Abstract
Objective
Brain-derived neurotrophic factor (BDNF) and high sensitive C-reactive protein (hs-CRP) have been reported to play roles in depression and bipolar disorder (BD). However, the probable discriminatory properties of these biologic markers are less investigated. We aimed to assess the serum BDNF and hs-CRP levels among Iranian patients with major depressive disorder (MDD) and BD during a depressive episode and investigate the optimum cut-off point for differential diagnosis of BD and MDD.
Methods
We recruited 30 patients with MDD, 30 with BD in depressive mood and 30 healthy comparators. Blood sample was taken from each participant to measure BDNF and hs-CRP levels. We also used receiver operating characteristic (ROC) curve analysis to find an optimal cut-off point for differentiating MDD from BD according to pre-defined variables.
Results
The mean age of total study population was 37.3 ± 5.0 years (males 49%). BDNF was significantly lower in patients with BD, followed by MDD subjects and healthy controls 541.0 ± 601.0 pg/ml vs. 809.5 ± 433.3 pg/ml vs. 1,482.1 ± 519.8, respectively, p < 0.001). The area under curve of ROC curve analysis for BD versus MDD was 0.704 (95% confidence interval 0.564−0.844, p = 0.007). We also found that the BDNF cut-off value of 504 could appropriately distinguished BD from MDD (sensitivity 73%, specificity 70%). No significant association were identified in terms of hs-CRP levels.
Conclusion
Patients suffering from BD had lowest BDNF levels compared to MDD or healthy adults and this biomarker could play a practical role differentiating MDD from BD. Several studies are required confirming our outcomes.
Keywords: Brain-derived neurotrophic factor, C-reactive protein, Major depressive disorder, Bipolar disorder
INTRODUCTION
Bipolar disorder (BD) and major depressive disorder (MDD) also known as unipolar depression are two common mental diseases with considerable economic burden. These diseases negatively affect patients’ quality of life. Although the prevalence of BD and MDD are reported to be 1.06% and 4.7% in general population, they are associated with higher likelihood of complications including suicide [1,2]. The total expenditure of BD and MDD is estimated to be USD 202.1 and USD 210.5 billion, respectively [3,4]. Despite the genetic and environmental factors play roles in pathophysiology of BD and MDD, the exact mechanism needs to be more investigated. Moreover, proper diagnosis of these two entities is pivotal in order to prevent delayed implementation of therapeutic interventions and occurrence of subsequent complications due to misdiagnosis [5,6]. Depression in BD is the major remaining psychiatric morbidity with existing treatments. Some studies mentioned that treatments used for MDD patients may lead to increased risk of manic episodes, more frequent mood episodes, cycle acceleration, and antidepressant-treatments-induced-suicidality in BD patients [7-10]. Furthermore, untreated depression, is associated with morbidities like metabolic syndrome and cardiovascular disease, with increased mortality. Recently, the role of different biomarkers has been studied in diagnosis, treatment and prognosis of depression. As the measurement and analyzing of the biomarkers in peripheral blood are simple, less invasive and less expensive, they are suitable for assessing depression, prognosis, evaluate the therapeutic responses and also differentiating depression sub-types and separating them from other disease [10-13].
There are numerous biomarkers related to depression which are mainly categorized as cytokines and inflam-matory markers, growth factors (GFs), endocrine, and metabolic markers. Inflammation plays an important role in etiopathogenesis of depression and inflammatory markers are crucial to upkeep natural function of the brain (e.g., interleukin [IL] 1β, tumor necrosis factor [TNF]-β, IL-2, interferon [IFN]-β, IFN-gama, IL-10, and high sensitive C-reactive protein [hs-CRP]). Moreover, proinflammatory markers (e.g., IL-1β, IL-6, TNF-β) are higher in depression and tends to decrease with antidepressants. GFs like Brain-derived neurotrophic factor (BDNF), insulin- like growth factor-1, vascular endothelial growth factor (VEGF), glial cell derived neurotrophic factor, fibroblast growth factor (FGF)-2, nerve growth factor have been studied to be related to depression. Some of these factors are studied to be increased (e.g., VEGF, basic fibroblast growth factor [bFGF], and epidermal growth factor), while others such as BDNF are decreased [10-15].
BDNF is a 13-k Dalton protein originated from pro- BDNF in endoplasmic reticulum [16]. The main location for production of this biologic marker is brain, especially hippocampus and cerebral cortex. However, other tissues including liver, smooth muscles as well as endothelial cells have been reported to secrete BDNF [17]. This protein has been reported to be effective in memory process, nervous tissue survival, synaptic plasticity as well as neurogenesis [18,19]. BDNF has been shown to promote the survival and morphological differentiation of 5-HT neurons both in culture and in vivo [20]. Several studies indicate that BDNF is reduced in both manic and depressive episodes of BD [21-23]. On the other hand, this biologic marker could be raised by anti-depressant and anti-psychotic medications [21-24]. Additionally, this factor has been shown to be lower in patients suffering from MDD [25,26]. Therefore, appropriate differentiation of BD and MDD based on BDNF might be a practical tool in this regard.
Another marker is an acute phase reactant, named C-reactive protein (CRP). This inflammatory molecule is mostly secreted from liver cells induced by IL-6. However, CRP can be produced by atherosclerotic plaques, monocytes, nervous tissue and lymphocytes [27,28]. CRP is reported to be elevated in cardiovascular diseases, in addition, psychiatric disorders including BD and depression have been associated with raised CRP levels [29-31]. Huang and Lin [32] suggested that BD individuals had significantly higher high sensitive-CRP (hs-CRP) levels compared to healthy patients, but this relation was insignificant among MDD patients.
Additionally, hypothalamic pituitary-adrenal (HPA) axis abnormalities are suggested in the pathogenesis of de-pression. This is an interactive neuroendocrine unit comprising of the hypothalamus, the pituitary gland, and the adrenal glands. The HPA axis plays an important role in response to stress. Function of the axis results in the production and secretion of glucocorticoid. It’s shown that the activity of HPA axis function is increased in both depressed males and females. Moreover, HPA-axis dysfunction in depression correlates with a 4−6-fold higher risk for relapse [14,33].
Till now, few articles assessed discriminatory capabilities of BDNF and hs-CRP in mood disorders, especially BD. One study suggested BDNF level of 6.74 ng/ml could be better distinguish BD from healthy individuals [34]. Another study reported the cut-off point of 0.26 pg/mg for differentiating between MDD and BD [5]. With respect to CRP, this cut-off was found to be 621.6 ng/ml for appropriate differential diagnosis of BD from MDD [35]. However, performing a complementary study to assess discriminatory properties of these aforementioned biomarkers in proper diagnosis of BD from MDD or healthy persons seems necessary.
Remedies for bipolar depression is far less developed than for unipolar depression, probably reflecting lack of discernment in distinction between bipolar and unipolar depression. Due to difficult distinguishing MDD from BD in depressive episode and presence of controversial findings, we aimed to define the probable relation of BDNF and hs-CRP levels in Iranian patients suffered from BD during a depressive episode and unipolar depression in comparison to healthy comparators and assess the discriminatory properties of BDNF and hs-CRP levels for appropriate differential diagnosis of BD and MDD.
METHODS
Study Population
This observational exploratory study took place from March 2020 to October 2020. Patients with diagnosis of MDD (unipolar depression) or BD during a depressive episode aged 18−65 years admitted to governmental psychiatric hospitals located in Isfahan, Iran were eligible for enrollment in current project. Definite diagnosis of MDD and BD were made through structural clinical interview for the Diagnostic and Statistical Manual of Mental Disorders 5th edition-axis I disorder done by skillful psychiatrists [36]. We also used Persian validated version of Beck depression inventory (BDI) scores for assessment of depression severity [37]. All patients were drug free and presence of prior drug usages including anti-depressant, anti-psychotic, antibiotics and corticosteroid agents excluded subjects from study. Moreover, patients did not have any prior positive findings in terms of previous shock therapy or psychotherapeutic interventions. We also excluded patients if they had any of the followings: prior history of definite diagnosis of mental disorders, history of malignancy or any positive findings attributable to current infectious diseases, body mass index (BMI) of at least 24 kg/m2, positive pregnancy test or history of parturition during the past year for females as well as any other medical issues including diabetes mellitus, dyslipidemia, coronary artery diseases or chronic inflammatory disorders. After implementation of all inclusion and exclusion criteria, a total of 30 patients with MDD and 30 ones with BD in depressive episode were selected for this study. We also recruited 30 age and sex matched healthy controls. All procedures performed in studies involving human participants were under the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee affiliated to Baqiyatallah University of Medical Sciences (IR.BMSU.BAQ.REC.1398.027) and written informed consent for participation was obtained from the patients.
Assessment of Variables
Data on age and sex were gathered through each patient’s medical profile. BMI was measured by division of weight over height (kg/m2). BDI is a 21-item questions with a 4-point scale ranges 0 (absent symptom) to 4 (severe symptoms), which is proved as a questionnaire with good reliability, validity, and excellent internal consistency among Iranian population [38]. The minimum and maximum scores would be 0 and 63, respectively. After fasting for approximately 8 hours, a blood sample was taken from each participant by a venipuncture in a free-coagulated tube. After clotting for 15 minutes at room temperature, the tubes were centrifuged at 3,000 round per minute for 10 minutes and serum was kept frozen at −20°C. We measured BDNF levels with sandwich-enzyme linked immunosorbent assay (ELISA) kit based on manufacturer’s instructions (ZellBio GmbH, Ulm, Germany). The optical density was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm, which was proportional to BDNF concentration. The intra-assay and inter-assay of BDNF variations were less than 10% and 12%, respectively. The lowest detection dose of BDNF was 18.7 pg/ml. In terms of hs-CRP, ELISA kit (Roche Diagnostic, Meylan, France) was used according to instructions provided by the manufacture. The minimum detection range of hs-CRP was 0.1 mg/L with intra-assay and inter-assay coefficient variation of less than 10%.
Statistical Analysis
Continuous and categorical variables were reported as mean ± standard deviation and frequency, respectively. Normality test was used to assess the distribution of our recruited variables. The null hypothesis was set to be there was not any differences between recruited subjects in terms of BDNF and hs-CRP levels. The alternative hypothesis was defined as there was a difference. In order to evaluate the differences between groups of study population, analysis of variance (ANOVA)/Kruskal−Wallis with least significant difference (LSD) post hoc test and chi-square tests were used, as indicated. BDI scores were analyzed using ttest or Mann−Whitney test based on normality results. Due to previously reported effects of age and sex on BDNF levels, we used analysis of covariance (ANCOVA) with post hoc LSD test with adjustment of age, sex and BMI as covariates to evaluate BDNF differences between groups [39]. Pearson correlation test was used to investigate the correlation between BDI scores and BDNF and hs-CRP means. Finally, we used receiver operating characteristic (ROC) curve to analyze the capability of pre-defined variables to appropriately differentiate MDD, BD, and healthy controls. We used Statistical Package for Social Sciences (SPSS) version 22 (IBM Corp., Armonk, NY, USA) for implementation of all data analysis. p values < 0.05 were considered statistically significant.
RESULTS
The mean age of population was 37.3 ± 5.0 years and 49% of participants were males. Results of normality tests revealed data were not normally distributed. Characteristics of study participants according to mental diseases are shown in Table 1. BMI was uniformly distributed between groups (p = 0.156). Mean BDI score was 42.0 ± 7.2. However, there was no significant differences between MDD and BD with depression patients. Our findings showed BDNF was significantly lower in patients with BD in depressive episode followed by MDD participants and healthy controls (p < 0.001). Post hoc LSD test revealed the same significant difference between groups in a way that patients with BD had significantly lower BDNF means in comparison to MDD ones (541.0 ± 601.0 pg/ml vs. 809.5 ± 433.3 pg/ml, p = 0.021). Also, this serum index was remarkably lower among both BD and MDD subjects rather than healthy controls (541.0 ± 601.0 pg/ml vs. 1,482.1 ± 519.8 pg/ml, p < 0.001 and 809.5 ± 433.3 pg/ml vs. 1,482.1 ± 519.8 pg/ml, p < 0.001). We found no significant association in terms of hs-CRP levels between groups (p = 0.087). Based on BDI scores, BD and MDD patients were confirmed to suffer from severe depression with no remarkable difference between groups. Our further analysis revealed that depression severity, as assessed by BDI scores, had a significant quite negative correlation with BDNF levels. No correlation had been found in terms of hs-CRP (p = 0.122) (Table 2).
Table 1.
Characteristics of study population according to mental disease
| Characteristics | Total (n = 90) | Healthy controls (n = 30) | Major depression (n = 30) | Bipolar disorder with depressive episode (n = 30) | p value* |
|---|---|---|---|---|---|
| Age (yr) | 37.3 ± 5.0 | 37.1 ± 5.2 | 37.9 ± 4.3 | 37 ± 5.6 | 0.733 |
| Male | 44 (49) | 12 (40) | 16 (53) | 16 (53) | 0.491 |
| BMI (kg/m2) | 20.9 ± 1.1 | 21.2 ± 1.0 | 20.6 ± 1.1 | 21.0 ± 1.2 | 0.176 |
| BDI score | 42.0 ± 7.2 | - | 43.0 ± 6.6 | 41.4 ± 7.7 | 0.383 |
| BDNF (pg/ml) | 944.2 ± 654.4 | 1,482.1 ± 519.8 | 809.5 ± 433.3 | 541.0 ± 601.0 | < 0.001 |
| hs-CRP (mg/l) | 9.2 ± 5.2 | 7.9 ± 4.0 | 8.9 ± 5.5 | 10.8 ± 5.7 | 0.087 |
Values are presented as mean ± standard deviation or number (%).
BMI, body mass index; BDI, Beck depression inventory; BDNF, brain derived neurotrophic factor; hs-CRP, high sensitive C-reactive protein.
*p values resulted from Kruskal−Wallis (age, BMI, hs-CRP), analysis of covariance (ANCOVA) (BDNF), Mann−Whitney (BDI score) and chi-square (male) tests.
Table 2.
Correlation analysis between serum BDNF and hs-CRP levels versus BDI scores
| Variable | BDI score | |
|---|---|---|
|
| ||
| r | p value | |
| BDNF | −0.346 | 0.001 |
| hs-CRP | −0.164 | 0.122 |
BDNF, brain derived neurotrophic factor; hs-CRP, high sensitive C-reactive protein; BDI, Beck depression inventory.
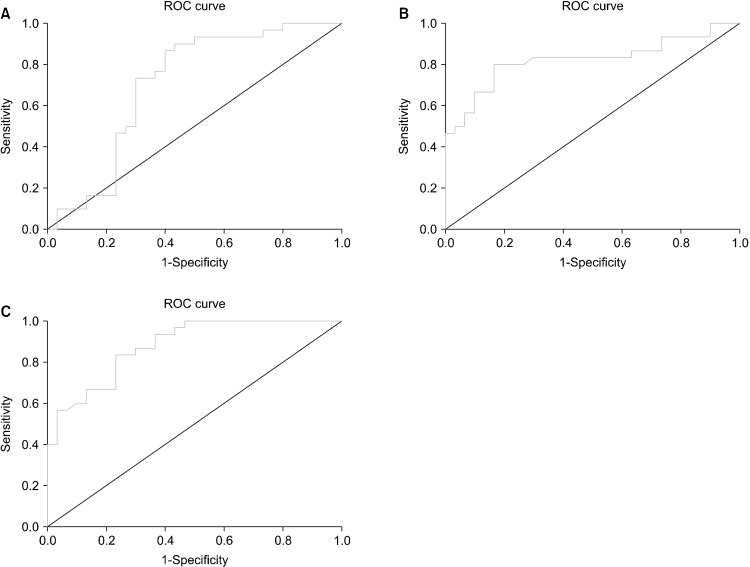
Table 3 represents data on ROC curve analysis for comparison of participants based on presence/absence of mental disease. ROC curve analysis in terms of discrimination of MDD and BD based on BDNF showed an area under curve (AUC) of 0.704 (95% confidence interval [CI]: 0.564−0.844, p = 0.007). Moreover, we figured out the optimal cut-off point of 504 (sensitivity: 73%, specificity: 70%) could appropriately differentiate BD from MDD (Fig. 1A). AUC of ROC curve for differential diagnosis of MDD vs. controls was 0.823 (95% CI: 0.711−0.935, p < 0.001) with BDNF cut-off value of 1,206 (sensitivity: 80%, specificity: 83%) (Fig. 1B). Finally, data analysis of ROC curve for differentiating BD patients from healthy subjects revealed the AUC of 0.879 (95% CI: 0.797−0.962, p < 0.001). We found that BDNF cut-off point of 918 with sensitivity of 83% and specificity of 76% could differentiate these two entities (Fig. 1C).
Table 3.
ROC curve analysis for comparison of participants according to mental disease status
| ROC analysis | BD vs. MDD | MDD vs. control | BD vs. control |
|---|---|---|---|
| AUC (95% CI) | 0.704 (0.564−0.844) | 0.823 (0.711−0.935) | 0.879 (0.797−0.962) |
| p value | 0.007 | < 0.001 | < 0.001 |
| Cut-off | 504 | 1,206 | 918 |
| Sensitivity | 73% | 80% | 83% |
| Specificity | 70% | 83% | 76% |
ROC, receiver operating curve; AUC, area under curve; BD, bipolar disorder; MDD, major depressive disorder.
Fig. 1.
Receiver operating curve of BD vs. MDD (A), MDD vs. controls (B) and BD vs. controls (C).
BD, bipolar disorder; MDD, major depressive disorder; ROC, receiver operating curve.
DISCUSSION
In this study we aimed to evaluate the association of two biologic markers, BDNF and CRP, among patients suffered from BD during a depressive episode and MDD as well as investigation of discriminatory capabilities of BDNF and CRP for differential diagnosis among BD and MDD and healthy individuals. We found that BD patients in a depressive episode had significantly lower BDNF means in comparison to either MDD patients or healthy controls. Also, a cut-off point of 504 might be a good discriminator between BD and MDD individuals. Since differentiation of these two mental disorders is essential, usage of the former cut-off value might be practical in clinical settings.
Our findings were in favor of several studies indicate decreased BDNF levels in BD. Chiou and Huang [34] performed a study to investigate the possible association of BDNF in BD patients. They recruited 83 subjects with BD and 222 healthy adults over a 12-year duration. Their outcomes revealed that BDNF means were remarkably lower in BD individuals compared to controls (5.7 ± 4.2 ng/ml vs. 12.2 ± 7.5 ng/ml, p < 0.001) [34]. A cross-sectional study was implemented to compare BDNF means in older BD patients with controls. 91 subjects with euthymic bipolar 1 disorder (B1D) and 76 healthy comparators aged at least 50 years were eligible for enrollment in the study. They suggested that serum BDNF levels were significantly decreased in comparison to those with no mental disease (8 ± 5.5 pg/mg vs. 12.3 ± 8.9 pg/mg, p < 0.001) [40]. Wu and colleagues [41] enrolled 102 patients in different episodes of BD (acute mania: 44, acute depressive episode: 20, and remission: 38) and measured serum BDNF means in all participants and reported that the level of this biomarker differed significantly in those with manic or depressive episode rather than patients in remission (acute mania: 5.5 ± 0.5 ng/ml vs. remission: 7.7 ± 0.6 ng/ml, p = 0.010, acute depressive episode: 4.3 ± 0.9 ng/ml vs. remission: 7.7 ± 0.6 ng/ml, p = 0.003). However, there was an insignificant association in terms of BDNF between patients with manic and depressive episode (p = 0.244) [41]. A systematic review and meta-analysis on total of 1,113 participants including 548 ones suffered from BD with different mood episodes (mania, depression and euthymia) and 565 healthy controls revealed that in individuals with manic or depressive episodes of B1D, the BDNF means were significantly lower compared to control comparators (effect size [ES]: −0.81, 95% CI: −1.11 to −0.52, p < 0.0001 and ES: −0.97, 95% CI: −1.79 to −0.51, p = 0.02, respectively). However, euthymic participants had no significant different BDNF levels compared to healthy adults (ES: −0.20, 95% CI: −0.61 to 0.21, p = 0.33) [42].
Likewise, the reduced level of this neurotropic biomarker in depression has been reported till now. For instance, Karege et al. [43] measured blood BDNF levels in 30 major depressed individuals and 30 controls and their findings indicated that those depressive participants had remarkably lower BDNF means in comparison to healthy ones (22.6 ± 3 ng/ml vs. 26.5 ± 7 ng/ml, p < 0.01) [43]. Another observational study was performed on 52 patients with first depressive episode and 50 healthy adults aged 18−65 years to evaluate the alterations in BDNF levels between groups. A blood sample was taken from all participants and the concentration of BDNF was measured. The mean levels of BDNF was significantly reduced in depressed participants rather than the other group (2.176 ± 0.753 ng/dl vs. 2.556 ± 1.014 ng/dl, p = 0.034) [44].
BDNF has been suggested to have different ranges between unipolar depression and BD patients during a depressive episode and might be a useful index for appropriate differential diagnosis of these two mental disorders. Li et al. [45] implemented a prospective longitudinal study in order to evaluate the BDNF levels for diagnosis of MDD or B1D with depression. They recruited 203 patients with their first major depressive episode and after a 3-year duration 164 participants including 143 ones with final MDD diagnosis and 21 ones with diagnosis of B1D with depression completed the study. Their data were compared with 167 controls. They finally suggested that both B1D with depression and MDD groups had lower plasma BDNF levels rather than healthy participants (p = 0.002 and p = 0.01, respectively). Also the mean BDNF was lower in B1D patients compared to MDD ones [45]. Fernandes and colleagues [5] selected 40 patients with B1D with depressive episode, 10 with MDD and 30 healthy controls in order to investigate the levels of BDNF between groups. They figured out that B1D patients had lower BDNF means in comparison to MDD subjects and healthy comparators (0.15 ± 0.08 pg/mg vs. 0.35 ± 0.08 pg/mg vs. 0.38 ± 0.12 pg/mg, p = 0.001, respectively) [5].
In terms of discriminatory properties of BDNF, several studies were performed with different results. For instance, a longitudinal study was performed to assess the optimal BDNF cut-off point between BD patients and healthy adults. The AUC resulted from ROC curve analysis was found to be 0.801. The suggested that BDNF of 6.74 ng/ml with sensitivity of 82% and specificity of 63.9% could be categorized as the best cut-off point differentiating these two aforementioned groups [34]. Another observational study was conducted to investigate the capability of serum BDNF for differential diagnosis of B1D, MDD and controls. The AUC resulted from ROC curve analysis for B1D with depression versus MDD was 0.95 (95% CI: 0.89−1.00) and they proposed the optimum cut-off point of 0.26 (sensitivity: 88%, specificity: 90%) for differentiation between B1D with depression and MDD. Likewise, they analyzed the data regarding discrimination of B1D versus healthy subjects and found the AUC of 0.94 (95% CI: 0.89 − 1.00). The optimum cut-off point for distinguishing between these two states was set to be 0.27 (sensitivity: 90%, specificity: 87%). Finally they reported BDNF levels of 0.23 with sensitivity of 85% and specificity of 95% could appropriately differentiate BD form total of MDD and healthy adults [5].
BDNF and HPA axis abnormalities are suggested in the pathogenesis of depression. There are several mechanisms which might be considered for explanation of decreased or malfunctioned BDNF serum level in depression. The most investigated [46] BDNF gene variation causes in a single nucleotide polymorphism (SNP) of A758G (rs6265) leads to a change in one amino acid, Val66Met, in proBDNF protein which is finally resulting in abnormal hippocampal activation and predisposition for depressive disorder. Another SNP within the p75 receptor gene which is led to decreased in the amount of allele (L205) that is protective against major depression. Also, the effect of excessive glucocorticoid on BDNF expression and function has been suggested via main mechanisms including stress-induced hyperactivity of HPA axis which might lead to glucocorticoid over production and decrease BDNF expression in the hippocampus and excessive glucocorticoid produc-tion interaction with TrkB-bound glucocorticoid receptor, resulting in decreased BDNF signaling (interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression) [46,47].
BDNF would be altered after medical therapy initiation with controversial results. A record on 23 and 19 patients with B1D in manic episode and depression, respectively showed that although BDNF levels raised from baseline after a one-month treatment, the differences were not remarkably significant [41]. Oliveira et al. [6] enrolled 22 drug free B1D individuals and 22 treated ones as well as 22 age and sex matched controls. They reported that despite the lower means of BDNF in both drug free (0.23 ± 0.09 pg/ml) and medicated (0.29 ± 0.19 pg/ml) patients compared to healthy participants (0.40 ± 0.12 pg/ml), this biomarker was not differently distributed according to presence or absence of treatment [6]. On the other hand, a meta-analysis study on eight records with a total of 220 MDD patients showed that BDNF increased significantly after initiation of therapy (pre-treatment: 46.9 ± 9.9 mg/ml, post-treatment: 53.1 ± 10.1 mg/ml, p = 0.003) [24]. It seems that prior usage of anti-depressant medications might distort the possible association of BDNF with aforementioned mental disorders. Therefore, we just included drug free patients in current study.
BDNF plays an important role in survival of neurons during hippocampal development, and this may relate to its assumed role in depression. Decreased levels of BDNF in depressed patients may contribute to the atrophy of hippocampus that has been observed in patients. In addition, data suggest that serum BDNF levels are reduced in depressed patients and are negatively correlated with depression severity [48]. Our findings showed a negative correlation between BDNF levels and BDI scores. However, data are limited in this regard and other studies are necessary. A cross-sectional study on 52 patients with first depression event showed the mean BDI scores of 23.57 ± 7.476. However, they failed to prove any signifi-cant correlation between BDI scores and BDNF means (Pearson c: 0.19, p = 0.16) [44]. On the other hand, Karege et al. [43] recruited 30 MDD patients and 30 healthy controls to assess the correlation of BDNF with depression score via another depression score, named Montgomery−Asberg-Depression Rating Scale (MADRS). Their final results were in favor of our outcomes in a way that serum BDNF had a significant negative correlation with MADRS (r = −0.55, p < 0.02) [42]. It seems that more severe status of depression was associated with lower BDNF levels. However, complementary studies are manda-tory. Another analytical cross-sectional study which is done by Sjahrir and Roesyanto-Mahadi [49] on 23 Psoriasis vulgaris patients. This study revealed there is a strong negative correlation between serum BDNF level examined with ELISA and depression severity assessed with BDI (r = −0.667 with significant value p = 0.001) [49].
Although our findings showed insignificant findings in terms of hs-CRP among desired groups, reports are con-troversial. Huang et al. implemented a study to assess hs-CRP levels in patients with MDD and B1D. They enrolled 23 MDD patients and 13 ones suffered from B1D in manic episode as well as 31 healthy comparators. The mean hs-CRP levels in aforementioned groups were 2 ± 3.5 mg/L, 5.8 ± 9.6 mg/L and 1.5 ± 1.8 mg/L, respectively. After adjustment for ages, their final outcomes suggested that hs-CRP means remained significantly higher in B1D subjects in comparison to healthy adults (p = 0.043). However, the difference between MDD and healthy groups did not show remarkable association (p = 0.172) [32]. Jeenger and colleagues [44] failed to prove any significant association between CRP levels among patients with depression episode and healthy control (1.936 ± 1.85 mg/dl vs. 1.713 ± 1.32 mg/dl, p = 0.489). Chang and colleagues [35] performed a cross-sectional study on 88 BD, 72 MDD and 96 healthy subjects and reported hs-CRP levels of 621.6 ng/ml (AUC: 0.816, sensitivity: 69%, specificity: 88%) could discriminate between BD and MDD patients. Although we found no significant correlation in terms of hs-CRP and depression score, data are controversial in literature. For instance, a study suggested that patients with higher depression scores had increased levels of hs-CRP. However, the correlation was just significant among women. It seems several contributing factors including age, BMI or minor inflammation as well as resting heart rate as an indicator of physical fitness might play role on CRP serum levels [50]. Moreover, genetic susceptibility might play an important role. In support of this, genetic polymorphisms in the CRP have been shown to influence CRP levels in different cohorts of psychiatric and non-psychiatric patients, as well as the general popula-tion [51]. Therefore, the exact role of hs-CRP in either MDD or BD with depressive episode needs to be more clarified in future studies.
To best of our knowledge, this study is the first in literature evaluated simultaneous BDNF and hs-CRP levels in Iranian patients with MDD and BD with depression. All patients were drug free and we excluded those previously consumed any mood stabilizer or anti-depressant agents due to probable effect of these drugs on BDNF and hs-CRP levels. By the way, there are several limitations. The main one is the study design disabling us from deducting any cause and effect relation between pre-defined variables. The patients were not followed for occurrence of any other mental episodes. We also did not consider the probable effects of sleep problems, sociodemographic factors including rural versus urban living status, seasonality or duration of patients’ illness. The aforementioned factors have been reported to be effective in BDNF expression [52,53]. Also, the insignificant result in terms of hs-CRP might be attributed to our small sample size indicating the probable occurrence of type II error. Due to illegal state of alcohol consumption in Iran and controversial reportage of alcohol usages by participants, this factor was not assessed. Also, we were unable to assess the genomic polymorphism of BDNF gene including Val66Met polymorphism, as recently suggested, to better clarifying our outcomes to understand whether this factor is a trait or state biomarker [54-56]. Finally, we measured serum BDNF levels. However, BDNF is able to cross blood brain barrier and a positive correlation has been suggested between serum and cortical BDNF means.
In conclusion, this study indicates that serum BDNF is decreased more robustly among BD with depression patients followed by MDD individuals and healthy controls. Also, this biomarker might have a good discriminatory capability for appropriate differentiation between MDD and BD with depressive episode. Several longitudinal studies are warranted confirming our findings.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Study concept and design: Shima Shahyad, Gila Pirzad Jahromi, Gholam Reza Kheirabadi, Muhammad Massaly. Acquisition of data: Muhammad Massaly, Gholam Reza Kheirabadi. Analysis and interpretation of data: Shima Shahyad, Gholam Reza Kheirabadi, Muhammad Massaly. Drafting of the manuscript: Shima Shahyad, Gila Pirzad Jahromi, Gholam Reza Kheirabadi, Muhammad Massaly. Critical revision of the manuscript for valuable intellectual content: Shima Shahyad, Gila Pirzad Jahromi, Gholam Reza Kheirabadi, Muhammad Massaly. Statistical analysis: Shima Shahyad, Gila Pirzad Jahromi. Administrative, technical, and material support: Gila Pirzad Jahromi, Gholam Reza Kheirabadi, Muhammad Massaly. Supervision: Shima Shahyad, Gila Pirzad Jahromi, Gholam Reza Kheirabadi.
References
- 1.Clemente AS, Diniz BS, Nicolato R, Kapczinski FP, Soares JC, Firmo JO, et al. Bipolar disorder prevalence: a systematic review and meta-analysis of the literature. Braz J Psychiatry. 2015;37:155–161. doi: 10.1590/1516-4446-2012-1693. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–481. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- 3.Cloutier M, Greene M, Guerin A, Touya M, Wu E. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. 2018;226:45–51. doi: 10.1016/j.jad.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes BS, Gama CS, Kauer-Sant'Anna M, Lobato MI, Belmonte-de-Abreu P, Kapczinski F. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: a potential adjunctive tool for differential diagnosis. J Psychiatr Res. 2009;43:1200–1204. doi: 10.1016/j.jpsychires.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira GS, Ceresér KM, Fernandes BS, Kauer-Sant'Anna M, Fries GR, Stertz L, et al. Decreased brain-derived neurotrophic factor in medicated and drug-free bipolar patients. J Psychiatr Res. 2009;43:1171–1174. doi: 10.1016/j.jpsychires.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Murray ML, Thompson M, Santosh PJ, Wong IC. Effects of the Committee on Safety of Medicines advice on antidepressant prescribing to children and adolescents in the UK. Drug Saf. 2005;28:1151–1157. doi: 10.2165/00002018-200528120-00009. [DOI] [PubMed] [Google Scholar]
- 8.Perlis RH, Uher R, Ostacher M, Goldberg JF, Trivedi MH, Rush AJ, et al. Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry. 2011;68:351–360. doi: 10.1001/archgenpsychiatry.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Praag HM. Why has the antidepressant era not shown a significant drop in suicide rates? Crisis. 2002;23:77–82. doi: 10.1027//0227-5910.23.2.77. [DOI] [PubMed] [Google Scholar]
- 10.Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8:1. doi: 10.1186/s40345-019-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strawbridge R, Young AH, Cleare AJ. Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat. 2017;13:1245–1262. doi: 10.2147/NDT.S114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skibinska M, Kapelski P, Dmitrzak-Weglarz M, Lepczynska N, Pawlak J, Twarowska-Hauser J, et al. Elevated epidermal growth factor (EGF) as candidate biomarker of mood disorders-longitudinal study in adolescent and young adult patients. J Clin Med. 2021;10:4064. doi: 10.3390/jcm10184064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HY, Kim YK. Different mechanisms between melancholic and atypical depression. In: Kim YK, editor. Major depressive disorder: cognitive and neurobiological mechanisms. IntechOpen; London: 2015. [DOI] [Google Scholar]
- 14.Malik S, Singh R, Arora G, Dangol A, Goyal S. Biomarkers of major depressive disorder: knowing is half the battle. Clin Psychopharmacol Neurosci. 2021;19:12–25. doi: 10.9758/cpn.2021.19.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziani PR, Feiten JG, Goularte JF, Colombo R, Antqueviezc B, Géa LP, et al. Potential candidates for biomarkers in bipolar disorder: a proteomic approach through systems biology. Clin Psychopharmacol Neurosci. 2022;20:211–227. doi: 10.9758/cpn.2022.20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148–158. doi: 10.1053/jhep.2001.20793. [DOI] [PubMed] [Google Scholar]
- 18.Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi: 10.1016/j.neuroscience.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, et al. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol. 2014;71:55–61. doi: 10.1001/jamaneurol.2013.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 21.Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 22.Gama CS, Andreazza AC, Kunz M, Berk M, Belmonte-de-Abreu PS, Kapczinski F. Serum levels of brain-derived neurotrophic factor in patients with schizophrenia and bipolar disorder. Neurosci Lett. 2007;420:45–48. doi: 10.1016/j.neulet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Monteleone P, Serritella C, Martiadis V, Maj M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord. 2008;10:95–100. doi: 10.1111/j.1399-5618.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 24.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunoni AR, Lopes M, Fregni F. A systematic review and meta- analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Jialal I, Devaraj S, Venugopal SK. C-reactive protein: risk marker or mediator in atherothrombosis? Hypertension. 2004;44:6–11. doi: 10.1161/01.HYP.0000130484.20501.df. [DOI] [PubMed] [Google Scholar]
- 28.Kuta AE, Baum LL. C-reactive protein is produced by a small number of normal human peripheral blood lymphocytes. J Exp Med. 1986;164:321–326. doi: 10.1084/jem.164.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J Affect Disord. 2013;150:456–459. doi: 10.1016/j.jad.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 30.Koosha P, Roohafza H, Sarrafzadegan N, Vakhshoori M, Talaei M, Sheikhbahaei E, et al. High sensitivity C-reactive protein predictive value for cardiovascular disease: a nested case control from Isfahan Cohort Study (ICS) Glob Heart. 2020;15:3. doi: 10.5334/gh.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Al-Sayegh H, Jabrah R, Wang W, Yan F, Zhang J. Asso-ciation between C-reactive protein and depression: modulated by gender and mediated by body weight. Psychiatry Res. 2014;219:103–108. doi: 10.1016/j.psychres.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Huang TL, Lin FC. High-sensitivity C-reactive protein levels in patients with major depressive disorder and bipolar mania. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:370–372. doi: 10.1016/j.pnpbp.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Chiou YJ, Huang TL. Brain-derived neurotrophic factor (BDNF) and bipolar disorder. Psychiatry Res. 2019;274:395–399. doi: 10.1016/j.psychres.2019.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Chang HH, Wang TY, Lee IH, Lee SY, Chen KC, Huang SY, et al. C-reactive protein: a differential biomarker for major depressive disorder and bipolar II disorder. World J Biol Psychiatry. 2017;18:63–70. doi: 10.3109/15622975.2016.1155746. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; Arlington: 2013. [DOI] [Google Scholar]
- 37.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the Beck Depression Inventory--second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185–192. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 38.Hossein Kaviani H, Mousavi A. Psychometric properties of the Persian version of Beck Anxiety Inventory (BAI) Tehran Univ Med J. 2008;66:136–140. [Google Scholar]
- 39.Elfving B, Buttenschøn HN, Foldager L, Poulsen PH, Andersen JH, Grynderup MB, et al. Depression, the Val66Met polymorphism, age, and gender influence the serum BDNF level. J Psychiatr Res. 2012;46:1118–1125. doi: 10.1016/j.jpsychires.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Soares AT, Andreazza AC, Rej S, Rajji TK, Gildengers AG, Lafer B, et al. Decreased brain-derived neurotrophic factor in older adults with bipolar disorder. Am J Geriatr Psychiatry. 2016;24:596–601. doi: 10.1016/j.jagp.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 41.Wu YS, Chiou YJ, Huang TL. Associations between serum brain-derived neurotrophic factors and bipolar disorder. Neuropsychiatry. 2017;7:968–973. doi: 10.4172/Neuropsychiatry.1000304. [DOI] [Google Scholar]
- 42.Fernandes BS, Gama CS, Ceresér KM, Yatham LN, Fries GR, Colpo G, et al. Brain-derived neurotrophic factor as a state- marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res. 2011;45:995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 44.Jeenger J, Singroha V, Sharma M, Mathur DM. C-reactive protein, brain-derived neurotrophic factor, interleukin-2, and stressful life events in drug-naive first-episode and recurrent depression: a cross-sectional study. Indian J Psychiatry. 2018;60:334–339. doi: 10.4103/psychiatry.IndianJPsychiatry_169_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z, Zhang C, Fan J, Yuan C, Huang J, Chen J, et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study. Br J Psychiatry. 2014;205:29–35. doi: 10.1192/bjp.bp.113.134064. [DOI] [PubMed] [Google Scholar]
- 46.Kunugi H, Hori H, Adachi N, Numakawa T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry Clin Neurosci. 2010;64:447–459. doi: 10.1111/j.1440-1819.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto K. A BDNF Val66Met polymorphism and ketamine-induced rapid antidepressant action. Clin Psychophar-macol Neurosci. 2012;10:59–60. doi: 10.9758/cpn.2012.10.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjahrir M, Roesyanto-Mahadi ID, Effendy E. Correlation between serum brain-derived neurotrophic factor level and depression severity in psoriasis vulgaris patients. Open Access Maced J Med Sci. 2019;7:583–586. doi: 10.3889/oamjms.2019.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y, Chiriboga DE, Pagoto SL, Rosal MC, Li W, Merriam PA, et al. Association between depression and C-reactive protein. Cardiol Res Pract. 2010;2011:286509. doi: 10.4061/2011/286509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evers AK, Veeh J, McNeill R, Reif A, Kittel-Schneider S. C-reactive protein concentration in bipolar disorder: association with genetic variants. Int J Bipolar Disord. 2019;7:26. doi: 10.1186/s40345-019-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giese M, Unternährer E, Hüttig H, Beck J, Brand S, Calabrese P, et al. BDNF: an indicator of insomnia? Mol Psychiatry. 2014;19:151–152. doi: 10.1038/mp.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molendijk ML, Haffmans JP, Bus BA, Spinhoven P, Penninx BW, Prickaerts J, et al. Serum BDNF concentrations show strong seasonal variation and correlations with the amount of ambient sunlight. PLoS One. 2012;7:e48046. doi: 10.1371/journal.pone.0048046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Min HJ, Cho HS, Kim SJ, Seok JH, Lee E, Jon DI. Association of the brain-derived neurotrophic factor gene and clinical features of bipolar disorder in Korea. Clin Psychopharmacol Neurosci. 2012;10:163–167. doi: 10.9758/cpn.2012.10.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71:651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sklar P, Gabriel SB, McInnis MG, Bennett P, Lim Y, Tsan G, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7:579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]