Abstract
Paroxetine and Sertraline are the only medications approved in posttraumatic stress disorder (PTSD). However, about 60% of traumatized patients fail to show an adequate clinical response. Second generation antipsychotics are recommended as second-line monotherapy or third-line augmentation strategies and quetiapine appears as one of the most used and promising agents. Up to date, no reviews assessed the efficacy of quetiapine in the treatment of PTSD. We aimed to assess the effectiveness and general safety of quetiapine on PTSD. A systematic review was conducted following Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) and Cochrane guidelines, selecting studies that evaluated the efficacy of quetiapine on global or specific PTSD symptomatology. Ten studies (n = 894) were considered eligible for qualitative synthesis: one case report, one case series, one prospective cohort study, 3 open-label trials, 3 retrospective studies, one randomized controlled trial. Quetiapine was effective on global PTSD symptomatology assessed in 6 studies as well as on re-experiencing (4/4 studies), avoidance (4/3 studies) and hyperarousal (4/4 studies), flashbacks (2/2 studies), depressive (4/4 studies), anxiety (1/1 studies), psychotic (3/3 studies), insomnia (4/5 studies), nightmares (3/3 studies) specific symptoms and PTSD domains. Sedation was among the most frequently observed adverse effects and the main cause of drug discontinuation. Preliminary findings support the efficacy of quetiapine in ameliorating symptoms relative to PTSD and its overall safety. However, quetiapine use in PTSD cannot be recommended yet as studies mainly rely on open-label, retrospective studies or case series.
Keywords: PTSD, Drug resistance, Quetiapine, Pharmacotherapy
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a common mental disorder consequence of exposure to traumatic events. Its symptoms consist of 4 major diagnostic clusters: intrusion/re-experiencing symptoms, avoidance symptoms, negative cognitions, symptoms of hyperarousal [1,2]. Psychotherapy and pharmacotherapy recommendations differ across PTSD treatment guidelines. In a recent systematic review of 14 guidelines, all of them recommend cognitive behavior therapy (CBT) as a first-line psychological treatment while 6 guidelines recommend Eye Movement Desensitization and Reprocessing (EMDR) therapy. Thirteen guidelines recommend an selective serotonin reuptake inhibitors (SSRI) as first-line pharmacological option for PTSD and 10 include venlafaxine as a first-line pharmacological treatment option [3]. However, SSRIs are associated with an overall response rate of ≈ 60% and only ≈ 25% of patients achieve a complete remission [4]. For this reason, several studies evaluated the efficacy of alternative and combination treatments to enhance the therapeutic response [5]. For instance, some guidelines recommend antipsychotics as second-line monotherapy or third-line augmentation strategies, others recommend avoiding their use [6]; however, quetiapine appears to be one of the more promising, investigated and likely more used agents in PTSD. From 1999 to 2018, quetiapine remained the most commonly prescribed antipsychotic in Veterans Health Administration care [7-9]. This primacy is confirmed also by large sample studies on traumatized civilians in which quetiapine prescription rate achieved 25−30% [10,11]. Although off-label quetiapine is widely prescribed in clinical practice and its efficacy in treating PTSD symptoms has been described, to the best of our knowledge no systematic collection of the available findings has yet supported or disconfirmed clinical recommendations [6]. The aim of our study is hence to evaluate the efficacy of quetiapine in the treatment of PTSD through a systematic review of the literature. As a secondary outcome, reports concerning adverse effects of quetiapine in the selected studies will be collected and discussed.
MATERIAL AND METHODS
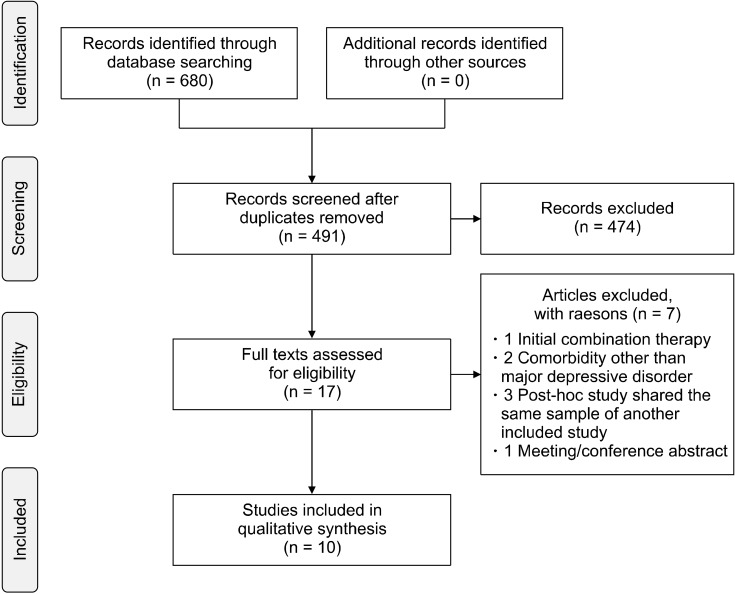
We conducted a systematic review of the literature available between 1998 and 22 February 2022. PubMed and Web of Science (all databases) were searched using the following search builder: (quetiapine AND (PTSD or posttrauma* or trauma* or combat or stress)). Population, Intervention, Comparison, Outcomes and Study (PICOS) design criteria [12] for study selection were applied and are reported in Table 1. Articles not in English were ex-cluded. Due to heterogeneous designs of the available studies and the complex clinical phenomenology of this mental disorder, we also included non-randomized controlled trial (RCT) studies to better report on a broader field of research. A total of 680 (437 Web of Science, 243 PubMed) items were retrieved from the search databases and reference cross-check. Duplicates (189) were re-moved. The remaining studies were independently evaluated by 2 reviewers (C.C. and C.A.) and included or excluded after reaching a final consensus. Quality of studies was evaluated by means of AMSTAR 2 scores, a 16-item assessment tool to check the quality of a systematic review: High (no or one non-critical weakness), Moderate (more than one non-critical weakness), Low (one critical flaw with or without non-critical weaknesses), Critically low (more than one critical flaw with or without non-critical weaknesses) [13]. Figure 1 reports the Preferred Re-porting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart regarding the different phases of this review [14].
Table 1.
PICOS criteria for study selection
| Parameter | Inclusion | Exclusion |
|---|---|---|
| Patients | Diagnosis of PTSD; Age ≥ 18 years | Presence of psychiatric comorbidities except major depressive disorder |
| Intervention | Quetiapine, any dose as monotherapy or augmenting agent (sequential therapy) | Initial combination therapy including quetiapine and other drugs |
| Comparator | None or placebo or other active treatments | |
| Outcomes | Quantitative or qualitative evaluation of overall or specific symptoms of PTSD | |
| Study design | Case reports, case series, retrospective, prospective, cohort, cross sectional studies, randomized and non-randomized controlled trial | Reviews, expert opinion, comments, conference report, post-hoc studies, study published in any other language than English |
PICOS, Population, Intervention, Comparison, Outcomes and Study; PTSD, posttraumatic stress disorder.
Fig. 1.
PRISMA flowchart of infor-mation through the different phases of the review.
RESULTS
Effectiveness
Ten studies (n = 894) with different designs and population (7 military and 3 civilian samples) were included in this systematic review (Table 2). In a case report of a 49-year-old patient with resistant PTSD, the addition of modest dosage of quetiapine to 40 mg/d of paroxetine resulted in the improvement of PTSD symptoms [15]. In a case series of 5 patients (civilian; 60% male; mean duration treatment “not available”) the addition of quetiapine 150−200 mg/d to a stable SSRI/SNRI and gabapentin combination therapy markedly reduced persistent flashbacks [16]. In an open-label trial (n = 20 veterans; 95% male; mean age of 53 years; mean duration treatment of 6 weeks; “Moderate” quality according to the AMSTAR 2 tool), the addition of quetiapine 25−300 mg/d to a psychotropic medication (except antipsychotics and at constant dose for at least 1 month before baseline visit) provided a significant improvement on global PTSD severity by week 2, and specifically on re-experiencing domains as well on insomnia, depression, and negative/positive psychotic symptoms [17]. In another open-label trial (n = 15 civilians; 100% male; mean age of 49 years, mean duration treatment of 8 weeks; “Moderate” quality according to the AMSTAR 2 tool) the addition of quetiapine 100−400 mg/d to a stable SSRI therapy provided a significant improvement on global PTSD severity and on re-experiencing, hyperarousal and avoidance domains, depression and insomnia [18]. In a prospective cohort study (n = 270 veterans, 97% male; mean age of 53,7 years; “Low” quality according to the AMSTAR 2 tool) the addition of quetiapine 25−600 mg/d or prazosin 1−25 mg/d to a psychotropic medication for treating nighttime symptoms was evaluated as measured by continued therapy for 0−6 months and 3−6 years. While short-term effectiveness was comparable, patients in the quetiapine group were significantly more likely to discontinue long-term therapy to the study end date (48.4% vs. 24%) due to adverse effects or ineffectiveness [19]. In a retrospective study (n = 327 veterans, 95% male; mean age of 53.7 years; mean duration treatment of 4 years; “Low” quality according to the AMSTAR 2 tool) the efficacy of various medications used to treat nightmares was evaluated. The three most prescribed agents as monotherapy were: prazosin 32.4%, risperidone 24.7% and quetiapine 22%. Efficacy was determined using three levels of response (absent, partial and full response). Quetiapine administered at a dosing range of 12.5−800 mg/d obtained a success rate of 50% while prazosin and risperidone had a success rate of 49% and 77% respectively [20]. In an open-label trial quetiapine monotherapy 25−400 mg/d (n = 53 veterans, 100% male; mean age of 55 years; mean duration treatment of 6 weeks; “Moderate” quality according to the AMSTAR 2 tool) provided a significant improvement on global PTSD symptoms severity, on re-experiencing, avoidance and hyperarousal domains and on negative and positive psychotic symptoms [21]. In a retrospective study the addition of quetiapine 25−700 mg/d was evaluated in 68 resistant veterans with PTSD (100% male; mean age of 55 years; mean duration treatment of 25 weeks; “Moderate” quality according to the AMSTAR 2 tool), 74% of patients were judged much or very much improved in at least one refractory symptom of PTSD: re-experiencing, avoidance/numbing, arousal, sleep disturbance, nightmares, depressed mood, flashbacks [22]. The only RCT (n = 80 veterans, 100% male; mean age of 53 years; mean duration treatment of 12 weeks; “Moderate” quality according to the AMSTAR 2 tool) conducted on quetiapine in PTSD demonstrated that quetiapine monotherapy 50−800 mg/d was more effective than placebo on global PTSD symptoms severity and on re-experiencing and hyperarousal domains as well on anxiety, depression, and positive psychotic symptoms, but not on the avoidance/ numbing domains and insomnia [23]. In an observational retrospective study of 50 resistant PTSD veterans (96% male; mean duration treatment of 10−12 weeks; “Low” quality according to the AMSTAR 2 tool) and declined trauma-focused therapy, 21 over 24 patients taking quetiapine 50−400 mg/d and 0 over 11 taking either risperidone (1−4 mg/d) or valproate (500−2,000 mg/d) monotherapy, were engaged in treatment and completed it. Quetiapine was also associated to greater improvements in sleep interruptions and nightmares [24]. Regarding concomitant psychotherapy, initiation or change in psychotherapy within 3 months of randomization was an exclusion criterion in Villarreal et al. [23], need for concurrent psychotherapy was an exclusion criterion in Pivac and Kozarić-Kovacić [21]. No concomitant psychotherapy was provided in Filteau et al. [16], Hamner et al. [17], Ahearn et al. [18], Byers et al. [19], Detweiler et al. [20], Sokolski et al. [22]. In Sattar et al. [15], the patient that at first declined group therapy was engaged in psychosocial treatment 3 days after quetiapine initiation.
Table 2.
Descriptive comparison between studies considered
| Source | Study design (add-on or monotherapy) |
Sample and sex (civilians or veterans) |
Range (and mean dose) (mg/d) | Duration | Effectiveness - changes from baseline assessment scores | AMSTAR 2 |
|---|---|---|---|---|---|---|
| Sattar et al. [15], 2002 | Case report (add-on to paroxetine) | 1 (100% male) (civilian) | 150 | 12 months | QTP associated with marked improvements in HAM-D and CAPS scores | NA |
| Filteau et al. [16], 2003 | Case series (add-on to SRI and GBP) | 5 (60% male) (civilians) | 150−200 | NA | NA | NA |
| Hamner et al. [17], 2003 | Open-label trial (add-on to psychotropic medication [SRI, anticonvulsants, sleep agents]) | 20 (95% male) (veterans) | 25−300 (100 ± 70) | 6 weeks | QTP associated with significant improvements in HAM-D, PANSS and CAPS scores | Moderate |
| Ahearn et al. [18], 2006 | Open-label trial (add-on to SSRI) | 15 (53% male) (civilians) | 100−400 (216) | 8 weeks | QTP associated with significant improvement in HAM-D, CGI-I/S, PSQI, CAPS scores | Moderate |
| Byers et al. [19], 2010 | Prospective cohort study (add-on to SSRI, sleep agents) | 270 (97% male) (veterans) | QTP 25−600 PRZ 1-25 |
≤ 6 months 3−6 years |
NA | Low |
| Detweiler et al. [20], 2016 | Retrospective study (monotherapy) | 327 (95% male) (veterans) | QTP 12.5−800 PRZ 1−20 RIS 0.25−6.0 |
4 years | NA | Low |
| Pivac and Kozarić-Kovacić [21], 2006 | Open-label trial (monotherapy) | 53 (100% male) (veterans) | 25−400 | 6 weeks | QTP associated with significant improvement in PANS, CGI-S, CAPS scores | Moderate |
| Sokolski et al. [22], 2003 | Retrospective study (add- on to SRI, anticonvulsants, antipsychotics) | 68 (100% male) (veterans) | 25−700 (155 ± 130) | 25 weeks | QTP associated with a marked improvement in CGI-I scores | Moderate |
| Villarreal et al. [23], 2016 | Randomized, double-blind, placebo-controlled trial (monotherapy) | 80 (100% male) (veterans) | 50−800 (258) | 12 weeks | QTP superior to PLC for CGI-S/I, PANSS, HAM-A, HAM-D, CAPS | Moderate |
| Baig et al. [24], 2019 | Retrospective study (add-on to SRI, mood stabilizers, sleep agents) | 50 (96% male) (veterans) | QTP 50−400 (180) VPA 500−2,000 (1,625) RIS 1−4 (2) |
10−12 weeks | NA | Low |
QTP, quetiapine; HAM-D, Hamilton Depression Rating Scale; PTSD, posttraumatic stress disorder; CAPS, Clinician Administered PTSD Scale; SRI, serotonin reuptake inhibitor; GBP, gabapentin; VPA, valproate; PLC, placebo; SSRI, selective serotonin reuptake inhibitors; PANSS, Positive and Negative Syndrome Scale; CGI-I/S, Clinical Global Impression-Improvement/Severity; PSQI, Pittsburgh Sleep Quality Index; PRZ, prazosin; RIS, risperidone; NA, not available.
Reported Adverse Effects
No adverse effects were reported in Sattar et al. [15] and this topic was not evaluated in Filteau et al. [16], Ahearn et al. [18], and Detweiler et al. [20]. In Hamner et al. [17] the most common adverse effects reported were sedation (37%), dizziness and dry mouth (16%), hypersalivation, diarrhea, headache and amblyopia (5.3%), without significant changes in weight, vital sign measurements, or neurologic ratings. In Byers et al. [19] sedation and metabolic effects occurred more frequently in the quetiapine group (21% and 9.1% respectively) than in the prazosin group (1.6% and 0% respectively). Disconti-nuation rate was higher in the quetiapine group compared to prazosin group due to adverse effects, especially sedation (34.9% vs. 17.7%) In Pivac and Kozarić-Kovacić [21] side effects were not evaluated, although a post hoc study sharing the same sample reported the following side effects frequency: hypotension (50.9 %), sedation (39.6%), anticholinergic effect (9.4%) and weight gain (9.4%) [25]. In Sokolski et al. [22] the side effects reported were: asthenia (13.6%), at least one among nausea, dizziness, dry mouth, muscle twitching or weight gain (0.68%); four patients (5.9%) discontinued quetiapine due to excessive sedation. In Villarreal et al. [23] the side effects in the quetiapine group were dry mouth (15.8%), somnolence (13.4%), and sedation (7.4%). Nine patients (11.2%) in the quetiapine and three (3.7%) in the placebo group dropped out because of adverse effects. No significant differences in weight, pulse, or blood pressure measurements occurred between quetiapine and placebo. In Baig et al. [24], the frequency of side effects was not reported although in the risperidone and valproate groups were observed more daytime sedative effects compared to quetiapine group.
DISCUSSION
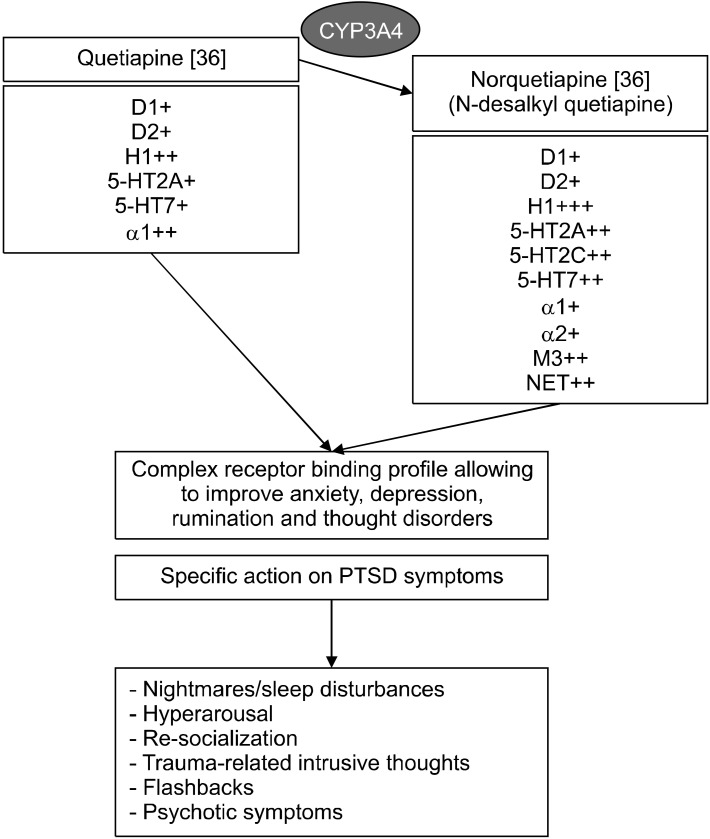
PTSD symptoms make the treatment of this disorder particularly challenging. In addition, different subtypes have been characterized (reexperiencing/hyperaroused, externalizing, internalizing, dissociative, psychotic, high- symptom, combat and noncombat-related subtypes) and are related to different history of disease and prognosis [26-31]. Additionally, several putative factors contribu-ting to SSRI resistance were reported: severity of illness, previous multiple and/or combat traumas, chronicity of illness, male sex, comorbidities [5] and sleep disturbances appear the most associated to refractory symptoms [23]. Despite this and its high heterogeneous clinical presenta-tion, the only medications approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for PTSD are sertraline and paroxetine [32] although other drugs reported significant evidence in the treatment of PTSD [33,34]. The first case report describing use of quetiapine in a patient with PTSD is reported by Sattar and colleagues [15]. In this report as well as in Filteau et al. [16], Hamner et al. [17], Ahearn et al. [18], Sokolski et al. [22], Byers et al. [19], quetiapine was evaluated as augmenting agent (≈ 50% of the whole sample) while in other studies it was evaluated as monotherapy agent [20,21,23,24]. In two studies a comorbidity with major depressive disorder may be present [19,23]. Global PTSD symptomatology was assessed only in 6 articles [15,17,18,21-23] where quetiapine provided a beneficial effect. In four studies quetiapine was effective on specific symptoms and PTSD domains [16,19,20,24]. Nightmares were evaluated in three studies, quetiapine was effective and in two of them comparable or inferior to prazosin [19,20,22]. Quetiapine was effective on re-experiencing, avoidance and hyperarousal domains in three studies [18,21,22] while in one study, the single RCT available [23], quetiapine was also effective on re-experiencing and hyperarousal domains but not on avoidance. Insom-nia was evaluated in five studies and quetiapine was effective in all studies [17,18,22,24] except one [23]. Of note, in a post hoc analysis of the latter study [23] quetiapine was effective on insomnia by week 2 [35]. Quetia-pine was effective on flashbacks, evaluated in two studies [16,22] and in anxiety, evaluated in one study [23]. Que-tiapine was also effective on depressive and psychotic symptoms in all the studies that evaluated them [17,18, 21-23]. The dose of quetiapine across studies encomprises the whole dose range (25−800 mg/d). However, dose mean values—provided by five studies—are generally low and range from 100 to 258 mg/d, suggesting that quetiapine may be effective on symptoms even at low/moderate dosages (Table 2). Sedation was the most reported adverse effect: it occurred more frequently in the quetiapine group than the prazosin group [19] and less frequently than valproate and risperidone [24]. The pharmacological mechanism by which quetiapine may ameliorate PTSD symptoms is undefined. Quetiapine is a multifunctional, second-generation antipsychotic, a thienobenzodiazepine derivative. It is approved by the FDA for the treatment of schizophrenia and bipolar disorder in adult patients, but it has shown to be effective in other conditions such as anxiety disorders, dementia, and deli-rium. It is a characterized by antagonism for D2, H1, 5- HT2A, 5-HT2C, α1 receptors while norquetiapine—its main metabolite—is a norepinephrine reuptake transporter inhibitor and characterized by antagonism for the H1, 5-HT1A, 5-HT1E, 5-HT2A, 5-HT2B, 5-HT7, α1, M1, M3, M5 receptors (Fig. 2) [36,37]. The D1/H1/5HT2A/5HT2C/ α1 antagonist activity, underling its sedative and anxiolytic properties, may contribute to i) ameliorate nightmares/sleep disturbances and hyperarousal [37-43] although its anti-arousal effects appears to be direct and unrelated to psychosedation [24] ii) help re-socialization (decreasing levels of anxiety) and improve therapeutic alliance like oxytocin promotes socially-oriented behaviors (normalizing amygdalar and insular activity) (Fig. 2) [24, 44,45]. The anti-D2/5-HT2A activity of quetiapine may contribute to treatment of trauma-related intrusive thoughts, flashbacks, and psychotic symptoms [16-18,21,22,36]. Included studies reported that quetiapine might be an effective and safe pharmacological tool improving a wide range of psychiatric symptoms relative to PTSD.
Fig. 2.
Potential mechanisms by which quetiapine could exert a positive impact in PTSD.
Limitations
This study is not without limitations. Data available were derived mainly from non-RCT trials and, as a potential confounding factor, less than 3% of the patients included in this review were civilians or females. Therefore, the strength of the evidence is too weak to recommend quetiapine in PTSD and more randomized controlled studies are needed. Such studies would enroll patients characterized by specific clinical features that have been linked to inhibitor serotonin transporter resistance. Fur-thermore, it may be useful to know and recognize which PTSD sub-phenotypes may specifically benefit from a treatment with quetiapine (as monotherapy or additional therapy to serotonin reuptake inhibitors [SRI]) to develop more effective treatment strategies. Finally, other atypical antipsychotics with a different receptor profile are reported as reasonable therapy option in patients with PTSD (such as aripiprazole that has a very low affinity toward M1 and H1 recptors), leaving open questions on how the major neurobiological pathways related to PTSD are affected by different antipsychotics [46,47].
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Calogero Crapanzano. Supervision: Chiara Amendola. Writing-original draft: Calogero Crapanzano. Writing review & Editing: Ilaria Casolaro, Stefano Damiani.
References
- 1.Lee HS, Min D, Baik SY, Kwon A, Jin MJ, Lee SH. Association between dissociative symptoms and morning cortisol levels in patients with post-traumatic stress disorder. Clin Psychophar-macol Neurosci. 2022;20:292–299. doi: 10.9758/cpn.2022.20.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo HJ, Jung YE, Bahk WM, Jun TY, Chae JH, Seo HJ, et al. A Comparison of mirtazapine and paroxetine for the treatment of patients with posttraumatic stress disorder: a randomized open-label trial. Clin Psychopharmacol Neurosci. 2010;8:84–89. [Google Scholar]
- 3.Martin A, Naunton M, Kosari S, Peterson G, Thomas J, Christenson JK. Treatment guidelines for PTSD: a systematic review. J Clin Med. 2021;10:4175. doi: 10.3390/jcm10184175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:169–180. doi: 10.1016/j.pnpbp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamner MB, Robert S, Frueh BC. Treatment-resistant posttraumatic stress disorder: strategies for intervention. CNS Spectr. 2004;9:740–752. doi: 10.1017/S1092852900022380. [DOI] [PubMed] [Google Scholar]
- 6.Ehret M. Treatment of posttraumatic stress disorder: focus on pharmacotherapy. Ment Health Clin. 2019;9:373–382. doi: 10.9740/mhc.2019.11.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardy NC, Lund BC, Alexander B, Friedman MJ. Prescribing trends in veterans with posttraumatic stress disorder. J Clin Psychiatry. 2012;73:297–303. doi: 10.4088/JCP.11m07311. [DOI] [PubMed] [Google Scholar]
- 8.Bauer MS, Lee A, Li M, Bajor L, Rasmusson A, Kazis LE. Off-label use of second generation antipsychotics for post-traumatic stress disorder in the Department of Veterans Affairs: time trends and sociodemographic, comorbidity, and regional correlates. Pharmacoepidemiol Drug Saf. 2014;23:77–86. doi: 10.1002/pds.3507. [DOI] [PubMed] [Google Scholar]
- 9.Holder N, Woods A, Neylan TC, Maguen S, Seal KH, Bernardy N, et al. Trends in medication prescribing in patients with PTSD from 2009 to 2018: a national veterans administration study. J Clin Psychiatry. 2021;82:20m13522. doi: 10.4088/JCP.20m13522. [DOI] [PubMed] [Google Scholar]
- 10.Delapaz NR, Hor WK, Gilbert M, La AD, Liang F, Fan P, et al. An emulation of randomized trials of administrating antipsychotics in PTSD patients for outcomes of suicide-related events. J Pers Med. 2021;11:178. doi: 10.3390/jpm11030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reinhard MA, Seifert J, Greiner T, Toto S, Bleich S, Grohmann R. Pharmacotherapy of 1,044 inpatients with posttraumatic stress disorder: current status and trends in German-speaking countries. Eur Arch Psychiatry Clin Neurosci. 2021;271:1065–1076. doi: 10.1007/s00406-020-01223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattar SP, Ucci B, Grant K, Bhatia SC, Petty F. Quetiapine therapy for posttraumatic stress disorder. Ann Pharmacother. 2002;36:1875–1878. doi: 10.1345/aph.1C040. [DOI] [PubMed] [Google Scholar]
- 16.Filteau MJ, Leblanc J, Bouchard RH. Quetiapine reduces flashbacks in chronic posttraumatic stress disorder. Can J Psychiatry. 2003;48:282–283. doi: 10.1177/070674370304800416. [DOI] [PubMed] [Google Scholar]
- 17.Hamner MB, Deitsch SE, Brodrick PS, Ulmer HG, Lorberbaum JP. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psycho-pharmacol. 2003;23:15–20. doi: 10.1097/00004714-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ahearn EP, Mussey M, Johnson C, Krohn A, Krahn D. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol. 2006;21:29–33. doi: 10.1097/01.yic.0000182116.49887.ae. [DOI] [PubMed] [Google Scholar]
- 19.Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30:225–229. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- 20.Detweiler MB, Pagadala B, Candelario J, Boyle JS, Detweiler JG, Lutgens BW. Treatment of post-traumatic stress disorder nightmares at a veterans affairs medical center. J Clin Med. 2016;5:117. doi: 10.3390/jcm5120117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pivac N, Kozarić-Kovacić D. Pharmacotherapy of treatment-resistant combat-related posttraumatic stress disorder with psychotic features. Croat Med J. 2006;47:440–451. [PMC free article] [PubMed] [Google Scholar]
- 22.Sokolski KN, Denson TF, Lee RT, Reist C. Quetiapine for treatment of refractory symptoms of combat-related post-traumatic stress disorder. Mil Med. 2003;168:486–489. doi: 10.1093/milmed/168.6.486. [DOI] [PubMed] [Google Scholar]
- 23.Villarreal G, Hamner MB, Cañive JM, Robert S, Calais LA, Durklaski V, et al. Efficacy of quetiapine monotherapy in posttraumatic stress disorder: a randomized, placebo-controlled trial. Am J Psychiatry. 2016;173:1205–1212. doi: 10.1176/appi.ajp.2016.15070967. [DOI] [PubMed] [Google Scholar]
- 24.Baig MR, Wilson JL, Lemmer JA, Beck RD, Peterson AL, Roache JD. Enhancing completion of cognitive processing therapy for posttraumatic stress disorder with quetiapine in veterans with mild traumatic brain injury: a case series. Psychiatr Q. 2019;90:431–445. doi: 10.1007/s11126-019-09638-z. [DOI] [PubMed] [Google Scholar]
- 25.Kozaric-Kovacic D, Pivac N. Quetiapine treatment in an open trial in combat-related post-traumatic stress disorder with psychotic features. Int J Neuropsychopharmacol. 2007;10:253–261. doi: 10.1017/S1461145706006596. [DOI] [PubMed] [Google Scholar]
- 26.Brinker M, Westermeyer J, Thuras P, Canive J. Severity of combat-related posttraumatic stress disorder versus noncombat- related posttraumatic stress disorder: a community-based study in American Indian and Hispanic veterans. J Nerv Ment Dis. 2007;195:655–661. doi: 10.1097/NMD.0b013e31811f4076. [DOI] [PubMed] [Google Scholar]
- 27.Campbell-Sills L, Sun X, Choi KW, He F, Ursano RJ, Kessler RC, et al. Dissecting the heterogeneity of posttraumatic stress disorder: differences in polygenic risk, stress exposures, and course of PTSD subtypes. Psychol Med. 2021 doi: 10.1017/s0033291721000428. doi: 10.1017/ S0033291721000428. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compean E, Hamner M. Posttraumatic stress disorder with secondary psychotic features (PTSD-SP): diagnostic and treatment challenges. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:265–275. doi: 10.1016/j.pnpbp.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen M, Ross J, Armour C. Evidence of the dissociative PTSD subtype: a systematic literature review of latent class and profile analytic studies of PTSD. J Affect Disord. 2017;213:59–69. doi: 10.1016/j.jad.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevlin M, Armour C, Murphy J, Houston JE, Adamson G. Evidence for a psychotic posttraumatic stress disorder subtype based on the National Comorbidity Survey. Soc Psychiatry Psychiatr Epidemiol. 2011;46:1069–1078. doi: 10.1007/s00127-010-0281-4. [DOI] [PubMed] [Google Scholar]
- 32.Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post- traumatic stress disorder (PTSD) Int J Neuropsychophar-macol. 2012;15:825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- 33.de Moraes Costa G, Zanatta FB, Ziegelmann PK, Soares Barros AJ, Mello CF. Pharmacological treatments for adults with post-traumatic stress disorder: a network meta-analysis of comparative efficacy and acceptability. J Psychiatr Res. 2020;130:412–420. doi: 10.1016/j.jpsychires.2020.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Hoskins MD, Sinnerton R, Nakamura A, Underwood JFG, Slater A, Lewis C, et al. Pharmacological-assisted psychotherapy for post-traumatic stress disorder: a systematic review and meta-analysis. Eur J Psychotraumatol. 2021;12:1853379. doi: 10.1080/20008198.2020.1853379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villarreal G, Hamner MB, Qualls C, Cañive JM. Characterizing the effects of quetiapine in military post-traumatic stress disorder. Psychopharmacol Bull. 2018;48:8–17. [PMC free article] [PubMed] [Google Scholar]
- 36.López-Muñoz F, Alamo C. Active metabolites as antidepres-sant drugs: the role of norquetiapine in the mechanism of action of quetiapine in the treatment of mood disorders. Front Psychiatry. 2013;4:102. doi: 10.3389/fpsyt.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crapanzano C, Damiani S, Guiot C. Quetiapine in the anxiety dimension of mood disorders: a systematic review of the literature to support clinical practice. J Clin Psychopharmacol. 2021;41:436–449. doi: 10.1097/JCP.0000000000001420. [DOI] [PubMed] [Google Scholar]
- 38.Crapanzano C, Amendola C, Politano A, Laurenzi PF, Casolaro I. Olanzapine for the treatment of somatic symptom disorder: biobehavioral processes and clinical implications. Psychosom Med. 2022;84:393–395. doi: 10.1097/PSY.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 39.Cohrs S, Rodenbeck A, Guan Z, Pohlmann K, Jordan W, Meier A, et al. Sleep-promoting properties of quetiapine in healthy subjects. Psychopharmacology (Berl) 2004;174:421–429. doi: 10.1007/s00213-003-1759-5. [DOI] [PubMed] [Google Scholar]
- 40.Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170:1003–1010. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- 41.Crapanzano C, Casolaro I, Damiani S, Amendola C. Efficacy of olanzapine in anxiety dimension of schizophrenia: a systematic review of randomized controlled trials. Clin Psycho-pharmacol Neurosci. 2022;20:592–599. doi: 10.9758/cpn.2022.20.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crapanzano C, Amendola A, Conigliaro C, Casolaro I. Clothiapine: highlights of the pharmacological and clinical profile of an undervalued drug. Swiss Arch Neurol Psychiatr Psychother. 2022;173:w10065. doi: 10.4414/sanp.2022.03243. [DOI] [Google Scholar]
- 43.Ketenci S, Acet NG, Sarıdoğan GE, Aydın B, Cabadak H, Gören MZ. The neurochemical effects of prazosin treatment on fear circuitry in a rat traumatic stress model. Clin Psycho-pharmacol Neurosci. 2020;18:219–230. doi: 10.9758/cpn.2020.18.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giovanna G, Damiani S, Fusar-Poli L, Rocchetti M, Brondino N, de Cagna F, et al. Intranasal oxytocin as a potential therapeutic strategy in post-traumatic stress disorder: a systematic review. Psychoneuroendocrinology. 2020;115:104605. doi: 10.1016/j.psyneuen.2020.104605. [DOI] [PubMed] [Google Scholar]
- 45.De Cagna F, Fusar-Poli L, Damiani S, Rocchetti M, Giovanna G, Mori A, et al. The role of intranasal oxytocin in anxiety and depressive disorders: a systematic review of randomized controlled trials. Clin Psychopharmacol Neurosci. 2019;17:1–11. doi: 10.9758/cpn.2019.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Britnell SR, Jackson AD, Brown JN, Capehart BP. Aripiprazole for post-traumatic stress disorder: a systematic review. Clin Neuropharmacol. 2017;40:273–278. doi: 10.1097/WNF.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 47.Crapanzano C, Laurenzi PF, Amendola C, Casolaro I. Com-bining aripiprazole and haloperidol: Focus on D2 Receptor. J Clin Pharmacol. 2022;62:918. doi: 10.1002/jcph.2026. [DOI] [PubMed] [Google Scholar]