Abstract
The last 10 years have revolutionized our basic understanding of nonalcoholic fatty liver disease and consequent liver cancer. It has become clear that several innate and adaptive immune cells play an important role in initiating, maintaining, or exacerbating nonalcoholic steatohepatitis (NASH)—a disease that has been recently defined as autoaggressive. Despite improved disease management aimed at reducing the progression of fibrosis, NASH is set to become a leading cause for hepatocellular carcinoma (HCC). Preliminary data from preclinical studies suggest that immunotherapy efficacy may be reduced in NASH-related HCC compared with viral HCC; however, conclusive evidence supporting clinical translation of these findings is lacking. Comprehensive clinical and immunologic phenotyping of mechanisms linking NASH progression with carcinogenesis and therapeutic resistance is key to prevent progression to cirrhosis, improve monitoring and stratification of NASH according to predicted cancer risk, and ultimately increase survival of patients with NASH-HCC. In this review, we summarize the state of the art in the field of NASH and NASH-HCC with focus on immunobiology. We discuss preclinical and clinical findings underpinning NASH as an immunologically distinct pro-tumorigenic disease entity, and explore areas of potential therapeutic vulnerabilities in NASH-associated HCC.
Introduction
Nonalcoholic fatty liver diseases (NAFLD) and in particular its specific disease stage, nonalcoholic steatohepatitis (NASH), represent an increasingly prevalent global healthcare problem tightly associated with metabolic syndrome, including type 2 diabetes and obesity (1). The prevalence of NAFLD is estimated to be 25% of the overall global population and this number is predicted to increase up to 56% in most of European countries, USA and China within the next 10 years (2). Being associated with complex metabolic disturbances, NAFLD develops through a chronic inflammatory process that is responsible for promoting and maintaining a pro-carcinogenic environment leading to liver cancer. Hepatocellular carcinoma (HCC), the most common form of primary liver cancer, is globally recognized as the fourth cause of cancer-related death and the World Health Organization projected more than a million annual deaths of liver cancer in 2030 (3). Whereas hepatitis B virus (HBV) infection remains the most relevant risk factor for HCC globally as of today, NAFLD has arisen to be the fastest growing cause of HCC in the United States, Europe as well as in South-East Asia in the last two decades (4). In this review, we provide an insight into recent findings that have deepened our knowledge on the hepatic immune microenvironment and analyze how preclinical evidence is changing our approach to the treatment of HCC in the frame of NAFLD. Following on from pathophysiology of the disease, we present concisely how the efficacy of current systemic and immune therapies for HCC may be differentially influenced according to etiology, focusing in particular on the challenges and opportunities of harnessing therapeutic vulnerabilities that are enriched in the immune microenvironment of NASH-associated HCC.
Pathophysiology of NAFLD-Associated HCC
NAFLD encompasses a wide spectrum of pathologic conditions ranging from simple fatty liver (steatosis) to steatohepatitis and fibrosis, leading to cirrhosis or HCC as end-stage liver diseases. Although initially fatty liver was not really acknowledged as a pathologic condition, the current opinion of many experts in the field indicates that liver steatosis frequently correlates with insulin resistance and the predisposition to a prediabetic status. Nevertheless, with time, disturbances of hepatic metabolism result in increased lipotoxicity, endoplasmic reticulum (ER), and oxidative stress causing hepatocellular death and activation of the immune system. This leads to a condition defined as necro-inflammation, the driving force in NAFL to NASH progression (5). However, it has also become apparent that potentially different qualitative states of steatosis might exist, triggered by different metabolite–lipid combinations (6). This different state of quality might also affect the transition from steatosis to NASH and is an important field of research, a field that still needs to be investigated in the future in more detail.
Whereas it is now well established that chronic inflammation is an essential trigger of hepatocyte transformation and carcinogenesis, growing evidence indicates that in the NAFLD setting HCC can develop also in absence of cirrhosis (25%–30%) unlike in other etiologies such as alcoholic liver disease or chronic viral infection (7–10). This high incidence could be related to the multifactorial nature of NAFLD, where many risk factors (e.g., genetics, obesity, systemic comorbidities) may synergistically promote tumor initiation. Interestingly, a recent systematic review and meta-analysis indicates that NAFLD-related HCC is associated with higher frequency in patients without cirrhosis than patients with HCC with other etiologies (38.5% vs. 14.6%) but also accompanied by other metabolic comorbidities (e.g., diabetes, hyperlipidemia; ref. 11). Of note, this was also reported to relate to lower surveillance rates as compared with other etiologies. However, results of another recent systemic review including 18 studies with a total of 470,404 patients revealed an annual incidence rate of HCC equal to 0.03 per 100 persons in patients with NAFLD without cirrhosis, whereas 3.78 per 100 person-years in patients with cirrhosis (12). Nevertheless, the authors encountered a high heterogeneity in pooled HCC estimates, only marginally reduced by sensitivity analyses. Therefore, there is an urgent need to identify biomarkers and prognostic tools enabling personalized surveillance of patients with NAFLD, also without cirrhosis. Although a plethora of factors was reported to influence the progression of the disease (e.g., genetic, environmental, nutritional, and lifestyle habits), further clinical and experimental studies are required to address this still poorly understood issue. One exemplary factor is certainly represented by the consumption of alcohol in patients with NAFLD. In fact, whereas chronic alcohol consumption represents a major etiology for chronic liver disease and HCC, potential overlapping mechanisms in the context of NAFLD are still poorly defined. Clinical studies showed that alcohol consumption aggravates liver histology and fibrosis progression in patients with NAFLD (13, 14). However, a few studies also indicate that moderate amount of alcohol intake seems to improve liver steatosis and overall cardiovascular disease, but this beneficial effects seem to depend mainly on the quality of alcohol consumed (e.g., red wine; ref. 15). Since the social problematic of alcohol consumption tightly relates to diffuse behavioral habits, it is not uncommon that NAFLD diagnosed patients might also experience a history of alcohol intake with widely variable consumption patterns and quality. This might certainly influence the disease progression as well as the risk of developing HCC. Given the systemic impact of alcohol as well as its immunosuppressive effects, more preclinical data and longitudinal clinical studies are required not only to identify possible biomarkers for further patient stratification but also to evaluate the responsiveness to therapy in individuals with these specific metabolic conditions. Finally, it is estimated that about 30% to 40% of patients with NASH develop fibrosis and about 15% of them progress to cirrhosis (16). It will be thus of highest importance to identify those patients with NASH without advanced fibrosis who are at risk to develop HCC to save resources and costs. In particular, given the large amount of patients with NAFLD worldwide, the surveillance of NAFLD progression to HCC should be optimized and improved in terms of sensitivity and cost-effectiveness. HCC surveillance should be performed in patients with cirrhosis, and progression of fibrosis should be assessed in non-cirrhotic individuals on a regular periodic basis. In this direction novel noninvasive test stratifying patients with NAFLD at risk to develop HCC have been proposed (e.g., FIB4, BARD, and GALAD scores, single-nucleotide polymorphisms analysis; refs. 17–20). The combination of classical biomarker detection like α-fetoprotein (AFP) with ultrasound has recently shown increased sensitivity as compared with the use of this noninvasive test alone (21). A novel intriguing diagnostic potential also emerged by the analysis of microbioma composition (22), serum lipidomic (23) as well as by the adoption of liquid biopsies (e.g., miRNAs, extracellular vesicles, circulating tumor cells; ref. 24), but these methods are still in their experimental stage. The integration of many of these approaches by artificial intelligence algorithms may not only enable individuation of patients at high risk of disease progression but it may also offer more stringent criteria for patients’ stratification for individualized therapies.
Similarly, a complex cellular network between resident non-parenchymal cells in the liver and the immune system triggers critical changes defining the progression from simple steatosis to NASH as well as its precipitation to fibrosis and HCC development. In this setting, an important contribution is also given by the adipose tissue and the gut microbiota, which fuel the inflammatory milieu in response to changes of tissue homeostasis (25, 26). In the early stages of the disease, myeloid populations seem to exert a pivotal role in orchestrating the immune response to increased oxidative stress, scavenging of death hepatocytes and bacterial products deriving from increased intestinal permeability. Kupffer cells, the liver resident macrophages, represent a first-line defense force in the liver, but they have been shown to lack effective turnover in NAFLD and over time are likely to be replaced by infiltrating inflammatory monocytes that are recruited through the chemokine CCR2 (27, 28). Therapeutically, CCR2 inhibition has revealed beneficial effects in NASH and NASH-induced fibrosis in experimental murine models and in distinct phase II clinical trials (29, 30). Genetic and pharmacologic targeting of CCR2 in murine HCC models inhibited tumor growth and metastatic spreading by reducing infiltration of tumor-associated macrophages and re-boosting CD8+ T-cell antitumor activity (31). However, the actual therapeutic effects of anti-CCR2 in NASH-induced HCC necessitate further investigations.
The interaction of Kupffer cells with infiltrating platelets via Gp1bα receptor was recently shown to initiate the inflammatory process responsible for CD8+ T-cell recruitment in the steatotic liver (32). Indeed, prophylactic aspirin/clopidrogel treatment and genetic antiplatelet therapy improved NAFLD activity score (NAS) and fibrosis, and reduced HCC incidence in nutritional mouse models of NASH. Interestingly, preliminary clinical evidence exists suggests improved liver damage as well as reduced fibrosis and HCC incidence in patients receiving aspirin (33, 34). However, given the associational nature of these studies and the widely debated use of anticoagulants for chronic liver disease (35), further preclinical investigations may throw light on specific platelets–immune cells interaction mechanisms that might allow targeting key cross-talk molecules preserving platelet functionality thereby increasing therapeutic safety.
Besides, other innate immune cells, such as dendritic cells, were shown to participate to the initial phases of NAFLD (36). In fact, the number of a particular subclass of CXCR1+ conventional dendritic cells (cDC), so called DC1 cells, increases in the liver of patients diagnosed with NASH as well as in experimental models of NASH, contributing to CD8+ T-cell activation (potentially through antigen independent mechanisms). Moreover, pharmacologic depletion of this cell population turned out to improve NAS score and liver injury in this model (36). The actual influence of this population in the development of NASH-associated HCC remains to be explored.
Recently, a promising therapeutic strategy targeting infiltrating neutrophils has been identified, including the context of NASH. CXR2+ neutrophils were found in tumors of human and mouse models of NASH-related HCC (37). A combined anti—programmed cell death protein 1 (PD-1) and anti-CXCR2 therapy resulted in improved survival and reduced tumor burden in mice by repolarizing neutrophils toward an anti-tumorigenic phenotype and sustaining CD8+ T-cell antitumor activity (38). Notably, conventional XCR1+ cDC1 were found increased in number in these tumors.
Emerging data indicate a central role for CD8+ T cells in tumor development in NAFLD. The inflammatory and metabolic environment characterizing this disease drives an over-activation of resident CXCR6+CD8+ T cells, which seem to acquire an autoaggressive character responsible for NASH progression. This process was illustrated in two recent papers showing (i) on the one hand the acquisition of an autoaggressive phenotype related to aberrant metabolism present in the NASH microenvironment and (ii) on the other hand the reduced efficacy of the anti–PD-1 treatment in NASH-related HCC. In the first paper, the authors showed in experimental models of NASH that IL15 produced in the hepatic microenvironment downregulates FOXO1 in CD8 T cells enabling them to acquire a resident character by upregulating CXCR6. In addition, the presence of acetate shapes the autoaggressive phenotype characterized by production of pro-inflammatory cytokines in a MHC-I dependent manner (39). The second study demonstrated that this immune-phenotype is likely responsible for the lack of responsiveness of NASH-related HCC to immune checkpoint inhibitors (ICI) in murine models (40). Anti–PD-1 treatment failed to reduce tumor burden in preclinical models of NASH-related HCC and indeed resulted in increased accumulation of aggressive CXCR6+PD-1+CD8+ T cells. These results were in line with a study showing that colon cancer cell metastasis in the liver was responsive to immunotherapy in a metabolically “normal” liver but not in a liver with NASH (41). Moreover, retrospective analyses of a small cohort of patients with HCC showed reduced overall survival in patients with NASH-HCC who received anti–PD-1 or anti—programmed cell death ligand 1 (PD-L1) treatment compared with patients with other HCC etiologies (40).
Conversely, naïve CD4+ T cells were shown to be more vulnerable to the NAFLD microenvironment where they display higher mortality rate due to the oxidative stress–related cytotoxic effects exerted by free fatty acids (42). Nevertheless, the CD4+ cell fraction of regulatory T cells (Treg) increased in the liver of experimental models of NASH-induced HCC as a consequence of the interaction neutrophils-CD4+ T cells. Tregs would then promote carcinogenesis by supporting an immunosuppressive microenvironment (43).
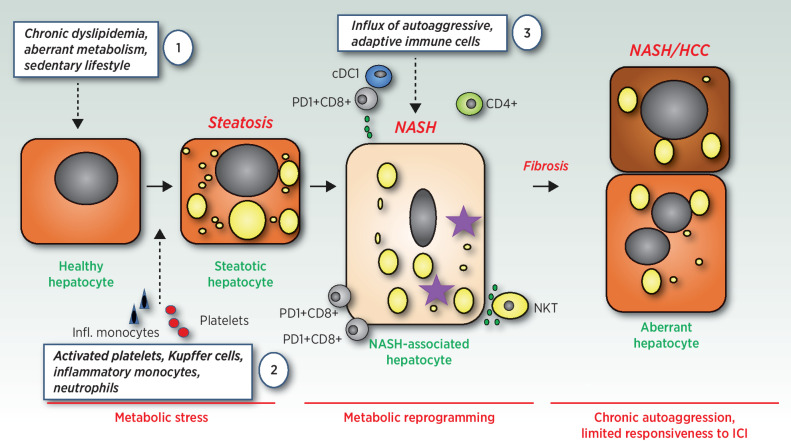
The understanding of these cellular interactions as well as a meticulous sifting of several components in the hepatic microenvironment (Fig. 1) allowed a reconsideration of the classical anticancer therapies adopted so far and is offering new rationale for the development of novel strategies based on combinatorial approaches.
Figure 1.
On the role of adaptive and innate immune cells in NASH and NASH-HCC transition (1). Chronic dyslipidemia and sedentary lifestyle cause aberrant hepatocyte metabolism, increased ER-, and mitochondrial stress. This over time leads to a metabolic catastrophe in hepatocytes – (2) driving first local inflammation by Kupffer cells; hepatocyte damage; and influx of platelets, neutrophils, and inflammatory monocytes. Over time different adaptive immune cells (e.g., CD8+PD-1+ T cells) as well as innate immune cells (e.g., CXCR1+ cDCs) infiltrate the liver and support the development of autoaggressive CD8+ T cells (3). This type of inflammation, either triggered by CD8+PD-1+ T cell or NKT-cell secreted cytokines (e.g., LIGHT) or cell–cell contact with hepatocytes, causes a downregulation of the metabolic machinery in hepatocytes and a consequent increase of lipid accumulation, lipid toxicity, and further liver damage. NASH as well as the autoaggressive T cells further develop and induce a fibrotic response as well as an inflammatory hepatic environment that in the context of NASH-HCC does not respond well to ICI.
Systemic Therapy for HCC
Systemic treatment has played a comparatively modest role in the management of HCC due to the lack of active agents and the limited survival benefit offered by tyrosine kinase inhibitor (TKI) therapy. Except for ramucirumab, whose efficacy is demonstrated in sorafenib-experienced patients with AFP ≥ 400 ng/mL, no systemic therapy is approved in the context of a biomarker-defined subgroup of patients (44). Whilst preliminary evidence suggests a role for β-catenin activation in driving immune escape in patients with HCC (45), the choice of TKIs versus ICIs and their combinations remains driven by clinical assessment of patients characteristics, rather than by utilization of predictive biomarkers. Toxicity profile (i.e., risk of immunotoxicity, bleeding) alongside general patients’ fitness and individual preference dominate therapeutic decision-making in absence of solid molecular predictors of benefit to either therapeutic modality (46).
ICIs in Advanced HCC
Systemic treatment has profoundly changed over the last decade, particularly with the addition of ICIs to the treatment armamentarium of HCC (47, 48). While PD-1–targeted monotherapies failed to meet prespecified significance levels for survival endpoints in phase III trials (49, 50), the combination of atezolizumab plus bevacizumab was the first ICI-based therapy to be added to the treatment armamentarium of HCC based on a successful phase III trial, and represents the new standard of care in systemic first-line (refs. 51–53; Table 1). A phase II/III study (ORIENT-32) from China successfully followed a similar concept by combining sintilimab and a bevacizumab biosimilar in systemic first-line of mainly HBV-associated patients with HCC (ref. 54; Table 1).
Table 1.
Summary of efficacy results from phase III trials testing ICIs in advanced HCC.
| Overall survival, months | Progression-free survival, months | ORR | ||||
|---|---|---|---|---|---|---|
| Study (Reference) | Arm (N of patients) | Median (95% CI) | HR (95% CI) | Median (95% CI) | HR (95% CI) | % |
| FIRST-LINE | ||||||
| CheckMate 459 (50) | Nivolumab (371) | 16.4 (13.9–18.4) | 0.85 (0.72–1.02) | 3.7 (3.1–3.9) | 0.93 (0.79–1.10) | 15 |
| Sorafenib (372) | 14.7 (11.9–17.2) | 3.8 (3.7–4.5) | 7 | |||
| IMbrave150a (52, 53) | Atezolizumab,b bevacizumab (336) | 19.2 (17.0–23.7) | 0.66 (0.52–0.85) | 6.9 (5.7–8.6) | 0.65 (0.53–0.81) | 30 |
| Sorafenib (165) | 13.4 (11.4–16.9) | 4.3 (4.0–5.6) | 11 | |||
| ORIENT-32 (54) | Sintilimab,b bevacizumab biosimilar (380) | NR (NR-NR) | 0.57 (0.43–0.75) | 4.6 (4.1–5.7) | 0.56 (0.46–0.70) | 21 |
| Sorafenib (191) | 10.4 (8.5–NR) | 2.8 (2.7–3.2) | 4 | |||
| COSMIC-312 (57) | Atezolizumab,b cabozantinib (432) | 15.4 (13.7–17.7)c | 0.90 (0.69–1.18)c | 6.8 (5.6–8.3)d | 0.63 (0.44–0.91)d | 11 |
| Sorafenib (217) | 15.5 (12.1-NE)c | 4.2 (2.8–7.0) d | 4 | |||
| HIMALAYA (55) | Durvalumab,b tremelimumab (393) | 16.4 (14.2–19.6) | 0.78 (0.65–0.93)e | 3.8 (3.7–5.3) | 0.90 (0.77–1.05)e | 20 |
| Durvalumab (389) | 16.6 (14.1–19.1) | 0.86 (0.73–1.03)f | 3.7 (3.2–3.8) | 1.02 (0.88–1.19)f | 17 | |
| Sorafenib (389) | 13.8 (12.3–16.1) | 4.1 (3.8–5.5) | 5 | |||
| SECOND-LINE | ||||||
| KEYNOTE-240g (49) | Pembrolizumab (278) | 13.9 (11.6–16.0) | 0.78 (0.61–1.0) | 3.0 (2.8–4.1) | 0.72 (0.57–0.90) | 18 |
| Placebo (135) | 10.6 (8.3–13.5) | 2.8 (1.6–3.0) | 4 | |||
| KEYNOTE-394h (58) | Pembrolizumab (300) | 14.6 (12.6–18.0) | 0.79 (0.63–0.99) | 2.6 (1.5–2.8) | 0.74 (0.60–0.92) | 13 |
| Placebo (153) | 13.0 (10.5–15.1) | 2.3 (1.4–2.8) | 1 | |||
Abbreviation: CI, confidence interval.
aUpdated analysis 12 months after primary analysis.
bPhase II/III study that included only patients from China.
cNumbers in parentheses represent 96% CI.
dNumbers in parentheses represent 99% CI.
eVersus sorafenib, numbers in parentheses represent 96.02% CI for overall survival and 95% CI for progression-free survival.
fVersus sorafenib, numbers in parentheses represent 95.67% CI for overall survival and 95% CI for progression-free survival.
gPretreatment with sorafenib (100%).
hIncluded only patients from Asia; pretreatment with sorafenib (91%) or oxaliplatin-based chemotherapy (9%).
Only recently, the combination of durvalumab and tremelimumab met the primary endpoint in a phase III study (HIMALAYA) by significantly improving overall survival (OS) versus sorafenib, and durvalumab alone demonstrated non-inferiority regarding OS compared with sorafenib (Table 1; ref. 55). Both the combinatorial regimen and durvalumab monotherapy have already been added as first-line options to the latest treatment recommendations of HCC (56).
Despite demonstrating superiority over their comparators in recently reported phase III trials, both atezolizumab plus cabozantinib in first-line and pembrolizumab monotherapy in second-line will likely only play a minor role in the treatment landscape of HCC for different reasons and shortcomings (i.e., lack of OS benefit and low response rate for atezolizumab/cabozantinib; Asian-only cohort with obsolete sorafenib-pretreatment for pembrolizumab; Table 1; refs. 57, 58). Rather than using pembrolizumab as a monotherapy, it is more likely that this ICI will be used in combinatory regimens. Indeed, based on encouraging response data from a phase Ib study (59), the combination of pembrolizumab plus lenvatinib is currently evaluated in a phase III trial (NCT03713593). Numerous other clinical studies are currently testing ICIs alone or in combination with other agents in the systemic front- and second-line setting (60).
Despite the progress and scientific activity in this field, biomarkers to predict outcome of patients with HCC undergoing immunotherapy are still an unmet medical need. Indicators of response used in other tumors, including PD-L1 expression or tumor mutational burden, have not proven their value in HCC yet (47). Novel scores like the recently published CRAFITY score, which is based on the two-serum parameters alpha-fetoprotein and C-reactive protein and predicts survival and radiologic outcome, require prospective validation before being implemented into clinical routine (61). In the quest of subgroups that may experience better outcomes with immunotherapy, the underlying liver disease etiology was brought into the focus of discussion (40).
Underlying Liver Disease Etiology and Efficacy of ICIs
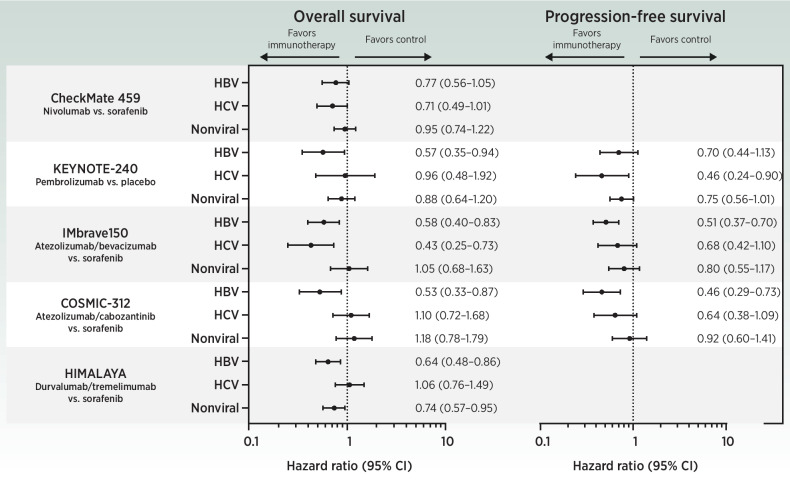
Clinical data from subgroup analysis of several phase III trials suggests that ICI-based therapy tends to be more effective versus control arm (TKI or placebo) in patients with HCC with underlying viral liver disease [HBV or hepatitis C virus (HCV)] than in those with nonviral etiologies (mainly alcohol, NAFLD/NASH and unknown; refs. 49, 50, 53, 57; Fig. 2).
Figure 2.
Forest plots of overall survival and progression-free survival according to underlying liver disease etiology. Data are shown from phase III trials testing immunotherapies in patients with advanced HCC.
A meta-analysis including eight studies with a total of 3,739 patients with HCC corroborated these data, finding that ICIs were less effective in patients with nonviral than viral etiology. In contrast, etiology was not associated with altered efficacy in patients receiving TKIs or VEGF-targeted therapies (62). However, these data need to be interpreted with caution as they have some limitations. Post hoc subgroup analyses were not subject to stratification for other relevant prognostic factors, which poses a risk for unbalances between treatment arms. Moreover, the meta-analysis was not based on individual patients’ data, and the trials included were heterogeneous in terms of treatment line and control arm (62). It also needs to be acknowledged, that the subgroup of viral etiologies were heterogeneous in terms of infection status, because patients with both resolved or active HCV infection were usually eligible in these studies, as were patients with resolved HBV infection or chronic HBV under effective antiviral therapy. Chronic infection as well as antiviral treatment affect the hepatic immune environment and immune surveillance (63–65). Whether patients with active viral disease respond differently to immunotherapy than those with resolved infection therefore requires further research.
Notably, even though median OS of patients treated with atezolizumab/bevacizumab was shorter in nonviral-related HCC (17.0 months) compared with HBV- (19.0 months) and HCV-related HCC (24.6 months) in the IMbrave150 trial, the lack of an OS benefit in the nonviral group was driven by the favorable median OS in the sorafenib arm (18.1 months; ref. 53). This is somewhat surprising, as previous studies reported better outcomes with sorafenib in HCV-positive patients (66, 67). The objective response rate (ORR) of 27% with atezolizumab/bevacizumab and 12% with nivolumab in patients with nonviral diseases (compared with 32% and 19% for HBV, and 30% and 17% for HCV) suggests that immunotherapy is also effective in patients of this etiologic subgroup (50, 53). This is supported by two meta-analyses that found no meaningful difference in ORR between patients with viral and nonviral etiology, and viral etiology had no relevant effect on the tumor immune microenvironment in HCC (68, 69). In addition, post hoc subgroup analysis from the HIMALAYA trial demonstrated improved OS for durvalumab plus tremelimumab versus sorafenib in patients with nonviral liver disease and HBV, but not in HCV-related HCC (55).
These partially conflicting data may result from the fact that the nonviral group is heterogenous and includes NASH- and alcohol-related HCC as well as unknown etiologies, which may respond differently to immunotherapy. Indeed, in two independent small, retrospective cohorts of patients with cirrhotic advanced HCC receiving ICIs, subjects with NAFLD/NASH had a worse survival than patients suffering from other underlying etiologies (40). Another retrospective study with limited sample size reported a lower disease control rate in immunotherapy-treated patients with HCC and NAFLD-related cirrhosis (NAFLD vs. non-NAFLD, 64% vs. 89%; ref. 70).
Mechanistically, loss of antitumor CD4+ T cells and accumulation of exhausted, unconventionally activated CD8+PD-1+ T cells in NASH hamper tumor immune surveillance as well as immunotherapy efficacy, as shown in preclinical NASH models with HCC or other intrahepatic tumors (40–42). NAFLD also hinders antigen-specific T-cell immunity against HCC, which seems to be related to an accumulation of macrophages in the liver environment (71). In addition, altered gut microbiota in patients with NASH-related HCC is associated with peripheral immunosuppression, which could also impair the efficacy of ICIs (72). Strategies to influence gut dysbiosis, such as fecal microbiota transplant, may make tumors more susceptible to immunotherapy, but these approaches are still in their infancy (73, 74).
Taken together, preliminary data suggests that ICIs may be less effective in patients with NASH-related HCC. As data came from preclinical studies and retrospective clinical analyses, the evidence level is very low. Thus, these data can only be considered as hypothesis-generating, and in the absence of firm evidence from prospective trials, decisions on immunotherapy initiation should not be based on etiology.
Toward NASH-HCC Targeted Therapeutics: Opportunities and Challenges for Drug Development
Whilst consistently included as preplanned stratification factor in the statistical analysis plan of contemporary phase III trials, etiology of chronic liver disease is often broadly grouped into viral versus nonviral categories. This leaves uncertainty as to the true nature of nonviral cases where NASH-HCC is grouped with other etiologies including alcohol excess, autoimmune hepatitis, and inherited causes of chronic liver disease. In the clinic, NASH remains a diagnosis of exclusion, requiring evidence of histological steatohepatitis as opposed to simple steatosis and no coexisting causes of chronic liver disease (75). Enrichment of NASH-associated HCC in clinical trials and differentiation from cases arising in the context of NAFLD or other etiologies would require more stringent inclusion criteria, which may affect efficiency of patient recruitment and retention within development programs. Compelling evidence suggests that the evolution of NAFLD/NASH is a continuous process of redundant and often nonoverlapping pathogenic mechanisms. These include metabolic dysfunction hallmarked by insulin resistance and increased liver lipogenesis, inflammation, characterized by Kupffer cell activation, gut dysbiosis and immune cell recruitment, as well as fibrosis, where hepatic stellate cell activation ultimately leads to collagen deposition and altered hepatic architecture (76). Targeting of altered lipid metabolism, inflammation and fibrogenesis is at the focus of experimental pharmacotherapy of NASH, with some evidence of activity for certain approaches such as farnesoid X receptor targeting (77). Whether reversal of the NASH-associated immune-suppressive microenvironment may lead to augmented antitumor immunity in patients with NASH-HCC needs to be proven prospectively in clinical studies (78).
To complicate the qualification of NASH-specific immune-biotherapeutic approaches in the clinic, several pathophysiologic characteristics that accompany the progression of NAFLD/NASH are known to differentially impact outcomes from immunotherapy even in absence of chronic liver disease. An elevated body mass index, for instance, predicts for adverse outcome from immunotherapy in unselected cancer types (79), although a paradoxically favorable role has been seen in patients with lung, renal cancer, and melanoma (80). This highlights a multifaceted relationship between patients’ metabolic status and immune dysfunction. Amongst other host factors, diabetes may also impair responsiveness to ICI (81), as a likely consequence of T-cell exhaustion (82). Enrichment in certain gut bacterial strains such as Faecalibacterium and Akkermansia (83, 84) have been linked with increased responsiveness to ICI, suggesting that the process of gut dysbiosis that is often seen in NAFLD/NASH may independently precondition responsiveness to ICI. Polypharmacy (85), a problem often encountered in patients with underlying metabolic syndrome, may lead to worse outcomes from ICI, although the precise mechanistic foundations of this association are not fully understood (86).
The multidimensional and codependent nature of factors that are both associated with NASH pathogenesis and responsiveness to ICI in patients with cancer make it particularly difficult to prioritize avenues for therapeutic targeting in NASH-associated HCC.
Conclusions and Future Perspectives
Innate and adaptive immune dysfunction has been increasingly recognized as a mechanism of NASH progression and overlaps with the mechanism of action of ICIs, a therapeutic modality that has now become the new standard of care for advanced HCC and which is likely to expand to earlier stages of the disease as a “chemopreventive” strategy, albeit necessitating thorough screening of patients (87). Preliminary data suggests that immunotherapy could be less effective in patients with HCC with underlying NAFLD, which may result from altered immune microenvironment, gut dysbiosis, and other pathophysiologic factors associated with NAFLD. If confirmed in large prospective clinical trials, this could become a major concern for HCC management, as NAFLD is on the rise globally and one of the leading underlying causes of HCC. Concerted efforts between industry and academia should prioritize systematic integration of biomarker development alongside drug development programs so that therapeutic vulnerabilities restricted to NASH-HCC can be discovered and prioritized for further preclinical and clinical testing. Qualification of CXCR2 as a putative therapeutic target in this subset of patients stands as an important paradigmatic example (38).
The evolving changes in the epidemiology of HCC call for the development of strategies to prevent the progression from NAFLD to HCC and to improve treatment efficacy by reprogramming metabolic and immune dysfunction in NASH-HCC.
Acknowledgments
We thank Bernhard Scheiner for his support in preparing the figures.
D.J. Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT); the NIHR Imperial Biomedical Research Centre; and the AIRC MFAG Grant No. 25697, Associazione Italiana per la Ricerca sul Cancro Foundation, Milan, Italy.
M. Heikenwalder was supported by an ERC Consolidator grant (HepatoMetaboPath), SFBTR179 Project-ID 272983813, SFB/TR 209 Project-ID 314905040, SFBTR1335 Project-ID 360372040, the Wilhelm Sander-Stiftung, a Horizon 2020 grant (Hepcar), Research Foundation Flanders (FWO) under grant 30826052 (EOS Convention MODEL-IDI), Deutsche Krebshilfe projects 70113166 and 70113167, the Rainer Hoenig Stiftung, the German-Israeli Cooperation in Cancer Research (DKFZ-MOST) and the Helmholtz-Gemeinschaft, Zukunftsthema “Immunology and Inflammation” (ZT-0027), and the Rainer Hoenig Stiftung.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authors' Disclosures
M. Pinter reports personal fees from AstraZeneca, Bayer, Eisai, BMS, Ipsen, Lilly, MSD, and Roche outside the submitted work. D.J. Pinato reports personal fees from ViiV Healthcare, Bayer Healthcare, Roche, Mursla, MiNa Therapeutics, Eisai, H3B, AstraZeneca, DaVolterra, Exact Sciences, and Ipsen; grants and personal fees from BMS; grants from GSK; and grants and nonfinancial support from MSD outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors, and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors, and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380:1450–62. [DOI] [PubMed] [Google Scholar]
- 4. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 5. Gehrke N, Schattenberg JM. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology 2020;158:1929–47. [DOI] [PubMed] [Google Scholar]
- 6. Masoodi M, Gastaldelli A, Hyotylainen T, Arretxe E, Alonso C, Gaggini M, et al. Metabolomics and lipidomics in NAFLD: biomarkers and noninvasive diagnostic tests. Nat Rev Gastroenterol Hepatol 2021;18:835–56. [DOI] [PubMed] [Google Scholar]
- 7. Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in nonalcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology 2016;63:827–38. [DOI] [PubMed] [Google Scholar]
- 9. Ertle J, Dechene A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Nonalcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer 2011;128:2436–43. [DOI] [PubMed] [Google Scholar]
- 10. Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of nonalcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol 2022;20:283–92. [DOI] [PubMed] [Google Scholar]
- 13. Ajmera V, Belt P, Wilson LA, Gill RM, Loomba R, Kleiner DE, et al. Among patients with nonalcoholic fatty liver disease, modest alcohol use is associated with less improvement in histologic steatosis and steatohepatitis. Clin Gastroenterol Hepatol 2018;16:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jarvis H, O'Keefe H, Craig D, Stow D, Hanratty B, Anstee QM. Does moderate alcohol consumption accelerate the progression of liver disease in NAFLD? a systematic review and narrative synthesis. BMJ Open 2022;12:e04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aberg F, Puukka P, Salomaa V, Mannisto S, Lundqvist A, Valsta L, et al. Risks of light and moderate alcohol use in fatty liver disease: follow-up of population cohorts. Hepatology 2020;71:835–48. [DOI] [PubMed] [Google Scholar]
- 16. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 2019;16:411–28. [DOI] [PubMed] [Google Scholar]
- 17. Loosen SH, Kostev K, Keitel V, Tacke F, Roderburg C, Luedde T. An elevated FIB-4 score predicts liver cancer development: a longitudinal analysis from 29,999 patients with NAFLD. J Hepatol 2022;76:247–8. [DOI] [PubMed] [Google Scholar]
- 18. Best J, Bechmann LP, Sowa JP, Sydor S, Dechene A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728–35. [DOI] [PubMed] [Google Scholar]
- 19. Namjou B, Lingren T, Huang Y, Parameswaran S, Cobb BL, Stanaway IB, et al. GWAS and enrichment analyses of nonalcoholic fatty liver disease identify new trait-associated genes and pathways across eMERGE network. BMC Med 2019;17:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vincentis A, Tavaglione F, Jamialahmadi O, Picardi A, Antonelli Incalzi R, Valenti L, et al. A polygenic risk score to refine risk stratification and prediction for severe liver disease by clinical fibrosis scores. Clin Gastroenterol Hepatol 2022;20:658–73. [DOI] [PubMed] [Google Scholar]
- 21. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sydor S, Best J, Messerschmidt I, Manka P, Vilchez-Vargas R, Brodesser S, et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin Transl Gastroenterol 2020;11:e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lewinska M, Santos-Laso A, Arretxe E, Alonso C, Zhuravleva E, Jimenez-Aguero R, et al. The altered serum lipidome and its diagnostic potential for nonalcoholic fatty liver (NAFL)-associated hepatocellular carcinoma. EBioMedicine 2021;73:103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Povero D, Yamashita H, Ren W, Subramanian MG, Myers RP, Eguchi A, et al. Characterization and proteome of circulating extracellular vesicles as potential biomarkers for NASH. Hepatol Commun 2020;4:1263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigues RM, Guan Y, Gao B. Targeting adipose tissue to tackle NASH: SPARCL1 as an emerging player. J Clin Invest 2021;131:e153640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol Med 2019;11:e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 2012;302:G1310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gelineau A, et al. Impaired kupffer cell self-renewal alters the liver response to lipid overload during nonalcoholic steatohepatitis. Immunity 2020;53:627–40. [DOI] [PubMed] [Google Scholar]
- 29. Krenkel O, Puengel T, Govaere O, Abdallah AT, Mossanen JC, Kohlhepp M, et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018;67:1270–83. [DOI] [PubMed] [Google Scholar]
- 30. Pedrosa M, Seyedkazemi S, Francque S, Sanyal A, Rinella M, Charlton M, et al. A randomized, double-blind, multicenter, phase IIb study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp Clin Trials 2020;88:105889. [DOI] [PubMed] [Google Scholar]
- 31. Li XG, Yao WB, Yuan Y, Chen PZ, Li B, Li JQ, et al. Targeting of tumor-infiltrating macrophages via CCL2/CCR2 signaling as a therapeutic strategy against hepatocellular carcinoma. Gut 2017;66:157–67. [DOI] [PubMed] [Google Scholar]
- 32. Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med 2019;25:641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simon TG, Henson J, Osganian S, Masia R, Chan AT, Chung RT, et al. Daily aspirin use associated with reduced risk for fibrosis progression in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:2776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med 2020;382:1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chien N, Yeo YH, Nguyen MH. Reduced hepatocellular carcinoma risk vs bleeding risk associated with aspirin. JAMA Oncol 2019;5:911. [DOI] [PubMed] [Google Scholar]
- 36. Deczkowska A, David E, Ramadori P, Pfister D, Safran M, Li B, et al. XCR1(+) type 1 conventional dendritic cells drive liver pathology in nonalcoholic steatohepatitis. Nat Med 2021;27:1043–54. [DOI] [PubMed] [Google Scholar]
- 37. Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ, et al. CXCR2-CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res 2015;34:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leslie J, Mackey JBG, Jamieson T, Ramon-Gil E, Drake TM, Fercoq F, et al. CXCR2 inhibition enables NASH-HCC immunotherapy. Gut 2022;71:2093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dudek M, Pfister D, Donakonda S, Filpe P, Schneider A, Laschinger M, et al. Autoaggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021;592:444–9. [DOI] [PubMed] [Google Scholar]
- 40. Pfister D, Nunez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, et al. NASH limits antitumor surveillance in immunotherapy-treated HCC. Nature 2021;592:450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heinrich B, Brown ZJ, Diggs LP, Vormehr M, Ma C, Subramanyam V, et al. Steatohepatitis Impairs T-cell–directed immunotherapies against liver tumors in mice. Gastroenterology 2021;160:331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Zhang H, Wang Y, Brown ZJ, Xia Y, Huang Z, et al. Regulatory T-cell and neutrophil extracellular trap interaction contributes to carcinogenesis in nonalcoholic steatohepatitis. J Hepatol 2021;75:1271–83. [DOI] [PubMed] [Google Scholar]
- 44. Fulgenzi CAM, D'Alessio A, Talbot T, Gennari A, Openshaw MR, Demirtas CO, et al. New frontiers in the medical therapy of hepatocellular carcinoma. Chemotherapy 2022;67:164–72. [DOI] [PubMed] [Google Scholar]
- 45. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, et al. β-Catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinoma. Cancer Discov 2019;9:1124–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pinato DJ, Fessas P, Cortellini A, Rimassa L. Combined PD-1/VEGFR blockade: a new era of treatment for hepatocellular cancer. Clin Cancer Res 2021;27:908–10. [DOI] [PubMed] [Google Scholar]
- 47. Pinter M, Jain RK, Duda DG. The current landscape of immune checkpoint blockade in hepatocellular carcinoma: a review. JAMA Oncol 2021;7:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 2022;19:151–72. [DOI] [PubMed] [Google Scholar]
- 49. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 50. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomized, multicenter, open-label, phase III trial. Lancet Oncol 2022;23:77–90. [DOI] [PubMed] [Google Scholar]
- 51. Vogel A, Martinelli E., clinicalguidelines@esmo.org EGCEa, Committee EG. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO clinical practice guidelines. Ann Oncol 2021;32:801–5. [DOI] [PubMed] [Google Scholar]
- 52. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–905. [DOI] [PubMed] [Google Scholar]
- 53. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862–73. [DOI] [PubMed] [Google Scholar]
- 54. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomized, open-label, phase II–III study. Lancet Oncol 2021;22:977–90. [DOI] [PubMed] [Google Scholar]
- 55. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 2022;1:EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 56. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicenter, open-label, randomized, phase III trial. Lancet Oncol 2022;23:995–1008. [DOI] [PubMed] [Google Scholar]
- 58. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo B-Y, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase III KEYNOTE-394 study. J Clin Oncol 2022;40(4_suppl):383. [Google Scholar]
- 59. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020;38:2960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scheiner B, Pomej K, Kirstein MM, Hucke F, Finkelmeier F, Waidmann O, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—development and validation of the CRAFITY score. J Hepatol 2022;76:353–63. [DOI] [PubMed] [Google Scholar]
- 62. Haber PK, Puigvehi M, Castet F, Lourdusamy V, Montal R, Tabrizian P, et al. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020). Gastroenterology 2021;161:879–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reig M, Boix L, Mariño Z, Torres F, Forns X, Bruix J. Liver cancer emergence associated with antiviral treatment: an immune surveillance failure? Semin Liver Dis 2017;37:109–18. [DOI] [PubMed] [Google Scholar]
- 64. Debes JD, Janssen HL, Boonstra A. Hepatitis C treatment and liver cancer recurrence: cause for concern? Lancet Gastroenterol Hepatol 2017;2:78–80. [DOI] [PubMed] [Google Scholar]
- 65. Rehermann B, Bertoletti A. Immunological aspects of antiviral therapy of chronic hepatitis B virus and hepatitis C virus infections. 2015;61:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kolamunnage-Dona R, Berhane S, Potts H, Williams EH, Tanner J, Janowitz T, et al. Sorafenib is associated with a reduced rate of tumor growth and liver function deterioration in HCV-induced hepatocellular carcinoma. J Hepatol 2021;75:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67:999–1008. [DOI] [PubMed] [Google Scholar]
- 68. Ho WJ, Danilova L, Lim SJ, Verma R, Xavier S, Leatherman JM, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J Immunother Cancer 2020;8:e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ding Z, Dong Z, Chen Z, Hong J, Yan L, Li H, et al. Viral status and efficacy of immunotherapy in hepatocellular carcinoma: a systematic review with meta-analysis. Front Immunol 2021;12:733530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chang J, Ryan JS, Ajmera V, Ting S, Tamayo P, Burgoyne A. Responses to immunotherapy in hepatocellular carcinoma patients with nonalcoholic steatohepatitis cirrhosis. J Clin Oncol 2022;40:389. [Google Scholar]
- 71. McVey JC, Green BL, Ruf B, McCallen JD, Wabitsch S, Subramanyam V, et al. NAFLD indirectly impairs antigen-specific CD8(+) T-cell immunity against liver cancer in mice. iScience 2022;25:103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, et al. Gut microbiota impact on the peripheral immune response in nonalcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun 2021;12:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021;371:602–9. [DOI] [PubMed] [Google Scholar]
- 74. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-- PD-1 therapy in melanoma patients. Science 2021;371:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ando Y, Jou JH. Nonalcoholic fatty liver disease and recent guideline updates. Clin Liver Dis 2021;17:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 2018;68:362–75. [DOI] [PubMed] [Google Scholar]
- 77. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, nonalcoholic steatohepatitis (FLINT): a multicenter, randomized, placebo-controlled trial. Lancet 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Horn P, Newsome PN. Emerging therapeutic targets for NASH: key innovations at the preclinical level. Expert Opin Ther Targets 2020;24:175–86. [DOI] [PubMed] [Google Scholar]
- 79. Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw Open 2021;4:e213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-- PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer 2019;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Catenacci CL D V, Maron S, Solomon BJ, Mahipal A, Johnson ML, Carbone D, et al. 960MO - Clinical outcomes and immune responses in a phase I/II study of personalized, neoantigen-directed immunotherapy in patients with advanced MSS-CRC, GEA and NSCLC. ESMO Congress. Ann Oncol; 2021;32:S829–S66. [Google Scholar]
- 82. Mallardo D, Cortellini A, Capone M, Madonna G, Pinato DJ, Warren S, et al. Concomitant type 2 diabetes mellitus (T2DM) in metastatic melanoma patients could be related to lower level of LAG-3: a transcriptomic analysis of a retrospective cohort. Ann Oncol 2022;33:445–7. [DOI] [PubMed] [Google Scholar]
- 83. Naqash AR, Kihn-Alarcon AJ, Stavraka C, Kerrigan K, Maleki Vareki S, Pinato DJ, et al. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann Transl Med 2021;9:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- 85. Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer 2021;142:18–28. [DOI] [PubMed] [Google Scholar]
- 86. Hussain N, Naeem M, Pinato DJ. Concomitant medications and immune checkpoint inhibitor therapy for cancer: causation or association? Hum Vaccin Immunother 2021;17:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pinato DJ, Fessas P, Sapisochin G, Marron TU. Perspectives on the neoadjuvant use of immunotherapy in hepatocellular carcinoma. Hepatology 2021;74:483–90. [DOI] [PubMed] [Google Scholar]