Abstract
Objectives
During the acute phase of infection, IV antibiotics are preferred to ensure adequate systemic exposure. To assess whether adequate exposure may also be achieved with oral antibiotics, we investigated exposure to oral antibiotics and PTA during the acute phase of infection and after defervescence.
Methods
We enrolled hospitalized, non-critically ill febrile patients treated with IV antibiotics other than amoxicillin or ciprofloxacin. The study consisted of two visits: when patients had received <24 h IV treatment; and when patients had become afebrile. On both visits, patients received one additional dose of 750 mg amoxicillin, or 500 mg ciprofloxacin, depending on the presumed infection, after which serial blood samples were obtained. The primary endpoint was the ratio of the AUC during the febrile and the afebrile phase. The AUCs were considered to be equivalent when the ratio of the mean AUCs and its 90% CI was contained within the acceptance interval of 80%–125%. The secondary endpoint was PTA.
Results
Forty-four patients (15 amoxicillin, 29 ciprofloxacin) completed both study visits. The median time between the two study visits was 65.8 h (range 33.8–427.4). The ratio of the mean AUCs (study visit 1/study visit 2) was 97% (90% CI of 80%–117%) for amoxicillin and 112% (90% CI of 108%–116%) for ciprofloxacin. The PTA for amoxicillin and ciprofloxacin did not differ between the two phases and was adequate to treat common pathogens.
Conclusions
The acute phase of infection in non-critically ill febrile patients does not influence the exposure to, or PTA of, orally administered amoxicillin and ciprofloxacin. This might justify earlier IV-to-oral switching.
Introduction
IV-administered antibiotics are preferred over orally administered antibiotics during the acute phase of a systemic infection to ensure adequate antibiotic exposure.1,2 Even for antibiotic agents that are known to have good bioavailability, physicians are reluctant to treat serious infections orally, because of the belief that the systemic response to an infection may alter the absorption, distribution, metabolism and/or clearance of antibiotics.3,4
In critically ill patients it has indeed been demonstrated that acute infection-induced pathophysiological changes lead to an increase of volume of distribution and augmented or impaired renal clearance.3,5,6 Data on bioavailability, however, are scarce and contradictory and may not apply to non-critically ill patients.4,7 In a recently published systematic review, the very limited number of available studies on this topic suggested that the bioavailability of orally administered antibiotics in non-critically ill patients was not altered during the acute phase.8–11 Yet, included studies were small and had a high risk of bias. Consequently, sound evidence is lacking whether adequate antibiotic levels can be reached in the systemic circulation when antibiotics are administered orally during the initial stage of an infectious illness.
As a consequence, in hospitalized febrile patients who require IV antibiotic treatment it is recommended to switch to oral therapy only when the patient has been treated IV for at least 48–72 h and is recovering.12 Switching to oral therapy has been shown to shorten the length of hospital stay and lower the risk of new infections and healthcare costs, without compromising clinical outcome.13 The cut-off duration of 48–72 h of IV therapy is, however, arbitrary. Patients may therefore be unnecessarily exposed to prolonged IV treatment.
The primary aim of this study was therefore to compare the exposure to orally administered amoxicillin and ciprofloxacin in hospitalized non-critically ill patients during the acute phase of infection and after defervescence, to determine whether the acute phase of infection has an effect on antibiotic exposure after oral administration. A secondary aim was to compare PTA. This knowledge contributes to assessing the possibility of an earlier IV-to-oral switch therapy, in order to gain the benefits of the switch as soon as possible.
Methods
Study design and setting
The EXPO-AB study was a multicentre, prospective intervention study. Non-critically ill, febrile patients treated with IV antibiotics were recruited from August 2019 to December 2021 on the general wards of three acute care hospitals in Amsterdam, the Netherlands: OLVG West, a large non-academic teaching hospital; and the Amsterdam University Medical Centres (locations VUmc and AMC), two academic teaching hospitals. Ethical approval for the EXPO-AB study was given by the Medical Ethical Committee of the Amsterdam University Medical Centres, location AMC. All included subjects signed informed consent. The trial is registered at the Netherlands Trial Register (NTR): NL7782.
Study procedures and data collection
Non-critically ill patients were defined as patients admitted to a general, non-ICU ward. Patients were eligible for inclusion if they were aged ≥18 years and were diagnosed with an acute febrile illness, with a body temperature ≥38.3°C measured at least once since admission, and in need of IV antibiotic therapy. Furthermore, patients had to be able to take medication orally, defined as the absence of abdominal pathology that may alter absorption, like vomiting, severe diarrhoea, malabsorption syndrome, short bowel syndrome, severe gastroparesis, continuous nasogastric suction, ileus or history of resection surgery of the gastrointestinal tract, i.e. oesophagectomy, pylorus-preserving pancreaticoduodenectomy. The decision to start IV antibiotics and the choice of antibiotics were at the discretion of the treating physician, following local guidelines. The IV therapy had to be other than amoxicillin or ciprofloxacin, but prescribed for an indication for which amoxicillin or ciprofloxacin is a registered treatment, for instance community-acquired pneumonia or urosepsis.14,15 This enabled us to safely investigate exposure to oral amoxicillin and ciprofloxacin without affecting standard patient care for the febrile illness. Patients were excluded when they had a glomerular filtration rate of <30 mL/min estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation,16 or were diagnosed with liver cirrhosis, active hepatitis or liver failure, to exclude a gross effect of altered clearance on antibiotic exposure. Patients were also excluded if they were neutropenic (<1000 neutrophils/µL), were treated with chemotherapy within the past 28 days, were pregnant, or when they had a history of alcohol or drug abuse.
The study consisted of two visits: study visit 1 (SV1, ‘febrile phase’), when patients were febrile (temperature ≥38.3°C measured at least once since admission) and had received less than 24 h empirical IV treatment; and study visit 2 (SV2, ‘afebrile phase’), when patients were recovering from their infectious illness and were afebrile (temperature <38.3°C) for at least 24 h, or when they qualified for an IV-to-oral switch.17 In addition to the IV antibiotic treatment, at both study visits the subjects received a single oral dose of amoxicillin 750 mg, if the presumed infection at that moment was a registered indication for amoxicillin, or ciprofloxacin 500 mg, if the infection was a registered indication for ciprofloxacin.14,15 Thereafter, a maximum of four blood samples were obtained per study visit to measure the antibiotic plasma concentrations: three samples randomly during the first 4 h after administration, focusing on the absorption part of the concentration–time curve, and one sample around 6 and 8 h for, respectively, amoxicillin and ciprofloxacin. The time of drug administration and blood sample collection were carefully documented.
Demographic results, medical history, co-medication, vital parameters and body temperature were documented by the coordinating investigator and plasma creatinine, AST, ALT, alkaline phosphatase (AP), GGT, albumin and bilirubin were measured on both study visits.
Sample handling
The blood samples were obtained in heparinized tubes, either via an IV catheter or by direct venepuncture, and immediately transferred on dry ice to the laboratory of the Department of Hospital Pharmacy & Clinical Pharmacology of the Amsterdam UMC or to the Clinical Chemistry Laboratory of the OLVG, where they were centrifuged and stored at −80°C until analysis. Total and unbound amoxicillin and ciprofloxacin concentrations were analysed using a validated LC-MS/MS method. Unbound concentrations were measured in a random selection of 20% of the samples. For amoxicillin total and unbound concentrations, the lower limit of quantification (LLQ) was 0.5 mg/L with an accuracy of 96.7% and a precision of 14.1%. The higher limit of quantification (HLQ) was 40 mg/L with an accuracy of 104% and a precision of 7.8%. For the corresponding parameters for ciprofloxacin we refer to De Vroom et al.18
Endpoints
The primary endpoint was the ratio of the mean area-under-the-plasma-concentration versus time curve (AUC) of the febrile and afebrile phase, for both amoxicillin (AUC0–8) and ciprofloxacin (AUC0–12). The secondary endpoints were the ratios of the mean maximum plasma concentrations (Cmax) and the difference in PTA of the febrile and afebrile phase. For amoxicillin, target attainment was defined as exceeding the MIC during more than 50% of a dosing interval of 8 h. MICs used for this purpose were the EUCAST epidemiological cut-off (ECOFF) values for Streptococcus pneumoniae (0.06 mg/L), Streptococcus pyogenes (0.06 mg/L) and Haemophilus influenzae (2.0 mg/L).19 For ciprofloxacin, PTA was defined as achieving an AUC0–24/MIC ratio ≥125, considering ECOFF values of Escherichia coli (0.064 mg/L) and Pseudomonas aeruginosa (0.5 mg/L).19 The AUC0–24 for ciprofloxacin was pragmatically obtained by multiplying the AUC0–12 by 2. Target attainment was deemed sufficient when PTA was >90%.20,21
Population pharmacokinetic modelling
Individual AUC, Cmax and PTA values for amoxicillin and ciprofloxacin were calculated using a population pharmacokinetic (PPK) model developed with non-linear mixed-effects modelling (NONMEM) Version 7.3 (ICON Development Solutions, Hanover, MD, USA). Detailed methodological information on model development is presented in the Supplementary methods, available as Supplementary data at JAC Online. In short, first a structural PPK model was developed. Next, a covariate analysis was performed in which patient demographics and pathophysiological factors were tested for their correlation with the identified pharmacokinetic parameters from the structural model, which yielded the final model. Last, the validity and robustness of the model was tested by preforming a visual predictive check (VPC) and a bootstrap analysis.
Sample size calculation and statistical analysis
AUC0–8 for amoxicillin and AUC0–12 for ciprofloxacin were considered to be equivalent when the ratio of the mean AUCs in the febrile and afebrile phase was contained within the acceptance interval of 80%–125%, which was adapted from the bioequivalence criteria.22 In order to achieve 90% power at a 5% significance level, 13 patients were required per study visit for amoxicillin, assuming a mean AUC0–inf of 22.6 mg·h/L and an SD of 4.9.23 We aimed to include 15 patients. For ciprofloxacin, 32 patients were required, considering a mean AUC0–inf of 11.05 mg·h/L and an SD of 3.99.11,24 Patients who started the study but in whom zero concentration–time measurements were collected in either the febrile or afebrile phase of the illness were considered non-evaluable for the endpoint measurements and were to be replaced by additional patients. Pharmacokinetics data of these patients collected in one of the two phases were used for the development of the PPK model.
Baseline categorical patient characteristics were summarized by presenting numbers and percentages. Continuous baseline characteristics were summarized by presenting the mean and SD or the median and minimum–maximum ranges, as appropriate.
The ratio of the mean AUC and mean Cmax was obtained by logarithmic transformation of the AUC and Cmax data, followed by a paired t-test and logarithmic back transformation.22 Differences in PTA were illustrated by descriptive statistics. These statistical analysis were performed in IBM SPSS Statistics for Windows, version 26.0.
Results
Patient characteristics
A total of 52 participants were included in the study: 19 receiving amoxicillin, of whom 15 patients completed both study visits, and 33 receiving ciprofloxacin, of whom 29 patients completed both study visits. The intended sample size of 32 patients for ciprofloxacin was not achieved due to slow inclusion resulting from the coronavirus pandemic. The reasons for the patients to discontinue the study were: discomfort and possible side effects (n = 3); early discharge or transferal to another hospital (n = 3); and one patient died due to the underlying febrile illness. In addition, one patient in the ciprofloxacin arm was switched to oral ciprofloxacin by the treating physician before SV2.
The characteristics of the patients who completed both study visits are presented in Table 1; the characteristics of all included patients are presented in Table S1. The majority of patients who received amoxicillin were empirically diagnosed with community-acquired pneumonia and those who received ciprofloxacin with a complicated urinary tract infection or an intra-abdominal infection. The median time between the two study visits was 47.3 h (range 43.7–185.7) for amoxicillin and 67.1 h (33.8–427.4) for ciprofloxacin. The wide range was caused by the variety of underlying diseases, causing some patients to be infectious for a prolonged period, e.g. in case of disseminated streptococcal infection or endocarditis, which was diagnosed after the first study visit.
Table 1.
Patient characteristics
| Amoxicillin (n = 15) | Ciprofloxacin (n = 29) | |||||
|---|---|---|---|---|---|---|
| Age (years) | 67 (21–80) | 65 (18–87) | ||||
| Gender (male) | 12 (80) | 13 (49) | ||||
| Height (cm) | 175 (157–195) | 168 (1.55–1.89) | ||||
| Weight (kg) | 77.9 (53.3–121) | 79.9 (45–130) | ||||
| BMI (kg/m2) | 23.8 (18.8–40.9) | 27.2 (18.0–39.3) | ||||
| Presumed site of infection at admission | 11 respiratory tract infection 4 urinary tract infection |
18 urinary tract infection 8 Intra-abdominal infection 3 bone/joint infection |
||||
| Definitive site of infection | 9 respiratory tract infection 4 urinary tract infection 1 gastrointestinal infection 1 disseminated streptococcal infection |
16 urinary tract infection 8 Intra-abdominal infection 1 bone/joint infection 3 skin and soft tissue infection 1 endocarditis |
||||
| Time between the study visitsa | 47.3 h (43.7–185.7) | 67.1 h (33.8–427.4) | ||||
| BL | SV1 | SV2 | BL | SV1 | SV2 | |
|---|---|---|---|---|---|---|
| Body temperature (°C) | 38.9 (38.3–40.5) |
37.2 (36.6–38.6) |
36.7 (36–37.8) |
39 (38.3–40) | 37.2 (36.1–40.5) | 36.7 (35.9–37.6) |
| Antipyretic use | 12 (80) | 12 (80) | 8 (53) | 21 (72) | 22 (76) | 18 (62) |
| Plasma creatinine (µmol/L) | 82 (47–176) | 98 (47–176) | 84 (40–152) | 90 (42–145) | 86 (42–145) | 75 (41–143) |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 78 (31–131) | 78 (31–131) | 89 (32–139) | 63 (44–129) | 65 (44–129) | 74 (40–154) |
| AST (U/L) | 30 (16–52) | 25 (16–75) | 31 (17–159) | 27 (12–118) | 26 (12–119) | 32 (9–119) |
| ALT (U/L) | 19 (11–51) | 19 (11–137) | 27 (12–96) | 23 (10–134) | 23 (10–134) | 29 (5–140) |
| GGT (U/L) | 40 (13–178) | 41 (13–194) | 42 (19–269) | 38 (16–1267) | 39 (16–913) | 66 (19–574) |
| Albumin (g/L) | 35 (30–43) | 35 (28–43) | 33 (25–42) | 37 (28–45) | 36 (28–45) | 34 (20–38) |
| Bilirubin (µmol/L) | 9 (3–29) | 9 (3–29) | 5 (3–9) | 11 (2–555) | 11 (2–555) | 6 (2–470) |
Data are presented as n (%) or median (range). Most SV1 results were the laboratory results from baseline. In the case of no recent (<24 h) results being available, (new) blood samples were obtained for SV1. BL, baseline; eGFR, estimated glomerular filtration rate.
The wide range was caused by the variety of underlying diseases, causing some patients to be infectious for a prolonged period, e.g. in the case of disseminated streptococcal infection or endocarditis.
PPK analysis
For the PPK analysis the blood samples of all subjects were included, which yielded a total of 121 amoxicillin and 219 ciprofloxacin plasma samples for analysis. Of these, 67 and 115 samples were obtained at SV1, of which 53 and 88 samples were obtained during the first 4 h after administration, and 54 and 104 samples at SV2, of which 43 and 80 samples were obtained during the first 4 h after administration. The measured total antibiotic plasma concentrations are presented in Figure 1 and Figure 2 for amoxicillin and ciprofloxacin, respectively. Less than 2% of samples were below the LLQ, for which we imputed the value of LLQ/2. The total and unbound plasma concentrations of both amoxicillin and ciprofloxacin were strongly correlated, both r: 0.99, 95% CI 0.98–0.99, indicating linear plasma protein binding. We therefore used total plasma concentrations to build the PPK model.
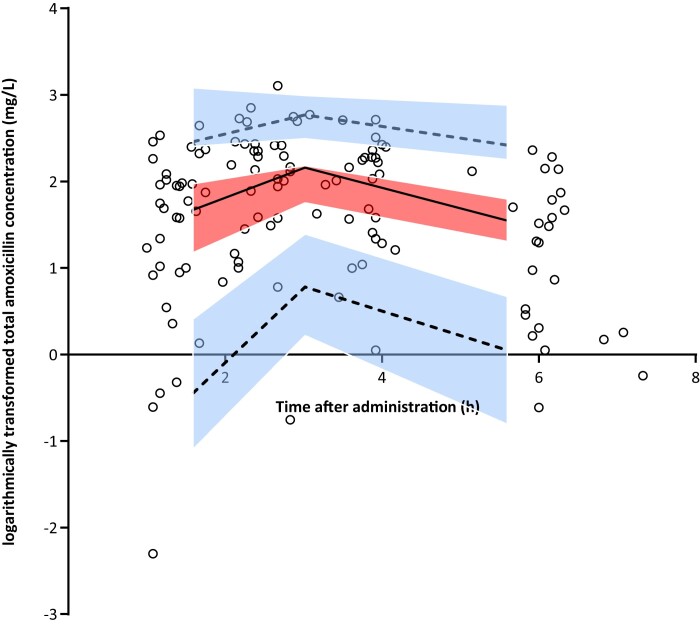
Figure 1.
VPC for logarithmically transformed total amoxicillin concentrations versus time based on 1000 simulations of the final model. The black open circles are the observed concentrations. The solid line represents the median and the dashed lines the 5th and 95th percentiles of the observed data. The centre, red shaded area is the 95% CI of the model-predicted median and the outer, blue shaded areas are the 95% CIs of the model-predicted 5th and 95th percentiles. The solid and dashed lines run within their respective shaded areas, thereby demonstrating adequate fit of the model. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
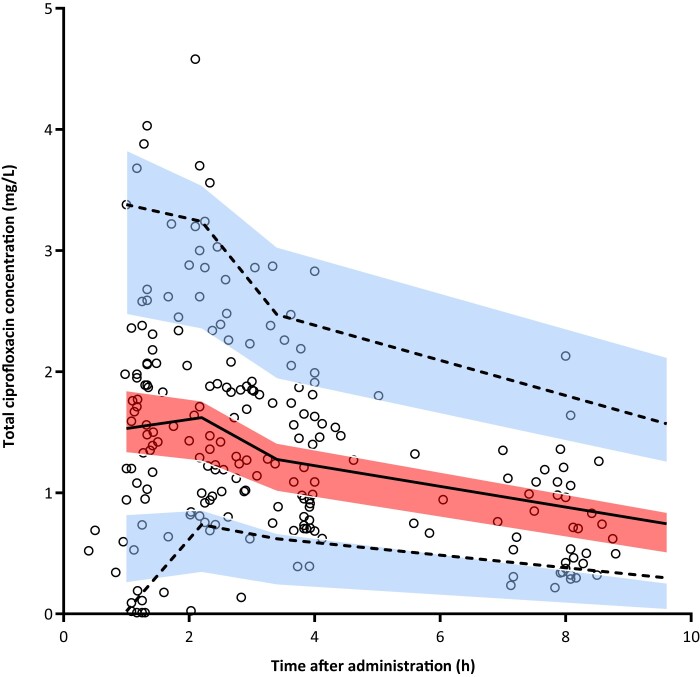
Figure 2.
VPC for the total ciprofloxacin concentrations versus time based on 1000 simulations of the final model. The black open circles are the observed concentrations. The solid line represents the median and the dashed lines the 5th and 95th percentiles of the observed data. The centre, red shaded area is the 95% CI of the model-predicted median and the outer, blue shaded areas are the 95% CIs of the model-predicted 5th and 95th percentiles. In the 5th percentile (lower blue shaded area) there is a minor overestimation of observed concentrations with time after administration <2 h and a minor underestimation at the end of the dosing interval. The overestimation was likely to be caused by three observed concentrations that were <LLQ and which were imputed with a value of LLQ/2, which the final model cannot predict. Overall, these model misspecifications are small and all other solid and dashed lines run within their respective shaded areas, demonstrating sufficient fit of the model. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Detailed information of the PPK model development is presented in Tables S2 and S3. In short, the pharmacokinetics of amoxicillin was best described by a one-compartment model, using logarithmic transformed data with non-linear absorption (Michaelis–Menten model) and an absorption lag time (Tlag). Interindividual variability (IIV) could be estimated for clearance (CL) and interoccasion variability for maximum absorption rate (Vmax). Multivariate analyses showed that CKD-EPI was significantly associated with CL. The VPC plot (Figure 1) shows that the final model was able to predict the range of observed amoxicillin concentrations without bias and was therefore valid to be used for the AUC0–8, Cmax and PTA calculations.
The pharmacokinetics of ciprofloxacin was best described by a one-compartment model, using first-order absorption, a Tlag and first-order elimination, without logarithmic transformation of the data. IIV could be estimated for CL and volume of distribution (Vd). Multivariate analyses showed that body temperature was significantly associated with Vd and CKD-EPI with CL. The VPC plot (Figure 2) showed adequate fit of the final model predicting the vast majority of observed ciprofloxacin concentrations without bias and was therefore valid to be used for the AUC0–12, Cmax and PTA calculations.
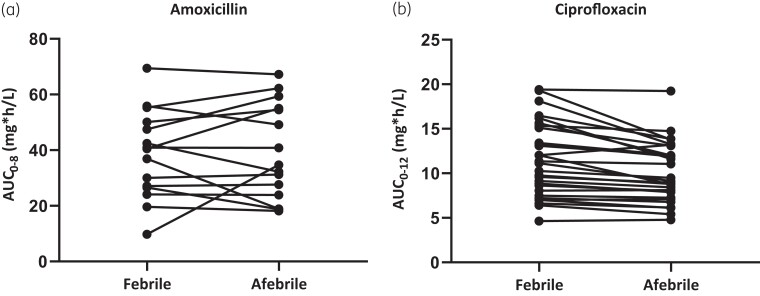
Amoxicillin and ciprofloxacin AUC and Cmax equivalence
Results of the calculated AUC and Cmax values of SV1 versus SV2 are presented in Table 2. The before/after slope line plots in Figure 3(a and b) show the individual changes in AUC from SV1 to SV2 for amoxicillin and ciprofloxacin, respectively. The ratios of the mean AUC0–8 of orally administered amoxicillin and the mean AUC0–12 of orally administered ciprofloxacin were, respectively, 97% (90% CI 80%–117%) and 112% (90% CI 108%–116%), and therefore equivalent between the febrile and afebrile phase of infection. For the ratios of the mean Cmax, this only accounted for ciprofloxacin: 111% (90% CI 106%–117%). Patients who received amoxicillin had a slightly lower mean peak concentration when they were febrile compared with when they were afebrile, with a 90% CI that did not meet the equivalence criteria (ratio 94%, 90% CI 71%–124%).
Table 2.
AUC and Cmax of amoxicillin and ciprofloxacin during the febrile and afebrile phase of infection
| Febrile | Afebrile | Ratio (%) | 90% CI | |
|---|---|---|---|---|
| Amoxicillin | ||||
| ȃAUC0–8 (mg·h/L) | 34.79 (1.64) | 36.0 (1.59) | 97 | 80–117 |
| ȃCmax (mg/L) | 8.86 (1.56) | 9.45 (1.42) | 94 | 71–124 |
| Ciprofloxacin | ||||
| ȃAUC0–12 (mg·h/L) | 10.74 (1.46) | 9.58 (1.40) | 112 | 108–116 |
| ȃCmax (mg/L) | 1.82 (1.34) | 1.63 (1.29) | 111 | 106–117 |
Data are presented as mean (SD) unless otherwise indicated.
Figure 3.
Before/after slope line plot for individual AUC change from the febrile to the afebrile phase.
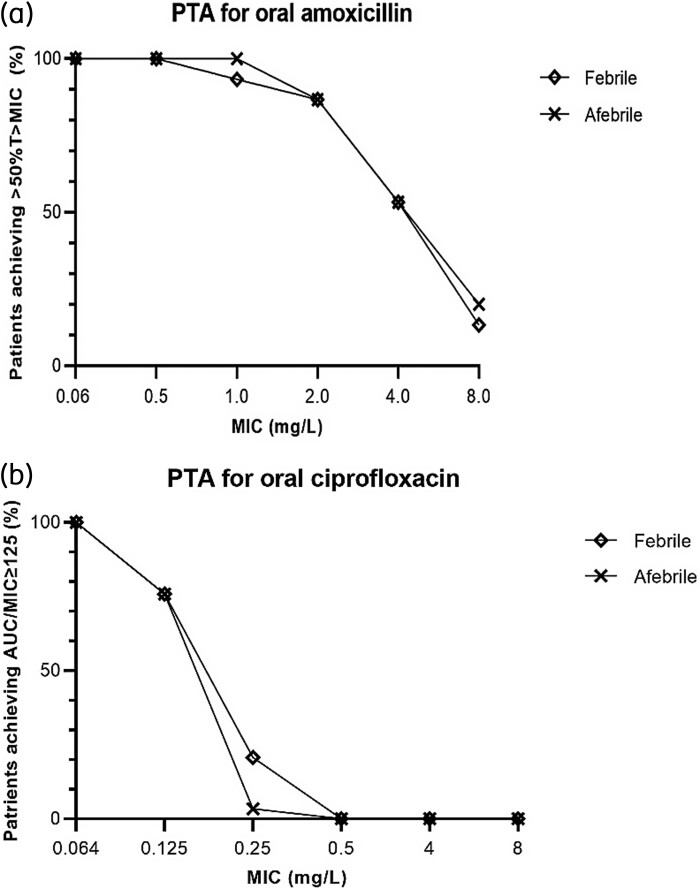
Pharmacokinetic/pharmacodynamic (PK/PD) target attainment
Figure 4(a and b) shows the PTA for amoxicillin and ciprofloxacin. For amoxicillin, assuming MICs of 0.06 and 2.0 mg/L, the PTA was 100% and 86.7%, respectively, for the febrile and the afebrile phase. For ciprofloxacin, assuming MICs of 0.064 and 0.5 mg/L, the PTA was, respectively, 100% and 0% for the febrile and the afebrile phase.
Figure 4.
PTA for oral amoxicillin and ciprofloxacin. (a) PTA for oral amoxicillin 750 mg, defined as achieving an amoxicillin plasma concentration above the MIC during half of the dosing interval (50%T>MIC); (b) PTA for oral ciprofloxacin 500 mg, defined as achieving an AUC0–24/MIC ratio ≥125 (calculated as AUC0–12 multiplied by 2). PTA was calculated for a range of MICs, for the febrile phase and the afebrile phase separately.
Discussion
With this study we have shown that the exposure to orally administered amoxicillin and ciprofloxacin is not different during the acute phase of infection compared with the afebrile phase in hospitalized, non-critically ill patients. We were able to develop a valid PPK model for both amoxicillin and ciprofloxacin, as was shown by the VPC plots and bootstrap results, enabling reliable calculation of individual AUC, Cmax and PTA values. In addition, we have shown that for both phases the probability of PK/PD target attainment is high for amoxicillin in the case of microorganisms with MIC ≤ 1.0 mg/L and for ciprofloxacin in the case of microorganisms with MIC ≤ 0.064 mg/L. These results suggest that from a pharmacokinetic point of view reluctance for oral administration of amoxicillin and ciprofloxacin during the acute phase of infection is not necessary.
To our knowledge this is the first PPK study investigating the absorption of and resulting exposure to oral antibiotics in non-critically ill patients.8 Studies regarding antibiotic exposure have predominantly been performed in critically ill patients using IV antibiotics, as the gastrointestinal tract is usually impaired or not accessible in that population.4 In our previously reported systematic review, we showed that only three studies truly addressed the absorption of and exposure to oral ciprofloxacin and clarithromycin during the febrile and afebrile phase of infection in non-critically ill patients.8 Although these studies also concluded that the exposure was not altered during the acute phase of infection, they had a small sample size and used now-outdated laboratory and pharmacokinetic methodology to assess the AUC and Cmax, questioning the reliability and generalizability of the results.9–11
Our PPK model showed that the absorption of amoxicillin was best described by non-linear saturable absorption (Michael–Menten kinetics), confirming previous studies.25–27 No significant effect of the acute phase of infection on pharmacokinetic parameters could be found as study visit was tested as a categorical covariate. Although the mean Cmax was numerically slightly lower during the acute phase of infection, the wide 90% CI (71%–124%) indicates that the sample size may have been too small to accurately measure Cmax (non-)equivalence. In addition, Cmax is not the PK/PD target in the case of amoxicillin. For ciprofloxacin, which followed first-order absorption as previously described,28 higher body temperature was significantly associated with lower Vd. This might lead to increased peak concentrations, as was confirmed by the (non-significant) higher Cmax of ciprofloxacin on SV1 (ratio of mean Cmax: 111%). This did not result in a different AUC between the two study visits. As both drugs follow first-order elimination and no association was identified between body temperature and CL, indeed no effect of the febrile phase on AUC was expected.
When comparing our PPK modelling results with those of studies performed in critically ill21,24,29,30 and burn patients,31 CLCR was likewise associated with CL of both amoxicillin29,31 and ciprofloxacin.21,24,30 The IIV of CL was lower in our study population, suggesting that exposure is more predictable in non-critically ill patients, also in the acute phase of infection. Therefore, the acute phase of infection only had a marginal effect on the pharmacokinetics of the investigated antibiotics in non-critically ill patients. This is in contrast with critically ill patients, where CL and Vd may differ considerably between patients: decreased and increased CL and increased Vd are observed due to (extremely) decreased or (extremely) increased renal function, altered fluid balance and organ support.6
Based on our results, the recommended oral dosing regimen of amoxicillin 750 mg three times a day would suffice during the acute phase of most infections for which amoxicillin is the preferred treatment. This is in line with the PK/PD simulation study of de Velde and colleagues25 performed in healthy volunteers. Most amoxicillin-susceptible microorganisms have an MIC of <2 mg/L and the PTA for amoxicillin was only just below 90% for microorganisms with an MIC of 2 mg/L (Figure 3).19 For ciprofloxacin, the PK/PD target was not sufficiently attained for microorganisms with an MIC of >0.064 mg/L, also not during the afebrile phase. In previous studies it was already shown that a ciprofloxacin dose of 500 mg twice a day is often not enough to attain the PK/PD target of AUC0–24/MIC > 125 for bacteria with MIC values >0.125 mg/L, nor for ciprofloxacin 750 mg twice a day in the case of difficult-to-treat infections such as P. aeruginosa (MIC = 0.5 mg/L).21,28,32,33 These results suggest that if oral treatment is initiated during the acute phase of infection, rapid microbiological test results should be used as a basis for potential dose adjustments to ensure that sufficient bacterial killing can be achieved, or a higher ciprofloxacin starting dose should be considered.28,32
Strength and limitations
This is the first study investigating PPK during the acute phase of infection in non-critically ill febrile patients, in a group of patients covering a wide range of ages, renal function and infectious diseases. Our results therefore provide new and relevant data applicable to the majority of hospitalized, febrile patients. Furthermore, patients were their own control, which eliminated residual variation between the febrile and afebrile phase. Also, the time of administration and blood sampling were carefully registered. A limitation is that special patient populations, for example patients with severe renal impairment or neutropenic patients, were excluded. Although it is to be expected that equivalent exposure also accounts for these patients, this was not investigated. Our results can therefore not be automatically extrapolated to these patients. In addition, although the first study visit was performed within the first 24 h of initiation of IV antibiotics, the body temperature in most of our patients had already declined, albeit not normalized, at that time. This may have limited the power to identify associations between pharmacokinetics parameters and body temperature. Also, the primary focus of the study was to investigate exposure, for which we investigated single administrations only instead of repeated dosages. Our results on PTA for the investigated antibiotics, especially ciprofloxacin, are therefore lower than those reported after multiple dosing and at steady-state.28,32 This is caused by the fact that we were forced to multiply the AUC0–12 of ciprofloxacin by two to estimate AUC0–24 (assuming twice-daily dosing). Nonetheless, even when presenting a worst-case scenario, our results showed that exposure to ciprofloxacin was sufficient to treat common infections (e.g. E. coli). A final limitation is that we focused on the PTA for ciprofloxacin 500 mg, instead of 750 mg, which is used for difficult-to-treat infections including P. aeruginosa. However, simulations using the developed PPK model showed that with 750 mg ciprofloxacin the PTA to effectively treat P. aeruginosa (MIC = 0.5 mg/L) infections was still insufficient (PTA = 0%).
Conclusions
With this study we have shown that the differences in antibiotic exposure (AUC) between the febrile and afebrile phase of infection are contained within the acceptance interval of 80%–125% in hospitalized non-critically ill infectious patients. In addition, the PK/PD target was equally attained during both phases and sufficient to treat common pathogens. Herewith, we have provided a pharmacokinetics base for an IV-to-oral switch within 48 h of IV therapy for a large patient population, as our study population was heterogeneous in age, febrile illness and renal function. The next step is to actually shorten the IV treatment duration. Next to increasing patient comfort, an earlier switch may (further) reduce length of hospital stay and healthcare costs.
Supplementary Material
Acknowledgements
We thank the patients who were willing to participate to this study, all the staff of the general wards of the participating hospitals for contributing to the study, and Marcel Pistorius, Dennis van der Laan and colleagues from the Laboratory of Clinical Pharmacology of the Amsterdam UMC for the amoxicillin and ciprofloxacin concentration measurements. We also thank Tingjie Guo, Assistant Professor of Pharmacometrics, Leiden Amsterdam Centre for Drug Research, Leiden University, for his guidance on NONMEM.
Contributor Information
A K Van Den Broek, Department of Internal Medicine, Division of Infectious Diseases, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
C E Visser, Department of Medical Microbiology and Infection Prevention, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
J Veenstra, Department of Internal Medicine, OLVG, location West, Jan Tooropstraat 164, 1061 AE, Amsterdam, The Netherlands.
B T J Van Den Berg, Department of Pulmonology, OLVG, location West, Jan Tooropstraat 164, 1061 AE, Amsterdam, The Netherlands.
J M Prins, Department of Internal Medicine, Division of Infectious Diseases, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
R M Van Hest, Department of Hospital Pharmacy, Division of Clinical Pharmacology, Amsterdam UMC, University of Amsterdam, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands.
Funding
This work was funded by the Amsterdam UMC, University of Amsterdam.
Transparency declarations
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Author contributions
A. K. van den Broek, J. M. Prins, C. E. Visser and R. M. van Hest contributed to the study conception and design. A. K. van den Broek, J. M. Prins, J. Veenstra, B. T. J. van den Berg and R. M. van Hest contributed to data collection. A. K. van den Broek and R. M. van Hest contributed to data analysis. A. K. van den Broek, J. M. Prins, C. E. Visser and R. M. van Hest contributed to interpretation of data. All authors contributed to drafting the manuscript and/or reviewing the manuscript. All authors read and approved the final manuscript.
Supplementary data
Supplementary Methods and Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother 2014; 5: 83–7. 10.4103/0976-500X.130042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SL, Azmi S, Wong PS. Clinicians’ knowledge, beliefs and acceptance of intravenous-to-oral antibiotic switching, Hospital Pulau Pinang. Med J Malaysia 2012; 67: 190–8. [PubMed] [Google Scholar]
- 3. Jager NG, van Hest RM, Lipman Jet al. . Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 2016; 9: 961–79. 10.1586/17512433.2016.1172209 [DOI] [PubMed] [Google Scholar]
- 4. Roberts DJ, Hall RI. Drug absorption, distribution, metabolism and excretion considerations in critically ill adults. Expert Opin Drug Metab Toxicol 2013; 9: 1067–84. 10.1517/17425255.2013.799137 [DOI] [PubMed] [Google Scholar]
- 5. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 2014; 77: 3–11. 10.1016/j.addr.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 6. Roberts JA, Abdul-Aziz MH, Lipman Jet al. . Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis 2014; 14: 498–509. 10.1016/S1473-3099(14)70036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarwari AR, Mackowiak PA. The pharmacologic consequences of fever. Infect Dis Clin North Am 1996; 10: 21–32. 10.1016/S0891-5520(05)70283-X [DOI] [PubMed] [Google Scholar]
- 8. van den Broek AK, Prins JM, Visser CEet al. . Systematic review: the bioavailability of orally administered antibiotics during the initial phase of a systemic infection in non-ICU patients. BMC Infect Dis 2021; 21: 285. 10.1186/s12879-021-05919-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guay DR, Awni WM, Peterson PKet al. . Pharmacokinetics of ciprofloxacin in acutely ill and convalescent elderly patients. Am J Med 1987; 82: 124–9. [PubMed] [Google Scholar]
- 10. Offman E, Varin F, Nolan Tet al. . Oral absorption of clarithromycin in acute illness and during convalescence in patients with community-acquired pneumonia. Chest 2000; 117: 1090–3. 10.1378/chest.117.4.1090 [DOI] [PubMed] [Google Scholar]
- 11. Patel KB, Belliveau PP, Nightingale CHet al. . Absorption of ciprofloxacin in febrile and afebrile patients. Int J Antimicrob Agents 1995; 6: 119–22. 10.1016/0924-8579(95)00030-6 [DOI] [PubMed] [Google Scholar]
- 12. Nathwani D, Lawson W, Dryden Met al. . Implementing criteria-based early switch/early discharge programmes: a European perspective. Clin Microbiol Infect 2015; 21Suppl 2: S47–55. 10.1016/j.cmi.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 13. Schuts EC, Hulscher M, Mouton JWet al. . Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16: 847–56. 10.1016/S1473-3099(16)00065-7 [DOI] [PubMed] [Google Scholar]
- 14. emc. Ciprofloxacin 500 mg film-coated tablets. https://www.medicines.org.uk/emc/product/3484/smpc.
- 15. emc. Amoxicillin 500 mg capsules BP. https://www.medicines.org.uk/emc/product/6109/smpc.
- 16. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . http://ckdepi.org/.
- 17. Akhloufi H, Hulscher M, Melles DCet al. . Development of operationalized intravenous to oral antibiotic switch criteria. J Antimicrob Chemother 2017; 72: 543–6. 10.1093/jac/dkw470 [DOI] [PubMed] [Google Scholar]
- 18. de Vroom SL, Pistorius MCM, Bijleveld YAet al. . Development and validation of a Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) assay for the determination of total and unbound ciprofloxacin concentrations in human plasma. Ther Drug Monit 2022; 44: 552–7. 10.1097/FTD.0000000000000969 [DOI] [PubMed] [Google Scholar]
- 19. MIC EUCAST . Antimicrobial wild type distributions of microorganisms. https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=2&search%5Bspecies%5D=-1&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50.
- 20. Mouton JW, Brown DF, Apfalter Pet al. . The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 2012; 18: E37–45. 10.1111/j.1469-0691.2011.03752.x [DOI] [PubMed] [Google Scholar]
- 21. Roberts JA, Alobaid AS, Wallis SCet al. . Defining optimal dosing of ciprofloxacin in patients with septic shock. J Antimicrob Chemother 2019; 74: 1662–9. 10.1093/jac/dkz069 [DOI] [PubMed] [Google Scholar]
- 22. Morais JAG, do Rosário Lobato M. The new European Medicines Agency guideline on the investigation of bioequivalence. Basic Clin Pharmacol Toxicol 2010; 106: 221–5. 10.1111/j.1742-7843.2009.00518.x [DOI] [PubMed] [Google Scholar]
- 23. Li M, Andrew MA, Wang Jet al. . Effects of cranberry juice on pharmacokinetics of beta-lactam antibiotics following oral administration. Antimicrob Agents Chemother 2009; 53: 2725–32. 10.1128/AAC.00774-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khachman D, Conil JM, Georges Bet al. . Optimizing ciprofloxacin dosing in intensive care unit patients through the use of population pharmacokinetic-pharmacodynamic analysis and Monte Carlo simulations. J Antimicrob Chemother 2011; 66: 1798–809. 10.1093/jac/dkr220 [DOI] [PubMed] [Google Scholar]
- 25. de Velde F, de Winter BC, Koch BCet al. . Non-linear absorption pharmacokinetics of amoxicillin: consequences for dosing regimens and clinical breakpoints. J Antimicrob Chemother 2016; 71: 2909–17. 10.1093/jac/dkw226 [DOI] [PubMed] [Google Scholar]
- 26. Paintaud G, Alván G, Dahl MLet al. . Nonlinearity of amoxicillin absorption kinetics in human. Eur J Clin Pharmacol 1992; 43: 283–8. 10.1007/BF02333024 [DOI] [PubMed] [Google Scholar]
- 27. Sjövall J, Alván G, Westerlund D. Dose-dependent absorption of amoxycillin and bacampicillin. Clin Pharmacol Ther 1985; 38: 241–50. 10.1038/clpt.1985.166 [DOI] [PubMed] [Google Scholar]
- 28. de Vroom SL, van Hest RM, van Daalen FVet al. . Pharmacokinetic/pharmacodynamic target attainment of ciprofloxacin in adult patients on general wards with adequate and impaired renal function. Int J Antimicrob Agents 2020; 56: 106166. 10.1016/j.ijantimicag.2020.106166 [DOI] [PubMed] [Google Scholar]
- 29. Carlier M, Noë M, De Waele JJet al. . Population pharmacokinetics and dosing simulations of amoxicillin/clavulanic acid in critically ill patients. J Antimicrob Chemother 2013; 68: 2600–8. 10.1093/jac/dkt240 [DOI] [PubMed] [Google Scholar]
- 30. Guo T, Abdulla A, Koch BCPet al. . Pooled population pharmacokinetic analysis for exploring ciprofloxacin pharmacokinetic variability in intensive care patients. Clin Pharmacokinet 2022; 61: 869–79. 10.1007/s40262-022-01114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fournier A, Goutelle S, Que YAet al. . Population pharmacokinetic study of amoxicillin-treated burn patients hospitalized at a Swiss tertiary-care center. Antimicrob Agents Chemother 2018; 62: e00505-18. 10.1128/AAC.00505-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haeseker M, Stolk L, Nieman Fet al. . The ciprofloxacin target AUC:MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br J Clin Pharmacol 2013; 75: 180–5. 10.1111/j.1365-2125.2012.04337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zelenitsky S, Ariano R, Harding Get al. . Evaluating ciprofloxacin dosing for Pseudomonas aeruginosa infection by using clinical outcome-based Monte Carlo simulations. Antimicrob Agents Chemother 2005; 49: 4009–14. 10.1128/AAC.49.10.4009-4014.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.