Abstract
Researchers discovered that diets rich in anthocyanin-rich fruits and vegetables significantly impacted gut flora. To conclude, large-scale randomized controlled clinical trials are challenging to conduct; therefore, merging data from multiple small studies may aid. A systematic review collects and analyses all research on a particular subject and design. This comprehensive review and meta-analysis examined the influence of dietary anthocyanins on Firmicutes/Bacteroide (Fir/Bac) and short-chain fatty acids (SCFAs) content. The current meta-analysis followed the guidelines of PRISMA—the preferred reporting items for systematic reviews and meta-analyses. Diets high in anthocyanins substantially reduced the Fir/Bac ratio in the assessed trials. Among three SCFAs, the highest impact was observed on acetic acid, followed by propionic acid, and then butanoic acid. The meta-analysis results also obtained sufficient heterogeneity, as indicated by I2 values. There is strong evidence that anthocyanin supplementation improves rodent gut health biomarkers (Fir/Bac and SCFAs), reducing obesity-induced gut dysbiosis, as revealed in this systematic review/meta-analysis. Anthocyanin intervention duration and dosage significantly influenced the Fir/Bac ratio and SCFA. Anthocyanin-rich diets were more effective when consumed over an extended period and at a high dosage.
Subject terms: Biotechnology, Health care
Introduction
Polyphenols are phytochemicals in various foods, including fruits and vegetables, tea, coffee, chocolate, legumes, and cereals. The primary function of polyphenols is to act as antioxidants and quench free radicals1. Dietary polyphenols are gaining scientific attention due to their health benefits. Several clinical studies have found that polyphenols can help protect against cancer, cardiovascular disease, aging, and neurodegenerative diseases2–4. Anthocyanins are among the most potent polyphenols due to their chemical structure, i.e., the abundance of hydroxyl groups. Anthocyanins are pigments that give plants vibrant color [purple, blue, and red] and have antioxidant properties5. Several studies have discovered that they can aid in preventing obesity, diabetes, and metabolic disorders by improving gut health and microbiota6–9. An individual's gut microbiota is complex, containing thousands of different bacterium species and trillions of microbes10. The gut microbiota varies with the dietary pattern, co-evolves with the host, and has a symbiotic relationship11. The majority of the gut microbiota is considered non-pathogenic. Scientific data from numerous experimental and clinical studies have established the health benefits of healthy gut microbiota12,13. However, specific stimuli may change their composition over time, leading to a condition known as dysbiosis, favoring pathogenic microbes and negatively affecting the gastrointestinal tract, immune system, central nervous system, and metabolic machinery. These conditions lead to irritable bowel syndrome [IBS], inflammatory bowel diseases, allergies, Alzheimer's and Parkinson's, and type 1 diabetes, among others14–16. As a result, it is vital to identify potentially beneficial bacteria that could aid in developing treatments that protect people from the adverse effects of gut dysbiosis. The ratio of two major microbial phyla, Firmicutes/Bacteroidetes [Fir/Bac], and the level of short-chain fatty acids [SCFAs] are frequently regarded as vital indicators of an individual's gut health status. Obese people, for example, have a higher Fir/Bac ratio than lean people17,18. The healthy gut microbiota metabolizes indigestible dietary components to SCFAs17,18. SCFAs such as acetic acid, propionic acid, and butyric acid, acidify the intestinal pH and inhibit pathogenic bacteria such as Enterobacteriaceae from propagating19. Propionate is essential for gluconeogenesis, whereas acetate is important for lipogenesis20. Butyrate gives energy to colon cells, keeps the structure of the biological membrane stable, and encourages the growth of colonocytes21.
Anthocyanins can pass through the gastrointestinal mucosa in their natural state. Hydrolytic enzymes in the small intestine absorb them as phenolic aglycone, especially in the jejunum. The anthocyanins do not pass through the colon. The colonic microbiota metabolizes unabsorbed anthocyanins into simpler metabolites. These metabolites have been shown to influence the proliferation of beneficial bacteria such as bifidobacterium22, Fir/Bac ratio, and SCFA production22–27.
The health effects of a molecule are usually concluded based on large-scale randomized controlled clinical trials, which are notoriously difficult to conduct. However, combining data from several small studies can aid in the conclusion. A systematic review compiles all possible studies on a specific topic and design, then reviews and analyses their findings. During the systematic review process, the quality of the studies is evaluated, and a statistical meta-analysis of the study results is performed based on their quality. A meta-analysis is a legal, objective, and scientific method of analyzing and combining different results. Previous meta-analysis studies looked into the effects of anthocyanin-rich diets on cardiovascular health and oxidative stress28,29. Nonetheless, the effect of anthocyanins on the gut microbiota, particularly the Fir/Bac ratio and SCFA concentration, has yet to be thoroughly reviewed. Thus, this systematic review and meta-analysis aim to conclude the effect of dietary anthocyanins on the Fir/Bac ratio and SCFA content.
Materials and methods
The current meta-analysis study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (PRISMA)30.
Literature search
Scientific databases, including Scopus, PubMed, Science Direct, Web of Science, and MEDLINE, were searched up to 2022. The search terms or keywords included gut microbiota and anthocyanins; Gut microbiota, anthocyanins, and animal study; Anthocyanin-rich fruits and gut microbiota; Anthocyanin-rich vegetables and gut microbiota; and Anthocyanin-rich vegetables and gut microbiota; Anthocyanins, Firmicutes, Bacteroidetes; Anthocyanins and in vivo gut microbiota; Anthocyanins, gut microbiota, short-chain fatty acids. We have also formulated the search using the PICO framework for evidence-based practice (STable 1). Because the PICO framework is used in systematic reviews to create literature search tactics that are both thorough and objective.
Criteria for study selection, inclusion, and exclusion
Revised ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments)31 designed to help researchers and publishers identify the minimum necessary information for scientific reporting of in vivo experiments, such as inclusion and exclusion criteria, were followed. The titles of the collected articles were examined first, followed by the selection of abstracts and confirmation of manuscript content. The following inclusion criteria were specified during the study selection process. (a) Clearly stated study design; (b) Animal studies [mice and rats]; (c) A minimum of three subjects (d) Anthocyanin supplementation in purified, extract, whole fruit or juice form; (e) Control mentioned (f) Intervention duration > one week; (g) Data for Firmicutes to Bacteroidetes ratio, acetic acid, propionic acid, and butyric acid. English-language studies were preferred.
Two authors reviewed the studies from the initial search to identify those relevant to the study. This allowed us to exclude studies that did not address the purpose of the study or the previously stated requirements. A kappa analysis can be performed to check for consistency in interpreting the selection criteria between the two reviewers. Using Cohen's kappa coefficient32, we could determine if there was substantial agreement between reviewers in each study. The formula for Cohen's kappa is calculated as follows:
where po: Relative observed agreement among raters, pe: Hypothetical probability of chance agreement.
Data extraction
The following information was extracted from each article: author, year of publication, subjects’ clinical characteristics, sample size, study duration, source of anthocyanins, daily dosage, means, and standard deviations (SD) of the Fir-Bac ratio and SCFAs33. If the trial included standard errors (SE), the SE was converted to SD by multiplying the SE by the square root of the sample size. The unit of SCFAs (acetic acid, propionic acid, butyric acid) was µmol/gm. Different anthocyanin interventions given to animals were formalized to mg/kg body weight in all studies. For dose conversion in mg/kg of body weight, average weight and diet considered for mice were 22 g and 2.5 g, and for rats, 200 g and 11 g, respectively (STable 2, 3).
Statistical analysis
The standardized mean difference (SMD) was calculated using Hedges' adjusted g. The weighted mean differences (MD) for net change and 95 percent confidence intervals (CI) were used to estimate the effect of anthocyanins on the Fir/Bac ratio and SCFA concentration34. The Forest plots were created to display the SMDs and CIs, which represent each study's observed effect, confidence interval, and weight35. Statistical tests for heterogeneity I2, Chi2, and Tau2 were used to assess the consistency of the study's results. The I2 values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively. The treatment groups receiving low and high doses of anthocyanin were chosen for dose comparison through meta-analysis. In studies with more than two anthocyanin treatments or anthocyanin-rich food interventions, each treatment group was compared to the control group36. The influence of anthocyanins on the Fir/Bac ratio and SCFAs was calculated using a random-effects analysis model. Subgroup analyses were performed to identify potential contributory variables37. In the Fir/Bac analysis, studies were classified into subgroups based on the duration (less than ten weeks vs. equal to and more than ten weeks), dose (higher and lower doses as per respective studies), an animal model type (High fat diet-induced obesity, diabetes, and other diseases). For SCFA analysis, studies were classified similarly into subgroups based on the duration (less than four weeks vs. equal to and more than 4 weeks), dose, and animal model type. The RevMan 5.4 package38 and the R script (meta-package) were used for all statistical studies39.
Data evaluation/testing
GRADEprofiler (GRADEpro) tool was used for the analysis of data quality. For systematic reviews and recommendations in healthcare, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) provides a transparent and structured approach for creating and presenting evidence summaries, including the quality of that evidence40. Using GRADE, we classified the quality of our meta-analysis results into two categories: higher and lower.
Publication bias test
Begg's and Egger's regression asymmetry tests were used for estimating publication bias in various forms, such as time-lag bias (caused by delayed publication), duplicate or multiple publications, outcome reporting bias (only reporting good results), and language bias. Egger test used linear regression to test asymmetry with numbers by examining the relationship between the standardized effect estimates and the standard error41. Begg's test assessed the significance of the correlation between the ranks of the effect estimates and the ranks of their variances42. The minor corrections were implemented using the Trim-Fill correction method in all the studies, including Fir/Bac and SCFA.
Bibliometric analysis
A bibliometric study was conducted by selecting the articles published (indexed in the Pubmed database) till March 2022 using the search terms *anthocyanin*, *gut microbiota*, and/or *SCFA* to know the research output progress on anthocyanin. The publications were downloaded in the Medline file format. After selection, visualization of the thematic contiguity of the articles was carried out using the Vos Viewer tool, which enabled the network charts. The network visualization consists of multiple-colored bubbles. Each bubble with a single color belongs to a ‘cluster.’ Bubbles that are distantly located from others have a weak relationship among them. Moreover, the number of links between two bubbles depicted the level of interaction between the items under consideration.
Result
Literature search
Detailed information on the search strategy and the process followed for the meta-analysis has been displayed in the PRISMA flowchart (Fig. 1). We identified 605 articles using various search engines through a literature survey. Of these articles, 173 were review articles, and 432 were research articles. From the total research articles selected for the study, 298 were duplicates and therefore removed. Afterward, article abstracts and the full text of 133 articles were read thoroughly and checked to determine whether they met the eligibility criteria. Those studies that did not meet the eligibility criteria (“Criteria for study selection, inclusion, and exclusion” section) were also removed. Thirty-four studies met the eligibility criteria. Out of it, 20 and 14 articles examining the effect of anthocyanins on the Fir/Bac ratio and the concentration of SCFAs, respectively, were included for meta-analysis. Figure 1 shows the flow and data extraction of the current study.
Figure 1.
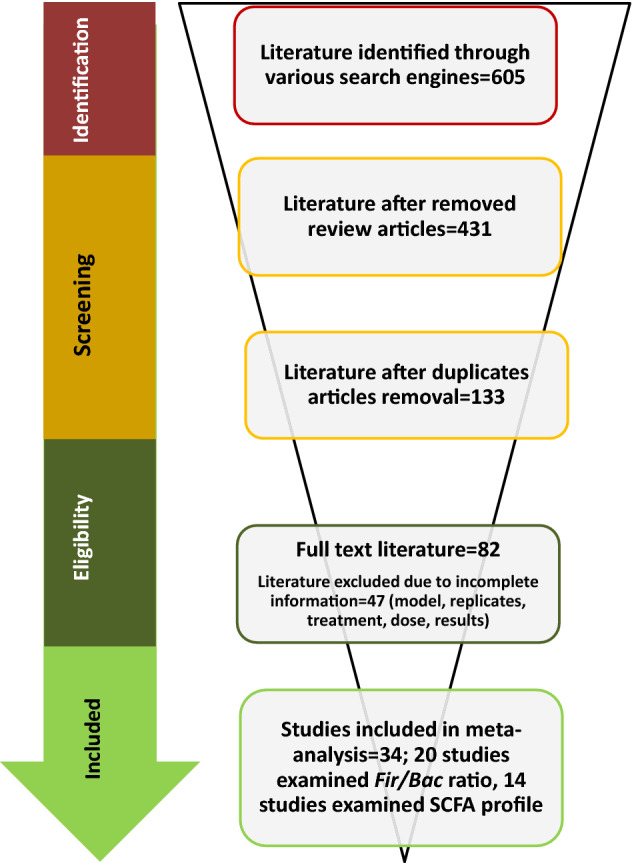
Meta-Analysis flow chart as per PRISM guidelines.
The first criteria we applied for screening total studies was 605; in that case % of the agreement was 96.89%, Cohen’s k: 0.91. The second screening was performed on 432 selected articles; in that case % of the agreement was 97.85%, and Cohen’s k was 0.95. In the third screening, the % of agreement: was 96.33%, Cohen’s k: 0.91, and in the fourth screening total of 34 studies were selected with % of agreement: 97.47% and Cohen’s k: 0.93. To pass the test, you should aim for a kappa score of 0.5 or higher. Near-perfect agreement in the selection and filtering of studies was observed, with values over 0.9; differences were discussed and resolved by consensus.
Study characteristics
The characteristics of the studies examining the effects of anthocyanins on the Fir/Bac ratio are mentioned in Table 1. Of the total studies, 14 investigated the impact of the intervention of anthocyanins from various berry fruits. The remaining studies included interventions from other sources like cereals and pulses (Table 1). Seventeen studies were conducted on males, one was conducted on female mice models, and two were performed on male rats.
Table 1.
Characteristics of the studies used to investigate the effect of anthocyanins on the Fir/Bac ratio.
| S.no. | Animal | Age [weeks] | Model type | Source | Intervention compound | Dose | Intervention duration [weeks] | References | Doi |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male C57BL/6 J mice | 7–9 | High fat diet | Roselle | Flower water extract [Phenolic extract] | 1 mg/kg body weight [L] | 6 | 43 | https://doi.org/10.1016/j.foodres.2019.108722 |
| 10, mg/kg body weight [M] | |||||||||
| 25 mg/kg body weight [H] | |||||||||
| 2 | Male C57BL/6Cnc mice | 4 | Highfat, highfructose diet | Grape | Fruit ethanol extract [Phenolic extract] | 405 mg/kg body weight | 12 | 44 | https://doi.org/10.1002/mnfr.202000149 |
| 3 | Male C57BL/6 J mice | 4 | High fat diet | Russian box thorn | Fruit based commercial powder [Anthocyanins] | 50 mg/kg body weight [L] | 12 | 24 | https://doi.org/10.1002/mnfr.202000745 |
| 100 mg/kg body weight [M] | |||||||||
| 200 mg/kg body weight [H] | |||||||||
| 4 | Male Wistar rats | NA | High fat diet | Blackberry | Fruit acidified ethanol extract [Anthocyanins] | 25 mg/kg body weight | 17 | 45 | https://doi.org/10.1038/s41598-018-29744-5 |
| 5 | Male C57BL/6 mice | 6 | High fat diet | Blueberry | Fruit fermented juice | 4 ml/kg | 17 | 46 | https://doi.org/10.1039/D0FO00334D |
| 6 | Male C57BL/6JbomTac mice | 6 | High fat diet | Lingonberries | Fruit freeze dried | 22725 mg/kg body weight | 11 | 47 | https://doi.org/10.3402/fnr.v60.29993 |
| Phenolic compound | 138.6 mg/kg body weight | ||||||||
| 7 | Male C57BL/6 J mice | 6 | High fat, high sucrose diet | Blueberries | Fruit hydro-ethanolic extract [Fraction rich in anthocyanin and phenolic acids] | 32 mg/kg body weight [L] | 8 | 48 | https://doi.org/10.1038/s41598-020-58863-1 |
| Fraction rich in oligomeric PACs, phenolic acids and flavonols | 53 mg/kg body weight [H] | ||||||||
| Fraction rich in polymeric PACs | 37 mg/kg body weight [M] | ||||||||
| 8 | Male C57BL/6 J | 6 | High fat diet | Tea [Purple-leaf] | Leaves dried | 1137 mg/kg body weight [L] | 10 | 49 | https://doi.org/10.1186/s12906-020-03171-4 |
| 3409 mg/kg body weight[H] | |||||||||
| Phenolic compounds | 12.6 [L], 37.8[H] mg/kg body weight | ||||||||
| Anthocyanins | 1.8 [L], 5.4 [M]mg/kg body weight | ||||||||
| 9 | Male C57BL/6N mice | 5 | Western diet | Bilberry | Fruitsdried | 2273 mg/kg body weight | 18 | 50 | https://doi.org/10.3390/nu12113252 |
| Anthocyanins | 5.7 mg/kg body weight | ||||||||
| 10 | Male C57BL6/J mice | 6 | Cholesterol diet | Black rice | Fruit based commercial extract [Anthocyanins] | 13.6 mg/kgbody weight [L] | 12 | 51 | https://doi.org/10.1002/mnfr.201900876 |
| 27.3 mg/kg body weight [M] | |||||||||
| 54.4 mg/kg body weight [H] | |||||||||
| 11 | Male C57BL/6 mice | 5 | Dextran sodium sulfate induced colitis | Russian box thorn | Fruithydro-acidic ethanolic extract [Anthocyanins] | 200 mg/kg body weight | 2.2 | 23 | https://doi.org/10.1016/j.freeradbiomed.2019.04.005 |
| 12 | Male C57BL/6 J mice | 4 | High fat diet | Black currant | Fruithydro-acidic ethanolic extract [Anthocyanins] | 150 mg/kg body weight | 14 | 52 | https://doi.org/10.1002/mnfr.202001090 |
| 13 | Female C57BL/6 mice | 8 | Colon cancer | Bilberry | Fruit based commercial powder [Anthocyanins] | 25 mg/kg body weight | 2 | 53 | https://doi.org/10.3390/microorganisms8020175 |
| 14 | Male diabetic Zucker rats | 3 | High fat diet | Bilberries and purple potato | Bilberry fruit [a,b] and potato tuber [c,d] commercial extract [50:50 ratio] [Anthocyanins] | 25 mg/kg body weight [L:a,c] | 8 | 54 | https://doi.org/10.1016/j.foodres.2022.110978 |
| 50 mg/kg body weight [H: b,d] | |||||||||
| 15 | Male C57BL/6 J mice | 5 | High fat diet | Russian box thorn | Fruithydro-acidic ethanolic extract [Anthocyanins] | 100 mg/kg body weight | 11 | 55 | https://doi.org/10.3390/foods11010098 |
| 16 | Male C57BL/6 J mice | NA | High fat diet | Undefined | Commercial powder [Anthocyanins] | 40 mg/kg body weight | 14 | 56 | https://doi.org/10.1016/j.redox.2019.101269 |
| 17 | Male C57BL/6 J mice | 5 | High fat diet | Blueberry [a,b] and cranberry [c,d] | Commercial powder[50:50 ratio] [Anthocyanins] | 1137 mg/kg body weight [L:a,c] | 24 | 57 | https://doi.org/10.1007/s00394-020-02446-3 |
| 2273 mg/kg body weight [H:b,d] | |||||||||
| 18 | Male C57BL/6 mice | 7 | High fat diet | Jamun [black plum] | Fruit pulp hydro-ethanol/acetone extract [Phenolic extract] | 100 mg/kg body weight | 8 | 58 | https://doi.org/10.1002/mnfr.201801307 |
| 19 | Male C57BL/6 J mice | 6 | High fat, cholesterol diet | Purple sweet potato | Tuber acidified methanolic extract [Anthocyanins] | 340 mg/kg body weight [L] | 12 | 59 | https://doi.org/10.1111/1750-3841.16130 |
| 681.8 mg/kg body weight [M] | |||||||||
| 1022.6 mg/kg body weight [H] | |||||||||
| 20 | Male Swiss-albino mice | 6–8 | Normal | Purple and black wheat | Seed powder [a,c] and cooked chapatti powder [b,d] | 96.6 g/kg body weight [L: a, c] | 11 | 60 | https://doi.org/10.1016/j.jcs.2022.103433 |
| 104.5 g/kg body weight [H: b,d] | |||||||||
| Anthocyanins |
Purple Flour = 5.7 [a] Black flour = 15.4 [b] Purple Chapatti = 2.0 [c] Black chapatti = 10.3 [d] [mg/kg body weight] |
#For understanding the effect of dose, different interventions given to animals were uniformalised to mg/kg body weight. For dose conversion in mg/kg of body weight, the average weight and diet considered for mice were 22 g and 2.5 g, and for rats were 200 g and 11 g, respectively.
Study characteristics examining the effect of anthocyanins on SCFA profile (acetic, propionic, and butyric acid) were mentioned in Table 2. Ten studies were conducted on male mice, one on female mice, and three on male rats. Twelve studies that looked at the effect of the anthocyanins-rich diet intervention on the concentration of SCFAs in the cecal matter of the different subjects looked at the effect of berries, and one study each looked at the effect of black rice and purple sweet potatoes.
Table 2.
Characteristics of the studies used to investigate the effect of anthocyanins on the short chain fatty acids [SCFA’s].
| Animal | Age [weeks] | Model type | Source | Intervention compound | Dose | Duration [weeks] | References | Doi | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male C57BL/6 J mice | 8 | High fat, high sucrose diet | Blueberry | Fruits dried | 727.2 mg/kg body weight | 8 | 61 | https://doi.org/10.1152/ajpendo.00560.2019 |
| Anthocyanins [Size based fractionation] | 77.3 mg/kg body weight | ||||||||
| Proanthocyanins [Size based fractionation] | 4.6 mg/kg body weight | ||||||||
| 2 | Kunming mice | NA | Diphenoxylate induced constipation | Mulberry | Fruit dried | 142 mg/kg body weight [L] | 2 | 62 | https://doi.org/10.1039/C9FO00132H |
| 284.1 mg/kg body weight [M] | |||||||||
| 568.1 mg/kg body weight [H] | |||||||||
| Anthocyanins | 0.7 [L], 1.4 [M], and 6.82 [H] mg/kg body weight | ||||||||
| 3 | Male C57BL/6 mice | 6 | Western diet | Montmorency tart cherry | Fruit dried | 5681.3 mg/kg of body weight [L] | 12 | 63 | https://doi.org/10.1016/j.nutres.2021.10.003 |
| 11,362 mg/kg of body weight [H] | |||||||||
| Anthocyanins | 1.58 [L], 3.16 [H] mg/kg of body weight | ||||||||
| 4 | Male C57BL/6 mice | 4–5 | High fat diet | Raspberry | Fruit pulp hydro-acidified methanolic extract [Anthocyanins] | 22.8 mg/kg body weight | 12 | 64 | https://doi.org/10.1039/C7FO02061A |
| 5 | Male db/db mice with C57BL/6 J background | 6 | Diabetic Mice | Wild raspberry | Fruit hydro-acidified methanolic extract [Anthocyanins] | 150 mg/kg of body weight | 8 | 65 | http://doi.org/10.1021/acs.jafc.9b03338 |
| 6 | Male C57BL6/J mice | 6 | High fat, cholesterol diet | Black rice | Fruit based commercial extract [Anthocyanins] | 13.6 mg/kg body weight [L] | 12 | 51 | http://doi.org/10.1002/mnfr.201900876 |
| 27.3 mg/kg body weight [M] | |||||||||
| 54.6 mg/kg body weight [H] | |||||||||
| 7 | Male Wistar rats | 3 | Normal | Brazilian berry | Fruit peel water extract [Phenolic extract] | Undefined | 7 | 66 | https://doi.org/10.1111/jfbc.12705 |
| 8 | Male Wistar rats | 3 | Colitis model | Brazilian berry | Fruit peel water extract [Phenolic extract] | 141 and 151 mg/kg of body weight mg/kg of body weight [Short term treatment-L] | 7 | 67 | https://doi.org/10.3390/nu11112776 |
| 215 and 208 mg/kg of body weight mg/kg of body weight [Long term treatment-H] | |||||||||
| 9 | Male Wistar rats | NA | High fat diet | Blueberry | Fruit Dried | 113.63 mg/kg body weight | 8 | 68 | https://doi.org/10.1093/jn/nxx027 |
| Phenolic + anthocyanin extract | 6.79 mg/kg body weight | ||||||||
| 10 | Male C57BL/6 mice | 5 | Normal | Russian box thorn | Fruit hydro-ethanolic extract [Phenolic extract] | 200 mg/kg of body weight | 12 | 69 | https://doi.org/10.1016/j.foodres.2019.108952 |
| 11 | Male C57BL/6 J mice | 4 | High fat diet | Russian box thorn | Fruit Anthocyanins extract | 50 mg/kg body weight [L] | 12 | 24 | https://doi.org/10.1002/mnfr.202000745 |
| 100 mg/kg body weight [M] | |||||||||
| 200 mg/kg body weight [H] | |||||||||
| 12 | Male C57BL/6 mice | 5 | DSS-colitis model | Russian box thorn | Fruit hydro- ethanolic extract [Phenolic extract] | 200 mg/kg body weight [ACN:a, P3G:b] | 2.4 | 23 | https://doi.org/10.1016/j.freeradbiomed.2019.04.005 |
| 13 | Female C57BL/6 mice | 8 | Colon Cancer | Bilberry | Fruit based commercial powder [Anthocyanins] | 25 mg/kg body weight | 2 | 53 | https://doi.org/10.3390/microorganisms8020175 |
| 14 | Male C57BL/6 J mice | 6 | High fat, cholesterol diet | Sweet potato | Tuber based commercial powder [Anthocyanins] | 340.8 mg/kg body weight [L] | 12 | 59 | https://doi.org/10.1111/1750-3841.16130 |
| 681.8 mg/kg body weight [M] | |||||||||
| 1022.6 mg/kg body weight [H] |
#For understanding the effect of dose, different interventions given to animals were uniformalised to mg/kg body weight. For dose conversion in mg/kg of body weight, the average weight and diet considered for mice were 22 g and 2.5 g, and for rats were 200 g and 11 g, respectively.
Effect of anthocyanins on the Fir/Bac
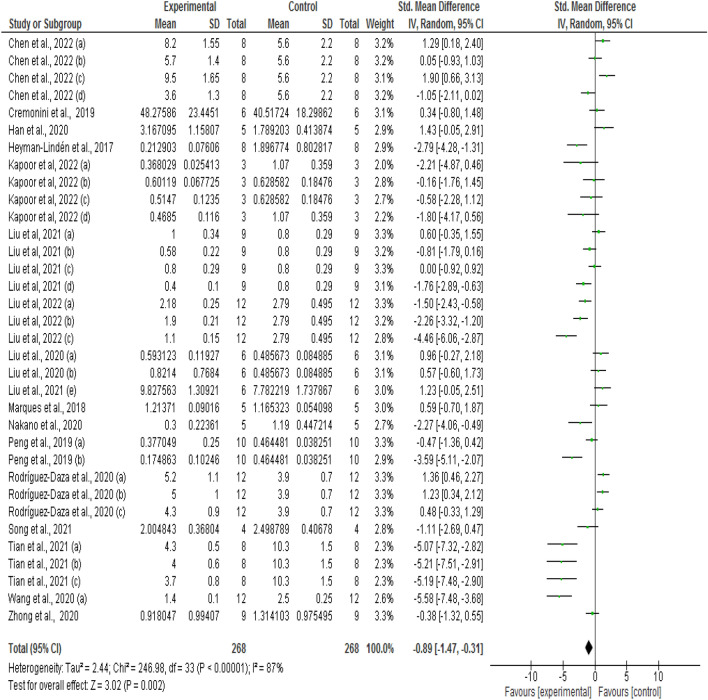
The anthocyanin-rich diet intervention significantly decreased the Fir/Bac ratio (SMD: − 1.80; 95% CI − 2.48, − 1.12; I2 = 90%; P < 0.00001) in all the studies under consideration (Table 3 and Supplemental Fig. 1). The meta-analysis result also obtained sufficient heterogeneity, as indicated by I2 values. Regarding the contribution of individual studies, some showed non-significant results, and others had a relatively higher influence on overall value than others. However, a comprehensive Fir/Bac ratio study produced statistically significant positive results. Four studies, including Diez-Echave et al.43, Wang et al.51 (Medium and High doses); Lin et al.49; and Xu et al.58, had more comprehensive cumulative interval ranges, which means there was more uncertainty about the usefulness of these interventions. When the studies mentioned above were deleted before analysis, the Fir/Bac ratio significantly reduced, but overall values changed (SMD: − 0.89; 95% CI − 1.47, − 0.31; I2 = 87%; P 0.002) (Fig. 2).
Table 3.
All-inclusive, high-influencer subtracted and sub-group [intervention duration, dose, and animal model type] analysis to understand the effects of anthocyanins on the Fir/Bac ratio.
| Study/ Subgroup type | SMD | 95% CI | P value | I2[%] |
|---|---|---|---|---|
| Whole study | − 1.80 | − 2.48, − 1.12 | 0.00001 | 90 |
| After removing Highly influencing studies | − 0.89 | − 1.47, − 0.31 | 0.002 | 87 |
| Study duration | ||||
| < 10 weeks | − 1.49 | − 2.64, − 0.33 | 0.01 | 92 |
| After removing Highly influencing studies | 0.30 | − 0.43, 1.03 | 0.42 | 82 |
| ≥ 10 weeks | − 2.16 | − 2.96, − 1.36 | 0.0001 | 87 |
| After removing Highly influencing studies | − 1.81 | − 2.56, − 1.05 | 0.00001 | 86 |
| Dose of anthocyanin | ||||
| Lower Dose | − 1.12 | − 2.47,0.23 | 0.11 | 91 |
| After removing Highly influencing studies | − 0.60 | − 1.74,0.54 | 0.30 | 89 |
| Higher Dose | − 2.33 | − 3.69, − 0.98 | 0.0007 | 91 |
| After removing Highly influencing studies | − 1.79 | − 2.95, − 0.64 | 0.002 | 89 |
| Study model type | ||||
| High fat diet model | − 2.31 | − 3.31, − 1.31 | 0.0001 | 92 |
| After removing Highly influencing studies | − 0.94 | − 1.78, − 0.11 | 0.03 | 90 |
| Other models [Western diet, tumour, colitis] | − 2.25 | − 3.64, − 0.86 | 0.002 | 89 |
| After removing Highly influencing studies | − 1.72 | − 2.97, − 0.46 | 0.007 | 86 |
Pooled effect sizes and 95% CI were determined using random effects model.
SMD Standardised mean difference, I2 Heterogeneity.
Figure 2.
Forest plot of studies investigating the effect of anthocyanin supplementation on the Firmicutes to Bacteroidetes ratio [Fir/Bac]. Pooled effect estimates [diamonds] for Fir/Bac are shown after removing highly influencing studies. Values are standardized mean differences with 95% CIs determined with the use of random-effects models. Heterogeneity was quantified by I2, inverse variance and standardised mean difference [SMD].
Similar meta-analyses, i.e., without highly influencing studies with wider cumulative interval ranges, were performed in each sub-group. Forest plots in Supplemental Figs. 2 and 3 show subgroup analyses investigating the effect of anthocyanin-rich diet intervention on the Fir/Bac ratio based on the duration, anthocyanin dose, and study model type. The meta-analyses results indicated that intervention duration of the more extended period, i.e., ≥ 10 weeks, significantly reduced the Fir/Bac ratio (SMD = − 1.81; 95% CI − 2. 56, − 1.05; I2 = 86%; P < 0.0001), whereas intervention study for a shorter period, i.e., less than 10 weeks had no effect (SMD = 0.30; 95% CI − 0.43, 1.03; I2 = 82%; P < 0.42). Similarly, the effect of higher intervention doses was more pronounced (SMD = − 1.79; 95% CI − 2.95, − 0.64; I2 = 89%; P < 0.002) as compared to lower doses (SMD = − 0.60; 95% CI − 1.74, − 0.54; I2 = 89%; P < 0.30). There was no effect of the type of study model. Anthocyanin-rich intervention remarkably reduced the Fir/Bac ratio irrespective of the study model type. It reduced in high fat/cholesterol diet-induced obese subjects (SMD = − 0.94; 95% CI − 1.78, − 0.11; I2 = 90%; P < 0.03) as well as in other model studies including western diet, dextran sodium sulphate [DSS]-induced colitis, and tumor (SMD = − 1.72; 95% CI − 2.97, − 0.46; I2 = 86%; P < 0.007) (Table 3, Supplemental Fig. 2 and 3). Finalized data quality was evaluated by Grade Tool (Supplemental Fig. 4) and showed moderate heterogeneity that is serious inconsistency.
Effect of anthocyanins on the short chain fatty acids (SCFA’s) production
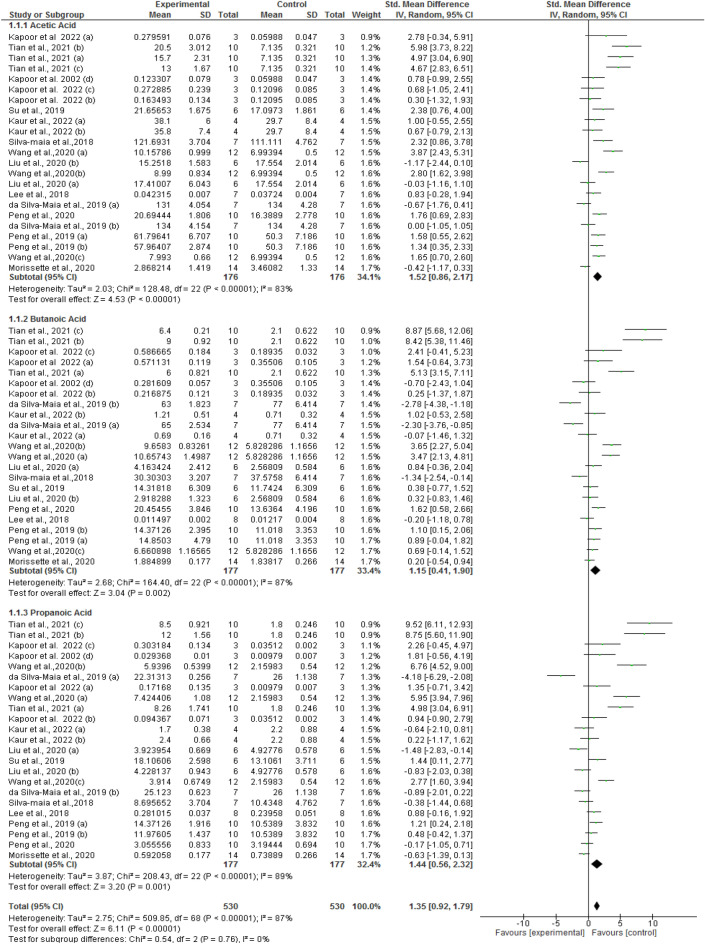
The meta-analyses showed a significant effect of the anthocyanin-rich diet intervention on acetic, propionic, and butanoic acid concentration (Table 4, Fig. 3, and Supplemental Fig. 5). Of the three SCFAs, the highest impact was observed on the acetic acid (SMD:1.52; 95% CI 0.86,2.17 µmole/gm; I2 = 83%; P < 0.00001]; followed by propionic acid (SMD:1.44; 95% CI 0.56, 2.32 µmole/gm; I2 = 89%; P = 0.001) and then butanoic acid (SMD: 1.15; 95% CI 0.41, 1.90 µmole/gm; I2: 87%; P value = 0.002). High heterogeneity was obtained, as indicated by I2 values.
Table 4.
Pooled effects of anthocyanins from various sources on short chain fatty acid profile including all-inclusive and high-influencer studies.
| Parameters | Acetic acid | After removing Highly influencing studies | Butanoic acid | After removing Highly influencing studies | Propionic acid | After removing Highly influencing studies |
|---|---|---|---|---|---|---|
| SMD | 2.69 | 1.52 | 1.60 | 1.15 | 2.33 | 1.44 |
| 95% CI [µmole/gm] | 1.88, 3.50 | 0.86, 2.17 | 0.82, 2.39 | 0.41, 1.90 | 1.45, 3.22 | 0.56, 2.32 |
| P value | 0.00001 | 0.00001 | 0.0001 | 0.002 | 0.00001 | 0.001 |
| I2[%] | 90 | 83 | 90 | 87 | 92 | 89 |
Pooled effect sizes and 95% CI were determined using Random effects model.
SMD Standardised mean difference, I2 Heterogeneity.
Figure 3.
Forest plot of studies investigating the effect of anthocyanin supplementation on the SCFA profile, sub-grouped by short chain fatty acid type. Pooled effect estimates are shown by diamonds after removing highly influencing studies. Values are standardized mean differences with 95% CIs determined with the use of random-effects models. Heterogeneity was quantified by I2, inverse variance and standardised mean difference [SMD].
Each short-chain fatty acid was sub-grouped based on intervention duration, anthocyanin dose, and model type. We found a considerable increase in acetic acid concentration when the intervention was continued for ≥ 4 weeks (SMD: 1.78; 95% CI 1.01, 2.54 µmole/gm; I2: 84%; P < 0.00001) as compared to the nonsignificant effect of intervention followed for less than 4 weeks (SMD:0.47; 95% CI − 0.73, 1.68µmole/gm; I2: 79%; P < 0.44). (Table 5 and Supplemental Figs. 6 and 7). The intervention of anthocyanin at a higher dose imparted a remarkable impact on acetic acid (SMD: 2.58; 95% CI 0.92, 4.24 µmole/gm, I2: 76%; P = 0.002) compared to a lower dose (SMD: 1.65; 95% CI 0.33, 2.98 µmole/gm, I2: 74%; P = 0.01). The anthocyanins exerted a significant effect on acetic acid concentration in high fat/cholesterol diet model type (SMD: 2.89; 95% CI 1.40, 4.37µmole/gm, I2: 91%; P = 0.0.0001) as compared to another model type (SMD: 0.81; 95% CI 0.24, 1.37µmole/gm, I2: 62%; P = 0.005) (Table 5; Supplemental Fig. 6 and 7).
Table 5.
All-inclusive, high-influencer subtracted and sub-group [intervention duration, dose, and animal model type] analysis to understand the effects of anthocyanins on the acetic acid.
| Study/ Subgroup type | SMD | 95% CI | P value | I2[%] |
|---|---|---|---|---|
| Study duration | ||||
| < 4 weeks | 2.97 | 1.01, 4.93 | 0.003 | 92 |
| After removing Highly influencing studies | 0.47 | − 0.73, 1.68 | 0.44 | 79 |
| ≥ 4 weeks | 2.63 | 1.73, 3.54 | 0.00001 | 89 |
| After removing Highly influencing studies | 1.78 | 1.01, 2.54 | 0.00001 | 84 |
| Dose of anthocyanin | ||||
| Lower Dose | 2.65 | 1.12, 4.19 | 0.0007 | 86 |
| After removing Highly influencing studies | 1.65 | 0.33, 2.98 | 0.01 | 74 |
| Higher Dose | 3.81 | 1.41, 6.20 | 0.002 | 92 |
| After removing Highly influencing studies | 2.58 | 0.92, 4.24 | 0.002 | 76 |
| Study model type | ||||
| High fat diet model | 4.36 | 2.80, 5.92 | 0.00001 | 93 |
| After removing Highly influencing studies | 2.89 | 1.40, 4.37 | 0.0001 | 91 |
| Other models [Western diet, tumour, colitis] | 1.63 | 0.80, 2.47 | 0.0001 | 83 |
| After removing Highly influencing studies | 0.81 | 0.24, 1.37 | 0.005 | 62 |
Pooled effect sizes and 95% CI were determined using Random effects model.
SMD Standardised mean difference, I2 Heterogeneity.
We have found a higher rise in the butanoic acid concentration for a more extended period of study duration (SMD: 1.30; 95% CI 0.36, 2.25µmole/gm; I2: 89%; P < 0.007) as compared to a shorter period, i.e., < 4 weeks (SMD:0.82; 95% CI 0.30, 1.34 µmole/gm; I2: 0%; P < 0.002) (Table 6; Supplemental Fig. 8 and 9). Also, the butanoic acid concentration was significantly higher in the subjects taking a higher dose of anthocyanins (SMD: 3.32; 95% CI 1.53, 5.11 µmole/gm, I2: 79%; P = 0.0003) compared to the subjects administered lower dose (SMD: 0.97; 95% CI − 0.57, 2.50 µmole/gm, I2: 83%; P = 0.22). The study subjects showed a remarkable rise in butanoic acid concentration in the high-fat diet-induced obesity model (SMD: 3.34; 95% CI 1.65, 5.03µmole/gm, I2: 93%; P = 0.0001) compared to other model types (SMD: 0.17; 95% CI − 0.49, 0.83µmole/gm, I2: 72%; P = 0.61).
Table 6.
All-inclusive, high-influencer subtracted and sub-group [intervention duration, dose, and animal model type] analysis to understand the effects of anthocyanins on the Butanoic acid.
| Study/ Subgroup type | SMD | 95% CI | P value | I2[%] |
|---|---|---|---|---|
| Study duration | ||||
| < 4 weeks | 0.93 | − 0.80, 2.56 | 0.29 | 91 |
| After removing Highly influencing studies | 0.82 | 0.30, 1.34 | 0.002 | 0 |
| ≥ 4 weeks | 1.81 | 0.90, 2.73 | 0.0001 | 90 |
| After removing Highly influencing studies | 1.30 | 0.36, 2.25 | 0.007 | 89 |
| Dose of anthocyanin | ||||
| Lower Dose | 0.31 | − 1.27, 1.89 | 0.70 | 90 |
| After removing Highly influencing studies | 0.97 | − 0.57, 2.50 | 0.22 | 83 |
| Higher Dose | 3.08 | 1.50, 4.66 | 0.0001 | 86 |
| After removing Highly influencing studies | 3.32 | 1.53, 5.11 | 0.0003 | 79 |
| Study model type | ||||
| High fat diet model | 3.64 | 2.31, 4.97 | 0.00001 | 92 |
| After removing Highly influencing studies | 3.34 | 1.65, 5.03 | 0.0001 | 93 |
| Other models [Western diet, tumour, colitis] | 0.33 | − 0.57, 1.24 | 0.47 | 86 |
| After removing Highly influencing studies | 0.17 | − 0.49,0.83 | 0.61 | 72 |
Pooled effect sizes and 95% CI were determined using Random effects model.
SMD Standardised mean difference, I2 Heterogeneity.
A remarkable rise in propionic acid was observed in the studies followed for a longer period i.e., ≥ 4 weeks of anthocyanin intervention (SMD: 2.40, 95% CI 1.34, 3.47 µmole/gm; I2: 90%; P = 0.0001) compared to the studies followed for less than 4 weeks (SMD: − 0.08, 95% CI − 1.22, 1.06 µmole/gm; I2: 77%; P = 0.89) (Table 7; Supplemental Fig. 10 and 11). The study subjects showed a significant rise in propionic acid when a higher dose was supplemented (SMD: 4.15, 95% CI 0.73, 7.57 µmole/gm; I2: 90%; P = 0.02) compared to the lower dose (SMD: 2.03, 95% CI 0.16, 3.91 µmole/gm; I2: 83%; P = 0.03). The propionic acid levels were significantly increased in the subjects with high-fat diet-induced obesity (SMD: 4.60, 95% CI 2.30, 6.90 µmole/gm; I2: 95%; P = 0.0001) in comparison to other study model type (SMD: 0.19, 95% CI − 0.56, 0.93 µmole/gm; I2: 79%; P = 0.62).
Table 7.
All-inclusive, high-influencer subtracted and sub-group [intervention duration, dose, and animal model type] analysis to understand the effects of anthocyanins on the propionic acid.
| Study/ Subgroup type | SMD | 95% CI [µmole/gm] | P value | I2[%] |
|---|---|---|---|---|
| Study duration | ||||
| < 4 weeks | 1.52 | 0.01, 3.03 | 0.05 | 90 |
| After removing Highly influencing studies | − 0.08 | − 1.22, 1.06 | 0.89 | 77 |
| ≥ 4 weeks | 2.82 | 1.74, 3.91 | 0.00001 | 92 |
| After removing Highly influencing studies | 2.40 | 1.34, 3.47 | 0.00001 | 90 |
| Dose of anthocyanin | ||||
| Lower Dose | 2.39 | 0.48, 4.30 | 0.01 | 90 |
| After removing Highly influencing studies | 2.03 | 0.16, 3.91 | 0.03 | 83 |
| Higher Dose | 4.24 | 1.60, 6.87 | 0.002 | 93 |
| After removing Highly influencing studies | 4.15 | 0.73, 7.57 | 0.02 | 90 |
| Study model type | ||||
| High fat diet model | 5.27 | 3.38, 7.16 | 0.00001 | 94 |
| After removing Highly influencing studies | 4.60 | 2.30, 6.90 | 0.0001 | 95 |
| Other models [Western diet, tumour, colitis] | 0.65 | − 0.50, 1.45 | 0.11 | 84 |
| After removing Highly influencing studies | 0.19 | − 0.56, 0.93 | 0.62 | 79 |
Pooled effect sizes and 95% CI were determined using Random effects model.
SMD Standardised mean difference, I2 = Heterogeneity.
The data quality of the SCFA meta-analysis was also evaluated by Grade Tool (Supplemental Fig. 12) and showed moderate heterogeneity that is serious inconsistency.
Publication bias
The publication bias in our study was predicted by applying Egger’s test for a regression intercept and Begg and Mazumdar’s test for rank correlation by using the Trim-Fill method. The funnel plot for Figure S1 shows no evidence of publication bias. For FIR/BAC ratio Egger’s test for a regression intercept gave a p-value of 0.6873. Begg and Mazumdar’s test for rank correlation showed a p-value of 0.6612, indicating no evidence of publication bias. In the case of SCFA, the p-value of Egger’s test for a regression intercept and Begg and Mazumdar’s test for rank correlation is > 0.05, which indicates no publication bias exists in the studies (Table 8 and Supplemental Fig. 13A–D).
Table 8.
Data stability analysis of the studies using Egger’s regression and Beggs test.
| Treatment | SMD | Reg.P | Begg.P |
|---|---|---|---|
| FIR/BAC | − 0.1051 | 0.6873 | 0.6612 |
| Acetic Acid | 0.7288 | 0.7827 | 0.9700 |
| Butanoic Acid | 0.7407 | 0.7498 | 0.6072 |
| Propionic Acid | 0.0418 | 0.9816 | 0.9715 |
*The test is not significant [Reg.P < 0.001], indicating funnel plot asymmetry.
Research trends related to anthocyanin
To depict the active collaborations in anthocyanin, gut microbiota, and SCFA research, we tried to detect the network level among the authors (Supplemental Fig. 14A,B). We selected authors with minimum criteria of 10 articles in the chosen field and observed 14 clusters represented in the author network. Out of 46,427 authors, 168 fulfilled the minimum criteria. Supplemental Fig. 14A represents the network visualization among authors, while Supplemental Fig. 14B represents the overlay visualization year-wise work. It indicates that Chen, and Zhang, are the leading researcher in anthocyanin, gut microbiota, and SCFA-related studies, with 37 and 35 articles. Most research work relevant to anthocyanin and gut studies has been carried out recently, i.e., between 2018 and 2022 (Supplemental Fig. 14B).
We also attempted to track the institution and department collaborations through visualization analysis (Supplemental Fig. 15A,B). Out of 21,846 organizations, only 16 met the threshold criteria, i.e., each with a minimum of two articles. These constituted 5 clusters (Supplemental Fig. 15A). This analysis shows that the microbiology laboratory at Wageningen University in the Netherlands published the most articles18, followed by the State key laboratory of animal nutrition at China Agricultural University in Beijing, which published 17 papers. Both are among the top institutions working on anthocyanin and gut microbiota (Supplemental Fig. 15A).
On the other hand, when we performed the independent analysis of the same organizations, 905 out of 21,846 fulfilled the criteria. The results reveal the same observations, even with no linkages (Supplemental Fig. 15A,B). On visualizing the year-wise work of organizations, it depicted that most of the collaborative studies were carried out in the 2014–2016 year by top working institutes, and independent research was carried out in recent years (Supplemental Fig. 15B and 14B).
Discussion
Edible parts of plants carry several health promoting compounds like, proteins, minerals, vitamins and coloured anthocyanins70–72. Numerous studies have discovered the health-promoting properties of anthocyanin-rich foods. Anthocyanins have anti-obesity properties, as they help to maintain energy balance and satiety while inhibiting the accumulation of body fat and the development of insulin resistance, dyslipidemia, and inflammation73,74. A diet of anthocyanin-rich fruits and vegetables substantially influences the gut flora13,75. After being consumed, anthocyanins have limited bioavailability in the body due to their resistance to complete absorption. Five percent to ten percent of total polyphenol consumption is absorbed in the small intestine. More importantly, most dietary anthocyanins arrive intact in the colon, where they may interact with the microbiota and undergo biotransformation before being absorbed via the intestinal mucosa76. This systematic review and meta-analysis demonstrated that dietary anthocyanin supplementation profoundly improves rodent models' gut health biomarkers (Fir/Bac and SCFAs). This finding was supported by studies carried out after cut off time limit of this studies77–81.
Several studies have shown that obesity is associated with the gut microbiome, which differs between obese and lean animals. The gut health biomarker Fir/ Bac ratio is relevant in human gut microbiota composition. According to certain research articles, the Fir/Bac ratio is a defining characteristic of obesity. Current meta-analyses revealed that anthocyanins effectively reduced the Fir/Bac ratio and mitigated the gut dysbiosis induced by high-fat diet-induced obesity and other factors. Anthocyanin intervention time and dose had a substantial impact on the Fir/Bac ratio in a variety of ways. The impact was more pronounced when the anthocyanin-rich diet was followed for a more extended period and at larger dosages. Our data analysis from rodent models will also help future investigators with the utility of rodent research in understanding the effect of anthocyanins on human models and planning such clinical trials.
Gut health biomarker SCFAs also have significant relevance in human gut microbiota composition. The healthy gut microbiota metabolizes indigestible dietary components to SCFAs82,83. The present meta-analysis of laboratory studies on rodents found that anthocyanin-rich diet interventions efficiently improved the gut's SCFAs, including acetic, propionic, and butyric acid profiles. Here also, the longer duration of the anthocyanin-rich diet intervention was more efficient in enhancing the levels of all three main SCFAs. Similarly, the higher dosage of the anthocyanin-rich food intervention was more effective. Aside from that, anthocyanins had more significant impacts on the concentrations of all SCFAs in high-fat diet-induced obesity models than in other disease models.
During meta-analysis, it was observed that a few studies with wider cumulative interval values had more influence on the overall results than a large number of normal studies. Therefore, additional analysis was carried out after removing such studies. Thus, all the analyses were carried out without such studies, and we recommend the same. This improved the outcomes of the meta-analysis. We also noticed substantial methodological and experimental variances in the research. Animal care procedures, oral dosing, and water purification protocols are some examples of unbiased observed variables that must be recorded. Since these factors significantly affect therapy outcomes9.
Publication bias is an important parameter in meta-analysis. It includes time lag, duplication, outcome reporting, linguistics, etc. Many electronic databases are examined to eliminate the likelihood of publication bias. To eliminate data supply bias, we employ individual searches and extractions. Participant differences, as well as the intervention's intensity and duration, all contributed to variability. The individuals' health, other therapies they were receiving simultaneously, supplement doses and contents, follow-up durations, treatment modalities, and so on all differed significantly among the trials. These variations may have had a significant role in the funnel plot's original asymmetry. The appearance of an asymmetrical funnel plot is purely coincidental84,85. The Trim-Fill correction method made minor changes to all studies, and associated funnel plots revealed a symmetrical distribution of SE and SMD with p-values greater than 0.05. The funnel plot indicated that the studies chosen for our research are not biased. Additionally, both Begg's and Egger's tests produced non-significant P-values [P > 0.05], further supporting the non-existence of any substantial systematic publishing bias in our study. It has also been observed that the discrepancy displayed by the GRADE tool is significant only when it affects confidence in the results concerning a specific decision. Even if the inconsistency is significant, it may still maintain confidence in the conclusion of a particular decision86.
The variability is significant, but the disparities between small and large treatment effects could be the source of the substantial heterogeneity.
For the first time, a comprehensive meta-analysis of the influence of anthocyanins on the Fir/Bac ratio and the concentrations of three main SCFAs, acetic acid, propionic acid, and butanoic acid, was performed. Bibliographic coupling analysis of leading researchers and institutes indicated that most research work relevant to anthocyanin and gut studies had recently been carried out in animal models, i.e., between 2018 and 2022. It is envisaged that several such human studies will be published in the near future to validate that current finding.
However, some important qualifiers to this study should be mentioned. As a limitation, PROSPERO, a central international database platform that helps to eliminate data duplication and reduces the chance for reporting bias by permitting comparison of the finished review with what was planned in the protocol, was not notified that this study was being conducted. Furthermore, the substantial amount of missing data for published studies and the exclusion of studies with incomplete data diminish the statistical power of our meta-analysis.
Supplementary Information
Acknowledgements
The authors are thankful to Dr. Humira Sonah for providing valuable data analysis advice. Authors are also thankful to Computational Biology Lab Facility provided by NABI-CDAC collaboration and DeLcO library facility. Authors are also thankful to National Agri-Food Biotechnology Institute Mohali, DBT, GOI for providing fellowships.
Abbreviations
- Fir/Bac
Firmicutes/Bacteroides
- SCFAs
Short chain fatty acids
- SMD
Standardized mean difference
- CI
Confidence interval
Author contributions
M.G.—Conceptualization; Project administration; Supervision and review; P.K., A.T. and S.S.-Data curation; Formal analysis, Investigation and Methodology; P.K.—original draft; A.T.—Methodology and review; S.S.—review and editing. V.T., B.S. and U.A. rechecked the data and, reviewed the paper and references. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data we used can be found in the references listed and also given in the attached supplementary files. All the figures represented in this manuscript have been produced by authors itself.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-28764-0.
References
- 1.Gutiérrez-Del-Río I, et al. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants. 2021;10(8):1264. doi: 10.3390/antiox10081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vauzour D, Rodriguez-Mateos A, Corona G, Jose Oruna-Concha M, Spencer JPE. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients. 2010;2:1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. 2020;25(17):3809. doi: 10.3390/molecules25173809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlos S, Nikiforou C. Medicinal plants against obesity: A met-analysis of literature. J. Complem. Med. Res. 2022;12(4):244–244. doi: 10.5455/jcmr.2021.12.04.36. [DOI] [Google Scholar]
- 5.Roy S, Rhim JW. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021;61(14):2297–2325. doi: 10.1080/10408398.2020.1776211. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015;145(4):742–748. doi: 10.3945/jn.114.205674. [DOI] [PubMed] [Google Scholar]
- 7.Naseri R, et al. Anthocyanins in the management of metabolic syndrome: A pharmacological and biopharmaceutical review. Front Pharmacol. 2018;4(9):1310. doi: 10.3389/fphar.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayarathne S, et al. Protective effects of anthocyanins in obesity-associated inflammation and changes in gut microbiome. Mol. Nutr. Food Res. 2019;63(20):e1900149. doi: 10.1002/mnfr.201900149. [DOI] [PubMed] [Google Scholar]
- 9.Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe. 2019;26(2):265–272. doi: 10.1016/j.chom.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jandhyala SM, et al. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21(29):8787. doi: 10.3748/wjg.v21.I29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibbò S, et al. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016;20(22):4742–4749. [PubMed] [Google Scholar]
- 12.Anhê FF, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2014;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 13.Jamar G, Estadella D, Pisani LP. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors. 2017;43(4):507–516. doi: 10.1002/biof.1365. [DOI] [PubMed] [Google Scholar]
- 14.Bravo JA, et al. Communication between gastrointestinal bacteria and the nervous system. Curr. Opin. Pharmacol. 2012;12(6):667–672. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26(1):26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young VB. The role of the microbiome in human health and disease: An introduction for clinicians. BMJ. 2017;356:j831. doi: 10.1136/bmj.j831. [DOI] [PubMed] [Google Scholar]
- 17.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in lactobacillus in obese patients and methanogens in anorexic patients. PLoS ONE. 2009;4(9):e7125. doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Ringø E, Deborah GS, Rahiman KM, Hatha AA. Enterobacter hormaechei bac 1010 from the gut of flathead grey mullet as probable aquaculture probiont. J. Nat. Sci. Sustain. Tech. 2011;5(3):189. [Google Scholar]
- 20.Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamer HM, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nut. 2009;28(1):88–93. doi: 10.1016/j.clnu.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo M, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012;60(15):3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y, et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lyciumruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019;136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Tian B, et al. Lyciumruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food Res. 2021;65(8):2000745. doi: 10.1002/mnfr.202000745. [DOI] [PubMed] [Google Scholar]
- 25.Faria A, Fernandes I, Norberto S, Mateus N, Calhau C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014;62(29):6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 26.Kim KN, Yao Y, Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: A systematic review and meta-analysis. Nutrients. 2019;11(10):2512. doi: 10.3390/nu11102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma G, Chen Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods. 2020;66:103829. doi: 10.1016/j.jff.2020.103829. [DOI] [Google Scholar]
- 28.Fallah AA, Sarmast E, Jafari T. Effect of dietary anthocyanins on biomarkers of oxidative stress and antioxidative capacity: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods. 2020;68:103912. doi: 10.1016/j.jff.2020.103912. [DOI] [Google Scholar]
- 29.Yang L, et al. Effects of anthocyanins on cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2017;8(5):684–693. doi: 10.3945/an.116.014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Res. Meth. Rep. 2021;10(1):1–1. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.du Sert NP, et al. The arrive guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;40(9):1769–1777. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez J, Díaz J, Garcia-Martin J, Tabuenca B. Systematic literature reviews in software engineering—Enhancement of the study selection process using Cohen’s kappa statistic. J. Syst. Softw. 2020;168:110657. doi: 10.1016/j.jss.2020.110657. [DOI] [Google Scholar]
- 33.Tena N, Martín J, Asuero AG. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants. 2020;9(5):451. doi: 10.3390/antiox9050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L, Aloe AM. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat. Med. 2021;40:403–426. doi: 10.1002/sim.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lortie CJ. Doing meta-analysis with R: A hands-on guide. J. Stat. Softw. 2022;102:1–4. doi: 10.18637/jss.v102.b02. [DOI] [Google Scholar]
- 36.von Hippel PT. The heterogeneity statistic I 2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015;15(1):1–8. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderWeele TJ, Knol MJ. Interpretation of subgroup analyses in randomized trials: Heterogeneity versus secondary interventions. Ann. Intern. Med. 2011;154(10):680–683. doi: 10.7326/0003-4819-154-10-201105170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cochrane. Review Manager (RevMan) (Computer program). Version 5.4. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration. 2020.
- 39.RStudio Team. RStudio | Open source & professional software for data science teams - RStudio. RStudio Inc. 2020.
- 40.Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: The GRADE approach. Res. Synth. Methods. 2019;10(3):312–329. doi: 10.1002/jrsm.1313. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test measures of funnel plot asymmetry. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1088–101 (1994). [PubMed]
- 43.Diez-Echave P, et al. The prebiotic properties of Hibiscus sabdariffa extract contribute to the beneficial effects in diet-induced obesity in mice. Food Res. Int. 2020;127:108722. doi: 10.1016/j.foodres.2019.108722. [DOI] [PubMed] [Google Scholar]
- 44.Han X, et al. Grape extract activates brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Mol. Nut. Food Res. 2020;64(10):2000149. doi: 10.1002/mnfr.202000149. [DOI] [PubMed] [Google Scholar]
- 45.Marques C, et al. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-29744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong H, et al. Probiotic-fermented blueberry juice prevents obesity and hyperglycemia in high fat diet-fed mice in association with modulating the gut microbiota. Food Funct. 2020;11:9192. doi: 10.1039/D0FO00334D. [DOI] [PubMed] [Google Scholar]
- 47.Heyman-Lindén L, et al. Lingonberries alter the gut microbiota and prevent low-grade inflammation in high-fat diet fed mice. Food Nutr. Res. 2016;60(1):29993. doi: 10.3402/fnr.v60.29993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodríguez-Daza MC, et al. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020;10(1):1–6. doi: 10.1038/s41598-020-58863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin YC, et al. Purple-leaf tea [Camellia sinensis L.] ameliorates high-fat diet-induced obesity and metabolic disorder through the modulation of the gut microbiota in mice. BMC Complem. Med. Ther. 2020;20(1):1–2. doi: 10.1186/s12906-020-03171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakano H, et al. Bilberry anthocyanins ameliorate NAFLD by improving dyslipidemia and gut microbiome dysbiosis. Nutrients. 2020;12:1–16. doi: 10.3390/nu12113252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, et al. Dietary supplementation of black rice anthocyanin extract regulates cholesterol metabolism and improves gut microbiota dysbiosis in C57BL/6J mice fed a high-fat and cholesterol diet. Mol. Nutr. Food Res. 2020;64(8):1900876. doi: 10.1002/mnfr.201900876. [DOI] [PubMed] [Google Scholar]
- 52.Song H, Shen X, Wang F, Li Y, Zheng X. Black current anthocyanins improve lipid metabolism and modulate gut microbiota in high-fat diet-induced obese mice. Mol. Nutr. Food Res. 2021;65(6):2001090. doi: 10.1002/mnfr.202001090. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Wang L, Jing N, Jiang G, Liu Z. Biostimulating gut microbiome with bilberry anthocyanin combo to enhance antiPD-l1 efficiency against murine colon cancer. Microorganisms. 2020;8(2):175. doi: 10.3390/microorganisms8020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, et al. Effects of acylated and nonacylated anthocyanins extracts on gut metabolites and microbiota in diabetic Zucker rats: A metabolomic and metagenomic study. Food Res. Int. 2022;153:110978. doi: 10.1016/j.foodres.2022.110978. [DOI] [PubMed] [Google Scholar]
- 55.Liu P, Zhou W, Xu W, Peng Y, Yan Y, Lu L, Mi J, Zeng X, Cao Y. 2022. The main anthocyanin monomer from Lycium ruthenicum murray fruit mediates obesity via modulating the gut microbiota and improving the intestinal barrier. Foods. [DOI] [PMC free article] [PubMed]
- 56.Cremonini E, et al. Anthocyanins protect the gastrointestinal tract from high fat diet-induced alterations in redox signaling, barrier integrity and dysbiosis. Redox. Biol. 2019;26:101269. doi: 10.1016/j.redox.2019.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, et al. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6 J mice fed with a high-fat diet. Eur. J. Nutr. 2021;60:2735–2746. doi: 10.1007/s00394-020-02446-3. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, et al. Jamun (Eugenia jambolana Lam.) fruit extract prevents obesity by modulating the gut microbiome in high-fat-diet-fed mice. Mol. Nutr. Food Res. 2019;63(9):e1801307. doi: 10.1002/mnfr.201801307. [DOI] [PubMed] [Google Scholar]
- 59.Liu D, et al. Purple sweet potato anthocyanin extract regulates redox state related to gut microbiota homeostasis in obese mice. J. Food Sci. 2022;87:2133–2146. doi: 10.1111/1750-3841.16130. [DOI] [PubMed] [Google Scholar]
- 60.Kapoor P, et al. Anthocyanin biofortified colored wheat modifies gut microbiota in mice. J. Cereal Sci. 2022;104:103433. doi: 10.1016/j.jcs.2022.103433. [DOI] [Google Scholar]
- 61.Morissette A, et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2020;318:965–980. doi: 10.1152/ajpendo.00560.2019. [DOI] [PubMed] [Google Scholar]
- 62.Hu TG, et al. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019;10:1513–1528. doi: 10.1039/C9FO00132H. [DOI] [PubMed] [Google Scholar]
- 63.Kaur A, et al. Montmorency tart cherry supplementation improved markers of glucose homeostasis but has modest effects on indicators of gut health in mice fed a Western diet. Nutr. Res. 2022;99:66–77. doi: 10.1016/j.nutres.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Wu T, et al. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018;9:2112–2120. doi: 10.1039/C7FO02061A. [DOI] [PubMed] [Google Scholar]
- 65.Su H, et al. Pelargonidin-3- O-glucoside derived from wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. J. Agric. Food Chem. 2020;68(46):13025–13037. doi: 10.1021/acs.jafc.9b03338. [DOI] [PubMed] [Google Scholar]
- 66.da Silva-Maia JK, et al. Aqueous extract of berry (Plinia jaboticaba) byproduct modulates gut microbiota and maintains the balance on antioxidant defense system in rats. J Food Biochem. 2019;43:e12705. doi: 10.1111/jfbc.12705. [DOI] [PubMed] [Google Scholar]
- 67.da Silva-Maia JK, et al. Aqueous Extract of Brazilian Berry (Myrciaria jaboticaba) Peel Improves Inflammatory Parameters and Modulates Lactobacillus and Bifidobacterium in Rats with Induced-Colitis. Nutrients. 2019;11:2776. doi: 10.3390/nu11112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, et al. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. J Nutr. 2018;148(2):209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng Y, et al. Effects of long-term intake of anthocyanins from Lycium ruthenicum Murray on the organism health and gut microbiota in vivo. Food Res Int. 2020;130:108952. doi: 10.1016/j.foodres.2019.108952. [DOI] [PubMed] [Google Scholar]
- 70.Garg M, Rao YS, Goyal A, Singh B. Variations in seed storage protein triticin among diploid Triticum and Aegilops species. Biotechnology. 2007;6(3):444–446. doi: 10.3923/biotech.2007.444.446. [DOI] [Google Scholar]
- 71.Garg M, Singh H, Kaur H, Dhaliwal HS. Genetic control of high protein content and its association with bread-making quality in wheat. J. Plant Nutr. 2006;29(8):1357–1369. doi: 10.1080/01904160600830134. [DOI] [Google Scholar]
- 72.Sharma N, et al. Anthocyanin biofortified black, blue and purple wheat exhibited lower amino acid cooking losses than white wheat. LWT. 2022;154:112802. doi: 10.1016/j.lwt.2021.112802. [DOI] [Google Scholar]
- 73.Sivamaruthi BS, Kesika P, Chaiyasut C. The influence of supplementation of anthocyanins on obesity-associated comorbidities: A concise review. Foods. 2020;9(6):687. doi: 10.3390/foods9060687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma S, et al. Anthocyanin-biofortified colored wheat prevents high fat diet-induced alterations in mice: Nutrigenomics studies. Mol. Nutr. Food Res. 2020;64(13):1900999. doi: 10.1002/mnfr.201900999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T, et al. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food Funct. 2018;9(4):2112–2120. doi: 10.1039/c7fo02061a. [DOI] [PubMed] [Google Scholar]
- 76.Gonçalves AC, Nunes AR, Falcão A, Alves G, Silva LR. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals. 2021;14(7):690. doi: 10.3390/ph14070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen J, et al. Evaluation of antioxidant capacity and gut microbiota modulatory effects of different kinds of berries. Antioxidants. 2022;11(5):1020. doi: 10.3390/antiox11051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, et al. Purple sweet potato anthocyanin extract regulates redox state related to gut microbiota homeostasis in obese mice. J. Food Sci. 2022;87(5):2133–2146. doi: 10.1111/1750-3841.16130. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, et al. Anthocyanins from opuntia ficus-indica modulate gut microbiota composition and improve short-chain fatty acid production. Biology. 2022;11(10):1505. doi: 10.3390/biology11101505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mo J, et al. Mulberry anthocyanins ameliorate DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Antioxidants. 2022;11(9):1674. doi: 10.3390/antiox11091674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T, et al. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022;397:133768. doi: 10.1016/j.foodchem.2022.133768. [DOI] [PubMed] [Google Scholar]
- 82.Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015;74(1):13–22. doi: 10.1017/S0029665114001463. [DOI] [PubMed] [Google Scholar]
- 83.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem. J. 2017;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Light JR, Pillemer BD. Summing Up: The Science of Reviewing Research Harvard University Press: Cambridge, MA, 1984, xiii+191 pp. Educ. Res. 1986;15(8):16–17. doi: 10.3102/0013189X015008016. [DOI] [Google Scholar]
- 85.Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat. Med. 2003;22(13):2113–2126. doi: 10.1002/sim.1461. [DOI] [PubMed] [Google Scholar]
- 86.Guyatt GH, et al. GRADE guidelines: 7: Rating the quality of evidence—inconsistency. J. Clin. Epidemiol. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Liu P, Zhou W, Xu W, Peng Y, Yan Y, Lu L, Mi J, Zeng X, Cao Y. 2022. The main anthocyanin monomer from Lycium ruthenicum murray fruit mediates obesity via modulating the gut microbiota and improving the intestinal barrier. Foods. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data we used can be found in the references listed and also given in the attached supplementary files. All the figures represented in this manuscript have been produced by authors itself.