Abstract
Purpose:
Tumoral programmed cell death ligand-1 (PD-L1) expression is common in human papillomavirus (HPV)–associated head and neck squamous cell carcinoma (HNSCC). We assessed whether a DNA vaccine targeting HPV-16/18 E6/E7 with IL12 adjuvant (MEDI0457) combined with the PD-L1 inhibitor durvalumab could enhance HPV-specific T-cell response and improve outcomes in recurrent/metastatic HPV-16/18–associated HNSCC.
Patients and Methods:
In this phase Ib/IIa study, immunotherapy-naïve patients with ≥1 previous platinum-containing regimen (neoadjuvant/adjuvant therapy or for recurrent/metastatic disease) received MEDI0457 7 mg intramuscularly with electroporation on weeks 1, 3, 7, and 12, then every 8 weeks, plus durvalumab 1,500 mg intravenously on weeks 4, 8, and 12, then every 4 weeks, until confirmed progression and/or unacceptable toxicity. Coprimary objectives were safety and objective response rate (ORR; H0: ORR ≤ 15%); secondary objectives included 16-week disease control rate (DCR-16), overall survival (OS), and progression-free survival (PFS).
Results:
Of 35 treated patients, 29 were response evaluable (confirmed HPV-associated disease; received both agents). ORR was 27.6% [95% confidence interval (CI), 12.7–47.2; four complete responses, four partial responses]; responses were independent of PD-L1 tumor-cell expression (≥25% vs. <25%). DCR-16 was 44.8% (95% CI, 26.5–64.3). Median PFS was 3.5 months (95% CI, 1.9–9.0); median OS was 29.2 months (15.2–not calculable). Twenty-eight (80.0%) patients had treatment-related adverse events [grade 3: 5 (14.3%); no grade 4/5], resulting in discontinuation in 2 (5.7%) patients. HPV-16/18–specific T cells increased on treatment; 4 of 8 evaluable patients had a >2-fold increase in tumor-infiltrating CD8+ T cells.
Conclusions:
MEDI0457 plus durvalumab was well tolerated. While the primary efficacy endpoint was not reached, clinical benefit was encouraging.
Translational Relevance.
In patients with human papillomavirus (HPV)–associated recurrent/metastatic head and neck squamous cell carcinoma who had been previously treated with a platinum-containing regimen, the HPV-16/18 DNA vaccine MEDI0457 was well tolerated when combined with durvalumab. Although the primary efficacy endpoint of the study was not reached (lower bound of 95% confidence interval for objective response rate was ≤15%), early and durable clinical responses were observed. The responses were independent of programmed cell death ligand-1 (PD-L1) tumor cell expression (≥25% vs. <25%). Peripheral expansion of HPV-16/18 E6/E7-specific T cells was frequently seen but did not predict tumor response. Tumor immune responses were also observed. Targeted immunotherapy using an HPV-16/18–specific vaccine strategy may be complementary to durvalumab, enhancing PD-L1 blockade in this treatment setting.
Introduction
Human papillomavirus (HPV) infection is causative in a high proportion of patients with head and neck squamous cell carcinoma (HNSCC), particularly oropharyngeal tumors (1, 2). Most cases of HPV-positive HNSCC are caused by HPV-16 (3), and HPV-18 is the next most frequent oncogenic HPV type (primarily in oral and laryngeal squamous cell carcinomas; ref. 3), with the HPV viral oncoproteins E6 and E7 playing a pivotal role in driving oncogenesis (4). E6 promotes degradation of p53, disrupting cell-cycle checkpoints and the critical response to DNA damage, while E7 inactivates the retinoblastoma protein causing cellular proliferation and malignant transformation (1, 2). While currently available prophylactic HPV vaccines are highly effective in preventing persistent infection and the subsequent development of dysplasia or cancer caused by HPV-16, HPV-18, and other HPV types, they have no therapeutic effect upon existing HPV infection or existing HPV-related neoplasia (5). Thus, in those who have developed disease secondary to HPV, a therapeutic vaccine may provide a virus-mediated disease-directed treatment. As tumor-associated antigens (6), E6 and E7 represent ideal therapeutic targets, and several HPV-16 or HPV-16/18 E6- or E7-specific vaccines are under development for the treatment of HPV-related cancers (7–9).
HPV-associated HNSCC tumors are also characterized by a high level of immune infiltration, but the microenvironment is often immunosuppressive (10, 11). Up to 70% of HPV-associated HNSCC tumors express programmed cell death ligand-1 (PD-L1), and the programmed cell death-1 (PD-1)/PD-L1 pathway has been shown to create an immune-privileged site for HPV infection and to promote tumoral adaptive immune resistance (12). Immunotherapies targeting PD-1/PD-L1 have provided clinically meaningful antitumor efficacy with improved overall survival (OS) versus standard-of-care treatment for previously treated recurrent/metastatic (R/M) HNSCC (13, 14). PD-1 inhibitors are now among the standard of care for first-line (1L) and second-line (2L) R/M HNSCC (13–17), including single-agent pembrolizumab for 1L treatment of PD-L1–expressing tumors [based on combined positive score (CPS); ref. 15], while PD-L1–directed therapies alone have not yet demonstrated superior OS compared with existing combination chemotherapy and cetuximab (18–20). Only a small subset of patients receives durable benefit from immune checkpoint inhibitors (15).
We hypothesized that an HPV vaccine-immunotherapy combination strategy would enhance the HPV-specific T-cell response and improve therapeutic outcomes in R/M HPV-associated HNSCC. T-cell responses against HPV-16 and HPV-18 generated by therapeutic vaccines may benefit from a complementary PD-1/PD-L1 blockade. Induction of antitumor cellular immunity is an important modality for effective cancer therapy, with PD-1/PD-L1 inhibitors demonstrating durable therapeutic benefit against a spectrum of cancers (21). However, a tumor microenvironment devoid of immunologic effector cells may limit the potential benefit of PD-1/PD-L1 inhibitors; multiple tumors lack detectable lymphocytes within the tumor itself and may thus have a lower rate of benefit from PD-1/PD-L1 blockade (22, 23). Vaccination against tumor antigens has been shown to increase T-cell infiltration into tumors both in preclinical models and in clinical trials and may lead to enhanced antitumor activity when combined with immune checkpoint inhibitors (24–26). In addition, treatment with tumor vaccines has been shown to upregulate PD-L1 tumor expression in preclinical models (27).
The proof of principle of combining a PD-1/PD-L1 inhibitor and HPV vaccine targeting E6/E7 has been demonstrated in previous phase II studies. An objective response rate (ORR) of 33% was reported in patients with incurable HPV-16–positive oropharyngeal cancer who received the PD-1 inhibitor nivolumab plus the synthetic long-peptide ISA101 vaccine, which was higher than that reported with PD-1 inhibitors alone in similar patients (28). Preliminary antitumor activity was also demonstrated with the PD-L1 inhibitor avelumab plus the TG4001 recombinant Modified Vaccinia Ankara vaccine, along with the development of specific immunity and remodeling of a more favorable tumor microenvironment, in patients with R/M HPV-16–positive cancers including oropharyngeal cancer (29).
Durvalumab is a PD-L1 inhibitor that selectively blocks PD-L1 binding to PD-1 and CD80 (30) and has demonstrated a manageable safety profile with signals of clinical activity as monotherapy for R/M HNSCC. In phase I–III studies of patients with previously treated R/M HNSCC, primarily in platinum-refractory patients, durvalumab resulted in ORRs of 6.5%–29.4% (18, 31, 32) across PD-L1 tumor cell (TC) expression subgroups and median OS of 9.8 and 7.6 months in patients with PD-L1 TC expression ≥25% and <25%, respectively (18); response rate was greater and survival was longer in patients with HPV-positive versus HPV-negative status (29.4% vs. 10.9%; ref. 32). In a phase III study of durvalumab as 1L therapy for R/M HNSCC, the ORR was 17.2% (19, 20).
MEDI0457 (INO-3112) is a DNA vaccine consisting of three plasmids expressing HPV 16/18 E6 and E7 oncoproteins and IL12 as a molecular adjuvant to increase the immune response. The vaccine is administered by intramuscular (i.m.) injection followed by electroporation, in which three controlled electrical pulses are delivered in three different orientations directly at the plasmid injection site, promoting transfection and antigen expression to increase the vaccine's immunogenicity (33). In a phase Ib/II study, MEDI0457 generated durable HPV-16/18 antigen-specific peripheral and tumor immune responses, including induction of HPV-specific T cells, in patients with locally advanced, p16-positive HNSCC (33). A separate phase II study showed that MEDI0457 plus durvalumab was well tolerated in patients with R/M HPV-associated anogenital cancers, with an ORR of 21% (34).
To further investigate simultaneous targeting of HPV-16/18 E6 and E7 oncoproteins with a novel therapeutic DNA vaccine and targeting of the PD-L1 pathway, this phase Ib/IIa study (NCT03162224) was conducted to evaluate the safety and efficacy of MEDI0457 plus durvalumab in patients with HPV-associated R/M HNSCC.
Patients and Methods
Study design and patients
This open-label, multicenter phase Ib/IIa study enrolled patients ≥18 years old with histologically or cytologically confirmed R/M HNSCC associated with HPV-16 or HPV-18 who had received ≥1 platinum-containing regimen for neoadjuvant/adjuvant therapy or for treatment of R/M disease and who had no curative option (platinum-ineligible patients could be enrolled if they had progressed on another approved treatment). Local assessment of p16 on IHC as a surrogate marker of HPV status or HPV status based on nucleic acid testing was used to enroll patients; HPV-16 or HPV-18 positivity was confirmed by central laboratory testing using HPV-16 or HPV-18 E6/E7 RNAscope analysis (Advanced Cell Diagnostics; ref. 35) and SPF10 Line Probe assay (DDL Diagnostic Laboratory; ref. 36). Other key inclusion criteria included measurable disease (defined as ≥1 lesion with a minimum size of 10 mm by CT except lymph nodes which must have had a minimum short axis of 15 mm), ≥2 HNSCC tumor lesions, a World Health Organization or Eastern Cooperative Oncology Group (ECOG) performance status of 0/1, and adequate organ and bone marrow function within 28 days of study treatment. Key exclusion criteria included nasopharyngeal cancer as the primary site, current or prior use of anticancer treatment within 21 days (or 5 half-lives) of study treatment, active or prior documented autoimmune or inflammatory disorders, and prior exposure to immune-mediated therapy, defined as T-cell–directed or natural killer cell–directed therapy such as anti-PD-1, anti-PD-L1, anti-CD137, and anti-CTLA4 therapies.
A safety run-in phase assessed the first 3–12 patients with a limit of four MEDI0457 doses (limited schedule). MEDI0457 7 mg i.m. was administered followed by electroporation with a CELLECTRA®-5P device on day 1 of weeks 1, 3, 7, and 12, and durvalumab 1,500 mg was administered by intravenous infusion on day 1 of weeks 4, 8, and 12 and then every 4 weeks. If the safety profile was acceptable in the initial patients who completed the 7-week dose-limiting toxicity (DLT) evaluation period (i.e., no reported DLTs), then the planned dosing schedule could commence.
In the planned dosing schedule, MEDI0457 and durvalumab were administered as in the safety run-in, with MEDI0457 continuing every 8 weeks and durvalumab every 4 weeks until confirmed disease progression, unacceptable toxicity, or consent withdrawal. Patients who weighed <30 kg received durvalumab 20 mg/kg every 4 weeks.
The study was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Council for Harmonization/Good Clinical Practice, applicable regulatory requirements, and the AstraZeneca policy on Bioethics and Human Biological Samples. All patients provided informed written consent.
Endpoints and assessments
The coprimary endpoints were safety assessed by adverse events (AE) in the as-treated population, and ORR, defined as complete responses (CR) or partial responses (PR) according to RECIST version 1.1 (37) in the response-evaluable population. Key secondary endpoints included ORR by RECIST version 1.1 in the as-treated population, disease control rate [DCR, defined as CRs plus PRs plus stable disease (SD) at 16 weeks] and progression-free survival (PFS) by RECIST version 1.1, and OS in the as-treated and response-evaluable populations. Exploratory endpoints included HPV-16 and HPV-18 E6/E7 antigen-specific cellular immune responses and the correlation between response and PD-L1 TC expression.
Procedures
AEs were monitored throughout the study and for 90 days after the last dose of study treatment and graded according to the NCI Common Terminology Criteria for Adverse Events, version 4.03. AEs of special interest included diarrhea or colitis; pneumonitis; alanine aminotransferase (ALT) increase, aspartate aminotransferase (AST) increase, hepatitis or hepatotoxicity; neuropathy or neuromuscular toxicity; endocrinopathies; dermatitis, rash or pruritus; nephritis; pancreatitis (or suggestive laboratory tests); myocarditis or pericarditis; uveitis; infusion-related reactions, hypersensitivity or anaphylactic reactions; and administration-site reactions. Tumors were assessed by CT and/or MRI every 8 weeks for 1 year. Thereafter, patients with a CR, PR, or SD were assessed every 12 weeks until treatment end. For patients who discontinued treatment prior to progression, tumor assessments continued until confirmed disease progression or start of subsequent anticancer therapy.
Blood samples for analyses of immune responses were taken on day 1 of weeks 1, 4, 8, and 10, week 16, and every 8 weeks until discharge, and then at the follow-up visit, 28 days after the last dose. Peripheral blood mononuclear cells (PBMC) were cryopreserved for immune analysis. An IFNγ enzyme-linked immunospot (ELISpot) assay was used to assess HPV-16 and HPV-18 E6/E7-specific immune responses, as described previously (38). Briefly, a standard protocol with 24-hour peptide stimulation and using two sets of peptides (containing 15 amino acid residues overlapping by eight amino acids representing the E6/E7 fusion protein sequence of HPV-16 or HPV-18) was used, with average spot-forming unit (SFU) numbers in R10 media wells subtracted from average SFU numbers in HPV peptide wells and adjusted to a value per million PBMCs.
Fresh tumor core biopsies were obtained during screening and at week 10 from consenting patients and stained separately by IHC for CD8 (SP239, Spring Bioscience; RRID:AB_2756374) and for PD-L1 [VENTANA PD-L1 (SP263) assay, Roche Tissue Diagnostics; RRID: AB_2819099]; intensity of CD8+ T-cell infiltrates and PD-L1+ TCs was determined using quantitative digital image analysis. For PD-L1, the proportion of TCs with PD-L1 expression was assessed microscopically using a cutoff of ≥25% TC.
Statistical methods
A sample size of 50 patients was chosen to yield 40 patients with evaluable disease to provide 80% power to reject H0: ORR ≤15% (Ha: ORR >15%) if the true ORR was 30%, based on type 1 error (α) of 0.1, using a one-sided exact test for a single proportion in nQuery Advisor 7.0. The primary efficacy endpoint would thus be met if the lower bound of the 95% confidence interval (CI) for ORR excluded the null hypothesis ORR of ≤15%.
The as-treated population included all patients who received ≥1 dose of either study drug. The response-evaluable population included all patients with confirmed HPV-16– or HPV-18–associated disease who received ≥1 dose of both study drugs and (i) had ≥1 on-treatment scan or (ii) discontinued because of disease progression, or (iii) died without an on-treatment scan.
Data were evaluated for the overall population and for three subgroups according to line and response to prior treatment. Platinum-refractory disease was defined as progression within 6 months of platinum-based chemoradiotherapy. The three subgroups comprised: patients who had received a prior platinum-containing treatment in the neoadjuvant/adjuvant setting (the 1L therapy R/M group, 1L R/M) and who were either nonrefractory to platinum (the 1L R/M platinum-nonrefractory group) or refractory to it (the 1L R/M platinum-refractory group); and patients who had received ≥1 prior line of platinum-containing therapy in the R/M setting (2L therapy and above group, 2L+ R/M). Summary statistics are presented for ORR and DCR. Median PFS and OS and their 95% CIs were estimated using the Kaplan–Meier method.
Geometric mean counts of HPV-16 and HPV-18 E6/E7-specific T lymphocytes are reported. PD-L1 TC expression levels were analyzed descriptively. Association of ORR with PD-L1 expression was explored using analyses of subgroups defined by PD-L1 expression level.
Data availability statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org; data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/.
Results
Patients
From June 26, 2017, to January 10, 2020, 43 patients were screened, and 35 patients were enrolled at 12 sites in the United States (Supplementary Fig. S1). Median duration of treatment exposure was 19.1 weeks (range, 2–155) for MEDI0457 and 20.0 weeks (range, 0–148) for durvalumab, with 13 (37.1%) patients and 12 (34.3%) patients receiving ≥1 year of MEDI0457 and durvalumab treatment, respectively. Median duration of follow-up was 20.0 months (range, 0–35). At the data cutoff date (March 19, 2021), 9 (25.7%) patients remained on study, and 26 (74.3%) patients had discontinued the study.
Baseline demographics and disease characteristics are shown in Table 1 (representativeness of study participants is described in Supplementary Table S1). Fifteen (42.9%) patients were 1L R/M platinum-nonrefractory, 9 (25.7%) patients were 1L R/M platinum-refractory, and 11 (31.4%) patients were 2L+ R/M. Patient and disease characteristics were generally comparable across the three groups (data not shown). PD-L1 TC expression ≥25% was most prevalent in 2L+ R/M patients (n = 6, 54.5%) and least prevalent in those who were 1L R/M platinum-nonrefractory (n = 2, 13.3%). In the total population at baseline, 29 (82.9%), 1 (2.9%), and 5 (14.3%) patients had tumors in the oropharynx, hypopharynx, and oral cavity, respectively. By consolidated central laboratory assessment (HPV E6/E7 RNA Scope and/or SPF10 Line Probe assay), 26 (74.3%) and 3 (8.6%) patients were HPV-16 and HPV-18 positive, respectively, and 1 (2.9%) was positive for both HPV-16 and HPV-18; all 6 patients with tumors in the hypopharynx or oral cavity were HPV-16/18 positive. Twenty-five (71.4%) patients were current or former smokers. Visceral metastases were present in 27 (77.1%) patients.
Table 1.
Baseline patient demographics and disease characteristics (as-treated population).
| Characteristic | N = 35 | |
|---|---|---|
| Sex, n (%) | ||
| Male | 34 (97.1) | |
| Female | 1 (2.9) | |
| Median age (range), years | 59 (41–81) | |
| Race | ||
| White | 33 (94.3) | |
| Black/African American | 1 (2.9) | |
| Native Hawaiian or other Pacific Islander | 1 (2.9) | |
| Smoking history, n (%) | ||
| Current | 2 (5.7) | |
| Former | 23 (65.7) | |
| Never | 10 (28.6) | |
| ECOG performance status, n (%) | ||
| 0 | 19 (54.3) | |
| 1 | 16 (45.7) | |
| Anatomical location, n (%) | ||
| Oropharynx | 29 (82.9) | |
| Hypopharynx | 1 (2.9) | |
| Oral cavity | 5 (14.3) | |
| Metastases at study entry/baseline, n (%) | ||
| Brain | 0 | |
| Visceral | 27 (77.1) | |
| Lymph node-only disease at study entry/baseline, n (%) | 4 (11.4) | |
| HPV-positive status at study entry, n (%) | 35 (100) | |
| Based on p16 assay | 30 (85.7) | |
| Based on nucleic acid testing | 5 (14.3) | |
| HPV-16/18 status by central laboratorya, n (%) | ||
| HPV-16-positive | 26 (74.3) | |
| HPV-18-positive | 3 (8.6) | |
| HPV-16- and HPV-18-positive | 1 (2.9) | |
| HPV-16/18-negative | 5 (14.3) | |
| p16 status by central laboratory, n (%) | ||
| p16-positive | 19 (54.3) | |
| p16-negative | 0 | |
| Not evaluable | 16 (45.7) | |
| PD-L1 TC expression | ||
| ≥25% | 11 (31.4) | |
| <25% | 13 (37.1) | |
| Not evaluable | 11 (31.4) | |
| Prior platinum therapy, n (%) | ||
| Cisplatin | 26 (74.3) | |
| Carboplatin | 13 (37.1) | |
| None | 3 (8.6) | |
| Line of therapy and response to prior platinum treatmentb, n (%) | ||
| 1L R/M platinum-nonrefractory | 15 (42.9) | |
| 1L platinum-naïve | 3 (8.6) | |
| 1L R/M platinum-refractory | 9 (25.7) | |
| 2L+ R/M | 11 (31.4) | |
| Prior cetuximab therapy and disease setting, n (%) | 12 (34.3) | |
| Primary | 5 (14.3) | |
| Recurrent | 3 (8.6) | |
| Metastatic | 3 (8.6) | |
| Other | 1 (2.9) | |
| Prior radiation, n (%) | 31 (88.6) | |
Abbreviations: 1L/2L, first-line/second-line; ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; PD-L1, programmed cell death ligand 1; R/M, recurrent/metastatic HNSCC; TC, tumor cell.
aOn the basis of HPV-16 or HPV-18 E6/E7 RNAscope analysis, Advanced Cell Diagnostics, and/or SPF10 Line Probe assay, DDL Diagnostic Laboratory. In addition, 22 (62.9%) and 3 (8.6%) patients were HPV-16– and HPV-18–positive, respectively, based on central laboratory Roche cobas HPV PCR testing.
bPatients in the 1L subgroups received platinum-containing therapy in the neoadjuvant or adjuvant setting. Platinum-refractory status was defined as disease recurrence within 6 months of receiving platinum-containing therapy.
Safety
In the overall population, treatment-emergent AEs (TEAE) were reported in all 35 patients and were grade 3 or 4 in 17 (48.6%) patients. Treatment-emergent serious AEs were reported in 14 (40.0%) patients and AEs of special interest in 19 (54.3%) patients. TEAEs led to discontinuation of both study medications in 4 (11.4%) patients and to dose interruption in 1 (2.9%) patient. One TEAE led to death (respiratory failure, not treatment related).
Treatment-related AEs (TRAE) were reported in 28 patients (80.0%; Table 2). The most common TRAEs related to either treatment were fatigue (n = 13, 37.1%), injection site pain (n = 9, 25.7%), and arthralgia (n = 5, 14.3%), and all cases of these three TRAEs were grade 1 or 2 severity. TRAEs related specifically to MEDI0457 were reported in 25 patients (71.4%), the most common of which were fatigue (n = 9, 25.7%), injection site pain (n = 7, 20.0%), arthralgia (n = 4, 11.4%), myalgia, and pruritis (each n = 3, 8.6%). Grade 3 TRAEs were reported in 5 (14.3%) patients: wheezing (n = 1; 2.9%) related to MEDI0457, and AST increased (n = 2; 5.7%), myocarditis, ophthalmic herpes zoster, and lipase increased (each n = 1; 2.9%) related to durvalumab. Grade 3 myocarditis occurred in one of the patients with grade 3 AST increased and grade 2 ALT increased (all serious AEs related to durvalumab) and led to treatment discontinuation. The only other TRAE that led to treatment discontinuation was grade 2 arthralgia in 1 (2.9%) patient, which was related to both MEDI0457 and durvalumab. All patients with grade 3 TRAEs recovered (1 patient with AST increased recovered with sequelae). No grade 4 or 5 TRAEs were reported. One (2.9%) patient had a TRAE resulting in dose interruption (an infusion-related reaction; Table 2).
Table 2.
Safety summary—TRAEs in the safety population.
| Total population (N = 35), n (%) | ||
|---|---|---|
| AE | Any grade | Grade 3–4 |
| Any TRAE | 28 (80.0) | 5 (14.3)a |
| Related to MEDI0457 | 25 (71.4) | 1 (2.9) |
| Related to durvalumab | 20 (57.1) | 4 (11.4) |
| Any treatment-related AESI | 17 (48.6) | |
| Any treatment-related SAE | 1 (2.9) | |
| Any TRAE leading to dose interruption | 1 (2.9) | |
| Any TRAE leading to discontinuation of both study medications | 2 (5.7) | |
| Most common (≥5%) any-grade TRAEs and all grade 3 TRAEsa | ||
| Fatigue | 13 (37.1) | 0 |
| Injection-site pain | 9 (25.7) | 0 |
| Arthralgia | 5 (14.3) | 0 |
| Administration-site pain | 4 (11.4) | 0 |
| Myalgia | 4 (11.4) | 0 |
| Rashb | 4 (11.4) | 0 |
| AST increasedb | 3 (8.6) | 2 (5.7) |
| Headache | 3 (8.6) | 0 |
| Hypothyroidism | 3 (8.6) | 0 |
| Pruritus | 3 (8.6) | 0 |
| Lipase increasedb | 2 (5.7) | 1 (2.9) |
| Administration-site reaction | 2 (5.7) | 0 |
| ALT increased | 2 (5.7) | 0 |
| Dermatitis acneiform | 2 (5.7) | 0 |
| Erythema | 2 (5.7) | 0 |
| Injection-site discomfort | 2 (5.7) | 0 |
| Neutrophil count decreased | 2 (5.7) | 0 |
| Pain in extremity | 2 (5.7) | 0 |
| WBC count decreased | 2 (5.7) | 0 |
| Lymphocyte count decreased | 1 (2.9) | 1 (2.9) |
| Myocarditis | 1 (2.9) | 1 (2.9) |
| Ophthalmic herpes zoster | 1 (2.9) | 1 (2.9) |
| Wheezing | 1 (2.9) | 1 (2.9) |
Abbreviations: AESI, adverse event of special interest; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event; TRAE, treatment-related adverse event; WBC, white blood cell.
aNo grade 4 or 5 TRAEs were reported.
bAESI. Other AESIs occurring in >1 patient included hypothyroidism (n = 3, 8.6%), ALT increased, dermatitis acneiform, injection site discomfort, and pruritus (n = 2, 5.7%).
Efficacy
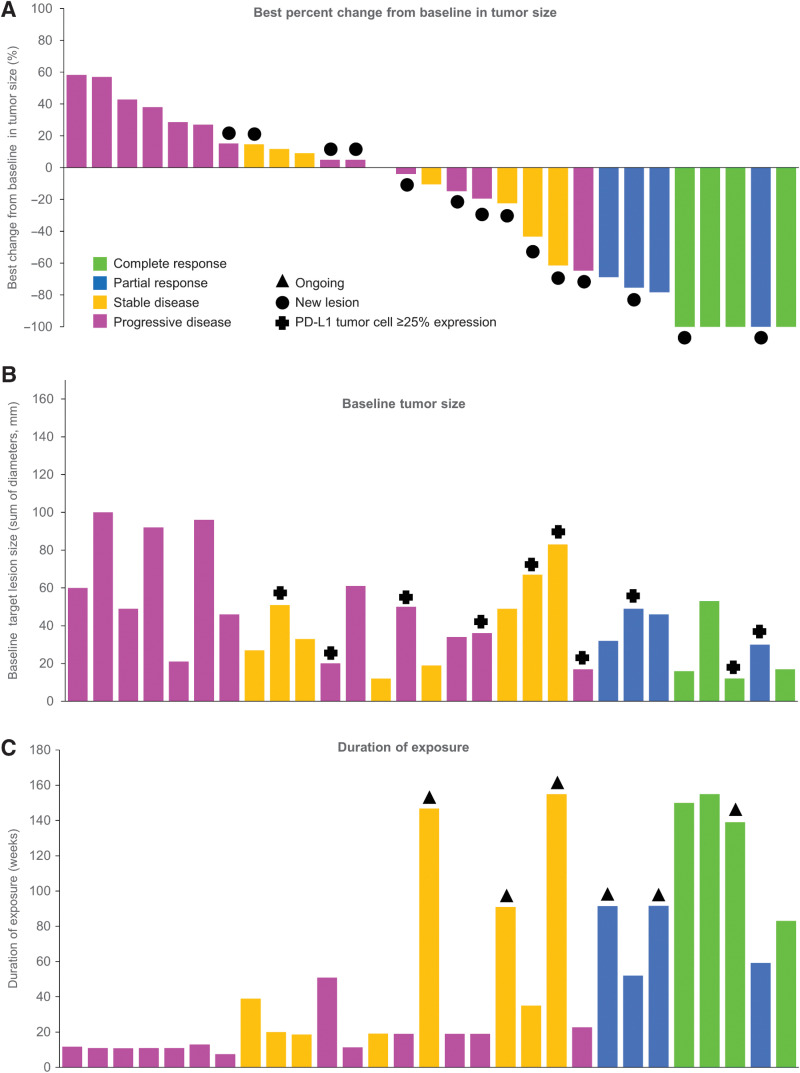
The response-evaluable population comprised 29 patients, including 3 patients from the safety run-in phase. Six patients were not included in the response-evaluable population due to being HPV-16/18 negative (n = 5) or not receiving durvalumab (n = 1). Of the 29 patients in the response-evaluable population, 8 had objective responses (ORR 27.6%; 95% CI, 12.7–47.2), half of which were CRs (Table 3). All responders were HPV-16 positive. As the 95% CI overlapped 15.0%, Ho was not rejected and the primary efficacy endpoint was not reached. The DCR was 44.8% (95% CI, 26.5–64.3) at 16 weeks and 37.9% (95% CI, 20.7–57.7) at 24 weeks. The median time to response was 1.9 months (95% CI, 1.8–3.5). The best percent change from baseline in tumor size and duration of treatment exposure in the response-evaluable population are shown in Fig. 1. Of the 8 responding patients, 5 had not progressed at data cutoff, with ongoing response durations of 14.1, 15.8, 16.8, 25.8, and 31.5 months, and 3 had progressed, with response durations of 7.2, 7.7, and 25.0 months. Thus, the median duration of response had not been reached (interquartile range, 16.3 months–not calculated).
Table 3.
Best overall response and survival.
| Response-evaluable N = 29 | As-treated N = 35 | |
|---|---|---|
| Best overall response | ||
| Confirmed objective response rate, % (95% CI) | 27.6 (12.7–47.2) | 25.7 (12.5–43.3) |
| Complete response, n (%) | 4 (13.8) | 4 (11.4) |
| Partial response, n (%) | 4 (13.8) | 5 (14.3) |
| Stable disease, n (%) | 8 (27.6) | 11 (31.4) |
| Progressive disease, n (%) | 13 (44.8) | 14 (40.0) |
| Not evaluable, n (%) | 0 | 1 (2.9) |
| DCR, % (95% CI) | ||
| At 16 weeks | 44.8 (26.5–64.3) | 45.7 (28.8–63.4) |
| At 24 weeks | 37.9 (20.7–57.7) | 37.1 (21.5–55.1) |
| Time to response, median (95% CI), months | 1.9 (1.8–3.5) | 1.9 (1.8–5.3) |
| Survival | ||
| PFS, median (95% CI), months | 3.5 (1.9–9.0) | 3.8 (2.0–8.9) |
| OS, median (95% CI), months | 29.2 (15.2–NC) | 29.2 (15.2–NC) |
Abbreviations: CI, confidence interval; DCR, disease control rate (complete responses + partial responses + stable disease); NC, not calculated; OS, overall survival; PFS, progression-free survival.
Figure 1.
Best percent change from baseline in tumor size and duration of exposure (response-evaluable population). A, Best change from baseline in tumor size, with bars color coded according to best response achieved; black filled circles indicate new lesions. B, Baseline tumor size; crosses indicate patients with PD-L1 tumor cell expression of ≥25%. C, Duration of exposure (any treatment); black triangles indicate patients ongoing on treatment at data cutoff. Data for individual patients are shown in the same order on the x-axis in each panel.
The confirmed ORRs for patients with PD-L1 TC expression ≥25% (n = 10) and those with PD-L1 TC expression <25% (n = 10) were 30.0% (95% CI, 6.7–65.3) and 20.0% (95% CI, 2.5–55.6), respectively. Of the 4 patients with CRs, 1 had PD-L1 TC expression ≥25%, 2 had PD-L1 TC expression <25%, and 1 had a biopsy that was not evaluable (Supplementary Fig. S2; Supplementary Table S2). Of the 4 patients with PRs, 2 had PD-L1 TC expression ≥25% and 2 patients had biopsies that were not evaluable (Supplementary Fig. S2). The association of PD-L1 expression as assessed by immune cell expression and CPS with best antitumor response is also shown in Supplementary Fig. S2. Subgroup analysis by prior line of therapy showed similar ORRs in 1L R/M platinum-nonrefractory patients [n = 12; 33.3% (95% CI, 9.9–65.1), including two CRs and two PRs] and 1L R/M platinum-refractory patients [n = 7; 28.6% (95% CI, 3.7–71.0), including one CR and one PR], and an ORR of 20.0% (95% CI, 5.7–43.7), including 1 CR and 1 PR, in 2L+ R/M patients (n = 10; Supplementary Table S3).
In the as-treated population, there was one additional PR (Table 3) that was ongoing at data cutoff after a duration of response of 12.4 months. Kaplan–Meier distribution curves for PFS and OS are shown in Supplementary Fig. S3; the estimated medians were similar for as-treated and response-evaluable patients (Table 3). Subgroup analysis showed longer PFS and OS for 1L R/M platinum-nonrefractory versus platinum-refractory patients (median PFS: 9.5 vs. 2.3 months; median OS: not reached vs. 29.2 months; Supplementary Table S3).
Cellular T-cell responses to MEDI0457 plus durvalumab
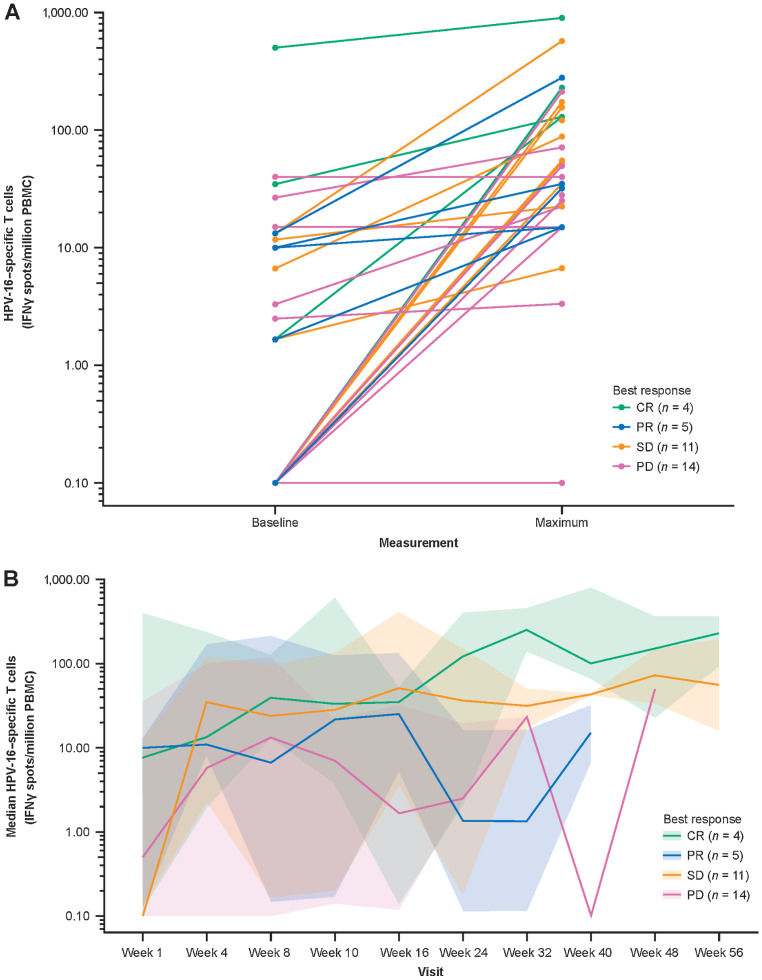
Total peripheral blood HPV-16–specific and HPV-18–specific IFNγ T-cell responses were available for 34 patients; most had increases on treatment, and the median (mean) spot-forming count per million PBMCs increased from 1.67 (105.5) at baseline to 69.6 (223.5) at the maximum value on treatment (Fig. 2A). There was no significant correlation between baseline tumor size and baseline HPV-16–specific or HPV-18–specific T-cell count (data not shown). Median spot-forming counts per million PBMCs increased over time regardless of RECIST response (Fig. 2B). The time to peak HPV-16–specific T-cell response varied among patients, with no apparent trends related to the pre-durvalumab (prior to week 4) and concomitant durvalumab (week ≥4) periods. Similar results were observed for HPV-18–specific T-cell responses (Supplementary Fig. S4), as well as for HPV-16 (Supplementary Fig. S5) and HPV-18 (Supplementary Fig. S6) E6-specific and E7-specific T-cell responses. There was no association with response; a trend toward higher HPV-16–specific and HPV-16 E6-specific and E7-specific T cells at baseline was observed in patients who had CRs compared with those who had progressive disease or SD (Supplementary Fig. S7), but no differences were seen for HPV-18–specific T cells (Supplementary Fig. S8). There was a high degree of interpatient and intrapatient variability in HPV-16–specific and HPV-18–specific T cells over the course of treatment, within and between response groups (Supplementary Fig. S9).
Figure 2.
Peripheral HPV-16–specific T cells on IFNγ ELISpot assay of PMBCs. Baseline and maximum T-cell count by patient and response type (n = 34; A), and median T-cell count over time by response category (n = 34), plotted on a log10y-axis (B). To facilitate visualization, T-cell counts of <0.1 were floored to 0.1. In A, each dot-to-dot line represents an individual patient, color coded according to best response. In B, the median lines are color coded according to best response, and the shaded areas represent the 95% CIs around the medians. Week 1 is the start of MEDI0457 dosing. Week 4 is the start of durvalumab dosing. CR, complete response; ELISpot, enzyme-linked immunospot; HPV, human papillomavirus; IFNγ, interferon gamma; PBMC, peripheral blood mononuclear cell; PD, progressive disease; PR, partial response, SD, stable disease.
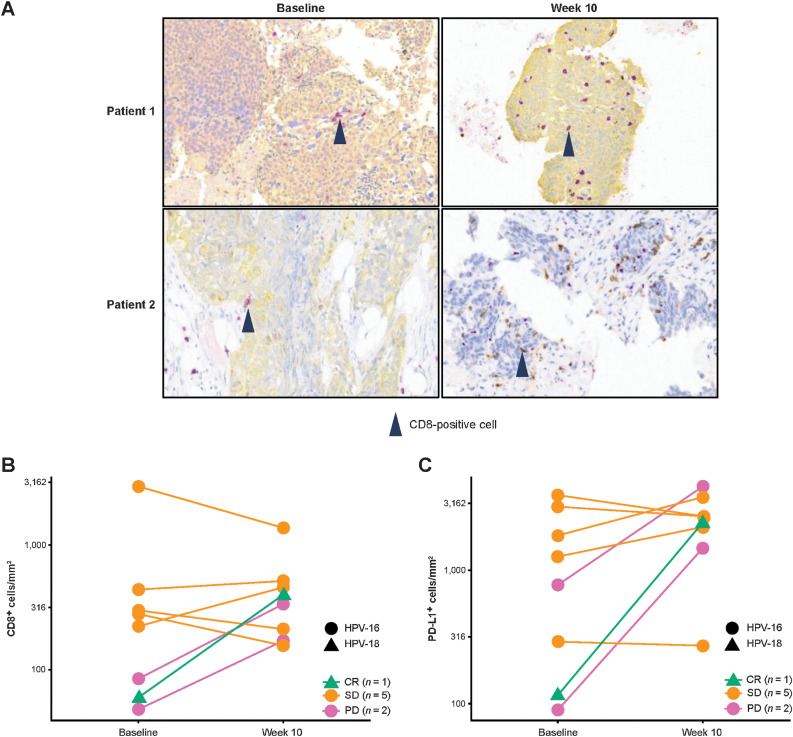
Of 8 evaluable patients with pretreatment and/or posttreatment tumor biopsies, 4 patients (who had best responses of 1 CR, 1 SD, and 2 PD) had a >2-fold increase from baseline to week 10 in tumoral CD8+ T cells (Fig. 3A and B). There were no significant correlations between tumoral CD8+ T cells and HPV-16–specific and/or HPV-18–specific T cells at weeks 1 and/or 10 (data not shown). Five of 8 patients (1 CR, 2 SD, 2PD) had a >50% increase in PD-L1+ TCs at week 10 (Fig. 3C).
Figure 3.
Intratumoral immunomodulation: CD8 IHC images from tumors of 2 patients showing increased numbers of CD8+ T cells at week 10 versus baseline—CD8+ T cells are stained purple and cytokeratin is stained yellow in the top two and the bottom left images, and CD8+ T cells are stained brown in the bottom right image (A); change in tumoral CD8+ T-cell count from baseline to week 10 (n = 8; B) and change in tumoral PD-L1+ cell count from baseline to week 10 (n = 8; C) by HPV status on IHC analysis of individual patients with paired tumor biopsies, with lines color coded according to best response.
Discussion
In this study, MEDI0457 in combination with durvalumab was well tolerated and showed meaningful clinical benefit in patients with HPV-associated R/M HNSCC. The AEs related to durvalumab in this study were broadly similar to previous reports, with fatigue and hypothyroidism being among the most common (18, 31, 32, 39). Consistent with the previous study of MEDI0457 monotherapy in patients with locally advanced HNSCC (33), mild injection-site pain was among the most common TRAEs. No TRAE led to death. The most common TRAEs of special interest were rash, hypothyroidism, pruritus, and AST increased. One TRAE of special interest (myocarditis) led to treatment discontinuation in 1 patient.
In patients with R/M HNSCC, single-agent immunotherapy has been associated with ORRs of up to approximately 18% and generally limited long-term outcomes. For example, durvalumab monotherapy has resulted in an ORR of 9.2%–17.9% and median PFS and OS of 1.9–2.1 and 6.0–7.6 months, respectively (18, 32, 39), nivolumab has resulted in an ORR of 13.3% and median PFS and OS of 2.0 and 7.5 months, respectively, and ORRs of 16%–18% have been reported with pembrolizumab, with median PFS and OS of 2.1–2.3 and 8–11.6 months, respectively (15–17, 40). Responses (14, 16, 39–41) and OS (14, 32, 41) have appeared somewhat better in HPV-positive versus HPV-negative patients, reflecting the generally more favorable prognosis of HPV-positive HNSCC (42). However, these differences between subgroups and across studies should be interpreted cautiously, as the studies had different designs and patient populations, and patient numbers were low in some subgroups, resulting in wide CIs for ORRs. In addition, rates of PD-L1 expression differed between populations, a factor that may impact response to treatment; for example, subgroup analyses of HPV-positive patients receiving durvalumab monotherapy in previous studies have shown ORRs of 29.4% and 16.7% in patients with PD-L1 TC ≥25% (32) and <25% (39), respectively, while ORRs of 23%, 19%, and 17% in patients with PD-L1 CPS of ≥20 or ≥1 and in the overall population were reported with pembrolizumab monotherapy in the KEYNOTE-048 study (15).
Notwithstanding the better prognosis compared with HPV-negative HNSCC, improving the relatively low response rates in patients with HPV-positive HNSCC using combined immunotherapeutic approaches is an area of active study, with a focus on the immune landscape in this setting and the mechanisms by which HPV evades recognition and clearance by the host immune system (43, 44). These strategies include regulation of the inflammatory response and antigen-presentation machinery, promotion of immunosuppressive mechanisms, and suppression of T-cell function through PD-L1 expression (12, 43), as well as mechanisms involving the PI3K pathway (45). In this context, various strategies to improve patient outcomes have been explored, including combining HPV vaccines targeting E6/E7 with a PD-1/PD-L1 inhibitor. For example, the HPV-16 synthetic long peptide vaccine ISA101, in combination with nivolumab, resulted in an ORR of 36%, similar to that reported in the current study, and a median PFS and OS of 2.7 and 17.5 months, respectively, in 22 patients with incurable oropharyngeal cancer (28).
Our current study demonstrates immunologic proof-of-concept findings for the combination of MEDI0457 (a plasmid DNA vaccine targeting HPV-16/18 E6/E7) with the PD-L1 inhibitor durvalumab. It confirms the clinical feasibility of administering a plasmid DNA vaccine with electroporation to promote transfection and antigen expression and the utility of IL12 as an adjuvant for stimulating the immune response. Intratumoral delivery of IL12 alone with electroporation has been shown to induce tumor regression as well as systemic T-cell responses and T-cell recruitment to the tumor microenvironment in patients with advanced melanoma, alone and in combination with PD-1 inhibition (46–48). Building on this and the findings of our previous phase Ib/II study of MEDI0457 monotherapy (33), here we have shown that MEDI0457 plus durvalumab increased peripheral HPV-specific T cells, with a trend for patients with CRs having higher levels of HPV-16–specific T cells at baseline. The varying time to peak HPV-16/18–specific T-cell responses did not indicate any trend in T-cell numbers associated with the initiation of durvalumab after 4 weeks. It was not feasible to compare these data with those of MEDI0457 monotherapy (33) to determine whether durvalumab affected T-cell responses. Increased tumoral infiltration of CD8+ cells and increased PD-L1+ tumor cells were also noted from baseline to week 10, demonstrating pharmacodynamic activity and evidence of a cellular response. However, baseline PD-L1 TC expression (≥25% or <25%) was not associated with antitumor response.
The ORR in the current study, in which 31.4% of patients had PD-L1 TC ≥25%, was 27.6%, which included 13.8% CRs (n = 4/29). Acknowledging that comparisons with other studies must be interpreted with caution, due to the heterogeneous nature of the patient population in the current study, this compares with an ORR of 17.9%, including 2.5% CRs, in EAGLE, in which 28.3% of patients treated with durvalumab monotherapy had PD-L1 TC ≥25% (18), and an ORR of 29.4%, including only 0.9% CRs (n = 1/111), in patients with PD-L1 TC ≥25% treated with durvalumab monotherapy in HAWK (32), suggesting potential clinical benefit with the combination of MEDI0457 and durvalumab. As expected, ORR was numerically highest in 1L R/M platinum-nonrefractory patients (33.3%) and lowest in 2L+ R/M patients (20.0%). Although the primary endpoint was not met due to the lower bound of the 95% CIs for ORR not excluding the null hypothesis ORR of ≤15%, many responses were durable, and more than one-third of patients remained on study treatment for ≥1 year. Furthermore, median PFS was 3.5 months and median OS was 29.2 months in the current study. These data compare favorably with outcomes from studies of standard-of-care treatments in this setting, as reviewed above, including the CheckMate-141 study of nivolumab (14) and the KEYNOTE-048 study of pembrolizumab (15), as well as with outcomes from studies of durvalumab monotherapy and durvalumab plus tremelimumab [median PFS of 1.4–2.1 months; median OS of 6.0–8.4 months (18, 31, 32, 39)], and from the study of nivolumab plus ISA 101 [median PFS of 2.7 months; median OS of 17.5 months (28)]. As noted earlier, differences between studies should be interpreted cautiously due to differing study designs and patient populations; for example, an important difference between our study and other studies of anti-PD-(L)1 immunotherapies is that durvalumab treatment was started only at week 4 to allow the vaccine, MEDI0457, to induce an HPV-specific immune response.
Several limitations of the study should be noted, including enrollment of populations of patients across different lines of therapy, and heterogeneity in platinum refractoriness, prior lines of treatment, tumor PD-L1 expression, and extent of disease. This study had an open-label design and low patient numbers, both overall and particularly in the subgroups assessed; a sample size of 40 patients with evaluable disease was determined to provide 80% power to reject H0 (ORR ≤ 15%) but only 35 patients were enrolled, of whom only 29 were evaluable for response. Another study limitation is that bias may have been introduced in selecting patients using p16 as a surrogate marker. Of the 30 p16-positive patients, 25 were positive for HPV-16/18 E6/E7 on central laboratory nucleic acid testing, suggesting that, even though p16 is a good surrogate marker for an HPV-16/18 tumor, additional HPV-specific testing should be done in the context of clinical trials. In addition, during the course of this clinical trial, pembrolizumab alone (for PD-L1–expressing tumors) or combination chemoimmunotherapy with pembrolizumab was established as a standard of care in patients with R/M HNSCC (15). These results have dramatically changed the management landscape for patients with metastatic disease in the 1L setting and may impact further development of HPV-specific strategies. Finally, although not a limitation of the current study, recent analyses of B-cell and T-cell responses in patients with HNSCC suggest that a therapeutic vaccination approach targeting not only E6 and E7, per MEDI0457, but also E2 and E5 may result in a greater breadth of immune response (49, 50). This aspect may be of interest for future studies.
In conclusion, these findings indicate that combining MEDI0457 with durvalumab in patients with HPV-associated R/M HNSCC is well tolerated and offers potential clinical benefit. Responses occurred early and were durable, and median OS was notable in the context of OS data previously observed in this patient population. Targeting E6 and E7 of HPV-16/18 may therefore be a complementary immune activation strategy to PD-L1 blockade, warranting further investigation in larger, randomized clinical trials.
Supplementary Material
Supplementary Table S1. Representativeness of Study Participants Supplementary Table S2. Disease response as assessed by RECIST v1.1 by PD-L1 tumor cell expression (response-evaluable population). Supplementary Table S3. Disease response as assessed by RECIST v1.1 in prior line of therapy subgroups (response-evaluable population) and survival (as-treated population).
Supplementary Figure S1. Patient disposition. Supplementary Figure S2. Association of PD-L1 expression and HPV status with best antitumor response. Supplementary Figure S3. Kaplan-Meier distribution curves for (A) PFS and (B) OS in the as-treated population according to line of treatment and platinum-refractory status, including estimates of medians. Supplementary Figure S4. Peripheral HPV-18-specific T-cells on IFNγ ELISpot assay of PMBCs. Supplementary Figure S5. Peripheral HPV-16 (A, C) E6-specific and (B, D) E7-specific T-cell responses on IFNγ ELISpot assay of PMBCs. Supplementary Figure S6. Peripheral HPV-18 (A, C) E6-specific and (B, D) E7-specific T-cell responses on IFNγ ELISpot assay of PMBCs. Supplementary Figure S7. Baseline peripheral (A) HPV-16-specific and HPV-16 (B) E6-specific and (C) E7-specific T-cell counts on IFNγ ELISpot assay of PMBCs according to best response to treatment. Supplementary Figure S8. Baseline peripheral (A) HPV-18-specific and HPV-18 (B) E6-specific and (C) E7-specific T-cell counts on IFNγ ELISpot assay of PMBCs according to best response to treatment. Supplementary Figure S9. (A) HPV-16-specific and (B) HPV-18-specific T-cell count measured by IFNγ ELISpot assay of PMBCs over time per individual patient (n = 34), color-coded by best response, plotted on a log10 y-axis.
Acknowledgments
The authors would like to thank the patients who participated in the study, and their families and caregivers. They would also like to thank all study sites and personnel.
This study (NCT03162224) was funded by AstraZeneca, the manufacturer of MEDI0457 and durvalumab.
Medical writing support, under the direction of the authors, was provided by Susanne Gilbert of Ashfield MedComms, an Ashfield Health company, and was funded by AstraZeneca.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 503
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
C. Aggarwal reports personal fees from Genentech, Lilly, Celgene, Merck, AstraZeneca, Blueprint Genetics, Shionogi, Daiichi Sankyo/AstraZeneca, Regeneron/Sanofi, Eisai, Turning Point, Pfizer, Janssen, and Boehringer Ingelheim; and grants from Genentech/Roche, Incyte, Macrogenics, Merck Sharp & Dohme, and AstraZeneca/MedImmune outside the submitted work. N.F. Saba reports advisory roles to Merck, BMS, Eisai, Exelixis, AstraZeneca, Novartis, Vaccinex, and GSK. A. Algazi reports other support from AstraZeneca during the conduct of the study; personal fees and other support from OncoSec, Onchilles, Valitor, and Sensei; and personal fees from Worldwide Clinical Trials, Radmetrix, Venn, and IAG outside the submitted work. T.Y. Seiwert reports grants and personal fees from Merck/MSD, Nanobiotix, and Kura; personal fees from Innate, CUE-101, VIR, Eisai, Bayer, Sanofi, BostonGene, and Nektar; and grants from Roche, AstraZeneca, Regeneron, BMS, and Dracen outside the submitted work. M. Haigentz reports personal fees from AstraZeneca, Blueprint Medicines, Coherus Biosciences, Jazz Pharmaceuticals, and Takeda Pharmaceuticals outside the submitted work. M. Porosnicu reports other support from AstraZeneca during the conduct of the study; and other support from AstraZeneca and Boehringer Ingelheim outside the submitted work. M. Bonomi reports grants from Regeneron and personal fees from Merck outside the submitted work. M.T. Esser is an employee of and owns stock in AstraZeneca. S. Agrawal reports personal fees from AstraZeneca outside the submitted work. E.C. Jennings reports personal fees from AstraZeneca outside the submitted work. N.M. Durham reports other support from AstraZeneca during the conduct of the study. D. Lissa reports personal fees from AstraZeneca during the conduct of the study; and personal fees from AstraZeneca outside the submitted work. M. Gong reports personal fees from AstraZeneca during the conduct of the study; and personal fees from AstraZeneca outside the submitted work. N. Ceaicovscaia reports other support from AstraZeneca during the conduct of the study; and other support from AstraZeneca outside the submitted work. R. Kumar is an employee of AstraZeneca. No disclosures were reported by the other authors.
Authors' Contributions
C. Aggarwal: Conceptualization, resources, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, writing–review and editing. N.F. Saba: Resources, data curation, formal analysis, writing–original draft, writing–review and editing. A. Algazi: Supervision, investigation, writing–review and editing. A. Sukari: Data curation, formal analysis, supervision, investigation, writing–review and editing. T.Y. Seiwert: Investigation, project administration, writing–review and editing. M. Haigentz: Data curation, investigation, writing–review and editing. M. Porosnicu: Data curation, investigation, writing–review and editing. M. Bonomi: Data curation, investigation, writing–review and editing. J. Boyer: Data curation, writing–review and editing. M.T. Esser: Conceptualization, data curation, formal analysis, writing–review and editing. L.I. Cheng: Investigation, writing–review and editing. S. Agrawal: Formal analysis, visualization, writing–review and editing. E.C. Jennings: Formal analysis, visualization, writing–review and editing. N.M. Durham: Conceptualization, data curation, formal analysis, supervision, investigation, visualization, writing–review and editing. K. Fraser: Conceptualization, formal analysis, writing–review and editing. D. Lissa: Data curation, formal analysis, writing–review and editing. M. Gong: Formal analysis, visualization, writing–review and editing. N. Ceaicovscaia: Formal analysis, visualization, writing–review and editing. A. Gascó Hernández: Conceptualization, data curation, formal analysis, writing–review and editing. R. Kumar: Conceptualization, resources, supervision, project administration, writing–review and editing.
References
- 1. Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A review of HPV-related head and neck cancer. J Clin Med 2018;7:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75. [DOI] [PubMed] [Google Scholar]
- 4. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers 2020;6:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Future IIS Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915–27. [DOI] [PubMed] [Google Scholar]
- 6. Chandra J, Dutton JL, Li B, Woo WP, Xu Y, Tolley LK, et al. DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J Immunother 2017;40:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shibata T, Lieblong BJ, Sasagawa T, Nakagawa M. The promise of combining cancer vaccine and checkpoint blockade for treating HPV-related cancer. Cancer Treat Rev 2019;78:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang C, Dickie J, Sutavani RV, Pointer C, Thomas GJ, Savelyeva N. Targeting head and neck cancer by vaccination. Front Immunol 2018;9:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beyaert S, Machiels JP, Schmitz S. Vaccine-based immunotherapy for head and neck cancers. Cancers 2021;13:6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandal R, Senbabaoglu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016;1:e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Partlova S, Boucek J, Kloudova K, Lukesova E, Zabrodsky M, Grega M, et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 2015;4:e965570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013;73:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 14. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 16. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- 17. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol 2017;35:1542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. [DOI] [PubMed] [Google Scholar]
- 19. NIH. AstraZeneca. Phase III open label study of MEDI 4736 with/without tremelimumab versus standard of care (SOC) in recurrent/metastatic head and neck cancer (KESTREL). Available from: https://clinicaltrials.gov/ct2/show/NCT02551159; 2021.
- 20. Wildsmith S, Ye J, Franks A, Melillo G, Armstrong J, Whiteley J, et al. Association of PD-L1 expression on tumor and immune cells with survival in recurrent or metastatic head and neck squamous cell carcinoma and assay validation. Cancer Res Commun 2022;2:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 2015;42:587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lutz ER, Kinkead H, Jaffee EM, Zheng L. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology 2014;3:e962401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 2016;34:1330–8. [DOI] [PubMed] [Google Scholar]
- 26. Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res 2013;73:3591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res 2014;74:4042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Massarelli E, William W, Johnson F, Kies M, Ferrarotto R, Guo M, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le Tourneau C, Cassier P, Rolland F, Salas S, Limacher JM, Capitain O, et al. TG4001 (Tipapkinogene sovacivec) and avelumab for recurrent/metastatic (R/M) human papilloma virus (HPV)-16+ cancers: clinical efficacy and immunogenicity. J Immunother Cancer 2020;8:793. [Google Scholar]
- 30. Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015;3:1052–62. [DOI] [PubMed] [Google Scholar]
- 31. Segal NH, Ou SI, Balmanoukian A, Fury MG, Massarelli E, Brahmer JR, et al. Safety and efficacy of durvalumab in patients with head and neck squamous cell carcinoma: results from a phase I/II expansion cohort. Eur J Cancer 2019;109:154–61. [DOI] [PubMed] [Google Scholar]
- 32. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with >/=25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019;107:142–52. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res 2019;25:110–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morris VK, Jazaeri AA, Westin SN, Pettaway CA, George S, Huey R, et al. Phase II trial of MEDI0457 and durvalumab for patients with recurrent/metastatic HPV-associated cancers. J Clin Oncol 39: 15s, 2021. (suppl; abstr 2595). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Wang MX, Su N, Wang LC, Wu X, Bui S, et al. RNAscope for in situ detection of transcriptionally active human papillomavirus in head and neck squamous cell carcinoma. J Vis Exp 2014:51426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Doorn LJ, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol 2006;44:3292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 38. Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012;4:155ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siu LL, Even C, Mesia R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol 2019;5:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer 2018;119:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brennan S, Baird AM, O'Regan E, Sheils O. The role of human papilloma virus in dictating outcomes in head and neck squamous cell carcinoma. Front Mol Biosci 2021;8:677900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang HF, Wang SS, Tang YJ, Chen Y, Zheng M, Tang YL, et al. The double-edged sword-how human papillomaviruses interact with immunity in head and neck cancer. Front Immunol 2019;10:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Julian R, Savani M, Bauman JE. Immunotherapy approaches in HPV-associated head and neck cancer. Cancers 2021;13:5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chitsike L, Duerksen-Hughes PJ. Targeted therapy as a potential de-escalation strategy in locally advanced HPV-associated oropharyngeal cancer: a literature review. Front Oncol 2021;11:730412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greaney SK, Algazi AP, Tsai KK, Takamura KT, Chen L, Twitty CG, et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol Res 2020;8:246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Algazi AP, Twitty CG, Tsai KK, Le M, Pierce R, Browning E, et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin Cancer Res 2020;26:2827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han M, Nguyen B, Lee JY, Browning E, Zhang J, Mukhopadhyay A, et al. Intratumoral electroporation of plasmid encoded IL-12 and membrane-anchored anti-CD3 increases systemic tumor immunity. Mol Cancer Res 2022;20:983–95. [DOI] [PubMed] [Google Scholar]
- 49. Eberhardt CS, Kissick HT, Patel MR, Cardenas MA, Prokhnevska N, Obeng RC, et al. Functional HPV-specific PD-1(+) stem-like CD8 T cells in head and neck cancer. Nature 2021;597:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wieland A, Patel MR, Cardenas MA, Eberhardt CS, Hudson WH, Obeng RC, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021;597:274–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Representativeness of Study Participants Supplementary Table S2. Disease response as assessed by RECIST v1.1 by PD-L1 tumor cell expression (response-evaluable population). Supplementary Table S3. Disease response as assessed by RECIST v1.1 in prior line of therapy subgroups (response-evaluable population) and survival (as-treated population).
Supplementary Figure S1. Patient disposition. Supplementary Figure S2. Association of PD-L1 expression and HPV status with best antitumor response. Supplementary Figure S3. Kaplan-Meier distribution curves for (A) PFS and (B) OS in the as-treated population according to line of treatment and platinum-refractory status, including estimates of medians. Supplementary Figure S4. Peripheral HPV-18-specific T-cells on IFNγ ELISpot assay of PMBCs. Supplementary Figure S5. Peripheral HPV-16 (A, C) E6-specific and (B, D) E7-specific T-cell responses on IFNγ ELISpot assay of PMBCs. Supplementary Figure S6. Peripheral HPV-18 (A, C) E6-specific and (B, D) E7-specific T-cell responses on IFNγ ELISpot assay of PMBCs. Supplementary Figure S7. Baseline peripheral (A) HPV-16-specific and HPV-16 (B) E6-specific and (C) E7-specific T-cell counts on IFNγ ELISpot assay of PMBCs according to best response to treatment. Supplementary Figure S8. Baseline peripheral (A) HPV-18-specific and HPV-18 (B) E6-specific and (C) E7-specific T-cell counts on IFNγ ELISpot assay of PMBCs according to best response to treatment. Supplementary Figure S9. (A) HPV-16-specific and (B) HPV-18-specific T-cell count measured by IFNγ ELISpot assay of PMBCs over time per individual patient (n = 34), color-coded by best response, plotted on a log10 y-axis.
Data Availability Statement
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org; data for studies not listed on Vivli may be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/.