Abstract
In the oral cavity, indigenous bacteria are often associated with two major oral diseases, caries and periodontal diseases. These diseases seem to appear following an inbalance in the oral resident microbiota, leading to the emergence of potentially pathogenic bacteria. To define the process involved in caries and periodontal diseases, it is necessary to understand the ecology of the oral cavity and to identify the factors responsible for the transition of the oral microbiota from a commensal to a pathogenic relationship with the host. The regulatory forces influencing the oral ecosystem can be divided into three major categories: host related, microbe related, and external factors. Among host factors, secretory immunoglobulin A (SIgA) constitutes the main specific immune defense mechanism in saliva and may play an important role in the homeostasis of the oral microbiota. Naturally occurring SIgA antibodies that are reactive against a variety of indigenous bacteria are detectable in saliva. These antibodies may control the oral microbiota by reducing the adherence of bacteria to the oral mucosa and teeth. It is thought that protection against bacterial etiologic agents of caries and periodontal diseases could be conferred by the induction of SIgA antibodies via the stimulation of the mucosal immune system. However, elucidation of the role of the SIgA immune system in controlling the oral indigenous microbiota is a prerequisite for the development of effective vaccines against these diseases. The role of SIgA antibodies in the acquisition and the regulation of the indigenous microbiota is still controversial. Our review discusses the importance of SIgA among the multiple factors that control the oral microbiota. It describes the oral ecosystems, the principal factors that may control the oral microbiota, a basic knowledge of the secretory immune system, the biological functions of SIgA, and, finally, experiments related to the role of SIgA in oral microbial ecology.
The indigenous microbiota plays an important role in health and diseases of humans and animals. It contributes to the development of the immune system and provides resistance to colonization by allochthonous or pathogenic microorganisms (95, 299, 323, 420, 495). It also constitutes a reservoir of potentially pathogenic bacteria that may infect host tissues (44, 299, 495).
In the oral cavity, indigenous bacteria are often associated with the etiology of two major oral diseases, which are endemic in industrialized societies and are increasing in developing countries (514). Oral diseases seem to appear after an inbalance among the indigenous microbiota, leading to the emergence of potentially pathogenic bacteria. To define the process involved in caries and periodontal diseases, it is necessary to understand the ecology of the oral cavity and to identify the factors responsible for the transition of the oral microbiota from a commensal to a pathogenic relationship with the host (299, 322). The regulatory forces influencing the oral ecosystem can be divided into three major categories: host related, microbe related, and external factors (299).
Secretory immunoglobulin A (SIgA) constitutes the predominant immunoglobulin isotype in secretions, including saliva. It is considered to be the first line of defense of the host against pathogens which colonize or invade surfaces bathed by external secretions (320, 328). The main function of SIgA antibodies seems to be to limit microbial adherence as well as penetration of foreign antigens into the mucosa (59, 320, 323, 328). Naturally occurring SIgA antibodies reactive with a variety of indigenous bacteria have been detected in saliva (55, 59, 108, 174, 293, 296). Furthermore, indigenous bacteria of the oral cavity have been found to be coated with SIgA (55, 108). The role of these antibodies in the colonization and the regulation of the indigenous microbiota is still controversial. Despite the presence of SIgA antibodies, a resident microbiota persists in the oral cavity. Indigenous bacteria can survive in the oral cavity because they are less susceptible to or can avoid immune mechanisms (30, 44, 87, 141, 142). It is also possible that SIgA has an effect on indigenous bacteria but that it is only a minor force among the multiple factors that maintain the homeostasis of the indigenous microbiota (87).
Since caries and periodontal diseases are associated with indigenous bacteria, defining the role of SIgA in the control of the oral indigenous microbiota is a prerequisite for the elaboration of effective vaccines against these diseases. Until now, studies that evaluated the role of SIgA in the microbial ecology of the oral cavity gave contradictory results. In vitro experiments have shown that SIgA may inhibit (222, 383) or promote (222, 270) the adherence of oral bacteria to teeth. Experiments with animal models showed that salivary IgA induced against Streptococcus mutans leads to a reduction in the colonization of this bacterium and to the prevention of caries (328). More recent studies indicate that the immunity is not maintained (392). IgA-deficient humans were found to be more or less susceptible to caries and periodontal diseases (90, 393, 394).
The present review describes the oral ecosystems, the major factors that might control the oral microbiota, the basic aspects of the secretory immune system, and the biological functions of SIgA and finally focuses on experiments related to the role of IgA in the microbial ecology of the oral cavity. To present an overall picture of the current knowledge of oral microbial ecology, this review is not limited to human studies but includes in vitro systems such as the chemostat and the artificial mouth, as well as results obtained with animal models, such as rodents and primates, including our study on a murine model.
MICROBIAL ECOLOGY OF THE ORAL CAVITY
Ecology is the science that studies interrelationships between organisms and their living (biotic) and nonliving (abiotic) environment (20).
An ecosystem consists of the microbial community living in a defined habitat and the abiotic surroundings composed of physical and chemical elements. In its simplest expression, the oral ecosystem is thus composed of the oral microorganisms and their surroundings, the oral cavity (495).
Within an ecosystem, the development of a community usually involves a succession of populations. The process begins with the colonization of the habitat by pioneer microbial populations. In the oral cavity of newborns, streptococci (S. mitis biovar 1, S. oralis, and S. salivarius) are the pioneer organisms (73, 367a). Pioneer microorganisms fill the niche of this new environment and modify the habitat, and as a result, new populations may develop. As the process continues, the diversity and the complexity of the microbial community increase. Succession ends when no additional niche is available for new populations. At this stage, a relatively stable assemblage of bacterial populations is achieved. It is called a climax community.
The concept of a stable or climax community does not imply static conditions. The stability is based upon homeostasis, which implies compensating mechanisms that act to maintain steady-state conditions by a variety of controls aimed at counteracting perturbations that would upset the steady state. The concepts of homeostasis and bacterial succession are important in oral microbiology. Some factors, such as a high-sucrose diet, may cause an irreversible breakdown in the homeostasis of the oral ecosystem, resulting in the initiation of caries (299).
ORAL ECOSYSTEM OF MAMMALS
Habitats
The oral cavity is a moist environment which is kept at a relatively constant temperature (34 to 36°C) and a pH close to neutrality in most areas and thus supports the growth of a wide variety of microorganisms. However, the mouth must not be considered a uniform environment. There are several habitats in the oral cavity, each being characterized by different physicochemical factors and thus supporting the growth of a different microbial community. This is partly due to the great anatomical diversity of the oral cavity and the interrelationship between the different anatomic structures. The oral cavity possesses both hard (teeth) and soft (mucosa) tissues. The tooth can be described as a nonshedding hard surface that offers many different sites for colonization by bacteria below (subgingival) and above (supragingival) the gingival margin. In contrast, the oral mucosa is characterized by a continuous desquamation of its surface epithelial cells, which allows rapid elimination of adhering bacteria. The mucosa that covers the cheek, tongue, gingiva, palate, and floor of the mouth varies according to the anatomical site. The epithelium may be keratinized (palate) or nonkeratinized (gingival crevice) (495). The tongue, with its papillary surface, provides sites of colonization that are protected from mechanical removal. The area between the junctional epithelium of the gingiva and teeth, referred to as the gingival crevice, also provides a unique colonization site that includes both hard and soft tissues.
The oral surfaces are also constantly bathed by two important physiological fluids, the saliva and the gingival crevicular fluid. These fluids are essential for the maintenance of the oral ecosystems by providing water, nutrients, adherence, and antimicrobial factors. The supragingival environment is bathed by saliva, while the subgingival environment (gingival crevice) is bathed mainly by the gingival crevicular fluid. Saliva is a complex mixture that enters the oral cavity via the ducts of three pairs of major salivary glands, the parotid, the submandibular, and the sublingual, and the minor salivary glands. Saliva contains 99% water but also contains glycoproteins, proteins, hormones, vitamins, urea, and several ions. The concentrations of these components will vary according to the salivary flow. Generally, a slight increase in the secretion rate leads to an increase in sodium, bicarbonate, and pH and a decrease in potassium, calcium, phosphate, chloride, urea, and proteins (103, 347). At higher secretion rates, the concentrations of sodium, calcium, chloride, bicarbonate, and proteins increase while the concentration of phosphate decreases. Saliva helps maintain tooth integrity by providing ions such as calcium, phosphate, magnesium, and fluoride for the remineralization of tooth enamel.
Gingival fluid is an exudate originating from plasma that passes through the gingiva (junctional epithelium) to reach the gingival crevice and flows along teeth. The diffusion of gingival fluid in healthy gingiva is slow but increases during inflammation. The composition of the gingival fluid is similar to that of plasma; it contains proteins, albumin, leukocytes, Igs, and complement.
Oral Microbiota in Healthy Individuals
Humans.
The oral microbiota of humans is highly complex and diverse. It is composed of more than 300 bacterial species, to which may be added protozoa, yeasts, and mycoplasmas. Some of the more frequently isolated microorganisms are listed in Table 1. Their distributions vary qualitatively and quantitatively according to the habitat. Mutans streptococci (S. mutans, S. sobrinus, S. cricetus, and S. rattus) and S. sanguis are found in larger numbers on teeth, while S. salivarius is isolated mainly from the tongue (446). S. mutans and S. sanguis appear in the oral cavity only after eruption of the teeth (446).
TABLE 1.
Comparative oral microbiota in human and animals
| Group | Microbial genus | Present in:
|
|||
|---|---|---|---|---|---|
| Humansa | Monkeysb | Ratsc | Miced | ||
| Gram-positive cocci | |||||
| Aerobic or facultative | Streptococcus | ++e | ++ | ++ | ++ |
| Staphylococcus | − | + | + | ++ | |
| Enterococcus | + | + | + | ++ | |
| Micrococcus | + | − | − | − | |
| Obligate anaerobes | Peptostreptococcus | + | + | − | − |
| Peptococcus | + | + | − | − | |
| Gram-positive rods | |||||
| Aerobic or facultative | Lactobacillus | + | + | ++ | ++ |
| Corynebacterium | + | + | + | − | |
| Actinomyces | ++ | + | + | − | |
| Arachnia | + | − | − | − | |
| Rothia | + | − | − | − | |
| Alcaligenes | − | − | + | − | |
| Obligate anaerobes | Eubacterium | + | + | − | − |
| Propionibacterium | + | + | + | − | |
| Bifidobacterium | + | − | − | − | |
| Bacillus | ± | ± | ++ | ± | |
| Clostridium | ± | ± | ± | ± | |
| Gram-negative cocci | |||||
| Aerobic or facultative | Neisseria/Branhamella | + | + | ++ | − |
| Obligate anaerobes | Veillonella | ++ | + | ++ | − |
| Gram-negative rods | |||||
| Aerobic or facultative | Enterobacteriaceae | − | + | + | ± |
| Campylobacter | + | + | − | − | |
| Eikenella | + | − | − | − | |
| Actinobacillus | + | + | − | − | |
| Capnocytophaga | + | + | − | − | |
| Haemophilus | + | + | − | − | |
| Simonsiella | + | − | − | − | |
| Obligate anaerobes | Bacteroides | ++ | + | + | − |
| Fusobacterium | + | + | + | − | |
| Porphyromonas | + | ++ | − | − | |
| Prevotella | + | ++ | − | − | |
| Leptotrichia | + | + | − | − | |
| Wolinella/Selenomonas | + | + | − | − | |
| Other microorganisms | Mycoplasma | + | NA | NA | − |
| Candida | + | NA | NA | − | |
| Spirochetes | + | + | + | − | |
| Protozoa | + | + | + | − | |
Data from reference 204.
Combined data from six mouse strains (BALB/c, CD-1, C3H/He, C57BL/6, DBA/2, and C57BL/10) (150, 396, 500).
++, isolated frequently and may constitute a high percentage of the total oral microbiota; +, isolated; ±, appears as transient; −, not isolated; NA, data not available.
(i) Teeth.
On teeth, microorganisms colonize in a dense mass forming dental plaque (299, 350, 495). Dental plaque consists of microbial communities organized in a complex matrix composed of microbial extracellular products and salivary compounds. The microbial composition of dental plaque varies according to the site and the sampling time. Dental plaque develops preferentially on surfaces protected from mechanical friction, such as the area between two teeth (approximal surface), the subgingival area (gingival crevice), and the pits and fissures of the biting surfaces. The predominant organisms isolated from supragingival dental plaque are gram-positive, facultatively anaerobic bacteria, particularly Actinomyces spp. and streptococci (299, 350, 495). Gram-negative bacteria of the group Veillonella, Haemophilus, and Bacteroides are regularly isolated but in lower proportions (45, 495).
In a healthy subgingival crevice, the total number of cultivable bacteria is relatively small (103 to 106 CFU/crevice). The subgingival plaque is also dominated by gram-positive organisms (Actinomyces and streptococci). It seems that the microbiota from the gingival crevice is an extension of supragingival plaque (44). Black-pigmented gram-negative rods including Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella melaninogenica, Prevotella intermedia, Prevotella loescheii, and Prevotella denticola are rarely isolated from a healthy gingival crevice (299, 437).
(ii) Mucosal surfaces.
Little information is available on the microbiota of mucosal surfaces. The oral mucosa of the gingiva, palate, cheeks, and floor of the mouth are colonized with few microorganisms (0 to 25 CFU/epithelial cell) (495). Streptococci constitute the highest proportion of the microbiota in these sites, with a predominance of S. oralis and S. sanguis. The genera Neisseria, Haemophilus, and Veillonella have also been isolated (495). On the tongue, a higher bacterial density (100 CFU/epithelial cell) and diversity is found (44, 495). In all studies, Streptococcus spp. (S. salivarius and S. mitis) and Veillonella spp. were the predominant members of the microbiota (44, 495). Other major groups isolated include Peptostreptococcus spp., gram-positive rods (mainly Actinomyces spp.), Bacteroides spp., and other gram-negative rods. Black-pigmented obligate anaerobic rods and spirochetes, which are closely associated with periodontal diseases, have been recovered in small numbers (511). It has been suggested that the tongue could act as a reservoir for microorganisms that are implicated in periodontal diseases (511).
Organisms that are found in saliva are derived from the dislodgement of bacteria colonizing the various oral sites. The microbial composition of saliva is similar to that of the tongue (347, 500).
Animal models.
Animal models have been widely used in dental research (347, 350, 475). In general, information on their resident oral microbiota has been only partial or has been obtained during experimental protocols (44, 347, 350, 533). However, some studies have characterized the oral resident microbiota of monkeys (26, 61, 438), rats (204), and mice (150, 396, 500) more extensively. These results are summarized in Table 1. It is interesting that two genera, Lactobacillus and Streptococcus, are common to all laboratory animals.
Nonhuman primates have a dentition and an oral microbiota similar to that of humans and, for this reason, represent the most suitable model for dental research (347, 350). Several primates, such as the macaque (306, 438), the marmoset (61), and the squirrel monkey (26, 84), have been used in dental research. Studies of their oral microbiota have been limited principally to their subgingival plaque, and the predominant groups isolated were streptococci, Actinomyces spp. and obligate anaerobic gram-negative rods (26, 306, 438). Among the gram-negative rods, black-pigmented species dominated while high proportions of Fusobacterium spp. and Alcaligenes spp. were also recovered (26, 306, 438).
Although the oral anatomic structures of humans and rodents differ in certain respects, rats and mice are often used in dental research due to their availability at low cost and the ability to use inbred animals. Isogai et al. (204) isolated more than 15 bacterial species from the oral cavity of rats (Wistar Kyoto strains). The predominant types of bacteria isolated from the saliva, tongue dorsum, buccal mucosa, and gingival crevice of rats were Streptococcus spp., Lactobacillus spp., Veillonella spp., and Neisseria spp. S. salivarius was found in higher proportions in saliva, on the tongue dorsum, and the buccal mucosa. S. mitis was found in high proportions on the tongue dorsum and the buccal mucosa. S. sanguis and S. mutans were found in low proportions and only in the gingival crevice. Gram-negative bacteria, such as Bacteroides spp., were found in low proportions. Studies of the oral microbiota of other rat strains were less detailed, but similar results were obtained for the rice rat (347).
We have extensively studied the composition of the oral microbiota of mice (34, 94, 150, 294, 295, 296, 396, 398, 500). We have isolated more than 20 species from six different mouse strains (BALB/c, CD-1, C3H/He, C57BL/6, DBA/2, and C57BL/10) originating from different suppliers. Generally, no more than four or five species predominated at any one time in the oral cavity of any one group of mice. The most predominant and frequently isolated bacteria from the whole oral cavity (cheeks, tongue, and teeth) of mice were Lactobacillus murinus, Streptococcus spp., Enterococcus faecalis, and staphylococci (Staphylococcus epidermidis, S. conhii, and S. sciuri). L. murinus was found in higher proportions on the mucosa, and E. faecalis was found in higher proportions on teeth (500). In contrast other experimental models, no obligate anaerobic bacteria were isolated. Wolff et al. (533) isolated obligate anaerobic bacteria from the gingival plaque of two mice strains (STR/N and Swiss-Webster). In our studies, a less diversified microbiota was found, probably because specific-pathogen-free (SPF) mice were used. An SPF mouse colony is initiated from germfree mice inoculated with a defined bacterial cocktail from a supplier. The original cocktail contains nonpathogenic bacteria that have been previously isolated from conventional mice. We are currently using this mouse model to study the effect of different factors on the homeostasis of the oral microbiota. Although the oral microbiota is not representative of that of humans, the basic principles that govern the mechanisms involved in the maintenance of homeostasis in the oral cavity are probably similar in the mouse model and in humans. It could also be argued that because of their coprophageous habits, the microbiota of the rodents would be significantly modified. However, our results (295) show that this does not appear to be the case. It would be interesting, however, to study more systematically the influence of coprophagy on the oral microbial biota of rodents.
Oral Microbiota Associated with Oral Diseases
It is now well established that caries and periodontal diseases are infectious diseases associated with resident microorganisms of the dental plaque (299). In deciding upon therapy, such as vaccination, it is important to determine if one or several microorganisms cause the diseases. There are two major hypotheses about the role of plaque in oral diseases (301). The specific plaque hypothesis proposes that only a few microorganisms are involved in the oral disease process, while the other hypothesis (nonspecific) considers that diseases result from the interaction of the whole plaque with the host. Several experiments have attempted to describe the oral microbiota associated with caries and periodontal diseases and to identify the specific etiological agents. The current knowledge of the microorganisms involved in caries and periodontal diseases process has been obtained from the studies of humans and of laboratory animals, such as monkeys, hamsters, rats, and mice.
Caries.
Dental caries is a bacterial disease of the dental hard tissues; it is characterized by a localized, progressive, molecular disintegration of the tooth structure. The development of caries is associated with dental plaque of smooth coronal surfaces, pits, and fissures. Caries may also appear on root surfaces that are exposed to the oral environment as a result of gingival recession. The demineralization of teeth (enamel, dentine, and cementum) is caused by organic acid produced from the bacterial fermentation of dietary carbohydrates. The frequent ingestion of carbohydrates may lead to the selection of bacteria that are acidogenic (capable of producing acid from carbohydrates) and aciduric (capable of tolerating acid) and concurrently to a low-pH environment. These conditions favor the solubilization of tooth minerals. The pH at which this demineralization begins is known as the critical pH and ranges between pH 5.0 and 5.5 (275).
Laboratory animals have been particularly valuable in elucidating the microbiological origin of dental caries. In a series of experiments, Keyes and his collaborators demonstrated that rodents developed caries when infected with cariogenic streptococci and fed a high sucrose diet (139, 221). The “caries-inducing streptococci” isolated by Keyes and colleagues were subsequently identified as a mutans streptococcus (S. cricetus). Many bacterial species have been similarly tested for their cariogenic potential in conventional animals (monkeys, rats, gerbils, hamsters, and mice) and in gnotobiotic rodents (germfree rodents monoassociated with a known bacterial species). Among 30 bacterial species tested, mutans streptococci including S. mutans, S. sobrinus, S. cricetus, and S. rattus were shown to be the most cariogenic. Other cariogenic bacterial species include Lactobacillus acidophilus, Lactobacillus casei, Actinomyces naeslundii, A. naeslundii genospecies 2 (formerly Actinomyces viscosus), S. salivarius, S. sanguis, and E. faecalis (275). It may be misleading to extrapolate the results obtained in animals to the situation prevailing in humans. In contrast to humans, the caries process is induced rapidly in animals by feeding them a high bacterial inoculum and a high-sucrose diet. Furthermore, the use of gnotobiotic animals does not account for the multiple microbial interactions that occur among the human oral resident microbiota. Bacteria that are less acidogenic, such as Actinomyces, may be cariogenic under such experimental conditions but not in humans.
The complexity of the bacterial community in dental plaque of humans has made it difficult to determine the single bacterial agent of caries. However, there is considerable evidence that mutans streptococci (particularly S. mutans and S. sobrinus) and Lactobacillus are involved in the initiation and progression of caries (275). These two bacterial groups are able to rapidly metabolize carbohydrates into acid, primarily lactic acid, and to tolerate a low-pH environment. Cross-sectional studies demonstrated that a large number and isolation frequency of mutans streptococci and Lactobacillus are associated with increasing prevalence of enamel lesions. Most of the longitudinal studies revealed that the appearance of enamel caries is preceded by an increased level of mutans streptococci (275). The increase in Lactobacillus is generally slower, and Lactobacillus reaches a high level only after the lesion can be detected clinically (69). These findings suggest a microbial succession in which mutans streptococci are implicated in caries initiation and Lactobacillus is implicated in caries progression (353). However, coronal caries also appears to develop in the absence of mutans streptococci and lactobacilli. Species such as Actinomyces spp., S. mitis, Veillonella spp., and Candida spp. have been associated with enamel caries (353). Other studies also suggest that the microflora of root surface caries is complex (43). In addition to mutans streptococci and lactobacilli, a broad range of microorganisms may be isolated from root lesions, and Actinomyces occasionally constitutes the predominant species (43, 353). Furthermore, a high level of S. mutans has been found in dental plaque without evidence of caries (353).
To reconcile these findings, Marsh proposed the “ecological plaque hypothesis” (299, 301, 303). A factor(s) will trigger a shift in the proportions of the resident microbiota and therefore predispose a site to disease. At neutral pH, mutans streptococci and lactobacilli are weakly competitive and constitute only a small percentage of the total plaque microbial community. The frequent consumption of fermentable carbohydrates may lead to frequent conditions of low pH in the plaque. Such conditions lead to decreased proportions of acid-sensitive bacteria such as S. sanguis, S. oralis, and S. mitis and to increased proportions of mutans streptococci and lactobacilli. Such a population shift predisposes a surface to dental caries. The increased numbers of S. mutans and Lactobacillus lead to the production of acid at a higher rate, enhancing the demineralization of the tooth. The sequence of events explains the lack of total specificity in the microbial etiology of caries and the bacterial succession observed in longitudinal studies. The apparent absence of caries observed in the presence of high levels of S. mutans may be due to differences in flow rate, buffer capacity, or composition of saliva or to the presence of a high level of lactate-metabolizing and base-generating bacterial species in dental plaque (299). Some studies suggest that the presence of Veillonella, a lactate-metabolizing bacteria, is associated with a lower prevalence of caries (353).
Periodontal diseases.
Periodontal diseases is a general term describing the inflammatory pathologic state of the supporting tissues of teeth. Periodontal diseases can be grouped into two major categories, gingivitis and periodontitis. Each can be further divided according to the age of the patient (prepubertal, juvenile, adult), disease activity and severity (rapid, acute, chronic), and distribution of lesions (localized or generalized) (299, 439). Gingivitis is defined as an inflammation of gingival tissues which does not affect the attachment of teeth. Periodontitis involves the destruction of the connective tissue attachment and the adjacent alveolar bone (439). In periodontitis, the gingival crevice is deepened to form a periodontal pocket due to the apical migration of the junctional epithelium along the root surface (495). The induction and progression of periodontal tissue destruction is a complex process involving plaque accumulation, release of bacterial substances, and host inflammatory response (156, 157, 299). Although bacteria rarely invade tissues, they may release substances that penetrate the gingivae and cause tissue destruction directly, by the action of enzymes and endotoxins, or indirectly, by induction of inflammation. The host inflammatory response to bacterial antigens is both protective and destructive in periodontal diseases. Tissue damage may be caused by the release of lysosomal enzymes from phagocytes and by the production of cytokines that stimulate connective tissue cells to release metalloproteinases (including collagenases) or cytokines that activate bone resorption. Among the bacteria regularly isolated from periodontal pockets, those producing such virulence factors are generally gram-negative rods and include Porphyromonas, Prevotella, Fusobacterium, Actinobacillus actinomycetemcomitans, Capnocytophaga, and Wolinella.
Experimental models have been widely used to understand the etiology of periodontal diseases. Rodents do not represent an attractive model for human periodontal diseases, in part because hair, food, and litter accumulated in their gingival crevices may induce inflammation and destruction of periodontal tissues. However, it has been demonstrated that oral inoculation of germfree rodents with several suspected periodontal pathogens including A. actinomycetemcomitans, Porphyromonas gingivalis, Capnocytophaga sputigena, Eikenella corrodens, and Fusobacterium nucleatum increases alveolar bone destruction. Antibiotic therapy was effective in preventing or arresting periodontal destruction, thus confirming the role of microorganisms in periodontal diseases (232). Other species, usually not recognized as periodontopathogens (such as Streptococcus and Actinomyces), also cause bone loss in gnotobiotic rodents, suggesting that the spectrum of periodontal pathogens in humans may be wider than generally accepted. Periodontal diseases associated with accumulations of indigenous plaque may also develop in rodents, dogs, and monkeys. Primates appear to be more suitable models for studies of periodontal diseases because of the similarity of the inflammatory response to that of humans. These animals develop periodontal diseases naturally, but the disease process may be accelerated by the subgingival placement of a silk ligature around the teeth. The destruction of periodontal tissues in primates is associated with an increase in gram-negative anaerobic rods including Fusobacterium, Capnocytophaga, A. actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia (26).
In humans, gingivitis is also associated with plaque accumulation around the gingival margin. When oral hygiene is restored, the gingival tissue quickly returns to a state of health, demonstrating that dental plaque is responsible for gingival inflammation and is not a result of the disease. In a healthy gingival crevice, the total number of microorganisms is small and the microbiota is dominated by facultative gram-positive bacteria. The number of bacterial cells in plaque associated with gingivitis is 10- to 20-fold larger than in healthy sites. The facultative gram-positive bacteria still dominate, but there is an increase in the proportion obligately anaerobic gram-negative bacteria (439). The microbiota increases in diversity, but no specific group seems associated with the diseases (336, 339). The predominant gram-positive bacteria are Actinomyces naeslundii genospecies 2 (formerly A. viscosus), A. naeslundii, S. sanguis, S. mitis, and Peptostreptococcus micros. Gram-negative rods include F. nucleatum, P. intermedia, Veillonella, Wolinella, Capnocytophaga, and Haemophilus. Although it is not clear whether gingivitis is essential for the development of more advanced forms of periodontitis, some species that predominate in periodontitis have been found in small numbers in gingivitis. Chronic adult periodontitis is the most common form of advanced periodontal disease. The microbiota is extremely diverse and may be composed of more than 150 different species, among which are large numbers of obligately anaerobic gram-negative rods and spirochetes (337). The microbiota differs in composition between pockets within a patient and between patients (337, 338). The predominant species include P. gingivalis, P. intermedia, Bacteroides forsythus, A. actinomycetemcomitans, W. recta, E. corrodens, Treponema denticola, and P. micros. Chronic periodontitis probably results from the microbial activity of a mixture of microorganisms, particularly the obligately anaerobic gram-negative rods. Other more acute and rapid forms of periodontal diseases may also arise due to different predisposing conditions such as hormonal changes or depressed immune systems. These diseases seem more associated with particular microbial groups, such as in localized juvenile periodontitis which is closely associated with high numbers of A. actinomycetemcomitans (440).
The plaque ecologic hypothesis may also be applied to explain the role of microorganisms in periodontal diseases (299, 301, 303). In the healthy gingival crevice, suspected periodontopathogens such as P. intermedia, A. actinomycetemcomitans, P. gingivalis, and spirochetes are undetectable or found in very small numbers. In the absence of oral hygiene, the accumulation of plaque can lead to inflammation and an increase in the flow of gingival crevicular fluid. This fluid may provide nutrients for bacteria and favor the growth of fastidious obligately anaerobic gram-negative bacteria implicated in periodontal destruction. It has been demonstrated that cultures of subgingival plaque in serum allowed the enrichment of suspected periodontopatogens that were previously undetected in the primary inoculum (493). This finding might explain the observed succession of microorganisms from healthy gingiva and gingivitis to periodontitis and the difficulty in identifying specific etiologic agents in periodontal diseases.
FACTORS INFLUENCING THE ORAL ECOSYSTEM
The growth of oral microorganisms is influenced by a variety of factors such as temperature, pH, oxidation-reduction potential, the availability of nutrients and water, the anatomy of the oral structures, salivary flow, and antimicrobial substances. Each factor in a given oral habitat influences the selection of oral microorganisms and helps maintain the equilibrium among bacterial populations. The result of these selective pressures has already been observed in the differences in the oral microbiota among the different sites of the oral cavity.
The formation of dental plaque on smooth surfaces has been widely studied in vitro and in vivo and represents a good example of the force involved to maintain the homeostasis of the oral ecosystems. The development of dental plaque follows a general bacterial succession pattern under the control of several factors (reviewed in reference 353). After tooth brushing, dental plaque is initiated by the deposition of an acellular proteinaceous film, termed the acquired pellicle (353). The major constituents of the pellicle are components of saliva and gingival crevicular fluid such as proteins (albumin, lysozyme, proline-rich proteins), glycoproteins (lactoferrin, IgA, IgG, amylase), phosphoproteins, and lipids. Bacterial components such as glucosyltransferase are also present. The bacteria colonize the pellicle within the first 2 to 4 hours after cleaning. The pioneer microorganisms consist mainly of streptococci (S. sanguis, S. oralis, and S. mitis) and, in smaller numbers, Neisseria and Actinomyces. The bacteria with low affinity for the pellicle are eliminated by salivary flow. After initial colonization, the pioneer microorganisms grow rapidly, forming microcolonies that are embedded in an extracellular matrix composed of bacterial and host molecules. During this process, the alteration of the environment by pioneers allows the colonization by other bacterial groups such as Veillonella and Haemophilus (48 h after tooth brushing). Several bacterial interrelationships including coaggregation, production of antibacterial substances, and food chains contribute to increase the diversity of the bacterial community. Also, the consumption of oxygen by aerobic species favors the colonization of obligately anaerobic microorganisms such as Fusobacterium, Bacteroides, and spirochetes (1 to 2 weeks). If the plaque is left to accumulate, the complexity of the microflora increases until a climax community has been established (2 to 3 weeks). As described above, an inbalance in the plaque ecosystem may lead to the development of oral diseases. For example, the uncontrolled development of supragingival plaque in the absence of oral hygiene may cause gingivitis and subsequent colonization of the subgingival crevice by periodontopathogens. A high sucrose intake may lead to a higher colonization of plaque by S. mutans and Lactobacillus and to the development of caries.
In the following sections, each factor that may influence the physicochemical environment and the colonization of the oral cavity will be described in detail. All these factors are interrelated and depend on host and microbial activities as well as external factors such as diet or oral hygiene. For better clarity, these factors have been divided into four categories: physicochemical, host related, microbial, and external.
Physicochemical Factors
The physicochemical factors result from the combined action of host, microbial, and external factors. In all in vivo and in vitro systems, the growth of microorganisms is influenced by five important variables: temperature, pH, availability of water, availability of nutrients, and oxidation-reduction potential (20). As the mouth is constantly bathed by saliva and crevicular fluid, water is not considered to be a limiting factor.
Temperature.
The temperature in the oral cavity is relatively constant (34 to 36°C), which allows a wide range of microorganisms to grow. The temperature may be more variable on the mucosal and tooth supragingival surface. During food intake, microorganisms colonizing these sites are exposed to hot and cold meals and probably must adapt to these extreme variations of temperature. However, to our knowledge, no data are available on the effect of this short period of temperature variation on the metabolism of oral bacteria.
pH.
The pH or hydrogen ion concentration of an environment affects microorganisms and microbial enzymes directly and also influences the dissolution of many molecules that indirectly influence microorganisms. Microorganisms generally cannot tolerate extreme pH values. In the oral cavity, the pH is maintained near neutrality (6.7 to 7.3) by saliva. The saliva contributes to maintenance of the pH by two mechanisms. First, the flow of saliva eliminates carbohydrates that could be metabolized by bacteria and removes acids produced by bacteria. Second, acidity from drinks and foods, as well as from bacterial activity, is neutralized by the buffering activity of saliva. Bicarbonate is the major salivary buffering system of saliva, but peptides, proteins, and phosphates are also involved. Increases in pH also result from bacteria that metabolize sialine and urea into ammonia. Acids that are produced by the microbial metabolism of carbohydrates may accumulate in dental plaque because of the slow diffusion of saliva through dental plaque. Following sugar intake, the pH of dental plaque may decrease to below 5.0.
The pH is an important parameter in oral microbial ecology (42, 47, 186). Frequent sugar intake favours the growth of aciduric bacteria such as Lactobacillus and S. mutans and predisposes to caries formation (299). An increased colonization by S. mutans was demonstrated by simply rinsing the mouth with low-pH buffers (462). In vitro studies have also shown that gradual decreases in pH in glucose-pulsed cultures favored S. mutans and Lactobacillus while populations of S. sanguis, S. mitior, P. intermedia, and F. nucleatum were reduced (42, 317). When the pH of the chemostat was controlled at 7.0, the glucose pulse had little effect on the microbial populations, suggesting that the low pH generated from carbohydrate metabolism, rather than carbohydrate availability per se, is responsible for the shift in composition of the oral microbiota in vivo (47, 322).
The subgingival area is bathed by gingival fluid and is not controlled by the buffering salivary activity. The pH in the gingival crevice may vary between 7.5 and 8.5, while the crevicular fluid ranges from pH 7.5 to 7.9. An alkaline pH in gingival crevices and periodontal pockets may exert a selective force towards the colonization of periodontopathogens (186, 316).
Oxidation-reduction potential and anaerobiosis.
Many enzymatic reactions are oxidation-reduction reactions in which one compound is oxidized and another compound is reduced. The proportion of oxidized to reduced components constitutes the oxidation-reduction potential or redox potential (Eh). The Eh is greatly influenced by the presence or absence of molecular oxygen, which is the most common electron acceptor. Anaerobic bacteria need a reducing environment (negative Eh) for growth, while aerobic bacteria need an oxidizing environment (positive Eh). The mouth is characterized by a wide range of oxidation-reduction potentials, allowing the growth of aerobic, facultative anaerobic, and anaerobic bacteria (495). In general, the dorsum of the tongue and the buccal and palatal mucosa are aerobic environments with positive Eh, thus better supporting the growth of facultative anaerobic bacteria. The gingival crevice and the approximal surfaces of the teeth (surfaces between teeth) possess the lowest Eh and the highest concentration of obligately anaerobic bacteria. The Eh values vary between +158 to +542 mV in saliva but may reach −300 mV in gingival crevices (495). The Eh also varies during plaque formation, changing from positive values (+294 mV) on clean tooth surfaces to negative values (−141 mV) after 7 days (218). The fall in Eh during plaque formation is the result of oxygen consumption by facultative anaerobic bacteria as well as a reduction in the ability of oxygen to diffuse through the plaque. This explains in part the increased in number of obligately anaerobic bacteria during plaque formation.
Nutrients.
Chemostat studies (42, 145, 186, 316) and a study with mice (34) suggest that the levels of most bacterial populations are strongly controlled by substrate availability. Each bacterial species must be more efficient than the rest in utilizing one or a few particular substrates under certain conditions. According to Liebig’s law of the minimum, the growth of each organism is limited by the required substrate that is present in the lowest concentration (20). In the oral cavity, microorganisms living in the supragingival environment have access to nutrients from both endogenous (saliva) and exogenous (diet) origin. Saliva is an important source of nutrients and can sustain normal growth of microorganisms in the absence of exogenous nutrients (105, 454). Saliva contains water, carbohydrates, glycoproteins, proteins, amino acids, gases, and several ions including sodium, potassium, calcium, chloride, bicarbonate, and phosphate. Among exogenous dietary components, carbohydrates and proteins have the greatest influence on the composition of the oral microbiota (34, 335, 457).
The gingival crevice is not exposed to dietary components and saliva, and its principal source of nutrients is the gingival crevicular fluid. The crevicular fluid originates from plasma and is an excellent source of nutrients for fastidious microorganisms. It contains growth factors such as hemin and vitamin K required by P. gingivalis, a gram-negative rod associated with adult periodontitis.
Many nutritional interrelationships also occur between microorganisms. Some microorganisms cooperate for the degradation of nutrients. Some bacteria also use nutrients and other substances produced by other microorganisms.
Host Factors
Host defense mechanisms.
The supragingival environment of the oral cavity is controlled primarily by saliva. The continuous flow of saliva increased by the muscular activity of the lips and tongue removes a large number of bacteria from teeth and mucosal surfaces. Saliva also contains several specific and nonspecific defense factors. SIgA is the principal specific defense factor of saliva, and its role is discussed more extensively below. The nonspecific defense factors include mucins, nonimmune salivary glycoproteins, lactoferrin, lysozyme, peroxidase, histatins, and cystatins.
Mucins are high-molecular-weight glycoproteins produced by submandibular, sublingual, and numerous minor salivary glands. They are the principal organic constituent of mucus, the slimy viscoelastic material that envelopes all mucosal surfaces of the body. Saliva contains two forms of mucins, MG1 and MG2. The MG1 mucin, which has a molecular mass greater then 1,000 kDa, is involved mainly in tissue coating; MG2, which has a molecular mass of 125 kDa, affects the aggregation and adherence of streptococci. In the oral cavity, mucins provide a protective coating for both soft and hard tissues. The mucins form a viscous slime layer on oral mucosa that traps microorganisms and antigens, limiting their penetration into the tissues (466, 467). Potentially harmful microorganisms are thus eliminated by the continuous renewal of the mucous layer combined with the washing action of salivary flow. Mucins are also constituents of the acquired pellicle and may protect teeth from acid demineralization.
The role of mucins and other nonimmune salivary glycoproteins in bacterial adherence is complex. When salivary glycoproteins are adsorbed on solid surfaces, they may bind to bacteria and promote bacterial adherence. Conversely, some of these glycoproteins, when free in saliva, may prevent bacterial colonization by binding to their adhesins or by agglutinating bacteria in saliva (50, 344, 422). This type of aggregation may facilitate the removal of oral bacteria by swallowing. Saliva from subjects with low levels of mutans streptococci aggregate these bacteria more efficiently, suggesting a protective role for salivary agglutinins (128). In vitro, pretreatment of S. sanguis and S. mutans with salivary glycoproteins prevented their attachment to hydroxyapatite (267). The phenomena of adherence and aggregation may be mediated by two different binding mechanisms. Saliva agglutinin (300 kDa), which acts as a receptor for antigen I/II of S. mutans, mediates both aggregation and adherence. However, the interaction involves different regions of the antigen I/II and different binding specificities (50, 344).
Saliva also possesses defense factors with direct antimicrobial activity in vitro. A group of salivary proteins, lysozyme, lactoferrin, and peroxidase, act in conjunction with other components of saliva to limit the growth of bacteria or kill them directly.
Lysozyme is a small cationic protein that is present in all major body fluids; it is secreted by intercalated duct cells (290, 377). Lysozyme can lyse some bacterial species by hydrolyzing glycosidic linkages in the cell wall peptidoglycan. It may also cause lysis of bacterial cells by interacting with monovalent anions, such as thiocyanate, perchlorate, iodide, bromide, bicarbonate, nitrate, and fluoride, and with proteases found in saliva. The combination leads to destabilization of the cell membrane probably by activation and deregulation of endogenous bacterial autolysins (290, 377). In vitro, the bacteriolytic activity of the lysozyme-protease-monovalent anion system has been demonstrated against S. mutans, L. casei, and F. nucleatum (376, 377). In addition, lysozyme can aggregate oral bacterial cells and inhibit their colonization on mucosal surfaces and teeth (378). In vivo, an inverse correlation has been found between the concentration of lysozyme and the accumulation of dental plaque (208).
Lactoferrin is an iron-binding glycoprotein produced by intercalated duct cells. It inhibits microbial growth, probably by sequestering iron in the environment. In addition, iron-free lactoferrin (apolactoferrin) possesses a direct, iron-independent bactericidal effect against various oral bacterial strains including S. mutans (17, 18). Apolactoferrin was shown to agglutinate S. mutans but not other species of streptococci, P. gingivalis, or A. actinomycetemcomitans (456). Recent results with bovine lactoferrin indicate a bactericidal activity of the N-terminal portion of the lactoferrin molecule (111a, 195a).
Salivary peroxidase is an enzyme secreted by salivary gland acinar cells. It is part of an antimicrobial system that involves the oxidation of salivary thiocyanate to hypothiocyanite and hypothiocyanous acid by hydrogen peroxide, generated by oral bacteria. These oxidizing agents react with sulfhydryl groups of the enzymes involved in glycolysis and sugar transport (489). Salivary peroxidase removes toxic hydrogen peroxide produced by oral microorganisms and can reduce acid production in dental plaque (113). Salivary peroxidase has been shown to retain activity when adsorbed on hydroxyapatite and so should be effective at the enamel-plaque interface (487). The activation of peroxidase systems in vivo by addition of appropriate amounts of exogenous hydrogen peroxide reduces plaque accumulation, gingivitis and caries (198, 199).
Histatins (histidine-rich peptides) are a family of small basic peptides (3 to 5 kDa), with a high content of histidine, that are produced by acinar cells (359). They inhibit the development of Candida albicans from the noninfective to the infective form (359). They can also inhibit coaggregation between P. gingivalis and S. mitis (343), aggregate oral streptococci, and inhibit the growth of S. mutans (367).
Cystatins are a family of cysteine-containing phosphoproteins that are secreted by acinar cells (190, 430). These proteins are also present in plasma and may reach the oral cavity via the gingival crevicular fluid (190). Cystatins act mainly as thiol protease inhibitors and can inhibit proteases produced by suspected periodontopathogens.
Saliva does not gain access to the gingival crevice, and this area of the oral cavity is almost essentially controlled by the antimicrobial factors of plasma. Cellular and humoral components of blood can reach the gingival crevice of the oral cavity by the flow of gingival fluid through the junctional epithelium. Even in the healthy state, there is a continuous flow of small quantities of fluid and leukocytes from the gingival capillaries through the crevicular epithelium into the gingival crevice. This flow increases greatly with inflammation induced by plaque accumulation (258). The continuous flow of gingival fluid from the crevice to the oral cavity removes nonadherent bacterial cells. Gingival fluid also contains antimicrobial substances including IgM, IgG, IgA, complement, and leukocytes. These factors are primarily protective against microbial invasion, but, as seen above, the inflammation may become destructive, resulting in loss of periodontal attachment.
The leukocytes in gingival crevicular fluid are composed of 90% polymorphonuclear leukocytes (PMNs) and 10% mononuclear cells. Among the mononuclear cells, 60% are B lymphocytes, 20 to 30% are T lymphocytes, and 10 to 15% are macrophages (478). About 80% of PMNs are viable and functional within the crevice. The cells are capable of phagocytosis and of killing microorganisms, although the efficiency of phagocytosis is reduced compared with that of blood neutrophils (258). The PMNs perhaps remain functional at a short distance from the gingival margin by the flow of gingival fluid along the tooth surface, but once neutrophils are in saliva, they degenerate, due to osmotic lysis (274). Lysozyme and peroxidase that are released from the lysosome of PMNs during phagocytosis might also control microbial growth in the gingival crevice.
Components of the complement cascade are present in the gingival crevicular fluid. In subjects with healthy gingivae, C3 and C4 components of complement can be detected. During gingival inflammation, C3a, C3b, and C5a appear, suggesting that complement activation may have occurred in vivo (258, 441). Complement factors may initiate bacterial cell lysis or enhance phagocytosis of microorganisms.
The IgG, IgM, and IgA antibodies directed against a variety of oral microorganisms have been detected in plasma and crevicular fluid even in healthy individuals (120, 219, 278, 342, 447). These antibodies may influence the oral microbiota by interfering with adherence or by inhibiting bacterial metabolism (258, 447). Furthermore, the IgG antibodies may enhance phagocytosis and killing of oral microorganisms through activation of complement or opsonization (12, 258, 313, 428). It has been demonstrated that systemic immunization of animals with periodontopathogens may reduce the colonization of these bacteria in the gingival crevice and reduce periodontal destruction (83, 131, 314, 371). However, since periodontal diseases are of multifactorial origin, systemic immunization with periodontopathogens may also enhance the destruction of alveolar bone (67, 122). The immune response itself may contribute significantly to the periodontal destruction, sometimes even more than the pathogens!
Some indications suggest that serum antibodies may also regulate the bacterial colonization of supragingival surfaces of teeth. Although conflicting evidence exists, some studies have reported an inverse correlation between the level of serum IgG antibodies against S. mutans and the level of this bacterium in dental plaque or the frequency of caries (2, 447). Also, systemic immunization of nonhuman primates with S. mutans antigens led to reduced levels of S. mutans and less caries (261, 262).
All specific and nonspecific antimicrobial factors in the oral cavity do not act in isolation. Synergistic and antagonistic interactions among antimicrobial factors may influence their actions. Mucins serve to concentrate other antimicrobial substances, including lysozyme, IgA, and cystatins, at the mucosal surface (466). SIgA enhances the antimicrobial activity of lactoferrin, salivary peroxidase, agglutinins, and mucins. Similarly, the polycationic antimembrane effect of lysozyme may be enhanced by salivary peroxidase (377) and histatins (282). In contrast, salivary peroxidase may block the bactericidal effect of lactoferrin (255).
Age.
The composition of the oral microbiota varies with the age of the host. Age-related changes in the oral microflora include those due to teeth eruption, changes in dietary habits, hormones, salivary flow, the immune system, or other factors.
The human oral cavity is usually sterile at birth. However, within 6 to 10 h after birth, microorganisms from the mother and to a lesser extent microorganisms from those present in the environment become established in the oral cavity. The pioneer species are usually streptococci, especially S. mitis biovar 1, S. oralis, and S. salivarius (73, 367a, 455). During the first year of life, the oral microbiota contains Streptococcus, Neisseria, Veillonella, Staphylococcus, and, to a lesser degree, Actinomyces, Lactobacillus, Rothia, Fusobacterium, and Prevotella (238, 455, 524). Some species, such as S. sanguis, S. mutans, and A. naeslundii genospecies 2 (formerly A. viscosus), colonize the oral cavity only after tooth eruption (446). Following tooth eruption, the number and isolation frequency of obligately anaerobic bacteria increase. The number of black-pigmented anaerobes and spirochetes in the gingival crevice increases more extensively during adolescence, and this could be due to hormonal changes (180, 455). Studies indicate that the next most important changes occur in the elderly and include an increased prevalence of staphylococci, lactobacilli, and A. naeslundii genospecies 2 (formerly A. viscosus) after the age of 70 years and an increase in the proportion of Candida albicans after 80 years (305, 369). The change in the oral microbiota of elderly individuals were not related to denture wearing, medication, or disease but may be caused by a decrease in salivary flow, an impaired immune system, or nutritional deficiencies (369, 370, 488).
Our research has focused on the development of the oral microbiota of BALB/c mice from birth to the age of 2 months (397). Staphylococci have been identified as the first colonizers, which are immediately followed by lactobacilli. The appearance of Enterococcus faecalis and members of the Enterobacteriaceae appeared to correspond to tooth eruption and the beginning of coprophagy. The proportion of Lactobacillus murinus sharply increased at weaning (20 days), probably due to changes in the composition and texture of the diet, from maternal milk to solid food (397). After the weaning period, no other significant changes were observed and the oral microbiota appeared to be completely stabilized when the mice reached the age of 6 to 8 weeks.
Hormonal changes.
It is well known that in humans, puberty and pregnancy are characterized by increased levels of steroid hormones in plasma and subsequently in the crevicular fluid and saliva (130, 252). It is also well documented that pregnancy and puberty are associated with an increase in gingival inflammation which is accompanied by an increase in gingival exudate (539). It has been proposed that the exacerbations in gingival inflammation may be due to hormone-induced alterations in the microbiota of the gingival crevice (211, 241, 539). Microorganisms in the subgingival area that use hormones as growth factors may be favored during the period of hormone increase associated with puberty and pregnancy (242). Several investigators have described a transient increase in the number of black-pigmented gram-negative anaerobic bacteria in the subgingival microbiota during puberty (107, 180, 337, 529) or pregnancy (211, 242). Kornman and Loeshe (242) reported an increased proportion of Prevotella intermedia in the subgingival microbiota of pregnant woman, corresponding to an increased levels of estrogens and progesterone in plasma. They also demonstrated in vitro that progesterone or estradiol can substitute for vitamin K as an essential growth factor for P. intermedia (242). In contrast, other studies were unable to find any changes in the subgingival microbiota during puberty (536) and pregnancy (212).
In mice, we did not find any modifications in the oral microbiota during puberty (397) or pregnancy (94). However, in contrast to humans, mice do not usually harbor obligately anaerobic gram-negative rods. Furthermore, since the periods of puberty and pregnancy in mice are short, the bacteria might not be exposed to the hormones long enough to induce modifications in the oral microbiota.
Stress.
Host stress may be associated with changes in hormones, salivary flow, dietary habits, and immune response (23, 51, 104, 254, 503). Few studies on the effect of stress on the indigenous microbiota have been performed, and most data have been obtained from studies of the intestinal microbiota of rodents. Stress in rodents is considered to be related to stimulation of the hypothalamic-pituitary-adrenocortical axis, which can lead to an elevation in corticosterone concentration. Crowding, fighting, and husbandry changes are some of the factors that are known to induce an increase in cortisol levels in plasma in mice and might play an important role in animal stress (51, 197, 254, 368, 503). These factors affect the behavior of mice in terms of feeding, sexual habits, grooming, rearing, and biting (503). The composition of the intestinal microbiota of rodents may be altered by a variety of unrelated disturbances, such as changes in environmental temperature, crowding in cages, and fighting among animals (239, 424). These changes in the intestinal microbiota include a reduction in numbers of lactobacilli (424, 474) as well as fusiform and segmented filamentous bacteria (230, 231, 239). In our studies, some evidence suggested that the oral microbiota of mice may also be influenced by stress. Various factors such as shipping to our animal facility, husbandry modifications, and low temperature (in nude mice) led to decreases in the proportions of Lactobacillus murinus among the total cultivable oral microbiota (150, 294, 295).
Genetic factors.
The genetic background appears to influence the susceptibility to caries (249, 379) and periodontal diseases (161). This could in part be because the host genetic factors select for a microbiota with varying potential for causing oral diseases. The selection of a certain microbiota by the host is dependent on inherited immune factors, physiology, metabolism, mucus composition, or receptor-ligand interactions (337). Malamud et al. (289) produced evidence for the inheritability of agglutinin activity and parotid flow rate. Genetic factors also seem to influence the intestinal (230, 231, 308, 506) and oral (337, 425, 476) microbiota. Moore et al. (337) reported that the composition of subgingival microbiotas of monozygotic twins (11 to 14 years of age) was more similar than that of dizygotic twins. In contrast, we found that the genetic background does not seem to be an important factor in the composition of the oral microbiota of mice and that its influence is probably masked by environmental and husbandry factors (150). On the other hand, the oral indigenous microbiota of SPF mice is simple and the effect of the genetic background is perhaps observed only when the host is exposed to a more complex microbiota.
Bacterial Factors
Adherence.
To get established in the oral cavity, microorganisms must first adhere to teeth or to mucosal surfaces. Adherence is essential for providing resistance to the flow of saliva. Adherence is mediated by adhesins on the surface of bacteria and by receptors on the oral surface. Microbial adhesins consist of polysaccharides, lipoteichoic acids, glucosyltransferases, and carbohydrate-binding proteins (lectins). These adhesins are found as cell wall components or are associated with cell structures, such as fimbriae, fibrils or capsules. The receptors may be salivary components (mucins, glycoproteins, amylase, lysozyme, IgA, IgG, proline-rich proteins, and statherins) or bacterial components (glucosyltransferases and glucans) that are bound to oral surfaces (160, 405, 422). The adherence may result from nonspecific physicochemical interactions between the bacteria and the oral surfaces. For example, lipoteicoic acids on microbial surfaces may interact with negatively charged host components through calcium ions or through hydrogen or hydrophobic bonding. However, these interactions cannot alone explain the selective attachment of bacteria to various oral surfaces. It is believed that another mechanism accounts for this selective colonization, perhaps involving specific or stereochemical interactions between bacterial adhesins and host receptors. In this case, the same physicochemical forces intervene but now act between extremely small, highly localized, spatially well organized opposing molecular groups (70). It is probable that the bacteria first adhere by nonspecific interactions which are followed by stronger stereochemical interactions. A number of specific interactions have been identified in adhesion to human tooth surfaces (reviewed in reference 422). The stereochemical interactions involved in bacterial adhesion in the oral cavity are analogous to the interactions between antigen and antibody or between an enzyme and its substrate. For example, type 1 fimbriae of Actinomyces naeslundii genospecies 2 and surface antigen I/II (protein P1) of S. mutans can bind to proline-rich proteins (407), Streptococcus gordonii can bind to α-amylase (423), and A. naeslundii genospecies 2 and Fusobacterium nucleatum can interact with statherin (159, 160, 535). Another adherence strategy involves a lectin-like bacterial protein with the complementary carbohydrate receptor located on glycoproteins (344). The interaction may be inhibited in vitro by adding the specific carbohydrate. S. sanguis can bind to sialic acid-containing oligosaccharides of the low-molecular-weight salivary mucin (MG2) (345). Type 2 fimbriae of Actinomyces binds to the beta-linked galactose glycoprotein on epithelial cell surfaces (60).
Bacteria may also colonize host surfaces by adhering to other bacteria, and several examples of coaggregation between human oral bacterial species have been demonstrated in vitro (235–237). Streptococcus spp. aggregates with Actinomyces spp., F. nucleatum, Veillonella, and Haemophilus parainfluenzae. F. nucleatum binds with A. actinomytemcomitans, Porphyromonas gingivalis, H. parainfluenzae, and Treponema spp. Most coaggregates that have been studied in detail involve two strains from different genera; this is referred to as intergeneric coaggregation. Intrageneric coaggregation is seen almost exclusively within oral viridans streptococci (235, 237). Many of these interactions appear to be mediated by a lectin from one cell type that interacts with a complementary carbohydrate receptor from the other cell type. Coaggregation may be important in the development of dental plaque because it allows the colonization of bacteria that are not able to adhere directly to the acquired pellicle. However, almost all the data on coaggregation derive from in vitro experiments. Indications that the phenomenon exist in vivo are the microscopic observations of dental plaque revealing the presence of two types of structures: gram-positive filaments covered by gram-positive cocci, referred to as corn cobs; and large filaments surrounded by gram-negative rods or short filaments, referred to as bristle brushes (353).
Another example of coaggregation is the synthesis of extracellular polysaccharides from sucrose by mutans streptococci. The glucosyltransferases that are bound to the surface of mutans streptococci synthesize glucans in the presence of sucrose. Thus, the polymers are cell associated and can bind to the tooth surface or to other bacteria via other glucosyltransferases or via independent glucan-binding components. These polysaccharides consolidate bacterial attachment to teeth and contribute to an increased stability of the plaque matrix. The synthesis of polymers by mutans streptococci in the presence of sucrose is probably one of the factors implicated in caries formation (299).
Bacterial interactions.
A variety of beneficial and antagonistic interactions may help in maintaining the homeostasis of the oral microbiota. Most of these bacterial interrelationships have been characterized in vitro or in animal models, and it is assumed that they operate in the same way in the human oral cavity.
Coaggregation is one exemple of commensalism and synergism that occurs between microbial species. Coaggregation allows the indirect adherence of some bacteria on oral surfaces. In addition, it was demonstrated that coaggregated cells were more resistant to phagocytosis and killing by neutrophils in vitro and in vivo (354). Several other examples of positive interactions are likely to occur in the oral cavity. The utilization of oxygen by facultative anaerobic bacteria reduces the oxygen concentration and the Eh to levels that allow the colonization of anaerobic bacteria (495). Different bacterial species may also cooperate in the utilization of substrates that they could not metabolize alone. In laboratory studies, P. gingivalis and F. nucleatum were shown to hydrolyze casein synergistically (158). Chemostat studies indicated that glycoprotein degradation may involve the synergistic action of several species possessing complementary patterns of glycosidase and protease activities (509). The development of complex food chains also contributes to the diversity and stability of oral ecosystems (299, 495). For example, the metabolism of carbohydrates by Streptococcus and Actinomyces generates lactate, which may be used by Veillonella. The utilization of lactic acid by Veillonella produces vitamin K, required by black-pigmented gram-negative rods, and H2, used by Wolinella.
Competition and antagonism mechanisms among oral resident bacteria may help to maintain the ecological balance by preventing the overgrowth of some resident bacterial species or the establishment of allochthonous bacteria (300, 495). It is more difficult to implant a bacterium in conventional animals than in axenic or antibiotic-treated animals (508). The barrier effect of the autochthonous microbiota against allochthonous and pathogenic species is known as colonization resistance (512). The competition for adhesion receptors, nutritional competition (322, 510), and the production of inhibitory substances (antagonism) are among the mechanisms involved in reducing bacterial colonization and preventing bacterial overgrowth. Recently, an inverse correlation was found between the proportion of salivary bacteria inhibiting Streptococcus mutans and the percentage of untreated carious teeth, suggesting a possible role of inhibitory substances in the maintenance of oral health (168). Inhibitory substances include organic fatty acids (112), hydrogen peroxide (296), lactic acid (365), antibiotics (417), enzymes (21), and bacteriocins (206, 366, 468, 469). The production of lactic acid by S. mutans and Lactobacillus generates low pH and inhibits the growth of S. sanguis and S. oralis as well as gram-negative bacteria. It has been suggested that the production of hydrogen peroxide by oral streptococci may reduce the growth of periodontopathogens. Bacteriocins are bactericidal proteinaceous substances, and those that are produced by streptococci may have a wide spectrum of activity against other gram-positive bacterial species (206, 366, 468). It has been argued that bacteriocins play a limited role in the ecology of the oral cavity because they are probably rapidly inactivated by the numerous proteases found in saliva (468). However, it has been found that some bacteriocins are not affected by human saliva (366). Many in vivo experiments with humans and animals indicate that the production of bacteriocins confers an ecological advantage. It is easier to implant strains of S. mutans that produce bacteriocins in the oral cavity than non-bacteriocin-producing strains (194, 228, 508).
External Factors
Diet.
It is well documented that frequent consumption of a high-sucrose diet enhances the development of S. mutans and Lactobacillus (335, 347, 457). The fermentation of sucrose into lactate generates a low pH, favoring acidogenic and acidophilic bacteria. The substitution of sucrose with weakly fermentable sugar alcohols such as xylitol results in a reduction of S. mutans numbers and caries (205, 287). Xylitol appears to selectively inhibit carbohydrate metabolism in S. mutans, which reduces acid production and thereby stabilizes the composition of the oral microbiota (49). Apart from the effect of sucrose, very few studies have addressed the effect of other dietary components on the oral microbiota. During our research on the effects of various dietary components on the oral microbiota of mice (34), we found that a high-starch diet favored an increase in the proportion of Enterococcus faecalis while a high-protein diet favored an increase in Lactobacillus murinus. Variations in the vitamin, lipid and mineral content of the diet had no direct effect on the oral microbiota of mice.
Oral hygiene and antimicrobial agents.
Oral hygiene is one of the most important factors in the maintenance of oral homeostasis and oral health. The mechanical removal of plaque by tooth brushing and flossing can almost completely prevent caries and periodontal diseases (307, 460). The addition of antimicrobial agents to dentifrices, mouthwashes, and varnishes increases the effect of mechanical oral hygiene procedures. Antimicrobial agents may assist in protection by reducing bacteria adhesion to the tooth surface, by reducing the growth of microorganisms and plaque accumulation, by selectively inhibiting only those bacteria directly associated with oral diseases, or by inhibiting the expression of virulence determinants, such as acid production or protease activity (302). Fluoride is found in most toothpastes and mouth rinses and is well known for its anti-caries properties. The principal caries-preventive effect of fluoride is attributed to the formation of fluoroapatite and calcium fluoride, which lead to an increase in the resistance of enamel to demineralization (48). It can also inhibit bacterial growth by reducing the sugar transport, glycolytic activity, and acid tolerance of many gram-positive species (48, 301). Fluoride can help stabilize the composition of the microflora by reducing the rate of acid production and the fall in pH during frequent carbohydrate intake (43). Other agents that have been formulated for commercial toothpastes and/or mouth rinses include chlorhexidine, quaternary ammonium compounds, plant extracts, metal ions, and phenolic compounds (97, 304). These antimicrobial agents have been shown to reduce dental plaque formation, caries, and gingivitis (97, 302).
Drugs and diseases.
Salivary gland hypofunction and xerostemia may result from the intake of xerogenic medication, irradiation treatments for head and neck cancer, and Sjøgren’s syndrome. Patients with xerostomia have a decrease capacity to eliminate sugars and buffer the acids found in plaque. In addition to suffering from the reduction in saliva protection, patients with xerostomia generally consume soft, high-sucrose diets and suck sour candies to keep their mouths moist (299). Patients suffering from xerostemia have higher levels of mutans streptococci, lactobacilli, staphylococci, and Candida, while the levels of S. sanguis, Neisseria, Bacteroides, and Fusobacterium are reduced compared with those in a healthy individual. They are also more susceptible to dental caries and candidiasis (63, 273).
Antibiotics that are given orally or systemically for the treatment of different infections may enter the oral cavity via saliva and gingival crevicular fluid and lead to a inbalance in the oral microbiota (416, 417). Antibiotics may suppress some resident bacterial populations which can result in overgrowth of antibiotic-resistant bacteria, infections by opportunist pathogens such as Candida, and colonization by exogenous potential pathogens such as yeasts and members of the Enterobacteriaceae.
Other factors.
Many other external factors may affect the oral microbiota; these include the wearing of dentures or partial dentures (305), smoking, oral contraceptives usage (539), malnutrition (474), host macroenvironment (150, 294, 295), and various exposures to exogenous bacterial species (150, 396).
SECRETORY IGA SYSTEM
IgA Structure
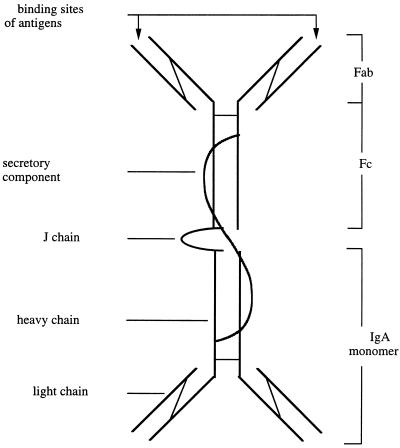
SIgA is the principal immunoglobulin isotype found in saliva and all other secretions. It exists as a polymeric molecule composed of two (or more) IgA monomers (300,000 Da), a J (joining) chain (15,600 Da), and a secretory component (SC) (70,000 Da) (reviewed in references 54, 81, and 220) (Fig. 1). Each monomeric IgA is formed of four polypeptides, two α-heavy chains and two light chains (kappa or lambda) linked covalently by disulfide bonds. The J chain and SC are disulfide linked to the Fc region of the IgA molecule (220). The J chain is a polypeptide synthesized within plasma cells that is involved in initiating the polymerization of IgA. The SC is a heavily glycosylated protein produced by mucosal epithelial cells. The SC stabilizes the structure of polymeric IgA and protects the molecule from proteolytic attack in secretions (224). It is referred to as the polyimmunoglobulin receptor (PIgR) in its membrane-bound molecular form. It is present on basolateral epithelial cell membranes and acts as a receptor for transepithelial transport of polymeric IgA (and IgM) (81, 497).
FIG. 1.
Schematic representation of SIgA. SIgA consists of at least two IgA monomers linked to a J chain and a secretory component (SC). The J chain and SC are disulfide linked to the Fc region of the IgA molecule. Each IgA monomers consist of two α-heavy chains and two light chains linked covalently by disulfide bonds. The wavy line represents the SC.
In humans, there are two IgA subclasses, IgA1 and IgA2, which occur in similar proportions in saliva and other secretions. The IgA1 and IgA2 heavy chains differ in only 22 amino acids, predominantly due to a deletion of 13 amino acids in the hinge region of IgA2; these amino acids are present in IgA1 (220, 498, 501). This structural difference renders IgA2 resistant to the action of a number of bacterial proteases that specifically cleave IgA1 in the hinge region (220). These IgA1 proteases are produced by several mucosal pathogens as well as by a large number of resident bacteria of the oral cavity and are thought to interfere with most of the protective properties of IgA antibodies (224). Salivary IgA antibodies against proteins and carbohydrates of bacteria occur predominantly in the IgA1 subclass, and the antibodies against lipoteichoic acid and lipopolysaccharide are more prevalent in the IgA2 subclass (64).
Two subclasses of IgA, IgA1 and IgA2, similar to those in humans have been identified in chimpanzees, gorillas, and gibbons (215, 216). Generally, only one IgA isotype is found in other primates, rats, and mice, with its structure differing from IgA1 and IgA2 (215, 216, 395, 502). Only IgA1 from gorillas and chimpanzees and IgA from orangutans are susceptible to cleavage by IgA1 proteases (380, 384).
Synthesis and Transport of Salivary IgA
Salivary IgA is produced by plasma cells that are located adjacent to the duct and acini of salivary glands (243). IgA-secreting plasma cells predominate in the major and minor salivary glands over plasma cells producing other Ig isotypes (328). Polymeric IgA containing J chain, secreted by plasma cells, is specifically recognized by the PIgR located on the basolateral surface of the ductal and acinar cells (497). The polymeric IgA-PIgR complex is internalized into endocytic vesicles and transported to the apical surface of the epithelial cells. After fusion of the vesicles with the cell membrane, the PIgR is proteolytically cleaved, which releases a portion of PIgR, called SC, and polymeric IgA into the secretions as SIgA (81, 497). During the external translocation, disulfide bonds covalently link SC with polymeric IgA, which in turn stabilizes the IgA-SC complex (54).
Induction of Salivary IgA Response
The salivary IgA response against oral antigens may be induced by two mechanisms. First, oral antigens may stimulate the proliferation and differentiation of lymphoid cells locally in the salivary glands. Salivary glands contain lymphoid tissues consisting of macrophages, T cells, and B cells, which may be directly accessible to oral antigens (110, 346, 363, 364). Oral antigens may enter into the duct gland by natural retrograde flow and gain access to underlying immune system cells through endocytosis by ductal epithelium (92). The antigens may then be captured by macrophages and presented to T and B cells (364). The indirect evidence for the presence of a local immune system associated with salivary glands was derived from topical immunization studies. The instillation of antigens into the parotid duct of monkeys has been shown to induce salivary IgA antibodies (129, 133, 346, 517). In humans, topical application of glucosyltransferase from S. sobrinus to the labial mucosa results in a salivary IgA antibody response (443). The minor salivary glands have short ducts and are located superficially throughout the lamina propria of the lips, cheeks, and soft palate mucosa. They may be continuously exposed to oral antigens by natural retrograde flow (346). The presence of bacteria deep within the secretory ducts of labial salivary glands of primates suggests that antigens can naturally infiltrate these ducts (346). However, the duct length of the major salivary glands probably precludes retrograde stimulation under natural conditions.
The second important mechanism involves the migration of antigen-sensitized IgA precursor B cells from gut-associated lymphoid tissues (GALT) to salivary glands (320, 324, 328). The GALT, including numerous solitary lymphoid nodules and particularly Peyer’s patches, are a rich source of precursor IgA B cells that have the potential to populate distant lymphoid tissues. These lymphoid follicules are covered by a specialized epithelium termed follicule-associated epithelial cells (FAE cells) or microfold cells (M cells) that take up and transport antigens from intestinal lumen into the underlying lymphoid tissue. Following antigen presentation by accessory cells, precursor IgA B cells and T cells leave the GALT via the efferent lymphatics and reach peripheral blood through the thoracic duct. The circulating B and T cells then migrate to the lamina propria of the intestine, the lungs, the genitourinary tract, and the secretory glands, where they are selectively retained (54, 81, 324). In these mucosal and glandular tissues, the precursor IgA B cells clonally expand and mature into IgA plasma cells under the influence of T cells. This cell distribution pathway from inductive tissues such as GALT to mucosal and glandular distant tissues has been termed the common mucosal immune system. Support for the existence of a common mucosal immune system is the finding of naturally specific IgA in secretions of glands not directly stimulated by antigens (10, 72, 123). Furthermore, experimental work with animal and humans has demonstrated that oral administration of bacterial antigens induces specific SIgA antibodies in saliva and other secretions (99, 101, 170, 325, 327, 333). Although GALT constitutes the major part of the mucosa-associated lymphoid tissues (MALT), the nasal mucosa-associated lymphoid tissues (NALT), which are represented by the tonsils and other organized lymphoid tissues of Waldeyer’s pharyngeal ring, may also constitute an important IgA inductive site (54). SIgA antibodies against inhaled antigens have been detected in various secretions including saliva.
Thus, the salivary glands contain the lymphoid cells that are necessary for generating a SIgA response independent from MALT and for pursuing the last stage of differentiation of antigen-sensitized IgA precursor B cells arising from GALT or NALT. It is possible that the induction of SIgA antibodies in saliva involves an initial antigenic stimulation in major inductive sites (GALT and NALT) followed by further local antigenic stimulation in the salivary glands to expand the pool of plasma cells.
Biological Functions of Secretory IgA
SIgA is considered the first line of defense against pathogens which colonize and invade surfaces bathed by secretions (323, 328). It is known that SIgA plays an important role in protection against infections caused by enteropathogens and viruses both in human and in animal models (147, 323, 328, 334, 387). The intrinsic resistance of IgA to proteolysis, which is reinforced by the presence of the SC, preserves the biological functions of the molecule in secretions (320). The multivalence of SIgA enhances its potential to agglutinate bacteria and neutralize toxins, enzymes, and viruses (224, 320). The decreased ability of SIgA to activate complement and opsonize bacteria for phagocytosis may limit local inflammatory reactions and mucosal tissue damage (54).
Inhibition of bacterial adherence.
The inhibition of bacterial adherence by SIgA is considered one of the most important defense mechanisms against mucosal bacterial invasion. In vitro, SIgA has been shown to limit the attachment of bacteria to epithelial cells isolated from buccal (525), intestinal (286, 530), urinary (463, 464), genital (499), nasal (250), and bronchial mucosa (461). SIgA antibodies to Vibrio cholerae reduce the adsorption of this bacterium to rabbit intestinal sections (286). Human salivary IgA inhibits the adherence of oral streptococci and Candida albicans to buccal epithelial cells (515, 525). Although SIgA may inhibit the attachment of oral streptococci to teeth (183, 222, 225), they may also promote the adsorption of some strains (222, 270). An explanation for these contradictory results will be given below. Evidence for the antiadherence properties of SIgA was obtained in studies involving passive immunization (32). Specific monoclonal SIgA antibodies directed against microbial surface components protected mice against intestinal infection caused by Salmonella typhimurium (334), V. cholerae (14, 528), and Shigella flexneri (373).
SIgA interferes with bacterial adherence to host surfaces by preventing both nonspecific and stereochemical interactions. The binding of SIgA to adhesins can reduce the negative surface charge and the hydrophobicity of bacteria, thus limiting the potential for ionic and hydrophobic interactions between bacteria and host receptors (283–285). The reduction of the hydrophobicity of bacteria is probably due to the heavy glycosylation of Fc and SC components, which confer hydrophilic properties to the SIgA molecule (224). Studies have also found that SIgA can sterically block the binding of adhesins to complementary surface receptors on host surfaces (183, 224). SIgA antibodies against fimbriae of gonococci and members of the Enterobacteriaceae reduced the adhesion of these bacteria to epithelial cells (464, 499). In addition, SIgA may impair bacterial adherence by agglutinating bacteria, thereby facilitating their clearance by secretions (59, 270, 286, 358). In vitro, the aggregation of E. coli by SIgA prevents its passage across intestinal tissue sections (9). The mechanisms involving aggregation of members of the Enterobacteriaceae by SIgA may be independent of antibody specificity. SIgA was shown to possess a carbohydrate receptor for the mannose-specific type 1 fimbrial lectin of E. coli (530).
Inactivation of bacterial enzymes and toxins.
SIgA can neutralize toxins by blocking their binding to cell receptors. SIgA antibodies prevent the binding of cholera toxin to intestinal epithelial cells (286) and partially protect animals against diarrheal diseases (14, 505). SIgA can also inhibit a variety of enzymes, perhaps by blocking their binding to substrates or by destabilizing the enzyme-substrate complex (35, 148, 162, 171, 176, 233). SIgA directed against glucosyltransferases of streptococci has been shown to inhibit the synthesis of extracellular polysaccharides and reduce dental plaque accumulation (233). Purified colostral SIgA inhibits IgA1 proteases that are produced by Neisseria meningitidis, N. gonorrhoeae, and Streptococcus sanguis (162). If IgA1 proteases play a role in the infectious process, the ability to neutralize IgA1 proteases may constitute a defense mechanism against mucosal invasion by some pathogens (162).
Synergism with other defense mechanisms.
SIgA may also act synergistically with innate immune factors present in secretions. It has been shown that the activity of the lactoperoxidase system against S. mutans was enhanced by the presence of SIgA (485). This improved activity was not dependent on the presence of specific antibodies against S. mutans. It has been suggested that the interaction between the Fc part of IgA and lactoperoxidase is responsible for stabilizing the enzymatic and antimicrobial activity of lactoperoxidase (224). SIgA may enhance the bacteriostatic effect of lactoferrin, and this effect can be inhibited by the addition of iron (149, 399). Although the mechanism by which SIgA increases the ability of lactoferrin to take iron from microorganisms is not clear, some evidence suggests that SIgA antibodies suppress the production of bacterial siderophores, which are able to remove iron from lactoferrin (149, 399). SIgA antibodies also appear to bind to lactoferrin and target the protein against microorganisms. The presence of complexes between SIgA and lactoferrin has been observed in secretions (224).
Some studies have suggested a synergistic action between SIgA and mucins. The binding of SIgA antibodies may render bacteria highly mucophilic, which facilitates their removal from the mucosa by the continuous renewal of the mucous layer (283). Furthermore, the low-molecular-weight salivary mucin, MG2, may participate in noncovalent interactions with SIgA. The binding of MG2 to Pseudomonas aeruginosa and Staphylococcus aureus is dependent on heterotypic complexing between MG2 and SIgA (33). The interaction occurs in solution but not when proteins are immobilized onto a solid surface. Thus, the complex MG2-SIgA does not promote bacterial attachment and can facilitate bacterial removal from the oral cavity by enhancing bacterial aggregation (33). The association between SIgA and agglutinins in saliva may also enhance bacterial aggregation (358, 406).
Virus neutralization.
SIgA plays an important role in viral immunity because of its presence at the site of initial contact between virions and host cells (81). A protective effect of SIgA against respiratory and enteric viral infections has been demonstrated (348, 387, 483). Mice that had been passively immunized with SIgA antibodies against influenzae virus were protected against experimental nasal infection with this virus (387). In vitro, it has been demonstrated that human immunodeficiency virus type 1 can be neutralized by SIgA (66). The mechanisms involved in viral inactivation are complex and not fully understood. Traditionally, SIgA was thought to prevent the penetration of virions into epithelial cells by sterically blocking their adhesins. SIgA antibodies to hemagglutinin of the influenza virus reduce viral attachment to cells and penatration of virions into cells (483). However, SIgA antibodies enhance the binding of the gastroenteritis virus but block its subsequent penetration into the cells (348). It has been suggested that SIgA antibodies induce agglutination of the gastroenteritis virus by enhancing nonspecific viral attachment to epithelial cells (81, 224). Armstrong and Dimmock (15) provided evidence in support of different mechanisms for viral neutralization, according to the number of antibodies per virion. High concentrations of anti-hemagglutinin polymeric IgA inhibited the attachment and internalization of influenza virus virions to the tracheal epithelial monolayer, while lower concentrations of IgA did not prevent virus attachment and internalization but did inhibit the fusion and transcriptase activities of the virus. The mechanism of neutralization appears to involve conformational changes in the structure of the hemagglutinin following antibody binding, which subsequently transduces a signal that affects a crucial molecular function within the virion (15, 111, 484). Thus, dimeric IgA can neutralize influenza virus by the inhibition of fusion, internalization, and attachment as the ratio of IgA to virus increases (111).
Recent in vitro cell culture experiments suggested that dimeric IgA can neutralize virus within infected mucosal epithelial cells, if the epithelial cells express the polymeric Ig receptor (transmembrane secretory component) (310–312). The epithelial cells used were MDCK cells (derived from canine renal tubule cells) that have been transfected to express the rabbit polymeric Ig receptor and therefore were capable of endocytozing dimeric IgA. It was shown that dimeric IgA antibodies that were actively transported through epithelial cells by the polymeric Ig receptor, bind to viral proteins within the cells and prevent viral replication (253, 312). This mechanism of neutralization may also be effective during the transport of dimeric IgA into secretions in vivo (253). What remains to be determined is in which subcellular compartment this process takes place.
Complement activation.
The ability of serum or SIgA to activate complement remains controversial. The use of different assays and different sources of IgA and complement have resulted in contradictory reports. Compared with IgG and IgM, IgA is a poor activator of complement, and it is not clear whether IgA may efficiently induce complement-mediated bacterial death via opsonization or cell lysis. There is general agreement that IgA does not activate the classical complement pathway in vitro (401, 408). Human serum or SIgA does not bind the C1q (193, 401) component, while rat polymeric and dimeric IgA can bind the C1q component but does not activate the C1 component (193).
There is evidence that IgA might activate the alternative complement pathway (191, 192, 372, 388, 389, 459). The ability of IgA to activate the alternative pathway in vitro has been demonstrated in experiments with aggregated SIgA and monoclonal IgA. It has also been shown that the alternative pathway can be activated, as revealed by C3 deposition and solubilization of the immune complex, by immune precipitates composed of mouse or rat monoclonal IgA and the corresponding antigen (191, 388, 389). Bohnsack et al. (37) reported that murine IgA monoclonal antibodies directed against group B streptococci mediated C3 deposition through the alternative pathway and enhanced phagocytosis of the bacteria. In patients with nephropathy, IgA deposition is frequently associated with the C3 component, suggesting that IgA may also activate the alternative pathway in vivo (31). In contrast, others have reported that all forms of human IgA antibodies bound to corresponding antigens fail to fix C3 component by the alternative pathway (93, 203, 349, 408). It has been postulated that denaturation of IgA might be responsible for its alternative-pathway-activating property (349, 408).
In general, studies have revealed that complement activation by IgA results in consumption of the C3 component but rarely in consumption of the C5 component (220, 224, 372). Thus, the C5a component, a major anaphylatoxic and inflammatory peptide, is probably rarely released in vivo (224). Furthermore, the assembly of the membrane attack complex (C5 to C9) and bacteriolysis are probably rare events (220, 224). Several studies suggest that IgA antibodies inhibit the activation of the classical pathway by IgG and IgM antibodies recognizing the same antigens (178, 349, 411). IgA antibodies reduce IgG and IgM complement-mediated bacteriolysis in vitro and Arthus reactions in mice (414). This inhibition might be attributed to competition between IgA and other isotypes for antigen binding sites or to interference with the availability of the initial classical pathway components (193, 349, 411). Since it is known that SIgA is a poor activator of complement and that secretions are generally deficient in complement components, complement-mediated microbial killing is probably of limited immunological importance at mucosal surfaces.
IgA-dependent cell-mediated functions.
Receptors for the Fc portion of IgA (FcαR) are expressed on several cell types including polymorphonuclear neutrophils (PMNs) (136), monocytes and macrophages (25, 136, 155), eosinophils (3), and lymphocytes (4, 200, 277). The expression of FcαR seems to be influenced by the level of IgA in the environment surrounding the cells. The proportion of PMNs and monocytes expressing FcαR cells is higher in the oral cavity than in the peripheral blood (25, 136). The expression of FcαR on blood neutrophils, monocytes, and lymphocytes can be enhanced by incubation with SIgA or myeloma IgA (136). However, the exact role of IgA as modulator of the leukocytes effector functions is still unclear. Some studies suggest that IgA is able to induce phagocytosis and a respiratory burst in monocytes, macrophages, and PMNs (25, 136, 167, 213, 429, 520, 537). Fanger et al. (136) observed phagocytosis of ox erythrocytes that had been opsonized with SIgA antibodies by PMNs from the oral cavity but not by peripheral blood PMNs. Human serum IgA and SIgA were found to enhance the phagocytosis of Staphylococcus aureus by PMNs and elicit a respiratory burst as measured by the release of H2O2 (167). The capability of IgA to induce antibody-dependent cellular cytotoxicity (ADCC) was also reported in experiments with monocytes (276) and T lymphocytes (470–472) as effector cells. However, these experiments have not been independently confirmed. Intraepithelial CD4+ lymphocytes from the gut mucosa exerted antimicrobial activity against Salmonella and Shigella in the presence of specific SIgA antibodies (471). The number of T lymphocytes mediating IgA-dependent ADCC increased when individuals were orally immunized with Salmonella (470, 472).
In contrast, other studies have found that the interaction between IgA and FcαR leads to inhibition of phagocytosis, chemotaxis, and ADCC (415, 434, 513, 526, 531, 532). It was reported that SIgA and serum IgA inhibited the phagocytosis of Staphylococcus epidermidis and Candida albicans by PMNs (415, 526). It appears that IgA reduces PMN function by interfering with the binding of other opsonins such as IgG and the C3b component to their receptor (415, 531). In addition, other studies have suggested that IgA may directly inhibit phagocyte activation, induced by a variety of chemotactic stimuli (lipopolysaccharide, Haemophilus influenzae type B, and n-formyl-methionyl-leucyl-phenylalanine [fMLP]) without influencing receptor-ligand interaction. It has been shown that human serum IgA down-regulates the induction and release of inflammatory cytokines (tumor necrosis factor alpha and interleukin-6) in activated monocytes (531). Human serum IgA also inhibited the generation of respiratory burst and release of reactive oxygen intermediates, such as superoxide anion, H2O2, and the hydroxyl radical, in activated monocytes and PMNs (531). Thus, IgA has a dual effect on phagocytes which appears to be dependent on their state of activation (465, 531). Sybille et al. (465) demonstrated that the interaction of human IgA with FcαR on human PMNs stimulated chemotaxis and increased PMN migration in response to suboptimal concentrations of fMLP but decreased the PMN responses at optimal fMLP concentrations.
It is probable that SIgA-dependent cell-mediated activity does not constitute a primary line of defense at mucosal surfaces due to the small number of functional leukocytes in secretions and the large number of microorganisms that colonize mucosal surfaces (81, 224). However, epithelium-associated phagocytes and cytotoxic cells may act as a secondary line of defense against pathogens that invade intestinal epithelium, such as Salmonella and Shigella (470–472). Furthermore, by modulating the release of inflammatory mediators, IgA both in secretions and within the mucosa may interfere with the development of chronic inflammation initiated by microbial pathogens and antigens (54, 56, 531).
Immune exclusion.
One of the major functions of SIgA is to perform immune exclusion, which consists of limiting the penetration of antigenic materials through the mucosal epithelium (54). This involves the binding of SIgA antibodies with antigens, which facilitates their removal from mucosal surfaces. Furthermore, polymeric IgA containing immune complexes formed within the mucosal lamina propria may bind to the polymeric IgA receptor and be transported across the epithelium by the same mechanism as free polymeric IgA (253, 312). This antigen-handling mechanism is particularly important in the intestinal tract, where components of food and microbiota may gain access to the lamina propria. It has been established that previous enteric exposure to foreign proteins diminishes subsequent intestinal adsorption of the same antigen in association with the production of specific SIgA antibodies (13, 518). It is also known that IgA-deficient subjects have an increased intestinal permeability to macromolecules that lead to the formation of circulating immune complexes (98, 188, 268). Since SIgA is relatively ineffective in activating complement and promoting phagocytosis, immune exclusion is primarily a noninflammatory mechanism (54). By competing with proinflammatory IgM, IgG, and IgE antibodies, SIgA at mucosal surfaces, as well as locally produced dimeric IgA and serum-derived IgA within the lamina propria, has been shown to control levels of inflammation in mucosal tissues (54, 253, 312). Experimental studies with rodent models suggest that an IgG or IgE immune response to antigens may damage intestinal mucosal tissues, rendering them more susceptible to penetration by a variety of macromolecules through mucosal epithelium (36, 496). A decrease in the number of IgA-producing cells and an increase in the number of IgG-producing cells have been observed in gastrointestinal inflammatory lesions (54, 106, 268). The mucosal integrity may be damaged by lysosomal enzymes and oxygen intermediates released from PMNs that are attracted by complement-activating IgG immune complexes. Some studies reported a higher incidence of inflammatory and autoimmune diseases in IgA-deficient patients (268).
ROLE OF SECRETORY IGA IN ORAL MICROBIAL ECOLOGY
It is well established that SIgA is the predominant Ig in whole saliva and is considered to be the main specific defense mechanism in the oral cavity. SIgA antibodies could help maintain the integrity of the oral surfaces by limiting microbial adherence to epithelial and tooth surfaces; by neutralizing enzymes, toxins and virus; or by acting in synergy with other antibacterial factors such as lysozyme, lactoferrin, salivary peroxidase, and mucins. SIgA may also prevent the penetration of antigens in the oral mucosa. Since saliva is generally deficient in complement components and functional effector cells (monocytes, PMNs, and lymphocytes), it is unlikely that other functions associated with SIgA, such as complement activation, opsonization, and SIgA-dependent ADCC, occur in the supragingival environment. However, inflammatory cells and complement are present in the subgingival environment, and these functions may be accomplished by serum IgA.
One of the major intriguing questions concerning the role of SIgA in oral microbial ecology is whether it has an effect on indigenous bacteria. Despite the presence of the secretory immune system and high levels of SIgA in saliva, the resident microbiota still persists in the oral cavity. The survival of indigenous bacteria in the oral cavity has been attributed to their reduced susceptibility to and their ability to avoid immune mechanisms (30, 44, 87). Dubos et al. (114) postulated that autochthonous bacteria were not immunogenic for their host, perhaps because these microorganisms had achieved a state of symbiosis with their host over a long period of evolutionary adaptation. Other microorganisms of the resident microbiota that are potentially pathogenic would elicit a protective response and be eliminated or kept at low levels under normal circumstances. Several experimental studies support the hypothesis that the immune system is relatively tolerant to its autochthonous microbiota (28, 30, 74, 115, 141, 142, 281, 375). Germ-free mice monoassociated with indigenous Escherichia coli or Bacteroides isolated from conventional mice exhibited a lower systemic immune response than did germ-free mice monoassociated with E. coli and B. fragilis derived from human sources (30). Oral administration of Salmonella paratyphi to germ-free mice resulted in the development of germinal centers in Peyer’s patches and serum antibody response, while oral administration of Enterococcus faecalis, a member of the resident microbiota of mice, failed to induce an immune response (375). Duchmann et al. (115) found that humans were tolerant to their own intestinal microbiota but responded to the intestinal microbiota of other individuals.
Tolerance could be the result of clonal elimination (cell death), clonal anergy (functional inactivation without cell death), or active suppression of antigen-reactive B and T cells (115, 146, 281, 427). It has also been suggested that indigenous bacteria could share surface antigens with host tissues (142) or become coated with host-derived molecules so as to not be recognized as foreign (44, 87). Although logical and appealing, these hypotheses have unfortunately often become part of established theories without the required support from a wide range of experiments. Furthermore, most of these studies compared the systemic immune response between indigenous and nonindigenous bacteria (28, 30, 141, 142) and only a few data reported on the mucosal IgA response (74, 375, 433). A comparison of germfree and conventional mice revealed that the presence of resident microbiota on mucosal surfaces is essential for the normal development of IgA-bearing plasma cells in Peyer’s patches and lamina propria of the gut (95, 340, 507). Evidence indicated that the ability to induce the mucosal immune response may vary among indigenous bacterial species (340, 432, 433). In support of this view, Moreau et al. (340) reported that monoassociation of germfree mice with Bacteroides and E. coli elicited a higher development of intestinal IgA plasmocytes than did monoassociation with Lactobacillus, Streptococcus, Actinobacillus, Eubacterium, and Clostridium, while Corynebacterium and Micrococcus were not immunogenic. In that study, the specificities of the SIgA response were not tested.
Although indigenous bacteria may induce a lower mucosal immune response than allochthonous bacteria, many studies have revealed the presence of naturally occurring SIgA antibodies directed against a variety of resident bacteria in saliva and other secretions of humans (7, 10, 55, 59, 64, 151, 426, 436) and animals (108, 109, 293, 294, 296, 297). These antibodies have been detected against whole cells or purified antigens including polysaccharides, proteins, lipoteichoic acids, and glucosyltransferases. Oral bacteria are generally coated with SIgA, as demonstrated in both humans (55) and mice (108). Salivary IgA antibodies have also been detected in the acquired pellicle and dental plaque (361, 400, 477).
The development of the salivary IgA antibody response against oral indigenous bacteria has been investigated in humans. Smith and colleagues (153, 444, 452) found that the appearance of salivary IgA antibodies to oral streptococci in infants and children correlated well with the sequence of colonization of these bacteria in the oral cavity. Salivary IgA antibodies to antigens of S. salivarius and S. mitis were detected in infants as young as 5 weeks old, which reflects the early colonization of the mouth by these bacteria (444, 452). The specificities of the IgA antibodies were suggested by the absence of reaction against S. mutans and S. sanguis, which do not colonize predentate infants. Salivary IgA antibodies to glucosyltransferase of S. sanguis appeared only after 1 year of age, while salivary IgA antibody to glucosyltransferase of S. mutans, an organism that colonizes later than S. sanguis, was detected only at 3 to 4 years of age. The same study also found that salivary antibodies specificities to oral streptococci continued to increase in intensity and quantity with the length of exposure to these microorganisms (451). These findings suggest that the mucosal immune system is capable of responding to indigenous microorganisms early in life and that SIgA antibodies may influence the colonization patterns of the indigenous microbiota (441).
In contrast, other studies suggest that a major part of IgA antibodies directed against resident bacteria are generated by cross-reactive antigens from other bacteria (24). For example, lipoteichoic acids from a wide range of bacterial genera are cross-reactive (57, 413) and glucosyltransferases of oral streptococci are antigenically related (450). Some investigators have reported that salivary IgA antibodies react with S. mutans in saliva of infants without any detectable S. mutans in their mouths (24, 71, 390, 492). These antibodies may have resulted from a challenge with mutans streptococci that did not lead to permanent colonization. Alternatively, antibodies may have been induced by cross-reactive antigens from food or other indigenous oral or intestinal microorganisms. Cole et al. (91) found that adult conventional rats that were free of mutans streptococci exhibited salivary IgA antibodies reactive with whole cells, glucosyltransferase, and serotype carbohydrate (g) of S. sobrinus. Thus, the naturally occurring SIgA antibodies to indigenous bacteria may reflect a response to a number of different cell wall antigens, some of which are specific to bacteria and others of which are shared with other bacteria (24, 79, 522, 523).
It is thought that naturally occurring SIgA antibodies may play an important role in the homeostasis of oral resident microbiota and in the prevention against caries and periodontal diseases (328, 444). Naturally occurring SIgA antibodies have been detected against S. mutans, A. actinomycetemcomitans, and P. gingivalis which are strongly associated with oral diseases (64, 426). To gain insight into the role of SIgA in controlling the resident microbiota, we have reported a wide range of studies evaluating the role of SIgA and bacterial IgA1 proteases in bacterial adherence to teeth, the association of natural SIgA antibodies with caries and periodontal diseases, the oral microbiota and oral health status in patients with IgA deficiency, and the effect of active and passive immunization on oral bacteria and oral diseases.
Role in Bacterial Adherence
The adherence to oral mucosa and teeth is the first important step for bacteria in colonizing the oral cavity. SIgA may interfere with this process by blocking adhesins, reducing hydrophobicity or aggregating bacteria. SIgA has been shown to inhibit the adherence of oral bacteria to oral epithelial cells (525). Salivary IgA may also reduce bacterial adhesion to hydroxyapatite and enamel and thus reduce the formation of the dental plaque. SIgA constitutes about 2% of the dry weight of human dental plaque. SIgA is found in the salivary pellicle in considerable quantities and in a biologically active state (270, 361, 477). Saliva-coated hydroxyapatite particles or beads are frequently used in model studies of dental research to mimic tooth surfaces (85). Studies in vitro have shown that SIgA and other salivary proteins are rapidly adsorbed by hydroxyapatite when this mineral is exposed to saliva (222, 360, 400). Studies have evaluated the adherence of bacteria to saliva-coated hydroxyapatite in the presence of various SIgA preparations, such as salivary IgA-containing fractions and colostrum SIgA (154, 183, 270, 383). The results were inconclusive and varied according to the strain tested (154, 183, 222, 270, 383). Although studies demonstrated that SIgA antibodies in solution inhibited the binding of some strains of oral streptococci (S. sanguis and S. oralis) to saliva-coated hydroxyapatite (183, 222, 291, 383), they enhanced the binding of other strains (222). Some investigators also found an inhibitory effect of IgA antibodies on S. mutans adherence (183, 256, 383), while others did not find such an effect (154, 222).
The difference in the size of bacterial aggregates formed by each bacterial strain may explain the contrasting results (269). It has been shown that the formation of large aggregates results in decreased numbers of adhered bacteria whereas the formation of small aggregates results in increased numbers (269). However, this parameter has not been measured in the above-mentioned studies. In most experiments, the antigen specificity of the antibodies was unknown and the lack of adherence-inhibiting effect could be explained by the lack of IgA antibodies against adhesins or by a difference in adhesins expressed on the surface of bacteria. One study demonstrated that only SIgA antibodies directed against antigen I/II inhibited the initial adherence of S. mutans to saliva-coated hydroxyapatite (183). The growth conditions of the bacteria prior to the adherence assay may influence antigen expression, reactivity with antibodies, and binding to hydroxyapatite. Kilian et al. (222) found that SIgA antibodies inhibited the binding of S. mutans only when they had been propagated in the absence of sucrose. The synthesis of glucan from sucrose may have enhanced adherence to hydroxyapatite. However, these results were not confirmed in a following study by the same research group (383). Under certain experimental conditions, bacteria may also release SIgA antibodies bound to cell surfaces and enhance their ability to adhere to the surface. It has been demonstrated that SIgA- and IgG-coated S. mutans may release bound antibodies in the form of an antibody-antigen immune complex, which reduces the inhibitory effect of antibodies on the adherence to salivary agglutinin-coated hydroxyapatite (256). The active release of bound antibodies may be a strategy used by bacteria to counteract the neutralizing effect of naturally occurring antibodies in the oral cavity.
Salivary IgA antibodies in the salivary pellicle may expose the Fab fragments, which can act as receptors for bacteria. The net effect of IgA antibodies on bacterial adherence to hydroxyapatite may be the result of the reaction of IgA antibodies in the fluid phase inhibiting bacterial adherence and those present in the salivary pellicle promoting bacterial binding (154, 222). The presence of SIgA antibodies adsorbed to hydroxyapatite enhances the sorption of some strains of S. sanguis (222, 270) but, in contrast, inhibits the adherence of S. oralis and S. salivarius and does not significantly influence the adherence of S. mutans (154, 222). The difference among bacterial species is difficult to explain and may be due to the antigenic specificity of antibodies bound to hydroxyapatite. The orientation of the SIgA molecule in the pellicle may allow the binding to only certain antigens. The active release of bound antibodies by bacteria may affect their desorption from hydroxyapatite, and this process may be specific only for certain antigens or bacteria. However, these hypotheses have not been verified.
Other salivary components, including mucins and other salivary agglutinins, are also important in inhibiting or promoting adhesion to hydroxyapatite. In most studies evaluating the effects of salivary IgA antibodies on bacterial adherence to saliva-coated hydroxyapatite, the bacteria were treated with IgA preparations before the adherence assay (183, 222, 256, 383). The effect of SIgA antibodies on bacterial adherence in relation to other salivary factors could be determined by treating bacteria with the different salivary fractions to which SIgA is added. Alternatively, SIgA could be added to saliva from IgA-deficient patients. Unfractionated parotid saliva and some salivary fractions with no detectable amounts of IgA have a more pronounced inhibitory effect on S. mutans adherence than SIgA-containing fractions (154). It cannot be excluded that in vivo, the influence of SIgA antibodies on initial bacterial adherence to hydroxyapatite is dependent on other adherence-promoting and adherence-inhibiting factors including salivary receptors, antimicrobial factors, bacterial antigen expression, active release of bound antibodies, and availability of sucrose.
Role of IgA Proteases in Bacterial Adherence
Several bacterial species that colonize mucosal and tooth surfaces produce IgA proteases. Most of these enzyme are known as IgA1 proteases, extracellular products that cleave serum IgA1 and secretory IgA1 in the hinge region and release intact Fab and Fc (or Fc.SC) fragments. The unique enzyme that is capable of cleaving both IgA1 and IgA2 of the A2m(1) allotype is produced by an intestinal isolate of Clostridium ramosum. The cleavage of SIgA1 may lead to a loss of protective properties of the antibody molecule. IgA1 proteases are produced by various mucosal pathogens including Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and N. gonorrhoeae, suggesting that they are important virulence factors. However, the production of IgA1 protease does not necessarily confer invasive properties to bacteria; other virulence factors such as capsules, toxins, and proteolytic activities are probably also required to cause diseases. IgA proteases are produced by some oral indigenous bacterial species including S. sanguis, S. oralis, and S. mitis biovar 1, which are particularly successful in initiating the colonization of oral mucosa and teeth (223, 225, 374). These enzymes are also produced by Prevotella and Capnocytophaga species, which may be involved in periodontal diseases (143, 144, 223). In addition, suspected periodontopathogens, such as Porphyromonas gingivalis and Prevotella intermedia, produce proteases that are capable of completely degrading IgA1 and IgA2. However, this type of degradation does not appear to involve an initial cleavage in the hinge region (223).
An analysis of the role of IgA1 proteases in bacterial adherence may indirectly provide information on the importance of salivary IgA in oral microbial ecology. The production of IgA1 proteases may counteract the adherence-inhibiting activity of SIgA antibodies and enhance the ability of indigenous bacteria to colonize oral surfaces. The effect of IgA1 proteases on bacterial adherence has been investigated in vitro by treating bacteria with SIgA alone or with SIgA and IgA proteases. In these studies, IgA1 proteases enhanced the adherence of oral streptococci, including S. sanguis, S. oralis, and S. mutans, to saliva coated-hydroxyapatite (183, 383). Although S. mutans does not synthesize IgA proteases, the production of this enzyme by primary colonizers may cleave SIgA1 antibodies directed against other bacteria and enhance their colonization to oral surfaces (183).
The cleavage of SIgA may interfere with the functions attributed to the Fc part of the molecule (224). As in the intact molecule, Fabα fragments retain their capacity to bind to antigens (291, 383). These fragments may bind to bacteria after the cleavage of SIgA1 molecules, or, alternatively, SIgA1 may bind to bacterial surfaces before being exposed to IgA protease, and Fabα fragments are left on bacterial surfaces (383). Although Fabα fragments retain their full antigen-binding capacity and may block adhesins (183, 291, 383), they are probably unable to aggregate or to reduce the hydrophobicity of bacteria. Fabα fragments are dominated by hydrophobic residues, whereas (Fcα)2SC is more hydrophilic due to the large amount of carbohydrate within the Fcα region and SC (224). Furthermore, as hydrophobic interactions appear to be important in bacterial adherence to hydroxyapatite, Fabα fragments left on the bacterial surfaces may enhance bacterial adherence (183, 383). It has been demonstrated that Fabα fragments bind more readily than SIgA1 to saliva-coated hydroxyapatite (183). Although SIgA may be incorporated into the pellicle during its formation, it interacts poorly with preformed pellicle (183). Finally, Fabα fragments that are bound to surface antigens could protect bacteria from the mucosal immune system by blocking access to intact IgA antibodies (224).
Because IgA1 proteases exclusively cleave human, chimpanzee, orangutan, and gorilla IgA (88, 240), this precludes the use of animal models to study the role of these enzymes in oral microbial ecology. However, human studies may reveal the potential ecological significance of IgA1 proteases. The IgA proteases are not susceptible to physiological protease inhibitors, and some experiments suggest that the cleavage of SIgA occurs in saliva and dental plaque from humans (6, 201, 224). The detection of Fabα fragments on dental plaque bacteria suggests that IgA1 protease activity promotes the formation of dental plaque in humans (6). Other observations indirectly support the role of IgA proteases in bacterial colonization of the oral cavity. The IgA1 protease activity can be described as being associated almost exclusively with the streptococci that adhere to a clean tooth surface and to the oral mucosa, a shedding epithelial surface necessitating a constant recolonization by the bacteria (144, 225). The proportion of streptococci with IgA1 protease activity is much lower in mature supragingival and subgingival plaque, and on the tongue. Among the streptococci that initiate the colonization of clean dental enamel, nearly 90% have IgA1 protease activity compared with only 17% of the streptococci in mature dental plaque (144, 225). In support of these results, salivary SIgA antibodies that are reactive with oral streptococci or suspected streptococcal adhesins (proteins and glycoproteins) were found to be primarily of the IgA1 subclass (7, 64).
However, no difference in the proportions of IgA1 protease-producing streptococci was found between normal and IgA-deficient individuals (386). This finding suggests that evasion of the adherence-inhibiting effect of SIgA is not essential for the colonization of oral surfaces by members of the indigenous microbiota (144). Since IgA1 proteases are extracellular enzymes, they may not cleave IgA directed only against protease-producing bacteria (6). Thus, IgA1 proteases do not necessarily confer an ecological advantage to the protease-producing bacteria but may promote the colonization of all members of the oral indigenous microbiota (386). The equilibrium between the indigenous oral bacteria may depend on the balance between IgA-inhibiting adherence and IgA protease-promoting adherence activities, and the net effect of these factors may not be detectable in vivo. In addition to this complex interaction, there are salivary IgA antibodies directed against IgA1 proteases (162) and glycosidase activity which may increase or decrease the susceptibility of IgA1 antibodies to cleavage (385). As well, IgA proteases may have salivary substrates other than IgA which favor the colonization of oral streptococci to pellicle-coated enamel and oral mucosa (386). Salivary IgA antibodies and, subsequently, the degradation of these antibodies may not be important factors in the initial adherence of indigenous bacteria to oral surfaces. Other factors may also favor the colonization of IgA1 protease-producers such as the presence of large numbers of specific adhesion receptors on oral surfaces. In our research, it was shown that mouse IgA was resistant to IgA1 protease activity but that the ability to completely degrade IgA did not confer an ecological advantage to oral resident bacteria. Our studies showed that Lactobacillus murinus, the indigenous oral bacterial species with the lowest proteolytic activity against IgA and other salivary defense components, predominated in the oral cavity of mice (116).
Correlation between Secretory IgA and Oral Diseases
The role of salivary IgA in the protection against dental caries has been investigated in many studies both in children and adults. Earlier experiments attempted to correlate total salivary IgA concentrations with caries susceptibility, as recorded by an index of decayed, missing, filled teeth (DMFT) or surfaces (DMFS) (reviewed in reference 53). However, the results of these studies were variable, with positive, negative, or no correlation found between total salivary IgA and dental caries (53). Such correlation analyses are complicated by the high intrinsic variability in levels of salivary IgA between individuals and within the same individual over short and long periods (58, 109, 151). The level of salivary IgA may vary according to salivary flow rate (52), age (248, 486), hormonal factors (166, 521), smoking habits (27), emotional states (210), physical activity (494), and genetic background (179). Since the concentration of salivary IgA is inversely correlated with the salivary flow rate (52), experiments with uncontrolled salivary flow rate are subject to increased variability in levels of salivary IgA. Furthermore, this type of experiment does not take into account the antibody specificities of salivary IgA.
Since mutans streptococci are considered to be the primary agents of caries, other studies have examined the relationship between the level of naturally occurring salivary IgA antibodies against mutans streptococci (S. mutans and S. sobrinus) and the level of mutans streptococci or dental caries. Most of these studies were cross-sectional designs that attempted to correlate IgA antibodies with caries susceptibility (DMFT or DMFS) (1, 71, 72, 75, 134, 135, 169, 171, 173, 195, 265, 390, 402, 491) or the presence of active lesions (without considering past caries experience) (10, 38, 39, 72, 75, 202, 491). These studies also produced confusing and conflicting data. The correlation between salivary IgA antibodies and DMF index was found to be positive (75), negative (71, 72, 134, 171, 173, 265, 402), or not significant (1, 135, 169, 195, 390, 491) (Table 2). Similarly, studies found positive (202), negative (38, 39, 72, 75, 491), or no (1, 10, 169) correlation between salivary IgA antibodies to mutans streptococci and active caries (Table 3). A negative correlation indicated that low levels of salivary IgA antibodies were associated with high caries activity or caries susceptibility.
TABLE 2.
Relationship between natural salivary IgA antibodies to mutans streptococci and caries experience
| No. of subjects in study group (age [yr]) | Sample | Antigens of mutans streptococci in immunoassay (serotype) | Correlation of salivary IgA antibodies with DMFSa | Protection against caries | Reference |
|---|---|---|---|---|---|
| 17 (2.5–5.5) | Whole saliva | Whole cells (b, c) | − | Yes | 134 |
| 289 (military recruits) | Whole saliva | Whole cells (c, g) | − | Yes | 265 |
| 42 (22–39) | Whole and parotid saliva | Whole cells (c), antigen I/II, carbohydrate (c) | − | Yes | 72 |
| 64 (3–6) | Whole saliva | Whole cells (c, d), antigen I/II, carbohydrate (c, d) | − | Yes | 71 |
| 131 (17–44) | Parotid saliva | Whole cells (c), purified antigens | − | Yes | 171 |
| 41 (7–11) | Whole and parotid saliva | Whole cells (c) | − | Yes | 402 |
| 40 (dental school students) | Whole and parotid saliva | Whole cells (c), antigens | − | Yes | 173 |
| 96 (18–24) | Parotid saliva | Whole cells (c) | + | No | 75 |
| 100 (20–30) | Whole saliva | Whole cells (a, c, d) | No | No | 135 |
| 63 (6–13) | Whole saliva | Whole cells (b–g) | No | No | 390 |
| 50 (19–21) | Whole saliva | Whole cells (c) | No | No | 169 |
| 67 (4–9) | Whole saliva | Whole cells (c) | No | No | 1 |
| 59 (22–32) | Whole saliva | Whole cells (c) | No | No | 491 |
| 43 (20–64) | Whole saliva | Whole cells (g), antigen I/II, carbohydrate (g) | No | No | 195 |
Negative (−), positive (+), or no correlation.
TABLE 3.
Relationship between natural salivary IgA antibodies to mutans streptococci and the presence of active caries lesions
| No. of subjects in study group (age [yr]) | Sample | Antigens of mutans streptococci in immunoassay (serotype) | Correlation of salivary IgA antibodies with caries activitya | Protection against caries | Reference |
|---|---|---|---|---|---|
| 100 (20–30) | Whole saliva | Lipoteichoic acid | − | Yes | 38 |
| 53 (3–14) | Whole saliva | Whole cells (c), glucan, lipoteichoic acid | − | Yes | 39 |
| 42 (22–39) | Whole and parotid saliva | Whole cells (c), antigen I/II, carbohydrate (c) | − | Yes | 72 |
| 59 (22–32) | Whole saliva | Whole cells (c) | − | Yes | 491 |
| 96 (18–24) | Parotid saliva | Whole cells (c) | − | No | 75 |
| 55 (18–20) | Parotid saliva | Whole cells (b, c) | + | No | 202 |
| 79 (mothers) | Whole saliva | Whole cells (c) | No | No | 10 |
| 50 (19–21) | Whole saliva | Whole cells (c) | No | No | 169 |
| 67 (4–9) | Whole saliva | Whole cells (c) | No | No | 1 |
Negative (−), positive (+), or no correlation.
One major difficulty encountered in interpreting the results came from the fact that some studies attempted to correlate salivary IgA antibodies with DMF scores while others correlated salivary IgA antibodies with the presence of active caries. The correlation of salivary IgA antibodies with DMF scores is questionable. The DMF score provides information on present and past dental caries (missing and filled teeth) and does not specifically assess the dental health condition at the time of evaluation. It was found that subjects with large numbers of missing and filled teeth and active caries had lower levels of salivary IgA antibodies than did subjects with large numbers of missing and filled teeth without active lesions (75). It has therefore been recommended that subjects with present caries experience and those with previous caries experience be analyzed separately (71, 75). Furthermore, some studies measured a coefficient of correlation between salivary IgA antibodies and caries experience (DMF) (1, 134, 135, 491) while other studies compared salivary antibodies in caries-resistant (low DMF) and caries-susceptible (high DMF) subjects (71, 72, 75, 171, 173, 195, 265, 390, 402). The criteria used for separating subjects into caries-resistant and caries-susceptible groups were shown to vary among studies, and this may also have contributed to the contradictory reports. In general, the limit of DMFS scores for caries-resistant subjects was equal to zero or lower than 2 or 3, while the limit for caries-susceptible subjects was higher than 5 or 10.
The correlation of salivary IgA antibodies with active caries may also lead to erroneous conclusions. Investigators who observed a lower level of IgA antibodies to S. mutans in patients with initial or active lesions concluded that salivary IgA plays a protective role against caries (38, 39, 491). Another explanation is that the level of salivary IgA antibodies becomes depressed following the development of caries. An increase in the levels of IgG and IgM antibodies in serum has been observed during the development of caries, and immune complexes composed of serum antibodies and S. mutans antigens may suppress the stimulation of the mucosal immune system (75). This suggestion was supported by sequential studies which revealed that the treatment of caries was followed by an increase in the level of IgA antibodies to S. mutans, concurrent with a decrease in the levels of IgG and IgM antibodies in serum (75, 135).
Studies with cross-sectional designs may not reveal correlations between salivary IgA antibodies and caries. Salivary IgA antibodies are measured only at a single point in time, while the caries score measure a disease process that may take several months to develop. The level of salivary IgA antibodies varies considerably over time (59, 109, 151). The exact stage of development of the SIgA immune response in relation to an antigenic challenge at the moment of sampling is not known. The salivary IgA antibodies measured may reflect present or past stimulation by S. mutans antigens. The SIgA antibody response may be influenced by the age at which the challenge with S. mutans occurred and by the period of exposure to S. mutans (71, 444). Saliva of children recently colonized (<6 months) with S. mutans contained higher levels of IgA antibodies than did saliva of children who had carried S. mutans for longer periods (24 months) (71). These results may also reflect a short duration of the secretory immune system to antigenic challenges and may account for the difficulties in correlating salivary IgA antibody levels and caries. Although more expensive, a longitudinal study which could monitor levels of salivary antibodies directed against S. mutans as a function of caries development would better clarify the potential of salivary IgA antibodies to protect from caries.
Other studies did not demonstrate a decisive role of salivary IgA antibodies in the resistance against the initial colonization by indigenous S. mutans in children or in the control of the level of indigenous S. mutans at adult ages. Studies that attempted to find a correlation between the level of salivary IgA antibodies and the level of indigenous mutans streptococci led to variable results with positive (202, 266, 451), negative (453), or no (1, 24, 71, 72, 169, 491, 492) correlation (Table 4). In contrast, other investigators found that exogenous implanted mutans streptococci were more rapidly eliminated from the oral cavity of subjects with high salivary IgA antibody activities (177, 246, 357). However, indigenous mutans streptococci may be better adapted to environmental conditions in the oral cavity than are laboratory strains. It was demonstrated that cultivated strains were less hydrophobic than freshly isolated strains and adhered to teeth to a lesser extent (234, 315, 357).
TABLE 4.
Relationship between natural salivary IgA antibodies to mutans streptococci and the level of mutans streptococci
| No. of subjects in study group (age [yr]) | Sample | Antigens of mutans streptococci for immunoassay (serotype) | Correlation of salivary IgA antibodies with the level of mutans streptococcia | Reference |
|---|---|---|---|---|
| ? (children) | Whole saliva | Glucosyltransferase, antigen I/II, glucan-binding protein, 110-kDa antigen | − | 453 |
| 10 (22–59) | Mouth rinses | Whole cells (c, d) | −b | 246 |
| 12 (27–43) | Parotid saliva | Carbohydrate (a–g) | −b | 177 |
| 20 (young adults) | Mouth rinses | Whole cells (c) | −b | 357 |
| 55 (18–20) | Parotid saliva | Whole cells (c, d) | + | 202 |
| 19 (adults) | Whole saliva | Whole cells (c) | + | 266 |
| 21 (0.3–35) | Whole saliva | Glucosyltransferase, antigen I/II, glucan binding protein | + | 451 |
| 12 (0.5–44) | Whole saliva | Whole cells | No | 24 |
| 42 (22–39) | Whole and parotid saliva | Whole cells (c), antigen I/II, carbohydrate (c) | No | 72 |
| 64 (3–6) | Whole saliva | Whole cells (c, d), antigen I/II, carbohydrate (c, d) | No | 71 |
| 31 (0.8–3.8) | Whole saliva | Whole cells (c) | No | 492 |
| 50 (19–21) | Whole saliva | Whole cells (c) | No | 169 |
| 67 (4–9) | Whole saliva | Whole cells (c) | No | 1 |
| 59 (22–32) | Whole saliva | Whole cells (c) | No | 491 |
Negative (−), positive (+), or no correlation.
Negative correlation with implanted mutans streptococci.
One of the difficulties in correlating salivary IgA antibodies with the level of S. mutans and caries is the standardization of the measurement of salivary IgA antibodies. The type of sample analyzed varies between studies and includes unstimulated or stimulated whole and parotid saliva and mouth rinses. The use of whole saliva may be more relevant clinically, since it is the secretion that bathes the mouth (404). However, the bacteria in whole saliva (or mouth rinses) may adsorb antibodies prior to measurement, and this may lead to underestimated antibody values (72, 151, 265, 402). The sampling of parotid or submandibular saliva by placing a collector over the major salivary gland ducts provides an uncontaminated source of saliva, but the amount of salivary IgA from each gland may differ (522). Some investigators reported a lower level of antibodies to mutans streptococci in whole saliva of subjects with active caries or high caries history, but they did not find this relationship in the parotid saliva of the same subjects (72, 402). The level of salivary S. mutans was found to be higher in caries-susceptible subjects and may have adsorbed a greater amount of salivary IgA antibodies (72, 402). Another explanation could be that caries-resistant subjects produce more SIgA antibodies in minor, submandibular, or sublingual salivary glands than in the parotid (402).
In immunoassay methods, the use of whole cells of mutans streptococci as antigen measures antibodies against all surface components including cell wall carbohydrates, glucosyltransferases, highly immunogenic proteins (antigens I/II, I, II, III, C, and D) and other wall-associated proteins, glucans, glucan-binding proteins, and lipoteichoic acids (410). The reference strains used in immunoassays may be antigenically different from the indigenous strain present in the oral cavity. The expression of cell surface protein antigens may vary according to the type of strains, the culture conditions, and repeated subculturing of microorganisms (234, 315, 523). Furthermore, the antibodies detected may be not specific to mutans streptococci and may also have been induced by cross-reactive antigens from other oral and intestinal bacteria (24, 71, 202). It has been reported that many salivary IgA antibodies to S. mutans were directed against lipoteichoic acids, which are found on all gram-positive microorganisms (57), or against glucans and polysaccharides, which are synthesized by a variety of oral microorganisms (24). Finally, the use of whole cells may not discriminate between protective antibodies directed against adhesins or enzymes from antibodies directed against all surface antigens (357).
A more accurate approach would be to determine which antigens may be responsible for the induction of the protective salivary IgA antibodies response against S. mutans (173, 195). However, studies in which purified antigens were used to measure antibodies also have led to contradictory results (Tables 2 through 4). The target antigens were primarily those considered to be involved in the adherence of mutans streptococci to tooth surfaces including glucosyltranferase, antigen I/II, glucan, and lipoteichoic acid but also serotype-specific cell wall carbohydrates. Bolton (38) reported a protective role of salivary IgA antibodies to lipoteichoic acid against dental caries in adult subjects. In contrast, Bolton and Hlava (39) observed that salivary IgA antibodies to lipoteichoic acid and glucan were lower in children free from caries, indicating no protective role for these antibodies. However, they observed that levels of salivary IgA antibodies to whole cells of S. mutans were higher in caries-free groups and concluded that surface antigens that induced a protective response remained to be identified. Gregory et al. (171) observed higher levels of IgA antibodies to glucosyltransferase, antigen I/II, and glucan in caries-resistant subjects than in caries-susceptible subjects and no difference in the level of IgA antibodies directed against antigen III, lipoteichoic acid, or carbohydrate serotype. In contrast, Camling and Köhler (71) found that salivary IgA antibody levels to the polysaccharide serotype antigen, but not the antigen I/II adhesin, were significantly higher in children without caries. The experiments of Hocini et al. (195) revealed no protective role for salivary IgA antibodies directed against carbohydrate serotype g and protein antigen I/II.
The sodium dodecyl sulfate-polyacrylamide gel electrophoresis/immunoblot technique has also been used to identify the specificities of salivary IgA antibodies directed against mutans streptococci antigens (173, 195, 522, 523). The immunoblot analyses have revealed that salivary IgA antibodies react with several antigens of mutans streptococci. More recently, some investigators used this method to correlate the reaction pattern of salivary IgA antibodies to S. mutans antigens with caries susceptibility or the degree of colonization by S. mutans. This approach seems to be more promising because the specificities of several salivary IgA antibodies are compared simultaneously, but more research is necessary before any conclusions can be reached. Smith et al. (453) observed in a longitudinal study that salivary antibodies of children not colonized by mutans streptococci were more frequently reactive with mutans streptococci components whose migration corresponded to antigen I/II, glucosyltransferase, glucan-binding protein, and a 110-kDa component, suggesting a regulatory role of these antibodies in the primary colonization of mutans streptococci. These results contradict other results by the same authors demonstrating that salivary IgA antibodies to S. mutans antigens were detected only in children colonized with S. mutans (444, 451). Gregory et al. (173) reported that caries-susceptible subjects had an impaired salivary IgA antibody response against the 94-, 40-, and 35-kDa S. mutans antigens. In contrast, Hocini et al. (195) showed no qualitative difference in the specificity of the IgA antibodies to S. sobrinus antigens between caries-susceptible and caries-resistant groups.
It has been postulated that SIgA2 antibodies are more efficient antibodies in secretion since they are resistant to IgA1 proteases. Gregory et al. (171) found higher levels of salivary IgA2 antibodies (but not IgA1) to whole cells of S. mutans in caries-resistant adult subjects than in caries-susceptible subjects. However, others reported no difference in the proportion of IgA2 antibodies directed against mutans streptococci between caries-susceptible and caries-resistant subjects (195, 402). Also, the avidity of IgA antibodies does not seem to play a major role in protection against caries (1, 171, 195, 265). Some studies reported no difference in avidity of salivary IgA antibodies between caries-susceptible and caries-resistant subjects (171, 195), while others observed higher levels of high-avidity IgA antibodies in caries-susceptible subjects (1, 265). Although the frequent contact of the secretory immune system with high doses of S. mutans in subjects with a long caries history may select for high-avidity antibodies, these antibodies do not seem to confer a higher protection level (265).
Little information exists on the role of salivary IgA in the development of gingivitis and periodontitis. It is more difficult to see how salivary IgA could control subgingival plaque, since secretory IgA antibodies do not penetrate the crevice or pocket. It is possible that salivary IgA antibodies, by modulating the accumulation of supragingival plaque, control the formation and composition of subgingival plaque and its potential for causing disease (527). Salivary IgA could prevent potential periodonthopathogens that inhabit the tongue from colonizing the gingival crevice. It could limit the spread of the disease by preventing bacterial transmission from infected to healthy gingival sites. Past studies have reported positive (181, 182, 189, 271, 362, 419) or no significant (80, 169, 298, 421, 426) correlations between total salivary IgA levels and periodontal diseases (Table 5). The increased release of serum IgA due to an increased permeability of the crevicular epithelium during gingival inflammation may contribute to the rise in IgA levels observed in whole saliva (181, 271, 381). However, higher SIgA levels have also been observed in parotid saliva of subjects with gingival inflammation (80, 189, 208, 209, 362). These studies suggest that an increased antigenic load from dental plaque induces an SIgA response (169, 209). In contrast, no direct correlation was found between the concentration of total salivary IgA and plaque accumulation (208, 209, 404, 435).
TABLE 5.
Relationship between total salivary IgA and periodontal diseases
| No. of subjects in study group (age [yr]) | Sample | Correlation of total salivary IgA with periodontal diseasesa | Reference |
|---|---|---|---|
| 18 | Whole saliva, parotid saliva | +b/+c | 189 |
| 27 (20–33) | Parotid saliva | + | 362 |
| 60 (19–63) | Whole saliva | + | 181 |
| 66 (11–43) | Whole saliva | + | 419 |
| 11 | Saliva from different glands | + | 182 |
| 19 (17–53) | Whole saliva, parotid saliva | +/No | 271 |
| 50 (19–30) | Whole saliva, parotid saliva | +/No | 381 |
| 66 (adults) | Parotid saliva | No | 80 |
| 56 (23–57) | Whole saliva | No | 298 |
| 40 (19–21) | Whole saliva | No | 169 |
| 28 (15–38) | Whole saliva | No | 421 |
| 12 (22–37) | Parotid saliva | No | 426 |
Positive (+) or no correlation.
Correlation with salivary IgA in whole saliva.
Correlation with salivary IgA in parotid saliva.
In a few studies, the level of salivary IgA antibodies to dental plaque bacteria was measured in relation to periodontal diseases. Salivary IgA antibodies to suspected periodonthopathogens were detected in both healthy subjects and subjects with periodontal diseases. Some studies observed no change in the levels of salivary IgA antibodies to Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans, and Bacteroides asaccharolyticus between healthy subjects and subjects with periodontal diseases (182, 292). Other studies reported an increased level of IgA antibodies to A. actinomycetemcomitans in some patients with adult and juvenile periodontitis (124, 418, 445). Others observed that treatment of gingivitis and periodontitis by root scaling and polishing was accompanied by an increased level of salivary IgA antibodies not only against suspected pathogens such as Actinomyces naeslundii genospecies 2, A. actinomycetemcomitans, P. gingivalis, and Treponema denticola but also against S. salivarius (124, 418, 504). This treatment may remove large amounts of microorganisms that may induce a salivary SIgA response via minor salivary glands or GALT. However, this does not explain the increase in the level of salivary IgA antibodies against S. salivarius, a microorganism which does not colonize teeth. This observation could, however, be explained by cross-reactivity between bacterial antigens. Experimental gingivitis may provide more meaningful results than cross-sectional studies because it is possible to measure the level of salivary IgA antibodies before the development of inflammation. In subjects who stopped using oral hygiene, the development of gingivitis was delayed when high levels of IgA antibody activities against A. actinomycetemcomitans, P. gingivalis, Eubacterium saburreum, and S. mutans were present in parotid saliva, suggesting a protective role of SIgA antibodies (426). However, the secretory immune system did not seem to respond to the increased oral antigenic stimulus that was provoked by plaque accumulation. In both groups, a slight significant decrease in the levels of SIgA antibodies to the bacteria tested was observed during the course of gingivitis. The level of antibodies in serum remained unchanged. The experiment should be repeated with more individuals and salivary IgA antibodies should be measured over a longer period after resumption of oral hygiene to verify if they will return to initial levels.
Caries and periodontal diseases are multifactorial diseases, and it is difficult to isolate the role of each factor separately. In studies that attempted to correlate SIgA levels with caries or periodontal diseases, other factors such as diet, oral hygiene, ethnic background, diseases, medications, tobacco, menstrual cycle, or salivary flow rate were often not controlled. As seen above, the level of salivary IgA is inversely correlated with salivary flow rate. In experiments that do not take into account the salivary flow rate, it is difficult to determine if the relationship with caries or periodontal diseases is caused by variations in salivary IgA or flow rate (403). Salivary flow alone has been negatively correlated with the S. mutans level and caries experience (169, 370). It should also be kept in mind that all antimicrobial factors in the oral cavity interact with each other (403, 404). A positive correlation has been reported between the levels of salivary IgA, lysozyme, and lactoferrin, and total protein in whole saliva (404). The analyses of many antimicrobial factors simultaneously may provide further information on the role of salivary IgA in the prevention of dental caries and periodontal diseases (169, 208, 209, 403, 404). A concomitant decrease in the level of salivary IgA to S. mutans and hypothiocyanite has been observed in patients with initial dental caries (491). Others have reported low levels of salivary IgA and serum IgG antibodies to S. mutans in subjects with high caries susceptibility (171, 173).
A more recent approach is the use of multivariate and cluster analyses to define groups of subjects with similar profiles of salivary flow rate and antimicrobial factors (hypothiocyanite ions, lysozyme, lactoferrin, salivary peroxidase, and total protein) (208, 209, 403, 404). The subject clusters with similar salivary protein profiles are compared for short-term outcome variables such as incipient caries lesions, plaque accumulation, or microbial counts. Subjects with different profiles of antimicrobial protein concentrations may show different patterns of antimicrobial protein interactions and, consequently, different patterns of antimicrobial activity (208, 209, 403, 404). Jalil et al. (208, 209) found that although there was no straightforward correlation between salivary IgA levels and plaque accumulation, the plaque accumulation was greater in a group of individuals with both a low concentration of hypothiocyanite and a high concentration of total salivary IgA. It would also be interesting to include IgA antibodies to some oral bacteria, e.g., S. sanguis, S. mutans, A. naeslundii genospecies 2, A. actinomycetemcomitans, and P. gingivalis, as a variable in the cluster analysis. However, the number of antimicrobial factors assayed is probably limited by the volume of saliva harvested.
As an alternative to analyzing the concentrations of any antimicrobial factor in saliva, it has been proposed that studies be carried out on the functional aspects of all the specific and nonspecific defense systems together, such as the effect of saliva on the growth, agglutination, and adhesion of bacteria (490). The rate of plaque formation was found to be inversely related to the bacterial aggregation capacity of parotid saliva, but this could not be explained by the salivary content of IgA (435). Another study demonstrated that the capacity of saliva to support or inhibit the growth of mutans streptococci could not be explained by the levels of salivary IgA antibodies and other antimicrobial factors. In contrast, it has been reported that parotid saliva from caries-resistant subjects inhibited S. mutans growth, adherence, acid production, glucosyltransferase, and glucosephosphotransferase activities to a greater extent than did saliva from caries-susceptible individuals, and this difference was related to salivary IgA antibody activity (171). However, the interactions between antimicrobial factors as well as the content in salivary-promoting binding proteins and salivary nutrients were not considered.
Oral Health in Patients with IgA Deficiencies
In past studies, Ig-deficient patients have been used as potential models for elucidating the role of host immunity in the control of diseases. Selective IgA deficiency is the most common primary immunodeficiency and can occur at a frequency within a population varying from 1:300 to 1:3,000 depending on the screened population (268). Patients with antibody deficiencies show an increased susceptibility to microbial infections, more particularly in the intestinal and upper respiratory tracts (11, 106). Ig-deficient patients, especially those deficient in IgA, represent a useful model for studying the role of salivary IgA in oral health and diseases. If salivary IgA plays a major role in the maintenance of the homeostasis of the oral microbiota, Ig-deficient individuals should show an increased susceptibility to caries and periodontal diseases. Only a few studies have attempted to correlate humoral immune deficiency with oral microbiological changes or oral diseases, because these conditions appear at low frequencies within a population and therefore a large number of patients may not always be available for statistical analysis (Table 6). Some investigators reported a higher frequency of harboring S. mutans and a greater susceptibility to dental caries in Ig-deficient patients (90, 257). In contrast, others found only minor changes in the oral microbiota of Ig-deficient individuals (62, 102, 351) and no increased susceptibility to caries and periodontal diseases (102, 393, 394). In a 2-year study of Ig-deficient patients, Robertson et al. (393, 394) reported a lower caries experience and gingival inflammation in Ig-deficient patients compared with normal individuals.
TABLE 6.
Oral health status in Ig-deficient patients compared with normal subjects
| No. of subjects in study group (age [yr]) | Plaque bacterial countsa
|
Clinical index scorea
|
Reference(s) | ||||
|---|---|---|---|---|---|---|---|
| S. mutans | Suspected periodontal pathogens | Caries experience | Plaque accumulation | Gingival inflammation | Periodontal destruction | ||
| 23 (3–43) | Lower | Similar | Lower | Similar | Lower | Similar | 62, 393, 394 |
| 25 (20–69) | NTb | Similar | Similar | Similar | Similar | Similar | 102 |
| 45 (3–50) | NT | NT | Higher | Similar | Similar | Similar | 257 |
| 22 (3–36) | Higher | NT | Higher | Similar | Similar | Similar | 90 |
| 25 (4–36) | NT | NT | Higher | NT | NT | NT | 19 |
| 12 (5–12) | Similar | NT | Lower | NT | NT | NT | 137 |
Given for Ig-deficient patients as a comparison with normal subjects.
NT, not tested.
There are numerous variables which could account for the discrepancies between the different studies. Age, level of education, dietary habits, oral hygiene, dentist visit frequency, and fluoride exposure may influence the severity of the disease. Furthermore, individuals with recurrent infections may receive prolonged antibiotic therapy and Ig prophylaxis that may suppress microorganisms in dental plaque (102, 394). While not statistically significant, caries experience has been shown to be lower in immunodeficient patients with a history of antibiotic therapy (394). The relatively good oral health of IgA-deficient patients may also be attributed in part to compensatory IgM antibodies (16, 90, 137). An enhanced local synthesis of IgM at mucosal surfaces is frequently observed in patients with selective IgA deficiency (19, 352). It has been found that patients with selective IgA deficiency who compensate by producing salivary SIgM antibodies to S. mutans exhibit a caries susceptibility similar to that of normal individuals (16, 19, 90). However, other investigators did not find more caries or periodontal diseases in patients with agammaglobulinemia who were unable to compensate in this manner (393). The possibility of compensation by nonspecific defense factors must also be considered (226). Higher levels of lysozyme and lactoferrin have been reported in subjects with immune system dysfunction (19). Parotid saliva from IgA-deficient patients demonstrated greater agglutinating activity for S. sanguis and S. mutans than did saliva from control subjects. The compensatory increase in agglutination may be mediated by IgM antibodies but also by other salivary molecules such as parotid salivary agglutinins (288).
Immunodeficient rodents may represent a more simple model for studying the role of Igs in the control of oral microbiota, in part because external factors such as diet and host environment can be controlled more easily. Thymectomized and congenitally athymic nude rodents with thymic dysgenesis have been used as models for immunological studies of dental caries (119, 121, 458). The thymus is essential for the maturation of T lymphocytes, and the interaction between T and B cells is necessary for the generation of a normal humoral response. Consequently, nude and thymectomized rodents have a T-lymphocyte defect and a severely depressed mucosal and systemic antibody response (118, 119, 121, 481). Decreases in salivary and serum antibody responses in these animals have been associated with increased colonization by mutans streptococci and increased dental caries (121, 458). These results suggest that naturally occurring salivary IgA and IgG antibodies may reduce the colonization by allochthonous bacteria. We have also reported a change in the resident oral microbiota and a lower salivary IgA response to some indigenous bacteria in nude mice compared with phenotypically normal (nu/+) mice (294). Nude mice have a lower proportion of Lactobacillus murinus and a higher proportion of Enterococcus faecalis than normal mice. However, our results do not necessarily indicate that the modification of the oral microbiota of nude mice is due to the lower IgA antibody response. Nude mice are affected in so many immune functions including enhanced macrophage and natural killer cell activities. The differences in the oral microbiota detected between nude and heterozygous mice may also be attributed to stress. It was observed that the proportion of Lactobacillus spp. decreased in stressed mice (150, 424, 474). The skin of nude mice is defective in keratinization of hair, which results in hairlessness and thermoregulatory problems (197). At ambient temperature under the thermoneutral zone, nude mice must maintain their basal metabolism up-regulated, and this phenomenon may be associated with the release of hormones, physiological changes, and higher food and water intake (197, 519).
Since nude mice do not appear to be a good model for studying the effect of antibodies on the control of the indigenous microbiota, we have analyzed the oral and intestinal microbiota of B-cell-deficient knockout mice. These mice were produced by disrupting one of the genes encoding the membrane form of the μ chain of IgM (227). B-cell development in homozygous (μMT/μMT) mice is stopped at the stage of pre-B-cell maturation, while it is normal in heterozygous (μMT/+) mice. The levels of salivary IgA and serum IgA and IgG were normal in μMT/+ mice, while no Igs were detected in μMT/μMT mice (227, 295). The acquisition and proportions of the different species of the oral and intestinal indigenous bacterial populations were not significantly different between the two groups of mice (295). Our results thus suggest that SIgA and other Ig isotypes do not play a major role in the acquisition and the regulation of the indigenous microbiota of mice. The role of SIgA may be masked by more predominant factors such as bacterial interactions, environmental factors, host physiological factors, or other host defense mechanisms. The coadaptation between oral resident microbes and their host probably begins immediately after birth, and the absence of one factor, such as SIgA, may be compensated for by other salivary defense factors (5, 403). No attempt was made to measure the level of innate defense factors in Ig-deficient (μMT/μMT) mice. On the other hand, the oral and intestinal microbiota of SPF mice is simple, and the effect of SIgA antibodies is perhaps observed only when the host is exposed to a more complex microbiota.
Active Immunization against Oral Diseases
Numerous studies have focused on the development of a vaccine that could induce salivary IgA antibodies and be effective in protecting against caries (reviewed in reference 328). These studies may also lead to new insights into the SIgA immune system and its ability to respond to antigenic challenges from oral bacteria and to control the equilibrium of the oral resident microbiota. Two major approaches have been used to induce the SIgA response to S. mutans in rodents, monkeys, and humans. The first approach consists of the induction of a local SIgA response in lymphoid tissues of salivary glands. Earlier studies attempted to elicit a salivary SIgA response in animal models by the injection of antigens in the vicinity of the major salivary glands, instillation of antigens into the parotid duct, or submucosal injections in the oral cavity. However, these immunizations may also induce serum antibodies that reach the oral cavity through the gingival crevicular fluid. Topical application of antigens to the oral mucosa also induces a local SIgA antibody response, probably via the entry of antigens to the salivary duct of minor salivary glands and stimulation of duct-associated lymphoid tissues (245, 247, 259, 260, 346, 443). The application of antigens to the gingival mucosa may also induce IgG antibodies in the crevicular gingival fluid (259, 260). It is difficult to determine the role of SIgA antibodies with immunization schedules which also give rise to serum and gingival crevicular fluid IgG antibodies. Studies with rodents and monkeys revealed that parenteral administration of S. mutans, which induces serum IgG but not SIgA antibodies, was protective against caries (46, 207, 261–263). The second approach to inducing salivary IgA antibodies originated from the evidence for a common mucosal immune system and consists of inducing a generalized secretory IgA response via the stimulation of GALT. Oral immunization elicits predominantly SIgA antibodies in saliva and other secretions with a poor serum antibody response. The induction of SIgA by oral immunization with nonviable antigens requires large and repeated doses of the antigens. This is probably due to enzymatic degradation of antigens in the stomach, poor adsorption of antigens by the intestinal mucosa, poor uptake by the GALT, or complexing of antigen with preexisting antibodies or mucins (324). Incorporation of antigens in liposomes or gelatin capsules may provide protection against digestive enzymes and result in a higher SIgA response (82, 89, 175, 325, 516). Animal studies demonstrated the ability of adjuvants such as muramyl dipeptide, concanavalin A, aluminum phosphate, or cholera toxin to increase the secretory immune system response when given orally with mutans streptococci antigens (332, 341, 409, 482). Cholera toxin has been shown to elicit a strong mucosal and systemic response against itself as well as against unrelated antigens (99, 100, 126). The adjuvant property of cholera toxin includes the ability of cholera toxin B subunit to bind specifically to a receptor on the surface of intestinal epithelial cells, to increase the permeability of the mucosal epithelia, to stimulate antigen presentation by enhancing major histocompatibility complex class II molecule expression and interleukin-1 production, and to promote B-cell isotype switch differentiation toward IgG1 and IgA (320).
Live recombinant avirulent Salmonella spp. expressing cloned gene products of mutans streptococci or periodontopathogens have also been evaluated as an oral vaccine (117, 382). The selection of Salmonella as the carrier is based on the fact that this genus may persist in the intestinal tract and continuously invade the host via Peyer’s patches of the GALT. More recently, it has been suggested that the mucosal immune system is not uniform and that intranasal immunization, which stimulates the NALT and tonsil-associated lymphoid tissues, is more effective than oral immunization in generating antibody responses in salivary glands (214, 412, 534).
Most studies on inducing salivary IgA against mutans streptococci to confer protection against dental caries have been performed in rodents (Table 7). Different antigen preparations were used for immunization and include whole cells (245, 321, 331, 333, 341, 392, 479), cell walls (331, 332), glucosyltransferase (448–450, 480, 482), surface proteins (100, 214, 382, 409, 516, 534), ribosomal preparations (172), synthetic peptides (260), and anti-idiotypic antibodies (207). It has been demonstrated that germ-free and conventional rodents injected in the salivary gland region with mutans streptococcus whole cells or glucosyltransferase in complete Freund’s adjuvant showed higher salivary IgA and IgG antibodies, lower mutans streptococcus colonization, and fewer carious lesions (321, 448, 449, 479, 480). The oral immunization of germ-free rodents with mutans streptococci whole cells or cell wall elicited salivary SIgA antibodies with no detectable serum antibodies (331–333, 341). Salivary IgA antibodies were directed against several cell antigens including glucan, serotype carbohydrate, and lipoteichoic acid (331, 332, 341). The rise in the level of salivary IgA antibodies correlated with a reduction in the level of mutans streptococcus colonization and dental caries, suggesting that SIgA antibodies alone are protective (333, 449). These studies demonstrated that a critical dose of orally administrated antigen was required to induce a secretory response. Also, the oral administration of S. mutans vaccine consisting of particulate antigens (whole cells or cell wall) seemed more effective in inducing salivary IgA antibodies and providing protection against caries than does administration of soluble forms (331, 332). Rodents that were given soluble antigens such as wall-associated antigens, antigen I/II, serotype carbohydrate, or anti-idiotypic antibodies exhibited only low salivary IgA antibody levels and caries protection (175, 207, 331, 332, 516). This is probably due to the intestinal digestion and lack of uptake by the GALT. The incorporation of soluble antigens in liposomes or the addition of adjuvant (muramyl dipeptide, aluminum phosphate), which renders the antigens particulate, resulted in higher salivary SIgA antibody levels and higher serum antibody response, which led to increased protection against caries (175, 207, 482, 516). Peroral immunization with the protein antigen I/II induced salivary IgA antibodies only when the antigen was conjugated with cholera toxin B subunit, which allowed binding to the host cellular membrane receptors (100, 184, 409). Oral immunization with a live avirulent recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus (analogous to antigen I/II of S. mutans) or glucosyltransferase elicited salivary and serum antibodies and reduced caries lesions in rodents (382). It was suggested that SIgA antibodies were also implicated in protection against caries in topical applications of whole S. mutans to the oral mucosa (245) or intranasal instillation of antigen I/II conjugated to cholera toxin B subunit (214).
TABLE 7.
Active immunity against mutans streptococci in rodents
| Immunization route | Antigens | Adjuvant or vehicule | Increase in:
|
Reduction in S. mutans counts | Protection against caries | Reference(s) | |
|---|---|---|---|---|---|---|---|
| Salivary IgA antibody levels | Salivary or serum IgG antibody levels | ||||||
| Subcutaneous | Whole cells, glucosyltransferase | Complete Freund adjuvant | Yes | Yes | Yes | Yes | 321, 448, 449, 479, 480 |
| Topical | Whole cells | None | Yes | No | Yes | NTa | 245 |
| Intranasal | Antigen I/II | Cholera toxin | Yes | Yes | Yes | NT | 214, 544 |
| Oral | Whole cells, cell wall, ribosomal extract, antigen I/II, glucosyltransferase, anti-idiotype | Muramyldipeptide, peptidoglycan, concanavalin A, cholera toxin, liposome, aluminum phosphate, cloned Salmonella | Yes | Yes/nob | Yes | Yes | 100, 175, 207, 331–333, 341, 382, 409, 449, 482, 516 |
NT, not tested.
In general, immunization with whole cells gives rise only to salivary IgA antibodies while immunization with soluble antigens and adjuvant, which render the antigens particulate, may also lead to an increase in the level of antibodies in serum.
A few investigations have studied the effects of stimulation of salivary IgA antibodies on the progress of periodontal bone loss in rodents. Germfree rats were immunized in the salivary gland region with whole cells of Actinomyces naeslundii genospecies 2 or A. naeslundii and then inoculated orally with the live homologous organism (96, 356). Crawford et al. (96) found that high salivary IgA and IgG responses limited the colonization of A. naeslundii and the development of periodontal bone loss but the immunization with A. naeslundii genospecies 2 increased periodontal loss. In another study, salivary antibodies were found to be capable of reducing the initial implantation of A. naeslundii genospecies 2 but had limited capability in suppressing the establishment of this organism (356). The injection of whole cells or fimbrial components of Porphyromonas gingivalis in the vicinity of submaxillary glands of germfree rats led to increased levels of salivary antibodies and protected against periodontal destruction (131, 132). The mechanism by which this mode of immunization provides protection is not known and may involve both humoral and cellular immunity. SIgA antibodies may have limited the adherence of P. gingivalis, but the level of P. gingivalis colonization was not measured. A mucosal immune response was also induced against P. gingivalis by oral immunization of rodents with liposomes containing adhesive fimbriae and adjuvant (117, 251, 355) or with attenuated Salmonella expressing a cloned P. gingivalis adhesin hemagglutinin (117). However, these studies did not evaluate the effect of salivary IgA antibodies on P. gingivalis colonization and the destruction of periodontal tissues.
Monkeys may be useful models of dental caries since they may develop caries similar to those of humans. However, monkeys rarely harbor mutans streptococci as part of their resident oral microbiota and do not usually develop caries in the wild (133). Caries can be produced in monkeys by infecting them with mutans streptococci and by feeding them with a cariogenic diet (133, 262). It was shown that macaque monkeys may also be naturally colonized by S. mutans (serotype c) when fed a carbohydrate-rich diet, but the origin of the bacteria was unknown (259, 260, 263). Furthermore, experiments on the effect of salivary SIgA antibodies on caries protection in nonhuman primates are limited in number (Table 8). Some studies demonstrated that the instillation of S. mutans whole cells or crude glucosyltransferase into the duct of parotid glands induced a salivary and serum antibody response (129, 133, 272, 517). The presence of salivary IgA antibodies correlated with a reduction in the colonization of implanted S. sobrinus (133), but changes in caries were not tested. Bowen et al. (46) reported that submucosal injection of whole cells or crude glucosyltransferase in the oral cavity of monkeys increased the level of salivary and serum antibodies. Animals that were intraorally immunized with whole cells were protected against caries, but animals that were immunized with crude glucosyltransferase had higher counts of S. mutans in dental plaque and were not protected. Topical gingival immunization by brushing live S. mutans cells onto the gingiva in rhesus monkeys failed to induce salivary antibodies or to prevent the development of caries (264). However, the application of antigen I/II in 50% dimethyl sulfoxide on monkey gingival tissues resulted in whole-saliva IgA antibodies, crevicular IgG antibodies, and a reduction in the proportion of S. mutans in plaque (259, 260). Since only whole saliva was examined, the source of IgA could not be determined. The IgA antibodies may have been produced locally by the gingival lymphoid cells or via the minor oral salivary glands that are scattered under the oral mucosa of the lips and cheeks (260). The protection of monkeys against dental caries has been principally related to IgG antibodies in serum (46, 78, 261, 263, 264), and it is difficult to estimate the importance of SIgA antibodies in these studies. The oral immunization of monkeys with mutans streptococcus whole cells or antigens failed to induce SIgA antibodies in saliva (272, 517) or induced only a short-lived SIgA response (78, 264, 326). The administration of live S. mutans in drinking water elicited low levels of salivary IgA antibodies, but no reduction in the level of colonized S. mutans or in caries frequency was observed (264).
TABLE 8.
Active immunity against mutans streptococci in nonhuman primates
| Immunization route | Antigens | Adjuvant or vehicle | Increase in:
|
Reduction in S. mutans counts | Protection against caries | Reference(s) | |
|---|---|---|---|---|---|---|---|
| Salivary IgA antibody levels | Salivary or serum IgG antibody level | ||||||
| Subcutaneous | Whole cells | Complete Freund adjuvant | No | Yes | Yes | Yes | 78, 261–264 |
| Submucosal | Whole cells, glucosyltransferase | None | Yes | Yes | Yes/noa | Yes/noa | 46 |
| Intraductal | Whole cells, glucosyltransferase | None | Yes | Yes | Yes | NTb | 129, 133, 272, 517 |
| Topical | Antigen I/II | Dimethyl sulfoxide | Yes | Yes | Yes | NT | 259, 260 |
| Intranasal | Antigen I/II | Cholera toxin | Yes | Yes | NT | NT | 412 |
| Oral | Whole cells, antigen I/II | Capsule, aluminum phosphate, cholera toxin | Yes/noc | Yes/noc | No | No | 78, 264, 272, 326, 517 |
Immunization with whole cells was protective, but immunization with crude glucosyltransferase resulted in an increase in colonization by S. mutans and no protection against caries.
NT, not tested.
Slight or no increase in salivary and serum antibody levels.
The immunization studies in humans were conducted mainly by oral administration of whole cells of mutans streptococci (Table 9). Mestecky et al. (325, 330) were the first to demonstrate on SIgA response in the saliva of humans who ingested capsules containing whole cells of a laboratory strain of S. sobrinus. Subjects with no preexisting antibody activity to the strain before immunization were selected. Subsequent investigators used a similar oral immunization protocol and then challenged the subjects with the homologous streptomycin-resistant mutans streptococci strain. No increase in preexisting levels of antibodies to S. mutans was reported, but the number of implanted strains in saliva and dental plaque was reduced in immunized subjects (40, 89, 152, 247). Due to the high variability in the level of natural antibodies, the method may have not been sensitive enough to detect any change in the level of salivary IgA antibodies. Gahnberg and Krasse (152) observed no difference in the elimination of implanted bacteria after a second challenge 2 weeks after the last immunization, suggesting that if such a response exists, it is of very short duration. Furthermore, no difference in the level of indigenous mutans streptococci was observed during these experiments (89, 152). Czerkinsky et al. (101) observed an SIgA response in four subjects who were orally immunized with indigenous S. mutans whole cells but not in one subject who had high levels of SIgA antibodies against S. mutans before immunization. The presence of preexisting antibodies may interfere with the adsorption of antigens and with further stimulation of the secretory immune system. A few studies reported an effect of SIgA antibodies against indigenous S. mutans. The oral administration of glucosyltransferase from S. sobrinus with aluminum-based adjuvant led to an increased level of SIgA antibodies in parotid saliva and interfered with the reaccumulation of mutans streptococci after dental prophylaxis (442). A second series of immunization also increased the level of SIgA antibodies but did not affect the level of indigenous S. mutans. Gregory and Filler (170) reported an increase in the level of salivary IgA antibodies and a decrease in the level of indigenous S. mutans after both first and second oral immunizations with whole cells of S. mutans previously isolated from each individual. This finding suggests that the secretory immune system may respond to a greater indigenous antigenic challenge and that salivary SIgA antibodies may control the colonization of indigenous mutans streptococci and protect against dental caries. Topical application of glucosyltransferase in aluminum phosphate delayed the reaccumulation of indigenous mutans streptococci and was correlated with an increase in the level of SIgA antibody in the parotid but not the labial saliva. The rise in the level of parotid salivary IgA antibody was not likely to have resulted from ingestion of antigens or local immunization of the major salivary glands (443). The lymphoid cells stimulated in the minor salivary glands may have migrated to the parotid glands; however, this hypothesis has never been verified. The difficulty in collecting labial saliva and in measuring labial salivary flow rate may in part explain these results.
TABLE 9.
Active immunity against mutans streptococci in humans
| No. of subjects in study group | Immunization route | Antigens (serotype) | Vehicle or adjuvant | Increase in:
|
Decrease in the level of S. mutans | Reference | |
|---|---|---|---|---|---|---|---|
| Salivary IgA antibodies against S. mutans | Serum antibodies against S. mutans | ||||||
| 3 | Topical (lower lips) | Whole cells (c) | Capsule | No | NTa | Yesb | 247 |
| 11 | Oral | Whole cells (d) | None | No | NT | Yesb | 152 |
| 4 | Oral | Whole cells (d) | None | No | No | Yesb | 40 |
| 8 | Oral | Whole cells (d) | Capsule | No | No | Yesb | 89 |
| 5 | Oral | Whole cells (indigenous) | Capsule | Yes | No | Yes | 170 |
| 25 | Oral | Glucosyltransferase (g) | Aluminum phosphate, capsule | Yes | No | Yes | 442 |
| 23 | Topical (lower lips) | Glucosyltransferase (g) | Aluminum phosphate | Yes | No | Yes | 443 |
| 4 | Oral | Whole cells (d) | Capsule | Yes | No | NT | 325 |
| 5 | Oral | Whole cells (c) (indigenous) | Capsule | Yes | No | NT | 101 |
| 7 | Oral | Glucosyltransferase (c) | Liposome | Yes | NT | NT | 82 |
NT, not tested.
Decrease in implanted S. mutans.
For all routes of immunization tested, the SIgA response was generally of short duration and rarely exceeded 2 to 3 months in humans (101, 170, 325, 443) and animals (78, 129, 133, 264, 272, 326). The antibody level dropped quickly to the preimmune level when immunization stopped, even though the oral cavity had been infected by the immunizing strain (129, 264, 272). Only Hajishengallis et al. (184, 185) were able to induce a SIgA response that persisted for at least 11 months by oral immunization of mice with a chimeric protein in which the toxic A1 subunit of cholera toxin had been genetically replaced by a 42-kDa segment representing the saliva-binding region of antigen I/II. In most of the studies, a second immunization procedure resulted in a faster but generally not in a greater or longer response than did the primary immunization (78, 129, 185, 262, 272, 391, 392, 409, 443, 534). Only a few studies demonstrated an anamnestic SIgA response against mutans streptococcal antigens in rodents (516) and humans (170, 325). These findings suggest that there is little or no evidence of immune memory from the mucosal immune system and that a persistent antigenic challenge seems necessary to maintain salivary IgA antibody levels. The difficulty in inducing long-lasting and anamnestic responses may be due to interference with antigen adsorption by antibodies that are synthesized in response to the preceding stimulation (13), or it may be due to the induction of suppressor cells (125, 200, 319). Animals that were fed protein antigens were shown to develop a state of systemic unresponsiveness or oral tolerance which would have been mediated by suppressor cells in GALT and other lymphoid tissues. Some studies suggested that these cells do not inhibit the mucosal SIgA response and demonstrated that protein antigen feeding led simultaneously to the induction of SIgA antibodies and systemic unresponsiveness to these antigens (76, 77). In contrast, other results indicated that oral feeding of antigens may also lead to the emergence of T suppressor cells that inhibit the SIgA response (4, 125, 127, 200, 319). The development of unresponsiveness of the mucosal immune system to specific bacterial antigens has not been extensively studied (115, 281, 432, 433). However, studies with rodents showed that the intestinal microbiota may induce nonspecific suppressive cells, in lymphoid tissues, that regulate the secretory and systemic immune response (22, 309, 318). These nonspecific suppressor cells are not found in germfree rats and mice unless they are colonized by bacteria. Suppression appears to be mediated by macrophage-like cells or by T suppressor cells. Lipopolysaccharides from resident gram-negative bacteria may be involved in the maturation of precursor suppressor cells in GALT which are subsequently stimulated by antigens to become the antigen-specific suppressor cells that mediate tolerance (125, 229, 329). The phenomenon of oral tolerance is a complex and controversial area which needs more research. Its application to indigenous microbiota is largely unknown. The oral ecosystem could be an interesting ecosystem for such studies.
Most of the studies on vaccines against caries were performed in germfree animals or in animal models that did not harbour mutans streptococci as part of the resident oral microbiota and were infected with a human strain. The infection with S. mutans can be induced rapidly by a high-sucrose diet, and dental caries develop within 2 months. Under these conditions, a short SIgA antibody response (2 to 3 months) may limit the colonization of S. mutans and reduce caries. Although these experiments are valuable for the development of a vaccine, they do not provide additional information about the role of SIgA in the control of indigenous microbiota. Experiments with animals do not reflect the situation in humans, where dental caries are caused by indigenous bacteria and may take many months to develop. The persistence of resident bacteria in the oral cavity may result from a long-term adaptation between the host and the bacteria. As stated earlier, there is evidence that the host is more tolerant to autochthonous than allochthonous bacteria (115, 281). The chronic exposure of the host immune system to resident bacteria may result in the suppression of the antibody response (391, 392, 433). Riviere et al. (391, 392) demonstrated that primary oral immunization with S. mutans may induce SIgA antibodies in rats but that continued exposure to S. mutans whole cells led to progressive suppression of the SIgA response and the level of salivary IgA antibodies returned to baseline (391, 392). The acquired suppression of the response to mutans streptococci was long-lasting and could not be reversed by restimulation with antigens (392). The effect of long-term oral immunization on S. mutans colonization has not been studied in animals. The role of the mucosal immune system in long-lasting protection against caries and periodontal diseases could be studied by using rodents that are orally immunized with recombinant bacteria that persist in the gut expressing gene products from mutans streptococci or periodontopathogens.
Studies with our mouse model suggest that salivary IgA antibodies play a minor role in controlling the indigenous oral microbiota. In these studies, SPF BALB/c mice that naturally harbored a strain of streptococcus (Streptococcus sp. strain TG) exhibited a fourfold-lower salivary IgA antibody response than did another mouse colony that was inoculated with this bacterium at adult age (296). However, the increase in the level of salivary IgA antibodies in the inoculated group did not affect the colonization and persistence of Streptococcus sp. strain TG. In both the inoculated and naturally colonized groups, the proportion of Streptococcus sp. strain TG among the total cultivable microbiota reached similar levels (296). In another study, we found that the oral immunization of SPF mice with an indigenous bacterium (Lactobacillus murinus) elicited SIgA antibodies to whole bacteria in 50% of the mice and did not affect the proportion of L. murinus among the oral bacterial populations of these mice (297). It is known that indigenous bacteria may evade SIgA immune control by continually changing their antigenic composition (59, 196), by masking their epitopes with host molecules, or by releasing IgA-coated antigens (256). It has been shown that pathogenic bacteria may persist in the host through molecular mimicry and antigenic variations, but this phenomenon has not been widely studied for indigenous bacteria (65, 86). Only three immunization studies of humans suggest that salivary IgA antibodies may limit the colonization of indigenous S. mutans (170, 442, 443). A second immunization regimen was shown to affect the proportion of S. mutans in one study (170) but not in another (442). Other experiments should be performed with humans, using more individuals and over longer periods to evaluate if a protective secretory IgA response may be maintained against indigenous mutans streptococci and potential periodontopathogens. Until now, the short duration of salivary IgA response has failed to support the argument that the SIgA system plays an important role in maintaining the homeostasis of the oral resident microbiota.
The progress in vaccine research has been limited primarily by the difficulty in inducing an SIgA response. It is thus necessary to develop new immunization strategies for inducing SIgA antibodies over long periods (184, 382), e.g., by immunizing against synthetic peptide vaccines including epitopes that induce the protective response (B-cell, adhesion, and T-helper cell epitopes) but not the epitopes that induce the suppressive response (217). Other approaches may be used to prevent caries and periodontal diseases such as systemic immunization or passive immunization with monoclonal antibodies (41, 46, 261–263, 279, 280). These modes of immunization avoid the difficulties encountered in stimulating the secretory immune system. However, systemic immunization is difficult to justify because of its undesirable side effects. There is evidence for cross-reacting antigens between S. mutans and human heart tissues. Furthermore, systemic immunization induces mainly IgG antibodies, which may promote an inflammatory response at the gingival margin, where plaque accumulates (410). Local oral passive immunization against caries and periodontal diseases presents a lower risk and seems more promising (138, 279, 280). It has been demonstrated that the topical application of IgG monoclonal antibodies onto human teeth prevented the recolonization of resident S. mutans following elimination of plaque with chlorhexidine. Protection against colonization by S. mutans, lasting up to 2 years, was observed in immunized subjects, although IgG monoclonal antibodies was applied over a period of only 3 weeks (280). These results are surprising considering the complexity of the oral ecosystem. It has been suggested that this long-term protection could have been due to a shift in the equilibrium of the oral microbiota which prevented recolonization by S. mutans. In the presence of monoclonal antibodies to S. mutans, S. mutans colonization is prevented and other bacteria take over the niche that is vacated by S. mutans. However, a shift in the proportions of the oral bacterial populations has not been demonstrated. Long-term evaluations of immunized subjects will demonstrate if the elimination of S. mutans prevents the initiation of caries.
Caries and periodontal diseases are multifactorial diseases that appear following a disequilibrium of the oral microbiota. It is possible that the induction of antibodies against only the principal etiological agents will not be sufficient to reverse the disease process. Diseases may result from other bacteria that will fill the niche vacated by the target species. This is principally the case in periodontal diseases, which seem to result from the microbial activity of a mixture of microorganisms (obligately anaerobic gram-negative rods).
Passive Immunity with Milk Secretory IgA
The oral cavity and the gastrointestinal tract are colonized immediately after birth. Breast milk is thought to play an important role in the protection of mucosa of breast-fed infants. The severity of gastrointestinal and respiratory infections is much lower in breast-fed infants than in bottle-fed infants (187). Breast milk is characterized by a complex host defense system that includes Igs, lactoferrin, lactoperoxidase, lysozyme, leukocytes, anti-adherent oligosaccharides, antiviral lipids, and anti-inflammatory agents (165). SIgA levels are low in saliva during the first few months, and stable levels of SIgA have seldom been found before 12 to 24 months (8, 68). Before weaning, the potentially protective effect of SIgA would be provided by mother’s milk, which is a rich source of SIgA. Infants who are breast fed may get around 0.5 g of SIgA per day via the milk (187). Milk SIgA antibodies are directed against microbes and food proteins to which the mother has been exposed and to which the infants will also be exposed (187). These antibodies appear in milk mainly via the antigenic exposure of lymphoid tissues associated with gut and upper respiratory tract followed by the migration of antigen-sensitized lymphocytes to mammary glands. It is generally agreed that milk IgA antibodies protect infants against microbial infections and food allergies (431). Milk IgA antibodies have been detected against pathogens from the gastrointestinal tract (enterotoxigenic E. coli, Shigella, Vibrio cholerae, and Salmonella) and the respiratory tract (pneumococci, Haemophilus influenzae, respiratory syncytial virus). The protection against cholera in the breast-fed baby could be related to the level of the mother’s milk IgA antibodies against cholera toxin and lipopolysaccharide (163).
Colostrum and milk also contain antibodies that are directed against resident oral streptococci (S. mitis, S. salivarius, S. mutans, and S. sanguis), including those that colonize the oral cavity of neonates, as well as mutans streptococci (10, 72, 123). It has been found that breast-fed infants are less caries susceptible than are bottle-fed infants (473). Although S. mutans colonizes the oral cavity only after teeth eruption and after breast feeding has been discontinued, milk IgA antibodies may interfere with the colonization of pioneer streptococci and subsequently with the colonization of other oral resident bacteria. Breast feeding may also influence the maturation of the infant mucosal immune system and the infant SIgA response against indigenous bacteria, but this remains a subject of controversy (140, 164, 244). To our knowledge, no study has yet compared the development of the oral microbiota between breast-fed and bottle-fed human infants. However, the development of the intestinal microbiota was found to be different between these two groups (538). In rodents, teeth are present before weaning, and this model proved to be useful for demonstrating the role of milk IgA antibodies in colonization of teeth. Rat dams that were fed S. mutans antigen exhibited IgA antibodies in their colostrum and milk which conferred protection against caries to their offspring infected with the S. mutans homologous strain (327). In contrast, we did not observe any differences in the changes of oral resident microbiota between pups from Ig-deficient (μMT) mothers and pups from phenotypically normal mothers (295). In addition to Igs, other properties of milk could influence the resident microbiota, including its nutrient contents, buffering capacity, and presence of other defense mechanisms (538). In the intestinal tract, the poor buffering capacity of breast milk could favor the growth of Lactobacillus and Bifidobacterium, which lead to conditions that are unfavorable for the growth of the Enterobacteriaceae (538).
CONCLUSION
The oral microbiota is controlled by several factors. At present, experiments have not succeeded in demonstrating a significant role for SIgA in the control of the indigenous microbiota. In vitro, SIgA antibodies reduce the colonization of bacteria to the oral mucosa and teeth, but attempts to demonstrate a role for SIgA in vivo have led to contradictory reports. Salivary IgA is part of a complex and interdependent immune defense system. Immunodeficient subjects do not seem to be more susceptible to oral diseases, and the absence of SIgA may be compensated by other defense factors. SIgA antibodies may play a major role in the homeostasis of the oral microbiota and reverse the disease process only if the mucosal immune system responds to an increased bacterial antigenic load by producing higher levels of IgA antibodies. However, correlation studies in humans have not demonstrated that a higher level of S. mutans is associated with higher levels of naturally occurring SIgA antibodies. Local and oral immunization experiments have revealed that high antigen doses are necessary to induce a secretory IgA response and that this response is relatively short-lasting. Only longitudinal studies in humans can show that SIgA antibodies may help to reestablish the initial equilibrium of the oral microbiota and prevent oral diseases. If a change in the bacterial population is not associated with a corresponding change in salivary IgA antibodies, the salivary IgA plays a no more important role than other salivary glycoproteins in the prevention of oral diseases.
It has been demonstrated in animal models that a protective SIgA antibody response may be induced against allochthonous (nonresident) bacteria. However, only a few studies have been able to induce a protective SIgA responses against resident bacteria. Indigenous bacteria might survive in the oral cavity because they are less susceptible to or are able to avoid immune mechanisms (30, 44, 87, 142). Although immune responses against true pathogens consist of a rapid and protective SIgA antibody response, hyporesponsiveness seems to develop against the ever-present resident bacteria (147). There is much to be learned about the mechanisms by which resident bacteria may persist in the oral cavity. Fortunately, investigators have recently demonstrated an interest in understanding the regulation of the IgA response to resident bacteria and the cellular and molecular interactions leading to hyporesponsiveness to chronically present bacteria (115, 217, 433). It will also be necessary to gain further insights into the possible mechanisms by which resident bacteria can avoid reacting with SIgA antibodies (256). This information may help in the selection of target antigens for immunization and for the manipulation of the mucosal immune system in order to provide a long-term immunity against the principal agents of oral diseases.
The main role of SIgA antibodies in the oral cavity might be to prevent the colonization of pathogenic microorganisms. Resident bacteria and the host may have developed mechanisms to live together in a relatively commensal interrelationship. It must be kept in mind that the SIgA system not only is a mechanism for eliminating bacteria but also acts as a selective force in the interaction between the host and indigenous bacteria (30). SIgA antibodies may select for antigenic variants of bacteria with low antigenicity and pathogenicity. The great complexity of the oral microbiota makes it difficult to detect such subtle changes.
REFERENCES
- 1.Aaltonen A S, Tenovuo J, Lehtonen O-P, Saksala R. Maternal caries incidence and salivary close-contacts with children affect antibody levels to Streptococcus mutans in children. Oral Microbiol Immunol. 1990;5:12–18. doi: 10.1111/j.1399-302x.1990.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 2.Aaltonen A S, Tenovuo J, Lehtonen O-P. Increased dental caries activity in pre-school children with low baseline levels of serum IgG antibodies against the bacterial species Streptococcus mutans. Arch Oral Biol. 1987;32:55–60. doi: 10.1016/0003-9969(87)90154-3. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Ghazaleh R I, Fujisawa T, Mestecky J, Kyle R A, Gleich G J. IgA induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 4.Adachi M, Yodoi J, Noro N, Masuda T, Uchino H. Murine IgA binding factors produced by FcαR(+) T cells: role of FcγR(+) cells for the induction of FcαR and formation of IgA-binding factor in Con A-activated cells. J Immunol. 1984;133:65–71. [PubMed] [Google Scholar]
- 5.Aguirre A, Testa-Weintraub L A, Banderas J A, Haraszthy G G, Reddy M S, Levine M J. Sialochemistry: a diagnostic tool. Crit Rev Oral Biol Med. 1993;4:343–350. doi: 10.1177/10454411930040031201. [DOI] [PubMed] [Google Scholar]
- 6.Ahl T, Reinholdt J. Detection of immunoglobulin A1 protease-induced Fabα fragments on dental plaque bacteria. Infect Immun. 1991;59:563–569. doi: 10.1128/iai.59.2.563-569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahl T, Reinholdt J. Subclass distribution of salivary secretory immunoglobulin A antibodies to oral streptococci. Infect Immun. 1991;59:3619–3625. doi: 10.1128/iai.59.10.3619-3625.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaluusua S. Longitudinal study of salivary IgA in children from 1 to 4 years old with reference to dental caries. Scand J Dent Res. 1983;91:163–168. doi: 10.1111/j.1600-0722.1983.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 9.Albanese C T, Smith S D, Watkins S, Kurkchubasche A, Simmons R L, Rowe M I. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in-vitro. J Am Coll Surg. 1994;179:679–688. [PubMed] [Google Scholar]
- 10.Aldred M J, Wade W G, Llewelyn D R, Walker D M. Class-specific antibodies to Streptococcus mutans in human serum, saliva and breast milk. J Immunol Methods. 1986;87:103–108. doi: 10.1016/0022-1759(86)90349-2. [DOI] [PubMed] [Google Scholar]
- 11.Amman A J, Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine. 1980;50:223–236. [PubMed] [Google Scholar]
- 12.Anderson D M, Ebersole J L, Novak M J. Functional properties of nonhuman primate antibody to Porphyromonas gingivalis. Infect Immun. 1995;63:3245–3252. doi: 10.1128/iai.63.9.3245-3252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.André C, Lambert R, Bazin H, Heremans J F. Interference of oral immunization with the intestinal absorption of heterologous albumin. Eur J Immunol. 1974;4:701–704. doi: 10.1002/eji.1830041013. [DOI] [PubMed] [Google Scholar]
- 14.Apter F M, Michetti P, Winner L S, III, Mack J A, Mekalanos J J, Neutra M R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong S J, Dimmock N J. Neutralization of influenza virus by low concentrations of hemagglutinin-specific polymeric immunoglobulin A inhibits viral fusion activity, but activation of the ribonucleoprotein is also inhibited. J Virol. 1992;66:3823–3832. doi: 10.1128/jvi.66.6.3823-3832.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold R R, Pruitt K M, Cole M F, Adamson J M, McGhee J R. Salivary antibacterial mechanisms in immunodeficiency. In: Kleinberg I, Ellison S A, Mandel I D, editors. Saliva and dental caries. New York, N.Y: Information Retrieval Inc.; 1979. pp. 449–462. [Google Scholar]
- 17.Arnold R R, Russell J E, Champion W J, Brewer M, Gauthier J J. Bactericidal activity of human lactoferrin: influence of physical conditions and metabolic state of target microorganisms. Infect Immun. 1981;32:655–660. doi: 10.1128/iai.32.2.655-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold R R, Russell J E, Champion W J, Brewer M, Gauthier J J. Bactericidal activity of human lactoferrin: differentiation from the status of iron deprivation. Infect Immun. 1982;35:792–799. doi: 10.1128/iai.35.3.792-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold R R, Cole M F, Prince S, McGhee J R. Secretory IgM antibodies to Streptococcus mutans in subjects with selective IgA deficiency. Clin Immunol Immunopathol. 1977;8:475–486. doi: 10.1016/0090-1229(77)90011-3. [DOI] [PubMed] [Google Scholar]
- 20.Atlas R M, Bartha R. Microbial ecology: fundamentals and applications. 3rd ed. Redwood City, Calif: The Benjamin/Cummings Publishing Co., Inc.; 1992. [Google Scholar]
- 21.Baba H. Lysis of Streptococcus sanguis by an extracellular enzyme from the bacterium Streptococcus mutans from human dental plaque. Arch Oral Biol. 1986;31:849–853. doi: 10.1016/0003-9969(86)90140-8. [DOI] [PubMed] [Google Scholar]
- 22.Babb J L, McGhee J R. Mice refractory to lipopolysaccharide manifest high immunoglobulin A responses to orally administered antigen. Infect Immun. 1980;29:322–328. doi: 10.1128/iai.29.2.322-328.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballieux R E. Impact of mental stress on the immune response. J Clin Periodontol. 1991;18:427–430. doi: 10.1111/j.1600-051x.1991.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 24.Bammann L L, Gibbons R J. Immunoglobulin A antibodies reactive with Streptococcus mutans in saliva of adults, children, and predentate infants. J Clin Microbiol. 1979;10:538–543. doi: 10.1128/jcm.10.4.538-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbeau J, Bourget J, Deslauriers N. RFc alpha bearing cells and IgA-mediated phagocytosis in the mouse oral mucosa. Adv Exp Med Biol. 1987;216A:563–572. doi: 10.1007/978-1-4684-5344-7_67. [DOI] [PubMed] [Google Scholar]
- 26.Beem J E, Hurley C G, Magnusson I, McArthur W P, Clark W B. Subgingival microbiota in squirrel monkeys with naturally occurring periodontal diseases. Infect Immun. 1991;59:4034–4041. doi: 10.1128/iai.59.11.4034-4041.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennet K R, Reade P C. Salivary immunoglobulin A levels in normal subjects, tobacco smokers, and patients with minor aphthous ulceration. Oral Surg. 1982;53:461–465. doi: 10.1016/0030-4220(82)90457-1. [DOI] [PubMed] [Google Scholar]
- 28.Berg R D. Host immune response to antigens of the indigenous intestinal flora. In: Hentges D J, editor. Human intestinal flora in health and disease. New York, N.Y: Academic Press, Inc.; 1983. pp. 101–120. [Google Scholar]
- 29.Berg R D, Savage D C. Immunological responses and microorganisms indigenous to the gastrointestinal tract. Am J Clin Nutr. 1972;25:1364–1371. doi: 10.1093/ajcn/25.12.1364. [DOI] [PubMed] [Google Scholar]
- 30.Berg R D, Savage D C. Immune responses of specific-pathogen-free and gnotobiotic mice to antigens of indigenous and non-indigenous microorganisms. Infect Immun. 1975;11:320–329. doi: 10.1128/iai.11.2.320-329.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger J. IgA glomerular deposits in renal disease. Transplant Proc. 1979;1:939–944. [PubMed] [Google Scholar]
- 32.Bessen D, Fischetti V A. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988;167:1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biesbrock A R, Reddy M S, Levine M J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blais J F, Lavoie M C. Effect of dietary components on the indigenous oral bacterial flora of BALB/c mice. J Dent Res. 1990;69:868–873. doi: 10.1177/00220345900690030801. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard T G, Czinn S J, Maurer R, Thomas W D, Soman G, Nedrud J G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect Immun. 1995;63:1394–1399. doi: 10.1128/iai.63.4.1394-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloch K J, Walker W A. Effect of locally induced intestinal anaphylaxis on the uptake of a bystander antigen. J Clin Immunol. 1981;67:312–316. doi: 10.1016/0091-6749(81)90027-0. [DOI] [PubMed] [Google Scholar]
- 37.Bohnsack J F, Hawley M M, Pritchard D G, Egan M L, Shigeoka A O, Yang K D, Hill H R. An IgA monoclonal antibody directed against type III antigen on group B streptococci acts as an opsonin. J Immunol. 1989;143:3338–3342. [PubMed] [Google Scholar]
- 38.Bolton R W. Naturally-occurring antibodies to glycerol-teichoic acid in human saliva. Correlation with caries activity. J Dent Res. 1981;60:878–882. doi: 10.1177/00220345810600050401. [DOI] [PubMed] [Google Scholar]
- 39.Bolton R W, Hlava G L. Evaluation of salivary IgA antibodies to cariogenic microorganisms in children. Correlation with dental caries activity. J Dent Res. 1982;61:1225–1228. doi: 10.1177/00220345820610110201. [DOI] [PubMed] [Google Scholar]
- 40.Bonta C Y, Linzer R, Emmings F, Evans R T, Genco R J. Human oral infectivity and immunization studies with Streptococcus mutans strain B13. J Dent Res. 1979;58:143. . (Abstract.) [Google Scholar]
- 41.Booth V, Ashley F P, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–427. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowden G H, Hamilton I R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and “S. mitior” growing in continuous culture. Can J Microbiol. 1987;33:824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- 43.Bowden G H W. Microbiology of root surface caries in humans. J Dent Res. 1990;69:1205–1210. doi: 10.1177/00220345900690051701. [DOI] [PubMed] [Google Scholar]
- 44.Bowden G H W, Ellwood D C, Hamilton I R. Microbial ecology of the oral cavity. Adv Microb Ecol. 1979;3:135–217. [Google Scholar]
- 45.Bowden G H, Hardie J M, Slack G L. Microbial variations in approximal dental plaque. Caries Res. 1975;9:253–277. doi: 10.1159/000260162. [DOI] [PubMed] [Google Scholar]
- 46.Bowen W H, Cohen B, Cole M F, Colman G. Immunization against dental caries. Br Dent J. 1975;139:45–58. [PubMed] [Google Scholar]
- 47.Bradshaw D J, McKee A S, Marsh P D. Effect of carbohydrate pulses and pH on population shifts within oral microbial communities in vitro. J Dent Res. 1989;68:1298–1302. doi: 10.1177/00220345890680090101. [DOI] [PubMed] [Google Scholar]
- 48.Bradshaw D J, McKee A S, Marsh P D. Prevention of population shifts in oral microbial communities in vitro by low fluoride concentrations. J Dent Res. 1990;69:436–441. doi: 10.1177/00220345900690020301. [DOI] [PubMed] [Google Scholar]
- 49.Bradshaw D J, Marsh P D. Effect of sugar alcohols on the composition and metabolism of a mixed culture of oral bacteria grown in a chemostat. Caries Res. 1994;28:251–256. doi: 10.1159/000261977. [DOI] [PubMed] [Google Scholar]
- 50.Brady L J, Piacentini D A, Crowley P J, Oyston P C F, Bleiweis A S. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesine P1. Infect Immun. 1992;60:1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brain P F, Nowell N W. The effect of differential grouping on endocrine function of mature male albino mice. Physiol Behav. 1970;5:907–910. doi: 10.1016/0031-9384(70)90180-0. [DOI] [PubMed] [Google Scholar]
- 52.Brandtzaeg P. Human secretory immunoglobulins. VII. Concentrations of parotid IgA and other secretory proteins in relation to the rate of flow and duration of secretory stimulus. Arch Oral Biol. 1971;16:1295–1310. doi: 10.1016/0003-9969(71)90033-1. [DOI] [PubMed] [Google Scholar]
- 53.Brandtzaeg P. The oral and secretory immune system with special emphasis on its relation to dental caries. Proc Finn Dent Soc. 1983;79:71–84. [PubMed] [Google Scholar]
- 54.Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. Acta Pathol Microbiol Immunol Scand. 1995;103:1–19. doi: 10.1111/j.1699-0463.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 55.Brandtzaeg P, Fjellanger I, Gjeruldsen S T. Adsorption of immunoglobulin A onto oral bacteria in vivo. J Bacteriol. 1968;96:242–249. doi: 10.1128/jb.96.1.242-249.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brandtzaeg, P., K. Valnes, H. Scott, T. O. Rognum, K. Bjerke, and K. Baklien. 1985. The human gastrointestinal secretory immune system in health and disease. Scand. J. Gastroenterol. 20(Suppl. 114):17–38. [DOI] [PubMed]
- 57.Bratthall D, Carlen A, Knox K W, Wicken A J. Examination of parotid saliva for antibodies reacting with Streptococcus mutans, lipoteichoic acid and peptidoglycan by the enzyme-linked immunosorbent assay. Acta Pathol Microbiol Scand Sect C. 1979;87:251–255. [PubMed] [Google Scholar]
- 58.Bratthall D, Widerström L. Ups and downs for salivary IgA. Scand J Dent Res. 1985;93:128–134. doi: 10.1111/j.1600-0722.1985.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 59.Bratthall D, Gibbons R J. Changing agglutination activities of salivary immunoglobulin A preparations against oral streptococci. Infect Immun. 1975;11:603–606. doi: 10.1128/iai.11.3.603-606.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brennan M J, Cesar J O, Sanberg A L. A 160-kilodalton epithelial cell surface glycoprotein recognized by plant lectins that inhibit the adherence of Actinomyces naeslundii. Infect Immun. 1986;52:840–845. doi: 10.1128/iai.52.3.840-845.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown L R, Handler S, Allen S S, Shea C, Wheatcroft M G, Frome W J. Oral microbial profile of the marmoset. J Dent Res. 1972;52:815–822. doi: 10.1177/00220345730520042801. [DOI] [PubMed] [Google Scholar]
- 62.Brown L R, Mackler B F, Levy B M, Wright T E, Handler S F, Moylan J S, Perkins D H, Keene H J. Comparison of the plaque microflora in immunodeficient and immunocompetent dental patients. J Dent Res. 1979;58:2344–2352. doi: 10.1177/00220345790580120301. [DOI] [PubMed] [Google Scholar]
- 63.Brown L R, Dreizen S, Handler S, Johnston D. Effect of radiation-induced xerostomia on human oral microflora. J Dent Res. 1975;54:740–750. doi: 10.1177/00220345750540040801. [DOI] [PubMed] [Google Scholar]
- 64.Brown T A, Mestecky J. Immunoglobulin A subclass distribution of naturally occurring salivary antibodies to microbial antigens. Infect Immun. 1985;49:459–462. doi: 10.1128/iai.49.2.459-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunham R C, Plummer F A, Stephens R S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bukawa H, Sekigawa K I, Hamajima K, Fukushima J, Yamada Y, Kiyono H, Okuda K. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nat Med. 1995;1:681–685. doi: 10.1038/nm0795-681. [DOI] [PubMed] [Google Scholar]
- 67.Burckhardt J J, Gaegauf-Zollinger R, Schmid R, Guggenheim B. Alveolar bone loss in rats after immunization with Actinomyces viscosus. Infect Immun. 1981;31:971–977. doi: 10.1128/iai.31.3.971-977.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burgio G R, Lanzavecchia A, Plebani A, Jayakar S, Ugazio A G. Ontogeny of secretory immunity: levels of secretory IgA and natural antibodies in saliva. Pediatr Res. 1980;14:1111–1114. doi: 10.1203/00006450-198010000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Burt B A, Loesche W J, Eklund S A. Stability of selected plaque species and their relationship to caries in a child population over 2 years. Caries Res. 1985;19:193–200. doi: 10.1159/000260843. [DOI] [PubMed] [Google Scholar]
- 70.Busscher H J, Cowan M M, van der Mei H C. On the relative importance of specific and non-specific approaches to oral microbial adhesions. FEMS Microbiol Rev. 1992;88:199–209. doi: 10.1111/j.1574-6968.1992.tb04988.x. [DOI] [PubMed] [Google Scholar]
- 71.Camling E, Köhler B. Infection with the bacterium Streptococcus mutans and salivary IgA antibodies in mothers and their children. Arch Oral Biol. 1987;32:817–823. doi: 10.1016/0003-9969(87)90009-4. [DOI] [PubMed] [Google Scholar]
- 72.Camling E, Gahnberg L, Krasse B. The relationship between IgA antibodies to Streptococcus mutans antigens in human saliva and breast milk and the numbers of indigenous oral Streptococcus mutans. Arch Oral Biol. 1987;32:21–25. doi: 10.1016/0003-9969(87)90149-x. [DOI] [PubMed] [Google Scholar]
- 73.Carlsson J, Grahnen H, Jonsson G, Wilkner S. Early establishment of Streptococcus salivarius in the mouths of infants. J Dent Res. 1970;49:415–418. doi: 10.1177/00220345700490023601. [DOI] [PubMed] [Google Scholar]
- 74.Carter B, Pollard M. Host responses to normal microbial flora in GF mice. J Reticuloendothel Soc. 1971;9:580–587. [PubMed] [Google Scholar]
- 75.Challacombe S J. Serum and salivary antibodies to Streptococcus mutans in relation to the development and treatment of human dental caries. Arch Oral Biol. 1980;25:495–502. doi: 10.1016/0003-9969(80)90058-8. [DOI] [PubMed] [Google Scholar]
- 76.Challacombe S J. Salivary antibodies and systemic tolerance in mice after oral immunization with bacterial antigens. Ann N Y Acad Sci. 1983;409:177–193. doi: 10.1111/j.1749-6632.1983.tb26868.x. [DOI] [PubMed] [Google Scholar]
- 77.Challacombe S J, Tomasi T B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980;152:1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Challacombe S J, Lehner T. Salivary antibody responses in rhesus monkeys immunized with Streptococcus mutans by the oral, submucosal, or subcutaneous routes. Arch Oral Biol. 1980;24:917–925. doi: 10.1016/0003-9969(79)90217-6. [DOI] [PubMed] [Google Scholar]
- 79.Challacombe S J, Bergmeier L A, Czerkinsky C, Rees A S. Natural antibodies in man to Streptococcus mutans: specificity and quantification. Immunology. 1984;52:143–150. [PMC free article] [PubMed] [Google Scholar]
- 80.Chandler D C, Silverman M S, Lundblad R L, McFall W T. Human parotid IgA and periodontal disease. Arch Oral Biol. 1974;19:733–735. doi: 10.1016/0003-9969(74)90144-7. [DOI] [PubMed] [Google Scholar]
- 81.Childers N K, Bruce M G, McGhee J R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- 82.Childers N K, Zhang S S, Michalek S M. Oral immunization of humans with dehydrated liposomes containing Streptococcus mutans glucosyltransferase induces salivary immunoglobulin A2 antibody responses. Oral Microbiol Immunol. 1994;9:146–153. doi: 10.1111/j.1399-302x.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 83.Clark W B, Magnusson I, Beem J E, Jung J M, Marks R G, McArthur W P. Immune modulation of Prevotella intermedia colonization in squirrel monkeys. Infect Immun. 1991;59:1927–1931. doi: 10.1128/iai.59.6.1927-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clark W B, Magnusson I, Abee C, Collins B, Beem J E, McArthur W P. Natural occurrence of black-pigmented Bacteroides species in the gingival crevice of the squirrel monkey. Infect Immun. 1988;56:2392–2399. doi: 10.1128/iai.56.9.2392-2399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark W B, Bammann L L, Gibbons R J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978;19:846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cohen I R, Young D B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 87.Cole M F. Influence of secretory immunoglobulin A on ecology of oral bacteria. In: Mergenhagen S E, Rosan B, editors. Molecular basis of oral microbial adhesion. Washington, D.C: American Society for Microbiology; 1985. pp. 131–135. [Google Scholar]
- 88.Cole M F, Hale C A, Sturzenegger S. Identification of two subclasses of IgA in the chimpanzee (Pan troglodytes) J Med Primatol. 1992;21:275–278. [PubMed] [Google Scholar]
- 89.Cole M F, Emilson C-G, Hsu S D, Li S-H, Bowen W H. Effect of peroral immunization of humans with Streptococcus mutans on induction of salivary and serum antibodies and inhibition of experimental infection. Infect Immun. 1984;46:703–709. doi: 10.1128/iai.46.3.703-709.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cole M F, Arnold R R, Rhodes M J, McGhee J R. Immune dysfunction and dental caries. A preliminary report. J Dent Res. 1977;56:198–204. doi: 10.1177/00220345770560030201. [DOI] [PubMed] [Google Scholar]
- 91.Cole M F, Hsu S D, Sheridan M J, Stiles H M. Natural transmission of Streptococcus sobrinus in rats: saliva and serum antibody responses to colonization. Infect Immun. 1992;60:778–783. doi: 10.1128/iai.60.3.778-783.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coleman R, Hand A R. Endocytosis of native and cationized ferritin by rat parotid duct cells. J Dent Res. 1985;64:223. doi: 10.1007/BF00217329. . (Abstract.) [DOI] [PubMed] [Google Scholar]
- 93.Colten H R, Bienenstock J. Lack of C3 activation through classical or alternative pathways by human secretory IgA anti-blood group A antibody. Adv Exp Med Biol. 1974;45:305–309. doi: 10.1007/978-1-4613-4550-3_36. [DOI] [PubMed] [Google Scholar]
- 94.Coulombe C, Lavoie M C. Evolution of resident oral bacterial biota in BALB/c mice during pregnancy and lactation. Microb Ecol. 1995;30:219–225. doi: 10.1007/BF00172576. [DOI] [PubMed] [Google Scholar]
- 95.Crabbé P A, Bazin H, Eyssen H, Heremans J F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. Int Arch Allergy. 1968;34:362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- 96.Crawford J M, Taubman M A, Smith D J. The effects of local immunization with periodontopathic microorganisms on periodontal bone loss in gnotobiotic rats. J Periodontal Res. 1978;13:445–459. doi: 10.1111/j.1600-0765.1978.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 97.Cummins D, Creeth J E. Delivery of antiplaque agents from dentifrices, gels, and mouthwashes. J Dent Res. 1992;71:1439–1449. doi: 10.1177/00220345920710071601. [DOI] [PubMed] [Google Scholar]
- 98.Cunningham-Rundles C, Brandeis W E, Good R A, Day N K. Milk precipitins, circulating immune complexes, and IgA deficiency. Proc Natl Acad Sci USA. 1978;75:3387–3389. doi: 10.1073/pnas.75.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Czerkinsky, Svennerholm A-M, Quiding M, Jonsson R, Holmgren J. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect Immun. 1991;59:996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Czerkinsky C, Prince S J, Michalek S M, Jackson S, Russell M W, Moldoveanu Z, McGhee J R, Mestecky J R. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci USA. 1987;84:2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dahlén G, Björkander J, Gahnberg L, Slots J, Hanson L-Å. Periodontal disease and dental caries in relation to primary IgG subclass and other humoral immunodeficiencies. J Clin Periodontol. 1991;20:7–13. doi: 10.1111/j.1600-051x.1993.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 103.Dawes, C. 1970. Effects of diet on salivary secretion and composition. J. Dent. Res. 49(Suppl. 6):1263–1273. [DOI] [PubMed]
- 104.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 105.De Jong M H, Van der Hoeven J S. The growth of oral bacteria on saliva. J Dent Res. 1987;66:498–505. doi: 10.1177/00220345870660021901. [DOI] [PubMed] [Google Scholar]
- 106.De Laat P C J, Weemaes M R, Gonera R, van Munster P J J, Bakkeren J A J M, Stoelinga G B A. Clinical manifestations in selective IgA deficiency in childhood. Acta Paediatr Scand. 1991;80:798–804. doi: 10.1111/j.1651-2227.1991.tb11952.x. [DOI] [PubMed] [Google Scholar]
- 107.Delaney J E, Ratzan S K, Korman K S. Subgingival microbiota associated with puberty: studies of pre-, circum-, and postpubertal human females. Pediatr Dent. 1986;8:268–275. [PubMed] [Google Scholar]
- 108.Deslauriers N, Séguin J, Trudel L. Differential recognition of oral indigenous bacteria by salivary immunoglobulin A and G. Microbiol Immunol. 1987;31:199–209. doi: 10.1111/j.1348-0421.1987.tb03084.x. [DOI] [PubMed] [Google Scholar]
- 109.Deslauriers N, Oudghiri M, Seguin J, Trudel L. The oral immune system: dynamics of salivary immunoglobulin production in the inbred mouse. Immunol Invest. 1986;15:339–349. doi: 10.3109/08820138609052953. [DOI] [PubMed] [Google Scholar]
- 110.Deslauriers N, Néron S, Mourad W. Immunobiology of the oral mucosa in the mouse. Immunology. 1985;55:391–397. [PMC free article] [PubMed] [Google Scholar]
- 111.Dimmock N J. Neutralization of animal viruses. Curr Top Microbiol Immunol. 1993;183:99–101. doi: 10.1007/978-3-642-77849-0. [DOI] [PubMed] [Google Scholar]
- 111a.Dionysius D A, Milne J M. Antibacterial peptides of bovine lactoferrin: purification and characterization. J Dairy Sci. 1997;80:667–674. doi: 10.3168/jds.S0022-0302(97)75985-X. [DOI] [PubMed] [Google Scholar]
- 112.Donoghue H D, Tyler J E. Antagonisms amongst streptococci isolated from the human oral cavity. Arch Oral Biol. 1975;20:381–387. doi: 10.1016/0003-9969(75)90031-x. [DOI] [PubMed] [Google Scholar]
- 113.Donoghue H D, Hudson D E, Perrons C J. Effect of the lactoperoxidase system on streptococcal acid production and growth. J Dent Res. 1987;66:616–618. doi: 10.1177/00220345870660024701. [DOI] [PubMed] [Google Scholar]
- 114.Dubos R, Schaedler R W, Costello R, Hoet P. Indigenous, normal, and autochthonous flora of the gastrointestinal tract. J Exp Med. 1965;122:67–75. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde K-H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dumas C, Champagne A, Lavoie M C. Proteolytic activity of bacteria isolated from the oral cavities of BALB/c mice toward salivary proteins. J Dent Res. 1987;66:62–64. doi: 10.1177/00220345870660011301. [DOI] [PubMed] [Google Scholar]
- 117.Dusek D M, Progulske-Fox A, Brown T A. Systemic and mucosal immune responses in mice orally immunized with avirulent Salmonella typhimurium expressing a cloned Porphyromonas gingivalis hemagglutinin. Infect Immun. 1994;62:1652–1657. doi: 10.1128/iai.62.5.1652-1657.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ebersole J L, Taubman M A, Smith D J. Thymic control of secretory antibody responses in the rat. J Immunol. 1979;123:19–24. [PubMed] [Google Scholar]
- 119.Ebersole J L, Taubman M A, Smith D J. Effect of neonatal thymectomy on dental caries in rats. Infect Immun. 1982;38:1130–1136. doi: 10.1128/iai.38.3.1130-1136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ebersole J L, Taubman M A, Smith D J. Gingival crevicular fluid antibody to oral microorganisms. II. Distribution and specificity of local antibody responses. J Periodontal Res. 1985;20:349–356. doi: 10.1111/j.1600-0765.1985.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 121.Ebersole J L, Taubman M A, Smith D J, Frey D E. Effect of neonatal thymectomy on immune responses of rats to Streptococcus mutans. Infect Immun. 1982;37:993–1000. doi: 10.1128/iai.37.3.993-1000.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ebersole J L, Brunsvold M, Steffensen B, Wood R, Holt S C. Effects of immunization with Porphyromonas intermedia and Prevotella intermedia on progression of ligature-induced periodontitis in the nonhuman primate Macaca fascicularis. Infect Immun. 1991;59:3351–3359. doi: 10.1128/iai.59.10.3351-3359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eggert F M, Gurner B W. Reaction of human colostral and early milk antibodies with oral streptococci. Infect Immun. 1984;44:660–664. doi: 10.1128/iai.44.3.660-664.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eggert F M, Maenz L, Tam Y-C. Measuring the interaction of human secretory glycoproteins with oral bacteria. J Dent Res. 1987;66:610–612. doi: 10.1177/00220345870660024501. [DOI] [PubMed] [Google Scholar]
- 125.Elson, C. O. 1985. Induction and control of the gastrointestinal immune system. Scand. J. Gastroenterol. 20(Suppl. 114):1–15. [DOI] [PubMed]
- 126.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1985;132:2736–2741. [PubMed] [Google Scholar]
- 127.Elson C O, Woogen S, Gaspari M, Ealding W. Induction of secretory IgA responses to protein antigens. Adv Exp Med Biol. 1987;216B:877–884. [PubMed] [Google Scholar]
- 128.Emilson C G, Ciardi J E, Olsson J, Bowen W H. The influence of saliva on infection of the human mouth by mutans streptococci. Arch Oral Biol. 1989;34:335–340. doi: 10.1016/0003-9969(89)90106-4. [DOI] [PubMed] [Google Scholar]
- 129.Emming F G, Evans R T, Genco R J. Antibody response in the parotid fluid and serum of irus monkeys (Macaca fascicularis) after local immunization with Streptococcus mutans. Infect Immun. 1975;12:281–292. doi: 10.1128/iai.12.2.281-292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Evans J J, Wilkinson A R, Aickin D R. Salivary estriol concentration during normal pregnancy, and a comparison with plasma estriol. Clin Chem. 1984;30:120–121. [PubMed] [Google Scholar]
- 131.Evans R T, Klausen B, Sojar H T, Bedi G S, Sfintescu C, Ramamurthy N S, Golub L M, Genco R J. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992;60:2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evans R T, Klausen B, Genco R J. Immunization with fimbrial protein and peptide protects against Porphyromonas gingivalis-induced periodontal tissue destruction. Adv Exp Med Biol. 1992;327:255–262. doi: 10.1007/978-1-4615-3410-5_27. [DOI] [PubMed] [Google Scholar]
- 133.Evans R T, Emmings F G, Genco R J. Prevention of Streptococcus mutans infection of tooth surfaces by salivary antibody in irus monkeys (Macaca fascicularis) Infect Immun. 1975;12:293–302. doi: 10.1128/iai.12.2.293-302.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Everhart D L, Rothenberg K, Carter W H, Jr, Klapper B. The determination of antibody to Streptococcus mutans serotypes in saliva for children ages 3 to 7 years. J Dent Res. 1978;57:631–635. doi: 10.1177/00220345780570041701. [DOI] [PubMed] [Google Scholar]
- 135.Everhart D L, Bamgboye P O, Schwartz M S. Salivary anti-Streptococcus mutans changes over a six-month period in children ages two–five years. J Dent Res. 1982;61:386–390. doi: 10.1177/00220345820610020301. [DOI] [PubMed] [Google Scholar]
- 136.Fanger M W, Goldstine S N, Shen L. Cytofluorographic analysis of receptors for IgA on human polymorphonuclear cells and monocytes and the correlation of receptor expression with phagocytosis. Mol Immunol. 1983;20:1019–1027. doi: 10.1016/0161-5890(83)90043-3. [DOI] [PubMed] [Google Scholar]
- 137.Fernandes F R C, Nagao A T, Mayer M P A, Zelante F, Carneiro-Sampaio M M S. Compensatory levels of salivary IgM anti-Streptococcus mutans antibodies in IgA-deficient patients. J Invest Allergy Clin Immunol. 1995;5:151–155. [PubMed] [Google Scholar]
- 138.Filler S J, Gregory R L, Michalek S M, Katz J, McGhee J R. Effect of immune bovine milk on Streptococcus mutans in human dental plaque. Arch Oral Biol. 1991;36:41–47. doi: 10.1016/0003-9969(91)90052-v. [DOI] [PubMed] [Google Scholar]
- 139.Fitzgerald R J, Keyes P H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- 140.Fitzsimmons S P, Evans M K, Pearce C L, Sheridan M J, Wientzen R, Cole M F. Immunoglobulin A subclasses in infants’ saliva and in saliva and milk from their mothers. J Pediatr. 1994;124:566–573. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]
- 141.Foo M C, Lee A. Immunological response of mice to members of the autochthonous intestinal microflora. Infect Immun. 1972;6:525–532. doi: 10.1128/iai.6.4.525-532.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Foo M C, Lee A. Antigenic cross-reaction between mouse intestine and a member of the autochthonous microflora. Infect Immun. 1974;9:1066–1069. doi: 10.1128/iai.9.6.1066-1069.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frandsen E V G, Reinholdt J, Kilian M. Enzymatic and antigenic characterization of immunoglobulin A1 proteases from Bacteroides and Capnocytophaga spp. Infect Immun. 1987;55:631–638. doi: 10.1128/iai.55.3.631-638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frandsen E V G, Pedrazzoli V, Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991;6:129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 145.Freter R, Brickner H, Botney M, Cleven D, Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983;39:676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Friedman A, Al-Sabbagh A, Santos L M B, Fishman-Lobell J, Polanski M, Das M P, Khoury S J, Weiner H L. Oral tolerance: a biologically relevant pathway to generate peripheral tolerance against external and self antigens. Chem Immunol. 1994;58:259–290. [PubMed] [Google Scholar]
- 147.Fubara E S, Freter R. Source and protective function of coproantibodies in intestinal disease. Am J Clin Nutr. 1972;25:1357–1363. doi: 10.1093/ajcn/25.12.1357. [DOI] [PubMed] [Google Scholar]
- 148.Fukui Y, Fukui K, Moriyama T. Inhibition of enzymes by human salivary immunoglobulin A. Infect Immun. 1973;8:335–340. doi: 10.1128/iai.8.3.335-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Funakoshi S, Doi T, Nakajima T, Suyama T, Tokuda M. Antimicrobial effect of human serum IgA. Microbiol Immunol. 1982;26:227–239. doi: 10.1111/j.1348-0421.1982.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 150.Gadbois T, Marcotte H, Rodrigue L, Coulombe C, Goyette N, Lavoie M C. Distribution of the resident oral bacterial populations in different strains of mice. Microb Ecol Health Dis. 1993;6:245–251. [Google Scholar]
- 151.Gahnberg L, Krasse B. Salivary immunoglobulin A antibodies reacting with antigens from oral streptococci: longitudinal study in humans. Infect Immun. 1981;33:697–703. doi: 10.1128/iai.33.3.697-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gahnberg L, Krasse B. Salivary immunoglobulin A antibodies and recovery from challenge of Streptococcus mutans after oral administration of Streptococcus mutans vaccine in humans. Infect Immun. 1983;39:514–519. doi: 10.1128/iai.39.2.514-519.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gahnberg L, Smith D J, Taubman M A, Ebersole J L. Salivary-IgA antibody to glucosyltransferase of oral microbial origin in children. Arch Oral Biol. 1985;30:551–556. doi: 10.1016/0003-9969(85)90056-1. [DOI] [PubMed] [Google Scholar]
- 154.Gahnberg L, Olsson J, Krasse B, Carlén A. Interference of salivary immunoglobulin A antibodies and other salivary fractions with adherence of Streptococcus mutans to hydroxyapatite. Infect Immun. 1982;37:401–406. doi: 10.1128/iai.37.2.401-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gauldie J, Richards C, Lamontagne L. Fc receptors for IgA and other immunoglobulins on resident and activated alveolar macrophages. Mol Immunol. 1983;20:1029–1037. doi: 10.1016/0161-5890(83)90044-5. [DOI] [PubMed] [Google Scholar]
- 156.Genco, R. J. 1992. Host responses in periodontal diseases:current concepts. J. Periodontol. 63(Suppl.):338–355. [DOI] [PubMed]
- 157.Genco R J, Slots J. Host responses in periodontal diseases. J Dent Res. 1989;63:441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- 158.Gharbia S E, Shah H N, Welch S G. The influence of peptides on the uptake of amino acids in Fusobacterium: predicted interactions with Porphyromonas gingivalis. Curr Microbiol. 1984;19:231–235. [Google Scholar]
- 159.Gibbons R J, Hay D I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988;56:439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gibbons R J, Hay D I, Cisar J O, Clark W B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988;56:2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gilbert A D, Sofaer J A. Neutrophil function, genotype and periodontal bone loss in the mouse. J Periodontal Res. 1989;24:412–414. doi: 10.1111/j.1600-0765.1989.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert J V, Plaut A G, Longmaid B, Lamm M E. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol Immunol. 1983;20:1039–1049. doi: 10.1016/0161-5890(83)90045-7. [DOI] [PubMed] [Google Scholar]
- 163.Glass R I, Svennerholm A-M, Stoll B J, Khan M R, Belayet Hossain K M, Huq M I, Holmgren J. Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med. 1983;308:1389–1392. doi: 10.1056/NEJM198306093082304. [DOI] [PubMed] [Google Scholar]
- 164.Gleeson M, Cripps A W, Clancy R L, Hensley M J, Dobson A J, Firman D W. Breast feeding conditions a differential developmental pattern of mucosal immunity. Clin Exp Immunol. 1986;66:216–222. [PMC free article] [PubMed] [Google Scholar]
- 165.Goldman A S, Garza C, Nichols B L, Goldblum R M. Immunological factors in human milk during the first year of lactation. J Pediatr. 1982;100:563–567. doi: 10.1016/s0022-3476(82)80753-1. [DOI] [PubMed] [Google Scholar]
- 166.Gómez E, Ortiz V, Saint-Martin B, Boeck L, Díaz-Sánchez V, Bourges H. Hormonal regulation of the secretory IgA (sIgA) system: estradiol- and progesterone-induced changes in sIgA in parotid saliva along the menstrual cycle. Am J Reprod Immunol. 1993;29:219–223. doi: 10.1111/j.1600-0897.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 167.Gorter A, Hiemstra P S, Leijh P C J, van der Sluys M E, van den Barselaar M T, van Es L A, Daha M R. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987;61:303–309. [PMC free article] [PubMed] [Google Scholar]
- 168.Goyette N, Parrot M, Sutzescu D, Leduc M, Dufour L, Trahan L, Lavoie M C. Inverse correlation between the proportion of salivary bacteria inhibiting Streptococcus mutans and the percentage of untreated carious teeth. J Oral Pathol Med. 1995;24:462–467. doi: 10.1111/j.1600-0714.1995.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 169.Gråhn E, Tenovuo J, Lehtonen O-P, Eerola E, Vilja P. Antimicrobial systems of human whole saliva in relation to dental caries, cariogenic bacteria, and gingival inflammation in young adults. Acta Odontol Scand. 1988;46:67–74. doi: 10.3109/00016358809004749. [DOI] [PubMed] [Google Scholar]
- 170.Gregory R L, Filler S J. Protective secretory immunoglobulin A antibodies in humans following oral immunization with Streptococcus mutans. Infect Immun. 1987;55:2409–2415. doi: 10.1128/iai.55.10.2409-2415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gregory R L, Kindle J C, Hobbs L C, Filler S J, Malmstrom H S. Function of anti-Streptococcus mutans antibodies: inhibition of virulence factors and enzyme neutralization. Oral Microbiol Immunol. 1990;5:181–188. doi: 10.1111/j.1399-302x.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 172.Gregory R L, Michalek S M, Shechmeister I L, McGhee J R. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with a ribosomal preparation from Streptococcus mutans. Microbiol Immunol. 1983;27:787–800. doi: 10.1111/j.1348-0421.1983.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 173.Gregory R L, Gfell L E, Malmstrom H S. Differences in secretory IgA and serum antibodies to Streptococcus mutans isolated from caries-resistant and caries-susceptible subjects. Adv Exp Med Biol. 1995;371B:1149–1152. [PubMed] [Google Scholar]
- 174.Gregory R L, Shöller M, Filler S J, Crago S S, Prince S J, Allansmith M R, Michalek S M, Mestecky J, McGhee J R. IgA antibodies to oral and ocular bacteria in human external secretions. Protides Biol Fluids Proc Colloq. 1985;32:53–56. [Google Scholar]
- 175.Gregory R L, Michalek S M, Richardson G, Harmon C, Hilton T, McGhee J R. Characterization of immune response to oral administration of Streptococcus sobrinus ribosomal preparations in liposomes. Infect Immun. 1986;54:780–786. doi: 10.1128/iai.54.3.780-786.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gregory R L, Michalek S M, Shechmeister I L, McGhee J R. Function of anti-Streptococcus mutans antibodies: anti-ribosomal antibodies inhibit acid production, growth, and glucose phosphotransferase activity. Infect Immun. 1984;45:286–289. doi: 10.1128/iai.45.1.286-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gregory R L, Michalek S M, Filler S J, Mestecky J, McGhee J R. Prevention of Streptococcus mutans colonization by salivary IgA antibodies. J Clin Immunol. 1985;5:55–62. doi: 10.1007/BF00915169. [DOI] [PubMed] [Google Scholar]
- 178.Griffiss J M, Goroff D K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983;130:2882–2885. [PubMed] [Google Scholar]
- 179.Grundbacher F J. Variation in levels of immunoglobulins A, G, E in human saliva. Arch Oral Biol. 1988;33:121–126. doi: 10.1016/0003-9969(88)90055-6. [DOI] [PubMed] [Google Scholar]
- 180.Gusberti F A, Mombelli A, Lang N P, Minder C E. Changes in subgingival microbiota during puberty: a 4-year longitudinal study. J Clin Periodontol. 1990;29:1114–1124. doi: 10.1111/j.1600-051x.1990.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 181.Güven O, De Vissher J G A M. Salivary IgA in periodontal disease. J Periodontol. 1982;53:334–335. doi: 10.1902/jop.1982.53.5.334. [DOI] [PubMed] [Google Scholar]
- 182.Hägewald S, Jancke M, Bernimoulin J-P, Kage A. Specific and total IgA from salivary glands in periodontitis patients. J Dent Res. 1996;75:310. . (Abstract.) [Google Scholar]
- 183.Hajishengallis G, Nikolova E, Russell M W. Inhibition of Streptococcus mutans adherence to saliva-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5064. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 185.Hajishengallis G, Michalek S M, Russell M W. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect Immun. 1996;64:665–667. doi: 10.1128/iai.64.2.665-667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hamilton I R, McKee A S, Bowden G H. Growth and metabolic properties of Bacteroides intermedia in anaerobic continuous culture. Oral Microbiol Immunol. 1989;4:89–97. doi: 10.1111/j.1399-302x.1989.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 187.Hanson, L. Å., S. A. Ahlstedt, B. Andersson, B. Carlsson, S. P. Fällström, L. Mellander, O. Porras, T. Söderström, and C. Svanborg Edén. 1985. Protective factors in milk and the development of the immune system. Pediatrics 75(Suppl.):172–176. [PubMed]
- 188.Hanson L A, Björkander J, Oxelius V A. Selective IgA deficiency. In: Chandra R K, editor. Primary and secondary immunodeficiency disorders. New York, N.Y: Churchill-Livingstone, Inc.; 1983. pp. 62–84. [Google Scholar]
- 189.Harding J, Berry W C, Jr, Marsh C, Jolliff C R. Salivary antibodies in acute gingivitis. J Periodontol. 1980;51:63–69. doi: 10.1902/jop.1980.51.2.63. [DOI] [PubMed] [Google Scholar]
- 190.Henskens Y M, van der Velden U, Veerman E C, Nieuw Amerongen A V. Protein, albumin, and cystatin concentrations in saliva of healthy subjects and of patients with gingivitis or periodontitis. J Periodontal Res. 1993;28:43–48. doi: 10.1111/j.1600-0765.1993.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 191.Hiemstra P S, Gorter A, Stuurman M E, van Es L A, Daha M R. Activation of the alternative pathway of complement by human serum IgA. Eur J Immunol. 1987;17:321–326. doi: 10.1002/eji.1830170304. [DOI] [PubMed] [Google Scholar]
- 192.Hiemstra P S, Biewenga J, Gorter A, Stuurman M E, Faber A, van Es L A, Daha M R. Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol Immunol. 1988;25:527–533. doi: 10.1016/0161-5890(88)90074-0. [DOI] [PubMed] [Google Scholar]
- 193.Hiemstra P S, Rits M, Gorter A, Stuurman M E, Hoekzema R, Bazin H, Vaerman J-P, van Es L A, Daha M R. Rat polymeric IgA bind C1q, but does not activate C1. Mol Immunol. 1990;27:867–874. doi: 10.1016/0161-5890(90)90153-q. [DOI] [PubMed] [Google Scholar]
- 194.Hillman J D, Dzuback A L, Andrews S W. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res. 1987;66:1092–1094. doi: 10.1177/00220345870660060101. [DOI] [PubMed] [Google Scholar]
- 195.Hocini H, Iscaki S, Bouvet J-P, Pillot J. Unexpectedly high levels of some presumably protective secretory immunoglobulin A antibodies to dental plaque bacteria in salivas of both caries-resistant and caries-susceptible subjects. Infect Immun. 1993;61:3597–3604. doi: 10.1128/iai.61.9.3597-3604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195a.Hoek K S, Milne J M, Grieve P A, Dionysius D A, Smith R. Antibacterial activity in bovine lactoferrin-derived peptides. Antimicrob Agents Chemother. 1997;41:54–59. doi: 10.1128/aac.41.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hohwy J, Kilian M. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol Immunol. 1994;10:19–25. doi: 10.1111/j.1399-302x.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 197.Holub M. The athymic nude mouse. In: Ríhová B, Vetvicka V, editors. Immunological disorders in mice. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 23–47. [Google Scholar]
- 198.Hoogendoorn H. Activation of the salivary peroxidase antimicrobial system: clinical studies. In: Pruitt K M, Tenovuo J, editors. The lactoperoxidase system: chemistry and clinical significance. New York, N.Y: Marcel Dekker, Inc.; 1985. pp. 217–227. [Google Scholar]
- 199.Hoogendoorn H, Moorer W R. Lactoperoxidase in the prevention of plaque accumulation, gingivitis and dental caries. II. Odontol Revy. 1973;24:367–372. [PubMed] [Google Scholar]
- 200.Hoover R G, Lynch R G. Isotype-specific suppression of IgA: suppression of IgA responses in BALB/c by Tα cells. J Immunol. 1983;130:521–523. [PubMed] [Google Scholar]
- 201.Hsu S D, Cole M F. Structural integrity of host defense factors in dental plaque. Infect Immun. 1985;50:398–402. doi: 10.1128/iai.50.2.398-402.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huis in’t Veld J H, van Palenstein Helderman W H, Backer Dirks O. Streptococcus mutans and dental caries in humans: a bacteriological and immunological study. Antonie Leeuwenhoek. 1979;45:25–33. doi: 10.1007/BF00400775. [DOI] [PubMed] [Google Scholar]
- 203.Imai H, Chen A, Wyatt R J, Rifai A. Lack of complement activation by human IgA immune complexes. Clin Exp Immunol. 1988;73:479–483. [PMC free article] [PubMed] [Google Scholar]
- 204.Isogai E, Isogai H, Sawada H, Kaneko H, Ito N. Microbial ecology of plaque in rats with naturally occurring gingivitis. Infect Immun. 1985;48:520–527. doi: 10.1128/iai.48.2.520-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Isokangas P, Tenovuo J, Söderling E, Mannisto H, Mäkinen K K. Dental caries and mutans streptococci in the proximal areas of molars affected by the habitual use of xylitol chewing gum. Caries Res. 1991;25:444–448. doi: 10.1159/000261408. [DOI] [PubMed] [Google Scholar]
- 206.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jackson S, Mestecky J, Childers N K, Michalek S M. Liposomes containing anti-idiotypic antibodies: an oral vaccine to induce protective secretory immune responses specific for pathogens of mucosal surfaces. Infect Immun. 1990;58:1932–1936. doi: 10.1128/iai.58.6.1932-1936.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jalil R A, Ashley F P, Wilson R F. The relationship between 48-h dental plaque accumulation in young human adults and the concentrations of hypothiocyanite, ‘free‘ and ‘total‘ lysozyme, lactoferrine and secretory immunoglobulin A in saliva. Arch Oral Biol. 1992;37:23–28. doi: 10.1016/0003-9969(92)90148-2. [DOI] [PubMed] [Google Scholar]
- 209.Jalil R A, Ashley F P, Wilson R F, Wagaiyu E G. Concentrations of thiocyanate, hypothiocyanite, ‘free‘ and ‘total‘ lysozyme, lactoferrine and secretory IgA in resting and stimulated whole saliva of children aged 12–14 years and the relationship with plaque accumulation and gingivitis. J Periodontal Res. 1993;28:130–136. doi: 10.1111/j.1600-0765.1993.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 210.Jemmott J B, Boryensko J Z, Boryensko M, McClelland C, Chapman R, Meyer D. Academic stress, power motivation, and decrease in secretion rate of salivary immunoglobulin A. Lancet. 1983;i:1400–1402. doi: 10.1016/s0140-6736(83)92354-1. [DOI] [PubMed] [Google Scholar]
- 211.Jensen J, Liljemark W, Bloomquist C. The effect of female sex hormones on subgingival plaque. J Periodontol. 1981;52:599–602. doi: 10.1902/jop.1981.52.10.599. [DOI] [PubMed] [Google Scholar]
- 212.Jonsson R, Howland B E, Bowden G H W. Relationships between periodontal health, salivary steroids, and Bacteroides intermedia in males, pregnant and non-pregnant women. J Dent Res. 1988;67:1062–1069. doi: 10.1177/00220345880670080101. [DOI] [PubMed] [Google Scholar]
- 213.Kashem A, Endoh M, Nomoto Y, Sakai H, Nakasawa H. Monocyte superoxyde generation and its IgA-receptor in IgA nephropathy. Clin Nephrol. 1996;45:1–9. [PubMed] [Google Scholar]
- 214.Katz J, Harmon C C, Buckner G P, Richardson G J, Russell M W, Michalek S M. Protective salivary immunoglobulin A responses against Streptococcus mutans infection after intranasal immunization with S. mutans antigen I/II coupled to the B subunit of cholera toxin. Infect Immun. 1993;61:1964–1971. doi: 10.1128/iai.61.5.1964-1971.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kawamura S, Omoto K, Ueda S. Evolutionary hypervariability in the hinge region of the immunoglobulin alpha gene. J Mol Biol. 1990;215:201–206. doi: 10.1016/S0022-2836(05)80336-5. [DOI] [PubMed] [Google Scholar]
- 216.Kawamura S, Saitou N, Ueda S. Concerted evolution of the primate immunoglobulin α-gene through gene conversion. J Biol Chem. 1992;267:7359–7367. [PubMed] [Google Scholar]
- 217.Kelly C G, Todryk S, Kendal H L, Munro G H, Lehner T. T-cell, adhesion, and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect Immun. 1995;63:3649–3658. doi: 10.1128/iai.63.9.3649-3658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kenny E B, Ash M M. Oxidation reduction potential of developing plaque, periodontal plaque, periodontal pockets and gingival sulci. J Periodontol. 1969;40:630–633. doi: 10.1902/jop.1969.40.11.630. [DOI] [PubMed] [Google Scholar]
- 219.Kent R, Smith D J, Joshipura K, Soparkar P, Taubman M A. Humoral IgG antibodies to oral microbiota in a population at risk for root-surface caries. J Dent Res. 1992;71:1399–1407. doi: 10.1177/00220345920710070801. [DOI] [PubMed] [Google Scholar]
- 220.Kerr M A. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Keyes P H. The infectious and transmissible nature of experimental dental caries: findings and implications. Arch Oral Biol. 1960;1:304–320. doi: 10.1016/0003-9969(60)90091-1. [DOI] [PubMed] [Google Scholar]
- 222.Kilian M, Rolland K, Mestecky J. Interference of secretory immunoglobulin A with sorption of oral bacteria to hydroxyapatite. Infect Immun. 1981;31:935–941. doi: 10.1128/iai.31.3.942-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kilian M, Thomsen B, Peterson T E, Bleeg H S. Occurrence and nature of bacterial IgA proteases. Ann N Y Acad Sci. 1983;409:612–624. doi: 10.1111/j.1749-6632.1983.tb26903.x. [DOI] [PubMed] [Google Scholar]
- 224.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kilian M, Reinholdt J, Nyvad B, Frandsen E V G, Mikkelsen L. IgA1 proteases of oral streptococci: ecological aspects. Immunol Invest. 1989;18:161–170. doi: 10.3109/08820138909112235. [DOI] [PubMed] [Google Scholar]
- 226.Kirstila V, Tenovuo J, Ruuskanen O, Nikoskelainen J, Irjala K, Vilja P. Salivary defense factors and oral health in patients with common variable immunodeficiency. J Clin Immunol. 1994;14:229–236. doi: 10.1007/BF01552309. [DOI] [PubMed] [Google Scholar]
- 227.Kitamura D, Roes J, Kühn R, Rajewsky K. A B-cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature (London) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 228.Kitamura K, Masuda N, Kato K, Sobue S, Hamada S. Effect of a bacteriocin-producing strain of Streptococcus sobrinus on infection and establishment of Streptococcus mutans on tooth surfaces in rats. Oral Microbiol Immunol. 1989;4:65–70. doi: 10.1111/j.1399-302x.1989.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 229.Kiyono H, McGhee J R, Wannemuehler M J, Michalek S M. Lack of oral tolerance in C3H/HeJ mice. J Exp Med. 1982;155:605–610. doi: 10.1084/jem.155.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Klaasen H L B, Peters B, Koopman J P, Poelma F G J, van den Brink M E, Bakker M H, Beynen A C. Different degree of ileal colonization by segmented, filamentous bacteria in two strains of mice. J Exp Anim Sci. 1992;35:103–109. [PubMed] [Google Scholar]
- 231.Klaasen H L B M, Koopman J P, Beynen A C. Effect of age, strain and social hierarchy on colonization of autochthonous segmented filamentous bacteria in the ileum of mice. Microecol Ther. 1990;20:15–18. [Google Scholar]
- 232.Klausen B. Microbiological and immunological aspects of experimental periodontal disease in rats: a review article. J Periodontol. 1991;62:59–73. doi: 10.1902/jop.1991.62.1.59. [DOI] [PubMed] [Google Scholar]
- 233.Klein J P, Schöller M, Frank R M. Inhibition of glucosyltransferase by human salivary immunoglobulin A. Infect Immun. 1977;15:329–331. doi: 10.1128/iai.15.1.329-331.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Koga T, Asakawa H, Okahashi N, Takahashi I. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J Gen Microbiol. 1989;135:3199–3207. doi: 10.1099/00221287-135-12-3199. [DOI] [PubMed] [Google Scholar]
- 235.Kolenbrander, P. E. 1993. Coaggregation of human oral bacteria: potential role in the accretion of dental plaque. J. Appl. Bacteriol. 74(Suppl.):79S–86S. [DOI] [PubMed]
- 236.Kolenbrander P E, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kolenbrander P E, Ganeshkumar N, Classels F J, Hughes C V. Coaggregation: specific adherence among human oral plaque bacteria. FASEB J. 1993;7:406–413. doi: 10.1096/fasebj.7.5.8462782. [DOI] [PubMed] [Google Scholar]
- 238.Könönen E, Jousimies-Somer H, Asikainen S. Relationship between oral gram-negative anaerobic bacteria in saliva of the mother and the colonization of her edentulous infant. Oral Microbiol Immunol. 1992;7:273–276. doi: 10.1111/j.1399-302x.1992.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 239.Koopman J P, van den Brink M E, Scholten P M, van der Heyden M, van Schie F W, Hectors M P C, Nagengast F. The influence of stress and cheese-whey on intestinal parameters in mice. Vet Q. 1989;11:24–29. doi: 10.1080/01652176.1989.9694192. [DOI] [PubMed] [Google Scholar]
- 240.Kornfeld S J, Plaut A G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981;3:521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- 241.Kornman K S, Loesche W J. The subgingival microbial flora during pregnancy. J Periodontal Res. 1980;15:111–122. doi: 10.1111/j.1600-0765.1980.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 242.Kornman K S, Loesche W J. Effects of estradiol and progesterone on Bacteroides melaninogenicus and Bacteroides gingivalis. Infect Immun. 1982;35:256–263. doi: 10.1128/iai.35.1.256-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Korsrud F R, Brandtzaeg P. Quantitative immunochemistry of immunoglobulin and J-chain-producing cells in human parotid and submandibular salivary glands. Immunology. 1980;39:129–140. [PMC free article] [PubMed] [Google Scholar]
- 244.Kramer D R, Cebra J J. Early appearance of “natural” mucosal IgA responses and germinal centers in suckling mice developing in the absence of maternal antibodies. J Immunol. 1995;150:2051–2062. [PubMed] [Google Scholar]
- 245.Krasse B, Jordan H V. Effect of orally applied vaccines on oral colonization by Streptococcus mutans in rodents. Arch Oral Biol. 1977;22:479–484. doi: 10.1016/0003-9969(77)90041-3. [DOI] [PubMed] [Google Scholar]
- 246.Krasse B, Gahnberg L. Available immunoglobulin A antibodies in mouth rinses and implantation of Streptococcus mutans. Infect Immun. 1983;41:1360–1362. doi: 10.1128/iai.41.3.1360-1362.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Krasse B, Gahnberg L, Brathall D. Antibodies reacting with Streptococcus mutans in secretions from minor salivary glands in humans. In: McGhee J R, Mestecky J, Babb J L, editors. Secretory immunity and infections. New York, N.Y: Plenum Publishing Corp.; 1978. pp. 349–354. [DOI] [PubMed] [Google Scholar]
- 248.Kugler J, Hess M, Haake D. Secretions of salivary immunoglobulin A in relation to age, saliva flow, mood states, secretion of albumin, cortisol, and catecholamines in saliva. J Clin Immunol. 1992;12:45–49. doi: 10.1007/BF00918272. [DOI] [PubMed] [Google Scholar]
- 249.Kurihara Y, Naito T, Obayashi K, Hirasawa M, Kurihara Y, Moriwaki K. Caries susceptibility in inbred mouse strains and inheritance patterns in F1 and backross (N2) progeny from strains with high and low caries susceptibility. Caries Res. 1991;25:341–346. doi: 10.1159/000261389. [DOI] [PubMed] [Google Scholar]
- 250.Kurono Y, Fujiyoshi T, Mogi G. Secretory IgA and bacterial adherence to nasal mucosal cells. Ann Otol Rhinol Laryngol. 1989;98:273–278. doi: 10.1177/000348948909800407. [DOI] [PubMed] [Google Scholar]
- 251.Kusumoto Y, Ogawa T, Hamada S. Generation of specific antibody-secreting cells in salivary glands of BALB/c mice following parenteral or oral immunization with Porphyromonas gingivalis fimbriae. Arch Oral Biol. 1993;38:361–367. doi: 10.1016/0003-9969(93)90206-2. [DOI] [PubMed] [Google Scholar]
- 252.Lachelin G C L, McGarrigle H H G. A comparison of saliva, plasma, unconjugated and plasma total oestriol levels throughout. Br J Obstet Gynaecol. 1984;91:1203–1209. doi: 10.1111/j.1471-0528.1984.tb04738.x. [DOI] [PubMed] [Google Scholar]
- 253.Lamm M E, Nedrud J G, Kaetzel C S, Mazanec M B. IgA and mucosal defense. Acta Pathol Microbiol Immunol Scand. 1995;103:241–246. doi: 10.1111/j.1699-0463.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 254.Landi M S, Kreider J W, Lang C M, Bullock L P. Effects of shipping on the immune function in mice. Am J Vet Res. 1982;9:1654–1657. [PubMed] [Google Scholar]
- 255.Lassiter M O, Newsome A, Sams L D, Arnold R R. Characterization of lactoferrin interaction with Streptococcus mutans. J Dent Res. 1987;66:480–485. doi: 10.1177/00220345870660021601. [DOI] [PubMed] [Google Scholar]
- 256.Lee S G. Active release of bound antibody by Streptococcus mutans. Infect Immun. 1995;63:1940–1946. doi: 10.1128/iai.63.5.1940-1946.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Legler D W, McGhee J R, Lynch D P, Mestecky J F, Schaefer M E, Carson J, Bradley E L., Jr Immunodeficiency disease and dental caries in man. Arch Oral Biol. 1981;26:905–910. doi: 10.1016/0003-9969(81)90150-3. [DOI] [PubMed] [Google Scholar]
- 258.Lehner T. Immunology of oral diseases. 3rd ed. Oxford, United Kingdom: Blackwell Scientific Publications; 1992. [Google Scholar]
- 259.Lehner T, Mehlert A, Caldwell A J. Local active gingival immunization by a 3,800-molecular-weight streptococcal antigen in protection against dental caries. Infect Immun. 1986;52:682–687. doi: 10.1128/iai.52.3.682-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lehner T, Haron J, Bergmeier L A, Mehlert A, Beard R, Dodd M, Mielnik B, Moore S. Local oral immunization with synthetic peptides induces a dual mucosal IgG and salivary IgA antibody response and prevents colonization of Streptococcus mutans. Immunology. 1989;67:419–424. [PMC free article] [PubMed] [Google Scholar]
- 261.Lehner T, Russell M W, Caldwell J. Immunization with a purified protein from Streptococcus mutans against dental caries in rhesus monkeys. Lancet. 1980;i:995–996. doi: 10.1016/s0140-6736(80)91437-3. [DOI] [PubMed] [Google Scholar]
- 262.Lehner T, Russell M W, Caldwell J, Smith R. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect Immun. 1981;34:407–415. doi: 10.1128/iai.34.2.407-415.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lehner T, Challacombe S J, Caldwell J. An immunological investigation into the prevention of caries in deciduous teeth of rhesus monkeys. Arch Oral Biol. 1975;20:305–310. doi: 10.1016/0003-9969(75)90019-9. [DOI] [PubMed] [Google Scholar]
- 264.Lehner T, Challacombe S J, Caldwell J. Oral immunization with Streptococcus mutans in rhesus monkeys and the development of immune responses and dental caries. Immunology. 1980;41:857–864. [PMC free article] [PubMed] [Google Scholar]
- 265.Lehtonen O-P J, Gråhn E M, Ståhlberg T H, Laitinen L A. Amount and avidity of salivary and serum antibodies against Streptococcus mutans in two groups of human subjects with different dental caries susceptibility. Infect Immun. 1984;43:308–313. doi: 10.1128/iai.43.1.308-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lenander-Lumikari M, Tenovuo J, Emilson C G, Vilja P. Viability of Streptococcus mutans and Streptococcus sobrinus in whole saliva with varying concentrations of indigenous antimicrobial agents. Caries Res. 1992;26:371–378. doi: 10.1159/000261471. [DOI] [PubMed] [Google Scholar]
- 267.Levine M J, Herzberg M C, Levine M S, Ellison S A, Stinson M W, Li H C, Van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and S. mutans. Infect Immun. 1978;19:107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Liblau R S, Bach J-F. Selective IgA deficiency and autoimmunity. Int Arch Allergy Immunol. 1992;99:16–27. doi: 10.1159/000236330. [DOI] [PubMed] [Google Scholar]
- 269.Liljemark W F, Bloomquist C G, Germaine G R. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981;31:935–941. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Liljemark W F, Bloomquist C G, Ofstehage J C. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979;26:1004–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Lindström F, Folke L E A. Salivary IgA in periodontal disease. Acta Odontol Scand. 1973;31:31–34. doi: 10.3109/00016357309004610. [DOI] [PubMed] [Google Scholar]
- 272.Linzer R, Evans R T, Emmings F G, Genco R J. Use of combined immunization routes in induction of a salivary immunoglobulin A response to Streptococcus mutans in Macaca fascicularis monkeys. Infect Immun. 1981;31:345–351. doi: 10.1128/iai.31.1.345-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Llory H, Dammron A, Gioanni M, Frank R M. Some population changes in oral anaerobic microorganisms, Streptococcus mutans and yeasts following irradiation of the salivary glands. Caries Res. 1972;6:298–311. doi: 10.1159/000259809. [DOI] [PubMed] [Google Scholar]
- 274.Loesche W J. Dental caries: a treatable infection. Springfield, Ill: Charles C Thomas Publisher; 1982. [Google Scholar]
- 275.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lowell G H, Smith L F, Griffiss J M, Brandt B L, MacDermott R P. Antibody-dependent mononuclear cell-mediated anti-meningococcal activity. Comparison of the effects of convalescent and postimmunization immunoglobulins G, M, A. J Clin Invest. 1980;66:260–267. doi: 10.1172/JCI109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lum L G, Muchmore A V, Keren D, Decker J, Koski I, Strober W, Blaese R M. A receptor for IgA on human T lymphocytes. J Immunol. 1979;122:65–69. [PubMed] [Google Scholar]
- 278.Luo Z, Smith D J, Taubman M A, King W F. Cross-sectional analysis of serum antibody to oral streptococcal antigens in children. J Dent Res. 1988;67:554–560. doi: 10.1177/00220345880670030601. [DOI] [PubMed] [Google Scholar]
- 279.Ma, J. K.-C., M. Hunjan, R. Smith, C. Kelly, and T. Lehner. 1990. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. 58:3407–3414. [DOI] [PMC free article] [PubMed]
- 280.Ma J K-C, Smith R, Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987;55:1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.MacDonald T T. Breakdown of tolerance to the intestinal bacterial flora in inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:445–447. doi: 10.1111/j.1365-2249.1995.tb03835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.MacKay B J, Denepitiya L, Iacono V J, Krost S B, Pollock J J. Growth-inhibitory and bactericidal effects of human parotid salivary histidine-rich polypeptides on Streptococcus mutans. Infect Immun. 1984;44:695–701. doi: 10.1128/iai.44.3.695-701.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Magnusson K-E, Stjernstrom I. Mucosal barrier mechanisms. Interplay between secretory IgA (SIgA), IgG and mucins on the surface properties and association of salmonellae with intestine and granulocytes. Immunology. 1982;45:239–248. [PMC free article] [PubMed] [Google Scholar]
- 284.Magnusson K-E, Stendahl O, Stjernström I, Edebo L. Reduction of phagocytosis, surface hydrophobicity and charge of Salmonella typhimurium 395 MR10 by reaction with secretory IgA (SIgA) Immunology. 1979;36:439–447. [PMC free article] [PubMed] [Google Scholar]
- 285.Magnusson K-E, Stendhal O, Stjernström I, Edebo L. The effect of colostrum and colostral antibody SIgA on the physico-chemical properties and phagocytosis of Escherichia coli O86. Acta Pathol Microbiol Scand Sect B. 1978;86:113–116. doi: 10.1111/j.1699-0463.1978.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 286.Majumdar A S, Ghose A C. Evaluation of the biological properties of different classes of human antibodies in relation to cholera. Infect Immun. 1981;32:9–14. doi: 10.1128/iai.32.1.9-14.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mäkinen K K. Latest dental studies on xylitol and mechanism of xylitol in caries limitation. In: Grenby T H, editor. Progress in sweeteners. London, United Kingdom: Elsevier; 1989. pp. 331–362. [Google Scholar]
- 288.Malamud D. Influence of salivary proteins on the fate of oral bacteria. In: Mergenhagen S E, Rosan B, editors. Molecular basis of oral microbial adhesion. Washington, D.C: American Society for Microbiology; 1985. pp. 117–124. [Google Scholar]
- 289.Malamud D, Christensen C M, Navazesh M, Davis C. Bacterial agglutinin activity in the saliva of human identical and fraternal twins. Arch Oral Biol. 1988;33:801–805. doi: 10.1016/0003-9969(88)90104-5. [DOI] [PubMed] [Google Scholar]
- 290.Mandel I D. The functions of saliva. J Dent Res. 1987;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- 291.Mansa B, Kilian M. Retained antigen-binding activity of Fabα fragments of human monoclonal immunoglobulin A1 (IgA1) cleaved by IgA1 protease. Infect Immun. 1986;52:171–174. doi: 10.1128/iai.52.1.171-174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mansheim B J, Stenstrom M L, Low S B, Clark W B. Measurement of serum and salivary antibodies to the oral pathogen Bacteroides asaccharolyticus in human subjects. Arch Oral Biol. 1980;25:553–557. doi: 10.1016/0003-9969(80)90067-9. [DOI] [PubMed] [Google Scholar]
- 293.Marcotte H, Lavoie M C. Evaluation of mouse salivary IgA directed against indigenous oral bacteria. J Immunoassay. 1993;14:63–81. doi: 10.1080/15321819308019841. [DOI] [PubMed] [Google Scholar]
- 294.Marcotte H, Lavoie M C. Comparison of the indigenous oral microbiota and immunoglobulin responses of athymic (nu/nu) and euthymic (nu/+) mice. Oral Microbiol Immunol. 1996;12:141–147. doi: 10.1111/j.1399-302x.1997.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 295.Marcotte H, Lavoie M C. Influence of immunoglobulins on the indigenous oral and intestinal microbiota of mice. Infect Immun. 1997;64:4694–4699. doi: 10.1128/iai.64.11.4694-4699.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Marcotte H, Rodrigue L, Coulombe C, Goyette N, Lavoie M C. Colonization of the oral cavity by an unidentified streptococcus. Oral Microbiol Immunol. 1995;10:168–174. doi: 10.1111/j.1399-302x.1995.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 297.Marcotte H, Rodrigue L, Gamache M, Lavoie M C. Immunization against the oral indigenous bacteria of the BALB/c mouse. Adv Exp Med Biol. 1995;371B:1173–1176. [PubMed] [Google Scholar]
- 298.Markkanen H, Syrjänen S M, Alalakuijala P. Salivary IgA, lysozyme and β2-microglobulin in periodontal disease. Scand J Dent Res. 1986;94:115–120. doi: 10.1111/j.1600-0722.1986.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 299.Marsh P, Martin M. Oral microbiology. 3rd ed. London, United Kingdom: Chapman & Hall, Ltd.; 1992. [Google Scholar]
- 300.Marsh P D. Host defenses and microbial homeostasis: role of microbial interactions. J Dent Res. 1989;68:1567–1575. [Google Scholar]
- 301.Marsh P D. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc Finn Dent Soc. 1991;87:515–525. [PubMed] [Google Scholar]
- 302.Marsh P D. Microbiological aspects of the chemical control of plaque and gingivitis. J Dent Res. 1992;71:1431–1438. doi: 10.1177/00220345920710071501. [DOI] [PubMed] [Google Scholar]
- 303.Marsh P D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 304.Marsh P D, Bradshaw D J. Microbial effects of new agents in dentifrices for plaque control. Int Dent J. 1993;43:399–406. [PubMed] [Google Scholar]
- 305.Marsh P D, Percival R S, Challacombe S J. The influence of denture-wearing and age on the oral microflora. J Dent Res. 1992;71:1374–1381. doi: 10.1177/00220345920710070501. [DOI] [PubMed] [Google Scholar]
- 306.Mashimo P A, Ellison S A, Slots J. Microbial composition of monkey dental plaque (Macaca arctoides and Macaca fascicularis) Scand J Dent Res. 1979;87:24–31. doi: 10.1111/j.1600-0722.1979.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 307.Mathiesen A T, Øgaard B, Rølla G. Oral hygiene as a variable in dental caries experience in 14-year-olds exposed to fluoride. Caries Res. 1996;30:29–33. doi: 10.1159/000262133. [DOI] [PubMed] [Google Scholar]
- 308.Matsumoto S, Setoyama H, Umesaki Y. Differential induction of major histocompatibility complex molecules on mouse intestine by bacterial colonization. Gastroenterology. 1992;103:1777–1782. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- 309.Mattingly J A, Eardly D D, Kemp J D, Gershon R K. Induction of suppressor cells in rat spleen: influence of microbial stimulation. J Immunol. 1979;122:787–790. [PubMed] [Google Scholar]
- 310.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mazanec M B, Nedrud J G, Kaetzel C S, Lamm M E. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 313.McArthur W P, Clark W B. Specific antibodies and their potential role in periodontal diseases. J Periodontol. 1993;64:807–818. doi: 10.1902/jop.1993.64.8s.807. [DOI] [PubMed] [Google Scholar]
- 314.McArthur W P, Magnusson I, Marks R G, Clark W B. Modulation of colonization black-pigmented Bacteroides species in squirrel monkeys by immunization with Bacteroides gingivalis. Infect Immun. 1989;57:2313–2317. doi: 10.1128/iai.57.8.2313-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.McBride B C, Song M, Krasse B, Olsson J. Biochemical and immunological differences between hydrophobic and hydropholic strains of Streptococcus mutans. Infect Immun. 1984;44:68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.McDermid A S, McKee A S, Marsh P D. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun. 1988;56:1096–1100. doi: 10.1128/iai.56.5.1096-1100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.McDermid A S, McKee A S, Ellwood D C, Marsh P D. The effect of lowering the pH on the composition and metabolism of a community of nine oral bacteria grown in a chemostat. J Gen Microbiol. 1986;132:1205–1214. doi: 10.1099/00221287-132-5-1205. [DOI] [PubMed] [Google Scholar]
- 318.McGhee J R, Kiyono H, Michalek S M, Babb J L, Rosenstreich D L, Mergenhagen S E. Lipopolysaccharide (LPS) regulation of the immune response: T lymphocytes from normal mice suppress mitogenic and immunogenic responses to LPS. J Immunol. 1980;124:1603–1611. [PubMed] [Google Scholar]
- 319.McGhee J R, Mestecky J, Elson C O, Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989;9:175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- 320.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 321.McGhee J R, Michalek S M, Webb J, Navia J M, Rahman A F R, Legler D W. Effective immunity to dental caries: protection of gnotobiotic rats by local immunization with Streptococcus mutans. Infect Immun. 1975;114:300–305. [PubMed] [Google Scholar]
- 322.McKee A S, McDermid A S, Elwood D C, March P D. The establishment of reproducible, complex communities of oral bacteria in the chemostat using defined inocula. J Appl Bacteriol. 1985;59:263–275. doi: 10.1111/j.1365-2672.1985.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 323.McNabb P C, Tomasi T B. Host defense mechanisms at mucosal surfaces. Annu Rev Microbiol. 1981;35:477–496. doi: 10.1146/annurev.mi.35.100181.002401. [DOI] [PubMed] [Google Scholar]
- 324.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 325.Mestecky J, McGhee J R, Arnold R R, Michalek S M, Prince S J, Babb J L. Selective induction of an immune response in human external secretions by ingestion of bacterial antigen. J Clin Invest. 1978;61:731–737. doi: 10.1172/JCI108986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Michalek S M, Lackner A A, Katz J, Russell M W, Eldridge J H, Mestecky J, Lallone R, Moldoveanu Z. Oral immunization studies with Streptococcus mutans and influenzae vaccines in rhesus macaque monkeys. Adv Exp Med Biol. 1995;371B:1423–1429. [PubMed] [Google Scholar]
- 327.Michalek S M, McGhee J R. Effective immunity to dental caries: passive transfer to rats of antibodies to Streptococcus mutans elicits protection. Infect Immun. 1977;17:644–650. doi: 10.1128/iai.17.3.644-650.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Michalek S M, Childers N K. Development and outlook for a caries vaccine. Crit Rev Oral Biol Med. 1990;1:37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- 329.Michalek S M, Kiyono H, Wannemuehler M J, Mosteller L M, McGhee J R. Lipopolysaccharide (LPS) regulation of the immune response: LPS influence on oral tolerance induction. J Immunol. 1982;128:1992–1998. [PubMed] [Google Scholar]
- 330.Michalek S M, Gregory R L, Scholler-Guinard M, Kimura S, Mestecky J, Curtis R, III, McGhee J R. Immunity to dental caries: induction of protective salivary IgA antibodies to Streptococcus mutans. In: Revillard J P, Voisin C, Wierzbicki N, editors. Mucosal immunity IgA and polymorphonuclear neutrophils. Suresnes Cedex, France: Fondation Franco-Allemande; 1985. pp. 99–105. [Google Scholar]
- 331.Michalek S M, Morisaki I, Harmon C C, Hamada S, McGhee J R. Effective immunity to dental caries: gastric intubation of Streptococcus mutans whole cells or cell walls induces protective immunity in gnotobiotic rats. Infect Immun. 1983;39:645–654. doi: 10.1128/iai.39.2.645-654.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Michalek S M, Morisaki I, Gregory R L, Kiyono H, Hamada S, McGhee J R. Oral adjuvants enhance IgA responses to Streptococcus mutans. Mol Immunol. 1983;20:1009–1018. doi: 10.1016/0161-5890(83)90042-1. [DOI] [PubMed] [Google Scholar]
- 333.Michalek S M, McGhee J R, Mestecky J, Arnold R R, Bozzo L. Ingestion of Streptococcus mutans induces secretory IgA and caries immunity. Science. 1976;192:1238–1240. doi: 10.1126/science.1273589. [DOI] [PubMed] [Google Scholar]
- 334.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Minah G E, Solomon E S, Chu K. The association between dietary sucrose consumption and microbial population shifts at six oral sites in man. Arch Oral Biol. 1985;30:397–401. doi: 10.1016/0003-9969(85)90066-4. [DOI] [PubMed] [Google Scholar]
- 336.Moore L V H, Moore W E C, Cato E P, Smibert R M, Burmeister J A, Best A M, Ranney R R. Bacteriology of human gingivitis. J Dent Res. 1987;66:989–995. doi: 10.1177/00220345870660052401. [DOI] [PubMed] [Google Scholar]
- 337.Moore W E C, Burmeister J A, Brooks C N, Ranney R R, Hinkelmann K H, Schieken R M, Moore L V H. Investigation of the influences of puberty, genetics, and environment on the composition of subgingival periodontal floras. Infect Immun. 1993;61:2891–2898. doi: 10.1128/iai.61.7.2891-2898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Moore W E C, Holdeman L V, Cato E P, Good J, Smith E P, Ranney R R, Palcanis K G. Variation in periodontal floras. Infect Immun. 1984;46:720–726. doi: 10.1128/iai.46.3.720-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Moore W E C, Holdeman L V, Smibert R M, Good I J, Burmeister J A, Palcanis K G, Ranney R R. Bacteriology of experimental gingivitis in young adult humans. Infect Immun. 1982;38:651–667. doi: 10.1128/iai.38.2.651-667.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Moreau M C, Ducluzeau R, Guy-Grand D, Muller M C. Increase in the population of duodenal immunoglobulin A plasmocytes in axenic mice associated with different living or dead bacterial strains of intestinal origin. Infect Immun. 1978;21:532–539. doi: 10.1128/iai.21.2.532-539.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Morisaki I, Michalek S M, Harmon C C, Torii M, Hamada S, McGhee J R. Effective immunity to dental caries: enhancement of salivary anti-Streptococcus mutans antibody responses with oral adjuvants. Infect Immun. 1983;40:577–591. doi: 10.1128/iai.40.2.577-591.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Mouton C, Hammond P G, Slots J, Genco R J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periodontal disease. Infect Immun. 1981;31:182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Murikami Y, Nagata H, Amano A, Takagaki M, Shizukuishi S, Tsunemitsu A, Aimoto S. Inhibitory effects of human salivary histatins and lysozyme on coaggregation between Porphyromonas gingivalis and Streptococcus mitis. Infect Immun. 1991;59:3284–3286. doi: 10.1128/iai.59.9.3284-3286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Murray P A, Prakobphol A, Lee T, Hoover C I, Fisher S J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992;60:31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Murray P A, Levine M J, Tabak L A, Reddy M S. Specificity of salivary-bacterial interactions. II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAcα2, 3Galβ1, 3GalNac sequence. Biochem Biophys Res Commun. 1982;106:390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- 346.Nair P N R, Shroeder H E. Local immune response to repeated topical antigen application in the simian labial mucosa. Infect Immun. 1983;41:399–409. doi: 10.1128/iai.41.1.399-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Navia J M. Animal models in dental research. Birmingham: The University of Alabama Press; 1977. [Google Scholar]
- 348.Nguyen T D, Bottreau E, Bernard S, Lantier I, Aynaud J M. Neutralizing secretory IgA and IgG do not inhibit attachment of transmissible gastroenteritis virus. J Gen Virol. 1986;67:939–943. doi: 10.1099/0022-1317-67-5-939. [DOI] [PubMed] [Google Scholar]
- 349.Nikolova E B, Tomana M, Russell M W. All forms of human IgA antibodies bound to antigen interfere with complement (C3) fixation induced by IgG or by antigen alone. Scand J Immunol. 1994;39:275–280. doi: 10.1111/j.1365-3083.1994.tb03371.x. [DOI] [PubMed] [Google Scholar]
- 350.Nolte W A. Oral microbiology: with basic microbiology and immunology. 4th ed. St. Louis, Mo: The C.V. Mosby Co.; 1982. [Google Scholar]
- 351.Norhagen G E, Engström P-E, Hammarström L, Smith C I E, Nord C E. Oral and intestinal microflora in individuals with different immunoglobulin deficiencies. Eur J Clin Microbiol Infect Dis. 1990;9:631–633. doi: 10.1007/BF01967224. [DOI] [PubMed] [Google Scholar]
- 352.Norhagen G, Engström P-E, Hammarström L, Söder P-, Smith C I E. Immunoglobulin levels in saliva in individuals with selective IgA deficiency: compensatory IgM secretion and its correlation with HLA and susceptibility to infections. J Clin Immunol. 1989;9:279–286. doi: 10.1007/BF00918659. [DOI] [PubMed] [Google Scholar]
- 353.Nyvad B. Microbial colonization of human tooth surfaces. Acta Pathol Microbiol Immunol Scand. 1993;101:7–45. [PubMed] [Google Scholar]
- 354.Ochiai K, Kurita-Ochiai T, Kamino Y, Ikeda T. Effect of co-aggregation on the pathogenicity of oral bacteria. J Med Microbiol. 1993;39:183–190. doi: 10.1099/00222615-39-3-183. [DOI] [PubMed] [Google Scholar]
- 355.Ogawa T, Shimauchi H, Hamada S. Mucosal and systemic immune responses in BALB/c mice to Bacteroides gingivalis fimbriae administered orally. Infect Immun. 1989;57:3466–3471. doi: 10.1128/iai.57.11.3466-3471.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Olsson J, Hsu S D, Kingman A, Cole M F. Effect of local and parenteral immunization on implantations of Actinomyces viscosus T6 in rats. Infect Immun. 1985;47:301–305. doi: 10.1128/iai.47.1.301-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Olsson J, Svanberg M. Oral implantation in man of Streptococcus mutans in relation to salivary IgA activity. Scand J Dent Res. 1991;99:489–497. doi: 10.1111/j.1600-0722.1991.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 358.Olsson J, Bratthall D, Carlén A. Association between bacterial agglutinins and immunoglobulin A in human saliva. Acta Odontol Scand. 1981;39:61–66. doi: 10.3109/00016358109162260. [DOI] [PubMed] [Google Scholar]
- 359.Oppenheim F G, Xu T, McMillan F M, Levitz S M, Diamond R D, Offner G D, Troxler R F. Histatins, a novel family of histidine-rich proteins in human parotid secretions. J Biol Chem. 1988;263:7472–7477. [PubMed] [Google Scholar]
- 360.Örstavik D, Kraus F W. The acquired pellicle: immunofluorescent demonstration of specific proteins. J Oral Pathol. 1973;2:68–76. doi: 10.1111/j.1600-0714.1973.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 361.Örstavik D, Kraus F W. The acquired pellicle: enzyme and antibody activities. Scand J Dent Res. 1974;82:202–205. doi: 10.1111/j.1600-0722.1974.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 362.Örstavik D, Brandtzaeg P. Secretion of parotid IgA in relation to gingival inflammation and dental caries experience in man. Arch Oral Biol. 1975;20:701–704. doi: 10.1016/0003-9969(75)90037-0. [DOI] [PubMed] [Google Scholar]
- 363.Oudghiri M, Séguin J, Deslauriers N. The cellular basis of salivary immunity in the mouse: incidence and distribution of B cells, T cells and macrophages in single-cell suspensions of the major salivary glands. Eur J Immunol. 1986;16:281–285. doi: 10.1002/eji.1830160313. [DOI] [PubMed] [Google Scholar]
- 364.Pappo J, Ebersole J L, Taubman M A. Resident salivary gland macrophages function as accessory cells in antigen-dependent T-cell proliferation. Immunology. 1988;63:99–104. [PMC free article] [PubMed] [Google Scholar]
- 365.Parrot M, Charest M, Lavoie M C. Production of mutacin like-substances by Streptococcus mutans. Can J Microbiol. 1989;35:366–372. doi: 10.1139/m89-056. [DOI] [PubMed] [Google Scholar]
- 366.Parrot M, Caufield P W, Lavoie M C. Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol. 1990;36:123–130. doi: 10.1139/m90-022. [DOI] [PubMed] [Google Scholar]
- 367.Payne J B, Iacono V J, Crawford I T, Lepre B M, Bernzweig E, Grossbard B L. Selective effects of histidine-rich polypeptides on the aggregation and viability of Streptococcus mutans and Streptococcus sanguis. Oral Microbiol Immunol. 1991;6:169–176. doi: 10.1111/j.1399-302x.1991.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 367a.Pearce C, Bowden G H, Evans M, Fitzsimmons S P, Johnson J, Sheridan M J, Wientzen R, Cole M F. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J Med Microbiol. 1995;42:67–72. doi: 10.1099/00222615-42-1-67. [DOI] [PubMed] [Google Scholar]
- 368.Peng X, Lang C M, Drozdowicz C K, Ohlsson-Wilhelm B M. Effect of cage population density on plasma corticosterone and peripheral lymphocyte populations of laboratory mice. Lab Anim. 1989;23:302–306. doi: 10.1258/002367789780746042. [DOI] [PubMed] [Google Scholar]
- 369.Percival R S, Challacombe S J, Marsh P D. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J Med Microbiol. 1991;35:5–11. doi: 10.1099/00222615-35-1-5. [DOI] [PubMed] [Google Scholar]
- 370.Percival R S, Challacombe S J, Marsh P D. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res. 1994;73:1416–1420. doi: 10.1177/00220345940730080401. [DOI] [PubMed] [Google Scholar]
- 371.Persson G R, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page R C. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–1031. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Pfaffenbach G, Lamm M, Giggli I. Activation of the guinea pig alternative complement pathway by mouse IgA immune complexes. J Exp Med. 1982;155:231–247. doi: 10.1084/jem.155.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Phalipon A, Kaufmann M, Michetti P, Cavaillon J-M, Huerre M, Sansonetti P, Kraehenbuhl J-P. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Plaut A. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 375.Pollard M, Sharon N. Responses of the Peyer’s patches in germ-free mice to antigenic stimulation. Infect Immun. 1970;2:96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Pollock J J, Goodman H, Elsey P K, Iacono V J. Synergism of lysozyme, proteases and inorganic monovalent anions in the bacteriolysis of oral Streptococcus mutans GS5. Arch Oral Biol. 1983;28:865–873. doi: 10.1016/0003-9969(83)90045-6. [DOI] [PubMed] [Google Scholar]
- 377.Pollock J J, Lotardo S, Gavai R, Grossbard B. Lysozyme protease-inorganic monovalent anion lysis of oral bacteria strains in buffers and stimulated whole saliva. J Dent Res. 1987;66:467–474. doi: 10.1177/00220345870660021401. [DOI] [PubMed] [Google Scholar]
- 378.Pollock J J, Iacono V J, Bicker H G, MacKay B J, Katona L I, Taichman L B, Thomas E. The binding, aggregation and lytic properties of lysozyme. In: Stiles H M, Loesche W J, O’Brien T C, editors. Microbial aspects of dental caries. II. Washington, D.C: Information Retrieval Inc.; 1976. pp. 325–352. [Google Scholar]
- 379.Potter R H. Twin half-sibs: a research design for genetic epidemiology of common dental disorders. J Dent Res. 1990;69:1527–1530. doi: 10.1177/00220345900690081601. [DOI] [PubMed] [Google Scholar]
- 380.Qiu J, Brackee G P, Plaut A G. Analysis of the specificity of bacterial immunoglobulin A (IgA) proteases by a comparative study of ape serum IgAs as substrates. Infect Immun. 1996;64:933–937. doi: 10.1128/iai.64.3.933-937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ranney R R, Ruddy S, Tew J G, Welshimer H J, Palcanis K G, Segreti A. Immunological studies of young adults with severe periodontitis. J Periodontol Res. 1981;16:390–402. doi: 10.1111/j.1600-0765.1981.tb00990.x. [DOI] [PubMed] [Google Scholar]
- 382.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral responses in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Reinholdt J, Kilian M. Interference of IgA protease with the effect of secretory IgA on adherence of oral streptococci to saliva-coated hydroxyapatite. J Dent Res. 1987;66:492–497. doi: 10.1177/00220345870660021801. [DOI] [PubMed] [Google Scholar]
- 384.Reinholdt J, Kilian M. Lack of cleavage of immunoglobulin A (IgA) from rhesus monkeys by bacterial IgA1 proteases. Infect Immun. 1991;59:2219–2221. doi: 10.1128/iai.59.6.2219-2221.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Reinholdt J, Tomana M, Mortensen S B, Kilian M. Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect Immun. 1990;58:1186–1194. doi: 10.1128/iai.58.5.1186-1194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Reinholdt J, Friman V, Kilian M. Similar proportions of immunoglobulin A1 (IgA1) protease-producing streptococci in initial dental plaque of selectively IgA-deficient and normal individuals. Infect Immun. 1993;61:3998–4000. doi: 10.1128/iai.61.9.3998-4000.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 388.Rits M, Hiemstra P S, Bazin H, van Es L A, Vaerman J P, Daha M R. Activation of rat complement by soluble and insoluble rat IgA immune complexes. Eur J Immunol. 1988;18:1873–1880. doi: 10.1002/eji.1830181202. [DOI] [PubMed] [Google Scholar]
- 389.Rits M, Hiemstra P S, van Es L A, Bazin H, Vaerman J P, Daha M R. Complement-mediated solubilization of rat IgA immune precipitates. Mol Immunol. 1987;24:1047–1053. doi: 10.1016/0161-5890(87)90072-1. [DOI] [PubMed] [Google Scholar]
- 390.Riviere G R, Papagiannoulis L. Antibodies to indigenous and laboratory strains of Streptococcus mutans in saliva from children with dental caries and from caries-free children. Pediatr Res. 1987;9:216–220. [PubMed] [Google Scholar]
- 391.Riviere G R, Bohaty B S, Pratt S Y, Wagoner M A. Chronic peroral administration of Streptococcus sobrinus to conventional laboratory rats produces cycling levels of salivary antibodies. Oral Microbiol Immunol. 1991;6:30–33. doi: 10.1111/j.1399-302x.1991.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 392.Riviere G R, Wagoner M A, Freeman I L. Chronic peroral immunization of conventional laboratory rats with mutans streptococci leads to stable acquired suppression of salivary antibodies. Oral Microbiol Immunol. 1992;7:137–141. doi: 10.1111/j.1399-302x.1992.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 393.Robertson P B, Mackler B F, Wright T E, Levy B M. Periodontal status of patients with abnormalities of the immune system. II. Observations over a 2-year period. J Periodontol Res. 1980;51:70–73. doi: 10.1902/jop.1980.51.2.70. [DOI] [PubMed] [Google Scholar]
- 394.Robertson P B, Wright T E, Mackler B F, Lenertz D M, Levy B M. Periodontal status of patients with abnormalities of the immune system. J Periodont Res. 1978;13:37–45. doi: 10.1111/j.1600-0765.1978.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 395.Robinson E A, Appella E. Complete amino acid sequence of a mouse immunoglobulin α chain (MOPC 511) Proc Natl Acad Sci USA. 1980;77:4909–4913. doi: 10.1073/pnas.77.8.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Rodrigue L, Lavoie M C. Comparison of the proportions of oral bacterial species in BALB/c mice from different suppliers. Lab Anim. 1996;30:108–113. doi: 10.1258/002367796780865853. [DOI] [PubMed] [Google Scholar]
- 397.Rodrigue L, Barras M J, Marcotte H, Lavoie M C. Bacterial colonization of the oral cavity of the BALB/c mouse. Microb Ecol. 1993;26:267–275. doi: 10.1007/BF00176958. [DOI] [PubMed] [Google Scholar]
- 398.Rodrigue L, Marion D, Trudel L, Barthe C, Lavoie M C. Comparison of methods for evaluation of the oral bacterial flora of mice. J Microbiol Methods. 1989;10:71–82. [Google Scholar]
- 399.Rogers H J, Synge C. Bacteriostatic effect of human milk on Escherichia coli: the role of IgA. Immunology. 1978;34:19–28. [PMC free article] [PubMed] [Google Scholar]
- 400.Rölla G, Ciardi J E, Bowen W H. Identification of IgA, IgG, lysozyme, albumin, α-amylase and glucosyltransferase in the protein layer adsorbed to hydroxyapatite from whole saliva. Scand J Dent Res. 1983;91:186–190. doi: 10.1111/j.1600-0722.1983.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 401.Römer W, Rother U, Roelke D. Failure of IgA cold agglutinin to activate C. Immunobiology. 1980;157:41–46. doi: 10.1016/S0171-2985(80)80060-X. [DOI] [PubMed] [Google Scholar]
- 402.Rose P T, Gregory R L, Gfell L E, Hughes C V. IgA antibodies to Streptococcus mutans in caries-resistant and -susceptible children. Pediatr Dent. 1994;16:272–275. [PubMed] [Google Scholar]
- 403.Rudney J D. Does variability in salivary protein concentrations influence oral microbial ecology and oral health. Crit Rev Oral Biol Med. 1995;6:343–367. doi: 10.1177/10454411950060040501. [DOI] [PubMed] [Google Scholar]
- 404.Rudney J D, Krig M A, Neuvar E K, Soberay A H, Iverson L. Antimicrobial proteins in human unstimulated whole saliva in relation to each other, and to measures of health status, dental plaque accumulation and composition. Arch Oral Biol. 1991;36:497–506. doi: 10.1016/0003-9969(91)90142-h. [DOI] [PubMed] [Google Scholar]
- 405.Rudney J D, Ji Z, Larson C J, Liljemark W F, Hickey K L. Saliva protein binding to layers of oral streptococci in vitro and in vivo. J Dent Res. 1995;74:1280–1288. doi: 10.1177/00220345950740060701. [DOI] [PubMed] [Google Scholar]
- 406.Rundegren J, Arnold R R. Bacteria-agglutinating characteristics of secretory IgA and a salivary agglutinin. Adv Exp Med Biol. 1987;216:1005–1013. [PubMed] [Google Scholar]
- 407.Russell M W, Rahemmtulla B M-B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989;4:106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 408.Russell M W, Mansa B. Complement-fixing properties of human IgA antibodies. Scand J Immunol. 1989;30:175–183. doi: 10.1111/j.1365-3083.1989.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 409.Russell M W, Wu H-Y. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect Immun. 1991;59:4061–4070. doi: 10.1128/iai.59.11.4061-4070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Russell M W, Mestecky J. Potential for immunological intervention against dental caries. J Biol Buccale. 1986;14:159–175. [PubMed] [Google Scholar]
- 411.Russell M W, Reinholdt J, Kilian M. Anti-inflammatory activity of human IgA antibodies and their Fabα fragments: inhibition of IgG-mediated complement activation. Eur J Immunol. 1989;19:2243–2249. doi: 10.1002/eji.1830191210. [DOI] [PubMed] [Google Scholar]
- 412.Russell M W, Moldaveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Russell R R B, Beighton D. Specificity of natural antibodies reactive with Streptococcus mutans in monkey. Infect Immun. 1982;35:741–744. doi: 10.1128/iai.35.2.741-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Russell-Jones G J, Ey P L, Reynolds B L. Inhibition of cutaneous anaphylaxis and Arthus reactions in the mouse by antigen-specific IgA. Int Arch Allergy Appl Immunol. 1981;66:316–325. doi: 10.1159/000232836. [DOI] [PubMed] [Google Scholar]
- 415.Saito K, Kato C, Katsuragi H, Komatsuzaki A. IgA-mediated inhibition of human leucocyte function by interference with Fcγ and C3b receptors. Immunology. 1991;74:99–106. [PMC free article] [PubMed] [Google Scholar]
- 416.Sanders C C, Sanders W E., Jr Enocin: an antibiotic produced by Streptococcus salivarius that may contribute to protection against infections due to group A streptococci. J Infect Dis. 1982;146:683–689. doi: 10.1093/infdis/146.5.683. [DOI] [PubMed] [Google Scholar]
- 417.Sanders W E, Sanders C C. Modification of normal flora by antibiotics: effects on individuals and the environment. In: Koot R K, Sande M A, editors. New dimensions in antimicrobial therapy. New York, N.Y: Churchill Livingstone, Inc.; 1984. pp. 217–241. [Google Scholar]
- 418.Sandholm L, Tolo K, Olsen I. Salivary IgG, a parameter of periodontal disease activity. J Clin Periodontol. 1987;14:289–294. doi: 10.1111/j.1600-051x.1987.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 419.Sandholm L, Grönblad E. Salivary immunoglobulins in patients with juvenile periodontitis and their healthy siblings. J Periodontol. 1984;55:9–12. doi: 10.1902/jop.1984.55.1.9. [DOI] [PubMed] [Google Scholar]
- 420.Savage D C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 421.Saxén L, Tenovuo J, Vilja P. Salivary defense mechanisms in juvenile periodontitis. Acta Odontol Scand. 1990;48:399–407. doi: 10.3109/00016359009029071. [DOI] [PubMed] [Google Scholar]
- 422.Scannapieco F A. Saliva-bacterium interactions in oral microbial ecology. Crit Rev Oral Biol Med. 1994;5:203–248. doi: 10.1177/10454411940050030201. [DOI] [PubMed] [Google Scholar]
- 423.Scannapieco F A, Torres G I, Levine M J. Salivary amylase promotes adhesion of oral streptococci to hydroxyapatite. J Dent Res. 1995;74:1360–1366. doi: 10.1177/00220345950740070701. [DOI] [PubMed] [Google Scholar]
- 424.Schaedler R W, Dubos R J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962;115:1149–1159. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Scheinkein H A, Burmeister J A, Koertge T E, Brooks C N, Best A M, Moore L V H, Moore W E C. The influence of race and gender on periodontal flora. J Periodontol. 1993;64:292–296. doi: 10.1902/jop.1993.64.4.292. [DOI] [PubMed] [Google Scholar]
- 426.Schenck K, Poppelsdorf D, Denis C, Tollefsen T. Levels of salivary IgA antibodies reactive with bacteria from dental plaque are associated with susceptibility to experimental gingivitis. J Clin Periodontol. 1993;20:411–417. doi: 10.1111/j.1600-051x.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 427.Schwartz R H. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 428.Scully C M, Lehner T. Opsonization, phagocytosis and killing of Streptococcus mutans by polymorphonuclear leukocytes, in relation to dental caries in the rhesus monkey (Macaca mulatta) Arch Oral Biol. 1979;24:307–312. doi: 10.1016/0003-9969(79)90093-1. [DOI] [PubMed] [Google Scholar]
- 429.Shen L, Collins J. Monocyte superoxyde secretion triggered by human IgA. Immunology. 1989;68:491–496. [PMC free article] [PubMed] [Google Scholar]
- 430.Shomers J P, Tabak L A, Levine M J, Mandel I D, Hay D I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982;61:397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- 431.Shope S R, Schiemann D A. Passive secretory immunity against Salmonella typhimurium demonstrated with foster mouse pups. J Med Microbiol. 1991;35:53–59. doi: 10.1099/00222615-35-1-53. [DOI] [PubMed] [Google Scholar]
- 432.Shroff K E, Cebra J J. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371A:441–446. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 433.Shroff K E, Meslin K, Cebra J J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Shute J K, Lindley I, Peichl P, Holgate S T, Church M K, Djukanovic R. Mucosal IgA is an important moderator of eosinophil responses to tissue-derived chemoattractants. Int Arch Allergy Immunol. 1995;107:340–341. doi: 10.1159/000237022. [DOI] [PubMed] [Google Scholar]
- 435.Simonsson T, Rönström A, Rundegren J, Birkhed D. Rate of plaque formation-some clinical and biochemical characteristics of “heavy” and “light” plaque formers. Scand J Dent Res. 1986;95:97–103. doi: 10.1111/j.1600-0722.1987.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 436.Sirisinha S, Charupatana C. Antibodies to indigenous bacteria in human serum, secretions, and urine. Can J Microbiol. 1971;17:1471–1473. doi: 10.1139/m71-233. [DOI] [PubMed] [Google Scholar]
- 437.Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977;85:245–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 438.Slots J, Hausmann E. Longitudinal study of experimentally induced periodontal disease in Macaca arctoides: relationship between microflora and alveolar bone loss. Infect Immun. 1979;23:260–269. doi: 10.1128/iai.23.2.260-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Slots J, Rams T E. Microbiology of periodontal diseases. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby Year Book; 1992. pp. 425–443. [Google Scholar]
- 440.Slots J, Schonfeld S E. Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. In: Hamada S, Holt S C, McGhee J R, editors. Periodontal disease: pathogens and host immune response. Tokyo, Japan: Quintessence; 1991. pp. 53–64. [Google Scholar]
- 441.Smith D J. The oral cavity as an immunological entity. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby Year Book; 1992. pp. 524–532. [Google Scholar]
- 442.Smith D J, Taubman M A. Oral immunization of humans with Streptococcus sobrinus glucosyltransferase. Infect Immun. 1987;55:2562–2569. doi: 10.1128/iai.55.11.2562-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Smith D J, Taubman M A. Effect of local deposition of antigen on salivary immune responses and reaccumulation of mutans streptococci. J Clin Immunol. 1990;10:273–281. doi: 10.1007/BF00916703. [DOI] [PubMed] [Google Scholar]
- 444.Smith D J, Taubman M A. Ontogeny of immunity to oral microbiota in humans. Crit Rev Oral Biol Med. 1992;3:109–133. doi: 10.1177/10454411920030010201. [DOI] [PubMed] [Google Scholar]
- 445.Smith D J, Ebersole J L, Taubman M A, Gadalla L. Salivary IgA antibody to Actinobacillus actinomycetemcomitans in a young adult population. J Periodontal Res. 1985;20:8–11. doi: 10.1111/j.1600-0765.1985.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 446.Smith D J, Anderson J M, King W F, van Houte J, Taubman M A. Oral streptococcal colonization of infants. Oral Microbiol Immunol. 1993;8:1–4. doi: 10.1111/j.1399-302x.1993.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 447.Smith D J, van Houte J, Kent R, Taubman M A. Effect of antibody in gingival crevicular fluid on early colonization of exposed root surfaces by mutans streptococci. Oral Microbiol Immunol. 1994;9:65–69. doi: 10.1111/j.1399-302x.1994.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 448.Smith D J, Taubman M A, Ebersole J L. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in hamsters caused by homologous and heterologous serotypes of Streptococcus mutans. Infect Immun. 1978;21:843–851. doi: 10.1128/iai.21.3.843-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Smith D J, Taubman M A, Ebersole J L. Effect of oral administration of glucosyltransferase antigens on experimental dental caries. Infect Immun. 1979;26:82–89. doi: 10.1128/iai.26.1.82-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Smith D J, Taubman M A, Ebersole J L. Effects of local immunization of hamsters with glucosyltransferase antigens on infection with Streptococcus sanguis. Infect Immun. 1983;42:156–162. doi: 10.1128/iai.42.1.156-162.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Smith D J, Taubman M A. Development of salivary IgA antibody to oral streptococcal antigens associated with virulence. Adv Exp Med Biol. 1995;371B:1141–1143. [PubMed] [Google Scholar]
- 452.Smith D J, King W F, Taubman M A. Salivary IgA antibody to oral streptococcal antigens in predente infants. Oral Microbiol Immunol. 1990;5:57–62. doi: 10.1111/j.1399-302x.1990.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 453.Smith D J, King W F, Imelmann C, Akita H, Taubman M A. Longitudinal association of salivary IgA antibody and initial mutans streptococcal infection in children. J Dent Res. 1994;73:153. . (Abstract.) [Google Scholar]
- 454.Smith K, Beighton D. Proteolytic activities in the supragingival plaque of monkeys (Macaca fascicularis) Arch Oral Biol. 1987;32:473. doi: 10.1016/s0003-9969(87)80007-9. [DOI] [PubMed] [Google Scholar]
- 455.Socransky S S, Manganiello S D. The oral microbiota of man from birth to senility. J Periodontol. 1971;42:485–494. doi: 10.1902/jop.1971.42.8.485. [DOI] [PubMed] [Google Scholar]
- 456.Souka T, Tenovuo J, Rundegren J. Agglutination of Streptococcus mutans serotype c but not inhibition of Porphyromonas gingivalis autoaggregation by human lactoferrin. Arch Oral Biol. 1993;38:227–232. doi: 10.1016/0003-9969(93)90032-h. [DOI] [PubMed] [Google Scholar]
- 457.Staat R H, Gawronski T H, Cressey D E, Harris R S, Folke L E A. Effects of dietary sucrose levels on the quantity and microbial composition of human dental plaque. J Dent Res. 1975;54:872–880. doi: 10.1177/00220345750540042801. [DOI] [PubMed] [Google Scholar]
- 458.Stack W E, Taubman M A, Tsukuda T, Smith D J, Ebersole J L, Kent R. Dental caries in congenitally athymic rats. Oral Microbiol Immunol. 1990;5:309–314. doi: 10.1111/j.1399-302x.1990.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 459.Stad R K, Bogers W M J M, Thoomes-van der Sluys M E, van Es L A, Daha M R. In vivo activation of complement by IgA in rat model. Clin Exp Immunol. 1992;87:138–143. doi: 10.1111/j.1365-2249.1992.tb06427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Stecksen-Blicks C, Gustafsson L. Impact of oral hygiene and use of fluorides on caries increment in children during one year. Community Dent Oral Epidemiol. 1986;14:185–189. doi: 10.1111/j.1600-0528.1986.tb01530.x. [DOI] [PubMed] [Google Scholar]
- 461.Stokes C R, Soothill J F, Turner M W. Immune exclusion is a function of IgA. Nature (London) 1975;255:745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 462.Svanborg M. Streptococcus mutans in plaque after mouth rinsing with buffers at varying pH values. Scand J Dent Res. 1980;88:76–78. doi: 10.1111/j.1600-0722.1980.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 463.Svanborg-Edén C, Svennerholm A M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Svanborg-Edén C, Andersson B, Hagberg L, Hanson L Å, Leffler H, Magnusson G, Noori G, Dahmén J, Söderström T. Receptor analogues and anti-pili antibodies as inhibitors of bacterial attachment in vivo and in vitro. Ann N Y Acad Sci. 1983;409:580–592. doi: 10.1111/j.1749-6632.1983.tb26900.x. [DOI] [PubMed] [Google Scholar]
- 465.Sybille Y, Delacroix D L, Merill W W, Chatelain B, Vaerman J P. IgA-induced chemokinesis of human polymorphonuclear neutrophils: requirement of their Fc-alpha receptor. Mol Immunol. 1987;24:551–559. doi: 10.1016/0161-5890(87)90035-6. [DOI] [PubMed] [Google Scholar]
- 466.Tabak L A. Structure and function of human salivary mucins. Crit Rev Oral Biol Med. 1990;4:229–234. doi: 10.1177/10454411900010040201. [DOI] [PubMed] [Google Scholar]
- 467.Tabak L A, Levine M J, Mandel I D, Ellison S A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982;11:1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 468.Tagg J R, Dajani A S, Wannamaker L W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976;40:722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Tagg J R, Russell C. Role of bacteriocin during plaque formation by Streptococcus salivarius and Streptococcus sanguis on a tooth in an artificial mouth. J Appl Bacteriol. 1981;50:305–313. doi: 10.1111/j.1365-2672.1981.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 470.Tagliagabue A. Immune response to oral Salmonella vaccines. Curr Top Microbiol Immunol. 1989;146:225–231. doi: 10.1007/978-3-642-74529-4_24. [DOI] [PubMed] [Google Scholar]
- 471.Tagliagabue A, Boraschi D, Villa L, Keren D F, Lowell G H, Rappuoli R, Nencioni L. IgA-dependent cell-mediated activity against enteropathogenic bacteria: distribution, specificity, and characterization of the effector cells. J Immunol. 1984;133:988–992. [PubMed] [Google Scholar]
- 472.Tagliagabue A, Villa L, De Magistris M T, Romano M, Silvestri S, Boraschi D, Nencioni L. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J Immunol. 1986;137:1504–1510. [PubMed] [Google Scholar]
- 473.Tank G, Storvick C A. Caries experience of children one to six years old in two Oregon communities (Corvallis and Albany). III. Relation of diet to variation of dental caries. J Am Dent Assoc. 1965;70:394–403. doi: 10.14219/jada.archive.1965.0302. [DOI] [PubMed] [Google Scholar]
- 474.Tannock G W, Savage D C. Influence of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Tanzer J M. Animal models in cariology. Washington, D.C: Information Retrieval Inc.; 1981. [Google Scholar]
- 476.Tappuni A, Roberts G, Challacombe S J. A longitudinal study of selected oral flora in twins and singletons. J Dent Res. 1989;68:594. . (Abstract.) [Google Scholar]
- 477.Taubman M A. Immunoglobulins of human dental plaque. Arch Oral Biol. 1974;19:439–446. doi: 10.1016/0003-9969(74)90149-6. [DOI] [PubMed] [Google Scholar]
- 478.Taubman M A. Immunological aspects of periodontal diseases. In: Slots J, Taubman M A, editors. Contemporary oral microbiology and immunology. St. Louis, Mo: Mosby Year Book; 1992. pp. 542–554. [Google Scholar]
- 479.Taubman M A, Smith D J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974;9:1079–1090. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Taubman M A, Smith D J. Effects of local immunization with glucosyltransferase fractions from Streptococcus mutans on dental caries in rats and hamsters. J Immunol. 1977;118:710–720. [PubMed] [Google Scholar]
- 481.Taubman M A, Ebersole J L, Smith D J. Immunoglobulin levels of congenitally athymic rats immunized with thymus-dependent-independent antigens. Immunology. 1986;58:145–150. [PMC free article] [PubMed] [Google Scholar]
- 482.Taubman M A, Ebersole J L, Smith D J, Reger R C. The immunological approach to dental caries prevention: secretory immune system adjuvant studies. J Dent Res. 1980;59:2163–2170. [Google Scholar]
- 483.Taylor H P, Dimmock N J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J Exp Med. 1985;161:198–209. doi: 10.1084/jem.161.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Taylor H P, Armstrong S J, Dimmock N J. Quantitative relationships between an influenza virus and neutralizing antibody. Virology. 1987;159:288–298. doi: 10.1016/0042-6822(87)90466-1. [DOI] [PubMed] [Google Scholar]
- 485.Tenovuo J, Moldoveanu Z, Mestecky J, Pruitt K M, Mansson-Rahemtulla B. Interaction of specific and innate factors of immunity: IgA enhances the antimicrobial effect of the lactoperoxidase system against Streptococcus mutans. J Immunol. 1982;128:726–731. [PubMed] [Google Scholar]
- 486.Tenovuo J, Lehtonen O-P, Aaltonen A S, Vilja P, Tuohimaa P. Antimicrobial factors in whole saliva of humans infants. Infect Immun. 1986;51:49–53. doi: 10.1128/iai.51.1.49-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Tenovuo J, Valtakoski J, Knuuttila M L E. Antibacterial activity of lactoperoxidase adsorbed by human salivary sediment and hydroxyapatite. Caries Res. 1977;11:257–262. doi: 10.1159/000260277. [DOI] [PubMed] [Google Scholar]
- 488.Tenovuo J. Oral defense factors in the elderly. Endod Dent Traumatol. 1992;8:93–98. doi: 10.1111/j.1600-9657.1992.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 489.Tenovuo J, Mansson-Rahemtulla B, Pruitt K M, Arnold R R. Inhibition of dental plaque acid production by the salivary lactoperoxidase antimicrobial system. Infect Immun. 1981;34:208–214. doi: 10.1128/iai.34.1.208-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Tenovuo J, Gråhn E, Lehtonen O-P, Hyyppä T, Karhuvaara L, Vilja P. Antimicrobial factors in saliva: ontogeny and relation to oral health. J Dent Res. 1987;66:475–479. doi: 10.1177/00220345870660021501. [DOI] [PubMed] [Google Scholar]
- 491.Tenovuo J, Jentsch H, Soukka T, Karhuvaara L. Antimicrobial factors of saliva in relation to dental caries and salivary levels of mutans streptococci. J Biol Buccale. 1992;20:85–90. [PubMed] [Google Scholar]
- 492.Tenovuo J, Lehtonen O-P, Aaltonen A S. Serum and salivary antibodies against Streptococcus mutans in young children with and without detectable oral S. mutans. Caries Res. 1987;21:289–296. doi: 10.1159/000261032. [DOI] [PubMed] [Google Scholar]
- 493.Ter Steeg P F, Van der Hoeven J S, de Jong M H, van Munster P J J, Jansen M J H. Modelling the gingival pocket by enrichment of subgingival microflora in human serum in chemostats. Microb Ecol Health Dis. 1988;1:73–84. [Google Scholar]
- 494.Tharp G D. Basketball exercise and secretory immunoglobulin A. Eur J Appl Physiol. 1991;63:312–314. doi: 10.1007/BF00233868. [DOI] [PubMed] [Google Scholar]
- 495.Theilade E. Factors controlling the microflora of the healthy mouth. In: Hill M J, Marsh P D, editors. Human microbial ecology. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 2–56. [Google Scholar]
- 496.Tolo K, Brandtzaeg P, Jonsen J. Mucosal penetration of antigen in the presence or absence of serum-derived antibody. An in vitro study of rabbit oral intestinal mucosa. Immunology. 1977;33:733–743. [PMC free article] [PubMed] [Google Scholar]
- 497.Tomasi T B. Regulation of the mucosal IgA response: an overview. Immunol Invest. 1989;18:1–15. doi: 10.3109/08820138909112223. [DOI] [PubMed] [Google Scholar]
- 498.Toraño A, Putnam F W. Complete amino acid sequence of the 2 alpha heavy chains of a human IgA2 immunoglobulin of the A2m(2) allotype. Proc Natl Acad Sci USA. 1978;75:966–969. doi: 10.1073/pnas.75.2.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Tratmont E C, Ciak J, Boslego J, McChesney D G, Brington C C, Zollinger W. Antigenic specificity of antibodies in vaginal secretions during infection with Neisseria gonorrhoae. J Infect Dis. 1980;142:23–31. doi: 10.1093/infdis/142.1.23. [DOI] [PubMed] [Google Scholar]
- 500.Trudel L, St. Amand L, Bareil M, Cardinal P, Lavoie M C. Bacteriology of the oral cavity of the BALB/c mice. Can J Microbiol. 1986;32:673–678. doi: 10.1139/m86-124. [DOI] [PubMed] [Google Scholar]
- 501.Tsuzukida Y, Wang C-C, Putnam F W. Structure of the A2m(1) allotype of human IgA-α recombinant molecule. Proc Natl Acad Sci USA. 1979;76:1104–1108. doi: 10.1073/pnas.76.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Tucker P W, Slightom J L, Blattner F R. Mouse IgA heavy chain gene sequence: implications for evolution of immunoglobulin hinge exons. Proc Natl Acad Sci USA. 1981;78:7684–7688. doi: 10.1073/pnas.78.12.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Tuli J S, Smith J A, Morton D B. Stress measurements in mice after transportation. Lab Anim. 1994;29:132–138. doi: 10.1258/002367795780740249. [DOI] [PubMed] [Google Scholar]
- 504.Tynelius-Bratthall G, Ellen R P. Fluctuations in crevicular and salivary anti-A. viscosus antibody levels in response to treatment of gingivitis. J Clin Periodontol. 1985;12:762–773. doi: 10.1111/j.1600-051x.1985.tb01402.x. [DOI] [PubMed] [Google Scholar]
- 505.Vaerman J P, Derijck-Langendries A, Rits M, Delacroix D. Neutralization of cholera toxin by rat bile secretory IgA antibodies. Immunology. 1985;54:601–603. [PMC free article] [PubMed] [Google Scholar]
- 506.Van de Merwe J P, Stegeman J H, Hazenberg M P. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn’s disease? Antonie Leeuwenhoek. 1983;49:119–124. doi: 10.1007/BF00393669. [DOI] [PubMed] [Google Scholar]
- 507.Van der Heijden P J, Bianchi A T J, Heidt P J, Stok W, Bokhout B A. Background (spontaneous) immunoglobulin production in the murine small intestine before and after weaning. J Reprod Immunol. 1989;15:217–227. doi: 10.1016/0165-0378(89)90013-2. [DOI] [PubMed] [Google Scholar]
- 508.Van der Hoeven J S, Rogers A H. Stability of the resident microflora and the bacteriocinogeny of Streptococcus mutans as factors affecting its establishment in specific pathogen-free rats. Infect Immun. 1979;23:206–212. doi: 10.1128/iai.23.2.206-212.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 509.Van der Hoeven J S, Camp P J M. Synergistic degradation of mucin by Streptococcus oralis and Streptococcus sanguis in mixed chemostat cultures. J Dent Res. 1991;70:1041–1044. doi: 10.1177/00220345910700070401. [DOI] [PubMed] [Google Scholar]
- 510.Van der Hoeven J S, de Jong M H, Camp P J M, van den Kieboom C W A. Competition between oral Streptococcus species in the chemostat under alternating conditions of glucose limitation and excess. FEMS Microbiol Ecol. 1985;31:373–379. [Google Scholar]
- 511.Van der Velden U, van Winkelhoff A J, Abbas F, de Graaff J. The habitat of periodontopathic micro-organisms. J Clin Periodontol. 1986;13:243–248. doi: 10.1111/j.1600-051x.1986.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 512.Van der Waaij D, Berghuis de Vries J M, Lekkerkerk van der Wees J E C. Colonisation resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg. 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Van Epps D E, Reed K, William R C., Jr Suppression of human polymorphonuclear bactericidal activity by human IgA preparation. Cell Immunol. 1978;36:363–376. doi: 10.1016/0008-8749(78)90280-0. [DOI] [PubMed] [Google Scholar]
- 514.Van Houte J. Role of micro-organisms in caries etiology. J Dent Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 515.Vudhichamnong K, Walker D M, Ryley H C. The effect of secretory immunoglobulin A on the in vitro adherence of the yeast Candida albicans to human oral epithelial cells. Arch Oral Biol. 1982;27:617–621. doi: 10.1016/0003-9969(82)90184-4. [DOI] [PubMed] [Google Scholar]
- 516.Wachsmann D, Klein J P, Schöller M, Frank R M. Local and systemic immune response to orally administered liposome-associated soluble S. mutans cell wall antigens. Immunology. 1985;54:189–193. [PMC free article] [PubMed] [Google Scholar]
- 517.Walker J. Antibody responses of monkeys to oral and local immunization with Streptococcus mutans. Infect Immun. 1981;31:61–70. doi: 10.1128/iai.31.1.61-70.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Walker W A, Isselbacher K J, Bloch K J. Intestinal uptake of macromolecules: effect of oral immunization. Science. 1972;177:608–610. doi: 10.1126/science.177.4049.608. [DOI] [PubMed] [Google Scholar]
- 519.Weihe W H. The thermoregulation of the nude mouse. Exp Cell Biol. 1984;52:140–144. [PubMed] [Google Scholar]
- 520.Weisbart R H, Kacena A, Schuh A, Golde D W. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature (London) 1988;332:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 521.Widerström L, Bratthall D. Increased IgA levels in saliva during pregnancy. Scand J Dent Res. 1984;92:33–37. doi: 10.1111/j.1600-0722.1984.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 522.Widerström L, Bratthall D, Hamberg K. Immunoglobulin A antibody activity to mutans streptococci in parotid, submandibular, and whole saliva. Oral Microbiol Immunol. 1992;7:326–331. doi: 10.1111/j.1399-302x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 523.Widerström L, Bratthall D, Hamberg K. Immunoglobulin A antibodies to mutans streptococci in human saliva and serum comparing fresh and subcultivated strains and activity in repeated saliva samples. Oral Microbiol Immunol. 1994;9:278–283. doi: 10.1111/j.1399-302x.1994.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 524.Willett N P, White R R, Rosen S. Essential dental microbiology. Norwalk, Conn: Appleton & Lange; 1991. [Google Scholar]
- 525.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 526.Wilton J M A. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978;34:423–428. [PMC free article] [PubMed] [Google Scholar]
- 527.Wilton J M A, Curtis M A, Gillet I R, Griffiths G S, Maiden M F J, Sterne J A C, Wilson D T, Johnsson N W. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers from analysis of saliva. J Clin Periodontol. 1989;16:475–483. doi: 10.1111/j.1600-051x.1989.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 528.Winner L, III, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J-P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Wojcicki C J, Harper D S, Robinson P J. Differences in periodontal disease-associated microorganisms of subgingival plaque in prepubertal, pubertal and postpubertal children. J Periodontol. 1986;58:219–223. doi: 10.1902/jop.1987.58.4.219. [DOI] [PubMed] [Google Scholar]
- 530.Wold A E, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, Svanborg Edén C. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Wolf H M, Vogel E, Fischer M B, Rengs H, Schwarz H-P, Eibl M M. Inhibition of receptor-dependent and receptor-independent generation of the respiratory burst in human neutrophils and monocytes by human serum IgA. Pediatr Res. 1994;36:235–243. doi: 10.1203/00006450-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 532.Wolf H M, Fischer M B, Pühringer H, Samstag A, Vogel E, Eibl M M. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-α, interleukin-6) in human monocytes. Blood. 1994;83:1278–1288. [PubMed] [Google Scholar]
- 533.Wolff L F, Krupp M J, Liljemark W F. Microbial changes associated with advancing periodontis in STR/N mice. J Periodontal Res. 1985;20:378–385. doi: 10.1111/j.1600-0765.1985.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 534.Wu H-Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Xie H, Gibbons R J, Hay D I. Adhesive properties of strains of Fusobacterium nucleatum of the subspecies nucleatum, vincentii and polymorphum. Oral Microbiol Immunol. 1991;6:257–263. doi: 10.1111/j.1399-302x.1991.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 536.Yanover L, Ellen R P. A clinical and microbiologic examination of gingival disease in parapubescent females. J Periodontol. 1986;57:562–567. doi: 10.1902/jop.1986.57.9.562. [DOI] [PubMed] [Google Scholar]
- 537.Yeaman G R, Kerr M A. Opsonization of yeast by human serum IgA anti-mannan antibodies and phagocytosis by human polymorphonuclear leukocytes. Clin Exp Immunol. 1987;68:200–208. [PMC free article] [PubMed] [Google Scholar]
- 538.Yoshioka H, Iseki K-I, Fujita K. Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics. 1983;72:317–321. [PubMed] [Google Scholar]
- 539.Zachariasen R D. The effect of elevated ovarian hormones on periodontal health: oral contraceptives and pregnancy. Women Health. 1993;20:21–30. doi: 10.1300/J013v20n02_02. [DOI] [PubMed] [Google Scholar]