Abstract
Background
Early hearing detection and intervention (EHDI) measures initiated in high-income countries (HICs) were attempted in low-income and middle-income countries (L&MICs). However, information regarding the models of EHDI, context-specific adaptations made to strategies and outcomes are not known.
Aims
The aims of this systematic review were to identify the various models of EHDI used in Asian L&MICs in the published scientific literature and to describe their efficacy and validity.
Methods
The studies were eligible if the programme was from Asian L&MICs, implemented for children below 6 years of age and published between 2010 and 2021. Google Scholar, PubMed, Web of Science, Scopus, EBSCOHost and EBSCO–CINAHL were used to find articles. Data were extracted from each selected article, and the risk of bias was assessed. The search results were summarised using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. For primary outcomes, narrative synthesis was used, and forest plots were generated for secondary outcomes.
Results
In all, 82 studies were included, and these studies were divided into two categories: newborn and infant screening programmes and screening programmes for older children. Predominantly, a two-stage objective otoacoustic emission (Distortion Product/Transient Evoked) or automated auditory brainstem response screening, followed by a detailed auditory brainstem response to confirm the hearing loss, was used in newborn and infant screening programmes. Audiologists were the most frequent screening personnel. Screening of older children was mostly done by otolaryngologists, school instructors and nurses. They performed a single-stage pure tone audiometry screening followed by a detailed examination.
Conclusion
The screening tools and protocols used were similar to those used in HICs. However, no uniform protocols were followed within each country. Long-term viability of EHDI programmes was not known as there was limited information on impact outcomes such as cost–benefit.
PROSPERO registration number
CRD42021240341.
Keywords: Deafness, Neonatology, Health services research, Audiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Early hearing detection and intervention (EHDI) programmes are mandated in several high-income countries (HICs) for over two decades. These screening programmes are based on guidelines and standards provided by the Joint Committee on Infant Hearing, the American Audiology Association, the Newborn Hearing Screening Programme England, WHO, the European Consensus Statement on Neonatal Hearing Screening, etc. Systematic reviews have documented screening protocols and programme outcomes predominantly in the context of HICs.
WHAT THIS STUDY ADDS
Unlike several HICs, EHDI programmes are not mandated in many low-income and middle-income countries (L&MICs). In this context, we conducted a systematic review and gathered information on hearing screening programmes mainly to identify different models of EHDI that were implemented in the context of Asian L&MICs. This review provides information on various screening protocols, tools, personnel, diagnostic tools, use of information and communication technology, barriers and facilitators in different EHDI programmes of L&MICs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
We found that the screening tools and protocols used were similar to those used in HICs, yet no uniform protocols were followed within each country. Long-term viability of EHDI programmes is not known in this context due to limited impact outcome-based studies(eg, cost–benefit, rate of intervention, etc); hence, future research should focus on these aspects. Further, policy makers and programme planners in these countries should build consensus to implement uniform countrywise protocols suited to the context.
Introduction
Currently, 34 million children below 15 years are estimated to have hearing loss, with a higher prevalence in low-income and middle-income countries (L&MICs) (2.4%) than in high-income countries (HICs) (0.5%).1 Early hearing detection and intervention (EHDI) for children with hearing loss is critical to maximise linguistic competence and literacy development. EHDI is a concept that emanated in the USA in the 1990s and is intended as an at-birth hearing screening of newborns prior to hospital discharge. Infants who do not pass the screening are recommended for diagnostic evaluation and, when confirmed to have hearing loss, are enrolled in early intervention programmes. Subsequently, the Joint Committee on Infant Hearing (2007) in the USA recommended that all infants be screened for hearing by 1 month of age and diagnosed by 3 months and receive intervention by 6 months of age.2 It is practised as a mandatory universal screening in the entire country.
The concept was subsequently adopted in the UK and practised as universal screening since 2006. Subsequently, several other HICs (Australia and Canada, to name a few) adopted this strategy. Alternative strategies for EHDI have been implemented in L&MICs due to financial, human resource and infrastructural challenges.3 These include high risk-based screening,4 screening during immunisation,5 community-based hearing screening by health workers6 7 and school entry-level screening.8 9 Several of these programmes have also integrated telepractice to either improve coverage of screening or provide better diagnostic follow-up.10 11 However, there remains a lack of clarity on the range of strategies implemented in L&MICs and which should be promoted.
The aims of this systematic review were to identify different models of EHDI that have been implemented in the context of Asian L&MICs in the published scientific literature and to describe evidence of their efficacy and validity.
Method
The protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (registration number CRD42021240341).
Patient and public involvement statement
This systematic review did not involve any subject/patient and public directly.
Inclusion criteria
All types of study designs were eligible for this review, including (1) cross-sectional, (2) cohort, (3) case–control, (4) randomised controlled trials, (5) quasi-experimental and (6) field trials. Both qualitative and quantitative types of studies were included.
The EHDI model is operationally defined for the purpose of this systematic review as programmes for identification and referral of young children with hearing loss. Studies that described EHDI programmes related to triaging children suspected with hearing loss using methods such as objective or subjective screening, parental questionnaire-based screening, implemented in the context of low-income countries (LICs), lower middle-income countries (LMICs) and upper middle-income countries (UMICs) including hospital, community, school based or any other alternative approach were included.
Studies were eligible regardless of screening strategies (eg, at birthing hospital/community/school), protocol used (eg, single stage/two-stage), provider stakeholder (eg, private/public) involved, tools for screening (eg, checklist, otoacoustic emission (OAE), automated auditory brainstem response (AABR) etc), or personnel involved in screening, diagnosis and intervention (eg, nurse, audiometrists, audiologists and ENT). We also included studies that explored evidence of validity (eg, sensitivity/specificity) and reported implementation barriers and facilitators to EHDI.
According to World Bank classification (2021), LICs, LMICs and UMICs (L&MICs) in the Asian continent (South East Asia, Central Asia and Western Asia/Middle East) were considered as eligible for the review. In the L&MICs, 6 years and below was predominantly considered as the age band for ‘early’ detection and intervention. Therefore, this review included studies describing EHDI among neonates, infants and children below 6 years of age. Studies were eligible if they had been published from 2010 to 2022.
Exclusion criteria
We excluded studies that described hearing screening programmes for individuals older than 6 years of age or for other disabilities not including hearing. In addition, studies from HICs, studies published in languages other than English and studies published before the year 2010 were excluded.
Search strategy
Since EHDI is an interdisciplinary programme often implemented by ENT/paediatrics/neonatology/audiology/nursing, databases that captured articles from multiple disciplines was preferred. The primary databases used for the search include PubMed, Scopus, Web of science, EBSCOHost, EBSCO–CINAHL (humanities and social sciences) and Google scholar. Hand searching was conducted for the International Journal of Audiology (2015–2022) and bibliographies of the selected papers based on the eligibility criteria. Grey literature search included ProQuest Dissertations & Theses Global (nterdisciplinary) and first 500 searches for articles/reports in Google Search. We excluded social media articles, newspaper articles, editorials and website information.
A search strategy for each of the aforementioned databases was designed using 2Dsearch online tool.12 The search strategy included Medical Subject Headings terms and Boolean operators. A pilot search was conducted in each database to identify the keywords. Synonyms of the keywords were then identified and included in the search strategy.
Screening for eligibility and quality
Title screening was conducted as per the inclusion and exclusion criteria using database search. The Rayyan software13 was used to screen the abstract and full texts. Screening was conducted by two reviewers (DJ and VR), and any discrepancies were discussed between the reviewers and decisions were made. Joanna Briggs Quality assessment tools specific to the research design were used to assess the quality of the articles.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart14 was used to represent the search results.
Data extraction and synthesis
A Google Sheet was used for data extraction, which was undertaken by two authors (DJ and LSN) and verified by another author (VR).
Narrative synthesis of available data was conducted using textual approach to describe strategies adopted for EHDI including screening methods, service delivery points, use of information and communications technology (ICT), the target age groups of such programmes, personnel involved in delivery of the programme, and reported barriers and facilitators of the programme. The Joanna Briggs Institute (JBI) tool for critical appraisal15 was used for quality assessment. The Synthesis Without Meta-analysis (SWiM) guideline was used for analysis of secondary outcomes.16 If a country had at least three studies that reported data on children with confirmed hearing loss, then that country was included for estimation of prevalence per 1000 using forest plots.
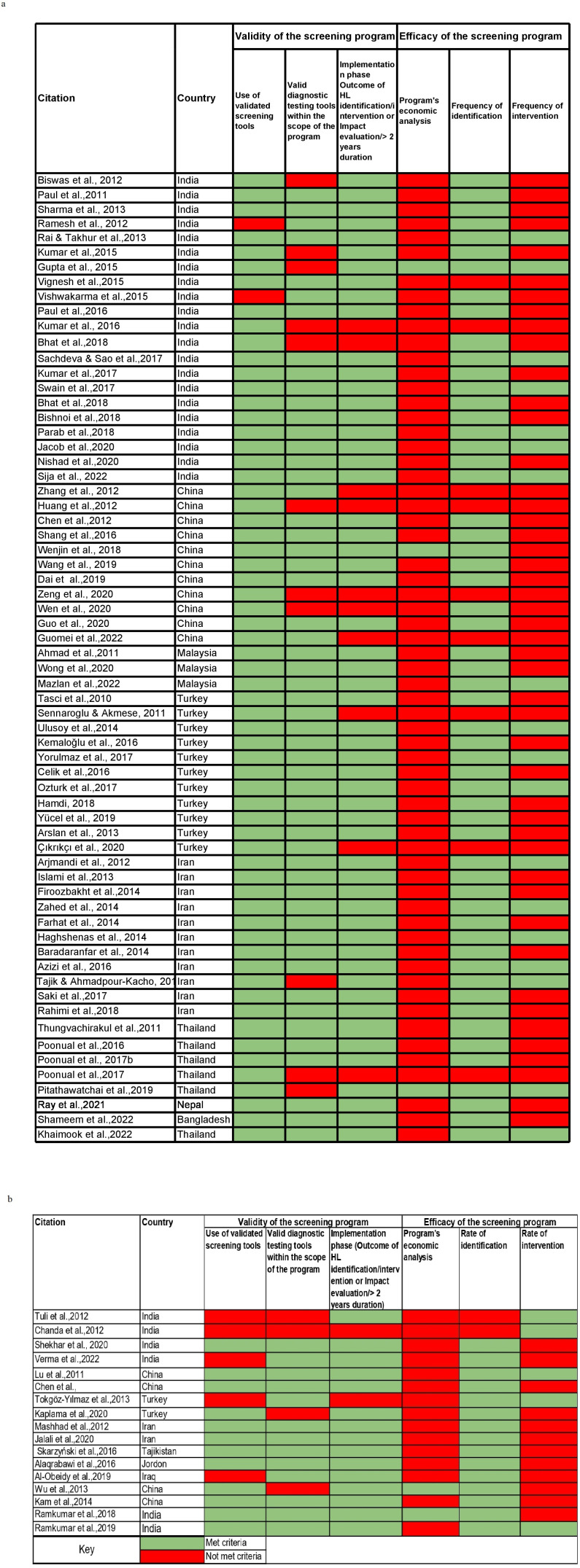
The primary outcomes of interest were the validity and efficacy of the screening programmes. We developed a checklist (figure 1A, B) to assess the validity and efficacy using three criteria each. The items in the validity checklist included (1) the use of a validated screening tool, (2) the use of a validated diagnostic tool, whether the screening programme reported was in the (3) design phase (eg, pilot/feasibility/validity/only reported coverage rate or referral rate or follow-up rate) or implementation phase (eg, scale programme). The efficacy was assessed if the study reported (1) evidence of early identification, (2) evidence of early intervention and (2) inclusion of an economic analysis.
Figure 1.
Validity and efficacy of screening programmes (A) for newborns and infants and (B) for older children. HL, hearing loss.
The secondary outcome of interest was to estimate the incidence and prevalence outcomes of EHDI programmes in the Asian L&MICs. For secondary outcomes analysis, in screening programmes for newborns and infants, the prevalence of hearing loss in infants reported in each country was analysed using the SWiM guidelines. Using a random effect model, Forest plots (figure 2A–E) were constructed for each country based on two criteria: if more than five studies in a country reported prevalence outcomes and if the number of children screened was more than 1000.
Figure 2.
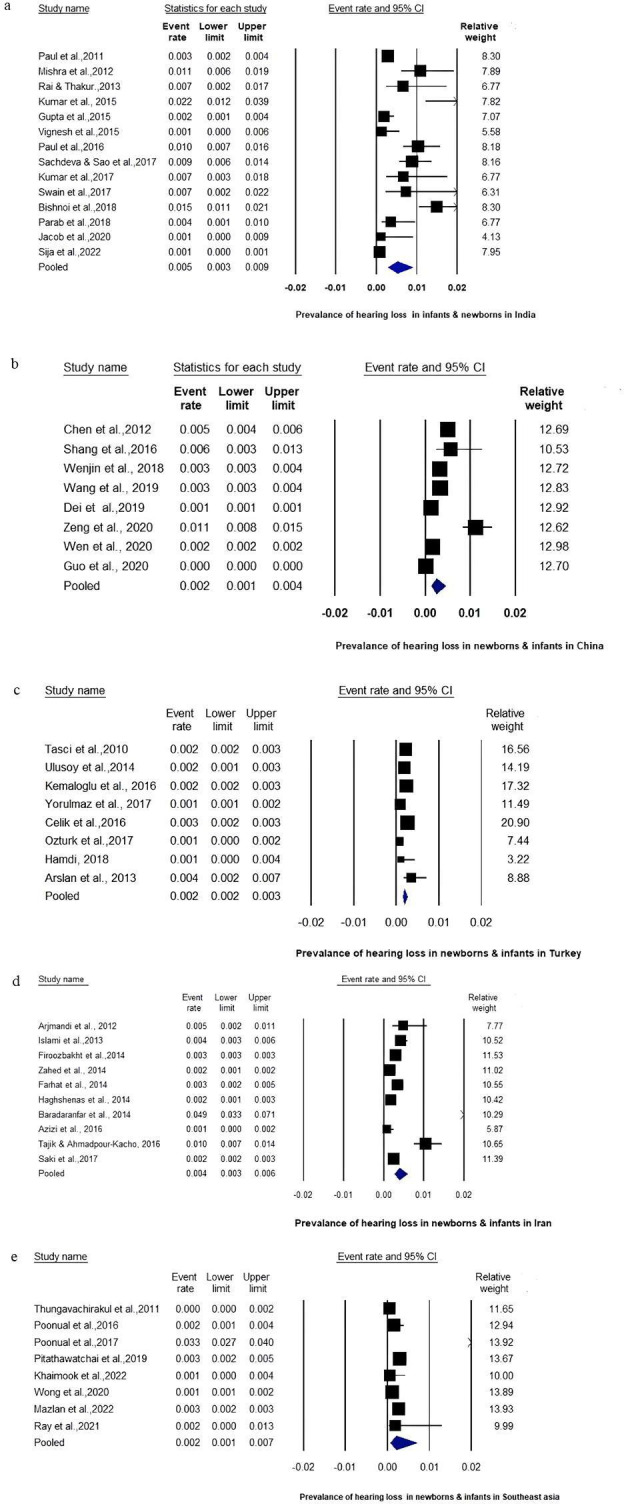
Forest plot of prevalence of hearing loss in (A) newborns and infants in India; (B) newborns and infants in China; (C) newborns and infants in Turkey; (D) newborns and infants in Iran; and (E) newborns and infants in other Asian countries (Thailand, Malaysia and Nepal).
Results
Our electronic search yielded 1312 citations. Based on the inclusion/exclusion criteria and multiple levels of screening by the two reviewers independently, a total of 82 studies qualified for the current review. The article selection process is presented in the PRISMA flowchart (figure 3). Sixty-five studies (79%) reported on newborn hearing screening (NHS), and only 17 studies (21%) reported hearing screening among older children. Predominantly, studies were conducted in India (n=27), followed by Turkey (n=13), Iran (n=13), China (n=15), Thailand (n=6), Malaysia (n=3), Nepal (n=1), Bangladesh (n=1), Iraq (n=1), Jordan (n=1) and Tajikistan (n=1).
Figure 3.
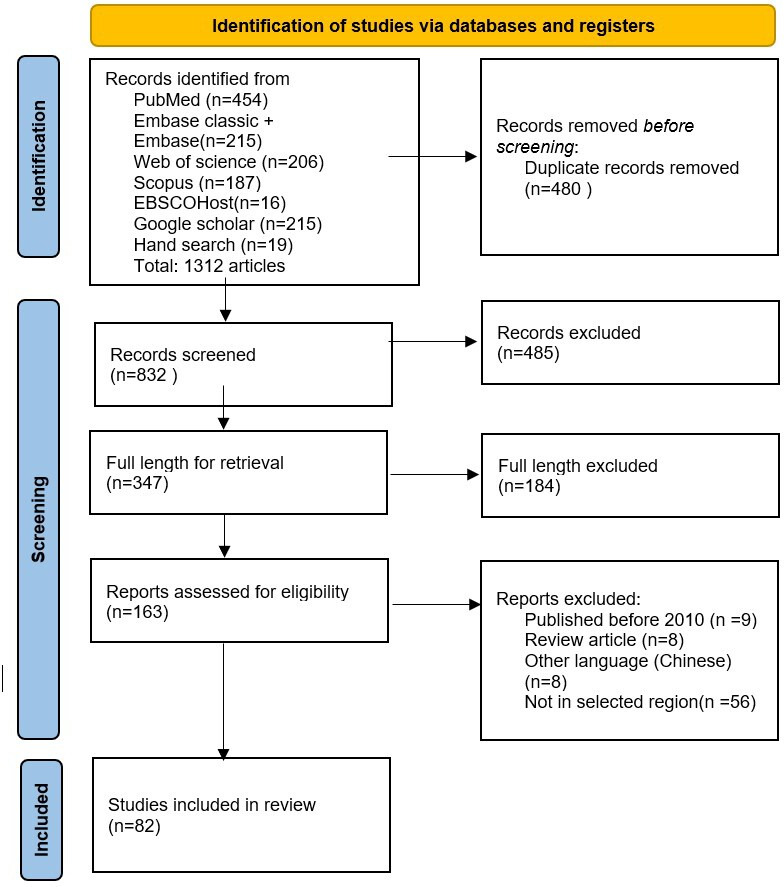
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart representing the selection of article at each stage.
These studies included 75 cross-sectional studies and 7 cohort studies. Results of quality appraisal using appropriate JBI tool are provided in online supplemental file 1.
bmjpo-2022-001752supp001.pdf (80.4KB, pdf)
The screening programmes identified in this review were grouped based on the age group of the children: (1) screening programmes for newborns and infants (0–3 years of age) and (2) screening programmes for older children even beyond 6 years of age.
Hearing screening programmes for newborns and infants (below 2 years) included 65 studies. Most studies (49) reported single-hospital programmes, whereas others (16 studies) reported multiple-centre programmes. Of these studies, 55 were undertaken in the private sector and 10 in the public sector. There were 17 studies of hearing screening programmes for older children aged 3–17. Fifteen of these studies were school-based hearing screenings, while two were community-based. Of these studies, nine were undertaken in the private sector and eight in the public sector. Table 1A–E represents the summary of included studies describing hearing screening programmes for newborns and infants in each country. Table 2 represents the summary of included studies hearing screening programmes for older children.
Table 1.
Hearing screening programmes for (A) newborns and infants in India (LMIC), (B) newborns and infants in China (UMIC), (C) newborns and infants in other Asian countries, (D) newborns and infants in Turkey (UMIC), and (E) newborns and infants in Iran (LMIC)
| (A) | ||||||||
| Author and year | Citation | Duration of programme | Population screened | Screened (n) | Screening protocol | Screening test used | Screening personnel | Diagnostic test |
| Biswas et al 2012 | 104 | 2 years | Newborns | 490 | 1 stage | DPOAE | Not mentioned | Not mentioned |
| Paul 2011 | 81 | 7 years | Newborns | 10 165 | 2 stage | OAE+OAE (non-mentioned DP/TE) | Person with basic knowledge in computer with training on NHS | Diagnostic ABR |
| Mishra et al 2013 | 32 | 3 years | 0–2 years | 1101 | <6 months of age, 5 stage; 6 months–1 year, 4 stage; 1–2 years, 3 stage | DPOAE | Not mentioned | Diagnostic ABR |
| Ramesh et al 2012 | 85 | 2 years | Newborns | 425 | 1 stage | Calibrated noise maker-based Behavioral Observation Audiometry (BOA) | Trained health workers (30 hours of training) | Diagnostic ABR, OAE and BOA |
| Rai et al 2013 | 63 | 1 year | Newborns | 500 | 3 stage | TEOAE+TEOAE+TEOAE | ENT Specialist (Ear Nose Throat) | Diagnostic ABR |
| Kumar et al 2015 | 17 | 1 year 8 months | High risk <2 years of age | 500 | 2 stage | TEOAE+AABR | Audiologist | Not mentioned |
| Gupta et al 2015 | 50 | 1 year | Newborns | 2265 | 2 stage | AABR+AABR | Single specialist staff | Not mentioned |
| Vignesh et al 2015 | 18 | 1.5 years | Newborns | 1405 | 2 stage | TEOAE+AABR | Not mentioned | Diagnostic ABR |
| Vishwakarma et al 2015 | 54 | 1 year 8 months | Newborns | Well babies: 2000 High risk:1020 |
3 stage | TEOAE+TEOAE+AABR | Nurse, resident doctor/certified audiologist | Diagnostic ABR |
| Paul et al 2016 | 33 | 11 years | Newborns | Well babies: 84 774 High risk: 16 914 |
2 stage | OAE+OAE (non-mentioned DP/TE) | Person with basic training in hearing screening | Diagnostic ABR |
| Sharma et al 2018 | 86 | 3 years | Newborns | 2534 | 2 stage | DPOAE | Not mentioned | Diagnostic ABR |
| Kumar et al 2016 | 105 | 2 years | Newborns | 1537 | 2 stage | TEOAE+TEOAE+AABR | Not mentioned | Not mentioned |
| Sachdeva et al 2017 | 87 | 10 months | Newborns | 2254 | 2 stage | (HRR+BOA+DPOAE)+DPOAE | Not mentioned | Confirmatory, diagnostic ABR |
| Kumar et al 2017 | 82 | No info | Newborns | 600 | 2 stage | TEOAE+DPOAE | Not mentioned | Not mentioned |
| Swain et al 2017 | 88 | 1.5 years | Newborns | 410 | 2 stage | DPOAE+DPOAE | Not mentioned | Diagnostic ABR |
| Bhat et al 2018 | 19 | 1 year | High-risk newborns | 195 | 1 stage | TEOAE | Not mentioned | Diagnostic ABR |
| Bishnoi et al 2018 | 89 | No info | Newborns | 2000 | 2 stage | (OAE and Tymp)+OAE (non-mentioned DP/TE) | Not mentioned | Diagnostic ABR |
| Parab et al 2018 | 34 | 3 years | Newborns | 8192 | 2 stage | TEOAE+TEOAE | Audiologist | Diagnostic ABR |
| Jacob et al 2020 | 35 | 2 years | Newborns | 773 | 2 stage | TEOAE+TEOAE | Not mentioned | Diagnostic ABR |
| Nishad et al 2020 | 36 | 1 year | Newborns | 1000 | 2 stage | OAE+OAE (non-mentioned DP/TE) | Not mentioned | Diagnostic ABR |
| Sija et al 2022 | 37 | 4 years | Newborns | 16 265 | 2 stage | DPOAE+DPOAE | Trained nurse | Diagnostic ABR |
| (B) | ||||||||
| Author and year | Citation | Duration of programme | Population screened | Screened (n) | Screening protocol | Screening test used | Screening personnel | Diagnostic test |
| Zhang et al 2012 | 106 | 1.5 years | Newborns | 10 043 | 2 stage+genetic screening | TEOAE+(TEOAE and AABR) | Nurse | Not mentioned |
| Tobe et al 2013 | 83 | 2 years | Newborns | Not mentioned | 2 stage | OAE+AABR (non-mentioned DP/TE) | Trained personnel, no info | Not mentioned |
| Chen et al 2012 | 38 | 2 years | Newborns | 11 568 | 2 stage | TEOAE | Audiologist | Diagnostic ABR, TFT, impedance, ASSR at hospital |
| Shang et al 2016 | 52 | 6 months | Newborns | 1064 | 2 stage | First protocol: TEOAE+TEOAE Second protocol: (TEOAE and ABR screen)+TEOAE |
Not mentioned | Diagnostic ABR |
| Wenjin et al 2018 | 20 | 2 years | Newborns | 19 098 | 2 stage | Well babies: DPOAE+ABR screening High-risk babies: (DPOAE and ABR screening) (DPOAE and ABR screening) |
Nurse | Otoscopy, diagnostic ABR at 30 dBHL, Tymp; DPOAEs |
| Wang et al 2019 | 21 | 5 years | Newborns | 55 977 | 2 stage | OAE+AABR (non-mentioned DP/TE) | Nurse | Comprehensive diagnostic audiometry around 3 months of age |
| Dai et al 2019 | 22 | 1 year | Newborns | 180 469 | 2 stage+genetic screening | TEOAE+(TEOAE and AABR) | Not mentioned | Diagnostic ABR, ASSR, DPOAE, immitance |
| Zeng et al 2020 | 23 | 1 year | Newborns | 4205 | 2 stage+genetic screening | OAE+AABR screening (non-mentioned DP/TE) | Not mentioned | No |
| Wen et al 2020 | 24 | 2 years | Newborns | 467 980 | 2 stage | OAE+(OAE and AABR) (non-mentioned DP/TE) | Not mentioned | Not mentioned |
| Guo et al 2020 | 25 | 2 years 4 months | Infants >3 months | 287 430 | 2 stage+genetic | OAE+AABR (non-mentioned DP/TE) | Not mentioned | Diagnostic ABR |
| Guomei et al 2022 | 53 | 9 months | Newborns | 2174 | 2 stage+genetic | OAE+OAE (non-mentioned DP/TE) | Not mentioned | Diagnostic ABR |
| (C) | |||||||||
| Author and year | Citation | Country | Duration of programme | Population screened | Screened (n) | Screening protocol | Screening test used | Screening personnel | Diagnostic test |
| Ahmad et al 2011 | 64 | Malaysia (MIC) | 5 years | Newborns | 16 000 | 3 stage | DPOAE+DPOAE+DPOAE | Technician, staff nurse, ward attendants | Diagnostic ABR |
| Wong et al 2021 | 26 | Malaysia (UMIC) | 2 years | Newborns | 28 432 | 1 and 2 stage | 1 stage: AABR 2 stage: DPOAE+AABR |
Nurses | Diagnostic ABR |
| Tungvachirakul et al 2011 | 39 | Thailand (UMIC) | 1 year 11 months | Newborns | 4043 | 2 stage | OAE+OAE (not mentioned DP/TE) | Not mentioned | ASSR |
| Poonual et al 2016 | 107 | Thailand (UMIC) | 1 year 7 months | Newborns | 3120 | 2 stage | Automated TEOAE+conventional TEOAE | Not mentioned | Diagnostic ABR |
| Poonual et al 2017 | 27 | Thailand (UMIC) | Not mentioned | Newborns | 3120 | 3 stage | COBRA HRR tool+TEOAE+AABR | Not mentioned | Not mentioned |
| Poonual et al 2017 | 96 | Thailand (UMIC) | 1 year | Newborns | 3120 | 2 stage | TEOAE+AABR | Not mentioned | ABR at 3 and 8 months |
| Pitathawatchai et al 2019 | 40 | Thailand (UMIC) | 1 year 7 months | Newborns | 6140 | 2 stage | TEOAE+TEOAE | Nurses | Not mentioned |
| Ray et al 2021 | 41 | Nepal (LMIC) | 2 years | Newborns | 540 | 2 stage | OAE+OAE (not mentioned DP/TE) | Not mentioned | Diagnostic OAE and diagnostic ABR |
| Mazlan et al 2022 | 28 | Malaysia (UMIC) | 10 years | Newborns | 50 633 | 2 stage | TEOAE+AABR | Trained nurses and medical technologists | Diagnostic ABR |
| Shameem et al 2022 | 42 | Bangladesh (LMIC) | 2 years | High-risk newborns | 426 | 2 stage | TEOAE+TEOAE | Not mentioned | Diagnostic ABR |
| Khaimook et al 2022 | 43 | Thailand (UMIC) | 6 months | Newborns | 1696 | 2 stage | TEOAE+TEOAE | Trained nurse and audiologist | Diagnostic ABR+Tymp |
| (D) | ||||||||
| Author and year | Citation | Duration of programme | Population screened | Screened (n) | Screening protocol | Screening test used | Screening personnel | Diagnostic test |
| Tasci et al 2010 | 55 | 14 months | Newborns | 16 975 | 3 steps | TEOAE+TEOAE+ ABR | Audiology technician | Diagnostic ABR |
| Sennaroglu et al 2011 | 44 | 1 year | Newborns | 1840 | 2 stage | TEOAE | Audiologist or audiometrist | Diagnostic ABR; |
| Ulusoy et al 2014 | 56 | 3 years | Newborns | 11 575 | 3 stage | TEOAE+AABR | 2 audiometrists and 1 nurse | Diagnostic ABR, the level 3 centre |
| Kemaloğlu et al 2016 | 57 | 10 years | Newborns | 19 436 (I/P) 2083 (O/P) |
3 stage | TEOAE+TEOAE+(TEOAE and AABR) | Audiology technicians and audiology students | Diagnostic ABR |
| Yorulmaz et al 2017 | 58 | 5 years | Newborns | 13 693 | 3 stage | TEOAE+TEOAE+AABR | Audiometrist | Diagnostic ABR, Tymp, acoustic reflexes, ASSR |
| Çelik et al 2016 | 45 | 6 years | Newborns | 142 128 | 2 stage | TEOAE (twice same day)+TEOAE | Not mentioned | Diagnostic ABR |
| Ozturk et al 2017 | 59 | 2 years | Newborns | 7502 | 3 stage | Wellbabies: DPOAE+DPOAE+ ABR screening Highrisk babies: Direct ABR |
Audiologist | Diagnostic ABR |
| Hamdi 2018 | 60 | 2 years | Newborns | 1808 | 3 stage | TEOAE+TEOAE+ABR screening | Nurses (trained) | Diagnostic ABR |
| Yücel et al 2019 | 65 | 2 years | Newborns | 786 Syrian and 7230 turkish | 3 stage | (TEOAE and Tymp)+TEOAE+ABR | Not mentioned | Detailed testing |
| Arslan et al 2013 | 46 | 8 months | Newborns | 2229 | 2 stage | TEOAE+TEOAE | Nurse | Diagnostic ABR |
| Çıkrıkçı et al 2020 | 51 | 1.5 years | Newborns | 702 turkish 172 syrian |
2 stage | AABR+AABR | Not mentioned | Diagnostic ABR |
| (E) | ||||||||
| Author and year | Citation | Duration of programme | Population screened | Screened (n) | Screening protocol | Screening test used | Screening personnel | Diagnostic test |
| Arjmandi et al 2012 | 47 | 1 year | Newborns | 1232 | 2 stage | TEOAE+TEOAE | Not mentioned | Diagnostic ABR |
| Islami et al 2013 | 48 | 1.5 years | Newborns | 7250 | 2 stage | TEOAE+TEOAE | Audiologists | Diagnostic ABR |
| Firoozbakht et al 2014 | 29 | 8 years | Newborns | 3 350 995 | 2 stage | TEOAE+AABR | Audiologists, nurses, midwives and trained health technicians. | Comprehensive test |
| Zahed et al 2014 | 30 | 8 years | Newborns | 40 930 | 2 stage | TEOAE+ABR | Audiologists | ABR/ASSR and immittance audiometry, |
| Farhat et al 2014 | 90 | 2 years | Newborns | 8987 | 2 stage | TEOAE+TEOAE | Not mentioned | ASSR |
| Haghshenas et al 2014 | 61 | 2 years | Newborns | 15 165 | 3 stage | OAE+OAE+(OAE and AABR) (not mentioned DP/TE) | Audiologist | ABR screening |
| Baradaranfar et al 2014 | 108 | 1 year | Newborns | 514 | 2 stage | TEOAE+TEOAE | Not mentioned | Diagnostic ABR |
| Azizi et al 2016 | 49 | 1.5 years | Newborns | 3818 | 2 stage | TEOAE+TEOAE | not mentioned | ABR, |
| Tajik et al 2016 | 31 | 4 years | Newborns | 3362 | 2 stage | TEOAE+(TEOAE and ABR) | Not mentioned | Not mentioned |
| Saki et al 2017 | 84 | 3 years | Newborns | 92 521 | 2 stage | First and second:TEOAE+AABR | Audiologists | Diagnostic OAE and ABR |
| Rahimi et al 2018 | 62 | 5 years | Newborns | 4729 | 3 stage | TEOAE+TEOAE+AABR | Audiologist | Diagnostic ABR |
AABR, automated auditory brainstem response; ABR, auditory brainstem response; ASSR, auditory steady-state response; BOA, behavioral observation audiometry; DPOAE, distortion product otoacoustic emission; LMIC, lower middle-income country; MIC, middle-income country; NHS, newborn hearing screening; OAE, otoacoustic emission; PTA, pure tone audiometry; TEOAE, transient evoked otoacoustic emission; Tymp, tympanometry; UMIC, upper middle-income country.
Table 2.
Hearing screening programmes for older children
| Author and year | Citation | Country | Duration of programme | Age of screening (years) | Screened (n) | Screening protocol | Screening test used | Pass/fail criteria | Screening personnel | Diagnostic test | Diagnostic person |
| Tuli et al 2012 | 66 | India | 2 years | 5–16 years | 111 | 1 stage | Case history, audiological and ENT evaluation, awareness and SIFTER | Not mentioned | Not mentioned | ENT and PTA and diagnostic ABR | Audiologist |
| Chadha et al 2013 | 67 | India | 3 years | 5–12 years | 15 718 | 1 stage | Otoscopy, 10-Question Screening Index for Disabilities in English and Hindi |
Positive history of hearing or speech defects, a positive finding on examination | Proforma: parents, otoscopy: otolaryngologists |
Not mentioned | Not mentioned |
| Ramkumar et al 2018 | 77 | India | 2 years | Birth–5 years | 1335 | 2 stages | DPOAE+DPOAE | >SNR 3 dB | Trained village health worker | Telediagnostic ABR | Audiologist |
| Ramkumar et al 2019 | 76 | India | 2 years | Birth–5 years | 2815 | 2 stages | DPOAE+DPOAE | >SNR 3 dB | Trained village health Worker | Diagnostic ABR—in person and telediagnostic ABR | Audiologist |
| Verma et al 2022 | 91 | India | 6 months | 6–17 years | 597 | 1 stage | Tuning fork test | Not mentioned | Not mentioned | PTA and Tymp | Audiologist |
| Shekhar et al 2022 | 68 | India | Not mentioned | 5–14 years | 474 | 1 stage | PTA | Not mentioned | ENT specialist | ENT examination | ENT specialist |
| Lü et al 2011 | 69 | China | 1 year | 3–6 years | 21 427 | 1 stage | PTA | 1, 2 and 4 kHz >20 dB | Screening person with training (training programme with certificate) | PTA (5–6 years) VRA or play PTA (3–4 years) |
Not mentioned |
| Chen et al 2013 | 80 | China | 1 year 5 months | 3–6 years | 28 546 | 1 stage | TEOAE | >SNR 3 dB | School nurses and doctors 2 hours of training |
Comprehensive test | Not mentioned Audiologist |
| Wu et al 2014 | 70 | China | Not mentioned | 3–6 years | 6288 | 1 stage | Software-based new PTA | >30 dBHL at 1, 2 and 4 kHz | Preschool teachers—minimally trained | Not mentioned | Not mentioned |
| Kam et al 2014 | 71 | China | Not mentioned | 3–7 years | 6231 | 1 stage | Automated PTA | >30 dBHL at 1, 2 and 4 kHz | Automatic test: nurses with 2 hours’ training as facilitator | Tymp, DPOAE and PTA (0.25–8.0 kHz) | Not mentioned |
| Tokgöz-Yılmaz et al 2013 | 72 | Turkey | 3 years | 3–5 years | 239 | 1 stage | PTA | Not mentioned | Audiologist and SLP | ENT examination | ENT specialist |
| Kaplama et al 2020 | 79 | Turkey | 1 year | 69–84 months | 23 664 | 2 stage | PTA, 10 questionnaire |
500, 1000, 2000 and 4000 Hz >20 dB 10 questions—refer in 1 question |
Certified nurses, midwives, health officers or audiometrists, | ENT examination | ENT specialist |
| TarvijEslami et al 2017 | 78 | Iran | 1 year | 6–7 years | 2237 | Not mentioned | PTA | Not mentioned | Not mentioned | PTA, Weber, Rinne test |
Not mentioned |
| Jalali et al 2020 | 73 | Iran | 4 months | 6–13 years | 2019 | 1 stage | PTA | 0.5–4.0 kHz >15 dBHL | Not mentioned | ENT examination and comprehensive audiological examination | Not mentioned |
| Skarzyński et al 2016 | 8 | Tajikistan (LMIC) | Not mentioned | 6–8 years | 143 | 1 stage | Questionnaire, PTA using SZOK telemed model |
PTA module (500–8 kHz) >25 dB at one frequency | Medical doctors Other specialists |
Detailed PTA | Audiologists |
| Alaqrabawi et al 2016 | 74 | Jordan (UMIC) | 4 years | 5–15 years | 1649 | 1 stage | PTA | 500 Hz, 1, 2 and 4 kHz >25 dB | Not mentioned | Audiometry, otoscopy and Tymp |
Audiologists |
| Al-Obeidy et al 2019 | 75 | Iraq (UMIC) | 1 year | 6 years | 425 | 1 stage | HR questionnaire | Not mentioned | Not mentioned | ENT examination, TFT (Weber, Rinne and absolute bone conductio). HRR children: PTA |
Not mentioned |
ABR, auditory brainstem response; BOA, behavioral observation audiometry; DPOAE, distortion product otoacoustic emission; HRR, high-risk register; LMIC, lower middle-income country; PTA, pure tone audiometry; SIFTER, Screening Identification For Targeting Educational Risk; SNR, signal-to-noise ratio; TEOAE, transient evoked otoacoustic emission; TFT, Tuning Fork Test; Tymp, tympanometry; UMIC, upper middle-income country.
Screening protocol and tests
Newborn and infant hearing screening
Two-stage hearing screening protocols were employed most frequently for newborn and infant hearing screening (n=47), followed by three-stage protocols (n=13) and one-stage protocols (n=4). One study reported employing a five-step hearing screening protocol.
Sixteen studies that reported a two-stage hearing screening protocol, employed OAE (TE/DP-OAE) or AABR as screening tests (individually or combined in either stage).17–31 The other 25 studies used only OAEs (DP/TE)32–49 or AABR screening50 51 for testing in both stages. Those studies that reported the use of AABR in the initial stage of screening either employed AABR solely for both stages50 or a combination of AABR and OAE to screen only high-risk newborns.20 52 Four studies from China used two-stage screening coupled with genetic hearing screening.21–23 25 53
When a three-stage protocol was used, generally the first two stages included OAE (DP/TE) screening followed by AABR/auditory brainstem response (ABR) screening54–62 or included OAE (DP/TE) for all three stages.63 64 Only one study reported combining tympanometry and TEOAE in the initial stage of its three-stage screening protocol.65 Studies from Turkey (n=7) reported a three-stage screening protocol.55–60 65
Screening for older children
Fourteen studies for older children employed a single-stage screening protocol8 66–75 with three employing a two-stage protocol.3 76 Ten studies reported using subjective hearing screening tests, two studies used questionnaire or otoscopy for screening67 75 and another three studies used TEOAE.76 77 Pure tone audiometry (PTA) was the most commonly used subjective test for screening older children.68 69 72–74 78 Two studies reported the use of automated software-based PTA.70 71 PTA was combined with questionnaires8 79 or otoscopy.67 75 Only one study reported the use of TEOAE screening.80
Pass/refer criteria
In several programmes for newborn and infant screening, screening results were based on data generated from the screening instrument automatically. The pass criteria for DP/TEOAE was between 3 dB and 6 dB signal-to-noise ratio,19 20 25 37 38 40 43 45 49 54 57 63 64 81 82 and for AABR, it varied between 30, 35 and 40 dB neural hearing loss (NHL).20 52 56 58 61 Predominantly, refer results in one ear was considered for follow-up screening.
For screening older children, the pass criteria for PTA ranged from 15 dB HL to 30 dB HL. All studies used the four frequencies from 0.5 kHz to 4.0 kHz for pure tone testing. In questionnaire-based studies, failing one item or a family history of hearing loss was the referral criterion.67 68
Screening personnel
Audiologists were the primary screening personnel in many newborn and infant programmes,17 30 34 38 44 48 54 59 61 62 83 84 followed by nurses.20 21 26 28 29 37 40 43 46 54 56 60 64 In five studies, the training provided for nurses to perform hearing screening was also briefly mentioned,28 29 40 46 60 including some certifications.56 Other than nurses, some studies reported audiometrists44 56 58 and audiologist technicians55 as personnel involved in screening. Other non-specialists that were engaged in hearing screening were technicians,64 ward attendants,64 trained health workers29 85 social workers83 and midwives.29 33 81 In a few programmes, otolaryngologists63 performed the hearing screening. Out of 59 studies, 29 did not provide any information regarding the screening individual.
Screening for older children was conducted by otorhinolaryngologists8 67 68 audiologists72 and audiometrists.79 Other non-specialists involved in the hearing screening included trained nurses/midwives,38 71 79 trained village health workers or volunteers,76 77 and school teachers with training.70
Studies have reported a variety of training programmes. They included hearing screening certification,67 79 2 hours of TEOAE training,38 TEOAE training and telediagnostic testing facilitation,76 and minimal training/2 hours of training for facilitating automated PTA.70 71
Confirmation of hearing loss
Diagnostic ABR was the only testing carried out to confirm the hearing loss in studies in newborns and infants.25 28 32 35–37 42 54 63 86–89 Comprehensive test battery including the diagnostic BERA, OAE, and tympanometry was mentioned only in 11 studies.20 29 58 Four studies also reported the inclusion of the auditory steady-state response (ASSR) in the test battery.30 58
Two programmes used solely ASSR,39 90 and studies also used ABR screening at 3020 or 35 dB NHL61 for hearing loss diagnosis.
However, 11 of the 65 programmes made no mention of the diagnostic confirmatory test used for confirmation of hearing loss. More than half of the studies (n=37), reported that the diagnostic confirmatory test was performed at the same hospital where screening was conducted. In another 18 studies, children were referred to more specialist or tertiary care facilities for diagnostic confirmatory tests. The diagnostic site was not mentioned or could not be inferred in 10 studies.
In studies reporting screening for older children, a test battery approach was used in three studies where they included PTA with tympanometry and DPOAE71 or PTA with otoscopy and tympanometry74 or PTA and detailed ABR.66 Two studies reported the use of comprehensive test battery but did not mention the tests included.38
PTA was frequently included in the diagnostic test battery,71 74 91 but in three studies, PTA was the only diagnostic test used.8 73 78 Of the studies that reported the use of PTA for diagnosis, only four studies72–74 78 mentioned information related to bone conduction testing. Apart from these studies, ENT examination was included in five studies.68 72 73 75 79 The diagnostic testing sites included a hospital,73 a school,68 a speech and hearing centre,71 and a telemedicine platform.8 76
Use of ICT
In studies related to newborn and infant hearing screening, three programmes reported the use of ICT for storing and forwarding results,34 database management28 83 and sending reminders for follow-up screening.
In studies reporting screening of older children, five studies reported using telepractice for screening, diagnosis or both. Telediagnostic ABR76 77 was reported in India. Use of m-health-based automated hearing screening was reported in China.70 71.A telesensory screening platform including hearing screening was reported (SZOK - (Sense Examination Platform) paradigm) in Tajikistan, where both screening and diagnosis were carried out via telemedicine.8
Validity and efficacy of the screening programmes
Validity of screening programmes as reported in the studies was evaluated based on three criteria: use of a validated screening tool, use of a validated diagnostic tool, and whether the programme was in the design phase or in the implementation phase.
Among the studies that reported newborn and infant hearing screening, 48 studies fulfilled all three criteria of the validity tool; 11 studies fulfilled two out of three criteria; and 6 studies fulfilled one out of three criteria (figure 1A). The validated screening tool was used by 63 studies and 54 studies used a validated diagnostic tool. As per the criteria we used, 55 studies could be classified to be in the implementation phase and 10 studies were in the design phase.
Economic analysis, frequency of identification and intervention were the three criteria included to assess efficacy. Only 2 studies fulfilled all the three efficacy criteria; 17 studies fulfilled two out of the three criteria; and 37 studies fulfilled only one of the three criteria, whereas the remaining 9 studies did not fulfil any of the criteria. Fifty-one studies reported only the frequency of identification, whereas 14 reported both the frequency of identification and intervention. Twelve per cent of the studies did not mention either of these outcomes. Economic analysis was very limited (n=3) and was reported majorly in public programmes.
Among the studies that reported screening programmes for older children, 10 studies fulfilled all the three criteria; 3 studies fulfilled two out of three criteria; and 3 studies fulfilled one out of three criteria. Only one study did not meet any of the criteria67 since only a questionnaire and an otoscopic examination were used to estimate the incidence of conductive hearing loss in older children.
With respect to efficacy, it was observed that none of the studies among older children fulfilled all the three criteria. Only five studies fulfilled two out of three criteria, whereas the remaining 12 studies fulfilled only one criterion.
Fourteen studies have reported frequency of identification, but only five studies have reported the frequency of intervention (eg, medical intervention for conductive pathology). The intervention-related screening programmes were reported from India, China and Turkey. The economic analysis was reported in only two studies.71 77 Except for the economic analysis, only 2 of the 17 studies fulfilled all validity and efficacy criteria.69 76
Prevalence of hearing loss
Across 48 studies, the mean prevalence of hearing loss among newborns and infants was 5/1000 in India, 2/1000 in China, 2/1000 in other Southeast Asian nations (Thailand, Malaysia and Nepal), 2/1000 in Turkey, and 4/1000 in Iran. Figure 2A–E shows the forest plots for prevalence of each country.
In screening programmes for older children, 11 studies reported number of cases with hearing loss including conductive and sensori neural hearing losses. However, in four studies,67 68 79 80 the specific audiological tests conducted to diagnose were not mentioned, and in seven studies,69 72–75 78 details of diagnostic audiometry were provided. In this age group, the percentage of conductive hearing loss reported was higher compared with sensori neural hearing loss across all the studies. In two studies, the type of loss was not differentiated.8 67 The percentage of children identified with a certain type of hearing loss was calculated based on the information on the number of children diagnosed that was provided in each of the studies. The study outcomes are reported in table 3.
Table 3.
Secondary outcomes: studies reporting number of cases identified with CDHL/SNHL in older children in each country
| Country | Author and year | Screened (n) | CDHL identified (n) | % of CDHL | NHL identified (n) | % of SNHL | Overall HL identified (n) | % of HL | 95% CI (LB to UP) |
| India | Chadha et al.67 2013 | 15 718 | NA | NA | NA | NA | 1578 | 10.30 | 9.57 to 10.52 |
| Shekhar et al.68 2020 | 474 | 146 | 30.80 | 1 | 0.21 | 147 | 31.01 | 26.87 to 35.39 | |
| Turkey | Tokgöz-Yılmaz et al.72 2013 | 239 | 25 | 10.46 | 1 | 0.42 | 26 | 10.88 | 7.23 to 15.53 |
| Kaplama et al.79 2020 | 23 664 | 186 | 0.79 | 89 | 0.37 | 275 | 1.16 | 1.03 to 1.31 | |
| Iran | TarvijEslami et al.78 2017 | 2284 | 28 | 1.23 | 8 | 0.35 | 36 | 1.58 | 1.11 to 2.18 |
| Jalali et al.73 2020 | 2019 | 19 | 0.94 | 8 | 0.39 | 27 | 1.33 | 0.88 to 1.94 | |
| Tajikistan | Skarzyński et al. 2016 | 143 | NA | NA | NA | NA | 34 | 23.70 | 17.06 to 31.61 |
| Jordan | Alaqrabawi et al.74 2016 | 1649 | 54 | 3.27 | 36 | 2.18 | 90 | 5.45 | 4.41 to 6.61 |
| Iraq | Al-Obeidy et al.75 2019 | 425 | 28 | 6.59 | 2 | 0.47 | 30 | 7.06 | 4.81 to 9.92 |
| China | Lu et al.44 2011 | 21 547 | 285 | 1.32 | 16 | 0.07 | 301 | 1.39 | 1.24 to 1.56 |
| Chen et al. 38 2012 | 28 546 | 344 | 1.21 | 22 | 0.08 | 366 | 1.29 | 1.15 to 1.42 |
CDHL, conductive hearing loss; HL, hearing loss; LB, lower bound; NA, not mentioned; NHL, neural hearing loss; SNHL, sensorineural hearing loss; UB, upper bound.
Barriers and facilitators
Barriers
Loss to follow-up for second screening and diagnostics20 29 35–37 40 43 48 54 56 59 81 87 was reported as a major challenge. Loss to follow-up was linked to parental rejection for diagnosis,33 43 50 poor tracking system,20 29 financial burden of parents, low socioeconomic status51 and travel distance to testing distance. Other major challenges highlighted in relation to outcomes included limited coverage35 82 and a high referral rate,18 37 54 poor long-term outcomes with respect to coverage and referral rate.24
Other factors that had an indirect impact on programme outcomes included the lack of dedicated screening personnel,50 lack of professional resources/audiologists,29 84 high ambient noise in the testing environment82 and the absence of diagnostic facilities.56 A few studies mentioned challenges affecting programme implementation, such as the use of a three-step protocol only with OAE,55 the difficulties of centralised programme implementation in remote locations29 and delay in diagnosis in remote locations due to referral to regional facilities.84
In screening for older children, children’s attention was regarded as a major challenge resulting in poor accuracy.71 Other key factors influencing programme outcomes included inadequate internet connectivity8 76 and poor follow-up due to social stigma.
Facilitators
Use of appropriate tracking or data management systems, were reported to be helpful in minimising loss to follow-up.20 28 33 35 81 Combining hearing screening with other screenings improved follow-up rates.25 62 Several studies highlighted strategies to minimise false referral rates, including (1) employing a conducive environment and trained individuals,54 (2) adding AABR in the initial stage of screening protocol,52 (3) screening between 3 days and 5 days of age62 and (4) incorporating tympanometry into the screening protocol.89 Financial assistance in the form of funding28 37 83 and centralised hearing screening facilities or grouping more centres33 81 were strategies reported in studies to improve coverage rates. Multicentre-based or a centralised hearing screening programme was reported to be resource efficient with respect to cost, infrastructure and professionals.81
Discussion
The primary purpose of this review was to describe the models of hearing screening programmes implemented in young children in various Asian L&MICs in the published scientific literature. The inclusion of countries was based on the World bank classification rather than culturally defined regions; this led to a heterogenous inclusion with central Asian and middle eastern countries as well. Out of 61 L&MICs in Asia, only 14 countries reported hearing screening programmes that fit our inclusion criteria. In a recent systematic review, high-quality literature with hearing screening programmes was reported to be primarily in HICs92; yet, it is also likely that resources for research and publication are low and hence are also low on priority in the L&MICs context. Though studies from both L&MICs were included, our results show that most of the studies reporting on hearing screening were from the middle-income countries and more specifically from UMICs. This suggests greater adoption of EHDI measures in UMICs, possibly due to greater availability of resources in comparison to LMICs and LICs.
Our review gathered evidence on hearing screening programmes in general, including screening protocols, screening tests, pass/fail criteria, screening personnel, diagnostic tests, use of ICT, and programme validity and efficacy. The hearing screening tools and protocols used for newborns, infants and older children were similar to those used in HICs.93 Despite the fact that the majority of programmes used a two-stage OAE (DP/TE) and ABR screening as preferred screening tools across countries, there was no consistency in protocol stages or screening tests undertaken. This was consistent with Kanji et al ’s assessment of NHS protocols, which revealed non-uniformity in the protocols followed.
It was also noted that objective hearing screening was most commonly reported over subjective hearing screening for newborns and infants. Only one study85 found good sensitivity and specificity for behavioural hearing assessment for neonates and infants using calibrated noise makers. The use of objective screening in L&MICs implies a preference for international best practices based on Western contexts and guidelines.2 However, it is important to assess the sustainability and long-term outcomes of these efforts. Subjective single-stage PTA screening, on the other hand, was extensively used in various screening programmes for older children above the age of 3. This is comparable to HICs where PTA screening is mandatory for children over the age of 3.94 95 In contrast, the current review found a few public initiatives75 87 96 that used questionnaire methods, and this implies that mass screening was being done by low-cost tools like questionnaires where resources were limited.
Audiologists were the most common screening personnel in newborn screening programmes across Asian L&MICs. This is in contrast to HICs, where nurses mostly performed hearing screening.97 While the majority of NHS programmes in Asian L&MICs were started by audiologists or otolaryngologists in private hospitals, in most HICs, the screening programmes were generally universal and followed as a part of other normal newborns screening before discharge. Screening of older children was mostly done by otolaryngologists, school instructors and nurses. This could be because many of the screening programmes for older children were conducted in schools or community settings in the absence of audiologists on site. In contrast, hearing screenings are carried out at child health clinics by a dedicated school nurse/audiologist in HICs.97
Use of the test battery was limited in diagnostic confirmation of hearing loss. Detailed ABR testing was considered as the standard diagnostic tool in many countries as it examines the entire peripheral auditory pathway responsible for hearing. Apart from this, studies from China employed a test battery containing a variety of tests altogether (eg, ASSR, ABR and tympanometry) to confirm hearing loss. In WHO guidelines for hearing screening, diagnostic test battery including ABR/ASSR, tympanometry, acoustic reflex, otoscopic examination and medical evaluation was suggested.98 Therefore, in HICs, the diagnostic test battery approach is mostly preferred.97 In screening programmes for older children, medical (ENT) examination in cases of conductive pathology and routine PTA with or without tympanometry were prioritised as tests to confirm hearing loss. This is inconsistent with the WHO guidelines98 and with the programmes from HICs97 It is important to note that PTA is a crucial test to differentiate CDHL and sensorineural hearing loss. However information on bone conduction testing was was limited.
Few studies reported the use of ICT to screen, manage data or perform diagnostic tests.8 76 Lack of use of ICT could be due to lack of adequate infrastructure, skills to support use of such tools. Yet, this is not unique to L&MICs as evidence on use of ICT is limited even among HICs.92 93 97 99
We assessed the validity and efficacy of the screening programme for infants and older children using a purposively developed tool. None of the programmes reported met all of the criteria. The majority of programmes made use of validated screening and diagnostic tools and reported the rate of hearing loss identification. However, information on economic analysis was scarce, even though cost effectiveness is a key variable for determining programme success.100 Furthermore, studies predominantly reported only identification but not intervention. The importance of EHDI programmes is to intervene children so that the pervasive impact of childhood hearing loss can be mitigated101 102; therefore, it is pertinent to know whether such programmes resulted in early intervention.
Mean prevalence of hearing loss in newborns and infants was identified to be high in India (5/1000), followed by Iran (3/1000) and China (2/1000). This is similar to the findings of Bussé and colleagues (2021) where the highest prevalence was found in India and Nigeria, followed by Iran. In another review, prevalence was found to be highest in Asian countries compared with other regions.99 A world report on hearing also stated that prevalence of congenital hearing loss in L&MICs is high compared with HICs.
Barriers identified from our review were similar to those previously identified and discussed in various studies including L&MICs.97 101–103 However, a recent study in HICs found that when hearing screening programmes were integrated as part of national screening with a dedicated screening person, database management system and appropriate guidelines, they were more successful. Therefore, EHDI in L&MICs is also likely to be more successful when implemented through the government.
There were some limitations to the review which must be considered. No article was excluded based on quality assessment owing to the limited literature available from L&MICs, yet the risk of bias in many included studies was moderate to high. Furthermore, due to heterogeneity in the information obtained across studies, no meta-analysis was performed. The generalisability of the findings was limited to Asian L&MICs. Further, there were potential for publication bias as not all programmes would have published their results. The coverage of EHDI in these countries was not assessed.
From this study, it is evident that strategies for EHDI in Asian L&MICs were similar to those recommended in HICs. However, there is inadequate evidence related to the intended outcome of early intervention in this context. Therefore, programme planners and researchers must focus on impact evaluations that demonstrate the long-term viability of EHDI programmes in the L&MI context.
Supplementary Material
Footnotes
Contributors: VR, DJ are responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. World Health Organization (WHO) . Deafness and hearing loss; 2021.
- 2. Busa J, Harrison J, Chappell J. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007;120:898–921. 10.1542/peds.2007-2333 [DOI] [PubMed] [Google Scholar]
- 3. Krishnan LA, Donaldson LK. Newborn hearing screening in developing countries: understanding the challenges and complexities of implementation. Perspect Glob Iss CSD 2013;3:54–61. 10.1044/gics3.2.54 [DOI] [Google Scholar]
- 4. Kanji A, Khoza-Shangase K. In pursuit of successful hearing screening: an exploration of factors associated with follow-up return rate in a risk-based newborn hearing screening programme. Iran J Pediatr 2018;28. 10.5812/ijp.56047 [DOI] [Google Scholar]
- 5. Swanepoel DW, Hugo R, Louw B. Infant hearing screening at immunization clinics in south africa. Int J Pediatr Otorhinolaryngol 2006;70:1241–9. 10.1016/j.ijporl.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 6. de Kock T, Swanepoel D, Hall JW. Newborn hearing screening at a community-based obstetric unit: screening and diagnostic outcomes. Int J Pediatr Otorhinolaryngol 2016;84:124–31. 10.1016/j.ijporl.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 7. Friderichs N, Swanepoel D, Hall JW. Efficacy of a community-based infant hearing screening program utilizing existing clinic personnel in Western Cape, South Africa. Int J Pediatr Otorhinolaryngol 2012;76:552–9. 10.1016/j.ijporl.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 8. Skarzyński PH, Świerniak W, Piłka A, et al. A hearing screening program for children in primary schools in tajikistan: a telemedicine model. Med Sci Monit 2016;22:2424–30. 10.12659/msm.895967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monica SD, Ramkumar V, Krumm M, et al. School entry level tele-hearing screening in a town in south india - lessons learnt. Int J Pediatr Otorhinolaryngol 2017;92:130–5. 10.1016/j.ijporl.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 10. Dharmar M, Simon A, Sadorra C, et al. Reducing loss to follow-up with tele-audiology diagnostic evaluations. Telemed J E Health 2016;22:159–64. 10.1089/tmj.2015.0001 [DOI] [PubMed] [Google Scholar]
- 11. Yousuf Hussein S, Swanepoel DW, Mahomed F, et al. Community-based hearing screening for young children using an mHealth service-delivery model. Glob Health Action 2018;11:1467077. 10.1080/16549716.2018.1467077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. 2Dsearch. Available: https://www.2dsearch.com/ [Accessed 08 Oct 2022].
- 13. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. JBI . Critical appraisal tools. Available: https://jbi.global/critical-appraisal-tools [Accessed 08 Oct 2022].
- 16. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368:l6890. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar A, Shah N, Patel KB, et al. Hearing screening in a tertiary care hospital in India. J Clin Diagn Res 2015;9:MC01–4. 10.7860/JCDR/2015/11640.5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vignesh SS, Jaya V, Sasireka BI, et al. Prevalence and referral rates in neonatal hearing screening program using two step hearing screening protocol in chennai - a prospective study. Int J Pediatr Otorhinolaryngol 2015;79:1745–7. 10.1016/j.ijporl.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 19. Bhat J, Kurmi R, Kumar S, et al. Targeted screening for hearing impairment in neonates: a prospective observational study. Indian J Otol 2018;24:42. 10.4103/indianjotol.INDIANJOTOL_10_18 [DOI] [Google Scholar]
- 20. Wenjin W, Xiangrong T, Yun L, et al. Neonatal hearing screening in remote areas of China: a comparison between rural and urban populations. J Int Med Res 2018;46:637–51. 10.1177/0300060517706643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q, Xiang J, Sun J, et al. Nationwide population genetic screening improves outcomes of newborn screening for hearing loss in China. Genet Med 2019;21:2231–8. 10.1038/s41436-019-0481-6 [DOI] [PubMed] [Google Scholar]
- 22. Dai P, Huang L-H, Wang G-J, et al. Concurrent hearing and genetic screening of 180,469 neonates with follow-up in beijing, china. Am J Hum Genet 2019;105:803–12. 10.1016/j.ajhg.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng X, Liu Z, Wang J, et al. Combined hearing screening and genetic screening of deafness among hakka newborns in china. Int J Pediatr Otorhinolaryngol 2020;136:110120. 10.1016/j.ijporl.2020.110120 [DOI] [PubMed] [Google Scholar]
- 24. Wen C, Li X, Huang L, et al. Current status of universal newborn hearing screening program at 26 institutions in china. Int J Pediatr Otorhinolaryngol 2020;138:110131. 10.1016/j.ijporl.2020.110131 [DOI] [PubMed] [Google Scholar]
- 25. Guo L, Xiang J, Sun L, et al. Concurrent hearing and genetic screening in a general newborn population. Hum Genet 2020;139:521–30. 10.1007/s00439-020-02118-6 [DOI] [PubMed] [Google Scholar]
- 26. Wong YA, Mazlan R, Abdul Wahab NA, et al. Quality measures of a multicentre universal newborn hearing screening program in Malaysia. J Med Screen 2021;28:238–43. 10.1177/0969141320973060 [DOI] [PubMed] [Google Scholar]
- 27. Poonual W, Navacharoen N, Kangsanarak J, et al. Outcome of early identification and intervention on infants with hearing loss under universal hearing screening program. J Med Assoc Thai 2017;100:197–206. [PubMed] [Google Scholar]
- 28. Mazlan R, Raman K, Abdullah A. A 10-year retrospective analysis of newborn hearing screening in a tertiary hospital in Malaysia. Egypt J Otolaryngol 2022;38. 10.1186/s43163-022-00331-w [DOI] [Google Scholar]
- 29. Firoozbakht M, Mahmoudian S, Alaeddini F, et al. Community-based newborn hearing screening programme for early detection of permanent hearing loss in iran: an eight-year cross-sectional study from 2005 to 2012. J Med Screen 2014;21:10–7. 10.1177/0969141314522992 [DOI] [PubMed] [Google Scholar]
- 30. Zahed Y, Zamani M, Hashemi A, et al. Screening of hearing in newborn infants: follow-up and outcome after 40 930 births in babol, northern iran. Arch Iran Med 2018. [PubMed] [Google Scholar]
- 31. Tajik S, Ahmadpour-Kacho M. Early diagnosis and intervention for hearing loss in newborns discharged from intensive care units: A four-year follow-up study in north of iran. Int J Pediatr 2016;4:3283–91. 10.22038/ijp.2016.7154 [DOI] [Google Scholar]
- 32. Mishra G, Sharma Y, Mehta K, et al. Efficacy of distortion product oto-acoustic emission (OAE)/auditory brainstem evoked response (ABR) protocols in universal neonatal hearing screening and detecting hearing loss in children <2 years of age. Indian J Otolaryngol Head Neck Surg 2013;65:105–10. 10.1007/s12070-012-0553-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul AK. Centralized newborn hearing screening in ernakulam, Kerala, experience over a decade. Indian Pediatr 2016;53:15–7. 10.1007/s13312-016-0782-7 [DOI] [PubMed] [Google Scholar]
- 34. Parab SR, Khan MM, Kulkarni S, et al. Neonatal screening for prevalence of hearing impairment in rural areas. Indian J Otolaryngol Head Neck Surg 2018;70:380–6. 10.1007/s12070-018-1386-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacob J, Kurien M. Challenges of universal newborn hearing screening in a developing country-a double-edged sword. Indian J Otolaryngol Head Neck Surg 2022;74(Suppl 1):395–401. 10.1007/s12070-020-02170-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishad A, Gangadhara Somayaji KS, Mithun HK, et al. A study of incidence of hearing loss in newborn, designing aa protocol and methodology to detect the same in aa tertiary health-care center. Indian J Otol 2020;26:85–8. 10.4103/indianjotol.INDIANJOTOL_63_20 [DOI] [Google Scholar]
- 37. Sija S, Gireesan VK, Kumar A, et al. Outcome of a newborn hearing screening program in a tertiary care center, south india. J Early Hear Detect Interv 2022;7:101–7. 10.26077/6021-102c [DOI] [Google Scholar]
- 38. Chen G, Yi X, Chen P, et al. A large-scale newborn hearing screening in rural areas in china. Int J Pediatr Otorhinolaryngol 2012;76:1771–4. 10.1016/j.ijporl.2012.08.021 [DOI] [PubMed] [Google Scholar]
- 39. Tungvachirakul V, Boonmee S, Nualmoosik T, et al. Newborn hearing screening at rajavithi Hospital, Thailand: hearing loss in infants not admitting in intensive care unit. J Med Assoc Thai 2011;94 Suppl 2:S108–12. [PubMed] [Google Scholar]
- 40. Pitathawatchai P, Khaimook W, Kirtsreesakul V. Pilot implementation of newborn hearing screening programme at four hospitals in southern Thailand. Bull World Health Organ 2019;97:663–71. 10.2471/BLT.18.220939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ray P, Thakali S, Prajapati S. Newborn hearing screening: experience from a tertiary level hospital in Nepal. Nepal Med Jor 2021;4:33–6. 10.37080/nmj.152 [DOI] [Google Scholar]
- 42. Shameem M, Saha KL, Uddin MB, et al. Hearing screening to evaluate the status of newborn hearing impairment in the NICU of a tertiary hospital. TAJ: J of Teachers Assoc 2022;35:77–82. 10.3329/taj.v35i1.61159 [DOI] [Google Scholar]
- 43. Khaimook W, Suwanno R, Dindamrongkul R, et al. An early hearing detection and intervention program in songklanagarind hospital. J Health Sci Med Res 2022;40:551–9. 10.31584/jhsmr.2022867 [DOI] [Google Scholar]
- 44. Sennaroglu G, Akmese PP. Risk factors for hearing loss and results of newborn hearing screening in rural area. J Int Adv Otol 2011;7:343. [Google Scholar]
- 45. Çelik O, Eskiizmir G, Uz U. A comparison of thresholds of auditory steady-state response and auditory brainstem response in healthy term babies. J Int Adv Otol 2016;12:277–81. 10.5152/iao.2016.2397 [DOI] [PubMed] [Google Scholar]
- 46. Arslan S, Işik AÜ, Imamoǧlu M, et al. Universal newborn hearing screening; automated transient evoked otoacoustic emissions. B-ENT 2013;9:123–31. [PubMed] [Google Scholar]
- 47. Arjmandi F, Farhangfar B, Mehrabi S, et al. Prevalence of deafness and hearing screening in newborns in isfahan. J Res Med Sci 2012;17:S233–6. [Google Scholar]
- 48. Islami Z, Baradaranfar M-H, Mehrparvar A-H, et al. Frequency of hearing impairment among full-term newborns in yazd, iran. Iran J Pediatr 2013;23:349–52. [PMC free article] [PubMed] [Google Scholar]
- 49. Azizi A, Amirian F, Dargahi A, et al. Evaluation of universal newborn hearing screening with TEOAE and ABR: aa cross-sectional study with the literature review. Int J Trop Med 2016;11:84–9. 10.36478/ijtmed.2016.84.89 [DOI] [Google Scholar]
- 50. Gupta S, Sah S, Som T, et al. Challenges of implementing universal newborn hearing screening at a tertiary care centre from India. Indian J Pediatr 2015;82:688–93. 10.1007/s12098-015-1688-4 [DOI] [PubMed] [Google Scholar]
- 51. Çıkrıkçı S, Deni Z H, Gülşen S. Comparison of hearing screening results of syrian refugees and turkish newborns. Int J Pediatr Otorhinolaryngol 2020;135:110095. 10.1016/j.ijporl.2020.110095 [DOI] [PubMed] [Google Scholar]
- 52. Shang Y, Hao W, Gao Z, et al. An effective compromise between cost and referral rate: a sequential hearing screening protocol using teoaes and aabrs for healthy newborns. Int J Pediatr Otorhinolaryngol 2016;91:141–5. 10.1016/j.ijporl.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 53. Guomei C, Luyan Z, Lingling D, et al. Concurrent hearing and genetic screening among newborns in ningbo, China. Comput Math Methods Med 2022;2022:1713337. 10.1155/2022/1713337 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54. Sinha V, Mathur R, Jindal S, et al. Universal hearing screening vs targetted hearing screening: make a choice. Indian J Otol 2015;21:179. 10.4103/0971-7749.161023 [DOI] [Google Scholar]
- 55. Tasci Y, Muderris II, Erkaya S, et al. Newborn hearing screening programme outcomes in a research Hospital from turkey. Child Care Health Dev 2010;36:317–22. 10.1111/j.1365-2214.2009.01029.x [DOI] [PubMed] [Google Scholar]
- 56. Ulusoy S, Ugras H, Cingi C, et al. The results of national newborn hearing screening (NNHS) data of 11,575 newborns from west part of turkey. Eur Rev Med Pharmacol Sci 2014;18:2995–3003. [PubMed] [Google Scholar]
- 57. Kemaloğlu YK, Gökdoğan Ç, Gündüz B, et al. Newborn hearing screening outcomes during the first decade of the program in a reference hospital from turkey. Eur Arch Otorhinolaryngol 2016;273:1143–9. 10.1007/s00405-015-3654-1 [DOI] [PubMed] [Google Scholar]
- 58. Yorulmaz A, Genç U, Yılmaz FH, et al. Evaluation and importance of our newborn hearing screening results. Haseki 2017;55:111–8. 10.4274/haseki.3469 [DOI] [Google Scholar]
- 59. Öztürk SEA, Aktaş S, Karakurt LT, et al. The follow-up results of newborn hearing screening of gaziosmanpasa taksim research and training hospital. Turk Pediatri Ars 2018;53:10–6. 10.5152/TurkPediatriArs.2018.5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hamdi A. Evaluation of 1808 newborns hearing screening outcome. EJMO 2018. 10.14744/ejmo.2018.58076 [DOI] [Google Scholar]
- 61. Haghshenas M, Zadeh P, Javadian Y, et al. Auditory screening in infants for early detection of permanent hearing loss in northern Iran. Ann Med Health Sci Res 2014;4:340–4. 10.4103/2141-9248.133456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rahimi V, Mohammadkhani G, Javadi F. Improving universal newborn hearing screening outcomes by conducting it with thyroid screening. Int J Pediatr Otorhinolaryngol 2018;111:111–4. 10.1016/j.ijporl.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 63. Rai N, Thakur N. Universal screening of newborns to detect hearing impairment--is it necessary? Int J Pediatr Otorhinolaryngol 2013;77:1036–41. 10.1016/j.ijporl.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 64. Ahmad A, Mohamad I, Mansor S, et al. Outcome of a newborn hearing screening program in a tertiary hospital in Malaysia: the first five years. Ann Saudi Med 2011;31:24–8. 10.4103/0256-4947.75774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yücel A, Alataş N, Yücel H, et al. Newborn hearing screening results of refugees living in our city and the factors affecting the results. Int J Pediatr Otorhinolaryngol 2019;123:187–90. 10.1016/j.ijporl.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 66. Tuli I, Pal I, Sengupta S, et al. Role of early audiological screening and intervention. Indian J Otol 2012;18:148. 10.4103/0971-7749.103443 [DOI] [Google Scholar]
- 67. Chadha SK, Sayal A, Malhotra V, et al. Prevalence of preventable ear disorders in over 15,000 schoolchildren in northern India. J Laryngol Otol 2013;127:28–32. 10.1017/S0022215112002691 [DOI] [PubMed] [Google Scholar]
- 68. Shekhar H, Khokhar A, Motwani G, et al. Prevalence of ear morbidities among school children in delhi, india: a cross-sectional study. Int J Adolesc Med Health 2022;34:289–95. 10.1515/ijamh-2020-0088 [DOI] [PubMed] [Google Scholar]
- 69. Lü J, Huang Z, Yang T, et al. Screening for delayed-onset hearing loss in preschool children who previously passed the newborn hearing screening. Int J Pediatr Otorhinolaryngol 2011;75:1045–9. 10.1016/j.ijporl.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 70. Wu W, Lü J, Li Y, et al. A new hearing screening system for preschool children. Int J Pediatr Otorhinolaryngol 2014;78:290–5. 10.1016/j.ijporl.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 71. Kam ACS, Li LKC, Yeung KNK, et al. Automated hearing screening for preschool children. J Med Screen 2014;21:71–5. 10.1177/0969141314528322 [DOI] [PubMed] [Google Scholar]
- 72. Tokgöz-Yılmaz S, Özcebe E, Türkyılmaz MD, et al. Evaluation of hearing and speech-language in preschool children: how important, why we should perform? Turk J Pediatr 2013;55:606–11. [PubMed] [Google Scholar]
- 73. Jalali MM, Nezamdoust F, Ramezani H, et al. Prevalence of hearing loss among school-age children in the North of Iran. Iran J Otorhinolaryngol 2020;32:85–92. 10.22038/ijorl.2019.36090.2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Alaqrabawi WS, Alshawabka AZ, Al-Addasi ZM, et al. What are the predictive causes of conductive hearing loss in school-age children in jordan? Jordan Med J 2016;50:187–94. [Google Scholar]
- 75. Al-Obeidy SH, Abdulrahman ZN, Zaradwy IAR. School-entry screening program for ear and hearing problems in tikrit, Iraq. ME-JFM 2019;17:91–4. 10.5742/MEWFM.2019.93717 [DOI] [Google Scholar]
- 76. Ramkumar V, Nagarajan R, Shankarnarayan VC, et al. Implementation and evaluation of a rural community-based pediatric hearing screening program integrating in-person and tele-diagnostic auditory brainstem response (ABR). BMC Health Serv Res 2019;19:1. 10.1186/s12913-018-3827-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ramkumar V, John KR, Selvakumar K, et al. Cost and outcome of a community-based paediatric hearing screening programme in rural India with application of tele-audiology for follow-up diagnostic hearing assessment. Int J Audiol 2018;57:407–14. 10.1080/14992027.2018.1442592 [DOI] [PubMed] [Google Scholar]
- 78. TarvijEslami S, Nassirian H, Bayesh S. Impact on performance of hearing screening program through prevalence and diagnostic age evaluation in elementary school students in north-eastern city of Iran, Mashhad. Pediatria Polska 2017;92:705–10. 10.1016/j.pepo.2017.07.005 [DOI] [Google Scholar]
- 79. Kaplama ME, Ak S. The results of hearing screening in refugee school children living in şanliurfa /turkey and the related risk factors. Int J Pediatr Otorhinolaryngol 2020;134:110041. 10.1016/j.ijporl.2020.110041 [DOI] [PubMed] [Google Scholar]
- 80. Chen G, Fu S, Luo S, et al. Screening of delayed-onset hearing loss in preschool children in the mid-south of china. Int J Audiol 2013;52:568–71. 10.3109/14992027.2013.796408 [DOI] [PubMed] [Google Scholar]
- 81. Paul AK. Early identification of hearing loss and centralized newborn hearing screening facility-the cochin experience. Indian Pediatr 2011;48:355–9. 10.1007/s13312-011-0067-0 [DOI] [PubMed] [Google Scholar]
- 82. Kumar A, Gupta SC, Sinha VR. Universal hearing screening in newborns using otoacoustic emissions and brainstem evoked response in eastern Uttar Pradesh. Indian J Otolaryngol Head Neck Surg 2017;69:296–9. 10.1007/s12070-017-1081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tobe RG, Mori R, Huang L, et al. Cost-effectiveness analysis of a national neonatal hearing screening program in China: conditions for the scale-up. PLoS One 2013;8:e51990. 10.1371/journal.pone.0051990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Saki N, Bayat A, Hoseinabadi R, et al. Universal newborn hearing screening in southwestern iran. Int J Pediatr Otorhinolaryngol 2017;97:89–92. 10.1016/j.ijporl.2017.03.038 [DOI] [PubMed] [Google Scholar]
- 85. Ramesh A, Jagdish C, Nagapoorinima M, et al. Low cost calibrated mechanical noisemaker for hearing screening of neonates in resource constrained settings. Indian J Med Res 2012;135:170–6. [PMC free article] [PubMed] [Google Scholar]
- 86. Sharma Y, Bhatt SH, Nimbalkar S, et al. Non-compliance with neonatal hearing screening follow-up in rural Western India. Indian Pediatr 2018;55:482–4. 10.1007/s13312-018-1338-9 [DOI] [PubMed] [Google Scholar]
- 87. Sachdeva K, Sao T. Outcomes of newborn hearing screening program: a hospital based study. Indian J Otolaryngol Head Neck Surg 2017;69:194–8. 10.1007/s12070-017-1062-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Swain SK, Das A, Sahu MC, et al. Neonatal hearing screening: our experiences at a tertiary care teaching hospital of eastern India. Pediatria Polska 2017;92:711–5. 10.1016/j.pepo.2017.08.002 [DOI] [Google Scholar]
- 89. Bishnoi R, Baghel S, Agarwal S, et al. Newborn hearing screening: time to act! Indian J Otolaryngol Head Neck Surg 2019;71:1296–9. 10.1007/s12070-018-1352-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Farhat AS, Ghasemi MM, Akhondian J, et al. Assessment of the prevalence of hearing impairment in neonates born in imam reza, ghaem and OM-albanin hospitals of mashhad. Iran J Neonatol 2014;5:17–20. 10.22038/IJN.2014.2642 [DOI] [Google Scholar]
- 91. Verma PK, Chopra D, Khwaja M, et al. Prevalence of hearing impairment in school children in aa rural area of lucknow- aa cross sectional study. Int J Pharm Clin Res 2022;14:80–4. 10.1007/s12070-019-01651-9 [DOI] [Google Scholar]
- 92. Yoshinaga-Itano C, Manchaiah V, Hunnicutt C. Outcomes of universal newborn screening programs: systematic review. J Clin Med 2021;10:2784. 10.3390/jcm10132784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kanji A, Khoza-Shangase K, Moroe N. Newborn hearing screening protocols and their outcomes: a systematic review. Int J Pediatr Otorhinolaryngol 2018;115:104–9. 10.1016/j.ijporl.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 94. Bright K, Greeley CO, Eichwald J. American academy of audiology childhood hearing screening guidelines. Reston, VA: American Academy of Audiology Task Force, 2011. [Google Scholar]
- 95. Childhood hearing screening. Available: https://www.asha.org/practice-portal/professional-issues/childhood-hearing-screening/ [Accessed 07 Oct 2022].
- 96. Poonual W, Navacharoen N, Kangsanarak J, et al. Hearing loss screening tool (cobra score) for newborns in primary care setting. Korean J Pediatr 2017;60:353–8. 10.3345/kjp.2017.60.11.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bussé AML, Mackey AR, Carr G, et al. Assessment of hearing screening programmes across 47 countries or regions III: provision of childhood hearing screening after the newborn period. Int J Audiol 2021;60:841–8. 10.1080/14992027.2021.1897170 [DOI] [PubMed] [Google Scholar]
- 98. World Health Organization . Hearing screening: considerations for implementation; 2021.
- 99. Butcher E, Dezateux C, Cortina-Borja M, et al. Prevalence of permanent childhood hearing loss detected at the universal newborn hearing screen: systematic review and meta-analysis. PLoS ONE 2019;14:e0219600. 10.1371/journal.pone.0219600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Colgan S, Gold L, Wirth K, et al. The cost-effectiveness of universal newborn screening for bilateral permanent congenital hearing impairment: systematic review. Acad Pediatr 2012;12:171–80. 10.1016/j.acap.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Neumann K, Chadha S, Tavartkiladze G, et al. Newborn and infant hearing screening facing globally growing numbers of people suffering from disabling hearing loss. IJNS 2019;5:7. 10.3390/ijns5010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Olusanya B. Screening for neonatal deafness in resource-poor countries: challenges and solutions. RRN 2015:51. 10.2147/RRN.S61862 [DOI] [Google Scholar]
- 103. Galhotra A, Sahu P. Challenges and solutions in implementing hearing screening program in India. Indian J Community Med 2019;44:299–302. 10.4103/ijcm.IJCM_73_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Biswas AK, Goswami SC, Baruah DK, et al. The potential risk factors and the identification of hearing loss in infants. Indian J Otolaryngol Head Neck Surg 2012;64:214–7. 10.1007/s12070-011-0307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kumar P, Adhisivam B, Vishnu Bhat B, et al. Screening for hearing loss among high risk neonates– experience from a tertiary care center. Current Pediatric Research 2016;20:43–6. [Google Scholar]
- 106. Zhang Z, Ding W, Liu X, et al. Auditory screening concurrent deafness predisposing genes screening in 10,043 neonates in Gansu province, China. Int J Pediatr Otorhinolaryngol 2012;76:984–8. 10.1016/j.ijporl.2012.03.016 [DOI] [PubMed] [Google Scholar]
- 107. Poonual W, Navacharoen N, Kangsanarak J, et al. Risk factors for hearing loss in infants under universal hearing screening program in Northern Thailand. J Multidiscip Healthc 2016;9:1–5. 10.2147/JMDH.S92818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baradaranfar MH, Mehrparvar AH, Mostaghaci M, et al. Hearing abnormality in neonate intensive care unit (NICU) yazd-iran. Int J Pediatr 2014;2:113–7. 10.22038/ijp.2014.2345 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2022-001752supp001.pdf (80.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.