Abstract
The objective of this study was to determine the dose of folate and vitamin B12 in beef heifers fed rumen protected methionine and choline required to maintain increased B12 levels and intermediates of the methionine-folate cycle in circulation. Angus heifers (n = 30; BW = 392.6 ± 12.6 kg) were individually fed and assigned to one of five treatments: 0XNEG: Total mixed ration (TMR) and saline injections at day 0 and 7 of the estrous cycle, 0XPOS: TMR, rumen protected methionine (MET) fed at 0.08% of the diet DM, rumen protected choline (CHOL) fed at 60 g/d, and saline injections at day 0 and 7, 0.5X: TMR, MET, CHOL, 5 mg B12, and 80 mg folate at day 0 and 7, 1X: TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7, and 2X: TMR, MET, CHOL, 20 mg B12, and 320 mg folate at day 0 and 7. All heifers were estrus synchronized but not bred, and blood was collected on day 0, 2, 5, 7, 9, 12, and 14 of a synchronized estrous cycle. Heifers were slaughtered on day 14 of the estrous cycle for liver collection. Serum B12 concentrations were greater in the 0.5X, 1X, and 2X, compared with 0XNEG and 0XPOS on all days after treatment initiation (P < 0.0001). Serum folate concentrations were greater for the 2X treatment at day 5, 7, and 9 of the cycle compared with all other treatments (P ≤ 0.05). There were no differences (P ≥ 0.19) in hepatic methionine-cycle or choline analyte concentrations by treatment. Concentrations of hepatic folate cycle intermediates were always greater (P ≤ 0.04) in the 2X treatment compared with the 0XNEG and 0XPOS heifers. Serum methionine was greater (P = 0.04) in the 0.5X and 2X heifers compared with 0XNEG, and S-adenosylhomocysteine (SAH) tended (P = 0.06) to be greater in the 0.5X heifers and the S-adenosylmethionine (SAM):SAH ratio was decreased (P = 0.05) in the 0.5X treatment compared with the 0XNEG, 0XPOS, and 2X heifers. The hepatic transcript abundance of MAT2A and MAT2B were decreased (P ≤ 0.02) in the 0.5X heifers compared with the 0XNEG, 0XPOS, and 2X heifers. These data support that beef heifers fed rumen protected methionine and choline require 20 mg B12 and 320 mg folate once weekly to maintain increased concentrations of B12 and folate in serum. Furthermore, these data demonstrate that not all supplementation levels are equal in providing positive responses, and that some levels, such as the 0.5X, may result in a stoichiometric imbalance in the one-carbon metabolism pathway that results in a decreased SAM:SAH ratio.
Keywords: estrous cycle, heifers, one-carbon metabolism
This study determined the optimal doses for vitamin B12 (20 mg injected weekly) and folate (320 mg injected weekly) injection to beef heifers during the periconceptual period when rumen protected methionine and choline were fed at 0.08% of dry matter and 60 g/d, respectively. These doses set the basis upon which future studies can be based when feeding and injecting one-carbon metabolites.
Introduction
One-carbon metabolites (OCM) include choline, B-vitamins (vitamin B12, vitamin B6, riboflavin [vitamin B2], and folate [vitamin B9]), minerals (cobalt [component of vitamin B12], sulfur [component of methionine], and amino acids (methionine, serine, and glycine; Clare et al., 2019). At its core, one-carbon metabolism centers around the methionine and folate cycles which are responsible for the transfer of methyl groups to DNA, RNA, and proteins, but branch into pathways for the synthesis of nucleotides, polyamines, amino acids, creatine, and phospholipids (Mason, 2003; Clare et al., 2019). Because of the interconnected nature of the cycles involved in one-carbon metabolism, it is inherently understood that the perturbation of a metabolite(s) in one part of the pathway results in compensatory changes to metabolite synthesis and availability in other portions of the pathway (Zeisel, 2011).
Epigenetic modifications including DNA methylation rely on the availability of methyl donors such as those generated via OCM (Van den Veyver, 2002). Furthermore, during early gestation, the bovine conceptus undergoes intensive epigenetic reprogramming with global demethylation taking place shortly after fertilization, followed by a wave of DNA methylation beginning at the blastocyst stage (Morgan et al., 2005; Dobbs et al., 2013; Jiang et al., 2018; Duan et al., 2019; Ivanova et al., 2020). Multiple studies have determined that the presence or absence of OCM during the periconceptual period of gestation results in immediate effects on the embryo with lasting phenotypic effects on the offspring in sheep (Sinclair et al., 2007) and cattle (Peñagaricano et al., 2013; Acosta et al., 2016; Estrada-Cortés et al., 2020, 2021) such as altered fat metabolism and accretion and insulin resistance in male offspring of methyl deficient ewes as well as increased birth and 205-d adjusted weaning weights in brahman calves supplemented as embryos.
In beef cattle, the knowledge base surrounding the role of OCM supplementation during the periconceptual period is severely limited. In fact, the committee that drafted the 2016 version of the Nutrient Requirements of Beef Cattle (National Academies of Sciences and Medicine, 2016) indicated that our current understanding of the roles of one-carbon metabolites in beef cattle is “insufficient”, and therefore, “additional research in one-carbon metabolism, folic acid, and other factors affecting the metabolic one-carbon pool would likely provide needed insight into developmental and epigenetic events during the fetal and perinatal periods of growth.”
Previous work in ruminants investigating the roles of OCM on embryonic development and postnatal performance have been limited to the supplementation or depletion of one or two OCM. Our laboratory recently reported that the supplementation of methionine, choline, vitamin B12, and folate to bovine embryonic fibroblasts cultured in divergent energy supplies (media containing low or high glucose) improved growth rate and mitochondrial respiration in cells treated with increasing concentrations of OCM (Crouse et al., 2022). Therefore, we hypothesized that increasing OCM supplementation to heifers during the first 14 d of the estrous cycle would increase circulating OCM concentrations and improve methylation potential in a dose dependent manner. The objective of the present study aimed to translate our in vitro model to an in vivo model and determine the optimal supplementation rate of the same four OCM (methionine, choline, vitamin B12, and folate) to heifers during the first 14 d of the estrous cycle to maintain increased circulating concentrations of OCM for use as a model in early pregnancy studies.
Materials and Methods
This experiment was approved by the United States Meat Animal Research Center Institutional Animal Care and Use Committee (EO # 128.1) in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching.
Animals, housing, and treatments
Angus heifers (n = 30, ~ 15 mo of age; average initial BW = 392.6 ± 12.6 kg) were trained to consume feed from individual feeders (American Calan, Northwood, NH) for at least one month prior to the start of the study. Heifers were stratified across five pens with six heifers per pen. Each treatment was represented in each pen with one treatment being represented twice in each pen. Heifers were fed a total mixed ration (TMR) consisting of 75% grass/alfalfa hay, 21% corn silage, and 4% mineral pellet on a dry matter basis which met or exceeded the recommended metabolizable energy and metabolizable protein requirements for heifers fed to target 0.45 kg/d gain (actual gain = 0.44 ± 0.11 kg) as established in NASEM (National Academies of Sciences, Engineering, and Medicine, 2016).
All heifers were administered 100 µg Gonadotropin Releasing Hormone (GnRH; Cystorelin, Boehringer Ingelheim, Ingelheim am Ehin, Germany), followed in 7 d by 25 mg Prostaglandin (Lutalyse, Zoetis, Parsippany-Troy Hills, NJ), followed in 48 h by 100 µg GnRH, with the final injection of GnRH considered day 0 of the study. None of the heifers were bred as part of the synchronization protocol. Heifers were randomly assigned to one of five treatments (n = 6/treatment) which were initiated after day 0 (referred to as day 0 of the estrous cycle) of the study and are described in Table 1. These treatments were: 0XNEG: Total mixed ration (TMR) and injections of saline at day 0 and 7 of the estrous cycle, 0XPOS: TMR, rumen protected methionine (MET; Smartamine M, Adisseo, Alpharetta, GA) fed at 0.08% of the diet DM, rumen protected choline (CHOL; ReaShure, Balchem Inc., New Hampton, NY) fed at 60 g/d, and injections of saline at day 0 and 7 of the estrous cycle, 0.5X: TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle, 1X: TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle, 2X: TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle. Injections of saline (0.9% NaCl; 10 mL), vitamin B12 (5,000 µg/mL; Neogen Vet, Lansing, MI) and/or folic acid (5 mg/mL; Fresenius Kabi, Bad Homburg, Germany) were administered intramuscularly with no more than 10 mL of solution provided in a single injection site. Vitamin B12 and folate injection levels were based on previously published literature in Holsteins (Preynat et al., 2009b) where the injection dosage of vitamin B12 and folate, equivalent to the 1X treatment, increased milk yield as well as folate and B12 concentrations in milk. Methionine and CHOL were fed at the same inclusion level across all supplemented treatments as per manufacturer’s recommendations.
Table 1.
Treatment structure
| 0XNEG | 0XPOS | 0.5X | 1X | 2X | |
|---|---|---|---|---|---|
| Feed1 | TMR | TMR + 0.08% MET + 60 g/d CHOL | TMR + 0.08% MET + 60 g/d CHOL | TMR + 0.08% MET + 60 g/d CHOL | TMR + 0.08% MET + 60 g/d CHOL |
| Injection2 | 0.9% Saline | 0.9% Saline | 5mg B12 + 80 mg FA | 10 mg B12 + 160 mg FA | 20 mg B12 + 320 mg FA |
1TMR, 75% grass/alfalfa hay, 21% corn silage, and 4% mineral pellet on a dry matter basis fed to gain 0.45 kg/d. MET, Rumen Protected Methionine fed at 0.08% inclusion on a Dry Matter Basis. CHOL, Rumen Protected Choline.
2B12, Vitamin B12 (Cyanocobalamin) injected after blood collection on day 0 and 7 of the synchronized estrous cycle. FA, Folic Acid (pteroylglutamic acid) injected after blood collection on day 0 and 7 of the synchronized estrous cycle.
Sample collection and analysis
Serum and plasma samples were collected on day 0, 2, 5, 7, 9, 12, and 14 of the estrous cycle via jugular venipuncture using 10-mL serum and EDTA vacutainer tubes (Becton Dickinson HealthCare, Franklin Lakes, NJ). On injection days (day 0 and 7) and the day of slaughter (day 14), serum and plasma were collected prior to injection or transport to the abattoir, respectively. The serum was allowed to set for 20 min at room temperature and both serum and plasma were centrifuged at 1,500 × g for 20 min, separated from blood constituents, and were stored at -80 °C. Heifers were slaughtered at the U.S. Meat Animal Research Center abattoir on day 14 of the synchronized estrous cycle. Livers were collected at slaughter and 500 mg of sample collected from the left hepatic lobe and snap frozen in liquid nitrogen and stored at −80 °C.
Methionine-folate cycle intermediates
Serum vitamin B12 and folate analysis was conducted at IDEXX Laboratories (Westbrook, ME). For vitamin B12, the IMMULITE 2000 (Siemens Medical Solutions, Malvern, PA) performs a one-cycle sample treatment with dithiothreitol and a sodium hydroxide/potassium cyanide solution in a reaction tube containing no bead. After a 30-min incubation, the treated sample was transferred to a second reaction tube containing a vitamin B12-coated polystyrene bead and hog intrinsic factor (HIF). During this subsequent 30-min incubation, the vitamin B12 released from the endogenous binding proteins during sample treatment competes with immobilized vitamin B12 for binding with HIF. Alkaline phosphatase-labeled anti-hog intrinsic factor was then introduced, which binds to any HIF that was immobilized on the B12-coated bead in the final 30-min incubation. Unbound enzyme conjugate was removed by centrifugal wash. Substrate was added and the procedure continued as described for typical immunoassays. For folate, the IMMULITE 2000 performs a 2-cycle, on-board sample treatment of serum. The sample, along with ligand-labelled folic acid was first treated with dithiothreitol in a reaction tube containing no bead, and then sodium hydroxide/potassium cyanide in a second treatment cycle. The treated sample was transferred to a second reaction tube containing a murine anti-folate binding protein antibody-coated polystyrene bead and folate binding protein (FBP). During a 30-min incubation, folic acid released from binding proteins in the sample competes with ligand-labeled folic acid for binding with FBP. The bead was washed, and alkaline phosphatase labeled anti-ligand was added. During the final 30-min incubation, the alkaline phosphatase labeled anti-ligand binds to the ligand-labeled folate that was bound to the bead during the first incubation. The unbound enzyme conjugate was removed by centrifugal wash. Substrate was added and the procedure continues as describe for typical immunoassays.
Hepatic folic acid intermediates were measured as previously described by Nandania et al. (2018) with 80 mg of initial tissue for homogenization. Folate cycle intermediates: folic acid, dihydrofolic acid (DHF), tetrahydrofolate (THF), 5,10-methylene-THF, and 5-methyl-THF were purchased from Schircks Laboratories (Switzerland). Quality control standards (QC standards containing all five metabolite standards of interest) were analyzed between samples within runs (intra run CV = 9.14%; across run CV = 14.27%).
Hepatic methionine cycle intermediates were measured by the Duke University Proteomics and Metabolomics Shared Resource (Duke University, Durham, NC). For liver tissue, 500 microliters of a 50% methanol solution was added to 50 mg of liver samples and homogenized with a Percellys 24 bead blaster (Bertin instruments, Montingny-le-Bretonneux, France) at 4 °C for 3 cycles of 10 s each at 10,000 rpm with a 20-s pause between each burst. All sample extracts were centrifuged at 20,000 rcf for 5 min at 4 °C. Fifty microliters of supernatant and standard solutions were transferred into the well of a 1 mL 96-well NUNC plate (Nalge Nunc Internation Corporation, Roskilde, Denmark) and mixed with 20 microliters of 1 µg/mL L-methionine methyl-D3 (Cambridge Isotope Laboratories, Inc, Tewksbury, MA) and S-adenosylhomocystein-d4 (Cayman Chemical, Ann Arbor, MI), then subjected to complete dryness at room temperature under nitrogen. One hundred microliters of methanol solution was added into the dried wells and incubated at room temperature while shaking for 20 min at 500 rpm, and then 10 min for 2,000 rpm. Finally, the plate was centrifuged at 500 rcf for 5 min before instrument injection. Samples were analyzed with a 6500+ QTRAP LC-MS/MS system (Sciex, Framingham, MA). Software Analyst 1.7.1 was used for data acquisition and analysis. The Sciex ExionLC UPLC system includes a degasser, an AD autosampler, an AD column oven, a controller, and an AD pump. The LC separation was performed on an Agilent SB-Aq RRHD column (2.1 × 50 mm, 1.8 μm) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The flow rate was 0.4 mL/min. The linear gradient was as follows: 0–1 min, 100% A; 2–3.5 min, 0% A; 3.6–4 min, 50% A; 4.1–5.5 min, 100% A. The autosampler was set at 10 °C and the column was kept at 40 °C. The injection volume was 1 μL. Mass spectra were acquired under positive electrospray ionization with the ion spray voltage of 4,500 V. The source temperature was 450 °C. The curtain gas, ion source gas 1, and ion source gas 2 were at 32, 60, and 60 psi, respectively. Multiple reaction monitoring was used for quantitation: methionine (MET; m/z 150.0 → m/z 56.0), S-adenosylhomocysteine (SAH; m/z 385.1 → m/z 136.0), S-adenosylmethionine (SAM; m/z 399.0 → m/z 97.1), DL-homocysteine (m/z 136.0 → m/z 56.0), and internal standards L-methionine methyl-d3 (m/z 153.0 → m/z 107.0), S-adenosylhomocysteine-d4 (m/z 389.1 → m/z 136.0). The average assay CV for all 4 analytes in hepatic tissue was 2.29%.
Plasma methionine cycle intermediates were analyzed on samples from day 14 of the estrous cycle by the Duke University Proteomics and Metabolomics Shared Resource. Fifty microliters of plasma was mixed with 200 microliters of methanol and 20 microliters 1 µg/mL L-methionine-d3 and S-adenosylhomocysteine-d4 followed by vigorous vortexing. Samples were then kept at −20 °C for 20 min, centrifuged at 15,000 rcf for 5 min at 4 °C, and the supernatant was transferred into the well of a 1 mL 96-well NUNC plate and subjected to complete dryness at room temperature by nitrogen. One hundred microliters of 50% methanol solution was added into the dried wells and incubated at room temperature while shaking for 20 min at 500 rpm, and then for 5 min at 2,000 rpm. Finally, the plate was centrifuged at 500 rcf for 5 min before instrument injection. After injection, mass spectrometry methods follow the same protocols as outlined for hepatic tissue. The average assay coefficient of variation for all 4 analytes in plasma was 1.78%.
Hepatic choline was measured with the Abcam Total Choline Assay Kit (Abcam, Cambridge, UK). Twenty mg of liver tissue was used and was assayed according to the manufacturer’s protocol except for being read by absorbance instead of fluorescence and thus the OD being measured at 576 nm (intra assay CV = 9.90%).
Methionine-folate cycle enzyme transcript abundance
Ribonucleic acid extraction was performed using the RNeasy Plus Universal Mini Kit (Qiagen, Hilden, Germany) and quantified using the Qubit 3.0 (Invitrogen, Waltham, MA). The RNA was diluted to 2 µg and cDNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, Waltham, MA). The efficiency of the primer pairs for the 13 genes (ACTB, DNMT1, DNMT3A, GAPDH, GNMT, HPRT1, MAT1A, MAT2A, MAT2B, MTHFR, MTR, MTRR, and PRMT1) were tested (Supplementary Table S1). Samples were analyzed in triplicate with a 1:5 serial dilution. Out of the three reference genes selected (ACTB, GAPDH, and HPRT1), GAPDH was the most stably expressed across all samples and therefore was the only gene used as a reference gene. Real-time polymerase chain reaction (rt-PCR) was performed using SYBR Green with 1.75 microliters water, 7.5 microliters SYBR Green, 0.375 microliters of the 10 µM forward primer, and 0.375 microliters of the 10 µM reverse primer were included per well. The PCR reaction was performed using the QuantStudio Real-Time PCR system (ThermoFisher Scientific). The program was set to run at 95 °C for 20–30 s, at 95 °C for 1–3 s, and finally at 60 °C for 20–30 s for 35–40 cycles. The primer information can be found in Supplementary Table S1.
The average Ct value of the triplicates was used for final analysis. First, one animal from the control treatment was designated as the control sample for calculations, and its relative quantification (RQ) was set to 1. Relative quantification for all other genes were calculated as RQ = 2-ΔΔCt where ΔΔCt = (sample gene of interest Ct—sample reference gene Ct) − (control gene of interest Ct—control reference gene Ct; Livak and Schmittgen, 2001).
Statistical analysis
Serum vitamin B12 and folate concentrations were analyzed using the MIXED procedure of SAS 9.4 (SAS Institute, Cary, NC) with repeated measures using Spatial Power as the covariate, with day, treatment, and the interaction in the model as fixed effects and animal nested within treatment as the random effect. Net area under the curve (AUC) analysis was conducted to account for the variation in starting B12 and folate concentrations across heifers using Proc SQL and macro as previously described by Shiang et al. (2010). Measurements include 1st Week = area under the curve for the first week of the estrous cycle (day 0 to 7), 2nd Week = area under the curve for day 7 to 14 of the estrous cycle using day 0 as the baseline value, and Total = total area under the curve for the entire sampling period.
Methionine-folate cycle intermediates in serum and liver as well as the methionine-folate cycle transcript abundance were analyzed using PROC GLM and means were separated using LSMEANS. Polynomial contrast coefficients were generated using PROC IML for unequal spacing between treatments. Contrasts performed were Negative vs. Positive (0XNEG vs. 0XPOS), Met + Choline vs. Folate (0XPOS vs. 0.5X, 1X, and 2X) as well as linear quadratic, and cubic contrasts from 0XPOS, 0.5X, 1X, and 2X for increasing folate and B12 concentrations.
Results
Serum vitamin B12and folate
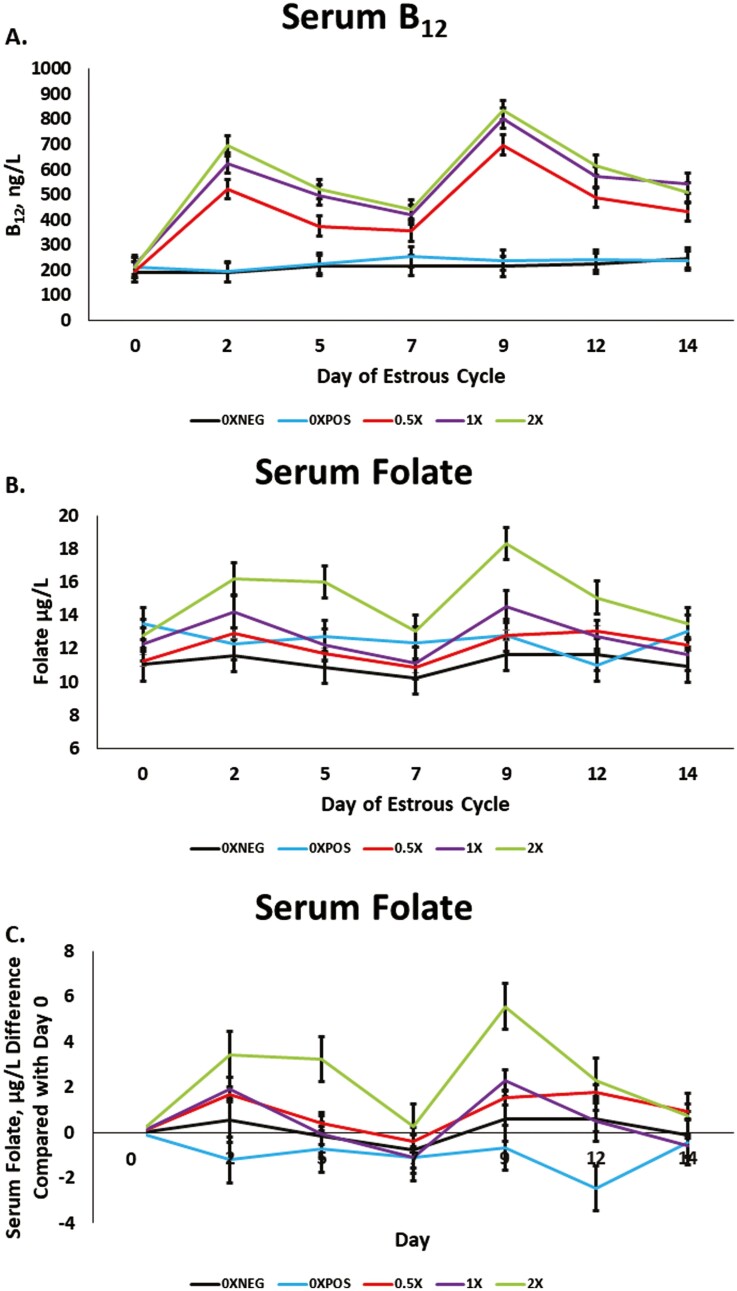
Serum vitamin B12 was influenced by the day × treatment interaction (P < 0.0001; Figure 1A). On day 0, there were no differences (P ≥ 0.47) in serum vitamin B12 concentrations across nutritional treatment. Across all days, serum vitamin B12 concentrations were not different (P ≥ 0.48) between the 0XNEG and 0XPOS treatments, and the 0.5X, 1X, and 2X were always greater (P < 0.05) than both the 0XNEG and 0XPOS from day 2 to day 14 of the study (Figure 1A). On day 2, 5, 9, and 12, both the 1X and 2X treatments had greater (P ≤ 0.04) concentrations of vitamin B12 compared with the 0.5X. On day 7, 2X was greater than 0.5X (P = 0.03) with 1X being intermediate and equal (P ≥ 0.10) to both 0.5X and 2X. Lastly, on day 14, 1X was greater than 0.5X (P < 0.01) with the 2X treatment being intermediate and equal (P ≥ 0.06) to both the 0.5X and 1X treatments (Figure 1A).
Figure 1.
The serum concentrations of vitamin B12 (A), folate (B), and folate normalized to day 0 concentrations (C) as influenced by day of the estrous cycle, and methyl donor treatments. Probability values for Vitamin B12: Day: P < 0.0001, Trt: P < 0.0001, Day × Trt: P < 0.0001. Probability values for Folate: Day: P < 0.0001, Trt: P = 0.008, and Day × Trt: P = 0.0004. Panel C is solely for visualization of AUC data and does not have statistical analysis directly applied to it. 0XNEG: TMR and sham injections of saline at day 0 and 7 of the estrous cycle, 0XPOS: TMR, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle; 0.5X, TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle; 1X, TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle; 2X, TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle.
Serum folate was influenced by the day × treatment interaction (P = 0.0004; Figure 1B). There were no differences in serum folate concentrations among treatments on day 0 and 14 of the study (P ≥ 0.07). On day 2, the concentration of folate was greater (P ≤ 0.02) in serum of the 2X heifers compared with the 0.5X, 0XPOS, and 0XNEG with 1X being intermediate and equal (P ≥ 0.15) to all other treatments (Figure 1B). On day 5, the concentration of folate was greater (P ≤ 0.02) in serum of the 2X-treated heifers compared with all other treatments. On day 7, the concentration of folate was greater (P = 0.05) in serum of the 2X-treated heifers compared with 0XNEG with 0XPOS, 0.5X, and 1X being intermediate and equal (P ≥ 0.14) to both 0XNEG and the 2X-treated heifers. On day 9, the concentration of folate was greater (P ≤ 0.01) in serum of the 2X-treated heifers compared with all other treatments (Figure 1B). Furthermore, the concentration of folate was greater (P = 0.04) in serum of the 1X-treated heifers compared with 0XNEG with 0XPOS and 0.5X being intermediate and equal (P ≥ 0.23) to both the 0XNEG- and 1X-treated heifers. Lastly, on day 12, the concentration of folate was greater (P ≤ 0. 05) in serum of the 2X-treated heifers compared with all other treatments (Figure 1B).
Net AUC data for both vitamin B12 and folate are presented in Table 2. Figure 1C is prepared for visualization of folate data with day 0 as a baseline. Serum vitamin B12 net AUC was greater (P < 0.01) for the first week (day 1 to 7) in the 1X- and 2X-treated heifers compared with the 0.5X heifers, which were greater than the 0XNEG- and 0XPOS-treated heifers. There was also a quadratic increase (P < 0.01) in net AUC for vitamin B12 with increasing supplementation from the 0XPOS to 2X supplementation levels. There was no difference (P = 0.77) between the 0XNEG and 0XPOS treatments which received injections of saline. The second week AUC followed a similar trend as the first week, being greater (P < 0.01) in the 1X- and 2X-treated heifers compared with the 0.5X treated heifers which were greater than the 0XNEG- and 0XPOS-treated heifers. Furthermore, there was a quadratic (P < 0.01) increase in net AUC with increasing supplementation level, and no difference (P = 0.88) between the 0XNEG and 0XPOS treatments.
Table 2.
Area under the curve analysis of vitamin B12 and folate through the first 14 d of the estrous cycle
| Vitamin3 | Time4 | Treatment1 | SEM5 | Probability Values2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0XNEG | 0XPOS | 0.5X | 1X | 2X | Trt | Neg vs. Pos | Met + Chol vs. Vitamin | Linear | Quadratic | Cubic | |||
| B12 | 1st Week | 86.3a | 38.1a | 1300.3b | 1932.2c | 2216.9c | 124.1 | <0.01 | 0.77 | <0.01 | <0.01 | <0.01 | 0.52 |
| 2nd Week | 313.8a | 358.8a | 2728.9b | 3631.2c | 3908.3c | 193.8 | <0.01 | 0.88 | <0.01 | <0.01 | <0.01 | 0.18 | |
| Total | 310.9a | 247.6a | 3512.1b | 4851.9c | 5319.2c | 233.4 | <0.01 | 0.85 | <0.01 | <0.01 | <0.01 | 0.18 | |
| Folate | 1st Week | −1.13a | −6.05a | 4.83a | 3.75a | 16.89b | 4.92 | 0.04 | 0.41 | 0.03 | 0.01 | 0.91 | 0.39 |
| 2nd Week | −3.31 | −13.47 | 6.09 | 3.04 | 21.50 | 9.14 | 0.13 | 0.27 | 0.05 | 0.03 | 0.77 | 0.40 | |
| Total | −0.35a | −15.55a | 13.86a | 9.28a | 37.45b | 10.74 | 0.03 | 0.20 | 0.02 | 0.01 | 0.72 | 0.29 | |
10XNEG, TMR and sham injections of saline at day 0 and 7 of the estrous cycle; 0XPOS, TMR, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle; 0.5X, TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle; 1X, TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle; 2X, TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle.
2Probability values for: Trt, main effect of treatment. Probability values for contrast statements: Neg vs. Pos, 0XNEG vs. 0XPOS; Met + Chol vs. Vitamin, 0XPOS vs. 0.5X, 1X, and 2X; Linear, Quadratic, and Cubic, Polynomial contrasts with increasing folate level of 0XPOS to 2X.
3Vitamin B12 (Cyanocobalamin). Folic Acid (Vitamin B9).
4Area under the curve measurements for: 1st Week, area under the curve for the first week of the estrous cycle (day 0 to 7), 2nd Week, area under the curve for day 7 to 14 of the using d 0 as the baseline value.
5The average standard error of the mean for a specific measurement.
a–cMeans within row without a common superscript differ by a main effect of treatment.
Serum folate net AUC during the first week was greater (P = 0.04) in the 2X compared with all other treatments. Furthermore, there was a linear (P = 0.01) increase in net AUC during the first week with increasing supplementation level from the 0XPOS to the 2X treatments, with no difference (P = 0.41) in folate AUC between the 0XNEG and 0XPOS treatments. There were no differences (P = 0.13) in net AUC for folate in the second week; however, there was a linear increase (P = 0.03) in net folate AUC. The total net AUC was greater (P = 0.03) in the 2X treatment compared with all other treatments. Moreover, there was a linear increase (P = 0.01) in total net AUC with increasing supplementation from 0XPOS to 2X.
Hepatic methionine-folate cycle intermediates
All hepatic methionine-folate cycle analyte data are presented in Table 3. There were no treatment differences (P ≥ 0.19) in any methionine cycle intermediates or choline in liver at day 14 of the estrous cycle. The concentration of methionine, homocysteine, or the SAM:SAH ratio was not different (P ≥ 0.13) in any contrasts measured. The concentrations of SAM and SAH tended (P = 0.08 and P = 0.07, respectively) to increase in liver with increasing supplementation level from 0XPOS to 2X. The concentration of SAH also decreased (P = 0.03) in the 0XPOS compared to the 0XNEG treatment. The concentration of choline tended (P = 0.09) to be greater in the 0XPOS compared with 0XNEG treated heifers and tended (P = 0.08) to be affected by a cubic response from 0XPOS to 2X, where the 0.5X treatment decreased in choline abundance compared with the 0XPOS, and subsequently increased to be equal to the 0XPOS at 1X and 2X.
Table 3.
Hepatic methionine-folate cycle analyte abundance at day 14 of the synchronized estrous cycle
| Analyte | Treatment1 | SEM3 | Probability Values2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0XNEG | 0XPOS | 0.5X | 1X | 2X | Trt | Neg vs. Pos | Met + Chol vs. Vitamin | Linear | Quadratic | Cubic | ||
| Methionine, ng/mg | 76.7 | 80.9 | 83.3 | 82.5 | 87.3 | 3.66 | 0.41 | 0.44 | 0.40 | 0.24 | 0.82 | 0.66 |
| S-adenosyl Methionine (SAM), ng/mg | 7.52 | 5.91 | 5.52 | 4.99 | 8.56 | 1.15 | 0.19 | 0.33 | 0.73 | 0.08 | 0.12 | 0.66 |
| S-adenosyl Homocysteine (SAH), ng/mg | 1.09 | 0.84 | 0.92 | 0.95 | 1.13 | 0.10 | 0.27 | 0.03 | 0.21 | 0.07 | 0.85 | 0.76 |
| Homocysteine, ng/mg | 1.48 | 1.48 | 1.34 | 1.26 | 1.38 | 0.10 | 0.49 | 0.99 | 0.17 | 0.54 | 0.13 | 0.87 |
| SAM:SAH | 7.08 | 7.47 | 5.88 | 5.54 | 8.33 | 1.33 | 0.57 | 0.84 | 0.58 | 0.52 | 0.14 | 0.99 |
| Choline, µmol/g | 5642 | 7559 | 4741 | 7025 | 7315 | 975 | 0.22 | 0.09 | 0.31 | 0.63 | 0.26 | 0.08 |
| Folate, ng/mg | 0.28a | 0.35a | 1.07b | 1.13b | 2.03c | 0.23 | <0.01 | 0.71 | <0.01 | <0.01 | 0.82 | 0.31 |
| Dihyrdrofolate, ng/mg | 31.7a | 35.6a | 58.4ab | 68.0b | 109.8c | 10.1 | <0.01 | 0.50 | <0.01 | <0.01 | 0.88 | 0.61 |
| Tetrahydrofolate, ng/mg | 0.26a | 0.33a | 0.33a | 0.40ab | 0.63b | 0.08 | 0.04 | 0.24 | 0.30 | 0.02 | 0.44 | 0.89 |
| 5,10-Methylene-THF, ng/mg | 0.10a | 0.12a | 0.90a | 2.00b | 2.82b | 0.32 | <0.01 | 0.92 | <0.01 | <0.01 | 0.29 | 0.53 |
| 5-Methyl-THF, ng/mg | 1.05a | 1.57a | 4.20ab | 6.63bc | 9.35c | 1.67 | 0.01 | 0.55 | 0.03 | 0.01 | 0.60 | 0.94 |
10XNEG, TMR and sham injections of saline at day 0 and 7 of the estrous cycle; 0XPOS, TMR, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle; 0.5X, TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle; 1X, TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle; 2X, TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle.
2Probability values for: Trt, main effect of treatment. Probability values for contrast statements: Neg vs. Pos, 0XNEG vs. 0XPOS; Met + Chol vs. Vitamin, 0XPOS vs. 0.5X, 1X, and 2X; Linear, Quadratic, and Cubic, Polynomial contrasts with increasing folate level of 0XPOS to 2X.
3The average standard error of the mean for a specific measurement.
a–cMeans within row without a common superscript differ by a main effect of treatment.
The concentrations of folate cycle intermediates in liver were affected by treatment. Folate concentrations in liver were greater (P < 0.01) in 2X compared with the 0.5X and 1X-treated heifers which were greater than the 0XNEG- and 0XPOS-treated heifers. The concentration of DHF in liver was greater (P < 0.01) in 2X-treated heifers compared with the 1X-treated heifers, which were greater than the 0XNEG and 0XPOS, with 0.5X being intermediate and equal to 0XNEG-, 0XPOS-, and the 1X-treated heifers. The concentration of THF was greater (P = 0.04) in 2X heifers compared with both the negative and positive control as well as the 0.5X-treated heifers, with 1X-treated heifers being intermediate and equal to all other treatments. The concentration of 5,10-methylene-THF in liver was greater (P < 0.01) in the 1X- and 2X-treated heifers compared with the 0XNEG-, 0XPOS-, and 0.5X-treated heifers which were all equal. Finally, the concentration of the physiological methyl donor 5-methyl-THF was greater in the 2X-treated heifers (P = 0.01) than the 0XNEG, 0XPOS, and 0.5X. The concentration of 5-methyl-THF was greater (P = 0.05) in 1X than 0XNEG, and 0XPOS with 0.5X being intermediate and equal to the others. The concentrations of folate, DHF, 5,10-methylene-THF, and 5-methyl-THF were greater (P ≤ 0.03) in the folate-treated heifers compared with the 0XPOS and increased linearly (P ≤ 0.01) with increasing folate supplementation from 0XPOS to 2X-treated heifers. The concentration of THF in liver was not different (P = 0.30) between the 0XPOS-treated heifers and the folate-treated heifers, but THF increased linearly (P = 0.02) with increasing folate supplementation from 0XPOS to 2X.
Serum methionine cycle intermediates
All day 14 serum analyte data are presented in Table 4. Because of the homeostasis in methionine cycle analyte abundance in liver tissue, methionine cycle analytes were measured in serum at day 14 of the estrous cycle. The concentration of methionine was increased (P = 0.04) in 0.5X- and 2X-treated heifers compared with 0XNEG with 0XPOS, with 1X being intermediate and equal to all other treatments. The concentration of SAM was not affected (P = 0.18) by nutritional treatment. The concentration of SAH tended (P = 0.06) to be greater in the 0.5X-treated heifers compared with 0XNEG. The concentration of homocysteine in serum was not affected (P = 0.98) by treatment at day 14 of the estrous cycle. Finally, the SAM:SAH ratio was greater (P = 0.05) in the 0XNEG-, 0XPOS-, and 2X-treated heifers compared with the 0.5X-treated heifers with the 1X-treated heifers being intermediate and equal to all other treatments. Concentration differences in methionine and homocysteine were not detected by any contrasts investigated (P ≥ 0.12). The concentration of SAM tended (P = 0.06) to be affected quadratically, decreasing from 0XPOS to 0.5X and increasing from 1X- to 2X-treated heifers. In contrast, the concentration of SAH tended (P = 0.07) to be affected cubically such that the concentration in 0.5X-treated heifers was increased compared to 0XPOS and subsequently decreased in 1X and 2X which were equal to the 0XPOS heifers. Lastly, the SAM:SAH ratio tended (P = 0.08) to decrease with folate and B12 supplementation compared to heifers receiving only methionine and choline. Furthermore, the SAM:SAH ratio was affected quadratically (P = 0.03) such that it decreased from 0XPOS to 0.5X and increased from 0.5X to 1X and further increased to 2X to be equivalent to the concentration of the 0XPOS treated heifers.
Table 4.
Serum methionine cycle analyte abundance at day 14 of the synchronized estrous cycle
| Analyte | Treatment1 | SEM3 | Probability Values2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0XNEG | 0XPOS | 0.5X | 1X | 2X | Trt | Neg vs. Pos | Met + Chol vs. Vitamin | Linear | Quadratic | Cubic | ||
| Methionine, ng/mL | 3178a | 3688ab | 4150b | 3635ab | 4398b | 267 | 0.04 | 0.30 | 0.23 | 0.12 | 0.52 | 0.14 |
| S-adenosyl Methionine, ng/mL | 20.5 | 21.2 | 17.8 | 18.8 | 20.4 | 1.07 | 0.18 | 0.55 | 0.11 | 0.95 | 0.06 | 0.26 |
| S-adenosyl Homocysteine, ng/mL | 5.51 | 6.10 | 7.48 | 6.42 | 6.07 | 0.45 | 0.06 | 0.36 | 0.29 | 0.44 | 0.15 | 0.07 |
| Homocysteine, ng/mL | 568 | 560 | 572 | 558 | 589 | 38.3 | 0.98 | 0.90 | 0.74 | 0.57 | 0.79 | 0.70 |
| SAM:SAH | 3.48b | 3.52b | 2.50a | 3.02ab | 3.37b | 0.25 | 0.05 | 0.91 | 0.08 | 0.69 | 0.03 | 0.07 |
10XNEG, TMR and sham injections of saline at day 0 and 7 of the estrous cycle; 0XPOS, TMR, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle; 0.5X, TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle; 1X, TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle; 2X, TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle.
2Probability values for: Trt, main effect of treatment. Probability values for contrast statements: Neg vs. Pos, 0XNEG vs. 0XPOS; Met + Chol vs. Vitamin, 0XPOS vs. 0.5X, 1X, and 2X; Linear, Quadratic, and Cubic, Polynomial contrasts with increasing folate level of 0XPOS to 2X.
3The average standard error of the mean for a specific measurement.
a–cMeans within row without a common superscript differ by a main effect of treatment.
Methionine-folate cycle enzyme transcript abundance
Enzyme transcript abundance data can be found in Table 5. The transcript abundance of DNMT1, DNMT3A, DNMT3B, MAT1A, MTHFR, and PRMT1 were not affected (P ≥ 0.12) by nutritional treatment. Transcript abundance of MAT2A was greater (P = 0.01) in 2X-treated heifers compared with 0XNEG, 0.5X, and 1X treated heifers. Additionally, MAT2A transcript abundance was greater in 0XNEG and 0XPOS compared with 0.5X, with 1X being intermediate and equal to 0XNEG, 0XPOS, and 0.5X. The transcript abundance of MAT2B was greatest (P < 0.01) in 2X, intermediate in 0XNEG and 0XPOS, and lower in 0.5X. The transcript abundance of the 1X treatment was intermediate and equal to both the 0XNEG, 0XPOS, and 0.5X treatments. The transcript abundance of MTR tended (P = 0.08) to be greater in the 2X-treated heifers compared with the 0.5X- and 1X-treated heifers. Finally, the transcript abundance of MTRR tended (P = 0.07) to be greater in the 2X treatment compared with all other treatments. The abundance of DNMT1 was affected quadratically (P = 0.02), where the transcript abundance decreased from 0XPOS to 1X and increased from 1X to 2X. The transcript abundance of MAT2A, MAT2B, and MTR was affected quadratically (P ≤ 0.02) such that the abundance decreased from 0XPOS to 0.5X and 1X, with a subsequent increase to 2X. Finally, the transcript abundance of MTRR increased linearly (P = 0.04) with increasing supplementation of folate and vitamin B12.
Table 5.
Hepatic methionine-folate cycle enzyme transcript abundance at day 14 of the synchronized estrous cycle
| Genes3 | Treatment1 | Probability values2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0XNEG | 0XPOS | 0.5X | 1X | 2X | SEM4 | Trt | Neg vs. Pos | Met + Chol vs. Vitamin | Linear | Quadratic | Cubic | |
| DNMT1 | 1.30 | 1.39 | 1.27 | 0.97 | 1.56 | 0.16 | 0.12 | 0.76 | 0.49 | 0.43 | 0.02 | 0.29 |
| DNMT3A | 0.68 | 0.76 | 0.81 | 0.69 | 0.92 | 0.11 | 0.55 | 0.67 | 0.74 | 0.37 | 0.38 | 0.41 |
| GNMT | 0.98 | 1.73 | 0.92 | 1.25 | 2.25 | 0.56 | 0.42 | 0.49 | 0.70 | 0.33 | 0.18 | 0.61 |
| MAT1A | 0.73 | 0.93 | 0.91 | 0.73 | 0.90 | 0.10 | 0.36 | 0.25 | 0.34 | 0.64 | 0.13 | 0.26 |
| MAT2A | 0.93b | 1.25bc | 0.70a | 0.91ab | 1.55c | 0.18 | 0.02 | 0.35 | 0.31 | 0.06 | <0.01 | 0.24 |
| MAT2B | 1.26b | 1.18b | 0.85a | 1.04ab | 1.85c | 0.12 | <0.01 | 0.68 | 0.62 | <0.01 | <0.01 | 0.36 |
| MTHFR | 0.37 | 0.26 | 0.30 | 0.46 | 0.45 | 0.10 | 0.56 | 0.47 | 0.23 | 0.14 | 0.55 | 0.51 |
| MTR | 1.61 | 1.71 | 1.33 | 1.34 | 2.02 | 0.20 | 0.08 | 0.79 | 0.52 | 0.12 | 0.02 | 0.81 |
| MTRR | 1.16 | 1.41 | 1.10 | 1.30 | 2.07 | 0.27 | 0.07 | 0.53 | 0.78 | 0.04 | 0.11 | 0.68 |
| PRMT1 | 0.47 | 0.72 | 0.50 | 0.48 | 0.61 | 0.18 | 0.83 | 0.40 | 0.76 | 0.34 | 0.84 | 0.36 |
10XNEG, TMR and sham injections of saline at day 0 and 7 of the estrous cycle; 0XPOS, TMR, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle; 0.5X, TMR, MET, CHOL, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle; 1X, TMR, MET CHOL, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle; 2X, TMR, MET, CHOL, 20 mg vitamin B12, and 320 mg folate at d 0 and 7 of the estrous cycle.
2Probability values for: Trt, main effect of treatment. Probability values for contrast statements: Neg vs. Pos, 0XNEG vs. 0XPOS; Met + Chol vs. Vitamin, 0XPOS vs. 0.5X, 1X, and 2X; Linear, Quadratic, and Cubic, Polynomial contrasts with increasing folate level of 0XPOS to 2X.
3Genes are DNMT1, DNA methyltransferase 1; DNMT3A, DNA methyltransferase 3A; GNMT, glycune N-methyltransferase; MAT1A, methionine adenosyltransferase 1A; MAT2A, methionine adenosyltransferase 2A; MAT2B, methionine adenosyltransferase 2B; MTHFR, methylenetetrahydrofolate reductase; MTR, 5-methyltetrahydrofolate-homocysteine methyltransferase; MTRR, 5-methyltetrahydrofolate-homocysteine methyltransferase reductase; PRMT1, protein arginine methyltransferase 1.
4The average standard error of the mean for a specific measurement.
a–cMeans without a common superscript differ by a main effect of treatment.
Discussion
It is well established that cattle with functioning rumens have microbiota that are very active in the synthesis of B-vitamins including choline, folic acid, and vitamin B12 (McDowell, 2000), and that under normal conditions, the synthesis of these B vitamins should be adequate for normal metabolic functions. In the case of vitamin B12, its synthesis requires the presence of cobalt, which is at the center of its corrin ring structure, and in some areas of the United States, New Zealand, and Australia, the concentrations of cobalt in consumed forages may be inadequate for microbial vitamin B12 synthesis in the rumen (National Academies of Sciences, Engineering, and Medicine, 2016). Furthermore, there are times within the normal beef cattle production cycle, such as during drought, that culminates in limited forage quantity and/or forage quality, resulting in nutrient restriction and decreases in circulating methionine concentrations (Crouse et al., 2019). Syring et al., (2022) reported that restricting beef heifers to 60% of CON intake resulted in increased folate and vitamin B12 concentrations in allantoic fluid, which may be a compensatory mechanism to maintain fetal growth and the fetal epigenome during times of aberrant maternal nutrition. Reports using ruminant models demonstrated the negative responses to OCM restriction on offspring performance (Sinclair et al., 2007) such as altered fat metabolism and accretion, and insulin resistance in male offspring of methyl deficient ewes. Contrarily, recent studies have demonstrated the positive responses to maternal OCM supplementation on beef calf development when supplemented during the periconceptual period of gestation or in the culture medium during in vitro fertilization (Liu et al., 2020; Estrada-Cortés et al., 2021; Silva et al., 2021) such as increased rate of gain and gain:feed (Silva et al., 2021), and increased birth and adjusted weaning weights (Estrada-Cortés et al., 2021). It should be noted that while positive responses to late gestation OCM supplementation to the cow or calf have been documented in dairy cattle (Preynat et al., 2009a; Batistel et al., 2017; Alharthi et al., 2018, 2019; Batistel et al., 2019; Palombo et al., 2021), there are discrepancies in the data as to the benefit of supplementation of methionine in beef cattle. Feeding methionine hydroxy analogues to beef cows prior to and after calving increased weaning weights in studies from Varner (1974), Varner et al., (1975) and Thomas and Langford (1978) but failed to affect calf weaning weights in studies reported from Clanton and England (1980) and Clements et al., (2017). It is important to note when comparing these studies that metabolic stressors such as lactation, nutrient intake and forage quality, breeding/calving season, and limiting/meeting/exceeding protein requirements also play an important role in the responses to methionine supplementation. Furthermore, there are multiple methionine supplement sources and the type (methionine hydroxy analog or rumen protection) influences the bioavailability which should be accounted for prior to supplementation. A recent focus in beef cattle has been placed on early-pregnancy (Liu et al., 2020; Estrada-Cortés et al., 2021; Silva et al., 2021), a time when there is epigenetic reprogramming that is taking place during early gestation and thus substantiates the specific need for OCM during this time (Dobbs et al., 2013). Therefore, these critical events and timing of nutrient supplementation require the additional investigation of OCM supplementation during early gestation as well as defining the optimal supplementation dose of OCM during early pregnancy.
Data presented in this manuscript demonstrate that the concentrations of folate, vitamin B12, and methionine-folate cycle intermediates in serum and liver tissue in response to supplemental B12, folate, methionine, and choline are dose dependent, and that not all supplementation levels yield positive responses to increasing the circulating concentrations of methyl donors. It is important to note that while choline and methionine are stored in the body either via phospholipids or proteins, water soluble vitamins such as folate and vitamin B12 are not stored in substantial quantities. Therefore, these data support the injection of folate and B12 at 160 to 320 mg folate and 10 to 20 mg of vitamin B12 to yearling beef heifers fed rumen protected methionine and choline at the manufacturer recommended intakes (Smartamine inclusion: 0.08% of DMI; Reashure inclusion: 60 g/d) to maintain increased circulating concentrations of OCM particularly folate and vitamin B12.
Regardless of injection dosage, vitamin B12 concentration was greater in all supplemented heifers compared with negative and positive controls for both concentrations and area under the curve. The concentration of folate, however, was not increased until 320 mg of folate was injected weekly. This is likely due to the different roles of vitamin B12 and folate in metabolism with vitamin B12 being a cofactor for enzymes such as methionine synthase and methylmalonyl-CoA-mutase and folate being an intermediate in one-carbon metabolism and nucleotide synthesis (McDowell, 2000; Kräutler, 2012; Clare et al., 2019; Aggett et al., 2020). Similar to previously published work in dairy cattle (Preynat et al., 2009a), our work demonstrated that supplementation of methionine without vitamin B12 and folate injections resulted in decreased folate concentrations in circulation. Increases in SAM result in the downregulation of MTHFR enzyme (Selhub, 1999; Lucock, 2000; Clare et al., 2019) and thus a decrease in circulating 5-methyl-THF, which is the major form of folates found in circulation of humans (Selhub, 1999) and cattle (Preynat et al., 2009b). Therefore, the coordinated increase in both methionine and SAM as presented herein in coordination with the decrease in circulating folates in the 0XPOS-treated heifers is supported from previous research.
The concentrations of methionine cycle intermediates in liver at day 14 of the estrous cycle did not differ by treatment. A recent publication from Clare et al. (2021) determined that in tissues with betaine homocysteine methyltransferase, such as liver, the net flux of methionine would be maintained regardless of substrate supply. Therefore, the lack of differences seen in hepatic methionine cycle intermediates in this study was to be expected. Furthermore, all folate cycle intermediates measured were increased in folate supplemented heifers compared with the saline injected heifers. Like the circulating total folates in serum, only the 2X treatment was greater in all folate metabolites measured in liver suggesting a similarity between hepatic and circulating folate responses.
Serum methionine cycle metabolites in the present study demonstrate that there are doses of folate and vitamin B12 that alter the methylation potential in beef heifers. Methylation potential is the ability of cells to methylate DNA, RNA, or proteins and is measured by the ratio of SAM:SAH, that are the substrates and products, respectively, for methyltransferases. A decreased SAM:SAH ratio is associated with decreased methylation potential and hypomethylated DNA, primarily driven by increased SAH (Caudill et al., 2001). The tendency to increase serum SAH and decrease the SAM:SAH ratio in heifers in the present study demonstrates a decreased methylation potential in heifers receiving the 0.5X treatment.
Recently, Crouse et al. (2022) reported cubic effects on embryonic cell growth, mitochondrial respiration, and differential methylation in bovine embryonic fibroblasts cultured with a similar model of doubling OCM in the media. These cubic effects seen both in vitro (increase in mitochondrial respiration and cell growth from control to 2.5X, decrease at 5X, and subsequent increase at 10X) and in vivo (decrease in SAM:SAH ratio in 0.5X) support the interconnected nature and stoichiometry of the methionine-folate cycle and the need to establish an optimal substrate level for each OCM in relationship to the others to keep the balance of substrates and products in each cycle and their connected pathways. It should also be noted that the concentration of circulating homocysteine was not altered by supplementing OCM in the current study. This may be due to the basal diet already meeting protein requirements and the fact that this study did not aim to induce a methyl deficiency either directly via the basal diet, or indirectly by the the inclusion of methyl substrate consumers or inhibitors as has been previously demonstrated (Sinclair et al., 2007; Ardalan et al., 2020).
To further understand the effects of OCM supplementation on concentrations of circulating OCM, hepatic methionine-folate cycle enzyme transcript abundance was measured. Interestingly, enzymes responsible for the synthesis of SAM as well as the folate cycle remethylation of homocysteine to methionine decreased in the 0.5X treatment and subsequently increased to be equal to or greater than the 0XPOS treated heifers by the 2X supplemented group. The differences in transcript abundance of MAT2A and MAT2B accompany a similar quadratic response in the concentrations of SAM decreasing from 0XPOS to 0.5X and subsequently increasing to the 2X supplemented level. Methionine adenosyl transferase 2A is one of the enzymes that synthesizes SAM from methionine whereas MAT2B encodes a regulatory subunit for MAT2A (Nordgren et al., 2011). The change in MAT2B transcript abundance in Crouse et al., (2022) and in the current report demonstrates similar effects between in vitro culture of an immortalized cell line of bovine embryonic fibroblasts and in vivo derived tissues from this study. Increases in SAM typically result in inhibition of MTHFR and activate the transsulfuration pathway to decrease the amount of 5-mTHF available for the remethylation of homocysteine to methionine but also remove homocysteine via transsulfuration (Neidhart, 2016; Clare et al., 2019). While there were no changes in the magnitude of SAM due to nutritional treatment, our data support these findings with no change in MTHFR transcript abundance either. The decrease in MTR and MTRR at 0.5X do not follow the same pattern as the increase in their substrate concentrations. Further work in cattle would need to be conducted to determine the regulatory effects of methionine-folate intermediates on enzyme transcript abundance as well as to determine the enzyme function in the presence of increased or decreased substrate.
It is important to note that data from this study was conducted in cyclic heifers, and that further work using these doses are needed to determine whether dietary and injectable OCM may be used in ruminants to alter the conceptus or fetal epigenomes and lead to developmental programming responses. Furthermore, not all concentrations of vitamin B12 and folic acid used in this study yielded positive responses in terms of methyl donor availability/metabolism, and that more folate and vitamin B12 may not be better for the conceptus. Therefore, further mechanistic studies should be conducted with heifers/cows at different physiological stages to better understand the stoichiometric balance of methyl donors in the methionine-folate cycle in beef cattle thereby more appropriately supplementing cattle of different breeds and at different physiological stages in an effort to improve growth, reproductive performance, and developmental programming outcomes.
In conclusion, these data support the injection of 20 mg of vitamin B12 and 320 mg of folic acid injected once weekly when cyclic heifers are fed rumen protected methionine and rumen protected choline at manufacturer recommended feed intakes to increase circulating concentrations of B12 and folate. These doses set the basis upon which future studies can be based when feeding and injecting one-carbon metabolites.
Supplementary Material
Acknowledgments
We thank Chad Engle, Kris Kotinek, Nikki Krupicka, Ken Ostdiek, and the USMARC Cattle Operations for expert care and handling of the animals; Sarah Knox and Darrell Light for management of the data; Ashley Lutz for expert technical assistance in the laboratory; and Donna Griess for assistance with preparation of the manuscript. This project was funded in part by ARS project number 3040-31000-097-00D.
Glossary
Abbreviations
- 0XNEG
total mixed ration and sham injections of saline at day 0 and 7 of the estrous cycle
- 0XPOS
total mixed ration, rumen protected methionine fed at 0.08% of the diet DM, rumen protected choline; fed at 60 g/d, and sham injections of saline at day 0 and 7 of the estrous cycle
- 0.5X
total mixed ration, rumen protected methionine, rumen protected choline, 5 mg vitamin B12, and 80 mg folate at day 0 and 7 of the estrous cycle
- 1X
total mixed ration, rumen protected methionine, rumen protected choline, 10 mg vitamin B12, and 160 mg folate at day 0 and 7 of the estrous cycle
- 2X
total mixed ration, rumen protected methionine, rumen protected choline, 20 mg vitamin B12, and 320 mg folate at day 0 and 7 of the estrous cycle
- ACTB
beta actin
- AUC
area under the curve
- CHOL
rumen protected choline
- DHF
dihydrofolic acid
- DNMT1
DNA methyltransferase 1
- DNMT3A
DNA methyltransferase 3A
- FBP
folate binding protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GNMT
glycine N-methyltransferase
- GnRH
gonadotropin releasing hormone
- HIF
hog intrinsic factor
- HPRT1
hypoxanthine phosphoribosyltransferase
- MAT1A
methionine adenosyltransferase 1A
- MAT2A
methionine adenosyltransferase 2A
- MAT2B
methionine adenosyltransferase 2B
- MET
rumen protected methionine
- MTHFR
methyltretrhydrofolate reductase
- MTR
methionine synthase
- MTRR
methionine synthase reductase
- OCM
one-carbon metabolites
- PRMT1
protein methyltransferase
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- THF
tetrahydrofolic Acid
- TMR
total mixed ration
Mention of a trade name, proprietary product, or specific agreement does not constitute a guarantee or warranty by the USDA and does not imply approval to the inclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
Contributor Information
Matthew S Crouse, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Harvey C Freetly, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Amanda K Lindholm-Perry, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Bryan W Neville, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
William T Oliver, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Robert T Lee, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Jessica G Syring, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Layla E King, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Lawrence P Reynolds, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Carl R Dahlen, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Joel S Caton, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Alison K Ward, Department of Animal Sciences, Center for Nutrition and Pregnancy, North Dakota State University, Fargo, ND 58108, USA.
Robert A Cushman, USDA, ARS, U.S. Meat Animal Research Center, Clay Center, NE 68933, USA.
Conflict of Interest Statement
The authors declare no known or perceived conflicts of interest.
Literature Cited
- Acosta, D. A. V., Denicol A. C., Tribulo P., Rivelli M. I., Skenandore C., Zhou Z., Luchini D., Corrêa M. N., Hansen P. J., and Cardoso F. C... 2016. Effects of rumen-protected methionine and choline supplementation on the preimplantation embryo in Holstein cows. Theriogenology. 85:1669–1679. doi: 10.1016/j.theriogenology.2016.01.024 [DOI] [PubMed] [Google Scholar]
- Aggett, P. J., Ahnen R. T., Aydemir T. B., Bailey L. B., Bettendorff L., Blaner W. S., Borum P. R., Bruno R. S., Calder P. C., Caudill M. A.,. et al. 2020. Contributors to volume 1. In: Marriott, B. P., Birt D. F., Stallings V. A., and Yates A. A., editors. Present knowledge in nutrition. 11th ed.Amsterdam, Netherlands: Academic Press; p. xi–xiii. [Google Scholar]
- Alharthi, A. S., Batistel F., Abdelmegeid M. K., Lascano G., Parys C., Helmbrecht A., Trevisi E., and Loor J. J... 2018. Maternal supply of methionine during late-pregnancy enhances rate of Holstein calf development in utero and postnatal growth to a greater extent than colostrum source. J. Anim. Sci. Biotechnol. 9:83. doi: 10.1186/s40104-018-0298-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharthi, A. S., Coleman D. N., Liang Y., Batistel F., Elolimy A. A., Yambao R. C., Abdel-Hamied E., Pan Y. X., Parys C., Alhidary I. A.,. et al. 2019. Hepatic 1-carbon metabolism enzyme activity, intermediate metabolites, and growth in neonatal Holstein dairy calves are altered by maternal supply of methionine during late pregnancy. J. Dairy Sci. 102:10291–10303. doi: 10.3168/jds.2019-16562 [DOI] [PubMed] [Google Scholar]
- Ardalan, M., Batista E. D., and Titgemeyer E. C... 2020. Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 98:skaa072. doi: 10.1093/jas/skaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistel, F., Alharthi A. S., Yambao R. R. C., Elolimy A. A., Pan Y. X., Parys C., and Loor J. J... 2019. Methionine supply during late-gestation triggers offspring sex-specific divergent changes in metabolic and epigenetic signatures in bovine placenta. J. Nutr. 149:6–17. doi: 10.1093/jn/nxy240 [DOI] [PubMed] [Google Scholar]
- Batistel, F., Arroyo J. M., Bellingeri A., Wang L., Saremi B., Parys C., Trevisi E., Cardoso F. C., and Loor J. J... 2017. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 100:7455–7467. doi: 10.3168/jds.2017-12689 [DOI] [PubMed] [Google Scholar]
- Caudill, M. A., Wang J. C., Melnyk S., Pogribny I. P., Jernigan S., Collins M. D., Santos-Guzman J., Swendseid M. E., Cogger E. A., and James S. J... 2001. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine β-synthase heterozygous mice. J. Nutr. 131:2811–2818. doi: 10.1093/jn/131.11.2811 [DOI] [PubMed] [Google Scholar]
- Clanton, D. C., and England M. E... 1980. Methionine hydroxy analog in supplements for lactating beef cows. J. Anim. Sci. 51:539–543. doi: 10.2527/jas1980.513539x [DOI] [Google Scholar]
- Clare, C. E., Brassington A. H., Kwong W. Y., and Sinclair K. D... 2019. One-carbon metabolism: linking nutritional biochemistry to epigenetic programming of long-term development. Annu. Rev. Anim. Biosci. 7:263–287. doi: 10.1146/annurev-animal-020518-115206 [DOI] [PubMed] [Google Scholar]
- Clare, C. E., Pestinger V., Kwong W. Y., Tutt D. A. R., Xu J., Byrne H. M., Barrett D. A., Emes R. D., and Sinclair K. D... 2021. Interspecific variation in one-carbon metabolism within the ovarian follicle, oocyte, and preimplantation embryo: consequences for epigenetic programming of DNA methylation. Int. J. Mol. Sci. 22:1838. doi: 10.3390/ijms22041838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements, A. R., Ireland F. A., Freitas T., Tucker H., and Shike D. W... 2017. Effects of supplementing methionine hydroxy analog on beef cow performance, milk production, reproduction, and preweaning calf performance. J. Anim. Sci. 95:5597–5605. doi: 10.2527/jas2017.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse, M. S., Caton J. S., Claycombe-Larson K. J., Diniz W. J. S., Lindholm-Perry A. K., Reynolds L. P., Dahlen C. R., Borowicz P. P., and Ward A. K... 2022. Epigenetic modifier supplementation improves mitochondrial respiration and growth rates and alters DNA methylation of bovine embryonic fibroblast cells cultured in divergent energy supply. Front. Genet. 13:812764. doi: 10.3389/fgene.2022.812764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse, M. S., Greseth N. P., McLean K. J., Crosswhite M. R., Pereira N. N., Ward A. K., Reynolds L. P., Dahlen C. R., Neville B. W., Borowicz P. P.,. et al. 2019. Maternal nutrition and stage of early pregnancy in beef heifers: impacts on hexose and AA concentrations in maternal and fetal fluids. J. Anim. Sci. 97:1296–1316. doi: 10.1093/jas/skz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs, K. B., Rodriguez M., Sudano M. J., Ortega M. S., and Hansen P. J... 2013. Dynamics of DNA methylation during early development of the preimplantation bovine embryo. PLoS One. 8:e66230. doi: 10.1371/journal.pone.0066230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J. E., Jiang Z. C., Alqahtani F., Mandoiu I., Dong H., Zheng X., Marjani S. L., Chen J., and Tian X. C... 2019. Methylome dynamics of bovine gametes and in vivo early embryos. Front. Genet. 10:512. doi: 10.3389/fgene.2019.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Cortés, E., Negrón-Peréz V. M., Tríbulo P., Zenobi M. G., Staples C. R., and Hansen P. J... 2020. Effects of choline on the phenotype of the cultured bovine preimplantation embryo. J. Dairy Sci. 103:10784–10796. doi: 10.3168/jds.2020-18598 [DOI] [PubMed] [Google Scholar]
- Estrada-Cortés, E., Ortiz W., Rabaglino M. B., Block J., Rae O., Jannaman E. A., Xiao Y., and Hansen P. J... 2021. Choline acts during preimplantation development of the bovine embryo to program postnatal growth and alter muscle DNA methylation. FASEB J. 35:e21926. doi: 10.1096/fj.202100991R [DOI] [PubMed] [Google Scholar]
- Ivanova, E., Canovas S., Garcia-Martínez S., Romar R., Lopes J. S., Rizos D., Sanchez-Calabuig M. J., Krueger F., Andrews S., Perez-Sanz F.,. et al. 2020. DNA methylation changes during preimplantation development reveal inter-species differences and reprogramming events at imprinted genes. Clin. Epigenet. 12:64. doi: 10.1186/s13148-020-00857-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z., Lin J., Dong H., Zheng X., Marjani S. L., Duan J., Ouyang Z., Chen J., and Tian X. C... 2018. DNA methylomes of bovine gametes and in vivo produced preimplantation embryos. Biol. Reprod. 99:949–959. doi: 10.1093/biolre/ioy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräutler, B. 2012. Biochemistry of B12-cofactors in human metabolism. Subcell. Biochem. 56:323–346. doi: 10.1007/978-94-007-2199-9_17 [DOI] [PubMed] [Google Scholar]
- Liu, L., Amorín R., Moriel P., DiLorenzo N., Lancaster P. A., and Peñagaricano F... 2020. Differential network analysis of bovine muscle reveals changes in gene coexpression patterns in response to changes in maternal nutrition. BMC Genom. 21:684. doi: 10.1186/s12864-020-07068-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D... 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 24:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lucock, M. 2000. Folic acid: nutrition biochemistry, molecular biology, and role in disease processes. Mol Genet. Metab. 71:121–138. doi: 10.1006/mgme.2000.3027 [DOI] [PubMed] [Google Scholar]
- Mason, J. B. 2003. Biomarkers of nutrient exposure and status in one-carbon (methyl) metabolism. J. Nutr. 133:941S941s–941S947S. doi: 10.1093/jn/133.3.941s [DOI] [PubMed] [Google Scholar]
- McDowell, L. R. 2000. Vitamins in animal and human nutrition. 2nd ed.AMES, IA: Iowa State University Press. [Google Scholar]
- Morgan, H. D., Santos F., Green K., Dean W., and Reik W... 2005. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14Review Issue 1:R47–58. doi: 10.1093/hmg/ddi114 [DOI] [PubMed] [Google Scholar]
- Nandania, J., Kokkonen M., Euro L., and Velagapudi V... 2018. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1092:168–178. doi: 10.1016/j.jchromb.2018.06.008 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2016. Nutrient requirements of beef cattle. 8th rev. ed.Washington, DC: The National Academies Press. doi: 10.17226/19014 [DOI] [Google Scholar]
- Neidhart, M. 2016. Chapter 27 - methyl donors. In: Neidhart M., editor. DNA methylation and complex human disease. Oxford: Academic Press; p. 429–439. [Google Scholar]
- Nordgren, K. K., Peng Y., Pelleymounter L. L., Moon I., Abo R., Feng Q., Eckloff B., Yee V. C., Wieben E., and Weinshilboum R. M... 2011. Methionine adenosyltransferase 2A/2B and methylation: gene sequence variation and functional genomics. Drug Metab. Dispos. 39:2135–2147. doi: 10.1124/dmd.111.040857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo, V., Alharthi A., Batistel F., Parys C., Guyader J., Trevisi E., D’Andrea M., and Loor J. J... 2021. Unique adaptations in neonatal hepatic transcriptome, nutrient signaling, and one-carbon metabolism in response to feeding ethyl cellulose rumen-protected methionine during late-gestation in Holstein cows. BMC Genom. 22:280. doi: 10.1186/s12864-021-07538-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagaricano, F., Souza A. H., Carvalho P. D., Driver A. M., Gambra R., Kropp J., Hackbart K. S., Luchini D., Shaver R. D., Wiltbank M. C.,. et al. 2013. Effect of maternal methionine supplementation on the transcriptome of bovine preimplantation embryos. PLoS One. 8:e72302. doi: 10.1371/journal.pone.0072302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preynat, A., Lapierre H., Thivierge M. C., Palin M. F., Matte J. J., Desrochers A., and Girard C. L... 2009a. Effects of supplements of folic acid, vitamin B12, and rumen-protected methionine on whole body metabolism of methionine and glucose in lactating dairy cows. J. Dairy Sci. 92:677–689. doi: 10.3168/jds.2008-1525 [DOI] [PubMed] [Google Scholar]
- Preynat, A., Lapierre H., Thivierge M. C., Palin M. F., Matte J. J., Desrochers A., and Girard C. L... 2009b. Influence of methionine supply on the response of lactational performance of dairy cows to supplementary folic acid and vitamin B12. J. Dairy Sci. 92:1685–1695. doi: 10.3168/jds.2008-1572 [DOI] [PubMed] [Google Scholar]
- Selhub, J. 1999. Homocystein metabolism. Annu. Rev. Nutr. 19:217–246. doi: 10.1146/annurev.nutr.19.1.217 [DOI] [PubMed] [Google Scholar]
- Shiang, K. 2010. The SAS® calculations of areas under the curve (AUC) for multiple metabolic readings [accessed April 4, 2022]. https://www.lexjansen.com/wuss/2004/posters/c_post_the_sas_calculations_.pdf.
- Silva, G. M., Chalk C. D., Ranches J., Schulmeister T. M., Henry D. D., DiLorenzo N., Arthington J. D., Moriel P., and Lancaster P. A... 2021. Effect of rumen-protected methionine supplementation to beef cows during the periconception period on performance of cows, calves, and subsequent offspring. Animal. 15:100055. doi: 10.1016/j.animal.2020.100055 [DOI] [PubMed] [Google Scholar]
- Sinclair, K. D., Allegrucci C., Singh R., Gardner D. S., Sebastian S., Bispham J., Thurston A., Huntley J. F., Rees W. D., Maloney C. A.,. et al. 2007. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. U.S.A. 104:19351–19356. doi: 10.1073/pnas.0707258104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syring, J. S., Neville T. L., Crouse M. S., Ward A. K., Dahlen C. R., Reynolds L. P., Borowicz P. P., McLean K. J., Neville B. W., and Caton J. S... 2022. O23 Maternal nutrient restriction in beef heifers during early gestation increases B12 and folate concentrations in fetal allantoic fluids. Animal. 13:271–272. doi: 10.1016/j.anscip.2022.07.033 [DOI] [Google Scholar]
- Thomas, O. O., and Langford W. J... 1978. Methionine hydroxy analog in beef cow supplements given pre- and post calving. Proc. West. Sec. Amer. Soc. Anim. Sci. 29:454. [Google Scholar]
- Van den Veyver, I. B. 2002. Genetic effects of methylation diets. Annu. Rev. Nutr. 22:255–282. doi: 10.1146/annurev.nutr.22.010402.102932 [DOI] [PubMed] [Google Scholar]
- Varner, L. W. 1974. Supplementary diets of beef cows with methionine hydroxy analog. Montana Agric. Exp. Sta. Res. Rep. 52:34. [Google Scholar]
- Varner, L. W., Denham A. H., McCone W. C., Nelms G. E., Smith W. H., Ward J. K., and Cash E. H... 1975. Methionine hydroxy analog additions to diets of beef cows. J. Anim. Sci. 41:278. [Google Scholar]
- Zeisel, S. H. 2011. The supply of choline is important for fetal progenitor cells. Semin. Cell Dev. Biol. 22:624–628. doi: 10.1016/j.semcdb.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.