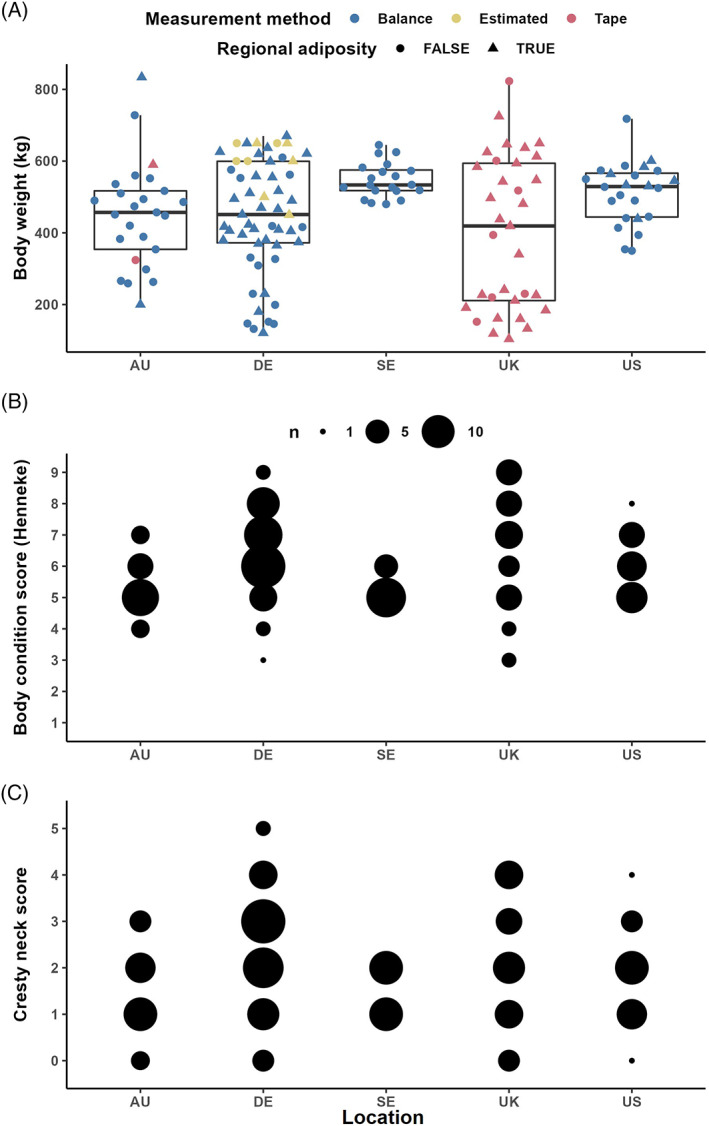
Bodyweight (kg) was measured using an electronic balance or weight tape (or estimated in 9 cases) in horses and ponies (n = 157) with a wide range of phenotypes receiving an oral glycemic challenge (A). While the differences in bodyweight between study location were essentially attributable to the breeds included in each cohort, the ponies and horses had body condition scores (B) mostly above 5/9, with more evenly distributed cresty neck scores (C)
An owner‐reported history of PPID was recorded in 5 subjects (3.2% of the cohort) with an additional 13 PPID‐positive subjects identified using a combination of clinical examination and basal ACTH concentration (using relevant cut‐offs for the season when sampled). There were 4 subjects where a diagnosis of PPID was equivocal. Overall, 11.5% of the cohort was diagnosed with PPID. Of the cohort 10.2% had been diagnosed with EMS before entering the study (again noting that not all animals had previously been tested for ID) and 19.1% had a history of laminitis. Overall, 49.7% of subjects had 1 or more of the following clinical signs: BCS ≥6/9, a CNS ≥3/5 or a history of laminitis. The median resting blood glucose and serum insulin concentrations for the cohort were 4.8 [4.6‐5.3] mmol/L and 2 2 , 3 , 4 , 5 , 6 μIU/mL, respectively. For the cohort, resting blood glucose concentration was positively correlated with CNS (r = .18; P = .03) and BCS (r = .36; P < .001). However, while resting insulin concentration was positively correlated with CNS (r = .26; P = .006), it was not correlated with BCS (r = .16; P = .08).
3.2. Palatability of pellets
Acceptance of the pellets was graded as 1 (very good) for 75.8% of the cohort, and 2 (good) for 8.3%, making the pellets acceptable to 84.1% of subjects. The pellets were more readily accepted by ponies, compared to horses (P = .004; Figure 3A). Further, there was a difference in acceptance between the geographical regions (P = .02; Figure 3B). Of the 25 subjects that found the pellets to be unacceptable, 15 showed no aversion (grade 1) to eating the pellets and either did not finish the full amount within the 10 minutes timeframe (67%) or did not touch the pellets at all (33%). The remaining 10 subjects tasted the pellets but showed either moderate (grade 2; 60%) and marked (grade 3; 40%) aversive behavior and did not consume an adequate amount.
FIGURE 3.
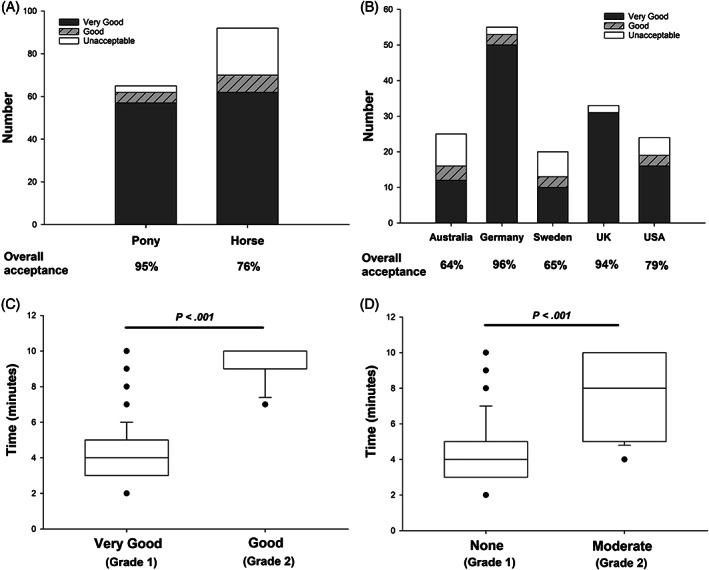
Glycemic carbohydrate pellets fed to a cohort of ponies and horses across 5 geographical regions (n = 157) were more palatable (P = .004) to ponies than horses (A) and the acceptance varied by location (B; P = .02). The rate of intake of the pellets was correlated with the degree of acceptance (C) and aversion (D). Subjects with very good acceptance of the pellets, and ones that showed no aversion to eating them, consumed the pellets faster (P < .001) that subjects with only good acceptance or those that showed mild‐moderate aversion to eating them
The median time to full intake of the pellets for the “acceptance” subset was 4 [3–6] minutes, although within this group, the intake time was faster for subjects graded 1, compared to 2 (Figure 3C). As such, the intake time was positively correlated with the acceptance grade (r = .51; P < .0001). Ponies (4 [3–5] minutes) and horses (4.5 [4‐6.25] minutes) consumed the pellets in comparable time (P = .09) and the median intake time did not differ between geographical locations (Australia 5 [4‐9.5] minutes, Germany 5 [4–6] minutes, Sweden 4 [3.5‐6] minutes, the United Kingdom 4 [3–6] minutes, and the United States 3 [3–5] minutes).
Consumption of the pellets did not induce adverse gastrointestinal effects or lameness and did not appear to induce major aversion with 87.1% graded as 1 (no aversion), 12.9% graded 2 (moderate aversion), and no subject graded as 3 (marked aversion) when offered the pellets. However, aversive behaviors did negatively affect intake time, which was longer for subjects graded 2, compared to 1 (Figure 3D). Accordingly, there was a positive correlation between intake time and the aversion score (r = .41; P < .0001). There was no difference between horses and ponies for aversion score.
3.3. Metabolic responses to the pellets
For the cohort, the median maximum (C max) blood glucose concentration in response to consuming the pellets was 6.6 [5.8‐7.8] mmol/L and the serum insulin C max was 40.5 [19‐99.8] μIU/mL (Figure 4). The timepoint at which the C max was reached (T max) was calculated for subjects with data for all 4 timepoints and for blood glucose (n = 87) occurred most frequently at 120 minutes (52.9%), then at the 60 minutes timepoint (28.7%). Similarly, the T max for serum insulin (n = 60) occurred most frequently at 120 minutes (46.7%), then at the 60 minutes timepoint (38.3%). When examining overall hormone responses to the pellets the AUC for blood glucose was correlated to the AUC for insulin in the cohort (r = .4; P < .001). However, the intake time for the pellets was not correlated with either blood glucose AUC (r = .15; P = .1) or insulin AUC (r = −.03; P = .78).
FIGURE 4.
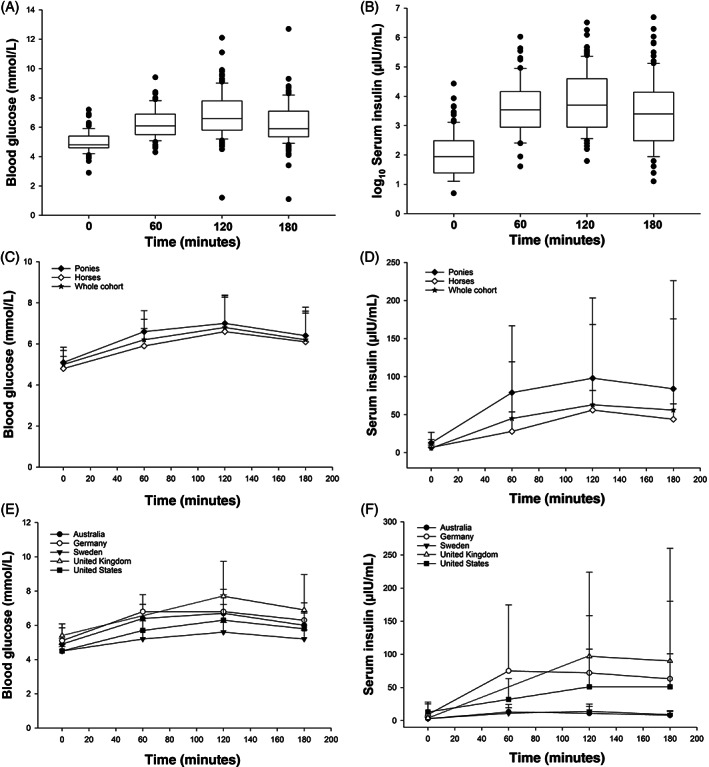
The median (range) blood glucose (A; mmol/L) and log10 serum insulin concentration (B; μIU/mL) in horses and ponies before (0 minutes) and after (at 60, 120, and 180 minutes) being fed a glycemic carbohydrate pellet. When shown by type, the mean (±SD) blood glucose (C) and serum insulin (D) concentrations were higher (P < .05) in the ponies, compared to horses. Further, the blood glucose (E) and serum insulin (F) concentrations differed (P < .05) among the geographical regions
The blood glucose responses (AUC) were higher (Figure 4C; P = .002) in ponies (6.4 [5.65‐7.05] mmol/L min), compared to horses (5.7 [5.3‐6.58] mmol/L min), and this was affected (P = .05) by whether the subject had clinical signs of EMS or not. Having clinical signs of EMS did not result in a larger blood glucose response in ponies, but it did for horses (P = .02), compared to subjects without clinical signs. Ponies had a larger insulin response (AUC; Figure 4D; P = .008) to the pellets than horses, and both ponies and horses with clinical signs of EMS had a larger insulin response (P = .002) than those without clinical signs. Both the blood glucose (Figure 4E) and serum insulin (Figure 4F) responses differed (P < .001) among the locations.
3.4. Assays and derivation of test cut‐off values
The use of 2 clusters was deemed optimal by most of the algorithms provided by the NbClust package, resulting in a group of individuals with high insulin (n = 16 and n = 20 for the IMMULITE 2000XPi and Mercodia Equine Insulin ELISA, respectively) and a group with low insulin (n = 84 and n = 70). Regarding cluster sizes, it should be noted that while there was substantial overlap between the individuals included in the IMMULITE 2000XPi and the Mercodia Equine Insulin ELISA dataset (n = 83), they were not equal. In the overlapping parts of the study population, the agreement for classification was 93% with K Cohen = 0.78.
At 120 minutes the optimal cut‐off was 83 μIU/mL (95% CI: 70‐99 μIU/mL) for the IMMULITE 2000XPi (Figure 5A). In comparison, the same cut‐off using the insulin measurements from the Mercodia Equine Insulin ELISA was 92 μIU/mL (95% CI: 81‐108 μIU/mL; Figure 5B). Accordingly, the insulin measurements were slightly higher with this assay than with the IMMULITE 2000XPi although the correlation between both assays was very strong (r[300] = .97, P < .0001; Figure 5C). The performance estimates for the cut‐off analyses for both assays are reported in Table 3. The limits of agreement for the 2 assays are shown in Supporting information item 3.
FIGURE 5.
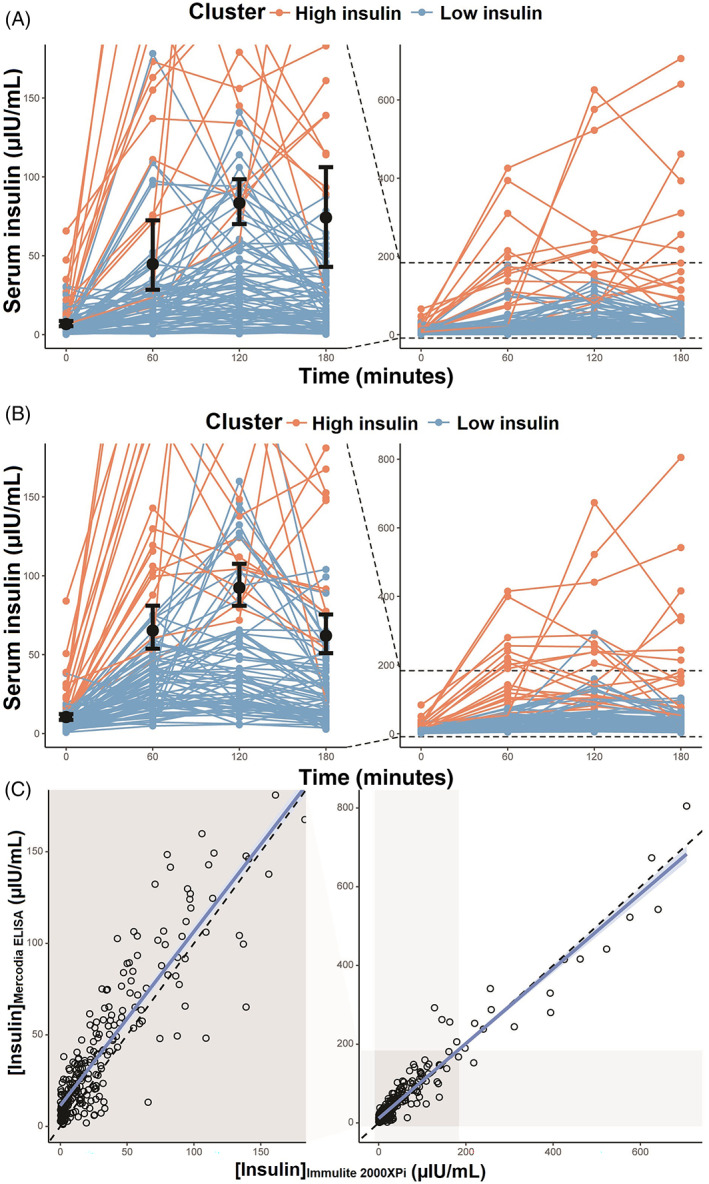
Individual serum insulin concentrations used to derive cut‐off values for the diagnosis of insulin dysregulation (shown in the left [enlarged] panel with 95% CI) for the IMMULITE 2000XPi assay (A) and the Mercodia Equine Insulin assay (B). The correlation between the 2 assays (C), with the identity line, was estimated using simple linear regression. In the range of 0 to 150 μIU/mL, insulin concentrations measured with the Mercodia assay were slightly higher which was consistent with the higher cut‐off value
TABLE 3.
Cut‐off values and out‐of‐bag performance estimates for different target metrics for the datasets for the IMMULITE 2000XPi and Mercodia Equine Insulin ELISA assays
| Assay | Timepoint (min) | Cut‐off | Accuracy | Sensitivity | Specificity | Cohen's Kappa |
|---|---|---|---|---|---|---|
| IMMULITE | 0 | 6.69 [5.31‐8.66] | 0.81 [0.72‐0.89] | 0.74 [0.33‐1] | 0.83 [0.73‐0.93] | 0.43 [0.15‐0.68] |
| IMMULITE | 60 | 44.7 [28.4‐72.42] | 0.87 [0.76‐0.95] | 0.81 [0.5‐1] | 0.88 [0.72‐1] | 0.58 [0.36‐0.8] |
| IMMULITE | 120 | 83.41 [70.1‐98.51] | 0.88 [0.8‐0.95] | 0.82 [0.43‐1] | 0.89 [0.79‐0.97] | 0.6 [0.36‐0.84] |
| IMMULITE | 180 | 74.2 [42.94‐106.17] | 0.95 [0.86‐1] | 0.89 [0.5‐1] | 0.96 [0.84‐1] | 0.83 [0.56‐1] |
| Mercodia | 0 | 10.43 [8.63‐12.33] | 0.75 [0.63‐0.87] | 0.73 [0.43‐1] | 0.76 [0.59‐0.92] | 0.4 [0.18‐0.64] |
| Mercodia | 60 | 65.25 [53.8‐81.04] | 0.91 [0.83‐0.97] | 0.87 [0.67‐1] | 0.92 [0.8‐1] | 0.74 [0.560.91] |
| Mercodia | 120 | 92.42 [80.98‐107.55] | 0.83 [0.74‐0.91] | 0.77 [0.44‐1] | 0.85 [0.73‐0.96] | 0.55 [0.33‐0.77] |
| Mercodia | 180 | 62 [50.95‐75.46] | 0.89 [0.81‐0.97] | 0.85 [0.62‐1] | 0.91 [0.77‐1] | 0.71 [0.51‐0.9] |
3.5. Test outcomes
With the binary decision limit of 83 μIU/mL, 24 out of 100 subjects would have been considered positive for ID after successfully undergoing the glycemic challenge. Of these animals, 5 had a reported history of EMS and 8 had a history of laminitis before enrollment. There was good agreement between the decision limit and the clinical sign of CNS, which was examined because it is known to be a strong clinical marker of EMS, 25 with up to 80% agreement with a classification based on CNS alone (Figure 6A). The proportion of high insulin responses was positively correlated with CNS (Figure 6B). As the number of negative and positive cases (high vs low insulin responses) are unbalanced, these data should be interpreted with care.
FIGURE 6.
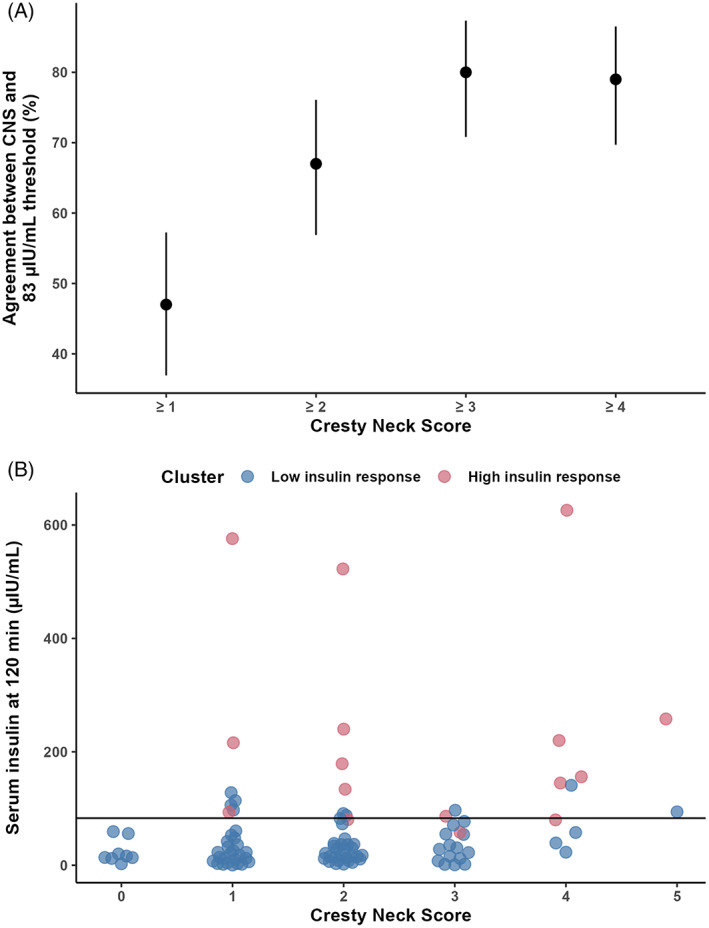
There was 80% agreement with the binary decision limit for a diagnosis of ID of 83 μIU/mL at 120 minutes after a glycemic challenge test and a having a CNS of ≥3 (A). Two clusters were used to determine the decision limit (—) and the proportion of high insulin responders (pink circle) was positively correlated with CNS (B), compared to low responders (blue circle)
4. DISCUSSION
Using a diverse cohort of both horses and ponies comprised of many breeds that were located across different regions of the world and with variable metabolic health, the glycemic pellet tested here had acceptable palatability and therefore could be a suitable carbohydrate source for the OGT. When used in an OGT the novel pellet formulation also enabled the detection of ID using a diagnostic threshold of 83 μIU/mL when using the IMMULITE 2000XPi immunoassay for insulin quantification. This diagnostic threshold is essentially the same as the recommended threshold at 120 minutes post glycemic challenge for the 1 g/kg BW in‐feed OGT of 80 to 90 μIU/mL. 1 However, the diagnostic threshold for the OGT was determined using the IMMULITE 1000 chemiluminescent assay for insulin analyses and the pellet provides half the carbohydrate “dose” compared to dextrose powder in this comparative form of the test. The similar threshold value for diagnosis of ID identified here using the lower carbohydrate dose could be associated with faster intake and gastrointestinal transit or digestion for the small volume of pellets, compared to the chaff, bran and dextrose meal or, alternatively, could result from a difference in the assays used as IMMULITE 2000XPi results are generally higher than for the IMMULITE 1000 assay. In achieving these 2 main aims, this study has successfully assessed a novel alternative carbohydrate source for the OGT that could provide an improved end‐user experience and reduce the number of test formats used globally.
Use of pelletized carbohydrate has been suggested as a preferable test substrate to powdered sugar for the OGT previously, because of its familiarity to horses, and particularly, ponies. 10 , 26 This study reports the successful use of a custom produced glycemic pellet in an OGT in a diverse cohort. Currently, the OGT can be performed using different formats with the choice of test protocol often depending on the geographical location of the veterinarian performing the test, 1 which underpinned the rationale for undertaking this study across several sites. All available sources of carbohydrate used in an OST or OGT have been the subject of debate, with issues including ease of administration, palatability and test repeatability. 10 , 27 , 28 Neophobic responses to novel feedstuffs can occur in horses, 29 and use of a pellet that mimicked other concentrated horse feeds aimed to reduce the neophobic response to the test “dose.” Given the prevalence of ID and the need for ongoing dynamic testing in animals with ID, an OGT format where the carbohydrate is simple to administer as demonstrated here, is ideal. Voluntary intake of the pellets used in this study was ~4 minutes, which is faster than consumption of a meal, such as in the in‐feed OGT, and could be advantageous for field‐based testing.
Despite using a similar protocol to the in‐feed form of the OGT, the dose and intake time in the current study share similarities with the OST. 7 The pellets were offered at 0.5 g/kg BW soluble carbohydrate, and this dose is the lowest end of the rates suggested for the OGT, and just above the upper end of an OST, where the dose of sugar ranges from 0.15 to 0.45 mL(~mg)/kg BW. 7 This moderate dose rate seeks to reduce the variability seen with the lower end of the dosing spectrum, 28 while trying to lessen the risk of inducing insulin responses well above the diagnostic threshold in more severely insulin‐dysregulated individuals. Given the transient nature of the insulin peak in dynamic tests the risk of inducing laminitis seems to be low in the collective experience of the authors. The other main way that this test differs from an in‐feed OGT is the short intake time to ingest the carbohydrate. The rapid ingestion of the pellets (~4 minutes) is more akin to the administration of syrup via dosing syringe, or dextrose solution via a naso‐gastric tube, compared to the much slower (~15‐30 minutes) ingestion of the OGT meal. Further, the in‐feed form of the test includes other unquantified forms of carbohydrate present in the bran and chaff. Faster intake of the carbohydrate might reduce intra‐animal variability associated with gastrointestinal transit time, which would be advantageous for repeated testing over time. Oral challenge tests have not shown high repeatability for insulin responses, 10 so future studies should investigate test repeatability with a focus on binary test outcomes.
The speed of pellet intake was correlated with both the level of acceptance and signs of aversion. Overall, palatability of the pellet was acceptable at 84%, based on the European Medicines Agency's 2021 “Guideline on the demonstration of palatability of veterinary medicinal products” recommendation of ≥70%. Based on this minimum requirement, the pellet was palatable to both horses and ponies, although ponies found it more palatable than horses. Appetite differences, or familiarity with pelleted feeds may have contributed to this difference in palatability between ponies and horses. There is a preference for sweet taste in horses 29 , 30 , 31 and ponies, 32 and sweet taste receptors are present in horses. 33 Two‐thirds of ponies selected an oats plus sucrose combination over oats alone, 32 and adding a sweet taste (nonnutritive sweetener) encouraged food intake in horses, 29 supporting that sweet feeds are attractive to horses and ponies. However, whether ponies have a greater variety or density of oral or intestinal sweet taste receptors compared to horses has not been reported, and this knowledge could be valuable in helping to determine whether ponies differ to horses in their perception and acceptance of nonstructural carbohydrates.
Minimum palatability requirements were not reached in Australia (64%) or Sweden (65%). It is unknown why failure of acceptance occurred in these regions although unfamiliarity with the type of pellet is 1 possible reason. In Australia, changes to the pellet because of increased travel time and processing of the pellet in quarantine (eg, irradiation) could have occurred and affected palatability. It has been suggested anecdotally that refusal to eat the test meal in the in‐feed OGT is associated with the overly sweet taste of the test diet. It is possible that some animals also found the pellets to be overly sweet. Future studies could examine whether offering the pellets with other feedstuffs improves palatability (and possibly familiarity), but this would reintroduce the need for additional meal components in the test and change the interpretation. Levels of neophobia in ungulates differ between individuals 34 and overall, the pellets were unacceptable to 15.9% of the animals tested. In these instances, the test could not be adequately performed, so selection of a different form of the OGT where voluntary intake is not required is suggested for these animals.
The pellets induced a glycemic and insulinemic response in both horses and ponies of varying magnitude. Further, the metabolic responses and decision limit generated for the test agreed with clinical indicators of EMS (both generalized and regional adiposity) in this cohort. Assessment of the clinical signs associated with EMS is subjective, and application of the scoring systems for adiposity could have differed between the investigators. Because of geographical isolation of each location, validation of the assessors' scores against one another was not possible. However, this limitation is inherent to any clinical examination of a patient with EMS, and supports the need to refine the key diagnostic test used for assessing ID.
The most frequent T max of 2 hours (followed by 1 hour) means that no change to the protocol for the OGT is required, where sampling between 1 and 2 hours is recommended to capture the C max. 1 Insulin and glucose responses to an oral glycemic challenge are affected by the fed state of the animal being tested. 35 However, many horses and ponies are housed exclusively on pasture, as supported by the current study where the greatest proportion of the cohort was housed predominantly outside, and therefore cannot be fasted before an OGT. Access to grazing before the test (in animals normally housed on pasture) is not thought to markedly affect OGT outcomes, and thus some access to forage before the test is generally recommended so that the subject is tested in a “resting” state. 28 , 36 The current study design opted to allow access to hay, rather than pasture, because of the variable feeding and housing strategies in each study location, to align the results with current recommendations on test preparation.
Because of the difficulties associated with measuring equine insulin with assays that are designed for other species using an assay that has been validated for measuring equine insulin is paramount. 37 Further, discrepancies between the various assays available for measuring equine insulin mean that direct comparison of results derived from different assays is not possible, and multiple reference intervals are required for test interpretation. This problem, which is currently without an obvious resolution, was addressed in this study by performing interlaboratory and interassay comparisons. In this study the IMMULITE 2000XPi and Mercodia Equine Insulin ELISA showed excellent concordance, and similar threshold values for the diagnosis of ID. The provision of a diagnostic threshold for both assays is a strength of the current study and should aid in making this test more accessible to field practitioners.
Undertaking a multicenter study, with different investigators, animal husbandry practices and pellet shipping conditions for each site, introduced more variability into the dataset than if the study had been performed under controlled conditions at 1 location. However, as this variability is reflective of field conditions, we felt that the study design provided a more realistic assessment of how the test might perform in practice and despite the approach, the pellets proved to be a comparable, easy to use alternative carbohydrate source for an OGT. The fact that the same insulin assay was not available at each study site was also a study limitation. The requirement to subject the samples to different transport conditions before insulin analyses introduced a further source of variability. This was addressed by undertaking the interassay validation of the data and analyzing multiple samples with more than 1 assay. The determination of palatability using simple observational grading scales is also a limitation inherent to veterinary studies. However, as a basic assessment of acceptance (or not) was considered likely to reflect the potential for voluntary uptake under field conditions in the study cohort, and would therefore be indicative of the target population, the grading scales used were considered adequate for fulfilling this requirement.
A novel, pelletized carbohydrate source designed to be familiar to ponies and horses, and therefore more readily acceptable, was successfully used in a glycemic challenge test. The pellet was considered to have acceptable palatability, and because of the rapid voluntary intake, might provide a simplified version of the OGT for end‐users, particularly those working in the field. A diagnostic threshold of 83 μIU/mL (for the IMMULITE 2000XPi) at 120 minutes after consuming the pellets was deemed suitable for diagnosing ID. A diagnostic threshold was also provided for the Mercodia Equine Insulin assay (92 μIU/mL) and the choice of assay for insulin analyses remains important when using any form of the OGT with use of the diagnostic threshold relevant to the selected assay paramount.
CONFLICT OF INTEREST DECLARATION
Tobias Warnken, Dania B. Reiche and Johanna Sonntag are employees of Boehringer Ingelheim Vetmedica GmbH, Germany. During the time of the study Tobias Warnken was employed by the Clinic for Horses at the University of Veterinary Medicine Hannover, Germany. No other authors have a conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the Animal Ethics Committees at the institutions in the study locations. The studies performed in Australia were approved by the University Animal Ethics Committee of Queensland University of Technology (1900000217). The studies performed in Germany were approved by the State Office for Consumer Protection and Food Safety in accordance with the German Animal Welfare Law (33.19‐42 502‐04‐19/3202 and 33.19‐42 502‐04‐18/3006). The studies performed in Sweden were approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden (5.8.18‐15 663/2019). The studies performed in the United Kingdom were approved by the University of Liverpool Veterinary Research Ethic committee (VREC797). The studies performed in the United States of America were approved by the University of Massachusetts IACUC (#2019‐0033).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting information
ACKNOWLEDGMENT
Funding provided by Boehringer Ingelheim Vetmedica GmbH. We acknowledge the Riding for the Disabled Associations at Anstead, Arundel Park and Sunshine Coast in Australia and the Animal Research Centre at the Swedish University of Agricultural Sciences.
de Laat MA, Warnken T, Delarocque J, et al. Carbohydrate pellets to assess insulin dysregulation in horses. J Vet Intern Med. 2023;37(1):302‐314. doi: 10.1111/jvim.16621
Melody A. de Laat and Tobias Warnken contributed equally to this work.
Funding information Boehringer Ingelheim
REFERENCES
- 1. Durham AE, Frank N, McGowan CM, et al. ECEIM consensus statement on equine metabolic syndrome. J Vet Intern Med. 2019;33:335‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frank N, Tadros EM. Insulin dysregulation. Equine Vet J. 2014;46:103‐112. [DOI] [PubMed] [Google Scholar]
- 3. Karikoski NP, McGowan CM, Singer ER, et al. Pathology of natural cases of equine Endocrinopathic laminitis associated with hyperinsulinemia. Vet Pathol. 2015;52:945‐956. [DOI] [PubMed] [Google Scholar]
- 4. Treiber KH, Kronfeld DS, Hess TM, Byrd BM, Splan RK, Staniar WB. Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture‐associated laminitis in ponies. J Am Vet Med Assoc. 2006;228:1538‐1545. [DOI] [PubMed] [Google Scholar]
- 5. Coleman MC, Belknap JK, Eades SC, et al. Case‐control study of risk factors for pasture‐ and endocrinopathy‐associated laminitis in north American horses. J Am Vet Med Assoc. 2018;253:470‐478. [DOI] [PubMed] [Google Scholar]
- 6. Bertin FR, de Laat MA. The diagnosis of equine insulin dysregulation. Equine Vet J. 2017;49:570‐576. [DOI] [PubMed] [Google Scholar]
- 7. Jocelyn NA, Harris PA, Menzies‐Gow NJ. Effect of varying the dose of corn syrup on the insulin and glucose response to the oral sugar test. Equine Vet J. 2018;50:836‐841. [DOI] [PubMed] [Google Scholar]
- 8. Schuver A, Frank N, Chameroy KA, Elliott SB. Assessment of insulin and glucose dynamics by using an oral sugar test in horses. J Equine Vet. 2014;34:465‐470. [Google Scholar]
- 9. Warnken T, Delarocque J, Schumacher S, Huber K, Feige K. Retrospective analysis of insulin responses to standard dosed oral glucose tests (OGTs) via naso‐gastric tubing towards definition of an objective cut‐off value. Acta Vet Scand. 2018;60:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Laat MA, Sillence MN. The repeatability of an oral glucose test in ponies. Equine Vet J. 2017;49:238‐243. [DOI] [PubMed] [Google Scholar]
- 11. Warnken T, Huber K, Feige K. Comparison of three different methods for the quantification of equine insulin. BMC Vet Res. 2016;12:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henneke DR, Potter GD, Kreider JL, et al. Relationship between condition score, physical measurements and body‐fat percentage in mares. Equine Vet J. 1983;15:371‐372. [DOI] [PubMed] [Google Scholar]
- 13. Carter RA, Geor RJ, Staniar WB, et al. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet J. 2009;179:204‐210. [DOI] [PubMed] [Google Scholar]
- 14. Hackett ES, McCue PM. Evaluation of a veterinary glucometer for use in horses. J Vet Intern Med. 2010;24:617‐621. [DOI] [PubMed] [Google Scholar]
- 15. Copas VE, Durham AE. Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction. Equine Vet J. 2012;44:440‐443. [DOI] [PubMed] [Google Scholar]
- 16. Öberg J, Bröjer J, Wattle O, Lilliehöök I. Evaluation of an equine‐optimized enzyme‐linked immunosorbent assay for serum insulin measurement and stability study of equine serum insulin. Comp Clin Pathol. 2012;21:1291‐1300. [Google Scholar]
- 17. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37‐46. [Google Scholar]
- 18. Carstensen B, Gurrin L, Ekstrøm C, et al. MethComp: Functions for Analysis of Agreement in Method Comparison Studies. R Package Version 122; 2013.
- 19. Chiu D, Talhouk A. diceR: Diverse Cluster Ensemble in R. 1.1.0 edR Package; 2021.
- 20. Charrad M, Ghazzali N, Boiteau V, et al. NbClust: Determining the Best Number of Clusters in a Data Set. 3.0 edR Package; 2015.
- 21. Thiele C. cutpointr: Determine and Evaluate Optimal Cutpoints in Binary Classification Tasks . 1.1.1 edR Package; 2021.
- 22. Jackson C. Multi‐State Modelling with R: The msm Package ; 2007.
- 23. Lee L. NADA: Nondetects and Data Analysis for Environmental Data. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 24. Hastie T, Tibshirani R, Narasimhan B, et al. Imputation for Microarray Data. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 25. Fitzgerald DM, Anderson ST, Sillence MN, de Laat MA. The cresty neck score is an independent predictor of insulin dysregulation in ponies. PLoS One. 2019;14:e0220203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warnken T, Schaub C, Delarocque J, et al. Evaluation of Glycemic Carbohydrate Formulations for Assessment of Insulin Dysregulation in Equines. Phoenix Arizona: American College of Veterinary Medicine Forum; 2019. [Google Scholar]
- 27. Smith S, Harris PA, Menzies‐Gow NJ. Comparison of the in‐feed glucose test and the oral sugar test. Equine Vet J. 2016;48:224‐227. [DOI] [PubMed] [Google Scholar]
- 28. Knowles EJ, Harris PA, Elliott J, Menzies‐Gow NJ. Use of the oral sugar test in ponies when performed with or without prior fasting. Equine Vet J. 2017;49:519‐524. [DOI] [PubMed] [Google Scholar]
- 29. Van den Berg M, Giagos V, Lee C, et al. The influence of odour, taste and nutrients on feeding behaviour and food preferences in horses. Appl Anim Behav Sci. 2016;184:41‐50. [Google Scholar]
- 30. Jankunis ES, Whishaw IQ. Sucrose bobs and quinine gapes: horse (Equus caballus) responses to taste support phylogenetic similarity in taste reactivity. Behav Brain Res. 2013;256:284‐290. [DOI] [PubMed] [Google Scholar]
- 31. Randall RP, Schurg WA, Church DC. Response of horses to sweet, salty, sour and bitter solutions. J Anim Sci. 1978;47:51‐55. [DOI] [PubMed] [Google Scholar]
- 32. Hawkes J, Hedges M, Daniluk P, et al. Feed preferences of ponies. Equine Vet J. 1985;17:20‐22. [DOI] [PubMed] [Google Scholar]
- 33. Daly K, Al‐Rammahi M, Arora DK, et al. Expression of sweet receptor components in equine small intestine: relevance to intestinal glucose transport. Am J Physiol Regul Integr Comp Physiol. 2012;303:R199‐R208. [DOI] [PubMed] [Google Scholar]
- 34. Schaffer A, Caicoya AL, Colell M, et al. Neophobia in 10 ungulate species—a comparative approach. Behav Ecol Sociobiol. 2021;75:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacob SI, Geor RJ, Weber PSD, Harris PA, McCue ME. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet J. 2018;50:249‐254. [DOI] [PubMed] [Google Scholar]
- 36. Banse HE, McFarlane D. Comparison of three methods for evaluation of equine insulin regulation in horses of varied body condition score. J Equine Vet. 2014;34:742‐748. [Google Scholar]
- 37. McFarlane D. Diagnostic testing for equine endocrine diseases: confirmation versus confusion. Vet Clin North Am Equine Pract. 2019;35:327‐338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


