Abstract
The recognition of compatible mating partners in the basidiomycete fungi requires the coordinated activities of two gene complexes defined as the mating-type genes. One complex encodes members of the homeobox family of transcription factors, which heterodimerize on mating to generate an active transcription regulator. The other complex encodes peptide pheromones and 7-transmembrane receptors that permit intercellular signalling. Remarkably, a single species may have many thousands of cross-compatible mating types because the mating-type genes are multiallelic. Different alleles of both sets of genes are necessary for mating compatibility, and they trigger the initial stages of sexual development—the formation of a specialized filamentous mycelium termed the dikaryon, in which the haploid nuclei remain closely associated in each cell but do not fuse. Three species have been taken as models to describe the molecular structure and organization of the mating-type loci and the genes sequestered within them: the pathogenic smut fungus Ustilago maydis and the mushrooms Coprinus cinereus and Schizophyllum commune. Topics addressed in this review are the roles of the mating-type gene products in regulating sexual development, the molecular basis for multiple mating types, and the molecular interactions that permit different allelic products of the mating type genes to be discriminated. Attention is drawn to the remarkable conservation in the mechanisms that regulate sexual development in basidiomycetes and unicellular ascomycete yeasts, Saccharomyces cerevisiae and Schizosaccharomyces pombe, a theme which is developed in the general conclusion to include the filamentous ascomycetes Neurospora crassa and Podospora anserina.
Mating is an essential step in the life cycle of sexually reproducing organisms. The function of the mating-type genes in the fungi is to impose barriers on self-mating and thereby maintain genetic variability within the population. In the basidiomycete fungi, the subject of this review, somatic cell fusion is sufficient for mating and no specialized cells are required. The mating-type genes ensure that only nuclei from genetically different individuals will fuse to give a diploid nucleus that will undergo meiosis prior to the formation of the sexual spores. Self-sterile species are said to be heterothallic, whereas those that can self-mate are said to be homothallic (14). The basidiomycete fungi are largely heterothallic, and a remarkable feature of this group is that they have evolved multiallelic mating type genes; as a result, some have many thousands of different mating types. The molecular interactions that permit mating cells to distinguish self from nonself not only are of great interest to fungal biologists but also give exciting insights into the complex interactions that govern development in higher eukaryotic organisms.
The basidiomycetes constitute a large fungal group encompassing many diverse forms including the rusts and smuts that cause plant disease, the mushrooms and other large forms such as boletes, puffballs, and bracket fungi, and the yeast-like Cryptococcus neoformans, which is an opportunistic pathogen that causes meningitis in immunocompromised humans. The basidiomycete fungi are so called because meiosis occurs in specialized cells called basidia and the resulting spores, the basidiospores, are produced outside the cell. This is in contrast to the other major group of fungi, the ascomycetes, where meiosis occurs in a cell called the ascus and the resulting ascospores develop inside the cell. We shall concentrate chiefly on three basidiomycete species, the corn smut Ustilago maydis and the mushrooms Coprinus cinereus and Schizophyllum commune, since these are the model organisms that were originally used to study the genetics of basidiomycete fungi. Over the past few years, our knowledge of mating in these fungi has progressed rapidly, and it is now appropriate to bring the different aspects of this work together.
MATING-TYPE GENES
In our model species, mating type is determined by genes at two unlinked loci. These are known as A and B in the mushroom fungi (for a historical review, see reference 97) and a and b in U. maydis (43, 104, 105). A compatible mating is one in which the mates have different alleles of genes at both mating-type loci, e.g., A1B1 × A2B2 in C. cinereus and S. commune or a1b1 × a2b2 in U. maydis. These fungi are said to be tetrapolar, since four mating types can segregate in the sexual progeny as a consequence of meiosis. Other species may be bipolar, in which case mates have different alleles of genes at a single mating-type locus (e.g., A1 × A2) and only two mating types segregate in the sexual progeny.
The locus designations were given long before the natures of the mating-type genes were known, and it is now rather unfortunate to discover that the mushroom A genes are equivalent to the U. maydis b genes and the mushroom B genes are equivalent to the U. maydis a genes! Molecular analysis has revealed that one set (the A and b genes) encodes the two protein subunits of a heterodimeric regulatory protein whereas the other set (the B and a genes) encodes both peptide pheromones and transmembrane receptors. There is no reason to believe that bipolar species with only a single mating-type locus lack any of these functions. Recent studies have shown that the bipolar smut Ustilago hordei has genes for the regulatory protein and the pheromones and receptors sequestered into its single complex locus rather than separated into two loci (3, 4).
With genes separated into different loci, large numbers of mating types are easily generated. In U. maydis, there are at least 25 alleles of the b locus (94) and 2 alleles of the a locus, giving 50 different mating types overall. In the mushroom fungi, both sets of mating-type genes are multiallelic and as a consequence there are many more mating types—more than 12,000 in C. cinereus and more than 20,000 in S. commune (97). Clearly it is of interest not just to know what the different mating-type genes encode but also to elucidate how such large numbers of different A and B allelic specificities are generated and how they are distinguished at the molecular level.
LIFE CYCLE
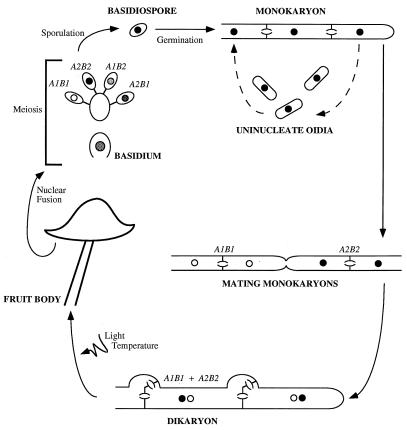
To familiarize the reader with some of the terms we shall need to use, we will begin by describing the life cycle of C. cinereus (Fig. 1). A single basidiospore germinates to give rise to a mass of monokaryotic hyphal filaments, each cell of which contains a single haploid nucleus. The complex interwoven mat of branching hyphae arising from a single spore is called the mycelium. Part of the mycelium grows submerged within the medium on which the fungus is growing, but aerial hyphae exist and produce abundant uninucleate haploid spores (oidia), which can germinate to complete the asexual cycle. The mycelium continues to grow as a monokaryon until it encounters a hypha from another fungus. At this point, hyphae from the two separate fungi fuse, and determination of whether the mates are sexually compatible occurs intracellularly.
FIG. 1.
Life cycle of C. cinereus.
If the two fungi are compatible, reciprocal nuclear migration occurs after cell fusion. The nuclei from the fused cell become the “donors” and migrate through the cells of the “recipient” compatible monokaryon (19). The septa which separate cells within a hyphal filament contain a complex pore apparatus known as the dolipore (79), which normally prevents nuclear movement between cells. Nuclear migration after cell fusion is facilitated by the degradation of the dolipores (35), and after nuclear migration has occurred, septa are re-formed between cells. Migration of nuclei may be very rapid and occurs much more quickly than does hyphal growth. The rate of nuclear migration in S. commune has been estimated at up to 3 mm/h (83), but the fastest migration recorded occurs in C. congregatus, at up to 4 cm/h (103). Once two compatible nuclei are present within the hyphal tip cell, all subsequent growth is in the form of a mycelial dikaryon rather than as a diploid. This prolonged dikaryotic phase is characteristic of basidiomycete fungi and can be maintained indefinitely. In contrast, cellular fusion between compatible mates in the life cycle of ascomycetes (both unicellular and filamentous) is followed rapidly by nuclear fusion.
After dikaryotization, asexual sporulation no longer occurs and appropriate environmental stimuli can induce formation of the fruit body (mushroom). The mushroom remains dikaryotic, and nuclear fusion and meiosis occur only in the specialized basidia, which are protected within the gills located underneath the mushroom cap. Haploid nuclei migrate into a tetrad of basidiospores, external to the basidium, which is left enucleate. The spores are released as the fruit body deliquesces, turning the mushroom black, which gives C. cinereus its common name—the ink-cap mushroom.
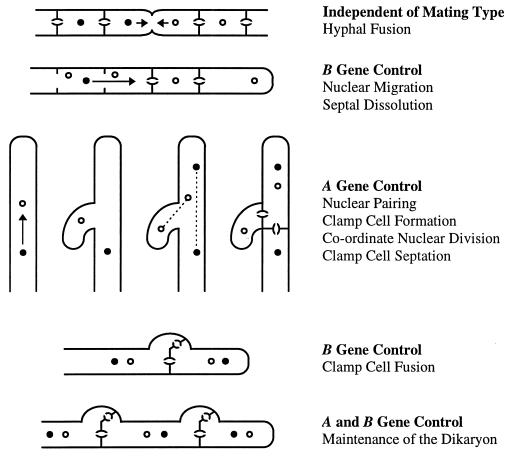
The morphology of the dikaryon differs from that of the monokaryon in several respects, but most distinctively, cells of the dikaryon undergo a complex form of cell division involving the formation of clamp connections to preserve one copy of each haploid nucleus within every dikaryotic cell (Fig. 2). Upon cell division, one of the nuclei moves into a protrusion (clamp) from the apical cell. The other nucleus moves into position near the clamp, and coordinate nuclear division occurs. Septa are formed across the mitotic spindles, locking the first nucleus into the clamp cell and the second into the subapical cell. Finally, the clamp and subapical cells fuse and the clamp cell nucleus is released into the subapical cell to restore the dikaryotic association. Because of the way the mitotic spindles separate the daughter nuclei, the nucleus that enters the clamp cell alternates at each successive conjugate division (45).
FIG. 2.
Roles of the A and B mating-type genes in regulating the formation and maintenance of the dikaryon of C. cinereus.
Both A and B mating-type genes of the mushroom fungi are required for the development and maintenance of the dikaryotic state. Genes encoded at the A locus are responsible for repressing asexual sporulation in C. cinereus (127), regulating pairing of nuclei within the dikaryotic tip cell, and coordinating nuclear division, clamp cell formation, and septation from the subapical cell (97, 125). B genes regulate the initial nuclear migration to the apical cell, which, in S. commune, has been shown to involve the induction of cell wall-degrading enzymes that disrupt the septa (133). The B genes also regulate fusion of the clamp cell with the subapical cell (97, 125).
Fusion between two monokaryons occurs irrespective of mating type and can generate a heterokaryotic mycelium in which both nuclear types exist but not in the characteristic organization of a dikaryon. If only the A alleles differ between mates (common B), clamp cells form but are unable to fuse with subapical cells, leaving a nucleus trapped in each clamp cell of the mycelium. If only the B alleles are different (common A), in S. commune a distinctive “flat” phenotype is observed in which aerial growth is lacking, the hyphae branch frequently, and nuclear migration and septal disruption occur continuously (97). There is no obvious heterokaryotic phenotype in common A matings of C. cinereus. Finally, if two mates share both A and B gene alleles, no nuclear exchange occurs and the fungi resume monokaryotic growth.
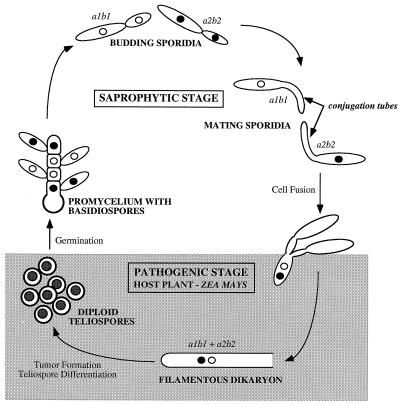
C. cinereus, S. commune, and other species which produce protected basidia within a fruit body are termed homobasidiomycetes. All other species are hemibasidiomycetes which produce naked basidia. We can use U. maydis to illustrate features of a hemibasidiomycete life cycle (Fig. 3). In its asexual form, U. maydis exists as elongated unicellular sporidia which are uninucleate and divide by budding. These cells are saprophytic. Fusion is dependent upon mating type and can occur only between sporidia with different alleles at the a locus (44, 93). The first stage in mating is the development of long mating filaments, called conjugation tubes, that fuse at their tips. If sporidia have different b alleles, dikaryotic filaments are formed after cell fusion. These dikaryotic hyphae are pathogenic on maize and can grow only in planta. Hyphal proliferation within the plant induces the formation of tumors, within which each dikaryotic cell differentiates to form a unicellular diploid teliospore. When the tumor bursts, black teliospores are scattered in clouds which resemble soot, giving the fungus its common name of smut fungus. Meiosis occurs within the teliospore, and four haploid cells (including the original teliospore cell) are formed as a promycelial structure which buds to form sporidia (reviewed in references 6 and 7).
FIG. 3.
Life cycle of the pathogenic smut fungus U. maydis.
It is not known whether the dikaryotic mycelium of U. maydis produces clamp connections, and assignment of roles to the a and b genes is complicated by the fact that plant signals are believed to play an unknown role in dikaryotic growth and tumor development. In common with the homobasidiomycetes, however, both a and b gene functions are required for maintenance of dikaryotic growth.
HOMEOBOX GENES
Conserved Domains of the Homeobox Proteins
The genes of the A and b mating-type loci encode the two subunits of a heterodimeric regulatory protein. The characteristic feature of the two proteins and what marks them as potential transcription factors is the presence of a DNA-binding motif known as the homeodomain. These proteins are modular in structure and may also have several other functional domains. Heterodimerization may serve to bring these functional domains together to influence DNA target site selection and to regulate target gene transcription.
The DNA-binding domain, the homeodomain, is generally defined as a 60- to 63-amino-acid sequence and characterizes a large family of transcription factors ubiquitous in eukaryotic organisms. Homeodomain proteins often play important roles in development, e.g., those encoded by the clustered homeotic genes of the fruit fly Drosophila melanogaster and the corresponding hox genes of vertebrates that specify body segmentation (20, 77). The interaction of the homeodomain with DNA has been determined by nuclear magnetic resonance spectroscopy and X-ray crystallography (reviewed in reference 33). The homeodomain folds into three helices, and the third helix (the recognition helix) inserts into the major groove of DNA, where the main protein-DNA contacts are made. While the overall sequence of the homeodomain may be variable between different proteins, the residues of the recognition helix are strongly conserved.
The mating-type proteins of the A/b loci fall into two distinct subgroups on the basis of the homeodomain sequence, and these have been termed HD1 and HD2 (61). The homeodomains of the HD1 class are considered to be atypical due to deviation from the consensus homeodomain sequence (particularly in the recognition helix) and alterations in the spacing of the helices. Members of the HD2 class have a sequence motif that more closely resembles the consensus (20). Intracellular recognition of sexual compatibility occurs when an HD1 protein from one mate heterodimerizes with an HD2 protein from the other mate to form a functional regulatory protein (5, 50, 72). The heterodimer is assumed to be a transcription factor that binds unique target sites within the promoters of genes whose activity commits cells to a new developmental pathway. Unmated cells are unable to form this transcription factor and hence unable to undergo sexual development.
Obviously, intracellular recognition of compatible proteins is very important. An HD1 protein must not be allowed to heterodimerize with any HD2 proteins encoded by the same unmated cell, or the A/b-regulated pathway would be constitutively active without a requirement for mating! The region amino-terminal to the homeodomain has been shown to be responsible for discrimination between compatible and noncompatible interactions, and substitution of critical residues within this recognition domain has been shown to affect protein specificity (5, 50, 58, 72, 135, 137).
Dimerization between compatible protein subunits can occur in the absence of DNA and most probably occurs in the cytoplasm. However, to bind DNA, the active transcription factor must be transported into the nucleus. The HD1 protein family contains classic bipartite nuclear localization signals characterized by clusters of basic amino acids (59, 127). Similar nuclear localization signals are not found in the HD2 proteins. Thus, heterodimerization is likely to be essential for function—we predict that the HD2 protein cannot enter the nucleus without first associating with an HD1 protein, and although the HD1 protein can enter the nucleus without its HD2 partner, once there it lacks the specificity to recognize their joint target site on DNA (120).
Molecular Organization of the b Locus of U. maydis
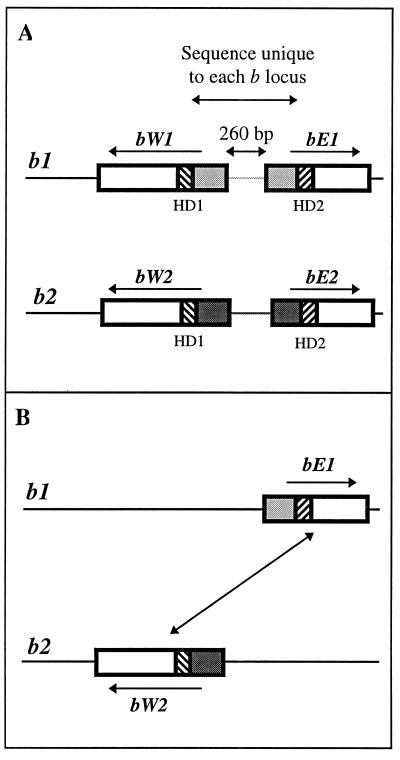
The organization of the b locus of U. maydis is illustrated in Fig. 4. There are two genes in each b locus, designated bE and bW, which are divergently transcribed. bE genes encode HD1 proteins, and bW genes encode HD2 proteins. bE and bW proteins have no sequence similarity other than limited conservation in the homeodomain region. However, different allelic versions of bE (or bW) are nearly identical in the homeodomain and carboxy-terminal domains, but the predicted amino-terminal ends of the proteins are highly variable (36, 59, 108). As we have already mentioned, these amino-terminal recognition domains determine the specificity of the proteins encoded at each b locus. The DNA sequence encoding the variable amino-terminal regions of the b proteins and the intervening 260 bp containing the gene promoters is different for every b allele. Thus, the two genes in any one b locus are inseparable by normal recombination events and remain together as a single genetic unit. This pattern of variable genes or regions of genes bordered by homologous sequences is conserved throughout the locus structures of both mating-type loci in each of our model species.
FIG. 4.
Molecular structure and organization of the b mating-type locus of U. maydis. (A) The multiallelic b locus contains two divergently transcribed genes, bE and bW, which encode polypeptides of 473 and 644 amino acids, respectively (36, 108). The sequence of the regions amino-terminal to the homeodomains of the two proteins and the intervening 260-bp promoter region is unique to each b specificity. The b1 and b2 loci are illustrated, and the variable DNA sequence specific to each is represented by the light grey and dark grey regions, respectively. Striated boxes indicate the homeodomain-encoding sequences. (B) Gene deletion experiments showed that the compatible gene combination that promotes b-regulated sexual development is a bE gene from one locus and a bW gene from the other (diagonal arrow). For b1 and b2, the compatible gene combinations are bE1 + bW2 and bE2 + bW1.
When a b1 strain and a b2 strain fuse, four different b polypeptides are present in the fusion cell: bE1, bE2, bW1, and bW2 (Fig. 4A). By deleting genes from mating partners, it was shown that only two polypeptides are necessary for a successful mating. This pair of polypeptides must include one HD1 protein and one HD2 protein, i.e., one bE protein and one bW protein, and the polypeptides must come from different mates, i.e., bE1 plus bW2 or bE2 plus bW1. Figure 4B shows the compatible mating between two strains which encode only the bE1 or the bW2 protein; the complementary mating between strains which encode only the bE2 or the bW1 protein would also be fully compatible (36).
Molecular Organization of the A Locus of C. cinereus and S. commune.
The same rules govern the homeodomain protein interaction which allows intracellular recognition of compatible mating partners in the mushroom fungi. However, the mushroom A loci contain many more genes than the b locus of U. maydis, and there are correspondingly more compatible and incompatible gene products to be distinguished within a cell.
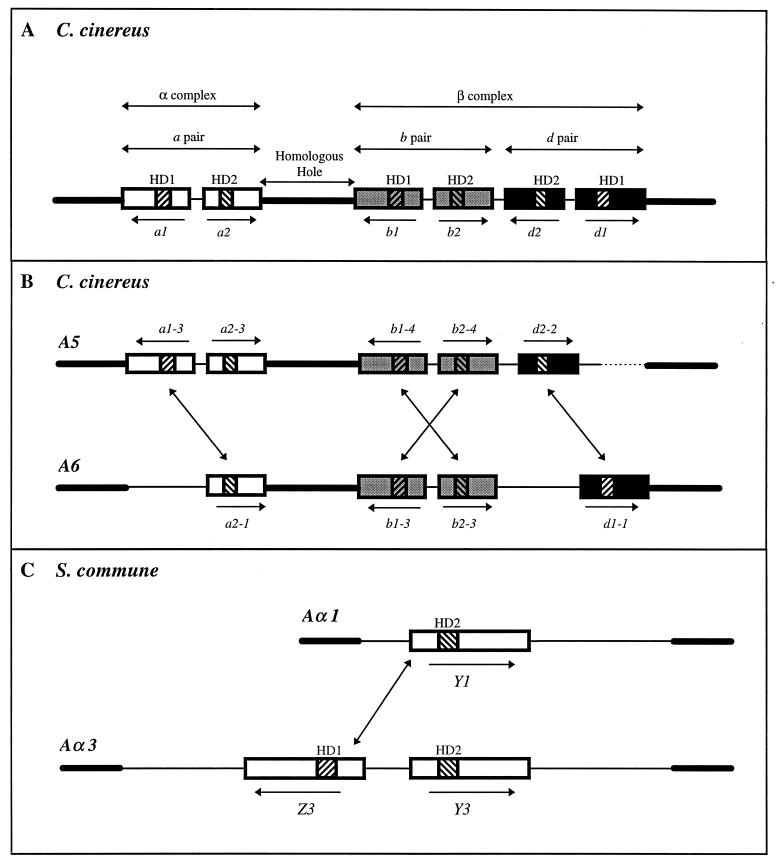
Classical recombination analysis identified two closely linked A loci, which were termed Aα and Aβ, in both S. commune and C. cinereus (24, 88, 100). These two loci are functionally redundant in that alleles at only one of them need be different between mates in order to have a compatible A gene interaction. Nine versions of Aα and a predicted 32 of Aβ means that there are potentially 288 different A mating specificities in S. commune (99). In C. cinereus, there are an estimated 160 A specificities but the actual numbers of α and β alleles are unknown (97, 134). However, considerably more is known about the molecular organization of the C. cinereus genes because the Aα and Aβ loci are very close together (only 0.07 map unit apart) (24, 25) and the complex has been isolated and characterized in its entirety (65, 76, 82, 91).
Each A locus of C. cinereus contains a variable number of genes, but taken together, it can be seen how each is derived from an ancestral locus (Fig. 5A) with three pairs of genes, each equivalent to a bE-bW pair in the U. maydis b locus (62). The C. cinereus genes have been designated the a, b, and d gene pairs. Unfortunately, the first locus to be sequenced had a nonfunctional gene between the b and d pairs, and this was thought to represent a fourth, c pair (62, 65)! The entire A locus is bounded by sequences homologous in all A specificities. In addition, the a gene pair is separated from the other two pairs by approximately 7.0 kb of DNA sequence (equivalent to 0.07 map unit) that is homologous in all A loci. This a pair of genes represents the classical Aα locus, while the b and d gene pairs constitute the Aβ locus (65, 69). Because there are so many functionally redundant genes encoded within the A locus, some mating specificities have lost several genes through evolution; indeed, it is rare to find all the genes present in a single locus. Of nine loci that have been examined, only one (A44) has all six genes (91). In the A6 locus, illustrated in Fig. 5B, one of the a genes (a1) and one of the d genes (d2) are missing, whereas in the A5 locus, just one of the d genes (d1) is missing.
FIG. 5.
Molecular structure and organization of the A mating-type locus of C. cinereus and S. commune. (A) The predicted archetypal A locus of C. cinereus contains three pairs of divergently transcribed multiallelic genes (a, b, and d). The genes in each pair encode two dissimilar proteins with distinctive homeodomain motifs designated HD1 and HD2. HD1 genes (a1, b1, and d1) encode proteins of ∼680 amino acids, and HD2 genes (a2, b2, and d2) encode proteins of ∼520 amino acids. Thick black lines indicate homologous DNA sequences flanking the locus and the common 7-kb sequence (the homologous hole) between the a and b genes which divides the genes into two complexes, α and β (65, 69). Thin lines indicate nonhomologous DNA sequences that do not permit recombination between genes. The a genes are represented by open boxes, the b genes are represented by grey boxes, and the d genes are represented by solid boxes. The homeodomain-encoding sequences are represented by striated boxes. (B) Organization of the A5 and A6 loci. The events that led to gene deletion may explain why d2-2 is not oriented as predicted. Diagonal arrows indicate the compatible HD1-HD2 gene combinations that promote A-regulated sexual development. The dashed line indicates that the region of nonhomology has not been fully defined. (C) The Aα locus of S. commune contains a single pair of divergently transcribed multiallelic HD1 and HD2 genes designated Z and Y, respectively, which encode proteins of 890 to 930 amino acids (122). The Aα1 locus contains only the Y gene (Y1), but the other eight versions of this locus are predicted to contain both genes. The diagonal arrow indicates a compatible gene combination that promotes A-regulated development.
By deleting most of the genes from an A locus of C. cinereus and reintroducing single genes to test for compatible gene interactions, it was shown that the three gene pairs are functionally independent. As in U. maydis, just one compatible HD1 and HD2 gene combination is sufficient to promote sexual development (91), but they must come from the same subset of genes; e.g., a genes work only with a genes, not with b or d genes. Thus, the three gene pairs can be considered to belong to separate subfamilies (a, b, and d), in which an HD1 protein may interact with all other HD2 proteins within its subfamily, except the HD2 protein encoded by the same locus, and vice versa.
To summarize, two A loci will produce a compatible gene interaction on mating if they encode different alleles of a single subfamily of genes, even if they have the same alleles of the other two pairs of genes. Between A5 and A6, there are four compatible gene combinations, as indicated by diagonal arrows in Fig. 5B. The large numbers of A mating-type specificities in C. cinereus are derived from different combinations of a, b, and d gene alleles. It would require only five or six alleles of each pair of genes to generate the estimated 160 unique A gene combinations (5 × 6 × 6). Clearly, having three pairs of genes with just a few alleles of each is a far more efficient evolutionary strategy for generating multiple mating types than is having many alleles of just a single pair of genes, as occurs in U. maydis (62).
Only the organization of the Aα locus of S. commune is fully known (Fig. 5C). This locus corresponds to a single gene pair in the C. cinereus complex and encodes a pair of divergently transcribed HD1 and HD2 genes designated Y and Z, respectively. In the Aα1 locus, only the Y gene is present (117, 122), but since the other eight Aα loci appear to contain a Z gene (116), the AαY1 gene will always find a compatible Z partner in other loci. To date, only a single HD2 gene from the Aβ locus has been identified (110), and it will be interesting to see how similar the structures of the C. cinereus and S. commune Aβ loci are.
Protein Heterodimerization
What determines compatibility between HD1 and HD2 proteins? With 25 alleles of each pair of b genes in U. maydis, there are potentially 625 possible heterodimers; 25 are “self” interactions that are unsuccessful, but the 600 “nonself” interactions are all assumed to be possible and equally capable of promoting dikaryotic growth. In C. cinereus, there are many more incompatible combinations, because as well as proteins encoded by “self” genes, proteins encoded by paralogous genes (genes from different subfamilies) cannot dimerize. For example, in a mating between A5 and A6 monokaryons of C. cinereus, four HD1 and five HD2 proteins are present in the fused cell. Of the 20 potential HD1-HD2 protein interactions, only 4 can heterodimerize and trigger development.
Clearly, the amino acid sequence of the amino-terminal dimerization domain is critical for determining compatibility. In U. maydis, chimeric genes which exchanged 5′ sequences between bE1 and bE2 (or bW1 and bW2) were used to demonstrate that the amino-terminal domains are sufficient to confer b1 or b2 allele specificity. These chimeric experiments also identified a particularly critical region in which changing the amino acid sequence could generate proteins with recognition specificities that were neither b1 nor b2 (58, 135).
The S. commune and C. cinereus genes have no obvious conserved and variable regions as found in U. maydis, and they can be quite dissimilar in overall sequence (only 40 to 70% identity [22, 122]). Nonetheless, chimeric genes generated between different Y alleles of S. commune (137) or between different a, b, and d genes of C. cinereus (63) confirmed that the 5′ ends of the A genes are sufficient to confer the specificity required to distinguish between allelic versions of the genes and also to determine to which subfamily a gene belongs.
One way in which dimerization between two polypeptides may be mediated is by coiled-coil interactions. Coiled coils are supercoiled α-helical regions which play many roles in protein structure (71) and have been shown to mediate both homo- and heterodimerization of transcription factors in yeasts and mammals. The structure of a coiled coil is such that it can generate a hydrophobic interface for dimerization flanked by charged hydrophilic regions which may act to stabilize the interaction (12, 23, 86). Two coiled-coil domains are predicted in the amino-terminal domain of the C. cinereus HD1 proteins (5, 34). Significantly, the relative positions of these are different in the a, b, and d proteins, which would provide a physical basis for discrimination between different protein subfamilies (91). Of more general interest is why certain pairs of proteins belonging to the same subfamily are unable to form heterodimers. For the U. maydis bE-bW pair, it was found that a single amino acid substitution at one of several positions was sufficient to convert a normally incompatible pair into a pair that could dimerize. Significantly these substitutions caused either an increase in hydrophobicity or a change in charge, which would be consistent with changes affecting coiled-coil interactions (47, 50).
Roles for Heterodimerization
Heterodimerization of A gene products in basidiomycetes plays an important role in bringing together the various domains required for the formation of a functional transcription factor. In attempts to isolate monokaryons with altered A mating specificities, several A mutants which were constitutive for clamp cell development were isolated from C. cinereus and S. commune (26, 98). The A mutations are dominant and thus completely overcome the need for a mating partner to have a different A mating specificity, although a different B specificity is still required.
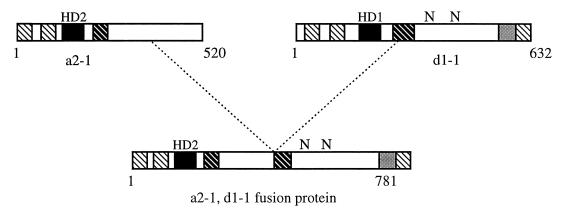
Molecular analysis of one of these self-compatible mutations in C. cinereus (64) revealed that most of the A locus had been deleted, leaving a single chimeric gene which had been generated by fusion of an HD2 gene and an HD1 gene (Fig. 6). This unusual gene can be translated to give a protein that is essentially a fused heterodimer and is sufficient to promote sexual development. Analysis of this constitutive fusion protein highlights the essential domains brought together by heterodimerization.
FIG. 6.
Predicted structural features of the HD1 and HD2 A mating-type proteins of C. cinereus, and the constitutively active protein encoded by an HD2-HD1 gene fusion. Diagonally striped boxes indicate α-helical domains, solid boxes indicate the homeodomains, and grey boxes indicate a negatively charged region that is a putative activation domain. N indicates the position of bipartite NLSs.
The fusion protein contains most of the HD2 sequence but only the carboxy-terminal half of the HD1 protein. Thus, the amino-terminal dimerization domain and the HD1 homeodomain are not essential for heterodimer function. Assuming that this protein binds DNA, the HD2 homeodomain is sufficient for specific binding to target sites. The carboxy-terminal domain of the HD1 protein contains the two predicted NLSs which are sufficient for nuclear targeting (120) and also contains an essential negatively charged sequence which is thought to be the activation domain required for transcriptional activation of target genes (2). Therefore, while it seems that all functional domains of the heterodimer can quite easily be expressed as a single protein, the separation of functional domains into two proteins represents an elegant strategy to ensure that mating-dependent developmental pathways are activated only after fusion between compatible mates. By generating further deletions of this fusion protein, it was shown that none of the sequences amino-terminal to the homeodomain or carboxy-terminal to the proposed activation domain were necessary for function (2).
a1/α2 Heterodimer of S. cerevisiae
As yet, we know nothing about the DNA-binding properties of the basidiomycete heterodimer. However, a very similar interaction between two homeodomain mating-type proteins regulates sexual development in the budding yeast Saccharomyces cerevisiae, and we can learn about the importance of cooperative DNA binding from these studies (reviewed in references 28, 42, and 46).
S. cerevisiae is an ascomycete fungus and has only two mating types, a and α. The mating type of haploid cells is determined by alternative genes at a single mating-type locus known as MAT. The MATa locus encodes the a1 polypeptide, which contains a domain similar to the HD2 motif, and the MATα locus encodes the α2 polypeptide with an HD1-type domain (20, 61, 65, 111). The a1 and α2 proteins are small, and while the amino-terminal domains are similar in length to the basidiomycete proteins, the homeodomain is at the carboxy terminus of both yeast proteins; thus by comparison, the yeast proteins lack the predicted activation domain present in the basidiomycete proteins.
Interestingly, the a1-α2 heterodimer which forms in diploid cells acts as a transcriptional repressor by recruiting the general transcription factors Tup1p and Ssn6p to form a repressor complex (51). This repressor binds specific target sites upstream of haploid cell-specific genes to ensure that only diploid cell functions are expressed after mating. Bearing in mind the modular structure of these proteins, it is not surprising that while the DNA-binding domains of the yeast and basidiomycete proteins are quite similar, the rest of the proteins may include domains with very different functions. Rather than recruiting other proteins to form a functional repressor or activator complex, the basidiomycete proteins would seem to have incorporated within themselves an activation or a repressor domain appropriate for regulation of the downstream pathway.
The homeodomain sequences are sufficient for highly specific recognition of target sites in vitro. However, the a1 homeodomain has no affinity for the target site in the absence of α2. The α2 protein has a 20-amino-acid carboxy-terminal tail which interacts directly with the a1 homeodomain to effect a conformational change in the a1 homeodomain; in cooperation with α2, the a1 homeodomain binds DNA tightly and provides the major part of the binding specificity (73). Similarly, the constitutively active fusion protein from C. cinereus requires only the HD2 homeodomain for DNA binding. Binding of the a1-α2 heterodimer causes a pronounced bend in the DNA, making additional protein-DNA contacts possible (68). Interestingly, if the 20-amino-acid tail of α2 is fused to the end of the a1 homeodomain, the α2 homeodomain is no longer essential and the single a1 homeodomain binds DNA with the same affinity and specificity as both protein domains together (123).
Interestingly, interaction of the α2 protein with a totally different partner, the general transcription factor MCM1, permits it to bind a different DNA target site and to regulate a different subset of genes (112). In α cells, the α2-MCM1 complex binds upstream of genes expressed only in haploid a cells and acts as a transcriptional repressor to ensure that only α cell-type functions are expressed in haploid α cells.
Although the HD1 homeodomain does not appear to be required for DNA binding in the basidiomycetes (2, 70), this sequence is very highly conserved in the A proteins of both C. cinereus and S. commune. This would not be expected if the homeodomain sequence were truly redundant. The HD1 homeodomain probably binds or contacts the DNA target sequence to some extent, because alterations at critical positions within the homeodomain lead to loss of A-regulated development, indicating that mutant residues interfere with normal binding of the heterodimer (2, 107). It is interesting to speculate whether the basidiomycete HD1 proteins, like the α2 protein in S. cerevisiae, may have other regulatory functions not requiring association with HD2 proteins.
PHEROMONES AND RECEPTORS
Pheromone Response Pathway in Yeasts
Signaling by means of pheromones plays an important role in mating in basidiomycetes, and the genes that encode pheromones and receptors are sequestered in the second (a/B) mating-type locus. Although not encoded by mating-type genes, similar pheromones and receptors are found in S. cerevisiae. Yeast, in fact, provides us with the most completely understood model of how mating pheromones and receptors interact with one another to stimulate an intracellular signaling cascade which ultimately activates genes required for mating competence. Many of the important components of this signaling pathway were originally identified by classical genetic analysis of sterile (ste) mutants that were unable to mate. The pheromone response pathway in yeast has been extensively reviewed (66, 67, 75, 121; for the most recent review, see reference 8), and here we shall simply present an overview appropriate as an introduction to the signaling pathway in basidiomycetes.
A yeast cell produces a single type of pheromone depending on its mating type as well as the receptor for the pheromone produced by cells of the opposite mating type. Thus, a cells produce a-factor (pheromone) and the α-factor receptor (encoded by STE2), while α cells produce α-factor and the a-factor receptor (STE3). Fusion can occur only between yeast cells of opposite mating types. After initial pheromone reception, the unicellular yeasts form a protrusion in the direction of the pheromone source, and this elongated cell is referred to as a schmoo. Pheromone receptors and agglutinins (proteins which facilitate cell adhesion) concentrate at the tip of the protrusion, where cell fusion eventually occurs. Similarly, in the unicellular yeast-like sporidia of U. maydis, secretion and concomitant reception of pheromones induce the formation of conjugation tubes (118), which extend along a gradient of pheromones produced by sporidia of the opposite mating type (113).
In contrast to the basidiomycete life cycle, nuclear fusion follows immediately after cell fusion in S. cerevisiae. To ensure that fusing nuclei will be at the same stage of the cell cycle, the pheromone response induces cell cycle arrest (at the G1 phase) in mating cells. Because of this, mutations which constitutively activate the pheromone response pathway in yeast ultimately lead to cell death. Recently, mutant yeast lines in which the pheromone response no longer induces cell cycle arrest have been constructed to examine such constitutively acting mutations (48, 92).
In some instances, yeast cells undergo pheromone stimulation and concomitant cell cycle arrest but do not fuse with a compatible mate. To prevent cell death, a variety of mechanisms are involved in adaptation to and recovery from pheromone stimulation. Both haploid cell types produce proteases which degrade pheromone to prevent further stimulation of unmated cells. In addition, activated pheromone receptors can be silenced via ligand-induced hyperphosphorylation of the carboxy-terminal tail or removed from the cell membrane altogether via ligand-induced endocytosis. Further downstream, the Sst2 protein is involved in silencing the Gα protein involved in the intracellular signaling cascade. Thus, by suppressing the pheromone response pathway, unmated cells which have been stimulated by pheromone can resume the normal haploid life cycle.
The two yeast pheromones, a-factor and α-factor, are processed and secreted by very different pathways. α-factor is a 13-amino-acid peptide produced by proteolytic cleavage of two large precursors, encoded by the genes MFα1 and MFα2, which consist of four and two repeats, respectively, of the α-factor peptide separated by cleavage sites. Five of the pheromone repeats are identical, but one of the MFα2 pheromones differs by two amino acids. The α-factors are glycosylated and secreted from α cells via the normal vesicle-based secretory pathway.
In contrast, active a-factor is a mixture of two 12-amino-acid lipopeptides which differ at a single residue. Large precursor proteins are encoded by the MFa1 and MFa2 genes and consist of the 12-amino-acid pheromone sequence, a large amino-terminal region, and a CaaX motif at the carboxyl terminus, where C is cysteine, a is aliphatic, and X is one of several amino acids (21). Posttranslational processing includes farnesylation of the cysteine residue, removal of the terminal 3 amino acids, carboxymethylation of the now carboxy-terminal cysteine, and, finally, removal of the amino-terminal precursor region. a-factor is externalized via the Ste6p transporter protein, which is located in the cell membrane and actively transports the pheromone outside the cell (60, 78).
Yeast pheromone receptors belong to the rhodopsin-like superfamily of seven transmembrane (7-TM) receptors which couple to heterotrimeric guanine nucleotide-binding proteins (G-proteins) to effect intracellular signaling (Fig. 7). Although the two yeast receptors are quite dissimilar in primary sequence, probably because the pheromones they recognize are of different classes, they have a common tertiary structure, namely, a short amino-terminal extracellular domain, seven hydrophobic α-helices (the transmembrane domains), three extracellular and three intracellular loops, and a long carboxy-terminal intracellular tail. This receptor tertiary structure is shared by pheromone receptors in other fungal systems.
FIG. 7.
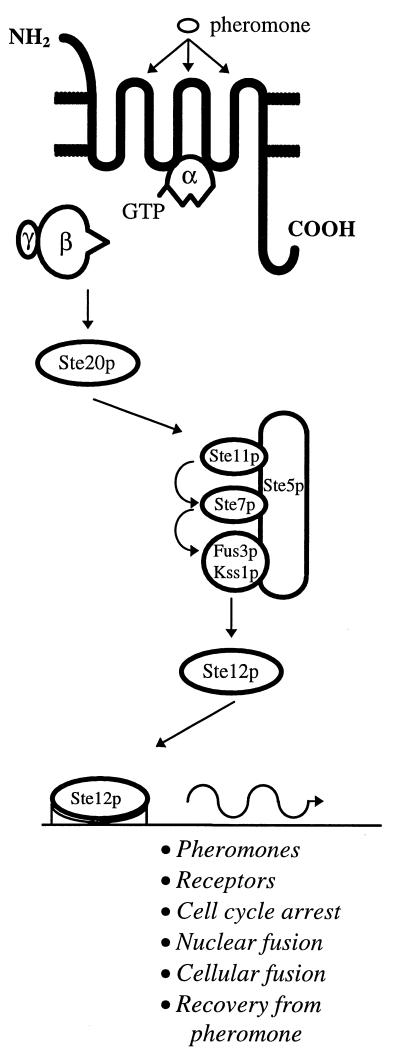
Overview of the pheromone response pathway in S. cerevisiae. The pheromone binds to the extracellular loops of the receptor. The heterotrimeric G-protein is entirely membrane bound throughout the signalling process but is drawn free for simplicity. The Gα subunit is anchored to the cell membrane and interacts with the receptor through its carboxy-terminal domain and with the Gβγ subunit through its amino-terminal domain. Gγ is responsible for anchoring Gβγ to the cell membrane, whereas Gβ interacts with the Gα subunit (inactive state) and activates Ste20p (active state). Ste5p acts as a scaffold to anchor the members of the MAP kinase module. Ste12p binds to PREs in the promoters of a wide range of genes.
The same G-protein binds to both yeast receptors and consists of three subunits, α, β, and γ, although the β and γ subunits act together as a single entity. The entire G-protein complex is entirely membrane bound via the α and γ subunits throughout receptor activation and intracellular signaling. The Gα subunit interacts with the second and third intracellular loops of the 7-TM receptor through its carboxy-terminal domain and with the β subunit through its amino-terminal domain (reviewed in reference 17).
In its inactive conformation, the Gα subunit is bound to the Gβγ subunit and to a GDP moiety and is not associated with the receptor. On pheromone stimulation, the receptor undergoes a conformational change which allows it to associate with Gα. The GDP bound to Gα is replaced with GTP, which induces a conformational change in the Gα subunit that releases the Gβγ subunit. The Gβγ subunit is then free to stimulate the downstream member of the signaling cascade, thought to be the Ste20 protein. Signaling through the Gβγ subunit was thought to be a peculiarity of the yeast mating-type pathway, since G-protein signaling in eukaryotic systems usually occurs through the Gα subunit but has since been shown to occur in a variety of different systems (18, 32, 81, 132). Finally, GTP is converted to GDP through the intrinsic GTPase activity of the Gα subunit, which allows the Gα subunit to disassociate from the receptor and to reassociate with and silence the active Gβγ subunit.
The downstream signaling pathway involves a mitogen-activated protein (MAP) kinase module. Ste20p is a serine/threonine kinase which triggers the activation of a MAP kinase cascade consisting of a MAP kinase kinase kinase (Ste11p), a MAP kinase kinase (Ste7p), and two MAP kinases (Fus3p and Kss1p). The MAP kinase module is tethered via the Ste5p scaffold protein (74). Either of the MAP kinases can phosphorylate the transcription factor Ste12p, which interacts with other transcription factors to activate transcription of genes in response to pheromone. These include genes required for cell cycle arrest (e.g., cyclins), for cellular and nuclear fusion (e.g., agglutinins), and for recovery from pheromone (e.g., Sst2p); in addition, the pheromones and receptor genes themselves are upregulated in response to pheromone.
Pheromone Response Pathway in Basidiomycetes
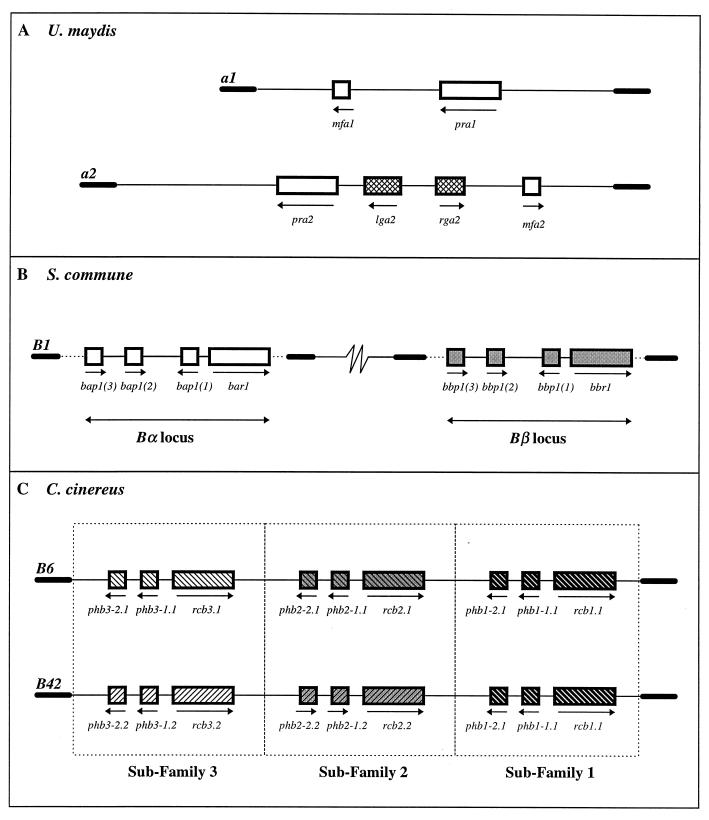
Our three model species present us with three levels of complexity in terms of the numbers of genes found at the mating-type loci that encode the mating pheromones and receptors. U. maydis, like S. cerevisiae, has only two versions of pheromones and receptors (Fig. 8A). The two alleles of the a locus, a1 and a2, consist of very dissimilar DNA sequences bordered by regions of homology, and each locus contains two genes that encode a mating-type-specific pheromone and the corresponding receptor (16). Genes encoding pheromone receptors are designated pra1 and pra2, and genes encoding pheromones, or mating factors, are designated mfa1 and mfa2. In addition, the a2 locus contains two genes, lga2 and rga2, whose functions are unknown but which are activated by the pheromone response pathway (128).
FIG. 8.
Molecular structure and organization of mating-type loci encoding pheromones and pheromone receptors. (A) The a1 locus of U. maydis spans ∼4.5 kb and contains two genes, mfa1 (encoding the a1 pheromone) and pra1 (encoding the receptor). The a2 locus spans ∼8 kb and contains the corresponding genes, mfa2 (encoding the a2 pheromone) and pra2 (encoding the receptor). Two additional genes with unknown function, lga2 and rga2, reside in the a2 locus. Arrows indicate the directions of gene transcription, and thick lines indicate the homologous flanking regions bordering the mating-type-specific DNA sequences. (B) Each locus of S. commune spans ∼8 kb of allele-specific DNA sequence which contains four genes, three (small boxes) encoding pheromone precursors and one (large box) encoding a receptor. The Bα genes are shown as open boxes and the Bβ genes are shown as solid boxes to indicate that they belong to two functionally independent subfamilies. The Bα and Bβ loci may be separated by up to 3.5 map units (97). Dashed lines indicate that the borders of the homologous region have not yet been defined. (C) The B locus of C. cinereus spans ∼17 kb of locus-specific DNA sequence that contains three subfamilies of functionally independent genes represented by the open, grey, and solid boxes. Small boxes represent pheromone genes, and large boxes represent receptor genes. The B6 and B42 loci encode different alleles of the genes in subfamilies 2 and 3, as indicated by the upward or downward diagonals. The alleles of the genes in subfamily 1 are shared.
In S. commune, the B mating-type genes are separated into two discrete loci, Bα and Bβ (90) (Fig. 8B). Recombination can theoretically combine one of nine Bα alleles with any one of nine Bβ alleles to generate a potential 81 different B mating specificities. Recombination between the two loci does not lead to self-compatible responses, indicating that the Bα and Bβ genes are functionally independent. In addition, genes at the two loci are functionally redundant, in that compatible mates may share alleles of one locus; i.e., a cross between Bα1β1 and Bα1β2 strains will be fully compatible. This is similar to the situation found with the A mating-type genes, where two loci contain functionally independent yet redundant sets of genes.
Based on the sequence of one locus of each type (Bα1 [131] and Bβ1 [130]) it appears that each contains a receptor (bar, bbr) and three pheromone (bap, bbp) genes. Since the Bα and Bβ loci appear to represent two independent subfamilies of genes, it is convenient to consider the sets of genes as functional cassettes which must remain undisturbed and unbroken. A pheromone with a given Bα specificity can stimulate Bα receptors from other cassettes but cannot stimulate its own resident receptor or any Bβ receptors. Likewise, a Bβ pheromone can stimulate other Bβ receptors except its own and cannot activate Bα receptors. Interestingly, any one receptor must be able to recognize and respond to a large number of pheromones yet be able to discriminate between them so as not to be activated by its own.
Unlike S. commune, the B mating-type genes of C. cinereus are sequestered into a single locus encompassing approximately 17 kb unique to each specificity (87) (Fig. 8C). Like the A locus, the B locus derives its many specificities from three sets of functionally independent genes. Again, each of these sets of genes belongs to an independent subfamily, and each allele within a subfamily consists of a “cassette” of one receptor and two pheromone genes. Two mates need only contain a different (allelic) cassette within one subfamily to be compatible. Like S. commune, the pheromones from a single subfamily can stimulate only all the receptors within that subfamily except, of course, their own. It is not known how many different cassettes of genes occur in each subfamily, but four or five different alleles of each would be sufficient to generate the estimated 79 B mating specificities of C. cinereus.
Transformation studies have shown that in C. cinereus, introduction of a single nonself pheromone or receptor gene into a mating where the two mates would otherwise encode the same B specificity is sufficient to trigger B-regulated development. A far more extensive analysis in S. commune has tested each of the Bα1 and Bβ1 genes in hosts having each of the other eight Bα and Bβ specificities. Not all of the pheromone-receptor combinations were active, yet all the genes could be shown to be functional in at least some backgrounds (130, 131). Although the levels of functional redundancy within any one locus appear to be excessive, they are obviously necessary to achieve the remarkable precision required for mating recognition.
It is interesting that all basidiomycete pheromones so far described, including pheromones from Cryptococcus neoformans (80) and Rhodosporidium toruloides (49), as well as those from our model species, encode CaaX motifs at the carboxy terminus of the protein. It is thus not surprising that the pheromone receptors from these fungi are more similar in primary sequence to the a-factor receptor of S. cerevisiae, which responds to the CaaX-modified a-factor. All of these fungal pheromones encode relatively large precursor molecules, which are processed to small peptides of 9 to 15 amino acids (15, 129). Where studied, further maturation of the pheromones is known to include, among other modifications, the addition of a farnesyl group to the carboxy-terminal cysteine residue, which aids membrane localization and may direct the pheromones to a transmembrane transporter protein (21). Interestingly, a gene encoding a protein with predicted homology to multidrug transporters has been identified within the B locus of C. cinereus (39).
The specificity of the pheromone-receptor interaction is truly remarkable. Somehow, receptors can distinguish between pheromones to the extent that they reject pheromones encoded within the same locus yet are stimulated by compatible pheromones from within their own subfamily. Sequence analysis of pheromones from the same and different subfamilies does not immediately suggest a mechanism by which pheromone discrimination is effected (Fig. 9), but it is likely that the secondary or tertiary structure of the receptors plays an important role in mediating specificity. An alignment of the C. cinereus B6 pheromone precursors reveals a conserved ER/DR amino acid motif, which may represent the cleavage site used to generate the short mature peptides (87). Significantly, if we examine the sequences of the S. commune pheromones (130), we can see that they also contain a similar charged amino acid motif (ER/DR/EH) at a comparable position.
FIG. 9.
Predicted amino acid sequences of pheromone precursors encoded by genes of the B mating-type loci of C. cinereus and S. commune. The amino acid sequences encoded by the six genes in the B6 locus of C. cinereus (Phb 1.1, Phb 1.2, Phb 2.1, Phb 2.2, Phb 3.1, and Phb 3.2) and the six genes in the Bα1 [Bap1(1), Bap1(2), and Bap1(3)] and Bβ1 [Bbp1(1), Bbp1(2), and Bbp1(3)] loci of S. commune have been aligned. Each sequence encodes a carboxy-terminal CaaX motif (CVIA/CVIS/CVCH/CVRG/CVVA) which acts as a signal for post-translational isoprenylation, and most encode a two-residue charged motif (ER/DR/EH/NH, indicated by arrows) that may be a signal for cleavage to generate the mature peptide pheromone. The two pheromones [Bap1(1) and Bbp1(2)] which lack this doubly charged motif have a comparable motif (SR/NR) at a similar position.
Chimeric experiments performed with the yeast Ste2p receptor and some human receptors indicate that specificity determinants reside in the first and third extracellular loops (109), implying that the receptor forms a three-dimensional structure within the membrane that creates a “pocket” lined with residues involved in pheromone reception. However, the precise residues involved have not been delineated for fungal receptors, and the mushroom fungi offer us a wonderful tool for exploring this specificity. Receptors which have different specificities but which belong to the same subfamily may have over 90% sequence similarity (131; our unpublished data). Most of the unconserved differences reside in the third extracellular loop, indicating that this loop is probably involved in pheromone reception. Chimeras generated from these receptors may resolve the precise regions of the receptor that determine specificity.
As yet, little is known about the downstream pheromone response pathway in basidiomycetes. It is thought to be analogous to that of S. cerevisiae, since a Ste7p homolog (Fuz7) (10), a Gα homolog (101), and a transcription factor analogous in function to Ste12p (Prf1, for pheromone response factor) (40) have recently been identified in U. maydis. Interestingly, an alignment of the three C. cinereus B6 receptors shows good conservation of the sequence of the third intracellular loop, perhaps indicating that, as in yeast, all pheromone receptors may bind to the same G-protein. However, a significant difference between the S. cerevisiae and basidiomycete pheromone response pathway is that activation of the MAP kinase pathway appears to occur through the Gα rather than through the Gβγ subunit, in common with other eukaryotic systems including that of the ascomycete Schizosaccharomyces pombe (85).
Self-Compatible Mutations
Although single mutations have never given rise to new B mating specificities within the mushroom fungi, such mutations may give rise to self-compatible phenotypes. Several self-compatible mutations have been mapped to the B mating-type locus (41, 54, 56, 89, 96). A monokaryon harboring such a mutation is able to form a dikaryon when mated to a strain which carries different A gene alleles but the same mutated or wild-type version of the B genes. Self-compatible mutations have been identified in both the Bα and Bβ loci of S. commune, and all mutants resemble the common A heterokaryon in having a “flat” phenotype caused by activation of the pheromone response pathway. Similar self-compatible mutations have been identified in C. cinereus, although, as with the common A heterokaryon in this species, there is no distinctive phenotype. These B mutations await analysis but, by mapping to the genes of the B loci, probably give rise to altered receptors or pheromones. Such mutations would be lethal in S. cerevisiae because a constitutively activated pheromone response pathway would lead to cell cycle arrest and cell death; they are viable in the basidiomycetes because the pheromone response is necessary for dikaryotic growth.
It will be exciting to see if any of the mutations parallel those found in genes encoding mammalian 7-TM receptors which alter the conformation of the receptor such that constitutive activation or repression of the G-protein occurs (106, 115, 126). Inappropriate signaling from such 7-TM receptors is responsible for several human diseases such as retinitis pigmentosa, color blindness, nephrogenic diabetes insipidus, and hyperthyroidism (reviewed in references 95 and 119).
COORDINATING THE ACTIVITIES OF MATING-TYPE GENES
Pheromone Response Element in U. maydis
Growth of the basidiomycete dikaryon requires the coordinated activities of genes activated by the pheromone response and those regulated by the newly formed homeobox transcription factor. We now have to consider how this may be achieved. The coordinated effects of the mating-type genes on downstream targets has been most widely studied in U. maydis.
Unmated sporidia of U. maydis produce only low levels of pheromone and receptor gene transcripts, but after reception of compatible pheromones from another sporidium, transcription of these genes increases dramatically (10- to 50-fold) (128). Pheromone stimulation also leads to induction of other genes whose transcripts are barely detectable in unmated sporidia. These include lga2 and rga2 (Fig. 8A), but, most significantly, expression of the b mating-type genes is also upregulated in response to pheromone (40, 128).
Pheromone-inducible gene expression is mediated by the presence of pheromone response elements (PREs) in the promoters of target genes. The consensus PRE repeat sequence is ACAAAGGGA and is sufficient to confer pheromone inducibility when placed within a totally unrelated gene promoter. The function of this element is orientation independent, as is typical of many sequences that can enhance gene transcription at a distance, but it appears that multiple copies are required (128).
PREs are found in promoters of S. cerevisiae pheromone-induced genes and also in those of the fission yeast Schizosaccharomyces pombe. The U. maydis element matches more closely the TR-box of S. pombe, a 10-bp consensus sequence, TTCTTTGTTY (or, inverted, RAACAAAGAA), that is bound by the Ste11 protein (1, 124) rather than the (A)TGAAACA element bound by the S. cerevisiae Ste12p transcription factor (29, 31). Although they apparently have similar functions, Ste12p and Ste11 belong to different families of transcription factors; Ste12p is considered to be a degenerate member of the homeodomain family (136), whereas Ste11 belongs to the HMG-box family (high-mobility group) of DNA-binding proteins (38, 124). With this in mind, efforts were made to look for a gene encoding an HMG-box protein in U. maydis that might have a function analogous to that of Ste11; this led to the isolation of the prf1 gene (pheromone response factor 1) (40).
The prf1 gene encodes an HMG-box protein that specifically binds U. maydis PREs in vitro. This gene is expressed at low levels in haploid cells but is induced approximately 20-fold after pheromone stimulation. There are two PREs about 700 bp upstream of prf1 itself, indicating that Prf1 regulates its own transcription in response to pheromone stimulation. Evidence indicates that Prf1 function is required both for the basal levels of transcription of the receptor and pheromone genes found in unmated cells and for the induced levels that occur on pheromone stimulation. Accordingly, if prf1 is inactivated, sporidia are sterile, because they cannot express any pheromones or receptors at all (40).
The presence of PREs in the b locus and their resultant pheromone inducibility has identified a role for Prf1 in b gene expression. It also suggests how a and b mating-type gene activity in U. maydis may be coordinated during sexual development. prf1 mutants are not only sterile but also nonpathogenic; they are unable to produce dikaryotic filaments or tumors, two functions that require an active bE-bW heterodimer. It is known that pheromone activity in itself is not required for pathogenicity, since transgenic sporidia which encode components of a functional heterodimer but only one pheromone-receptor pair can induce tumors on host plants without mating (9). In addition, by constitutively activating the transcription of b genes, filamentous growth and pathogenicity can be restored to prf1 mutant dikaryons (40). Thus, it appears that Prf1 is essential and plays a pivotal role in coordinating the regulation of both the a and b genes in U. maydis.
The model that has been presented (40, 113, 128) is that sporidia secrete low levels of pheromones that enable them to sense a compatible mating partner. Once partner recognition is established by pheromone binding to the appropriate receptor, the pheromone response pathway amplifies the signal through induction of prf1. At the same time, induction of bE and bW (via Prf1) prepares cells for the switch from sporidial to dikaryotic growth. Binding of the bE-bW heterodimer to its specific target sites promotes the growth of a filamentous dikaryon and eventual differentiation into diploid teliospores. Dikaryotic growth is thus dependent on elevated Prf1 levels and concomitant b gene expression.
Regulation in the Mushroom Fungi
The U. maydis model is not entirely applicable to the mushroom fungi because pheromone signaling is not required to attract mates or to promote mating structures in the already filamentous mating partners. A compatible B gene interaction activates the pheromone response only after cell fusion (130; our unpublished data). A gene transcription is not pheromone dependent and is constitutive in monokaryons, with no evidence for increased transcription levels being required to maintain the dikaryon (102). Nonetheless, pheromone stimulation plays an essential role in maintaining the organized growth of the dikaryon; B gene function is required for clamp cell fusion, a process that resembles in many respects the mating of two haploid sporidia of U. maydis. Therefore, while pheromone stimulation is not required before the initial fusion between two hyphae, it is required for fusion of cells within the dikaryon once it has formed. However, some species such as Coprinus congregatus have dikaryotic mycelia which lack clamp connections yet still require the activities of both sets of mating-type genes for maintenance of the dikaryon. Despite the slight peculiarities of the different fungal species, it is unlikely that U. maydis and the mushroom fungi have evolved markedly different ways of regulating dikaryotic growth. Now that the complexity of the mushroom mating-type loci has been resolved, the free-living dikaryon offers us a wonderful opportunity to combine the tools of molecular biology and microscopy to finally determine how mating-type genes act together to coordinate dikaryotic growth.
It is always interesting to ponder why the prolonged dikaryotic phase exists in the basidiomycete life cycle. In S. commune, matings between monokaryons having the recessive dik mutation do not form a typical dikaryon; instead, the compatible nuclei fuse almost immediately to give a diploid mycelium that has uninucleate cells and no clamp connections but that can differentiate normal fruit bodies at the appropriate time (55). Species such as Armellaria mellea, the honey fungus, produce diploid rather than dikaryotic cells in the long rhizomorphs that infect trees (57). Thus, there appears to be no barrier to maintaining a diploid nucleus within the mated filaments; unfortunately, nothing is yet known about the genes responsible for keeping compatible nuclei apart.
SOME GENERAL CONCLUSIONS
A study of mating recognition in the basidiomycetes has drawn attention to the remarkable conservation of the mechanisms that regulate sexual development in these fungi and the unicellular yeasts. We have seen how the HD1 and HD2 proteins of the basidiomycete fungi resemble the a1 and α2 homeodomain mating-type proteins of S. cerevisiae. Transcription factors are modular in structure, having separate activation/repressor domains, dimerization domains, and DNA-binding domains. The a1 and α2 proteins act as transcriptional repressors but recruit other proteins to fulfill this function. It is not yet known how the homeodomain proteins of the basidiomycetes regulate transcription, but putative activation domains have been described in the HD1 proteins of C. cinereus (2).
We have also described how S. cerevisiae identifies a compatible mating partner by means of mating pheromones and how the transduced signal makes cells competent to mate. U. maydis and presumably the other basidiomycetes more closely resemble S. pombe in transmitting the signal via the α subunit of the G-protein and effecting the pheromone response with a member of the HMG-box family rather than an Ste12p homolog. While it is clear that pheromone stimulation leads to phosphorylation of Ste12p in S. cerevisiae (114), this has not been shown for Ste11, and the situation is complicated by the fact that Ste11 is involved in regulating genes in response to nitrogen starvation (124), a metabolic trigger that induces mating in S. pombe. There is evidence that nitrogen starvation also affects mating in U. maydis (11), so it will be interesting to see whether Prf1 is also implicated in nitrogen regulation (40). It is not clear whether Prf1 is the direct target for phosphorylation by the pheromone response pathway (as is Ste12p) or whether there is an alternative target that must interact with Prf1.
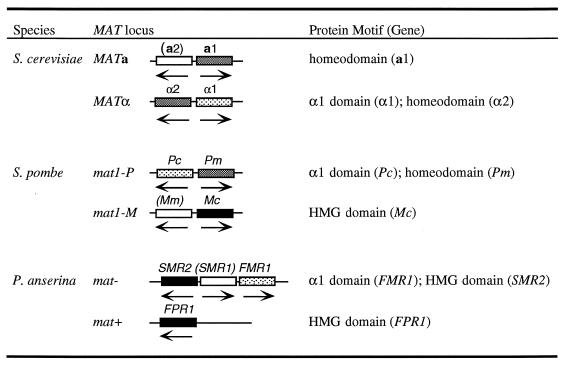
The mechanisms of recognition and response to a compatible mating partner are thus highly conserved among the unicellular ascomycetes, the hemibasidiomycetes, and the homobasidiomycetes; they are also conserved in the filamentous ascomycetes such as Neurospora crassa and Podospora anserina. Although all these fungi have dedicated mating-type loci, the genes found in these loci may not at first appear to be related. Table 1 summarizes what we currently know about the genes in the alternate mating-type loci of the two ascomycete species we have mentioned and the filamentous ascomycete fungi represented by P. anserina (similar genes are present in the equivalent mating type loci of N. crassa) (37).
TABLE 1.
Mating-type loci and mating-type proteins of ascomycetesa
These ascomycetes have only one mating-type locus, and the genes within each allelic version of the locus are mating-type specific. The mat− locus of P. anserina contains three genes, two of which have identifiable motifs that mark them as transcriptional regulators (27). One encodes a protein with a motif that is conserved in the α1 mating-type protein of S. cerevisiae and in the Pc mating-type protein of S. pombe (84). In both S. cerevisiae and S. pombe, the α1 domain protein binds DNA cooperatively with another protein (MCM1 and its homolog Map3, respectively) to activate the transcription of haploid mating-type specific genes (e.g., pheromone and pheromone receptors) (28, 42, 46, 84). As yet, no targets have been found for the protein in P. anserina or N. crassa. Both mating-type loci of P. anserina encode proteins with HMG domains. The P. anserina mat+ FPR1 HMG domain identifies it as a likely homolog of the S. pombe Mc protein (30), which determines M cell-type-specific gene expression (53), but the sequence of the P. anserina mat− SMR2 HMG domain suggests that this protein may be a homolog of Ste11, which could imply a role in regulating a pheromone response.
Pheromones undoubtedly play a role in mating in filamentous ascomycetes, but the genes that encode these have not been identified. Unlike the basidiomycetes, some species such as P. anserina and N. crassa differentiate special cells for mating. Circumstantial evidence suggests that male and female cells are attracted by diffusible pheromones (13). Like the basidiomycetes, nuclear fusion is delayed and a short-lived dikaryotic mycelium develops (the ascogenous hyphae) with binucleate cells and clamp connections (croziers) within the developing fruit body. We have seen how the coordinated division and distribution of nuclei in the basidiomycete dikaryon requires the activity of pheromones, and so it is significant that mutations in the mating-type genes of P. anserina interfere with regular nuclear pairing in the ascogenous hyphae and result in abnormal meiosis and sporulation (138).
In conclusion, the mating processes of the fungi have been particularly valuable for determining the individual steps in cell signaling pathways and for demonstrating the critical role that protein-protein interactions play in influencing DNA target site selection and hence gene transcription. In reviewing our current knowledge of these processes in basidiomycete fungi, we have shown how partner recognition demands a great degree of specificity on the part of the proteins and peptides involved. The protein families are ancient, and we find their homologs in all eukaryotic organisms. In particular, the mammals have evolved exceptionally large families of homeodomain proteins and 7-TM receptors. The basidiomycetes provide us with a marvelous genetic system in which to ask how protein structure, function, and specificity have coevolved—a question that has far-reaching biological relevance, particularly in clarifying the roles of similar proteins in the development of organisms such as humans.
ACKNOWLEDGMENTS
We acknowledge John Halsall for contributing unpublished data on the B42 locus of C. cinereus. We also thank our colleagues Tom Brutnell, Mike Milner, and John Halsall for critical comments on the manuscript.
Work in our laboratory is supported by the Biotechnology and Biological Sciences Research Council and the Gatsby Charitable Foundation. L.A.C. is a BBSRC Senior Research Fellow, and N.S.O. is supported by a Rhodes Scholarship.
REFERENCES
- 1.Aono T, Yanai H, Miki F, Davey J, Shimoda C. Mating pheromone-induced expression of the mat1-Pm gene of Schizosaccharomyces pombe: identification of signalling components and characterization of upstream controlling elements. Yeast. 1994;10:757–770. doi: 10.1002/yea.320100607. [DOI] [PubMed] [Google Scholar]
- 2.Asante-Owusu R N, Banham A H, Böhnert H U, Mellor E J C, Casselton L A. Heterodimerization between two classes of homeodomain proteins in the mushroom Coprinus cinereus brings together potential DNA-binding and activation domains. Gene. 1996;172:25–31. doi: 10.1016/0378-1119(96)00177-1. [DOI] [PubMed] [Google Scholar]
- 3.Bakkeren G, Kronstad J W. Conservation of the b mating-type gene complex among bipolar and tetrapolar smut fungi. Plant Cell. 1993;5:123–136. doi: 10.1105/tpc.5.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakkeren G, Kronstad J W. Linkage of mating-type loci distinguishes bipolar from tetrapolar mating in basidiomycetous smut fungi. Proc Natl Acad Sci USA. 1994;91:7085–7089. doi: 10.1073/pnas.91.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banham A H, Asante-Owusu R N, Göttgens B, Thompson S A J, Kingsnorth C S, Mellor E J C, Casselton L A. An N-terminal dimerization domain permits homeodomain proteins to choose compatible partners and initiate sexual development in the mushroom Coprinus cinereus. Plant Cell. 1995;7:773–783. doi: 10.1105/tpc.7.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banuett F. Ustilago maydis, the delightful blight. Trends Genet. 1992;8:174–179. doi: 10.1016/0168-9525(92)90220-x. [DOI] [PubMed] [Google Scholar]
- 7.Banuett F. Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu Rev Genet. 1995;29:179–208. doi: 10.1146/annurev.ge.29.120195.001143. [DOI] [PubMed] [Google Scholar]
- 8.Banuett, F. Signalling in the yeasts: an informational cascade with links to the filamentous fungi. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 9.Banuett F, Herskowitz I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc Natl Acad Sci USA. 1989;86:5878–5882. doi: 10.1073/pnas.86.15.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banuett F, Herskowitz I. Identification of Fuz7, a Ustilago maydis MEK/MAPKK homologue required for a-locus dependent and a-locus independent steps in the fungal life cycle. Genes Dev. 1994;8:1367–1378. doi: 10.1101/gad.8.12.1367. [DOI] [PubMed] [Google Scholar]
- 11.Banuett F, Herskowitz I. Morphological transitions in the life cycle of Ustilago maydis and their genetic control by the a and b loci. Exp Mycol. 1994;18:247–266. [Google Scholar]
- 12.Baxevanis A D, Vinson C R. Interactions of coiled coils in transcription factors: where is the specificity? Curr Opin Genet Dev. 1993;3:278–285. doi: 10.1016/0959-437x(93)90035-n. [DOI] [PubMed] [Google Scholar]
- 13.Bistis G N. Evidence for diffusible mating-type-specific trichogyne attractants in Neurospora crassa. Exp Mycol. 1983;7:292–295. [Google Scholar]
- 14.Blakeslee A F. Sexual reproduction in the Mucorineae. Proc Am Acad Sci. 1904;40:206–319. [Google Scholar]
- 15.Bölker M, Kahmann R. Sexual pheromones and mating responses in fungi. Plant Cell. 1993;5:1461–1469. doi: 10.1105/tpc.5.10.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bölker M, Urban M, Kahmann R. The a mating type locus of U. maydis specifies cell signalling components. Cell. 1992;68:441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- 17.Bourne H R. How receptors talk to heterotrimeric G proteins. Curr Opin Genet Dev. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 18.Braselmann S, Palmer T M, Cook S J. Signalling enzymes: bursting with potential. Curr Biol. 1997;7:R470–R473. doi: 10.1016/s0960-9822(06)00239-9. [DOI] [PubMed] [Google Scholar]
- 19.Buller A H R. Researches on fungi. IV. London, United Kingdom: Longmans Green; 1931. [Google Scholar]
- 20.Bürglin T R. A comprehensive classification of homeobox genes. In: Duboule D, editor. Guidebook to the homeobox genes. Oxford, United Kingdom: Oxford University Press; 1994. pp. 25–72. [Google Scholar]
- 21.Caldwell G A, Naider F, Becker J M. Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol Rev. 1995;59:406–422. doi: 10.1128/mr.59.3.406-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casselton L A, Kües U. Mating type genes in homobasidiomycetes. In: Wessels J G H, Meinhardt F, editors. The Mycota. I. Growth, differentiation and sexuality. Berlin, Germany: Springer-Verlag KG; 1994. pp. 307–322. [Google Scholar]
- 23.Cohen C, Parry D A D. Proteins: structure, function and genetics. α-helical coiled coils and bundles: how to design an α-helical protein. Protein. 1990;7:1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- 24.Day P R. The structure of the A mating-type locus in Coprinus lagopus. Genetics. 1960;45:641–650. doi: 10.1093/genetics/45.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day P R. The structure of the A mating type factor in Coprinus lagopus. Wild alleles. Genet Res. 1963;4:323–325. [Google Scholar]
- 26.Day P R. Mutations affecting the A mating-type factor in Coprinus lagopus. Genet Res. 1963;4:55–64. [Google Scholar]
- 27.Debuchy R, Arnaise S, Lecellier G. The mat− allele of Podospora anserina contains three regulatory genes required for the development of fertilized female organs. Mol Gen Genet. 1993;241:667–673. doi: 10.1007/BF00279909. [DOI] [PubMed] [Google Scholar]
- 28.Dolan J W, Fields S. Cell-type-specific transcription in yeast. Biochim Biophys Acta. 1991;1088:155–169. doi: 10.1016/0167-4781(91)90051-m. [DOI] [PubMed] [Google Scholar]
- 29.Dolan J W, Kirkman C, Fields S. The yeast STE12 protein binds to the DNA sequence mediating pheromone induction. Proc Natl Acad Sci USA. 1989;86:5703–5707. doi: 10.1073/pnas.86.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooijes D, van de Wetering M, Knippels L, Clevers H. The Schizosaccharomyces pombe mating-type gene mat-Mc encodes a sequence-specific DNA-binding high mobility group box protein. J Biol Chem. 1993;268:24813–24817. [PubMed] [Google Scholar]
- 31.Errede B, Ammerer G. STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein-DNA complexes. Genes Dev. 1989;3:1349–1361. doi: 10.1101/gad.3.9.1349. [DOI] [PubMed] [Google Scholar]
- 32.Federman A D, Conklin B R, Schrader K A, Reed R R, Bourne H R. Hormonal stimulation of adenylyl cyclase through Gi-protein βγ subunits. Nature. 1992;356:159–161. doi: 10.1038/356159a0. [DOI] [PubMed] [Google Scholar]
- 33.Gehring W J, Qian Y Q, Billeter M, Furukubo-Tokunaga K, Schier A F, Resendez-Perez D, Affolter M, Otting G, Wüthrich K. Homeodomain-DNA recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 34.Gieser P T, May G. Comparison of two b1 alleles from within the A mating-type of the basidiomycete Coprinus cinereus. Gene. 1994;146:167–176. doi: 10.1016/0378-1119(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 35.Giesy R M, Day P R. The septal pores of Coprinus lagopus (Fr.) sensu Buller in relation to nuclear migration. Am J Bot. 1965;52:287–293. [Google Scholar]
- 36.Gillissen B, Bergemann J, Sandmann C, Schroeer B, Bölker M, Kahmann R. A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell. 1992;68:647–657. doi: 10.1016/0092-8674(92)90141-x. [DOI] [PubMed] [Google Scholar]
- 37.Glass N L, Nelson M A. Mating type genes in mycelial ascomycetes. In: Wessels J G H, Meinhardt F, editors. The Mycota. I. Growth, differentiation and sexuality. Berlin, Germany: Springer-Verlag KG; 1994. pp. 295–306. [Google Scholar]
- 38.Grosschedl R, Giese K, Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 39.Halsall, J. R., and P. T. Chaure. Personal communication.
- 40.Hartmann H A, Kahmann R, Bölker M. The pheromone response factor coordinates filamentous growth and pathogenicity in Ustilago maydis. EMBO J. 1996;15:1632–1641. [PMC free article] [PubMed] [Google Scholar]
- 41.Haylock R W, Economou A, Casselton L A. Dikaryon formation in Coprinus cinereus: selection and identification of B factor mutants. J Gen Microbiol. 1980;121:17–26. [Google Scholar]
- 42.Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988;52:536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holliday R. The genetics of Ustilago maydis. Genet Res. 1961;2:204–230. [Google Scholar]
- 44.Holliday R. Induced mitotic crossing-over in Ustilago maydis. Genet Res. 1961;2:231–248. doi: 10.1017/s0016672300003979. [DOI] [PubMed] [Google Scholar]
- 45.Iwasa, M., S. Tanabe, and T. Kamada. The two nuclei in the dikaryon of the homobasidiomycete Coprinus cinereus change position after each conjugate division. Fungal Genet. Biol. in press. [DOI] [PubMed]
- 46.Johnson A D. Molecular mechanisms of cell-type determination in budding yeast. Curr Opin Genet Dev. 1995;5:552–558. doi: 10.1016/0959-437x(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 47.Kahmann R, Romeis T, Bölker M, Kämper J. Control of mating and development in Ustilago maydis. Curr Opin Genet Dev. 1995;5:559–564. doi: 10.1016/0959-437x(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 48.Kajkowski E M, Price L A, Pausch M H, Young K H, Ozenberger B A. Investigation of growth hormone releasing hormone receptor structure and activity using yeast expression technologies. J Recept Signal Transduct Res. 1997;17:293–303. doi: 10.3109/10799899709036610. [DOI] [PubMed] [Google Scholar]
- 49.Kamiya Y, Sakurai A, Tamura S, Takahashi N, Abe K, Tsuchiya E, Fukui S, Kitada C, Fujino M. Structure of rhodotorucine A, a novel lipopeptide inducing mating tube formation in Rhodosporidium toruloides. Biochem Biophys Res Commun. 1978;83:1077–1083. doi: 10.1016/0006-291x(78)91505-x. [DOI] [PubMed] [Google Scholar]
- 50.Kämper J, Reichmann M, Romeis T, Bölker M, Kahmann R. Multiallelic recognition: nonself-dependent dimerization of the bE and bW homeodomain proteins in Ustilago maydis. Cell. 1995;81:73–83. doi: 10.1016/0092-8674(95)90372-0. [DOI] [PubMed] [Google Scholar]
- 51.Keleher C A, Redd M J, Schulz J, Carlson M, Johnson A D. SSN6/TUP1 is a general repressor of transcription in yeast. Cell. 1992;68:709–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 52.Kelly M, Burke J, Smith M, Klar A, Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988;7:1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kjaerulff S, Dooijes D, Clevers H, Nielsen O. Cell differentiation by interaction of two HMG-box proteins: Mat1-Mc activates M cell-specific genes in S. pombe by recruiting the ubiquitous transcription factor Ste11 to weak binding sites. EMBO J. 1997;16:4021–4033. doi: 10.1093/emboj/16.13.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koltin Y. The genetic structure of the incompatibility factors of Schizophyllum commune: comparative studies of primary mutations in the B factor. Mol Gen Genet. 1968;102:196–303. doi: 10.1007/BF00385974. [DOI] [PubMed] [Google Scholar]
- 55.Koltin Y, Raper J R. Dikaryosis: genetic determination in Schizophyllum. Science. 1968;160:85–86. doi: 10.1126/science.160.3823.85. [DOI] [PubMed] [Google Scholar]
- 56.Koltin Y, Stamberg J, Bawnik N, Tamarkin A, Werczberger R. Mutational analysis of natural alleles in and affecting the B incompatibility factor of Schizophyllum. Genetics. 1979;93:383–391. doi: 10.1093/genetics/93.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korhonen K, Hintikka V. Cytological evidence for somatic diploidization in dikaryotic cells of Armillariella mellea. Arch Microbiol. 1974;95:187–192. [Google Scholar]
- 58.Kronstad, J., G. Bakkeren, S. Gold, A. Yee, C. Laity, G. Duncan, K. Barrett, L. Giasson, R. Campbell, and G. Athwal. 1995. Control of filamentous growth by mating and cyclic-AMP in Ustilago. Can. J. Bot. 73(Suppl. 1):S258–S265.
- 59.Kronstad J W, Leong S A. The b mating-type locus of Ustilago maydis contains variable and constant regions. Genes Dev. 1990;4:1384–1395. doi: 10.1101/gad.4.8.1384. [DOI] [PubMed] [Google Scholar]
- 60.Kuchler K, Sterne R E, Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989;8:3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kües U, Casselton L A. Homeodomains and regulation of sexual development in basidiomycetes. Trends Genet. 1992;8:154–155. doi: 10.1016/0168-9525(92)90207-k. [DOI] [PubMed] [Google Scholar]
- 62.Kües U, Casselton L A. The origin of multiple mating types in mushrooms. J Cell Sci. 1993;104:227–230. [Google Scholar]
- 63.Kües U, Asante-Owusu R N, Mutasa E S, Tymon A M, Pardo E H, O’Shea S F, Göttgens B, Casselton L A. Two classes of homeodomain proteins specify the multiple A mating types of the mushroom Coprinus cinereus. Plant Cell. 1994;6:1467–1475. doi: 10.1105/tpc.6.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kües U, Göttgens B, Stratmann R, Richardson W V J, O’Shea S F, Casselton L A. A chimeric homeodomain protein causes self-compatibility and constitutive sexual development in the mushroom Coprinus cinereus. EMBO J. 1994;13:4054–4059. doi: 10.1002/j.1460-2075.1994.tb06722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kües U, Richardson W V J, Tymon A M, Mutasa E S, Göttgens B, Gaubatz S, Gregoriades A, Casselton L A. The combination of dissimilar alleles of the Aα and Aβ gene complexes, whose proteins contain homeodomain motifs, determines sexual development in the mushroom Coprinus cinereus. Genes Dev. 1992;4:568–577. doi: 10.1101/gad.6.4.568. [DOI] [PubMed] [Google Scholar]
- 66.Kurjan J. The pheromone response pathway in Saccharomyces cerevisiae. Annu Rev Genet. 1993;27:147–179. doi: 10.1146/annurev.ge.27.120193.001051. [DOI] [PubMed] [Google Scholar]
- 67.Leberer E, Thomas D Y, Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr Opin Genet Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- 68.Li T, Stark M R, Johnson A D, Wolberger C. Crystal structure of the MATa1/MATα2 homeodomain heterodimer bound to DNA. Science. 1995;270:262–269. doi: 10.1126/science.270.5234.262. [DOI] [PubMed] [Google Scholar]
- 69.Lukens L, Yicun H, May G. Correlation of genetic and physical maps at the A mating type locus of Coprinus cinereus. Genetics. 1996;144:1471–1477. doi: 10.1093/genetics/144.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo Y H, Ullrich R C, Novotny C P. Only one of the paired Schizophyllum commune Aα mating-type putative homeobox genes encodes a homeodomain essential for Aα regulated development. Mol Gen Genet. 1994;244:318–324. doi: 10.1007/BF00285460. [DOI] [PubMed] [Google Scholar]
- 71.Lupas A. Coiled coils—new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 72.Magae Y, Novotny C, Ullrich R. Interaction of the A alpha Y mating-type and Z mating-type homeodomain proteins of Schizophyllum commune detected by the two-hybrid system. Biochem Biophys Res Commun. 1995;211:1071–1076. doi: 10.1006/bbrc.1995.1920. [DOI] [PubMed] [Google Scholar]
- 73.Mak A, Johnson A D. The carboxy-terminal tail of the homeodomain protein α2 is required for function with a second homeodomain protein. Genes Dev. 1993;7:1862–1870. doi: 10.1101/gad.7.10.1862. [DOI] [PubMed] [Google Scholar]
- 74.Marcus S, Polverino A, Barr M, Wigler M. Complexes between STE5 and components of the pheromone-responsive mitogen-activated protein kinase module. Proc Natl Acad Sci USA. 1994;91:7762–7766. doi: 10.1073/pnas.91.16.7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marsh L, Neiman A M, Herskowitz I. Signal transduction during pheromone response in yeast. Annu Rev Cell Biol. 1991;7:699–728. doi: 10.1146/annurev.cb.07.110191.003411. [DOI] [PubMed] [Google Scholar]
- 76.May G, Le Chevanton L, Pukkila P J. Molecular analysis of the Coprinus cinereus mating type A factor demonstrated an unexpectedly complex structure. Genetics. 1991;128:529–538. doi: 10.1093/genetics/128.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 78.McGrath J P, Varchavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989;340:400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- 79.Moore R T, McAlear J H. Fine structure of Mycota. 7. Observations on septa of Ascomycetes and Basidiomycetes. Am J Bot. 1962;49:86–94. [Google Scholar]
- 80.Moore T D E, Edman J C. The α mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol Cell Biol. 1993;13:1962–1970. doi: 10.1128/mcb.13.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murthy K S, Coy D H, Makhlouf G M. Somatostatin receptor-mediated signalling in smooth muscle. J Biol Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 82.Mutasa E S, Tymon A M, Göttgens B, Mellon F M, Little P F R, Casselton L A. Molecular organisation of an A mating type factor of the basidiomycete fungus Coprinus cinereus. Curr Genet. 1990;18:223–229. [Google Scholar]
- 83.Niederpruem D J. Direct studies of dikaryotization in Schizophyllum commune. 1. Live intercellular migration patterns. Arch Microbiol. 1980;128:162–171. doi: 10.1007/BF00406154. [DOI] [PubMed] [Google Scholar]
- 84.Nielsen O, Friis T, Kjaerulff S. The Schizosaccharomyces pombe map1 gene encodes an SRF/MCM1-related protein required for P-cell specific gene expression. Mol Gen Genet. 1996;253:387–392. doi: 10.1007/pl00008604. [DOI] [PubMed] [Google Scholar]
- 85.Obara T, Nakafuku M, Yamamoto M, Kaziro Y. Isolation and characterization of a gene encoding a G-protein α subunit from Schizosaccharomyces pombe: involvement in mating and sporulation pathways. Proc Natl Acad Sci USA. 1991;88:5877–5881. doi: 10.1073/pnas.88.13.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Shea E K, Rutowski R, Kim P S. Mechanism of specificity in the Fus-Jun oncoprotein heterodimer. Cell. 1992;68:699–708. doi: 10.1016/0092-8674(92)90145-3. [DOI] [PubMed] [Google Scholar]
- 87.O’Shea, S. F., P. T. Chaure, J. H. Halsall, N. S. Olesnicky, A. Leibbrandt, I. F. Connerton, and L. A. Casselton. A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus. Genetics, in press. [DOI] [PMC free article] [PubMed]
- 88.Papazian H P. Incompatibility factors and a related gene in Schizophyllum commune. Genetics. 1951;36:441–459. doi: 10.1093/genetics/36.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parag Y. Mutations in the B incompatibility factor of Schizophyllum commune. Proc Natl Acad Sci USA. 1962;48:743–750. doi: 10.1073/pnas.48.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parag Y, Koltin Y. The structure of the incompatibility factors of Schizophyllum commune: constitution of the three classes of B factors. Mol Gen Genet. 1971;112:43–48. [Google Scholar]
- 91.Pardo E H, O’Shea S F, Casselton L A. Multiple versions of the A mating type locus of Coprinus cinereus are generated by three paralogous pairs of multiallelic homeobox genes. Genetics. 1996;144:87–94. doi: 10.1093/genetics/144.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Price L A, Kajkowski E M, Hadcock J R, Ozenberger B A, Pausch M H. Functional coupling of a mammalian somatostatin receptor to the yeast pheromone response pathway. Mol Cell Biol. 1995;15:6188–6195. doi: 10.1128/mcb.15.11.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puhalla J E. The formation of diploids of Ustilago maydis on agar medium. Phytopathology. 1969;59:1771–1772. [PubMed] [Google Scholar]
- 94.Puhalla J E. Genetic studies of the b incompatibility locus of Ustilago maydis. Genet Res. 1970;16:229–232. [Google Scholar]
- 95.Rao V R, Oprian D D. Activating mutations of rhodopsin and other G protein-coupled receptors. Annu Rev Biophys Biomol Struct. 1996;25:287–314. doi: 10.1146/annurev.bb.25.060196.001443. [DOI] [PubMed] [Google Scholar]
- 96.Raper C A. Controls for development and differentiation in basidiomycetes. In: Bennett J W, Ciegler A, editors. Secondary metabolism and differentiation in fungi. New York, N.Y: Marcel Dekker, Inc.; 1983. pp. 195–238. [Google Scholar]
- 97.Raper J R. Genetics of sexuality in higher fungi. New York, N.Y: Ronald Press; 1966. [Google Scholar]
- 98.Raper J R, Boyd D H, Raper C A. Primary and secondary mutations at the incompatibility loci in Schizophyllum. Proc Natl Acad Sci USA. 1965;53:1324–1332. doi: 10.1073/pnas.53.6.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raper J R, Krongelb G S, Baxter M G. The number and distribution of incompatibility factors in Schizophyllum commune. Am Nat. 1958;92:221–232. [Google Scholar]
- 100.Raper J R, Baxter M G, Ellingboe A H. The genetic structure of the incompatibility factors of Schizophyllum commune: the A factor. Proc Natl Acad Sci USA. 1960;46:833–842. doi: 10.1073/pnas.46.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Regenfelder E, Spellig T, Hartmann A, Lauenstein S, Bölker M, Kahmann R. G proteins in Ustilago maydis: transmission of multiple signals? EMBO J. 1997;16:1934–1942. doi: 10.1093/emboj/16.8.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richardson W V J, Kües U, Casselton L A. The A mating-type genes of the mushroom Coprinus cinereus are not differentially transcribed in monokaryons and dikaryons. Mol Gen Genet. 1993;238:304–307. doi: 10.1007/BF00279559. [DOI] [PubMed] [Google Scholar]
- 103.Ross I K. Nuclear migration rates in Coprinus congregatus: a new record? Mycologia. 1976;68:418–422. [Google Scholar]
- 104.Rowell J B. Functional role of compatibility factors: an in vitro test for sexual compatibility with haploid lines of Ustilago zeae. Phytopathology. 1955;45:370–374. [Google Scholar]
- 105.Rowell J B, De Vay J E. Genetics of Ustilago zeae in relation to basic problems of its pathogenicity. Phytopathology. 1954;44:356–362. [Google Scholar]
- 106.Scheer A, Fanelli F, Costa T, de Benedetti P G, Cotecchia S. Constitutively active mutants of the α1B-adrenergic receptor: role of highly conserved polar amino acids in receptor activation. EMBO J. 1996;15:3566–3578. [PMC free article] [PubMed] [Google Scholar]
- 107.Schlesinger R, Kahmann R, Kämper J. The homeodomains of the heterodimeric bE and bW proteins of Ustilago maydis are both critical for function. Mol Gen Genet. 1997;254:514–519. doi: 10.1007/pl00008609. [DOI] [PubMed] [Google Scholar]
- 108.Schulz B, Banuett F, Dahl M, Schlesinger R, Schäfer W, Martin T, Herskowitz I, Kahmann R. The b alleles of U. maydis, whose combinations programme pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell. 1990;60:295–306. doi: 10.1016/0092-8674(90)90744-y. [DOI] [PubMed] [Google Scholar]
- 109.Sen M, Marsh L. Noncontiguous domains of the α-factor receptor of yeasts confer ligand specificity. J Biol Chem. 1994;269:968–973. [PubMed] [Google Scholar]
- 110.Shen G P, Park D C, Ullrich R C, Novotny C P. Cloning and characterization of a Schizophyllum gene with Aβ6 mating-type activity. Curr Genet. 1996;29:136–142. doi: 10.1007/BF02221577. [DOI] [PubMed] [Google Scholar]
- 111.Shepherd J C W, McGinnis W, Carrasco A E, DeRobertis E M, Gehring W J. Fly and frog homeo domains show homologies with yeast mating type regulatory proteins. Nature. 1984;310:70–71. doi: 10.1038/310070a0. [DOI] [PubMed] [Google Scholar]
- 112.Smith D L, Johnson A D. A molecular mechanism for combinatorial control in yeast: MCM1 protein sets the spacing and orientation of the homeodomains of an α2 dimer. Cell. 1992;68:133–142. doi: 10.1016/0092-8674(92)90212-u. [DOI] [PubMed] [Google Scholar]
- 113.Snetselaar K M, Bölker M, Kahmann R. Ustilago maydis mating hyphae orient their growth toward pheromone sources. Fungal Genet Biol. 1996;20:299–312. doi: 10.1006/fgbi.1996.0044. [DOI] [PubMed] [Google Scholar]
- 114.Song O, Dolan J W, Yuan Y O, Fields S. Pheromone-dependent phosphorylation of the yeast STE12 protein correlates with transcriptional activation. Genes Dev. 1991;5:741–750. doi: 10.1101/gad.5.5.741. [DOI] [PubMed] [Google Scholar]
- 115.Spalding T A, Burstein E S, Brauner-Osbourne H, Hill-Eubanks D, Brann M R. Pharmacology of a constitutively active muscarinic receptor generated by random mutagenesis. J Pharmacol Exp Ther. 1995;275:1274–1279. [PubMed] [Google Scholar]
- 116.Specht C A, Stankis M M, Novotny C P, Ullrich R C. Mapping the heterogeneous DNA region that determines the 9 A alpha mating-type specificities of Schizophyllum commune. Genetics. 1994;137:709–714. doi: 10.1093/genetics/137.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Specht C A, Stankis M M, Giasson L, Novotny C P, Ullrich R C. Functional analysis of the homeodomain-related proteins of the Aα locus of Schizophyllum commune. Proc Natl Acad Sci USA. 1992;89:7174–7178. doi: 10.1073/pnas.89.15.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spellig T, Bölker M, Lottspeich F, Frank R W, Kahmann R. Pheromones trigger filamentous growth in Ustilago maydis. EMBO J. 1994;13:1620–1627. doi: 10.1002/j.1460-2075.1994.tb06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spiegel A M. Defects in G protein-coupled signal transduction in human disease. Annu Rev Physiol. 1995;58:143–170. doi: 10.1146/annurev.ph.58.030196.001043. [DOI] [PubMed] [Google Scholar]
- 120.Spit, A., R. Hyland, E. J. C. Mellor, and L. A. Casselton. Heterodimerization targets a homeodomain protein complex to the nucleus. Submitted for publication.
- 121.Sprague G F, Thorner J W. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. In: Broach J R, Pringle J R, Jones E W, editors. The molecular and cellular biology of the yeast Saccharomyces: gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 657–744. [Google Scholar]
- 122.Stankis M M, Specht C A, Yang H L, Giasson L, Ullrich R C, Novotny C P. The Aα mating locus of Schizophyllum commune encodes two dissimilar, multiallelic, homeodomain proteins. Proc Natl Acad Sci USA. 1992;89:7169–7173. doi: 10.1073/pnas.89.15.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stark M P, Johnson A D. Interaction between two homeodomain proteins is specified by a short C-terminal tail. Nature. 1994;371:429–432. doi: 10.1038/371429a0. [DOI] [PubMed] [Google Scholar]
- 124.Sugimoto A, Lino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- 125.Swiezynski K M, Day P R. Heterokaryon formation in Coprinus lagopus. Genet Res. 1960;1:114–128. [Google Scholar]
- 126.Tseng C-C, Lin L. A point mutation in the glucose-dependent insulinotropic peptide receptor confers constitutive activity. Biochem Biophys Res Commun. 1997;232:96–100. doi: 10.1006/bbrc.1997.6231. [DOI] [PubMed] [Google Scholar]
- 127.Tymon A M, Kües U, Richardson W V J, Casselton L A. A fungal mating type protein that regulates sexual and asexual development contains a POU-related domain. EMBO J. 1992;11:1805–1813. doi: 10.1002/j.1460-2075.1992.tb05232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Urban M, Kahmann R, Bölker M. Identification of the pheromone response element in Ustilago maydis. Mol Gen Genet. 1996;251:31–37. doi: 10.1007/BF02174341. [DOI] [PubMed] [Google Scholar]
- 129.Vaillancourt L J, Raper C A. Pheromones and pheromone receptors as mating type determinants in basidiomycetes. In: Setlow J K, editor. Genetic engineering. New York, N.Y: Plenum Press; 1996. pp. 219–247. [DOI] [PubMed] [Google Scholar]
- 130.Vaillancourt L J, Raudaskoski M, Specht C A, Raper C A. Multiple genes encoding pheromones and a pheromone receptor define the Bβ1 mating-type specificity in Schizophyllum commune. Genetics. 1997;146:541–551. doi: 10.1093/genetics/146.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wendland J, Vaillancourt L J, Hegner J, Lengeler K B, Laddison K J, Specht C A, Raper C A, Kothe E. The mating-type locus Bα1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone genes. EMBO J. 1995;14:5271–5278. doi: 10.1002/j.1460-2075.1995.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weng G, Li J, Dingus J, Hildebrandt J D, Weinstein H, Iyengar R. Gβ subunit interacts with a peptide encoding region 956-982 of adenylyl cyclase 2. J Biol Chem. 1996;271:26445–26448. doi: 10.1074/jbc.271.43.26445. [DOI] [PubMed] [Google Scholar]
- 133.Wessels J G H, Niederpruem D J. Role of a cell-wall glucan-degrading enzyme in mating of Schizophyllum commune. J Bacteriol. 1967;94:1594–1602. doi: 10.1128/jb.94.5.1594-1602.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whitehouse H L K. Multiple allelomorph heterothallism in the fungi. New Phytol. 1949;48:212–244. [Google Scholar]
- 135.Yee A R, Kronstad J W. Construction of chimeric alleles with altered specificity at the b incompatibility locus of Ustilago maydis. Proc Natl Acad Sci USA. 1993;90:664–668. doi: 10.1073/pnas.90.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yuan Y O, Fields S. Properties of the DNA-binding domain of the Saccharomyces cerevisiae STE12 protein. Mol Cell Biol. 1991;11:5910–5918. doi: 10.1128/mcb.11.12.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yue C L, Osier M, Novotny C P, Ullrich R C. The specificity determinant of the Y mating-type proteins of Schizophyllum commune is also essential for Y-Z protein binding. Genetics. 1997;145:253–260. doi: 10.1093/genetics/145.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zickler D, Arnaise S, Coppin E, Debuchy R, Picard M. Altered mating-type identity in the fungus Podospora anserina leads to selfish nuclei, uniparental progeny and haploid meiosis. Genetics. 1995;140:493–503. doi: 10.1093/genetics/140.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]