Abstract
This review discusses the effects and mechanisms of a ketogenic diet on neurodegenerative diseases on the basis of available evidence. A ketogenic diet refers to a high-fat, medium-protein, and low-carbohydrate diet that leads to a metabolic shift to ketosis. This review systematically summarizes the scientific literature supporting this effective treatment approach for neurodegenerative diseases, including effects on mitochondrial function, oxidative stress, neuronal apoptosis, neuroinflammation, and the microbiota–gut-brain axis. It also highlights the clinical evidence for the effects of the ketogenic diet in the treatment of Alzheimer's disease, Parkinson's disease, and motor neuron disease. Finally, it discusses the common adverse effects of ketogenic therapy. Although the complete mechanism of the ketogenic diet in the treatment of neurodegenerative diseases remains to be elucidated, its clinical efficacy has attracted many new followers. The ketogenic diet is a good candidate for adjuvant therapy, but its specific applicability depends on the type and the degree of the disease.
Keywords: Ketogenic diet, neurodegenerative diseases, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, brain gut axis
1. INTRODUCTION
The adult brain accounts for approximately 2% of body weight and approximately 20–23% of the body's energy requirements, mainly in the form of glucose. When glucose supply is insufficient, the brain uses ketones (also known as ketone bodies [KBs]) as the main reserve fuel [1]. The ketogenic diet (KD) was developed in 1921 by Dr. Wilder as an effective treatment for children with refractory epilepsy, and it can improve cognition and behavior to a certain extent in children with drug-resistant epilepsy [2]. In recent years, KD has been demonstrated to be beneficial in several neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS) [3]. The classic high-fat, low-carbohydrate KD has an energy ratio (fat to nonfat) of 4:1. The most common KD is one based on long-chain triglycerides. Because of the high percentage of fats (approximately 90%) and the drastic change in dietary habits, classic KD is difficult to maintain for an extended period. To increase adaptability, a diet based on medium-chain triglycerides (MCT) [4] instead of long-chain triglycerides is preferred. The main fatty acids in MCT are octanoic acid and decanoic acid [5], which are more efficiently absorbed than long-chain fatty acids and quickly transported to the liver by albumin and then transformed into KBs by fatty acid oxidation in mitochondria [6]. The modified Atkins diet (MAD), first introduced in 2003, includes fewer restrictions [7]; it recommends limited consumption of carbohydrates, i.e., 10 and 20 g per day for children and adults, respectively, and encourages fat consumption to maintain the level of KBs. MAD consists of 27% protein, 39% carbohydrates, and 34% total fat, and is supplemented with (R)-3-hydroxybutyl (R)-3-hydroxybutytate, which supplies 30% of the calories in this diet. Murray et al. examined the effects of MAD on the physical and cognitive abilities of rats [8]. They concluded that MAD improved cognitive function and may help treat a range of nervous disorders with metabolic changes.
Mitochondrial dysfunction, the antioxidant system, anti-inflammatory response, anti-apoptotic signaling, and the brain-gut axis are the main pathophysiological features of neurodegenerative diseases. The neuroprotective effects of KD are mainly due to long-lasting metabolic changes in the central nervous system during its long-term application [9]. KD causes a shift in energy metabolism toward ketone production and fatty acid oxidation, which enhances mitochondrial function, anti-inflammatory ability, endogenous antioxidant effect, and anti-apoptotic activity, and improves the energy supply of the brain [10-12]. Long-term consumption of KD may alter mitochondrial function mainly by diminishing the production of reactive oxygen species (ROS), increasing the expression of uncoupling proteins (UCPs), and promoting the production of adenosine triphosphate (ATP) to improve mitochondrial activity [11]. Neuronal UCPs (UCP2, UCP4, BMCP1/UCP5) are integral membrane proteins located in the mitochondrial inner membrane, which positively affect neuronal function by preventing neuronal degeneration through regulating UCP-mediated proton transport and ROS production [13]. Neuronal mitochondrial UCP4 mediates the adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress [14].
KD improves the serum level of brain-derived neurotrophic factor (BDNF) both in humans [15] and in a rat model of irritable bowel syndrome [16]. BDNF promotes neuronal differentiation, increases synaptic plasticity, and influences dopaminergic neurotransmission and neurogenesis in the hippocampus [17]. The expression of several nerve growth factors, including neurotrophic protein 3 (NT-3) [18] and glial line-derived neurotrophic factor (GDNF) [19], has also been found to be increased in hippocampal neurons after calorie restriction.
Long aliphatic chain saturated, monosaturated, or polyunsaturated fatty acids (SAFAs, MUFAs, and PUFAs, respectively) are the main types of dietary fatty acids (FAs) [20]. Some of the neuroprotective effects of KD may be mediated by polyunsaturated fatty acids; treatment with KD increased the levels of the neuroprotective omega-3 (n-3) PUFA in the brain and serum [21]. However, PUFAs exhibit different properties regarding inflammatory potential: n-6 PUFAs promote inflammation and atherosclerosis, whereas n-3 PUFAs restore healthy microbiota composition and increase the production of anti-inflammatory compounds [22]. Moreover, n-3 PUFAs improve cognitive processes and maintain synaptic function and plasticity, and are thus considered to have anti-aging properties. Low levels of n-3 PUFAs are known to be associated with inflammatory outcomes in neuropsychiatric and neurological diseases [23-25].
Among PUFA derivatives, endocannabinoids (eCBs) are some of the oldest signaling molecules in vertebrates [26, 27], and there are many eCB-related pathways in the brain [28, 29]. Notably, eCB function underscores the role of lipids in synaptic activity, neuroplasticity, and neuroendocrine function [30-32], which highlights the importance of dietary lipids for maintaining specific molecular systems and mechanisms that regulate neural function and the possibility of preventing or treating brain diseases through dietary regulation (Fig. 1).
Fig. (1).
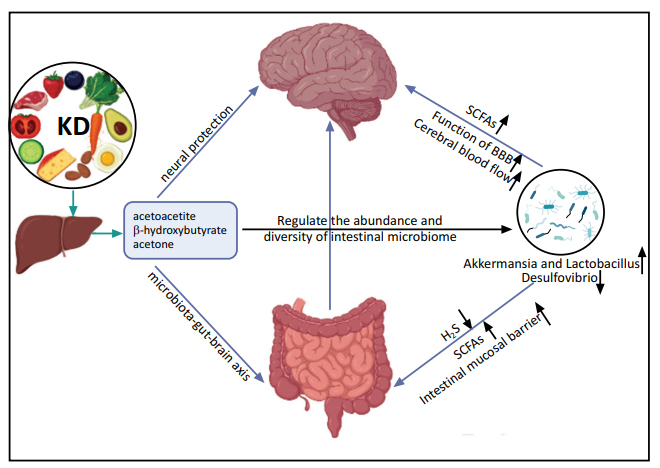
Direct and indirect (microbiota–gut-brain axis) effects of KD on neurodegenerative diseases.
2. MECHANISMS OF THE NEURAL PROTECTIVE EFFECTS OF THE KETOGENIC DIET
2.1. Regulation at the Mitochondrial Level: Antioxidation, Uncoupling Proteins, and ATP Generation
There is growing evidence that ketones play a neural protective role through various mechanisms at the mitochondrial level, including increased levels of UCPs, enhanced antioxidant activity (reduced production of ROS), and ATP synthesis [11, 33] (Fig. 2). KD exerts its antioxidant effects on mitochondria mainly by regulating the function of mitochondrial respiratory complexes, decreasing ROS, and increasing antioxidant levels. Rats fed with KD showed an increased level of glutathione in hippocampal mitochondria [34] and enhanced activity of antioxidant enzymes in the hippocampus [35].
Fig. (2).
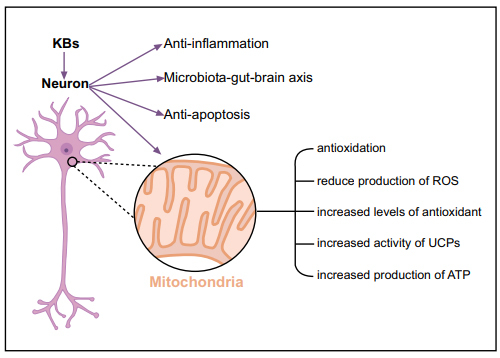
Neuroprotective mechanisms of the ketogenic diet.
The oxidative stress resistance conferred by KD is mainly attributable to KBs [36]. β-hydroxybutyrate (BHB) and acetoacetate together reduced the production of ROS by mitochondrial respiratory complex I, and acetoacetate increased the survival rate of hippocampal cell lines by reducing the production of ROS [37, 38]. BHB promotes oxidative stress resistance, which is critical for the improvement of cognitive function by decreasing the production of mitochondrial ROS (mtROS), increasing the expression of antioxidant enzymes, and reducing the expression of histone deacetylase (HDAC) [10, 39]. Similarly, in mice, BHB inhibited HDAC in a dose-dependent manner [40], which was associated with an increased expression of forkhead box O3(FOXO3a), superoxide dismutase (SOD)2, and catalase, thus increasing the antioxidant defense [41-43]. Moreover, BHB seems to inhibit HDAC2 in nerve cells, suggesting that further research on the protective effect of KD on neurons is warranted [44]. In addition to the upregulation of antioxidant defense induced by BHB, ketones also exert antioxidant function directly by increasing antioxidant activity [34]. In cultured hippocampal neurons, BHB or ACA reduced ROS, and infusion of BHB reduced hippocampal lipid peroxidation [45].
Moreover, ketones protect hippocampal neurons against mitochondrial respiratory complex dysfunction [46]. In rats with traumatic brain injury, KD treatment led to increased protein levels of NAD(P)H: quinone oxidoreductase 1 (NQO1), SOD1, and SOD2 in cortical homogenate, which prevented mitochondrial dysfunction mediated by oxidative stress [47]. KD regulates the ratio between the oxidized and reduced forms of nicotinamide adenine dinucleotide (NAD+/NADH) [48, 49]. A study reported that four weeks of exposure to KBs in association with glucose deprivation significantly restored the stability and activity of mitochondrial complex I and increased ATP synthesis and NAD+/NADH ratio, suggesting that ketones facilitate the repair of complex I damage [50]. Ketones also affect mitochondrial function by preventing the prolonged opening of mitochondrial permeability transition pore (mPTP), which attenuates mtROS-induced cell damage and alleviates neurodegenerative diseases [51]. In neurons isolated from rat brain sections, treatment with BHB + ACA reduced mtROS production, inhibited mPTP opening, and decreased H2O2-induced cell death [36].
The overall goal of mitochondrial uncoupling is to reduce ROS, and KD promotes mitochondrial respiration and significantly reduces ROS production by activating UCPs [52]. KD increases the expression and activity of UCPs in the hippocampus and enhances the efficiency of the mitochondrial electron transport chain by increasing UCP expression and reducing mitochondrial membrane potential [53], which leads to mitochondrial uncoupling and reduced production of mtROS [54-58].
Moreover, KD stimulates mitochondrial activity and increases ATP concentration in the brain [59, 60]. BHB has been shown to significantly increase ATP production in isolated brain mitochondria [61].
KD treatment activates the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway and attenuates oxidative stress [62, 63]. BHB may act as a cellular signaling molecule that regulates the Nrf2 signaling pathway, which is related to increased activity of the cellular endogenous antioxidant system and can be activated by KBs [40, 62, 64, 65]. Consistent with these findings, rats fed with KD initially exhibit decreased production of mtROS and increased expression of 4-hydroxy-2-nonenal (4-HNE), which activates the Nrf2 pathway, one of the cell detoxification pathways through the redox signaling system, leading to chronic cell adaptation and triggering the oxidative stress response [66, 67]. In addition, KD plays an antioxidant role by regulating the mammalian target of rapamycin (mTOR) signaling pathway [68]; the expression of pS6 and pAkt (markers of mTOR pathway activation) was decreased in the hippocampus and liver of rats fed with KD [69]. BHB activates the mTOR signaling pathway and increases the phosphorylation level of mTOR in cells [70].
2.2. Ketogenic Diet has an Anti-apoptotic Effect
Apoptosis is programmed cell death, and blocking apoptosis increases the survival rate of neurons in neurodegenerative diseases [71]. In addition to antioxidant and metabolic effects, anti-apoptotic effects also contribute to neuroprotection. Diminished expression of the pro-apoptotic factors clusterin or caspase-3 was detected in animals fed with KD, suggesting significant anti-apoptotic effects [12, 72-74]. Furthermore, KD increases the activity of calbindin [75, 76], which acts as an intracellular calcium buffer to enable neurons to survive, and inhibits the dissociation of the pro-apoptotic factor Bad from the chaperone 14-3-3 [45]. In addition, KD inhibits nerve cell apoptosis by reducing the expression levels of Bax [77], and decreases apoptosis in hippocampal cells by inhibiting the AMPK pathway and HSP70 expression [78].
2.3. Anti-inflammatory Role of the Ketogenic Diet
Neuroinflammatory pathways are associated with cognitive impairment and neurodegenerative diseases. The major components of long-chain triglycerides, such as docosahexaenoic acid (DHA) and arachidonic acid (ARA), and their mediators regulate several key cognitive processes, such as neurotransmission and neuroinflammation [23, 79, 80]. Previous studies report that several agents derived from DHA, which showed strong anti-inflammatory activity in vivo and in target microglial cells, were detected in the brain [81-85]. Impaired expression of DHA and its derived agents was observed in Alzheimer's disease [86, 87], which accelerated aging in mice [88].
Recent studies suggest that the role of KBs in reducing inflammatory responses contributes to their neuroprotective effect [89, 90] (Fig. 3). Several studies on neuroinflammation in humans or rodents demonstrate that KD inhibits the activation of microglia induced by 1-methyl-4-phenyl-1,2,3,6,tetrahydropyridine (MPTP), and exerts anti-inflammatory effects by reducing the level of proinflammatory cytokines and inflammatory markers [91-93]. An increase in BHB induced by KD inhibits inflammation and blocks the expression of interleukin (IL)-1β in neutrophils or in the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome, which controls the activation and release of caspase-1 [94-96]. Moreover, other studies have shown that KD activates peroxisome proliferator-activated receptors (PPARs), which inhibit the pro-inflammatory nuclear transcription factor κB (NF-κB) signaling pathway, downregulate the expression of COX2 involved in the inflammatory response, and induce nitric oxide and cytokine synthase [21, 65, 97-99]. BHB also regulates hydroxy carboxylic acid 2 (HCA 2) to limit neuroinflammation and plays a neuroprotective role [98, 100]. The interaction between BHB and HCA2 leads to increased prostaglandin D2 synthesis, which inhibits NF-κB and inflammatory signaling [11]. When BHB binds to HCA1 or FAs bind to GPR40, the expression of the scaffold protein β-arrestin-2 (β-Arr2, ARRB2) increases, resulting in the inhibition of ARRB2-dependent NRLP3 inflammasome function [11, 101, 102]. In addition, KD and BHB also have a direct effect on microglia associated with neuroinflammation, leading to microglial polarization toward the neuron-regenerative and protective M2-like phenotypes [102]. However, high concentrations of BHB increase the expression levels of inflammatory signaling molecules, such as NF-κB, tumor necrosis factor (TNF)-α, IL-6, and IL-1β [103]. Therefore, the overall effect of KD on inflammation may be influenced by the concentration of KBs in local tissues.
Fig. (3).
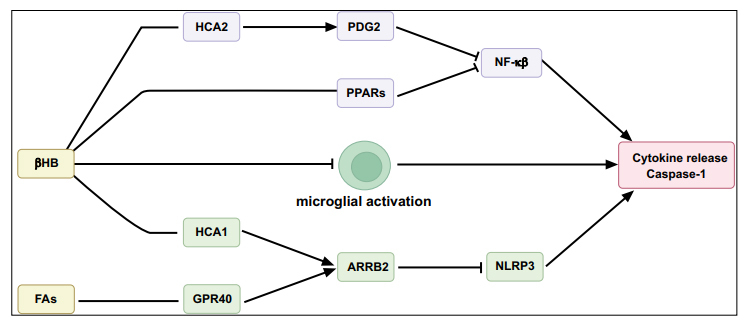
Anti-inflammatory mechanisms of the ketogenic diet.
2.4. Ketogenic Diet Plays an Indirect Protective Role through the Microbiota–gut-Brain Axis
Large cohort studies driven by advances in sequencing technology demonstrated a relationship between the intestinal microbiome and brain structure in healthy and diseased states [104, 105], which led to the establishment of the microbiota–gut-brain axis. There is a complex relationship between intestinal microbiome and KD, and specific microbiome/probiotics have been shown to be useful in animal models of nervous system disease in vivo [106]. The potential mechanisms of KD-based therapy targeting the gut microbiome in clinical studies of neurological diseases may provide new avenues for treatment. Furthermore, by altering the composition of the microbiome, it is possible to infer how KD contributes to a protective role in various CNS diseases. Studies have shown that the intake of fructooligosaccharides (FOS) and galactooligosaccharides (GOS) in soluble dietary fiber produces short-chain fatty acids (SCFAs); FOS and GOS increase the expression of brain-derived neurotrophic factor (BDNF) and N-methyl-D-aspartate receptor (NMDAR) subunits in the hippocampus, which are key factors in memory and synaptic plasticity [107]. Memory dysfunction and altered expression of neurotrophic factors in the hippocampus induced by stress can be restored with probiotic treatment [108]. The dysbiosis of the intestinal microbiome is associated with neurodegenerative diseases, such as AD [20, 109, 110], PD [111-113], and ALS [114-116]. The intestinal microbiome composition is affected by diet, which has a positive effect on metabolism and inflammation through changes in the intestinal microbiota or the production of SCFAs as metabolites of intestinal microorganisms [117, 118]. KDs have been demonstrated to affect the abundance and diversity of the intestinal microbiome [119, 120] and microbial-derived molecules that play a role in CNS homeostasis and neuroprotection [121]. Treatment with the modified Mediterranean KD (MMKD) for 6 weeks improved the levels of AD biomarkers in the cerebrospinal fluid of patients with mild cognitive impairment (MCI) by regulating the gut microbiome; the abundance of Enterobacteriaceae, Akkermansia, Slackia, Christensenellaceae, and Erysipelotriaceae increased, whereas that of Bifidobacterium and Lachnobacterium decreased [122].
The effect of KD intervention on the brain is mediated indirectly by short-chain fatty acids (SCFAs), which are produced by Akkermansia and Lactobacillus [123, 124], and transported by monocarboxylate transporters expressed on the astrocytes of blood-brain barrier (BBB). SCFAs have a neuroprotective effect and improve learning and memory [125, 126], similar to KD-related Akkermansia [127]. In patients with MCI, the abundance of Akkermansia was significantly increased after KD treatment [122]. Notably, the abundance of pro-inflammatory organisms, such as Desulfovibrio [128], which produces hydrogen sulfide and destroys the intestinal mucosal barrier, was reduced after KD treatment [129]. The reduction in Desulfovibrio and increase in Akkermansia and Lactobacillus may improve the function of the BBB and neurovascular function, including cerebral blood flow (CBF); improve metabolic condition; and reduce the risk of AD [119].
However, because carbohydrates are the basic source of energy for microbes, a low-carbohydrate KD reduces the overall diversity of gut microbes [130]. Moreover, the neuroprotective effect of KD exerted via the intestinal microbiome is affected by individual factors, such as gender, age and race, and further research is needed to explore the specific mechanism.
3. KETOGENIC DIET AND NEURODEGENERATIVE DISEASES
3.1. Mild Cognitive Impairment
DHA has a neuroprotective effect on cognitive decline [131, 132]. In particular, some studies reveal a positive correlation between n-3 PUFAs and basic neurobiological processes, especially cognitive processes [23, 133]. Notably, some clinical trials have reported improvements in cognitive performance in patients with MCI after increased intake of DHA. Dietary supplementation with N-3 PUFAs is correlated with improved total cognitive function, perceptual speed, space image efficiency, and working memory of MCI patients with MCI [134]. A randomized, double-blind, placebo-controlled trial demonstrated that DHA improved cognitive function and reduced the production of blood amyloid beta (Aβ) in MCI patients with MCI [132]. Remarkably, it has been reported that supplementation with PUFAs improved cognitive function in older adults with the lowest plasma DHA levels [135]. These promising studies further emphasize the importance of assessing the dietary intake of n-3 PUFAs and the effects of supplementation of these FAs in subjects at risk for cognitive decline.
The potential mechanisms for the effects of KD on patients with MCI are reported (Table 1). A randomized controlled trial showed improved Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) scores in APOE4(-) subjects after KD treatment, and higher ketone levels were strongly associated with the improved ability of paragraph recall [136]. In another study on MCI, after 6 weeks of intervention, the verbal memory of subjects on a low-carbohydrate diet improved, and ketone levels were positively correlated with memory performance [137]. Moreover, a randomized, placebo-controlled trial revealed that KD improved the serum level of KBs and the memory of patients with MCI after they received 56 g/day of MCT for 24 weeks [138]. In another study involving patients with MCI, compared to the group on a diet recommended by the National Institute on Aging, the group on the MAD showed a significantly higher memory composite score and improved mood compared to baseline [139]. Fortier et al. randomized 52 subjects with MCI to a 30 g/day ketogenic medium chain triglyceride (kMCT) or placebo treatment; a 230% increase in ketone metabolism and improved measures of episodic memory, language, executive function, and processing speed were observed in the kMCT group, suggesting a positive correlation between increased ketone uptake and several cognitive indicators. The authors concluded that this long-term ketogenic intervention is safe and feasible in patients with MCI, and significantly improves cognitive scores in multiple domains [140]. Moreover, an MMKD reduces tau protein levels and improves CSF Aβ42 levels, cerebral perfusion, cerebral ketone body uptake, and memory in patients with MCI [141]. Another study reported that a 57-year-old woman diagnosed with MCI and metabolic syndrome showed significant improvement in cognitive function after a 12-week KD intervention [142]. To date, few studies have investigated the effects of KD on MCI, and more clinical studies are needed to reveal the potential role of KD in the prevention and treatment of cognitive decline.
Table 1.
The clinical research on KD in MCI.
| Number | Subjects, n | Trial Type | KD Duration | Outcome | References |
|---|---|---|---|---|---|
| NR | 5 | Crossover RCT | 1 meal consumed 90 min before tests | Improved ADAS-COG scores in ApoE4(-) subjects and higher ketone levels were more correlated with the improved ability for paragraph recall after KD treatment | [136] |
| NCT00777010 | 23 | Parallel RCT | 6 weeks | Improved verbal memory performance | [137] |
| NCT01669200 | 6 | Parallel RCT | 24 weeks | Improved memory | [138] |
| NCT02521818 | 14 | Parallel RCT | 12 weeks | At 6th week, compared to the NIA group, the MAD group had a significantly higher memory composite score compared to baseline, and the mood of subjects in the MAD group was improved | [139] |
| NCT02551419 | 52 | Parallel RCT | 6 months | Episodic memory, language, executive function, and processing speed were improved in ketogenic medium chain triglycerides groups compared to baseline. Increased uptake of brain ketone was positively correlated to several cognitive scales. | [140] |
| NCT02984540 | 20 | Crossover RCT | 6 weeks | Increased CSF Aβ42 and reduced tau protein, increased cerebral perfusion and cerebroketone body uptake; improvement in memory after a modified Mediterranean-ketogenic diet (MMKD) compared to the American Heart Association diet (AHAD) |
[141] |
3.2. Alzheimer’s Disease
AD is a progressive neurodegenerative disease, the most common form of dementia, characterized by memory loss and a reduced ability to perform daily activities. Low glucose metabolism, mitochondrial dysfunction, extracellular Aβ plaque accumulation, and intracellular tau neurofibrillary tangles are the recognized biochemical and histopathological markers of AD [143-145]. KD treatment may have a positive effect on AD, mainly through the following molecular biological mechanisms: improvement of CBF in the hypothalamus; decrease in mTOR through an increase in endothelial nitric oxide synthase; upregulated expression of P-glycoprotein in transporter amyloid plaques; and improvement in the integrity of the BBB [119].
The ability of glucose utilization in the brains of patients with AD is pathologically reduced. Progressive cognitive decline in patients with AD is associated with decreased glucose uptake and metabolism [146]. In addition, decreased glucose uptake in these patients may be associated with the downregulation of the glucose transporter GLUT1 in the brain [89, 147]. Furthermore, a high glycemic diet is associated with the amyloid burden in the brain [148]; thus, using KBs instead of glucose to provide energy may prevent the accumulation of Aβ in the brain and further reduce the risk of AD. There is increasing preclinical evidence that KD acts as an effective treatment for AD. In an AD mouse model, KD improved the learning and memory functions, and reduced the level of Aβ and phosphorylated tau deposition [149]. A study on AD treatment showed that a Mediterranean diet improved metabolic status by reducing systemic inflammation [150]. In adult rats, KD affected the synaptic morphology of the CA1 and dentate gyrus of the hippocampus [151]. In addition, in young healthy mice, KD intervention seemed to enhance cerebrovascular function, increase the abundance of beneficial gut microbiota, improve metabolic status, and reduce the risk of AD [123].
As mentioned earlier, mitochondrial dysfunction is thought to be involved in the etiology of AD [152]. In elderly dogs, KD significantly improved mitochondrial function and decreased the level of amyloid precursor protein (APP) after MCT intervention at 2g/kg/d [153]. Mitochondrial dysfunction and the corresponding decline in respiratory chain function affected the processing of APP, leading to increased production of Aβ fragments [154]. However, it has been shown in mouse models that KD improves motor function but does not affect Aβ40 or Aβ42 levels [155, 156]. Ketones are also critical for alleviating the toxicity of Aβ, whereas β-hydroxybutyrate reduces the toxicity of substance Aβ42 in cultured hippocampal neurons and enables the cells to overcome amyloid-induced pyruvate dehydrogenase dysfunction [157]. In AD mouse models, ketones prevented Aβ42 (the pathological signature of AD) from entering neurons, thus reducing the burden of Aβ and greatly improving learning and memory [158].
Several clinical studies suggest that ketogenic therapy benefits patients with AD by increasing plasma ketone levels and improving cognition (Table 2). A study including 20 subjects with AD or mild cognitive impairment treated with drinks containing emulsified MCT or placebo reported that β-hydroxybutyric acid levels were significantly increased and cognitive function was significantly improved in subjects treated with MCT [136]. Henderson et al. administered MCT to subjects with mild and moderate AD, which increased serum levels of KBs and improved cognitive function, but no such effect was observed in subjects with the APOE4(+) genotype [159]. Moreover, APOE4(-) patients showed increased blood flow to specific brain regions, including the left upper lateral temporal cortex, anterior cerebellum, left lower temporal cortex, and hypothalamus [160]. Polymorphisms in APOE, IL1B, and IDE may influence cognitive responses to ketosis in patients with mild-to-moderate AD [161]. It has been revealed that patients on KD showed significantly higher Alzheimer's Disease Cooperative Study-activities of daily living (ADCS-ADL) and quality of life- Alzheimer's disease (QOL-AD) scores were significantly increased in patients on KD compared to those on a conventional diet; however, no improvement in memory was observed [162]. After 12 weeks of KD intervention in 20 patients with mild-to-moderate AD, significant improvements were observed in both the number–symbol coding test and the real-time logical memory test [163]. After administration of a potent ketogenic agent-ketone monoester (KME) for 20 months, one patient with AD showed improvement in mood, affect, self-care, and cognitive and daily activity performance [164]. A recent study on patients with mild-to-moderate AD treated with MCT supplements for 1 month showed increased brain uptake of acetoacetic acid, suggesting that KD compensated for the observed brain glucose deficiency in AD [165]. A single-arm study revealed that KD improved the mean ADAS-cog scores by 4.1 points in patients with AD [166].
Table 2.
The clinical research on KD in AD.
| Number | Subjects, n | Trial Type | KD Duration | Outcome | References |
|---|---|---|---|---|---|
| NR | 15 | Crossover RCT | 1 meal consumed 90 min before tests | Improved ADAS-COG scores in ApoE4(-) subjects and higher ketone levels were more correlated with the improved ability of paragraph recall after KD treatment | [136] |
| NCT00142805 | 152 | Parallel RCT | 90 days | AC-1202, an oral ketogenic compound, can increase serum ketone body rapidly in AD patients, resulting in a significant difference in ADAS-COG score between the placebo group and the population groups, whereas APOE4(-) subjects had the most significant effect after taking AC-1202. | [159] |
| NCT00142805 | 131 | Parallel RCT | 90 days | Variations of APOE, IL1B, and IDE may influence cognitive responses to ketosis in patients with mild to moderate AD | [161] |
| NR | 1 | Single-arm | 20 months | Improvement in mood, affect, self-care, and cognitive and daily activity performance | [164] |
| NCT02709356 | 15 | Parallel RCT | 1 month | Increased ketone supply without affecting brain glucose utilization to increase total brain energy metabolism | [165] |
| NR | 15 | Single-arm | 12 weeks | Increased ADAS-cog score by 4.1 point | [166] |
| NR | 16 | Parallel RCT | 45 days | Increased blood flow to specific brain regions in ApoE4(-) patients, including the left upper lateral temporal cortex, anterior cerebellum, left lower temporal cortex, and hypothalamus. | [160] |
| NR | 20 | Crossover RCT | 12 weeks | At 8 weeks, patients showed significant improvement on tests of immediate and delayed logical memory tests compared to baseline. At 12 weeks, they showed significant improvements in numerical digit-symbol coding test and immediate logical memory test compared to baseline. | [163] |
| ACTRN12618001450202 | 21 | Randomized crossover | 12 weeks | Improvement in daily function and quality of life, and no improvement in memory | [162] |
In summary, KD has both direct and indirect effects on the progression of AD. These studies suggest that ketogenic therapy does improve cognitive outcomes in patients with AD, but the APOE4 genotype may influence the response to metabolic ketosis [40, 159].
3.3. Parkinson's Disease
The main pathological mechanism in PD is the degeneration of dopaminergic neurons in the substantia nigra of the midbrain, resulting in motor and non-motor disorders. Decreased activity of mitochondrial complex I is thought to play a vital role in the death of dopaminergic neurons in the dense part of the substantia nigra. Ketones bypass complex I to provide an alternative fuel source for neurons at risk while enhancing mitochondrial function and increasing ATP production. A study in vitro demonstrated that mitochondrial respiratory chain dysfunction exogenously induced by complex I and II inhibitors rotenone and 3-nitropropionic acid, respectively, was improved by KBs [46]. 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine (MPTP) is mainly used to produce an animal model of PD because of its ability to induce the death of dopaminergic substantia nigra cells both in vivo and in vitro. In an in vitro experiment, 4 mM β-hydroxybutyrate was shown to protect cultured mesencephalic neurons from the toxic effects of MPTP on the mitochondrial respiratory chain [157]. Similarly, continuous subcutaneous infusion of β-hydroxybutyrate protected mice from dopaminergic neuronal degeneration upon MPTP injection mediated by a complex II-dependent mechanism that led to enhanced mitochondrial respiration and ATP production [167]. A study on a mouse model of PD revealed that octanoic acid (C8) significantly reduced MPTP-induced damage to dopaminergic neurotransmission in the striatum, which may be associated with the increased metabolic activity in the mitochondria of the striatum [168]. Moreover, KD protects dopaminergic neurons in rat models of PD by upregulating glutathione [169].
In addition, neuroinflammation and activated microglia appear to play a role in the pathological process and progression of PD [21, 170-172]. Recent studies indicate that several human leukocyte antigens (HLA)-DR-positive reactive microglia cells are found in the substantia nigra of patients with PD [173, 174]. Activated microglia release various inflammatory factors, such as IL-1, IL-6, TNF-α, and IFN-γ, and chemokines, such as macrophage inflammatory protein-1α, monocyte chemotactic protein-1, and prostaglandin E [175]. Compared to the control group, MPTP-treated mice fed with KD showed reduced microglial activation; down-regulated levels of IL-1 β, IL-6, and TNF-α proteins; and improved motor dysfunction and neuronal loss [91]. Prostaglandin E enhances the transmission of glutaminergic neurons by inhibiting astrocytes and glutamate reuptake, leading to excitatory neurotoxicity in the central nervous system [176, 177]. Nutritional ketosis may help in treating neurodegenerative diseases by reducing astrocyte proliferation [178]. Moreover, KD is reported to have anti-inflammatory effects on MPTP-induced neurotoxicity [91].
KD is indeed effective for the improvement of motor symptoms in PD [167, 168, 179]. A study demonstrated that KD did not significantly affect the pharmacokinetics of L-dopa [180]; however, Jabre et al. suggest that diet alters levodopa absorption rather than affecting neural function, which needs to be more thoroughly investigated [181]. Furthermore, rats with PD consuming a KD showed enhanced motor function, which was mainly manifested in the increased effect of pramipexole [182].
Several clinical studies have shown the effects of KD on patients with PD (Table 3). After 28 days of KD treatment, all the symptoms of patients with PD were moderately or significantly improved, and the movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS) scores of most patients improved (an average decline of 43.4%); however, a placebo effect could not be excluded. Therefore, further research on the role of KD in neuronal function in the maintenance of PD or other neurodegenerative diseases is needed [183]. A randomized controlled study, including 47 patients with PD who were administered a low-fat diet or KD, showed that MDS-UPDRS scores in both groups reduced significantly, whereas the KD group showed greater improvement in non-motor symptoms [184]. Nutritional ketosis has shown improvement in lexical access and memory in PD, but no differences in MDS-UPDRS-III or finger tapping scores [185].
Table 3.
The clinical research on KD in PD.
| Number | Subjects, n | Trial Type | KD Duration | Outcome | References |
|---|---|---|---|---|---|
| NR | 5 | Single-arm | 28 days | Decreases in total UPDRS | [183] |
| ACTRN12617000027314 | 47 | Parallel RCT | 8 weeks | Improvements in MDS-UPDRS I scores, improvement in both motor and nonmotor symptoms, greater improvement in non-motor symptoms | [184] |
| NCT00777010 | 14 | Parallel RCT | 8 weeks | Improvement in vocabulary access and working memory, no differences in MDS-UPDRS-III or finger tapping | [185] |
These findings need to be further confirmed in large-scale clinical trials. However, the application of KD is expected to be a new strategy for PD treatment.
3.4. Amyotrophic Lateral Sclerosis
ALS, the most common motor neuron disease [186], is mostly sporadic, and few patients have a family history of ALS [187]. This neurodegenerative disease is characterized by a progressive loss of upper and lower motor neurons in the brain and spinal cord [188], resulting in paralysis and death, which typically occurs 2–5 years after the onset of symptoms, usually because of respiratory paralysis [189]. Therefore, there is a strong need for more effective treatments for ALS. The pathogenesis of ALS mainly includes excessive production of free radicals, the toxicity of excitatory neurotransmitters, and a decrease in antioxidant capacity, leading to mitochondrial membrane dysfunction, which eventually leads to a change in energy balance and a decrease in the activity of mitochondrial electron transport chain enzymes [190].
KDs reduce the level of glutamate in the synaptic gap and improve disease progression by reducing hyperexcitability [191]. In addition, the antioxidant capacity of endogenous antioxidants is enhanced by KDs [192, 193]. Moreover, in vitro cell culture studies have shown that KDs restore the electron transport chain complex I activity. Recently, several in vitro studies have suggested that sirtuins (SIRTs) are also associated with ALS [194, 195]. SIRT3 prevents SOD1-G93A-induced mitochondrial fragmentation and neuronal apoptosis in vitro [196]. The expression of SIRTs in primary motoneurons increased after treatment with MCT, the main source of KBs, and SIRT3 had a protective effect on mitochondrial breakage and neuronal death induced by SOD1-G93A [197]. Thus, KBs regulate sirtuin-mediated mitochondrial stress response and neuronal cell survival.
In recent years, there have been several studies on the application of KBs in animal models of ALS (Table 4). In the first study published in 2006, the authors observed the effect of KD on motor neurons in the SOD1-G93A transgenic mouse model of ALS, which was found to be preserved by managing triglycerides (obtained by coconut oil fractionation), and mice with KD compared to control mice showed aggravated movement ability and an increase in mitochondrial weight and ATP synthesis [198]. In another study, the treatment of SOD1-G93A transgenic mice with caprylic acid triglycerides, a substitute energy source in neuronal metabolism, improved motor performance, prevented the loss of spinal motoneurons, significantly increased the mitochondrial oxygen consumption rate in vivo, and increased the survival rate of treated mice (they lived for 6 days longer than the control mice), suggesting that the improvement in motor function is associated with an increase in motor neurons [199]. In addition, after following the Deanna regimen based on the KD, KD-fed mice exhibited better performance in all motor function tests at 15 and 16 weeks of age than the control group, and KD treatment significantly extended the survival time of SOD1-G93A mice by 7.5% [200].
Table 4.
The preclinical research on KD in ALS.
| Model | Animals, n | Outcome | References |
|---|---|---|---|
| SOD1-G93A transgenic ALS mice | n=11 | Increased motor neuron survival and improvement in motor function; increased mitochondrial levels of weight and ATP synthesis | [198] |
| SOD1-G93A transgenic ALS mice | n=35 | relieved the progression of weakness, protected the loss of motor neurons in the spinal cord, and increased the rate of oxygen consumption in the body's mitochondria | [199] |
| SOD1-G93A transgenic ALS mice | n=48 | Mice fed with KD showed better motor performance on all motor function tests at 15 and 16 weeks of age compared to the control group. KD therapy significantly prolonged the survival time of SOD1-G93A mice by 4.2%. | [200] |
Given that the main causes of ALS are mitochondrial dysfunction and motor neuron degeneration, KDs seem to improve the symptoms by interfering with the main pathogenetic mechanisms of the disease.
3.5. Adverse Effects of Ketogenic Diets
Some adverse effects may occur during a long-term KD; moreover, the high-fat ingredients of KD also decrease the compliance of patients to the diet. However, under the guidance of doctors, adverse effects can be effectively reduced, and the safety of KD can be improved greatly through strict monitoring of indications. Hypoglycemia [201] and dehydration [202] may occur at the early stage. Furthermore, the most common adverse reactions are digestive problems, usually short-term, including nausea, vomiting, diarrhea, constipation, and anorexia [203, 204]. The main causes of anorexia include reduced secretion of the appetite-stimulating factor neuropeptide Y (NPY) in the hypothalamus, increased cholecystokinin secretion to reduce gastric motor function, and increased levels of circulating growth hormone-releasing peptide, which regulates appetite [205]. Therefore, patients diagnosed with anorexia should be cautious or prohibited from employing KD. However, these effects are usually transient; gastrointestinal side effects can be reduced through the gradual introduction of MCT and improved to some extent by increased intake of dietary fiber, sodium, and liquids, with little need for drug intervention or diet cessation. Patients also exhibit certain long-term adverse reactions, including hypercholesterolemia, hypertriglyceridemia, hyperuricemia, abnormal liver function, ion disorders, atherosclerosis, low bone mineral density, cardiomyopathy, kidney stones, optic neuropathy, anemia, vitamin and mineral deficiency, and rare pancreatitis [9, 204, 205]. Increased free fatty acids constitute an independent risk factor for insulin resistance in patients with type 2 diabetes who already have disorders of lipid metabolism, and they may increase the risk of cardiovascular disease [206, 207]. In addition, rats exposed to long-term KD have been shown to be at risk of for myocardial fibrosis and decreased mitochondrial biogenesis in myocardial cells [208]; however, there is a lack of relevant data from clinical trials. Clinically, the plan of KD can be evaluated and modified by replenishing dietary fiber and nutrients and regularly monitoring these biochemical indexes; however, patients with pancreatitis should be cautious or prohibited from using KD. There are individual differences in the adverse reactions to KD, and the influencing factors include gender, age, race, and underlying diseases. In future research, more detailed inclusion criteria should be formulated for clinical trials to reduce the incidence of adverse reactions.
Because it is a high-fat, low-carbohydrate diet, KD may result in metabolic effects and, ultimately, weight loss through energy value restriction [205]. Considering that some patients with neurological diseases are overweight or obese, weight loss may be beneficial. For those who wish to maintain or gain weight, it is recommended that calorie intake should be monitored and regulated during KD. Patients with poor blood glucose control in diabetes are more prone to serious side effects, such as acidosis caused by hyperketonemia, but the incidence is relatively low in clinical studies and may be further reduced through strict monitoring of indications [209].
For patients with neurodegenerative diseases, especially elderly patients, individualized KD treatment should be developed according to the existing underlying diseases of patients. Chronic KD treatment can lead to catabolic disorders and reduced synthesis of functional proteins, and individuals with neurodegenerative diseases are at high risk of malnutrition, the symptoms of which lead to reduced food intake [210]. In addition, these patients may experience food-related movement disorders, such as chewing and swallowing, taste and smell disorders, apraxia, and eating behavior disorders [205]. Therefore, long-term application of KD should be combined with the supplementation of dietary fiber and other nutrients to prevent adverse reactions. Dynamic monitoring of blood ketone and urinary ketone levels may help assess the compliance of a patient to KD [11]. A research that focused on brain fog during the menopausal transition showed that estrogen enhanced hippocampus-sensitive learning and memory by increasing glucose metabolism, but it damaged striatum-sensitive learning and memory by reducing lactate and ketone body levels [211]. Therefore, for menopausal women with cognitive impairment, if a ketogenic diet is needed for treatment, the dosage of the ketogenic diet should be adjusted according to whether estrogen is used, so as not to affect the ketogenic effect.
CONCLUSION
In conclusion,ketones freely cross the BBB and prevent mitochondrial dysfunction, protect neurons from free radical damage, and reduce oxidative stress and neuroinflammatory response. The neuroprotective effect of KD is exerted through the regulation of the intestinal microbiome. For patients with neurodegenerative diseases, in addition to drug therapy and rehabilitation, KD can also be considered as a potential treatment. Under the guidance of professional doctors to formulate diet plans, by reducing the percentage of carbohydrates, increasing the fat and protein content, and increasing the cellulose content of the diet, patients can transform their diets into a KD. KD reduces the speed and degree of neuronal degeneration, and alleviates the symptoms of neuronal degenerative diseases.
ACKNOWLEDGEMENTS
The figures in this manuscript were created using BioRender.com.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by funding from the Irma and Paul Milstein Medical Asian American Partnership (MMAAP) Foundation Program for Senior Health fellow supported by the MMAAP Foundation (http://www. mmaapf.org) to Dr. HZ.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Cunnane S.C., Courchesne-Loyer A., St-Pierre V., Vandenberghe C., Pierotti T., Fortier M., Croteau E., Castellano C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016;1367(1):12–20. doi: 10.1111/nyas.12999. [DOI] [PubMed] [Google Scholar]
- 2.Martin-McGill K.J., Bresnahan R., Levy R.G., Cooper P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2020;6(6):CD001903. doi: 10.1002/14651858.CD001903.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paoli A., Bianco A., Damiani E., Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. BioMed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kossoff E.H., Hartman A.L. Ketogenic diets. Curr. Opin. Neurol. 2012;25(2):173–178. doi: 10.1097/WCO.0b013e3283515e4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira L., Lisenko K., Barros B., Zangeronimo M., Pereira L., Sousa R. Influence of medium-chain triglycerides on consumption and weight gain in rats: a systematic review. J. Anim. Physiol. Anim. Nutr. (Berl.) 2014;98(1):1–8. doi: 10.1111/jpn.12030. [DOI] [PubMed] [Google Scholar]
- 6.Giordano C., Marchiò M., Timofeeva E., Biagini G. Neuroactive peptides as putative mediators of antiepileptic ketogenic diets. Front. Neurol. 2014;5:63–63. doi: 10.3389/fneur.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kossoff E.H., Krauss G.L., McGrogan J.R., Freeman J.M. Efficacy of the Atkins diet as therapy for intractable epilepsy. Neurology. 2003;61(12):1789–1791. doi: 10.1212/01.WNL.0000098889.35155.72. [DOI] [PubMed] [Google Scholar]
- 8.Murray A.J., Knight N.S., Cole M.A., Cochlin L.E., Carter E., Tchabanenko K., Pichulik T., Gulston M.K., Atherton H.J., Schroeder M.A., Deacon R.M.J., Kashiwaya Y., King M.T., Pawlosky R., Rawlins J.N.P., Tyler D.J., Griffin J.L., Robertson J., Veech R.L., Clarke K. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30(12):4021–4032. doi: 10.1096/fj.201600773R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choragiewicz T., Zarnowska I., Gasior M., Zarnowski T. Anticonvulsant and neuroprotective effects of the ketogenic diet. Przegl. Lek. 2010;67(3):205–212. [PubMed] [Google Scholar]
- 10.Miller V.J., Villamena F.A., Volek J.S. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J. Nutr. Metab. 2018;2018:1–27. doi: 10.1155/2018/5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gough S.M., Casella A., Ortega K.J., Hackam A.S. Neuroprotection by the ketogenic diet: Evidence and controversies. Front. Nutr. 2021;8:782657. doi: 10.3389/fnut.2021.782657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh H.S., Kim Y.S., Lee H.P., Chung K.M., Kim D.W., Kang S.S., Cho G.J., Choi W.S. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53(1-2):119–128. doi: 10.1016/S0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 13.Hoang T., Kuljanin M., Smith M.D., Jelokhani-Niaraki M. A biophysical study on molecular physiology of the uncoupling proteins of the central nervous system. Biosci. Rep. 2015;35(4):e00226. doi: 10.1042/BSR20150130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D., Chan S.L., de Souza-Pinto N.C., Slevin J.R., Wersto R.P., Zhan M., Mustafa K., de Cabo R., Mattson M.P. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol. Med. 2006;8(3):389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 15.Mohorko N., Černelič-Bizjak M., Poklar-Vatovec T., Grom G., Kenig S., Petelin A., Jenko-Pražnikar Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2019;62:64–77. doi: 10.1016/j.nutres.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Orlando A., Chimienti G., Notarnicola M., Russo F. The Ketogenic diet improves gut–brain axis in a rat model of irritable bowel syndrome: Impact on 5-HT and BDNF systems. Int. J. Mol. Sci. 2022;23(3):1098. doi: 10.3390/ijms23031098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colucci-D’Amato L., Speranza L., Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020;21(20):7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Seroogy K.B., Mattson M.P. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J. Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 19.Maswood N., Young J., Tilmont E., Zhang Z., Gash D.M., Gerhardt G.A., Grondin R., Roth G.S., Mattison J., Lane M.A., Carson R.E., Cohen R.M., Mouton P.R., Quigley C., Mattson M.P., Ingram D.K. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2004;101(52):18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chianese R., Coccurello R., Viggiano A., Scafuro M., Fiore M., Coppola G., Operto F.F., Fasano S., Laye S., Pierantoni R., Meccariello R. Impact of dietary fats on brain functions. Curr. Neuropharmacol. 2018;16(7):1059–1085. doi: 10.2174/1570159X15666171017102547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh S., Dupuis N., Auvin S. Ketogenic diet and neuroinflammation. Epilepsy Res. 2020;167:106454. doi: 10.1016/j.eplepsyres.2020.106454. [DOI] [PubMed] [Google Scholar]
- 22.Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G., Miggiano G.A.D., Gasbarrini A., Mele M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazinet R.P., Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014;15(12):771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- 24.Cederholm T. Salem, N., Jr; Palmblad, J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv. Nutr. 2013;4(6):672–676. doi: 10.3945/an.113.004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Layé S. Polyunsaturated fatty acids, neuroinflammation and well being. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82(4-6):295–303. doi: 10.1016/j.plefa.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Elphick M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fasano S., Meccariello R., Cobellis G., Chianese R., Cacciola G., Chioccarelli T., Pierantoni R. The endocannabinoid system: an ancient signaling involved in the control of male fertility. Ann. N. Y. Acad. Sci. 2009;1163(1):112–124. doi: 10.1111/j.1749-6632.2009.04437.x. [DOI] [PubMed] [Google Scholar]
- 28.Marsicano G., Lutz B. Neuromodulatory functions of the endocannabinoid system. J. Endocrinol. Invest. 2006;29(3) Suppl.:27–46. [PubMed] [Google Scholar]
- 29.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 31.Lafourcade M., Larrieu T., Mato S., Duffaud A., Sepers M., Matias I., De Smedt-Peyrusse V., Labrousse V.F., Bretillon L., Matute C., Rodríguez-Puertas R., Layé S., Manzoni O. J. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011;14(3):345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 32.Thomazeau A., Bosch-Bouju C., Manzoni O., Layé S. Nutritional n-3 PUFA deficiency abolishes endocannabinoid gating of hippocampal long-term potentiation. Cereb. Cortex. 2017;27(4):2571–2579. doi: 10.1093/cercor/bhw052. [DOI] [PubMed] [Google Scholar]
- 33.Maalouf M., Rho J.M., Mattson M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Brain Res. Rev. 2009;59(2):293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarrett S.G., Milder J.B., Liang L.P., Patel M. The ketogenic diet increases mitochondrial glutathione levels. J. Neurochem. 2008;106(3):1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler D.R., Ribeiro L.C., Hagenn M., Siqueira R., Araújo E., Torres I.L.S., Gottfried C., Netto C.A., Gonçalves C.A. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem. Res. 2003;28(12):1793–1797. doi: 10.1023/A:1026107405399. [DOI] [PubMed] [Google Scholar]
- 36.Kim D.Y., Davis L.M., Sullivan P.G., Maalouf M., Simeone T.A., Brederode J., Rho J.M. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 2007;101(5):1316–1326. doi: 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- 37.Maalouf M., Sullivan P.G., Davis L., Kim D.Y., Rho J.M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noh H.S., Hah Y.S., Nilufar R., Han J., Bong J.H., Kang S.S., Cho G.J., Choi W.S. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J. Neurosci. Res. 2006;83(4):702–709. doi: 10.1002/jnr.20736. [DOI] [PubMed] [Google Scholar]
- 39.Peixoto L., Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology. 2013;38(1):62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto A., Bonucci A., Maggi E., Corsi M., Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: New perspectives for neuroprotection in Alzheimer’s disease. Antioxidants (Basel) 2018;7(5):63. doi: 10.3390/antiox7050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., Grueter C.A., Lim H., Saunders L.R., Stevens R.D., Newgard C.B., Farese R.V., Jr, de Cabo R., Ulrich S., Akassoglou K., Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei T., Tian W., Liu F., Xie G. Protective effects of exogenous β-hydroxybutyrate on paraquat toxicity in rat kidney. Biochem. Biophys. Res. Commun. 2014;447(4):666–671. doi: 10.1016/j.bbrc.2014.04.074. [DOI] [PubMed] [Google Scholar]
- 43.Nagao M. Toh, R.; Irino, Y.; Mori, T.; Nakajima, H.; Hara, T.; Honjo, T.; Satomi-Kobayashi, S.; Shinke, T.; Tanaka, H.; Ishida, T.; Hirata, K. β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem. Biophys. Res. Commun. 2016;475(4):322–328. doi: 10.1016/j.bbrc.2016.05.097. [DOI] [PubMed] [Google Scholar]
- 44.Tanegashima K., Sato-Miyata Y., Funakoshi M., Nishito Y., Aigaki T., Hara T. Epigenetic regulation of the glucose transporter gene Slc2a1 by β-hydroxybutyrate underlies preferential glucose supply to the brain of fasted mice. Genes Cells. 2017;22(1):71–83. doi: 10.1111/gtc.12456. [DOI] [PubMed] [Google Scholar]
- 45.Noh H.S., Kim Y.S., Kim Y.H., Han J.Y., Park C.H., Kang A.K., Shin H.S., Kang S.S., Cho G.J., Choi W.S. Ketogenic diet protects the hippocampus from kainic acid toxicity by inhibiting the dissociation of bad from 14-3-3. J. Neurosci. Res. 2006;84(8):1829–1836. doi: 10.1002/jnr.21057. [DOI] [PubMed] [Google Scholar]
- 46.Kim D.Y., Vallejo J., Rho J.M. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J. Neurochem. 2010;114(1):130–141. doi: 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greco T., Glenn T.C., Hovda D.A., Prins M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016;36(9):1603–1613. doi: 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elamin M., Ruskin D.N., Masino S.A., Sacchetti P. Ketone-Based Metabolic Therapy: Is Increased NAD+ a Primary Mechanism? Front. Mol. Neurosci. 2017;10:377. doi: 10.3389/fnmol.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y., Sauve A.A. NAD + metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta. Proteins Proteomics. 2016;1864(12):1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frey S., Geffroy G., Desquiret-Dumas V., Gueguen N., Bris C., Belal S., Amati-Bonneau P., Chevrollier A., Barth M., Henrion D., Lenaers G., Bonneau D., Reynier P., Procaccio V. The addition of ketone bodies alleviates mitochondrial dysfunction by restoring complex I assembly in a MELAS cellular model. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(1):284–291. doi: 10.1016/j.bbadis.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94(3):909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis L.M., Rho J.M., Sullivan P.G. UCP-mediated free fatty acid uncoupling of isolated cortical mitochondria from fasted animals: Correlations to dietary modulations. Epilepsia. 2008;49(Suppl. 8):117–119. doi: 10.1111/j.1528-1167.2008.01854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan P.G., Rippy N.A., Dorenbos K., Concepcion R.C., Agarwal A.K., Rho J.M. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann. Neurol. 2004;55(4):576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- 54.Brand M.D., Affourtit C., Esteves T.C., Green K., Lambert A.J., Miwa S., Pakay J.L., Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 2004;37(6):755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 55.Echtay K. Mitochondrial uncoupling proteins - What is their physiological role? Free Radic. Biol. Med. 2007;43(10):1351–1371. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Mookerjee S.A., Divakaruni A.S., Jastroch M., Brand M.D. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010;131(7-8):463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mailloux R.J., Harper M.E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. 2011;51(6):1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Skulachev V.P. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q. Rev. Biophys. 1996;29(2):169–202. doi: 10.1017/S0033583500005795. [DOI] [PubMed] [Google Scholar]
- 59.Bough K.J., Wetherington J., Hassel B., Pare J.F., Gawryluk J.W., Greene J.G., Shaw R., Smith Y., Geiger J.D., Dingledine R.J. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 2006;60(2):223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 60.Devivo D.C., Leckie M.P., Ferrendelli J.S., McDougal D.B., Jr Chronic ketosis and cerebral metabolism. Ann. Neurol. 1978;3(4):331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki M., Suzuki M., Sato K., Dohi S., Sato T., Matsuura A., Hiraide A. Effect of beta-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn. J. Pharmacol. 2001;87(2):143–150. doi: 10.1254/jjp.87.143. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y., Yang Y.Y., Zhou M.W., Liu N., Xing H.Y., Liu X.X., Li F. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci. Lett. 2018;683:13–18. doi: 10.1016/j.neulet.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Liśkiewicz, A.D.; Kasprowska, D.; Wojakowska, A.; Polański, K.; Lewin-Kowalik, J.; Kotulska, K.; Jędrzejowska-Szypułka, H. Long-term high fat ketogenic diet promotes renal tumor growth in a rat model of tuberous sclerosis. Sci. Rep. 2016;6(1):21807. doi: 10.1038/srep21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chorley B.N., Campbell M.R., Wang X., Karaca M., Sambandan D., Bangura F., Xue P., Pi J., Kleeberger S.R., Bell D.A. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40(15):7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu B., Hong J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 66.Milder J.B., Liang L.P., Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol. Dis. 2010;40(1):238–244. doi: 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gano L.B., Patel M., Rho J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014;55(11):2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko A., Sim N.S., Choi H.S., Yang D., Kim S.H., Lee J.S., Kim D.S., Lee J.H., Kim H.D., Kang H.C. Efficacy of the ketogenic diet for pediatric epilepsy according to the presence of detectable somatic mTOR pathway mutations in the brain. J. Clin. Neurol. 2022;18(1):71–78. doi: 10.3988/jcn.2022.18.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDaniel S.S., Rensing N.R., Thio L.L., Yamada K.A., Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52(3):e7–e11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang Z., Li X., Wang S., Jiang Q., Loor J.J., Jiang X., Ju L., Yu H., Shen T., Chen M., Song Y., Wang Z., Du X., Liu G. Overactivation of hepatic mechanistic target of rapamycin kinase complex 1 (mTORC1) is associated with low transcriptional activity of transcription factor EB and lysosomal dysfunction in dairy cows with clinical ketosis. J. Dairy Sci. 2022;105(5):4520–4533. doi: 10.3168/jds.2021-20892. [DOI] [PubMed] [Google Scholar]
- 71.Reyes N.A., Fisher J.K., Austgen K., VandenBerg S., Huang E.J., Oakes S.A. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J. Clin. Invest. 2010;120(10):3673–3679. doi: 10.1172/JCI42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu Z.G., Wang H.D., Jin W., Yin H.X. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann. Clin. Lab. Sci. 2009;39(1):76–83. [PubMed] [Google Scholar]
- 73.Luan G., Zhao Y., Zhai F., Chen Y., Li T. Ketogenic diet reduces Smac/Diablo and cytochrome c release and attenuates neuronal death in a mouse model of limbic epilepsy. Brain Res. Bull. 2012;89(3-4):79–85. doi: 10.1016/j.brainresbull.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 74.McDougall A., Bayley M., Munce S.E.P. The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Inj. 2018;32(4):416–422. doi: 10.1080/02699052.2018.1429025. [DOI] [PubMed] [Google Scholar]
- 75.Noh H.S., Kang S.S., Kim D.W., Kim Y.H., Park C.H., Han J.Y., Cho G.J., Choi W.S. Ketogenic diet increases calbindin-D28k in the hippocampi of male ICR mice with kainic acid seizures. Epilepsy Res. 2005;65(3):153–159. doi: 10.1016/j.eplepsyres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Noh H.S., Kim D.W., Kang S.S., Cho G.J., Choi W.S. Ketogenic diet prevents clusterin accumulation induced by kainic acid in the hippocampus of male ICR mice. Brain Res. 2005;1042(1):114–118. doi: 10.1016/j.brainres.2005.01.097. [DOI] [PubMed] [Google Scholar]
- 77.Hu Z.G., Wang H.D., Qiao L., Yan W., Tan Q.F., Yin H.X. The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj. 2009;23(5):459–465. doi: 10.1080/02699050902788469. [DOI] [PubMed] [Google Scholar]
- 78.Jeon B.T., Lee D.H., Kim K.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Ketogenic diet attenuates kainic acid-induced hippocampal cell death by decreasing AMPK/ACC pathway activity and HSP70. Neurosci. Lett. 2009;453(1):49–53. doi: 10.1016/j.neulet.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 79.Karamikheirabad M., Behzadi G., Faghihi M., Raoofian R., Ejtemaei Mehr S., Zuure W.A., Sadeghipour H.R. A role for endocannabinoids in acute stress-induced suppression of the hypothalamic-pituitary-gonadal axis in male rats. Clin. Exp. Reprod. Med. 2013;40(4):155–162. doi: 10.5653/cerm.2013.40.4.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vauzour D., Martinsen A., Layé S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015;89:63–74. doi: 10.1016/j.neuint.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Orr S.K., Palumbo S., Bosetti F., Mount H.T., Kang J.X., Greenwood C.E., Ma D.W.L., Serhan C.N., Bazinet R.P. Unesterified docosahexaenoic acid is protective in neuroinflammation. J. Neurochem. 2013;127(3):378–393. doi: 10.1111/jnc.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Madore C., Nadjar A., Delpech J.C., Sere A., Aubert A., Portal C., Joffre C., Layé S. Nutritional n-3 PUFAs deficiency during perinatal periods alters brain innate immune system and neuronal plasticity-associated genes. Brain Behav. Immun. 2014;41:22–31. doi: 10.1016/j.bbi.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 83.Rey C., Nadjar A., Buaud B., Vaysse C., Aubert A., Pallet V., Layé S., Joffre C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 2016;55:249–259. doi: 10.1016/j.bbi.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Lukiw W.J., Bazan N.G. Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem. Res. 2000;25(9/10):1173–1184. doi: 10.1023/A:1007627725251. [DOI] [PubMed] [Google Scholar]
- 85.De Smedt-Peyrusse V., Sargueil F., Moranis A., Harizi H., Mongrand S., Layé S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008;105(2):296–307. doi: 10.1111/j.1471-4159.2007.05129.x. [DOI] [PubMed] [Google Scholar]
- 86.Zhu M., Wang X., Hjorth E., Colas R.A., Schroeder L., Granholm A.C., Serhan C.N., Schultzberg M. Pro-resolving lipid mediators improve neuronal survival and increase Aβ42 phagocytosis. Mol. Neurobiol. 2016;53(4):2733–2749. doi: 10.1007/s12035-015-9544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Freund-Levi Y., Hjorth E., Lindberg C., Cederholm T., Faxen-Irving G., Vedin I., Palmblad J., Wahlund L.O., Schultzberg M., Basun H., Eriksdotter Jönhagen M. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer’s disease: the OmegAD study. Dement. Geriatr. Cogn. Disord. 2009;27(5):481–490. doi: 10.1159/000218081. [DOI] [PubMed] [Google Scholar]
- 88.Wang X., Puerta E., Cedazo-Minguez A., Hjorth E., Schultzberg M. Insufficient resolution response in the hippocampus of a senescence-accelerated mouse model--SAMP8. J. Mol. Neurosci. 2015;55(2):396–405. doi: 10.1007/s12031-014-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koppel S.J., Swerdlow R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018;117:114–125. doi: 10.1016/j.neuint.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruskin D.N., Kawamura M., Masino S.A. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4(12):e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang X., Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J. Mol. Neurosci. 2010;42(2):145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- 92.Nandivada P., Fell G.L., Pan A.H., Nose V., Ling P.R., Bistrian B.R., Puder M. Eucaloric ketogenic diet reduces hypoglycemia and inflammation in mice with endotoxemia. Lipids. 2016;51(6):703–714. doi: 10.1007/s11745-016-4156-7. [DOI] [PubMed] [Google Scholar]
- 93.Benlloch M., López-Rodríguez M.M., Cuerda-Ballester M., Drehmer E., Carrera S., Ceron J.J., Tvarijonaviciute A., Chirivella J., Fernández-García D., de la Rubia Ortí J.E. Satiating effect of a ketogenic diet and its impact on muscle improvement and oxidation state in multiple sclerosis patients. Nutrients. 2019;11(5):1156. doi: 10.3390/nu11051156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., D’Agostino D., Planavsky N., Lupfer C., Kanneganti T.D., Kang S., Horvath T.L., Fahmy T.M., Crawford P.A., Biragyn A., Alnemri E., Dixit V.D. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldberg E.L. Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. β-hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18(9):2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bae H.R., Kim D.H., Park M.H., Lee B., Kim M.J., Lee E.K., Chung K.W., Kim S.M. Im, D.S.; Chung, H.Y. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–66454. doi: 10.18632/oncotarget.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cullingford T.E. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot. Essent. Fatty Acids. 2004;70(3):253–264. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 98.Rahman M., Muhammad S., Khan M.A., Chen H., Ridder D.A., Müller-Fielitz H., Pokorná B., Vollbrandt T., Stölting I., Nadrowitz R., Okun J.G., Offermanns S., Schwaninger M. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014;5(1):3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 99.Dupuis N., Curatolo N., Benoist J.F., Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56(7):e95–e98. doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 100.Wang X., Song Y., Chen J., Zhang S., Le Y., Xie Z., Ouyang W., Tong J. Subcutaneous administration of β-hydroxybutyrate improves learning and memory of sepsis surviving mice. Neurotherapeutics. 2020;17(2):616–626. doi: 10.1007/s13311-019-00806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin C., Chao H., Li Z., Xu X., Liu Y., Bao Z., Hou L., Liu Y., Wang X., You Y., Liu N., Ji J. Omega-3 fatty acids regulate NLRP3 inflammasome activation and prevent behavior deficits after traumatic brain injury. Exp. Neurol. 2017;290:115–122. doi: 10.1016/j.expneurol.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Harun-Or-Rashid M., Inman D.M. Reduced AMPK activation and increased HCAR activation drive anti-inflammatory response and neuroprotection in glaucoma. J. Neuroinflammation. 2018;15(1):313. doi: 10.1186/s12974-018-1346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi X. Li, X.; Li, D.; Li, Y.; Song, Y.; Deng, Q.; Wang, J.; Zhang, Y.; Ding, H.; Yin, L.; Zhang, Y.; Wang, Z.; Li, X.; Liu, G. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell. Physiol. Biochem. 2014;33(4):920–932. doi: 10.1159/000358664. [DOI] [PubMed] [Google Scholar]
- 104.Gilbert J.A., Blaser M.J., Caporaso J.G., Jansson J.K., Lynch S.V., Knight R. Current understanding of the human microbiome. Nat. Med. 2018;24(4):392–400. doi: 10.1038/nm.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Long-Smith C., O’Riordan K.J., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota-gut-brain axis: New therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020;60(1):477–502. doi: 10.1146/annurev-pharmtox-010919-023628. [DOI] [PubMed] [Google Scholar]
- 106.Rawat K., Singh N., Kumari P., Saha L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 2021;32(2):143–157. doi: 10.1515/revneuro-2020-0078. [DOI] [PubMed] [Google Scholar]
- 107.Savignac H.M., Corona G., Mills H., Chen L., Spencer J.P.E., Tzortzis G., Burnet P.W.J. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-d-aspartate receptor subunits and d-serine. Neurochem. Int. 2013;63(8):756–764. doi: 10.1016/j.neuint.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J., MacQueen G., Sherman P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 109.Sochocka M. Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease—a critical review. Mol. Neurobiol. 2019;56(3):1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Megur A. Baltriukienė D.; Bukelskienė V.; Burokas, A. The microbiota–gut–brain axis and Alzheimer’s disease: Neuroinflammation is to blame? Nutrients. 2020;13(1):37. doi: 10.3390/nu13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015;21(37):10609–10620. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., Chesselet M.F., Keshavarzian A., Shannon K.M., Krajmalnik-Brown R., Wittung-Stafshede P., Knight R., Mazmanian S.K. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021;172:105840. doi: 10.1016/j.phrs.2021.105840. [DOI] [PubMed] [Google Scholar]
- 114.Blacher E., Bashiardes S., Shapiro H., Rothschild D., Mor U., Dori-Bachash M., Kleimeyer C., Moresi C., Harnik Y., Zur M., Zabari M., Brik R.B.Z., Kviatcovsky D., Zmora N., Cohen Y., Bar N., Levi I., Amar N., Mehlman T., Brandis A., Biton I., Kuperman Y., Tsoory M., Alfahel L., Harmelin A., Schwartz M., Israelson A., Arike L., Johansson M.E.V., Hansson G.C., Gotkine M., Segal E., Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 115.Gotkine M., Kviatcovsky D., Elinav E. Amyotrophic lateral sclerosis and intestinal microbiota—toward establishing cause and effect. Gut Microbes. 2020;11(6):1833–1841. doi: 10.1080/19490976.2020.1767464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boddy S.L., Giovannelli I., Sassani M., Cooper-Knock J., Snyder M.P., Segal E., Elinav E., Barker L.A., Shaw P.J., McDermott C.J. The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021;19(1):13. doi: 10.1186/s12916-020-01885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leeming E.R., Johnson A.J., Spector T.D., Le Roy C.I. Effect of diet on the gut microbiota: Rethinking intervention duration. Nutrients. 2019;11(12):2862. doi: 10.3390/nu11122862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moszak M. Szulińska, M.; Bogdański, P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—a review. Nutrients. 2020;12(4):1096. doi: 10.3390/nu12041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paoli A., Mancin L., Bianco A., Thomas E., Mota J.F., Piccini F. Ketogenic diet and microbiota: Friends or enemies? Genes (Basel) 2019;10(7):534. doi: 10.3390/genes10070534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dowis K., Banga S. The potential health benefits of the ketogenic diet: A narrative review. Nutrients. 2021;13(5):1654. doi: 10.3390/nu13051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu S., Jiang Y., Xu K., Cui M., Ye W., Zhao G., Jin L., Chen X. The progress of gut microbiome research related to brain disorders. J. Neuroinflammation. 2020;17(1):25. doi: 10.1186/s12974-020-1705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagpal R., Neth B.J., Wang S., Craft S., Yadav H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine. 2019;47:529–542. doi: 10.1016/j.ebiom.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma D., Wang A.C., Parikh I., Green S.J., Hoffman J.D., Chlipala G., Murphy M.P., Sokola B.S., Bauer B., Hartz A.M.S., Lin A.L. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci. Rep. 2018;8(1):6670. doi: 10.1038/s41598-018-25190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang Y., Wang Q., Liu J. Microbiota-gut-brain axis: A novel potential target of ketogenic diet for epilepsy. Curr. Opin. Pharmacol. 2021;61:36–41. doi: 10.1016/j.coph.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 125.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mathewson N.D., Jenq R., Mathew A.V., Koenigsknecht M., Hanash A., Toubai T., Oravecz-Wilson K., Wu S.R., Sun Y., Rossi C., Fujiwara H., Byun J., Shono Y., Lindemans C., Calafiore M., Schmidt T.M., Honda K., Young V.B., Pennathur S., van den Brink M., Reddy P. Gut microbiome–derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 2016;17(5):505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olson C.A., Vuong H.E., Yano J.M., Liang Q.Y., Nusbaum D.J., Hsiao E.Y. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13. doi: 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Devkota S., Wang Y., Musch M.W., Leone V., Fehlner-Peach H., Nadimpalli A., Antonopoulos D.A., Jabri B., Chang E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487(7405):104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tagliabue A., Ferraris C., Uggeri F., Trentani C., Bertoli S., de Giorgis V., Veggiotti P., Elli M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN. 2017;17:33–37. doi: 10.1016/j.clnesp.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 130.Swidsinski A., Dörffel Y., Loening-Baucke V., Gille C., Göktas Ö., Reißhauer A., Neuhaus J., Weylandt K.H., Guschin A., Bock M. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front. Microbiol. 2017;8:1141. doi: 10.3389/fmicb.2017.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McGrattan A.M., McEvoy C.T., McGuinness B., McKinley M.C., Woodside J.V. Effect of dietary interventions in mild cognitive impairment: a systematic review. Br. J. Nutr. 2018;120(12):1388–1405. doi: 10.1017/S0007114518002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bai D., Fan J., Li M., Dong C., Gao Y., Fu M., Huang G., Liu H. Effects of folic acid combined with DHA supplementation on cognitive function and amyloid-β-related biomarkers in older adults with mild cognitive impairment by a randomized, double blind, placebo-controlled trial. J. Alzheimers Dis. 2021;81(1):155–167. doi: 10.3233/JAD-200997. [DOI] [PubMed] [Google Scholar]
- 133.Joffre C., Nadjar A., Lebbadi M., Calon F., Laye S. n-3 LCPUFA improves cognition: The young, the old and the sick. Prostaglandins Leukot. Essent. Fatty Acids. 2014;91(1-2):1–20. doi: 10.1016/j.plefa.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 134.Bo Y., Zhang X., Wang Y., You J., Cui H., Zhu Y., Pang W., Liu W., Jiang Y., Lu Q. The n-3 polyunsaturated fatty acids supplementation improved the cognitive function in the chinese elderly with mild cognitive impairment: A double-blind randomized controlled trial. Nutrients. 2017;9(1):54. doi: 10.3390/nu9010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yurko-Mauro K., McCarthy D., Rom D., Nelson E.B., Ryan A.S., Blackwell A., Salem N., Jr, Stedman M. Beneficial effects of docosahexaenoic acid on cognition in age‐related cognitive decline. Alzheimers Dement. 2010;6(6):456–464. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 136.Reger M.A., Henderson S.T., Hale C., Cholerton B., Baker L.D., Watson G.S., Hyde K., Chapman D., Craft S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging. 2004;25(3):311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 137.Krikorian R., Shidler M.D., Dangelo K., Couch S.C., Benoit S.C., Clegg D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging. 2012;33(2):425.e19–425.e27. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rebello C.J., Keller J.N., Liu A.G., Johnson W.D., Greenway F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015;3:123–125. doi: 10.1016/j.bbacli.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brandt J., Buchholz A., Henry-Barron B., Vizthum D., Avramopoulos D., Cervenka M.C. Preliminary report on the feasibility and efficacy of the modified atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J. Alzheimers Dis. 2019;68(3):969–981. doi: 10.3233/JAD-180995. [DOI] [PubMed] [Google Scholar]
- 140.Fortier M., Castellano C.A., Croteau E., Langlois F., Bocti C., St-Pierre V., Vandenberghe C., Bernier M., Roy M., Descoteaux M., Whittingstall K., Lepage M., Turcotte É.E., Fulop T., Cunnane S.C. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019;15(5):625–634. doi: 10.1016/j.jalz.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 141.Neth B.J., Mintz A., Whitlow C., Jung Y., Solingapuram Sai K., Register T.C., Kellar D., Lockhart S.N., Hoscheidt S., Maldjian J., Heslegrave A.J., Blennow K., Cunnane S.C., Castellano C.A., Zetterberg H., Craft S. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: a pilot study. Neurobiol. Aging. 2020;86:54–63. doi: 10.1016/j.neurobiolaging.2019.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dahlgren K., Gibas K.J. Ketogenic diet, high intensity interval training (HIIT) and memory training in the treatment of mild cognitive impairment: A case study. Diabetes Metab. Syndr. 2018;12(5):819–822. doi: 10.1016/j.dsx.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 143.Swerdlow R.H. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Biophys. Acta Mol. Basis Dis. 2011;1812(12):1630–1639. doi: 10.1016/j.bbadis.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Swerdlow R.H. Alzheimer’s disease pathologic cascades: who comes first, what drives what. Neurotox. Res. 2012;22(3):182–194. doi: 10.1007/s12640-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 146.Castellano C.A., Nugent S., Paquet N., Tremblay S., Bocti C., Lacombe G., Imbeault H., Turcotte É., Fulop T., Cunnane S.C. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J. Alzheimers Dis. 2014;43(4):1343–1353. doi: 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- 147.Winkler E.A., Nishida Y., Sagare A.P., Rege S.V., Bell R.D., Perlmutter D., Sengillo J.D., Hillman S., Kong P., Nelson A.R., Sullivan J.S., Zhao Z., Meiselman H.J., Wenby R.B., Soto J., Abel E.D., Makshanoff J., Zuniga E., De Vivo D.C., Zlokovic B.V. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat. Neurosci. 2015;18(4):521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Taylor M.K., Sullivan D.K., Swerdlow R.H., Vidoni E.D., Morris J.K., Mahnken J.D., Burns J.M. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am. J. Clin. Nutr. 2017;106(6):1463–1470. doi: 10.3945/ajcn.117.162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kashiwaya Y., Bergman C., Lee J.H., Wan R., King M.T., Mughal M.R., Okun E., Clarke K., Mattson M.P., Veech R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2013;34(6):1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gu Y., Luchsinger J.A., Stern Y., Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J. Alzheimers Dis. 2010;22(2):483–492. doi: 10.3233/JAD-2010-100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Balietti M., Giorgetti B., Fattoretti P., Grossi Y., Di Stefano G., Casoli T., Platano D., Solazzi M., Orlando F., Aicardi G., Bertoni-Freddari C. Ketogenic diets cause opposing changes in synaptic morphology in CA1 hippocampus and dentate gyrus of late-adult rats. Rejuvenation Res. 2008;11(3):631–640. doi: 10.1089/rej.2007.0650. [DOI] [PubMed] [Google Scholar]
- 152.Yao J., Diaz Brinton R. Targeting mitochondrial bioenergetics for Alzheimer’s prevention and treatment. Curr. Pharm. Des. 2011;17(31):3474–3479. doi: 10.2174/138161211798072517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Studzinski C.M., MacKay W.A., Beckett T.L., Henderson S.T., Murphy M.P., Sullivan P.G., Burnham W.M. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-β precursor protein (APP) levels in the aged dog. Brain Res. 2008;1226:209–217. doi: 10.1016/j.brainres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 154.Wilkins H.M., Swerdlow R.H. Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2017;133:71–79. doi: 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beckett T.L., Studzinski C.M., Keller J.N., Paul Murphy M., Niedowicz D.M. A ketogenic diet improves motor performance but does not affect β-amyloid levels in a mouse model of Alzheimer’s Disease. Brain Res. 2013;1505:61–67. doi: 10.1016/j.brainres.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brownlow M.L., Benner L., D’Agostino D., Gordon M.N., Morgan D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS One. 2013;8(9):e75713–e75713. doi: 10.1371/journal.pone.0075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kashiwaya Y. Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D -β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2000;97(10):5440–5444. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yin J.X., Maalouf M., Han P., Zhao M., Gao M., Dharshaun T., Ryan C., Whitelegge J., Wu J., Eisenberg D., Reiman E.M., Schweizer F.E., Shi J. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol. Aging. 2016;39:25–37. doi: 10.1016/j.neurobiolaging.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 159.Henderson S.T., Vogel J.L., Barr L.J., Garvin F., Jones J.J., Costantini L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. (Lond.) 2009;6(1):31. doi: 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Torosyan N., Sethanandha C., Grill J.D., Dilley M.L., Lee J., Cummings J.L., Ossinalde C., Silverman D.H. Changes in regional cerebral blood flow associated with a 45 day course of the ketogenic agent, caprylidene, in patients with mild to moderate Alzheimer’s disease: Results of a randomized, double-blinded, pilot study. Exp. Gerontol. 2018;111:118–121. doi: 10.1016/j.exger.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 161.Henderson S.T., Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med. Genet. 2011;12(1):137. doi: 10.1186/1471-2350-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Phillips M.C.L., Deprez L.M., Mortimer G.M.N., Murtagh D.K.J., McCoy S., Mylchreest R., Gilbertson L.J., Clark K.M., Simpson P.V., McManus E.J., Oh J.E., Yadavaraj S., King V.M., Pillai A., Romero-Ferrando B., Brinkhuis M., Copeland B.M., Samad S., Liao S., Schepel J.A.C. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimers Res. Ther. 2021;13(1):51. doi: 10.1186/s13195-021-00783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ota M., Matsuo J., Ishida I., Takano H., Yokoi Y., Hori H., Yoshida S., Ashida K., Nakamura K., Takahashi T., Kunugi H. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019;690:232–236. doi: 10.1016/j.neulet.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 164.Newport M.T., VanItallie T.B., Kashiwaya Y., King M.T., Veech R.L. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 2015;11(1):99–103. doi: 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Croteau E., Castellano C.A., Richard M.A., Fortier M., Nugent S., Lepage M., Duchesne S., Whittingstall K., Turcotte É.E., Bocti C., Fülöp T., Cunnane S.C. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer’s disease. J. Alzheimers Dis. 2018;64(2):551–561. doi: 10.3233/JAD-180202. [DOI] [PubMed] [Google Scholar]
- 166.Taylor M.K., Sullivan D.K., Mahnken J.D., Burns J.M., Swerdlow R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4(1):28–36. doi: 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tieu K. Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; Przedborski, S. D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Invest. 2003;112(6):892–901. doi: 10.1172/JCI200318797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Veyrat-Durebex C., Reynier P., Procaccio V., Hergesheimer R., Corcia P., Andres C.R., Blasco H. How can a ketogenic diet improve motor function? Front. Mol. Neurosci. 2018;11:15–15. doi: 10.3389/fnmol.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cheng B., Yang X., An L., Gao B., Liu X., Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009;1286:25–31. doi: 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 170.Wang S., Yuan Y.H., Chen N.H., Wang H.B. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int. Immunopharmacol. 2019;67:458–464. doi: 10.1016/j.intimp.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 171.Yilmaz R., Strafella A.P., Bernard A., Schulte C., van den Heuvel L., Schneiderhan-Marra N., Knorpp T., Joos T.O., Leypoldt F., Geritz J., Hansen C., Heinzel S., Apel A., Gasser T., Lang A.E., Berg D., Maetzler W., Marras C. Serum inflammatory profile for the discrimination of clinical subtypes in Parkinson’s disease. Front. Neurol. 2018;9:1123–1123. doi: 10.3389/fneur.2018.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Burguillos M.A., Deierborg T., Kavanagh E., Persson A., Hajji N., Garcia-Quintanilla A., Cano J., Brundin P., Englund E., Venero J.L., Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 173.Chuang Y.H., Lee P.C., Vlaar T., Mulot C., Loriot M.A., Hansen J., Lill C.M., Ritz B., Elbaz A. Pooled analysis of the HLA-DRB1 by smoking interaction in Parkinson disease. Ann. Neurol. 2017;82(5):655–664. doi: 10.1002/ana.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aliseychik M.P., Andreeva T.V., Rogaev E.I. Immunogenetic factors of neurodegenerative diseases: The role of HLA class II. Biochemistry (Mosc.) 2018;83(9):1104–1116. doi: 10.1134/S0006297918090122. [DOI] [PubMed] [Google Scholar]
- 175.Colonna M., Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017;35(1):441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lofrumento D.D., Saponaro C., Cianciulli A., De Nuccio F., Mitolo V., Nicolardi G., Panaro M.A. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation. 2011;18(2):79–88. doi: 10.1159/000320027. [DOI] [PubMed] [Google Scholar]
- 177.Lee E., Park H.R., Ji S.T., Lee Y., Lee J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-κB, ERK, and JNK. J. Neurosci. Res. 2014;92(1):130–139. doi: 10.1002/jnr.23307. [DOI] [PubMed] [Google Scholar]
- 178.Morris G., Maes M., Berk M., Carvalho A.F., Puri B.K. Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur. Psychiatry. 2020;63(1):e8–e8. doi: 10.1192/j.eurpsy.2019.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McDonald T.J.W., Cervenka M.C. Lessons learned from recent clinical trials of ketogenic diet therapies in adults. Curr. Opin. Clin. Nutr. Metab. Care. 2019;22(6):418–424. doi: 10.1097/MCO.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Elbarbry F., Nguyen V., Mirka A., Zwickey H., Rosenbaum R. A new validated HPLC method for the determination of levodopa: Application to study the impact of ketogenic diet on the pharmacokinetics of levodopa in Parkinson’s participants. Biomed. Chromatogr. 2019;33(1):e4382. doi: 10.1002/bmc.4382. [DOI] [PubMed] [Google Scholar]
- 181.Jabre M.G., Bejjani B.P.W., VanItallie T.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology. 2006;66(4):617–617. doi: 10.1212/01.wnl.0000216108.57529.b1. [DOI] [PubMed] [Google Scholar]
- 182.Shaafi S., Najmi S., Aliasgharpour H., Mahmoudi J., Sadigh-Etemad S., Farhoudi M., Baniasadi N. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran. J. Neurol. 2016;15(2):63–69. [PMC free article] [PubMed] [Google Scholar]
- 183.VanItallie T.B., Nonas C., Di Rocco A., Boyar K., Hyams K., Heymsfield S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology. 2005;64(4):728–730. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- 184.Phillips M.C.L., Murtagh D.K.J., Gilbertson L.J., Asztely F.J.S., Lynch C.D.P. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Mov. Disord. 2018;33(8):1306–1314. doi: 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Krikorian R., Shidler M.D., Summer S.S., Sullivan P.G., Duker A.P., Isaacson R.S., Espay A.J. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clin Park Relat Disord. 2019;1:41–47. doi: 10.1016/j.prdoa.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sorenson E.J., Stalker A.P., Kurland L.T., Windebank A.J. Amyotrophic lateral sclerosis in Olmsted County, Minnesota, 1925 to 1998. Neurology. 2002;59(2):280–282. doi: 10.1212/WNL.59.2.280. [DOI] [PubMed] [Google Scholar]
- 187.Miller R.G., Mitchell J.D., Lyon M., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology. Research Group on Motor Neuron Diseases. 2003;4(3):191–206. [PubMed] [Google Scholar]
- 188.Robberecht W., Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14(4):248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 189.Valko K., Ciesla L. Amyotrophic lateral sclerosis. Prog. Med. Chem. 2019;58:63–117. doi: 10.1016/bs.pmch.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 190.Caplliure-Llopis J., Peralta-Chamba T., Carrera-Juliá S., Cuerda-Ballester M., Drehmer-Rieger E., López-Rodriguez M.M., Rubia Ortí J.E. Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review. Food Sci. Nutr. 2020;8(1):23–35. doi: 10.1002/fsn3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Deng-Bryant Y., Prins M.L., Hovda D.A., Harris N.G. Ketogenic diet prevents alterations in brain metabolism in young but not adult rats after traumatic brain injury. J. Neurotrauma. 2011;28(9):1813–1825. doi: 10.1089/neu.2011.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kong G., Huang Z., Ji W., Wang X., Liu J., Wu X., Huang Z., Li R., Zhu Q. The ketone metabolite β-hydroxybutyrate attenuates oxidative stress in spinal cord injury by suppression of class i histone deacetylases. J. Neurotrauma. 2017;34(18):2645–2655. doi: 10.1089/neu.2017.5192. [DOI] [PubMed] [Google Scholar]
- 193.Veech R.L., Bradshaw P.C., Clarke K., Curtis W., Pawlosky R., King M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life. 2017;69(5):305–314. doi: 10.1002/iub.1627. [DOI] [PubMed] [Google Scholar]
- 194.Pasinetti G.M., Bilski A.E., Zhao W. Sirtuins as therapeutic targets of ALS. Cell Res. 2013;23(9):1073–1074. doi: 10.1038/cr.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hor J.H., Santosa M.M., Lim V.J.W., Ho B.X., Taylor A., Khong Z.J., Ravits J., Fan Y., Liou Y.C., Soh B.S., Ng S.Y. ALS motor neurons exhibit hallmark metabolic defects that are rescued by SIRT3 activation. Cell Death Differ. 2021;28(4):1379–1397. doi: 10.1038/s41418-020-00664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Song W., Song Y., Kincaid B., Bossy B., Bossy-Wetzel E. Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: Neuroprotection by SIRT3 and PGC-1α. Neurobiol. Dis. 2013;51:72–81. doi: 10.1016/j.nbd.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Körner S., Böselt S., Thau N., Rath K.J., Dengler R., Petri S. Differential sirtuin expression patterns in amyotrophic lateral sclerosis (ALS) postmortem tissue: neuroprotective or neurotoxic properties of sirtuins in ALS? Neurodegener. Dis. 2013;11(3):141–152. doi: 10.1159/000338048. [DOI] [PubMed] [Google Scholar]
- 198.Zhao Z., Lange D.J., Voustianiouk A., MacGrogan D., Ho L., Suh J., Humala N., Thiyagarajan M., Wang J., Pasinetti G.M. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7(1):29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao W., Varghese M., Vempati P., Dzhun A., Cheng A., Wang J., Lange D., Bilski A., Faravelli I., Pasinetti G.M. Caprylic triglyceride as a novel therapeutic approach to effectively improve the performance and attenuate the symptoms due to the motor neuron loss in ALS disease. PLoS One. 2012;7(11):e49191. doi: 10.1371/journal.pone.0049191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ari C., Poff A.M., Held H.E., Landon C.S., Goldhagen C.R., Mavromates N., D’Agostino D.P. Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS One. 2014;9(7):e103526. doi: 10.1371/journal.pone.0103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dhamija R., Eckert S., Wirrell E. Ketogenic diet. Can. J. Neurol. Sci. 2013;40(2):158–167. doi: 10.1017/s0317167100013676. [DOI] [PubMed] [Google Scholar]
- 202.Bansal S., Cramp L., Blalock D., Zelleke T., Carpenter J., Kao A. The ketogenic diet: initiation at goal calories versus gradual caloric advancement. Pediatr. Neurol. 2014;50(1):26–30. doi: 10.1016/j.pediatrneurol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 203.McDonald T.J.W., Cervenka M.C. Ketogenic diets for adult neurological disorders. Neurotherapeutics. 2018;15(4):1018–1031. doi: 10.1007/s13311-018-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ułamek-Kozioł M.; Pluta, R.; Bogucka-Kocka, A.; Czuczwar, S. To treat or not to treat drug-refractory epilepsy by the ketogenic diet? That is the question. Ann. Agric. Environ. Med. 2016;23(4):533–536. doi: 10.5604/12321966.1226841. [DOI] [PubMed] [Google Scholar]
- 205.Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients. 2019;11(1):169. doi: 10.3390/nu11010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han L., Liu J., Zhu L., Tan F., Qin Y., Huang H., Yu Y. Free fatty acid can induce cardiac dysfunction and alter insulin signaling pathways in the heart. Lipids Health Dis. 2018;17(1):185. doi: 10.1186/s12944-018-0834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yuan X., Wang J., Yang S., Gao M., Cao L., Li X., Hong D., Tian S., Sun C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr. Diabetes. 2020;10(1):38. doi: 10.1038/s41387-020-00142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Xu S., Tao H., Cao W., Cao L., Lin Y., Zhao S.M., Xu W., Cao J., Zhao J.Y. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct. Target. Ther. 2021;6(1):54. doi: 10.1038/s41392-020-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hashim S.A., VanItallie T.B. Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J. Lipid Res. 2014;55(9):1818–1826. doi: 10.1194/jlr.R046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rusek M., Pluta R. Ułamek-Kozioł M.; Czuczwar, S.J. Ketogenic diet in Alzheimer’s disease. Int. J. Mol. Sci. 2019;20(16):3892. doi: 10.3390/ijms20163892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jaff N.G., Maki P.M. Scientific insights into brain fog during the menopausal transition. Climacteric. 2021;24(4):317–318. doi: 10.1080/13697137.2021.1942700. [DOI] [PubMed] [Google Scholar]


