Abstract
Background
Bacterial meningitis (BM) and meningoencephalitis (BMEM) are associated with high case fatality rates and neurologic sequelae in people, but limited data exists on outcome in dogs.
Hypothesis/Objectives
To report the clinicopathologic features, treatment and outcome of BM/BMEM in dogs, with a focus on clinical presentation, relapse and long‐term neurological deficits.
Animals
Twenty‐four client‐owned dogs diagnosed with BM/BMEM without empyema.
Methods
Retrospective case series of dogs diagnosed with BM/BMEM from 5 veterinary referral hospitals between January 2010 and August 2020.
Results
Twenty‐four dogs were included. Median duration of clinical signs was 2 days (range ≤24 hours to 30 days) and signs recorded included pyrexia (3) and cervical hyperesthesia (10). Neurological deficits were present in 18 dogs including altered mentation (12), ataxia (8), nonambulatory status (8), head tilt (8), and cranial nerve deficits (13). Intracellular bacteria were visualized on cerebrospinal fluid (CSF) analysis in 15/24 dogs, with positive CSF bacteriological culture in 8/21. Otitis media/interna (OMI) was diagnosed in 15/24 dogs, of which 6/15 dogs underwent total ear canal ablation and lateral bulla osteotomy.
Twenty dogs survived to hospital discharge. Median duration of antibiotic administrations was 8 weeks (range, 2‐16 weeks). Glucocorticoids were administered to 15 dogs. Median follow‐up time was 92 days (range, 10‐2233 days). Residual neurological deficits were reported in 9 dogs, with a single case of suspected relapse.
Conclusions and Clinical Importance
Clinical signs were variable in dogs with BM/BMEM, the nidus of bacterial infection was often OMI and the majority of dogs made a full recovery with treatment.
Keywords: bacterial infection, canine, central nervous system, meningitis
Abbreviations
- BM
bacterial meningitis
- BMEM
bacterial meningoencephalomyelitis
- CNS
central nervous system
- CRP
C‐reactive protein
- CSF
cerebrospinal fluid
- CT
computed tomography
- FLAIR
fluid attenuation inversion recovery
- LBO
lateral bulla osteotomy
- MRI
magnetic resonance imaging
- NSAID
nonsteroidal anti‐inflammatory drugs
- SRMA
steroid‐responsive meningitis arteritis
- T2W
T2‐weighted
- TECA
total ear canal ablation
- TNCC
total nucleated cell count
- VBO
ventral bulla osteotomy
1. INTRODUCTION
Bacterial meningitis (BM) is characterized by inflammation of the meninges secondary to bacterial infection. 1 , 2 Concurrent involvement of the adjacent neuroparenchyma is referred to as bacterial meningoencephalomyelitis (BMEM). 1 Routes of infection include hematogenous spread, extension from a neighboring focus such as otitis media/interna (OMI), 3 , 4 foreign body migration, 5 traumatic or iatrogenic inoculation. 6 In human patients a “triad” of typical clinical signs is associated with BM; pyrexia, neck stiffness, and altered mental status. 7
BM/BMEM in dogs causes cervical hyperesthesia and pyrexia. 8 , 9 Diagnosis is based on clinical presentation, routine blood work, advanced imaging, and cerebrospinal fluid (CSF). Advanced imaging can confirm meningeal contrast enhancement and suggest the presence of parenchymal involvement or evidence of concurrent empyema, or a combination of these changes. 10 , 11 , 12 Cerebrospinal fluid (CSF) analysis often reveals a marked neutrophilic pleocytosis with intracellular bacteria. 13 In the more chronic cases, CSF analysis might be normal or yield a moderate, predominantly mononuclear pleocytosis. 4
Treatment of BM/BMEM involves aggressive administration of antibiotics, analgesia, and intensive nursing care. 14 In people, the choice of antibiotic agent used is based on antibiotic susceptibility patterns of the anticipated pathogens; however, no standardization exists for duration of antibiotic therapy (7 to ≥21 days). 14 A delay in commencing antibiotic therapy has been associated with reduced survival in human studies. 15 , 16 Surgical drainage and debridement of necrotic tissue might be indicated if a primary focus of infection has been identified, such as OMI. There is an improved outcome with surgical treatment of otogenic meningitis compared with medical treatment. 17 , 18
Case fatality rates are ~14% to 21% (increasing to 34% in elderly patients) in people with BM/BMEM. 2 , 7 , 19 , 20 , 21 Long‐term neurological deficits in surviving patients are varied and can include cognitive impairment, weakness, hearing deficits, other cranial nerve deficits, and aphasia. 7 , 22 A recent meta‐analysis documented neurological deficits in 19.9% of BM/BMEM patients after hospital discharge (range 12.3%‐35.3%). 22 Limited data is currently available on survival and long‐term outcome of BM in dogs. Outcome after prompt and aggressive medical or surgical treatment of otogenic intracranial infections is very good to excellent. 4 , 23
The aims of this study were to report the clinicopathologic features, treatment, and outcome of BM/BMEM without empyema in dogs.
2. MATERIALS AND METHODS
Ethical approval for this study was granted by the Royal Veterinary College's Ethical Review Board (URN SR2021‐0002). Medical records from 5 small animal referral hospitals were retrospectively reviewed to identify dogs with a diagnosis of BM/BMEM between January 2010 and August 2020. A diagnosis of BM was considered to include (1) the presence of intracellular bacteria on CSF cytological evaluation or (2) positive CSF bacteriological culture or (3) neutrophilic pleocytosis (specifically degenerate neutrophils) on CSF cytological evaluation with clinical improvement after initiation of administration of antibiotics, or these findings in combination. Cases were excluded if they had incomplete medical records or imaging findings consistent with empyema (evidence of accumulation of T1W‐hypointense, T2W‐ and FLAIR‐hyperintense extra‐axial material with peripheral heterogenous contrast enhancement) 10 , 12 or suspected intracranial abscess formation indicated by a well demarcated mass lesion with a T1W‐hypointense, T2W‐hyperintense center, and strongly contrast enhancing rim. 24
Data retrieved from the medical records included signalment, clinical history, physical and neurological examination findings, complete blood count (CBC), serum biochemistry, advanced imaging findings (reported by a board‐certified veterinary radiologist), CSF analysis including bacteriological culture and sensitivity, blood/urine/other bacteriological culture and sensitivity (if performed), treatment type, and duration. Data on outcome and quality of life were obtained from the referral hospital and primary care practice clinical records and telephone interview of owners where available.
3. RESULTS
3.1. Clinical presentation
Twenty‐four dogs were included in the study (clinical data summarized in Supplemental Table 1). The median age was 4.2 years (range, 2 months to 9.9 years). Fifteen dogs (63%) were male (11 neutered) and 9 (37%) were female (5 neutered). Breeds included French Bulldogs (8), British/English Bulldogs (3), Cocker Spaniels (2), Boxers (2), and cross‐breeds (2).
The onset of clinical signs was categorized as acute (≤48 hours) in 13 dogs (54%), subacute (3‐7 days) in 4 (17%), and chronic (>7 days) in 7 (29%), with a median duration of 2 days (range, ≤24 hours to 30 days). Six dogs (25%) were PO administered amoxicillin clavulanate before referral (median duration 5 days; range, 1‐7), 2 dogs were concurrently administered glucocorticoids (prednisolone 0.5 and 1 mg/kg PO q24h) for 5 days. Three dogs had received topical antibiotics in the ears. Despite antibiotic therapy, all 9 dogs had progression of clinical signs. A further 3 dogs (13%) received glucocorticoids (1: prednisolone 0.5 mg/kg PO q24h for 3 days, 2: dexamethasone 0.1 mg/kg IV q24h for 1 day), without antibiotics administered PO before to referral (median duration 1 day; range, 1‐3), and 6 dogs (25%) received nonsteroidal anti‐inflammatories (NSAIDs) or paracetamol (median duration 5 days; range, 1‐30) PO.
A potential source of infection was identified on physical examination in 11/24 (46%) dogs: otitis externa in 9 (38%), left elbow effusion in 1 (4%), and popliteal lymph node enlargement with nail bed (left hind, digit 3) inflammation in 1 (4%). Pyrexia (≥39.2°C) was recorded in 3 (13%) dogs.
Neurological examination revealed abnormalities in 18/24 (75%) dogs, which included altered mentation (12; obtundation [11], stupor [1]), ataxia (8), nonambulatory status (8) (because of severe vestibular ataxia [6] or obtundation and reluctance to walk [2]), head tilt (8), postural reaction deficits (3) and cranial nerve deficits (13) including pathological nystagmus (10), positional strabismus (6) and facial nerve paresis/paralysis (2). Cervical hyperesthesia was identified in 10 (42%) dogs; 5 of these without concurrent neurological deficits. One dog presented with no neurological deficits or pain detected on examination but with a history of head tremors and reluctance to open the mouth. Neuroanatomical localization was recorded in 17 dogs (71%); vestibular system (10; 6 with evidence of central involvement), brainstem (3), multifocal (3) and C1‐C5 spinal cord segments (1).
3.2. Diagnostic investigations
Twenty‐one dogs (88%) had CBC and serum biochemistry performed on admission, with abnormalities including neutrophilia (12), monocytosis (8), anemia (2), hyperglobulinemia (5), hypoalbuminemia (5), elevated ALP and ALT activity (4) and mild electrolyte disturbances (4). C‐reactive protein (CRP) concentration was assessed in 6 dogs (25%) and found to be elevated in all 6 (median 38 mg/L; range, 17‐103.2 mg/L).
Twenty‐two dogs (92%) underwent MRI (Supplemental Table 1, Figure 1): 12 of the brain, 8 of the brain and cervical region, 1 of the cervical region and 1 of the cervical to sacral vertebral column. Pachymeningeal contrast enhancement was present in 15 dogs (68%) with additional leptomeningeal enhancement in 3 (14%); this was confined to the brain in 13 dogs, with extension to the cervical meninges in 2. Brain contrast enhancement was diffuse in 5 dogs and focal in 10 (brainstem [8] or cerebellum [2]). The meninges appeared thickened on T2‐weighted images in 12 dogs (55%). Brain parenchymal changes were noted in 4 dogs (20%), characterized as single (2) or multifocal (2) ill‐defined T2W and T2W‐FLAIR hyperintensities without contrast enhancement. Additional periventricular T2W and T2W‐FLAIR hyperintensities likely to represent interstitial edema were noted in 4 dogs (20%) (1 with concurrent multifocal parenchymal changes described above). Changes consistent with otitis media/interna were described in 14 dogs (70%) including T2W and T2W‐FLAIR hyperintensity in the tympanic bulla (14), contrast enhancement of the bulla lining or contents, or a combination of both (12), and adjacent meningeal contrast enhancement (12). Contrast enhancement of para‐aural soft tissues were evident in 6 dogs (30%), with 3 also showing changes consistent with osteomyelitis of the temporomandibular joint, tympanic bulla or petrous temporal bone (heterogenous T2W and T2W‐FLAIR hyperintensity with T1W hypointensity and contrast enhancement), either separately or in combination. Three dogs (14%) had no evidence of central nervous system lesions; MRI was normal in 2, while 1 dog had T2‐weighted hyperintensity with associated contrast enhancement of the epaxial muscles adjacent to the spinous processes of C2‐C7.
FIGURE 1.
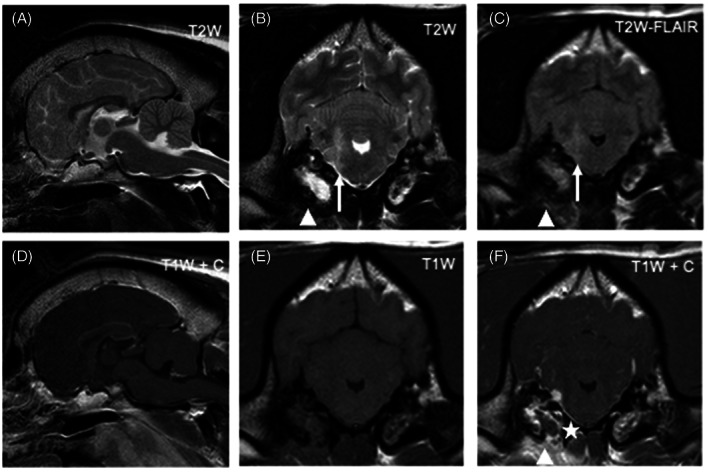
Magnetic resonance images of the head of a dog diagnosed with bacterial meningoencephalitis secondary to otogenic infection (dog 6). From left to right: Top row: sagittal T2W image (A), transverse T2W (B), transverse T2W‐FLAIR (C); Bottom row: sagittal T1W postgadolinium (D), transverse T1W pregadolinium (E), transverse T1W postgadolinium administration (F). Transverse images are at the level of the tympanic bullae and, by convention, the right side of the dog is displayed on the left of the image. There is an ill‐defined T2W and T2W‐FLAIR hyperintensity (compared to normal gray matter) of the right rostral medulla oblongata extending dorsally into the middle cerebellar peduncle (white arrow). The adjacent meninges show contrast enhancement and a focal area of thickening (asterisk). There is bilateral T2W and T2W‐FLAIR hyperintensity of the tympanic bullae contents (more pronounced and homogenous on the right), with bilateral contrast enhancement (again more pronounced on the right). Ventral to the right tympanic bulla there is ill‐defined T2W and T2W‐FLAIR hyperintensity of the soft tissues with marked contrast enhancement (arrowheads)
Computed tomography (without MRI) was performed in 2 dogs (8%). This revealed changes consistent with suspected bilateral chronic otitis externa and media with right bulla osteitis and right para‐aural abscessation in 1 dog, and changes consistent with pyelonephritis with renal infarcts, left hindlimb 4th digit cellulitis and multifocal lymphadenopathy in 1 dog.
Cerebrospinal fluid was collected from the cerebellomedullary cistern in all dogs. A neutrophilic pleocytosis was observed in all dogs with a median total nucleated cell count (TNCC) of 2760 cells/μL (range, 44‐43 200). Total protein concentration was evaluated in 17 dogs (71%) and found to be abnormally high in 16 (94%) (median of 189 mg/dL; range, 13‐725). Intracellular bacteria were identified in 15 (63%) (6 cocci, 4 rods, 3 rods and cocci, 2 undefined) and extracellular bacteria in 1 (4%) (rods and rare cocci). Cerebrospinal fluid bacteriological culture and sensitivity was performed in 21 dogs (88%) (Supplemental Table 2); this yielded bacterial growth in 8 (38%) including Staphylococcus species (5), Streptococcus species (2), and Bordetella bronchiseptica (1).
Further samples for bacteriological culture were obtained in 17 dogs (71%); external ear canal sampling (7, positive culture in 5), total ear canal ablation (TECA) and lateral bulla osteotomy (LBO) (5, positive culture in 4), para‐aural abscess fine‐needle aspiration (1, positive culture), arthrocentesis (1, negative culture), urine (7, all negative cultures), blood (4, all negative cultures) and a swab from a swollen digit (1, positive culture). Isolates from non‐CSF samples included Staphylococcus species (5), Enterococcus (2), Escherichia coli (1), Pseudomonas (1), Pasteurella (1) and Pluralibacter (1). From a total of 18 isolates, 6 were resistant to 2 or more antibiotics (1 isolated from CSF, 1 isolated from CSF and external ear canal swab, 4 from non‐CSF cultures). The suspected source of infection was recorded in 18 (75%) dogs including OMI (15), septic joint (1), digit cellulitis (1), and cervical myositis (1).
3.3. Treatment
Six dogs (25%) had surgery alongside medical treatment and 18 (75%) received solely medical treatment. All 6 surgically treated cases had OMI and underwent a TECA‐LBO. Medical treatment (with or without concurrent surgery) consisted of administration of antibiotics, provision of analgesia and variable administration of glucocorticoids. Eighteen (75%) dogs (13 medically treated and 5 surgically treated) received anti‐inflammatory doses of glucocorticoids at the referral center or in the 3 days preceding referral. The median duration of glucocorticoid therapy was 3 days (range, 2‐7 days).
Four (17%) dogs were euthanized during hospitalization; 2 because of lack of improvement despite medical therapy, and 2 because of severe aspiration pneumonia. Twenty dogs (83%) survived to discharge with a median hospitalization time of 2.5 days (range, 2‐7). Choice of antibiotic varied between surviving dogs and was chosen by the attending clinician pending culture and sensitivity results (Supplemental Table 2); 19 dogs (95%) received antibiotics by IV administration (13 amoxicillin clavulanate, 3 cefuroxime, 3 second‐generation fluoroquinolone [enrofloxacin 2, marbofloxacin 1], some with 1 or 2 additional agents) while hospitalized and were discharged with continued administration of oral formulations. One dog (5%) had antibiotics by oral administration only (cephalexin and metronidazole). A resistant bacterium (Enterococcus spp., resistant to amoxicillin clavulanate) was isolated from 1 dog, but this dog was euthanized before culture results were available. In all other dogs the isolated bacteria were sensitive to the chosen antibiotic. The number of antibiotics used varied between dogs: 1 agent in 8 dogs, 2 in 10 dogs and 3 in 6 dogs. Of the dogs receiving 2 or 3 antibiotics, only 1 had 2 bacterial isolates identified, both sensitive to amoxicillin clavulanate. All antibiotics were continued for the duration of treatment. The median duration of antibiotic therapy was 8 weeks (range, 2‐16). Dogs managed surgically had a median duration of antibiotic therapy of 3 weeks (range, 2‐6), compared to 9 weeks (range, 6‐16) for dogs managed medically.
3.4. Outcome
Median duration of follow up for dogs surviving to hospital discharge was 92 days (range, 10‐2233 days). One dog was reevaluated after 7 days of amoxicillin clavulanate treatment because of lack of improvement, at which time an 8‐week course of enrofloxacin was dispensed (Supplemental Table 2). At the end of this treatment course the dog was neurologically normal but was then lost to follow up. One dog was reevaluated because of recurrence of cervical hyperesthesia 7 days after completing a 6‐week course of cephalexin, metronidazole and clindamycin. Repeat culture was not performed. The original isolate was Streptococcus spp. (group G) sensitive to clindamycin and amoxicillin clavulanate (metronidazole and cephalexin were not tested in the sensitivity panel and the reason for their use is not recorded). A 14‐day course of amoxicillin clavulanate was dispensed to manage the suspected relapse of BMEM. The dog was reevaluated 14 days later at which time resolution of clinical signs was recorded but the dog was then lost to follow up.
One dog was reevaluated 24 weeks after diagnosis of BM for recurrence of cervical hyperesthesia. Investigations were suggestive of steroid‐responsive meningitis arteritis (SRMA) and the dog responded to immunosuppressive glucocorticoid therapy. The initial BM had been treated with amoxicillin clavulanate and clindamycin, without glucocorticoids.
Follow up data at >21 days after diagnosis was available for 19 (95%) dogs surviving to hospital discharge. Residual deficits were reported in 9/19 (47%), including head tilt (6), facial nerve paresis/paralysis (2) and intermittent ataxia (vestibular ataxia [3] and pelvic limb proprioceptive ataxia [1]). Eight dogs with residual deficits had OMI (89%), of which 4 had undergone surgical treatment. At the time of writing 2 dogs had been euthanized; 1 because of development of a nasal mass and the reason for euthanasia was not available for the second dog. One further dog died of unknown causes 319 weeks after initial diagnosis.
4. DISCUSSION
This UK‐based retrospective case series evaluated the clinical presentation, diagnostic findings, treatment, and outcome of dogs diagnosed with BM or BMEM without empyema. Of 24 included dogs, the onset of clinical signs was typically acute, the source of infection was most commonly OMI and the survival to hospital discharge was 83%. Relapses were uncommon and where long‐term neurological deficits were reported, they were typically mild. Bacterial mening(oencephal)itis is a relatively rare condition in dogs; over a 10‐year study period we identified 24 cases from 5 referral hospitals. In comparison, a study of dogs with inflammatory CNS disease presenting to 2 referral hospitals over a similar 10‐year period identified 350 cases of SRMA. 25 The classical presentation of acute BM in people is the triad of pyrexia, neck stiffness, and altered mental state. However, only 21% 19 to 44% 21 of patients present with the complete triad; most commonly older people. 7 In our study, 2 dogs (8%) presented with all 3 clinical signs of the triad, 4 dogs (17%) with 2 clinical signs, 12 dogs (50%) with 1 and 6 dogs (25%) with none. Therefore, BM should not be excluded in the absence of pyrexia, neck pain/stiffness, and altered mental status in dogs. Pyrexia (defined as ≥39.2°C) was detected at the referral hospital in only 3 (13%) dogs, and either not evaluated or not recorded in the majority of the referring veterinary surgeon's clinical records. Many of the dogs had received some form of treatment before referral (glucocorticoids [5] and antibiotics by oral administration [6] or NSAIDs or paracetamol by oral administration [6], or a combination of these) which might have reduced the prevalence of pyrexia on presentation.
Steroid‐responsive meningitis arteritis causes a similar combination of clinical signs including pyrexia, neck pain, and lethargy and hence is an important differential diagnosis for BM. Prompt initiation of glucocorticoid therapy at immunosuppressive doses typically generates a rapid, sustained improvement in dogs with SRMA. Bacterial meningitis, while much less common than SRMA, should be excluded before the initiation of glucocorticoid therapy. However, definitively excluding BM as the underlying cause can be challenging given the low diagnostic yield of CSF culture (38% in our study), and the time associated with obtaining culture and sensitivity results. In a recent study of 350 UK cases of SRMA, the median age was 11 months, sporting and hound breeds were most commonly affected, pyrexia was detected in 83%, the median CSF TNCC was 289 cells/μL, and total protein was 61 mg/dL. 25 In our study of BM/BMEM, dogs were older with a median age of 4.2 years, brachycephalic breeds were overrepresented, pyrexia was detected in only 13%, and CSF TNCC and protein were markedly higher (median 2760 cells/μL, protein 189 mg/dL). Furthermore, neurological deficits are uncommon in SRMA, and usually a consequence of hemorrhage secondary to the arteritis, 26 while neurological deficits were common in our study cohort (75%), particularly vestibular dysfunction (42%). Therefore, signalment, presence of pyrexia, neurological examination findings and results of CSF analysis (TNCC, protein concentration, cytological assessment for presence of bacteria, and bacteriological culture) can facilitate differentiation of SRMA and BM/BMEM. 27 Abnormally high serum and CSF concentrations of IgA can support a diagnosis of SRMA but it is not specific and hence would be expected to be elevated in BM/BMEM, future studies are needed to evaluate this. 28
In 1 dog the BM was suspected to be secondary to a nail bed infection. The dog showed a full recovery following initiation of amoxicillin clavulanate and clindamycin. However, 174 days after diagnosis the dog represented with neck pain and was subsequently diagnosed with SRMA. Treatment with glucocorticoids resulted in full recovery. We cannot definitively exclude the initial presentation being a misdiagnosed SRMA because of the natural waxing‐and‐waning of SRMA. An interesting possibility is that the initial BM acted as an immunogenic trigger for development of a secondary SRMA‐phenotype.
Eleven (46%) dogs presented with lateralized vestibular signs and 15 (63%) were diagnosed with a suspected otogenic cause of the BMEM (OMI), based on MRI and CT findings. Twelve (80%) of the dogs with OMI were brachycephalic breeds. Several factors are thought to predispose brachycephalic dogs to middle ear effusion including a thickened soft palate, reduced bulla volume and overlap between the tympanic bulla and the temporomandibular joint. These factors might compromise function of the auditory tube leading to inadequate draining of the tympanic bulla with subsequent middle ear effusion. 29 Presence of a middle ear effusion might increase susceptibility to secondary infections, 30 similar to “glue ear” in children, occurring as a result of Eustachian tube dysfunction. 31 Whether the brachycephalic conformation promotes intracranial extension of the infection remains to be studied.
Of 24 dogs included in the study, 21 (88%) had CSF bacteriological culture and sensitivity performed yielding bacterial growth in 8 (38%). This was higher than the 13% (1/8) reported in the previous largest retrospective analysis. 9 Interestingly, none of the blood and urine cultures performed yielded a positive culture. This is much lower than reported in a study of dogs with discospondylitis (19/45 positive blood cultures, 6/16 positive urine cultures). 32 Therefore, our study would suggest that urine and blood culture has a low diagnostic yield in dogs with BM/BMEM.
The optimal antibiotic and duration of treatment for BM remain unclear. In people, pending sensitivity results, the use of either penicillins or cephalosporins are common first line agents. 33 A wide variety of drug choice and duration of treatment in our study might reflect varying clinician preference, severity of clinical presentation, hospital protocols, and lack of consensus in the veterinary literature. The antibiotic used was not altered in light of culture and sensitivity results. Some sensitivity panels did not cover the antibiotics chosen (2 dogs) or covered only some of the antibiotics chosen (3 dogs). This might suggest a reliance on empirical choices and a prompt positive clinical response to those empirical choices. It is not possible to discern if a clinical relapse could have been avoided or long‐term outcome could have been improved had the antibiotic choice been modified to ensure sensitivity of the isolated microorganisms.
The median duration of antibiotic therapy was 8 weeks in this study but with a considerable range from 2 to 16 weeks. Regardless of treatment duration, the prognosis was good with 83% of dogs surviving to hospital discharge and 4% demonstrating clinical signs consistent with a relapse.
Median duration of antibiotic therapy was shorter for surgically treated dogs (3 weeks) compared to medically treated (9 weeks). The preference for a shorter course of antibiotic therapy for surgically managed dogs might reflect clinician confidence that a more aggressive treatment approach with the ability to remove necrotic tissues and promote drainage of infectious material facilitates faster resolution of the infection. Given the retrospective nature of this study we were unable to determine if the decision for shorter treatment courses was based on a more rapid clinical improvement, and this would be a worthwhile goal for future prospective studies.
The indications for, optimal dose and duration of glucocorticoid use in BM and BMEM remains controversial in human medicine. 34 A recent systematic review reported that short courses of glucocorticoids at anti‐inflammatory doses are associated with fewer residual neurological deficits without an elevated risk of adverse effects in people with BM/BMEM. 34 In our case series, glucocorticoids were administered to 18 dogs (75%), including 3 of the 4 dogs that were euthanized during hospitalization and the dog that relapsed. Of the 9 dogs with reported residual deficits, 6 received glucocorticoids either before or following referral. Therefore, clinical benefit of glucocorticoid use in dogs with BM/BMEM remains unclear and warrants further investigation.
Data on long‐term outcome in dogs with BM/BMEM limited largely to single case reports, 3 , 4 , 5 , 6 , 27 , 35 while outcome after prompt and aggressive medical or surgical treatment of otogenic intracranial infections is reported to be very good to excellent. 4 , 23 In a study of 10 cats and 2 dogs that survived to hospital discharge, 1 cat was reported to have a mild residual head tilt and facial paresis, while the remaining cases made a fully recovery. 4 In a single case report of ventriculitis associated with otogenic meningoencephalitis in a dog a persistent mild head tilt was documented 3 years after the initial presentation. 35 In our study, 9/19 dogs for which follow up data of more than 21 days was available were reported to have residual deficits. These were typically mild and more likely in dogs with otogenic infection (8/9, 89%).
Limitations of this study include its retrospective nature and multicenter approach, with the associated variability in clinical records, diagnostic investigations, treatment protocols and outcome measurement. Despite including animals over a 10‐year period from 5 referral centers, only 24 dogs met the inclusion criteria and thus our study cohort is small. We included dogs with a degenerate neutrophilic pleocytosis on CSF cytological evaluation that showed clinical improvement after initiation of antibiotic administration; given the lack of definitive identification of bacteria we cannot completely exclude the possibility of a self‐resolving inflammatory etiology. One of the study inclusion criteria was the presence of consistent findings on CSF analysis and hence the study cohort was limited to dogs in which CSF was collected and analyzed. Furthermore, we excluded dogs with empyema (accumulation of purulent material within the cranial cavity and vertebral canal) as these might represent a unique, severe disease subset with mass effect causing progressive and often marked neurological deterioration. Such cases can require rapid surgical decompression and studies exist in the veterinary literature to describe treatment and outcome. 10 , 11 , 36 Thus, in this study we elected to focus on dogs without empyema or intracranial abscess formation, but we recognize that our study cohort might represent an earlier time point in the pathogenesis compared to those dogs with empyema or intracranial abscess formation. Four dogs had brain parenchymal changes on MRI (1 of these dogs and 3 others also had evidence of periventricular edema), which might represent edema or parenchymal inflammation secondary to bacterial invasion. The latter would be consistent with a diagnosis of BMEM; however, the lack of histopathological analysis of these lesions prevents a definitive diagnosis. Another important limitation is the lack of long‐term outcome data for all 24 dogs included in the study, which might have resulted in an underestimation of long‐term neurological sequelae. Furthermore, owner telephone questionnaires were used to identify long‐term neurological deficits; however, this is subject to recall bias, subjectivity, and relies on owner interpretation and reporting.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the Royal Veterinary College Ethical Review Board (URN SR2021‐0002).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1: Summarized clinical data for 24 dogs diagnosed with bacterial meningitis or bacterial meningoencephalitis. Signalment, clinical signs, neuroanatomical localization, CSF analysis, and bacteriological culture where performed. CRP, C‐reactive protein; CSF, cerebrospinal fluid; FE, female entire; FN, female neutered; L, left; ME, male entire; MN, male neutered; MRI, magnetic resonance imaging; OD, oculus dextrus; OS, oculus sinister; PLR, pupillary light reflex; R, right; TNCC, total nucleated cell count
Supplemental Table 2: Clinical diagnosis, treatment, and outcome in 24 dogs with bacterial meningitis or meningoencephalitis. Nidus of infection where identified, bacteriological culture and sensitivity results, treatment before presentation, antimicrobial course and length, additional treatments, duration of hospitalization, and summarized long‐term follow‐up. Multidrug resistant (MDR) defined as resistance to two or more tested antimicrobials.
ACKNOWLEDGMENT
No funding was received for this study. Presented as a research abstract at BSAVA Symposium, 2021.
Rawson F, Foreman M, Mignan T, Galer J, Fraser A, Crawford A. Clinical presentation, treatment, and outcome of 24 dogs with bacterial meningitis or meningoencephalitis without empyema (2010‐2020). J Vet Intern Med. 2023;37(1):223‐229. doi: 10.1111/jvim.16605
REFERENCES
- 1. Swartz MN. Bacterial meningitis: more involved than just the meninges. N Engl J Med. 1984;311:912‐914. [DOI] [PubMed] [Google Scholar]
- 2. Tunkel AR, Scheld WM. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spangler EA, Dewey CW. Meningoencephalitis secondary to bacterial otitis media/interna in a dog. J Am Anim Hosp Assoc. 2000;36:239‐243. [DOI] [PubMed] [Google Scholar]
- 4. Sturges BK, Dickinson PJ, Kortz GD, et al. Clinical signs, magnetic resonance imaging features, and outcome after surgical and medical treatment of otogenic intracranial infection in 11 cats and 4 dogs. J Vet Intern Med. 2006;20:648‐656. [DOI] [PubMed] [Google Scholar]
- 5. Dennis MM, Pearce LK, Norrdin RW, Ehrhart EJ. Bacterial meningoencephalitis and ventriculitis due to migrating plant foreign bodies in three dogs. Vet Pathol. 2005;42:840‐844. [DOI] [PubMed] [Google Scholar]
- 6. Fletcher DJ, Snyder JM, Messinger JS, Chiu AG, Vite CH. Ventricular pneumocephalus and septic meningoencephalitis secondary to dorsal rhinotomy and nasal polypectomy in a dog. J Am Vet Med Assoc. 2006;229:240‐245. [DOI] [PubMed] [Google Scholar]
- 7. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Community‐acquired bacterial meningitis in older people. J Am Geriatr Soc. 2006;54:1500‐1507. [DOI] [PubMed] [Google Scholar]
- 8. Munana KR. Encephalitis and meningitis. Vet Clin North Am Small Anim Pract. 1996;26:857‐874. [PubMed] [Google Scholar]
- 9. Radaelli ST, Platt SR. Bacterial meningoencephalomyelitis in dogs: a retrospective study of 23 cases (1990‐1999). J Vet Intern Med. 2002;16:159‐163. [DOI] [PubMed] [Google Scholar]
- 10. Forward AK, Plessas IN, Guilherme S, de Decker S. Retrospective evaluation of the clinical presentation, magnetic resonance imaging findings, and outcome of dogs diagnosed with intracranial empyema (2008‐2015): 9 cases. J Vet Emerg Crit Care (San Antonio). 2019;29:431‐438. [DOI] [PubMed] [Google Scholar]
- 11. Laws EJ, Sanchez L, Beltran E, et al. Multicenter study of clinical presentation, treatment, and outcome in 41 dogs with spinal epidural empyema. Front Vet Sci. 2022;9:813316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Stefani A, Garosi LS, McConnell FJ, et al. Magnetic resonance imaging features of spinal epidural empyema in five dogs. Vet Radiol Ultrasound. 2008;49:135‐140. [DOI] [PubMed] [Google Scholar]
- 13. Nazifi S, Karimi I, Hosseini Nezhad M, et al. Evaluation of haematological, serum biochemical and cerebrospinal fluid parameters in experimental bacterial meningoencephalitis in the dog. Comp Clin Pathol. 2006;15:44‐48. [Google Scholar]
- 14. Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39:1267‐1284. [DOI] [PubMed] [Google Scholar]
- 15. Proulx N, Frechette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM. 2005;98:291‐298. [DOI] [PubMed] [Google Scholar]
- 16. Koster‐Rasmussen R, Korshin A, Meyer CN. Antibiotic treatment delay and outcome in acute bacterial meningitis. J Infect. 2008;57:449‐454. [DOI] [PubMed] [Google Scholar]
- 17. Bento R, de Brito R, Ribas GC. Surgical management of intracranial complications of otogenic infection. Ear Nose Throat J. 2006;85:36‐39. [PubMed] [Google Scholar]
- 18. Slovik Y, Kraus M, Leiberman A, Kaplan DM. Role of surgery in the management of otogenic meningitis. J Laryngol Otol. 2007;121:897‐901. [DOI] [PubMed] [Google Scholar]
- 19. Pizon AF, Bonner MR, Wang HE, Kaplan RM. Ten years of clinical experience with adult meningitis at an urban academic medical center. J Emerg Med. 2006;30:367‐370. [DOI] [PubMed] [Google Scholar]
- 20. Thigpen MC, Whitney CG, Messonnier NE, et al. Bacterial meningitis in the United States, 1998‐2007. N Engl J Med. 2011;364:2016‐2025. [DOI] [PubMed] [Google Scholar]
- 21. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849‐1859. [DOI] [PubMed] [Google Scholar]
- 22. Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect. 2016;73:18‐27. [DOI] [PubMed] [Google Scholar]
- 23. Moore SA, Bentley RT, Carrera‐Justiz S, Foss KD, da Costa RC, Cook LB. Clinical features and short‐term outcome of presumptive intracranial complications associated with otitis media/interna: a multi‐center retrospective study of 19 cats (2009‐2017). J Feline Med Surg. 2019;21:148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bersan E, Maddox T, Walmsley G, Piviani M, Burrow R. CT‐guided drainage of a brainstem abscess in a cat as an emergency treatment procedure. JFMS Open Rep. 2020;6:2055116919896111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goncalves R, De Decker S, Walmsley G, et al. Inflammatory disease affecting the central nervous system in dogs: a retrospective study in England (2010‐2019). Front Vet Sci. 2021;8:819945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tipold A, Schatzberg SJ. An update on steroid responsive meningitis‐arteritis. J Small Anim Pract. 2010;51:150‐154. [DOI] [PubMed] [Google Scholar]
- 27. Rylander H, Djani DM, Cameron S. Case report: Bordetella bronchiseptica meningoencephalomyelitis in a dog. Front Vet Sci. 2022;9:852982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maiolini A, Carlson R, Schwartz M, Gandini G, Tipold A. Determination of immunoglobulin A concentrations in the serum and cerebrospinal fluid of dogs: an estimation of its diagnostic value in canine steroid‐responsive meningitis‐arteritis. Vet J. 2012;191:219‐224. [DOI] [PubMed] [Google Scholar]
- 29. Salguero R, Herrtage M, Holmes M, et al. Comparison between computed tomographic characteristics of the middle ear in nonbrachycephalic and brachycephalic dogs with obstructive airway syndrome. Vet Radiol Ultrasound. 2016;57:137‐143. [DOI] [PubMed] [Google Scholar]
- 30. Owen MC, Lamb CR, Lu D, Targett MP. Material in the middle ear of dogs having magnetic resonance imaging for investigation of neurologic signs. Vet Radiol Ultrasound. 2004;45:149‐155. [DOI] [PubMed] [Google Scholar]
- 31. Watson P, Voss L, Barber C, Aickin R, Bremner D, Lennon D. The microbiology of chronic otitis media with effusion in a group of Auckland children. N Z Med J. 1996;109:182‐184. [PubMed] [Google Scholar]
- 32. Nye G, Liebel FX, Harcourt‐Brown T. C‐reactive protein in dogs with suspected bacterial diskospondylitis: 16 cases (2010‐2019). Vet Rec Open. 2020;7:e000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffman O, Weber RJ. Pathophysiology and treatment of bacterial meningitis. Ther Adv Neurol Disord. 2009;2:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brouwer MC, McIntyre P, Prasad K, van de Beek D, Cochrane Acute Respiratory Infections Group . Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2015;9:CD004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu CC, Chang YP. Cerebral ventriculitis associated with otogenic meningoencephalitis in a dog. J Am Anim Hosp Assoc. 2015;51:272‐278. [DOI] [PubMed] [Google Scholar]
- 36. Martin S, Drees R, Szladovits B, Beltran E. Comparison of medical and/or surgical management of 23 cats with intracranial empyema or abscessation. J Feline Med Surg. 2019;21:566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Summarized clinical data for 24 dogs diagnosed with bacterial meningitis or bacterial meningoencephalitis. Signalment, clinical signs, neuroanatomical localization, CSF analysis, and bacteriological culture where performed. CRP, C‐reactive protein; CSF, cerebrospinal fluid; FE, female entire; FN, female neutered; L, left; ME, male entire; MN, male neutered; MRI, magnetic resonance imaging; OD, oculus dextrus; OS, oculus sinister; PLR, pupillary light reflex; R, right; TNCC, total nucleated cell count
Supplemental Table 2: Clinical diagnosis, treatment, and outcome in 24 dogs with bacterial meningitis or meningoencephalitis. Nidus of infection where identified, bacteriological culture and sensitivity results, treatment before presentation, antimicrobial course and length, additional treatments, duration of hospitalization, and summarized long‐term follow‐up. Multidrug resistant (MDR) defined as resistance to two or more tested antimicrobials.


