Abstract
The arginine gingipains RgpA and RgpB of Porphyromonas gingivalis are well-documented virulence factors of this organism. Structurally, RgpA and RgpB have nearly identical catalytic domains, while RgpA possesses an additional hemagglutinin domain. In this study, we examined the abilities of these proteins to elicit protection against P. gingivalis-mediated oral bone loss in a murine oral challenge model. Mice immunized subcutaneously with heat-killed P. gingivalis or purified RgpA or RgpB possessed elevated levels of P. gingivalis-specific immunoglobulin G; however, only the animals immunized with P. gingivalis whole cells or RgpA were protected from maxillary bone loss. These data suggest that immunization with RgpA stimulates the production of hemagglutinin domain-specific antibodies, which contribute to the prevention of P. gingivalis-mediated periodontal disease.
Periodontal diseases in the past have been characterized as a broad group of infections with multiple bacterial etiologies (19, 26, 27). However, recent evidence strongly suggests that the primary pathogen responsible for adult progressive periodontal disease is Porphyromonas gingivalis. Following colonization of the gingival crevice, P. gingivalis initiates inflammation of the gingivae, destruction of periodontal tissue, loss of alveolar bone, and in severe cases, exfoliation of teeth. This organism possesses a variety of virulence factors, including fimbriae, lectin-like adhesins, capsular polysaccharide, lipopolysaccharide, hemagglutinins, and hemolysins, as well as numerous proteolytic enzymes (10, 12, 20, 31). Some of the proteases, the gingipains, a group of cysteine proteases produced by P. gingivalis, have received considerable attention. Gingipains are classified into two groups based on substrate specificity. Gingipains R (RgpA and RgpB) cleave proteins after arginine (R) residues and are encoded by two similar genes, rgpA and rgpB, while gingipain K (Kgp) cleaves proteins after lysine (K) residues (21, 28). The mature form of RgpA possesses both a catalytic domain and a hemagglutinin domain, while RgpB possesses only a catalytic domain (30). There is a high degree of homology between the catalytic domains of RgpA and RgpB at both the DNA and protein levels, while the hemagglutinin domain of RgpA is similar to both P. gingivalis hemagglutinin HagA and the tla gene product (9). In vitro studies have shown that gingipains are able to degrade both collagen and fibronectin, inactivate protease inhibitors, degrade immunoglobulins, and facilitate iron acquisition (10, 25, 29). Furthermore, they are able to destroy host coagulation cascade proteins, degrade complement, and digest various cytokines (3, 5, 10, 13–15).
Several studies have demonstrated that immunization of animals with relevant P. gingivalis antigens, including fimbriae and porphypain 2 (gingipain K), as well as HagA and HagB, may provide protection against subsequent P. gingivalis challenge in various animal models (6, 7, 16, 22). Genco et al. (9) demonstrated that treatment of P. gingivalis with various protease inhibitors prior to challenge of mice significantly reduced morbidity and mortality compared to the morbidity and mortality of animals challenged with untreated P. gingivalis. More recently, it was observed that mice immunized with RgpA or RgpB produced increased levels of immunoglobulin G (IgG) and were protected from subsequent P. gingivalis challenge when a chamber infection model was used (9). These observations correlate well with human studies, which have shown that patients with rapidly progressive periodontal disease possess elevated levels of serum antibody to the hemagglutinin domain of P. gingivalis RgpA (23).
Recently, Baker et al. (2) demonstrated that oral challenge of mice with P. gingivalis stimulated oral bone loss and that the observed bone loss occurred in a site-specific manner. Furthermore, it appears that oral bone loss is linked to T-cell activation (1). In the present study we assessed whether the arginine gingipains could be vaccine candidates for prevention of oral bone loss in a murine model.
P. gingivalis and gingipain preparation.
P. gingivalis A7A1-28 (obtained from Pamela Baker, Bates College, Lewiston, Maine) was grown anaerobically on anaerobic blood agar plates supplemented with hemin and menadione (BBL, Cockeysville, Md.). Bacterial growth was collected from plates and suspended in sterile phosphate-buffered saline (pH 7.2), and the optical density at 660 nm was adjusted to either 3.0 (approximately 1 × 1010 CFU/ml) for gavage of mice or 0.3 for immunizations and enzyme-linked immunosorbent assay (ELISA) plate coating. Heat-killed P. gingivalis was prepared by incubating 1 ml of cells, adjusted to an optical density at 660 nm of 0.3 in phosphate-buffered saline, at 60°C for 5 min, and an aliquot of the preparation was plated to confirm the loss of P. gingivalis viability. Gingipains RgpA and RgpB were isolated and purified as previously described (9) and were kindly provided by Jan Potempa (Jagiellowian University, Cracow, Poland).
Mouse immunization and challenge studies.
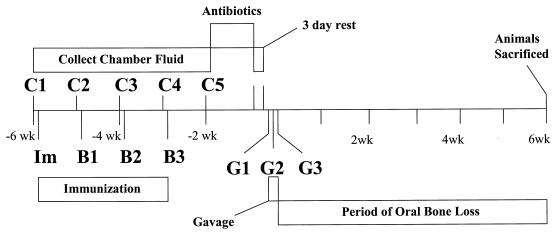
A stainless steel wire chamber was surgically implanted under the skin of each 6- to 8-week-old BALB/c mouse (Jackson Laboratories, Bar Harbor, Maine) (8). Preimmune chamber fluid samples were collected from each mouse, and the animals were separated into groups (eight animals per group), including a nonimmunized group and groups that were immunized subcutaneously (100 μl/injection) with Freund's complete adjuvant or with heat-killed P. gingivalis or adjuvant containing either RgpA and RgpB (100 μg/injection). The animals then received weekly booster doses for 3 weeks with the respective antigen suspended in incomplete adjuvant (Fig. 1). Prior to each immunization, chamber fluid samples were collected from each mouse, pooled by group, and stored frozen until P. gingivalis-specific IgM or IgG ELISAs were performed. Following immunization mice were challenged orally three times (approximately 1 × 109 CFU per challenge) with P. gingivalis A7A1-28 by the method of Baker et al. (2). P. gingivalis colonization of maxillary molars of mice was assessed with sterile paper points (2). Forty-two days after gavage, the mice were sacrificed, the heads were collected, and each skull was cleaned with hot water, 3% hydrogen peroxide, and 0.1% hypochlorite and was stained with 1% methylene blue. Seven linear (millimeter) and three area (square millimeter) measurements were obtained from the left and right sets of maxillary molars from each skull by using a stereomicroscope with an onscreen computer-aided measurement package (Image-Pro Plus V 3.0; Media Cybernetics, Silver Spring, Md.). These experiments were performed twice for a total of 16 animals per group. Means ± standard errors of the means were determined for all linear and area measurements. The Mann-Whitney nonparametric test was performed to compare groups (InStat V 2.0; Graphpad Software, San Diego, Calif.). Significant differences between groups were determined by using a P value of <0.05.
FIG. 1.
Time line of events during immunization-oral challenge experiments. Groups of BALB/c mice received immunizations (Im, B1 to B3), had their sera collected (C1 to C5), and were orally gavaged with P. gingivalis A7A1-28 (G1 to G3). After a 42-day oral bone loss period, mice were sacrificed.
Immunization of mice with RgpA and RgpB stimulates P. gingivalis-specific antibodies. (i) ELISA analysis.
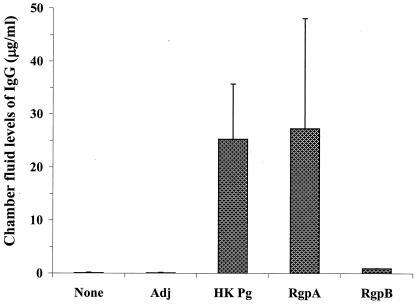
ELISAs were performed with all chamber fluid samples to detect the amounts of P. gingivalis-specific IgM and IgG in each sample. Immunol 4Hbx ELISA plates (Dynex, Chantilly, Va.) were coated with 50 μl of formaldehyde-fixed P. gingivalis A7A1-26 in carbonate-bicarbonate buffer (pH 9.6) per well and were blocked with 2% bovine serum albumin, serial twofold dilutions of chamber fluid samples were added to the wells, and the plates were incubated overnight. The wells were washed, and 100-μl portions of either goat anti-mouse IgM- or IgG-alkaline phosphatase conjugate (Sigma, St. Louis, Mo.) were added to the wells. The plates were developed with p-nitrophenyl phosphate and were read at 405 nm. The concentration of P. gingivalis-specific IgG was determined from a murine IgG standard curve ELISA preparation that was run in parallel with test samples. Immunization of mice with heat-killed P. gingivalis, RgpA, or RgpB resulted in maximal levels of P. gingivalis-specific IgM within 3 weeks (data not shown). The level of P. gingivalis-specific IgG present in chamber fluid samples from mice that were immunized with heat-killed P. gingivalis was robust (Fig. 2). Immunization with RgpA also resulted in a potent P. gingivalis-specific IgG response similar to that observed after immunization with heat-killed P. gingivalis (Fig. 2). Interestingly, animals immunized with RgpB produced only low levels of P. gingivalis-specific IgG. Nonimmunized animals, as well as animals given adjuvant alone, produced negligible amounts of P. gingivalis-specific IgM or IgG.
FIG. 2.
Quantification of P. gingivalis-specific IgG levels in week 4 chamber fluids of BALB/c mice following immunization. Chamber fluids were collected from nonimmunized (None) mice or from animals that received adjuvant only (Adj), heat-killed P. gingivalis (HK Pg), RgpA, or RgpB, and the levels of P. gingivalis-specific IgG were determined. The data are means based on two experiments (n = 16 mice), and the error bars indicate standard deviations.
(ii) Immunoprobe analysis.
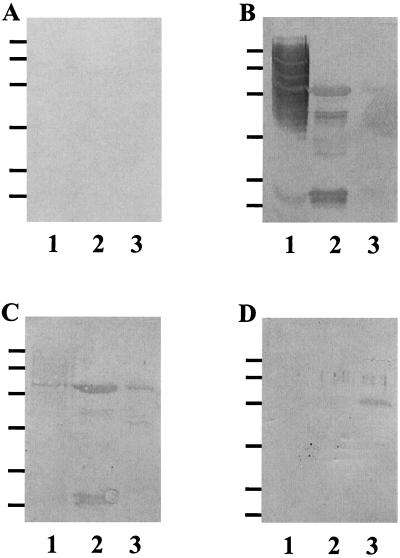
To determine which polypeptides of RgpA and RgpB were recognized by chamber fluid samples from each group of immunized animals, P. gingivalis whole-cell lysates, RgpA, and RgpB were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (18). When purified RgpA and RgpB are denatured and reduced and then separated on a SDS-PAGE gel, five bands, including a 45-kDa band (catalytic domain) and 44-, 27-, 17-, and 15-kDa bands (four polypeptides of the hemagglutinin domain), are resolved or a single 50-kDa band is resolved (30). The blots were either stained with Simply Blue SafeStain (Invitrogen, Carlsbad, Calif.) (Fig. 3A) or transferred to polyvinylidene difluoride membranes, probed with chamber fluid samples, and developed with goat anti-mouse IgG-alkaline phosphatase conjugate and with phosphatase substrate (Bio-Rad). As expected, week 4 chamber fluid samples obtained from untreated animals failed to recognize P. gingivalis whole-cell lysates, RgpA, or RgpB on immunoblots (Fig. 3A). Chamber fluid samples collected from animals immunized with heat-killed P. gingivalis recognized a broad range of high-molecular-mass antigens and several lower-molecular- mass bands in the P. gingivalis whole-cell lysate (Fig. 3B). Furthermore, these chamber fluid samples recognized each of the five polypeptide bands of RgpA, as well as RgpB (Fig. 3B). Chamber fluid samples obtained from mice immunized with RgpA recognized all polypeptides of the RgpA complex, as well as RgpB. Additionally, these chamber fluid samples recognized a 44-kDa band in the P. gingivalis whole-cell lysate corresponding to the high-molecular-mass hemagglutinin domain of RgpA (Fig. 3C). Chamber fluid from mice immunized with RgpB recognized purified RgpB but failed to recognize any domain of RgpA or any antigens in P. gingivalis whole-cell lysates (Fig. 3D).
FIG. 3.
Immunoprobe analysis of week 4 murine chamber fluid samples for P. gingivalis and each of the gingipains. A 10-μl aliquot of P. gingivalis whole cells (∼1 × 107 cells) (lanes 1) and 10-μl portions of 1-mg/ml solutions of RgpA (lane 2) and RgpB (lane 3) reduced in sample buffer were separated by SDS-PAGE and were transferred to Immobilon P membranes. The blots were probed with week 4 chamber fluid samples from nonimmunized animals (A) or from mice immunized with P. gingivalis (B), RgpA (C), or RgpB (D) and developed. The positions of molecular mass markers are indicated on the left (96, 67, 43, 30, 20.1, and 14.4 kDa from top to bottom).
Immunization of mice with RgpA but not RgpB protects the animals from P. gingivalis-induced bone loss.
Immunized animals were subsequently orally challenged with P. gingivalis, and the level of oral bone loss was assessed 42 days after the challenge (Fig. 1). Since oral bone loss during rapidly progressive periodontal disease occurs in both an overall manner and a site-specific manner, seven linear and three area measurements were obtained for each set of maxillary molars per animal. The overall bone loss in each group was determined by pooling the data obtained from each site, and comparisons between groups were subsequently made. The level of site-specific bone loss was also determined by separating data by site, and comparisons were made between groups (2). Overall mean linear (0.204 ± 0.003 mm) and overall area (0.226 ± 0.022 mm2) measurements were obtained for nonimmunized, unchallenged, age-matched BALB/c mice. Animals orally challenged with P. gingivalis showed a marked increase in the overall linear measurements compared to the measurements obtained for the unchallenged group (P < 0.0001) (Table 1). A comparison of linear and area measurements obtained for each site from unchallenged and P. gingivalis-challenged mice demonstrated that oral challenge induced significant bone loss primarily at linear sites 6 and 7 and area site 3 (Tables 1 and 2). Morphometric analysis of murine maxillary molars indicated that there was no significant bone loss induced by P. gingivalis oral challenge at linear sites 1 to 5 or area sites 1 and 2 (data not shown).
TABLE 1.
Linear measurements of maxillary molars obtained from BALB/c mice 42 days after gavage with P. gingivalis A7A1-28a
| Immunogen | P. gingivalis challenge | Linear measurements (mm)
|
||
|---|---|---|---|---|
| Overall | Linear site 6 | Linear site 7 | ||
| None | None | 0.204 ± 0.003 | 0.189 ± 0.005 | 0.228 ± 0.007 |
| None | + | 0.230 ± 0.003∗ | 0.243 ± 0.005∗ | 0.278 ± 0.005∗ |
| Heat-killed P. gingivalis | + | 0.204 ± 0.003∗∗ | 0.188 ± 0.004∗∗ | 0.222 ± 0.005∗∗ |
| RgpA | + | 0.203 ± 0.003∗∗ | 0.184 ± 0.006∗∗ | 0.221 ± 0.005∗∗ |
| RgpB | + | 0.226 ± 0.003 | 0.227 ± 0.006 | 0.262 ± 0.006 |
Means ± standard errors of the means were determined for overall linear measurements (grouping of sites 1 to 7) and for site-specific analyses of mice (n = 16). Statistical comparisons were made between groups within each measurement type. ∗, P < 0.0001 compared with nonimmunized, unchallenged mice; ∗∗, P < 0.0001 compared with animals orally gavaged with P. gingivalis A7A1-28.
TABLE 2.
Area measurements of maxillary molars obtained from BALB/c mice 42 days after gavage with P. gingivalis A7A1-28a
| Immunogen | P. gingivalis challenge | Area measurements (mm2)
|
|
|---|---|---|---|
| Overall | Area site 3 | ||
| None | None | 0.226 ± 0.022 | 0.111 ± 0.002 |
| None | + | 0.250 ± 0.021 | 0.128 ± 0.002∗ |
| Heat-killed P. gingivalis | + | 0.232 ± 0.020 | 0.115 ± 0.002∗∗ |
| RgpA | + | 0.219 ± 0.019 | 0.109 ± 0.003∗∗ |
| RgpB | + | 0.232 ± 0.019 | 0.122 ± 0.002 |
Means ± standard errors of the means were determined for overall area points (grouping of sites 1 to 3) or for area site 3 of mice (n = 16). Statistical comparisons were made between groups within each measurement type. ∗, P < 0.0001 compared with nonimmunized, unchallenged mice; ∗∗, P < 0.0001 compared with animals orally gavaged with P. gingivalis A7A1-28.
Immunization of mice with heat-killed P. gingivalis prior to oral challenge resulted in significant reductions in both overall and site-specific bone loss compared to the values obtained for the P. gingivalis-challenged group (P < 0.0001) (Table 1), and the results resembled the results obtained for unchallenged animals (P > 0.9) (Table 1). Likewise, immunization of mice with RgpA protected against oral bone loss. Interestingly, the level of protection observed after RgpA immunization was similar to that observed in animals immunized with heat-killed P. gingivalis (overall, P > 0.4; linear site 6, P > 0.8; linear site 7, P > 0.6) (Table 1). In contrast, immunization with RgpB failed to protect mice from P. gingivalis-induced oral bone loss. The area measurement data for site 3 confirmed the linear measurement data (Table 2).
Concluding remarks.
We previously demonstrated that RgpA and RgpB represent a group of vaccine candidates that could be used for prevention of P. gingivalis infection (9); however, it was recognized that the results obtained may not accurately represent what occurs during P. gingivalis infection in the oral cavity (11). The results obtained in the present study demonstrate that immunization of mice with RgpA or heat-killed P. gingivalis prevents oral bone loss stimulated by P. gingivalis. However, immunization of mice with RgpB failed to stimulate protection against P. gingivalis oral challenge. Animals immunized with RgpA possessed modest levels of P. gingivalis-specific IgM and high levels of P. gingivalis-specific IgG, while mice immunized with RgpB had a modest P. gingivalis-specific IgM response and a P. gingivalis-specific IgG titer significantly lower than that observed for mice immunized with RgpA. Low levels of P. gingivalis-specific IgM and high levels of P. gingivalis-specific IgG have also been observed following immunization of rats with P. gingivalis HagB (16). As RgpA and RgpB possess similar catalytic domains and only RgpA possesses a hemagglutinin domain, we concluded that protection and the enhanced P. gingivalis-specific IgG levels were directed to the hemagglutinin domain of RgpA. This conclusion was supported by our observation that chamber fluid samples from RgpA-immunized mice, but not chamber fluid samples from RgpB-immunized mice, recognized a 44-kDa band in P. gingivalis whole-cell lysates, which corresponded to the major hemagglutinin domain of RgpA. Similar blots probed with chamber fluid samples from mice immunized with RgpB failed to detect this band. There are two possible explanations for this: (i) RgpB is not exposed at high levels on the surface of P. gingivalis, or (ii) the presence of the hemagglutinin domain stimulates the production of an antibody subset that efficiently recognizes P. gingivalis. O'Brien-Simpson et al. have reported results similar to observations made in the present study, as immunization of mice with RgpA-Kgp hemagglutinin domain peptides induced a potent IgG response and stimulated a protective host response when a murine lesion model was used (24).
Previously, we reported that immunization of mice with either RgpA or RgpB stimulated protection against P. gingivalis challenge when the subcutaneous chamber model was used. It is not clear why RgpB immunization provided protection in the subcutaneous model but failed to provide protection in the oral challenge model. Based on our observations, a likely explanation is that immunization with RgpB did not result in a sufficiently high level of P. gingivalis-specific IgG in serum. As a result, the low levels of P. gingivalis-specific antibody elicited by RgpB were unable to modulate either P. gingivalis colonization or subsequent oral bone loss. It is possible that in the subcutaneous challenge model, RgpB immunization elicited elevated levels of antigen-primed immune cells which were localized at this site prior to challenge with P. gingivalis. This effect could enhance localized P. gingivalis killing and thus suggests that RgpB elicited a protective response. Booth et al. (4) reported that localized administration of a P. gingivalis-specific monoclonal antibody at severely infected subgingival sites significantly reduced subsequent P. gingivalis recolonization in periodontal patients. Although the protection observed was transient, this study demonstrated that a specific antibody is able to promote selective removal of P. gingivalis from the oral cavity. In addition, Klausen et al. (17) demonstrated that rats immunized with P. gingivalis produced elevated levels of serum and salivary antibodies and that immunized animals were protected from oral bone loss elicited by P. gingivalis challenge. Taken together, these observations, in conjunction with our data, may suggest that a critical level of P. gingivalis-specific antibody is necessary to prevent P. gingivalis colonization in the oral cavity.
In summary, we demonstrated that immunization of mice with RgpA can stimulate production of both P. gingivalis- and RgpA-specific IgG primarily directed to the hemagglutinin domain of this protein. The elevated levels of this antibody coincide with protection against oral bone loss elicited by P. gingivalis. Taken together, these data suggest that RgpA is a novel vaccine candidate that could be used for prevention of periodontal disease caused by P. gingivalis.
Acknowledgments
We thank Salomon Amar for the use of his light microscopy station to obtain linear and area measurements and Lee Wetzler for helpful criticism in preparing the manuscript.
This work was supported in part by Public Health Science grant 12517 awarded to C.A.G. and by National Research Service Award grant DE05739-02 awarded to F.C.G.
REFERENCES
- 1.Baker P J, Dixon M, Evans R T, Dufour L, Johnson E, Roopenian D C. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker P J, Evans R T, Roopenian D C. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch Oral Biol. 1994;39:1035–1040. doi: 10.1016/0003-9969(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Banbula A, Bugno M, Kuster A, Heinrich P C, Travis J, Potempa J. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1999;261:598–602. doi: 10.1006/bbrc.1999.1075. [DOI] [PubMed] [Google Scholar]
- 4.Booth V, Ashley F P, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–427. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calkins C C, Platt K, Potempa J, Travis J. Inactivation of tumor necrosis factor-alpha by proteinases (gingipains) from the periodontal pathogen, Porphyromonas gingivalis. Implications of immune evasion. J Biol Chem. 1998;273:6611–6614. doi: 10.1074/jbc.273.12.6611. [DOI] [PubMed] [Google Scholar]
- 6.Dusek D M, Progulske-Fox A, Brown T A. Systemic and mucosal immune responses in mice orally immunized with avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans R T, Klausen B, Sojar H T, Bedi G S, Sfintescu C, Ramamurthy N S, Golub L M, Genco R J. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992;60:2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genco C A, Cutler C W, Kapczynski D, Maloney K, Arnold R R. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genco C A, Odusanya B M, Potempa J, Mikolajczyk-Pawlinska J, Travis J. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infect Immun. 1998;66:4108–4114. doi: 10.1128/iai.66.9.4108-4114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genco C A, Potempa J, Mikolajczyk-Pawlinska J, Travis J. Role of gingipains R in the pathogenesis of Porphyromonas gingivalis-mediated periodontal disease. Clin Infect Dis. 1999;28:456–465. doi: 10.1086/515156. [DOI] [PubMed] [Google Scholar]
- 11.Genco C A, Van Dyke T, Amar S. Animal models for Porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998;6:444–449. doi: 10.1016/s0966-842x(98)01363-8. [DOI] [PubMed] [Google Scholar]
- 12.Holt S C, Kesavalu L, Walker S, Genco C A. Virulence factors of Porphyromonas gingivalis. Periodontology. 2000;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 13.Imamura T, Pike R N, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Invest. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imamura T, Potempa J, Tanase S, Travis J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J Biol Chem. 1997;272:16062–16067. doi: 10.1074/jbc.272.25.16062. [DOI] [PubMed] [Google Scholar]
- 15.Jagels M A, Travis J, Potempa J, Pike R, Hugli T E. Proteolytic inactivation of the leukocyte C5a receptor by proteinases derived from Porphyromonas gingivalis. Infect Immun. 1996;64:1984–1991. doi: 10.1128/iai.64.6.1984-1991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz J, Black K P, Michalek S M. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect Immun. 1999;67:4352–4359. doi: 10.1128/iai.67.9.4352-4359.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klausen B, Evans R T, Ramamurthy N S, Golub L M, Sfintescu C, Lee J Y, Bedi G, Zambon J J, Genco R J. Periodontal bone level and gingival proteinase activity in gnotobiotic rats immunized with Bacteroides gingivalis. Oral Microbiol Immunol. 1991;6:193–201. doi: 10.1111/j.1399-302x.1991.tb00477.x. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Landi L, Amar S, Polins A S, Van Dyke T E. Host mechanisms in the pathogenesis of periodontal disease. Curr Opin Periodontol. 1997;4:3–10. [PubMed] [Google Scholar]
- 20.Malek R, Fisher J G, Caleca A, Stinson M, van Oss C J, Lee J Y, Cho M I, Genco R J, Evans R T, Dyer D W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikolajczyk-Pawlinska J, Kordula T, Pavloff N, Pemberton P A, Chen W C, Travis J, Potempa J. Genetic variation of Porphyromonas gingivalis genes encoding gingipains, cysteine proteinases with arginine or lysine specificity. Biol Chem. 1998;379:205–211. doi: 10.1515/bchm.1998.379.2.205. [DOI] [PubMed] [Google Scholar]
- 22.Moritz A J, Cappelli D, Lantz M S, Holt S C, Ebersole J L. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–697. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien-Simpson N M, Black C L, Bhogal P S, Cleal S M, Slakeski N, Higgins T J, Reynolds E C. Serum immunoglobulin G (IgG) and IgG subclass responses to the RgpA-Kgp proteinase-adhesin complex of Porphyromonas gingivalis in adult periodontitis. Infect Immun. 2000;68:2704–2712. doi: 10.1128/iai.68.5.2704-2712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien-Simpson N M, Paolini R A, Reynolds E C. RgpA-Kgp peptide-based immunogens provide protection against Porphyromonas gingivalis challenge in a murine lesion model. Infect Immun. 2000;68:4055–4063. doi: 10.1128/iai.68.7.4055-4063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake D B, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- 26.Okuda K. Bacteriological diagnosis of periodontal disease. Bull Tokyo Dent Coll. 1994;35:107–119. [PubMed] [Google Scholar]
- 27.Oliver R C, Brown L J, Loe H. Periodontal diseases in the United States population. J Periodontol. 1998;69:269–278. doi: 10.1902/jop.1998.69.2.269. [DOI] [PubMed] [Google Scholar]
- 28.Pavloff N, Pemberton P A, Potempa J, Chen W C, Pike R N, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. A new member of an emerging family of pathogenic bacterial cysteine proteinases. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 29.Pike R N, Potempa J, McGraw W, Coetzer T H, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thogersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 31.Schifferle R E, Reddy M S, Zambon J J, Genco R J, Levine M J. Characterization of a polysaccharide antigen from Bacteroides gingivalis. J Immunol. 1989;143:3035–3042. [PubMed] [Google Scholar]