Abstract
In the current study, we hypothesized that an increase in dietary ileal indigestible protein concentration induces an increase in hindgut nitrogen utilization and nitrogen excretion and a shift in fecal microbiota in growing pigs, when compared to pigs given a high total protein diet. Three diets were prepared: 1) standard protein diet based on corn and soybean meal, 2) high-indigestible protein diet in which autoclaved, low-digestible soybean meal replaced soybean meal in the first diet, and 3) high protein diet where the inclusion rate of soybean meal was greater than that of the other diets. The 3 diets were fed to 18 barrows that were fitted with T-cannula at the ileo-cecal junction (initial body weight = 63.4 ± 8.0 kg) in a randomized complete block design with body weight as a blocking factor. Pigs were individually housed in pens and the experiment lasted for 23 d. On days 7 and 21, fecal samples were collected by rectal massage for microbiota analysis. Grab samples of feces were collected on days 20 and 21, and ileal digesta were collected on days 22 and 23 for the determination of energy and nitrogen utilization. Lower apparent ileal digestibility of nitrogen in the high-indigestible protein diet containing autoclaved soybean meal resulted in greater ileal indigestible nitrogen concentration (P < 0.05). Apparent total tract digestibility of nitrogen was lower (P < 0.05), and correspondingly nitrogen concentration and daily fecal nitrogen output were greater (P < 0.05) in the high-indigestible protein diet compared with the other diets. Apparent post-ileal digestibility and hindgut disappearance of nitrogen and gross energy were the greatest (P < 0.05) in the high protein diet, whereas a statistical difference was not observed in those variables between the standard protein diet and the high-indigestible protein diet. Beta diversity metrics of feces in the high-indigestible protein diet on day 21 were different (q < 0.05) from those in the other two diets, which indicates a shift in microbial communities. According to the results of the DESeq2, the direction of microbiota shift induced by the high-indigestible protein diet may have reduced fiber utilization in the hindgut. In conclusion, an increase in dietary ileal indigestible protein concentration increased fecal nitrogen excretion and shifted fecal microbial communities but did not increase nitrogen utilization in the hindgut.
Keywords: fermentation, indigestible protein, nitrogen excretion, swine
Changes in dietary ileal indigestible protein concentration may be more influential in fecal nitrogen excretion and fecal microbiota compared to changes in dietary total protein concentration.
Introduction
Swine nutritionists have been striving to reduce dietary protein concentration by replacing conventional protein sources such as soybean meal with crystalline amino acids (AA), which allows for meeting requirements of indispensable AA but reduces feed costs and nitrogen excretion (Wang et al., 2018). Reducing protein concentration in swine diets is also advantageous because energetic efficiency of protein is relatively devalued in the net energy system compared with the metabolizable energy system (Le Bellego and Noblet, 2002). Furthermore, reducing protein concentration in diets may be beneficial to the colonic microbial community and gut health of pigs (Heo et al., 2008). Proteins and peptides that are not absorbed by the end of the small intestine flow into the large intestine and are subsequently degraded into AA by microbial proteases and hydrolases in the hindgut which can be decarboxylated or deaminated (Lammers-Jannink, 2022). If AA are decarboxylated, amine and carbon dioxide are released. On the other hand, deamination of AA releases ammonia and keto acids. The resulting keto acids can be incorporated into bacteria as microbial proteins or enter catabolic pathways. Keto acids can provide energy to intestinal cells in the hindgut or be fermented into products such as fatty acids, indole, and phenol by hindgut microbiota. Although not all of these products are detrimental (Oliphant and Allen-Vercoe, 2019), efforts to minimize hindgut microbial nitrogen metabolism is ongoing because of the potential toxicity of some products such as amine, ammonia, indole, and phenol at high concentration. The intestinal nitrogenous compounds include undigested dietary protein, endogenous loss, and microbial protein. Among these factors, the amount of intestinal nitrogenous compounds is largely affected by diet in most cases. Hindgut nitrogen metabolism is likely to be lower in response to a decrease in dietary protein concentration, which leads to decreased proliferation of bacteria preferentially utilizing nitrogen such as coliforms and pathogenic E.coli, as well as decreased production of detrimental metabolites (Heo et al., 2008).
Bioavailability of dietary protein is another factor affecting hindgut nitrogen metabolism because the amount of nitrogen that flows into the large intestine is also affected by ileal digestibility of protein in diets. For this reason, hindgut nitrogen utilization and fecal nitrogen excretion may be more correlated with dietary ileal indigestible protein concentration compared with total protein concentration in diets. However, information on the effect of ileal indigestible protein on fecal nitrogen excretion and fecal microbiota is limited. In the most recent studies, ileal nitrogen flow was not measured, and experimental diets were formulated to reduce total protein concentrations (Yu et al., 2019; Zhang et al., 2020; Wang et al., 2021). Furthermore, dietary fiber concentration was slightly changed because dietary protein concentration was modified by partially replacing grain sources (e.g., corn and wheat) with a protein source (e.g., soybean meal). Considering that dietary fiber is one of the critical factors affecting nutrient digestibility and microbial communities (Jha and Berrocoso, 2016), the previous studies might have been confounded. For this reason, Elling-Staats et al. (2022) suggested that applying heat damage on feed ingredients may be a suitable model for contrasting indigestible protein in diets without changing dietary fiber, and autoclaving is widely used to induce heat damage of feeds in animal nutrition studies (Sung et al., 2022b). For these reasons, the objective of the current study was to test the hypothesis that an increase in dietary ileal indigestible protein concentration induces an increase in hindgut nitrogen utilization and fecal nitrogen excretion and a shift in fecal microbiota in growing pigs, when compared to pigs given a high total protein diet. Contrast in ileal indigestible protein concentration among diets was created by autoclaving.
Materials and Methods
All protocols used in the study were approved by the Purdue University Animal Care and Use Committee (West Lafayette, IN).
Diets, animals, and experimental design
Soybean meal from the same batch was divided into two portions and, one of these portions was autoclaved at 135 °C for 60 min, while the other was not autoclaved. Dietary treatments consisted of a standard protein diet, high-indigestible protein diet, and high protein diet (Table 1). The standard protein diet or high-indigestible protein diet contained 216 g/kg of non-autoclaved or autoclaved soybean meal, respectively. The standard protein diet met standardized ileal digestible AA requirements (NRC, 2012) by supplementing lysine, methionine, and threonine, which are the most widely used crystalline AA in the industry. The inclusion rate of non-autoclaved soybean meal in the high protein diet was 286 g/kg to increase total protein concentration by 30 g/kg compared to the other diets. Three diets were fed to 18 barrows that were fitted with T-cannula at the ileo-cecal junction (initial body weight = 63.4 ± 8.0 kg; Dilger et al., 2004) in a randomized complete block design with body weight as a blocking factor. Pigs were individually housed in pens equipped with a feeder and a nipple drinker. Daily feed allowance was provided at 3.5% of mean body weight of each block.
Table 1.
Ingredient and chemical composition of the experimental diets (as-fed basis)1
| Item | Standard protein | High-indigestible protein | High protein |
|---|---|---|---|
| Ingredient, g/kg | |||
| Corn | 418.7 | 418.7 | 418.7 |
| SBM | 216.0 | - | 286.0 |
| Autoclaved SBM2 | - | 216.0 | - |
| Cornstarch | 250.0 | 250.0 | 183.5 |
| Wheat bran | 50.0 | 50.0 | 50.0 |
| Soybean oil | 10.0 | 10.0 | 10.0 |
| MCP | 7.1 | 7.1 | 7.1 |
| Limestone | 10.8 | 10.8 | 10.8 |
| Salt | 4.0 | 4.0 | 4.0 |
| L-Lysine HCl | 2.1 | 2.1 | - |
| DL-Methionine | 0.7 | 0.7 | - |
| L-Threonine | 0.7 | 0.7 | - |
| Cr2O3 premix3 | 25.0 | 25.0 | 25.0 |
| Vit-min premix4 | 5.0 | 5.0 | 5.0 |
| Total | 1,000.0 | 1,000.0 | 1,000.0 |
| Analyzed composition5 | |||
| DM, g/kg | 866 | 862 | 867 |
| GE, kcal/kg | 3,840 | 3,799 | 3,872 |
| CP, g/kg | 137 | 138 | 166 |
| NDF, g/kg | 63 | 129 | 67 |
1MCP, monocalcium phosphate; SBM, soybean meal; Vit-min premix, vitamin-mineral premix.
2Autoclaved at 135 °C for 60 min.
35 g chromic oxide plus 20 g ground corn.
4Provided the following quantities per kg of complete diet: vitamin A, 8,575 IU; vitamin D3, 4,300 IU; vitamin E, 28.6 IU; menadione, 7.3 mg; riboflavin, 9.2 mg; D-pantothenic acid, 18.3 mg; niacin, 73.5 mg; choline chloride, 1,285 mg; vitamin B12, 0.02 mg; biotin, 0.09 mg; thiamine mononitrate, 3.7 mg; folic acid, 1.7 mg; pyridoxine hydrochloride, 5.5 mg; I, 1.9 mg as calcium iodate; Mn, 180 mg as manganese sulfate; Cu, 7.4 mg as copper sulfate; Fe, 73.5 mg as ferrous sulfate; Zn, 180 mg as zinc sulfate; Se, 0.4 mg.
5CP, crude protein; DM, dry matter; GE, gross energy; NDF, neutral detergent fiber.
Sampling
On the morning of days 7 and 21, fecal samples were collected by rectal massage, and subsamples were immediately transferred to MagAttract PowerMicrobiome DNA/RNA kit (Qiagen, Artois, CA). For energy and nitrogen digestibility estimation, grab samples of feces were collected on days 20 and 21. On days 22 and 23, plastic sample bags (Whirl-Pak bag, NASCO, Fort Atkinson, WI) containing 10 mL of 10% formic acid were attached to T-cannulas to collect ileal digesta samples for 9 h. Plastic sample bags were changed every 30 min and immediately stored at −20 °C. Frozen ileal digesta samples were slightly thawed, pooled within each pig, and stored again at −20 °C.
Chemical analysis
Frozen ileal digesta samples were lyophilized. Fecal samples collected by grab sampling were dried at 55 °C in a forced-air drying oven until constant weight. Samples of soybean meal, experimental diets, ileal digesta, and feces were finely ground. Ground samples were analyzed for dry matter by drying at 105 °C for 24 h in a forced-air drying oven (Precision Scientific Co., Chicago, IL; method 934.01; AOAC, 2005) and for nitrogen by the combustion method (model FP2000; LECO Corp., St. Joseph, MI; method 990.03; AOAC, 2005). The concentration of crude protein was calculated by multiplying the product of nitrogen concentration and 6.25. Ground samples of the experimental diets, ileal digesta, and feces were analyzed for the concentration of chromium as reported by Fenton and Fenton (1979). Ground samples were also analyzed for energy by isoperibol bomb calorimeter (model 6200; Parr Instrument Co., Moline, IL). In addition, ground samples of the experimental diets were analyzed for neutral detergent fiber (Van Soest et al., 1991).
Microbiome library preparation and analysis
Total DNA was extracted from fecal samples collected on days 7 and 21 using the MagAttract PowerMicrobiome DNA/RNA kit (Qiagen, Artois, CA) based on the manufacturer’s procedures. The V4 region of 16S rRNA gene library preparation was conducted based on the procedure of Abraham et al. (2021). Sequences of 16S amplicons were analyzed using Qiime2 (Version 2021.11). Overlapped paired-end reads were denoised by the DADA2 pipeline and by removing the first 13 bases in the forward and reverse reads. The representative sequences were extracted using “feature-table” plugins, and assigned by taxonomy via “feature-classifier”. Amplicon sequence variants (ASV) were classified with 99% similarity against the Silva 138 database (Quast et al., 2013). Samples were rarefied at 20,000 sequences based on rarefaction plotting. As a result, four samples at days 7 and 21 were excluded from the final analysis. Overall, the number of observations for microbiota analysis at days 7 and 21 in each diet was five (standard protein diet), five (high-indigestible protein diet), and four (high protein diet), respectively. The function of “qiime diversity core-metrics-phylogenetic” was used to generate alpha and beta diversity distance matrices. The functional profiles of metabolic pathways in each diet were investigated using PICRUSt2 (Version 2.4.2; phylogenetic investigation of communities by reconstruction of unobserved states; Douglas et al., 2020).
Calculation and statistical analysis
The apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) of nitrogen were calculated using the index method (Sung et al., 2020).
AID or ATTD of nitrogen (%) = 100 × [1 − (dietary chromium ÷ ileal or fecal chromium) ÷ (dietary nitrogen ÷ ileal or fecal nitrogen)].
Chromium and nitrogen concentrations are expressed as g/kg dry matter.
Apparent post-ileal digestibility (APID) of nitrogen defined as the proportion of ileal indigestible nitrogen that is digested and absorbed in the large intestine was also calculated as follows:
APID of nitrogen (%) = 100 × [1 − (ileal chromium ÷ fecal chromium) ÷ (ileal nitrogen ÷ fecal nitrogen)].
Hindgut disappearance of nitrogen was calculated as follows:
Hindgut disappearance, % = ATTD (%) − AID (%).
Daily fecal nitrogen output was calculated as follows:
Daily fecal nitrogen output (g/d) = nitrogen concentration in diet (g/kg) × daily feed intake (kg/d) × [100 − ATTD of nitrogen (%)] ÷ 100.
Values of AID, ATTD, APID, and hindgut disappearance of gross energy (GE) were also calculated using the aforementioned equations.
Energy and nitrogen utilization data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC) as a randomized complete block design using a pig as the experimental unit and model that included diet as a fixed variable and block as a random variable. Least square means for each treatment were calculated, and differences among least squares means were tested using the PDIFF option with Tukey’s adjustment.
For microbiome analysis, R (Version 4.1.3) was used. Analysis of variance followed by Tukey’s post hoc test was used if alpha diversity metrics were normally distributed, otherwise the Kruskal–Wallis test was conducted. Principal coordinate analysis was conducted to estimate dissimilarity of microbiota communities. Significance in dissimilarity was determined in Qiime2 using the permutational multivariate analysis of variance test, and the false discovery rate-adjusted P-value was used for multiple comparisons. Difference in abundance of genus and metabolic pathways were determined using the DESeq2 and PICRUSt2 functions, respectively. Detail in selected metabolic pathways was adapted from https://metacyc.org/. Alpha level of 0.05 was used to declare significance. Qiime2 commands and R scripts are available at https://github.com/Jung-purdue/2021-31-Indigestible-protein-in-pigs.
Results
The AID of GE was greater (P < 0.05) in the standard protein diet compared with the high-indigestible protein diet and high protein diet (Table 2). The AID of nitrogen was the lowest in the high-indigestible protein diet, which resulted in the highest ileal indigestible nitrogen concentration (P < 0.05). In terms of total tract digestibility, ATTD of GE and nitrogen in the high-indigestible protein diet was lower (P < 0.05) than that of the other diets. Fecal nitrogen concentration, daily fecal nitrogen output, and total tract indigestible nitrogen were the greatest (P < 0.05) in the high-indigestible protein diet. The APID of GE and nitrogen as well as hindgut disappearance were greater (P < 0.05) in the high protein diet compared with the other two diets.
Table 2.
Energy and nitrogen utilization in the experimental diets fed to growing pigs1
| Item2 | Standard protein | High-indigestible protein | High protein | SEM | P-value |
|---|---|---|---|---|---|
| Ileal | |||||
| AID of GE, % | 77.5a | 70.9b | 70.4b | 1.2 | 0.003 |
| AID of N, % | 75.5a | 48.6b | 72.6a | 1.5 | <0.001 |
| Ileal indigestible N, g/kg | 5.4c | 11.3a | 7.3b | 0.3 | <0.001 |
| Total tract | |||||
| ATTD of GE, % | 87.0a | 80.3b | 87.4a | 0.7 | <0.001 |
| ATTD of N, % | 82.3a | 54.6b | 85.0a | 1.4 | <0.001 |
| Fecal N concentration, g/kg | 32.1b | 58.8a | 33.7b | 0.9 | <0.001 |
| Total tract indigestible N, g/kg | 3.9b | 10.0a | 4.0b | 0.3 | <0.001 |
| Fecal N output, g/d | 9.5b | 24.5a | 9.8b | 1.1 | <0.001 |
| Hindgut, % | |||||
| Hindgut disappearance of GE | 9.5b | 9.4b | 17.0a | 1.1 | 0.001 |
| Hindgut disappearance of N | 6.8b | 5.9b | 12.6a | 1.6 | 0.020 |
| APID of GE | 41.5b | 32.1b | 57.5a | 2.9 | <0.001 |
| APID of N | 26.8b | 11.4b | 46.0a | 4.5 | 0.001 |
1Each least squares mean represents six observations except for AID in high protein diet (n = 5).
2AID, apparent ileal digestibility; APID, apparent post-ileal digestibility; ATTD, apparent total tract digestibility; GE, gross energy; N, nitrogen.
a,b,cLeast squares means within a row without a common superscript differ (P < 0.05).
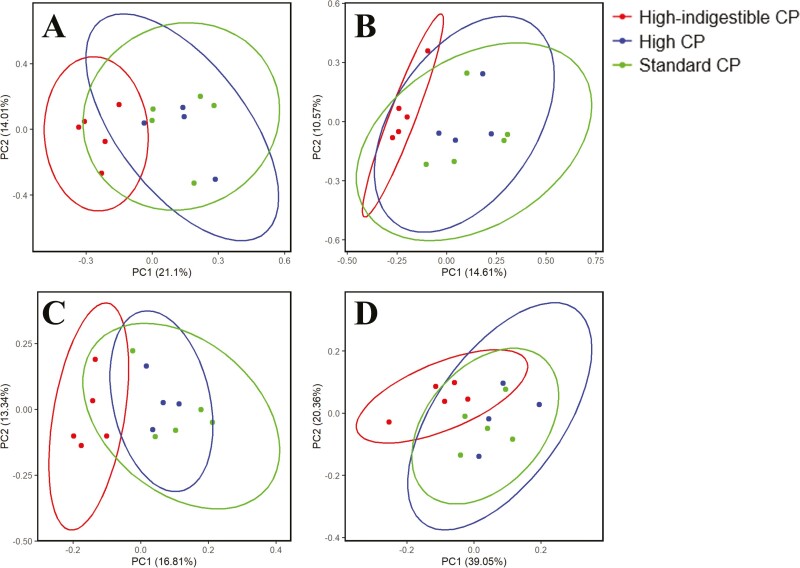
For fecal microbial communities, differences in alpha diversity metrics on days 7 and 21 (Shannon index, Pielou’s evenness, Observed features, and Faith PD) due to dietary treatments were not observed. Differences in beta diversity metrics (Bray–Curtis, Jaccard, Unweighted UniFrac, and Weighted UniFrac) were not observed on day 7, but on day 21, the high-indigestible protein diet group was different (q < 0.05) from those of the standard protein and high protein diet groups by all beta diversity metrics indicating that fecal microbiota was shifted by changing the ileal indigestible protein concentration (Figure 1).
Figure 1.
Beta diversity at day 21 (Standard CP = standard protein diet, High-indigestible CP = high-indigestible protein diet, and High CP = high protein diet). Fecal microbiome community (A: Bray-Curtis, B: Jaccard, C: Unweighted UniFrac, D: Weighted UniFrac) in the high-indigestible protein diet was statistically different from the standard protein diet and high protein diet (q < 0.05). No statistical difference was observed between the standard protein diet and high protein diet.
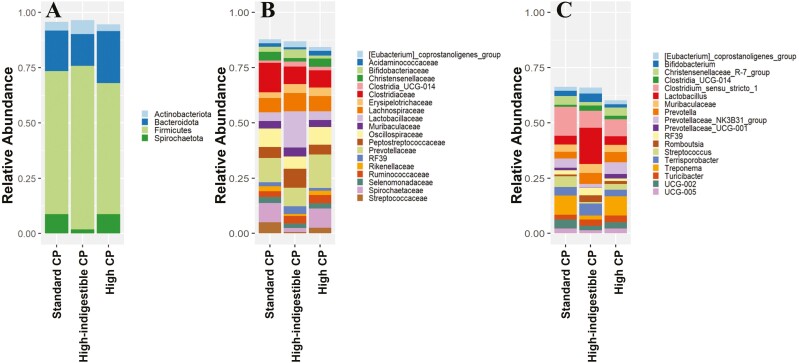
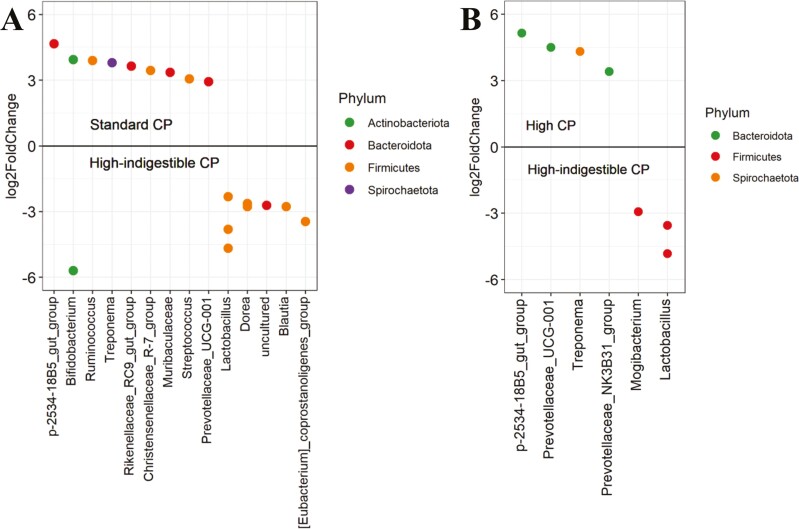
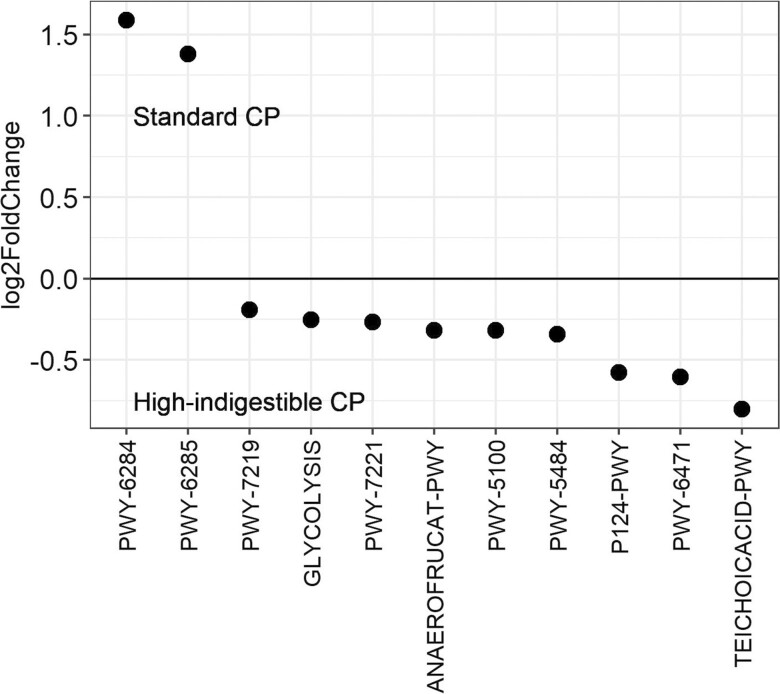
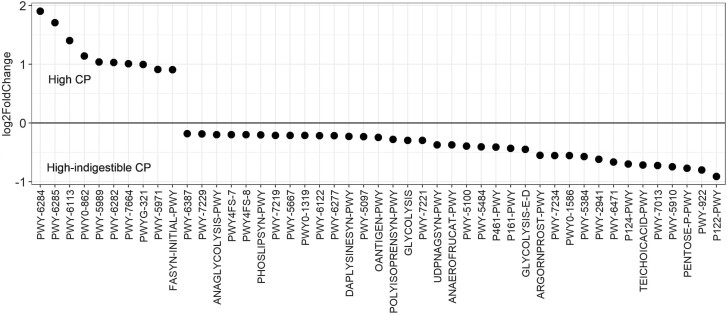
To see if this shift was beneficial or detrimental to the host animal, the average relative abundance of the fecal microbiota was compared, and changes in ASV of each genus were statistically compared. On day 21, the sum of the relative abundance of Bacteroidota and Firmicutes was greater in the high-indigestible protein diet (Figure 2A). At the genus level, multiple Lactobacillus ASV increased in relative abundance, whereas the relative abundance of p-2534-18B5_gut_group, Prevotellaceae_UCG-001, and Treponema ASV decreased in the high-indigestible protein diet compared with both the other diets (P < 0.05; Figure 3). Based on PICRUST2, pathways involved in long-chain fatty acids synthesis (PWY-6284, PWY-6285, PWY-6113, etc.) were less abundant (P < 0.05) in the high-indigestible protein diet compared with the other two diets on day 21 (Figures 4–6).
Figure 2.
Average relative abundance of the microbiota at day 21 classified at the phylum (A), family (B), and genus level (C); Standard CP = standard protein diet, High-indigestible CP = high-indigestible protein diet, and High CP = high protein diet. Taxa shown are those that are greater than 2% of the total community in at least one sample.
Figure 3.
Log2fold change in amplicon sequence variant at day 21 among the experimental diets (Standard CP = standard protein diet, High-indigestible CP = high-indigestible protein diet, and High CP = high protein diet). No statistical difference in genus level was observed between the standard protein diet and high protein diet. Values greater than zero indicate that ASV enriched in the standard protein diet (A) or the high protein diet (B) while values less than zero indicate ASV enriched in the high-indigestible protein diet.
Figure 4.
Comparison between the standard protein diet and high-indigestible protein diet at day 21 using PICRUSt2 (Standard CP = standard protein diet and High-indigestible CP = high-indigestible protein diet). Values greater than zero indicate enrichment in the standard protein diet, while values less than zero indicate enrichment in the high-indigestible protein diet.
Figure 6.
Detail in selected metabolic pathways from Figures 4 and 5 (Standard CP = standard protein diet, High-indigestible CP = high-indigestible protein diet, and High CP = high protein diet; detail was adapted from https://metacyc.org/).
Figure 5.
Comparison between the high-indigestible protein diet and high protein diet at day 21 using PICRUSt2 (High-indigestible CP = high-indigestible protein diet and High CP = high protein diet). Values greater than zero indicate enrichment in the high protein diet, while values less than zero indicate enrichment in the high-indigestible protein diet.
Discussion
In the current study, autoclaved soybean meal was included in the high-indigestible protein diet to induce a change in ileal indigestible protein concentration without altering dietary fiber concentration. Protein concentration in the high protein diet was formulated to be 30 g/kg greater compared with the standard protein diet, which has been widely used to formulate high protein diets for nursery and growing-finishing pigs (Fan et al., 2017; Chen et al., 2018; Yu et al., 2019). To our knowledge, the current study is the first to compare the effect of changes in ileal indigestible protein and total protein concentration in the diet of growing pigs. Because ileal indigestible protein concentration considers both protein concentration and quality of diets, an increase in dietary ileal indigestible protein concentration was expected to induce an increase in hindgut nitrogen utilization and fecal nitrogen excretion and a shift in fecal microbiota in growing pigs, when compared to pigs given a high total protein diet.
According to digestibility values of standard protein diet and high-indigestible protein diet, autoclaved soybean meal had lower AID and ATTD of GE and nitrogen compared to normal soybean meal as previously noted in a publication by González-Vega et al. (2011). Decreased nitrogen digestibility of autoclaved soybean meal is attributed to conversion of L-form AA into D-form AA (racemization) and binding between reducing sugars and free AA (Maillard reaction), which makes AA to be less biologically available (Sung et al., 2022b). The greater neutral detergent fiber concentration in the high-indigestible protein diet (129 g/kg) compared to the other two diets (63 and 67 g/kg, respectively) is also attributable to the Maillard reaction (Almeida et al., 2014). During autoclaving soybean meal, some of the products derived from the Maillard reaction form a lignin-like matrix which is contained in, and analyzed as neutral detergent fiber. For this reason, neutral detergent fiber concentration in soybean meal is one of the indicators of heat damage. However, there is no report on the biological function of heat damage-induced increase in neutral detergent fiber. The AID of GE was also less in the high protein diet compared with the standard protein diet, whereas there was no difference in ATTD of GE and nitrogen between the standard and high protein diets because hindgut disappearance was greater in pigs fed the high protein diet. Considering that fiber concentration would be expected to be greater in the high protein diet, the amount of undigested nutrients flowing into the large intestine would intuitively be greater because dietary fiber reduces the digestion and absorption of nutrients in the small intestine (Choi et al., 2020). In addition, microbes in the large intestine utilizes more dietary fiber compared to the small intestine, which also contributes to greater hindgut fermentation. However, there was little difference in dietary fiber concentration (63 and 67 g/kg neutral detergent fiber, respectively) and thus, the current numerical difference in hindgut disappearance of GE and nitrogen between the high protein diet and the other two diets was greater than expected. Furthermore, the proportion of ileal indigestible GE and nitrogen that is digested and absorbed in the large intestine was greater in the high protein diet considering greater values of APID of GE and nitrogen. The reason is not clear, but it is speculated that there was a synergetic interaction between nitrogen and fiber on hindgut fermentation in pigs fed the high protein diet. However, microbiota-related evidence (e.g., DESeq2 and PICRUSt2) of the greater hindgut utilization of GE and nitrogen in the high protein diet group compared to the standard protein group was not observed in the current study.
Fecal nitrogen concentration and daily fecal nitrogen output in the current study were more susceptible to change in ileal indigestible nitrogen compared to total nitrogen concentration in diets. Although formulating diets based on total tract indigestible nitrogen may be the most accurate to estimate fecal nitrogen excretion, using total tract indigestible nitrogen is not practical because ileal digestibility is the standard method of estimating nitrogen and AA digestibility (Sung et al., 2022a). Urinary nitrogen output generally increases with increasing total dietary protein concentration (Kim et al., 2022). However, urinary nitrogen is also dependent on the biological value of dietary nitrogen (NRC, 2012). To be specific, urinary nitrogen output increases when the efficiency of protein synthesis decreases because absorbed AA not used for synthesizing proteins are excreted through urine mostly as urea. Considering that lysine is heat-labile and a key AA for protein synthesis, urinary nitrogen output in pigs fed the high-indigestible protein diet in the current study would be expected to be the greatest because autoclaving soybean meal might induce a larger decrease in ileal digestible lysine compared to other AA, which causes AA imbalance (Sung et al., 2022b).
Hindgut disappearance and APID values in the high-indigestible protein diet were less compared to the high protein diet, and not different from those in the standard protein diet. The hypothesis of the current study, therefore, was not confirmed as hindgut nitrogen utilization was expected to be greatest in the high-indigestible protein diet group because of the greater amount of substrate. Rather, APID of nitrogen in the high-indigestible protein diet was numerically much less compared to the other two diets (11.4% vs. 26.8% and 46.0%), which indicates that the degree of nitrogen utilization in the large intestine was lower despite the greater amount of nitrogen flowing into the large intestine. Based on the current results, nitrogen utilization in the hindgut may be inhibited in an indigestible nitrogen-rich environment.
Nutrients flowing into the large intestine of pigs are extensively utilized by the hindgut microbial populations rather than the host animal (NRC, 2012). For this reason, the hindgut nutrient utilization may be largely dependent on the microbial population. The form or concentration of dietary protein fed to pigs at various stages of growth did not affect alpha diversity and beta diversity metrics in most of the previous studies (Luo et al., 2015; Chen et al., 2018; Zhang et al., 2020). Although increasing dietary protein concentration may increase the proliferation of some detrimental bacteria such as coliforms and pathogenic E.coli, the impact of this change might not be sufficient to induce overall change in microbial communities (Li et al., 2020). In the current study, there was no difference in alpha and beta diversity metrics except for beta diversity on day 21. The little change in diversity metrics on day 7 may be attributed to limited time for microbiota to adapt to diets because in some studies at least 3 wk of adaptation to a new diet are required for resilience of hindgut microbiota in growing pigs (Le Sciellour et al., 2018). Significant differences in beta diversity metrics on day 21 derived from the high-indigestible protein diet indicate that the impact of ileal indigestible protein on overall fecal microbial communities may be greater than that of total dietary protein concentration.
At the phylum level, the sum of the relative abundance of Bacteroidota and Firmicutes on day 21 was greater in the high-indigestible protein diet. Considering that many species in these phyla are anaerobic and nitrogen-fermenting (Wang et al., 2018), the direction of microbiota shift induced by the high-indigestible protein diet may have stimulated protein fermentation, which was not the case because of lower hindgut disappearance and APID of nitrogen. On day 21, three and two Lactobacillus ASV decreased in relative abundance in pigs fed the standard protein and high protein diets compared with the high-indigestible protein group. Generally, Lactobacillus is regarded to be beneficial (Zhang et al., 2020), but recent studies have revealed the potential role of Lactobacillus in proteolytic fermentation in the large intestine (Davila et al., 2013; Zhou et al., 2016; Yu et al., 2019). In the study of Zhou et al. (2016) and Yu et al. (2019), reducing protein concentrations in diets from 160 to 130 g/kg decreased relative abundance of Lactobacillus in the cecum and colonic digesta of growing-finishing pigs, respectively. From this point of view, it is not clear why a difference in the relative abundance of Lactobacillus between the standard protein (137 g/kg of protein) and high protein diet groups (166 g/kg of protein) was not observed in the current study. The discrepancy between the current and previous studies is partially explained by dietary fiber concentration. In the two previous studies (Zhou et al., 2016; Yu et al., 2019), the inclusion rate of wheat bran in the 160 g/kg-protein diets (60 g/kg) was greater than that in the 130 g/kg-protein diets (30 g/kg). Considering that wheat bran is a high-fiber ingredient (Choi et al., 2020), the difference in AID of nitrogen between the two diets is expected to have been greater in the previous studies compared with the current study, resulting in a greater difference in nitrogen flow to the hindgut. In contrast to the previous studies, the inclusion rate of wheat bran among diets was constant in the current study, and cornstarch in the standard protein diet was replaced by soybean meal to prepare the high protein diet. Despite the greater relative abundance of Lactobacillus in the high-indigestible protein diet group, which is a potential nitrogen-fermenting bacterium, nitrogen utilization was the numerically lowest in the high-indigestible protein diet group, which remains unclear.
On day 21, the relative abundance of p-2534-18B5_gut_group, Prevotellaceae_UCG-001, and Treponema ASV in feces was greater in pigs fed the standard protein and high protein diets compared to pigs fed the high-indigestible protein diet. These genera have been found to be abundant in an environment where fermentation or digestion of dietary fiber is active (Le Sciellour et al., 2018; Sutton et al., 2021; Qiu et al., 2022). Therefore, fiber utilization of the standard protein and high protein diets in the large intestine may have been greater compared to the high-indigestible protein diet.
Analysis of PICRUSt2 was conducted in the current study to predict the abundance of bacterial metabolic pathways based on community composition profiles (Douglas et al., 2020), and metagenome functions predicted by PIRCRUST2 may represent pathways that occurred in the swine intestinal tract bacterial community. Compared to the high-indigestible protein diet group, several pathways favoring biosynthesis of long-chain fatty acids (PWY-6284, PWY-6285, PWY-6113, etc.) were more abundant in the standard protein and high protein diet groups. Although intestinal microbiota can synthesize long-chain fatty acids by elongating short-chain fatty acids, these pathways may not be supportive of greater fiber fermentation because short-chain fatty acids such as acetate, propionate, and butyrate are major end-products of fiber fermentation rather than long-chain fatty acids (Jha and Berrocoso, 2016). Pathways TEICHOICACID-PWY and P122-PWY were more abundant in the high-indigestible protein group compared to the standard protein and high protein diet groups, respectively, which is supported by Kheirandish et al. (2022) where relative abundance of Lactobacillus was positively correlated with these pathways. The pathway P122-PWY is related to fermentation of carbohydrate to ethanol and lactate (Jensen et al., 2022). Considering that Lactobacillus is one of the bacteria which produces lactate, the increased relative abundance of multiple Lactobacillus ASV likely contributed to the increased abundance of P122-PWY in the high-indigestible protein group.
One of the limitations of the current research is a lack of equalization of ileal digestible indispensable AA. As AA digestibility of non-autoclaved and autoclaved soybean meal used in the current study had not been determined, additional crystalline AA were not included in the high-indigestible protein diet to compensate for reduced AA digestibility by autoclaving. Furthermore, autoclaving might affect fiber utilization of soybean meal in pigs, and subsequently, affect gut microbiota, which can be a potential confounding effect. Finally, from a practical point of view, it may be difficult to reduce ileal indigestible protein concentration of corn-soybean meal-based diets in growing-finishing pigs because AA digestibility of these ingredients in growing-finishing pigs is high. When considering lower nitrogen and AA digestibility due to an immature digestive system, nursery pig diets should be targeted to reduce indigestible protein concentration. More specifically, the use of high-quality soy protein and animal protein sources, exogenous protease or both may be necessary.
Conclusions
An increase in ileal indigestible protein concentration increased fecal nitrogen excretion and induced shifts in microbial communities. Nitrogen and fiber utilization in the hindgut may be inhibited in an indigestible nitrogen-rich environment. Based on the current results, formulating swine diets toward reducing indigestible protein concentration rather than reducing total dietary protein concentration may be a more sound approach to reduce fecal nitrogen excretion.
Acknowledgment
The authors appreciate Pat Jaynes for her technical assistance.
Glossary
Abbreviations
- AA
amino acids
- AID
apparent ileal digestibility
- APID
apparent post-ileal digestibility
- ASV
amplicon sequence variants
- ATTD
apparent total tract digestibility
- GE
gross energy
Contributor Information
Jung Yeol Sung, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA.
Timothy A Johnson, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA.
Darryl Ragland, Department of Veterinary Clinical Sciences, Purdue University, West Lafayette, IN 47907, USA.
Olayiwola Adeola, Department of Animal Sciences, Purdue University, West Lafayette, IN 47907, USA.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
LITERATURE CITED
- Abraham, M. E., Weimer S. L., Scoles K., Vargas J. I., Johnson T. A., Robison C., Hoverman L., Rocheford E., Rocheford T., and Ortiz D.. . 2021. Orange corn diets associated with lower severity of footpad dermatitis in broilers. Poult. Sci. 100:101054. doi: 10.1016/j.psj.2021.101054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, F. N., Htoo J. K., Thomson J., and Stein H. H.. . 2014. Effects of balancing crystalline amino acids in diets containing heat-damaged soybean meal or distillers dried grains with solubles fed to weanling pigs. Animal 8:1594–1602. doi: 10.1017/S175173111400144X [DOI] [PubMed] [Google Scholar]
- AOAC. 2005. Official methods of analysis. 18th ed.Washington (DC): Association of Official Analytical Chemists. [Google Scholar]
- Chen, X., Song P., Fan P., He T., Jacobs D., Levesque C. L., Johnston L. J., Ji L., Ma N., and Chen Y.. . 2018. Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model. Front. Cell. Infect. Microbiol. 8:246. doi: 10.3389/fcimb.2018.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H., Sung J. Y., and Kim B. G.. . 2020. Neutral detergent fiber rather than other dietary fiber types as an independent variable increases the accuracy of prediction equation for digestible energy in feeds for growing pigs. Asian-Australas. J. Anim. Sci. 33:615–622. doi: 10.5713/ajas.19.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, A. -M., Blachier F., Gotteland M., Andriamihaja M., Benetti P. -H., Sanz Y., and Tomé D.. . 2013. Re-print of “Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host”. Pharmacol. Res. 69:114–126. doi: 10.1016/j.phrs.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Dilger, R. N., Sands J. S., Ragland D., and Adeola O.. . 2004. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls. J. Anim. Sci. 82:715–724. doi: 10.2527/2004.823715x [DOI] [PubMed] [Google Scholar]
- Douglas, G. M., Maffei V. J., Zaneveld J. R., Yurgel S. N., Brown J. R., Taylor C. M., Huttenhower C., and Langille M. G.. . 2020. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38:685–688. doi: 10.1038/s41587-020-0548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elling-Staats, M. L., Gilbert M. S., Smidt H., and Kwakkel R. P.. . 2022. Caecal protein fermentation in broilers: a review. Worlds Poult. Sci. J. 78:103–123. doi: 10.1080/00439339.2022.2003170 [DOI] [Google Scholar]
- Fan, P., Liu P., Song P., Chen X., and Ma X.. . 2017. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 7:1–12. doi: 10.1038/srep43412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton, T. W., and Fenton M.. . 1979. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 59:631–634. doi: 10.4141/cjas79-081 [DOI] [Google Scholar]
- González-Vega, J. C., Kim B. G., Htoo J. K., Lemme A., and Stein H. H.. . 2011. Amino acid digestibility in heated soybean meal fed to growing pigs. J. Anim. Sci. 89:3617–3625. doi: 10.2527/jas.2010-3465 [DOI] [PubMed] [Google Scholar]
- Heo, J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. . 2008. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 62:343–358. doi: 10.1080/17450390802327811 [DOI] [PubMed] [Google Scholar]
- Jensen, E. A., Young J. A., Jackson Z., Busken J., Kuhn J., Onusko M., Carroll R. K., List E. O., Brown J. M., and Kopchick J. J.. . 2022. Excess growth hormone alters the male mouse gut microbiome in an age-dependent manner. Endocrinology 163:bqac074. doi: 10.1210/endocr/bqac074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha, R., and Berrocoso J. F.. . 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002 [DOI] [Google Scholar]
- Kheirandish, P., Petri R. M., Sener-Aydemir A., Schwartz-Zimmermann H. E., Berthiller F., Zebeli Q., and Pacífico C.. . 2022. Characterization of microbial intolerances and ruminal dysbiosis towards different dietary carbohydrate sources using an in vitro model. J. Appl. Microbiol. 133:458–476. doi: 10.1111/jam.15573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., Sung J. Y., and Kim B. G.. . 2022. The influence of protein concentrations in basal diet on metabolizable energy of full-fat soybeans and soy protein isolate determined by the difference procedure in pigs. Anim. Feed Sci. Technol. 288:115299. doi: 10.1016/j.anifeedsci.2022.115299 [DOI] [Google Scholar]
- Lammers-Jannink, K. C. M. 2022. Microbial protein metabolism in the monogastric gastrointestinal tract: a review. Understanding gut microbiomes as targets for improving pig gut health. In:Bailey M. and Stokes C., editors, Understanding gut microbiomes as targets for improving pig gut health. Cambridge (UK): Burleigh Dodds Science Publishing Limited; p. 435–466. doi: 10.19103/AS.2021.0089.23 [DOI] [Google Scholar]
- Le Bellego, L., and Noblet J.. . 2002. Performance and utilization of dietary energy and amino acids in piglets fed low protein diets. Livest. Prod. Sci. 76:45–58. doi: 10.1016/s0301-6226(02)00008-8 [DOI] [Google Scholar]
- Le Sciellour, M., Labussière E., Zemb O., and Renaudeau D.. . 2018. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS One 13:e0206159. doi: 10.1371/journal.pone.0206159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., Peng X., Burrough E. R., Sahin O., Gould S. A., Gabler N. K., Loving C. L., Dorman K. S., and Patience J. F.. . 2020. Dietary soluble and insoluble fiber with or without enzymes altered the intestinal microbiota in weaned pigs challenged with enterotoxigenic E. coli F18. Front. Microbiol. 11:1110. doi: 10.3389/fmicb.2020.01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Z., Li C., Cheng Y., Hang S., and Zhu W.. . 2015. Effects of low dietary protein on the metabolites and microbial communities in the caecal digesta of piglets. Arch. Anim. Nutr. 69:212–226. doi: 10.1080/1745039x.2015.1034521 [DOI] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed.Washington (DC): National Academic Press. [Google Scholar]
- Oliphant, K., and Allen-Vercoe E.. . 2019. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7:1–15. doi: 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, M., Hu J., Peng H., Li B., Xu J., Song X., Yu C., Zhang Z., Du X., and Bu G.. . 2022. Research note: the gut microbiota varies with dietary fiber levels in broilers. Poult. Sci. 101:101922. doi: 10.1016/j.anifeedsci.2021.114822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quast, C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., and Glöckner F. O.. . 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41:D590–D596. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, J. Y., Ji S. Y., and Kim B. G.. . 2020. Amino acid and calcium digestibility in hatchery byproducts fed to nursery pigs. Anim. Feed Sci. Technol. 270:114703. doi: 10.1016/j.anifeedsci.2020.114703 [DOI] [Google Scholar]
- Sung, J. Y., Ji S. Y., and Kim B. G.. . 2022a. Additivity of digestible energy and nutrient concentrations in hatchery byproducts fed to nursery pigs. Anim. Biosci 35:453–460. doi: 10.5713/ab.21.0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, J. Y., Wiltafsky-Martin M. K., González-Vega J. C., and Adeola O.. . 2022b. Autoclaving time-related reduction in metabolizable energy of poultry meal is greater in growing pigs compared with broiler chickens. J. Anim. Sci. 100:skac117. doi: 10.1093/jas/skac117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, T. A., O’Neill H. V. M., Bedford M. R., McDermott K., and Miller H. M.. . 2021. Effect of xylanase and xylo-oligosaccharide supplementation on growth performance and faecal bacterial community composition in growing pigs. Anim. Feed Sci. Technol. 274:114822. doi: 10.1016/j.anifeedsci.2021.114822 [DOI] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Wang, Z., Bai Y., Pi Y., Gerrits W. J., de Vries S., Shang L., Tao S., Zhang S., Han D., and Zhu Z.. . 2021. Xylan alleviates dietary fiber deprivation-induced dysbiosis by selectively promoting Bifidobacterium pseudocatenulatum in pigs. Microbiome 9:1–14. doi: 10.1186/s40168-021-01175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Zhou J., Wang G., Cai S., Zeng X., and Qiao S.. . 2018. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 9:1–14. doi: 10.1186/s40104-018-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, D., Zhu W., and Hang S.. . 2019. Effects of long-term dietary protein restriction on intestinal morphology, digestive enzymes, gut hormones, and colonic microbiota in pigs. Animals 9:180. doi: 10.3390/ani9040180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Wielen N. v. d., Hee B. v. d., Wang J., Hendriks W., and Gilbert M.. . 2020. Impact of fermentable protein, by feeding high protein diets, on microbial composition, microbial catabolic activity, gut health and beyond in pigs. Microorganisms 8:1735. doi: 10.3390/microorganisms8111735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., Fang L., Sun Y., Su Y., and Zhu W.. . 2016. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 38:61–69. doi: 10.1016/j.anaerobe.2015.12.009 [DOI] [PubMed] [Google Scholar]