Abstract
Background
Colorectal carcinoma is one of the most commonly diagnosed malignancies worldwide. Consumption of dietary supplements and nutraceuticals such as phenolic compounds may help combat colorectal carcinoma. The effect of two phenolic-rich extracts prepared from biotransformed grape pomace on the antioxidant properties and antiproliferative activity against two colorectal cancer cell lines (Caco-2 and SW620) were investigated.
Methods
A 15-day solid-state fermentation with the white-rot fungi Phanerochaete chrysosporium and Trametes gibbosa was used to biotransform grape pomace. Solid-liquid extraction was then performed to extract bioactive compounds. The extract was analyzed for the determination of phenolic compounds by ultra-high performance liquid chromatography and in vitro assays of biological activities (antioxidant activity, antiproliferative activity, cell cycle analysis).
Results
The 4 days of solid-state fermentation proved to be the optimal period to obtain the maximum yield of phenolic compounds. The tested extracts showed significant antioxidant and antiproliferative activities. Grape pomace treated with P. chrysosporium and T. gibbosa reduced cancer cell growth by more than 60% at concentrations (solid/liquid ratio) of 1.75 mg/mL and of 2.5 mg/mL, respectively. The cell cycle perturbations induced by the grape pomace extracts resulted in a significant increase in the number of cells in the S (9.8%) and G2/M (6.8%) phases of SW620 exposed to T. gibbosa after 48 hours, while P. chrysosporium increased the percentage of cells in the G1 phase by 7.7%. The effect of grape pomace extracts on Caco-2 was less pronounced.
Conclusions
The obtained results suggest the presence of bioactive compounds in biotransformed grape pomace as a residue from winemaking, which could be used to prevent colon cancer.
Keywords: Wine production residues, Phenolic compounds, Solid-state fermentation, Bioactivity, Colorectal cancer cell lines
Introduction
Colorectal carcinoma is the second deadliest and third most commonly diagnosed malignancy [1, 2] with over 1.9 million new cases (10% of all new cases regardless of gender and age) and more than 935,000 deaths in 2018 according to Bray et al. [3]. Although colorectal cancer has multifactorial causes, most of them are related to poor dietary habits. Among dietary supplements, phenolic compounds represent the most interesting and well-studied class of compounds that can be used as therapeutics for a variety of common diseases, including cancer [4]. In recent years, numerous studies [5–7] have shown a protective effect of plant phenolic compounds against colorectal carcinoma. The use of agro-food industry side streams to produce a wide range of value-added products, including phenolic compounds [8–11], is one of the key strategies of the biorefinery paradigm towards a sustainable circular bioeconomy. In this context, the cascade use of certain biomass is of great importance, as in this way several different products can be produced from the same waste feedstock within the same or different biorefineries [12]. Emphasis is placed on the introduction of environmentally friendly and cost-effective technologies to achieve zero-waste production, and on the development and commercialization of novel biobased formulations [13].
Grape pomace (GP) is the residue generated during wine production and consist mainly of the skin, pulp, seeds, and stems. It is a source of bioactive phenolic compounds, including flavonoids (flavan-3-ols, anthocyanins, and flavanols) and non-flavonoids (phenolic acids), which are being studied in the context of preventive measures against cardiovascular diseases and tumours, and in the treatment of nervous and brain disorders and arthritis [14, 15]. Due to the antioxidant and anti-inflammatory properties of these compounds, the extracts of GP may have a number of beneficial effects in health care [16–18]. Flavonoids have various anticancer effects, including introduction of apoptosis, cell cycle arrest, antiproliferative, antioxidative, antiangiogenic, and anti-metastatic activities against many human cancer cell lines [4]. Previous research suggests that grape seed extracts have a preventive effect on colorectal carcinoma due to their phenolic compounds and antioxidant activity, as they can scavenge reactive oxygen and nitrogen species, thereby reducing oxidative stress, increasing epithelial barrier integrity, and reducing inflammation in the gut [19, 20]. In addition, extracts of GP and/or grape stems may be effective in preventing certain cancers (prostate, leukaemia, breast, skin, and colon) through mechanisms such of inhibition of cell proliferation, cell cycle arrest, induction of apoptosis, and interruption of intracellular signal transduction [17, 21]. It is well known that the functionality of phenolic compounds is limited by the fact that many of them, such as polymers, glycosides and esters, are not always bioavailable. Enzymatic hydrolysis and bioprocesses such as fermentation can result in the release of phenolic components from their conjugates, leading to increased bioactivity [22]. The bioprocess that has been shown to be effective in releasing large amounts of phenolic compounds and increasing their bioactivity is solid-state fermentation (SSF) [23]. Due to its economic and sustainable properties, it has been widely explored and applied to obtain high-value compounds from organic wastes [24]. Usually, white-rot fungi are used in SSF, which produce a complex system of lignolytic enzymes during their growth, in which enzymatic hydrolysis occurs, free phenolic compounds are released, and those with lower molecular weight are formed, which contribute to the enhancement of antioxidant activity [24–26]. In this study, the antioxidant properties and antiproliferative properties of extracts from biologically treated GP were investigated on two human colon carcinoma cell lines (Caco-2, SW620). As far as we know, this is the first study in this field conducted with GP extracts obtained after SSF of GP with Phanerochaete chrysosporium and Trametes gibbosa. This research is intended to contribute to the cascading use of GP, as the same sample has already been used in our other extensive research to produce enzymes, biofuels, and animal feed.
Materials and methods
Standards and reagents
Ethanol (analytical grade) and sodium acetate were purchased from Gram-Mol Ltd. (Zagreb, Croatia), methanol (ultra-gradient grade) from J.T. Baker (Arnhem, The Netherlands), acetonitrile (HPLC grade) from Fisher Chemical (Loughborough, UK) and glacial acetic acid from Macron Fine Chemicals (Gliwice, Poland). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), ammonium persulfate, catechin, epicatechin, gallocatechin gallate, epicatechin gallate, gallic acid, ellagic acid, resveratrol, syringic acid, kaempferol, o-coumaric acid, ferulic acid, p-coumaric acid, p-hydroxybenzoic acid, and caffeic acid were purchased from Sigma Aldrich (Saint Louis, USA). Iron(III)chloride hexahydrate was purchased from Merck (Darmstadt, Germany). Procyanidin B1 and procyanidin B2 were purchased from Extrasynthese (Genay, France). Quercetin, protocatechuic acid and vanillic acid were purchased from Acros Organics (Geel, Belgium). Hydrochloric acid was purchased from Carlo Erba Reagents GmbH (Emmendingen, Germany). Dulbecco′s Modified Eagle′s Medium (DMEM) containing glutamine and fetal bovine serum (FBS) was purchased by GIBCO (EU), whereas trypan blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI), and RNase A were purchased from Sigma Aldrich (MERCK, Darmstadt, Germany). Phosphate-buffered saline (PBS) was purchased from Capricorn Scientific (Ebsdorfergrund, Germany).
Substrate and microorganisms
GP of the variety Cabernet Sauvignon (Vitis vinifera L.) was purchased from a local winery (Erdut, Croatia, 2016 harvest) and stored at − 20 °C before use in the experiments. Before each experiment, GP was thawed for 24 hours at + 25 °C and coarsely ground (HR 2860, Philips). The dry matter content of GP, determined using a radiation-infrared dryer HR-73, Mettler Toledo (Switzerland) using the standard method, drying temperature at 105 °C and process termination criterion (switch off 3:1 mg to 50 s weight loss) to constant mass, ranged from 91.62 to 93.52%.
Two species of white-rot fungi, P. chrysosporium and T. gibbosa, were cultivated on potato dextrose agar medium at 27 °C for 7 days and used for biological treatment of GP.
Biological treatment of grape pomace by Phanerochaete chrysosporium and Trametes gibbosa
Fifty grams (50 g) of the prepared GP was mixed with 30 mL of distilled water in laboratory jars, autoclaved (121 °C / 20 min) and cooled overnight. In this way, the initial moisture of GP was adjusted to 60%, since the growth of white rot fungi and their metabolism under SSF conditions require a substrate humidity of 60–80%. SSF was performed to degrade the complex lignocellulosic structure of the GP to release the simple phenolic compounds.
After adjusting the moisture content of the substrate, GP was inoculated with 5 mycelial plugs of white rot fungi (diameter 1 cm) suspended in 10 mL of sterile distilled water. Experiments were performed separately for P. chrysosporium and T. gibbosa in triplicate. After inoculation, biological treatments were performed at + 27 °C for 2, 4, 10, and 15 days in an incubator with a blower set at 10% (KB 115, BINDER GmbH, Germany). The biological treatment was terminated by sterilization (121 °C / 20 min). Control samples from GP were prepared according to the same protocol but without inoculation with white rot fungi.
After treatment, all samples were dried at room temperature for 48 hours, then milled to 1 mm particle size using an ultracentrifugal mill (Retsch ZM200, Germany) and stored at + 4 °C until extraction and further analysis.
Preparation of grape pomace extracts
Solid-liquid extraction was performed to obtain GP extracts rich in phenolic compounds. 1 gram (1 g) of the biologically treated GP was extracted using 50% aqueous ethanol as solvent with a solid/liquid ratio (S/L) of 0.025 g/mL in a water bath (SW-23, Julabo, Germany) by shaking at 200 rpm and 80 °C for 120 min. After extraction, samples were centrifuged at 10000 x g for 10 min (Hermle Z 326 K, HERMLE Labortechnik GmbH, Germany). Experiments were performed in duplicate. Extracts of untreated GP (control) were prepared according to the same protocol. The obtained liquid extracts were used for further analysis.
Determination of phenolic compounds by ultra-high performance liquid chromatography
Quantification of individual phenolic compounds (flavan-3-ols, flavonols, stilbenes, and phenolic acids) in the liquid extracts of GP was performed by ultra-high performance liquid chromatography (UHPLC) according to the previously published method [26]. Photodiode array detection was performed by scanning between 252 and 370 nm. Separation was performed using a reversed phase Kinetex® C18 core-shell column (100 × 4.6 mm, 2.6 μm, Phenomenex). Analysis was performed in triplicate and results were expressed as mass of individual polyphenolic compounds per dry weight of grape pomace (mg/gdb). The term total phenolic compound content determined by the UHPLC method (TPC) refers to the sum of the compounds quantified in this study.
In vitro assays of the biological activities of the grape pomace extract
Determination of antioxidant activity (AA)
The antioxidant activity of the extracts of GP, which were biologically treated with P. chrysosporium and T. gibbosa for 4 days, was determined by DPPH, FRAP, and ABTS assays using a UV-VIS spectrophotometer (UV/VIS Spectrophotometer UV-1800, Shimadzu, Japan). The DPPH assay was performed according to the method of Bucić-Kojić et al. [27]. The FRAP assay was performed according to the method of Benzie and Strain [28] with some modifications. Briefly, the samples were prepared by mixing 2.7 mL of FRAP reagent, 270 μL of distilled water and 150 μL of extract, and the absorbance was read at 592 nm after 40 min of incubation in the dark at 37 °C. The blank sample was prepared in the same way but distilled water was used instead of extract. The ABTS assay was performed according to the method of Re et al. [29], but with modifications. Briefly, 950 μl of a diluted ABTS• + radical solution was added to 50 μl of the extracts. The absorbance was measured after 10 min of incubation in the dark at 734 nm. The control sample was prepared in the same way, but ethanol was used instead of the sample. Absolute ethanol was used as a blank. All tests were performed in triplicate, and results were expressed in Trolox equivalents per dry basis of GP (gTROLOX/gdb).
Cell culturing
The antiproliferative effect of the extracts prepared from GP biologically treated with P. chrysosporium and T. gibbosa for 4 days was tested on two human colon carcinoma cell lines: Caco-2 (ATCC® HTB-37™) and SW-620 (ATCC® CCL-227™). Cells were cultured in DMEM supplemented with 10% FBS and 2 mM glutamine in tissue culture flasks (BD Falcon, Germany) in humidified atmosphere under the conditions of 37 °C / 5% CO2 gas in CO2 incubator (Shell Lab, Sheldon Manufacturing, USA). The trypan blue exclusion method was used to assess the viability of the cells.
Determination of antiproliferative activity
Extracts from GP were filtered through a 0.22 μm syringe filter and prepared in sterile deionized water at a concentration (S/L ratio) of 0.25 mg/mL, 1.00 mg/mL, 1.75 mg/mL, and 2.5 mg/mL.
The antiproliferative effect of the extracts of GP was determined by cell viability using the MTT assay [30]. Cells were seeded in 96-well flat-bottomed plates (Greiner, Frickenhausen, Austria) at a concentration of 2 × 104 cells/mL and left overnight in the CO2 incubator to attach to the plate surface. 72 hours (72 h) after addition of the extracts from GP, the growth medium was discarded and 5 mg/mL MTT was added. After 4 hours of incubation at 37 °C, the water-insoluble MTT formazan crystals were dissolved in DMSO. The absorbance was measured at 595 nm using an Elisa microplate reader (iMark, BIO RAD, Hercules, CA, USA). Control cells were grown under the same conditions. Blank means medium without cells containing MTT. All experiments were performed at least three times in triplicate. The percentage of cell viability (CV, %) was calculated according to eq. (1):
| 1 |
GI50 value, defined as compound concentration (mg/mL) that results in a 50% reduction in cell viability, was calculated and used as a parameter for comparing the inhibitory effect between GP samples.
Cell cycle analysis
Cells (1 × 105 cells/mL) were seeded in 6-well plates (Falcon, Durham, SAD). After 24 hours GP extracts were added at concentrations (S/L ratio) corresponding to GI50 (Table 1) and allowed to stand for 48 hours. Floating and adherent cells were collected separately, combined, washed with PBS fixed with 70% ethanol and stored at − 20 °C until analysis. Cell pellets were washed twice with PBS, resuspended in 1 mg/mL of PI and 0.2 mg/mL RNase A, and left in dark/cold atmosphere for 30 min. The stained cells were analysed using FACSanto II (Becton Dickinson, USA) flow cytometer (20.000 cells were analysed). The assays were performed in duplicate and repeated twice. FlowJo software, version 10.2, with the implementation of the Watson model was used to analyse the DNA histograms.
Table 1.
GI50 concentration (S/L ratio) of GP extracts applied to the cell cycle of Caco-2 and SW620 for 48 hours
| Cell line | Treatmenta | GI50 (mg/mL) |
| Caco-2 | PC | 1.08 |
| TG | 0.91 | |
| SW620 | PC | 0.98 |
| TG | 1.16 |
a TG - biological treatment by T. gibbosa; PC - biological treatment by P. chrysosporium
Statistical analysis
STATISTICA 13.5 (TIBCO Software Inc., Tulsa, USA) was used for statistical analysis of the results. All results were expressed as mean values. The mean values of phenolic compound content and antioxidant activity of the tested extracts were compared using Student’s t-test at a confidence level of at least 95%. Student’s t-test and non-parametric Mann-Whitney U-test were used to compare MTT data and cell data. Differences were considered statistically significant at p < 0.05.
Results and discussion
Phenolic content of biologically treated grape pomace
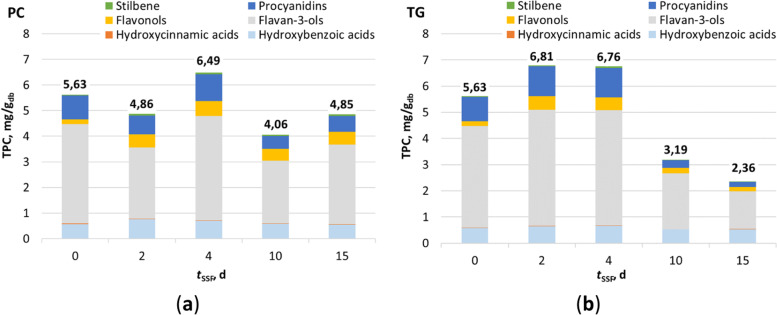
The biological treatment of GP was carried out under the initial assumption that the white-rot fungi tested synthesize enzymes during their growth at GP that degrade the lignocellulose structure and release simpler phenolic compounds that are more accessible for extraction. Figure 1 shows the content of total phenolic compounds (TPC) in extracts prepared from GP biologically treated with P. chrysosporium and T. gibbosa for 15 days. Day “0” represents a control sample that was not biologically treated with white-rot fungi. Flavan-3-ols, representing the sum of the catechin, epicatechin, gallocatechin gallate, and epicatechin gallate content, were the most abundant group of phenolic compounds (57.10–68.87% of TPC) in all GP extracts tested, followed by hydroxybenzoic acids (10.19–22.74% of TPC), procyanidins (8.31–16.84% of TPC) and flavonols (3.26–11.60% of TPC). The hydroxybenzoic acids include the sum of the contents of gallic acid, ellagic acid, syringic acid, protocatechuic acid, vanillic acid, and p-hydroxybenzoic acid. Procyanidins comprise the sum of the content of the oligomers procyanidin B1, and procyanidin B2 and flavonols represent the sum of the content of kaempferol and quercetin. Resveratrol (0.81–1.33% of TPC), representing a group of stilbenes, and hydroxycinnamic acids (0.19–0.43% of TPC), comprising the sum of the contents of ferulic acid, o-coumaric acid, p-coumaric acid, and caffeic acid, were determined in small amounts and with smaller contributions to TPC than the above phenolic compounds.
Fig. 1.
Total phenolic compound content (TPC) determined by UHPLC in extracts from GP, which where biologically treated with a P. chrysosporium (PC) and b T. gibbosa (TG) during the 15 days
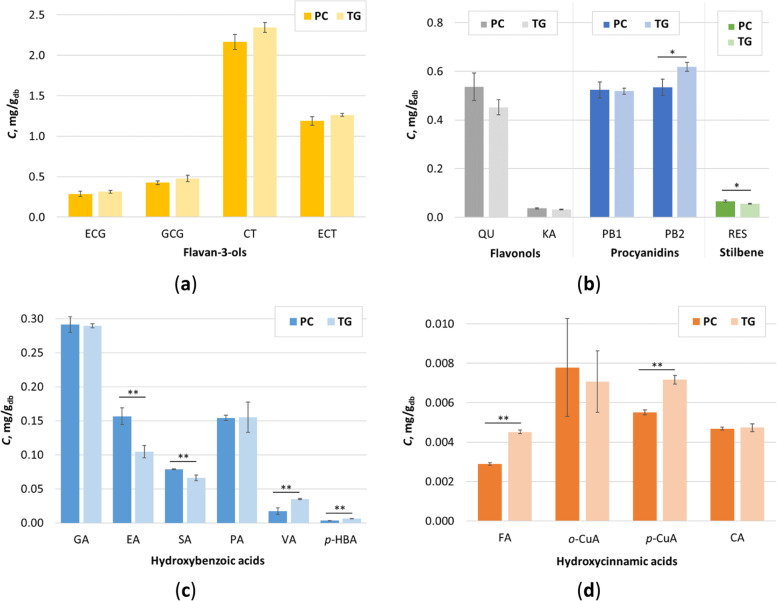
In both cases (Figure 1), it can be seen that SSF with white-rot fungi had a positive effect on the release of phenolic compounds and their extractability. In the case of P. chrysosporium, the highest TPC (6.49 mg/gdb) was reached after 4 days of fermentation, while in the case of T. gibbosa, the highest TPC was reached after 2 days (6.81 mg/gdb) of biological treatment. Compared to the control sample (5.63 mg/gdb), the observed increase in TPC was statistically significant (p < 0.05). It was also found that there was no statistically significant (p < 0.05) difference between the TPC of the samples treated with T. gibbosa for 2 and 4 days. Therefore, the GP samples treated with P. chrysosporium and T. gibbosa for 4 days under SSF conditions were selected for further studies to determine the biological activity (antioxidant and antiproliferative activities) of the GP extracts. 19 individual phenolic compounds were identified in the extracts obtained from GP after the fourth day of biological treatment by P. chrysosporium and T. gibbosa (Fig. 2).
Fig. 2.
Content of individual phenolic compounds (C) determined by UHPLC in extracts obtained from biologically treated GP by P. chrysosporium (PC) and T. gibbosa (TG) after 4 days of fermentation: a flavan-3-ols; b flavonols; c hydroxybenzoic acids and d hydroxycinnamic acids. Error bars correspond to SD. (*) - statistically significant difference between 95% extracts at confidence level (Student’s t-test); (**) - statistically significant difference between extracts at 99% confidence level (Student’s t-test)
Catechin was the dominant single phenolic compound in both extracts (2.17 mg/gdb for P. chrysosporium and 2.34 mg/gdb for T. gibbosa) followed by epicatechin (1.19 mg/gdb for P. chrysosporium and 1.26 mg/gdb for T. gibbosa) (Fig. 2a). These values are higher than those published by Negro et al. [31] for the biologically untreated GP variety Cabernet Sauvignon. In our study, other compounds were detected at lower concentrations and generally did not follow the same trend in both extracts (Fig. 2a-d). This can be explained by the specificity of each SSF process, which depends on a number of factors, but mainly on the microorganism and the properties of the substrate used. Statistical analysis (Student’s t-test) showed that there was no statistically significant difference between the determined contents of catechin, epicatechin, epicatechin gallate, gallocatechin gallate, quercetin, kaempferol, procyanidin B1, gallic acid, protocatechuic acid o-coumaric acid, and caffeic acid in both extracts. On the other hand, the content of procyanidin B2 and resveratrol in the extracts from GP treated with P. chrysosporium and T. gibbosa was statistically significant at 95% confidence level, and the content of ellagic acid, syringic acid, vanillic acid, p-hydroxybenzoic acid, ferulic acid, and p-coumaric acid was statistically significant at 99% confidence level (Fig. 2a-d).
Antioxidant activity of biologically treated grape pomace extracts
Phenolic compounds contribute to antioxidant activity and have a positive effect on human health by reducing the risk of developing chronic diseases such as cancer, diabetes, inflammatory and cardiovascular diseases. The antioxidant activity of phenolic compounds depends on their degree of hydroxylation and conjugation [32]. The antioxidant activity evaluated by the three assays (DPPH, ABTS, and FRAP) showed a similar trend for the extracts obtained after the fourth day of biological treatment of GP by P. chrysosporium and T. gibbosa, although it can be seen in Fig. 3 that the extracts obtained after SSF with PC showed a slightly lower AA. Student’s t-test showed that there is no statistically significant difference of AA between the tested extracts at 95% and 99% confidence level for the same assay. Dulf et al. [33] found an increase in antioxidant activity when GP was biologically treated with the filamentous fungi Actinomucor elegans (21.42% after 4 days SSF) and Umbelopsis isabellina (16% after 8 days SSF). Martínez-Ávila et al. [34] found that the SSF of GP with Aspergillus niger could improve the antioxidant activity of GP extracts by 5 to 20% during 18 hours. They observed that the decrease in antioxidant activity after 18 hours of biological treatment coincided with a decrease in gallic acid content. Leite et al. [35] showed that the antioxidant activity of extracts from winemaking residues (exhausted grape marc and vine trimming shoots) was most affected by SSF with Rhizopus oryzae among all filamentous fungi tested, while SSF with Aspergillus niger resulted in the highest antioxidant activity of extracts from grape stalks. Certain phenolic compounds can be bound to the structure of hemicellulose and lignin, and the action of fungal enzymes during SSF can influence the release of free phenolic compounds and consequently the increase of antioxidant activity [35]. Antioxidant activity can be correlated with the phenolic content of the extract and often results from the synergistic action of active substances. Therefore, the phenolic profile of the extract determines its efficacy, as the individual activity of the represented substances can vary greatly [36]. Since phenolic compounds have high antioxidant activity and their content changes during biological treatment, this also affects the radical scavenging properties of the fermented GP. The fact that antioxidants affect numerous important signaling pathways and targets associated with antiproliferative effects is well recognized, and numerous studies have been devoted to investigating the utility of certain bioactive compounds and their effectiveness as inhibitors of colon cancer cell growth [37, 38].
Fig. 3.
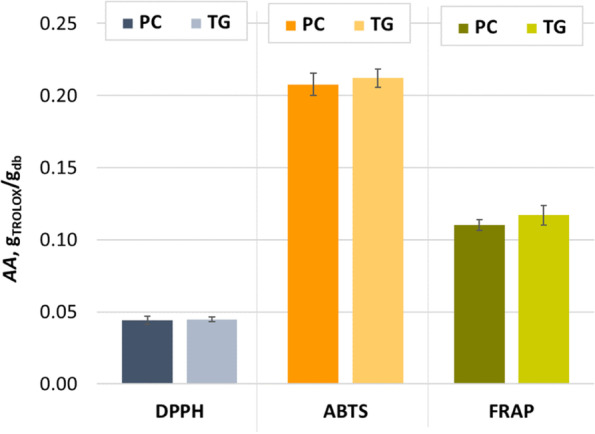
Antioxidant activities (AA) of extracts from GP after the fourth day of biological treatment by P. chrysosporium (PC) and T. gibbosa (TG) measured by DPPH, ABTS, and FRAP methods. Histogram bars represent the mean of six measurements and error bars represent SD
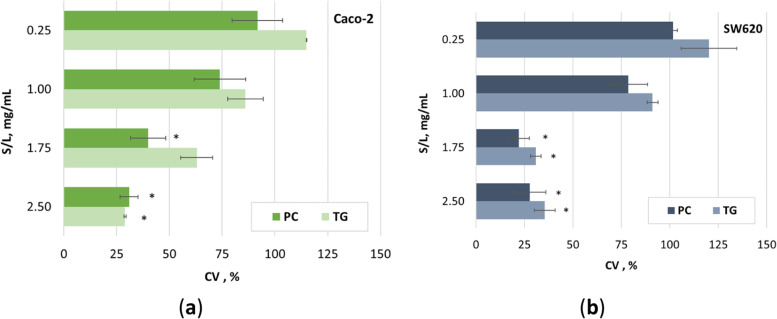
Antiproliferative effect of biologically treated grape pomace extracts
Phenolic compounds such as catechin, resveratrol, gallic acid, etc. are known to be radical scavengers, have antiproliferative effects on tumors, and protect membranes from oxidation. Numerous studies have demonstrated the key role of cancer therapy in identifying compounds that induce cell apoptosis and play a key role in eliminating mutated hyperproliferating cells from the system. Phenolic compounds, with their antiproliferative and antitumor activities, are involved in the proliferation, differentiation, and apoptosis of various cancer cells, including colon cancer [4, 39]. Also very important in terms of chemoprevention by phenolic compounds is their differential effect on cancer cells but not on normal cells [40]. In this study, the inhibitory effect of GP extracts on cell growth of two colon cancer cell lines was investigated. The results showed a difference in efficacy in cell growth regression between Caco-2 and SW620 cells depending on the concentration of the extract and the biological treatment (Fig. 4). Both types of colon cancer cell lines were more sensitive to the presence of extracts prepared from P. chrysosporium-treated GP than to extracts prepared from T. gibbosa-treated GP at the same concentration (S/L ratio), except at the highest concentration (2.5 mg/mL), where a slightly lower cell viability of Caco-2 cells was observed in the presence of extracts prepared from T. gibossa-treated GP. An inhibitory effect on Caco-2 cell growth was observed for GP treated with P. chrysosporium at the two highest concentrations (S/L ratio), 1.75 and 2.5 mg/mL, resulting in a 60.1 and 69.1% reduction in cell growth, respectively. On the other hand, the greatest effect on reducing Caco-2 cell viability was observed when Caco-2 cells were treated with T. gibbosa-treated extract GP at a concentration of 2.5 mg/mL, resulting in an inhibitory effect of 71.0%. Compared to the control sample, these three effects were statistically significant (p < 0.05) (Fig. 4a). Martins et al. [22] studied the antiproliferative effect of biotransformed grape pomace by tannase on Caco-2 cells and their results are less striking than ours, since they studied a lower concentration (100–500 μg/mL). Dükel et al. [41] investigated the antiproliferative effects of flavonoids (catechin, epicatechin, and naringenin) in colon cancer cells (Caco-2, DLD-1, and SW620) and normal colon epithelial cells (CCD18Co) and found that all flavonoids showed cytotoxic effects on all cell lines studied at a treatment of less than 25 μM according to IC50 values. In addition, all flavonoids used showed high cytotoxicity against the Caco-2 adenocarcinoma cell line at low concentrations, while naringenin inhibited proliferation in the SW620 metastatic cell line at low concentration (IC50 11 ± 1.9 μM). The study conducted by Parry et al. [21] investigated the cytotoxic activity of GP on Caco-2 without biotransformation. Their results suggest a less pronounced antiproliferative activity compared with our results. Possible reasons for the different results of the studies dealing with the antiproliferative effect on the Caco-2 cell line are the different geographical origin of the grape pomace, the manufacturing process, and the presence of a biotransformation step. Another possible reason for the different results is the form of the grape pomace extract studied. We tested liquid extracts without prior lyophilisation. All the previously mentioned reasons related to Caco-2 cells and GP origin are related to the cytotoxic results obtained for the extracts of GP on the SW620 cell line. However, the results are somewhat different compared to Caco-2 cells [21, 22]. Specifically, higher concentrations (S/L ratio) of both types of extracts (1.75 mg/mL and 2.5 mg/mL) showed a statistically significant (p < 0.05) inhibitory effect on SW620 cell growth, while lower concentrations (0.25 mg/mL; 1.00 mg/mL) had no effect on SW620 cell line growth (Fig. 4b). The extract of GP, treated with P. chrysosporium, had the greatest inhibitory effect at a concentration of 1.75 mg/mL (77.9%), followed by the same type of extract at a concentration of 2.5 mg/mL (72.3%), followed by GP extracts after treatment with T. gibbosa at a concentration of 1.75 mg/mL (69.1%) and 2.5 mg/mL (64.6%). Using the results of the antiproliferative assay, the growth inhibitory concentration (S/L ratio) leading to a 50% reduction in cell growth is calculated (GI50). These values were used to evaluate the effects of the extracts of GP on the cell cycle of Caco-2 and SW620 cells (Table 1).
Fig. 4.
Antiproliferative effect of extracts from GP after the fourth day of biological treatment by P. chrysosporium (PC) and T. gibbosa (TG) on: a Caco-2 and b SW620 cell line. Data are presented as percentage of cell viability (CV, %) as a function of concentration of the two different extracts. Histogram bars represent the mean of three independent experiments performed in triplicate, and error bars correspond to SD. Values marked with (*) are statistically significant (p < 0.05) with respect to the control group, as determined by the Mann-Whitney U-test
Further evaluation of the tested GP extracts in GI50 concentration (S/L ratio) showed an outstanding effect on SW620 and Caco-2 cell cycle after 48 hours of exposure. Both extracts cause different changes in cell cycle ratio compared to control and each other. GP treated with T. gibbosa reduces the percentage of SW620 cells in S phase by 9.8% and increases the percentage of cells in G2/M phase by 6.8% compared to control. GP treated with P. chrysosporium induces the change in G1 cell phase and increases the percentage of cells by 7.7% without significant change in the percentage of cells in S and G2/M phase (Table 2).
Table 2.
Cell cycle phases (G1, S, G2/M) of Caco-2 and SW620 cell lines (mean (SD))
| Cell line | Treatmenta | G1 (%) | S (%) | G2/M (%) |
| Caco-2 | Control | 55.9 (0.4) | 30.1 (0.4) | 9.4 (0.2) |
| PC | 58.3 (0.6) | 23.3 (2.2) | 11.7 (2.9) | |
| TG | 49.1 (0.6) | 34.0 (0.1) | 8.8 (0.4) | |
| SW620 | Control | 50.1 (0.7) | 26.7 (0.3) | 12.7 (0.2) |
| PC | 57.8 (0.4)* | 20.9 (1.7) | 13.4 (0.1) | |
| TG | 54.7 (0.6) | 16.9 (0.9)* | 19.5 (0.9)* |
a PC - biological treatment by P. chrysosporium; TG - biological treatment by T. gibbosa. * Statistically significant at confidence level at 95% (Student’s t-test)
The effect of GP, treated with P. chrysosporium, on the cell cycle of Caco-2 was complementary to the effect of SW620 but less pronounced. GP, which was treated with T. gibbosa, had a different effect on the cell cycle of Caco-2 cells than SW620 cells. The percentage of cells in G1 and G2/M phase was slightly reduced, while S phase was increased by 3.9% compared with control (Table 2). The difference between the total number of cells (100%) and the cells in the cell phases (4.6 to 10.5%) is probably due to the Watson model used, which does not include apoptotic or dead cells in the presentation of the results. Ho et al. [42] investigated the antiproliferative effect of Barringtonia racemosa leaf water extract (BLE) and gallic acid (GA) on Caco-2 cells for 48 h. For cell treatment, they used IC20 (69.1 μg/ml) and IC50 (325.5 μg/ml) concentrations of BLE and IC20 (3.7 μg/ml) and IC50 (10.6 μg/ml) concentrations of gallic acid. All treatments significantly decreased the percentage of cells in G0/G1 phase compared with control (Caco-2 cells grown only in MEM). Meanwhile, only BLE IC50 and GA IC50 caused a significant increase in the percentage of cells in S phase, and only cells treated with gallic acid had a significantly higher percentage of cells in G2/M phase. The cell cycle of mammalian cells is tightly controlled by several checkpoints and protein complexes formed by cyclins and cyclin dependent kinases. The cell cycle goes through four phases in a uniform order from G1 to S, followed by G2 and M [43], from which two new cells emerge. While most cells in the human body do not enter division until necessary, malignant cells divide rapidly because they lack the key control mechanisms of division. As a result, they proliferate rapidly and uncontrollably. Polyphenolic compounds affect the cell cycle through four possible mechanisms: antioxidant activity, regulation of p53 protein, inhibition of protein kinase activity [44], modulation of cyclin, cyclin dependent kinases or anaphase promoting complex or cyclosome (APC/C), which arrest the cell cycle [45], and apoptosis [4]. It has been reported that certain phenolic compounds cause cell cycle changes or cell arrest in cancer cells. For example, in colorectal cancer cells, quercetin causes cell cycle arrest in S phase and a reduction in the proportion of cells in G0/G1 phase (LoVo49) [46], whereas kaempferol causes cell cycle arrest in G1 and G2/M phase [47]. Rutin has also been reported to induce G2/M arrest in human neuroblastoma cells, LAN-5 [48]. Epigalocatechin inhibits the activity of cyclin-dependent kinases by inducing the expression of inhibitors of cyclin-dependent kinases p21 and p27 [29]. Resveratrol, one of the most abundant grape polyphenols, has the potential to stop colon cancer cell growth [2]. It inhibits tumour cell proliferation by stopping cell growth at different stages of the cell cycle depending on the cell line [49]. In malignant colorectal cancer cells, resveratrol induces apoptosis through activation of proapoptotic p53 and activation of caspase 3 and 8 through production of reactive oxygen species [50]. A study by Schneider et al. [51] showed that resveratrol generally has an inhibitory effect on cell growth of Caco-2 cells, with no toxic effect stopping cells in the G2/M phase of the cell cycle. Our results related to the cell cycle of SW620 cells exposed to an extract of GP treated with T. gibbosa are consistent with results indicating an increased number of cells in the G2/M phase. Although the concentration of resveratrol in our samples is not very high (0.07 mg/gdb for GP-treated with P. chrysosporium and 0.06 mg/gdb for GP-treated with T. gibbosa), other components such as catechin, epicatechin, procyanidin B1 and B2 are present in significant amounts. Considering the different effects of each polyphenolic compound on the cell cycle, it is likely that a synergistic effect occurs when these compounds are included as a mixture in the extracts used. Quercetin is another possible cause of cell cycle arrest of SW620 cells in G2/M phase. Quercetin disrupts the link between beta-catenin and transcription factor 4 while reducing the expression of survivin and cyclin D [50]. Quercetin in the extract prepared from GP treated with T. gibbosa and P. chrysosporium, is present at a concentration of 0.45 mg/gdb and 0.54 mg/gdb, respectively, indicating a possible cumulative effect on the antiproliferative activity and cell cycle impairment of Caco-2 and SW620 cell lines. Our data on the cell cycle of SW620 and Caco-2 cells suggest that there is no clear biological trend of effect between the samples studied treated with P. chrysosporium and T. gibbosa microorganisms. These discrepancies are likely the result of biological treatment with P. chrysosporium and T. gibbosa fungi.
Conclusions
The studied grape pomace extracts from biologically treated Cabernet Sauvignon are rich in phenolic compounds such as catechin, epicatechin, procyanidin B1, procyanidin B2 and quercetin. Most of the health-promoting effects of phenolic compounds are related to their antioxidant capabilities. By combining various mechanisms, including antioxidant activity, antiproliferation effect, cell cycle inhibition, induction of apoptosis, etc., they may play an important role in cancer prevention. The evaluation of the biological activity of grape pomace extracts against Caco-2 and SW620 cell lines suggests an antiproliferative activity and a reduction in cell growth of these two colorectal cancer cell lines. The studied grape pomace extracts induce changes in the cell cycle of the SW620 cell line, but the differences in the observed effect indicate a different mode of action, probably caused by fermentation in the presence of different fungi, i.e. P. chrysosporium and T. gibbosa. The obtained results suggest the presence of bioactive compounds in biotransformed grape pomace as a residue from winemaking, which could be used to prevent colon cancer. Future research will aim to determine the sequence of cascading utilization of biotransformed grape pomace to maximize its use for the production of high-value products, including phenolic-rich extracts, while approaching the zero-waste concept.
Acknowledgements
Not applicable.
Abbreviations
- AA
antioxidant activity
- ABTS
azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- CA
caffeic acid
- Caco-2
colorectal adenocarcinoma cells
- CT
catechin
- CV
cell viability
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DMSO
dimethyl sulfoxide
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- EA
cell viability
- ECG
epicatechin gallate
- ECT
epicatechin
- FA
ferulic acid
- FBS
fetal bovine serum
- GA
gallic acid
- GCG
gallocatechin gallate
- GI50
50% reduction in cell growth
- GP
grape pomace
- KA
kaempferol
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- o-CuA
o-coumaric acid
- PA
protocatechuic acid
- PB1
procyanidin B1
- PB2
procyanidin B2
- PBS
phosphate buffered saline
- PC
Phanerochaete chrysosporium
- p-CuA
p-coumaric acid
- p-HBA
p-hydroxybenzoic acid
- PI
propidium iodide
- QU
quercetin
- RES
resveratrol
- S/L
solid/liquid ratio, concentration (mg/mL)
- SA
syringic acid
- SD
standard deviation
- SSF
solid-state fermentation
- SW620
colon adenocarcinoma cells
- TPTZ
2,4,6-tris(2-pyridyl)-s-triazine
- TG
Trametes gibbosa
- TPC
total phenolic compounds
- UHPLC
ultra-high performance liquid chromatography
- VA
vanillic acid
Authors’ contributions
Co-first authors, writing original draft preparation and investigation, K.M Š. and G.Š.; formal analysis, E.P. and J.M.; data curation, M.T.; visualization and writing—review and editing, M.P.; conceptualization and supervision, A.B-K. All authors have read and approved the final manuscript.
Funding
This research was supported by the CROATIAN SCIENCE FOUNDATION, grant number: IP-2018-01-1227 (“Development of a sustainable integrated process for the production of bioactive isolates from food industry residues”, POPI-WinCEco), and by the EUROPEAN REGIONAL DEVELOPMENT FUND (ERDF), grant number: KK.01.1.1.04.0107 (“Bioconversion of lignocellulosic materials to value added feed”, Bio4Feed).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Araújo JR, Gonçalves P, Martel F. Chemopreventive effect of dietary polyphenols in colorectal cancer cell lines. Nutr Res. 2011;31(2):77–87. doi: 10.1016/j.nutres.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela M, Bastias L, Montenegro I, Werner E, Madrid A, Godoy P, et al. Autumn royal and Ribier grape juice extracts reduced viability and metastatic potential of Colon Cancer cells. Evid-Based Complement Altern Med ECAM. 2018;2018:2517080. doi: 10.1155/2018/2517080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelo S, Martino E, Ilisso CP, Bagarolo ML, Porcelli M, Cacciapuoti G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast Cancer cells. Int J Oncol. 2017;51(3):939–948. doi: 10.3892/ijo.2017.4088. [DOI] [PubMed] [Google Scholar]
- 5.Alam MN, Almoyad M, Huq F. Polyphenols in colorectal Cancer: current state of knowledge including clinical trials and molecular mechanism of action. Biomed Res Int. 2018;2018:4154185. doi: 10.1155/2018/4154185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yammine A, Namsi A, Vervandier-Fasseur D, Mackrill JJ, Lizard G, Latruffe N. Polyphenols of the Mediterranean diet and their metabolites in the prevention of colorectal Cancer. Molecules. 2021;26(12):3483. doi: 10.3390/molecules26123483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cory H, Passarelli S, Szeto J, Tamez M, Mattei J. The role of polyphenols in human health and food systems: a Mini-review. Front Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helkar PB, Sahoo A, Patil N. Review: food industry by-products used as a functional food ingredients. Int J Waste Resour. 2016;6:1–6. [Google Scholar]
- 9.Quero J, Ballesteros LF, Ferreira-Santos P, Velderrain-Rodriguez GR, Rocha CMR, Pereira RN, et al. Unveiling the antioxidant therapeutic functionality of sustainable olive pomace active ingredients. Antioxidants. 2022;11(5):828. doi: 10.3390/antiox11050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paesa M, Nogueira DP, Velderrain-Rodríguez G, Esparza I, Jiménez-Moreno N, Mendoza G, et al. Valorization of onion waste by obtaining extracts rich in phenolic compounds and feasibility of its therapeutic use on Colon Cancer. Antioxidants. 2022;11(4):733. doi: 10.3390/antiox11040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Vargas MC, Contreras M del M, Castro E. Avocado-derived biomass as a source of bioenergy and bioproducts. Appl Sci 2020;108195.
- 12.Bucić-Kojić A, Tišma M, Šelo G, Grgić J, Perković G, Planinić M. Winery production residues as Feedstocks within the biorefinery concept. Eng Power Bull Croat Acad Eng. 2022;17(1):11–17. [Google Scholar]
- 13.Tsegaye B, Jaiswal S, Jaiswal AK. Food waste biorefinery: pathway towards circular bioeconomy. Foods. 2021;10(6):1174. doi: 10.3390/foods10061174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh CK, Siddiqui IA, El-Abd S, Mukhtar H, Ahmad N. Combination chemoprevention with grape antioxidants. Mol Nutr Food Res. 2016;60(6):1406–1415. doi: 10.1002/mnfr.201500945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chojnacka K, Lewandowska U. Chemopreventive effects of polyphenol-rich extracts against Cancer invasiveness and metastasis by inhibition of type IV collagenases expression and activity. J Funct Foods. 2018;46:295–311. [Google Scholar]
- 16.Costa JR, Amorim M, Vilas-Boas A, Tonon RV, Cabral LMC, Pastrana L, et al. Impact of in vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah Grape Pomace Extract Food Funct. 2019;10(4):1856–1869. doi: 10.1039/c8fo02534g. [DOI] [PubMed] [Google Scholar]
- 17.Quero J, Jiménez-Moreno N, Esparza I, Osada J, Cerrada E, Ancín-Azpilicueta C, et al. Grape stem extracts with potential anticancer and antioxidant properties. Antioxidants. 2021;10(2):243. doi: 10.3390/antiox10020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bucić-Kojić A, Fernandes F, Silva T, Planinić M, Tišma M, Šelo G, et al. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes versicolor. Food Funct. 2020;11(1):680–688. doi: 10.1039/c9fo02296a. [DOI] [PubMed] [Google Scholar]
- 19.Nallathambi R, Poulev A, Zuk JB, Raskin I. Proanthocyanidin-rich grape seed extract reduces inflammation and oxidative stress and restores tight junction barrier function in Caco-2 Colon cells. Nutrients. 2020;12(6):1623. doi: 10.3390/nu12061623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian Q, Xu Z, Sun X, Deavila J, Du M, Zhu M. Grape pomace inhibits Colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J Nutr Biochem. 2020;84:108443. doi: 10.1016/j.jnutbio.2020.108443. [DOI] [PubMed] [Google Scholar]
- 21.Parry JW, Li H, Liu JR, Zhou K, Zhang L, Ren S. Antioxidant activity, Antiproliferation of Colon Cancer cells, and chemical composition of grape pomace. Food Nutr Sci. 2011;2(6):530–540. [Google Scholar]
- 22.Martins IM, Macedo GA, Macedo JA. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: effects in Caco-2 cells. Food Biosci. 2020;35:100607. [Google Scholar]
- 23.Šelo G, Planinić M, Tišma M, Grgić J, Perković G, Koceva KD. A comparative study of the influence of various fungal-based pretreatments of grape pomace on phenolic compounds recovery. Foods. 2022;10(10):1665. doi: 10.3390/foods11111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verduzco-Oliva R, Gutierrez-Uribe JA. Beyond enzyme production: solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustainability. 2020;12(2):495. [Google Scholar]
- 25.Martins S, Mussatto SI, Martínez-Avila G, Montañez-Saenz J, Aguilar CN, Teixeira JA. Bioactive phenolic compounds: production and extraction by solid-state fermentation. A review Biotechnol Adv. 2011;29(3):365–373. doi: 10.1016/j.biotechadv.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Bucić-Kojić A, Šelo G, Zelić B, Planinić M, Tišma M. Recovery of phenolic acid and enzyme production from corn silage biologically treated by Trametes versicolor. Appl Biochem Biotechnol. 2017;181(3):948–960. doi: 10.1007/s12010-016-2261-y. [DOI] [PubMed] [Google Scholar]
- 27.Bucić-Kojić A, Planinić M, Tomas S, Jakobek L, Šeruga M. Influence of solvent and temperature on extraction of phenolic compounds from grape seed, antioxidant activity and colour of extract. Int J Food Sci Technol. 2009;44(12):2394–2401. [Google Scholar]
- 28.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation Decolorization assay. Free Radic Biol Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 31.Negro C, Aprile A, Luvisi A, De Bellis L, Miceli A. Antioxidant activity and polyphenols characterization of four Monovarietal grape pomaces from Salento (Apulia, Italy) Antioxidants. 2021;10(9):1406. doi: 10.3390/antiox10091406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milinčić DD, Stanisavljević NS, Kostić AŽ, Soković Bajić S, Kojić MO, Gašić UM, et al. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT - Food Sci Technol. 2021;138:110739. [Google Scholar]
- 33.Dulf FV, Vodnar DC, Toşa MI, Dulf EH. Simultaneous enrichment of grape pomace with γ-Linolenic acid and carotenoids by solid-state fermentation with Zygomycetes Fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020;310:125927. doi: 10.1016/j.foodchem.2019.125927. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Ávila GC, Aguilera-Carbó AF, Rodríguez-Herrera R, Aguilar CN. Fungal enhancement of the antioxidant properties of grape waste. Ann Microbiol. 2012;62(3):923–930. [Google Scholar]
- 35.Leite P, Silva C, Salgado JM, Belo I. Simultaneous production of Lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind Crop Prod. 2019;137:315–322. [Google Scholar]
- 36.Maluf DF, Gonçalves M, D’Angelo R, Girassol A, Tulio A, Pupo Y, et al. Cytoprotection of antioxidant biocompounds from grape pomace: further Exfoliant Phytoactive ingredients for cosmetic products. Cosmetics. 2018;5(3):46. [Google Scholar]
- 37.Jilani H, Cilla A, Barberá R, Hamdi M. Antiproliferative activity of green, black tea and olive leaves polyphenols subjected to biosorption and in vitro gastrointestinal digestion in Caco-2 cells. Food Res Int. 2020;136:109317. doi: 10.1016/j.foodres.2020.109317. [DOI] [PubMed] [Google Scholar]
- 38.Saleh AJ, Othman L, Elchoueiry M, Ghanem R, Bazzi S, El-Sabban M, et al. Anti-proliferative activity of yerba mate (Ilex paraguariensis) aqueous extracts on human colorectal Cancer cell lines. Funct Foods Health Dis. 2021;11(10):499–511. [Google Scholar]
- 39.Caponio GR, Cofano M, Lippolis T, Gigante I, De Nunzio V, Difonzo G, et al. Anti-proliferative and pro-apoptotic effects of digested Aglianico grape pomace extract in human colorectal Cancer cells. Molecules. 2022;27(20):6791. doi: 10.3390/molecules27206791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rengarajan T, Yaacob NS. The flavonoid Fisetin as an anticancer agent targeting the growth signaling pathways. Eur J Pharmacol. 2016;789:8–16. doi: 10.1016/j.ejphar.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Dükel M, Tavsan Z, Kayali HA. Flavonoids regulate cell death-related cellular signaling via ROS in human Colon Cancer cells. Process Biochem. 2021;101:11–25. [Google Scholar]
- 42.Ho IYM, Abdul Aziz A, Mat JS. Evaluation of anti-proliferative effects of Barringtonia racemosa and Gallic acid on Caco-2 cells. Sci Rep. 2020;10(1):9987. doi: 10.1038/s41598-020-66913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costea T, Nagy P, Ganea C, Szöllősi J, Mocanu MM. Molecular mechanisms and bioavailability of polyphenols in prostate Cancer. Int J Mol Sci. 2019;20(5):E1062. doi: 10.3390/ijms20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin SY, Yoon H, Ahn S, Kim DW, Bae DH, Koh D, et al. Structural properties of polyphenols causing cell cycle arrest at G1 phase in HCT116 human colorectal Cancer cell lines. Int J Mol Sci. 2013;14(8):16970–16985. doi: 10.3390/ijms140816970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Losada-Echeberría M, Herranz-López M, Micol V, Barrajón-Catalán E. Polyphenols as promising drugs against Main breast Cancer signatures. Antioxid Basel Switz. 2017;6(4):E88. doi: 10.3390/antiox6040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Zhang M, Yu L, Zhao Y, He N, Yang X. Antitumor activities of quercetin and Quercetin-5′,8-disulfonate in human Colon and Breast Cancer cell lines. Food Chem Toxicol. 2012;50(5):1589–1599. doi: 10.1016/j.fct.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 47.Cho HJ, Park and JHY. Kaempferol induces cell cycle arrest in HT-29 human Colon. Cancer Cells. 2013;18(3):257–263. doi: 10.15430/JCP.2013.18.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian L, et al. Anti-tumor effect of Rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci World J. 2013;2013:e269165. doi: 10.1155/2013/269165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailon-Moscoso N, Cevallos-Solorzano G, Romero-Benavides JC, Orellana MIR. Natural compounds as modulators of cell cycle arrest: application for anticancer chemotherapies. Curr Genomics. 2017;18(2):106–131. doi: 10.2174/1389202917666160808125645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Temraz S, Mukherji D, Shamseddine A. Potential targets for colorectal Cancer prevention. Int J Mol Sci. 2013;14(9):17279–17303. doi: 10.3390/ijms140917279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider Y, Vincent F, Duranton B, Badolo L, Gossé F, Bergmann C, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic Cancer cells. Cancer Lett. 2000;158(1):85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.