Abstract
Objective
To examine the associations between five major adverse pregnancy outcomes and long term risks of ischemic heart disease in mothers.
Design
National cohort study.
Setting
Sweden.
Participants
All 2 195 266 women with a first singleton delivery in Sweden during 1973-2015.
Main outcome measures
The main outcome measure was incidence of ischemic heart disease from delivery to 2018, identified from nationwide inpatient and outpatient diagnoses. Cox regression was used to calculate hazard ratios for ischemic heart disease associated with preterm delivery, small for gestational age, pre-eclampsia, other hypertensive disorders of pregnancy, and gestational diabetes, adjusting for other adverse pregnancy outcomes and maternal factors. Co-sibling analyses assessed for confounding by shared familial (genetic and environmental) factors.
Results
During 53.6 million person years of follow-up, ischemic heart disease was diagnosed in 83 881 (3.8%) women. All five adverse pregnancy outcomes were independently associated with increased risk of ischemic heart disease. In the 10 years after delivery, adjusted hazard ratios for ischemic heart disease associated with specific adverse pregnancy outcomes were 2.09 (95% confidence interval 1.77 to 2.46) for other hypertensive disorders of pregnancy, 1.72 (1.55 to 1.90) for preterm delivery, 1.54 (1.37 to 1.72) for pre-eclampsia, 1.30 (1.09 to 1.56) for gestational diabetes, and 1.10 (1.00 to 1.21) for small for gestational age. The hazard ratios remained significantly increased even 30-46 years after delivery: 1.47 (1.30 to 1.66) for other hypertensive disorders of pregnancy, 1.40 (1.29 to 1.51) for gestational diabetes, 1.32 (1.28 to 1.36) for pre-eclampsia, 1.23 (1.19 to 1.27) for preterm delivery, and 1.16 (1.13 to 1.19) for small for gestational age. These findings were only partially (<45%) explained by shared familial (genetic or environmental) factors. Women who experienced multiple adverse pregnancy outcomes showed further increases in risk (eg, <10 years after delivery, adjusted hazard ratios associated with 1, 2, or ≥3 adverse pregnancy outcomes were 1.29 (1.19 to 1.39), 1.80 (1.59 to 2.03), and 2.26 (1.89 to 2.70), respectively)).
Conclusions
In this large national cohort, women who experienced any of five major adverse pregnancy outcomes showed an increased risk for ischemic heart disease up to 46 years after delivery. Women with adverse pregnancy outcomes should be considered for early preventive evaluation and long term risk reduction to help prevent the development of ischemic heart disease.
Introduction
Cardiovascular disease is the leading cause of mortality among women in the United States and worldwide.1 2 Adverse pregnancy outcomes, including preterm delivery, small for gestational age infant, pre-eclampsia, other hypertensive disorders of pregnancy, and gestational diabetes have been associated with higher future risks of cardiovascular disease or traditional cardiovascular risk factors.3 4 5 6 7 8 9 10 11 Adverse pregnancy outcomes share certain pathogenetic features, including abnormal placentation, inflammation, and vascular dysfunction, that may lead to subclinical cardiovascular disease that persists or progresses after delivery.12 Despite this evidence, however, adverse pregnancy outcomes remain under-recognized as potential cardiovascular risk factors and are often overlooked when assessing risks in women.13 14 About 85% of women in high income countries deliver at least one child,15 16 and up to one third of pregnancies are affected by at least one adverse pregnancy outcome,4 17 thus nearly 30% of women experience an adverse pregnancy outcome during their reproductive years.4 17 A better understanding of the long term risks associated with specific adverse pregnancy outcomes is needed to improve risk stratification and guide preventive strategies to reduce the burden of cardiovascular disease in women.
Most previous studies have examined a single adverse pregnancy outcome in relation to future cardiovascular risks. For example, preterm delivery has been associated with long term increased risks of ischemic heart disease,7 stroke,9 heart failure,10 hypertension,11 and cardiovascular specific and all cause mortality18 in women. Multiple adverse pregnancy outcomes, however, have rarely been assessed in the same cohort to evaluate their independent associations with cardiovascular disease. A US study of 48 113 women reported that hypertensive disorders of pregnancy and low birth weight, but not preterm delivery or gestational diabetes, were associated with 1.1-fold to 1.3-fold higher risks of atherosclerotic cardiovascular disease (a composite of ischemic heart disease, stroke, or peripheral artery disease), after adjusting for other risk factors.3 These results, however, were based on retrospectively self-reported pregnancy outcomes in women who had survived to approximately ages 70 to >95 years and thus were potentially affected by recall or survivorship biases. Few studies have examined multiple adverse pregnancy outcomes that were prospectively ascertained in the same cohort to understand the relative impacts on long term risks. Furthermore, a key unanswered question is whether previously reported associations are explained by adverse pregnancy outcomes unmasking pre-existent risk or eliciting new risk of cardiovascular disease. Family based designs that control for unmeasured shared familial (genetic and early life environmental) factors are needed to help further elucidate potential causality.
To address these gaps in knowledge, we conducted a national cohort study of more than two million women in Sweden. Our goals were to examine five major adverse pregnancy outcomes (preterm delivery, small for gestational age, pre-eclampsia, other hypertensive disorders of pregnancy, gestational diabetes) in relation to risk of ischemic heart disease using prospectively ascertained data in a large population based cohort; to assess changes in such risks across the life course, with up to 46 years of follow-up; and to assess for potential confounding by unmeasured shared genetic and environmental factors in families using co-sibling analyses. We hypothesized that women who experienced any of these five major adverse pregnancy outcomes would have long term increased risks of ischemic heart disease, and that such risks would be only partially explained by shared familial factors.
Methods
Study population
The Swedish Medical Birth Register contains prenatal and birth information for nearly all deliveries in Sweden since 1973.19 Using this registry, we identified 2 201 352 women who had a first delivery during 1973-2015. To improve internal comparability, only singleton deliveries were included in the analyses, given the higher prevalence of adverse pregnancy outcomes and different underlying causes in multiple gestation pregnancies. We excluded 401 (<0.1%) women with a previous diagnosis of ischemic heart disease and 5685 (0.3%) women with missing information for pregnancy duration or infant birth weight, leaving 2 195 266 women (99.7% of the original cohort) for inclusion in the study. Participant consent was not required because this study used only pseudonymized registry based secondary data.
Adverse pregnancy outcome ascertainment
Five major adverse pregnancy outcomes were identified from prenatal and birth records in the Swedish Medical Birth Register. Preterm delivery (gestational age <37 completed weeks) was based on maternal report of last menstrual period in the 1970s and ultrasound estimation starting in the 1980s and onward (>70% of the cohort). Small for gestational age was defined by infant birth weight <10th centile for gestational age. Pre-eclampsia, other hypertensive disorders of pregnancy, and gestational diabetes were identified from diagnostic codes in the Medical Birth Register (see supplemental table 1). In this register, information on gestational age and birth weight have been found to be highly reliable,20 21 but to our knowledge diagnoses have not been formally validated.
Ascertainment of ischemic heart disease
The study cohort was followed up for the earliest diagnosis of ischemic heart disease from first delivery to 31 December 2018 (maximum follow-up time 46 (median 24.8) years). Ischemic heart disease was identified using ICD-8, ICD-9, and ICD-10 (international classification of diseases, eighth, ninth, and 10th revisions, respectively) codes in the Swedish Hospital and Outpatient Registers and all deaths attributed to ischemic heart disease in the Swedish Death Register (see supplemental table 1). The Swedish Hospital Register contains all primary and secondary hospital discharge diagnoses from six populous counties in southern Sweden starting in 1964 and with nationwide coverage starting in 1987; these diagnoses are currently >99% complete, and the positive predictive value for ischemic heart disease has been reported to be 98%.22 23 The Swedish Outpatient Register contains all diagnoses from specialty clinics nationwide starting in 2001. The Swedish Death Register includes all deaths and causes of death for all people registered in Sweden since 1960, with compulsory reporting nationwide.
Covariates
We identified other maternal characteristics that may be associated with adverse pregnancy outcomes and ischemic heart disease using the Swedish Medical Birth Register and national census and diagnosis data, which were linked using a pseudonymous serial number. Maternal age was adjusted for in all analyses as the Cox model time scale. Covariates included several maternal factors, with all time varying factors updated for each pregnancy: calendar year of delivery (continuous and categorical in five year groups), parity (1, 2, 3, 4, ≥5), education level (≤9, 10-12, >12 years), employment status (yes or no), income (fourths) in the year before delivery, country of origin (Sweden or other), body mass index (BMI; continuous and categorical (<18.5, 18.5-24.9, 25.0-29.9, ≥30.0)), smoking (0, 1-9, ≥10 cigarettes/day), and history of hypertension, diabetes, or hyperlipidemia identified from nationwide diagnoses (see supplemental table 1).
Maternal BMI and smoking were assessed at the beginning of prenatal care starting in 1982 and were available for 56.2% and 67.3% of women, respectively. Data were >99% complete for all other variables. Missing data were multiple imputed with 20 imputations using all other covariates and ischemic heart disease as predictors.24 As alternatives to multiple imputation, sensitivity analyses were performed that restricted to women with complete data (n=1 201 724), or coded missing data for each variable as a separate category.
Statistical analysis
Cox proportional hazards regression was used to calculate hazard ratios and 95% confidence intervals for subsequent risk of ischemic heart disease associated with adverse pregnancy outcomes. These associations were examined across the maximum possible follow-up (≤46 years) and within narrower intervals of follow-up (<10, 10-19, 20-29, 30-46 years) among women still living in Sweden without a previous diagnosis of ischemic heart disease at the beginning of the respective interval. All adverse pregnancy outcomes were modeled as time dependent variables based on ever experiencing the respective outcome. For example, if a woman had no adverse pregnancy outcomes in her first delivery but had pre-eclampsia and a preterm infant in her second delivery, she entered the preterm and pre-eclampsia categories at the date of her second delivery. If a woman had a preterm infant in both her first and her second delivery, she entered the preterm delivery category at the date of the first delivery.
Maternal age was used as the Cox model time axis, with age at each delivery as time zero (ie, each singleton delivery was included as a separate observation). Women were right censored at death as identified in the Swedish Death Register (n=65 468; 3.0%) or emigration as determined by absence of a Swedish residential address in census data (n=93 098; 4.3%). Two adjusted Cox models were performed that adjusted for maternal sociodemographic factors, parity, and traditional cardiovascular risk factors, and further adjusted for all adverse pregnancy outcomes. The proportional hazards assumption was assessed by examining log-log survival plots,25 and no substantial departures from the assumption were found (see supplemental figure 1). Incidence rate differences and 95% confidence intervals, attributable fraction among the exposed, and population attributable fraction were calculated for each adverse pregnancy outcome. In addition, we performed a sensitivity analysis that was restricted to each woman’s first delivery instead of time dependent modeling of adverse pregnancy outcomes across all deliveries.
Co-sibling analyses were performed to assess for potential confounding by unmeasured shared familial (genetic and environmental) factors among the 1 188 730 (54.3%) women with at least one sister who had a singleton delivery.18 Shared environmental factors in families may include lifestyle factors such as diet and physical activity, or ambient exposures such as passive smoking and air pollution. Shared genetic factors could potentially act as mediators or confounders. These analyses included all sisters who each had a singleton delivery (regardless of adverse pregnancy outcome status), and the same analysis was performed as the main analyses except for stratifying on sets of sisters. Stratified Cox regression was used where each stratum was a unique set of sisters identified by their mother’s pseudonymous serial number and modeled with their own baseline hazard function that reflects shared genetic and environmental factors. Thus, associations between specific adverse pregnancy outcomes and ischemic heart disease were examined within the family, controlling for shared factors. In addition, these analyses were further adjusted for the same covariates as in the main analyses.
Other secondary analyses were performed: to examine associations between the total number of adverse pregnancy outcomes (0, 1, 2, ≥3; that is, different adverse pregnancy outcomes in the same or different pregnancies, or the same adverse pregnancy outcome in different pregnancies) and risk of ischemic heart disease, adjusting for parity and other covariates; to examine all two way interactions among adverse pregnancy outcomes in relation to ischemic heart disease on the additive and multiplicative scales (including adverse pregnancy outcomes in the same or different pregnancies, each modeled time dependently based on ever occurrence, adjusting for parity and other covariates)26; to examine associations between adverse pregnancy outcomes and ischemic heart disease within 10 years after delivery, stratified on calendar year of delivery (by decade) to assess for changes in estimates over time; and to examine associations between adverse pregnancy outcomes and ischemic heart disease after restricting to women with a first delivery in 1990 or later. In addition, sensitivity analyses were performed that used competing risks models to account for death as a competing event27 as an alternative to only right censoring at death. All statistical tests were two sided and used a significance level of 0.05. All analyses were conducted using Stata version 15.1.
Patient and public involvement
Patients and members of the public were not directly involved in the planning or conduct of this study because of data protection restrictions and the highly technical methods required to do a data linkage analysis. This study was, however, inspired by patients who will be invited to read the manuscript and contribute to the dissemination of findings.
Results
A total of 667 774 (30.4%) women experienced at least one adverse pregnancy outcome, and 181 783 (8.3%) experienced at least two adverse pregnancy outcomes (not necessarily in the same pregnancy). The most common adverse pregnancy outcomes were delivery of a small for gestational age infant (14.3% of women across all deliveries) and preterm delivery (8.8% of women). The most common two adverse pregnancy outcomes experienced by one woman were preterm delivery and delivery of a small for gestational age infant (n=31 822 (1.4%) women; see supplemental table 2).
Table 1 shows the characteristics of the women with specific adverse pregnancy outcomes. Women who delivered a preterm or small for gestational age infant were more likely to be younger at first delivery, have low education level or income, or smoke. Women with pre-eclampsia were more likely to have low education level or income, have high BMI, or smoke. Women with other hypertensive disorders of pregnancy or gestational diabetes were more likely to be older at first delivery, have higher income or BMI, or be a non-smoker. Women with any adverse pregnancy outcome except small for gestational age infant also were more likely to have a history of hypertension, diabetes, or hyperlipidemia.
Table 1.
Baseline maternal characteristics by ever occurrence of an adverse pregnancy outcome, Sweden, 1973-2015. Values are number (percentage) of participants
| Characteristics | All women (n=2 195 266) | Preterm delivery (n=194 751) | Small for gestational age (n=314 605) | Pre-eclampsia (n=132 543) | Other hypertensive disorders of pregnancy (n=34 186) | Gestational diabetes (n=36 269 | |
|---|---|---|---|---|---|---|---|
| Age at first delivery (years): | |||||||
| <20 | 119 054 (5.4) | 16 329 (8.4) | 20 027 (6.4) | 6759 (5.1) | 1418 (4.2) | 1859 (5.1) | |
| 20-24 | 602 321 (27.4) | 58 301 (29.9) | 90 898 (28.9) | 36 879 (27.8) | 7966 (23.3) | 8797 (24.2) | |
| 25-29 | 790 440 (36.0) | 64 634 (33.2) | 109 077 (34.7) | 46 754 (35.3) | 11 662 (34.1) | 11 856 (32.7) | |
| 30-34 | 483 575 (22.0) | 38 379 (19.7) | 66 712 (21.2) | 28 479 (21.5) | 8519 (24.9) | 8595 (23.7) | |
| 35-39 | 166 339 (7.6) | 14 009 (7.2) | 23 307 (7.4) | 11 017 (8.3) | 3639 (10.6) | 4020 (11.1) | |
| ≥40 | 33 537 (1.5) | 3099 (1.6) | 4584 (1.5) | 2655 (2.0) | 982 (2.9) | 1142 (3.2) | |
| Period of first delivery: | |||||||
| 1973-79 | 528 175 (24.1) | 42 646 (21.9) | 92 125 (29.3) | 51 021 (38.5) | 1817 (5.3) | 6031 (16.6) | |
| 1980-89 | 444 535 (20.2) | 46 315 (23.8) | 65 695 (20.9) | 27 365 (20.6) | 6926 (20.3) | 5060 (14.0) | |
| 1990-99 | 445 510 (20.3) | 42 426 (21.8) | 58 355 (18.5) | 19 738 (14.9) | 8668 (25.4) | 7625 (20.0) | |
| 2000-09 | 460 901 (21.0) | 40 717 (20.9) | 57 180 (18.2) | 20 278 (15.3) | 9601 (28.1) | 10 380 (28.6) | |
| 2010-15 | 316 145 (14.4) | 22 647 (11.6) | 41 250 (13.1) | 14 141 (10.7) | 7174 (21.0) | 18 557 (20.8) | |
| Education (years): | |||||||
| ≤9 | 304 998 (13.9) | 30 401 (15.6) | 54 082 (17.2) | 21 391 (16.1) | 2938 (8.6) | 6472 (17.8) | |
| 10-12 | 971 712 (44.3) | 91 177 (46.8) | 144 549 (45.9) | 62 838 (47.4) | 15 085 (44.1) | 15 990 (44.1) | |
| >12 | 918 556 (41.8) | 73 173 (37.6) | 115 974 (36.9) | 48 314 (36.5) | 16 163 (47.3) | 13 807 (38.1) | |
| Employed | 1 894 648 (86.3) | 165 857 (85.2) | 264 748 (84.2) | 119 997 (90.5) | 31 121 (91.0) | 28 609 (78.9) | |
| Income fourth in year before delivery: | |||||||
| 1st (highest) | 548 873 (25.0) | 44 134 (22.7) | 67 748 (21.5) | 26 942 (20.3) | 12 531 (36.7) | 10 545 (29.1) | |
| 2nd | 547 162 (24.9) | 46 520 (23.9) | 75 756 (24.1) | 33 549 (25.3) | 8520 (24.9) | 8587 (23.7) | |
| 3rd | 551 468 (25.1) | 49 411 (25.4) | 84 955 (27.0) | 39 390 (29.7) | 6696 (19.6) | 7552 (20.8) | |
| 4th (lowest) | 547 763 (25.0) | 54 686 (28.1) | 86 146 (27.4) | 32 662 (24.6) | 6439 (18.8) | 9585 (26.4) | |
| Swedish born | 1 802 283 (82.1) | 160 656 (82.5) | 251 510 (79.9) | 114 766 (86.6) | 29 952 (87.6) | 25 929 (71.5) | |
| Body mass index: | |||||||
| <18.5 | 52 906 (2.4) | 5730 (2.9) | 10 816 (3.4) | 1509 (1.1) | 477 (1.4) | 542 (1.5) | |
| 18.5-24.9 | 844 958 (38.5) | 68 996 (35.4) | 112 544 (35.8) | 32 402 (24.5) | 13 081 (38.3) | 11 010 (30.4) | |
| 25.0-29.9 | 244 114 (11.1) | 20 876 (10.7) | 27 176 (8.6) | 15.127 (11.4) | 6.971 (20.4) | 6558 (18.1) | |
| ≥30.0 | 92 201 (4.2) | 9194 (4.7) | 10 474 (3.3) | 8962 (6.8) | 4442 (13.0) | 5116 (14.1) | |
| Unknown | 961 087 (43.8) | 89 955 (46.2) | 153 595 (48.8) | 74 543 (56.2) | 9215 (27.0) | 13 043 (36.0) | |
| Smoking (cigarettes/day): | |||||||
| 0 | 1 257 933 (57.1) | 106 397 (54.4) | 150 527 (47.7) | 60 699 (45.6) | 26 701 (77.7) | 23 423 (64.2) | |
| 1-9 | 154 498 (7.0) | 16 158 (8.3) | 29 052 (9.2) | 5971 (4.5) | 2390 (7.0) | 2614 (7.2) | |
| ≥10 | 69 352 (3.2) | 8498 (4.3) | 15 518 (4.9) | 2575 (1.9) | 994 (2.9) | 1243 (3.4) | |
| Unknown | 713 483 (32.7) | 63 698 (33.0) | 119 508 (38.2) | 63 298 (47.9) | 4101 (12.4) | 8989 (25.2) | |
| Medical history: | |||||||
| Hypertension | 2966 (0.1) | 718 (0.4) | 685 (0.2) | 884 (0.7) | 1212 (3.5) | 351 (1.0) | |
| Diabetes | 10 045 (0.5) | 3058 (1.6) | 745 (0.2) | 1907 (1.4) | 517 (1.5) | 6119 (16.8) | |
| Hyperlipidemia | 1826 (0.1) | 296 (0.2) | 211 (0.1) | 203 (0.2) | 114 (0.3) | 415 (1.1) | |
During 53.6 million person years of follow-up, ischemic heart disease was diagnosed in 83 881 (3.8%) women. The median age at first delivery was 27.3 (mean 27.6 (SD 5.2) years), at diagnosis of ischemic heart disease was 58.6 (mean 57.8 (10.4) years), and at end of follow-up was 51.9 (mean 52.1 (13.2) years). Table 2 shows the absolute incidence rates for ischemic heart disease by adverse pregnancy outcomes and follow-up time, and supplemental figure 2 shows the cumulative incidences. Most diagnoses of ischemic heart disease were acute myocardial infarction (55.3%), 38.7% were angina, and the remainder were other types of acute or chronic ischemic heart disease (see supplemental table 1).
Table 2.
Associations between adverse pregnancy outcomes and subsequent risk of ischemic heart disease
| Adverse pregnancy outcomes and time after delivery | No with IHD | Incidence rate of IHD* | Hazard ratio (95% CI) | Incidence rate difference* (95% CI) | AFe (%) | PAF (%) | |
|---|---|---|---|---|---|---|---|
| Reduced model† | Full model‡ | ||||||
| ≤46 years: | |||||||
| Preterm delivery | 9424 | 210.0 | 1.39 (1.36 to 1.43) | 1.33 (1.30 to 1.37) | 66.2 (61.7 to 70.6) | 31.5 | 5.1 |
| Small for gestational age | 15 186 | 187.3 | 1.20 (1.18 to 1.22) | 1.18 (1.16 to 1.20) | 36.4 (33.2 to 39.6) | 19.4 | 3.5 |
| Pre-eclampsia | 10 043 | 269.8 | 1.44 (1.41 to 1.47) | 1.39 (1.37 to 1.42) | 121.9 (116.5 to 127.3) | 45.2 | 5.4 |
| Other hypertensive disorders of pregnancy | 1262 | 197.1 | 1.66 (1.56 to 1.76) | 1.56 (1.47 to 1.65) | 43.1 (31.4 to 54.9) | 21.9 | 0.3 |
| Gestational diabetes | 2340 | 266.2 | 1.58 (1.50 to 1.66) | 1.45 (1.38 to 1.52) | 113.0 (99.6 to 126.5) | 42.5 | 0.8 |
| <10 years: | |||||||
| Preterm delivery | 507 | 33.4 | 1.86 (1.68 to 2.05) | 1.72 (1.55 to 1.90) | 18.9 (16.0 to 21.9) | 56.6 | 11.9 |
| Small for gestational age | 548 | 18.7 | 1.14 (1.04 to 1.25) | 1.10 (1.00 to 1.21) | 1.6 (0.0 to 3.3) | 8.6 | 1.3 |
| Pre-eclampsia | 362 | 28.7 | 1.72 (1.54 to 1.93) | 1.54 (1.37 to 1.72) | 12.2 (9.2 to 15.2) | 42.4 | 4.4 |
| Other hypertensive disorders of pregnancy | 166 | 61.9 | 2.18 (1.85 to 2.57) | 2.09 (1.77 to 2.46) | 45.8 (35.6 to 55.9) | 73.9 | 3.1 |
| Gestational diabetes | 214 | 49.5 | 1.47 (1.23 to 1.76) | 1.30 (1.09 to 1.56) | 33.2 (24.4 to 42.0) | 67.1 | 2.4 |
| 10-19 years: | |||||||
| Preterm delivery | 1457 | 105.4 | 1.63 (1.54 to 1.73) | 1.51 (1.42 to 1.60) | 47.5 (41.9 to 53.2) | 45.1 | 9.6 |
| Small for gestational age | 1875 | 82.3 | 1.30 (1.24 to 1.37) | 1.27 (1.20 to 1.33) | 16.9 (12.9 to 20.9) | 20.5 | 3.7 |
| Pre-eclampsia | 1221 | 119.4 | 1.69 (1.59 to 1.80) | 1.55 (1.46 to 1.65) | 55.2 (48.3 to 62.0) | 46.2 | 5.4 |
| Other hypertensive disorders of pregnancy | 327 | 152.6 | 1.81 (1.61 to 2.04) | 1.68 (1.50 to 1.89) | 88.3 (70.1 to 106.4) | 57.8 | 1.6 |
| Gestational diabetes | 528 | 172.0 | 1.57 (1.40 to 1.76) | 1.43 (1.27 to 1.59) | 107.7 (87.5 to 128.0) | 62.7 | 1.8 |
| 20-29 years: | |||||||
| Preterm delivery | 3246 | 327.9 | 1.45 (1.40 to 1.51) | 1.37 (1.32 to 1.43) | 105.7 (93.9 to 117.5) | 32.2 | 5.8 |
| Small for gestational age | 4803 | 284.8 | 1.22 (1.18 to 1.26) | 1.19 (1.16 to 1.23) | 46.2 (37.5 to 54.9) | 16.2 | 2.9 |
| Pre-eclampsia | 3192 | 401.2 | 1.53 (1.48 to 1.59) | 1.47 (1.41 to 1.52) | 167.8 (153.5 to 182.0) | 41.8 | 5.0 |
| Other hypertensive disorders of pregnancy | 491 | 385.0 | 1.63 (1.49 to 1.79) | 1.52 (1.38 to 1.66) | 144.4 (107.7 to 181.1) | 37.5 | 0.6 |
| Gestational diabetes | 802 | 520.9 | 1.77 (1.63 to 1.92) | 1.61 (1.49 to 1.75) | 281.5 (236.3 to 326.6) | 54.0 | 1.1 |
| 30-46 years: | |||||||
| Preterm delivery | 4214 | 703.7 | 1.27 (1.23 to 1.31) | 1.23 (1.19 to 1.27) | 128.4 (106.1 to 150.8) | 18.3 | 2.5 |
| Small for gestational age | 7960 | 658.3 | 1.17 (1.14 to 1.20) | 1.16 (1.13 to 1.19) | 71.5 (55.8 to 87.2) | 10.9 | 2.0 |
| Pre-eclampsia | 5268 | 817.4 | 1.34 (1.30 to 1.38) | 1.32 (1.28 to 1.36) | 239.9 (217.1 to 262.7) | 29.4 | 3.6 |
| Other hypertensive disorders of pregnancy | 278 | 766.2 | 1.56 (1.38 to 1.76) | 1.47 (1.30 to 1.66) | 171.1 (75.6 to 266.6) | 22.3 | 0.1 |
| Gestational diabetes | 796 | 970.8 | 1.51 (1.40 to 1.63) | 1.40 (1.29 to 1.51) | 378.1 (299.9 to 456.3) | 38.9 | 0.5 |
AFe=attributable fraction among exposed; CI=confidence interval; IHD=ischemic heart disease; PAF=population attributable fraction.
Rate per 100 000 person years.
Adjusted for maternal age, year of delivery, parity, education, employment, income, country of origin, body mass index, smoking, and history of hypertension, diabetes, or hyperlipidemia.
Additionally adjusted for all other adverse pregnancy outcomes.
Adverse pregnancy outcomes and risk of ischemic heart disease
Across the full duration of follow-up (≤46 years after delivery), all five adverse pregnancy outcomes were independently associated with increased risk of ischemic heart disease. After adjusting for all other adverse pregnancy outcomes and other covariates, the hazard ratios for ischemic heart disease associated with specific adverse pregnancy outcomes were 1.56 (95% confidence interval 1.47 to 1.65) for other hypertensive disorders of pregnancy, 1.45 (1.38 to 1.52) for gestational diabetes, 1.39 (1.37 to 1.42) for pre-eclampsia, 1.33 (1.30 to 1.37) for preterm delivery, and 1.18 (1.16 to 1.20) for small for gestational age. The fully adjusted hazard ratios were only moderately lower than those adjusted for maternal factors but not for other adverse pregnancy outcomes (table 2, reduced model). The association between other hypertensive disorders of pregnancy and ischemic heart disease was driven by new onset hypertension during pregnancy (1.60, 1.51 to 1.70; 92% of diagnoses) rather than chronic hypertension (1.12, 0.91 to 1.38; 8% of diagnoses). When subtypes of ischemic heart disease were examined, all hazard ratios were slightly higher for acute myocardial infarction but also were significantly increased for angina (see supplemental results).
Most hazard ratios were highest in the first 10 years after delivery, then subsequently declined, whereas the incidence rate differences associated with adverse pregnancy outcomes increased with additional follow-up time (table 2). For example, the adjusted hazard ratio for ischemic heart disease in the first 10 years associated with preterm delivery was 1.72 (95% confidence interval 1.55 to 1.90), with pre-eclampsia was 1.54 (1.37 to 1.72), and with other hypertensive disorders of pregnancy was 2.09 (1.77 to 2.46). However, small for gestational age was associated with the highest risk of ischemic heart disease at 10-19 years after delivery (1.27, 1.20 to 1.33) and gestational diabetes with the highest risk at 20-29 years after delivery (1.61, 1.49 to 1.75).
After additional follow-up time, the adjusted hazard ratios for ischemic heart disease decreased but remained significantly increased even 30-46 years after delivery: 1.47 (1.30 to 1.66) for other hypertensive disorders of pregnancy, 1.40 (1.29 to 1.51) for gestational diabetes, 1.32 (1.28 to 1.36) for pre-eclampsia, 1.23 (1.19 to 1.27) for preterm delivery, and 1.16 (1.13 to 1.19) for small for gestational age. Figure 1 shows adjusted hazard ratios for ischemic heart disease by time since delivery for specific adverse pregnancy outcomes.
Fig 1.
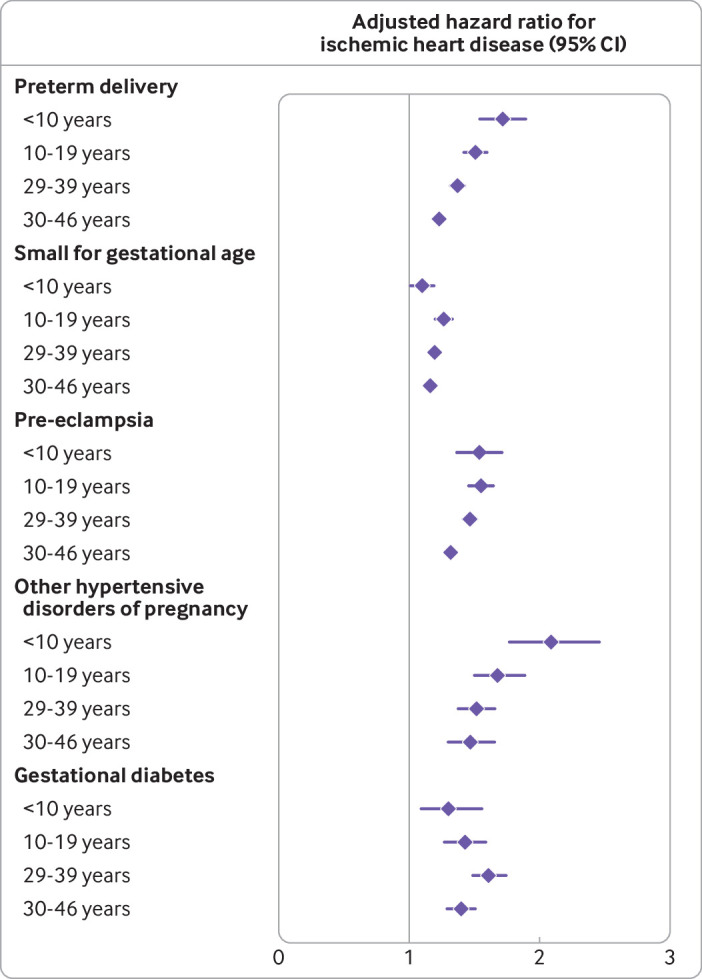
Adjusted hazard ratios for associations between adverse pregnancy outcomes and ischemic heart disease by time since delivery, Sweden, 1973-2018. Whiskers represent 95% confidence intervals
Incidence rate differences (ie, excess ischemic heart disease) associated with each adverse pregnancy outcome increased with additional follow-up to older ages. Across the entire follow-up period, pre-eclampsia and gestational diabetes were associated with the most excess ischemic heart disease (121.9 per 100 000 person years and 113.0 per 100 000 person years, respectively; table 2). Pre-eclampsia and preterm delivery accounted for the largest percentages (>5%) of women with ischemic heart disease in this population, whereas the population attributable fraction was 3.5% for small for gestational age and <1% for other hypertensive disorders of pregnancy and for gestational diabetes (table 2).
Co-sibling analyses
Co-sibling analyses to control for unmeasured shared familial (genetic and early life environmental) factors resulted in partial attenuation of most risk estimates (table 3). The co-sibling results should be interpreted with caution because they have lower precision and wider confidence intervals than the results of the main analyses. Across the entire follow-up period (≤46 years), the adjusted hazard ratio for ischemic heart disease associated with preterm delivery was 1.33 (95% confidence interval 1.30 to 1.37) in the primary analysis versus 1.28 (1.21 to 1.36) in the co-sibling analysis, with small for gestational age was 1.18 (1.16 to 1.20) versus 1.13 (1.08 to 1.19), with pre-eclampsia was 1.39 (1.37 to 1.42) versus 1.22 (1.15 to 1.30), with other hypertensive disorders of pregnancy was 1.56 (1.47 to 1.65) versus 1.33 (1.14 to 1.54), and with gestational diabetes was 1.45 (1.38 to 1.52) versus 1.37 (1.19 to 1.57).
Table 3.
Co-sibling analyses of adverse pregnancy outcomes and subsequent risk of ischemic heart disease
| Adverse pregnancy outcomes and time after delivery | No of women | Hazard ratio (95% CI)* |
|---|---|---|
| ≤46 years: | ||
| Preterm delivery | 5356 | 1.28 (1.21 to 1.36) |
| Small for gestational age | 8124 | 1.13 (1.08 to 1.19) |
| Pre-eclampsia | 5012 | 1.22 (1.15 to 1.30) |
| Other hypertensive disorders of pregnancy | 706 | 1.33 (1.14 to 1.54) |
| Gestational diabetes | 1333 | 1.37 (1.19 to 1.57) |
| <10 years: | ||
| Preterm delivery | 284 | 1.26 (0.77 to 2.04) |
| Small for gestational age | 329 | 1.07 (0.72 to 1.58) |
| Pre-eclampsia | 193 | 1.60 (0.93 to 2.76) |
| Other hypertensive disorders of pregnancy | 97 | 1.85 (0.83 to 4.16) |
| Gestational diabetes | 138 | 1.35 (0.56 to 3.26) |
| 10-19 years: | ||
| Preterm delivery | 894 | 1.52 (1.22 to 1.90) |
| Small for gestational age | 1059 | 1.34 (1.11 to 1.62) |
| Pre-eclampsia | 642 | 1.26 (0.99 to 1.60) |
| Other hypertensive disorders of pregnancy | 183 | 1.81 (1.13 to 2.89) |
| Gestational diabetes | 309 | 1.11 (0.68 to 1.80) |
| 20-29 years: | ||
| Preterm delivery | 1838 | 1.22 (1.07 to 1.38) |
| Small for gestational age | 2643 | 1.12 (1.02 to 1.24) |
| Pre-eclampsia | 1630 | 1.20 (1.05 to 1.37) |
| Other hypertensive disorders of pregnancy | 264 | 0.91 (0.67 to 1.23) |
| Gestational diabetes | 451 | 1.88 (1.40 to 2.52) |
| 30-46 years: | ||
| Preterm delivery | 2340 | 1.25 (1.12 to 1.39) |
| Small for gestational age | 4093 | 1.13 (1.05 to 1.23) |
| Pre-eclampsia | 2547 | 1.14 (1.03 to 1.27) |
| Other hypertensive disorders of pregnancy | 162 | 1.46 (0.98 to 2.19) |
| Gestational diabetes | 435 | 1.13 (0.85 to 1.49) |
CI=confidence interval.
Adjusted for shared familial (genetic and environmental) factors, and additionally adjusted for maternal age; year of delivery; parity; education; employment; income; country of origin; body mass index; smoking; history of hypertension, diabetes, or hyperlipidemia; and all other adverse pregnancy outcomes.
At 30-46 years after delivery, the adjusted hazard ratio for ischemic heart disease associated with preterm delivery was 1.23 (1.19 to 1.27) in the primary analysis versus 1.25 (1.12 to 1.39) in the co-sibling analysis, with small for gestational age was 1.16 (1.13 to 1.19) versus 1.13 (1.05 to 1.23), with pre-eclampsia was 1.32 (1.28 to 1.36) versus 1.14 (1.03 to 1.27), with other hypertensive disorders of pregnancy 1.47 (1.30 to 1.66) versus 1.46 (0.98 to 2.19), and with gestational diabetes was 1.40 (1.29 to 1.51) versus 1.13 (0.85 to 1.49). Certain estimates, however, showed greater attenuation; for example, at <10 years after delivery, the adjusted hazard ratio for ischemic heart disease associated with preterm delivery was 1.72 (1.55 to 1.90) in the primary analysis versus 1.26 (0.77 to 2.04) in the co-sibling analysis.
Secondary analyses
Women who experienced multiple adverse pregnancy outcomes showed further increases in the relative rate of ischemic heart disease (see supplemental table 3). For example, at <10 years after delivery, the adjusted hazard ratios for ischemic heart disease associated with 1, 2, or ≥3 adverse pregnancy outcomes were 1.29 (1.19 to 1.39), 1.80 (1.59 to 2.03), and 2.26 (1.89 to 2.70), respectively, compared with women who never experienced an adverse pregnancy outcome. At 30-46 years after delivery, the corresponding hazard ratios were 1.20 (1.18 to 1.23), 1.37 (1.32 to 1.42), and 1.62 (1.53 to 1.71).
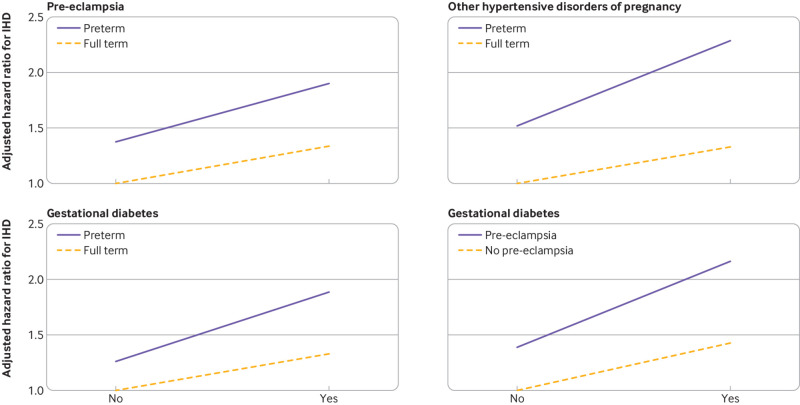
Four of 10 possible two way interactions between adverse pregnancy outcomes were positive on the additive scale or multiplicative scale, or both (see supplemental table 4). Preterm delivery had significantly positive additive interactions with pre-eclampsia, other hypertensive disorders of pregnancy, and gestational diabetes (ie, the combined effect on risk of ischemic heart disease was greater than the sum of the separate effects, P≤0.003 for each). These findings indicate that preterm delivery accounted for more ischemic heart disease diagnoses among those who also had pre-eclampsia, other hypertensive disorders of pregnancy, or gestational diabetes than among those who did not. In addition, pre-eclampsia and gestational diabetes had a positive additive interaction on the additive scale (P=0.003) but not on the multiplicative scale. These interactions are shown in figure 2 and in further detail in supplemental tables 5-8.
Fig 2.
Interactions between adverse pregnancy outcomes and risk of ischemic heart disease (IHD) at ≤46 years after delivery, 1973-2018, Sweden (P≤0.003 for each interaction on the additive scale)
When stratifying on calendar year of delivery, associations between delivery of a small for gestational age infant or gestational diabetes and ischemic heart disease were stronger among women who delivered in earlier years (1973-89) compared with later years (1990-2009), but other adverse pregnancy outcomes showed no consistent trend over time (see supplemental table 9). When restricting to women with a first delivery in 1990 or later, most hazard ratios for ischemic heart disease were similar to those from the main analyses, except that the association between delivery of a small for gestational age infant and ischemic heart disease was no longer significant (see supplemental results).
In sensitivity analyses that either assessed alternative approaches for missing data, restricted to each woman’s first delivery, or accounted for death as a competing event as an alternative to censoring at death, all results were similar to those of the main analyses and the conclusions were unchanged (see supplemental results).
Discussion
In this large national cohort, women who experienced any of five major adverse pregnancy outcomes showed an increased risk of subsequent ischemic heart disease. In the 10 years after delivery, the relative rates of ischemic heart disease were increased twofold in women with other hypertensive disorders of pregnancy, 1.7-fold in those with preterm delivery, 1.5-fold in those with pre-eclampsia, 1.3-fold in those with gestational diabetes, and 1.1-fold in those who delivered a small for gestational age infant, after adjusting for all other adverse pregnancy outcomes and maternal factors. Most relative rates decreased with longer follow-up but remained significantly increased (1.1-fold to 1.5-fold), even 30-46 years after delivery. Co-sibling analyses suggested that these findings were only partially explained by genetic or environmental factors that may be shared determinants of adverse pregnancy outcomes and ischemic heart disease within families.
This large study examined multiple adverse pregnancy outcomes and long term risk of ischemic heart disease using prospectively ascertained data and by assessing potential confounding from familial factors. A previous study of 48 133 participants in the Women’s Health Initiative reported that hypertensive disorders of pregnancy (including pre-eclampsia) and low birth weight were, respectively, associated with 27% and 12% increased odds of atherosclerotic cardiovascular disease, after adjusting for other risk factors.3 In contrast, preterm delivery and gestational diabetes were associated with modestly increased risks before but not after adjusting for other adverse pregnancy outcomes. However, that study differed in several important ways from the present study: it was based on retrospectively self-reported adverse pregnancy outcomes; assessed a composite outcome that included ischemic heart disease, stroke, or peripheral artery disease; and included only women who had survived to approximately ages 70 to >95 years (<50% of the cohort), and thus was potentially affected by recall or survivorship biases.3
Other studies have examined associations between a single adverse pregnancy outcome and risk of ischemic heart disease. For example, a meta-analysis of five studies of preterm delivery with more than two million women reported a pooled relative risk for ischemic heart disease of 1.5,6 which was similar to risk estimates from the present cohort in the first 30 years of follow-up. A recent investigation by our group also reported a strong gradient of higher risks of long term ischemic heart disease in women with shorter pregnancy duration.7 A meta-analysis of pre-eclampsia reported a pooled relative risk for ischemic heart disease of 2.5 (based on seven studies with >2 million women).8 Meta-analyses of gestational diabetes reported pooled relative risks for ischemic heart disease of 1.6 (based on four studies with >3 million women)28 and for a composite outcome that included ischemic heart disease and stroke of 2.0 (based on nine studies with >5 million women).29
The present study extends previous evidence by determining relative rates of ischemic heart disease associated with five major adverse pregnancy outcomes that were prospectively ascertained in a large national population, thus enabling assessment of their independent associations with good precision and validity. The findings suggest that all five major adverse pregnancy outcomes are independently associated with increased risks of ischemic heart disease that may persist for up to 46 years after delivery. Pre-eclampsia and gestational diabetes were associated with the most excess ischemic heart disease (ie, incidence rate differences), whereas pre-eclampsia and preterm delivery accounted for the largest proportion of ischemic heart disease diagnoses in this population (ie, population attributable fraction).
Women who experienced multiple adverse pregnancy outcomes showed further increases in risk of ischemic heart disease, with some synergistic effects noted. Significant additive interactions indicated that preterm delivery accounted for significantly more ischemic heart disease diagnoses among women who also had pre-eclampsia, other hypertensive disorders of pregnancy, or gestational diabetes. In addition, pre-eclampsia accounted for significantly more ischemic heart disease diagnoses among women who also had gestational diabetes (and vice versa). Early preventive interventions may be even more critical in women with these combinations of adverse pregnancy outcomes.
A key question from previous research is whether adverse pregnancy outcomes might unmask pre-existent risk versus elicit new risk for ischemic heart disease. In co-sibling analyses, we found that most of the associations persisted after controlling for familial (genetic and early life environmental) factors that may be shared determinants of adverse pregnancy outcomes and ischemic heart disease. Co-sibling analyses do not fully capture genetic risk, and it is possible that underlying genetic pathways may partly explain future risks of adverse pregnancy outcomes and ischemic heart disease or its precursors.30 31 32 Adverse pregnancy outcomes may be, at least partly, a signal for pre-existing or latent cardiometabolic risk. However, the present findings are also consistent with potential causal associations. Adverse pregnancy outcomes share key features that suggest shared pathogenesis, including abnormal placentation, inflammation, and maternal vascular dysfunction.12 Subclinical forms of cardiovascular disease, such as microvascular dysfunction, arterial stiffness, and myocardial remodeling, may emerge during an adverse pregnancy outcome and then progress or fail to resolve after delivery.12 Preterm delivery, for example, has been associated with endothelial specific inflammation that was undetectable before pregnancy.33 Pre-eclampsia has been associated with cardiac remodeling that continues well after the respective pregnancy.34 Gestational diabetes is strongly associated with future risk of type 2 diabetes and with cardiovascular disease, even in women without type 2 diabetes.29 Small for gestational age may result from poor placental implantation or vascular insufficiency—mechanisms that are also shared with preterm delivery and hypertensive disorders.35 36 Different adverse pregnancy outcomes thus share placental and maternal vascular characteristics that suggest overlapping pathophysiology.12 Preventive interventions to interrupt these processes soon after an adverse pregnancy outcome may be key to preventing future development of clinical cardiovascular disease. Additional genetic analyses and longitudinal studies with comprehensive prepregnancy and post-pregnancy phenotyping will be needed to clarify whether adverse pregnancy outcomes are indeed causally related to future development of ischemic heart disease.
The present study’s findings have important clinical implications. All major adverse pregnancy outcomes should now be recognized as lifelong independent risk factors for ischemic heart disease. The American Heart Association has recommended that evaluation of cardiovascular risk in women routinely include taking a history of adverse pregnancy outcomes.13 However, adverse pregnancy outcomes still remain under-recognized as potential cardiovascular risk factors, especially in primary care where most women are managed.14 Our findings underscore the importance of including adverse pregnancy outcomes in the evaluation of cardiovascular risk for women in primary care as well as in specialty clinic settings. Adverse pregnancy outcomes should be routinely tracked in electronic health records to facilitate transition of women from obstetric to primary care clinics where they should undergo early risk evaluation for cardiovascular disease. It is still uncertain whether specific adverse pregnancy outcomes add substantially to cardiovascular risk prediction beyond traditional risk factors that are more common.37 38 39 In the present study, the absolute risks of ischemic heart disease associated with adverse pregnancy outcomes were modest, albeit with an increasing trend as women reached older ages, when ischemic heart disease is more likely to manifest. Nonetheless, adverse pregnancy outcomes can provide an early window for identifying women who are at high risk potentially long before the development of ischemic heart disease, thus enabling earlier preventive actions. Women with a history of adverse pregnancy outcomes need early preventive evaluation and reduction of other cardiovascular risk factors, including obesity, physical inactivity, and smoking, and long term monitoring for timely detection and treatment of ischemic heart disease.
Strengths and limitations of this study
A key strength of the present study is its large national cohort design and up to 46 years of follow-up. The availability of highly complete nationwide birth and medical registry data, both inpatient and outpatient, helped minimize potential selection and ascertainment biases. The large sample size enabled high statistical power for simultaneous assessment of five major adverse pregnancy outcomes. The results were controlled for multiple other cardiovascular risk factors, as well as unmeasured shared familial factors using co-sibling analyses.
This study also had several limitations. First, detailed clinical records were unavailable to verify the diagnoses of ischemic heart disease, although a positive predictive value of 98% has been reported.23 Second, outpatient diagnoses were available starting in 2001, resulting in some underreporting of ischemic heart disease especially in earlier years. Consequently, the absolute risks of ischemic heart disease associated with adverse pregnancy outcomes may potentially be higher than those reported. Third, residual confounding by maternal smoking, obesity, or other risk factors during pregnancy cannot be excluded. Causality cannot be established, and future biological studies will be needed to elucidate the underlying pathophysiology. We also lacked information on other behavioral factors such as physical activity and diet, which would be useful to examine in future studies. Nevertheless, our results may help identify women who are at high risk and need long term clinical follow-up for prevention, early detection, and treatment of ischemic heart disease. Lastly, this study was limited to Sweden and will need replication in other countries when feasible, including racially diverse populations to explore for potential heterogeneity of findings. The public health impacts of our findings may be higher in minority populations in the US that have more restricted access to postpartum care and higher rates of adverse pregnancy outcomes and ischemic heart disease.1 2 12 40
Conclusions
In this large national cohort, women who experienced any of five major adverse pregnancy outcomes (preterm delivery, small for gestational age, pre-eclampsia, other hypertensive disorders of pregnancy, or gestational diabetes) showed an increased long term risk for ischemic heart disease. For each adverse pregnancy outcome, the relative rates of ischemic heart disease remained persistently increased up to 46 years after delivery. All major adverse pregnancy outcomes should be recognized as lifelong risk factors for ischemic heart disease. Women with adverse pregnancy outcomes should be considered for early preventive evaluation and long term risk reduction to help prevent the development of ischemic heart disease.
What is already known on this topic
Adverse pregnancy outcomes, such as preterm delivery and pre-eclampsia, have been associated with higher future risks of cardiovascular disease
Few studies have examined multiple adverse pregnancy outcomes that were prospectively ascertained in the same cohort to determine their independent associations with long term risks
What this study adds
In a large national cohort, women who experienced any of five major adverse pregnancy outcomes (preterm delivery, small for gestational age, pre-eclampsia, other hypertensive disorders of pregnancy, or gestational diabetes) showed increased risk for ischemic heart disease up to 46 years after delivery
Co-sibling analyses suggested that these findings were only partially explained by shared genetic and environmental factors within families
Women with adverse pregnancy outcomes should be considered for early preventive evaluation and long term risk reduction to help prevent the development of ischemic heart disease
Web extra.
Extra material supplied by authors
Web appendix: Supplemental material
Contributors: All authors contributed to the study concept and design, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. JS and KS acquired the data. CC drafted the manuscript. CC, JS, and KS obtained funding. CC and JS did the statistical analysis. KS and JS are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536), the Swedish Research Council, the Swedish Heart-Lung Foundation, and ALF (Avtal om Läkarutbildning och Forskning, or Agreement on Medical Training and Research) project grant, Region Skåne/Lund University, Sweden. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Heart, Lung, and Blood Institute at the National Institutes of Health, Swedish Research Council, Swedish Heart-Lung Foundation, and ALF project grant, Region Skåne/Lund University, Sweden for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The corresponding author (CC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, no important aspects of the study have been omitted, and any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results will be disseminated to patients and relevant communities on a research website and through press releases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study was approved by the ethics committee of Lund University in Sweden (No 2008/471 and later amendments).
Data availability statement
Owing to ethical concerns, supporting data cannot be made openly available. Further information about the data registries is available from the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/registers/).
References
- 1. Benjamin EJ, Muntner P, Alonso A, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56-528. 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2. Mehta LS, Beckie TM, DeVon HA, et al. American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016;133:916-47. 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 3. Søndergaard MM, Hlatky MA, Stefanick ML, et al. Association of Adverse Pregnancy Outcomes With Risk of Atherosclerotic Cardiovascular Disease in Postmenopausal Women. JAMA Cardiol 2020;5:1390-8. 10.1001/jamacardio.2020.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367-80. 10.1161/CIRCULATIONAHA.111.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grandi SM, Filion KB, Yoon S, et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019;139:1069-79. 10.1161/CIRCULATIONAHA.118.036748 [DOI] [PubMed] [Google Scholar]
- 6. Wu P, Gulati M, Kwok CS, et al. Preterm Delivery and Future Risk of Maternal Cardiovascular Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2018;7:e007809. 10.1161/JAHA.117.007809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crump C, Sundquist J, Howell EA, McLaughlin MA, Stroustrup A, Sundquist K. Pre-Term Delivery and Risk of Ischemic Heart Disease in Women. J Am Coll Cardiol 2020;76:57-67. 10.1016/j.jacc.2020.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes 2017;10:e003497. 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 9. Crump C, Sundquist J, Sundquist K. Preterm delivery and long-term risk of stroke in women: A national cohort and cosibling study. Circulation 2021;143:2032-44. 10.1161/CIRCULATIONAHA.120.052268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crump C, Sundquist J, McLaughlin MA, Dolan SM, Sieh W, Sundquist K. Pre-term delivery and long-term risk of heart failure in women: a national cohort and co-sibling study. Eur Heart J 2021;ehab789. 10.1093/eurheartj/ehab789 [DOI] [PubMed] [Google Scholar]
- 11. Crump C, Sundquist J, Sundquist K. Preterm Delivery and Long-term Risk of Hypertension in Women. JAMA Cardiol 2022;7:65-74. 10.1001/jamacardio.2021.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-Term Cardiovascular Risks Associated With Adverse Pregnancy Outcomes: JACC Review Topic of the Week. J Am Coll Cardiol 2019;73:2106-16. 10.1016/j.jacc.2018.12.092 [DOI] [PubMed] [Google Scholar]
- 13. Parikh NI, Gonzalez JM, Anderson CAM, et al. American Heart Association Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and the Stroke Council . Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021;143:e902-16. 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 14. Gogineni VSM, Manfrini D, Aroda SH, et al. Variations in Awareness of Association Between Adverse Pregnancy Outcomes and Cardiovascular Risk by Specialty. Cardiol Ther 2021;10:577-92. 10.1007/s40119-021-00220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15-44 years in the United States: National Survey of Family Growth, 2006-2010. Natl Health Stat Report 2012;(51):1-28. [PubMed] [Google Scholar]
- 16.United Nations. World Fertility Report 2015. In: Department of Economic and Social Affairs, Population Division. United Nations Publications, 2015. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/files/documents/2020/Feb/un_2015_worldfertilityreport_highlights.pdf [Google Scholar]
- 17. Rich-Edwards JW, Fraser A, Lawlor DA, Catov JM. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol Rev 2014;36:57-70. 10.1093/epirev/mxt006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crump C, Sundquist J, Sundquist K. Preterm delivery and long term mortality in women: national cohort and co-sibling study. BMJ 2020;370:m2533. 10.1136/bmj.m2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics Sweden. The Swedish Medical Birth Register. https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-medical-birth-register/
- 20. Cnattingius S, Ericson A, Gunnarskog J, Källén B. A quality study of a medical birth registry. Scand J Soc Med 1990;18:143-8. 10.1177/140349489001800209 [DOI] [PubMed] [Google Scholar]
- 21. Cnattingius S, Källén K, Sandström A, et al. The Swedish medical birth register during five decades: documentation of the content and quality of the register. Eur J Epidemiol 2023. 10.1007/s10654-022-00947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Linnersjö A, Hammar N, Gustavsson A, Reuterwall C. Recent time trends in acute myocardial infarction in Stockholm, Sweden. Int J Cardiol 2000;76:17-21. 10.1016/S0167-5273(00)00366-1 [DOI] [PubMed] [Google Scholar]
- 24. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley, 1987. 10.1002/9780470316696 . [DOI] [Google Scholar]
- 25. Grambsch PM. Goodness-of-fit and diagnostics for proportional hazards regression models. Cancer Treat Res 1995;75:95-112. 10.1007/978-1-4615-2009-2_5 [DOI] [PubMed] [Google Scholar]
- 26. VanderWeele TJ. Causal interactions in the proportional hazards model. Epidemiology 2011;22:713-7. 10.1097/EDE.0b013e31821db503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509 10.1080/01621459.1999.10474144 . [DOI] [Google Scholar]
- 28. Li J, Song C, Li C, Liu P, Sun Z, Yang X. Increased risk of cardiovascular disease in women with prior gestational diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract 2018;140:324-38. 10.1016/j.diabres.2018.03.054 [DOI] [PubMed] [Google Scholar]
- 29. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019;62:905-14. 10.1007/s00125-019-4840-2 [DOI] [PubMed] [Google Scholar]
- 30. Steinthorsdottir V, McGinnis R, Williams NO, et al. FINNPEC Consortium. GOPEC Consortium . Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun 2020;11:5976. 10.1038/s41467-020-19733-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honigberg MC, Chaffin M, Aragam K, et al. Genetic Variation in Cardiometabolic Traits and Medication Targets and the Risk of Hypertensive Disorders of Pregnancy. Circulation 2020;142:711-3. 10.1161/CIRCULATIONAHA.120.047936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Honigberg MC, Zekavat SM, Aragam K, et al. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol 2019;74:2743-54. 10.1016/j.jacc.2019.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lane-Cordova AD, Gunderson EP, Carnethon MR, et al. Pre-pregnancy endothelial dysfunction and birth outcomes: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Hypertens Res 2018;41:282-9. 10.1038/s41440-018-0017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011;58:709-15. 10.1161/HYPERTENSIONAHA.111.176537 [DOI] [PubMed] [Google Scholar]
- 35. Siddiqui N, Hladunewich M. Understanding the link between the placenta and future cardiovascular disease. Trends Cardiovasc Med 2011;21:188-93. 10.1016/j.tcm.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 36. Valdiviezo C, Garovic VD, Ouyang P. Preeclampsia and hypertensive disease in pregnancy: their contributions to cardiovascular risk. Clin Cardiol 2012;35:160-5. 10.1002/clc.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J 2019;40:1113-20. 10.1093/eurheartj/ehy863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Timpka S, Fraser A, Schyman T, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol 2018;33:1003-10. 10.1007/s10654-018-0429-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parikh NI, Jeppson RP, Berger JS, et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149-58. 10.1161/CIRCULATIONAHA.115.017854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crump C, Howell EA. Perinatal Origins of Cardiovascular Health Disparities Across the Life Course. JAMA Pediatr 2020;174:113-4. 10.1001/jamapediatrics.2019.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplemental material
Data Availability Statement
Owing to ethical concerns, supporting data cannot be made openly available. Further information about the data registries is available from the Swedish National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/registers/).