Abstract
We report the emergence of imipenem-relebactam nonsusceptible Pseudomonas aeruginosa in 5 patients treated for nosocomial pneumonia for 10–28 days. Genome sequence analysis identified treatment-emergent mutations in MexAB-OprM and/or MexEF-OprN efflux operons that arose independently in each patient across distinct P. aeruginosa sequence types. Testing with efflux-inhibitor PAβN restored imipenem-relebactam susceptibility.
Keywords: Pseudomonas, imipenem-relebactam, ceftolozane-tazobactam, efflux, resistance
Multidrug-resistant (MDR) Pseudomonas aeruginosa infections pose a serious threat to public health and a major challenge to effective treatment [1]. Ceftolozane-tazobactam and ceftazidime-avibactam are new β-lactam/β-lactamase inhibitor (BL/BLI) agents with enhanced in vitro activity against MDR P. aeruginosa [2]; however, treatment-emergent resistance has been reported [3, 4]. Imipenem-relebactam is the newest addition to the expanding armamentarium of BL/BLI agents. Relebactam is a diazabicyclooctane BLI that demonstrates potent inhibition of Ambler classes A and C serine β-lactamases, but not class B metallo-β-lactamases or class D oxacillinases. In surveillance studies, the addition of relebactam lowered modal imipenem minimum inhibitory concentrations (MICs) by 4- and 8-fold against imipenem-susceptible and nonsusceptible P. aeruginosa clinical isolates, respectively [5]. Imipenem-relebactam also retained high rates of susceptibility against P. aeruginosa harboring ampC mutations that confer resistance to ceftolozane-tazobactam and ceftazidime-avibactam [4]. Mechanisms leading to the emergence of imipenem-relebactam resistance in patients during treatment have not been described.
Treatment with imipenem-relebactam was previously assessed in 2 randomized clinical trials. The first demonstrated its efficacy and safety compared with piperacillin-tazobactam for hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) [6]. The second compared imipenem-relebactam with imipenem plus colistin for infections caused by imipenem-nonsusceptible pathogens [7]. Across both trials, 31 patients infected with P. aeruginosa were treated with imipenem-relebactam, and no cases of treatment-emergent resistance were reported. Here, we describe the emergence of imipenem-relebactam nonsusceptibility among 5 critically ill patients treated for MDR P. aeruginosa HAP/VAP. Serial isolates from each patient showed the emergence of mutations in efflux pump operons, including mutations in membrane transporter and transcriptional regulator genes.
METHODS
We identified patients between March 2020 and April 2021 who were infected with MDR P. aeruginosa and treated with imipenem-relebactam for more than 48 hours. Patients were included if an imipenem-relebactam nonsusceptible (MIC ≥4 mg/L) isolate was recovered during or within 30 days post-treatment. Serial P. aeruginosa isolates were collected before and after exposure to imipenem-relebactam and tested for susceptibility by broth microdilution in triplicate [8]. Imipenem and imipenem-relebactam MICs were further determined with and without 50 mg/L phenylalanine-arginine-β-naphthylamide (PAβN), a nonspecific efflux pump inhibitor [9]. Quality-control (QC) strains Klebsiella pneumoniae ATCC 700603 and P. aeruginosa ATCC 27853 were used throughout.
To investigate genetic mechanisms associated with imipenem-relebactam nonsusceptibility, whole-genome sequencing (WGS) was performed as previously described [4]. Genomes were assembled with CLC Genomics Workbench v11.0.1 (Qiagen) and were annotated with RAST [10]. Sequence types (STs) were identified using the Center for Genomic Epidemiology (https://cge.cbs.dtu.dk). Single nucleotide polymorphisms (SNPs) were identified by mapping sequencing reads from postexposure isolates to the genomes of baseline isolates from each patient. Each SNP had a minimum coverage of 10 reads and a minimum frequency of 90%. To assess mutations in genes of interest, sequences of each gene in the assembled genomes of all isolates from each patient were aligned to one another using BLAST, and alignments were examined using Geneious v11.1.5. Sequences were also compared to the P. aeruginosa PAO1 sequence. Illumina read data are available under BioProject PRJNA782612 (Available at: https://www.ncbi.nlm.nih.gov/bioproject/?term=782612).
RESULTS
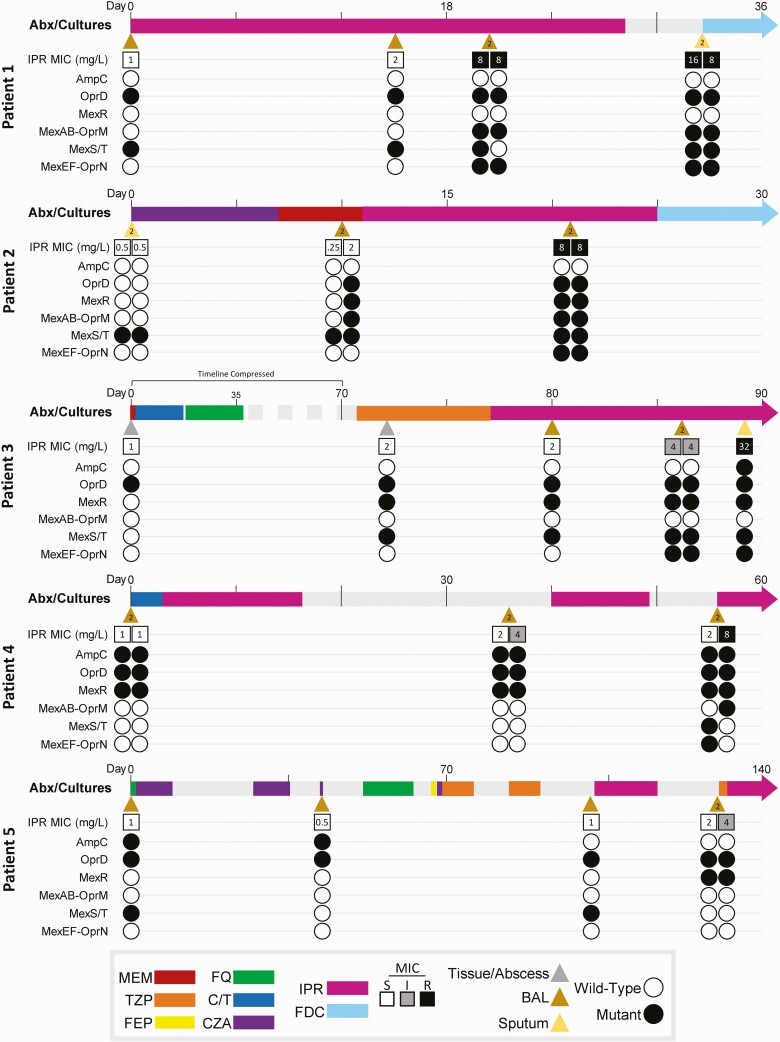
During the study period, 19 patients were treated with imipenem-relebactam for MDR P. aeruginosa infections. Imipenem-relebactam nonsusceptible isolates were recovered from 26% (5/19) of patients (Figure 1). Of these 5 patients, the median age was 56 years, 80% were male, and all were in the intensive care unit at treatment initiation. Each patient had failed prior antibiotic regimens for P. aeruginosa HAP/VAP, including 2 patients who received imipenem-relebactam after treatment-emergent resistance to ceftolozane-tazobactam (patients 4 and 5). No patient received concomitant intravenous antibiotics with imipenem-relebactam. At baseline, imipenem-relebactam MICs ranged from 0.5 to 1 mg/L. Following treatment courses of 10–28 days, postexposure imipenem-relebactam MICs ranged from 4 to 32 mg/L. Once imipenem-relebactam nonsusceptibility was identified, 2 patients were switched to cefiderocol, which remained active across serial isolates (median MIC = 0.25 mg/L; range: 0.06–2 mg/L) (Supplementary Table 1). Two other patients completed imipenem-relebactam treatment but experienced recurrent VAP. Treatment was discontinued in the final patient. No adverse events were attributed to imipenem-relebactam; however, all 5 patients had diarrhea during treatment. The overall in-hospital mortality was 60% (3/5).
Figure 1.
Timeline of serial P. aeruginosa clinical isolates for each patient, highlighting systemic anti-pseudomonal antibiotic exposures, imipenem-relebactam MICs, and mutation status of selected gene families for each isolate. Day 0 is defined as the day that baseline isolates were collected. Microbiologic cultures are indicated by triangles, and the color of each triangle specifies the source. A number “2” inside the triangle indicates that 2 different P. aeruginosa isolates were cultured from the same specimen; such culture “doublets” are organized side-by-side when depicting MICs and mutations. Timeline colored bars specify antibiotic type and duration of exposure. Imipenem-relebactam MICs are numerically shown inside small boxes, which are open if susceptible (S) and shaded if intermediate (I) or resistant (R), according to current CLSI guidelines. Gene families are depicted with small circles, which are open if all components of the gene family are wild-type (WT) compared with the reference, or shaded if any component harbors a mutation. Abbreviations: Abx, antibiotics; BAL, bronchoalveolar lavage; CLSI, Clinical and Laboratory Standards Institute; C/T, ceftolozane-tazobactam; CZA, ceftazidime-avibactam; FDC, cefiderocol; FEP, cefepime; FQ, fluoroquinolone (ciprofloxacin or levofloxacin); IPR, imipenem-relebactam; MEM, meropenem; MIC, minimum inhibitory concentration; TZP, piperacillin-tazobactam.
Twenty-nine P. aeruginosa isolates underwent WGS. No isolate harbored metallo- or other β-lactamase enzymes known to confer imipenem-relebactam resistance [5]. Each patient was infected by a unique P. aeruginosa ST, including 2 patients with high-risk MDR clones ST235 and ST244 [11]. Comparing baseline and postexposure isolates, the median number of SNPs was 8 (range: 1–285); 4 isolates from patient 2 harbored a mutation in mutS likely causing them to be hypermutators. Patients 4 and 5 developed sequential resistance to ceftolozane-tazobactam (after 32 and 16 days of treatment, respectively), followed by nonsusceptibility to imipenem-relebactam. Resistance to ceftolozane-tazobactam was mediated by previously described mutations within the catalytic center of AmpC [4, 12], which did not impact imipenem-relebactam MICs.
Imipenem-relebactam nonsusceptibility coincided with the emergence of mutations in P. aeruginosa efflux operons in all 5 patients (Supplementary Table 1). For patient 1, mutations in mexB and mexF emerged on day 19 of treatment, and corresponded with an imipenem-relebactam MIC shift from 1 to 8 mg/L. For patient 2, mutations arose simultaneously in dacB, mexR, mexB, and mexE/F after 10 days of treatment, resulting in an MIC shift from 0.5 to 8 mg/L. For patient 3, a stepwise MIC increase occurred in the presence of mexE/F mutations after 9 days (MIC = 4 mg/L), followed by a mutation upstream of ampC after 12 total days of treatment (MIC = 32 mg/L). For patient 4, a single mutation in mexB coincided with a mutation in the 2-component regulatory system creBC in an isolate with an MIC of 8 mg/L collected after 23 days of therapy. Isolates from patient 5 evolved a single mutation in mexR, but not other efflux genes, corresponding to an imipenem-relebactam MIC of 4 mg/L following 16 days of therapy. Across all patients, treatment-emergent mutations were identified within MexAB-OprM and/or MexEF-OprN operons, but not other efflux-related genes. There was no association between imipenem-relebactam MIC changes and mutations in porin genes (oprD) or genes encoding penicillin-binding proteins (ftsI, ponA).
These observations lead us to hypothesize that imipenem-relebactam nonsusceptibility was mediated by increased expression or structural changes of the MexAB-OprM and MexEF-OprN efflux pumps. To test this hypothesis, we compared imipenem and imipenem-relebactam MICs in the presence and absence of PAβN (Supplementary Table 1). Against imipenem-relebactam nonsusceptible isolates (n = 12), the presence of PAβN resulted in a median 8-fold MIC reduction, restoring susceptibility for 92% (11/12). The lone isolate for which susceptibility was not restored demonstrated an MIC reduction from 64 to 4 mg/L, and harbored a treatment-emergent mutation upstream of ampC that may have contributed to nonsusceptibility. Against the same isolates, imipenem MICs were only reduced by a median of 2-fold with PAβN, suggesting that relebactam may be preferentially impacted by increased efflux.
DISCUSSION
Our findings are the first to document the emergence of imipenem-relebactam nonsusceptibility following treatment of MDR P. aeruginosa infections and highlight the potential role of antimicrobial efflux in mediating resistance. These data are particularly notable given the dearth of effective treatment options for MDR P. aeruginosa infections among critically ill patients with HAP/VAP and underscore the remarkable propensity of P. aeruginosa to evolve resistance against all antibiotics, including novel BL/BLI agents [4].
Pseudomonas aeruginosa harbor numerous efflux systems; most notable are those from the resistance-nodulation-division (RND) family [13]. The first reported and best characterized RND efflux pump is MexAB-OprM, which has a broad substrate profile for β-lactams and is constitutively expressed in wild-type isolates. MexAB-OprM has the ability to export nearly all β-lactams, including meropenem, but not imipenem. Indeed, in OprD-deficient P. aeruginosa, imipenem MICs were unchanged in the presence of MexAB-OprM overexpression [5, 12]. Accordingly, relebactam was paired with imipenem to avoid antimicrobial efflux [5]; however, a recent in vitro study found that mutants selected by imipenem-relebactam through serial passage harbored mutations in mexR resulting in MexAB overexpression [14]. These findings align with our clinical observations showing the independent evolution of mutations in the MexAB-OprM operon, or the transcriptional repressor gene mexR, in all 5 patients infected by imipenem-relebactam nonsusceptible P. aeruginosa.
Mutations also evolved within the MexEF-OprN operon in 4 patients, including among nonsusceptible isolates from 3 patients. Unlike MexAB-OprM, the MexEF-OprN operon is not primarily controlled by a transcriptional repressor; rather, it is positively regulated by mexT, which further serves as a repressor of oprD [15], and mexAB-oprM transcription [16]. We hypothesize that MexAB-OprM and MexEF-OprN are interconnected [17] and work in concert to extrude relebactam. Mutations in mexT alone positively and negatively regulate MexAB-OprM and MexEF-OprN expression, respectively; however, it is unclear what specific role mutations in mexE/F play in regulating expression or function of either pump, or their impact on other regulatory genes. Further functional studies are underway in our laboratory. Interestingly, relebactam differs from other diazabyclooctane BLIs in that it carries a positively charged side chain believed to limit drug efflux [5]. Thus, secondary mechanisms identified by our analysis may have contributed to imipenem-relebactam nonsusceptibility, including ampC overexpression or mutations within the CreBC 2-component regulatory system that is interconnected with peptidoglycan recycling and ampC expression [18]. Multiple mechanisms were identified in a previously reported imipenem-relebactam–resistant P. aeruginosa isolate, including mexXY and ampC overexpression, and mutations in genes encoding PBP2 and PBP3 [12].
Consistent with prior in vitro studies [14], our data show that OprD inactivation appears to be a necessary first step in resistance development. Subsequent mutations in genes encoding cytoplasmic membrane transporter proteins (mexB, mexF) or regulatory genes that control expression (mexR, mexT) suggest that structural modifications and/or overexpression of P. aeruginosa efflux pumps are able to mediate imipenem-relebactam resistance. Previously against PAO1 isogenic mutants, OprD inactivation plus overexpression of MexAB-OprM resulted in a 4-fold increase in imipenem-relebactam MICs [12]. In support of this hypothesis, mutations in MexAB-OprM and/or MexEF-OprN efflux pumps evolved independently among 5 patients infected by P. aeruginosa with varying genetic backgrounds. Imipenem-relebactam treatment courses of 10–28 days preceded the emergence of resistance, which is in line with treatment-emergent resistance to other BL/BLI agents [3, 4]. Alarmingly, sequential emergence of resistance to ceftolozane-tazobactam followed by imipenem-relebactam was also identified. These data strongly support ongoing stewardship efforts to use newer BL/BLI agents judiciously and employ routine susceptibility testing to guide management decisions.
Finally, it is important to acknowledge that our analysis was centered upon a WGS-based approach to genetically characterize nonsusceptibility to imipenem-relebactam. Additional studies are needed to correlate gene mutations to expression and function of efflux pumps. Future studies are also needed to define the efficacy of imipenem-relebactam in real-world settings. Despite these limitations, our data shed new light on the role of antimicrobial efflux in mediating nonsusceptibility to imipenem-relebactam. It is possible that antimicrobial efflux may play an increased role in the emergence of resistance against other novel BL/BLIs [19]. Taken together, these findings attest to the need for continued drug-development efforts and exploration into novel approaches to overcome antimicrobial resistance in P. aeruginosa.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors gratefully acknowledge Hayley Nordstrom and the Microbial Genome Sequencing (MiGS) Center for assistance with whole-genome sequencing.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by research grants from the National Institutes of Health, including grant numbers R03AI144636 and R21AI151363 awarded to R. K. S. D. V. T. is supported by the University of Pittsburgh Department of Medicine.
Contributor Information
Ryan K Shields, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; XDR Pathogen Laboratory, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA; Antibiotic Management Program, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Madison E Stellfox, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Ellen G Kline, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Palash Samanta, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Antibiotic Management Program, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Daria Van Tyne, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA; Center for Evolutionary Biology and Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
References
- 1. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 2. Grupper M, Sutherland C, Nicolau DP. Multicenter evaluation of ceftazidime-avibactam and ceftolozane-tazobactam inhibitory activity against meropenem-nonsusceptible pseudomonas aeruginosa from blood, respiratory tract, and wounds. Antimicrob Agents Chemother 2017; 61:e00875–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamma PD, Beisken S, Bergman Y, et al. Modifiable risk factors for the emergence of ceftolozane-tazobactam resistance. Clin Infect Dis 2021; 73:e4599–e4606. [DOI] [PubMed] [Google Scholar]
- 4. Rubio AM, Kline EG, Jones CE, et al. In vitro susceptibility of multidrug-resistant Pseudomonas aeruginosa following treatment-emergent resistance to ceftolozane-tazobactam. Antimicrob Agents Chemother 2021; 65:e00084–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young K, Painter RE, Raghoobar SL, et al. In vitro studies evaluating the activity of imipenem in combination with relebactam against Pseudomonas aeruginosa. BMC Microbiol 2019; 19:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Titov I, Wunderink RG, Roquilly A, et al. A randomized, double-blind, multicenter trial comparing efficacy and safety of imipenem/cilastatin/relebactam versus piperacillin/tazobactam in adults with hospital-acquired or ventilator-associated bacterial pneumonia (RESTORE-IMI 2 study). Clin Infect Dis 2021; 73:e4539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: A multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis 2020; 70:1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 30th Edition, M100. Wayne, PA, 2020. [Google Scholar]
- 9. Sonnet P, Izard D, Mullie C. Prevalence of efflux-mediated ciprofloxacin and levofloxacin resistance in recent clinical isolates of Pseudomonas aeruginosa and its reversal by the efflux pump inhibitors 1-(1-naphthylmethyl)-piperazine and phenylalanine-arginine-beta-naphthylamide. Int J Antimicrob Agents 2012; 39:77–80. [DOI] [PubMed] [Google Scholar]
- 10. Aziz RK, Bartels D, Best AA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics 2008; 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Barrio-Tofino E, Lopez-Causape C, Oliver A. Pseudomonas aeruginosa epidemic high-risk clones and their association with horizontally-acquired beta-lactamases: 2020 update. Int J Antimicrob Agents 2020; 56:106196. [DOI] [PubMed] [Google Scholar]
- 12. Fraile-Ribot PA, Zamorano L, Orellana R, et al. Activity of imipenem-relebactam against a large collection of pseudomonas aeruginosa clinical isolates and isogenic beta-lactam-resistant mutants. Antimicrob Agents Chemother 2021; 65:e00084–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 2009; 22:582–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gomis-Font MA, Cabot G, Sanchez-Diener I, et al. In vitro dynamics and mechanisms of resistance development to imipenem and imipenem/relebactam in Pseudomonas aeruginosa. J Antimicrob Chemother 2020; 75:2508–15. [DOI] [PubMed] [Google Scholar]
- 15. Langendonk RF, Neill DR, Fothergill JL. The building blocks of antimicrobial resistance in pseudomonas aeruginosa: implications for current resistance-breaking therapies. Front Cell Infect Microbiol 2021; 11:665759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maseda H, Sawada I, Saito K, Uchiyama H, Nakae T, Nomura N. Enhancement of the mexAB-oprM efflux pump expression by a quorum-sensing autoinducer and its cancellation by a regulator, MexT, of the mexEF-oprN efflux pump operon in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2004; 48:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horna G, Lopez M, Guerra H, Saenz Y, Ruiz J. Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci Rep 2018; 8:16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zamorano L, Moya B, Juan C, Mulet X, Blazquez J, Oliver A. The Pseudomonas aeruginosa CreBC two-component system plays a major role in the response to beta-lactams, fitness, biofilm growth, and global regulation. Antimicrob Agents Chemother 2014; 58:5084–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomis-Font MA, Pitart C, Del Barrio-Tofino E, et al. Emergence of resistance to novel cephalosporin-beta-lactamase inhibitor combinations through the modification of the pseudomonas aeruginosa MexCD-OprJ efflux pump. Antimicrob Agents Chemother 2021; 65:e0008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.