Abstract
Migraine headache results from activation of meningeal nociceptors, however, the hypothalamus is activated many hours before the emergence of pain. How hypothalamic neural mechanisms may influence trigeminal nociceptor function remains unknown. Stress is a common migraine trigger that engages hypothalamic dynorphin/kappa opioid receptor (KOR) signalling and increases circulating prolactin. Prolactin acts at both long and short prolactin receptor isoforms that are expressed in trigeminal afferents. Following downregulation of the prolactin receptor long isoform, prolactin signalling at the prolactin receptor short isoform sensitizes nociceptors selectively in females. We hypothesized that stress may activate the kappa opioid receptor on tuberoinfundibular dopaminergic neurons to increase circulating prolactin leading to female-selective sensitization of trigeminal nociceptors through dysregulation of prolactin receptor isoforms.
A mouse two-hit hyperalgesic priming model of migraine was used. Repeated restraint stress promoted vulnerability (i.e. first-hit priming) to a subsequent subthreshold (i.e. second-hit) stimulus from inhalational umbellulone, a TRPA1 agonist. Periorbital cutaneous allodynia served as a surrogate of migraine-like pain. Female and male KORCre; R26lsl-Sun1-GFP mice showed a high percentage of KORCre labelled neurons co-localized in tyrosine hydroxylase-positive cells in the hypothalamic arcuate nucleus. Restraint stress increased circulating prolactin to a greater degree in females. Stress-primed, but not control, mice of both sexes developed periorbital allodynia following inhalational umbellulone. Gi-DREADD activation (i.e. inhibition through Gi-coupled signalling) in KORCre neurons in the arcuate nucleus also increased circulating prolactin and repeated chemogenetic manipulation of these neurons primed mice of both sexes to umbellulone. Clustered regularly interspaced short palindromic repeats–Cas9 deletion of the arcuate nucleus KOR prevented restraint stress-induced prolactin release in female mice and priming from repeated stress episodes in both sexes. Inhibition of circulating prolactin occurred with systemic cabergoline, a dopamine D2 receptor agonist, blocked priming selectively in females. Repeated restraint stress downregulated the prolactin receptor long isoform in the trigeminal ganglia of female mice. Deletion of prolactin receptor in trigeminal ganglia by nasal clustered regularly interspaced short palindromic repeats–Cas9 targeting both prolactin receptor isoforms prevented stress-induced priming in female mice.
Stress-induced activation of hypothalamic KOR increases circulating prolactin resulting in trigeminal downregulation of prolactin receptor long and pain responses to a normally innocuous TRPA1 stimulus. These are the first data that provide a mechanistic link between stress-induced hypothalamic activation and the trigeminal nociceptor effectors that produce trigeminal sensitization and migraine-like pain. This sexually dimorphic mechanism may help to explain female prevalence of migraine. KOR antagonists, currently in phase II clinical trials, may be useful as migraine preventives in both sexes, while dopamine agonists and prolactin/ prolactin receptor antibodies may improve therapy for migraine, and other stress-related neurological disorders, in females.
Keywords: stress, kappa opioid receptors, hypothalamus, prolactin, migraine
Watanabe, Kopruszinski et al. show that stress-induced activation of hypothalamic kappa opioid receptors increases circulating prolactin, resulting in dysregulation of trigeminal prolactin receptor isoforms and pain responses to normally innocuous stimuli. This sexually dimorphic mechanism may help explain the female predominance of migraine.
Introduction
Migraine is a complex neurological disease and is a leading cause of worldwide disability.1,2 Demonstration of clinical efficacy of new medications including therapies targeting the calcitonin-gene-related peptide (CGRP) pathway3–6 have been highly encouraging in improving outcomes for many patients. Nevertheless, many patients do not receive sufficient benefit from available therapies and migraine remains a major unmet medical need. Migraine has a strong sex component so that (i) it is highly female prevalent; (ii) women experience longer and more intense impairment, and have a greater number of migraine-related comorbid diseases; and (iii) structural and functional brain alterations are sexually dimorphic.7–10 Despite these characteristics, the underlying mechanisms leading to the sexually dimorphic features and female prevalence of migraine remain unclear.
Migraine attacks include multiple symptoms that often emerge in temporally specific migraine phases. The premonitory phase precedes and signals the impending headache phase and is thought to be critically important for migraine initiation. Imaging studies of spontaneous or provoked migraine attacks have shown that the hypothalamus is activated in the premonitory phase.11–14 Migraine has been recognized as an oscillating sensory threshold disorder.15 Thresholds for sensory afferents are lower in migraine patients even in the interictal phase and are lowered further as the pain phase emerges.15 Consistent with this, normally innocuous stimuli can provoke migraine pain through activation of sensitized trigeminal nociceptors in the cranial meninges. A significant knowledge gap in migraine research, however, is how hypothalamic activity ultimately sensitizes trigeminal nociceptors so that pain can result from subthreshold stimuli.
Migraine attacks are thought to be initiated by numerous intrinsic and/or environmental factors, often referred to as ‘triggers’. Stress or its relief is the most common self-reported migraine ‘trigger’.16–19 Women with migraine have reported higher levels of stress in comparison to men, suggesting that stress might be a key factor in promoting sexually dimorphic features of migraine.20 The hypothalamus integrates interoceptive and exteroceptive stimuli to elicit coordinated autonomic and sensory responses through the release of neurohormones. The hypothalamus has been shown to be activated by stress3,21–23 and individuals with migraine show higher plasma levels of cortisol, a stress-associated hormone controlled by the hypothalamus–pituitary–adrenal axis.3,24
The frequency of migraine attacks is the greatest predictor of transformation to chronic migraine, suggesting a ‘priming’ effect promoting increased vulnerability to future stimuli that promote pain attacks.17,25–29 Recent preclinical studies modelling migraine in rodents have demonstrated that repeated restraint stress (RS) also induces priming, a state of vulnerability termed latent sensitization (LS),30 demonstrated by pain responses to normally innocuous stimulation of trigeminal afferents.31,32 In mice with LS, exposure to a subthreshold dose of umbellulone (UMB), a TRPA1 agonist known to produce headache only in individuals with underlying headache disorders,33–36 elicits cutaneous allodynia (CA), a surrogate readout of migraine-like pain.32 Importantly, UMB-induced CA in primed animals was demonstrated after inhalational administration suggesting that stress priming decreased the thresholds for activation of trigeminal nociceptors consistent with observations of lowered sensory thresholds of migraine patients in the interictal phase37,38 and migraine pain that can result from subthreshold stimuli.32
Preclinical studies have revealed that stress engages the dynorphin–kappa opioid receptor (KOR) system throughout brain regions implicated in migraine including the amygdala, nucleus accumbens and the hypothalamus.27,39,40 In addition, both stress and the endogenous KOR agonist, dynorphin, increase circulating levels of prolactin (PRL) in humans41–47 and rodents.24,48–50 PRL is associated with increased headache in people with migraine51 and patients with prolactinoma-associated hyperprolactinemia show increased incidence of migraine.52,53 PRL is produced in the anterior pituitary by lactotroph cells that express Gi-coupled dopamine D2 receptors. Dopamine is the major PRL-inhibitory factor synthesized by tuberoinfundibular dopamine (TIDA) neurons in the arcuate nucleus (ARC).41,54–57 Cabergoline, a dopamine D2 receptor agonist, was shown to be effective in controlling prolactinoma-associated headache,52,53 supporting the conclusion that increased levels of circulating PRL can promote migraine. PRL signals through prolactin receptor long (PRLR-L) and short (PRLR-S) isoforms that are thought to be mutually inhibitory in function. Both PRLR isoforms are found on trigeminal nociceptors and PRL has been shown to selectively sensitize nociceptors in female mice.56,58–61 Our previous studies demonstrated that downregulation of PRLR-L in dorsal root ganglion (DRG) neurons promoted nociceptor sensitization through increased PRL signalling via PRLR-S only in female mice.
These observations led us to hypothesize that repetitive stress may engage hypothalamic KOR to increase levels of circulating PRL resulting in dysregulation of PRLR isoforms in trigeminal neurons and peripheral nociceptor sensitization in females. This mechanism links, for the first time, hypothalamic activation to trigeminal nociceptor sensitization.
Materials and methods
Animals
All experimental procedures were performed in accordance with the ARRIVE guidelines, the ethical guidelines of the International Association for the Study of Pain regulations on animal welfare and the National Institutes of Health guidelines for the care and use of laboratory animals. A total of 823 female and male 8–10-week-old mice were used in this study. Animals were housed 2–5 per cage under standard animal husbandry conditions of a temperature-, humidity- and light cycle-controlled environment, with free access to food and water, in the University of Arizona animal facility. The oestrous cycle was not monitored. The experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona. Experimenters were blinded for the conditions and/or treatments. Mice were randomly divided into control and experimental groups.
C57BL/6J mice were purchased from Jackson Laboratories. KORCre mice62 were kindly provided by Dr Sarah E. Ross (University of Pittsburgh, Pittsburgh, PA, USA) and were crossed with C57BL/6J mice for >6 generations; heterozygous mice are designated as KORCre, wild-type (WT) littermates as KORWT. B6; 129-Gt(ROSA)26Sortm5(CAG-Sun1/sfGFP)Nat/J63 mice were purchased from Jackson Laboratories (no. 021039) and are referred to as (R26lsl-Sun1-GFP) mice.
Drugs
UMB (AdipoGen) was prepared as a stock solution of 0.1 M in 100% dimethyl sulphoxide and freshly diluted to 0.01 M with PBS for delivery by inhalation. Nor-binaltorphimine dihydrochloride (Tocris Bioscience) was diluted in saline and administered into the ARC of the hypothalamus at 12.5 µg/µl (2.5 µg/site). Clozapine N-oxide (CNO; Tocris Bioscience) was diluted in saline and administered intraperitoneally (i.p.) at 5 mg/kg. Cabergoline (Tocris Bioscience) was diluted in 10% dimethyl sulphoxide, 10% Tween 80 and 80% saline and administered intraperitoneally at 1.2 mg/kg. The humanized monoclonal antibody that targets CGRP (‘anti-CGRP mAb’), fremanezumab (AJOVY, Teva Pharmaceuticals), was purchased from the hospital pharmacy at the University of Arizona, diluted in PBS immediately before use and administered intraperitoneally at 30 mg/kg; separate groups of mice received PBS vehicle as a control. As an additional control for the fremanezumab experiments, the appropriately matched human IgG2 isotype control antibody, anti-hapten 4-hydroxy-3-nitrophenyl acetyl (NP) [B1-8] (Absolute Antibody), was administered intraperitoneally at 30 mg/kg.64 Olcegepant (Tocris) was dissolved using 20% dimethyl sulphoxide in saline immediately before use and administered intraperitoneally at 1 mg/kg.
Viral vector, gRNA design, cloning and in vivo transfection for CRISPR–Cas9 targeting KOR and PRLR
Our strategy to delete the KOR and PRLR (PRLR-S and PRLR-L) aimed to target the second exon (ENSMUSG00000025905, gRNA: gTCCCCCATTCAGATCTTCCG, on-score 65.7, off-score 78.8) and the first exon (ENSMUST00000128921.7, gRNA total PRLR: GTGTCAGGGGAACGACATTTG, quality score 97), respectively. A complete description of clustered regularly interspaced short palindromic repeats (CRISPR)–Cas9 plasmids targeting KOR and PRLR is in the Supplementary material.
Stereotaxic drug and viral vector injections
Mice were anaesthetized with isoflurane (induction, 5%; maintenance 2%) and placed into a stereotaxic apparatus (David Kopf Instruments). After exposing the skull, a small hole was drilled for injection. A pulled-glass pipette with a 20–40 μm tip diameter (FIVEphoton Biochemicals; World Precision instruments) was inserted into the ARC (coordinates, bregma: anterior–posterior: −1.40 mm, dorsoventral: −5.80 mm, medio-ventral: +1.20 mm, angle: 10°) and drug (200 nl) or viral vector (100 nl) was injected using a Nanoliter2010 (World Precision instruments) at a flow rate of 30–40 nl/min. The glass pipette was kept in place for at least 5 min to ensure proper diffusion. The skin incision was closed with suture and surgical glue, the mouse was then allowed to recover on a heating pad. All experiments involving virus injection were performed at least 2 weeks after surgery to allow proper expression.
Intranasal CRISPR plasmid administration
The intranasal delivery of CRISPR plasmid solutions targeting the trigeminal ganglion (TG) was performed with minor modifications, as previously described.65 Mice were anaesthetized in a multi-station isoflurane anaesthesia board (Parkland Scientific) with isoflurane (induction, 5%; maintenance 2%), placed on a heating pad in a supine position and a total 30 µl of CRISPR–Cas9 or control plasmid was administered into each nasal cavity with a 2-min interval between administrations (3 µl each) over 20 min (15 µl in each nasal cavity). The administrations occurred in alternating fashion in the left and right nasal passages throughout the 20 min. This method allowed the animal to breathe through the other nasal cavity throughout the period of administration. Animals demonstrated a normal recovery time after the administration when compared to 20 min of inhalational anaesthesia alone. No unusual behaviours were observed after the intranasal administration of plasmids. This procedure was similar to that used by Meidahl and colleagues65 who also used intranasal administration with intervals and in small volumes at each administration to avoid adverse events. Consistent with a lack of adverse effects of nasal administration, baselines measurements for periorbital and hindpaw tactile stimulation performed before the intranasal treatment (Day −1 in Fig. 6G) and immediately before the RS exposures (Day 0 in Fig. 6G; i.e. 10 days after the intranasal treatment) were not significantly different [two-way ANOVA followed by Sidak’s multiple comparison test; P > 0.9999; F interaction (1,13) = 0.04494], suggesting no alteration of behaviour after intranasal administration of plasmids.
Figure 6.
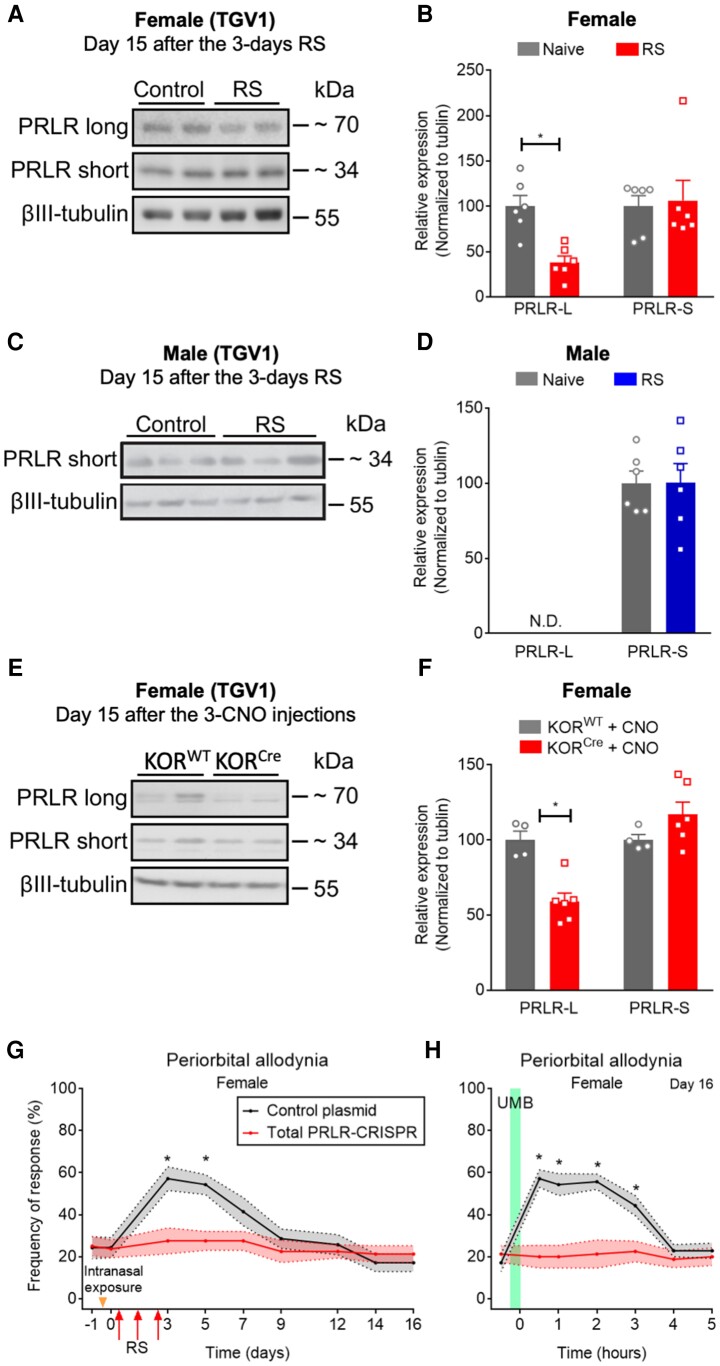
PRLRs in the TGV1 are required for stress-induced CA. Repeated RS or chemogenetic manipulation of KORCre neurons in the ARC induced down-regulation of PRLR-long form in trigeminal ganglia V1 region (TGV1) of female mice. (A and C) Representative western blot (WB) images of PRLR isoforms protein expression in TGV1 tissues collected on Day 15 after 3 days of RS episodes (2 h/day) from (A) female and (C) male mice. (B and D) Quantification of PRLR long and short isoform protein expression in TGV1 tissues from (B) female and (D) male mice after RS. (E) Representative WB images of PRLR isoforms protein expression in TGV1 tissues collected on Day 15 after 3 days of CNO injection (5 mg/kg, i.p.), 2 weeks after stereotaxic administration of AAV8-hSyn-DIO-hM4D(Gi)-mCherry (100 nl) in the ARC from KORCre or KORWT female mice. (F) Quantification of PRLR long and short isoform protein expression in TGV1 tissues from (E) female KORCre mice after CNO administration. (G) Intranasal administration of PRLR-CRISPR–Cas9 editing in the TGV1 blocked RS-induced periorbital CA in female mice. Tactile frequency of response at baseline (−1) was collected before 2 days of intranasal administration of PRLR-CRISPR–Cas9 or control plasmid (30 µl, each mouse/day) to female mice. Two weeks after the second intranasal administration, tactile frequency of response at baseline (0) was collected followed by three consecutive episodes of RS for 2 h, each. CA was assessed on indicated days after the first injection over a time course of 16 days. (H) PRLR-CRISPR–Cas9 editing in the TGV1 blocked RS-induced LS, revealed by a lack of effect of UMB exposure on restoring periorbital allodynia in female mice. On Day 16, baseline (0) response was collected, followed by inhalational exposure of UMB (0.01 M/500 µl, each) with measurements collected hourly for 5 h. Data are presented as mean ± SEM and analysed using a Mann–Whitney U-test with *P < 0.05 in comparison to control (B–F; n = 4–6) and two-way ANOVA followed by Sidak’s multiple comparison tests with *P < 0.05 (G and H; n = 7–8). Details of statistical analysis are found in Supplementary Table 1.
Fluorescence microscopy
KORCre; R26lsl-Sun1-GFP mice and KORCre mice injected with AAV8-hSyn-DIO-hM4D(Gi)-mCherry (Addgene viral services) were anaesthetized by isoflurane and transcardially perfused with 4% paraformaldehyde. The whole brain was collected and post-fixed by 4% paraformaldehyde and cryoprotected in 15–30 (w/v)% sucrose. Brain sections including the ARC were embedded in optimal cutting temperature compound (Sakura Finetek); 20-μm thick sections were cut on a cryostat (Microm, HM 525) and mounted on the slide glass (Surgipath X-tra microscope slides or Apex Superior Adhesive Slides; Leica Biosystems). Sections were permeabilized with 0.2% TritonX100 in PBS, blocked with 1% bovine serum albumin and 5% normal goat serum, and incubated overnight with rabbit polyclonal anti-TH (tyrosine hydroxylase) antibody (1:500; no. AB152, EMD Millipore) followed by anti-rabbit Alexa568 secondary antibody (1:1000, no. A-11036, Thermo Fisher Scientific). The sections were examined under an Olympus BX51 microscope (Olympus) equipped with a Hamamatsu C8484 digital camera using HC Image Live Imaging Software (Hamamatsu Corporation, v.4.1.6.0). Micrographs were processed using National Institutes of Health (NIH) ImageJ software. The number of KORCre; R26lsl-Sun1-GFP and TH coexpressing cells were counted manually. The number of cells in the ARC from three female and three male mice were averaged. Confocal micrographs were acquired on a Leica SP5-II confocal microscope using Leica Application Suite X (LASX) software, with an oil ×40/1.25 numerical aperture PL Apo objective.
Western blot analysis
Tissue lysates were prepared and analysed with western blot as previously described.58,66 The following primary antibodies were used (anti-OPRK1 antibody, 1:1000, no. 44-302G, Thermo Fisher Scientific; anti-PRLR-S, 1:1000, no. ab2772, Abcam; anti-PRLR-L, 1:1000 no. ab170935 Abcam and anti-βIII-tubulin: 1:5000, no. G7121, Promega). Horseradish peroxidase-conjugated light chain and Fc-specific secondary antibodies were from Jackson Immunoresearch (1:15000, nos 211-032-171 or 115-035-174).
Mouse prolactin ELISA
Whole blood was collected between 0900 and 1100 from isoflurane anaesthetized mice by inferior vena cava puncture and coagulated at room temperature for 1 h. Serum was isolated by centrifugation at 6000 rcf, for 10 min at 4°C and stored at −80°C. Serum prolactin level was quantified by a mouse prolactin ELISA kit according to the manufacturer’s instructions (ab100736; Abcam).
Periorbital cutaneous allodynia evaluation
CA was evaluated by measuring the frequency of response to tactile stimulation of the periorbital region as previously described.32 Measurements were performed before, and after, RS or UMB exposure. Briefly, a 0.4 g von Frey filament (Stoelting) was applied 10 times to the periorbital region until the filament slightly arched. Positive responses were scored as facial grooming, head shaking and/or turning away after filament application. Frequency response was calculated by [(number of positive responses × 100)/10].
Umbellulone inhalation exposure
Inhalational exposure to UMB was performed as previously described.32 A half square gauze was placed in each nose cone of the multi-station isoflurane anaesthesia board (Parkland Scientific) and 500 µl of UMB at 0.01 M was pipetted onto each gauze. Mice were individually placed in each nose cone under light isoflurane anaesthesia (1.5–2%), with exposure to UMB for 30 min.
Restraint stress
RS was performed according to Kopruszinski and colleagues.32 Mice were placed in plastic restrainers (Plas-labs INC, 551-BSRR). Single RS was performed for 30 min or 2 h. For repeated RS priming, mice underwent RS for 2 h each day consecutively for 3 days. Control animals were kept in their home cages without food and water during the same period. Periorbital response frequency was measured at baseline followed by three daily episodes of RS on Days 0, 1 and 2 and CA evaluation on Days 3, 5, 7, 9, 11, 14 and 16 after the first RS. On Days 16 and/or 21, baseline measurement was collected, animals were challenged with inhalational exposure to UMB, and CA was assessed at 30 min and hourly up to 5 h after the exposure.
General experimental design overview
See the detailed general experimental design overview in the Supplementary material.
Statistical analysis
Sample size was determined using GPower v.3.1 software, with an established significance level of P < 0.05. Data are expressed as means ± SEM. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software). Data for PRL levels were analysed using the Student t-test, Mann–Whitney U-test, one-way ANOVA with Tukey’s multiple comparisons post hoc test when two or more groups were compared, or two-way ANOVA with Tukey’s multiple comparisons post hoc test, respectively. Data for protein expression levels were analysed using the Mann–Whitney U-test. Mean differences of two or more group comparisons of time course experiments for sensory thresholds were analysed using two-way ANOVA with Sidak’s or Tukey’s multiple comparisons post hoc tests, respectively. Statistically significant differences were considered when P < 0.05. The statistical analysis, numbers of animals used (n), P-values and interaction F ratios are reported in Supplementary Table 1.
Data availability
All data are available upon reasonable request.
Results
Repeated RS produces transient cutaneous allodynia and latent sensitization in both female and male mice
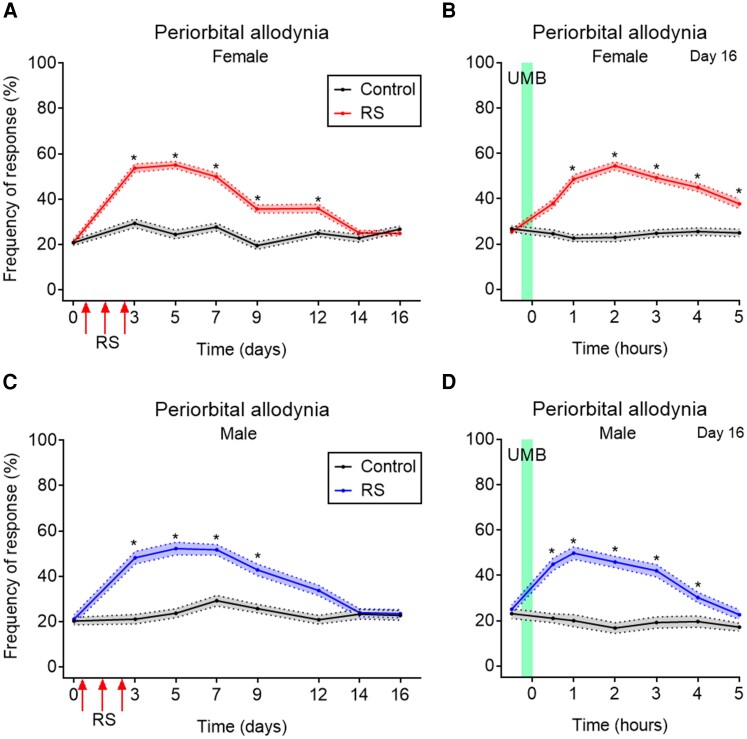
Our group had previously developed an injury-free mouse model of vulnerability to subthreshold stimuli in trigeminal afferents for evaluation of migraine mechanisms and potential therapies. Repeated RS was chosen to induce LS (i.e. priming) and to mimic vulnerability to normally innocuous stimuli observed in individuals with migraine. Inhalational exposure to a subthreshold concentration of UMB was used to provoke periorbital allodynia revealing stress-induced sensitization of trigeminal nociceptors. Additionally, we had demonstrated that the consequences of RS priming were prevented by systemic treatment with a KOR antagonist in both female and male mice.32 In the present study, we plotted together data from all experiments for all RS and sham groups of mice receiving vehicle treatments or control vectors. These results confirmed our previous observations that repeated RS, for three consecutive days, produced significant transient periorbital CA in both female (Fig. 1A) and male (Fig. 1C) mice. Tactile thresholds began to return to prestress baseline levels between Days 7 and 12 and there was no significant CA by Day 14 after stress. Following resolution of CA, mice were exposed to inhalational UMB on Day 16 resulting in CA only in RS-primed female (Fig. 1B) and male mice (Fig. 1D). Control (i.e. non-stressed) mice did not develop CA throughout the experiment and did not show CA to UMB (Fig. 1A–D). These findings reflect the development of LS of trigeminal nociceptors expressing TRPA1 that is induced by repeated stress episodes in both female and male mice.
Figure 1.
Repeated RS-induced CA and LS revealed by UMB inhalation in female and male mice. Data represent tactile responses pooled from all RS and sham female and male mice receiving vehicle treatment or control manipulation from a collection of all performed experiments. Tactile frequency of response was collected at baseline (0) in female (A) and male (C) mice, followed by three consecutive Days of RS for 2 h each. CA was assessed on indicated days after the initial RS over a time course of 16 days (A and C). On Day 16, baseline (0) response was collected, followed by inhalational exposure of UMB (0.01 M/500 µl, each) with measurements collected hourly for 5 h (B female and D male). Data are presented as mean ± SEM and analysed using two-way ANOVA followed by Sidak’s multiple comparison test with *P < 0.05 (n = 49–87). Details of statistical analysis are found in Supplementary Table 1.
KOR is expressed in the hypothalamus of female and male mice
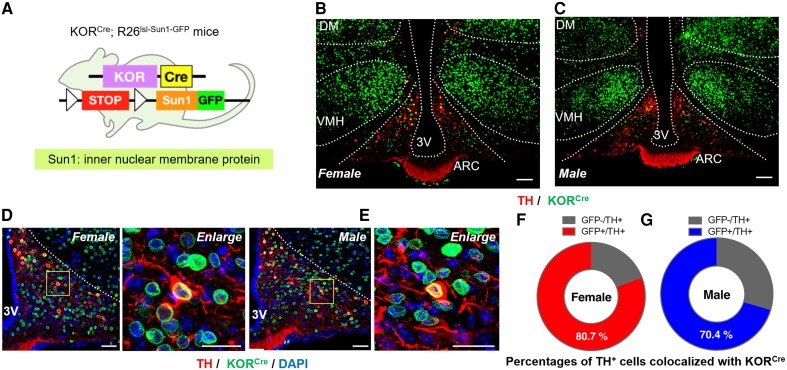
KORCre neurons in the ARC were visualized in KORCre mice62 crossed with genetic reporter mice enabling Cre-dependent expression of a Sun1-GFP fusion protein, which labels the nuclear envelope (Fig. 1A). Possible coexpression of KORCre in TIDA neurons was evaluated in hypothalamic brain slices from KORCre; R26lsl-Sun1-GFP mice following immunostaining with TH. Images from female (Fig. 2B) and male (Fig. 2C) mice demonstrated the presence of KORCre neurons (green) in different hypothalamic nuclei, including, dorsomedial, ventromedial hypothalamus and ARC. Immunohistochemistry also revealed TH (red) expression in the ARC of female (Fig. 2B) and male (Fig. 2C) mice. Higher magnification images from female (Fig. 2D) and male (Fig. 2E) KORcre; R26lsl-Sun1-GFP mice show expression of KORCre in TH-positive cells in the ARC that was quantified as ∼80 and 70% in female (Fig. 2F) and male (Fig. 2G) mice, respectively. These data identify high overlap of TH-positive (i.e. putative TIDA) cells with KORCre neurons in the ARC.
Figure 2.
KORCre and TH expression in the hypothalamus of female and male mice. (A) Schematic of Cre-dependent expression of Sun1-GFP fusion protein labelling KORCre neurons in KORCre; R26lsl-Sun1-GFP mice. (B and C) Representative images showing colocalization of KORCre labelled neurons (green) and TH+ neurons (red) in hypothalamus from (B) female and (C) male KORCre; R26lsl-Sun1-GFP mice. Scale bars, 100 μm. (D and E) Higher magnification images showing localization of KORCre (green), TH (red) and DAPI (blue) in the ARC from (D) female and (E) male KORCre; R26lsl-Sun1-GFP mice. Scale bars, 50 μm. (F and G) Percentages of TH-positive cells colocalized with KORCre neurons in the ARC from (F) female and (G) male mice.
Blockade of ARC KOR prevents increased circulating PRL following restraint stress
A single RS episode for 30 min increased circulating PRL in both female and male mice, with a greater magnitude in females (Supplementary Fig. 1). Right side ARC administration of the KOR antagonist, nor-binaltorphimine (2.5 µg/200 nl) given 24 h before RS challenge prevented the increased levels of circulating PRL in female mice (Supplementary Fig. 2). Administration of vehicle into the ARC did not modify increased levels of circulating PRL induced by RS (Supplementary Fig. 2). These findings confirm the functional role of ARC KOR-expressing cells in regulating PRL release in mice.
Chemogenetic activation of hM4D(Gi) in ARC KORCre neurons increases circulating PRL and elicits cutaneous allodynia and latent sensitization
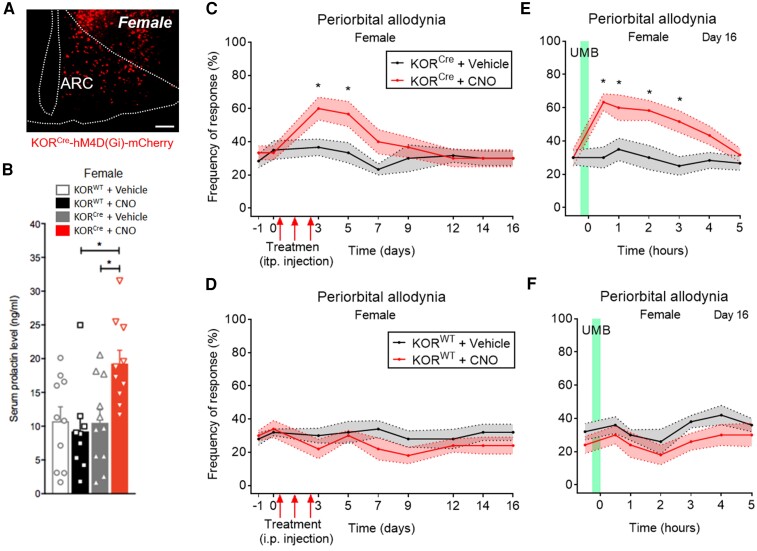
Figure 3 and Supplementary Fig. 3 show representative images demonstrating the expression of hM4D(Gi) (Gi-DREADD) (red) after administration of AAV8-hSyn-DIO-hM4D(Gi)-mCherry into the right ARC of KORCre female (Fig. 3A) and male (Supplementary Fig. 3A) mice. A significant increase in circulating PRL was observed following a single dose of CNO (5 mg/kg, i.p.), a Gi-DREADD specific agonist, in KORCre/Gi-DREADD female mice (Fig. 3B), but not in KORCre/Gi-DREADD male mice (Supplementary Fig. 3B); no significant increase was detected in KORWT/Gi-DREADD female (Fig. 3B) and male mice (Supplementary Fig. 3B). Three consecutive daily doses of CNO (5 mg/kg, i.p.) produced transient periorbital CA in both, KORCre/Gi-DREADD female (Fig. 3C) and male (Supplementary Fig. 3C) mice that resolved by Day 14. UMB exposure on Day 16 after the first CNO or vehicle injection elicited CA only in CNO-treated KORCre/Gi-DREADD female (Fig. 3E and F) and male (Supplementary Fig. 3E and F) mice. Vehicle-treated animals did not exhibit CA throughout the time course (Fig. 3C–F and Supplementary Fig. 3C–F). Repeated administration of CNO or vehicle (Fig. 3 and Supplementary Fig. 3), or UMB exposure, did not induce CA in KORWT female (Fig. 3D and F) and male (Supplementary Fig. 3D and F) mice. These data suggest that activation of hypothalamic KOR mediates circulating PRL and induces CA and LS to inhalational TRPA1 agonist in both female and male mice.
Figure 3.
Chemogenetic manipulation of KORCre neurons in the ARC produced increased levels of circulating PRL, transient CA and LS, mimicking effects of RS in female mice. (A) Representative images from KORCre female mice expressing Gi-DREADD (hM4D(Gi)-mCherry) demonstrating expression of hM4D(Gi)-mCherry (red) in the ARC. Scale bars, 100 μm. (B) Concentration of PRL determined from serum collected 30 min after intraperitoneal administration of CNO at 5 mg/kg or vehicle, 4 weeks after stereotaxic administration of AAV8-hSyn-DIO-hM4D(Gi)-mCherry (100 nl) in the ARC from KORCre or KORWT female mice. (C and D) Tactile frequency of response at baseline (−1) was collected right before injection of AAV8-hSyn-DIO-hM4D(Gi)-mCherry (100 nl) in the ARC of KORCre or KORWT female mice. Four weeks after stereotaxic surgery, tactile frequency of response at baseline (0) was collected followed by three consecutive days of intraperitoneal administration of CNO at 5 mg/kg or vehicle. CA was assessed on indicated days after the first injection over a time course of 16 days (C and D). On Day 16, baseline (0) response was collected, followed by inhalational exposure of UMB (0.01 M/500 µl, each) with measurements collected hourly for 5 h (E, heterozygous and F, wild-type mice). Data are presented as mean ± SEM and analysed using one-way ANOVA followed by Tukey’s (B) or two-way ANOVA followed by Sidak’s (E and F) multiple comparison tests with *P < 0.05 (n = 5–7). Details of statistical analysis are found in Supplementary Table 1.
Deletion of ARC KOR prevented RS-induced PRL release, as well as CA and LS in RS-primed female and male mice
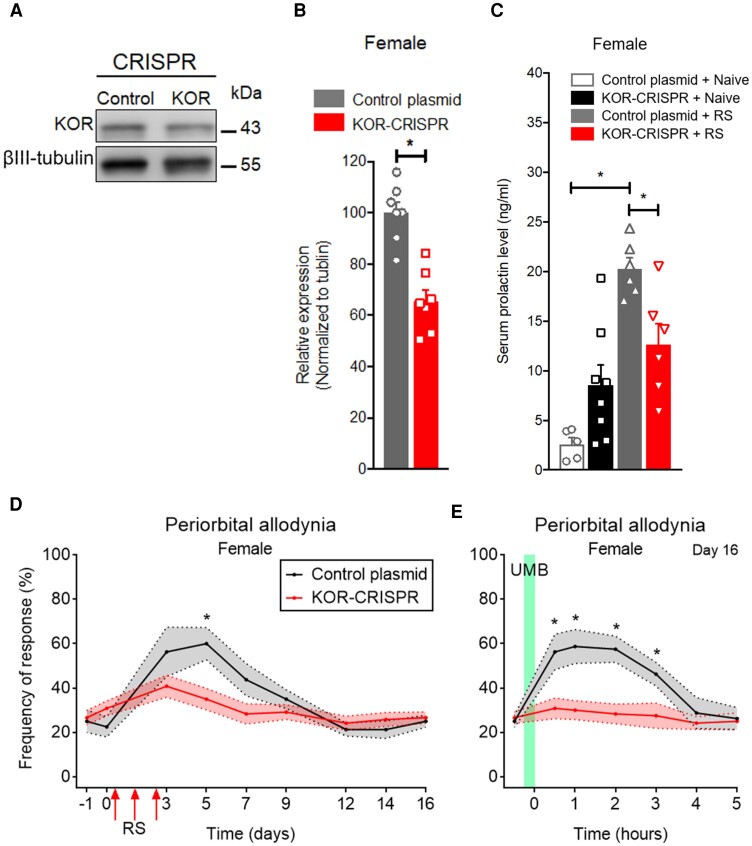
Bilateral administration of KOR-targeted CRISPR–Cas9 plasmid into the ARC produced significant deletion of KOR expression (Fig. 4A and B). Deletion of ARC KOR prevented repeated RS-induced increased circulating PRL in female (Fig. 4C). Deletion of ARC KOR also prevented repeated RS-induced transient CA (Fig. 4D and Supplementary Fig. 4A) and LS, so that precipitation of CA by UMB was not observed in female or male mice (Fig. 4E and Supplementary Fig. 4B). Bilateral administration of control plasmid into the ARC did not modify RS-induced release of circulating PRL (Fig. 4C) and repeated RS-induced CA (Fig. 4D and E and Supplementary Fig. 4A and B). These data confirm that hypothalamic KOR signalling is essential for RS-induced regulation of PRL in female mice and sensitization of trigeminal afferents in both female and male mice.
Figure 4.
KORs in the ARC are required for RS-induced periorbital CA and LS revealed by UMB in female mice. (A) Representative western blot of KOR-CRISPR–Cas9 gene editing in ARC of female mice. (B) Quantitative verification of KOR-CRISPR–Cas9 editing efficiency in ARC of female mice. (C) Stereotaxic administration of KOR-CRISPR–Cas9 plasmid (500 nl) in the bilateral ARC blocked RS-induced increased levels of circulating PRL in female mice. Serum was collected 2 h after RS. (D) KOR-CRISPR–Cas9 editing in the ARC blocked RS-induced periorbital CA in female mice. Baseline tactile frequency of response was collected before (−1) and 2 weeks after (0) KOR-CRISPR–Cas9 administration. Following three consecutive episodes of RS, CA was assessed on indicated days over a time course of 16 days. (E) KOR-CRISPR–Cas9 editing in the ARC blocked RS-induced LS revealed by lack of effect of UMB exposure. On Day 16, baseline (0) response was collected, followed by inhalational exposure of UMB with measurements collected hourly for 5 h. Data are presented as mean ± SEM and analysed using a Mann–Whitney U-test (B), one-way ANOVA with Tukey’s (C) or two-way ANOVA followed by Sidak’s (D and E) multiple comparison tests with *P < 0.05 (n = 5–12). Details of statistical analysis are found in Supplementary Table 1.
Circulating PRL following restraint stress priming promotes sexually dimorphic sensitization of trigeminal nociceptors
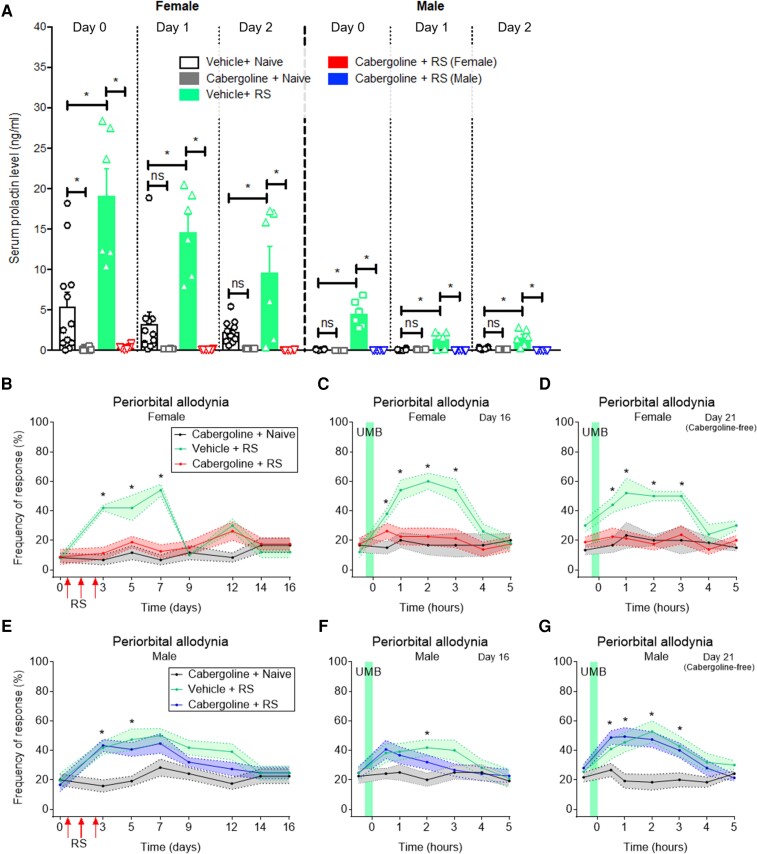
Increased levels of circulating PRL were observed following each of three consecutive episodes of RS (Fig. 5A), with a greater impact in female mice. It should be noted that the magnitude of the PRL increase diminished with the second and third RS episodes, probably reflecting reduced stress due to learning following increased exposure to the same stress condition (Fig. 5A). Systemic administration of cabergoline (1.2 mg/kg, i.p.), a dopamine D2 receptor agonist, 2 h before each RS episode, significantly inhibited RS-increased circulating PRL in female and male mice (Fig. 5A). Vehicle-RS treated animals continue to exhibit higher levels of PRL when compared to control and when compared to RS-cabergoline treated mice (Fig. 5A).
Figure 5.
Increased circulating levels of PRL are required for RS priming-induced periorbital CA and LS only in female mice. (A) RS on three consecutive days resulted in increased levels of circulating PRL in female and male mice, which was prevented by intraperitoneal administration of cabergoline at 1.2 mg/kg, 2 h before each RS episode. Serum was collected immediately after the 2 h of RS. (B and E) Tactile frequency of response was assessed at baseline (0) and on indicated days after the first RS over a time course of 16 days. On Day 16 and again on Day 21 (cabergoline-free), baseline (0) response was collected, followed by inhalational exposure of UMB and CA measurements for 5 h (C, female and F, male). Intraperitoneal treatment with cabergoline or vehicle, were performed 2 h before each RS (on Days 0, 1 and 2) and on Days 4, 6, 8, 10,12, 14, 16 and 17. Cabergoline administration blocked stress priming-induced CA and LS in (B–D) female, but not (E–G) male mice. Data are presented as mean ± SEM and analysed using one- or two-way ANOVA followed by Tukey’s multiple comparison tests *P < 0.05 (n = 5–15). Details of statistical analysis are found in Supplementary Table 1.
Repeated cabergoline treatment also blocked transient CA induced by three consecutive RS episodes in female mice (Fig. 5B). Following cabergoline, female mice primed with repeated RS (Fig. 5C) did not show UMB-induced CA, suggesting repeated cabergoline treatment also prevented LS. In contrast, cabergoline effects were not observed in male mice. Repeated cabergoline treatment did not prevent repeated RS-induced transient CA (Fig. 5E) and LS, so that UMB (Fig. 5F) still produced CA in male mice. Additionally, it should be noted that on Day 21 after RS (Fig. 5D and G) at a time point when cabergoline treatment had been discontinued, UMB elicited CA in male (Fig. 5G), but not female (Fig. 5D) mice, suggesting that cabergoline prevented the development of LS.
Repeated RS or activation of hypothalamic KOR downregulates PRLR-L in TGV1 only in females
Three consecutive RS episodes (Fig. 6A and B), or systemic administration of CNO in animals expressing Gi-DREADD in the right ARC of hypothalamus (Fig. 6E and F) downregulated PRLR-L expression in trigeminal ganglion V1 division (TGV1) tissues, in female mice, on Day 15 after priming (Fig. 6A, B, E and F), while levels of PRLR-S were not altered (Fig. 6A–F). In male mice, repeated RS (Fig. 6C and D) did not alter PRLR-S expression and PRLR-L was not detected in TGV1 tissues collected on Day 15 after the priming of male mice.
PRLR-gene editing in TGV1 blocked RS-induced periorbital allodynia and LS in female mice
Intranasal administration of PRLR-CRISPR–Cas9 plasmid targeting both PRLR-L and PRLR-S (i.e. total PRLR-CRISPR–Cas9) produced significant deletion of PRLR in the TGV1 region of female mice, as previously reported by our group.66 We also confirmed the lack of effect of intranasal administration of PRLR-CRISPR–Cas9 plasmid on producing alteration of PRLR expression in the ARC region of female mice (Supplementary Fig. 5A and B). Deletion of TGV1 PRLR with CRISPR–Cas9-gene editing prevented repeated RS-induced transient CA (Fig. 6G) and LS, so that precipitation of CA by UMB was not observed in female mice (Fig. 6H). Intranasal administration of control plasmid did not modify repeated RS-induced CA (Fig. 7K and L). These data confirm the pivotal role of circulating PRL and PRLR to sensitize peripheral TG neurons after RS priming in female mice.
Figure 7.
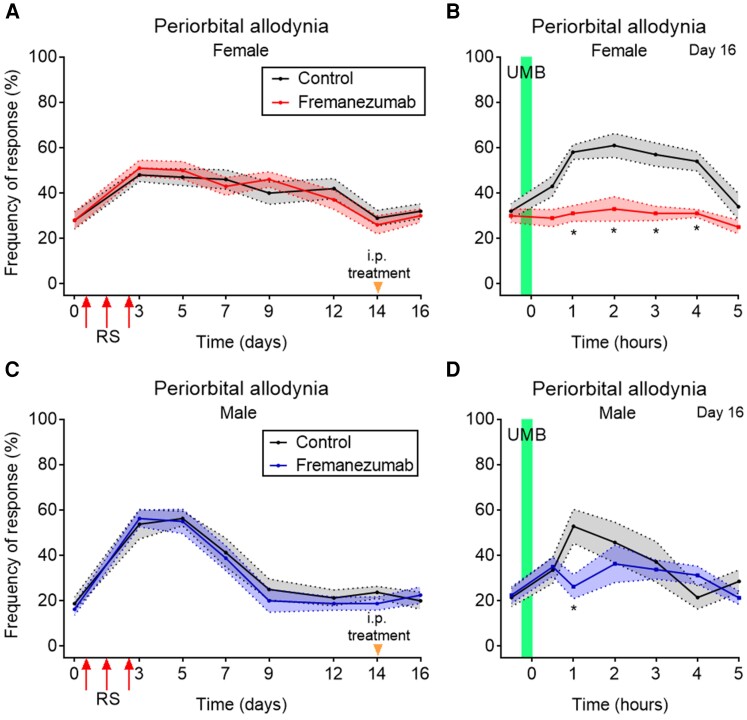
Anti-CGRP mAb fully abolished the RS-induced LS revealed by UMB in female, but partially reduced it in male mice. (A and C) Tactile frequency of response at baseline (0) was collected in (A) female and (C) male mice followed by three consecutive episodes of RS for 2 h, each. CA was assessed on indicated days after the first injection over a time course of 16 days. On Day 14, 48 h before the UMB inhalational exposure, mice were treated intraperitoneally with the anti-CGRP mAb, fremanezumab, at 30 mg/kg or control. (B and D) On Day 16, baseline (0) response was collected, followed by inhalational exposure of UMB (0.01M/500 µl, each) with measurements collected hourly for 5 h. The anti-CGRP mAb treatment fully abolished in (B) female and partially reduced in (D) male mice the RS-induced LS, revealed by lack of effect of UMB exposure on eliciting periorbital allodynia. Data are presented as mean ± SEM and analysed using two-way ANOVA followed by Sidak’s (A and B) multiple comparison tests with *P < 0.05 (n = 7–10). Details of statistical analysis are found in Supplementary Table 1.
Sexually dimorphic effect of anti-CGRP mAb in RS-induced LS
Repeated episodes of RS on three consecutive days produced significant CA in female (Fig. 7A and Supplementary Fig. 7A) and male (Fig. 7C and Supplementary Fig. 7C) mice. Animals were then treated intraperitoneally with anti-CGRP mAb, vehicle (Fig. 7) or the appropriately matched human IgG2 isotype control antibody (Supplementary Fig. 7A) on Day 14 after the first RS, 48 h before UMB exposure (Fig. 7A, C and Supplementary Fig. 7A and C). Additional groups of mice were treated intraperitoneally with olcegepant, a small molecule CGRP antagonist on Day 16 (i.e. after the first RS) 30 min before UMB exposure (Supplementary Fig. 7B and D). Both anti-CGRP mAb and olcegepant fully blocked the development of UMB-induced CA in repeated RS-primed female mice (Fig. 7B and Supplementary Fig. 7B). In contrast, anti-CGRP mAb and olcegepant only partially reduced the development of UMB-induced CA in repeated RS-primed male mice (Fig. 7D and Supplementary Fig. 7D). The isotype control antibody did not affect the allodynic response induced by UMB in mice previously subjected to RS (Supplementary Fig. 7B and D). These data demonstrate that, in female mice, CGRP plays an important role in repeated RS-induced CA and participates in the downstream mechanism mediating repeated RS-induced priming, possibly as a consequence of PRL-induced sensitization of trigeminal afferents to enhance release of CGRP.
Discussion
Imaging studies have demonstrated that the hypothalamus is activated prior to the pain phase of migraine implying a causal relationship with headache. However, the hypothalamic neural circuits and transmitters that may promote migraine pain remain unknown. We hypothesized that dynorphin/KOR signalling may be a strong candidate in linking the hypothalamus to migraine pain for several reasons. First, stress, or its relief, is strongly associated with migraine in both sexes. Second, stress is known to activate the hypothalamus and migraine patients show higher levels of circulating cortisol. Third, the frequency of migraine attacks is highly correlated with migraine chronification, suggesting a priming effect where pain episodes increase vulnerability to future attacks.17,25–29 Repeated stress has been shown to promote maladaptive changes in brain regions, including the hypothalamus, through dynorphin/KOR signalling.27,39,40 Fourth, dynorphin, the endogenous KOR agonist, increases PRL release in humans. Fifth, high PRL levels are associated with increased migraine frequency and chronification, while inhibition of circulating PRL with dopamine agonists decreases migraine in patients with PRL-secreting tumours. Finally, preclinical studies have consistently demonstrated that PRL selectively, but not exclusively, sensitizes female nociceptors through phosphorylation of TRP channels including TRPA1. TRPA1 mediates responses to environmental irritants including, for example, cigarette smoke and perfumes that promote migraine only in individuals with underlying headache disorders,67,68 probably reflecting activation of nociceptors from a subthreshold stimulus. Collectively, these observations suggest a neuroendocrine link between hypothalamic circuits that may be activated in the premonitory phase and sensitization of trigeminal nociceptors, a mechanism that can increase the likelihood of migraine pain in females. Additionally, these observations may partially explain the female prevalence of migraine.
Previous studies have explored the relationship between migraine and stress, but mechanistic understanding has been elusive. Relevant to migraine, acute and/or chronic stress were demonstrated to (i) decrease thresholds to induce cortical spreading depression69–71; (ii) increase dural vascular permeability and mast cell degranulation72,73; (iii) enhance migraine pain-like behaviour induced by nitroglycerin74,75; (iv) elicit pain-like behaviours40,76,77; and (v) prime animals to a subsequent subthreshold exposure of a migraine trigger.31,32 While these studies provide a strong link between experimental stress and migraine-like behaviours, the underlying circuits mediating these stress responses have not been identified. The maladaptive consequences of repeated stress including increases in anxiety and depression have been demonstrated to increase dynorphin/KOR signalling in multiple brain regions,78–82 including the hypothalamus, amygdala and the nucleus accumbens,27,39,40 all of which are areas that are also relevant to migraine.40,77
Importantly, both stress and dynorphin-A (1–13) increase circulating PRL in both humans and rodents.42,46,83–85 Consistent with these previous reports, we demonstrated that RS increased circulating PRL in both female and male mice. Notably, female mice had a higher baseline level of circulating PRL and a greater increase in circulating PRL level following RS than male mice. Likewise, baseline circulating PRL levels are higher in women than in men.42 PRL is a neurohormone that can be produced locally from neuronal and immune cell types, as well as from pituitary lactotrophs.41,54–57 PRL signals through formation of homodimers of two main receptor isoforms in mice termed PRLR-L and PRLR-S. We have previously reported that disruption of the balance of PRLR isoforms in the DRG by medications or by CRISPR–Cas9 gene editing can promote female-selective nociceptor sensitization resulting from increased signalling at the PRLR-S by circulating PRL.58 It should be noted that while PRL preferentially sensitizes female nociceptors, male nociceptors also respond at ∼40-fold higher concentrations, possibly explaining why both men and women with prolactinoma from PRL-secreting tumours suffer from increased migraine. PRL-induced nociceptor sensitization may result from intracellular modulation of ion channels including TRPV1, TRPM8 and, importantly, TRPA1.86,87 Thus, repeated stress-induced KOR signalling and PRL release could contribute to nociceptor sensitization and development of LS in female mice through dysregulation of PRLR isoforms.
Secretion of PRL from the pituitary gland is under inhibitory control of dopamine released from TIDA neurons in the ARC of the hypothalamus.88,89 Recent three-dimensional images of the mouse brain confirm KOR expression in stress-related regions including the hypothalamus.90 We found that KORCre was expressed throughout the hypothalamus and that there was a high degree of colocalization of the KORCre and TH+ neurons in the ARC in both sexes, providing an anatomical basis for stress-related dynorphinergic KOR activation and increased circulating PRL. As dopamine beta-hydroxylase and phenylethanolamine N-methyl transferase, the enzymes for noradrenaline and adrenaline synthesis, do not express or coexpress with TH+ neurons in the ARC,91,92 it is reasonable to assume that the TH+ neurons in the ARC are dopaminergic. Unilateral microinjection of nor-binaltorphimine, a long-acting KOR antagonist, in the ARC significantly, but not completely, blocked RS-induced increase in circulating PRL. Similar to RS, repeated Gi-DREADD activation (i.e. inhibition through Gi-coupled signalling) in KORCre neurons in the ARC induced LS so that subthreshold inhalational UMB produced allodynia in both female and male mice. It should be noted that we performed a unilateral injection of Gi-DREADD expressing virus. Previous electrophysiological studies have shown that TIDA cells are functionally interconnected and exhibit synchronized network rhythmic oscillatory discharge suggesting that manipulation of neuronal activity on one side of the hypothalamus may affect the activity of the other side.93 Deletion of ARC KOR expression with CRISPR–Cas9-gene editing prevented repeated RS-induced LS, as well as RS-induced increases of circulating PRL in female mice and also prevented repeated RS-induced LS in male mice. Thus, KOR-positive neurons in the ARC are critical in priming mice to subthreshold sensory stimuli known to promote headache in both sexes and represent a promising therapeutic target for migraine prevention.32,77 Recently, KOR antagonists including BTRX-33514024 and JNJ-67953964 (previously CERC-501 and LY2456302)94 have been advanced to clinical trials for the treatment of neuropsychiatric disorders suggesting the opportunity for evaluation as migraine preventives.
To determine whether circulating PRL was promoting trigeminal nociceptor sensitization following repeated RS or cell specific hypothalamic KORCre/Gi-DREADD activation, we administered cabergoline: a dopamine D2 receptor agonist to inhibit prolactin release from pituitary lactotroph cells. Systemic cabergoline fully blocked RS-induced increased levels of circulating PRL in both sexes, but only prevented the repeated RS-induced LS and, consequently, UMB-induced migraine pain-like behaviours in female mice. Therefore, pituitary PRL significantly promotes the consequences of repetitive ARC KOR activation to lower sensory thresholds in female mice. It should be noted that cabergoline has a modest affinity to serotonin 5-HT1 and 5-HT2 receptors.95,96 Further investigation is needed to conclude whether serotonergic mechanisms could contribute to the observed cabergoline effects. It should also be noted that migraine patients have lowered sensory thresholds in the interictal period and thresholds decrease further as the pain phase approaches, consistent with the characterization of migraine as a cyclical sensory threshold disorder that involves the hypothalamus.15 These findings are also consistent with clinical observations that dopamine D2 receptor agonists provide a beneficial anti-migraine effect in patients with hyperprolactinemia associated with prolactinomas.53,97,98
Our previous studies with opioid-induced hyperalgesia58 showed that disruption of the balance between PRLR-S and PRLR-L isoforms in the DRG promoted nociceptor sensitization in female mice by favouring PRL signalling at PRLR-S. This observation raises the possibility that repeated RS might also disrupt the balance between PRLR-S and PRLR-L isoforms in the TG, which could promote nociceptor sensitization from circulating PRL. We therefore determined whether repeated RS could dysregulate PRLR isoforms in the V1 region of the trigeminal ganglion. We found that repeated RS produced a downregulation of PRLR-L in the TG of female mice. To further test the requirement for PRLR in the TG we administered a CRISPR–Cas9 plasmid targeting both PRLR-L and PRLR-S by the nasal route. The CRISPR–Cas9 plasmid decreased expression of both PRLR isoforms and showed a blockade of repeated RS-induced nociceptor sensitization in female mice.
The mechanisms by which increased levels of circulating PRL and dysregulation of PRLR isoforms could result in increased likelihood of migraine in females remain to be elucidated. However, it is known that PRL promotes sensitization of responses to activation of TRPV1, TRPM8 and TRPA1-positive sensory neurons selectively in female DRG and TG neurons.87 Our studies provoked allodynia with a subthreshold exposure to UMB, a TRPA1 agonist, suggesting the possibility of modulation of TRPA1 expressing fibres. Furthermore, activation of trigeminal TRP channels increases the release of CGRP, a peptide known to promote migraine in humans. Our studies support the possibility that PRL-sensitization in female TG neurons lower thresholds to a TRPA1 agonist and promote release of CGRP selectively in females. We therefore evaluated whether targeting of CGRP with fremanezumab, an anti-CGRP monoclonal antibody, would block the allodynia induced by UMB in repeated RS-primed female and male mice. We found that targeting CGRP with anti-CGRP mAb or with a small molecule CGRP antagonist prevented UMB-induced allodynia in repeated RS-primed female mice but was only partially effective in male animals. These data are in agreement with recent reports that demonstrate increased effectiveness of CGRP applied to the dura mater of female rodents99 and an interaction between meningeal CGRP and PRL to induce migraine-like pain selectively in female mice.100 The effect of olcegepant on preventing allodynia induced by UMB in RS-primed mice has been previously reported by our group in female mice and our present study also shows the same effect in male mice.32 We did not see any effects of the human IgG2, anti-hapten 4-hydroxy-3-nitrophenyl acetyl (NP) [B1-8] on reducing RS-induced LS confirming that the mAb effects were CGRP specific. Previous reports by our group had also demonstrated that a different IgG control protein had no effect on mTBI-induced CA101 or in the current RS priming UMB model.102 This result is in contrast to nonspecific effects observed with the human IgG2 isotype control protein in studies of CSD in rats.64 Altogether, our data demonstrate that the effects observed with anti-CGRP mAb or small molecule CGRP antagonists in our model were CGRP dependent.
We found that repeated stress also promotes LS and vulnerability to migraine triggers in male mice, but these effects are independent of circulating PRL. The reasons for lack of effect of PRL in male animals could be due to possibly higher PRLR expression in DRGs58 and TG in females66 and the requirement for much higher PRL levels to sensitize nociceptors in males. However, the mechanisms that underlie stress priming in males remain to be elucidated and are a limitation of our study. Additional limitations of our study include the use of only a single stress mechanism for priming that may not be directly relevant to the intrinsic and environmental stressors, including physical and psychological events that are reported to trigger migraine in humans.16–19,26–28 A weakness of our studies is that intranasal administration of total PRLR-CRISPR–Cas9 may impact PRLR in the brain, however, we have confirmed that there is no effect on the expression of PRLR in the ARC by intranasal administration of total PRLR-CRISPR–Cas9. This possibility requires further investigation. Finally, we note that we did not consider the possible consequences of the oestrous cycle that is known to affect secretion of PRL.89 However, our results were robust and had a high reproducibility suggesting a limited impact of oestrous cycle-related changes of PRL levels in our model.
In summary, our study identifies a previously unknown mechanism that links the consequences of stress to hypothalamic activation of ARC KOR and sensitization of trigeminal nociceptors through a female-selective neuroendocrine mechanism. Stress-induced sensitization of trigeminal nociceptors can therefore promote migraine pain from normally innocuous stimuli and may additionally help to explain the female prevalence of migraine. It should be noted that interoceptive physiological processes that disrupt homeostasis, such as sleep disruption, missing meals, fluid imbalance and others that are often identified as ‘migraine triggers’, could also engage hypothalamic KOR circuits to promote nociceptor sensitization through this neuroendocrine mechanism. Similarly, psychological stress that arises from daily life and is most commonly perceived as a migraine trigger probably influences hypothalamic activity. Collectively, these findings may allow improved migraine therapies that can include sex-specific treatments. KOR antagonists may be useful as migraine preventives for both women and men, while dopamine D2 receptor agonists or PRL antibodies may provide novel preventive treatment options for migraine and other stress-related pain disorders, in women.
Supplementary Material
Acknowledgements
We kindly thank Dr Xu Yue, Dr Pavel Rychetsky, Juliana Swiokla and Luiz Henrique Moreira de Souza for the technical support.
Abbreviations
- ARC
arcuate nucleus
- CA
cutaneous allodynia
- CGRP
calcitonin-gene-related peptide
- CNO
clozapine N-oxide
- CRISPR
clustered regularly interspaced short palindromic repeats
- DRG
dorsal root ganglion
- KOR
kappa opioid receptor
- LS
latent sensitization
- PRLR-L/S
prolactin receptor long/short
- RS
restraint stress
- TG
trigeminal ganglion
- TGV1
trigeminal ganglion V1 division
- TIDA
tuberoinfundibular dopamine
- UMB
umbellulone
Contributor Information
Moe Watanabe, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Caroline M Kopruszinski, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Aubin Moutal, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Daigo Ikegami, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Rajesh Khanna, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Yanxia Chen, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Sarah Ross, Department of Anesthesiology and Perioperative Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA.
Kimberly Mackenzie, Teva Pharmaceutical Industries, Ltd., Biologics Discovery, Redwood City, CA 94063, USA.
Jennifer Stratton, Teva Pharmaceutical Industries, Ltd., Biologics Discovery, Redwood City, CA 94063, USA.
David W Dodick, Department of Neurology, Mayo Clinic, Phoenix, AZ 85054, USA.
Edita Navratilova, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA.
Frank Porreca, Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ 85724, USA; Department of Neurology, Mayo Clinic, Phoenix, AZ 85054, USA.
Funding
This work was supported by grants from the National Institutes of Health (NIH) to F.P. and E.N. and in part by research collaboration sponsored by Teva Pharmaceutical Industries, Ltd. (Redwood City, CA, USA).
Competing interests
M.W., C.M.K., D.I., Y.C., A.M. and E.N. declare that they have no personal, financial or relational conflicts of interest with this work. F.P. has served as a consultant or received research funding from Voyager, SiteOne Therapeutics, Nektar, Amgen, Acadia, Blackthorn, Teva, Eli Lilly, Hoba, Allergan, Ipsen and Proximagen, and has served as a founder of Catalina Pharma, Scientific Advisory Board Regulonix and Condor Pharma. R.K. is a stakeholder in Regulonix Holding Inc. D.W.D. reports the following conflicts within the past 12 months: Consulting: AEON, Amgen, Clexio, Cerecin, Ctrl M, Allergan, Alder, Biohaven, Linpharma, Lundbeck, Promius, Eli Lilly, eNeura, Novartis, Impel, Satsuma, Theranica, Vedanta, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Revance, Equinox. Honoraria: CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, MJH Lifesciences, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Ctrl M (options), Aural analytics (options), ExSano (options), Palion (options), Healint (Options), Theranica (Options), Second Opinion/Mobile Health (Options), Epien (Options/Board), Nocira (options), Matterhorn (Shares/Board), Ontologics (Shares/Board), King-Devick Technologies (Options/Board), Precon Health (Options/Board). Patent 17189376.1-1466: vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. K.M. and J.S. are employees of Teva Pharmaceutical Industries, Ltd.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. J Psychosom Res. 2015;78:228–236. [DOI] [PubMed] [Google Scholar]
- 2. Nahman-Averbuch H, Granovsky Y, Coghill RC, et al. Waning of “conditioned pain modulation”: A novel expression of subtle pronociception in migraine. Headache. 2013;53:1104–1115. [DOI] [PubMed] [Google Scholar]
- 3. Brennan KC, Pietrobon D. A systems neuroscience approach to migraine. Neuron. 2018;97:1004–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodick DW. Migraine. Lancet. 2018;391:1315–1330. [DOI] [PubMed] [Google Scholar]
- 5. Goadsby PJ, Holland PR. An update: pathophysiology of migraine. Neurol Clin. 2019;37:651–671. [DOI] [PubMed] [Google Scholar]
- 6. Puledda F, Messina R, Goadsby PJ. An update on migraine: Current understanding and future directions. J Neurol. 2017;264:2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borsook D, Erpelding N, Lebel A, et al. Sex and the migraine brain. Neurobiol Dis. 2014;68:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. [DOI] [PubMed] [Google Scholar]
- 9. Pavlovic JM, Akcali D, Bolay H, Bernstein C, Maleki N. Sex-related influences in migraine. J Neurosci Res. 2017;95:587–593. [DOI] [PubMed] [Google Scholar]
- 10. Wang SJ, Chen PK, Fuh JL. Comorbidities of migraine. Front Neurol. 2010;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karsan N, Goadsby PJ. Imaging the premonitory phase of migraine. Front Neurol. 2020;11:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karsan N, Prabhakar P, Goadsby PJ. Characterising the premonitory stage of migraine in children: A clinic-based study of 100 patients in a specialist headache service. J Headache Pain. 2016;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schulte LH, May A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–1993. [DOI] [PubMed] [Google Scholar]
- 14. Schulte LH, Menz MM, Haaker J, May A. The migraineur’s brain networks: Continuous resting state fMRI over 30 days. Cephalalgia. 2020;40:1614–1621. [DOI] [PubMed] [Google Scholar]
- 15. Peng KP, May A. Migraine understood as a sensory threshold disease. Pain. 2019;160:1494–1501. [DOI] [PubMed] [Google Scholar]
- 16. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. [DOI] [PubMed] [Google Scholar]
- 17. Lipton RB, Buse DC, Hall CB, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology. 2014;82:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache. 2013;53:81–92. [DOI] [PubMed] [Google Scholar]
- 19. Moon HJ, Seo JG, Park SP. Perceived stress in patients with migraine: A case-control study. J Headache Pain. 2017;18:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wacogne C, Lacoste JP, Guillibert E, Hugues FC, Le Jeunne C. Stress, anxiety, depression and migraine. Cephalalgia. 2003;23:451–455. [DOI] [PubMed] [Google Scholar]
- 21. Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. May A, Burstein R. Hypothalamic regulation of headache and migraine. Cephalalgia. 2019;39:1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci USA. 2016;113:8837–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerrero M, Urbano M, Kim EK, et al. Design and synthesis of a novel and selective kappa opioid receptor (KOR) antagonist (BTRX-335140). J Med Chem. 2019;62:1761–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bigal ME, Lipton RB. Modifiable risk factors for migraine progression. Headache. 2006;46:1334–1343. [DOI] [PubMed] [Google Scholar]
- 26. Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73:219–234. [DOI] [PubMed] [Google Scholar]
- 27. Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012;52:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santos IS, Brunoni AR, Goulart AC, et al. Negative life events and migraine: A cross-sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) baseline data. BMC Public Health. 2014;14:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Felice M, Ossipov MH, Wang R, et al. Triptan-induced latent sensitization: A possible basis for medication overuse headache. Ann Neurol. 2010;67:325–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avona A, Mason BN, Lackovic J, et al. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. Pain. 2020;161:2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kopruszinski CM, Navratilova E, Swiokla J, Dodick DW, Chessell IP, Porreca F. A novel, injury-free rodent model of vulnerability for assessment of acute and preventive therapies reveals temporal contributions of CGRP-receptor activation in migraine-like pain. Cephalalgia. 2021;41:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benemei S, De Cesaris F, Nicoletti P, Materazzi S, Nassini R, Geppetti P. Migraine models. Methods Mol Biol. 2010;617:105–114. [DOI] [PubMed] [Google Scholar]
- 34. Barrett SA. Bulletin of the public museum of the city of Milwaukee. Pomo Myths 1933;2:117–376. [Google Scholar]
- 35. Drake ME, Stuhr ET. Some pharmacological and bactericidal properties of umbellulone. J Am Pharmaceut Assoc. 1935;24:196–207. [Google Scholar]
- 36. Nassini R, Materazzi S, Vriens J, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. [DOI] [PubMed] [Google Scholar]
- 37. Schwedt TJ, Krauss MJ, Frey K, Gereau RW. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011;31:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: Clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. [DOI] [PubMed] [Google Scholar]
- 39. Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xie JY, De Felice M, Kopruszinski CM, et al. Kappa opioid receptor antagonists: A possible new class of therapeutics for migraine prevention. Cephalalgia. 2017;37:780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bernard V, Young J, Binart N. Prolactin - A pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15:356–365. [DOI] [PubMed] [Google Scholar]
- 42. Kreek MJ, Schluger J, Borg L, Gunduz M, Ho A. Dynorphin A1-13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: Gender differences and implications for modulation of dopaminergic tone in the treatment of addictions. J Pharmacol Exp Ther. 1999;288:260–269. [PubMed] [Google Scholar]
- 43. Lennartsson AK, Jonsdottir IH. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology. 2011;36:1530–1539. [DOI] [PubMed] [Google Scholar]
- 44. Levine S, Muneyyirci-Delale O. Stress-induced hyperprolactinemia: Pathophysiology and clinical approach. Obstet Gynecol Int. 2018;2018:9253083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schedlowski M, Wiechert D, Wagner TO, Tewes U. Acute psychological stress increases plasma levels of cortisol, prolactin and TSH. Life Sci. 1992;50:1201–1205. [DOI] [PubMed] [Google Scholar]
- 46. Sonino N, Navarrini C, Ruini C, Fallo F, Boscaro M, Fava GA. Life events in the pathogenesis of hyperprolactinemia. Eur J Endocrinol. 2004;151:61–65. [DOI] [PubMed] [Google Scholar]
- 47. Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. [DOI] [PubMed] [Google Scholar]
- 48. Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone release in male and female rats. Brain Res. 1998;807:228–233. [DOI] [PubMed] [Google Scholar]
- 49. Butelman ER, Kreek MJ. κ-Opioid receptor agonist-induced prolactin release in primates is blocked by dopamine D(2)-like receptor agonists. Eur J Pharmacol. 2001;423:243–249. [DOI] [PubMed] [Google Scholar]
- 50. Zullig KL, Murphree E, Reinscheid RK, Janik J, Callahan P. Effect of orphanin FQ/nociceptin (OFQ/N) and isoflurane on the prolactin secretory response in OFQ/N knockout mice. Peptides. 2007;28:1611–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cavestro C, Rosatello A, Marino MP, Micca G, Asteggiano G. High prolactin levels as a worsening factor for migraine. J Headache Pain. 2006;7:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bosco D, Belfiore A, Fava A, et al. Relationship between high prolactin levels and migraine attacks in patients with microprolactinoma. J Headache Pain. 2008;9:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kallestrup MM, Kasch H, Østerby T, Nielsen E, Jensen TS, Jørgensen JO. Prolactinoma-associated headache and dopamine agonist treatment. Cephalalgia. 2014;34:493–502. [DOI] [PubMed] [Google Scholar]
- 54. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. [DOI] [PubMed] [Google Scholar]
- 55. Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen Y, Navratilova E, Dodick DW, Porreca F. An emerging role for prolactin in female-selective pain. Trends Neurosci. 2020;43:635–648. [DOI] [PubMed] [Google Scholar]
- 57. Fitzgerald P, Dinan TG. Prolactin and dopamine: What is the connection? A review article. J Psychopharmacol. 2008;22:12–19. [DOI] [PubMed] [Google Scholar]
- 58. Chen Y, Moutal A, Navratilova E et al. The prolactin receptor long isoform regulates nociceptor sensitization and opioid-induced hyperalgesia selectively in females. Sci Transl Med. 2020;12:eaay7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Paige C, Barba-Escobedo PA, Mecklenburg J et al. Neuroendocrine mechanisms governing sex differences in hyperalgesic priming involve prolactin receptor sensory neuron signaling. J Neurosci. 2020;40:7080–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patil M, Belugin S, Mecklenburg J, et al. Prolactin regulates pain responses via a female-selective nociceptor-specific mechanism. iScience. 2019;20:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patil M, Hovhannisyan AH, Wangzhou A, et al. Prolactin receptor expression in mouse dorsal root ganglia neuronal subtypes is sex-dependent. J Neuroendocrinol. 2019;31:e12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cai X, Huang H, Kuzirian MS, et al. Generation of a KOR-Cre knockin mouse strain to study cells involved in kappa opioid signaling. Genesis. 2016;54:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mo A, Mukamel EA, Davis FP, et al. Epigenomic signatures of neuronal diversity in the mammalian brain. Neuron. 2015;86:1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Melo-Carrillo A, Schain AJ, Stratton J, Strassman AM, Burstein R. Fremanezumab and its isotype slow propagation rate and shorten cortical recovery period but do not prevent occurrence of cortical spreading depression in rats with compromised blood-brain barrier. Pain. 2020;161:1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meidahl AC, Klukinov M, Tzabazis AZ, Sorensen JC, Yeomans DC. Nasal application of HSV encoding human preproenkephalin blocks craniofacial pain in a rat model of traumatic brain injury. Gene Ther. 2017;24:482–486. [DOI] [PubMed] [Google Scholar]
- 66. Ikegami D, Navratilova E, Yue X, et al. A prolactin-dependent sexually dimorphic mechanism of migraine chronification. Cephalalgia. 2022;42(3):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dussor G, Yan J, Xie JY, Ossipov MH, Dodick DW, Porreca F. Targeting TRP channels for novel migraine therapeutics. ACS Chem Neurosci. 2014;5:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Balkaya M, Seidel JL, Sadeghian H, et al. Relief following chronic stress augments spreading depolarization susceptibility in familial hemiplegic migraine mice. Neuroscience. 2019;415:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shyti R, Eikermann-Haerter K, van Heiningen SH, et al. Stress hormone corticosterone enhances susceptibility to cortical spreading depression in familial hemiplegic migraine type 1 mutant mice. Exp Neurol. 2015;263:214–220. [DOI] [PubMed] [Google Scholar]
- 71. Yapici-Eser H, Donmez-Demir B, Kilic K, Eren-Kocak E, Dalkara T. Stress modulates cortical excitability via alpha-2 adrenergic and glucocorticoid receptors: As assessed by spreading depression. Exp Neurol. 2018;307:45–51. [DOI] [PubMed] [Google Scholar]
- 72. Kandere-Grzybowska K, Gheorghe D, Priller J, et al. Stress-induced dura vascular permeability does not develop in mast cell-deficient and neurokinin-1 receptor knockout mice. Brain Res. 2003;980:213–220. [DOI] [PubMed] [Google Scholar]
- 73. Theoharides TC, Spanos C, Pang X, et al. Stress-induced intracranial mast cell degranulation: A corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. [DOI] [PubMed] [Google Scholar]
- 74. Costa A, Smeraldi A, Tassorelli C, Greco R, Nappi G. Effects of acute and chronic restraint stress on nitroglycerin-induced hyperalgesia in rats. Neurosci Lett. 2005;383:7–11. [DOI] [PubMed] [Google Scholar]
- 75. Kaufmann D, Brennan KC. The effects of chronic stress on migraine relevant phenotypes in male mice. Front Cell Neurosci. 2018;12:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kopruszinski CM, Xie JY, Eyde NM, et al. Prevention of stress- or nitric oxide donor-induced medication overuse headache by a calcitonin gene-related peptide antibody in rodents. Cephalalgia. 2017;37:560–570. [DOI] [PubMed] [Google Scholar]
- 77. Nation KM, De Felice M, Hernandez PI, et al. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain. 2018;159:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cahill CM, Taylor AMW, Cook C, Ong E, Morón JA, Evans CJ. Does the kappa opioid receptor system contribute to pain aversion? Front Pharmacol. 2014;5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. DePaoli AM, Hurley KM, Yasada K, Reisine T, Bell G. Distribution of kappa opioid receptor mRNA in adult mouse brain: An in situ hybridization histochemistry study. Mol Cell Neurosci. 1994;5:327–335. [DOI] [PubMed] [Google Scholar]
- 80. Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: From addiction to depression, and back. Front Psychiatry. 2014;5:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: Anatomical and functional implications. Trends Neurosci. 1995;18:22–29. [DOI] [PubMed] [Google Scholar]
- 82. Minami M, Hosoi Y, Toya T, et al. In situ hybridization study of kappa-opioid receptor mRNA in the rat brain. Neurosci Lett. 1993;162:161–164. [DOI] [PubMed] [Google Scholar]
- 83. Kant GJ, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Effects of repeated stress on pituitary cyclic AMP, and plasma prolactin, corticosterone and growth hormone in male rats. Pharmacol Biochem Behav. 1983;18:967–971. [DOI] [PubMed] [Google Scholar]
- 84. Kirk SE, Xie TY, Steyn FJ, Grattan DR, Bunn SJ. Restraint stress increases prolactin-mediated phosphorylation of signal transducer and activator of transcription 5 in the hypothalamus and adrenal cortex in the male mouse. J Neuroendocrinol. 2017;29:1–9. [DOI] [PubMed] [Google Scholar]
- 85. McGivern RF, Rittenhouse P, Aird F, Van de Kar LD, Redei E. Inhibition of stress-induced neuroendocrine and behavioral responses in the rat by prepro-thyrotropin-releasing hormone 178–199. J Neurosci. 1997;17:4886–4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Diogenes A, Patwardhan AM, Jeske NA, et al. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patil MJ, Ruparel SB, Henry MA, Akopian AN. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: Contribution of prolactin receptor to inflammatory pain. Am J Physiol Endocrinol Metab. 2013;305:E1154–E1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bjorklund A, Nobin A. Fluorescence histochemical and microspectrofluorometric mapping of dopamine and noradrenaline cell groups in the rat diencephalon. Brain Res. 1973;51:193–205. [DOI] [PubMed] [Google Scholar]
- 89. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: Structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. [DOI] [PubMed] [Google Scholar]
- 90. Chen C, Willhouse AH, Huang P, et al. Characterization of a knock-in mouse line expressing a fusion protein of kappa opioid receptor conjugated with tdTomato: 3-dimensional brain imaging via CLARITY. eNeuro. 2020;7:ENEURO.0028-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Negishi K, Payant MA, Schumacker KS, et al. Distributions of hypothalamic neuron populations coexpressing tyrosine hydroxylase and the vesicular GABA transporter in the mouse. J Comp Neurol. 2020;528:1833–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruggiero DA, Ross CA, Anwar M, Park DH, Joh TH, Reis DJ. Distribution of neurons containing phenylethanolamine N-methyltransferase in medulla and hypothalamus of rat. J Comp Neurol. 1985;239:127–154. [DOI] [PubMed] [Google Scholar]
- 93. Lyons DJ, Horjales-Araujo E, Broberger C. Synchronized network oscillations in rat tuberoinfundibular dopamine neurons: switch to tonic discharge by thyrotropin-releasing hormone. Neuron. 2010;65:217–229. [DOI] [PubMed] [Google Scholar]
- 94. Rorick-Kehn LM, Witkin JM, Statnick MA et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology. 2014;77:131–144. [DOI] [PubMed] [Google Scholar]
- 95. Fariello RG. Pharmacodynamic and pharmacokinetic features of cabergoline. Rationale for use in Parkinson’s disease. Drugs. 1998;55:10–16. [DOI] [PubMed] [Google Scholar]
- 96. Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. [DOI] [PubMed] [Google Scholar]
- 97. Oliveira MDC, Barea L, Horn APK, et al. Resolution of headache after reduction of prolactin levels in hyperprolactinemic patients. Arq Neuropsiquiatr. 2020;78:28–33. [DOI] [PubMed] [Google Scholar]
- 98. Verhelst J, Abs R, Maiter D, et al. Cabergoline in the treatment of hyperprolactinemia: a study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518–2522. [DOI] [PubMed] [Google Scholar]
- 99. Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural calcitonin gene-related peptide produces female-specific responses in rodent migraine models. J Neurosci. 2019;39:4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Avona A, Mason BN, Burgos-Vega C, et al. Meningeal CGRP-prolactin interaction evokes female-specific migraine behavior. Ann Neurol. 2021;89:1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Navratilova E, Rau J, Oyarzo J, et al. CGRP-dependent and independent mechanisms of acute and persistent post-traumatic headache following mild traumatic brain injury in mice. Cephalalgia. 2019;39:1762–1775. [DOI] [PubMed] [Google Scholar]
- 102. Kopruszinski CM, Thornton P, Arnold J, et al. Characterization and preclinical evaluation of a protease activated receptor 2 (PAR2) monoclonal antibody as a preventive therapy for migraine. Cephalalgia. 2020;40:1535–1550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request.