Abstract
The adjuvanted recombinant glycoprotein E herpes zoster (HZ) vaccine is superior to the live attenuated HZ vaccine, with an efficacy >90% against HZ in healthy immunocompetent adults aged ≥50 years after vaccination. In pivotal studies, the efficacy of the new vaccine varied very little with the age of the vaccinee and decreased only by 5–10% in the 3.5 years after immunization. This nonlive vaccine was successfully administered to small cohorts of immunocompromised individuals; initial trials showed efficacy of >60–80% in several such settings. Potential drawbacks include the requirement for 2 vaccine doses separated by 2–6 months, local and systemic reactogenicity that is significantly greater than observed with commonly used vaccines, and the inclusion of a strong adjuvant that has been minimally studied in clinical settings where it might be problematic, such as in people with autoimmune diseases. Postmarketing studies are underway to address some of the drawbacks.
Keywords: recombinant gE vaccine, herpes zoster vaccine, VZV-specific immunity, herpes zoster, vaccine
The adjuvanted recombinant zoster vaccine is more effective than the live zoster vaccine, but more reactogenic. Efficacy declines minimally with increasing age of immunocompetent vaccines. Protection is maintained for ≥4 years. It is likely to be effective in people with many immunocompromising conditions.
FIRST HERPES ZOSTER VACCINE
Background and Evaluation
The first herpes zoster (HZ) vaccine, Zostavax (Merck; zoster vaccine live [ZVL]) has important limitations. Efficacy for prevention of HZ was limited to 69.8% for vaccinees aged 50–59 years, to 51.3 % at 60 or more years, and to 37.6% at 70 years or older [1, 2], reflecting a strong age effect on the vaccine response, such that efficacy declined with increasing age at vaccination. Two additional features of ZVL were problematic: (1) the protective effect declined significantly between 7 and 10 years after vaccination to ~20% [3] and (2) this live vaccine (Oka/Merck varicella-zoster virus [VZV] strain), although attenuated, was contraindicated in individuals with significant immune suppression [4]. However, subsequent large effectiveness studies found that the age of the vaccinee had less of an effect on the prevention of HZ and postherpetic neuralgia (PHN) than observed in the pivotal trial, and that protection was better maintained [5, 6].
Despite its shortcomings, ZVL provided a proof-of-concept critical to further research on the prevention of HZ and was an important attempt to solve an unmet medical need, and its availability accelerated the pressure to develop a vaccination platform for older adults. In 2017, when less than 35% of the target population in the United States received ZVL, it prevented ~135 000 cases of HZ and ~21 000 cases of PHN (M. J. Levin and M. N. Oxman, unpublished 2018). Zoster vaccine live remains an option for preventing HZ in the United States and Canada and is the only HZ vaccine available in most of the world.
ADJUVANTED RECOMBINANT HERPES ZOSTER VACCINE
Background
In October 2017, a second HZ vaccine, Shingrix (GlaxoSmithKline; recombinant zoster vaccine [RZV]), was approved for immunocompetent individuals aged 50 years or older. The Advisory Committee on Immunization Practices (ACIP) subsequently recommended RZV in preference over ZVL based on its superior efficacy against HZ in the target age group and the lack of an age effect on vaccine efficacy [7].
Description
Recombinant zoster vaccine consists of VZV glycoprotein E (gE) and an adjuvant system (AS01B) [8]. Glycoprotein E is the most abundant glycoprotein expressed during VZV infection [9], is a major component of the virion [10], is required for neurovirulence in animal models [11], and is essential for cell-to-cell spread [12]. Glycoprotein E–specific antibodies are readily detected in adults who had prior varicella and are a major component of the humoral immune response to VZV vaccines. T-cell–mediated immunity (CMI) directed against gE epitopes has been demonstrated but only as a limited component of the T-cell response to VZV [13, 14]. The gE component of RZV has the transmembrane anchor and the carboxyterminal domains removed to facilitate purification from Chinese hamster ovary cells.
Varicella-zoster virus–specific CMI is essential for control of VZV infections, for survival from varicella and HZ, and for prevention of HZ [15–17]. Decline in VZV CMI with age or immune compromise strongly correlates with increasing incidence and severity of HZ [18–20]. Given that gE administered by itself does not induce strong CMI responses, AS01B was added to gE in RZV to increase gE-specific CMI responses [21, 22]. AS01B is an adjuvant system that combines a plant saponin, QS21 (Quillaja saponaria Molina, fraction 21), and monophosphoryl lipid (MPL), a Toll-like receptor 4 agonist. Both adjuvant components stimulate antibody production and synergistically enhance gE-specific CMI. The adjuvant components are encapsulated in liposomes, which may enhance uptake by, and activation of, antigen-presenting cells, and protect tissues from the hemolytic activity of QS21 [21].
Preclinical and Early-phase (I/II) Studies
Preclinical studies in mice primed with live attenuated VZV were undertaken to determine the optimal concentrations of adjuvant components and gE for antibody and CMI responses. These experiments demonstrated synergy between the adjuvant components, and the importance of liposome encapsulation, in stimulating gE-specific CD4-positive (CD4+) T cells, as measured by flow cytometry [23]. Vaccination with RZV also induced strong gE-specific humoral responses measured by gE-specific enzyme-linked immunosorbent assay (ELISA) and neutralization assays.
Phase I and I/II studies in humans further delineated the optimal vaccine formulation. These experiments indicated that gE-specific T-cell responses to RZV were minimally affected by the age of the vaccinee and that induced immune responses persisted above prevaccination levels for at least 3 years [24, 25]. In the absence of adjuvant, both CMI and antibody responses to gE were lower with increasing age at vaccination, confirming the importance of the adjuvant in overcoming immune senescence. One study in mice demonstrated superior gE- and VZV-specific T-cell responses after administration of RZV compared with responses after live attenuated VZV [23]. These studies emphasized the polyfunctional nature of the T-cell responses to RZV, established the importance of the 2-dose schedule for immunogenicity, confirmed the synergistic interaction of the adjuvant components in stimulating immune responses, and documented that local and systemic reactions to RZV were greater than after other vaccines for older people.
Pivotal Clinical (Phase III) Trials
The final investigational vaccine contained 50 μg gE glycoprotein and AS01B (50 μg MPL and 50 μg QS21) in liposomes [8]. The adjuvant and antigen are mixed from separate vials prior to intramuscular administration and given as 2 doses separated by 2 months. There were 2 pivotal trials (started 2010–2011, completed 2015) of identical design so that the data could be pooled. The first study (termed ZOE-50) was for participants aged 50 years or older and the second (ZOE-70) for participants aged 70 years or older [26, 27]. These studies enrolled 70–75% Caucasian, 20% Asian, and 55–60% female participants. US and international sites were included; exclusions included prior HZ, VZV-containing vaccine, or autoimmune disease.
Participants aged 70 years or older were first randomly assigned to ZOE-50 or ZOE-70, after which they were randomly assigned to RZV or placebo. The trials were observer blinded. Key outcome measures were HZ and PHN. Polymerase chain reaction (PCR) confirmed 90% of HZ diagnoses; in the absence of PCR results, HZ was determined by an expert adjudication committee. Reactions to vaccination were recorded in subcohorts of recipients (9000 from ZOE-50 and 1000 from ZOE-70) for 7 days after each dose of vaccine. Postherpetic neuralgia was evaluated with the Zoster Brief Pain Index as utilized for the ZVL pivotal trial, and the criteria for the diagnosis of HZ were similar [1].
Efficacy of the Pivotal Trials
Table 1 indicates the sample size and efficacy of RZV against HZ for ZOE-50. The overall efficacy of 97.2% was strikingly superior to that achieved with ZVL and there was no age effect. Table 2 shows the results for participants aged 70 years or older in either trial, confirming the outstanding efficacy of RZV despite advanced age of vaccinees, which makes it unique among vaccines for older individuals, such as vaccines for influenza or pneumococcus. The pooled dataset confirmed the absence of an age effect, including among vaccines aged 80 years or older. Table 3 shows persistence of protection over a 4-year interval. The efficacy against PHN (~88%) (data not shown) was similar to the efficacy against HZ. PHN did not develop in anyone younger than 70 years. Moreover, RZV mitigated pain associated with breakthrough HZ, resulting in less severe pain, lower average pain, and less pain medication use [28].
Table 1.
Efficacy of Adjuvanted Glycoprotein E Vaccine (for ZOE-50 Clinical Trial)
| Glycoprotein E Vaccine | Placebo | |||||
|---|---|---|---|---|---|---|
| Age Group, years | HZ Cases | Rate/103 p-y | Age Group, years | HZ Cases | Rate/103 p-y | Vaccine Efficacy (95% CI) |
| All (N = 7344) |
6 | 0.3 | All (N = 7415) |
210 | 9.1 | 97.2 (93.7–99.1) |
| 50–59 (N = 3492) |
3 | 0.3 | 50–59 (N = 3525) |
87 | 7.8 | 96.6 (89.6–99.3) |
| 60–69 (N = 2141) |
2 | 0.3 | 60–69 (N = 2166) |
75 | 10.8 | 97.4 (90.1–99.70) |
| ≥70 (N = 1711) |
1 | 0.2 | ≥70 (N = 1724) |
48 | 9.4 | 97.9 (87.9–100.0) |
Adapted from reference [26].Abbreviations: CI, confidence interval; HZ, herpes zoster response over baseline response; p-y, person-years.
Table 2.
Efficacy of Adjuvanted Glycoprotein E Vaccine in Vaccinees ≥70 Years Old (from ZOE-50 + ZOE-70 Clinical Trials)
| Glycoprotein E Vaccine | Placebo | |||||
|---|---|---|---|---|---|---|
| Age Group, years | HZ Cases | Rate/103 p-y | Age Group, years | HZ Cases | Rate/103 p-y | Vaccine Efficacy (95% CI) |
| All (N = 8250) |
25 | 0.8 | All (N = 8346) |
284 | 9.3 | 91.3 (86.8–94.50) |
| 70–79 (N = 6468) |
19 | 0.8 | 70–79 (N = 6554) |
216 | 8.8 | 91.3 (86.0–94.9) |
| ≥80 (N = 1782) |
6 | 1 | ≥80 (N = 1792) |
68 | 11.1 | 91.4 (80.2–97.0) |
Adapted from reference [27].Abbreviations: CI, confidence interval; HZ, herpes zoster; p-y, person-years.
Table 3.
Four-Year Efficacy of Adjuvanted Glycoprotein E Vaccine in Vaccinees ≥70 Years Old (from ZOE-50 + ZOE-70 Clinical Trials)
| Glycoprotein E Vaccine | Placebo | |||||
|---|---|---|---|---|---|---|
| Year | HZ Cases | Rate/103 p-y | Year | HZ Cases | Rate/103 p-y | Vaccine Efficacy (95% CI) |
| Year 1 (N = 8250) |
2 | 0.2 | Year 1 (N = 8346) |
83 | 10.1 | 97.6 (90.9–99.8) |
| Year 2 (N = 8039) |
7 | 0.9 | Year 2 (N = 8024) |
87 | 11.1 | 92 (82.8–96.9) |
| Year 3 (N = 7736) |
9 | 1.2 | Year 3 (N = 7736) |
58 | 7.7 | 84.7 (69.0–93.4) |
| Year 4 (N = 7426) |
7 | 1 | Year 4 (N = 7267) |
56 | 8.2 | 87.9 (73.3–95.40) |
Adapted from reference [27].Abbreviations: CI, confidence interval; HZ, herpes zoster; p-y, person-years.
Safety
Safety information is essential for evaluating new vaccines, especially one that incorporates a strong adjuvant with limited prior use in older adults. In the 2 ZOE trials the number of serious adverse events was similar in RZV and placebo recipients. These events, which were recorded for a mean of ~3.5 years for the 2 studies, consisted mostly of illnesses expected in older participants. Events judged to be vaccine related were also similar in number for RZV and placebo recipients, as were the number of deaths and the proportion of immune-mediated diseases (1.3% or 1.4%) in either study. There were no anatomical, etiological, or temporal characteristics of serious adverse events that distinguished RZV and placebo recipients.
Reactogenicity
Recombinant zoster vaccine is significantly more reactogenic than other vaccines administered to older individuals. In both ZOE trials total adverse events were twice as common in RZV recipients as in placebo recipients (79–85% vs 30–38%). Injection site reactions were reported in 74–82% of vaccine recipients versus 10–12% of placebo recipients, and systemic reactions were reported in 53–66% versus 25–30%, respectively. The frequency of grade 3 events (preventing normal daily activity) was 8.5–9.5% versus 0.4–2% at the injection site and the frequency of systemic reactions was 6–11% versus 2–2.4% in vaccine compared with placebo recipients, respectively. Systemic reactions were mostly myalgia, fatigue, headache, and/or shivering. Reactions were transient: 2–3 days at injection sites and 1–2 days for systemic symptoms. There was a trend for reactions to be less severe in older versus younger vaccinees. Reactions to RZV in older individuals did not significantly alter their physical functioning or quality of life [29]. Reaction frequency and severity were not increased with the second dose [30]. Increased reactogenicity was also observed in trials using AS01B together with hepatitis B surface antigen, suggesting that the adjuvant was primarily responsible for the adverse events [31].
Immunologic Studies in the Pivotal Trials
A parallel effort was undertaken in the ZOE trials to understand the immunologic basis for the success of RZV and to identify a correlate of protection. A subset of 3300 participants (11%) in the ZOE trials were chosen to assess serum antibody responses measured by gE-specific ELISA, while 466 participants (1.5%) had gE-specific CMI responses measured in peripheral blood mononuclear cells by flow cytometry [32]. This assay, which measured 4 activation markers (CD40 ligand [CD40L], interleukin 2 [IL-2], tumor necrosis factor ɑ [TNF-α], and interferon γ [IFN-γ]), defined a positive result as the co-expression of 2 or more of these markers by T cells. Samples were evaluated prevaccination, 1 month after the second vaccine dose, and then annually for 3 years.
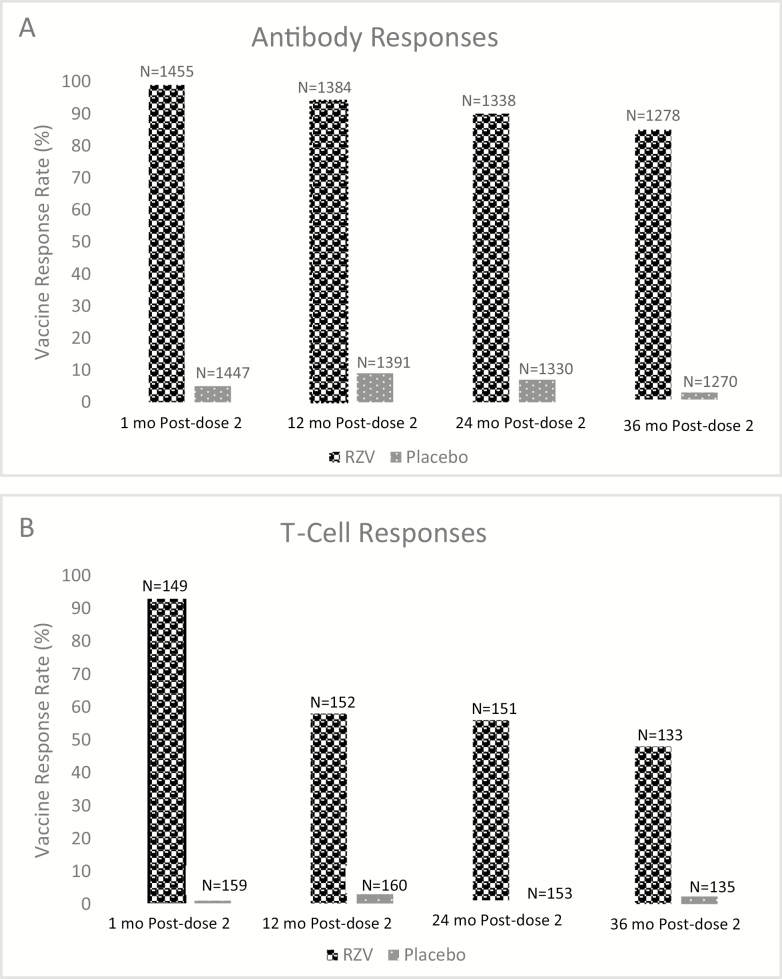
Almost all (97.8%) RZV recipients, but only 2% of placebo recipients, had an antibody response (Figure 1A). Positive responses were similar across age groups [32]. The peak antibody titers declined over the 3 years after immunization, but 70–90% of the participants still showed a response to vaccination after 3 years. A second measure of antibody response to the vaccine, the mean anti–gE antibody concentration, which had increased by 39-fold at the peak after vaccination, declined to 8.3-fold above baseline after 3 years.
Figure 1.
Immune responses induced by the adjuvanted recombinant herpes zoster vaccine. A, Vaccine response rate of gE-specific antibody determined by enzyme-linked immunosorbent assay. Response was defined as a ≥4-fold increase in antibody after vaccination compared with the prevaccination level (if seropositive prevaccination) or a ≥4-fold increase over the test cutoff value (97mIU) (if seronegative prevaccination). B. Vaccine response rate of gE-specific CD42+ T cells determined by flow cytometry after stimulation with gE peptides. Response was defined by a ≥2-fold increase in frequency of T cells that were detected by 2 of 4 surface markers (interferon γ, interleukin 2, tumor necrosis factor α, CD40 ligand) compared with prevaccination frequencies (if >320 positive cells/106 cells prevaccination) or a >2-fold increase above this level (if below this cutoff prevaccination). Abbreviations: ELISA, enzyme-linked immunosorbent assay; gE, glycoprotein E; N, number tested; RZV, recombinant glycoprotein E vaccine. Adapted from reference [32].
Glycoprotein E–specific T-cell responses were detected in less than 10% of study participants before vaccination and only at low frequency. At the peak response (1 month after the second dose) more than 93% of vaccinees in all age strata, and no placebo recipients, demonstrated boosted gE-specific CD4+ T-cell responses (Figure 1B) [32]. However, 1 year later, 60% or less of the vaccinees maintained these responses. The magnitude of mean T-cell responses declined by ~65% at 3 years postvaccination, with a trend for a greater decline in older versus younger vaccinees. Glycoprotein E–specific CD8+ T cells were rarely detected in the ZOE studies and did not increase after vaccination.
It is noteworthy that 1 year after immunization the CMI response rate was well below the 90% success achieved in preventing HZ, and there were trends for an age effect, suggesting that the immunologic measures utilized in these studies do not fully explain the protection provided by the vaccine or that they had insufficient sensitivity (lower limit of detection was higher than the level needed for protection). Given the small number of breakthrough cases of HZ in the CMI subset, a correlate of protection could not be identified. What may be an important feature of the CMI responses to RZV is its polyfunctional nature, defined by the presence of 2 or more activation markers. Within 2 years after RZV administration the proportion (not absolute number) of gE-specific T cells largely consisted of cells that possessed 3 or more activation markers, regardless of age of the vaccinee. Polyfunctional T-cell responses after vaccination have been associated with good protective responses against controlled human malaria and Leishmania major infections, suggesting that this may be a marker of protection against other intracellular pathogens [33, 34].
A small trial comparing CMI responses to ZVL or RZV, which included both VZV- and gE-specific responses, indicated that the superior immunogenicity of RZV is distinguished by the magnitude of the memory responses [14]. Memory T cells, which are programmed to persist for prolonged periods, ensure that a secondary immune response upon re-exposure to VZV is more rapid than the primary response. Effector T cells, which also increase after vaccination, mount an even faster response to antigen re-exposure compared with memory cells, but their half-life is shorter. Compared with ZVL, RZV recipients had similar VZV-specific effector responses but higher memory T-cell responses. Furthermore, the magnitude of the peak memory response to ZVL or RZV was the main determinant of the superior persistence of CMI responses at 1 year after RZV compared with ZVL. In contrast to the ZOE studies, using proliferation assays and activation markers, a CD8+ T-cell response to RZV was observed [14].
Basic Immunology
It is likely that the success of RZV is related to the adjuvant system. Some details of postadministration events, which were determined in murine models, emphasize that antigen and adjuvant must be colocalized with a short (or no) time interval between their administration [21, 35, 36]. The initial local response is characterized by activation of innate immune cells and cytokine production. Within hours, movement of both antigen and adjuvant to the draining lymph node occurs. This stimulates resident natural killer and unconventional CD8 T cells to produce IFN-γ, which is essential for optimizing recruitment and activation of dendritic cells to present gE to CD4 cells, and creates a T-helper 1 (Th1) polarizing environment. In animal models, including primates, IFN-γ in serum is a good biomarker for the activation events in the lymph node and subsequent antibody and CMI responses. It is not yet understood how these events abolish the age effect and lead to long-term persistence of immune responses.
Studies in Immunocompromised Individuals
Recombinant zoster vaccine has been administered in 5 clinical settings associated with immune compromise. With each medical condition the safety and reactogenicity after RZV administration were similar to that reported in immunocompetent vaccinees. Efficacy of RZV was demonstrated in autologous stem cell transplant recipients (n = 1751), with dose 1 being administered 50–70 days after transplantation. During a 21-month follow-up period, protection against HZ was 68%; efficacy was 89% against PHN, and was ~85% against complications and hospitalization related to HZ [37]. In patients with hematologic malignancies (n = 870), the efficacy against HZ was 87% when RZV was administered 10 days before (post-hoc) or 10–180 days after a chemotherapy cycle [38].
Additional trials with other immune-compromising diseases for which immunology and safety were the only outcome measures are shown in Table 4. In general, immune responses were robust and persisted to a significant extent for more than 12 months. The exception was reduced immunogenicity when vaccination was started at the time of chemotherapy for solid tumors. The efficacy of RZV and duration of protection in these settings are yet to be established.
Table 4.
Safety and Immunogenicity of Adjuvanted Glycoprotein E Vaccine in Immunocompromised Individuals
| Antibody | CMI | |||||
|---|---|---|---|---|---|---|
| Disease | Timing | Responsea | RZV/Placebo | Responsea | RZV/Placebo | Comment |
| Solid malignancy (N = 141 prechemotherapy; N = 40 on chemotherapy)b | 8–30 days prechemotherapy | 94% (prechemotherapy); | 23.2 | 50% (prechemotherapy) | 9.9 | 2 doses within 1–2 months; poor response when administered with chemotherapy |
| or on chemotherapy | 64% (on chemotherapy) | NA | NA | NA | ||
| Renal transplant (N = 121)c | ≥4 months stable post-transplant | 80% | 12.9 | 71% | 15.5 | No increase in rejection |
| HIV (N = 120)d | CD4+ e >200; stable ART | 92–98% | ~50 | >85% | ~16 | 3 doses: 0, 2, 6 months; third dose not needed; no impact on viral load or CD4+ e |
Abbreviations: ART, antiretroviral therapy; CMI, T-cell–mediated immunity; HIV, human immunodeficiency virus; NA, not available or not determined; RZV, recombinant zoster vaccine.
aProportion achieving predefined response.
bData from reference [39].
cData from reference [40].
dData from reference [41].
eCD4+ cells/mm3.
Recommendations
The major ACIP recommendations are shown in Table 5. Concomitant administration of RZV and nonadjuvanted quadrivalent influenza vaccine and pneumococcal polysaccharide vaccine demonstrated safety and lack of interference [42, 43]. Studies are ongoing with tetanus toxoid/reduced diphtheria toxoid/acellular pertussis vaccine. Coadministration with another adjuvanted vaccine has not been reported.
Table 5.
ACIP Recommendations and Commentsa
| • ≥50 years of age; immune competent |
| ◦Not necessary to verify prior varicella |
| ◦No recommendation on immunocompromised hosts |
| • Preferred over live attenuated zoster vaccine |
| • Administer if previously had live zoster vaccine or herpes zosterb |
| • Concomitant administration is acceptable |
| ◦Use different anatomic sites |
| • Contraindication/caution—allergic to vaccine contents; pregnancy |
Adapted from reference [7].Abbreviation: ACIP, Advisory Committee on Immunization Practices.aWhen recombinant vaccine follows live vaccine, wait at least 30 days.bWhen recombinant vaccine follows herpes zoster, wait until rash resolves.
KNOWN UNKNOWNS
Success in completion of the 2-dose vaccination schedule: Phase I/II clinical studies demonstrate that the optimal immune response to RZV requires 2 doses. Completion of multidose vaccines by older adults has been problematic, with rates approaching 50% for incomplete multidose series after 1 year [44, 45]. The second dose of RZV is to be administered between 2 and 6 months after the first. This could be hampered by the notable reactogenicity of the vaccine.
Safety of RZV assessed in large numbers of vaccinees: One concern is that the strong adjuvant system could alter immune regulation and stimulate (or worsen) autoimmune diseases (eg, rheumatologic conditions) or chronic/latent infections (eg, tuberculosis). This was not observed in the pivotal trials and in the first postlicensure safety report of the Vaccine Adverse Event Reporting System [46]. Postmarketing surveillance will include 2 additional years of active observation of 7500 individuals who received RZV during the ZOE trials and a 1-year prospective follow-up of 8700 former placebo recipients who are being immunized with RZV. As of the second quarter of 2019 more than 12 million doses of RZV have been delivered.
Duration of the protective effect: Mean follow-up was 3.2 years for ZOE 50 and 3.7 years for ZOE-70. From these trials, 7500 enrollees will be followed for a total of 6 years. Immune-response data are available from the phase II clinical trials at 6 years (n = 119) and 9 years (n = 70) [47, 48]. At both intervals, the majority of vaccinees had CMI responses higher than before vaccination. However, at 9 years, there was a trend for more vaccines aged 70 years or older to have values that overlapped the prevaccination range for T-cell responses. Modeling these data (combining both age cohorts) predicted that gE-specific CMI would remain above prevaccination levels for at least 15 years. However, given the absence of an immune surrogate for protection, the persistence of clinical protection remains undefined beyond 4 years.
Duration of efficacy in immunocompromised individuals: The information obtained from RZV administration in a variety of immune-compromising diseases is described in a preceding section. However, these studies were limited by sample size, duration of observation, and dependence on immune-response endpoints. Thus, uncertainty on the duration of protection remains in the absence of a surrogate of protection. It is clear that when immune suppression is severe, such as during some chemotherapy, the immune response may be compromised [39]. The current RZV recommendation is for people aged 50 years or older. This would exclude many younger immunocompromised patients for whom there is limited information with RZV. Furthermore, there is a large array of potential immune-suppressing agents for which RZV administration has not been evaluated. Some of these may work by mechanisms that inhibit immune responses in a manner that might limit RZV efficacy. Examples include Janus kinase inhibitors that interfere with the IFN-γ signaling pathway and sphingosine receptor inhibitors that sequester lymphocytes in lymph nodes.
Influence of VZV immune status on response to RZV- has been administered to individuals who previously had varicella. The nature of the immune response to RZV, and the extent and persistence of protection, is undefined for individuals who are VZV naive. This could also be relevant to individuals who have recovered from allogeneic stem cell transplantation. In addition, the need and potential value of RZV for a population of individuals who received varicella vaccine may become an issue in future decades (varicella vaccine was introduced in 1995).
Notes
Acknowledgments. The authors acknowledge the contribution by Deirdre Ferrall in assisting in the preparation of the manuscript and figures/tables, formatting the manuscript, and facilitating submission to the journal.
Potential conflicts of interest. M. J. L. receives research funds from GlaxoSmithKline (GSK) and is a member of a GSK advisory board. He also is a member of an advisory board for Merck Sharp & Dohme and Curevo. A. W. receives research funds from GSK and Merck, and her spouse has a patent on Zostavax. All authors have submitted the IMCJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Myron J Levin, Department of Pediatrics, University of Anschutz Medical Campus, Aurora, Colorado; Department of Medicine, University of Anschutz Medical Campus, Aurora, Colorado.
Adriana Weinberg, Department of Pediatrics, University of Anschutz Medical Campus, Aurora, Colorado; Department of Medicine, University of Anschutz Medical Campus, Aurora, Colorado; Department of Pathology, University of Anschutz Medical Campus, Aurora, Colorado.
References
- 1. Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group . A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. [DOI] [PubMed] [Google Scholar]
- 2. Schmader KE, Levin MJ, Gnann JW Jr, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54:922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrison VA, Johnson GR, Schmader KE, et al. ; Shingles Prevention Study Group . Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices ; Centers for Disease Control and Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30; quiz CE2–4. [PubMed] [Google Scholar]
- 5. Baxter R, Bartlett J, Fireman B, et al. Long-term effectiveness of the live zoster vaccine in preventing shingles: a cohort study. Am J Epidemiol 2018; 187:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis 2016; 213:1872–5. [DOI] [PubMed] [Google Scholar]
- 7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lecrenier N, Beukelaers P, Colindres R, et al. Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Rev Vaccines 2018; 17:619–34. [DOI] [PubMed] [Google Scholar]
- 9. Arvin A SO, Reichelt J, Moffat M, Zerboni L, Berarducci B, Analysis of the functions of glycoproteins E and I and their promoters during VZV replication in vitro and in skin and T-cell xenografts in the SCID mouse model of VZV pathogenesis. In: Abendroth A, Arvin A, Moffat J, eds. Varicella-zoster virus. Springer-Verlag, 2010:129–146. [DOI] [PubMed] [Google Scholar]
- 10. Maresova L, Pasieka TJ, Homan E, Gerday E, Grose C. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J Virol 2005; 79:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zerboni L, Berarducci B, Rajamani J, Jones CD, Zehnder JL, Arvin A. Varicella-zoster virus glycoprotein E is a critical determinant of virulence in the SCID mouse-human model of neuropathogenesis. J Virol 2011; 85:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berarducci B, Ikoma M, Stamatis S, Sommer M, Grose C, Arvin AM. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J Virol 2006; 80:9481–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sei JJ, Cox KS, Dubey SA, et al. Effector and central memory poly-functional CD4(+) and CD8(+) T cells are boosted upon ZOSTAVAX(®) vaccination. Front Immunol 2015; 6:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest 2018; 128:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol 2010; 342:341–57. [DOI] [PubMed] [Google Scholar]
- 16. Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med 2002; 347:26–34. [DOI] [PubMed] [Google Scholar]
- 17. Levin MJ, Oxman MN, Zhang JH, et al. ; Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators . Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis 2008; 197:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arvin AM. Immune responses to varicella-zoster virus. Infect Dis Clin North Am 1996; 10:529–70. [DOI] [PubMed] [Google Scholar]
- 19. Weinberg A, Lazar AA, Zerbe GO, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis 2010; 201:1024–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinberg A, Zhang JH, Oxman MN, et al. ; US Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators . Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis 2009; 200:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coccia M, Collignon C, Hervé C, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017; 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chlibek R, Smetana J, Pauksens K, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014; 32:1745–53. [DOI] [PubMed] [Google Scholar]
- 23. Dendouga N, Fochesato M, Lockman L, Mossman S, Giannini SL. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine 2012; 30:3126–35. [DOI] [PubMed] [Google Scholar]
- 24. Leroux-Roels I, Leroux-Roels G, Clement F, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis 2012; 206:1280–90. [DOI] [PubMed] [Google Scholar]
- 25. Chlibek R, Bayas JM, Collins H, et al. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis 2013; 208:1953–61. [DOI] [PubMed] [Google Scholar]
- 26. Lal H, Cunningham AL, Godeaux O, et al. ; ZOE-50 Study Group . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 27. Cunningham AL, Lal H, Kovac M, et al. ; ZOE-70 Study Group . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 28. Johnson R on behalf of the ZOE 50/70 Study Group. Impact of the adjuvanted recombinant zoster vaccine on pain and use of pain medication in adults aged ≥50 years. In: Canadian Pain Society 39th Annual Scientific Meeting. Montreal, Canada: Canadian Pain Society, 2018. [Google Scholar]
- 29. Curran D, Oostvogels, L, Heineman T, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine upon the physical functioning and quality of life of older adults: an open phase III trial. J Gerontol 2019. [Google Scholar]
- 30. Colindres RC, Wascotte V, Brecx A et al. Solicited adverse event intensity trends between dose 1 and dose 2 of the adjuvanted recombinant zoster vaccine: a post-hoc analysis of reactogenicity outcomes. In: ID Week 2019. Washington, DC, 2019. [Google Scholar]
- 31. Leroux-Roels G, Van Belle P, Vandepapeliere P, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS-21 induce strong humoral and cellular immune responses against hepatitis B surface antigen which persist for at least 4 years after vaccination. Vaccine 2015; 33:1084–91. [DOI] [PubMed] [Google Scholar]
- 32. Cunningham AL, Heineman TC, Lal H, et al. ; ZOE-50/70 Study Group . Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis 2018; 217:1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mordmüller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542:445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007; 13:843–50. [DOI] [PubMed] [Google Scholar]
- 35. Didierlaurent AM, Collignon C, Bourguignon P, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol 2014; 193:1920–30. [DOI] [PubMed] [Google Scholar]
- 36. Detienne S, Welsby I, Collignon C, et al. Central role of CD169+ lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci Rep 2016; 6:39475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batista A, de la Serna J, El Idrissi, M et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation. A randomized trial JAMA 2019; 322:123–33. [DOI] [PMC free article] [PubMed]
- 38.Dagnew AF, Ilhan O, Lee W-S et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 2019. doi:10/1016/S1473-3099(1(304163-X [DOI] [PubMed]
- 39. Vink P; Zoster-028 Study Group . Immunogenicity and safety of a candidate subunit adjuvanted herpes zoster vaccine in adults with solid tumors vaccinated before or during immunosuppressive chemotherapy treatment: a phase II/III, randomized clinical trial. In: ID Week 2018. San Francisco, 2018. [Google Scholar]
- 40. Vink P, Torrell JMR, Sanchez-Fructuoso AI, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase III, randomized clinical trial. Clin Infect Dis. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berkowitz EM, Moyle G, Stellbrink HJ, et al. ; Zoster-015 HZ/su Study Group . Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis 2015; 211:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwarz TF, Aggarwal N, Moeckesch B, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis 2017; 216:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Maréchal C, Lal H, Poder A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥50 years of age: a randomized trial. Vaccine 2018; 36:4278–86. [DOI] [PubMed] [Google Scholar]
- 44. Trantham L, Kurosky SK, Zhang D, Johnson KD. Adherence with and completion of recommended hepatitis vaccination schedules among adults in the United States. Vaccine 2018; 36:5333–9. [DOI] [PubMed] [Google Scholar]
- 45. Johnson KD, Lu X, Zhang D. Adherence to hepatitis A and hepatitis B multi-dose vaccination schedules among adults in the United Kingdom: a retrospective cohort study. BMC Public Health 2019; 19:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hesse EM, Shimabukuro TT, Su JR, et al. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix)—United States, October 2017-June 2018. MMWR Morb Mortal Wkly Rep 2019; 68:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chlibek R, Pauksens K, Rombo L, et al. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine 2016; 34:863–8. [DOI] [PubMed] [Google Scholar]
- 48. Schwarz TF, Volpe S, Catteau G, et al. Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum Vaccin Immunother 2018; 14:1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]