Abstract
Background:
The objective of this study was to categorize the presentation and treatment of acute limb ischemia (ALI) in young patients and compare adverse outcomes following revascularization when compared to older patients.
Methods:
All patients presenting to a multi-institution health system with ALI from 2016 to 2020 were identified. Presenting features, operative details, and outcomes were included in the analysis. Patients with existing peripheral arterial disease (acute on chronic) were analyzed separately from patients without (de novo thrombosis/embolus). Within these groups, young patients (age ≤ 50) were compared to older patients (age > 50). Three-month major adverse limb event-free survival (MALE-FS) was the primary outcome.
Results:
232 patients (60 ± 16 years, 44% female, 87% white race) were included in the analysis. 119 patients were in the acute on chronic cohort while 113 were in the de novo thrombosis/embolism cohort. Age did not affect outcomes overall (p = .45) or in acute on chronic patients (p = .17). However, in the de novo thrombosis/embolism cohort, patients ≤ 50 years old had worse MALE-FS compared to patients >50 years old (HR 2.47, 95% CI 1.08–5.68; p = .03) after adjusting for Rutherford class, time from presentation to the operating room, and smoking status. In this group, there were similar trends in operative approach across ages (endovascular 12% vs. 14%, open 48% vs. 41%; hybrid 41% vs. 45%; p = .78). In young patients, embolism was more likely from a proximal arterial source (71%) whereas in older patients, source of embolism was more often cardiac (86%). There were equal rates of hypercoagulable disease across age groups (10% vs. 10%; p = .95). In-hospital mortality was 3% overall (5% in acute on chronic patients, 3% in patients with de novo events).
Conclusions:
Despite advances in interventional options, in patients with ALI due to de novo thrombosis or embolus, young age is associated with worse short-term limb related outcomes.
Keywords: Acute limb ischemia, major adverse limb events, arterial thrombosis
Table of Contents Summary:
This retrospective study captured all patients who underwent operative treatment of acute limb ischemia. Short-term major adverse limb event-free survival was worse in young patients who did not have a previous diagnosis of peripheral artery disease. This represents a high-risk population and should warrant a lower threshold for anticoagulation after revascularization.
INTRODUCTION
Acute limb ischemia (ALI) affects an estimated 14 per 100,000 individuals per year.1 ALI is associated with high morbidity and mortality, with reported one-year amputation rates of 11% and one-year mortality rates as high as 40%.2 In recent years, endovascular interventions have shown comparable patency to open techniques with reduced morbidity and mortality.3,4 Despite these evolving interventional technologies, limb salvage following ALI remains poor.5,6
Younger patients are a particularly under-investigated group in acute limb ischemia and may have an elevated risk of adverse events compared to older counterparts. Those with an inherited thrombophilia, which may manifest in younger patients, have also been shown to have worse outcomes after revascularization for treatment of peripheral arterial disease.7 Furthermore, patients younger than 50 years old with peripheral arterial disease have been identified as a group that is under-prescribed indicated medical therapy.8 As with chronic disease, these factors may complicate the management of acute limb ischemia in young patients. There has yet to be a study that directly compares risk factors and outcomes in ALI between old and young patients.
Therefore, the objective of this study was to outline age related differences in acute limb ischemia presentation and outcomes, and to identify factors that may contribute to poor outcomes specifically in young patients. We hypothesized that young patients would have worse short-term outcomes following acute limb ischemia compared to older patients.
METHODS
Patient population
All patients who underwent an operation for ALI at UPMC, a multi-hospital healthcare system including both academic and community hospitals, from January 2016 to January 2020 were included. This time period was chosen to evaluate the use of current techniques, including endovascular, open, and hybrid techniques, for revascularization for ALI. The time frame also included ALI prior to the COVID pandemic, as the ALI observed in COVID patients seems to have exceptionally poor outcomes.9,10 The electronic medical record (EMR) was reviewed to capture patient demographics, co-morbidities, medications, in-hospital variables, operative variables, and outcome variables. Patients were deemed to be hypercoagulable if they had a positive hypercoagulable panel at any point. This study was approved by the University of Pittsburgh’s Human Research Protection Office (STUDY21100134).
Primary outcome
Major adverse limb event-free survival (MALE-FS) was the primary outcome. MALE-FS was a composite outcome that included ipsilateral vascular reintervention, major amputation (below-knee or above-knee), or death.11 MALE-FS was evaluated at three months as a marker of short-term outcomes following ALI.
The association between age and MALE-FS was moderated by prior history of PAD (p-value of interaction = .02). Therefore, the effect of age on MALE-FS for patients with prior documented PAD was analyzed separately from patients with no documented prior PAD (Supplemental Figure 1). Patients with documented prior PAD in the electronic health record were included in the acute on chronic cohort. Patients with no documented prior PAD were considered to have de novo thrombosis/embolism and included both in situ thrombosis and embolism. We excluded any patients with ALI due to aortic dissection. Within these groups, young patients (age ≤ 50) were compared to older patients (age > 50).
Statistical analysis
Data were analyzed with Stata version 16 (StataCorp LP, College Station, Texas). Continuous variables are reported as mean ± standard deviation or median (interquartile range [IQR]) and were compared between cohorts using Student’s T-tests for parametric variables and Mann-Whitney U tests for non-parametric variables. Categorical variables are presented as frequency (%). Chi-squared testing was used unless frequency (expected <5) required Fisher’s exact testing.
Outcomes were plotted with Kaplan-Meier curves comparing MALE-FS using log rank testing. Multivariable cox regression analyses were chosen to identify factors contributing to MALE-FS. All variables with a P ≤ .10 were included in the final model. Hazard Ratios (HR) and their corresponding 95% confidence intervals (CI) were reported as measures of associate factors. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.12
Sensitivity analyses
Several sensitivity analyses were performed on this patient population. Both the Kaplan-Meier and multivariable cox regression analyses were performed in a similar fashion to the primary analysis on patients with different age thresholds. Comparisons were made between acute on chronic and de novo thrombosis/embolism groups with age thresholds of both 40 years and 60 years old. Comparisons were also made after excluding patients who received a major amputation at the index operation.
RESULTS
232 patients (60 ± 16 years, 44% female, 87% white race) were included in the analysis. 119 patients had prior documented PAD which were categorized as acute on chronic patients. 113 patients did not have prior documented PAD and were characterized as the de novo thrombosis/embolism cohort. In the acute on chronic group, 32% of patients were ≤ 50 years old compared to 37% in the de novo thrombosis/embolism group (p = .48; Figure 1). Over the study timeline, there were no statistically significant changes in age distribution (p = .15) or type of intervention, endo, open, or hybrid, used (p = .85). Median follow-up was 11.5 months (IQR 2.8, 36.6 months).
Figure 1.
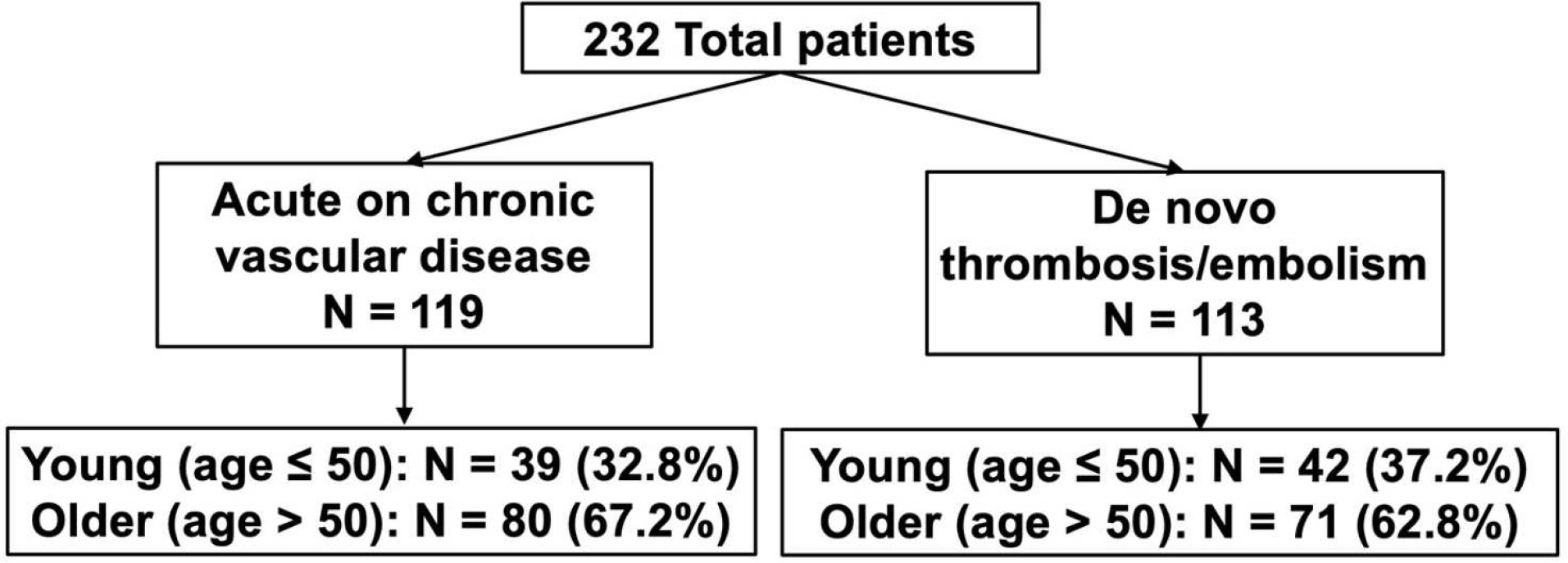
Diagram demonstrating the study population and relevant comparative groups.
Acute on chronic
Comorbidities and patient characteristics within the acute on chronic group are presented in Table I. In the acute on chronic group, most patients were male (61%), white (92%), with a prior (65%) or current (16%) history of smoking. More patients in the older cohort had a history of atrial fibrillation (21%) compared to the younger cohort (5%; p = .02). Additionally, there was a lower proportion of prior documentation of hypercoagulable disease in the older cohort (6%) compared to the young cohort (18%; p = .05). Fifteen percent of older patients carried an active cancer diagnosis, compared to 3% of young patients (p = .09). On presentation, most patients in both the young (54%) and older (46%) cohorts presented with Rutherford Class IIb ischemia (p = .73). Additionally, 36% of patients in the older cohort experienced a prolonged (>6 hours) time from presentation to the operating room, whereas 62% of patients in the young cohort had prolonged time to the operating room (p = .01).
Table I.
Demographics, comorbidities, presenting factors for each age group separated by etiology.
| Acute on chronic patients | |||
|
| |||
| Age ≤ 50 N = 39 | Age > 50 N = 80 | p-value | |
|
| |||
| Age a | 42.8 (6.2) | 57.3 (11.9) | <.01 |
|
| |||
| Gender (female) | 18 (46.2) | 29 (36.3) | .30 |
|
| |||
| Race (nonwhite) | 4 (10.3) | 6 (7.6) | .63 |
|
| |||
| BMI | 28.5 (6.2) | 28.2 (13.8) | .56 |
|
| |||
| Smoking history | |||
| Former | 33 (84.6) | 44 (55.0) | .01 |
| Current | 3 (7.7) | 16 (20.0) | |
|
| |||
| Atrial fibrillation | 2 (5.1) | 17 (21.3) | .02 |
|
| |||
| Diabetes | 14 (35.9) | 26 (32.5) | .71 |
|
| |||
| Chronic kidney disease | 8 (20.5) | 13 (16.3) | .57 |
|
| |||
| Creatinine | 1.11 (0.74) | 1.31 (1.41) | .21 |
|
| |||
| COPD | 8 (20.5) | 19 (23.8) | .69 |
|
| |||
| Hypercoagulable diagnosis | 7 (18.0) | 5 (6.3) | .05 |
|
| |||
| Cancer | 1 (2.6) | 12 (15.0) | .09 |
|
| |||
| Prior ipsilateral intervention | 25 (64.1) | 60 (75.0) | .22 |
|
| |||
| Preoperative anticoagulation | 11 (28.2) | 24 (30.0) | .84 |
|
| |||
| Preoperative antiplatelet | 18 (46.2) | 52 (65.0) | .05 |
|
| |||
| Rutherford Class | |||
| I | 4 (10.3) | 6 (7.5) | |
| IIa | 12 (30.8) | 33 (41.3) | .73 |
| IIb | 21 (53.9) | 37 (46.3) | |
| III | 2 (5.1) | 4 (5.0) | |
|
| |||
| Thrombus burden on initial imaging b | |||
| Suprainguinal | 9 (23.1) | 24 (30.0) | .95 |
| Femoral-popliteal | 26 (66.7) | 78 (97.5) | .08 |
| Tibial | 17 (43.6) | 45 (56.3) | .94 |
|
| |||
| > 6hrs to operating room | 24 (61.5) | 29 (36.3) | .01 |
|
| |||
| De novo thrombosis patients | |||
|
| |||
| Age ≤ 50 N = 42 | Age > 50 N = 71 | p-value | |
|
| |||
| Age | 42.7 (7.0) | 69.5 (12.4) | <.01 |
|
| |||
| Gender (female) | 22 (52.4) | 34 (47.9) | .64 |
|
| |||
| Race (nonwhite) | 9 (21.4) | 11 (15.5) | .49 |
|
| |||
| BMI | 29.8 (7.9) | 30.0 (7.5) | .55 |
|
| |||
| Smoking history | |||
| Former | 28 (66.7) | 26 (36.6) | <.01 |
| Current | 1 (2.4) | 13 (18.3) | |
|
| |||
| Atrial fibrillation | 7 (16.7) | 23 (32.4) | .07 |
|
| |||
| Diabetes | 9 (21.4) | 24 (33.8) | .16 |
|
| |||
| Chronic kidney disease | 1 (2.4) | 11 (15.5) | .03 |
|
| |||
| Creatinine | 1.10 (0.69) | 1.38 (1.06) | .14 |
|
| |||
| COPD | 2 (4.8) | 15 (21.1) | .02 |
|
| |||
| Hypercoagulable diagnosis | 4 (9.5) | 7 (9.9) | .95 |
|
| |||
| Cancer | 1 (2.4) | 7 (9.9) | .17 |
|
| |||
| Prior ipsilateral intervention | 4 (9.5) | 11 (15.5) | .37 |
|
| |||
| Preoperative anticoagulation | 5 (11.9) | 15 (21.1) | .22 |
|
| |||
| Preoperative antiplatelet | 6 (14.3) | 30 (42.3) | <.01 |
|
| |||
| Rutherford Class | |||
| I | 4 (9.5) | 6 (8.5) | |
| IIa | 16 (38.1) | 28 (39.4) | .26 |
| IIb | 17 (40.5) | 35 (49.3) | |
| III | 5 (11.9) | 2 (2.8) | |
|
| |||
| Thrombus burden on initial imaging b | |||
| Suprainguinal | 10 (23.8) | 17 (23.9) | .75 |
| Femoral-popliteal | 33 (78.6) | 69 (97.2) | .14 |
| Tibial | 18 (42.9) | 43 (60.6) | .23 |
|
| |||
| Source of embolism (N=57) c | |||
| Proximal arterial source | 26 (81.3) | 2 (7.8) | <.01 |
| Cardiac | 6 (18.8) | 24 (92.3) | |
|
| |||
| > 6hrs to operating | 9 (21.4) | 30 (42.3) | .02 |
Continuous variables are displayed as summary measure of mean (standard deviation) and P value from Student t-test when the variable is normally distributed. Categorical variables are summarized by N (%), and P values are calculated from the X2 test.
Thrombus identified on initial imaging – each level is not mutually exclusive
Source of embolism was identified on imaging of 57 de novo thrombosis/embolism patients
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Operative characteristics in this cohort are displayed in Table II. There were no significant differences in type of intervention. Twenty-eight percent of patients in the older cohort underwent endovascular only interventions compared to 31% of patients in the young cohort. Similarly, 21% of older patients underwent open interventions compared to 21% in the young cohort (p = .93). There were similar rates of stenting, bypass, and thrombectomy – both percutaneous and open – as well as similar operative times. However, while both older and young patients had similar rates of severe ischemia on presentation (5% vs. 5% Rutherford Class III ischemia; p=.73), young patients in this cohort had a significantly higher rate of amputation at index operation (1% vs. 13%, p = .01). Thirty-day complications were also similar between ages. While not statistically significant, there was a larger proportion of older patients requiring an amputation after index revascularization (20%) compared to young patients (9%; p = .08). In-hospital mortality was 0% for young patients and 8% for older patients. This was due to either multisystem organ failure secondary to cardiogenic shock (66%) or septic shock (33%). Three-month mortality was 3% for young patients and 19% for older patients. Cause of death in these patients were as follows: multisystem organ failure due to cardiogenic shock (33%), multisystem organ failure due to septic shock (33%), acute renal failure (22%), and unknown (11%).
Table II.
Operative details and 30-day complications for each age group separated by etiology.
| Acute on chronic patients | |||
|
| |||
| Age ≤ 50 N = 39 | Age > 50 N = 80 | p-value | |
|
| |||
| Type of intervention a | .93 | ||
| Endovascular only | 12 (30.8) | 22 (27.5) | |
| Open only | 8 (20.5) | 17 (21.3) | |
| Hybrid | 19 (48.7) | 41 (51.3) | |
|
| |||
| Stent b | 11 (29.2) | 23 (28.8) | .95 |
|
| |||
| Thrombolysis | 11 (28.2) | 29 (36.3) | .38 |
|
| |||
| Surgical thrombectomy | 19 (48.7) | 46 (57.5) | .37 |
|
| |||
| Percutaneous thrombectomy | 0 (0.0) | 4 (5.0) | .16 |
|
| |||
| Bypass | 8 (20.5) | 22 (27.5) | .41 |
|
| |||
| Stent + bypass | 1 (2.6) | 2 (2.5) | .83 |
|
| |||
| Stent + surgical thrombectomy | 5 (12.8) | 12 (15.0) | .87 |
|
| |||
| Four compartment fasciotomy at index operation | 26 (66.7) | 33 (41.3) | .77 |
|
| |||
| Major amputation at index operation | 5 (12.8) | 1 (13) | .01 |
|
| |||
| Operating room time (minutes) | 214.5 (144.5) | 182.3 (111.4) | .90 |
|
| |||
| Transfusion requirement | 11 (28.2) | 11 (13.8) | .06 |
|
| |||
| Length of stay | 10.9 (8.9) | 12.1 (11.9) | .71 |
|
| |||
| Discharge anticoagulation | 26 (66.7) | 49 (61.3) | .57 |
| Coumadin | 12 (46.2) | 14 (28.6) | |
| Lovenox | 2 (7.7) | 4 (8.2) | |
| DOAC | 8 (30.8) | 29 (59.2) | |
| Other | 4 (15.4) | 2 (4.1) | |
|
| |||
| Discharge antiplatelet | 33 (84.6) | 64 (80.0) | .54 |
|
| |||
| Ipsilateral majorc amputation | 2 (5.1) | 16 (20.0) | .08 |
|
| |||
| Renal failure | 2 (5.1) | 7 (8.8) | .46 |
|
| |||
| Respiratory failure | 1 (2.6) | 7 (8.8) | .21 |
|
| |||
| Congestive heart failure | 4 (10.3) | 5 (6.3) | .44 |
|
| |||
| Wound infection | 5 (12.8) | 10 (12.5) | .90 |
|
| |||
| In-hospital mortality | 0 (0.0) | 6 (7.5) | .79 |
|
| |||
| 3-month mortality | 1 (2.6) | 15 (18.8) | .32 |
|
| |||
| De novo thrombosis | |||
|
| |||
| Age ≤ 50 N = 42 | Age > 50 N = 71 | p-value | |
|
| |||
| Type of intervention | .78 | ||
| Endovascular only | 5 (11.9) | 10 (14.1) | |
| Open only | 20 (47.6) | 29 (40.9) | |
| Hybrid | 17 (40.5) | 32 (45.1) | |
|
| |||
| Stent | 6 (14.3) | 9 (12.7) | .81 |
|
| |||
| Thrombolysis | 5 (11.9) | 15 (21.1) | .22 |
|
| |||
| Surgical thrombectomy | 30 (71.4) | 52 (73.2) | .84 |
|
| |||
| Percutaneous thrombectomy | 0 (0.0) | 2 (2.8) | .27 |
|
| |||
| Bypass | 5 (11.9) | 6 (8.5) | .55 |
|
| |||
| Stent + bypass | 1 (2.3) | 0 (0.0) | .17 |
|
| |||
| Stent + surgical thrombectomy | 4 (9.5) | 7 (9.9) | .89 |
|
| |||
| Four compartment fasciotomy at index operation | 15 (35.7) | 33 (46.8) | .53 |
|
| |||
| Major amputation at index operation | 8 (19.1) | 1 (14) | <.01 |
|
| |||
| Operating room time (minutes) | 202.9 (130.6) | 157.6 (85.6) | .01 |
|
| |||
| Transfusion requirement | 14 (33.3) | 16 (22.5) | .21 |
|
| |||
| Length of stay | 15.0 (0.9) | 14.1 (16.1) | .38 |
|
| |||
| Discharge anticoagulation | 32 (76.2) | 47 (66.2) | .26 |
| Coumadin | 12 (37.5) | 10 (21.7) | |
| Lovenox | 5 (15.6) | 8 (17.0) | |
| DOAC | 14 (43.8) | 23 (48.9) | |
| Other | 1 (3.1) | 7 (14.9) | |
|
| |||
| Discharge antiplatelet | 24 (57.1) | 48 (67.6) | .26 |
|
| |||
| Ipsilateral major amputation | 2 (4.8) | 0 (0.0) | .18 |
|
| |||
| Renal failure | 0 (0.0) | 1 (14) | .44 |
|
| |||
| Respiratory failure | 0 (0.0) | 1 (14) | .44 |
|
| |||
| Cardiac failure | 0 (0.0) | 1 (14) | .44 |
|
| |||
| Wound infection | 2 (4.8) | 3 (4.2) | .89 |
|
| |||
| In-hospital mortality | 0 (0.0) | 3 (4.2) | .18 |
|
| |||
| 3-month mortality | 2 (4.8) | 9 (12.7) | .31 |
Continuous variables are displayed as summary measure of mean (standard deviation) and P value from Student t-test when the variable is normally distributed. Categorical variables are summarized by N (%), and P values are calculated from the X2 test.
Interventions reported are not mutually exclusive
Outcomes listed are 30-day events unless otherwise specified
At three months, there was no difference in MALE-FS between young and older patients. In patients ≤ 50 years old, MALE-FS was 82% at three months compared to 79% (log rank p value = .24; Figure 2A).
Figure 2.
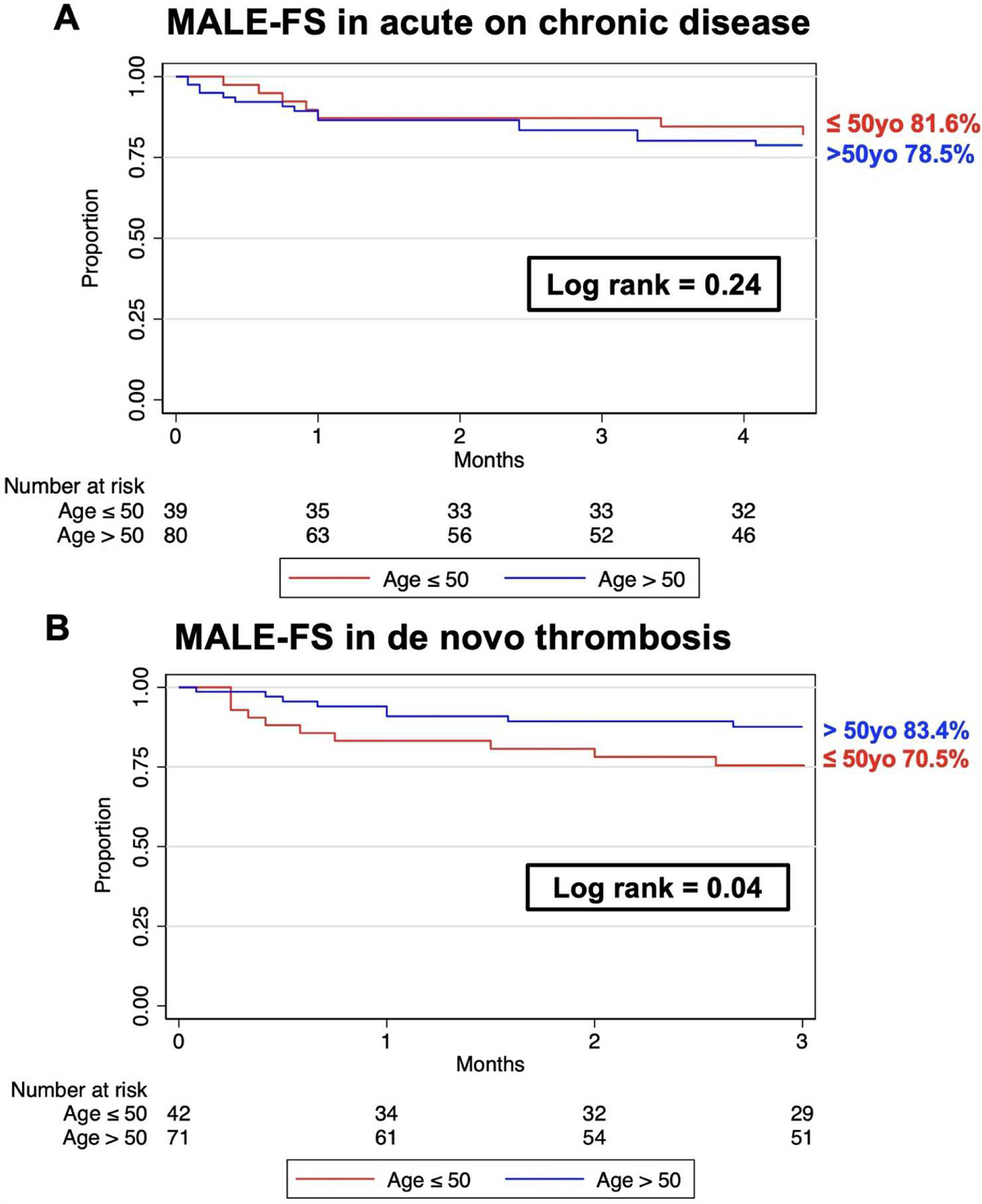
Kaplan-Meier curves of short-term MALE-FS after ALI in both acute on chronic and de novo thrombosis cohorts.
De novo thrombosis/embolism
Comorbidities and patient characteristics within the de novo group are presented in Table I. The proportion of male patients was similar to the proportion of female patients (50% male). 82% of patients were white with a prior (48%) or current (12%) history of smoking. There was a trend toward higher proportion of patients with a diagnosis of atrial fibrillation in the older cohort compared to the young cohort (32% vs. 17%; p = .07), but this difference was not statistically significant. There were no differences in incidence of hypercoagulable state across groups (older 10%, young 10%; p = .95) or active cancer diagnosis (older 10%, young 10%; p =.17). Thirty two (76%) of younger de novo thrombosis patients had a confirmed proximal source of embolism located. 26 (81%) of these were a proximal arterial source, whereas 6 (19%) were identified to be cardioembolic. On presentation, there were similar rates of both Rutherford Class IIa (38% vs. 39%) and IIb (41% vs. 49%) between age groups (p = .26). In in older patients, source of embolism was most commonly cardiogenic (86%) whereas in young patients, embolism was more likely from a proximal aortic or peripheral arterial source (71%, p < .01). Additionally, 42% of older patients had a prolonged (>6 hours) time from presentation to the operating room compared to 21% of patients in the young cohort (p = .02).
Operative characteristics in this cohort are displayed in Table II. There were no significant differences in type of intervention. Fourteen percent of patients in the older cohort underwent endovascular only interventions compared to 12% of patients in the young cohort. Similarly, 41% of older patients underwent open interventions compared to 48% of young patients (p = .78). There were also similar rates of stenting, bypass, and thrombectomy – both percutaneous and open. However, as with the acute on chronic group, young patients in this cohort had higher rates of major amputation at index operation (19% vs. 1%, p < .01). The younger cohort also had significantly longer operative times in the de novo thrombosis/embolism group (young 203 minutes vs. older 158 minutes; p = .01). Thirty-day complications were similar between cohorts. Rates of ipsilateral major amputation at thirty days were similar between cohorts (older 0% vs. younger 5%; p = .18). In hospital mortality was 0% for young patients and 4% for older patients. This was due to either cardiogenic shock (66%) or respiratory failure (33%). Three-month mortality was 5% for young patients and 13% for older patients. This was due to the following circumstances: multisystem organ failure due to septic shock (38%), cardiogenic shock (13%), respiratory failure (13%), trauma (13%) or unknown (25%).
Unlike acute on chronic patients, short-term MALE-FS at three months in older patients was 83% compared to 71% in the young cohort (log rank p-value = .04; Figure 2B).
Multivariable analyses
On multivariable cox regression, young age was not associated with MALE-FS in the total population (HR 1.12, 95% CI 0.75–1.91; p = .44). Age was also not associated with improved MALE-FS in the acute on chronic cohort (young age: HR 1.26, 95% CI 0.74–1.29; p = .37). However, when evaluating the de novo thrombosis/embolism cohort separately, the younger cohort was associated with worse short-term MALE-FS after controlling for Rutherford Class on presentation, time to the operating room, and previous smoking history (HR 2.47, 95% CI 1.08–5.68; p = .03).
Sensitivity analyses
Sensitivity analyses were performed by adjusting the age thresholds. Instead of stratifying the groups at age 50, an age threshold of 40 years old was considered, while controlling for Rutherford Class on presentation, prolonged time to the operating room, and smoking status. There were only 17 patients who were in the ≤ 40-year-old group, which makes the multivariable models difficult to interpret. Given such a low sample size, the higher age threshold of 50 years old was chosen.
An older age threshold of 60 years old was also considered. Young patients had improved MALE-FS in the acute on chronic group (HR 0.26, 95% CI 0.13–0.52; p = .04). Age did not have the same effects on the de novo thrombosis/embolism group when the age threshold was 60 years old (HR 1.59, 95% CI 0.93–2.73; p = .09). Given the effect seen at age 50 seemed to become negligible at 60 years old, the age threshold of 50 years was chosen. Furthermore, previous literature has used 50 years as an age threshold for consideration of young patients in the treatment of peripheral arterial disease.7,8,13
After excluding patients who required a major amputation at the initial operation, similar trends persisted. In acute on chronic patients, young age had a lower hazard of MALE-FS (HR 0.26, 95% CI 0.07–0.99; p = .05). Whereas in de novo thrombosis/embolism, young age had a higher hazard of MALE-FS, although not statistically significant (HR 1.64, 95% CI 0.84–3.19; p = .15). Patients who required amputation at index operation were included in the analysis as this was an evaluation of all patients who present with acute limb ischemia.
DISCUSSION
Acute limb ischemia is an incredibly morbid disease process, with one-year amputation rates as high as 37%.4,14 Despite the development of minimally invasive techniques and adjuncts, outcomes remain poor.5,6 Young patients presenting with ALI are of particular importance, as there is a paucity of data describing risk factors and outcomes in this group. Most reports in the literature are case reports, limited to describing an ambiguous presentation of ALI in a single young patient.15–17
While young patients are often thought to have better surgical outcomes due to less comorbidities and less frailty, we hypothesized that young patients would have worse short-term outcomes following acute limb ischemia compared to older patients for a number of reasons. First, young patients are less likely to have underlying peripheral arterial disease and may be less tolerant to an acute ischemic event. Second, young patients are less likely to have underlying atrial fibrillation and or other known risk factors for ALI. Thus, there may be a lower index of suspicion in these patients leading to delays in diagnosis and treatment. Third, prior studies suggest that young patients are known to have worse outcomes after treatment of peripheral arterial disease in the setting of hypercoagulability, therefore, we hypothesized that there are specific risk factors that portend worse outcomes in young patients.7 As reported here, patients who are ≤ 50 years old and do not have a history of documented PAD have worse MALE-FS compared to older patients who do not have a history of PAD.
In our cohort, observed short-term MALE-FS was highest in young patients with de novo thrombosis/embolism compared to older patients. The three-month MALE-FS in our study cohorts ranged from 71%−82% depending on the etiology and age range. This is similar to the large historical single-center reports in the literature,3,18 Medicare population,14 and most recent literature.19 Short-term limb salvage rates have not been reported in the literature. When evaluating the cohort with de novo thrombosis/embolism, there were higher rates of major amputation at index operation in younger patients. This was also reflected in lower MALE-FS at three months in patients with de novo thrombosis/embolism in young patients. While not significant, this trend persisted despite excluding patients that received an amputation at initial intervention. In a retrospective single center study of young patients with both chronic and acute ALI, Torrealba et al found that patients with inherited hypercoagulable states are reported to have worse limb related outcomes after revascularization.7 In our cohort, there was no difference in reported hypercoagulability, however this may be limited by the available hypercoagulability testing. In fact, there is data to suggest that some of the genetic variants commonly included in heritable thrombophilia testing are of little clinical relevance while there are some variants we do not test which should be included.20 It is difficult, therefore, to conclude that the young patients in our cohort definitively did not have a genetic thrombophilia. The poor outcomes seen in young patients were seen in patients without underlying peripheral artery disease, suggesting this population may have had prothrombotic tendencies. In addition, delays in diagnosis may have led to increased amputation rates at initial operation or lack of functional limb even after revascularization leading to subsequent amputation.
While there was no increased time to the operating room in the young patient cohort, many patients in our cohort were transferred from an outside facility. Potential delays in diagnosis may have contributed to increased amputation rates at the initial operation or lack of a functional limb even after revascularization leading to short term major amputation. Future studies will need to identify the effects of transfer on severity of disease progression on presentation and outcomes. Young patients in the de novo thrombosis/embolism cohort also had longer operating room times. The reasons for this are not completely clear as younger patients did not have significantly more extensive thrombus burden, hybrid techniques, or fasciotomies. This could reflect poor response to thrombectomy or lysis in younger patients, sometimes requiring redo thrombectomies or anastomosis revisions, which the authors do observe in clinical practice.
Finally, poor tolerance to ischemic insult likely plays a role in age disparity as well. The reduced limb salvage rates reported in young patients is limited to those without prior PAD. Previous studies have shown no differences in limb salvage outcomes for claudication in patients younger than 50 years old compared to older patients.13 There are several possible explanations for this. The pathology leading to poor limb outcomes is likely separate from underlying atherosclerosis, as patients with atherosclerosis have similar outcomes regardless of age. An alternative explanation could be that in the even in the absence of prior known PAD, older patients may have a degree of collateralization while their younger counterparts do not have that additional arterial reserve.. It could also follow that due to less collaterals, younger patients may have been closer to a Rutherford 3 than their older counterparts, with more severe ischemia. Regardless, young patients with no prior PAD represent a patient population that warrants targeted therapy such as early surgical intervention, close follow-up, and long-term anticoagulation. Future studies should evaluate to utility of these interventions.
While most patients in the de novo thrombosis/embolism cohort were discharged on anticoagulation, only 12% of young patients were on anticoagulation at time of presentation. The young patients were also significantly less likely to be on antiplatelet medication. Soudet et al performed a retrospective analysis of their single institution data and found that compared to older patients, patients younger than 50 years old with PAD were under-prescribed medical treatment such – leading to increased recurrence of symptoms and increased risk of major amputation.8 This younger cohort may not only have more severe risk factors, but they are less likely to be medically optimized. While this likely represents a reduced utilization of health care compared to their older counterparts, there are other factors that may lead to the under-prescription of anticoagulation or antiplatelets. For example, many hypercoagulable tests are not ordered until a patient has already presented with a thrombotic complication. This likely results in an under-diagnosis of hypercoagulability in this population. Identifying these patients earlier should be a priority of future studies.
This study intentionally included acute limb ischemia patients prior to the COVID-19 outbreak. Our institution, along with many other institutions across the world, have observed increased rates of ALI in COVID positive patients and at younger ages.9,21,22 This is likely a completely separate disease process than the ALI discussed in this report, and is thus out of the scope of this analysis.23 Future studies should aim to evaluate the risk factors and outcomes of ALI in the setting of COVID-19.
Our study has several limitations. First, this was a retrospective analysis and relied on retrospective review of electronic medical records. This introduces the possibility of selection bias between groups. To ameliorate this, the data collectors for this study were blinded to the planned analysis until after data collection. Additionally, much of the diagnostic variables were reliant on the information coded in the electronic medical record. While this captures the presence of a variable, it may not capture the absence of a variable, limiting measurement reliability. Finally, the median follow-up in this series of under a year limits long-term outcome data. Obtaining long-term follow-up data for ALI patients is difficult in the non-randomized control trial setting due to the high rates of loss to follow-up.4 However, most patients had follow-up at three months, which is the reporting threshold we chose.
CONCLUSIONS
In patients presenting with ALI, short-term MALE-FS was worse in patients ≤ 50 years old who do not have documented prior PAD independent of severity of presentation, comorbidities, and treatment modality. Early recognition and high clinical suspicion are necessary in this patient population. Further study is required to explore the increased morbidity and mortality in young patients with ALI.
Supplementary Material
Supplemental Figure 1. Amputation rate by age group.
Article Highlights:
Type of Research:
Multihospital single institution retrospective cohort study
Key Findings:
In acute limb ischemia, patients ≤50 years old had significantly worse short-term MALE-free survival compared to patients >50 years old (p=.02) despite similar interventional techniques and after controlling for degree of ischemia on presentation. This finding was seen in patients without prior diagnosis of peripheral artery disease.
Take Home Message:
In patients without a prior diagnosis of peripheral artery disease, young patients have a higher incidence of short term major adverse limb events. In treating younger patients, vascular surgeons should have a high suspicion for revascularization failure and consider adjunctive therapies such as anticoagulation after revascularization.
Funding sources:
This research was supported in part by grant T32HL98036 form the National Heart, Lung, and Blood Institute (Andraska, Phillips, Reitz) and L30 AG064730 from the National Institute on Aging (Reitz). The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund (Andraska).
Footnotes
Presented at the 35th annual meeting of the Eastern Vascular Society Charleston, South Carolina; September 23–26, 2021
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Araujo ST, Moreno DH, Cacione DG. Percutaneous thrombectomy or ultrasound-accelerated thrombolysis for initial management of acute limb ischaemia. Cochrane database Syst Rev. 2022. Jan;1(1):CD013486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2007;33 Suppl 1:S1–75. [DOI] [PubMed] [Google Scholar]
- 3.Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015. Jan;61(1):147–54. [DOI] [PubMed] [Google Scholar]
- 4.Genovese EA, Chaer RA, Taha AG, Marone LK, Avgerinos E, Makaroun MS, et al. Risk Factors for Long-Term Mortality and Amputation after Open and Endovascular Treatment of Acute Limb Ischemia. Ann Vasc Surg. 2016. Jan;30:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govsyeyev N, Malgor RD, Hoffman C, Harroun N, Sturman E, Al-Musawi M, et al. A systematic review and meta-analysis of outcomes after acute limb ischemia in patients with cancer. J Vasc Surg. 2021. Sep;74(3):1033–1040.e1. [DOI] [PubMed] [Google Scholar]
- 6.Lopez R, Yamashita TS, Neisen M, Fleming M, Colglazier J, Oderich G, et al. Single-center experience with Indigo aspiration thrombectomy for acute lower limb ischemia. J Vasc Surg. 2020. Jul;72(1):226–32. [DOI] [PubMed] [Google Scholar]
- 7.Torrealba JI, Osman M, Kelso R. Hypercoagulability predicts worse outcomes in young patients undergoing lower extremity revascularization. J Vasc Surg. 2019. Jul;70(1):175–80. [DOI] [PubMed] [Google Scholar]
- 8.Soudet S, Bultel L, Adnane L, Reix T, Sevestre MA. Under-Prescription of Medical Treatment for Peripheral Artery Disease in the Under 50s: A Retrospective Study. Angiology. 2021. Sep;33197211042155. [DOI] [PubMed] [Google Scholar]
- 9.Galyfos G, Sianou A, Frountzas M, Vasilios K, Vouros D, Theodoropoulos C, et al. Acute limb ischemia among patients with COVID-19 infection. J Vasc Surg. 2022. Jan;75(1):326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pena G, Fitridge R. Acute Limb Ischaemia in the COVID-19 Era: a Clinical and Organisational Challenge. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2021. Sep; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoner MC, Calligaro KD, Chaer RA, Dietzek AM, Farber A, Guzman RJ, et al. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. 2016. Jul;64(1):e1–21. [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014. Dec;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 13.Dermody M, Homsy C, Zhao Y, Goodney PP, Estes JM. Outcomes of infrainguinal bypass determined by age in the Vascular Study Group of New England. J Vasc Surg. 2015. Jul;62(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014. Sep;60(3):669–77.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernheim JW, Hanson J, Faries P, Kilaru S, Winchester P, Mousa A, et al. Acute lower extremity ischemia in a 7-year-old boy: an unusual case of popliteal entrapment syndrome. J Vasc Surg. 2004. Jun;39(6):1340–3. [DOI] [PubMed] [Google Scholar]
- 16.Nespola M, Sirignano P, Fermani N, Battocchio C, Tosti F, Pranteda C, et al. Treatment-Resistant Acute Upper Limb Ischemia in a Patient With Systemic Lupus Erythematous and Concomitant SARS-CoV-2 Infection: A Case Report. Vol. 76, Annals of vascular surgery. 2021. p. 289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus F, Claude EV, Josephine M, Teyang A. An Exceptional Cause of Acute Limb Ischemia: Nicolau Syndrome-Single-Center Experience with 4 Cases. Ann Vasc Surg. 2019. Jul;58:383.e7–383.e11. [DOI] [PubMed] [Google Scholar]
- 18.Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014. Apr;59(4):988–95. [DOI] [PubMed] [Google Scholar]
- 19.Poursina O, Elizondo-Adamchik H, Montero-Baker M, Pallister ZS, Mills JLS, Chung J. Safety and efficacy of an endovascular-first approach to acute limb ischemia. J Vasc Surg. 2021. May;73(5):1741–9. [DOI] [PubMed] [Google Scholar]
- 20.Gomez K Genomic Analysis for the Detection of Bleeding and Thrombotic Disorders. Semin Thromb Hemost. 2021. Mar;47(2):174–82. [DOI] [PubMed] [Google Scholar]
- 21.Bellosta R, Luzzani L, Natalini G, Pegorer MA, Attisani L, Cossu LG, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020. Dec;72(6):1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capell WH, Barnathan ES, Piazza G, Spyropoulos AC, Hsia J, Bull S, et al. Rationale and design for the study of rivaroxaban to reduce thrombotic events, hospitalization and death in outpatients with COVID-19: The PREVENT-HD study. Am Heart J. 2021. May;235:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoteit L, Deeb A-P, Andraska EA, Kaltenmeier C, Yazdani HO, Tohme S, et al. The Pathobiological Basis for Thrombotic Complications in COVID-19: a Review of the Literature. Curr Pathobiol Rep. 2021. Dec;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Amputation rate by age group.


