Abstract
Background
Nitrofurantoin has been re-introduced as a first-choice antibiotic to treat uncomplicated acute urinary tract infections in England and Wales. Highly effective against common uropathogens such as Escherichia coli, its use is accompanied by a low incidence (<10%) of antimicrobial resistance. Resistance to nitrofurantoin is predominantly via the acquisition of loss-of-function, step-wise mutations in the nitroreductase genes nfsA and nfsB.
Objective
To explore the in situ evolution of NitR in E. coli isolates from 17 patients participating in AnTIC, a 12-month open label randomized controlled trial assessing the efficacy of antibiotic prophylaxis in reducing urinary tract infections (UTIs) incidence in clean intermittent self-catheterizing patients.
Methods
The investigation of NitR evolution in E. coli used general microbiology techniques and genetics to model known NitR mutations in NitSE. coli strains.
Results
Growth rate analysis identified a 2%–10% slower doubling time for nitrofurantoin resistant strains: NitS: 20.8 ± 0.7 min compared to NitR: 23 ± 0.8 min. Statistically, these data indicated no fitness advantage of evolved strains compared to the sensitive predecessor (P-value = 0.13). Genetic manipulation of E. coli to mimic NitR evolution, supported no fitness advantage (P-value = 0.22). In contrast, data argued that a first-step mutant gained a selective advantage, at sub-MIC (4–8 mg/L) nitrofurantoin concentrations.
Conclusion
Correlation of these findings to nitrofurantoin pharmacokinetic data suggests that the low incidence of E. coli NitR, within the community, is driven by urine-based nitrofurantoin concentrations that selectively inhibit the growth of E. coli strains carrying the key first-step loss-of-function mutation.
Introduction
Nitrofurantoin is a broad-spectrum antibiotic that has been used since the mid-1950s to manage uncomplicated urinary tract infections (UTIs).1 With the introduction of trimethoprim and modern β-lactam antibiotics its popularity waned in the 1970s.1,2 However, due to increased antimicrobial resistance the 2015 England and Wales NICE recommendations replaced trimethoprim with nitrofurantoin as the front-line antibiotic treatment for uncomplicated lower UTIs. 3 Reassuringly, increases in nitrofurantoin prescriptions have not only been associated with decreased trimethoprim resistance, but also with no apparent changes in nitrofurantoin resistance, consistently, below 10%. 4
Nitrofurantoin is effective against a variety of common uropathogenic bacterial species, including E. coli, Enterococcus spp., Enterobacter spp. and Klebsiella spp.5,6 It has been established that many of these uropathogens carry genes encoding oxygen insensitive nitroreductases, which convert nitrofurantoin into electrophilic intermediates that attack bacterial ribosomal proteins, thereby inhibiting protein synthesis and facilitating microbial death.5 When nitrofurantoin is present at high concentrations, these intermediates can also interfere in nucleic acid synthesis and aerobic metabolism targeting the citric acid cycle.5 Bacterial nitroreductases are not essential proteins, allowing for the acquisition of chromosomally derived loss-of-function mutations generating nitrofurantoin resistance (NitR).7 In E. coli, the inactivation, via deletion or point mutation, of the genes nfsA and nfsB, is the common route to NitR.7 Other pathways leading to NitR include the horizonal acquisition of the efflux system oqxAB or chromosomal mutations in ribE.8
Inactivation of nfsA and nfsB follows a two-step evolutionary pathway: nfsA before nfsB.9 Supporting this evolutionary pathway, genetic surveillance studies of NitR uncovered nfsA− nfsB+ isolates, but seldom the reciprocal nfsA+ nfsB− combination.8,10 Sandegren et al. provided insight into potential factors contributing to the low incidence of NitR among E. coli isolates suggesting the growth rate of NitR isolates to be 3%–6% slower compared to NitS isolates.11 These authors concluded there was a fitness cost to uro-associated E. coli of inactivating nfsA/nfsB.11 This loss of fitness may impact the establishment of resistant isolates in the urinary tract, explaining the low incidence of nitrofurantoin resistance among urinary E. coli isolates.11
While genome surveillance studies support NitR being linked to point mutations and deletions of the nfsA/nfsB genes,8,10 they are unable to explain the factors that drive resistance phenotype selection. Understanding these factors is key to informing prescribing guidelines that underpin UTI treatments and future urology antibiotic stewardship programmes. AnTIC was an open label randomized controlled trial that assessed the efficacy of antibiotic prophylaxis in reducing the incidence of symptomatic UTIs in clean intermittent self-catheterized patients over a period of 12 months.12 Forty-five isolates from 17 patients experiencing persistent uro-associated E. coli colonization were analysed by WGS 13 and five isolates from two patients (PAT1646 and PAT2015) supported NitR acquisition during the trial period. Clinical data associated with these cases confirmed exposure to nitrofurantoin. One case provided an opportunity to investigate the in situ evolutionary dynamics driving nitrofurantoin resistance and address aspects of the NitR phenotype that underpin the low incidence of NitR observed among community isolates of E. coli.
Materials and methods
Bacterial strains
Strains used or constructed in this study are shown in the Supplementary materials (Table S1, available as Supplementary data at JAC Online). Antibiotics for selection were used at concentrations described previously.14
pBKK plasmid construction
Five pBKK plasmids carrying different DNA constructs of the nfsA or nfsB region (nfsA 1646B, ΔnfsA, ΔnfsA-rimK, ΔrimK and ΔnfsB) were generated using Gibson assembly, with pBKK as the vector backbone. The pBKK vector was constructed using Gibson assembly, with pBlueScript II SK (Ampicillin resistant) as the vector backbone and the kanamycin resistance gene from pKD4 as the insert. All plasmids were sequenced and primers are defined in Table S2.
Mutant generation
nfsA/nfsB mutants were generated using a two-step recombination strategy involving the lambda-red and CRISPR systems.15,16 The mutagenesis protocol has been previously described.17 During CRISPR mutagenesis, cat was replaced with a DNA construct carrying a modified version of the nfsA/nfsB region (ΔnfsA, ΔnfsA-rimK, ΔrimK, nfsA T37M, ΔnfsB, ΔnfsB30). Where necessary the target mutant was amplified from the pBKK plasmids or directly from chromosomal DNA (nfsA T37M and ΔnfsB30). Double mutants were generated by first replacing the nfsA region with the appropriate DNA construct followed by the nfsB region. Confirmation of mutants was conducted through colony PCR and sequencing.
MIC and growth assay and analysis
Using standard microbiology protocols MIC and growth curve assays were performed using sterile Greiner Bio-One clear 96-well flat-bottom plates. Technical details are reported in the Supplementary material. For MIC assays, no-growth was defined as an average optical density (OD600) reading over three independent repeats as <0.05. If an OD600 >0.05 was identified, the MIC was taken as the next upper concentration. The maximum doubling time of each isolate was derived from the maximum slope calculated using a moving ‘frame’ of eight timepoints using basic R coding.18 Statistical analysis of the doubling times was performed using either t-tests or ANOVA. For t-tests, the Bonferroni correction was considered to determine the threshold P-value for significance.
Viability and competition assays
Overnight bacterial cultures were diluted to achieve a starting density of 50–100 cfu/mL in 6 mL of MHB media and incubated for 16 hours at 37°C. Cultures were plated onto CPSE plates, incubated overnight at 37°C, photographed and colonies counted with the image processing package ImageJ. Competition assays were performed using the same protocol with adjusted volumes to incorporate the addition of two strains. Visual differentiation between strains was achieved by deleting uidA, the β-glucuronidase that leads to E. coli being red on CPSE plates. The competitive index was defined as log10(strain A cfu/mL: strain B cfu/mL).
Ethical approval
Permission to use clinical isolates and data from the AnTIC clinical study was derived from prior ethical approvals (ethics 19/NS/0024, IRAS Project ID 243903, Ref. 2586/2016).13
Results
In situ development of nitrofurantoin resistance amongst clinical E. coli isolates
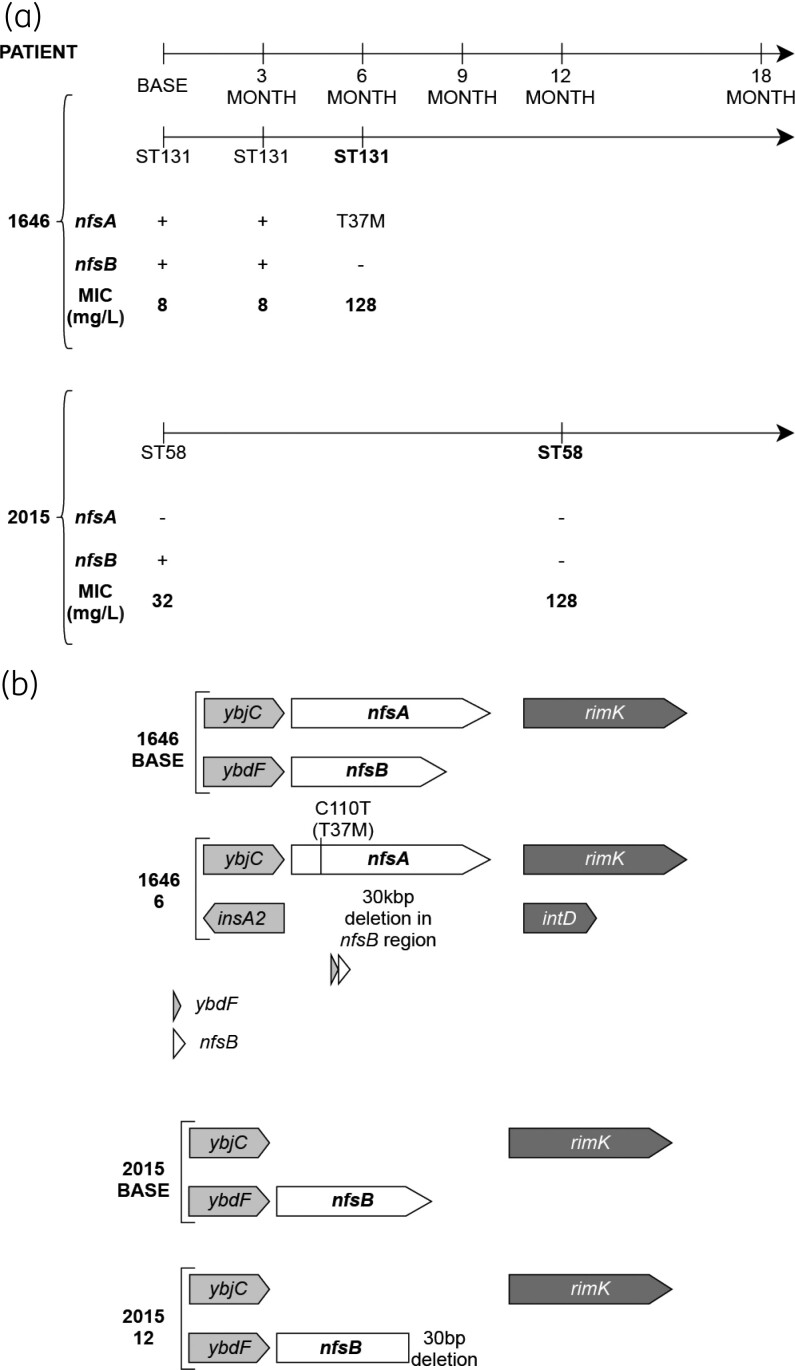
Forty-five (45) uro-associated isolates from 17 AnTIC patients with persistent E. coli colonization were previously analysed using WGS.13 Five isolates from two patients (PAT1646 and PAT2015) provided examples that supported in situ E. coli acquisition of nitrofurantoin resistance following exposure to the antibiotic. MLST and cgMLST genotyping confirmed stable E. coli colonization in both patients (Figure 1a). PAT1646 was colonized by ST131, whereas PAT2015 was colonized by ST58 (Figure S1). The NitS (MIC 8 mg/L) baseline isolate of PAT1646, 1646 BASE, possessed wild-type nfsA and nfsB genes (Figure 1b). The 6-month isolate, 1646 6, was NitR (MIC 128 mg/L: Figure 1a) and had a T37M point mutation in nfsA and a complete deletion of nfsB, spanning 30 kb upstream of the start codon. The baseline isolate of PAT2015, 2015 BASE, was NitS (MIC 32 mg/L) and carried a complete deletion of nfsA, but an intact, wild-type nfsB (Figure 1b). The 12-month isolate, 2015 12, was NitR (MIC 128 mg/L) having gained a partial deletion in nfsB (Figure 1b).
Figure 1.
Acquisition of the NitR phenotype in PAT1646 and PAT2015. (a) Timeline for each patient indicating the temporal isolation of each strain, the sequence type identified via MLST genotyping and the MIC for each isolate. (b) nfsA and nfsB genotype for the BASE and NitR isolates from PAT1646 and PAT2015.
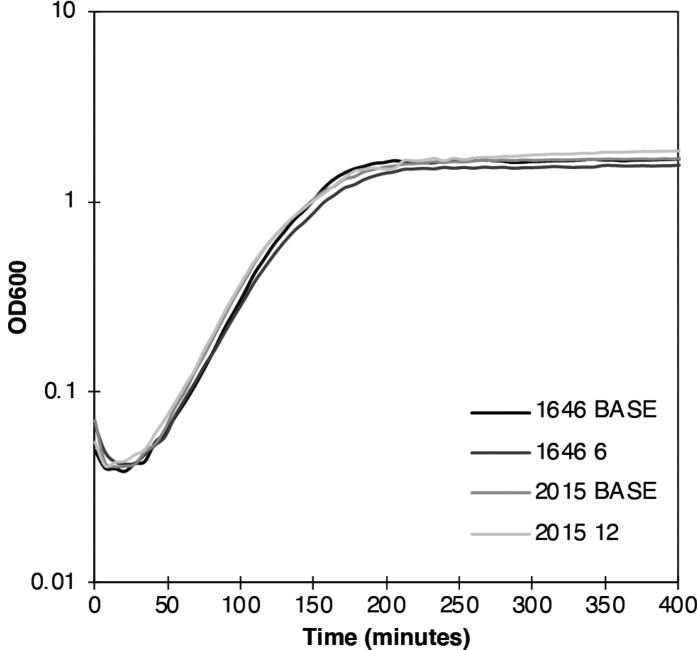
Growth kinetics of the BASE strains, and isolates 1646 6 and 2015 12, resulted in comparable growth curves (Figure 2), which suggested no difference in bacterial fitness. Calculation of the doubling time were comparable for all four strains: 1646 BASE: 20.8 ± 0.7 min; 1646 6 23 ± 0.8 min; 2015 BASE: 21.0 ± 0.7 and 2015 12: 21.4 ± 0.7 (ANOVA P-value = 0.13). In the absence of antibiotic, competition between 1646 BASE and 1646 6 was minimal, reflected by a calculated competitive index of 0.22 ± 0.16. These data therefore argued against a fitness advantage playing a significant role in driving the acquisition of NitR in UTI patients treated with nitrofurantoin.
Figure 2.
Growth characteristics of the BASE and NitR isolates from PAT1646 and PAT2015. The differences between the doubling times are consistent with previous studies of NitR but are not statistically significant (ANOVA P = 0.22). All data was calculated from n = 24 independent repeats of growth of each strain.
Generating nfsA and nfsB mutants from NitS isolates
PAT1646 E. coli isolates represent a chronological ‘snapshot’ of the same strain before and after developing nitrofurantoin resistance (Figure 1). To investigate the underlying advantage/disadvantage of acquiring the NitR phenotype, mutants in nfsA and/or nfsB, were generated in 1646 BASE and the NitS laboratory E. coli strain W3110, a commonly used ‘wild-type’ model strain (Figure S2).19 Comparing two isolates of contrasting genetic backgrounds (clinical versus laboratory), provided an opportunity to identify phenotypic effects, if any, of ΔnfsA/ΔnfsB combinations that were dependent or independent of genetic background. Mutants were generated by targeted mutagenesis rather than in vitro selection to minimize the acquisition of spontaneous, compensatory mutations linked to nitrofurantoin exposure, which could distort the phenotypic behaviour of ΔnfsA/ΔnfsB mutations.
rimK is in an operon with nfsA (Figure 1b) and encodes an enzyme involved in the post-translational modification of ribosomal protein S6 via the addition of glutamate residues.20 There is evidence of clinical NitR isolates carrying partial deletions in rimK, therefore ΔrimK and ΔnfsA-rimK mutations were also created (Figure S2).13 The flexibility of CRISPR–Cas technology allowed 1646 BASE to be engineered to model the impacts of the 1646 6 nfsA mutation (nfsA T37M) and the 30 kb nfsB deletion (ΔnfsB30). However, only nfsA T37M was modelled in W3110 (Figure S2).
Characterization of ΔnfsA/ΔnfsB mutants
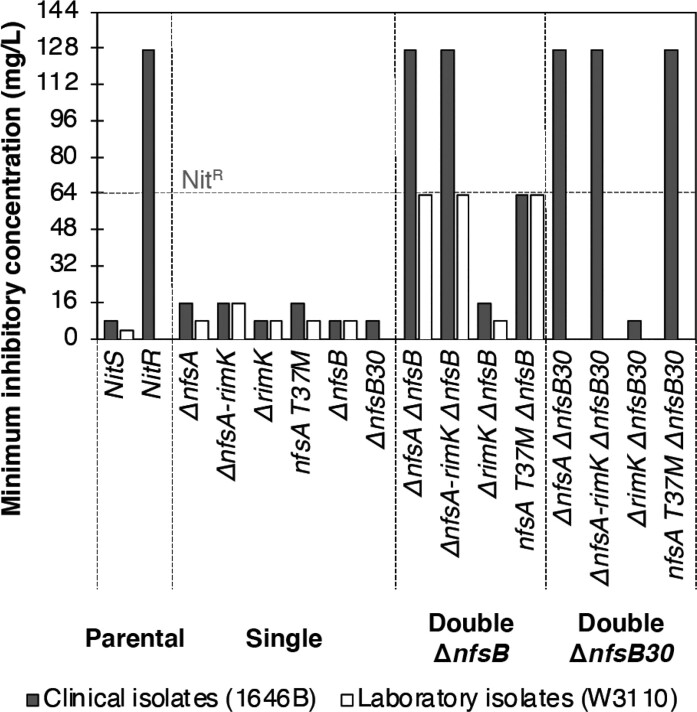
The NitR phenotypes of the strains generated were quantitatively assessed via MIC assays (Figure 3). Isolates with a MIC ≥ 64 mg/L were classified as NitR21,22 and strains 1646 BASE, W3110 and 1646 6 were included as NitS and NitR controls. Single 1646B mutants with ΔnfsA or nfsA T37M supported MIC values of 32 mg/L thus still clinically NitS (Figure 3). Single mutants ΔrimK, ΔnfsB and ΔnfsB30 showed a MIC comparable to their parental background. A similar pattern was observed for the W3110 although the NitS MIC of the parent and nfsA+ variants was 8 mg/L (Figure 3).
Figure 3.
MIC of ΔnfsA and ΔnfsB mutant combinations in 1646 BASE (filled bars) and the laboratory strain W3110 (open bars). The dashed horizontal line indicates the nitrofurantoin concentration defined as being the point at which strains are defined as clinically resistant to the antibiotic. Data represent a minimum of three independent repeats of the assay for each strain. MIC values represent the nitrofurantoin concentration where the average OD600 reached <0.05.
In vitro 1646 6 and its corresponding engineered mutant (nfsA T37M ΔnfsB) showed MICs of ≥64 mg/L respectively. In contrast, 1646 BASE double mutants in which nfsA was inactivated via complete deletion (ΔnfsA ΔnfsB, ΔnfsA-rimK ΔnfsB) displayed higher MICs (128 mg/L) (Figure 3). The MICs of the nfsA+ ΔrimK ΔnfsB variants were comparable to their single and parental derivatives (16 or 8 mg/L). Collectively, these data indicated that only single mutants with inactivated nfsA had a modest 2-fold MIC increase when compared to their parental isolate while double mutants, including ΔnfsA, had substantially higher MICs.
Plasmid-based complementation of NitR ΔnfsA ΔnfsB mutants
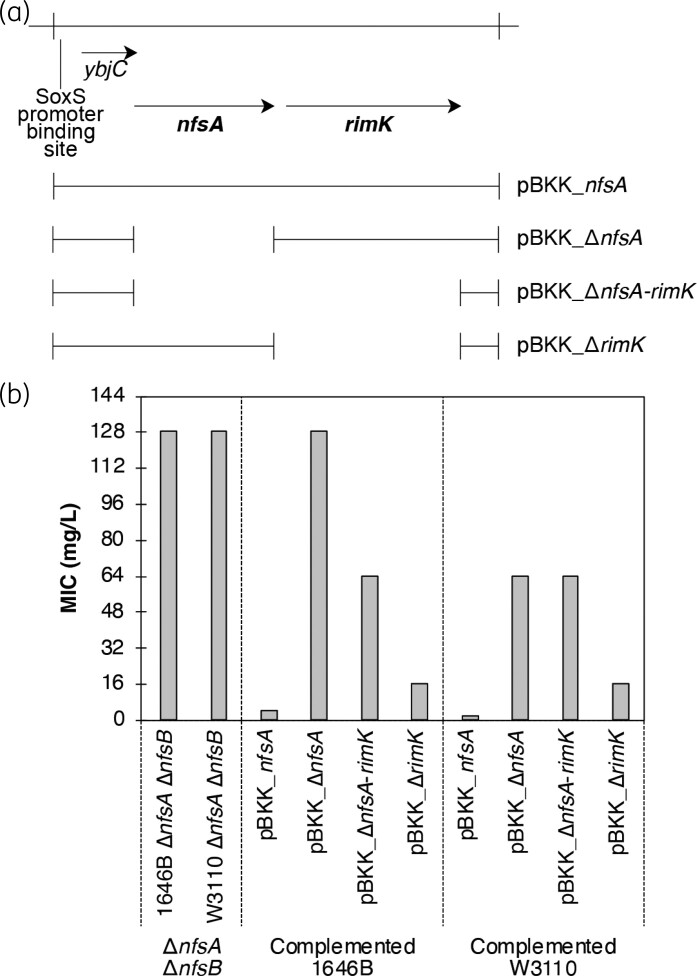
To verify that inactivating nfsA was critical in the acquisition of NitR, the engineered 1646 BASE and W3110 ΔnfsA ΔnfsB mutants were transformed with modified pBlueScript II SK (pBKK) plasmids carrying genetic variants of the nfsA region (Figure 4a). The pBKK empty vector was used as a negative control. MIC assays were performed on transformed strains (Figure 4b). Complementation was only observed when pBKK plasmids carried functional nfsA. Consistent with the role that nfsA plays in the NitR phenotype, complementation led to a strong 32-fold reduction in MIC compared to the negative control (Figure 4b).
Figure 4.
Complementation of ΔnfsA with ectopic expression of nfsA from pBKK. (a) Schematic diagram of plasmid constructs used in these complementation assays. (b) MIC data for strains used. Data represent a minimum of three independent repeats of the assay for each strain. MIC values represent the nitrofurantoin concentration where the average OD600 reached <0.05.
Fitness of nfsA/nfsB mutants
The relative fitness of the mutants was determined by maximum bacterial growth rate in terms of doubling time, with an assumed increase in fitness corresponding to shorter doubling times. In the absence of nitrofurantoin (0 mg/L), there was no significant difference in doubling time between any of the mutants and their corresponding parental isolates (ANOVA 1646 BASE: P = 0.22; W3110: P = 0.46) (Tables 1 and 2). Pairwise t-test analysis using a Bonferroni corrected significance threshold of P = 0.0009 agreed with the ANOVA analysis (Tables S3 and S4). These data are consistent with the initial growth analysis of 1646 and 2015 isolates (Figure 2)
Table 1.
Doubling times of mutants and clinical isolates relating to PAT1646 with or without nitrofurantoin
| Strains | Nitrofurantoin concentration (mg/L)a,b | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| 1646 BASE | 21.9 ± 0.9 | 24.7 ± 0.7 | 29.3 ± 1.1 | 43.2 ± 1.8 | ||||
| 1646 6 | 25.7 ± 1.1 | 25.1 ± 0.6 | 24.5 ± 1.2 | 25.3 ± 1.1 | 27.9 ± 0.9 | 35.2 ± 1 | 142 ± 11.3 | |
| 1646B ΔnfsA | 24.2 ± 0.6 | 24.3 ± 0.9 | 23.6 ± 1 | 31 ± 2 | 60.1 ± 1.6 | |||
| 1646B ΔnfsA-rimK | 23.7 ± 0.9 | 26.2 ± 1 | 25.4 ± 1.3 | 32.2 ± 1.9 | 64.7 ± 2.4 | |||
| 1646B nfsA T37M | 22.5 ± 0.8 | 24.2 ± 0.9 | 24.1 ± 1 | 30.3 ± 2.1 | 55.4 ± 2.2 | |||
| 1646B ΔrimK | 24.1 ± 1.2 | 25.3 ± 0.4 | 28.3 ± 1.3 | 45.9 ± 1.4 | ||||
| 1646B ΔnfsB | 22.2 ± 1.1 | 25.9 ± 0.8 | 29.6 ± 1 | 45 ± 0.9 | ||||
| 1646B ΔrimK ΔnfsB | 21.8 ± 1.8 | 25.3 ± 1.2 | 30.5 ± 0.8 | 43.7 ± 1.6 | ||||
| 1646B ΔnfsA ΔnfsB | 25.7 ± 0.4 | 25.6 ± 0.9 | 25 ± 0.5 | 24.3 ± 1 | 26.6 ± 0.3 | 34.2 ± 1.3 | 90.5 ± 5.9 | |
| 1646B ΔnfsA-rimK ΔnfsB | 21.9 ± 1.6 | 23.4 ± 0.8 | 22.6 ± 0.7 | 23.4 ± 1.3 | 27.7 ± 0.9 | 33.7 ± 0.8 | 88 ± 3.4 | |
| 1646B nfsA T37M ΔnfsB | 24.2 ± 0.8 | 25 ± 1.1 | 24.7 ± 0.5 | 27.7 ± 1 | 28.7 ± 0.5 | 37.6 ± 0.9 | 131.6 ± 10.8 | |
Italics and bold identify significant changes in the doubling time (Table S3).
Data relate to growth, while the MIC is defined as the first concentration having an OD600 < 0.05.
Table 2.
Doubling times of mutants and the parental strain W3110 with or without nitrofurantoin
| Strains | Nitrofurantoin concentration (mg/L)a,b | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | |
| W3110 | 34.9 ± 0.8 | 37.8 ± 1 | 37.7 ± 1.2 | 74.7 ± 2.9 | ||||
| W3110 ΔnfsA | 32.7 ± 1.7 | 35.4 ± 1.2 | 37.5 ± 1.8 | 50.9 ± 2.6 | ||||
| W3110 ΔnfsA-rimK | 33.1 ± 1.3 | 35.3 ± 1.1 | 40.4 ± 1.6 | 50.4 ± 2.1 | ||||
| W3110 nfsA T37M | 29 ± 2.3 | 35.8 ± 1.1 | 38.8 ± 1.3 | 51.9 ± 2.4 | ||||
| W3110 ΔrimK | 33.8 ± 1.2 | 40.3 ± 1.9 | 44.4 ± 2 | 79.5 ± 5.6 | ||||
| W3110 ΔnfsB | 31.5 ± 3.1 | 34.9 ± 1.7 | 41.5 ± 1.4 | 73.7 ± 3.2 | ||||
| W3110 ΔrimK ΔnfsB | 35.8 ± 2.1 | 37.3 ± 1.4 | 39.9 ± 2.1 | 72 ± 4.3 | ||||
| W3110 ΔnfsA ΔnfsB | 31 ± 1.8 | 33.9 ± 0.8 | 36 ± 2.1 | 44.6 ± 1.6 | 39 ± 2.7 | 54.4 ± 1.9 | 117.2 ± 5.8 | |
| W3110 ΔnfsA-rimK ΔnfsB | 32.5 ± 1 | 33.4 ± 0.9 | 34 ± 2 | 37.4 ± 1.6 | 32.8 ± 2.1 | 50.8 ± 1.4 | 97 ± 7.7 | |
| W3110 nfsA T37M ΔnfsB | 34.4 ± 0.5 | 37.7 ± 1.3 | 38.2 ± 2 | 44.1 ± 0.8 | 40.3 ± 1.3 | 56.7 ± 1.5 | ||
Italics and bold identify significant changes in the doubling time (Table S4).
Data relate to growth, while the MIC is defined as the first concentration having an OD600 < 0.05.
ANOVA analysis indicated that at 4 and 8 mg/L nitrofurantoin, there was significance within the bacterial growth data sets (P-value range <0.0001–0.02) (Tables 1 and 2) with pairwise comparisons using t-tests identifying specific trends. For example, at 4 mg/L nitrofurantoin an increase in doubling time from 21.9 ± 0.9 to 29.3 ± 1.1 min was observed for the parental strain 1646 BASE with comparable increases for nfsB and rimK single mutants (Table 1). This increase became statistically significant across all comparisons at 8 mg/L where a doubling time of 43–46 min was observed (Table 1: italics + bold and Table S3). The same trend was observed for W3110, which already had a lower intrinsic resistance to nitrofurantoin (Table 2 and Table S4). At concentrations >8 mg/L, most of these strains were unable to grow indicating that bacterial selection, rather than fitness, was the driving factor.
Similar data for the ΔnfsA, double mutants and the control strain 1646 6 was observed at higher concentrations of nitrofurantoin (Table 1). Interestingly, the higher the concentration of antibiotic, the stronger the impact on growth with, for example, 64 mg/L leading to a 5-fold increase in doubling time for 1646 6. The 1646 BASE ΔnfsA ΔnfsB variant exhibited a 3.5-fold increase in doubling time at this concentration, consistent with the difference defined by the MIC analysis.
Viability of nfsA/nfsB mutants
Growth experiments were initiated with an inoculation of between 0.01–0.05 OD600. This inoculum is equivalent to ∼1–5 × 106 cfu/mL, 1 log greater than the diagnostic threshold of microbes detected per millilitre of urine in an acute UTI (1 × 105 cfu/mL).23 It is well recognized that growth experiments can be influenced by the inoculum effect.24 Therefore, further growth analysis using a starting inoculum of 50–100 cfu/mL in 0 to 16 mg/L nitrofurantoin was investigated focussing on the viability of the 1646 BASE engineered strains rather than growth kinetics.
All strains tested grew well with or without 2 mg/L nitrofurantoin (Table 3). The 1646 BASE strain did not grow at 4 mg/L while the ΔnfsB mutant showed a significant (∼6 log-fold) reduction in viability (Table 3). Furthermore, single mutants of ΔnfsA and nfsA T37M behaved in a similar manner, but at double the antibiotic concentration i.e. 8 mg/L. All strains that possessed a NitR phenotype grew well at all nitrofurantoin concentrations tested. These data strengthen the argument for selective advantage over fitness for step-wise nfsA− intermediates.
Table 3.
Viability of test strains in low inoculum growth experiments with sub-MIC concentrations of nitrofurantoin
| Strains | Nitrofurantoin concentration (mg/L) | ||||
|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 16 | |
| 1646 BASE | 1.05E + 09 | 1.50E + 08 | 0 | 0 | 0 |
| 1646 6 | 6.67E + 08 | 4.03E + 08 | 4.63E + 08 | 2.88E + 08 | 1.56E + 08 |
| ΔnfsA | 8.37E + 08 | 1.83E + 08 | 3.13E + 08 | 1083 | 0 |
| nfsA T37M | 7.20E + 08 | 5.13E + 08 | 4.36E + 08 | 737 | 0 |
| ΔnfsB | 1.22E + 09 | 3.87E + 08 | 627 | 0 | 0 |
| ΔnfsA ΔnfsB | 5.47E + 08 | 2.80E + 08 | 2.53E + 08 | 2.62E + 08 | 9.67E + 07 |
| nfsA T37M ΔnfsB | 6.80E + 08 | 2.73E + 08 | 4.50E + 08 | 2.96E + 08 | 1.63E + 08 |
Discussion
High level nitrofurantoin resistance requires the inactivation of two genes, which are located so far apart from each other (287 kb distance) that the likelihood of both being simultaneously inactivated through a single natural genetic event is non-existent.9 Hence inactivation of these genes is far more likely to occur in a step-wise manner, starting with nfsA followed by nfsB.
To explore the evolutionary mechanism(s) underpinning antibiotic resistance NitS and NitR uro-associated E. coli isolates recovered from UTIs patients treated with nitrofurantoin were exploited. These strains were unique as they reflected in situ evolution of E. coli over 6 to 12 months in a clinical environment from NitSnfsAB+ (PAT1646) or nfsA−nfsB+ (PAT2015) genotypes to resistant genotypes.12,13 Consistent with the literature, the predominant mutations leading to NitR were deletions, although 1646 BASE to 1646 6 evolved NitR via the acquisition of a point mutation in nfsA: T37M. MIC and growth data provided strong evidence that T37M significantly reduced or inactivated NfsA. However, the behaviour of nfsA T37M ΔnfsB variants with respect to growth in sub-MIC conditions argues a low level of activity may be retained (Tables 1 and 2). Data also suggested that the method by which nfs genes were inactivated (ΔnfsA, nfsAT37M, ΔnfsB, ΔnfsB30) did not alter nitrofurantoin resistance with ≥64 mg/L MIC being observed for all combinations. Essentially, gene inactivation per se was more important than the mode of inactivation.
Sandegren et al. argued that fitness of NitR strains plays a key role in driving the low incidence of community AMR to nitrofurantoin.11 While the bacterial growth data reported in this study were consistent with Sandegren et al., in that a small change in doubling times (2%–10%) was observed, statistical analysis (ANOVA and pairwise t-tests) argued that these growth changes were not significant and, alone, could not explain AMR. Moreover, growth analysis relating to 1646 BASE and W3110 strains and their genetic variants in the presence of sub-MIC Nitrofurantoin concentration supports our conclusion against fitness playing a major role in NitR acquisition rates (Tables 1 and 2). Bacterial resistance has traditionally been linked to the ability of isolates to grow in antibiotic concentrations that exceed the MIC of sensitive isolates. However, several studies have isolated resistant mutants from growth conditions where antibiotic concentrations were low enough for the proliferation of sensitive isolates.25–27
Sub-MIC selective pressure may be a key factor in driving antibiotic resistant phenotypes to successfully establish themselves within a population. However, selective pressures will be determined not only by the pharmacodynamics of an antibiotic, but also by the genetic background of a bacterial species/strain. Findings reported here suggest that the nitrofurantoin selective window in relation to UTIs and uro-associated E. coli NitR, is wide (>8 mg/L), but this argument is only applicable when comparing NitR double mutants against their NitS parental isolates. From an evolutionary perspective, this comparison is biased as data suggests the emergence of NitR mutants is highly dependent on the ability of intermediate mutants with low level nitrofurantoin resistance to establish themselves within a population of sensitive isolates. In fact, comparing the growth of mutants (ΔnfsA, nfsA T37M) modelling these intermediate scenarios to their parental isolates (1646 BASE and W3110), argues for a very narrow selective window (4–8 mg/L). Despite these strong selective pressures favouring growth, bacteria may still fail to establish themselves in the urinary environment due to bladder voiding.28
Clinically, urinary nitrofurantoin concentrations rarely fall to within this narrow concentration range, which limits the selection of nfsA mutants.29 For example, extrapolating the urinary nitrofurantoin concentration data from Huttner et al. suggests the average urine concentration following a standard 50-mg dose may fluctuate between 20 and 40 mg/L during an 8-hour period.29 Essentially, these conditions inhibit intermediate mutants such as ΔnfsA and nfsA T37M establishing clinically, which in turn suppresses the emergence of double and hence NitR mutants. Therefore, the rarity of NitR intermediate mutants undermines the emergence of nitrofurantoin resistance in E. coli, explaining the low incidence of resistance.
Supplementary Material
Acknowledgements
The authors would like to thank all participants of the original AnTIC trial who agreed to their samples being banked for further analyses.
Contributor Information
Maxime Vallée, Biosciences Institute, Faculty of Medical Sciences, Newcastle University, UK; Department of Urology, Poitiers University Hospital, 2 Rue de la Milétrie, 86021 Poitiers, France.
Chris Harding, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, UK; Urology Department, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, UK.
Judith Hall, Biosciences Institute, Faculty of Medical Sciences, Newcastle University, UK.
Phillip D Aldridge, Biosciences Institute, Faculty of Medical Sciences, Newcastle University, UK.
Aaron TAN, Biosciences Institute, Faculty of Medical Sciences, Newcastle University, UK.
Funding
The AnTIC trial was funded by NIHR HTA (no. 11/72/01). Direct funding for this project has included support for M.V. through European Association of Urology and Association Française D’Urologie Funding; EAU (Ref. ESUP/Scholarship S-02-2018) and AFU (Ref. Bourse AFU 2017). A.T. was a self-funded PhD thesis aided by a Newcastle University Overseas Research Scholarship. C.H. reiceves funding outside the scope of this work from UK National Institute of Health Research and The Urology Foundation.
Transparency declaration
A.T., J.H. and P.D.A have no conflicts of interest to declare. M.V. has acted as a consultant for GSK outside the focus of this work, has received speakers fees from Astellas, IPSEN, Mylan and is on an Advisory board for IBSA PHARMA. C. H. has received speakers fees from Astellas, Allergan and Medtronic and is on Advisory boards for Astellas, Teleflex Medical, GSK, Viatris and Convatec. C.H. is also a member of the European Association of urology Female LUTS committee (chair) and the ICS Urodynamic, Mesh complications and Male LUTS committee.
Supplementary data
Figures S1 and S2 and Tables S1 to S4 are available as Supplementary data at JAC online.
References
- 1. Huttner A, Verhaegh EM, Harbarth Set al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70: 2456–64. 10.1093/jac/dkv147 [DOI] [PubMed] [Google Scholar]
- 2. Muller AE, Verhaegh EM, Harbarth Set al. Nitrofurantoin’s efficacy and safety as prophylaxis for urinary tract infections: a systematic review of the literature and meta-analysis of controlled trials. Clin Microbiol Infect 2017; 23: 355–62. 10.1016/j.cmi.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence . NICE Guideline [NG109] Urinary tract infection (lower): antimicrobial prescribing 2018.https://www.nice.org.uk/guidance/ng109.
- 4. Hammond A, Stuijfzand B, Avison MBet al. Antimicrobial resistance associations with national primary care antibiotic stewardship policy: primary care-based, multilevel analytic study. PLoS ONE 2020; 15: e0232903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother 1994; 33(Suppl A): 23–30. 10.1093/jac/33.suppl_A.23 [DOI] [PubMed] [Google Scholar]
- 6. Farfour E, Dortet L, Guillard Tet al. Antimicrobial resistance in Enterobacterales recovered from urinary tract infections in France. Pathogens 2022; 11: 356. 10.3390/pathogens11030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCalla DR, Kaiser C, Green MH. Genetics of nitrofurazone resistance in Escherichia coli. J Bacteriol 1978; 133: 10–6. 10.1128/jb.133.1.10-16.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wan Y, Mills E, Leung RCYet al. Alterations in chromosomal genes nfsA, nfsB, and ribE are associated with nitrofurantoin resistance in Escherichia coli from the United Kingdom. Microb Genom 2021; 7. 000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiteway J, Koziarz P, Veall Jet al. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J Bacteriol 1998; 180: 5529–39. 10.1128/JB.180.21.5529-5539.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Zhang Y, Wang Fet al. Unravelling mechanisms of nitrofurantoin resistance and epidemiological characteristics among Escherichia coli clinical isolates. Int J Antimicrob Agents 2018; 52: 226–32. 10.1016/j.ijantimicag.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 11. Sandegren L, Lindqvist A, Kahlmeter Get al. Nitrofurantoin resistance mechanism and fitness cost in Escherichia coli. J Antimicrob Chemother 2008; 62: 495–503. 10.1093/jac/dkn222 [DOI] [PubMed] [Google Scholar]
- 12. Fisher H, Oluboyede Y, Chadwick Tet al. Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial. Lancet Infect Dis 2018; 18: 957–68. 10.1016/S1473-3099(18)30279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mowbray C, Tan A, Vallée Met al. Multidrug-resistant uro-associated Escherichia coli populations and recurrent urinary tract infections in patients performing clean intermittent self-catheterisation. European Urology Open Science 2022; 37: 90–8. 10.1016/j.euros.2021.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonifield HR, Hughes KT. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J Bacteriol 2003; 185: 3567–74. 10.1128/JB.185.12.3567-3574.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Y, Chen B, Duan Cet al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol 2015; 81: 2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 2000; 97: 6640–5. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sim M, Koirala S, Picton Det al. Growth rate control of flagellar assembly in Escherichia coli strain RP437. Sci Rep 2017; 7: 41189. 10.1038/srep41189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall BG, Acar H, Nandipati Aet al. Growth rates made easy. Mol Biol Evol 2014; 31: 232–8. 10.1093/molbev/mst187 [DOI] [PubMed] [Google Scholar]
- 19. Hayashi K, Morooka N, Yamamoto Yet al. Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Mol Syst Biol 2006; 2: 2006.0007. 10.1038/msb4100049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao G, Jin Z, Wang Yet al. Structure and function of Escherichia coli RimK, an ATP-grasp fold, L-glutamyl ligase enzyme. Proteins 2013; 81: 1847–54. 10.1002/prot.24311 [DOI] [PubMed] [Google Scholar]
- 21. Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 2001; 48(Suppl 1):5–16. 10.1093/jac/48.suppl_1.5 [DOI] [PubMed] [Google Scholar]
- 22. Rodloff A, Bauer T, Ewig Set al. Susceptible, intermediate, and resistant—the intensity of antibiotic action. Dtsch Arztebl Int 2008; 105: 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113(Suppl 1A):5S–13S. 10.1016/S0002-9343(02)01054-9 [DOI] [PubMed] [Google Scholar]
- 24. Smith KP, Kirby JE. The inoculum effect in the era of multidrug resistance: minor differences in inoculum have dramatic effect on MIC determination. Antimicrob Agents Chemother 2018; 62: e000433–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nature Reviews Microbiology 2014; 12: 465–478. 10.1038/nrmicro3270 [DOI] [PubMed] [Google Scholar]
- 26. Hughes D, Andersson DI. Selection of resistance at lethal and non-lethal antibiotic concentrations. Curr Opin Microbiol 2012; 15: 555–60. 10.1016/j.mib.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 27. Andersson DI, Hughes D. Evolution of antibiotic resistance at non-lethal drug concentrations. Drug Resist Updat 2012; 15: 162–72. 10.1016/j.drup.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 28. Sintsova A, Frick-Cheng AE, Smith Set al. Genetically diverse uropathogenic Escherichia coli adopt a common transcriptional program in patients with UTIs. Elife 2019; 8: e49748. 10.7554/eLife.49748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huttner A, Wijma RA, Stewardson AJet al. The pharmacokinetics of nitrofurantoin in healthy female volunteers: a randomized crossover study. J Antimicrob Chemother 2019; 74: 1656–61. 10.1093/jac/dkz095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.