Abstract
Mapping the eco-evolutionary factors shaping the development of animals’ behavioural phenotypes remains a great challenge. Recent advances in ‘big behavioural data’ research—the high-resolution tracking of individuals and the harnessing of that data with powerful analytical tools—have vastly improved our ability to measure and model developing behavioural phenotypes. Applied to the study of behavioural ontogeny, the unfolding of whole behavioural repertoires can be mapped in unprecedented detail with relative ease. This overcomes long-standing experimental bottlenecks and heralds a surge of studies that more finely define and explore behavioural–experiential trajectories across development. In this review, we first provide a brief guide to state-of-the-art approaches that allow the collection and analysis of high-resolution behavioural data across development. We then outline how such approaches can be used to address key issues regarding the ecological and evolutionary factors shaping behavioural development: developmental feedbacks between behaviour and underlying states, early life effects and behavioural transitions, and information integration across development.
Keywords: behavioural development, big data, individuality, personality, tracking
1. Background
During the last decade, the rapid emergence of ‘big behavioural data’ research—the high-resolution tracking of individuals and the harnessing of that data with powerful machine learning techniques [1–6]—has spurred major advances in fields as diverse as behavioural genomics and transcriptomics [7–9], behavioural neuroscience [10–15], collective decision-making [16–19] and movement ecology [20–23]. Huge untapped potential remains, however, in applying big data approaches to understand the development of behaviour. In sharp contrast to previous data-limited approaches where only subsets of animals’ developmental trajectories are measured, big behavioural data research allows near-continuous monitoring of animals throughout their entire (or substantial parts of) development. We believe that this innovation, paired with timely advancements in computation, data storage, and tailored analytical tools, represents a watershed moment for understanding the ecology and evolution of behavioural development.
In our review, we begin (§2) by briefly reviewing the state-of-the-art approaches that allow the collection and analysis of behavioural data at increasing resolution. Then, we discuss in detail (§3) how these innovations in measuring and modelling behaviour can be productively applied to fundamental open questions regarding the rules governing the unfolding of behaviour: mapping the nature of developmental feedbacks between behaviour and underlying states, understanding early-life effects and behavioural transitions across development, and understanding information integration across development. Big behavioural data have greatly expanded the empirical toolkit with which to investigate these fundamental issues in behavioural development, and they have also spurred a new generation of conceptual models of behaviour. Throughout, we focus on the links between contemporary and potential future empirical advances and the set of theory and technical models that this new wealth of data can inform and test. Indeed, many of the state-of-the-art methods in behavioural analysis that we review are increasingly simple to use and ready to deploy, but their application to existing theory in behavioural development is largely unrealized; quick progress in this area is thus achievable, as we will outline. In sum, we believe that this mutual feedback between new data sources and novel theory will generate major new insights into one of the foundational elements of the study of behaviour, behavioural ontogeny.
2. New methods, measures and means of defining behaviour
One inherent difficulty with measuring the ontogeny of most traits—behaviour among them—is that, for many organisms, these traits often take considerable time to fully develop. Historically, behavioural researchers have tried to circumvent this issue in several ways (table 1): by limiting the period in development over which a trait is measured; by measuring a trait during relatively short periods during development interspersed by larger intervals of time without measurements; by manipulating early-life conditions and investigating the consequences later in life; and/or by adopting comparative approaches across individuals of different ages. These methods, while providing targeted information about certain periods during development or coarse-grained information about the arc of development, may miss critical time windows and complex nonlinearities in behavioural development, thereby hampering our ability to gain a fuller understanding of the factors shaping behavioural development. Additionally, constraints on data collection have also meant that most studies focus on the development of one or a few behavioural traits and thus miss out on quantifying biologically meaningful correlations in developmental time series of suites of traits.
Table 1.
A stylized overview of the main methods used to study the development of behaviour, including near-continuous behavioural measurements throughout development, that advances in tracking, computational and analytical tools have recently made possible.
| methods of quantifying behavioural development | example references |
|---|---|
(1) concentrated measurements early in life
|
[24,25] |
(2) periodic measurements throughout development 
|
[26–30] |
(3) experimental manipulation early in life, behavioural measurements before/after 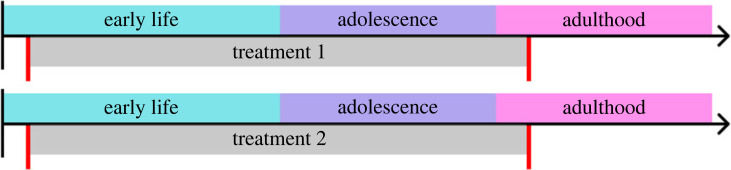
|
[31–35] |
(4) comparative approach across individuals of different ages 
|
[29,36–38] |
(5) near-continuous behavioural measurements throughout development 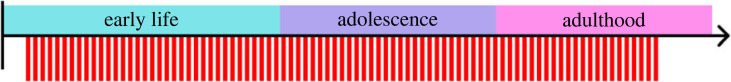
|
[39,40] |
Whereas collecting highly time-resolved data on behaviour through the entire course of development was once often prohibited by daunting logistics and limits on time and effort, the advent of sophisticated automated tracking technology, more advanced data storage infrastructure, and a host of powerful, data-hungry analytical tools can produce and parse these datasets with relatively low cost in terms of both person-hours and monetary investment [10,41,42]. We are now at a critical juncture where early studies have proven the worth of these new technologies for quantifying and conceptualizing behaviour in new ways [10,43–46], but where heretofore surprisingly limited consideration has been paid to the application of these tools to behavioural development. For the remainder of this section, we focus on recent innovations in both the measuring and modelling of behaviour that lay the foundation for the application of these technologies to answer major open questions in behavioural development.
(a) . Measurement innovations
Behavioural tracking technology has come a long way in the last decade, with sub-second temporal resolution in movement data now being standard for many automated real-time trackers. Such datasets, often as simple as a time series of two- or three-dimensional coordinate points in space, are commonly obtained via GPS devices, PIT tags, or video tracking, and numerous tracking software automate the process of converting coordinate point data into behavioural metrics such as activity rates, space use, velocity, etc. [2,6,47–50]. Crucially, many of these tools are specifically designed for keeping track of individuals through time, even when observed in groups, allowing behavioural patterns of many individuals to be tracked simultaneously [6,48,49]. Furthermore, rapid advances are being made in the identification and tracking of postural movements of body segments, allowing studies of fine motor development of social interactions, mating displays, resource manipulation, etc. [2,51]. Such approaches have vastly increased both the temporal resolution and overall duration of data that can be collected, allowing animals to be tracked throughout development with minimal intervention. Tracking technology is not just limited to tracking animal behaviour during development: some of the greatest promise lies in pairing high-resolution behavioural tracking data with complementary, non-behavioural data types with similar resolution. For instance, high-resolution behavioural data coupled with other high-throughput data sources have provided insight into both the neurological [14,52] and transcriptomic correlates of behaviour [8]. Internal state variables such as body condition can be estimated directly from video frames in the case of video tracking [53]. High-resolution physiological data can be obtained from a quickly expanding array of non-invasive biologging tools [54,55], such as heart rate monitors [56,57] or thermal imaging [58–60], and may help infer stress levels, respiration rate or energetic state when coupled with behavioural data. Other approaches use both supervised and unsupervised machine learning methods to infer underlying internal state variables from high-resolution behavioural tracking data themselves [61–64]. We believe there is also tremendous value in the simultaneous tracking of salient environmental variables and behaviour [65–67], allowing researchers to map detailed behavioural–experiential trajectories (i.e. interactions between behaviours and the sequence of experiences that result from interacting with the environment) and track environmental drivers of behaviour throughout development, getting us a step closer to understanding the ecological forces shaping behavioural development.
(b) . Data-driven definitions of behavioural axes
Behavioural biologists have long wrestled with how to identify and classify behavioural ‘traits’ [68,69], and while novel methods of measuring and conceptualizing behaviour may not be a panacea, they offer progress. Equipped with highly resolved behavioural time series over ontogeny, researchers have a quickly growing toolset of analytical techniques that permit, for example, high-throughput auto-labelling of observer-defined behaviours [70], unsupervised learning of new behavioural classes, detection of hierarchical sub-structure in behaviour, or modelling of behavioural transition rates across a wide range of timescales [10,12,42,71].
Big behavioural datasets are large and often inherently multi-dimensional, and while researchers may opt for dramatically reducing the dimensionality of these datasets by, for instance, calculating simple behavioural metrics directly from raw data (e.g. activity rates, velocity, position in relation to focal point or social partner, etc.), a range of tools can now be applied that retain the high dimensionality of these datasets to the degree that it is informative, thus moving a step closer to quantifying the development of integrated behavioural repertoires. While the specific toolkit in parsing big behavioural datasets will vary depending on the dataset and the questions to be answered, a common challenge among most analyses is reducing collinearities in the data structure while maintaining informative variation and interpretability. This task—determining the salient axes over which behaviours vary through ontogeny—sets the stage for then delimiting specific sets of related behaviours (also known as behavioural ‘classes’ or ‘clusters’) along these axes.
One of the simplest approaches for extracting behavioural axes in large, highly dimensional datasets is principal component analysis (PCA) [72]. More sophisticated techniques such as spectral analysis [73] or time frequency analysis [43,45] are specifically designed for time-series analysis and have also been productively applied to behavioural time series as a step towards defining the axes of behavioural variation across development. The important point is that, rather than researchers pre-defining which behavioural axes are important, the primary goal of these techniques is to leverage variation in the data themselves in order to determine axes of behavioural variation among a suite of (often correlated) behavioural metrics. In a dataset of bee behaviours across their adult lifetime, for instance, PCA was applied to a set of metrics derived from tracking data (e.g. location in the hive and movement metrics such as speed and space use), allowing the authors to avoid redundancies among many potentially non-independent metrics and instead define orthogonal behavioural axes ordered by the degree to which they explained variation in the overall dataset [39]. PC axes and more complex, nonlinear dimension reduction techniques [74,75] are also highly useful as visualization tools: high-dimensional behaviour such as specific posture patterns [43,44,46,76,77], social interactions [78] or microhabitat-related behaviours [79] have all been mapped using computational approaches to defining behavioural axes. The diversity of tools in this toolset allows researchers to accommodate the many unique challenges that might arise when, for example, behavioural covariance structures change across development (e.g. thus affecting the ‘loadings’ in a PCA), or when trade-offs between mapping ‘local’ versus ‘global’ behavioural variation are rebalanced [80].
Using statistical techniques to define the major axes of variation in behavioural time-series data has the potential to offer insights into fundamental questions about the structure and development of individual behavioural variation, that is, individuality. Traditionally, studies on behavioural individuality and animal personality have sought to describe behavioural variation along researcher-defined axes such as ‘boldness’, ‘exploration’ and ‘sociality’, a modification of the ‘Big Five’ model of personality variation imported from human psychology [81]. While this tradition is now firmly embedded in animal personality research, a steady flow of critique has questioned both the wisdom of defining traits a priori (e.g. whether something like ‘boldness’ or ‘exploration’ holds the same meaning across diverse species) and the methodological status quo of measuring behaviours along pre-defined axes (e.g. whether a certain assay actually measures ‘boldness’, ‘exploration’, etc.) [69,82–84]. Data-driven statistical approaches to defining behavioural axes have indeed been offered as alternatives to pre-defining such axes when quantifying individuality [85,86] (but see [87]), and the growing availability of high-dimensional behavioural datasets increases their appeal. Such methods may allow more comparability across periods of development, populations or even species, by first inferring the behavioural axes using data from all developmental time points/populations/species in an analysis and then asking how time points/populations/species differ across these ‘latent’ axes.
(c) . Identifying behavioural classes and quantifying transitions among classes
Once the axes of behavioural variation are identified, an important next step is delimiting how different behaviours distributed along these axes interrelate to each other, creating a ‘map’ of the structure of observed behavioural variation. Again, there are a diversity of methods to achieve this; however, one exciting set of methods, unsupervised behavioural classifiers (i.e. clustering algorithms), are increasingly being applied to big behavioural datasets as tools to define behavioural classes—groups of behavioural variables with high similarity [63,80,88,89]. Clustering, while not necessarily appropriate for all big behavioural datasets or questions, excels as a tool for dividing large, multi-dimensional behavioural data trait-spaces into interpretable subregions based on similarity, distance, or regions of high density in data space. In many cases, researchers may choose to forgo clustering: behavioural plasticity, for example, could be quantified without defining clusters and instead measuring ‘movement’ or ‘behavioural entropy’ in a multi-dimensional ‘behavioural space’. On the other hand, delineating clusters—or discrete groups of behaviours—may become useful when, for example, researchers are interested in understanding transition rates among distinct regions of behavioural trait-space throughout development. Used as tools to define behavioural diversity in entire behavioural repertoires, such methods are essentially a way to generate rich, data-driven ethograms.
A host of clustering algorithms has been deployed in unsupervised classification of behaviour, but many take as an input a specified and arbitrary number of clusters (i.e. behavioural classes), k, in a multi-dimensional data space [80]. While one might treat the decision about the magnitude of k as an optimization problem, many of the behavioural trait-spaces that are created with high-dimensional, dense behavioural data may not show clear, discrete boundaries between identified clusters [39]; in such cases, k is perhaps most useful as a parameter that is actively toggled to adjust the ‘graininess’ of behavioural partitioning. Indeed, creative, flexible use of k as a free parameter enables researchers to examine the structure or ‘architecture’ of behavioural repertoires. For example, Berman et al. [44] showed that, as they increased the number of clusters, k, new clusters were created largely by subdividing existing clusters in two (rather than more substantially reshuffling the behavioural map); this led the researchers to conclude that observed fly behaviours were structured hierarchically, testing decades-old theory in ethology [90,91].
Finally, once distinct behavioural clusters/classes are defined, the transitions among these classes can be quantified. One of the simplest and most common approaches to modelling behavioural transitions is to assume that behavioural transitions follow a Markovian process. These models assume that the probabilities of transitioning to any other behavioural state (i.e. cluster) depend only on an animal's current behaviour, rather than some sequence of behaviours that preceded the current behaviour. Common extensions of this concept are hidden Markov models (HMMs) whereby a sequence of behavioural patterns (e.g. movement) are linked to an underlying unmeasured (behavioural) state variable (e.g. a specific behavioural class such as foraging, hiding, walking and running). In this way, HMMs can be important tools in identifying behavioural modes [73,92], similar to behavioural clusters discussed earlier. Recently, HMMs have been extended to include nested latent states in order to explore hierarchical structures in behavioural modes (hierarchical hidden Markov models, HHMMs) [93,94]—an approach that is likely to be useful in high-resolution behavioural development data that can be parsed at a large range of temporal scales.
By determining the axes of behavioural variation, mapping onto these axes distinct behavioural classes or clusters, and then quantifying transitions among these classes, we can begin to ask questions that shed light on behavioural changes throughout development (figure 1): how do transitions between behaviours (i.e. clusters) change during development? Are there certain regions of this ‘behavioural map’ that are more frequently used by newborns versus adolescents versus adults? Are there certain areas of behavioural space that are only accessible to animals after a certain developmental time point? Do individuals decrease or increase in the amount of behavioural ‘space’ they use throughout their lifetime? Are individuals consistently different in how they ‘move through behavioural space’ during development? While many of these questions have been asked (and addressed) in some form previously, the degree of precision and depth with which these questions can now be addressed—not just for particular behaviours but for much of an animal's entire movement repertoire—is unprecedented.
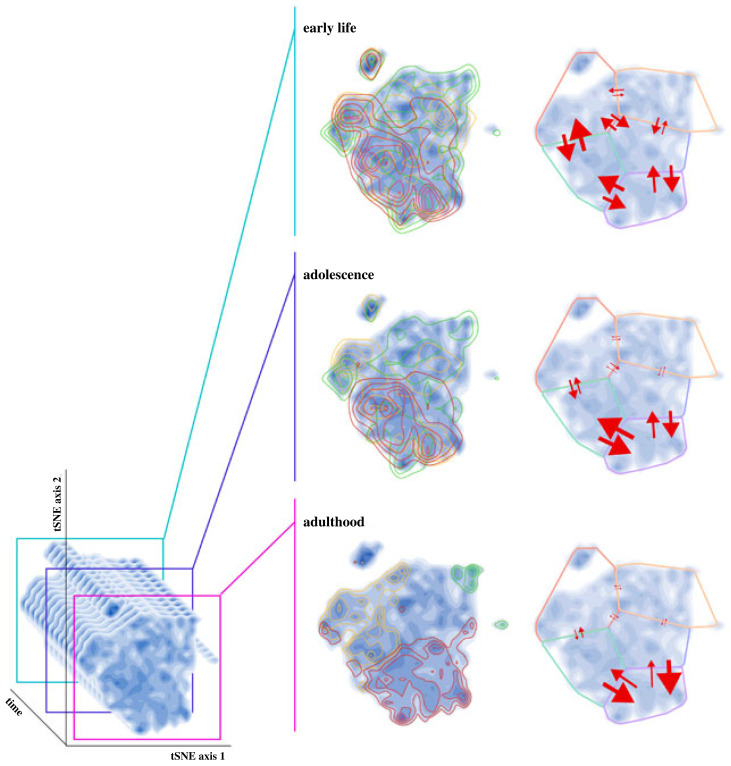
Figure 1.
After dominant behavioural axes have been calculated (e.g. via PCA), multi-dimensional behavioural data for all individuals and all developmental time points can be projected onto a two-dimensional behavioural/phenotypic space (e.g. via tSNE (t-distributed stochastic neighbour embedding), UMAP (uniform manifold approximation and projection) or other dimension reduction tools). Individual behavioural repertoires during subsets of development can then be characterized (here with contour plots of three individuals in green, yellow and red shown only for early life, adolescence and adulthood for ease of visualization; note that high-temporal resolution allows much finer-grained analyses across many more individuals), and further quantification of individual behavioural transition rates among distinct behavioural classes/clusters can be conducted (represented for the ‘red’ individual with arrows for which size and thickness are proportional to the magnitude of transition probabilities between different behavioural clusters). In this example, individuals explore a large range of behavioural space early in development but become more constrained—and distinct from each other—as development progresses. Such a pattern of decreasing behavioural plasticity across development is a major prediction of many Bayesian models of development, and a pattern of behavioural divergence among individuals across development is consistent with behavioural niche specialization [95,96]. See [43,44] for worked examples of many of the above approaches in a non-developmental context.
3. Using big behavioural data to uncover the principles governing behavioural development
We will now discuss how big behavioural data and the associated innovations in measuring and modelling behaviour discussed above can be productively applied to fundamental open questions regarding the principles governing behavioural development.
(a) . Mapping behaviour–state connections across ontogeny
Connections between behavioural decisions and an animal's underlying state (e.g. energetic state, informational state, social rank, etc.) may play a major role in the development of behaviour: such connections can help explain the evolution and development of a large range of behaviours and states (e.g. foraging tactics [97,98], anti-predator behaviours [99–101], emotions and self-awareness [102,103], developmental adjustments in response to environmental change [104,105]). While theoretical and conceptual models employing techniques such as stochastic dynamic programming [106] have hugely facilitated the investigation of behaviour–state connections and their role in the development of adaptive behaviour [107–112], empirical studies that simultaneously measure behaviour and state throughout development are relatively few. As discussed above (see §2a, ‘Measurement innovations’), behavioural tracking techniques provide a powerful way to directly measure state variables (or proxies of state variables) with as much automation as behaviour, allowing direct tests of the wealth of theory on behaviour–state connections with high-resolution behaviour and state time series across development.
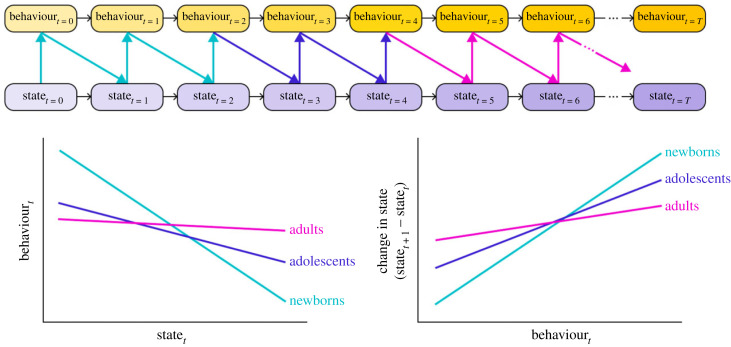
One major open question is how particular types of behaviour–state connections such as dynamic feedback loops (i.e. bidirectional linkages between state and behaviour) influence developmental trajectories [113–115]. So far, of the studies that have been designed to detect behaviour–state feedbacks, conclusions on the presence and consequences of these feedbacks have been mixed [116–119]. Nevertheless, behaviour–state feedbacks are commonly cited in the literature as a major causal factor in the formation of behavioural developmental trajectories. The now possible simultaneous high-resolution tracking of both behaviour and state can allow us to (i) detect the presence of feedbacks and (ii) measure the persistence of both the strength and direction of these feedbacks across development at a much finer temporal grain. To be concrete, consider a high-resolution time series of behaviours and states, where at each point in time t, Bt and St represent behavioural and state measurements, respectively (figure 2). Behaviour–state feedbacks would be evident if St correlated with Bt and Bt was then correlated with a change in state (i.e. ΔSt = St+ 1 – St) at some future point in time, t + 1. As an example, one could imagine that energetic state (e.g. fat reserves or hunger level) would affect foraging behaviour at one time point and that the intensity of any foraging behaviour would then subsequently be related to a further change in energetic state at the following time point. Furthermore, by testing for these correlations over subsets of a developmental time series, one could test for the degree to which feedbacks persist in both strength and direction throughout development. Such an investigation could test whether the slopes in the relationship between St and Bt and Bt and ΔSt vary as t increases, and whether any changes are symmetrically represented in both relationships.
Figure 2.
High-resolution behavioural tracking paired with similarly high-resolution tracking of state variables allow tests that detect behaviour–state feedbacks and quantify how both the strength and direction of these feedbacks change across development. In this schematic, for example, negative feedbacks between behaviour and state persist throughout development, but are stronger earlier in life (blue–green lines represent individuals in early development, purple in mid-development and magenta at maturity; note that with highly temporally resolved datasets, such an analysis could be done more continuously).
One commonly cited behaviour–state feedback mechanism is the asset-protection principle [120]: if having high ‘state’ (here, residual reproductive value) makes it less likely that an individual will take risks (here, actions that are associated with increased mortality but potentially increased state benefits, e.g. foraging under predation risk), a negative feedback might be generated [121,122]. Low-state individuals would thus take more risks compared with high-state individuals, thus increasing their state and becoming less likely to take further risks. The strength of this negative feedback may be expected to change over development (figure 2). For example, prey outgrowing a dominant gape-limited predator or gradually learning how to better evade predators may represent particular ecologies that generate systematic decreases in risk—and hence the strength of the negative feedback—through a prey's lifetime [123]. Figure 2 illustrates such a scenario where temporally high-resolution behaviour and state tracking allow us to test for the change in behaviour–state feedback strength across development: while negative feedbacks, indicated by slopes of the relationships between St and Bt and Bt and ΔSt having opposing signs, persist throughout development, the absolute values of the slopes of these lines—and thus the strength of feedbacks—decrease through life. Thus, the ability to detect behaviour–state feedbacks and quantify how the strengths of these feedbacks change through time will afford us greater potential to map the rules governing the unfolding of developmental trajectories to the specific selective forces particular to a species’ ecology.
(b) . Early-life effects and behavioural transitions
Continuous tracking of behaviour during development is particularly promising for understanding early-life effects [124,125]. Such early-life effects (e.g. parental imprinting or social experience during early life stages [126–128], the influence of early-life stressors on later behaviour [129,130], etc.) are typically studied experimentally by manipulating environmental stimuli early in life and then measuring outcomes (e.g. behaviour) later in life (table 1, example 3). The focus on early life has been particularly intense relative to other periods of animal life because (i) the developmental consequences of phenotypic adjustments made early in development may be outsized compared with those of later adjustments (e.g. the epiphenotype hypothesis [131]), (ii) animals may show greater developmental plasticity early in life [107], perhaps reflecting adaptive strategies in environments where animals gradually accrue fitness-related information from steady streams of imperfect cues [132], and (iii) early-life experiences often have long-lasting consequences for behaviour and fitness [133–136], with effects even persisting across multiple generations [137]. Despite their importance, however, the developmental routes connecting early-life experiences and their long-term outcomes often remain unmeasured and thus obscured.
Experiments based on near-continuous tracking throughout development will allow us to unravel the behavioural mechanisms that link early-life experiences to outcomes later in life. High-resolution tracking will provide both longer and more highly resolved time series of behavioural data that measure the entire developmental time course of responses to salient early-life stressors or stimuli. Whereas previous approaches might capture gross patterns or periods of sensitivity to cues in a few key periods of development, high-throughput behavioural measurements could more closely estimate the exact ‘cue–response curve’ [138]. Of key interest will be the periods of life near ‘switching points’ in cue sensitivity when animals switch from being sensitive to cue input to being unresponsive to cues. A highly temporally resolved dataset could then address, for example, whether these switching points represent a rapid switch, which may signal a threshold-mediated response or criticality in the underlying cue–response systems [139], or a more graded change in cue sensitivity, as one might observe during many learning processes [111,140]. Furthermore, it is around these switching points that individual variation is likely to exist; differences in the timing and magnitude of cue responses to ecological factors will further our understanding of the ecological and evolutionary factors driving individual variation.
While switching points often represent relatively brief and intense periods of behavioural transition, individuals are also transitioning among behavioural states on more frequent timescales second-by-second and day-by-day. Such state-switching is another important facet of behavioural development and—as we have discussed above (see §2c, ‘Identifying behavioural classes and quantifying transitions among classes’)—can now be captured by high-resolution behavioural tracking. And while state-switching models that calculate transition probabilities among behavioural states have been applied to behavioural data for decades, relatively large temporal graininess has only allowed transitions between high-level behavioural categories to be modelled [141,142]. By contrast, the richness of datasets produced through most automated tracking techniques allows much more sub-structure in behaviour to be modelled. For example, whereas a relatively low-resolution time series may allow the estimation of transition rates on the scale of a day (i.e. estimating the probability of behavioural shifts from one day to the next throughout development), the highly resolved datasets typical of automated tracking technology not only enable the estimation of those same day-to-day transition rates with greater accuracy, but also allow the estimation of transition rates between behaviours that occur on finer scales of hours or minutes [143–147].
Leveraging previously discussed approaches to define and classify behaviour (see §2b, ‘Data-driven definitions of behavioural axes’, and §2c, ‘Identifying behavioural classes and quantifying transitions among classes’), transition models (including Markov models) will allow us to address fundamental questions concerning the structure of behavioural development: when and to what extent are behavioural patterns during development path-dependent? Are behaviours operating at different timescales structured hierarchically (i.e. do distinct behavioural patterns consist of non-overlapping behavioural subclasses operating at shorter timescales)? Are individuals consistent not only in constituent behaviours but also in the transition rates among behaviours? Applied specifically to the study of individual behavioural variation across development, differences in individual-level behavioural plasticity can be quantified as changes to the structure of behavioural repertoires (i.e. behavioural transition matrices). Many state-switching models can also investigate how environmental cues influence behaviour by combining both behavioural and non-behavioural data as input [148] (see §2a, ‘Measurement innovations’). In total, these tools bring us closer to understanding the development of entire behavioural repertoires, the ‘architecture’ of behaviour throughout development and the factors that influence individual variation in developmental trajectories.
(c) . Information integration across development
Key to understanding behavioural development is understanding the cues to which developing organisms respond, how these cues are integrated with previous information available to animals, and how specific sequences of cues shape behavioural change [111,149–152]. In particular, Bayesian updating models have been very successful at describing behavioural development [111]. Briefly, Bayesian updating as it applies to developing animals is an information theoretic method for modelling an animal's informational state (and consequently, optimal behavioural responses), given its prior information (based, for example, on evolutionary history or epigenetically inherited information [153,154]) combined with incoming, often imperfect, information through its current experience (or a sequence of experiences) [155]. While there are some empirical findings that corroborate predictions from Bayesian models of behavioural development [156–158], in general, empirical tests of these predictions remain relatively rare [111]. We believe that new experimental possibilities associated with high-resolution behavioural tracking—allowing us to track the unfolding of behaviour under different cue regimes over prolonged periods of time—put us in the position to test many of the untested predictions of Bayesian models of development.
The key predictions in Bayesian models of development relate (i) the structure of environmental fluctuations and (ii) the availability and quality of information to the degree and duration of behavioural plasticity [111,159]. As long as the environment does not change and animals receive a steady stream of informative cues throughout development, the observed degree of behavioural plasticity at an individual level (and, in some cases, behavioural diversity at a population level) is predicted to decrease with age [107,108,136,160]. If, however, reliable cues are rare, if these reliabilities change through time, or if the environment fluctuates through time, periods of plasticity throughout development may be extended [109,110,160,161]. Likewise, cues received late in life might not elicit strong behavioural responses because they may be perceived to be associated with rare events or there may not be sufficient time left in life to capitalize on any benefits conferred through altering one's phenotype [162]. Experimenters could manipulate ‘priors’ (e.g. by manipulating parental experience, or sampling from evolutionarily divergent populations), the structure of environmental fluctuations, and/or the reliability of cues during development and then follow behavioural change over development. This sort of approach could produce large enough datasets to estimate the entire developmental time course of changes in behavioural plasticity at an individual level, offering direct tests of many of the core predictions stemming from Bayesian models of behavioural development.
4. Limitations and future challenges
Big behavioural data are powerful tools with great potential to test fundamental open questions in behavioural development in the context of ecology and evolution (box 1). The wealth of data that high-resolution tracking provides, however, is limited in its scope to advance our knowledge of behavioural development without the idea-rich scaffolding that theory and hypothesis generation provide [163,164]. Thus, throughout this review, we have paid particular attention (see §3, ‘Using big behavioural data to uncover the principles governing behavioural development’) to how big data tools can both test and advance current theory in this field. Nevertheless, limitations remain that researchers must confront.
Box 1. Representative major open questions in behavioural development and their big data approaches.
Behaviour–state connections
Approach: Detect presence of dynamic feedbacks between behaviour and state (i.e. bidirectional state–behaviour linkages); quantify both the strength and direction of state–behaviour feedbacks through development.
Q1 What are the relative rates of change in behaviour and state during development and what are the dynamic consequences when they are linked?
Q2 How common are state–behaviour feedbacks?
Q3 How is the strength and direction of feedbacks affected by certain ecological conditions/selective environments?
Q4 Are feedbacks more important in shaping developmental trajectories in particular developmental stages?
Early-life effects and behavioural transitions
Approach: Measure behavioural–experiential trajectories continuously throughout life; quantify fine-grained transitions between behavioural classes throughout development.
Q5 What are the behavioural–experiential mechanisms linking early-life experiences and long-term behavioural outcomes?
Q6 When and to what extent are behavioural patterns throughout development path-dependent?
Q7 To what extent are behaviours that operate at different timescales structured hierarchically?
Q8 Do individuals differ in their behavioural transition rates? Is this an ecologically important axis of behavioural variation?
Information integration across development
Approach: Pair high-resolution behavioural tracking data with similarly resolved monitoring of environmental cues.
Q9 How are developmental responses to recent cues moderated by the specific sequences of past cues?
Q10 How does behavioural development relate to the structure of environmental fluctuations?
Q11 How does the availability and quality of information affect behavioural development?
First, linking high-resolution behavioural tracking to metrics requiring terminal sampling (e.g. gene expression changes in the brain or experience-dependent epigenetic changes across development) will require creative experimental design and/or the development of new technologies (e.g. multi-electrode arrays or wireless neurotelemetry [165]). Further challenges arise when considering the combination of high-resolution behavioural and environmental time series. In some cases, salient environmental changes that have dramatic effects on behavioural development may occur only rarely (e.g. once or twice over the course of an individual's development), limiting the ability of researchers to estimate their effects. These types of scenarios would make studies that look for correlations among environmental metrics from passive sensors and high-resolution behavioural data difficult; on the other hand, they may offer great opportunities for experimentally manipulating candidate environmental variables/stressors (their timing or intensity, for example) while tracking behavioural development. Next, while methodological advances allowing the collection of temporally rich behavioural data across development vastly reduce the amount of work effort per datapoint, a fundamental limitation that will always remain is the relative lifespan (in particular, the duration of development) of research animals. For species with long developmental periods, there are inherent challenges to collecting near-continuous developmental data. This might be partially addressed, for example, by subsampling populations of longer-lived species by age class and conducting behavioural observations over shorter periods of development (relative to that species) but for individuals spread across age classes in order to get a fuller picture of development in the aggregate (e.g. combine approaches 4 and 5 in table 1). These and similar sorts of trade-offs will exist in most experiments in which researchers desire to test the whole of behavioural development, but the ever-growing technological advances in this field are also likely to continue to rebalance these trade-offs in favour of ever-richer behavioural developmental datasets across species.
5. Concluding remarks
For the last three-quarters of a century, a steady stream of technological advancement has allowed the discovery of new behaviours as well as innovations in the quantification of previously observed behaviours (e.g. audio recording of echolocation in bats [166] and cetaceans [167], spectrographic analysis of birdsong [168], high-speed cameras revealing feeding innovations [169,170], automatic sensing and social network analysis uncovering novel social and collective behaviours [65], machine learning algorithms as behavioural classifiers [89]). In turn, these technological advances in quantifying behaviour have been productively applied to understanding both the proximate and evolutionary causes and consequences of behaviour [171], helping to transform the field of animal behaviour from a largely descriptive science to an analytical and theoretical one [172]. Now, with the widening application of state-of-the-art tracking technologies, the time is ripe to continue in this tradition by applying these tools to major open questions in one of behavioural ecology's foundational research cores: the ontogeny of behaviour.
Acknowledgements
We thank L. Stärk for his help with visualizations included in figure 1. Two anonymous reviewers and the editor provided helpful edits during the review process.
Data accessibility
This article has no additional data.
Authors' contributions
S.M.E.: conceptualization, project administration, visualization, writing—original draft; writing—review and editing; U.S.: conceptualization; writing—review and editing; D.B.: conceptualization; writing—review and editing; F.F.: conceptualization; writing—review and editing; K.L.L.: conceptualization; writing—review and editing; J.K.: conceptualization, funding acquisition, project administration; writing—review and editing; M.W.: conceptualization, funding acquisition, project administration, writing—original draft; writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
S.M.E., M.W., R.S., D.B., J.K. and F.F. were supported by the Deutsche Forschungsgemeinschaft (DFG) under Germany's Excellence Strategy – EXC 2002/1 ‘Science of Intelligence’ – project number 390523135. K.L.L. was supported by NSF-IOS 2100625.
References
- 1.Nath T, Mathis A, Chen AC, Patel A, Bethge M, Mathis MW. 2019. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152-2176. ( 10.1038/s41596-019-0176-0) [DOI] [PubMed] [Google Scholar]
- 2.Lauer J, et al. 2022. Multi-animal pose estimation, identification and tracking with DeepLabCut. Nat. Methods 19, 496-504. ( 10.1038/s41592-022-01443-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francisco FA, Nührenberg P, Jordan A. 2020. High-resolution, non-invasive animal tracking and reconstruction of local environment in aquatic ecosystems. Mov. Ecol. 8, 27. ( 10.1186/s40462-020-00214-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcón-Nieto G, Graving JM, Klarevas-Irby JA, Maldonado-Chaparro AA, Mueller I, Farine DR. 2018. An automated barcode tracking system for behavioural studies in birds. Methods Ecol. Evol. 9, 1536-1547. ( 10.1111/2041-210X.13005) [DOI] [Google Scholar]
- 5.Haalck L, Mangan M, Webb B, Risse B. 2020. Towards image-based animal tracking in natural environments using a freely moving camera. J. Neurosci. Methods 330, 108455. ( 10.1016/j.jneumeth.2019.108455) [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, de Polavieja GG. 2014. idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743-748. ( 10.1038/nmeth.2994) [DOI] [PubMed] [Google Scholar]
- 7.McDiarmid TA, Yu AJ, Rankin CH. 2018. Beyond the response—high throughput behavioral analyses to link genome to phenome in Caenorhabditis elegans. Genes Brain Behav. 17, e12437. ( 10.1111/gbb.12437) [DOI] [PubMed] [Google Scholar]
- 8.Jones BM, et al. 2020. Individual differences in honey bee behavior enabled by plasticity in brain gene regulatory networks. eLife 9, e62850. ( 10.7554/eLife.62850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini P, Irisarri I, Fudickar A, Schmidt A, Meyer A, Wikelski M, Partecke J. 2017. Animal tracking meets migration genomics: transcriptomic analysis of a partially migratory bird species. Mol. Ecol. 26, 3204-3216. ( 10.1111/mec.14108) [DOI] [PubMed] [Google Scholar]
- 10.von Ziegler L, Sturman O, Bohacek J. 2020. Big behavior: challenges and opportunities in a new era of deep behavior profiling. Neuropsychopharmacology 46 33-44. ( 10.1038/s41386-020-0751-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez-Marin A, Ghazanfar AA. 2019. The life of behavior. Neuron 104, 25-36. ( 10.1016/j.neuron.2019.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta SR, Anderson DJ, Branson K, Perona P, Leifer A. 2019. Computational neuroethology: a call to action. Neuron 104, 11-24. ( 10.1016/j.neuron.2019.09.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh M, Rihel J. 2020. Hierarchical compression reveals sub-second to day-long structure in larval zebrafish behavior. eNeuro 7, ENEURO.0408-19.2020. ( 10.1523/ENEURO.0408-19.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn TW, et al. 2016. Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion. eLife 5, e12741. ( 10.7554/eLife.12741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Marin A, Paton JJ, Kampff AR, Costa RM, Mainen ZF. 2014. Big behavioral data: psychology, ethology and the foundations of neuroscience. Nat. Neurosci. 17, 1455–1462. ( 10.1038/nn.3812) [DOI] [PubMed] [Google Scholar]
- 16.Strandburg-Peshkin A, Farine DR, Couzin ID, Crofoot MC. 2015. Shared decision-making drives collective movement in wild baboons. Science 348, 1358-1361. ( 10.1126/science.aaa5099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy M, Vasarhelyi G, Pettit B, Roberts-Mariani I, Vicsek T, Biro D. 2013. Context-dependent hierarchies in pigeons. Proc. Natl Acad. Sci. USA 110, 13 049-13 054. ( 10.1073/pnas.1305552110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolles JW, Boogert NJ, Sridhar VH, Couzin ID, Manica A. 2017. Consistent Individual differences drive collective behavior and group functioning of schooling fish. Curr. Biol. 27, 2862-2868.e7. ( 10.1016/j.cub.2017.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolles JW, Laskowski KL, Boogert NJ, Manica A. 2018. Repeatable group differences in the collective behaviour of stickleback shoals across ecological contexts. Proc. R. Soc. B. 285, 20172629. ( 10.1098/rspb.2017.2629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westley PAH, Berdahl AM, Torney CJ, Biro D. 2018. Collective movement in ecology: from emerging technologies to conservation and management. Phil. Trans. R. Soc. B 373, 20170004. ( 10.1098/rstb.2017.0004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijeyakulasuriya DA, Eisenhauer EW, Shaby BA, Hanks EM. 2020. Machine learning for modeling animal movement. PLoS ONE 15, e0235750. ( 10.1371/journal.pone.0235750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dell AI, et al. 2014. Automated image-based tracking and its application in ecology. Trends Ecol. Evol. 29, 417-428. ( 10.1016/j.tree.2014.05.004) [DOI] [PubMed] [Google Scholar]
- 23.Nathan R, et al. 2022. Big-data approaches lead to an increased understanding of the ecology of animal movement. Science 375, eabg1780. ( 10.1126/science.abg1780) [DOI] [PubMed] [Google Scholar]
- 24.Boogert NJ, Farine DR, Spencer KA. 2014. Developmental stress predicts social network position. Biol. Lett. 10, 20140561. ( 10.1098/rsbl.2014.0561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bateson PPG, Jaeckel JB. 1976. Chicks’ preferences for familiar and novel conspicuous objects after different periods of exposure. Anim. Behav. 24, 386-390. ( 10.1016/S0003-3472(76)80048-6) [DOI] [Google Scholar]
- 26.Schürch R, Heg D. 2010. Life history and behavioral type in the highly social cichlid Neolamprologus pulcher. Behav. Ecol. 21, 588-598. ( 10.1093/beheco/arq024) [DOI] [Google Scholar]
- 27.Bell AM, Stamps JA. 2004. Development of behavioural differences between individuals and populations of sticklebacks, Gasterosteus aculeatus. Anim. Behav. 68, 1339-1348. ( 10.1016/j.anbehav.2004.05.007) [DOI] [Google Scholar]
- 28.Neave HW, Costa JHC, Weary DM, von Keyserlingk MAG. 2020. Long-term consistency of personality traits of cattle. R. Soc. Open Sci. 7, 191849. ( 10.1098/rsos.191849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kok EMA, Burant JB, Dekinga A, Manche P, Saintonge D, Piersma T, Mathot KJ. 2019. Within-individual canalization contributes to age-related increases in trait repeatability: a longitudinal experiment in red knots. Am. Nat. 194, 455-469. ( 10.1086/704593) [DOI] [PubMed] [Google Scholar]
- 30.Edenbrow M, Croft DP. 2011. Behavioural types and life history strategies during ontogeny in the mangrove killifish, Kryptolebias marmoratus. Anim. Behav. 82, 731-741. ( 10.1016/j.anbehav.2011.07.003) [DOI] [Google Scholar]
- 31.DiRienzo N, Pruitt JN, Hedrick AV. 2012. Juvenile exposure to acoustic sexual signals from conspecifics alters growth trajectory and an adult personality trait. Anim. Behav. 84, 861-868. ( 10.1016/j.anbehav.2012.07.007) [DOI] [Google Scholar]
- 32.Ehlman SM, Sandkam BA, Breden F, Sih A. 2015. Developmental plasticity in vision and behavior may help guppies overcome increased turbidity. J. Comp. Physiol. A 201, 1125-1135. ( 10.1007/s00359-015-1041-4) [DOI] [PubMed] [Google Scholar]
- 33.Urszán TJ, Garamszegi LZ, Nagy G, Hettyey A, Török J, Herczeg G. 2015. No personality without experience? A test on Rana dalmatina tadpoles. Ecol. Evol. 5, 5847-5856. ( 10.1002/ece3.1804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balsam JS, Stevenson PA. 2021. Agonistic experience during development establishes inter-individual differences in approach-avoidance behaviour of crickets. Scient. Rep. 11, 16702. ( 10.1038/s41598-021-96201-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer S, Bohn L, Oberhummer E, Nyman C, Taborsky B. 2017. Divergence of developmental trajectories is triggered interactively by early social and ecological experience in a cooperative breeder. Proc. Natl Acad. Sci. USA 114, E9300-E9307. ( 10.1073/pnas.1705934114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb G. 1961. Developmental age as a baseline for determination of the critical period in imprinting. J. Comp. Physiol. Psychol. 54, 422-427. ( 10.1037/h0049127) [DOI] [PubMed] [Google Scholar]
- 37.Petelle MB, McCoy DE, Alejandro V, Martin JG, Blumstein DT. 2013. Development of boldness and docility in yellow-bellied marmots. Anim. Behav. 86, 1147-1154. ( 10.1016/j.anbehav.2013.09.016) [DOI] [Google Scholar]
- 38.Wuerz Y, Krüger O. 2015. Personality over ontogeny in zebra finches: long-term repeatable traits but unstable behavioural syndromes. Front. Zool. 12, S9. ( 10.1186/1742-9994-12-S1-S9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith ML, Davidson JD, Wild B, Dormagen DM, Landgraf T, Couzin ID. 2022. Behavioral variation across the days and lives of honey bees. iScience 25, 104842. ( 10.1016/j.isci.2022.104842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laskowski KL, Bierbach D, Jolles JW, Doran C, Wolf M. 2022. The emergence and development of behavioral individuality in clonal fish. Nat. Commun. 13, 6419. ( 10.1038/s41467-022-34113-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy A. 2022. The what, how, and why of naturalistic behavior. Curr. Opin. Neurobiol. 74, 102549. ( 10.1016/j.conb.2022.102549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown AEX, de Bivort B. 2018. Ethology as a physical science. Nat. Phys. 14, 653-657. ( 10.1038/s41567-018-0093-0) [DOI] [Google Scholar]
- 43.Berman GJ, Choi DM, Bialek W, Shaevitz JW. 2014. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 11, 20140672. ( 10.1098/rsif.2014.0672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berman GJ, Bialek W, Shaevitz JW. 2016. Predictability and hierarchy in Drosophila behavior. Proc. Natl Acad. Sci. USA 113, 11 943-11 948. ( 10.1073/pnas.1607601113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, Datta SR. 2015. Mapping sub-second structure in mouse behavior. Neuron 88, 1121-1135. ( 10.1016/j.neuron.2015.11.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernández DG, Rivera C, Cande J, Zhou B, Stern DL, Berman GJ. 2021. A framework for studying behavioral evolution by reconstructing ancestral repertoires. eLife 10, e61806. ( 10.7554/eLife.61806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pereira TD, et al. 2022. SLEAP: a deep learning system for multi-animal pose tracking. Nat. Methods 19, 486-495. ( 10.1038/s41592-022-01426-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walter T, Couzin ID. 2021. TRex, a fast multi-animal tracking system with markerless identification, and 2D estimation of posture and visual fields. eLife 10, e64000. ( 10.7554/eLife.64000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson PL, Andersson M. 2018. ToxTrac: a fast and robust software for tracking organisms. Methods Ecol. Evol. 9, 460-464. ( 10.1111/2041-210X.12874) [DOI] [Google Scholar]
- 50.Jolles JW. 2021. Broad-scale applications of the Raspberry Pi: a review and guide for biologists. Methods Ecol. Evol. 12, 1562-1579. ( 10.1111/2041-210X.13652) [DOI] [Google Scholar]
- 51.Mathis MW, Mathis A. 2020. Deep learning tools for the measurement of animal behavior in neuroscience. Curr. Opin. Neurobiol. 60, 1-11. ( 10.1016/j.conb.2019.10.008) [DOI] [PubMed] [Google Scholar]
- 52.Pereira TD, Shaevitz JW, Murthy M. 2020. Quantifying behavior to understand the brain. Nat. Neurosci. 23, 1537-1549. ( 10.1038/s41593-020-00734-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monkman GG, Hyder K, Kaiser MJ, Vidal FP. 2019. Using machine vision to estimate fish length from images using regional convolutional neural networks. Methods Ecol. Evol. 10, 2045-2056. ( 10.1111/2041-210X.13282) [DOI] [Google Scholar]
- 54.Williams HJ, et al. 2020. Optimizing the use of biologgers for movement ecology research. J. Anim. Ecol. 89, 186-206. ( 10.1111/1365-2656.13094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chmura HE, Glass TW, Williams CT. 2018. Biologging physiological and ecological responses to climatic variation: new tools for the climate change era. Front. Ecol. Evol. 6, 92. ( 10.3389/fevo.2018.00092) [DOI] [Google Scholar]
- 56.Goldbogen JA, et al. 2019. Extreme bradycardia and tachycardia in the world's largest animal. Proc. Natl Acad. Sci. USA 116, 25 329-25 332. ( 10.1073/pnas.1914273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aoki K, Watanabe Y, Inamori D, Funasaka N, Sakamoto KQ. 2021. Towards non-invasive heart rate monitoring in free-ranging cetaceans: a unipolar suction cup tag measured the heart rate of trained Risso's dolphins. Phil. Trans. R. Soc. B 376, 20200225. ( 10.1098/rstb.2020.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reher S, Dausmann KH. 2021. Tropical bats counter heat by combining torpor with adaptive hyperthermia. Proc. R. Soc. B 288, 20202059. ( 10.1098/rspb.2020.2059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cilulko J, Janiszewski P, Bogdaszewski M, Szczygielska E. 2013. Infrared thermal imaging in studies of wild animals. Eur. J. Wildl. Res. 59, 17-23. ( 10.1007/s10344-012-0688-1) [DOI] [Google Scholar]
- 60.Demartsev V, Manser MB, Tattersall GJ. 2022. Vocalization-associated respiration patterns: thermography-based monitoring and detection of preparation for calling. J. Exp. Biol. 225, jeb243474. ( 10.1242/jeb.243474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andresen N, Wöllhaf M, Hohlbaum K, Lewejohann L, Hellwich O, Thöne-Reineke C, Belik V. 2020. Towards a fully automated surveillance of well-being status in laboratory mice using deep learning: starting with facial expression analysis. PLoS ONE 15, e0228059. ( 10.1371/journal.pone.0228059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C, Zhu W, Steibel J, Siegford J, Wurtz K, Han J, Norton T. 2020. Recognition of aggressive episodes of pigs based on convolutional neural network and long short-term memory. Comput. Electron. Agric. 169, 105166. ( 10.1016/j.compag.2019.105166) [DOI] [Google Scholar]
- 63.Calhoun AJ, Pillow JW, Murthy M. 2019. Unsupervised identification of the internal states that shape natural behavior. Nat. Neurosci. 22, 2040-2049. ( 10.1038/s41593-019-0533-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Briefer EF, et al. 2022. Classification of pig calls produced from birth to slaughter according to their emotional valence and context of production. Scient. Rep. 12, 3409. ( 10.1038/s41598-022-07174-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith JE, Pinter-Wollman N. 2021. Observing the unwatchable: integrating automated sensing, naturalistic observations and animal social network analysis in the age of big data. J. Anim. Ecol. 90, 62-75. ( 10.1111/1365-2656.13362) [DOI] [PubMed] [Google Scholar]
- 66.Frankenhuis WE, Nettle D, Dall SRX. 2019. A case for environmental statistics of early-life effects. Phil. Trans. R. Soc. B 374, 20180110. ( 10.1098/rstb.2018.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hawkins WD, DuRant SE. 2020. Applications of machine learning in behavioral ecology: quantifying avian incubation behavior and nest conditions in relation to environmental temperature. PLoS ONE 15, e0236925. ( 10.1371/journal.pone.0236925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levitis DA, Lidicker WZ Jr, Freund G. 2009. Behavioural biologists do not agree on what constitutes behaviour. Anim. Behav. 78, 103-110. ( 10.1016/j.anbehav.2009.03.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. 2013. Animal personality: what are behavioural ecologists measuring? Biol. Rev. Camb. Phil. Soc. 88, 465-475. ( 10.1111/brv.12007) [DOI] [PubMed] [Google Scholar]
- 70.Bohnslav JP, et al. 2021. DeepEthogram, a machine learning pipeline for supervised behavior classification from raw pixels. eLife 10, e63377. ( 10.7554/eLife.63377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kain J, Stokes C, Gaudry Q, Song X, Foley J, Wilson R, de Bivort B. 2013. Leg-tracking and automated behavioural classification in Drosophila. Nat. Commun. 4, 1910. ( 10.1038/ncomms2908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Budaev SV. 2010. Using principal components and factor analysis in animal behaviour research: caveats and guidelines. Ethology 116, 472-480. ( 10.1111/j.1439-0310.2010.01758.x) [DOI] [Google Scholar]
- 73.Heerah K, Woillez M, Fablet R, Garren F, Martin S, De Pontual H. 2017. Coupling spectral analysis and hidden Markov models for the segmentation of behavioural patterns. Mov. Ecol. 5, 20. ( 10.1186/s40462-017-0111-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Maaten L, Hinton G. 2008. Visualizing data using t-SNE. J. Machine Learn. Res. 9, 2579-2605. [Google Scholar]
- 75.McInnes L, Healy J. 2018. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv, 1802.03426. ( 10.48550/arXiv.1802.03426) [DOI]
- 76.Overman KE, Choi DM, Leung K, Shaevitz JW, Berman GJ. 2022. Measuring the repertoire of age-related behavioral changes in Drosophila melanogaster. PLoS Comput. Biol. 18, e1009867. ( 10.1371/journal.pcbi.1009867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang K, et al. 2021. A hierarchical 3D-motion learning framework for animal spontaneous behavior mapping. Nat. Commun. 12, 2784. ( 10.1038/s41467-021-22970-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klibaite U, Shaevitz JW. 2020. Paired fruit flies synchronize behavior: uncovering social interactions in Drosophila melanogaster. PLoS Comput. Biol. 16, e1008230. ( 10.1371/journal.pcbi.1008230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valletta JJ, Torney C, Kings M, Thornton A, Madden J. 2017. Applications of machine learning in animal behaviour studies. Anim. Behav. 124, 203-220. ( 10.1016/j.anbehav.2016.12.005) [DOI] [Google Scholar]
- 80.Todd JG, Kain JS, de Bivort BL. 2017. Systematic exploration of unsupervised methods for mapping behavior. Phys. Biol. 14, 015002. ( 10.1088/1478-3975/14/1/015002) [DOI] [PubMed] [Google Scholar]
- 81.Goldberg LR. 1993. The structure of phenotypic personality traits. Am. Psychol. 48, 26-34. ( 10.1037/0003-066X.48.1.26) [DOI] [PubMed] [Google Scholar]
- 82.Heyser CJ, Chemero A. 2012. Novel object exploration in mice: not all objects are created equal. Behav. Process. 89, 232-238. ( 10.1016/j.beproc.2011.12.004) [DOI] [PubMed] [Google Scholar]
- 83.Kaiser MI, Müller C. 2021. What is an animal personality? Biol. Philosophy 36, 1. ( 10.1007/s10539-020-09776-w) [DOI] [Google Scholar]
- 84.Groothuis TGG, Trillmich F. 2011. Unfolding personalities: the importance of studying ontogeny. Dev. Psychobiol. 53, 641-655. ( 10.1002/dev.20574) [DOI] [PubMed] [Google Scholar]
- 85.White SJ, Pascall DJ, Wilson AJ. 2020. Towards a comparative approach to the structure of animal personality variation. Behav. Ecol. 31, 340-351. ( 10.1093/beheco/arz198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter AJ, Feeney WE. 2012. Taking a comparative approach: analysing personality as a multivariate behavioural response across species. PLoS ONE 7, e42440. ( 10.1371/journal.pone.0042440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dingemanse NJ, Dochtermann N, Wright J. 2010. A method for exploring the structure of behavioural syndromes to allow formal comparison within and between data sets. Anim. Behav. 79, 439-450. ( 10.1016/j.anbehav.2009.11.024) [DOI] [Google Scholar]
- 88.Marques JC, Lackner S, Félix R, Orger MB. 2018. Structure of the zebrafish locomotor repertoire revealed with unsupervised behavioral clustering. Curr. Biol. 28, 181-195.e5. ( 10.1016/j.cub.2017.12.002) [DOI] [PubMed] [Google Scholar]
- 89.Clemens J, Coen P, Roemschied FA, Pereira TD, Mazumder D, Aldarondo DE, Pacheco DA, Murthy M. 2018. Discovery of a new song mode in Drosophila reveals hidden structure in the sensory and neural drivers of behavior. Curr. Biol. 28, 2400-2412.e6. ( 10.1016/j.cub.2018.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tinbergen N. 1951. The study of instinct. Oxford, UK: Clarendon Press. [Google Scholar]
- 91.Dawkins R. 1976. Hierarchical organisation: a candidate principle for ethology. In Growing points in ethology (eds PPG Bateson, RA Hinde), pp. 7–54. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 92.Conners MG, Michelot T, Heywood EI, Orben RA, Phillips RA, Vyssotski AL, Shaffer SA, Thorne LH. 2021. Hidden Markov models identify major movement modes in accelerometer and magnetometer data from four albatross species. Mov. Ecol. 9, 7. ( 10.1186/s40462-021-00243-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leos-Barajas V, Gangloff EJ, Adam T, Langrock R, van Beest FM, Nabe-Nielsen J, Morales JM. 2017. Multi-scale modeling of animal movement and general behavior data using hidden Markov models with hierarchical structures. J. Agric. Biol. Environ. Stat. 22, 232-248. ( 10.1007/s13253-017-0282-9) [DOI] [Google Scholar]
- 94.Adam T, Griffiths CA, Leos-Barajas V, Meese EN, Lowe CG, Blackwell PG, Righton D, Langrock R. 2019. Joint modelling of multi-scale animal movement data using hierarchical hidden Markov models. Methods Ecol. Evol. 10, 1536-1550. ( 10.1111/2041-210X.13241) [DOI] [Google Scholar]
- 95.Montiglio PO, Ferrari C, Réale D. 2013. Social niche specialization under constraints: personality, social interactions and environmental heterogeneity. Phil. Trans. R. Soc. B 368, 20120343. ( 10.1098/rstb.2012.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bergmüller R, Taborsky M. 2007. Adaptive behavioural syndromes due to strategic niche specialization. BMC Ecol. 7, 12. ( 10.1186/1472-6785-7-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nonacs P. 2001. State dependent behavior and the marginal value theorem. Behav. Ecol. 12, 71-83. ( 10.1093/oxfordjournals.beheco.a000381) [DOI] [Google Scholar]
- 98.Rands SA, Pettifor RA, Rowcliffe JM, Cowlishaw G. 2004. State-dependent foraging rules for social animals in selfish herds. Proc. R. Soc. Lond. B 271, 2613-2620. ( 10.1098/rspb.2004.2906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ehlman SM, Trimmer PC, Sih A. 2019. Prey responses to exotic predators: effects of old risks and new cues. Am. Nat. 193, 575-587. ( 10.1086/702252) [DOI] [PubMed] [Google Scholar]
- 100.Olsson O, Brown JS, Smith HG. 2002. Long- and short-term state-dependent foraging under predation risk: an indication of habitat quality. Anim. Behav. 63, 981-989. ( 10.1006/anbe.2001.1985) [DOI] [Google Scholar]
- 101.Tigreros N, Wang EH, Thaler JS. 2018. Prey nutritional state drives divergent behavioural and physiological responses to predation risk. Funct. Ecol. 77, 1116. ( 10.1111/1365-2435.13046) [DOI] [Google Scholar]
- 102.Budaev S, Jørgensen C, Mangel M, Eliassen S, Giske J. 2019. Decision-making from the animal perspective: bridging ecology and subjective cognition. Front. Ecol. Evol. 7, 164. ( 10.3389/fevo.2019.00164) [DOI] [Google Scholar]
- 103.Giske J, Eliassen S, Fiksen Ø, Jakobsen PJ, Aksnes DL, Jørgensen C, Mangel M. 2013. Effects of the emotion system on adaptive behavior. Am. Nat. 182, 689-703. ( 10.1086/673533) [DOI] [PubMed] [Google Scholar]
- 104.McHuron EA, Costa DP, Schwarz L, Mangel M. 2017. State-dependent behavioural theory for assessing the fitness consequences of anthropogenic disturbance on capital and income breeders. Methods Ecol. Evol. 8, 552-560. ( 10.1111/2041-210X.12701) [DOI] [Google Scholar]
- 105.Trimmer PC, Ehlman SM, Sih A. 2017. Predicting behavioural responses to novel organisms: state-dependent detection theory. Proc. R. Soc. B 284, 20162108. ( 10.1098/rspb.2016.2108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clark CW, Mangel M. 2000. Dynamic state variable models in ecology: methods and applications. Oxford, UK: Oxford University Press. [Google Scholar]
- 107.Frankenhuis WE, Panchanathan K. 2011. Balancing sampling and specialization: an adaptationist model of incremental development. Proc. R. Soc. B 278, 3558-3565. ( 10.1098/rspb.2011.0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Panchanathan K, Frankenhuis WE. 2016. The evolution of sensitive periods in a model of incremental development. Proc. R. Soc. B 283, 20152439. ( 10.1098/rspb.2015.2439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walasek N, Frankenhuis WE, Panchanathan K. 2021. An evolutionary model of sensitive periods when the reliability of cues varies across ontogeny. Behav. Ecol. 113, 101-114. ( 10.1093/beheco/arab113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walasek N, Frankenhuis WE, Panchanathan K. 2022. Sensitive periods, but not critical periods, evolve in a fluctuating environment: a model of incremental development. Proc. R. Soc. B. 289, 20212623. ( 10.1098/rspb.2021.2623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stamps JA, Frankenhuis WE. 2016. Bayesian models of development. Trends Ecol. Evol. 31, 260-268. ( 10.1016/j.tree.2016.01.012) [DOI] [PubMed] [Google Scholar]
- 112.Houston AI, McNamara JM. 1999. Models of adaptive behaviour: an approach based on state. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 113.Sih A, Mathot KJ, Moirón M, Montiglio PO, Wolf M, Dingemanse NJ. 2015. Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50-60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 114.Taborsky B. 2021. A positive feedback loop between sociality and social competence. Ethology 127, 774-789. ( 10.1111/eth.13201) [DOI] [Google Scholar]
- 115.Ehlman SM, Scherer U, Wolf M. 2022. Developmental feedbacks and the emergence of individuality. R. Soc. Open Sci. 9, 221189. ( 10.1098/rsos.221189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.MacGregor HEA, Cottage A, Ioannou CC. 2021. Suppression of personality variation in boldness during foraging in three-spined sticklebacks. Behav. Ecol. Sociobiol. 75, 71. ( 10.1007/s00265-021-03007-2) [DOI] [Google Scholar]
- 117.Mathot KJ, Dekinga A, Piersma T. 2017. An experimental test of state–behaviour feedbacks: gizzard mass and foraging behaviour in red knots. Funct. Ecol. 31, 1111-1121. ( 10.1111/1365-2435.12827) [DOI] [Google Scholar]
- 118.Petelle MB, Martin JGA, Blumstein DT. 2019. Mixed support for state maintaining risky personality traits in yellow-bellied marmots. Anim. Behav. 150, 177-188. ( 10.1016/j.anbehav.2019.02.008) [DOI] [Google Scholar]
- 119.Laskowski KL, Chang CC, Sheehy K, Aguiñaga J. 2022. Consistent individual behavioral variation: what do we know and where are we going? Annu. Rev. Ecol. Evol. Syst. 53, 161-182. ( 10.1146/annurev-ecolsys-102220-011451) [DOI] [Google Scholar]
- 120.Clark CW. 1994. Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159-170. ( 10.1093/beheco/5.2.159) [DOI] [Google Scholar]
- 121.Luttbeg B, Sih A. 2010. Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977-3990. ( 10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581-584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 123.Ferrari MCO, Brown GE, Bortolotti GR, Chivers DP. 2010. Linking predator risk and uncertainty to adaptive forgetting: a theoretical framework and empirical test using tadpoles. Proc. R. Soc. B 277, 2205-2210. ( 10.1098/rspb.2009.2117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stamps J, Groothuis T. 2010. The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301-325. ( 10.1111/j.1469-185X.2009.00103.x) [DOI] [PubMed] [Google Scholar]
- 125.Carlson BA. 2017. Early life experiences have complex and long-lasting effects on behavior. Proc. Natl Acad. Sci. USA 114, 11 571-11 573. ( 10.1073/pnas.1716037114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horn G. 1985. Memory, imprinting, and the brain. Oxford, UK: Oxford University Press. [Google Scholar]
- 127.Bateson P, Horn G. 1994. Imprinting and recognition memory: a neural-net model. Anim. Behav. 48, 695-715. ( 10.1006/anbe.1994.1289) [DOI] [Google Scholar]
- 128.Laskowski KL, Wolf M, Bierbach D. 2016. The making of winners (and losers): how early dominance interactions determine adult social structure in a clonal fish. Proc. R. Soc. B 283, 20160183. ( 10.1098/rspb.2016.0183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eachus H, Choi MK, Ryu S. 2021. The effects of early life stress on the brain and behaviour: insights from zebrafish models. Front. Cell Dev. Biol. 9, 657591. ( 10.3389/fcell.2021.657591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Langenhof MR, Komdeur J. 2018. Why and how the early-life environment affects development of coping behaviours. Behav. Ecol. Sociobiol. 72, 34. ( 10.1007/s00265-018-2452-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77-81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 132.Fawcett TW, Frankenhuis WE. 2015. Adaptive explanations for sensitive windows in development. Front. Zool. 12, S3. ( 10.1186/1742-9994-12-S1-S3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hayward AD, Rickard IJ, Lummaa V. 2013. Influence of early-life nutrition on mortality and reproductive success during a subsequent famine in a preindustrial population. Proc. Natl Acad. Sci. USA 110, 13 886-13 891. ( 10.1073/pnas.1301817110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rödel HG, von Holst D, Kraus C. 2009. Family legacies: short- and long-term fitness consequences of early-life conditions in female European rabbits. J. Anim. Ecol. 78, 789-797. ( 10.1111/j.1365-2656.2009.01537.x) [DOI] [PubMed] [Google Scholar]
- 135.Marshall HH, et al. 2017. Lifetime fitness consequences of early-life ecological hardship in a wild mammal population. Ecol. Evol. 7, 1712-1724. ( 10.1002/ece3.2747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.English S, Fawcett TW, Higginson AD, Trimmer PC, Uller T. 2016. Adaptive use of information during growth can explain long-term effects of early life experiences. Am. Nat. 187, 620-632. ( 10.1086/685644) [DOI] [PubMed] [Google Scholar]
- 137.Burton T, Metcalfe NB. 2014. Can environmental conditions experienced in early life influence future generations? Proc. R. Soc. B 281, 20140311. ( 10.1098/rspb.2014.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Groothuis TGG, Taborsky B. 2015. Introducing biological realism into the study of developmental plasticity in behaviour. Front. Zool. 12, S6. ( 10.1186/1742-9994-12-S1-S6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoke KL, Adkins-Regan E, Bass AH, McCune AR, Wolfner MF. 2019. Co-opting evo-devo concepts for new insights into mechanisms of behavioural diversity. J. Exp. Biol. 222, jeb190058. ( 10.1242/jeb.190058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salazar-Ciudad I, Jernvall J. 2005. Graduality and innovation in the evolution of complex phenotypes: insights from development. J. Exp. Zool. B Dev. Evol. 304B, 619-631. ( 10.1002/jez.b.21058) [DOI] [PubMed] [Google Scholar]
- 141.Herbert-Read JE, Krause S, Morrell LJ, Schaerf TM, Krause J, Ward AJW. 2013. The role of individuality in collective group movement. Proc. R. Soc. B 280, 20122564. ( 10.1098/rspb.2012.2564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hansen MJ, et al. 2020. Linking hunting weaponry to attack strategies in sailfish and striped marlin. Proc. R. Soc. B 287, 20192228. ( 10.1098/rspb.2019.2228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Berman GJ. 2018. Measuring behavior across scales. BMC Biol. 16, 23. ( 10.1186/s12915-018-0494-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eyjolfsdottir E, Branson K, Yue Y, Perona P.. 2016. Learning recurrent representations for hierarchical behavior modeling. arXiv, 1611.00094. ( 10.48550/arXiv.1611.00094) [DOI]
- 145.Katsov AY, Freifeld L, Horowitz M, Kuehn S, Clandinin TR. 2017. Dynamic structure of locomotor behavior in walking fruit flies. eLife 6, e26410. ( 10.7554/eLife.26410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahamed T, Costa AC, Stephens GJ. 2021. Capturing the continuous complexity of behaviour in Caenorhabditis elegans. Nat. Phys. 17, 275-283. ( 10.1038/s41567-020-01036-8) [DOI] [Google Scholar]
- 147.Stephens GJ, de Mesquita MB, Ryu WS, Bialek W. 2011. Emergence of long timescales and stereotyped behaviors in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 7286-7289. ( 10.1073/pnas.1007868108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bestley S, Jonsen ID, Hindell MA, Guinet C, Charrassin JB. 2013. Integrative modelling of animal movement: incorporating in situ habitat and behavioural information for a migratory marine predator. Proc. R. Soc. B 280, 20122262. ( 10.1098/rspb.2012.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fawcett TW, Johnstone RA. 2003. Optimal assessment of multiple cues. Proc. R. Soc. B 270, 1637-1643. ( 10.1098/rspb.2003.2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Munoz NE, Blumstein DT. 2012. Multisensory perception in uncertain environments. Behav. Ecol. 23, 457-462. ( 10.1093/beheco/arr220) [DOI] [Google Scholar]
- 151.Winkler DW, et al. 2014. Cues, strategies, and outcomes: how migrating vertebrates track environmental change. Mov. Ecol. 2, 10. ( 10.1186/2051-3933-2-10) [DOI] [Google Scholar]
- 152.Chmura HE, Kharouba HM, Ashander J, Ehlman SM, Rivest EB, Yang LH. 2018. The mechanisms of phenology: the patterns and processes of phenological shifts. Ecol. Monogr. 89, e01337. ( 10.1002/ecm.1337) [DOI] [Google Scholar]
- 153.Dall SRX, McNamara JM, Leimar O. 2015. Genes as cues: phenotypic integration of genetic and epigenetic information from a Darwinian perspective. Trends Ecol. Evol. 30, 327-333. ( 10.1016/j.tree.2015.04.002) [DOI] [PubMed] [Google Scholar]
- 154.McNamara JM, Dall SRX, Hammerstein P, Leimar O. 2016. Detection vs. selection: integration of genetic, epigenetic and environmental cues in fluctuating environments. Ecol. Lett. 19, 1267-1276. ( 10.1111/ele.12663) [DOI] [PubMed] [Google Scholar]
- 155.Trimmer PC, Houston AI, Marshall JAR, Mendl MT, Paul ES, McNamara JM. 2011. Decision-making under uncertainty: biases and Bayesians. Anim. Cogn. 12, 465-476. ( 10.1007/s10071-011-0387-4) [DOI] [PubMed] [Google Scholar]
- 156.Polverino G, Palmas BM, Evans JP, Gasparini C. 2019. Individual plasticity in alternative reproductive tactics declines with social experience in male guppies. Anim. Behav. 148, 113-121. ( 10.1016/j.anbehav.2018.12.014) [DOI] [Google Scholar]
- 157.Polverino G, Cigliano C, Nakayama S, Mehner T. 2016. Emergence and development of personality over the ontogeny of fish in absence of environmental stress factors. Behav. Ecol. Sociobiol. 70, 2027-2037. ( 10.1007/s00265-016-2206-z) [DOI] [Google Scholar]
- 158.Stamps JA, Biro PA, Mitchell DJ, Saltz JB. 2018. Bayesian updating during development predicts genotypic differences in plasticity. Evolution 72, 2167-2180. ( 10.1111/evo.13585) [DOI] [PubMed] [Google Scholar]
- 159.Stamps JA, Krishnan VV. 2014. Combining information from ancestors and personal experiences to predict individual differences in developmental trajectories. Am. Nat. 184, 647-657. ( 10.1086/678116) [DOI] [PubMed] [Google Scholar]
- 160.Fischer B, van Doorn GS, Dieckmann U, Taborsky B. 2014. The evolution of age-dependent plasticity. Am. Nat. 183, 108-125. ( 10.1086/674008) [DOI] [PubMed] [Google Scholar]
- 161.Stamps JA, Krishnan VV. 2017. Age-dependent changes in behavioural plasticity: insights from Bayesian models of development. Anim. Behav. 126, 53-67. ( 10.1016/j.anbehav.2017.01.013) [DOI] [Google Scholar]
- 162.Sherratt TN, Morand-Ferron J. 2018. The adaptive significance of age-dependent changes in the tendency of individuals to explore. Anim. Behav. 138, 59-67. ( 10.1016/j.anbehav.2018.01.025) [DOI] [Google Scholar]
- 163.Farley SS, Dawson A, Goring SJ, Williams JW. 2018. Situating ecology as a big-data science: current advances, challenges, and solutions. BioScience 68, 563-576. ( 10.1093/biosci/biy068) [DOI] [Google Scholar]
- 164.Leonelli S. 2019. The challenges of big data biology. eLife 8, e47381. ( 10.7554/eLife.47381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hauber ME, Louder MI, Griffith SC. 2021. Neurogenomic insights into the behavioral and vocal development of the zebra finch. eLife 10, e61849. ( 10.7554/eLife.61849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Griffin DR. 1946. Supersonic cries of bats. Nature 158, 46-48. ( 10.1038/158046a0) [DOI] [PubMed] [Google Scholar]
- 167.Schevill WE, McBride AF. 1956. Evidence for echolocation by cetaceans. Deep Sea Res. 3, 153-154. ( 10.1016/0146-6313(56)90096-X) [DOI] [Google Scholar]
- 168.Thorpe WH. 1958. The learning of song patterns by birds, with especial reference to the song of the chaffinch Fringilla coelebs. Ibis 100, 535-570. ( 10.1111/j.1474-919X.1958.tb07960.x) [DOI] [Google Scholar]
- 169.Versluis M. 2000. How snapping shrimp snap: through cavitating bubbles. Science 289, 2114-2117. ( 10.1126/science.289.5487.2114) [DOI] [PubMed] [Google Scholar]
- 170.Patek SN, Korff WL, Caldwell RL. 2004. Deadly strike mechanism of a mantis shrimp. Nature 428, 819-820. ( 10.1038/428819a) [DOI] [PubMed] [Google Scholar]
- 171.Tinbergen N. 1963. On aims and methods of ethology. Z. Tierpsychol. 20, 410-433. ( 10.1111/j.1439-0310.1963.tb01161.x) [DOI] [Google Scholar]
- 172.Dewsbury DA. 1989. A brief history of the study of animal behavior in North America. In Perspectives in ethology (eds Bateson P, Klopfer PH), pp. 85-122. New York, NY: Plenum Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.