In immunocompromised hosts, coronavirus disease 2019 (COVID-19) can span from asymptomatic to severe life-threatening disease. Moreover, chronic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inducing prolonged viral shedding and persistent and/or relapsing symptoms has been reported in these patients.1,2
In chronic SARS-CoV-2 infection, off-label use of remdesivir, convalescent plasma and/or SARS-CoV-2-specific monoclonal antibodies (mAbs) has been anecdotally attempted to reduce disease severity and to achieve viral clearance, with variable results.3,4
Nirmatrelvir/ritonavir is an oral antiviral, which has been proven to reduce by 89% the risk of progression to severe COVID-19 among high-risk patients with early-stage, symptomatic COVID-19.5 Early use of nirmatrelvir/ritonavir has also been shown to shorten time to viral clearance in patients who are immunocompromised and hospitalized with COVID-19.6
To date, there has been limited experience regarding nirmatrelvir/ritonavir efficacy in patients with prolonged and/or relapsing SARS-CoV-2 infection.7 Here we describe three cases of relapsing COVID-19 at the Careggi University Hospital, Florence, Italy, in patients undergoing anti-CD20 immunosuppressant therapy, who showed a successful clinical, virological and radiological response to nirmatrelvir/ritonavir treatment.
Patient 1
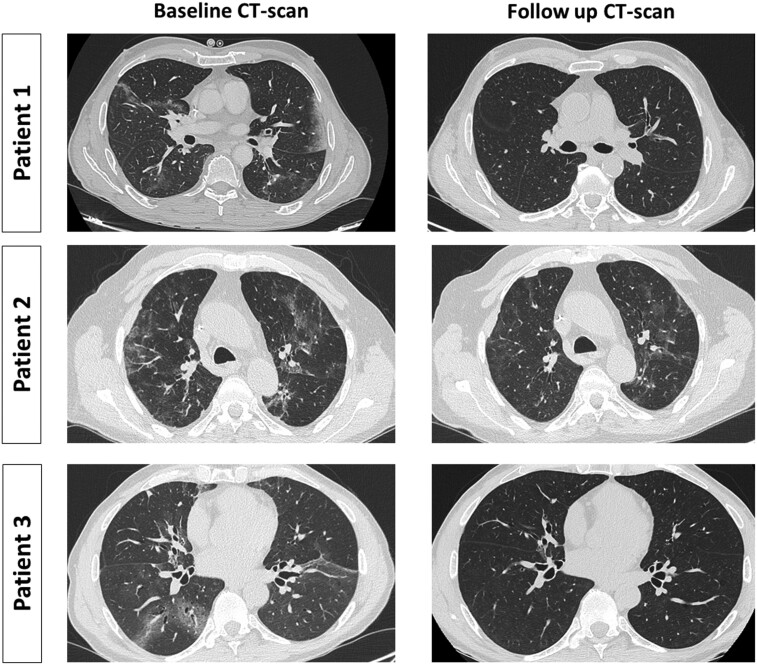
A 62-year-old Caucasian male with non-Hodgkin lymphoma on anti-CD20 therapy with obinutuzumab developed mild SARS-CoV-2 infection in December 2021, reporting fever, cough and myalgia. He was treated with sotrovimab, with a rapid clinical improvement. Five months later he presented to the hospital with a 10 day history of fever and dyspnoea; antigenic testing of a nasopharyngeal swab (NPS) at admission was negative. A CT scan showed bilateral ground glass opacities (GGOs) and PCR detected SARS-CoV-2 RNA in a bronchoalveolar lavage (BAL). IgG antibodies against the SARS-CoV-2 spike protein (S) (LIAISON® SARS-CoV-2 S1/S2 IgG, DiaSorin Inc., USA) were positive [154 binding antibody units (BAU)/mL], likely due to the previous infusion of mAbs. A central line-associated bloodstream infection was initially suspected but a complete microbiological work up did not identify infective foci. Despite multiple empirical antimicrobial regimens, including ceftriaxone, piperacillin/tazobactam, vancomycin and caspofungin, intermittent fever continued for 21 days, leading to discontinuation of antimicrobials and consideration of an off-label treatment with nirmatrelvir/ritonavir. Fever disappeared 24 h after the first nirmatrelvir/ritonavir dose, along with a rapid improvement of the respiratory parameters. The follow-up CT-scan 1 month later documented complete GGO resolution (Figure 1).
Figure 1.
Comparison of CT scan findings pre- and post-treatment with nirmatrelvir/ritonavir. Follow up CT scan performed 1 month after treatment conclusion showed complete resolution of GGOs in Patient 1 and Patient 3, and significant improvement in Patient 2. Radiological findings were consistent with the successful clinical response to antiviral drugs in all three cases.
Patient 2
A 76-year-old Caucasian male on anti-CD20 treatment with rituximab due to non-Hodgkin lymphoma experienced moderate COVID-19 in February 2022 with fever, dyspnoea, cough and radiological evidence of interstitial pneumonia, requiring hospitalization and low-flow oxygen supplementation. While hospitalized he received a 5 day remdesivir course and off-label sotrovimab infusion, owing to his seronegative status for anti-S IgG. Within 10 days he was discharged home. Three months later he presented to the hospital with fever, dyspnoea and cough. Radiological CT study showed GGOs; PCR on NPS and BAL detected SARS-CoV-2, while anti-S IgG dosage was still negative. Nirmatrelvir/ritonavir treatment was started and fever disappeared 24 h after the first dose; NPS for SARS-CoV-2 yielded a negative result 5 days after antiviral treatment conclusion and a follow-up CT scan 1 month later showed significant GGO reduction (Figure 1).
Patient 3
A 59-year-old Caucasian male with ongoing mycophenolate and low-dose steroids with a previous 2 year course of rituximab for eosinophilic granulomatosis with polyangiitis suffered from severe COVID-19 on January 2022, requiring non-invasive ventilation. During the hospital stay he was treated with remdesivir and tocilizumab. After a 30 day hospitalization he was discharged with complete symptom resolution. Four months later, while SARS-CoV-2 RNA was still detectable on NPS, he complained of dyspnoea; a CT scan reported new GGO appearance. Anti-S IgG was still undetectable at that time. He received a complete course of nirmatrelvir/ritonavir and sotrovimab infusion; after 14 days from treatment conclusion, SARS-CoV-2 RNA on NPS yielded a negative result, while 1 month later a CT scan showed complete resolution of GGOs (Figure 1).
In all three cases, infections were caused by the Omicron variant (B.1.1.529 lineage). Further genomic characterization demonstrated that latter episodes were sustained by sublineage BA.1, which had almost disappeared in Italy (<1% prevalent) when they occurred (May to June 2022), thus suggesting that they were due to SARS-CoV-2 relapse rather than reinfection.8 Complete demographics and clinical information about the three patients are reported in Table S1 (available as Supplementary data at JAC Online).
Although infections with the Omicron variant have been associated with reduced morbidity and mortality, immunocompromised patients remain at higher risk of severe COVID-19 outcomes, hospitalization and prolonged symptoms.9,10 In these patients, the humoral and cellular immune response after vaccination is reduced, and protection against severe COVID-19 is lower than in the general population.11 Antiviral agents proved their efficacy against COVID-19 when used in the first days after symptoms onset, during the viral replication phase.5 However, viable SARS-CoV-2 can persist for several months in immunocompromised hosts,1,2,12 thus warranting a late use of antiviral drugs in this population. Notably, in our cases, COVID-19 relapse occurred despite initial treatment with remdesivir and/or sotrovimab. At the time of writing, 5–6 months after nirmatrelvir/ritonavir administration, we have no evidence of further COVID-19 relapse in these patients.
Pending further studies on larger cohorts, the impressive response observed in the reported cases suggests that oral antiviral drugs may also have a role beyond the early stage of COVID-19, for the treatment of persistent and/or relapsing COVID-19 in immunocompromised hosts.
Ethics
The study was conducted in accordance with the Declaration of Helsinki. The patients provided signed informed consent for publication.
Supplementary Material
Contributor Information
Lucia Graziani, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Leonardo Gori, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Tommaso Manciulli, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Gregorio Basile, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Irene Campolmi, Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy.
Beatrice Borchi, Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy.
Marta di Dio, Internal Medicine Unit 2, Careggi University Hospital, Florence, Italy.
Marta Mattei, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Greta Ciurleo, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Maria Ciliberti, Internal Medicine Unit 2, Careggi University Hospital, Florence, Italy.
Francesca Malentacchi, Microbiology and Virology Unit, Careggi University Hospital, Florence, Italy.
Marco Coppi, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy.
Alessandro Morettini, Internal Medicine Unit 2, Careggi University Hospital, Florence, Italy.
Paola Parronchi, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Immunology and Cell Therapy Unit, Careggi University Hospital, Florence, Italy.
Gian Maria Rossolini, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Microbiology and Virology Unit, Careggi University Hospital, Florence, Italy.
Alessandro Bartoloni, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy.
Sara Tomassetti, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Interventional Pneumology Unit, Careggi University Hospital, Florence, Italy.
Michele Spinicci, Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy.
Funding
This work was supported by funds from the Ministry of Education, University and Research (Italy) Excellence Departments 2018–2022 (Project for the Department of Experimental and Clinical Medicine).
Transparency declarations
None to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC Online.
References
- 1. Choi B, Choudhary MC, Regan Jet al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 2020; 383: 2291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spinicci M, Mazzoni A, Coppi Met al. Long-term SARS-CoV-2 asymptomatic carriage in an immunocompromised host: clinical, immunological, and virological implications. J Clin Immunol 2022; 42: 1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Helleberg M, Niemann CU, Moestrup KSet al. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020; 222: 1103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark E, Guilpain P, Filip ILet al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol 2020; 190: e154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hammond J, Leister-Tebbe H, Gardner Aet al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386: 1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun F, Lin Y, Wang Xet al. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis 2022; 22: 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pérez Catalán I, García Muñoz S, Roig Martí Cet al. Nirmatrelvir/ritonavir as a potential treatment for prolonged SARS-CoV-2 infection in immunocompromised patients. Rev Esp Chemother 2022; 35: 589–91. Article in Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Istituto Superiore di Sanità . Prevalenza e distribuzione delle varianti di SARSCoV2 di interesse per la sanità pubblica in Italia. Rapporto n. 21 del 1 luglio 2022 (dati aggiornati al 27 giugno 2022). https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-1-luglio-2022.pdf.
- 9. Nyberg T, Ferguson NM, Nash SGet al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399: 1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malahe SRK, Hoek RAS, Dalm VASHet al. Clinical characteristics and outcome of immunocompromised patients with COVID-19 caused by the omicron variant: a prospective observational study. Clin Infect Dis 2022: ciac571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feikin DR, Higdon MM, Abu-Raddad LJet al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022; 399: 924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sepulcri C, Dentone C, Mikulska Met al. The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient - a case study. Open Forum Infect Dis 2021; 8: ofab217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.