Abstract
Purpose:
Watchful waiting (WW) can be considered for patients with metastatic clear-cell renal cell carcinoma (mccRCC) with good or intermediate prognosis, especially those with <2 International Metastatic RCC Database Consortium criteria and ≤2 metastatic sites [referred to as watch and wait (“W&W”) criteria]. The IMaging PAtients for Cancer drug SelecTion-Renal Cell Carcinoma study objective was to assess the predictive value of [18F]FDG PET/CT and [89Zr]Zr-DFO-girentuximab PET/CT for WW duration in patients with mccRCC.
Experimental Design:
Between February 2015 and March 2018, 48 patients were enrolled, including 40 evaluable patients with good (n = 14) and intermediate (n = 26) prognosis. Baseline contrast-enhanced CT, [18F]FDG and [89Zr]Zr-DFO-girentuximab PET/CT were performed. Primary endpoint was the time to disease progression warranting systemic treatment. Maximum standardized uptake values (SUVmax) were measured using lesions on CT images coregistered to PET/CT. High and low uptake groups were defined on the basis of median geometric mean SUVmax of RECIST-measurable lesions across patients.
Results:
The median WW time was 16.1 months [95% confidence interval (CI): 9.0–31.7]. The median WW period was shorter in patients with high [18F]FDG tumor uptake than those with low uptake (9.0 vs. 36.2 months; HR, 5.6; 95% CI: 2.4–14.7; P < 0.001). Patients with high [89Zr]Zr-DFO-girentuximab tumor uptake had a median WW period of 9.3 versus 21.3 months with low uptake (HR, 1.7; 95% CI: 0.9–3.3; P = 0.13). Patients with “W&W criteria” had a longer median WW period of 21.3 compared with patients without: 9.3 months (HR, 1.9; 95% CI: 0.9–3.9; Pone-sided = 0.034). Adding [18F]FDG uptake to the “W&W criteria” improved the prediction of WW duration (P < 0.001); whereas [89Zr]Zr-DFO-girentuximab did not (P = 0.53).
Conclusions:
In patients with good- or intermediate-risk mccRCC, low [18F]FDG uptake is associated with prolonged WW. This study shows the predictive value of the “W&W criteria” for WW duration and shows the potential of [18F]FDG-PET/CT to further improve this.
Translational Relevance.
This study shows that molecular imaging with [18F]FDG PET/CT identifies patients with metastatic clear-cell renal cell carcinoma with expected indolent disease. It improves the prognostic value of clinical parameters embedded in the International Metastatic RCC Database Consortium criteria, independent of the number of organ sites. While [18F]FDG PET/CT is currently not standard in the work-up of newly diagnosed metastatic clear-cell renal cell carcinoma, the IMaging PAtients for Cancer drug SelecTion-Renal Cell Carcinoma study provides a reason to reconsider the role of molecular imaging to select patients eligible for watchful waiting as an alternative to an immediate start of systemic treatment.
Introduction
Metastatic clear-cell renal cell carcinoma (mccRCC) has a variable course (1, 2). The International Metastatic RCC Database Consortium (IMDC) criteria, initially designed to predict survival of patients on first-line antiangiogenic drugs (3, 4), can be used to identify patients with mccRCC with indolent disease. In those patients, a period of watchful waiting (WW) can be considered (5, 6).
This WW strategy prevents overtreatment, unnecessary side effects, and treatment costs, but the current strategy to select patients for WW leaves room for improvement (3, 7, 8). Therefore, a prospective study was performed to improve the selection of patients for WW (9). It was reported that patients with <2 IMDC risk factors and ≤2 involved organs have a WW period of 22.2 months compared with 8.4 months in patients with ≥2 IMDC risk factors and/or >2 involved organ sites. These criteria, further referred to as watch and wait (“W&W”) criteria, have not been externally validated.
ccRCC biology has been studied intensively, especially the role of cellular homeostasis, glucose metabolism, and carbonic anhydrase IX (CAIX) expression (10–13). An increased glucose metabolism, as visualized by PET with [18F]FDG, has been associated with poor survival (14, 15). Overexpression of CAIX has been correlated with indolent disease although evidence is contradictory (16–20). The radiolabeled mAb girentuximab, [89Zr]Zr-DFO-girentuximab, can visualize CAIX expression using PET/CT, which potentially identifies patients with indolent disease (16, 21–24).
In the IMaging PAtients for Cancer drug SelecTion (IMPACT)-Renal Cell Carcinoma (RCC) study, we evaluated the predictive value of PET/CT using [18F]FDG and [89Zr]Zr-DFO-girentuximab in patients with mccRCC and a good or intermediate prognosis according to IMDC. The primary objective was to predict time to disease progression warranting systemic treatment. A secondary objective was to validate the “W&W criteria” (9).
Materials and Methods
Selection criteria
In this prospective multicenter cohort study (ClinicalTrials.gov: NCT02228954), patients aged ≥18 years with histologically proven RCC with a clear cell component, recently (<6 months) diagnosed metastases, and 0–2 IMDC risk factors were enrolled (3). Patients were eligible if a WW period of at least 2 months was expected. Patients with previous systemic treatment for mccRCC, untreated central nervous system metastases, or symptomatic intracerebral metastases were excluded. Because patients did not start treatment upon enrollment in this study, the IMDC criterion “time from diagnosis to treatment <1 year” was adapted into “time from primary diagnosis to first metastatic disease <1 year.” This study was conducted at four Dutch academic medical centers and approved by the Institutional Review Board. All patients provided written informed consent. Studies were conducted in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Procedures
[18F]FDG and [89Zr]Zr-DFO-girentuximab PET/CT.
At baseline, patients underwent whole-body [18F]FDG and [89Zr]Zr-DFO-girentuximab PET/CT. The [18F]FDG PET/CTs were performed according to European Association of Nuclear Medicine guidelines version 1.0 (25). The [89Zr]Zr-DFO-girentuximab imaging procedure was harmonized between participating, European Association Research GmbH (EARL)-accredited centers. Patients underwent [89Zr]Zr-DFO-girentuximab PET/CT 4 days after intravenous injection of 37 MBq [89Zr]Zr-DFO-girentuximab (protein dose 5 mg). Details on acquisition and reconstruction protocols, conjugation, radiolabeling, and quality control of [89Zr]Zr-DFO-girentuximab were described previously (26).
The [18F]FDG PET/CTs were assessed by local nuclear physicians according to standard clinical practice. The [89Zr]Zr-DFO-girentuximab PET/CTs were assessed by three independent nuclear physicians in a central reviewing system, to ensure reproducible lesion detection and interobserver agreement. The medical oncologist did not have access to the PET/CT images as these results were saved outside of the Electronic Patient Dossier. The nuclear medicine physicians were allowed to communicate findings that required (local) interventions.
Volumes-of-interest were defined using CT images coregistered to the PET/CT, drawn manually on the [18F]FDG and [89Zr]Zr-DFO-girentuximab PET images based on tracer accumulation exceeding mediastinal blood pool. Standardized uptake values (SUVmax) were measured using Inveon Research Workplace software (IRW, version 4.1). The geometric mean (gm) SUVmax values per patient were calculated for RECIST-evaluable lesions with visual tracer uptake. The volumetric parameters metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were determined for [18F]FDG PET/CT.
Contrast-enhanced CT scan.
Patients underwent contrast-enhanced CT (ceCT) of the chest, abdomen, and pelvis at baseline; at 2, 4, 6, 9, and 12 months; and every 4 months thereafter. To explore clinical usefulness of WW, progressive disease (PD) was adapted to “disease progression warranting systemic treatment.” Therefore, PD was defined according to RECIST 1.1 (27), except for:
(i) an increase of >20% of sum of diameters of target lesions, not exceeding an absolute increase of 10 mm;
(ii) a new lesion in lung or bones in patients with limited (<5 lesions) baseline disease located in lung and/or bones;
(iii) progression that warrants local therapy but not systemic therapy.
Outcomes
The primary aim was to assess the predictive value of [18F]FDG and/or [89Zr]Zr-DFO-girentuximab PET/CT to ceCT to predict time to disease progression warranting systemic treatment, also split into extremes of rapid progression (<2 months) and indolent disease (>12 months) in patients with good or intermediate prognosis mccRCC. Time to disease progression was defined as the time from baseline CT to disease progression warranting systemic treatment, based on radiological and clinical disease progression. Local therapy was permitted while on protocol.
Prespecified secondary endpoints reported in this article are progression-free survival (PFS) and overall survival (OS). Meanwhile, Rini and colleagues predicted indolent disease in patients with RCC with <2 IMDC risk factors and ≤2 involved organs (9). Therefore, the validation of these “W&W criteria” was added as an extra secondary aim. Finally, we report the time on first-line treatment after WW.
Statistical analyses
On the basis of the incidence of 450 patients with mccRCC yearly in the Netherlands, it was estimated that we could enroll 80 patients in 4 years’ time, including approximately 25 (30%) patients with rapid disease progression and another 25 (30%) with indolent disease. On the basis of a lower accrual than anticipated, the number of patients to be included was changed into 40 patients. To accommodate this lower number of patients and still provide meaningful results, we adapted the way to evaluate the primary outcome to a continuous instead of categorical assessment, thereby improving the statistical efficiency. Of note, no analyses related to the primary outcome were performed before making this decision.
Standard descriptive statistics were used to describe baseline patient characteristics, overall and according to [18F]FDG and [89Zr]Zr-DFO-girentuximab uptake. For the latter, we grouped patients into high and low uptake groups based on their gm SUVmax of RECIST-measurable PET-positive lesions, binning patients into equal groups (using the median across patients). We used gm values to account for the right-skewed SUVmax data, and assigned patients without visual PET-positive lesions a gm SUVmax of zero.
Standard Kaplan–Meier analyses served to describe WW time, time to RECIST PD and OS, and univariably evaluate IMDC, “W&W criteria,” and [18F]FDG and [89Zr]Zr-DFO-girentuximab high and/or low uptake groups, using log-rank tests for statistical inference. We used pointwise 95% confidence intervals (CI) for Kaplan–Meier curves and for median survival times. Furthermore, Firth penalized Cox regression was used to obtain small-sample bias-corrected HRs (28). Here, PET data (SUVmax, TLG, MTV) were analyzed dichotomously (using a median split) as well as continuously (after truncation at the 90th percentile to decrease the influence of extreme values), assuming linearity. Nonlinear relations or optimal grouping thresholds beyond a median split were not assessed due to the relatively small dataset and associated risk of overfitting.
The added value of predictors in multivariable analyses was assessed by likelihood-ratio tests, specifically focusing on the added value of gm SUVmax of the two PET scans beyond the (underlying variables of) “W&W criteria” as the current clinical standard (primary analyses in view of events-to-predictor ratio). In an exploratory analysis to evaluate more predictors (the two PET scans, IMDC, number of organ sites, lung-only disease, and sum of RECIST-measurable lesion diameters), LASSO penalized Cox regression was used (with 5-fold cross-validation optimizing the partial likelihood; ref. 29), combined with 2,500-fold bootstrap resampling to assess variable-selection robustness.
The ability to discriminate between patients with limited and prolonged WW time was assessed using Harrell C-index for the overall observation period (30), and using time-dependent ROC curves (for both measures, 0.50 denotes no discriminative value and 1.00 perfect discrimination; ref. 31). Here 2,000-fold bootstrapping was used to obtain confidence intervals and statistically test for differences in discrimination between models.
Bootstrapping (2,000-fold) was also used for internal validation of multivariable models to account for overoptimism and to yield predicted WW time probabilities that are more likely to validate when applied to new patients.
The data were analyzed with R 3.2.1 for Mac OS, particularly using coxphf (package coxphf 1.11), and cv.glmnet (package glmnet 2.0-3). Estimates are reported with corresponding 95% CIs, considering a P value <0.05 as statistically significant. All tests were two sided, except for the relation between IMDC and “W&W criteria” with WW time. These were evaluated one-sidedly focused on confirming the previously reported direction of these associations.
Data availability statement
The data generated in this study are available upon request from the corresponding author.
Results
Between February 2015 and March 2018, 48 patients with good- or intermediate-risk mccRCC eligible for WW, signed informed consent and were screened for study enrollment (Supplementary Fig. S1). Five patients were considered screen failures due to PD on baseline ceCT. Because this PD required systemic treatment before the initiation of the WW period, they did not meet the inclusion criteria of eligibility for a WW period of at least 2 months. One other patient withdrew consent for personal reasons before baseline imaging procedures. Two other patients withdrew their consent at the first CT evaluation without PD. Therefore, 40 patients were evaluable for the primary endpoint.
Baseline characteristics
In total, 14 of 40 patients (35%) had a good prognosis according to IMDC criteria. Intermediate IMDC prognosis was primarily due to the diagnosis of metastases <1 year after the primary diagnosis (81%). Most patients entered the study with metastases in one or two organs (75%, Table 1). No patient received prior adjuvant treatment.
Table 1.
Baseline characteristics and demographics.
| All patients | Baseline [18F]FDG uptakea | Baseline [89Zr)Zr-DFO-girentuximab uptakea | |||
|---|---|---|---|---|---|
| (n = 40) | Low uptake group (n = 20) | High uptake group (n = 20) | Low uptake group (n = 20) | High uptake group (n = 20) | |
| Age, years (mean ± SD) | 65.8 (± 9.1) | 67.4 (± 8.5) | 64.2 (± 9.7) | 66.7 (± 7.9) | 64.9 (± 10.4) |
| Male | 30 (75) | 16 (80) | 14 (70) | 17 (85) | 13 (65) |
| Synchronous metastasesb | 19 (48) | 9 (45) | 10 (50) | 7 (35) | 12 (60) |
| Nephrectomy | 35 (88) | 19 (95) | 16 (80) | 20 (100) | 15 (75) |
| Hb <LLN | 13 (33) | 7 (35) | 6 (30) | 4 (20) | 9 (45) |
| Neutrophils >ULN | 2 (5) | 1 (5) | 1 (5) | 1 (5) | 1 (5) |
| Platelets >ULN | 2 (5) | 0 (0) | 2 (10) | 1 (5) | 1 (5) |
| Calcium >ULN | 1 (3) | 0 (0) | 1 (5) | 1 (5) | 0 (0) |
| Karnofsky performance score < 80 | 1 (3) | 1 (5) | 0 (0) | 1 (5) | 0 (0) |
| Interval < 1 year between primary diagnosis and first metastases | 21 (53) | 10 (50) | 11 (55) | 7 (35) | 14 (70) |
| IMDC | |||||
| 0 | 14 (35) | 8 (40) | 6 (30) | 11 (55) | 3 (15) |
| 1 | 12 (30) | 5 (25) | 7 (35) | 3 (15) | 9 (45) |
| 2 | 14 (35) | 7 (35) | 7 (35) | 6 (30) | 8 (40) |
| Number of organ sites (on CT scan)c | |||||
| 1 | 14 (35) | 12 (60) | 2 (10) | 11 (55) | 3 (15) |
| 2 | 16 (40) | 5 (25) | 11 (55) | 8 (40) | 8 (40) |
| 3 | 8 (20) | 2 (10) | 6 (30) | 1 (5) | 7 (35) |
| 4 | 2 (5) | 1 (5) | 1 (5) | — | 2 (10) |
| W&W criteria, favorable | 21 (52.5) | 14 (61) | 7 (42) | 14 (70) | 7 (35) |
| Sum of diameter (cm) (median, range) | 7.7 (1.0–66.2) | 3.5 (1.0–41.9) | 11.3 (3.7–66.2) | 4.9 (1.0–66.2) | 11.3 (2.5–41.9) |
| If [18F]FDG positive, geometric mean per patient, (median, range)d | 4.8 (1.9–12.2) | — | — | 3.1 (1.9–3.7) | 6.7 (3.9–12.2) |
| If [89Zr]Zr-DFO-girentuximab positive, geometric mean per patient, (median, range)d | 15.4 (5.0–104.8) | 9.3 (5.0–12.8) | 20.1 (14.1–104.8) | — | — |
Abbreviations: LLN, lower limit of normal; ULN, upper limit of normal.
aPatients with no PET-positive and RECIST-measurable lesions were interpreted as having a very low SUVmax value and grouped accordingly. The per-patient uptake is expressed as the gm SUVmax of all RECIST-measurable PET-positive lesions. Patients are grouped on the basis of the median gm SUVmax of [18F]FDG- or [89Zr]Zr-DFO-girentuximab, respectively.
bTwo patients previously received local radiotherapy with curative intent. One patient underwent a metastasectomy of the only present metastasis with curative intent.
cBased on only RECIST-positive lesions as visualized by CT. Lesions were detected in lung, lymph nodes, bone, adrenal gland, kidney, and soft tissue.
dData available for 34 (85%) patients, based on RECIST-positive lesions only.
Baseline imaging detected a variable number of lesions considered RECIST-evaluable on ceCT and quantifiable on PET: 230 of 283 lesions on ceCT, 149 of 233 lesions on [18F]FDG PET/CT, and 163 of 272 on [89Zr]Zr-DFO-girentuximab PET/CT.
Overall, 30 patients had lesions with visual [18F]FDG and [89Zr]Zr-DFO-girentuximab accumulation. Two patients had no visual lesions on [18F]FDG and [89Zr]Zr-DFO-girentuximab-PET/CT. Four patients had a visual negative 89[Zr]Zr-DFO-girentuximab-PET/CT, and 4 other patients had a negative [18F]FDG PET/CT. Tracer accumulation within and between patients was heterogeneous for both [18F]FDG and [89Zr]Zr-DFO-girentuximab (Fig. 1A and B).
Figure 1.
Overview of the tracer uptake of [18F]FDG and [89Zr]Zr-DFO-girentuximab in the RECIST-measurable lesion per patient. A depicts a scatterplot of [18F]FDG-positive lesions. For each patient on the x-axis, per tumor lesion [18F]FDG SUVmax is shown in vertical direction. The size of the dot reflects the size of the lesion, and the color identifies the location of this lesion. B identical design, here with each patient on the x-axis and the y-axis depicts the tumor [89Zr]Zr-DFO-girentuximab SUVmax uptake per tumor lesion. In C, the duration of WW is depicted for each patient, highlighting the time with and without disease progression according to RECIST 1.1.
Additional imaging, clinicopathologic and demographic baseline characteristics for all patients and according to high and low [18F]FDG and [89Zr]Zr-DFO-girentuximab uptake groups are shown in Table 1, imaging examples in Fig. 2.
Figure 2.
Heterogeneity of tracer uptake. Representative images of [18F]FDG PET/CT (left) and [89Zr]Zr-DFO-girentuximab PET/CT (right) are shown of 2 patients. In patient 1, lymph node metastases as visualized on axial sections of [18F]FDG PET/CT (A) and [89Zr]Zr-DFO-girentuximab PET/CT (B). In patient 2, lung lesions as visualized on transverse sections of [18F]FDG PET/CT (C) and [89Zr]Zr-DFO-girentuximab PET/CT (D).
Follow-up of patients during and after WW
After a median follow-up of 48.0 months (95% CI: 44.8–56.5), 36 patients (90%) required systemic treatment. As of December 2021, 4 patients are still on WW (Fig. 1C). Two patients were censored prematurely during WW due to drop-out or surgical resection of all lesions without disease progression.
Four other patients underwent local therapy during WW but continued their WW period. This included 2 patients who underwent stereotactic radiotherapy for a newly detected asymptomatic solitary brain metastasis at baseline. One patient underwent radiotherapy for a growing pancreatic lesion. One other patient underwent radiotherapy for a symptomatic bone lesion. There was no clinical indication for systemic treatment in these patients
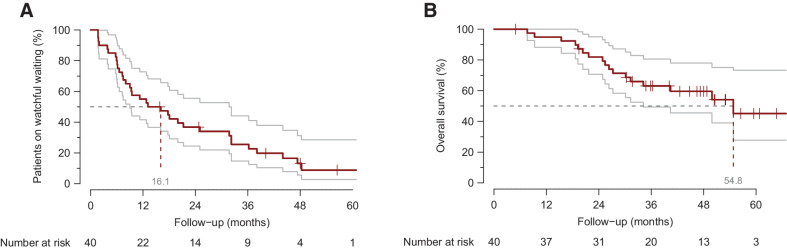
In total, 36 patients (90%) had RECIST PD; 3 patients had clinical disease progression without RECIST confirmation. The median time to RECIST PD was 7.8 months (95% CI: 6.1–12.1); the overall median WW period was 16.1 months (95% CI: 9.0–31.7; Fig. 3A) including 4 patients with rapid progression and 10 with indolent disease. The median OS was 54.8 months (95% CI: 34.2–NA; Fig. 3B). RECIST PD involved growth of known metastases (89%) and/or development of new lesions (31%), with 4 patients showing only new lesions (11%). In 11 (31%) patients, RECIST PD was the trigger to immediately start systemic treatment, while 24 other patients continued surveillance for an additional median time of 11.1 months (95% CI: 5.7–31.9).
Figure 3.
Kaplan–Meier curve for WW and OS. A shows the Kaplan–Meier curves for time under WW; median WW period is 16.1 months (95% CI: 9.0–31.7). B shows the Kaplan–Meier curves for the OS; median OS: 54.8 months (95% CI: 34.2–NA).
Four of 36 patients who required systemic treatment, also underwent radiotherapy or surgery of all target lesions and 7 patients received only best supportive care. Four patients were not fit for systemic treatment; in 3 patients clinical deterioration was due to rapid disease progression, 1 patient because of comorbidity and age. Three other patients did not want treatment. Of the remaining 25 patients, in first line 17 were treated with angiogenesis inhibitors (e.g., sunitinib, pazopanib, bevacizumab INF-alfa) and 8 patients with (combination) immunotherapy. The treatment-related PFS on first-line angiogenesis inhibitors was 10.6 months (95% CI: 6.1–15.0) and with (combination) immunotherapy 17.9 months (95% CI: 14.2–21.7). Five patients are still on active first-line treatment. An overview per patient is provided in Supplementary Fig. S2.
In total, 17 of 36 patients (47%) died following WW after a median time of 27.4 months from the end of WW (95% CI: 22.1–NA).
Predicting duration of WW
In univariable analyses, patients with above-median [18F]FDG gm SUVmax showed a shorter WW period compared with patients with below-median [18F]FDG gm SUVmax (9.0 vs. 36.2 months; HR, 5.6; 95% CI: 2.4–14.7; P < 0.001; Fig. 4A). This was also observed—to a lesser extent—when [18F]FDG uptake was expressed as MTV or TLG (Table 2A). The median WW period of patients with above-median [89Zr]Zr-DFO-girentuximab gm SUVmax was 9.3 versus 21.3 months in patients with below-median uptake (HR, 1.7; 95% CI: 0.9–3.3; P = 0.13; Fig. 4B). In contrast to [18F]FDG, the per-patient gm [89Zr]Zr-DFO-girentuximab SUVmax was not significantly associated with the time until RECIST PD (Table 2A).
Figure 4.
Kaplan–Meier curve. A shows the Kaplan–Meier curves for time on WW according to above or below median [18F]FDG uptake at baseline, with a median time under WW of 36.2 versus 9.0 months. B shows the Kaplan–Meier curves for time on WW according to above or below median [89Zr]Zr-DFO-girentuximab uptake at baseline, with a median time under WW of 21.3 versus 9.3 months. In both figures, the per-patient uptake is expressed as the gm SUVmax of all RECIST-measurable PET-positive lesions. The yellow graph depicts patients with below-median tracer uptake; the blue graph depicts patients with above-median tracer uptake. *One patient was censored because of surgical excision of all tumor lesions.
Table 2.
Univariable (A) and multivariable (B) Cox regression analysis of all patients.
| Table 2A | Univariable analysis | Univariable analysis | ||
|---|---|---|---|---|
| Watchful waiting time | Time until RECIST-defined PD | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| “W&W criteria”, < 2 IMDC and ≤ 2 organ sites vs. ≥ 2 IMDC and/or ≥ 2 organ sites | 1.9 (1.0–3.9) | 0.07a | 1.3 (0.7–2.5) | 0.48 |
| IMDC criteria, per unit increase in score | 1.4 (0.9–2.2) | 0.1a | 1.3 (0.9–2.0) | 0.21 |
| Number of organ sites, per involved site increase | 1.4 (1.0–1.9) | 0.087 | 1.0 (0.7–1.5) | 0.86 |
| [89Zr]Zr-DFO-girentuximab above-median split | 1.7 (0.9–3.3) | 0.14 | 1.3 (0.7–2.6) | 0.43 |
| [89Zr]Zr-DFO-girentuximab SUVmax, per 10 units increase in geometric mean | 1.3 (1.0–1.7) | 0.085 | 1.2 (0.9–1.5) | 0.3 |
| [18F]FDG above-median split | 5.6 (2.4–14.7) | <0.001 | 2.1 (1.0–4.4) | 0.046 |
| [18F]FDG SUVmax, per 3 units increase in geometric mean | 1.9 (1.4–2.6) | <0.001 | 1.4 (1.0–1.8) | 0.026 |
| [18F]FDG MTV above median split | 2.2 (1.1–4.5) | 0.03 | 2.5 (1.2–5.2) | 0.013 |
| [18F]FDG MTV, per 50 units increase | 1.3 (1.0–1.6) | 0.007 | 1.2 (0.9–1.5) | 0.17 |
| [18F]FDG TLG above median split | 2.5 (1.2–5.2) | 0.011 | 1.52 (0.8–3.1) | 0.23 |
| [18F]FDG TLG, per 200 units increase | 1.5 (1.1–2.0) | 0.006 | 1.3 (1.0–1.7) | 0.077 |
| Table 2B | Multivariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Watchful waiting time | Time until RECIST-defined PD | |||
| HR (95% CI) | P | HR (95% CI) | P | |
| IMDC criteria, per unit increase in score | 1.3 (0.81–2.00) | 0.29 | 1.3 (0.85–2.01) | 0.22 |
| Number of organ sites, per involved site increase | 0.9 (0.49–1.46) | 0.55 | 0.7 (0.40–1.23) | 0.22 |
| [89Zr]Zr-DFO-girentuximab SUVmax, per 10 units increase in geometric mean | 1.2 (0.84–1.78) | 0.28 | 1.3 (0.87–1.82) | 0.21 |
| [18F]FDG SUVmax, per 3 units increase in geometric mean | 1.9 (1.34–2.58) | <0.001 | 1.4 (1.04–1.80) | 0.025 |
Note: Continuous PET variables were truncated at the top 90% to reduce the influence of outliers, and were represented in the analyses in such a way that the resulting HRs correspond to an amount of increase in the respective variable corresponding with approximately one-fourth of its entire distribution. This was done to make the resulting HRs for the continuous PET variables comparable with each other. All HRs are based on Firth penalized regression.
aThe reported P value is two sided, according to Wald.
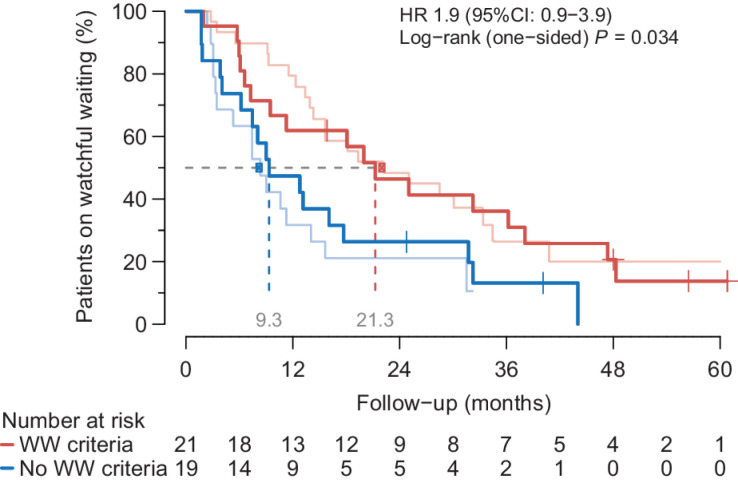
Patients without IMDC risk factors had a median WW period of 21.3 months, which was 16.1 and 7.5 for patients with one and two risk factors, respectively (one-sided likelihood-ratio Ptrend = 0.051). Patients with “W&W criteria” (n = 21) showed a median WW period of 21.3 months compared with 9.3 months in those without (n = 19; HR, 1.9; 95% CI: 0.9–3.9; one-sided log-rank P = 0.034; Fig. 5).
Figure 5.
Validation extended IMDC criteria. The WW period for the unfavorable (blue) versus favorable (red) WW group. The pink and light blue line correspond to the previously published observations by Rini and colleagues of unfavorable and favorable group, respectively (median time on WW of 8.4 vs. 22.2 months, P = 0.006). Patients that meet the “W&W criteria” (<2 IMDC criteria and ≤2 organ sites; red) have a longer WW period of 21.3 months compared with 9.3 months in patients with an unfavorable prognosis.
In multivariable analyses, adding [18F]FDG SUVmax to the “W&W criteria” significantly improved a Cox model for WW time. This was observed for [18F]FDG uptake as a continuous variable or based on the median split, and by analyzing “W&W criteria” in two groups or the underlying IMDC and involved organ sites as continuous variables (all P < 0.001). The addition of “W&W criteria” or its two underlying variables did not improve a Cox model with [18F]FDG-PET/CT (all P > 0.32). Similarly, the addition of [89Zr]Zr-DFO-girentuximab uptake did not improve a Cox model for WW based on the “W&W criteria” or its two underlying variables (all P > 0.27). In Table 2B, we report the multivariable analyses for WW time and time until RECIST-defined PD for a full multivariable model including both PET scans, number of organ sites, and IMDC criteria with accompanying Wald P values.
Regarding discriminating patients according to WW time, a Cox model for WW time including the underlying “W&W criteria” showed an overall C-index of 0.618 (95% CI: 0.506–0.729), which was 0.722 (95% CI: 0.610–0.834) for a model containing only [18F]FDG gm SUVmax continuously and 0.579 (95% CI: 0.466–0.691) for only [89Zr]Zr-DFO-girentuximab gm SUVmax continuously. Adding [18F]FDG uptake to the model with IMDC and number of organ sites increased the overall C-index to 0.732 (95% CI: 0.619–0.844; P = 0.086 for improvement in C-index), which was 0.615 (95% CI: 0.503–0.728; P = 1.0 for improvement in C-index) for adding [89Zr]Zr-DFO-girentuximab gm SUVmax. An exploratory LASSO model considering the two PET scans, IMDC, number of organ sites, lung only disease, and sum of RECIST-measurable lesion diameters resulted in a model that performed less than the above best model with an overall C-index of 0.711 (95% CI: 0.599–0.824; see Supplementary Data for more details about the LASSO model).
We also evaluated the discriminative ability to predict prolonged indolent disease (>12 months) using time-dependent ROC curves (Fig. 6). In our data, a subgroup of 12 patients (30%) could be identified on the basis of a [18F]FDG gm SUVmax <3 or a negative [18F]FDG PET/CT, who all had a WW period of >12 months [100% (95% CI: 76–100) positive predictive value and 64% (95% CI: 46–79) negative predictive value; data-driven threshold maximizing sensitivity at 100% specificity]. The prediction of rapid progression (<2 months after study enrolment) was not analyzed because of too few events.
Figure 6.
Time-dependent ROC curves at 12 months WW time. These figures show the discriminating value of the IMDC criteria together with the number of organ sites (the two variables underlying the “W&W criteria”) and gm [18F]FDG SUVmax (A) and [89Zr]Zr-DFO-girentuximab (B), respectively, individually and when combined. All variables were evaluated continuously, and for the curves that show the combined discriminating value of variables, we used Firth penalized Cox regression to obtain a combined prognostic score.
To allow further external validation and translation of our results toward clinical practice, we internally validated the predictive model with IMDC scores, the number of organ sites and the [18F]FDG gm SUVmax. A formula based on this over-optimism-corrected model yields predicted probabilities of remaining on WW at 12 months between 9% and 83%, depending on a particular patient's IMDC score, the number of organ sites and [18F]FDG-PET/CT results. An easy-to-use score chart showing these predicted probabilities and the underlying formula is provided in Supplementary Fig. S3.
Discussion
The prospective IMPACT-RCC study investigated the value of [89Zr]Zr-DFO-girentuximab and [18F]FDG PET/CT in patients with good- or intermediate-risk mccRCC to predict time to WW progression. We demonstrated that patients with low [18F]FDG uptake have a longer WW period. [89Zr]Zr-DFO-girentuximab uptake did not show prognostic value. Moreover, we confirmed that <2 IMDC risk factors and ≤2 involved organ sites (“W&W criteria”) identified patients with a prolonged WW period compared with other patients.
The only other prospective study on WW was published by Rini and colleagues. They identified a patient subgroup with a prolonged WW period based on the number of organ sites besides IMDC criteria (“W&W criteria”; ref. 9). On the basis of this study and to translate our results into standard clinical use outside studies, we used the number of involved organ sites rather than tumor burden as a marker for disease extent. Our study has a similar sample size, distribution of IMDC risk factors and disease extent, which allowed us to validate the “W&W criteria” to select patients for a WW strategy. Moreover, we highlighted that [18F]FDG SUVmax alone improved the selection of patients with indolent disease as compared with “W&W criteria.”
While CAIX in response to hypoxia has shown prognostic value across tumor types (32), no robust prospective data associated CAIX in ccRCC with prognosis (16, 18, 20). In contrast to other tumor types, the overexpression of CAIX in ccRCC is the result of a mutational loss of the Von Hipple Lindau gene (13), as opposed to hypoxic tumor microenvironment. Our data show no clinically meaningful prognostic value of CAIX as visualized by [89Zr]Zr-DFO-girentuximab PET/CT on a patient level in patients with mccRCC. Analyses on lesion level could help to better understand this lack of correlation. For example, we have previously reported that highest SUVmax values were observed in adrenal gland and kidney lesions, compared with lower SUVmax values in lung lesions (26).
On the basis of our data, [18F]FDG PET/CT has the potential to change clinical practice by providing guidance for decision making on WW as initial strategy. All patients with a gm [18F]FDG SUVmax <3 or negative [18F]FDG PET/CT (representing 30% of all patients) remained on WW at least 12 months. This is in agreement with a previous reported association between high [18F]FDG uptake and more aggressive disease (15). While our analyses should be confirmed in a larger prospective cohort and the optimal way to take [18F]FDG SUVmax into account is yet to be established, our results support the potential clinical relevance of baseline [18F]FDG PET/CT in patients with mccRCC considered for observation. To help validate our findings, we have included a formula and score chart to predict the 12-month WW duration based on our internally validated model with [18F]FDG PET/CT, IMDC criteria, and the number of organ sites. Such external validation is important as our results were derived from a small cohort of patients which may not only lead to overoptimistic results even after internal validation, but a small cohort also increases the chance of a nonrepresentative patient mix, which can affect the generalizability of the sensitivity, specificity, and the negative and positive predictive values we observed.
Recently, we reported a higher number of metastatic lesions detected by [18F]FDG PET/CT and [89Zr]Zr-DFO-girentuximab PET/CT plus CT compared with only CT in these patients (26). Although the clinical implications of those lesions are to be investigated, the analyses of lesional tracer uptake were limited to RECIST-measurable lesions thereby excluding potentially clinically relevant lesions (e.g., bone lesions). In addition, only 60% of all RECIST-measurable lesions were PET positive. As reported previously, tracer uptake was influenced by lesion size and to a lesser-extent lesion location (26). Lesion size introduces partial volume effects and so tracer uptake may not be observed as higher than the background. While tracer uptake on a patient level might be underestimated, including all lesions would not be feasible in daily practice upon clinical implementation. The definition of “disease progression warranting systemic treatment” is subject to interpretation of medical physician and patient which introduces subjectivity. However, it does best illustrate daily practice. Furthermore, due to the limited number of eligible patients and clinical trials offering first-line immunotherapy, the current article is published in an era where first-line (combination) immunotherapy is available for all patients with mccRCC which has changed the view on a WW strategy. The 4 patients in our study not eligible for systemic treatment upon disease progression also stress the relevance of optimal patient selection for the WW strategy.
With a median OS of 54.8 months in patients with mccRCC with WW as initial strategy in our study, WW should still be seen as a valid option, also in the current treatment landscape of mRCC (33, 34). So far, variable responses have been reported to new first-line treatment strategies (35). As illustrated by variable [18F]FDG and [89Zr]Zr-DFO-girentuximab uptake in our imaging results, the diverse tumor phenotypes might explain the heterogenous responses to first-line (combination) immunotherapy. Particularly in patients with a good-risk mccRCC with less efficacy of (combination) immunotherapy than intermediate- or poor-risk patients with mccRCC, WW could be an interesting treatment strategy. In our patient selection, the response to angiogenesis inhibitor monotherapy or (combination) immunotherapy after a WW period was comparable to what was reported in registration studies (36–38).
WW can be considered as initial treatment strategy in a subgroup of patients with mccRCC expected to have indolent disease. We confirmed the predictive value of <2 IMDC risk factors and ≤2 involved organ sites (or “W&W criteria”). Moreover, the current study establishes the potential of baseline [18F]FDG uptake for predicting WW time beyond these criteria, although this should be confirmed in an independent study.
Supplementary Material
Flow diagram of patient enrolment. * Four patients were unfit for systemic treatment due to clinical deterioration resulting from rapid disease progression (n=3) or comorbidity and age (n=1). Three other patients did not wat systemic treatment.
Score chart with predicted probability (%) to remain on WW at 12 months This model was based on the number of IMDC Risk factors (0, 1, 2), the number or involved organ sites (0 - 4) and the geometric mean [¹⁸F]FDG SUVmax as a continuous variable. The underlying formula is 100*(exp(-0.531)^exp(0.198*[IMDC score] + 0.039*[No of affected organ sites] + 0.170*[geometric mean [¹⁸F]FDG SUVmax] - 1.09))
Flow-chart patients according to RECIST-defined PD. *All patients had clinical disease progression; no CT-imaging was performed before initiation of systemic treatment ** In total 7 patients choose best supportive care. Three other patients underwent radiotherapy or surgery of all target lesions.
Lasso model
Acknowledgments
This work was supported by the Dutch Cancer Society (Alpe d'HuZes Grant RUG 2012-5400).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
S.F. Oosting reports grants from Dutch Cancer Foundation KWF during the conduct of the study, as well as other support from BMS and Merck outside the submitted work; S.F. Oosting is a member of study Steering and Safety Monitoring Committee for ALX Oncology. S.G. Elias reports grants from Dutch Cancer Society during the conduct of the study, as well as grants from European Union H2020 outside the submitted work. S. Heskamp reports grants from Dutch Cancer Society and Dutch Research Council during the conduct of the study, as well as grants and other support from Telix Pharma, Merck, and AstraZeneca outside the submitted work. I.M.E. Desar reports grants from Dutch Cancer Fund KWF during the conduct of the study. C.W. Menke-van der Houven van Oordt reports grants from Crystal Therapeutics, BMS, Pfizer, and Daiichi/AstraZeneca, as well as other support from GSK, Boehringer Ingelheim, Servier, and G1 therapeutics during the conduct of the study; C.W. Menke-van der Houven van Oordt also reports other support from Enterome, Sanofi, Eli Lilly, and Roche outside the submitted work. A.A.M. van der Veldt reports other support from BMS, MSD, Eisai, Roche, Merck, Sanofi, Pierre Fabre, Pfizer, Ipsen, and Novartis outside the submitted work. W.J.G. Oyen reports personal fees from Bayer, Novartis/AAA, and Astellas outside the submitted work. C.M.L. van Herpen reports consultant fees to the institution for advisory boards from Bayer, Bristol Myers Squibb, Ipsen, MSD, Regeneron, and Philips MPDx, as well as research grants from AstraZeneca, Bristol Myers Squibb, MSD, Merck, Ipsen, Novartis, and Sanofi. No disclosures were reported by the other authors.
Authors' Contributions
S.R. Verhoeff: Conceptualization, resources, data curation, software, formal analysis, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. S.F. Oosting: Investigation, methodology, writing–original draft, writing–review and editing. S.G. Elias: Conceptualization, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. S.C. van Es: Conceptualization, resources, data curation, investigation. S.L. Gerritse: Resources, data curation, investigation, writing–review and editing. L. Angus: Data curation, writing–review and editing. S. Heskamp: Formal analysis, supervision, methodology, writing–original draft, writing–review and editing. I.M.E. Desar: Conceptualization, supervision, writing–original draft. C.W. Menke-van der Houven van Oordt: Data curation, investigation, methodology, writing–review and editing. A.A.M. van der Veldt: Data curation, supervision, writing–review and editing. A.I.J. Arens: Conceptualization, data curation, software, writing–review and editing. A.H. Brouwers: Conceptualization, data curation, software, formal analysis, writing–review and editing. B. Eisses: Data curation, writing–review and editing. P.F.A. Mulders: Conceptualization, writing–original draft. O.S. Hoekstra: Software, formal analysis, writing–review and editing. G.J.C. Zwezerijnen: Software, formal analysis. W.T.A. van der Graaf: Conceptualization, funding acquisition, writing–original draft, writing–review and editing. E.H.J.G. Aarntzen: Software, formal analysis, supervision, investigation, methodology, writing–original draft, writing–review and editing. W.J.G. Oyen: Conceptualization, data curation, software, formal analysis, validation, methodology, writing–original draft, writing–review and editing. C.M.L. van Herpen: Conceptualization, data curation, supervision, funding acquisition, investigation, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58–68. [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–40. [DOI] [PubMed] [Google Scholar]
- 3. Heng DYC, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
- 4. Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med 2014;370:1769–70. [DOI] [PubMed] [Google Scholar]
- 5. Oliver RT, Nethersell AB, Bottomley JM. Unexplained spontaneous regression and alpha-interferon as treatment for metastatic renal carcinoma. Br J Urol 1989;63:128–31. [DOI] [PubMed] [Google Scholar]
- 6. Harrison MR, Costello BA, Bhavsar NA, Vaishampayan U, Pal SK, Zakharia Y, et al. Active surveillance of metastatic renal cell carcinoma: results from a prospective observational study (MaRCC). Cancer 2021;127:2204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bimbatti D, Ciccarese C, Fantinel E, Sava T, Massari F, Bisogno I, et al. Predictive role of changes in the tumor burden and international metastatic renal cell carcinoma database consortium class during active surveillance for metastatic renal cell carcinoma. Urol Oncol 2018;36:526 e13–8. [DOI] [PubMed] [Google Scholar]
- 8. Matsubara N, Mukai H, Naito Y, Itoh K, Komai Y, Sakai Y. First experience of active surveillance before systemic target therapy in patients with metastatic renal cell carcinoma. Urology 2013;82:118–23. [DOI] [PubMed] [Google Scholar]
- 9. Rini BI, Dorff TB, Elson P, Rodriguez CS, Shepard D, Wood L, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol 2016;17:1317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci 2013;104:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genega EM, Ghebremichael M, Najarian R, Fu Y, Wang Y, Argani P, et al. Carbonic anhydrase IX expression in renal neoplasms: correlation with tumor type and grade. Am J Clin Pathol 2010;134:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stillebroer AB, Mulders PF, Boerman OC, Oyen WJ, Oosterwijk E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur Urol 2010;58:75–83. [DOI] [PubMed] [Google Scholar]
- 14. Kayani I, Avril N, Bomanji J, Chowdhury S, Rockall A, Sahdev A, et al. Sequential FDG-PET/CT as a biomarker of response to sunitinib in metastatic clear cell renal cancer. Clin Cancer Res 2011;17:6021–8. [DOI] [PubMed] [Google Scholar]
- 15. Brouwers AH, Dorr U, Lang O, Boerman OC, Oyen WJG, Steffens MG, et al. 131 I-cG250 monoclonal antibody immunoscintigraphy versus [18 F]FDG-PET imaging in patients with metastatic renal cell carcinoma: a comparative study. Nucl Med Commun 2002;23:229–36. [DOI] [PubMed] [Google Scholar]
- 16. Leibovich BC, Sheinin Y, Lohse CM, Thompson RH, Cheville JC, Zavada J, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol 2007;25:4757–64. [DOI] [PubMed] [Google Scholar]
- 17. Ambrosetti D, Dufies M, Dadone B, Durand M, Borchiellini D, Amiel J, et al. The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. PLoS One 2018;13:e0193477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res 2003;9:802–11. [PubMed] [Google Scholar]
- 19. Sandlund J, Oosterwijk E, Grankvist K, Oosterwijk-Wakka J, Ljungberg B, Rasmuson T. Prognostic impact of carbonic anhydrase IX expression in human renal cell carcinoma. BJU Int 2007;100:556–60. [DOI] [PubMed] [Google Scholar]
- 20. Zhao Z, Liao G, Li Y, Zhou S, Zou H, Fernando S. Prognostic value of carbonic anhydrase IX immunohistochemical expression in renal cell carcinoma: a meta-analysis of the literature. PLoS One 2014;9:e114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grabmaier K, Vissers JLM, De Weijert MCA, Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH, et al. Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer 2000;85:865–70. [DOI] [PubMed] [Google Scholar]
- 22. Oosterwdk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EKJ, Jonas U, Zwartendijk I, et al. Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer 1986;38:489–94. [DOI] [PubMed] [Google Scholar]
- 23. Oosterwijk E, Ruiter DJ, Wakka JC, Huiskens-van der Meij JW, Jonas U, Fleuren GJ, et al. Immunohistochemical analysis of monoclonal antibodies to renal antigens. Application in the diagnosis of renal cell carcinoma. Am J Pathol 1986;123:301–9. [PMC free article] [PubMed] [Google Scholar]
- 24. Oosterwijk-Wakka JC, Boerman OC, Mulders PF, Oosterwijk E. Application of monoclonal antibody G250 recognizing carbonic anhydrase IX in renal cell carcinoma. Int J Mol Sci 2013;14:11402–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010;37:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verhoeff SR, van Es SC, Boon E, van Helden E, Angus L, Elias SG, et al. Lesion detection by [(89)Zr]Zr-DFO-girentuximab and [(18)F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur J Nucl Med Mol Imaging 2019;46:1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 28. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38. [Google Scholar]
- 29. Simon N, Friedman J, Hastie T, Tibshirani R. Regularization paths for Cox's proportional hazards model via coordinate descent. J Stat Softw 2011;39:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 31. Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–44. [DOI] [PubMed] [Google Scholar]
- 32. van Kuijk SJ, Yaromina A, Houben R, Niemans R, Lambin P, Dubois LJ. Prognostic significance of carbonic anhydrase IX expression in cancer patients: a meta-analysis. Front Oncol 2016;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rini BI, Battle D, Figlin RA, George DJ, Hammers H, Hutson T, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer 2019;7:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bedke J, Albiges L, Capitanio U, Giles RH, Hora M, Lam TB, et al. The 2021 updated European Association of Urology guidelines on renal cell carcinoma: immune checkpoint inhibitor-based combination therapies for treatment-naive metastatic clear-cell renal cell carcinoma are standard of care. Eur Urol 2021;80:393–7. [DOI] [PubMed] [Google Scholar]
- 35. Clark DJ, Dhanasekaran SM, Petralia F, Pan J, Song X, Hu Y, et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 2019;179:964–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 38. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of patient enrolment. * Four patients were unfit for systemic treatment due to clinical deterioration resulting from rapid disease progression (n=3) or comorbidity and age (n=1). Three other patients did not wat systemic treatment.
Score chart with predicted probability (%) to remain on WW at 12 months This model was based on the number of IMDC Risk factors (0, 1, 2), the number or involved organ sites (0 - 4) and the geometric mean [¹⁸F]FDG SUVmax as a continuous variable. The underlying formula is 100*(exp(-0.531)^exp(0.198*[IMDC score] + 0.039*[No of affected organ sites] + 0.170*[geometric mean [¹⁸F]FDG SUVmax] - 1.09))
Flow-chart patients according to RECIST-defined PD. *All patients had clinical disease progression; no CT-imaging was performed before initiation of systemic treatment ** In total 7 patients choose best supportive care. Three other patients underwent radiotherapy or surgery of all target lesions.
Lasso model
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.



![Figure 1. Overview of the tracer uptake of [18F]FDG and [89Zr]Zr-DFO-girentuximab in the RECIST-measurable lesion per patient. A depicts a scatterplot of [18F]FDG-positive lesions. For each patient on the x-axis, per tumor lesion [18F]FDG SUVmax is shown in vertical direction. The size of the dot reflects the size of the lesion, and the color identifies the location of this lesion. B identical design, here with each patient on the x-axis and the y-axis depicts the tumor [89Zr]Zr-DFO-girentuximab SUVmax uptake per tumor lesion. In C, the duration of WW is depicted for each patient, highlighting the time with and without disease progression according to RECIST 1.1.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4366/9890134/e99c926a9c70/592fig1.jpg)
![Figure 2. Heterogeneity of tracer uptake. Representative images of [18F]FDG PET/CT (left) and [89Zr]Zr-DFO-girentuximab PET/CT (right) are shown of 2 patients. In patient 1, lymph node metastases as visualized on axial sections of [18F]FDG PET/CT (A) and [89Zr]Zr-DFO-girentuximab PET/CT (B). In patient 2, lung lesions as visualized on transverse sections of [18F]FDG PET/CT (C) and [89Zr]Zr-DFO-girentuximab PET/CT (D).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4366/9890134/81eb5a1d9544/592fig2.jpg)
![Figure 4. Kaplan–Meier curve. A shows the Kaplan–Meier curves for time on WW according to above or below median [18F]FDG uptake at baseline, with a median time under WW of 36.2 versus 9.0 months. B shows the Kaplan–Meier curves for time on WW according to above or below median [89Zr]Zr-DFO-girentuximab uptake at baseline, with a median time under WW of 21.3 versus 9.3 months. In both figures, the per-patient uptake is expressed as the gm SUVmax of all RECIST-measurable PET-positive lesions. The yellow graph depicts patients with below-median tracer uptake; the blue graph patients with above median tracer uptake. *One patient was censored because of surgical excision of all tumor lesions.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4366/9890134/35e4fbf892f5/592fig4.jpg)
![Figure 6. Time-dependent ROC curves at 12 months WW time. These figures show the discriminating value of the IMDC criteria together with the number of organ sites (the two variables underlying the “W&W criteria”) and gm [18F]FDG SUVmax (A) and [89Zr]Zr-DFO-girentuximab (B), respectively, individually and when combined. All variables were evaluated continuously, and for the curves that show the combined discriminating value of variables, we used Firth penalized Cox regression to obtain a combined prognostic score.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/4366/9890134/4686743b72e8/592fig6.jpg)