Abstract
Mastitis occurrence in dairy cows is a broad topic that involves several sectors, from antimicrobial resistance and virulence of strains to economic implications and cattle management practices. Here, we assessed the molecular characterization (antimicrobial resistance determinants, virulence genes, sequences type, serotypes, and plasmid types) of 178 Escherichia coli strains isolated from milk samples from cows with clinical mastitis using a genome-based k-mers approach. Of these, 53 (29.8%) showed multidrug resistance by disc diffusion. We selected eight multidrug-resistant mastitis-associated E. coli for whole-genome sequencing and molecular characterization based on raw data using k-mers. We assessed antimicrobial resistance genes, virulence factors, serotypes, Multilocus Sequence Typing (MLST), and plasmid types. The most antimicrobial resistance gene found were blaTEM-1B (7/8), tetA (6/8), strA (6/8), strB (6/8), and qnrB19 (5/8). A total of 25 virulence factors were detected encoding adhesins, capsule, enzymes/proteins, increased serum survival, hemolysin, colicins, and iron uptake. These virulence factors were associated with Extraintestinal Pathogenic E. coli. Three pandemic clones were found: ST10, ST101, and ST69. Two E. coli were assigned in the O117 serogroup and one in the O8:H25 serotype. The most common plasmid groups were IncFII (7/8) and IncFIB (6/8). Our findings contribute to the knowledge of virulence mechanisms, epidemiological aspects, and antimicrobial resistance determinants of E. coli strains obtained from clinical mammary infections of cows.
Introduction
Mastitis is a growing and serious problem in dairy farms due to the effects it has on animal health, milk quality, and losses to the dairy industry. Among the infectious agents related to mammary infections of environmental origin, Escherichia coli is the most common, followed by Klebsiella and Enterobacter species [1]. In this context, antimicrobials have been routinely used to treat clinical mastitis cases (therapeutic use) and prevent future infections (prophylactic use) using the dry cow therapy [2].
Antimicrobial use on dairy farms has been associated with antimicrobial resistance, and these farms have been indicated as sources of antimicrobial resistance genes, acting as a dissemination hotspot together with human medicine and agriculture [3].
Classical molecular investigations use laborious and expensive methodologies for the characterization of bacteria, e.g., polymerase chain reaction (PCR) test, agglutination for serotyping, and enzyme restriction patterns. Usually, these approaches show incomplete results since they depend on the previous selection of genetic determinants.
Thus, we aimed to investigate the molecular characterization of E. coli strains isolated from clinical bovine mastitis using a genome-based approach by k-mers to assess antimicrobial resistance determinants, virulence genes, Multilocus Sequence Typing (MLST), serotypes, and plasmid types using whole-genome sequencing (WGS).
Materials and Methods
Escherichia coli strains were isolated from 4,275 milk samples of cows that showed clinical signs of mastitis on ten farms located in the states of São Paulo and Minas Gerais, in Southeast Brazil, from September 2017 to March 2019 (Ethics Committee on Animal Use—CEUA, São Paulo State University, protocol No. 2015/19688-8).
The calves were from medium-scale farms (20–200 hectares), with different average size of herds and breeding with similar nutrition, management, technical level, and sanitary conditions. All cows were housed in sand-bedded freestalls and were milked three times per day. Farms eligibility criteria included the following: (1) Holstein or Holstein crossbreed cows, (2) mastitis control programs with data recorded in management software, (3) somatic cell count (SCC) < 400,000 SCC/mL, (4) production > 20 L/cow/day, (5) at least 200 lactating cows in each farm, (6) mechanical milking system, and (7) history of clinical mastitis.
The diagnosis of clinical mastitis was performed at every milking. The first milk streams were visually inspected in strip cup test or deposited on the floor with black rubber to identify any abnormalities. Mild (Score 1) signs of clinical cases were characterized by any abnormal appearance in milk (presence of flakes, blood, pus, or color changes). Moderate clinical cases (Score 2) were characterized by macroscopic changes of milk and udder inflammation (local pain, swelling, or redness in the affected mammary gland). Cases with additional signs such as inappetence, fever, tachypnea, tachycardia, decubitus, or abnormal ruminal motility were identified as severe (Score 3) [4].
After milking, technicians carried out the udder hygiene procedures (examination of the first milk streams, pre-dipping, and drying of the teats), the teat end was disinfected using cotton pads soaked with 70% alcohol solution. Then, the first streams of milk were discarded, and 15 mL of milk were collected in a sterile plastic vial and kept in refrigerated conditions (4–8 °C) in isothermal boxes until transport to the laboratory for further bacteriological and molecular analyses.
The samples were cultivated onto MacConkey agar, and lactose-positive colonies were identified as E. coli using biochemical tests, namely, glucose fermentation, urea hydrolysis, hydrogen sulfide production, deamination of tryptophan, motility, lysine decarboxylase and indole production, and Simmons citrate utilization [5].
Antimicrobial susceptibility was performed by disk diffusion test according to Clinical and Laboratory Standards Institute (CLSI) guidelines [6, 7] using ampicillin (10 µg), amoxicillin (10 µg), ceftiofur (30 µg), cefoperazone (30 µg), ciprofloxacin (5 µg), enrofloxacin (5 µg), streptomycin (10 µg), florfenicol (30 µg), gentamicin (10 µg), neomycin (30 µg), sulfamethoxazole/trimethoprim (25 μg), tetracycline (30 µg), and erythromycin (15 μg). E. coli ATCC 25922 was used as a sensibility control. Multidrug-resistant E. coli (MDR E. coli) were characterized by showing resistance to three or more antimicrobial classes [8]. Intermediate results from the disk diffusion test were considered resistant and included in multidrug resistance analysis.
Genomic DNA was extracted from E. coli culture in brain heart infusion broth using AccuPrep® Genomic DNA Extraction Kit (Bioneer). DNA samples were sent to Life Sciences Core Facility (LaCTAD) of the University of Campinas (UNICAMP), Brazil, for whole-genome sequencing using Illumina MiSEQ platform 2 × 300 paired-end reads. The paired-end reads quality was verified using FastQC 0.11.4 [9]. Raw genome data were analyzed based on k-mers using tools from Center Genomic Epidemiology from Technical University of Denmark (CGE) (https://cge.food.dtu.dk/services): ResFinder 4.1, VirulenceFinder 2.0, MLST 2.0, SerotypeFinder 2.0, and PlasmidFinder 2.1. Reads were submitted to the NCBI, Sequence Read Archive—SRA (BioProject PRJNA831284).
Results and Discussion
In total, 178 E. coli were isolated from all clinical cases of bovine mastitis among the ten farms studied. Of these, 53 (29.8%) isolates revealed multidrug resistance in disk diffusion tests, mainly beta-lactam-aminoglycoside-tetracycline-macrolide resistant profile. The most commonly found antimicrobial resistances were erythromycin (44/53), streptomycin (37/53), ampicillin (35/53), amoxicillin (33/53), and neomycin (31/53). For the purpose of the study, we showed the genome-based results for eight multidrug-resistant E. coli isolates (Table 1). To our knowledge, this is the first whole-genome sequencing (WGS) report of mastitis-associated E. coli in Brazil.
Table 1.
Results genome-based of multidrug-resistant Escherichia coli isolated from bovine mastitis in Brazil
| Strain | Farm | State | Isolation date | Antimicrobial resistance1 | Antimicrobial resistance genes | Virulence genes | MLST3 | Serotype | Plasmid type |
|---|---|---|---|---|---|---|---|---|---|
| 01 T-32 | A | São Paulo | Sep-2017 | AMP, AMO, CFP, CIP, ENR, NEO, EST, FFC, SUT, TET, ERI | blaTEM-1B, tet(A), dfrA8, sul2, floR2, aadA1, strA, strB, parC:p.S80I, gyrA:p.D87N, gyrA:p.S83L | gad, iss, sitA, terC | ST10 | ND4 | IncFII, IncX1, p0111 |
| 23 T-166 | A | São Paulo | Mar-2018 | AMP, AMO, FFC, TET, ERI | blaTEM-1B, floR2, aadA22, qnrB19, qnrS1, tet(A) | gad, lpfA, terC, traT | ST101 | ND | IncR |
| 68 T-1 | B | Minas Gerais | Ago-2018 | AMP, AMO, EST, TET, ERI | blaTEM-1B, strA, strB, qnrB19, qnrB52, qnrB812, qnrB822, sul2, tet(A) | F17, gad, lpfA, ompT2, terC, traT2 | ST1049 | O117:H? | Col(pHAD28), IncFIB2, IncFII2 |
| 109 T-18 | C | Minas Gerais | Dec-2018 | AMP, AMO, EST, TET, ERI | blaTEM-1B, strA, strB, floR2, sul2, qnrB19, tet(A) | F17, gad, hra2, lpfA, ompT2, terC, traT2 | ST1049 | O117:H? | Col(pHAD28), IncFIB2, IncFII2, IncY2 |
| 111 T-10 | D | Minas Gerais | Dec-2018 | AMP, AMO, EST, TET | blaTEM-1B, qnrB812, qnrB19, qnrB822, strA2, strB, tet(B) | gad, iss, lpfA, ompT, terC | ST101 | ND | Col(pHAD28), IncFIB2, IncFII2, IncX12 |
| 112 T-8 | C | Minas Gerais | Jan-2019 | SUT, TET, ERI | tet(A), dfrA5, sitABCD2 | cia, cvaC, etsC, fyuA, gad, hlyF, iroN, irp2, iss, iucC, iutA, mchF, ompT, sitA, terC, traT | ST4138 | O8:H25 | IncFII, IncP1, IncFIB2 |
| 134 T-2 | E | São Paulo | Mar-2019 | AMP, AMO, EST, TET | blaTEM-1B, qnrB19, qnrB52, qnr822, tet(B), strA2, strB | gad, iss, lpfA, ompT, terC | ST101 | ND | Col(pHAD28), IncFIB2, IncFII2, IncX12 |
| 138 T-3 | F | Minas Gerais | Mar-2019 | AMP, AMO, EST, SUT, TET, ERI | blaTEM-1B, strA, strB, sul2, dfrA5, sitABCD2, tet(A) | air, chuA, cia, cvaC, eilA, etsC, gad, hlyF, iroN, iss, iucC2, iutA, kpsE, kpsMII_K1, lpfA, mchF, neuC, ompT, sitA, terC | ST69 | O17/O44: H18 or O17/O77:H18 | IncFII, IncFIB2 |
1: AMP: ampicillin; AMO: amoxicillin; CFP: cefoperazone; CIP: ciprofloxacin; ENR: enrofloxacin; NEO: neomycin; EST: streptomycin; FFC: florfenicol; SUT: sulfamethoxazole/trimethoprim; TET: tetracycline; ERI: erythromycin. 2: non-perfect match, identity < 100%. 3: MLST: Multilocus Sequence Typing. 4: ND: not determined. Antimicrobial resistance genes: beta-lactam: blaTEM-1B; quinolone: gyrA and parC mutations, qnrB19, qnrB5, qnrB81, qnrB82, qnrS1; aminoglycoside: strA, strB, aadA1, aadA2; trimethoprim: dfrA5, dfrA8; tetracycline: tetA, tetB; sulfonamide: sul2; phenicol: floR; hydrogen peroxide: sitABCD. Virulence genes: glutamate decarboxylase: gad; adhesins: lpfA, F17, air, eilA (regulator), hra; capsule: kpsE, kpsMII_K1, neuC; iron uptake: sitA, fyuA, irp2, iroN, iucC, chuA, iutA; colicin: cia, cvaC, mchF; increased serum survival: iss; tellurium iron resistance protein: terC; hemolysin: hlyF; outer membrane protein: ompT, traT, etsC
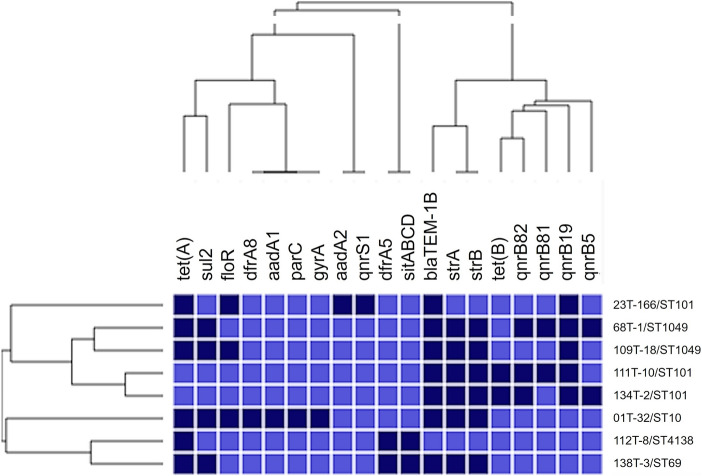
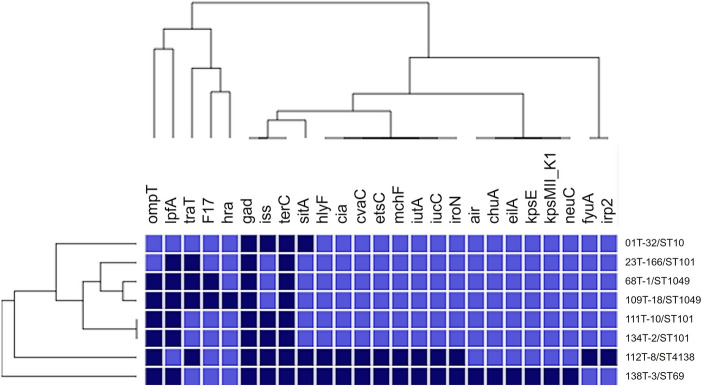
The k-mers approach, available on CGE services (https://cge.food.dtu.dk/services), allowed us to characterize the strains regarding antimicrobial resistance determinants, virulence genes, sequences type (ST), serotypes, and plasmid types. Table 1, Figs. 1 and 2 show the results.
Fig. 1.
Heatmap showing hierarchical clustering of antimicrobial resistance genes found in multidrug-resistant Escherichia coli isolated from bovine mastitis in Brazil. The columns represent genes, and the rows represent sample name and ST (sequence type from Multilocus Sequence Typing, MLST). Dark blue cells indicate gene presence, and light blue its absence. Hierarchical clustering was performed using the software Morpheus (https://software.broadinstitute.org/morpheus), binary distance matrix, and Pearson’s correlation
Fig. 2.
Heatmap showing hierarchical clustering of virulence genes found in multidrug-resistant Escherichia coli isolated from bovine mastitis in Brazil. The columns represent genes, and the rows represent sample name and ST (sequence type from Multilocus Sequence Typing, MLST). Dark blue cells indicate gene presence, and light blue its absence. Hierarchical clustering was performed using the software Morpheus (https://software.broadinstitute.org/morpheus), binary distance matrix, and Pearson’s correlation
Of the eight strains, the most frequent antimicrobial resistance genes were blaTEM-1B (7/8), tetA (6/8), strA (6/8), strB (6/8), and qnrB19 (5/8). Figure 1 shows two clusters based on antimicrobial resistance genes. The cluster with strains 23 T-166, 68 T-1, 109 T-18, 111 T-10, and 134 T-2 presents resistance genes to beta-lactam, aminoglycoside, quinolone, and tetracycline. All five strains on this cluster harbored blaTEM-1B and one qnrB gene. Indeed, blaTEM-1B gene is common in E. coli from animals, conferring resistance to beta-lactams, such as ampicillin, amoxicillin, and cefoperazone [10]. Moreover, an increase in the reports of quinolone resistance genes in Latin America has been observed, with the qnrB19 being the most detected qnrB variant [11].
The five strains that harbored plasmid-mediated quinolone resistance genes (qnr genes) did not show phenotype resistance by disk diffusion. The strain with gyrA and parC mutation (01 T-32, Table 1) was the only showing phenotype resistance to quinolone. The absence of the resistant phenotype can be due to the low level of resistance expressed in plasmid-mediated genes [12] or due to providing resistance to first-generation quinolones not tested in this study. The genes sul2 and aadA2 found in three strains (23 T-166, 68 T-1, 109 T-18) (Table 1) also did not show a sulfonamide and aminoglycoside resistant phenotype, respectively. This result could be attributed to plasmid-mediated resistance to other drugs not tested, or just non-expression of the genes. We believe that the erythromycin resistances found here were provided by low permeability in E. coli, since erythromycin-resistant determinants were not found [13].
We found five MLST types (ST10, ST101, ST1049, ST4138, ST69), and the ST10, ST101, and ST69 were found to be associated with pathogenicity and antimicrobial resistance spread [14–16].
The strain 01 T-32 (ST10) harbored ten of the 19 antimicrobial resistance genes detected (Table 1). These 10 genes encode beta-lactam, quinolone, aminoglycoside, trimethoprim, phenicol, sulfonamide, and tetracycline resistance.
The ST10 clone belongs to the group with worldwide spread. According to Fuga et al. [14]—which studied E. coli isolated from humans, animals, food, and the environment—this lineage has been circulating in Brazil since 1989, predominantly in environmental isolates. Here, we identified multidrug-resistant E. coli ST10 from bovine mastitis similar to those reported in other countries [17–19].
ST101 was found on three farms (A, D, and E). Strains from farm D (111 T-10) and farm E (134 T-2) showed the same plasmid profile (Inc group) (Table 1). These strains also showed a similar profile of antimicrobial resistance and virulence genes forming a cluster (Figs. 1 and 2). There are no reports of ST101 in mastitis isolates to date. However, this lineage is widespread among humans, animals, and the environment [15].
The strain belonging to the ST69 (138 T-3) harbored 20 of the 25 virulence genes detected (Table 1, Fig. 2); encoding adhesins, capsule, enzymes/proteins, increased serum survival, hemolysin, colicins, and iron uptake. The pandemic clone ST69 is associated with Extraintestinal Pathogenic E. coli (ExPEC) and has been isolated predominantly from human urinary tract infections [14, 16, 19]. Recently, the circulation of ST69 was reported in dairy cattle, suggesting the dissemination to other hosts [19]. On the other hand, the detection of air (enteroaggregative immunoglobulin repeat protein) and the eilA gene (Salmonella HilA homolog) can designate the strain ST69 (138 T-3) as Enteroaggregative E. coli (EAEC) harboring ExPEC typic virulence factors [20] or a combination of both pathotypes [21]. EAEC strains are associated with pathogenicity in humans, and there are no EAEC reports on animal infections. EAEC findings from environmental samples have been associated with human pollution [21].
Two strains were assigned to the O117 serogroup and one to the O8:H25 serotype, which harbored hlyF gene (hemolysin F) (112 T-8). This serotype has been associated with atypical enteropathogenic E. coli isolated from meat in Southeast Brazil [22], a relevant fact for local epidemiology.
The plasmid groups found were IncFII (7/8), IncFIB (6/8), Col(pHAD28) (4/8), IncX1 (3/8), p0111 (1/8), IncR (1/8), IncY (1/8), and IncP1 (1/8). The IncFIB and IncFII have been identified as the most frequent plasmids circulating in Brazil [14].
Genetic diversity was found for virulence genes, mainly in strains 112 T-8 (ST4138) and 138 T-3 (ST69) (Fig. 2).
Most virulence genes were related to the ExPEC pathotype, except for the genes hra (heat-resistant agglutinin), air, eilA, and terC (tellurium ion resistance protein) [23]. We highlight the identification of virulence genes associated with human pathogenic E. coli: hra (heat-resistant agglutinin, Enteroaggregative E. coli), hlyF (hemolysin F, Neonatal meningitis–associated E. coli), and air (enteroaggregative immunoglobulin repeat protein, Enteroaggregative E. coli).
Although our isolates did not harbor any of the genes proposed by Jung et al. [24] as mastitis pathogenic E. coli (MPEC) markers, our results showed some virulence factors reported in bovine mastitis-associated E. coli. These genes are iss (increased serum survival), lpfA (long polar fimbriae), traT (outer membrane protein complement resistance), F17 (fimbrial adhesin), fyuA (siderophore receptor), irp2 (yersiniabactin biosynthetic protein), and cva (microcin) [25]. This result supports the hypothesis of MPEC as a subgroup of ExPEC [25].
There are few data on virulence factors associated with E. coli mastitis in Brazil [26, 27] and few studies aimed at detecting specific markers. However, these markers may not be directly involved in the infection, given the diversity of virulence factors observed in these isolates [25]. In this context, broader studies using WGS raw data analysis can improve the monitoring and provide data for the etiology and epidemiology of mastitis in dairy herds.
We used k-mers to facilitate the molecular characterization of the mastitis-associated E. coli and thus contribute to the epidemiological studies. We showed that genome-based characterization using WGS raw data provides complete information that can be applied in the absence of resources for sophisticated bioinformatics analyses. Our objective is not to refute the analyses elaborated since they are crucial for the understanding of the genetic, evolutionary, and epidemiological contexts.
Conclusion
Overall, we observed that raw data evaluated by k-mers allowed us to characterize the antimicrobial resistance determinants, virulence genes, serotypes, MLST, and plasmid types of mastitis-associated E. coli strains. We found antimicrobial resistance genes for the main antimicrobial classes used in humans and animals, and virulence factors associated with ExPEC and EAEC. Additionally, three pandemic clones (ST10, ST101, and ST 69) were identified as well.
Our results may contribute to the knowledge of virulence, epidemiology, and antimicrobial susceptibility pattern of E. coli strains obtained from clinical cases of bovine mastitis. The use of raw data and online tools avoid complex bioinformatics processing and analysis that are not yet the expertise of many laboratories in Brazil and in other developing countries.
Acknowledgements
We thank the São Paulo Research Foundation (FAPESP), grant 2015/19688-8. The authors thank Espaço da Escrita—Pró-Reitoria de Pesquisa—UNICAMP—for the language services provided.
Author Contributions
Conceptualization: TdSA, DdSL. Formal analysis: TdSA. Methodology: TdSA, VSR, STG, SFJ, FFG. Resources: HL, JCdFP, SBL, VLMR, RTH, MGR, DdSL. Supervision: DdSL. Writing: TdSA. Review: MGR, RTH.
Funding
This study was funded by the São Paulo Research Foundation (FAPESP), grant 2015/19688-8.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval
Ethics Committee on Animal Use (CEUA) from School of Veterinary Medicine and Animal Science (FMVZ), São Paulo State University (UNESP), Botucatu, São Paulo State, Brazil; protocol number 2015/19688-8.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dego OK (2020) Bovine mastitis: part I. In Animal reproduction in veterinary medicine. IntechOpen. https://www.intechopen.com/online-first/bovine-mastitis-part-i, 10.5772/intechopen.93483.
- 2.Oliver SP, Murinda SE. Antimicrobial resistance of mastitis pathogens. Vet Clin North Am Food Anim Pract. 2012;28(2):165–185. doi: 10.1016/j.cvfa.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Cantas L, Shah SQ, Cavaco LM, Manaia CM, Walsh F, Popowska M, Garelick H, Bürgmann H, Sørum H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front Microbiol. 2013;4:96. doi: 10.3389/fmicb.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinzón-Sánchez C, Ruegg PL. Risk factors associated with short-term post-treatment outcomes of clinical mastitis. J Dairy Sci. 2011;94:3397–3410. doi: 10.3168/jds.2010-3925. [DOI] [PubMed] [Google Scholar]
- 5.Holt JG, editor. Bergey's manual of determinative bacteriology. 9. Baltimore: Williams & Wilkins; 1994. [Google Scholar]
- 6.CLSI . Performance standards for antimicrobial susceptibility testing: CLSI supplement M100S. 30. Wayne: Clinical and Laboratory Standards Institute; 2020. [Google Scholar]
- 7.CLSI . Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals (CLSI VET 015) 5. Wayne: Clinical and Laboratory Standards Institute; 2020. [Google Scholar]
- 8.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Liljequist BO, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 9.Andrews S (2010) FastQC—a quality control tool for high throughput sequence data. Babraham Bioinformatics. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 10.Poirel L, Madec JY, Lupo A, Schink AK, Kieffer N, Nordmann P, Schwarz S. Antimicrobial resistance in Escherichia coli. Microbiol Spectrum. 2018;6(4):ARBA-0026-2017. doi: 10.1128/microbiolspec.ARBA-0026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira DC, Lima WG, Paiva MC. Plasmid-mediated quinolone resistance (PMQR) among Enterobacteriales in Latin America: a systematic review. Mol Biol Rep. 2020;47:1471–1483. doi: 10.1007/s11033-019-05220-9. [DOI] [PubMed] [Google Scholar]
- 12.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22(4):664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes C, Martínez-Puchol S, Palma N, Horna G, Ruiz-Roldán L, Pons MJ, Ruiz J. Macrolide resistance mechanisms in Enterobacteriaceae: focus on azithromycin. Crit Rev Microbiol. 2017;43(1):1–30. doi: 10.3109/1040841X.2015.1136261. [DOI] [PubMed] [Google Scholar]
- 14.Fuga B, Sellera FP, Cerdeira L, Esposito F, Cardoso B, Fontana H, Moura Q, Cardenas-Arias A, Sano E, Ribas RM, Carvalho AC, Tognim MCB, Morais MMC, Quaresma AJPG, Santana ÂP, Reis JN, Pilonetto M, Vespero EC, Bonelli RR, Cerqueira AMF, Sincero TCM, Lincopan N. WHO critical priority Escherichia coli as one health challenge for a post-pandemic scenario: genomic surveillance and analysis of current trends in Brazil. Microbiol Spectr. 2022;10(2):e0125621. doi: 10.1128/spectrum.01256-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos ACM, Silva RM, Valiatti TB, Santos FF, Santos-Neto JF, Cayô R, Streling AP, Nodari CS, Gales AC, Nishiyama-Jr MY, Carvalho E, Gomes TAT. Virulence potential of a multidrug-resistant Escherichia coli strain belonging to the emerging clonal group ST101-B1 isolated from bloodstream infection. Microorganisms. 2020;8(6):827. doi: 10.3390/microorganisms8060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denamur E, Clermont O, Bonacorsi S, Gordon D. The population genetics of pathogenic Escherichia coli. Nat Rev Microbiol. 2021;19:37–54. doi: 10.1038/s41579-020-0416-x. [DOI] [PubMed] [Google Scholar]
- 17.Kempf F, Slugocki C, Blum SE, Leitner G, Germon P. Genomic comparative study of bovine mastitis Escherichia coli. PLoS ONE. 2016;11(1):e0147954. doi: 10.1371/journal.pone.0147954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leimbach A, Poehlein A, Vollmers J, Görlich D, Daniel R, Dobrindt U. No evidence for a bovine mastitis Escherichia coli pathotype. BMC Genomics. 2017;18(1):359. doi: 10.1186/s12864-017-3739-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nüesch-Inderbinen M, Käppeli N, Morach M, Eicher C, Corti S, Stephan R. Molecular types, virulence profiles and antimicrobial resistance of Escherichia coli causing bovine mastitis. Vet Rec Open. 2019;6(1):e000369. doi: 10.1136/vetreco-2019-000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paramita RI, Nelwan EJ, Fadilah F, Renesteen E, Puspandari N, Erlina L. Genome-based characterization of Escherichia coli causing bloodstream infection through next generation sequencing. PLoS ONE. 2020;15(12):e0244358. doi: 10.1371/journal.pone.0244358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins C. Enteroaggregative Escherichia coli. In: Frankel G, Ron E, editors. Escherichia coli, a versatile pathogen: current topics in microbiology and immunology. Switzerland: Springer; 2018. pp. 27–50. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe RHS, Vieira MA, Mariano NAB, Dias RCB, da Silva RV, Castro CM, Dos Santos LF, Camargo CH, Yamatogi RS, Rall VLM, Hernandes RT. Identification and characterization of atypical enteropathogenic and Shiga toxin-producing Escherichia coli isolated from ground beef and poultry breast purchased in Botucatu. Brazil Braz J Microbiol. 2019;50(4):1099–1103. doi: 10.1007/s42770-019-00101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G, Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung D, Park S, Ruffini J, Dussault F, Dufour S, Ronholm J. Comparative genomic analysis of Escherichia coli isolates from cases of bovine clinical mastitis identifies nine specific pathotype marker genes. Microb Genom. 2021;7(7):000597. doi: 10.1099/mgen.0.000597. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Goulart DB, Mellata M. Escherichia coli mastitis in dairy cattle: etiology, diagnosis, and treatment challenges. Front Microbiol. 2022;13:928346. doi: 10.3389/fmicb.2022.928346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerra ST, Paula CL, Bolaños CAD, Hernandes RT, Ribeiro MG. Virulence factors of Escherichia coli: an overview of animal and human infections with emphasis in bovine mastitis. Semin Cienc Agrar. 2019;40(5):2087–2100. doi: 10.5433/1679-0359.2019v40n5p2087. [DOI] [Google Scholar]
- 27.Guerra ST, Orsi H, Joaquim SF, Guimarães FF, Lopes BC, Dalanezi FM, Leite DS, Langoni H, Pantoja JCF, Rall VLM, Hernandes RT, Lucheis SB, Ribeiro MG. Investigation of extra-intestinal pathogenic Escherichia coli virulence genes, bacterial motility, and multidrug resistance pattern of strains isolated from dairy cows with different severity scores of clinical mastitis. J Dairy Sci. 2020;103:3606–3614. doi: 10.3168/jds.2019-17477. [DOI] [PubMed] [Google Scholar]