Abstract
Despite much research efforts being devoted to designing alternative pharmacological interventions, chronic pain remains to be an unresolved clinical problem. Quercetin, a compound that belongs to the flavonoids family, is abundantly found in fruits and vegetables. Emerging evidence indicates that quercetin possesses anti-nociceptive effects in different rodent models of chronic pain, including inflammatory pain, neuropathic pain and cancer pain. In this review, we summarize the mechanisms underlying the analgesic effect of quercetin in preclinical studies. These studies showed that quercetin exerts potent analgesic effects against chronic pain via suppressing neuroinflammation and oxidative stress as well as modulation of synaptic plasticity, GABAergic system, and opioidergic system. Considering that the safety of quercetin is well established, it has great potential for clinical use in pain treatment.
Keywords: Chronic pain, quercetin, neuroinflammation, oxidative stress, synaptic plasticity, GABAergic system
1. INTRODUCTION
Chronic pain is pain that lasts more than several months, which significantly affects an individual’s quality of life [1, 2]. Currently, non-steroidal anti-inflammatory drugs (NSAIDs) and opioids remain to be the first-line analgesics for the management of chronic pain in the clinic [3, 4]. However, long-term use of NSAIDs and opioids inevitably causes unacceptable side effects. Despite much research efforts being devoted to designing alternative pharmacological interventions, chronic pain remains to be an unresolved clinical problem [5-7]. Therefore, novel therapeutic targets and strategies are urgently needed for the management of chronic pain.
Quercetin, a compound that belongs to the flavonoids family, is abundantly found in fruits and vegetables [8, 9]. It has been reported that quercetin may play a critical role in the prevention or treatment of various diseases, such as neurological diseases and cancers [10, 11]. Emerging evidence indicates that quercetin possesses anti-nociceptive effects in different rodent models of chronic pain, including inflammatory pain, neuropathic pain, and cancer pain [12-14]. Anjaneyulu et al. provided the first evidence that chronic treatment with quercetin significantly attenuated streptozotocin-induced thermal hyperalgesia and cold allodynia [15]. In a rat model of chronic constriction injury (CCI)-induced neuropathic pain, Civi et al. showed that the analgesic effect of quercetin was significantly superior to gabapentin and morphine [13]. Moreover, administration of quercetin dose-dependently alleviated thermal hyperalgesia and mechanical allodynia in paclitaxel-induced neuropathic pain mice via inhibiting the expression of protein kinase C epsilon isoform and transient receptor potential vanilloid 1cation channel in the spinal cords and DRG [16]. In another study, Li et al. demonstrated that chronic treatment with quercetin considerably ameliorated bone cancer pain via suppressing peripheral and central sensitization [12]. It has been reported that the sigma-1 receptor plays a critical role in the generation and maintenance of chronic pain [17, 18]. Recently, Espinosa-Juarez et al. demonstrated that the sigma-1 receptor antagonist potentiated the anti-nociceptive effect of quercetin in neuropathic pain induced by CCI [19]. As its safety is well established, quercetin has great potential for clinical use in pain treatment. In this review, we summarized and discussed the preclinical evidence demonstrating the therapeutic potential of quercetin in chronic pain.
2. EFFECT OF QUERCETIN ON NEUROINFLAMMATION IN CHRONIC PAIN
Neuroinflammation is characterized by activation of glial cells such as microglia and astrocytes, overproduction of proinflammatory cytokines and chemokines, and infiltration of immune cells in the peripheral and central nervous system (CNS) [20-22]. A great deal of evidence from our laboratory and others has demonstrated that neuroinflammation plays a vital role in chronic pain [22-24]. Recent evidence indicates that quercetin exerts neuroprotective effects via suppression of neuroinflammation in the CNS [25-27]. In a rat model of spared nerve injury (SNI)-induced neuropathic pain, Muto et al. demonstrated that oral administration of quercetin attenuated mechanical allodynia via inhibiting the activation of satellite glial cells in the ipsilateral L5 dorsal root ganglions (DRG) [28]. In another study, Yang et al. showed that chronic treatment with quercetin significantly ameliorated mechanical allodynia and thermal hyperalgesia in a rat model of diabetic neuropathic pain [29]. Moreover, they found that quercetin suppressed the upregulation of the P2X4 receptor and activation of satellite glial cells as well as the upregulation of phosphorylated p38 mitogen-activated protein kinase (MAPK) in the DRG of diabetic neuropathic pain rats. Recently, Ye et al. reported that administration of quercetin considerably attenuated CCI-induced neuropathic pain via suppressing the activation of microglia in the spinal cord [30]. Taken together, these results demonstrate that quercetin exerts analgesic effects against chronic pain via inhibiting the activation of glial cells in DRG and spinal cord.
It is well-established that glial cell activation releases proinflammatory cytokines and chemokines, which play a critical role in the development of chronic pain [31-33]. Valerio et al. reported that intraperitoneal and oral treatment with quercetin dose-dependently ameliorated formalin- and carrageenan-induced inflammatory pain by suppressing the production of interleukin 1beta (IL-1β) in the paw skin [34]. Moreover, they also found that quercetin inhibited the hyper-nociception induced by intra-plantar injection of tumor necrosis factor α (TNF-α) and CXCL1. In a mice model of cancer pain, Calixto-Campos et al. showed that quercetin significantly reduced Ehrlich tumor-induced mechanical allodynia and thermal hyperalgesia via inhibiting the production of TNF-α and IL-1β as well as neutrophil recruitment [35]. In another study, Ji et al. showed that single or continuous oral administration of quercetin significantly attenuated thermal and cold hyperalgesia in spinal nerve ligation (SNL)-induced neuropathic pain [36]. Moreover, they found that quercetin administration inhibited the upregulation of TNF-α and IL-1β in the DRG from SNL rats. Furthermore, quercetin decreased the phosphorylation of transforming growth factor-β-activated kinase, inhibitor of nuclear factor-κB-kinase, and c-Jun N-terminal kinase 2 in cultured astrocytes. Palmitoylethanolamide (PEA), a naturally-occurring fatty acid amide, is well-known for its analgesic and anti-inflammatory properties [37, 38]. Britti et al. investigated the analgesic effect of PEA co-ultramicronized with the quercetin (PEA-Q) on carrageenan-induced inflammatory pain and sodium monoiodoacetate (MIA)-induced osteoarthritis pain [14]. They found that intragastrical (i.g.) administration of PEA-Q 30 minutes before carrageenan injection significantly improved thermal hyperalgesia and reduced activity of myeloperoxidase (MPO), a marker of inflammatory cell infiltration. Moreover, PEA-Q treatment also considerably alleviated mechanical allodynia and thermal hyperalgesia in MIA-treated rats by counteracting the increased serum levels of TNF-α, IL-1β, and nerve growth factor. These findings confirmed the analgesic and anti-inflammatory effects of the new composite PEA-Q in rodent inflammatory pain models. Moreover, Ruiz-Miyazawa et al. demonstrated that subcutaneous (s.c.) injection of quercetin markedly suppressed monosodium urate crystals (MSU)-induced mechanical hyperalgesia, leukocyte recruitment, activation of nuclear factor kappa B (NF-κB), and release of TNF-α and IL-1β [39]. Considering the pivotal role of the NLR family pyrin domain containing 3 (NLRP3) inflammasome-mediated neuroinflammation in chronic pain [40-42], they further determined the effect of quercetin on the activation of NLRP3 inflammasome. They found that quercetin treatment dramatically suppressed the upregulation of NLRP3, C-terminal caspase recruitment domain, and pro-caspase-1 in the knee joints after MSU injection. Their further study verified the analgesic effect of quercetin on titanium dioxide (TiO2)-induced arthritis pain [43]. In a rat model of CCI-induced neuropathic pain, Ye et al. reported that administration of quercetin for 21 days starting from 7 days after CCI surgery significantly improved the paw withdrawal threshold and paw withdrawal latency in response to von Frey filament stimuli in CCI rats, which was reversed by AMP-activated protein kinase (AMPK) inhibitor Compound C [30]. Moreover, they found that chronic treatment with quercetin not only reduced the inflammatory cells at the ligation site of the sciatic nerve, but also reduced the levels of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. Furthermore, quercetin treatment inhibited the activation of mitogen-activated protein kinase (MAPK), which was reversed by AMPK inhibitor Compound C. These results demonstrated that quercetin may attenuate neuropathic pain via activation of AMPK and inhibition of MAPK signaling pathways. Taken together, these studies indicate that quercetin exerts analgesic effects against chronic pain via suppressing the release of proinflammatory cytokines and chemokines in DRG and spinal cord.
3. EFFECT OF QUERCETIN ON OXIDATIVE STRESS IN CHRONIC PAIN
Quercetin is widely used in traditional Chinese medicine due to its potent antioxidant activity [45, 45]. Valerio et al. reported that quercetin dose-dependently reversed the downregulation of glutathione (GSH) in the paw skin of formalin- and carrageenan-induced inflammatory pain mice [34]. Moreover, Raygude et al. demonstrated that chronic treatment with quercetin for 10 weeks significantly ameliorated mechanical allodynia and thermal hyperalgesia in ethanol-induced neuropathic pain rats [46]. Additionally, quercetin treatment considerably decreased elevated levels of malondialdehyde (MDA), a biomarker of oxidative stress, and oxidative stress-induced DNA fragmentation and upregulation of nitric oxide (NO) in ethanol-treated rats. It is important to note that physiological amounts of NO are neuroprotective, while higher concentrations are obviously neurotoxic [47]. Furthermore, the preconditioning signal leading to cellular protection through hormesis is an important redox-dependent aging-associated with free radicals species accumulation, and inflammatory responses involved in cellular survival mechanisms [48, 49]. Interestingly, bilirubin, a final product of heme metabolism, can serve as an endogenous scavenger of both NO and reactive nitrogen species [50, 51]. In a mouse model of oxaliplatin-induced neuropathic pain, Azevedo et al. showed that quercetin significantly attenuated mechanical allodynia and thermal hyperalgesia via suppressing lipid peroxidation and protein nitrosylation in the spinal cord [52]. In another study, Calixto-Campos et al. showed that quercetin attenuated Ehrlich tumor-induced cancer pain by improving the antioxidant defense system as evidenced by restoration of GSH levels, free-radical scavenging ability, and ferric-reducing ability potential (FRAP) [35]. Similarly, Ruiz-Miyazawa et al. demonstrated that administration of quercetin remarkably ameliorated MSU-induced mechanical hyperalgesia and improved the antioxidant defense system [39]. Moreover, quercetin inhibited the production of superoxide anions and upregulation of gp91phox after MSU injection. Their further study showed that chronic treatment with quercetin also significantly attenuated TiO2-induced mechanical hyperalgesia via similar mechanisms [43]. Previously, our laboratory has demonstrated that activation of nuclear factor erythroid-2-related factor 2 (Nrf2), a critical regulator of endogenous antioxidant defense, exerts potent analgesic effects on chronic pain [53-56]. Borghi et al. reported that quercetin treatment showed an analgesic effect via activation of the nuclear Nrf2/heme oxygenase-1 (HO-1) signaling pathway [43]. Taken together, quercetin may attenuate chronic pain by suppressing oxidative stress due to an improved antioxidant defense system.
4. EFFECT OF QUERCETIN ON SYNAPTIC PLASTICITY IN CHRONIC PAIN
A great deal of evidence indicates that synaptic plasticity is a key cellular mechanism for the development and maintenance of chronic pain [57, 58]. Wang et al. reported that administration of quercetin once daily for 8 weeks significantly alleviated thermal hyperalgesia in diabetic neuropathic pain mice [59]. Moreover, chronic quercetin treatment reduced the total dendritic length, the number of dendritic branches, and the dendritic spine density in the spinal cord dorsal horn neurons of diabetic neuropathic pain mice. Furthermore, they found that quercetin treatment inhibited the upregulation of synaptic plasticity associated proteins and the phosphorylated levels of mammalian target of rapamycin and p70 ribosomal S6 kinase in the spinal cord of diabetic neuropathic pain mice. These results indicate that quercetin alleviates diabetic neuropathic pain by inhibiting mTOR/p70S6K -mediated changes in synaptic morphology and synaptic plasticity-associated proteins in the spinal cord.
5. EFFECT OF QUERCETIN ON GABAERGIC SYSTEM IN CHRONIC PAIN
γ-aminobutyric acid (GABA) is a well-known inhibitory neurotransmitter in the CNS. Emerging evidence from our laboratory and others has demonstrated that loss of GABAergic inhibition contributes to the development and maintenance of chronic pain [33, 60-62]. Filho et al. demonstrated that quercetin dose-dependently ameliorated formalin- and capsaicin-induced inflammatory pain, which was significantly reversed by GABAA receptor antagonist or GABAB receptor antagonist [63]. However, the underlying mechanisms remain unclear. Nevertheless, a recent study showed that quercetin displayed good binding affinities with GABA receptor subunits such as A5, B1, and B2 [64]. Overall, these results indicate that quercetin may exert analgesic effects via restoring GABAergic inhibition.
6. EFFECT OF QUERCETIN ON THE OPIOIDERGIC SYSTEM IN CHRONIC PAIN
It is well-established that the endogenous opioid system has been implicated in chronic pain [65, 66]. In a mice model of diabetic neuropathic pain, Anjaneyulu et al. provided the first evidence that quercetin significantly attenuated thermal hyperalgesia, which was reversed by naloxone, an opioid receptor antagonist [67]. In another study, Calixto-Campos et al. reported that the analgesic effect of quercetin on Ehrlich tumor-induced cancer pain was reversed by naloxone [35]. Notably, co-administration of morphine and quercetin at doses that were ineffective as a single treatment attenuated Ehrlich tumor-induced cancer pain. Furthermore, Ruiz-Miyazawa et al. demonstrated that quercetin substantially attenuated MSU-induced mechanical hyperalgesia, which was blocked by pre-treatment with naloxone [39]. Moreover, the inhibitory effects of quercetin on both neuroinflammation and oxidative stress in MSU-injected mice were abolished by pre-treatment with naloxone. Taken together, these results demonstrated that quercetin possesses anti-nociceptive property, probably through modulation of the opioidergic mechanism.
CONCLUSION AND FUTURE PERSPECTIVE
In this review, we summarized and discussed the therapeutic potential of quercetin against chronic pain in preclinical studies (Table 1). These studies showed that quercetin exerts potent analgesic effects against chronic pain via suppressing neuroinflammation and oxidative stress as well as modulation of synaptic plasticity, GABAergic system, and opioidergic system (Fig. 1). However, these findings raise further questions.
Table 1.
Summary of therapeutic potential of quercetin in chronic pain.
| Model | Treatment Strategy | Effects | Mechanisms | References |
|---|---|---|---|---|
| Carrageenan-induced inflammatory pain rats | PEA-Q (10 and 20 mg/kg, i.g.) was administered 30 min before carrageenan injection. | PWL↑ | MPO↓ | [8] |
| Formalin-induced inflammatory pain mice Capsaicin-induced inflammatory pain mice |
Quercetin (10-60 mg/kg, i.p. or 100-500 mg/kg, i.g.) was administered on 30 minutes before formalin or capsaicin injection | PWL↑ | Activation of GABAergic system Activation of 5-HT receptors |
[21] |
| Formalin-induced inflammatory pain mice Carrageenan-induced inflammatory pain mice |
Quercetin (3, 10, 30 and 100 mg/kg, i.p. or 30, 100 and 300 mg/kg, i.g.) was administered on 30 minutes before formalin or capsaicin injection | PWL↑ | IL-1β↓ GSH↓ |
[60] |
| MSU-induced gout arthritis pain mice | Quercetin (10, 30 and 100 mg/kg, s.c.) was administered on 30 minutes before MSU injection. | PWT↑ | Activation of opioidergic system Leukocyte recruitment↓ NF-κB, TNF-α, IL-1β↓ NLRP3, ASC, pro-caspase-1↓ GSH, FRAP, ABTS↑ Superoxide anion, gp91phox↓ |
[53] |
| TiO2-induced arthritis pain mice | Quercetin (10, 30 and 100 mg/kg, i.p.) was administered once daily for 30 days starting from the first day after TiO2 injection. | PWT↑ | Leukocyte recruitment↓ Neutrophil recruitment↓ TNF-α, IL-1β, IL-6, COX2↓ GSH, FRAP, ABTS↑ Superoxide anion, gp91phox↓ Nrf2, HO-1↑ |
[7] |
| MIA-induced osteoarthritis pain rats | PEA-Q (10 and 20 mg/kg, i.g.) was administered three times per week for 21 days starting from the third day after MIA injection. | PWT↑ PWL↑ |
TNF-α, IL-1β, NGF↓ MMP1, MMP3, MMP9↓ |
[8] |
| CCI-induced neuropathic pain rats | Quercetin (100 mg/kg, i.p.) was administered at 21 days after CCI surgery. Quercetin (100 mg/kg, i.p.) was administered for 4 consecutive days before CCI surgery. Quercetin (100 mg/kg, i.p.) was administered for 4 consecutive days after CCI surgery. |
PWT↑ PWL↑ |
/ | [16] |
| CCI-induced neuropathic pain rats | Quercetin (30, 60 and 120 mg/kg, i.g.) was administered for 21 days starting from 7 days after CCI surgery. | PWT↑ PWL↑ |
Microglial activation↓ p-JNK, p-p38, p-ERK↓ TNF-α, IL-6, IL-1β↓ |
[65] |
| CCI-induced neuropathic pain rats | Quercetin (5.6, 17.8, 56.2, 177.8 and 316 mg/kg, s.c.) was administered on 10 days after CCI surgery. | PWT↑ PWL↑ |
/ | [20] |
| SNL-induced neuropathic pain rats | A single administration of quercetin (100 mg/kg, i.g.) was performed on 14 days after SNL surgery. Quercetin (100 mg/kg, i.g.) was administered for 14 days starting from 0 day after SNL surgery. Quercetin (100 mg/kg, i.g.) was administered for 14 days before SNL surgery. |
PWL↑ | TNF-α, IL-1β↓ MMP-9, MMP-2, CCL2↓ NF-κB, TAK1, JNK2↓ |
[33] |
| SNI-induced neuropathic pain rats | MF diet containing 1% or 0.1% quercetin was given from 4 days before SNI surgery until sacrifice. MF diet containing 1% quercetin was given from 7 days after SNI surgery. |
PWT↑ | Activation of satellite glial cells↓ p-STAT3↓ |
[48] |
| STZ-induced diabetic neuropathic pain rats | Quercetin (10 mg/kg, i.g.) was administered for 4 weeks starting from 21 days after STZ-injection. | PWL↑ | / | [2] |
| STZ-induced diabetic neuropathic pain rats | Quercetin (50 mg/kg, i.p.) was administered for 14 days starting from 7 days after STZ-injection. | PWT↑ PWL↑ |
Activation of satellite glial cells↓ P2X4 receptor↓ p38 MAPK↓ |
[64] |
| STZ-induced diabetic neuropathic pain mice | A single administration of quercetin (50 and 100 mg/kg, i.p.) was performed on 4 weeks after STZ injection. | PWL↑ | Activation of opioidergic system | [1] |
| Diabetic neuropathic pain in db/db mice | Quercetin (50 and 100 mg/kg, i.g.) was administered once daily for 8 weeks. | PWL↑ | Changes of synaptic morphology↓ PSD-95, synaptophysin↓ mTOR, p70S6K↓ |
[62] |
| Oxaliplatin-induced neuropathic pain mice | Quercetin (25, 50 and 100 mg/kg) was administered 30 minutes before every oxaliplatin injection. | PWT↑ PWL↑ |
MDA↓ Fos, nitrotyrosine, iNOS↓ |
[3] |
| Paclitaxel-induced neuropathic pain mice | Quercetin (20, and 60 mg/kg) was administered once daily starting from the first day after the first injection of paclitaxel for 40 days in rats. Quercetin (20, and 60 mg/kg) was administered once daily starting from the first day after the first injection of paclitaxel for 12 days in mice. |
PWT↑ PWL↑ |
PKCε, TRPV1↓ | [23] |
| Ethanol-induced neuropathic pain rats | Quercetin (10, 20 and 40 mg/kg, i.g.) was administered for 10 week starting from 1 hour before ethanol administration. | PWT↑ PWL↑ |
MDA, MPO, NO↓ DNA fragmentation↓ |
[51] |
| TCI-induced bone cancer pain mice | Quercetin (5, 10 and 20 mg/kg, i.g.) was administered once daily for 21 days starting from the first day after TCI. | PWT↑ | TNF-α, IL-1β↓ RANKL, RANK, OPG↓ PAR2, TRPV1↓ |
[38] |
| Ehrlich tumor-induced cancer pain mice | Quercetin (10, 30 and 100 mg/kg, i.p.) was administered on 8 days after injection of tumor cells. Quercetin (10, 30 and 100 mg/kg, i.p.) was administered once daily for 12 days after injection of tumor cells. |
PWT↑ PWL↑ |
Activation of opioidergic system Neutrophil recruitment↓ TNF-α, IL-1β↓ GSH, FRAP, ABTS↑ |
[12] |
Abbreviations: ABTS: free radical scavenging ability; ASC: C-terminal caspase recruitment domain; CCI: chronic constriction injury; CCL2: C-C Motif chemokine ligand 2; COX-2: cyclooxygenase 2; DNA: deoxyribonucleic acid; ERK: extracellular regulated protein kinases; 5-HT: 5-hydroxytryptamine; FRAP: ferric reducing ability potential; GSH: glutathione; HO-1: heme oxygenase-1; IL-1β: interleukin 1beta; IL-6: interleukin 6; i.g.: intragastrically; iNOS: inducible nitric oxide synthase; i.p.: intraperitoneally; i.t.: intrathecally; JNK: c-Jun N-terminal kinase; MAPK: mitogen-activated protein kinase; MDA: malondialdehyde; MIA: monoiodoacetate; MPO: myeloperoxidase; MMP: matrix metallopeptidase; MSU: monosodium urate crystals; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa B; NGF: nerve growth factor; NO: nitric oxide; NLRP3: NLR family pyrin domain containing 3; Nrf2: nuclear factor erythroid-2-related factor 2; OPG: osteoprotegerin; PAR2: protease-activated receptor 2; PEA-Q: palmitoylethanolamide-quercetin; PI3K: phosphoinositide 3-kinase; PSD-95: postsynaptic density protein-95; p70S6K: 70 kDa ribosomal protein S6 kinase; PWL: paw withdrawal latency; PWT: paw withdrawal threshold; RANKL: receptor activator of nuclear factor-kappa B ligand; RANK: receptor activator of nuclear factor-kappa B; SNI: spared nerve injury; SNL: spinal nerve ligation; s.c.: subcutaneously; STAT3: signal transducer and activator of transcription 3; STZ: streptozotocin; TAK1: TGF-β activated kinase 1; TCI: tumor cell implantation; TiO2: titanium dioxide; TNF-α: tumor necrosis factor α; TRPV1: transient receptor potential vanilloid 1; ↑: upregulated; ↓: downregulated.
Fig. (1).
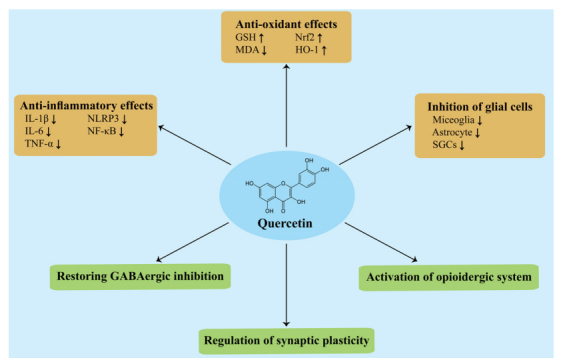
The mechanisms underlying the analgesic effect of quercetin in preclinical studies. Abbreviations: GSH: glutathione; HO-1: heme oxygenase-1; IL-1β: interleukin 1β; IL-6: interleukin 6; MDA: malondialdehyde; NF-κB: nuclear factor kappa B; NLRP3: NLR family pyrin domain containing 3; Nrf2: nuclear factor erythroid-2-related factor 2; SGCs: satellite glial cells. TNF-α: tumor necrosis factor-α;
First of all, current studies indicate that the analgesic effects of quercetin rely on mechanisms in the peripheral nervous system and spinal cord. It has been reported that quercetin showed medium permeability across the blood-brain barrier [68]. Moreover, supraspinal mechanisms are of great importance to chronic pain [69, 70]. Therefore, whether quercetin exerts analgesic effects via supraspinal mechanisms needs further exploration.
Secondly, the evidence mentioned above is based on studies mostly using male rodents, although most patients with chronic pain are women. It is becoming increasingly clear that sex difference exists in pain processing [71, 72], highlighting the necessity of including subjects of both sexes in preclinical pain research. It would be interesting to investigate whether quercetin possesses a similar analgesic effect on chronic pain in female rodents.
Additionally, it is well-established that hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels play a critical role in chronic pain [73, 74]. Interestingly, Liang et al. demonstrated that quercetin remarkably left-shifted the voltage-dependent activation curves of HCN channels and decelerated the deactivation process [75]. These results indicate that quercetin might be a potent HCN channels blocker, which sheds light on the mechanism of the analgesic effects of quercetin.
Finally, despite the promising future of quercetin in chronic pain, few clinical trials are available. Nevertheless, it should be noted that supplementation with a mango leaf extract (Zynamite®) in combination with quercetin attenuated muscle pain caused by exercise-induced muscle damage and accelerated the recovery of muscle performance in humans. Moreover, a randomized controlled trial reported that quercetin supplementation significantly improved the clinical symptoms in women with rheumatoid arthritis, including morning pain and after-activity pain. Overall, considering that the safety of quercetin is well established, it has great potential for clinical use in pain treatment.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- CCI
Chronic constriction injury
- CNS
Central nervous system
- DRG
Dorsal root ganglions
- FRAP
Ferric-reducing ability potential
- GABA
γ-aminobutyric acid
- GSH
Glutathione
- HCN
Hyperpolarization-activated cyclic-nucleotide-gated
- HO-1
Heme oxygenase-1
- IL-1β
Interleukin 1beta
- MAPK
Mitogen-activated protein kinase
- MDA
Malondialdehyde
- MPO
Myeloperoxidase
- MSU
Monosodium urate crystals
- NF-κB
Nuclear factor kappa B
- NLRP3
NLR family pyrin domain containing 3
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid-2-related factor 2
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PEA
Palmitoylethanolamide
- SNI
Spared nerve injury
- SNL
Spinal nerve ligation
- TNF-α
Tumor necrosis factor α
- TiO2
Titanium dioxide
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by grants from the National Natural Science Foundation of China 82001198, 82101310, 82071556, 81974170.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Goldberg D.S., McGee S.J. Pain as a global public health priority. BMC Public Health. 2011;11(1):770. doi: 10.1186/1471-2458-11-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge M.M., Zhou Y.Q., Tian X.B., Manyande A., Tian Y.K., Ye D.W., Yang H. Src-family protein tyrosine kinases: A promising target for treating chronic pain. Biomed. Pharmacother. 2020:110017. doi: 10.1016/j.biopha.2020.110017. [DOI] [PubMed] [Google Scholar]
- 3.Palop Larrea V., Martinez-Mir I. Use of opioids for chronic non-cancer pain. Med. Clin. (Barc.) 2021;156(2):96–98. doi: 10.1016/j.medcli.2019.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Eccleston C., Cooper T.E., Fisher E., Anderson B., Wilkinson N.M. Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst. Rev. 2017;8:CD012537. doi: 10.1002/14651858.CD012537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sideris-Lampretsas G., Malcangio M. Microglial heterogeneity in chronic pain. Brain Behav. Immun. 2021;96:279–289. doi: 10.1016/j.bbi.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Culp C., Kim H.K., Abdi S. Ketamine use for cancer and chronic pain management. Front. Pharmacol. 2021;11:599721. doi: 10.3389/fphar.2020.599721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y.Q., Liu D.Q., Chen S.P., Sun J., Zhou X.R., Xing C., Ye D.W., Tian Y.K. The role of CXCR3 in neurological diseases. Curr. Neuropharmacol. 2019;17(2):142–150. doi: 10.2174/1570159X15666171109161140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L., Wang H., Du X. The therapeutic use of quercetin in ophthalmology: Recent applications. Biomed. Pharmacother. 2021;137:111371. doi: 10.1016/j.biopha.2021.111371. [DOI] [PubMed] [Google Scholar]
- 9.Soofiyani S.R., Hosseini K., Forouhandeh H., Ghasemnejad T., Tarhriz V., Asgharian P., Reiner Ž., Sharifi-Rad J., Cho W.C. Quercetin as a novel therapeutic approach for lymphoma. Oxid. Med. Cell. Longev. 2021;2021:1–15. doi: 10.1155/2021/3157867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grewal A.K., Singh T.G., Sharma D., Sharma V., Singh M., Rahman M.H., Najda A., Walasek-Janusz M., Kamel M., Albadrani G.M., Akhtar M.F., Saleem A., Abdel-Daim M.M. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021;140:111729. doi: 10.1016/j.biopha.2021.111729. [DOI] [PubMed] [Google Scholar]
- 11.Ghafouri-Fard S., Shabestari F.A., Vaezi S., Abak A., Shoorei H., Karimi A., Taheri M., Basiri A. Emerging impact of quercetin in the treatment of prostate cancer. 2021;138:111548. doi: 10.1016/j.biopha.2021.111548. [DOI] [PubMed] [Google Scholar]
- 12.Li Z., Zhang J., Ren X., Liu Q., Yang X. The mechanism of quercetin in regulating osteoclast activation and the PAR2/TRPV1 signaling pathway in the treatment of bone cancer pain. Int. J. Clin. Exp. Pathol. 2018;11(11):5149–5156. [PMC free article] [PubMed] [Google Scholar]
- 13.Çivi S., Emmez G., Dere Ü.A., Börcek A.Ö., Emmez H. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir. (Wien) 2016;158(5):959–965. doi: 10.1007/s00701-016-2761-0. [DOI] [PubMed] [Google Scholar]
- 14.Britti D., Crupi R., Impellizzeri D., Gugliandolo E., Fusco R., Schievano C., Morittu V.M., Evangelista M., Di Paola R., Cuzzocrea S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017;13(1):229. doi: 10.1186/s12917-017-1151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anjaneyulu M., Chopra K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J. Exp. Biol. 2004;42(8):766–769. [PubMed] [Google Scholar]
- 16.Gao W., Zan Y., Wang Z.J., Hu X., Huang F. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol. Sin. 2016;37(9):1166–1177. doi: 10.1038/aps.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi S.R., Han H.J., Beitz A.J., Lee J.H. Intrathecal interleukin-1beta decreases sigma-1 receptor expression in spinal astrocytes in a murine model of neuropathic pain. Biomed. Pharmacother. 2021;144:112272. doi: 10.1016/j.biopha.2021.112272. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Cantero M.C., González-Cano R., Tejada M.Á., Santos-Caballero M., Perazzoli G., Nieto F.R., Cobos E.J. Sigma-1 receptor: A drug target for the modulation of neuroimmune and neuroglial interactions during chronic pain. Pharmacol. Res. 2021;163:105339. doi: 10.1016/j.phrs.2020.105339. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa-Juárez J.V., Jaramillo-Morales O.A., Déciga-Campos M., Moreno-Rocha L.A., López-Muñoz F.J. Sigma‐1 receptor antagonist (BD ‐1063) potentiates the antinociceptive effect of quercetin in neuropathic pain induced by chronic constriction injury. Drug Dev. Res. 2021;82(2):267–277. doi: 10.1002/ddr.21750. [DOI] [PubMed] [Google Scholar]
- 20.Jiang B.C., Liu T., Gao Y.J. Chemokines in chronic pain: Cellular and molecular mechanisms and therapeutic potential. Pharmacol. Ther. 2020;212:107581. doi: 10.1016/j.pharmthera.2020.107581. [DOI] [PubMed] [Google Scholar]
- 21.Ji R.R., Nackley A., Huh Y., Terrando N., Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. 2018;129(2):343–366. doi: 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu D.Q., Zhou Y.Q., Gao F. Targeting cytokines for morphine tolerance: A narrative review. Curr. Neuropharmacol. 2019;17(4):366–376. doi: 10.2174/1570159X15666171128144441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y.Q., Liu Z., Liu H.Q., Liu D.Q., Chen S.P., Ye D.W., Tian Y.K. Targeting glia for bone cancer pain. Expert Opin. Ther. Targets. 2016;20(11):1365–1374. doi: 10.1080/14728222.2016.1214716. [DOI] [PubMed] [Google Scholar]
- 24.Ji R.R., Xu Z.Z., Gao Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014;13(7):533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benameur T., Soleti R., Porro C. The potential neuroprotective role of free and encapsulated quercetin mediated by miRNA against neurological diseases. Nutrients. 2021;13(4):1318. doi: 10.3390/nu13041318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olayinka J., Eduviere A., Adeoluwa O., Fafure A., Adebanjo A., Ozolua R. Quercetin mitigates memory deficits in scopolamine mice model via protection against neuroinflammation and neurodegeneration. Life Sci. 2022;292:120326. doi: 10.1016/j.lfs.2022.120326. [DOI] [PubMed] [Google Scholar]
- 27.Lee B., Yeom M., Shim I., Lee H., Hahm D.H. Protective effects of quercetin on anxiety-like symptoms and neuroinflammation induced by lipopolysaccharide in rats. Evid. Based Complement. Alternat. Med. 2020;2020:4892415. doi: 10.1155/2020/4892415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muto N., Matsuoka Y., Arakawa K., Kurita M., Omiya H., Taniguchi A., Kaku R., Morimatsu H. Quercetin attenuates neuropathic pain in rats with spared nerve injury. Acta Med. Okayama. 2018;72(5):457–465. doi: 10.18926/AMO/56243. [DOI] [PubMed] [Google Scholar]
- 29.Yang R., Li L., Yuan H., Liu H., Gong Y., Zou L., Li S., Wang Z., Shi L., Jia T., Zhao S., Wu B., Yi Z., Gao Y., Li G., Xu H., Liu S., Zhang C., Li G., Liang S. Quercetin relieved diabetic neuropathic pain by inhibiting upregulated P2X 4 receptor in dorsal root ganglia. J. Cell. Physiol. 2019;234(3):2756–2764. doi: 10.1002/jcp.27091. [DOI] [PubMed] [Google Scholar]
- 30.Ye G., Lin C., Zhang Y., Ma Z., Chen Y., Kong L., Yuan L., Ma T. Quercetin alleviates neuropathic pain in the rat CCI model by mediating AMPK/MAPK pathway. J. Pain Res. 2021;14:1289–1301. doi: 10.2147/JPR.S298727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y.Q., Liu Z., Liu Z.H., Chen S.P., Li M., Shahveranov A., Ye D.W., Tian Y.K. Interleukin-6: An emerging regulator of pathological pain. J. Neuroinflammation. 2016;13(1):141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y.Q., Gao H.Y., Guan X.H., Yuan X., Fang G.G., Chen Y., Ye D.W. Chemokines and their receptors: Potential therapeutic targets for bone cancer pain. Curr. Pharm. Des. 2015;21(34):5029–5033. doi: 10.2174/1381612821666150831141931. [DOI] [PubMed] [Google Scholar]
- 33.Ge M.M., Chen S.P., Zhou Y.Q., Li Z., Tian X.B., Gao F., Manyande A., Tian Y.K., Yang H. The therapeutic potential of GABA in neuron-glia interactions of cancer-induced bone pain. Eur. J. Pharmacol. 2019;858:172475. doi: 10.1016/j.ejphar.2019.172475. [DOI] [PubMed] [Google Scholar]
- 34.Valério D.A., Georgetti S.R., Magro D.A., Casagrande R., Cunha T.M., Vicentini F.T.M.C., Vieira S.M., Fonseca M.J.V., Ferreira S.H., Cunha F.Q., Verri W.A. Jr Quercetin reduces inflammatory pain: Inhibition of oxidative stress and cytokine production. J. Nat. Prod. 2009;72(11):1975–1979. doi: 10.1021/np900259y. [DOI] [PubMed] [Google Scholar]
- 35.Calixto-Campos C., Corrêa M.P., Carvalho T.T., Zarpelon A.C., Hohmann M.S.N., Rossaneis A.C., Coelho-Silva L., Pavanelli W.R., Pinge-Filho P., Crespigio J., Bernardy C.C.F., Casagrande R., Verri W.A. Jr Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal. Cell. Pathol. (Amst.) 2015;2015:1–18. doi: 10.1155/2015/285708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji C., Xu Y., Han F., Sun D., Zhang H., Li X., Yao X., Wang H. Quercetin alleviates thermal and cold hyperalgesia in a rat neuropathic pain model by inhibiting Toll-like receptor signaling. 2017;94:652–658. doi: 10.1016/j.biopha.2017.07.145. [DOI] [PubMed] [Google Scholar]
- 37.D’Amico R., Impellizzeri D., Cuzzocrea S., Di Paola R. ALIAmides update: Palmitoylethanolamide and its formulations on management of peripheral neuropathic pain. Int. J. Mol. Sci. 2020;21(15):5330. doi: 10.3390/ijms21155330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mabou Tagne A., Fotio Y., Lin L., Squire E., Ahmed F., Rashid T.I., Karimian Azari E., Piomelli D. Palmitoylethanolamide and hemp oil extract exert synergistic anti-nociceptive effects in mouse models of acute and chronic pain. Pharmacol. Res. 2021;167:105545. doi: 10.1016/j.phrs.2021.105545. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Miyazawa K.W., Staurengo-Ferrari L., Mizokami S.S., Domiciano T.P., Vicentini F.T.M.C., Camilios-Neto D., Pavanelli W.R., Pinge-Filho P., Amaral F.A., Teixeira M.M., Casagrande R., Verri W.A., Jr Quercetin inhibits gout arthritis in mice: Induction of an opioid-dependent regulation of inflammasome. Inflammopharmacology. 2017;25(5):555–570. doi: 10.1007/s10787-017-0356-x. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Huang M., Wang W., Zhang Y., Ma X., Luo L., Xu X., Xu L., Shi H., Xu Y., Wang A., Xu T. Microglial TLR4-induced TAK1 phosphorylation and NLRP3 activation mediates neuroinflammation and contributes to chronic morphine-induced antinociceptive tolerance. Pharmacol. Res. 2021;165:105482. doi: 10.1016/j.phrs.2021.105482. [DOI] [PubMed] [Google Scholar]
- 41.Chen R., Yin C., Fang J., Liu B. The NLRP3 inflammasome: An emerging therapeutic target for chronic pain. J. Neuroinflammation. 2021;18(1):84. doi: 10.1186/s12974-021-02131-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S.P., Zhou Y.Q., Wang X.M., Sun J., Cao F. HaiSam, S.; Ye, D.W.; Tian, Y.K. Pharmacological inhibition of the NLRP3 in fl ammasome as a potential target for cancer-induced bone pain. Pharmacol. Res. 2019;147:104339. doi: 10.1016/j.phrs.2019.104339. [DOI] [PubMed] [Google Scholar]
- 43.Borghi S.M., Mizokami S.S., Pinho-Ribeiro F.A., Fattori V., Crespigio J., Clemente-Napimoga J.T., Napimoga M.H., Pitol D.L., Issa J.P.M., Fukada S.Y., Casagrande R., Verri W.A., Jr The flavonoid quercetin inhibits titanium dioxide (TiO 2)-induced chronic arthritis in mice. J. Nutr. Biochem. 2018;53:81–95. doi: 10.1016/j.jnutbio.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Han X., Xu T., Fang Q., Zhang H., Yue L., Hu G., Sun L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021;44:102010. doi: 10.1016/j.redox.2021.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu D., Hu M.J., Wang Y.Q., Cui Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24(6):1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raygude K.S., Kandhare A.D., Ghosh P., Ghule A.E., Bodhankar S.L. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20(6):331–341. doi: 10.1007/s10787-012-0122-z. [DOI] [PubMed] [Google Scholar]
- 47.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Giuffrida Stella A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 48.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: Novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trovato Salinaro A., Pennisi M., Di Paola R., Scuto M., Crupi R., Cambria M.T., Ontario M.L., Tomasello M., Uva M., Maiolino L., Calabrese E.J., Cuzzocrea S., Calabrese V. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: Modulation by nutritional mushrooms. Immun. Ageing. 2018:8. doi: 10.1186/s12979-017-0108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancuso C., Pani G., Calabrese V. Bilirubin: An endogenous scavenger of nitric oxide and reactive nitrogen species, Redox report : Communications in free radical research. 2006;11(5):207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 51.Miquel S., Champ C., Day J., Aarts E., Bahr B.A., Bakker M., Bánáti D., Calabrese V., Cederholm T., Cryan J., Dye L., Farrimond J.A., Korosi A., Layé S., Maudsley S., Milenkovic D., Mohajeri M.H., Sijben J., Solomon A., Spencer J.P.E., Thuret S., Vanden Berghe W., Vauzour D., Vellas B., Wesnes K., Willatts P., Wittenberg R., Geurts L. Poor cognitive ageing: Vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res. Rev. 2018;42:40–55. doi: 10.1016/j.arr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Azevedo M.I., Pereira A.F., Nogueira R.B., Rolim F.E., Brito G.A.C., Wong D.V.T., Lima-Júnior R.C.P., de Albuquerque Ribeiro R., Vale M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain. 2013;9:1744-8069-9-53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun J., Li J.Y., Zhang L.Q., Li D.Y., Wu J.Y., Gao S.J., Liu D.Q., Zhou Y.Q., Mei W. Nrf2 activation attenuates chronic constriction injury-induced neuropathic pain via induction of PGC-1α-mediated mitochondrial biogenesis in the spinal cord. Oxid. Med. Cell. Longev. 2021;2021:1–17. doi: 10.1155/2021/9577874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y., Liu D., Chen S., Chen N., Sun J., Wang X., Cao F., Tian Y., Ye D. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020;41(8):1041–1048. doi: 10.1038/s41401-020-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y.Q., Mei W., Tian X.B., Tian Y.K., Liu D.Q., Ye D.W. The therapeutic potential of Nrf2 inducers in chronic pain: Evidence from preclinical studies. Pharmacol. Ther. 2021;225:107846. doi: 10.1016/j.pharmthera.2021.107846. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y.Q., Liu D.Q., Chen S.P., Chen N., Sun J., Wang X.M., Li D.Y., Tian Y.K., Ye D.W. PPARgamma activation mitigates mechanical allodynia in paclitaxel-induced neuropathic pain via induction of Nrf2/HO-1 signaling pathway. 2020;129:110356. doi: 10.1016/j.biopha.2020.110356. [DOI] [PubMed] [Google Scholar]
- 57.Bliss T.V.P., Collingridge G.L., Kaang B.K., Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016;17(8):485–496. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 58.Luo C., Kuner T., Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014;37(6):343–355. doi: 10.1016/j.tins.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Wang R., Qiu Z., Wang G., Hu Q., Shi N., Zhang Z., Wu Y., Zhou C. Quercetin attenuates diabetic neuropathic pain by inhibiting mTOR/p70S6K pathway-mediated changes of synaptic morphology and synaptic protein levels in spinal dorsal horn of db/db mice. Eur. J. Pharmacol. 2020;882:173266. doi: 10.1016/j.ejphar.2020.173266. [DOI] [PubMed] [Google Scholar]
- 60.Zeilhofer H.U. Loss of glycinergic and GABAergic inhibition in chronic pain—contributions of inflammation and microglia. Int. Immunopharmacol. 2008;8(2):182–187. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Fu Q., Shi D., Zhou Y., Zheng H., Xiang H., Tian X., Gao F., Manyande A., Cao F., Tian Y., Ye D. MHC-I promotes apoptosis of GABAergic interneurons in the spinal dorsal horn and contributes to cancer induced bone pain. Exp. Neurol. 2016;286:12–20. doi: 10.1016/j.expneurol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y.Q., Chen S.P., Liu D.Q., Manyande A., Zhang W., Yang S.B., Xiong B.R., Fu Q.C., Song Z., Rittner H., Ye D.W., Tian Y.K. The role of spinal GABAB receptors in cancer-induced bone pain in rats. J. Pain. 2017;18(8):933–946. doi: 10.1016/j.jpain.2017.02.438. [DOI] [PubMed] [Google Scholar]
- 63.Filho A.W., Filho V.C., Olinger L., de Souza M.M. Quercetin: Further investigation of its antinociceptive properties and mechanisms of action. Arch. Pharm. Res. 2008;31(6):713–721. doi: 10.1007/s12272-001-1217-2. [DOI] [PubMed] [Google Scholar]
- 64.Hossain R., Al-Khafaji K., Khan R.A., Sarkar C., Islam M.S., Dey D., Jain D., Faria F., Akbor R., Atolani O., Oliveira S.M.R., Siyadatpanah A., Pereira M.L., Islam M.T. Quercetin and/or ascorbic acid modulatory effect on phenobarbital-induced sleeping mice possibly through GABAA and GABAB receptor interaction pathway. Pharmaceuticals (Basel) 2021;14(8):721. doi: 10.3390/ph14080721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruehl S., Chung O.Y. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124(3):287–294. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Malafoglia V., Ilari S., Vitiello L., Tenti M., Balzani E., Muscoli C., Raffaeli W., Bonci A. The interplay between chronic pain, opioids, and the immune system. Neuroscientist. 2021 doi: 10.1177/10738584211030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anjaneyulu M., Chopra K. Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(6):1001–1005. doi: 10.1016/S0278-5846(03)00160-X. [DOI] [PubMed] [Google Scholar]
- 68.Shimazu R., Anada M., Miyaguchi A., Nomi Y., Matsumoto H. Evaluation of blood–brain barrier permeability of polyphenols, anthocyanins, and their metabolites. J. Agric. Food Chem. 2021;69(39):11676–11686. doi: 10.1021/acs.jafc.1c02898. [DOI] [PubMed] [Google Scholar]
- 69.Sivanesan E., Maher D.P., Raja S.N., Linderoth B., Guan Y. Supraspinal mechanisms of spinal cord stimulation for modulation of pain. Anesthesiology. 2019;130(4):651–665. doi: 10.1097/ALN.0000000000002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bannister K., Dickenson A.H. Central nervous system targets: Supraspinal mechanisms of analgesia. Neurotherapeutics. 2020;17(3):839–845. doi: 10.1007/s13311-020-00887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mogil J.S. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020;21(7):353–365. doi: 10.1038/s41583-020-0310-6. [DOI] [PubMed] [Google Scholar]
- 72.Halievski K., Ghazisaeidi S., Salter M.W. Sex-dependent mechanisms of chronic pain: A focus on microglia and P2X4R. J. Pharmacol. Exp. Ther. 2020;375(1):202–209. doi: 10.1124/jpet.120.265017. [DOI] [PubMed] [Google Scholar]
- 73.He J.T., Li X.Y., Zhao X., Liu X. Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain. Rev. Neurosci. 2019;30(6):639–649. doi: 10.1515/revneuro-2018-0094. [DOI] [PubMed] [Google Scholar]
- 74.Liu F., Wuni G.Y., Bahuva R., Shafiq M.A., Gattas B.S., Ibetoh C.N., Stratulat E., Gordon D.K. Pacemaking activity in the peripheral nervous system: Physiology and roles of hyperpolarization activated and cyclic nucleotide-gated channels in neuropathic pain. Cureus. 2020;12(10):e11111. doi: 10.7759/cureus.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang Y., Xu Z., Wu X., Pang J., Zhou P., Cao Y. Inhibition of hyperpolarization-activated cyclic nucleotide-gated channels with natural flavonoid quercetin. Biochem. Biophys. Res. Commun. 2020;533(4):952–957. doi: 10.1016/j.bbrc.2020.09.102. [DOI] [PubMed] [Google Scholar]


