Abstract
Introduction
Obesity affects nearly 1 in 4 European adults increasing their risk for mortality and physical and psychological morbidity. Obesity is a chronic relapsing disease characterized by abnormal or excessive adiposity with risks to health. Medical nutrition therapy based on the latest scientific evidence should be offered to all Europeans living with obesity as part of obesity treatment interventions.
Methods
A systematic review was conducted to identify the latest evidence published in the November 2018–March 2021 period and to synthesize them in the European guidelines for medical nutrition therapy in adult obesity.
Results
Medical nutrition therapy should be administered by trained dietitians as part of a multidisciplinary team and should aim to achieve positive health outcomes, not solely weight changes. A diverse range of nutrition interventions are shown to be effective in the treatment of obesity and its comorbidities, and dietitians should consider all options and deliver personalized interventions. Although caloric restriction-based interventions are effective in promoting weight reduction, long-term adherence to behavioural changes may be better supported via alternative interventions based on eating patterns, food quality, and mindfulness. The Mediterranean diet, vegetarian diets, the Dietary Approaches to Stop Hypertension, portfolio diet, Nordic, and low-carbohydrate diets have all been associated with improvement in metabolic health with or without changes in body weight. In the November 2018–March 2021 period, the latest evidence published focused around intermittent fasting and meal replacements as obesity treatment options. Although the role of meal replacements is further strengthened by the new evidence, for intermittent fasting no evidence of significant advantage over and above continuous energy restriction was found. Pulses, fruit and vegetables, nuts, whole grains, and dairy foods are also important elements in the medical nutrition therapy of adult obesity.
Discussion
Any nutrition intervention should be based on a detailed nutritional assessment including an assessment of personal values, preferences, and social determinants of eating habits. Dietitians are expected to design interventions that are flexible and person centred. Approaches that avoid caloric restriction or detailed eating plans (non-dieting approaches) are also recommended for improvement of quality of life and body image perceptions.
Keywords: Medical nutrition therapy, Obesity, Guidelines, Weight loss
Introduction
The World Health Organization defines obesity as a chronic disease marked by abnormal and/or excessive accumulation of body fat (BF) that presents a risk to health [1]. At the population level, body mass index (BMI) is the most commonly used proxy measure to identify fat mass. A BMI between 25 and 29.9 kg/m2 indicates overweight and a BMI ≥30 kg/m2 is classified as obesity. Since 2021, the European Commission has prioritized obesity prevention and management in the non-communicable disease strategy for the region [2].
In 2021, on average, 23% of adults in the European Union were living with obesity, and combined overweight and obesity affected nearly 60% of all adults in Europe [3, 4]. The past 3 decades have seen a rise in the prevalence of obesity and although rates are decelerating the trends remain positive [5, 6]. By the year 2025, 1 in 4 Europeans could be living with obesity. In some European countries, the prevalence of obesity could be much higher. For example, by 2025 the prevalence of obesity in Ireland could reach 43% [5]. Obesity affects individuals across the lifespan, but by 2054 it is projected adults aged 60–69 years could be the most affected [6]. Globally, 2.4 million deaths and more than 70 million disability-adjusted life years were attributed to higher BMI in 2017 [7]. European economic studies indicate that obesity and its complications were associated with 8% of the annual national healthcare budgets across Europe in 2014 and that number is expected to increase in the years to come [3, 8].
Although BMI is widely used in population and health economic studies, it is not an accurate tool for identifying BF-related complications at the individual level [9]. Obesity is a complex chronic disease caused by an interaction between biological, genetic, psychosocial, behavioural, and environmental factors and contributes to increased morbidity and mortality [3, 10]. Obesity negatively affects both physical and psychological health, with higher risk of developing type 2 diabetes, cardiovascular disease, osteoarthritis, some cancers, dementia, and Alzheimer's disease [11]. Individuals living with obesity also experience weight stigma and weight-based discrimination across their lifespan and settings (e.g., home, school, workplaces, and healthcare), which contributes to a reduced quality of life and increased morbidity and mortality, independently of BMI [12, 13, 14].
Considering the impact of obesity on individuals, families, and societies, a unified framework for obesity prevention and treatment is required. The European guidelines for obesity management in adults present a general framework for healthcare professionals to assess and treat obesity [15]. The European guidelines are often supported by additional guidelines and position statements focusing on specific aspects of obesity prevention and treatment [16, 17].
The scope of this paper is to provide an update on evidence-based options for medical nutrition therapy (MNT) in the treatment of obesity. This document is aimed at healthcare professionals and policy makers in Europe and is focused on obesity in adults only. The conjunction of MNT with bariatric surgery or pharmacotherapy is covered by previously issued guidelines. This paper will focus on MNT options that can be integrated with obesity treatments. A review of obesity treatments is covered by previously issued guidelines [15].
Guideline Committee and Rationale for the European Guidelines
In 2004, the EU Platform for Action on Diet, Physical Activity and Health was created as a forum for stakeholders to agree on concrete actions that could help halt the obesity epidemic, to provide tools, and to address the current burden of the disease. Under the Platform, the European Association for the Study of Obesity (EASO) and the European Federation of the Associations of Dietitians (EFAD) made a joint commitment to map and address the needs of healthcare professionals and policy makers in the area of dietetic management of obesity.
A standing committee was created from members of the Nutrition Working Group (NWG) of EASO and the European Specialist Dietetic Network (ESDN) Obesity committee of EFAD, which include dietitians, physicians, and other health professionals. This committee collects data on available guidelines for dietetic management of obesity in Europe, conducts surveys, commissions literature reviews, and issues guidelines. In previous years, the committee has commissioned a series of reviews and surveys and has collaborated with similar organizations abroad for knowledge exchange and promotion of international collaboration.
Prior to the creation of the European guidelines, the committee conducted and published a survey of the available guidelines for dietetic treatment of obesity across Europe. Although several guidelines were identified, the majority of European countries did not have guidelines based on the latest scientific evidence and lacked regular updates. In a follow-up survey, it was highlighted that dietitians often consult guidelines from other countries or regions when designing obesity treatment plans. Based on these survey results, the committee identified the following priorities in the guideline creation process. The guidelines should
a. be based on latest available evidence
b. aim to expand on the existing state-of-the-art knowledge
c. act as a platform for collaboration across countries and regions
d. be transversal and applicable in all European countries
e. allow for local adaptation based on the needs and priorities of each country/region
In the lifetime of the project, the Canadian Adult Obesity Clinical Practice Guidelines (CPGs) were issued by Obesity Canada and the Canadian Association of Bariatric Surgeons and Physicians, which included an extensive review of literature from January 2006 to June 2018 [18]. The latest and most extensive literature review on the topic of MNT for the management of obesity was carried out as part of the creation of the Canadian CPGs [19]. The MNT chapter of the Canadian guidelines presents a point-by-point discussion on the evidence available for a long list of nutrition interventions commonly considered as part of an obesity management programme and aims to act as a platform of collaboration among healthcare professionals.
In the spirit of creating universal guidelines and building upon previous initiatives, the committee decided that the literature review that informed the Canadian CPGs would not be repeated but instead it would be expanded and updated with the latest evidence. A close collaboration with Canadian guideline committee was set in place to ensure alignment.
The current paper aims to undertake an update of the systematic review of nutrition interventions for the treatment of obesity in adults, expanding on the results of the Canadian CPG MNT chapter and using this chapter's PI/PECOT (Population, Intervention or Exposure, Comparison, Outcome, Time) questions and search strategy. The purpose of this paper is to adapt the MNT recommendations from the Canadian Adult Obesity Clinical Practice Guidelines to the European context and to update the evidence base with new studies published between 2018 and 2021.
Methods
Literature Review and Data Synthesis
A literature review for new evidence published in the period November 2018–March 2021 was conducted by the McMaster Evidence Review and Synthesis Team (MERST) in the MEDLINE and Embase databases via OVID, which aimed to identify the latest evidence published since the Canadian CPGs following the same search strategy [20]. Only systematic reviews and meta-analyses, individual randomized controlled trials (RCTs) with duration ≥3 months, and prospective cohort studies were included. Evidence from cross-sectional and/or retrospective studies, case series, and case studies was excluded. All human studies were included with the exception of acute and/or single-meal studies.
The overarching aim for the literature review was to map current dietary considerations that need to be addressed when designing an obesity management intervention. The overall question for the MNT chapter was “what are the dietary considerations for management of obesity in adults?”. This was further divided in subcategories as follows. Firstly, the review focused on elements of coordination/organization of care and whether there is an effect of MNT on obesity-related outcomes. Then a series of research questions were posed around seven themes: (1) caloric restriction, (2) macronutrient quantities, (3) macronutrient quality, (4) micronutrient supplementation, (5) low-calorie sweeteners, (6) meal replacements and liquid formula diets, and (7) eating approaches, dietary patterns, and food-based approaches. A detailed list of all research questions is presented in Table 1.
Table 1.
Detailed presentation of the research questions per theme of dietary considerations that should be taken into account when designing a MNT protocol for the management of obesity in adults
| Theme 1: caloric restriction Are there specific caloric recommendations? Are there negative consequences/harm for specific caloric restrictions or specific populations (e.g., people living with obesity, those that have lost weight, prevention of weight regain, minimizing weight gain)? |
| Theme 2: macronutrient quantity Are there specific macronutrient recommendations? Are there negative consequences/harm for specific macronutrient-based approaches or specific populations (e.g., people living with obesity, those that have lost weight, prevention of weight regain, minimizing weight gain)? |
| Theme 3: macronutrient quality What type of macronutrient is best and do they differ for specific populations (e.g., people living with obesity, those that have lost weight, prevention of weight regain, minimizing weight gain)? |
| Theme 4: micronutrient supplements Are there specific supplements needed for people living with obesity? Do they differ post-bariatric surgery? |
| Theme 5: LCS Should people living with obesity use/avoid LCS? |
| Theme 6: meal replacements/liquid formula diets Are meal replacements effective? Are they harmful? Who should use them? |
| Theme 7: eating approaches, dietary patterns, and food-based approaches What are the most effective eating approaches? Are there specific dietary patterns or diets that are better/worse? |
A PI/PECOT (Population, Intervention or Exposure, Comparison, Outcome, Time) process was followed to systematically address these research questions. The literature search focused on adults (≥18 years old) living with overweight or obesity using BMI categories (≥25 kg/m2 or ≥30 kg/m2, respectively) with or without comorbidities (P). Data on children and adolescents (<18 years) were excluded. The following interventions (I) were considered:
Counselling or “MNT” or “nutrition care process” provided by a dietitian/nutritionist
Counselling or “MNT” or “nutrition care process” provided by an interdisciplinary/multidisciplinary team or programme
Calorie restriction through low- or very low-calorie diets (all potential protocols included)
Macronutrient substitutions (all potential protocols of high-carbohydrate, low-carbohydrate, very low-carbohydrate, high-protein, “ketogenic” diets included)
Studies on carbohydrate quality. Carbohydrate quality was defined as content of dietary fibre (insoluble, soluble, viscous, psyllium, beta-glucan, glucomannan, konjac mannan), resistant starch, refined starches, total sugars, added/free sugars, and sugar-sweetened beverages. Glycaemic index was also used as a measure of carbohydrate quality
Studies on fat quality. Fat quality was defined as content of saturated fats, trans fats, monounsaturated fats, polyunsaturated fats (PUFAs), long chain polyunsaturated fatty acids, conjugated linoleic acid, n-3 PUFAs, n-6 PUFAs, and fish oil
Studies on protein quality, defined as plant- or animal-derived protein, the use of amino acid, or protein supplements
Dietary patterns, with or without calorie restriction, such as low-glycaemic index/low-glycaemic load diet, Mediterranean diet (MED), vegetarian/vegan diet, DASH diet, portfolio diet, Nordic diet, “Pure Prairie Eating Plan,” Canadian “traditional indigenous” diets, USDA (healthy eating index/alternative healthy eating index)
Diets emphasizing specific foods, with or without calorie restriction (e.g., whole grains, pulses, nuts and seeds, fruit and vegetables, dairy)
Use of micronutrient supplements, with or without calorie restriction, and especially vitamin D, calcium, B-complex, B-12, multivitamin, iron
Use of low-calorie sweeteners or non-nutritive sweeteners
Use of meal replacements or liquid formula diets (all potential protocols included)
Approaches to eating (“mindfulness” eating, “intuitive eating,” “portion control,” “volumetrics,” “intermittent fasting (IF),” meal frequency/timing/balance, “whole foods,” “clean eating,” “organics,” “eating out” approaches), with or without calorie restriction
Intensive lifestyle interventions, combination of low-risk lifestyle behaviours (achieving and maintaining a healthy body weight (BW), healthy diet, regular physical activity, smoking cessation, moderate alcohol consumption, and moderate sleep duration)
All studies performed comparisons between one nutrition intervention against another dietary or eating pattern (C). Studies without a control group or with a control group receiving no intervention were excluded. An intervention was defined as the provision of any counselling or dietary advice provided during the period of the study. Hence, free-living individuals that do not receive any counselling were not considered a suitable control group. Individual RCTs were only included if their duration was ≥3 months.
The outcomes of interest (O) were weight change/maintenance, waist circumference (WC) change, adiposity change, “hedonic hunger,” cravings, quality of life, mental health, nutritional deficiencies, malnutrition, cardiometabolic control (glycaemic control defined as changes in glycated haemoglobin A1c and fasting blood glucose, blood pressure [BP], and blood lipids), medication changes, and incidence of prediabetes, diabetes, and cardiovascular disease (hypertension, myocardial infarction, coronary heart disease, stroke, and other major adverse cardiovascular events). The detailed literature search terms and methodology can be made accessible upon request.
Evidence Grading
Updated literature found between November 2018 and March 2021 was reviewed and graded by authors (CC and CJS) from the MNT chapter of the Canadian guidelines. All evidence was graded for quality using the Shekelle tool [21]. Each individual paper was also appraised using the AGREE II methodology [22] by MERST. Evidence was then judged based on their quality and type of study and graded following international standards from level 1a to 4, as shown in Table 2.
Table 2.
Evidence classification and recommendation strength schemes based on the Obesity Canada methodology [18, 21]
| Evidence classification scheme Level 1a: evidence from meta-analysis of RCTs Level 1b: evidence from at least 1 RCT Level 2a: evidence from at least 1 controlled study without randomization Level 3: evidence from non-experimental descriptive studies, such as comparative studies, correlation studies, and case-control studies Level 4: evidence from expert committee reports or opinions or clinical experience of respected authorities, or both |
| Recommendation strength Grade A: directly based on level 1 evidence Grade B: directly based on level 2 evidence or extrapolated recommendation from category 1 evidence Grade C: directly based on level 3 evidence or extrapolated recommendation from level 1 or 2 evidence Grade D: directly based on level 4 evidence or extrapolated recommendation from level 1, 2, or 3 evidence |
As it was not possible to obtain access to the raw data included in the Canadian CPGs, the new studies identified were not co-synthesized with the previous evidence included in the Canadian CPGs. As a result, the current analysis cannot re-evaluate the grade of the previous guidelines and propose changes. In this aspect, the current analysis was aimed at grading the quality of new evidence available and identifying areas where there is room to strengthen the existing guidelines.
Results
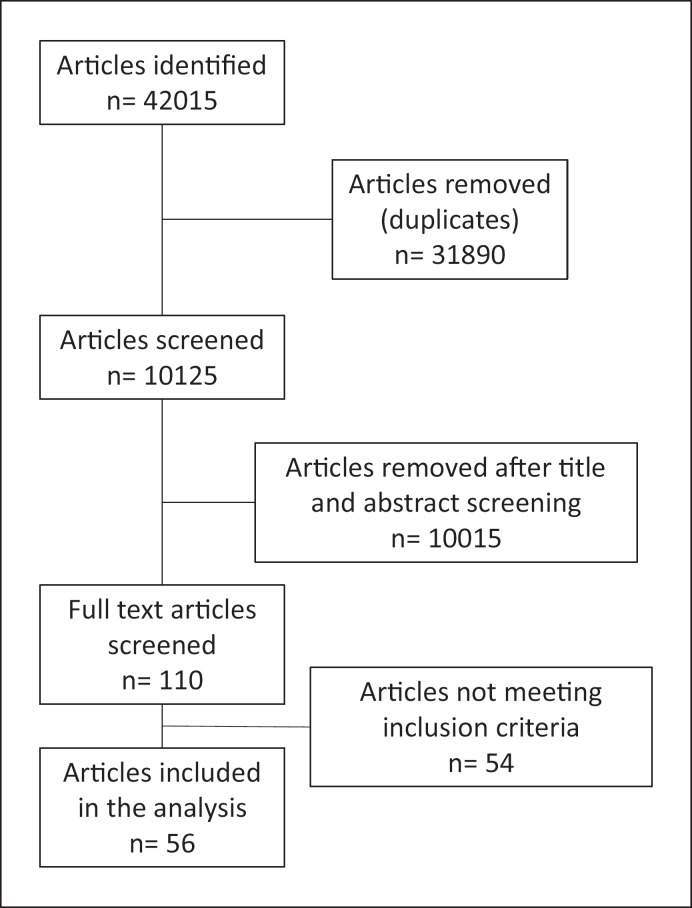
A detailed flow diagram of the article selection and screening process is presented in Figure 1. In total, n = 56 new studies were identified and analysed. The evidence available was mainly from RCTs (n = 33), followed by meta-analysis or systematic reviews of high-quality RCTs (n = 21). Only two studies reported data from non-randomized trials or cohorts and were graded as level 3. The RCTs covered the following intervention areas: meal replacement (n = 6), high protein dietary patterns/meals (n = 5) time-restricted eating/IF (n = 3), Mediterranean dietary pattern (n = 3), community or group intervention (n = 3), and then topics like vitamin C supplementation, calcium supplementation, flavonoid supplementation, extra virgin olive oil, flaxseed supplementation, wheat bran supplementation, vegan, paleolithic, flexible, central European dietary patterns, exercise without nutrition interventions, Web-based interventions, and replacement of low-calorie sweetened beverages with water were all populated by one study in each topic (n = 1 in each).
Fig. 1.
Flow diagram of article selection.
The main topics identified in meta-analyses and systematic reviews were time restricted/fasting interventions (n = 5), low-carbohydrate dietary patterns (n = 4), pre-/probiotic supplementation (n = 2), and meal replacements (n = 2). The remaining studies focused on exercise interventions, soy products, viscous fibre, primary care interventions, wholegrain products, educational weight loss programmes, and overall diet-induced weight loss with a special focus on psychological stress. A detailed presentation of the study designs, populations, and key findings is shown in Table 3.
Table 3.
Latest evidence in MNT for adult obesity from RCTs and systematic reviews/meta-analyses
| First author | Year | Population | Intervention | Duration | Main outcome | Evidence level | Conclusion |
|---|---|---|---|---|---|---|---|
|
RCTs Ghanwat and Sontakke [23] |
2019 | Adults with WC ≥80/90 cm | Vitamin C 500 mg 3/d | 3 months | FBG, insulin, HOMA-IR, and QUICKI | Level 2 | Improvement in FBG, insulin, HOMA-IR, and QUICKI |
|
| |||||||
| Romain et al. [24] | 2021 | Adults with BMI 25–40 kg/m2 | Flavonoid and vitamin B3-rich extract versus placebo | 16 weeks | BW, WC, BF, health-related QoL | Level 2 | Improvements in BW, WC, and BF, physical health QoL |
|
| |||||||
| Rohling et al. [41] | 2021 | Adults with BMI 27–35 kg/m2 and w/> 1 component of metabolic syndrome | Meal replacement by formula diet versus lifestyle | 52 weeks | BW | Level 2 | 12 weeks: meal replacement linked to larger BW (-2.39 kg) 52 weeks: no difference |
|
| |||||||
| Halle [42] | 2020 | Adults with BMI 27–35 kg/m2 and w/> 1 component of metabolic syndrome | Meal replacement by formula diet versus lifestyle | 52 weeks | FM, FFM, WC, FBG, SBP, DBP, TC, HDL-c, LDL-c, TG | Level 2 | Improvements in all parameters |
|
| |||||||
| Lowe et al. [53] | 2020 | Adults with BMI 27–43 kg/m2 | Time-restricted eating (16:8 h) | 12 weeks | BW, FM, LM, insulin, FBG, HbA1c | Level 2 | No evidence of difference |
|
| |||||||
| Hassapidou et al. [31] | 2020 | Adults with BMI ≥25 kg/m2 | MED versus usual care | 6 months | BW, WC, HC, % BF | Level 2 | Weight loss only in the intervention group |
|
| |||||||
| Headland et al. [51] | 2019 | Adults with BMI ≥25 kg/m2 | IF versus CER | 12 months | BW, body composition, TC, HDL-c, LDL-c, TG, FBG | Level 2 | No difference in any parameter |
|
| |||||||
| Brown et al. [45] | 2020 | Adults with T2DM (insulin) and obesity | Low-energy total meal replacement | 12 months | BW, HbA1c, FBG, C-peptide, serum lipids, BP, body composition, and QoL | Level 2 | Improved BW, HbA1c, insulin dosage, QoL |
|
| |||||||
| Gajewska [81] | 2019 | Adults with BMI ≥25 kg/m2 and hypertension | Nutrition education individual versus group | 6 months | BW, WC, BP, FBG, OGTT, HOMA-IR, lipids | Level 2 | Better BW, WC, BP, OGTT, HOMA-IR with individual education |
|
| |||||||
| Sundfør et al. [52] | 2019 | Adults with BMI 30–45 kg/m2 and w/> 1 component of metabolic syndrome | IER versus CER | 3 months | BW, energy intake | Level 2 | No differences in BW Better dietary habits with CER |
|
| |||||||
| Cai et al. [32] | 2019 | Adults >60 y with BMI ≥28 kg/m2 | Community intervention versus usual care | 24 months | BW, WC, BP, FBG, TC, HDL-c, LDL-c, TG | Level 2 | Community intervention linked to improved BW, WC, SBP, TG, HDL-c |
|
| |||||||
| Gepner et al. [35] | 2019 | Adults with WC ≥88/102 cm or TG≥150 mg/dL and HDL-c <40 mg/dL/<50 mg/dL | MED versus low-fat diet | 18 months | BW, WC, FBG, insulin, TC, LDL-c, HDL-c, TG, HOMA-IR, hepatic fat content | Level 2 | MED reduced hepatic fat content, TG, HDL-c, DBP further than low-fat diet |
|
| |||||||
| Mitra [82] | 2019 | Adults with BMI ≥23 kg/m2 | High-protein, vit E, and fibre, energy-restricted diet versus usual care | 6 months | BW, WC, HC, FM, FFM, TC, TG, HDL-c, FBG, insulin, CRP, HOMA-IR | Level 2 | The intervention led to significant improvements in BW, WC, FM, insulin, HOMA-IR, CRP |
|
| |||||||
| Otten [83] | 2019 | Postmenopausal women with BMI 27–41 kg/m2 | Paleolithic dietary pattern versus Nordic diet | 2 years | BW, FM, OGTT, Glucagon, GLP-1, GIP | 1A large | Paleolithic diet superior to Nordic in BW, FM, and GLP-1 |
|
| |||||||
| Salas-Salvado et al. [36] | 2019 | Adults with BMI 27–40 kg/m2 and metabolic syndrome | MED and physical activity weight loss intervention versus MED alone | 12 months | BW, WC, FM, FFM, FBG, HOMA-IR, DBP, SBP, HbA1c, TG, TC, HDL-c, CRP, IL, TNF-α | 1A large | Intensive MED intervention led to further improvements in BW, WC, insulin, FBG, HOMA-IR, blood lipids |
|
| |||||||
| Ilich et al. [25] | 2019 | Postmenopausal women with 26–45 kg/m2 | Calcium (1.5 g/d) and vitamin D (600 IU/d) via foods or supplements | 6 months | BW, WC, HC, FM, LM, BMD | Level 2 | Calcium and vitamin D supplementation through food associated with better BW and FM |
|
| |||||||
| Weaver [84] | 2019 | Adults >65 y with BMI ≥30 kg/m2 | Hypocaloric diet with 1 g/kg BW protein versus normocaloric 0.8 g/kg BW protein | 6 months | BW, BMD | 1A large | BW changes only in intervention without differences in bone density |
|
| |||||||
| Higgins [85] | 2019 | Adults with BMI ≥25 kg/m2 | Sucrose, aspartame, saccharin, sucralose, rebaudioside A | 12 weeks | BW, FM, FFM, OGTT | Level 2 | Sucrose and saccharin led to increased BW Sucralose associated with BW reduction No impact on OGTT |
|
| |||||||
| Phelan et al. [44] | 2018 | Women at 9–16 weeks gestation with pregestation BMI ≥25 kg/m2 | Partial meal replacement versus usual care | 26 weeks | Gestational weight gain | Level 2 | Partial meal replacement linked with better adherence to gestational weight gain guidelines |
|
| |||||||
| Guo et al. [43] | 2018 | Adults with BMI >24 kg/m2 | Meal replacement versus routine diet | 12 weeks | BW, WC, HC, FFM, FM, SBP, DBP, TC, TG, HDL-c, FBG | Level 2 | Meal replacements were associated with greater improvements in BW, WC, FFM, FM, and FBG |
|
| |||||||
| Watson [86] | 2018 | Adults with BMI ≥25 kg/m2 and T2DM | High-protein versus high-carbohydrate dietary patterns | 24 weeks | BW, WC Psychological wellbeing, QoL, sleep | Level 2 | High-protein diets expand the positive impact of weight loss on general health and vitality |
|
| |||||||
| Madjd [87] | 2018 | Adult women with overweight or obesity | Replacing low-calorie sweetened beverages with water | Weight loss, BMI, HOMA-IR, 2-h postprandial glucose | Level 2 | Replacement of low-calorie sweetened beverages with water after the main meal in women who were regular users of low-calorie sweetened beverages may cause further weight reduction during a 12-month weight maintenance programme. It may also offer benefits in carbohydrate metabolism including improvement of insulin resistance over the long-term weight maintenance period | |
|
| |||||||
| Gorostegi-Anduaga [88] | 2018 | Adults with overweight and obesity with primary hypertension and are sedentary | Aerobic exercise and nutritional intervention | Cardiovascular risk scores and vascular age | Level 2 | The improvement in CVR factors after 16-week lifestyle changes reduced the risk of suffering a cardiovascular event in adults with overweight/obesity with HTN through the FRS estimation tool but not with the ASCVD score. The risk score algorithms could underestimate CVR in women. In contrast, VA could be a useful and easier tool in the management of individuals with CVRfactors | |
|
| |||||||
| Kahleova [89] | 2018 | Adults with overweight or obesity | Plant-based high-carbohydrate, low-fat (vegan) dietary pattern or to maintain their current dietary pattern | A linear regression model was used to test the relationship between carbohydrate intake and body composition and insulin resistance | 1A large | Results suggest encouraging a flexible approach to eating behaviour and discouraging rigid adherence to a dietary pattern may lead to better intentional weight loss for older women with overweight and obesity | |
|
| |||||||
| Berg [90] | 2018 | Older women with overweight or obesity | Flexible eating behaviour following a diet and exercise intervention | Weight loss | Level 2 | ||
|
| |||||||
| Dus-Zuchowska et al. [37] | 2018 | Postmenopausal women with obesity and a high risk of metabolic syndrome | CED could be an alternative to the MED | CRP and asymmetrical dimethylarginine | Level 2 | In the central European postmenopausal women with obesity population, a well-designed, energy-restricted diet with the use of food items traditional for the region (CED) could be a good alternative to MED in terms of AT prevention | |
|
| |||||||
| Kikuchi [91] | 2018 | Japanese adults with visceral fat obesity | Refined wheat bread versus whole grain wheat bread | BW, BMI, WC, VFA, lipids, BP, diabetes measures | Level 2 | The WW group showed decrease (–4 cm2) in VFA (p < 0.05), whereas the RW group showed no significant changes. These time-dependent changes were significantly different between the groups. WW diet led to significant and safe reductions in VFA in subjects with BMI ≥23 kg/m2. WW diet may contribute to preventing visceral fat obesity | |
|
| |||||||
| Höchsmann et al. [33] | 2021 | Adults with obesity | Primary care: ILI | FBG and lipids, SBP, DBP, metabolic syndrome severity | Level 2 | A pragmatic ILI consistent with national guidelines and delivered by trained health coaches in primary care produced clinically relevant improvements in cardiometabolic health in an underserved population over 24 months | |
|
| |||||||
| Miller et al. [29] | 2021 | Adults 50–75 years old BMI ≥25 kg/m2 with T2DM | Whey protein (40 g/d) + vit D3 (2000 IU/d) combined with exercise versus exercise alone (n = 198) | 24 weeks | HbA1c, HOMA2-IR, FBG Body composition, BP, lipid profile, inflammatory markers | Level 2 | No effect on body composition, HbA1c, HOMA2-IR Improved FBG |
|
| |||||||
| Haghighat [92] | 2020 | Women with BMI between 18.5 and 24.9 kg/m2 and an excess in BF% >33.3% | Euengetic high protein versus standard protein | Appetite and body composition | 1A large | ||
|
| |||||||
| Aparecida Silveira [93] | 2020 | Adults with sarcopenia and severe obesity | EVOO and/or the traditional Brazilian diet | Level 2 | DieTBra contributes to improvements in handgrip strength, walking speed, and total BF in adults with obesity (BMI >40 kg/m2) | ||
|
| |||||||
| Lison [94] | 2020 | Patients with obesity and hypertension | Web-based exercise and nutritional education | BMI (primary outcome), FM, SBP, DBP, FBG, insulin, physical activity levels, and functional capacity for aerobic exercise | Level 2 | ||
|
| |||||||
| Rakvaag et al. [30] | 2019 | Adults with abdominal obesity (n = 65) | Whey protein (60 g/d) combined with wheat bran (30 g/d) | 12 weeks | Fasting and postprandial lipid profiles | 1A large | Whey protein increased fasting TC with or without wheat bran Whey protein improved TG only without wheat bran |
|
| |||||||
| Yari [95] | 2020 | Adults with overweight or obesity | Flaxseed | Anthropometric measurements, lipid profile, HOMA-IR, and inflammatory biomarkers | Level 2 | ||
|
| |||||||
| Meta-analysis or systematic review of high-quality RCT | |||||||
| Qu et al. [28] | 2021 | Adults BMI ≥25 kg/m2 |
Prebiotics supplements versus Placebo | BMI, BW, WC CRP, TN-a, IL-1β, LPS | Level 1a (meta-analysis) | No difference in adiposity parameters Improvements in inflammatory markets | |
|
| |||||||
| Enriquez Guerrero [96] | 2021 | Adult population (18–70 years old) with BMI ≥25 kg/ m2 | IF versus CER | BMI, BW, WC, FM TC, HDL-c, LDL-c, TG | Level 1a (meta-analysis) | IF showed higher weight loss and higher FM reduction but similar WC reduction IF improved lipid profile, but evidence is less conclusive | |
|
| |||||||
| Willems [97] | 2021 | Adults with obesity | Low-carbohydrate and low-fat dietary patterns | Markers of the metabolic syndrome | Level 2 | Higher fat and protein intakes associated with improved markers of MetS | |
|
| |||||||
| Park et al. [46] | 2020 | Adults BMI ≥25 kg/m2 |
ADF | BMI, BW, WC, FM, and LM TC, TG, HDL-c, LDL-c, FBG, insulin, HOMA-IR, CRP, SBP, and DBP | Level 1a | ADF effectively lowers BMI, BW, FM, and TC in adults with BMI ≥25–29.9 kg/m2 in interventions <6 months compared to control ADF lowers WC in adults with BMI ≥30 kg/m2, ≥40 years old | |
|
| |||||||
| Yan et al. [47] | 2020 | Adults with overweight or obesity | Fasting | BW, BMI, FFM, FM, WC, TC, LDL-c, HDL-c, TG, SBP, DBP, FBG, insulin | Level 1a | Our meta-analysis found that fasting was associated with a significant effect on the regulation of anthropometric (BW, BMI, FEM, FM, and WC) and metabolic parameters (LDL-C, TG, SBP, and DBP) in people with overweight or obesity | |
|
| |||||||
| Maula et al. [39] | 2020 | Adults with overweight or obesity and type 2 diabetes | Educational weight loss interventions | BW and BMI change | Level 1a | Low-calorie, low-carbohydrate meal replacements or dietary patterns combined with education appear the most promising interventions to achieve the largest weight and BMI reductions in people with type 2 diabetes | |
|
| |||||||
| Wang [98] | 2020 | Adults with overweight or obesity | Whole grain foods | Cardiovascular risk outcomes | Level 1a | This study suggests that whole grain food consumption can slightly reduce BW and CRP in people with overweight/obesity | |
|
| |||||||
| Mu [99] | 2019 | Asian women (non-menopausal) with overweight or obesity | Soy products | BW, BMI, % BF, FM, WC | Level 1a | This meta-analysis showed that soy products have weight loss effects, mainly due to soy protein, isoflavone, and soy fibre | |
|
| |||||||
| Suzumura et al. [27] | 2019 | Adults with overweight or obesity | Oral supplementation with probiotics or symbiotics | BW, BMI, WC | Level 1a | oral supplementation with probiotics or symbiotics has a small effect on WC reduction but no effect on BW or BMI Low to moderate quality of evidence | |
|
| |||||||
| Roman et al. [50] | 2019 | Adults with overweight or obesity | Intermittent versus continuous fasting | BW, BF, LM, WC, HC, and energy expenditure (kJ/day) | Level 1a | This systematic review in individuals with overweight and obesity showed that regular IF decreased LM compared to continuous fasting. There were no differences in effects for either intermittent versus continuous interventions across all other outcomes | |
|
| |||||||
| Bassatne et al. [26] | 2019 | Adults with obesity | Vitamin D supplementation | BMD, extra-skeletal parameters | Level 2 | No clear evidence for a beneficial effect of vitamin D supplementation on cardiometabolic parameters in adults living with obesity | |
|
| |||||||
| Gjuladin-Hellon [100] | 2019 | Adults BMI ≥25 kg/m2 | Very low- and low-carbohydrate dietary patterns versus low-fat dietary patterns | TC, TG, HDL-c, LDL-c | Level 1a (meta-analysis) | Carbohydrate restriction is superior to fat restriction in improving lipid profile | |
|
| |||||||
| Astbury et al. [40] | 2019 | Adults with overweight or obesity | Meal replacements | Weight loss | Level 1a | Programmes incorporating meal replacements led to greater weight loss at 1 year than comparator weight loss programmes and should be considered as a valid option for management of overweight and obesity in community and healthcare settings | |
|
| |||||||
| Booth [101] | 2018 | Adults with overweight or obesity | Diet-induced weight loss | Psychological stress | Level 1a | ||
|
| |||||||
| Castellana [102] | 2020 | Adults with overweight or obesity | VLCKD | BW, BMI, WC, body composition, BP, HbA1c, lipids, and markers of liver and kidney function | Level 1a | VLCKD proved to be a reliable option to achieve a significant weight loss in patients with overweight and obesity. Results were early obtained during the ketogenic phase and were stable over a follow-up of up to two years. In addition, VLCKD was associated with significant improvements in comorbidities, including hypertension, dyslipidaemia, T2DM, and NAFLD. However, an increase in serum sodium was found. VLCKD should thus be regarded as an effective intervention to be proposed to properly selected patients, as a part of a multicomponent strategy, and under strict medical supervision | |
|
| |||||||
| Noronha et al. [38] | 2019 | Adults with overweight or obesity and T2D | Liquid meal replacements | Cardiometabolic risk factors | Level 1a | Liquid meal replacements in weight loss diets lead to modest reductions in BW, BMI, and SBP and reductions of marginal clinical significance in BF, WC, HbA1c, fasting glucose, fasting insulin, and DBP | |
|
| |||||||
| Cheng [103] | 2018 | Postmenopausal women with obesity | Nutrition and exercise interventions | BW, BMI, WC, FM, LM | Level 1a (meta-analysis) | Diet and exercise combined results in greater weight loss, FM, and LM loss Diet alone leads to greater weight loss than exercise alone | |
|
| |||||||
| Jovanovski [104] | 2021 | Adults BMI ≥25 kg/m2 | Viscous fibre supplementation in calorie-restricted dietary pattern | BW, BMI, WC, BF | Level 1a (meta-analysis) | Moderate evidence for reduced BW, BMI, and BF Low evidence for reduced WC |
|
|
| |||||||
| Vitale and Kim [49] | 2020 | Adults with obesity and T2DM | IF | BW and glycaemic control | Level 1a (systematic review) | Insignificant differences between IF and CER on glycated haemoglobin A1c and body composition | |
|
| |||||||
| Canuto et al. [34] | 2021 | Adults with overweight and obesity | Primary care interventions | Weight reduction | Level 3 (meta-analysis) | No impact of diet alone or combined approaches on weight loss Similar findings for individual versus group sessions | |
|
| |||||||
| Churuangsuk [105] | 2018 | Adults with overweight or obesity | Low-carb dietary patterns | Weight loss | Level 3 (review of reviews) | Little or no difference in weight loss with low-carb dietary patterns versus control | |
TD2M, type 2 diabetes mellitus; BMI, body mass index; WC, waist circumference; HC, hip circumference; BF, body fat; BW, body weight; FM, fat mass; LM, lean mass; FFM, fat free mass; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; CRP, C-reactive protein; TN-a, tumour necrosis factor alpha; IL-1β, interleukin 1β; LPS, lipopolysaccharide; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment for insulin resistance; HbA1c, glycated haemoglobin; OGTT, oral glucose tolerance test; BMD, bone mineral density; ILI, intensive lifestyle intervention; EVOO, extra virgin olive oil; IER, intermittent energy restriction; CED, Central European diet; VFA, visceral fat area. ADF, alternate day fasting; VLCKD, very low-calorie ketogenic diet.
Three randomized trials were identified using vitamin supplements to reduce body adiposity and/or its metabolic comorbidities [23, 24]. The first study compared the effect of 3-month vitamin C supplementation on glucose homeostasis in adults with and without elevated WC showing improvements in both groups [23]. The second showed significant improvements in BW, WC, and % BF after a 16-week intervention with a commercial supplement rich in flavonoids and B vitamins for adults with a BMI ≥25 kg/m2 [24]. A study on calcium and vitamin D supplementation in postmenopausal women did not show any impact on weight loss or body composition; however, receiving the same calcium and vitamin D dose from dairy products was associated with greater weight loss and beneficial changes to body composition [25]. Reviews and meta-analyses on supplements showed no evidence of vitamin D supplementation on cardiometabolic parameters in adults with a BMI ≥30 kg/m2 [26], while probiotic, prebiotic, and symbiotic supplementation showed no effect on BMI and BF [27, 28]. However, limited low to moderate quality evidence was found for pro- and symbiotic supplementation on WC [27]. Similarly, prebiotics had a positive impact on inflammatory markers in adults with BMI ≥25 kg/m2 [28].
Whey protein supplementation at a dose of 40 g/d combined with vitamin D3 as part of physical activity intervention did not result in BW, body composition, or blood lipid changes compared to exercise alone in older adults after 24 weeks [29]. By contrast, a 12-week supplementation of 60 g/d whey protein in adults with abdominal circumference ≥80 cm (women) or ≥94 cm (men) and age ≥40 years was associated with better triglyceride profiles [30].
Evidence on community interventions supports the notion that the inclusion of a registered dietitian in community settings increases the likelihood of achieving positive changes in adiposity and metabolic factors compared to information provision by leaflets [31, 32, 33]. In these settings, the mode of delivery (individual or group session) did not appear to alter their effectiveness [34].
An 18-month long intervention with a low-carbohydrate Mediterranean dietary pattern in adults with increased WC and/or dyslipidaemia showed greater reduction in hepatic fat content compared to a low-fat diet, which mediated improvements in cardiometabolic risk factors [35]. Evidence from the PREDIMED-Plus trial indicated that intensive lifestyle intervention combined with physical activity and delivered by a dietitian leads to significant improvements in adiposity and cardiometabolic markers in individuals with increased WC up to 12 months follow-up compared to general advice on the MED [36]. In a 16-week intervention, MED and Central European diet gave similar results in improving inflammation and other blood markers of atherosclerosis risk in women living with obesity [37].
Systematic reviews and meta-analyses on partial meal replacements indicated modest reductions in BW and altered body composition, as well as improvements in markers of glycaemic control in individuals living with obesity and T2DM compared to traditional weight loss diets or educational programmes [38, 39]. These effects become larger in favour of partial meal replacement when weight status at 1 year postintervention is assessed in populations with and without T2DM [40]. More recent evidence from RCTs like the ACOORH trial further supports the use of partial meal replacements for improved weight loss and body composition changes compared to traditional lifestyle interventions [41, 42]. Evidence from smaller trials was also published showing a beneficial effect of single meal replacement products on adiposity and metabolic parameters compared to the habitual dietary pattern [43] but also a potential benefit from meal replacements in the management of gestational weight gain for women entering pregnancy with elevated BMI [44]. Only one study focused on total diet replacement in individuals living with obesity and receiving insulin therapy for T2DM, which showed greater weight change and even T2DM remission following total meal replacement compared to standard dietetic practice [45].
Fasting approaches were an important proportion of the new evidence identified. Meta-analyses of trials on adults with BMI >27 kg/m2 support the idea that fasting approaches either intermittent or alternate day fasting are associated with greater weight changes, greater changes in adiposity, and potentially larger benefits in lipid profiles, although the evidence for the latter is less conclusive [46, 47, 48]. However, when focusing on individuals living with obesity and T2DM the effect of IF is less clear [49]. Specific fasting techniques may be linked with larger lean mass loss, but evidence is currently inconclusive [50]. Three RCTs were published in the period researched and not all supported IF or time-restricted eating over continuous energy restriction (CER) for weight loss and improved body composition in adults with BMI >27 kg/m2 with or without comorbidities [51, 52, 53].
Discussion
Evidence Incorporation into MNT for Adult Obesity
Given the small amount of new evidence published in the period 2018–2021, the majority of the MNT recommendations in the Canadian Adult Obesity Clinical Practice Guidelines, published in 2020 [18], remain valid. A detailed presentation of guideline recommendations that remain unaffected by the latest evidence is given in Table 4.
Table 4.
MNT guidelines for adult obesity that remain unaffected by the latest evidence (adapted from Obesity Canada [19])
| Evidence level | MNT recommendation |
|---|---|
| Level 1a | Adults living with obesity should receive individualized MNT provided by a registered dietitian (when available) to improve weight outcomes (BW, BMI), WC, glycaemic control, established blood lipid targets, including LDL-c, TGs, and BP (grade A) |
| Adults living with obesity and impaired glucose tolerance (prediabetes) should consider intensive behavioural interventions that target 5–7% weight loss to improve glycaemic control, BP, blood lipids, reduce incidence of type 2 diabetes, microvascular complications, and cardiovascular and all-cause mortality (grade B) |
|
| Adults living with obesity and type 2 diabetes should consider intensive behavioural interventions that target 7–15% weight loss to increase the remission of type 2 diabetes, reduce the incidence of nephropathy, obstructive sleep apnoea, and depression (grade A) |
|
| Portfolio dietary pattern to improve established blood lipid targets, including LDL-c, apo B, and non-HDL-c (grade B) |
|
| DASH dietary pattern to reduce BW and WC (grade B) | |
|
| |
| Level 2 | Calorie-restricted dietary patterns emphasizing variable macronutrient distribution ranges (lower, moderate, or higher carbohydrate with variable proportions of protein and fat) to achieve similar BW reduction over 6–12 months (grade B) |
| Mediterranean dietary pattern to improve glycaemic control, HDL-cholesterol, and TGs, reduce cardiovascular events, reduce risk of type 2 diabetes, and increase reversion of metabolic syndrome with little effect on BW and WC (grade C) |
|
| Vegetarian dietary pattern to improve glycaemic control, established blood lipid targets, including LDL-c, and reduce BW (grade B) |
|
| Portfolio dietary pattern to improve established CRP, BP, and estimated 10-year coronary heart disease risk (grade B) Pulses (i.e., beans, peas, chickpeas, lentils) to improve BW, improve glycaemic control, established lipid targets, including LDL-c, systolic BP (grades B-C) |
|
| Vegetables and fruit to improve diastolic BP, glycaemic control (grade B) |
|
| Nuts to improve glycaemic control (grade B) |
|
| Whole grains (especially from oats and barley) to improve established lipid targets, including total cholesterol and LDL-c (grade B) |
|
| Low-GI dietary pattern to reduce BW, glycaemic control, established blood lipid targets, including LDL-c, and BP (grade B) |
|
| DASH dietary pattern to improve BP, established lipid targets, including LDL-c, CRP, glycaemic control (grade B) |
|
| Nordic dietary pattern to reduce BW and BW regain, improve BP and established blood lipid targets, including LDL-c, apo B, non-HDL-c (grade B) | |
|
| |
| Level 3 | Dairy foods to reduce BW, WC, BF and increase LM in calorie-restricted diets but not in unrestricted diets and reduce the risk of type 2 diabetes and cardiovascular disease (grade C) |
| Nuts to improve established lipid targets, including LDL-C, and reduce the risk of cardiovascular disease (grade C) |
|
| Vegetables and fruit to reduce the risk of type 2 diabetes and cardiovascular mortality (grade C) |
|
| Pulses (i.e., beans, peas, chickpeas, lentils) to reduce the risk of coronary heart disease (grade C) |
|
| Non-dieting approaches can improve quality of life, psychological outcomes (general well-being, body image perceptions), cardiovascular outcomes, BW, physical activity, cognitive restraint, and eating behaviours (grade C) | |
|
| |
| Level 4 | Nutrition recommendations for adults of all body sizes should be personalized to meet individual values, preferences, and treatment goals to support a dietary approach that is safe, effective, nutritionally adequate, culturally acceptable, and affordable for long-term adherence (grade D) |
DASH, Dietary Approaches to Stop Hypertension; Low-GI, low glycaemic index; CRP, C-reactive protein; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; HDL-c, high-density lipoprotein cholesterol; LM, lean mass.
The newest evidence for partial meal replacements further supports their role in MNT as elements with a positive impact on measures of BW, WC, and BP (Table 5). On the other hand, for IF the evidence highlights that although helpful, it does not provide significant advantages compared to CER. In more detail, the previous guidelines stated that “partial meal replacements (replacing one to two meals/day as part of a calorie-restricted intervention) could be used to reduce BW, WC, BP and improve glycaemic control,” but evidence was graded as level 1a, grade B. The addition of new evidence from literature reviews and meta-analyses indicates that meal replacements could be linked with modest to significant higher weight loss even at 1 year [38, 40], may indicate a greater role of meal replacements in adult obesity.
Table 5.
Potential updates in the MNT for adult obesity guidelines based on the latest evidence
| Current guideline | Impact of new evidence |
|---|---|
| Partial meal replacements (replacing one to two meals/day as part of a calorie-restricted intervention) to reduce BW, WC, BP and improve glycaemic control Level 1a, grade B |
New evidence in support of the guideline in both Level 2 (n = 3) and level 1 (n = 2) Evidence level to remain unchanged Strength of recommendation could be upgraded to grade A |
|
| |
| Intermittent or continuous calorie restriction achieved similar short-term BW reduction Level 2a, grade B |
New evidence includes both level 2 (n = 2) and level 1 (n = 5) New level 1 evidence indicates potential superiority of fasting approaches on weight outcomes and lipid profiles, but it is currently inconclusive No evidence to support a superiority in glucose metabolism Closer follow-up of the literature may warrant recommendation update in the near future |
Although the current recommendation is “intermittent or continuous calorie restriction achieved similar short-term BW reduction,” the latest evidence remains inconclusive to whether IF has additional benefits to CER. Data from meta-analyses available indicate that fasting approaches may have some benefit over CER for weight loss and body composition parameters but not for all patients. On the other hand, the latest RCTs show either no benefit of IF or even a disadvantage compared to CER. Overall, it seems that although the data from older trials are in support of IF, new trials are still challenging that notion. Hence, evidence in support of IF is considered to be inconclusive and an update of the existing recommendation deemed immature. Research on fasting would benefit greatly from better procedure standardization as there are currently multiple fasting protocols which makes their pooled analysis difficult and comparisons across various studies challenging.
Considerations for the European Region
Nutritional Assessment and Anthropometry
As mentioned in the European and the Canadian Adult Obesity Clinical Practice Guidelines [15, 18], screening for obesity is key in order to identify those at risk and proceed to a detailed nutrition diagnosis [54]. Although BMI is a commonly used index to screen for obesity, it is merely a measure of size, not health, and instead requires a combination of anthropometrics (BMI and WC) and a comprehensive medical assessment, including social, medical, functional, and mental health to diagnose obesity [15, 54]. This is particularly important for individuals with a lower BMI and/or older individuals [55, 56, 57]. Particularly in older individuals, the possibility of sarcopenic obesity should be considered and a combined WC and BMI measurement has been shown to better detect an increased risk of sarcopenic obesity [58]. According to the latest survey among European dietitians, 40% do not include WC as part of their clinical assessment and 60% opt for body composition assessment using bioelectric impedance analysis [59]. Body composition assessment is not essential for the management of obesity in routine clinical practice [15] and hence we do not recommend using it as a substitute for WC. By contrast, health professionals are strongly encouraged to train in measuring WC and to consider it an indispensable part of routine clinical practice [60]. At this point, we may add that according to the Canadian clinical practice guidelines, assessment of people living with obesity measuring WC in individuals with BMI 25–35 kg/m2 was graded as a level 2b, grade B recommendation [18]. The combination of BMI and WC is crucial as not all individuals with larger bodies have obesity; similarly, obesity may be found in individuals with smaller bodies.
Further to anthropometry, dietitians are strongly encouraged to utilize multiple tools to perform a thorough nutritional assessment which will include macro- and micronutrient intake (when needed) as well as the assessment of intake of specific food groups. As mentioned above, multiple dietary patterns should be considered as part of MNT. Dietitians are encouraged to assess whether a patient's current dietary habits are closer to any given dietary pattern. This consideration is especially relevant for Europe as both the Nordic and the Mediterranean dietary pattern have been identified as suitable options for the treatment of obesity and its comorbidities [18]. Future evidence might emerge for other traditional eating patterns in Europe. Adherence to these traditional dietary patterns shows a clear geographic distribution in Europe [61, 62, 63] and they should be considered first as they are likely to resonate with cultural values and traditions of patients from specific regions. However, it should not be assumed that all individuals in a given region would favour a traditional nutrition intervention. The inclusion of Food Frequency Questionnaires (FFQs) and adherence scores should be considered as part of the nutritional assessment to allow dietitians to investigate which nutrition interventions might be most feasible for their patients based on their current eating habits [64, 65, 66, 67, 68]. A final consideration often ignored in the context of obesity management is the assessment of water intake and hydration, especially for older individuals and those living in assisted care environments [69, 70]. A secondary analysis of the PREDIMED-Plus trial highlighted a link between baseline water intake in people >55 years of age and changes in BW and WC at 2-year follow-up [71], indicating that dietitians may need to consider further focus on promoting healthy hydration habits [72]. Any nutrition intervention should be patient centric and a detailed nutritional assessment should be conducted to allow the individual to better understand their current eating habits and decide with the dietitian the optimal intervention plan that is likely to provide long-term adherence. The dietitian needs the skills to include evidence-based nutrition interventions with the personal eating preferences, values, and social abilities of the individual.
Considerations for Training and Education
The current guidelines are an important step toward designing an inclusive flexible framework for the treatment of obesity in adults. Dietitians are invited to rethink their current practice and invest heavily in designing patient-centred nutrition interventions. This requires dietitians to feel comfortable with performing nutritional assessment using a variety of tools. Currently, more than 35 dietary assessment questionnaires are available in the literature to be used in clinical settings [73] and dietitians will require training and practice to choose the most suitable tool for their patients. Familiarity is not only an issue for nutritional assessment tools but for the nutrition interventions themselves. According to the current guidelines, obesity specialist dietitians are required to be familiar with more than 10 different nutrition interventions, all with their own intricacies and scientific rationale. A study among UK dietitians highlighted that dietitians' beliefs and barriers in delivering a specific nutrition intervention significantly impacted the degree to which this intervention was employed in clinical practice [74]. Historically, dietitians do not always report feeling sufficiently trained to treat obesity [75]. However, the latest data from Europe indicate that dietitians follow guidelines closely [59]. Based on the current guidelines, there is a significant risk that dietitians will invest heavily on the utilization of the nutrition treatments they feel comfortable with, and other treatment options may be underutilized, irrespectively of their effectiveness. As part of guideline implementation, audits and research should be carried out to understand the training needs of dietitians and dietetic students in delivering all the different nutrition interventions proposed and training should be organized at a local and regional level.
With respect to training and education, it is important that the European guidelines for MNT in adult obesity are incorporated in national guidance. As explained previously, national nutrition guidelines for the treatment of obesity vary widely among EU countries both in the context and the speed of update to include the latest evidence [59]. Dedicated training activities should be planned as part of a comprehensive guideline dissemination and implementation plan.
Finally, the Canadian CPGs refer to the importance of non-dieting, behavioural interventions in the treatment of obesity [19]. Although the evidence is still not strong for these interventions, it is evident that dietitians and healthcare professionals are likely to be expected to deliver such interventions. Both in clinical practice and research, this widening scope of nutrition interventions is met with a challenge for dietitians to engage in new training [76, 77]. In the USA, application of non-dieting approaches from dietitians was linked with greater experience in obesity management, working in the private sector, and having completed training in obesity management [78]. No similar data exist in Europe, but it is important to consider that even academic institutions should start incorporating modules that would equip dietitians with the skills to deliver novel nutrition interventions.
Considerations for Research
Apart from the need for closer audit and research in terms of the readiness of European dietitians to effectively implement the current guidelines, as discussed above, key research priorities have emerged as part of the current review, especially in the areas of meal replacements, fasting, and non-dieting approaches. The current and previous reviews of the literature however highlight that there is very limited evidence for mental health outcomes such as depression and quality of life. Another important topic for future research is the investigation of potential harms related to nutrition interventions in adult obesity treatment, including a deeper understanding of the conditions in which potential harms may be present. Research on mental health and general well-being should focus on identifying nutrition interventions in obesity management that might be linked with an increased risk for participant harm.
In the future, guidelines should consider incorporating methodologies that balance the relative weight reduction or health gain of an intervention with its potential risk of harm. This would be especially helpful in deciding the role of nutritional and obesity interventions that have marginal health benefits and allow for better personalization of treatments in clinical practice.
Finally, a recurrent topic of research in the area of obesity is the need for more studies targeted specifically to weight loss maintenance. Since obesity is classified as a chronic relapsing disease, treatments must be lifelong and healthcare professionals need guidance on how to support patients across different lifecycles of their disease. There is very little evidence available to guide healthcare professionals in the management of weight regain which impacts health outcomes, long-term adherence to obesity treatments, or in general studying the lifelong journey of obesity care.
Role of Obesity Stigma in the Treatment of Adult Obesity
Although the current review was focused on the latest evidence for the MNT in adult obesity, it is of extreme importance to discuss the role of weight bias in the treatment of adult obesity. Weight bias refers to negative attitudes and beliefs about weight and about people with higher weights or individuals living with obesity [79]. Weight bias has been measured among healthcare professionals such as physicians, nurses, and dietitians and can impact the delivery and quality of healthcare. A 2015 systematic review found that dietitians have significant weight-biased beliefs, including beliefs that weight is a controllable issue and that people with obesity are responsible for their obesity and related comorbidities [80]. Many healthcare professionals attribute excess weight to lack of physical activity and unhealthy eating and believe that people living with larger bodies simply lack willpower to manage their weight. The Canadian guidelines are considered pioneers in the field of obesity care as for the first time these guidelines included evidence-based and patient-centred recommendations for reducing weight bias in healthcare settings [79]. These recommendations are as follows [18]:
Healthcare providers should assess their own attitudes and beliefs regarding obesity and consider how they may influence care delivery (level 1a, grade A)
Healthcare providers may recognize that internalized weight bias in people living with obesity can affect behavioural and health outcomes (level 2a, grade B)
Healthcare providers should avoid using judgemental words (level 1a, grade A), images (level 2b, grade B), and practices (level 2a, grade B) when working with patients living with obesity
To address weight bias in obesity management, the Canadian guidelines also emphasized the need to refocus obesity interventions on improving health and wellbeing, rather than only focusing on weight loss [19]. A key message in the MNT chapter of the Canadian guidelines is that “healthy eating is important for all [individuals], regardless of body size, weight, or health status” (page 1 [19]).
Weight bias and sensitivity training should be considered locally and regionally to increase awareness and to help healthcare providers to assess and address any conscious or unconscious weight bias. Assessment of such attitudes and beliefs should be a priority for all healthcare providers and it should be discussed under the general umbrella of stigma and creating an inclusive environment.
In Europe, as in many other countries and regions, obesity presents a clear social gradient toward the less privileged in our societies [4]. In this context, it is also important to consider that individuals undergoing obesity management are likely to be affected by intersecting stigmas (e.g., poverty, racism, gender). This social gradient is also more pronounced in specific areas of Europe compared to other. Unfortunately, it is the areas with the higher obesity-related inequalities that also see higher inequalities in access in healthcare in general, lower education levels, and higher prevalence of social discrimination. It is also important for healthcare providers to deliver appropriate obesity care using evidence-based approaches that are based on patients' values and preferences. Any activity promoting guidelines for the treatment or prevention of obesity should always be put into the context of reducing obesity stigma and should take the opportunity to educate healthcare professionals and the public on the impact of weight bias and stigma on the health and social outcomes for individuals and populations.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The current activity received no funding.
Author Contributions
Tamara Brown and Jennifer Brown: data synthesis; Maria Hassapidou, Antonis Vlassopoulos, and Marianna Kalliostra: writing − first draft; Elisabeth Govers, Hilda Mary Mulrooney, Louissa Jane Ells, Ximena Ramos Salas, Giovanna Muscogiuri, Teodora Handjieva-Darlenska, Luca Busetto, Volkan Demirhan Yumuk, Dror Dicker, Jason C.G. Halford, Euan Woodward, and Pauline Douglas: writing − reviewing; and all authors have read and approved the final version of the manuscript.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the MERST (McMaster Evidence Review and Synthesis Team), Carol Clarke, and Carlene Johnson Stoklossa for their invaluable support in the collection of data.
Funding Statement
The current activity received no funding.
References
- 1.Obesity [cited 2022 Feb 16]. Available from: https://www.who.int/westernpacific/health-topics/obesity.
- 2.Burki T. European Commission classifies obesity as a chronic disease. Lancet Diabetes Endocrinol. 2021;9((7)):418. doi: 10.1016/S2213-8587(21)00145-5. [DOI] [PubMed] [Google Scholar]
- 3.OECD . The heavy burden of obesity: the economics of prevention. Paris: Organisation for Economic Co-operation and Development; 2019. [cited 2022 Feb 17]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/the-heavy-burden-of-obesity_67450d67-en. [Google Scholar]
- 4.World Health Organization. Regional Office for Europe . Regional Office for Europe. World Health Organization; 2022. WHO European regional obesity report 2022; p. p. 206. [Internet] [cited 2022 Jun 8]. Available from: https://apps.who.int/iris/handle/10665/353747. [Google Scholar]
- 5.Pineda E, Sanchez-Romero LM, Brown M, Jaccard A, Jewell J, Galea G, et al. Forecasting future trends in obesity across Europe: the value of improving surveillance. Obes Facts. 2018;11((5)):360–371. doi: 10.1159/000492115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen F, Bardoutsos A, Vidra N. Obesity prevalence in the long-term future in 18 European countries and in the USA. Obes Facts. 2020;13((5)):514–527. doi: 10.1159/000511023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. Plos Med. 2020;17((7)):e1003198. doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt AFJ. Health costs in the European Union: how much is related to EDCS? Brussels: Health and Environment Alliance; 2014. [cited 2022 Jun 8]. Available from: https://www.env-health.org/IMG/pdf/18062014_final_health_costs_in_the_european_union_how_much_is_realted_to_edcs.pdf. [Google Scholar]
- 9.Garvey WT, Mechanick JI. Proposal for a scientifically correct and medically actionable disease classification system (ICD) for obesity. Obesity (Silver Spring) 2020;28((3)):484–492. doi: 10.1002/oby.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . WHO European Ministerial Conference on Counteracting Obesity. 2006. [cited 2022 Feb 17]. Available from: https://www.euro.who.int/__data/assets/pdf_file/0009/87462/E89567.pdf. [Google Scholar]
- 11.Kinlen D, Cody D, O'Shea D. Complications of obesity. QJM: An Int J Med. 2018;111((7)):437–443. doi: 10.1093/qjmed/hcx152. [DOI] [PubMed] [Google Scholar]
- 12.Sutin AR, Stephan Y, Terracciano A. Weight discrimination and risk of mortality. Psychol Sci. 2015;26((11)):1803–1811. doi: 10.1177/0956797615601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsenkova VK, Carr D, Schoeller DA, Ryff CD. Perceived weight discrimination amplifies the link between central adiposity and nondiabetic glycemic control (HbA1c) Ann Behav Med. 2011;41((2)):243–251. doi: 10.1007/s12160-010-9238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadiveloo M, Mattei J. Perceived weight discrimination and 10-year risk of allostatic load among US adults. Ann Behav Med. 2017;51((1)):94–104. doi: 10.1007/s12160-016-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8((6)):402–424. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muscogiuri G, El Ghoch M, Colao A, Hassapidou M, Yumuk V, Busetto L, et al. European guidelines for obesity management in adults with a very low-calorie ketogenic diet: a systematic review and meta-analysis. Obes Facts. 2021;14((2)):222–245. doi: 10.1159/000515381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toplak H, Woodward E, Yumuk V, Oppert JM, Halford JCG, Frühbeck G. 2014 EASO position statement on the use of anti-obesity drugs. Obes Facts. 2014;8((3)):166–174. doi: 10.1159/000430801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192((31)):E875–91. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown J, Clarke C, Stoklossa CJ, Sievenpiper J. Medical nutrition therapy in obesity management. 2020;Vol. 25 [Google Scholar]
- 20.Obesity in adults: a clinical practice guideline | CMAJ [cited 2022 Feb 17]. Available from: https://www.cmaj.ca/content/192/31/E875.
- 21.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 1999;170((6)):348–351. [PMC free article] [PubMed] [Google Scholar]
- 22.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Agree II: advancing guideline development, reporting and evaluation in health care. Can Med Assoc J. 2010;182((18)):E839–842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghanwat GH, Sontakke AV. Effect of vitamin C supplementation on insulin resistance, β-cell function and insulin sensitivity in obese and non-obese individuals. Ind Jour Publ Health Rese Develop. 2019;10:183. [Google Scholar]
- 24.Romain C, Chung LH, Marín-Cascales E, Rubio-Arias JA, Gaillet S, Laurent C, et al. Sixteen weeks of supplementation with a nutritional quantity of a diversity of polyphenols from foodstuff extracts improves the health-related quality of life of overweight and obese volunteers: a randomized, double-blind, parallel clinical trial. Nutrients. 2021;13((2)):492. doi: 10.3390/nu13020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilich JZ, Kelly OJ, Liu PY, Shin H, Kim Y, Chi Y, et al. Role of calcium and low-fat dairy foods in weight-loss outcomes revisited: results from the randomized trial of effects on Bone and body composition in overweight/obese postmenopausal women. Nutrients. 2019;11((5)):E1157. doi: 10.3390/nu11051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassatne A, Chakhtoura M, Saad R, Fuleihan GEH. Vitamin D supplementation in obesity and during weight loss: a review of randomized controlled trials. Metabolism. 2019;92:193–205. doi: 10.1016/j.metabol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Suzumura EA, Bersch-Ferreira ÂC, Torreglosa CR, da Silva JT, Coqueiro AY, Kuntz MGF, et al. Effects of oral supplementation with probiotics or synbiotics in overweight and obese adults: a systematic review and meta-analyses of randomized trials. Nutr Rev. 2019;77((6)):430–450. doi: 10.1093/nutrit/nuz001. [DOI] [PubMed] [Google Scholar]
- 28.Qu H, Song L, Zhang Y, Gao ZY, Shi DZ. The effect of prebiotic products on decreasing adiposity parameters in overweight and obese individuals: a systematic review and meta- analysis. Curr Med Chem. 2020;28((2)):419–431. doi: 10.2174/0929867327666191230110128. [DOI] [PubMed] [Google Scholar]
- 29.Miller EG, Nowson CA, Dunstan DW, Kerr DA, Menzies D, Daly RM. Effects of whey protein plus vitamin D supplementation combined with progressive resistance training on glycaemic control, body composition, muscle function and cardiometabolic risk factors in middle-aged and older overweight/obese adults with type 2 diabetes: a 24-week randomized controlled trial. Diabetes Obes Metab. 2021;23((4)):938–949. doi: 10.1111/dom.14299. [DOI] [PubMed] [Google Scholar]
- 30.Rakvaag E, Fuglsang-Nielsen R, Bach Knudsen KE, Landberg R, Johannesson Hjelholt A, Søndergaard E, et al. Whey protein combined with low dietary fiber improves lipid profile in subjects with abdominal obesity: a randomized, controlled trial. Nutrients. 2019;11((9)):E2091. doi: 10.3390/nu11092091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassapidou M, Tziomalos K, Lazaridou S, Pagkalos I, Papadimitriou K, Kokkinopoulou A, et al. The nutrition health alliance (NutriHeAl) study: a randomized, controlled, nutritional intervention based on mediterranean diet in Greek municipalities. J Am Coll Nutr. 2020;39((4)):338–344. doi: 10.1080/07315724.2019.1660928. [DOI] [PubMed] [Google Scholar]
- 32.Cai R, Chao J, Li D, Zhang M, Kong L, Wang Y. Effect of community-based lifestyle interventions on weight loss and cardiometabolic risk factors in obese elderly in China: a randomized controlled trial. Exp Gerontol. 2019;128:110749. doi: 10.1016/j.exger.2019.110749. [DOI] [PubMed] [Google Scholar]
- 33.Höchsmann C, Dorling JL, Martin CK, Newton RL, Apolzan JW, Myers CA, et al. Effects of a 2-year primary care lifestyle intervention on cardiometabolic risk factors. Circulation. 2021;143((12)):1202–1214. doi: 10.1161/CIRCULATIONAHA.120.051328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canuto R, Garcez A, de Souza RV, Kac G, Olinto MTA. Nutritional intervention strategies for the management of overweight and obesity in primary health care: a systematic review with meta-analysis. Obes Rev. 2021;22((3)):e13143. doi: 10.1111/obr.13143. [DOI] [PubMed] [Google Scholar]
- 35.Gepner Y, Shelef I, Komy O, Cohen N, Schwarzfuchs D, Bril N, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71((2)):379–388. doi: 10.1016/j.jhep.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, Basora J, Fitó M, Corella D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Dia Care. 2018:dc180836. doi: 10.2337/dc18-0836. [DOI] [PubMed] [Google Scholar]
- 37.Duś-Żuchowska M, Department of Pediatric Gastroenterology and Metabolic Diseases Poznań University of Medical Sciences Poland. Bajerska J, Krzyżanowska P, Chmurzyńska A, Miśkiewicz-Chotnicka A. The Central European diet as an alternative to the Mediterranean diet in atherosclerosis prevention in postmenopausal obese women with a high risk of metabolic syndrome - a randomized nutrition-al trial. Acta Sci Pol Technol Aliment. 2018;17((4)):399–407. doi: 10.17306/J.AFS.0593. [DOI] [PubMed] [Google Scholar]
- 38.Noronha JC, Nishi SK, Braunstein CR, Khan TA, Blanco Mejia S, Kendall CWC, et al. The effect of liquid meal replacements on cardiometabolic risk factors in overweight/obese individuals with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2019;42((5)):767–776. doi: 10.2337/dc18-2270. [DOI] [PubMed] [Google Scholar]
- 39.Maula A, Kai J, Woolley AK, Weng S, Dhalwani N, Griffiths FE, et al. Educational weight loss interventions in obese and overweight adults with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabet Med. 2020;37((4)):623–635. doi: 10.1111/dme.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Astbury NM, Piernas C, Hartmann-Boyce J, Lapworth S, Aveyard P, Jebb SA. A systematic review and meta-analysis of the effectiveness of meal replacements for weight loss. Obes Rev. 2019;20((4)):569–587. doi: 10.1111/obr.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Röhling M, Stensitzky A, Oliveira CLP, Beck A, Braumann KM, Halle M, et al. Effects of a protein-rich, low-glycaemic meal replacement on changes in dietary intake and body weight following a weight-management intervention-the ACOORH trial. Nutrients. 2021;13((2)):376. doi: 10.3390/nu13020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meal replacement by formula diet reduces weight more than a lifestyle intervention alone in patients with overweight or obesity and accompanied cardiovascular risk factors: the ACOORH trial. Eur J Clin Nutr. doi: 10.1038/s41430-020-00783-4. [cited 2022 Feb 24]. Available from: https://www.nature.com/articles/s41430-020-00783-4. [DOI] [PubMed] [Google Scholar]
- 43.Guo X, Xu Y, He H, Cai H, Zhang J, Li Y, et al. Effects of a meal replacement on body composition and metabolic parameters among subjects with overweight or obesity. J Obes. 2018;2018:1–10. doi: 10.1155/2018/2837367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phelan S, Wing RR, Brannen A, McHugh A, Hagobian TA, Schaffner A, et al. Randomized controlled clinical trial of behavioral lifestyle intervention with partial meal replacement to reduce excessive gestational weight gain. The Am J Clin Nutr. 2018;107((2)):183–194. doi: 10.1093/ajcn/nqx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown A, Dornhorst A, McGowan B, Omar O, Leeds AR, Taheri S, et al. Low-energy total diet replacement intervention in patients with type 2 diabetes mellitus and obesity treated with insulin: a randomized trial. BMJ Open Diab Res Care. 2020;8((1)):e001012. doi: 10.1136/bmjdrc-2019-001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Seo YG, Paek YJ, Song HJ, Park KH, Noh HM. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. 2020;111:154336. doi: 10.1016/j.metabol.2020.154336. [DOI] [PubMed] [Google Scholar]
- 47.Yan S, Wang C, Zhao H, Pan Y, Wang H, Guo Y, et al. Effects of fasting intervention regulating anthropometric and metabolic parameters in subjects with overweight or obesity: a systematic review and meta-analysis. Food Funct. 2020;11((5)):3781–3799. doi: 10.1039/d0fo00287a. [DOI] [PubMed] [Google Scholar]
- 48.Effectiveness of an intermittent fasting diet versus continuous energy restriction on anthropometric measurements, body composition and lipid profile in overweight and obese adults: a meta-analysis | European Journal of Clinical Nutrition. [cited 2022 Feb 23]. Available from: https://www.nature.com/articles/s41430-020-00821-1. [DOI] [PubMed] [Google Scholar]
- 49.Vitale R, Kim Y. The effects of intermittent fasting on glycemic control and body composition in adults with obesity and type 2 diabetes: a systematic review. Metab Syndr Relat Disord. 2020;18((10)):450–461. doi: 10.1089/met.2020.0048. [DOI] [PubMed] [Google Scholar]
- 50.Roman YM, Dominguez MC, Easow TM, Pasupuleti V, White CM, Hernandez AV. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: a systematic review and meta-analysis of randomized controlled trials. Int J Obes. 2019 Oct;43((10)):2017–2027. doi: 10.1038/s41366-018-0204-0. [DOI] [PubMed] [Google Scholar]
- 51.Headland ML, Clifton PM, Keogh JB. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int J Obes. 2019;43((10)):2028–2036. doi: 10.1038/s41366-018-0247-2. [DOI] [PubMed] [Google Scholar]
- 52.Sundfør TM, Tonstad S, Svendsen M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur J Clin Nutr. 2019;73((7)):1006–1014. doi: 10.1038/s41430-018-0370-0. [DOI] [PubMed] [Google Scholar]
- 53.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180((11)):1491–1499. doi: 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rueda-Clausen CF, Poddar M, Lear A, Poirier P, Sharma AM. Assessment of People Living with Obesity. p. 17.
- 55.Vlassopoulos A, Combet E, Lean MEJ. Changing distributions of body size and adiposity with age. Int J Obes. 2014;38((6)):857–864. doi: 10.1038/ijo.2013.216. [DOI] [PubMed] [Google Scholar]
- 56.Højgaard B, Gyrd-Hansen D, Olsen KR, Søgaard J, Sørensen TIA. Waist circumference and body mass index as predictors of health care costs. PLoS One. 2008;3((7)):e2619. doi: 10.1371/journal.pone.0002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu S, Heshka S, Wang Z, Shen W, Allison DB, Ross R, et al. Combination of BMI and waist circumference for identifying cardiovascular risk factors in whites. Obes Res. 2004;12((4)):633–645. doi: 10.1038/oby.2004.73. [DOI] [PubMed] [Google Scholar]
- 58.Khor EQE, Lim JP, Tay L, Yeo A, Yew S, Ding YY, et al. Obesity definitions in sarcopenic obesity: differences in prevalence, agreement and association with muscle function. J Frailty Aging. 2020;9((1)):37–43. doi: 10.14283/jfa.2019.28. [DOI] [PubMed] [Google Scholar]
- 59.Vlassopoulos A, Govers E, Mulrooney H, Androutsos O, Hassapidou M. Dietetic management of obesity in Europe: gaps in current practice. Eur J Clin Nutr. 2021;75((7)):1155–1158. doi: 10.1038/s41430-020-00820-2. [DOI] [PubMed] [Google Scholar]
- 60.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16((3)):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vilarnau C, Stracker DM, Funtikov A, da Silva R, Estruch R, Bach-Faig A. Worldwide adherence to Mediterranean diet between 1960 and 2011. Eur J Clin Nutr. 2019;72((S1)):83–91. doi: 10.1038/s41430-018-0313-9. [DOI] [PubMed] [Google Scholar]
- 62.Fallaize R, Livingstone KM, Celis-Morales C, Macready AL, San-Cristobal R, Navas-Carretero S, et al. Association between diet-quality scores, adiposity, total cholesterol and markers of nutritional status in European adults: findings from the Food4Me study. Nutrients. 2018;10((1)):49. doi: 10.3390/nu10010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ocké MC. Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc. 2013;72((2)):191–199. doi: 10.1017/S0029665113000013. [DOI] [PubMed] [Google Scholar]
- 64.Roswall N, Olsen A, Boll K, Christensen J, Halkjær J, Sørensen TIA, et al. Consumption of predefined “Nordic” dietary items in ten European countries - an investigation in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Public Health Nutr. 2014;17((12)):2650–2659. doi: 10.1017/S1368980014000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassale C, Gunter MJ, Romaguera D, Peelen LM, Van der Schouw YT, Beulens JWJ, et al. Diet quality scores and prediction of all-cause, cardiovascular and cancer mortality in a pan-European cohort study. PLoS One. 2016;11((7)):e0159025. doi: 10.1371/journal.pone.0159025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168((7)):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 67.Panagiotakos DB, Milias GA, Pitsavos C, Stefanadis C. MedDietScore: a computer program that evaluates the adherence to the Mediterranean dietary pattern and its relation to cardiovascular disease risk. Computer Methods Programs Biomed. 2006;83((1)):73–77. doi: 10.1016/j.cmpb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 68.García-Conesa MT, Philippou E, Pafilas C, Massaro M, Quarta S, Andrade V, et al. Exploring the validity of the 14-item mediterranean diet adherence screener (MEDAS): a cross-national study in seven European countries around the Mediterranean region. Nutrients. 2020;12((10)):2960. doi: 10.3390/nu12102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bunn D, Jimoh F, Wilsher SH, Hooper L. Increasing fluid intake and reducing dehydration risk in older people living in long-term care: a systematic review. J Am Med Directors Assoc. 2015;16((2)):101–113. doi: 10.1016/j.jamda.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 70.Begum MN, Johnson CS. A review of the literature on dehydration in the institutionalized elderly. e-SPEN, Eur e-Journal Clin Nutr Metab. 2010;5((1)):e47–53. [Google Scholar]
- 71.Paz-Graniel I, Becerra-Tomás N, Babio N, Serra-Majem L, Vioque J, Zomeño MD, et al. Baseline drinking water consumption and changes in body weight and waist circumference at 2-years of follow-up in a senior Mediterranean population. Clin Nutr. 2021;40((6)):3982–3991. doi: 10.1016/j.clnu.2021.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Scientific Opinion on Dietary Reference Values for water - 2010 - EFSA Journal - Wiley Online Library. [cited 2022 Jun 8]. Available from: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2010.1459. [Google Scholar]
- 73.England CY, Andrews RC, Jago R, Thompson JL. A systematic review of brief dietary questionnaires suitable for clinical use in the prevention and management of obesity, cardiovascular disease and type 2 diabetes. Eur J Clin Nutr. 2015;69((9)):977–1003. doi: 10.1038/ejcn.2015.6. [DOI] [PubMed] [Google Scholar]
- 74.Brown A, Brosnahan N, Khazaei D, Wingrove J, Flint SW, Batterham RL. UK dietitians' attitudes and experiences of formula very low- and low-energy diets in clinical practice. Clin Obes. 2022;12((3)):e12509. doi: 10.1111/cob.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barr SI, Yarker KV, Levy-Milne R, Chapman GE. Canadian dietitians' views and practices regarding obesity and weight management. J Hum Nutr Diet. 2004;17((6)):503–512. doi: 10.1111/j.1365-277X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 76.Luo M, Allman-Farinelli M. Trends in the number of behavioural theory-based healthy eating interventions inclusive of dietitians/nutritionists in 2000–2020. Nutrients. 2021;13((11)):4161. doi: 10.3390/nu13114161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delahanty LM. An expanded role for dietitians in maximising retention in nutrition and lifestyle intervention trials: implications for clinical practice. J Hum Nutr Diet. 2010;23((4)):336–343. doi: 10.1111/j.1365-277X.2009.01037.x. [DOI] [PubMed] [Google Scholar]
- 78.Schaefer JT, Zullo MD. US registered dietitian nutritionists' knowledge and attitudes of intuitive eating and use of various weight management practices. J Acad Nutr Diet. 2017;117((9)):1419–1428. doi: 10.1016/j.jand.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 79.Kirk S, Salas XR, Alberga A, Russell-Mayhew S. Obesity Canada. 2020. Reducing Weight Bias in Obesity Management, Practice & Policy. [cited 2022 Jun 8]. Available from: https://obesitycanada.ca/guidelines/weightbias/. [Google Scholar]
- 80.Jung FUCE, Luck-Sikorski C, Wiemers N, Riedel-Heller SG. Dietitians and nutritionists: stigma in the context of obesity. A systematic review. PLoS One. 2015;10((10)):e0140276. doi: 10.1371/journal.pone.0140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.