Abstract
Background
The ALVAC/gp120 + MF59 vaccines in the HIV Vaccine Trials Network (HVTN) 702 efficacy trial did not prevent human immunodeficiency virus-1 (HIV-1) acquisition. Vaccine-matched immunological endpoints that were correlates of HIV-1 acquisition risk in RV144 were measured in HVTN 702 and evaluated as correlates of HIV-1 acquisition.
Methods
Among 1893 HVTN 702 female vaccinees, 60 HIV-1–seropositive cases and 60 matched seronegative noncases were sampled. HIV-specific CD4+ T-cell and binding antibody responses were measured 2 weeks after fourth and fifth immunizations. Cox proportional hazards models assessed prespecified responses as predictors of HIV-1 acquisition.
Results
The HVTN 702 Env-specific CD4+ T-cell response rate was significantly higher than in RV144 (63% vs 40%, P = .03) with significantly lower IgG binding antibody response rate and magnitude to 1086.C V1V2 (67% vs 100%, P < .001; Pmag < .001). Although no significant univariate associations were observed between any T-cell or binding antibody response and HIV-1 acquisition, significant interactions were observed (multiplicity-adjusted P ≤.03). Among vaccinees with high IgG A244 V1V2 binding antibody responses, vaccine-matched CD4+ T-cell endpoints associated with decreased HIV-1 acquisition (estimated hazard ratios = 0.40–0.49 per 1-SD increase in CD4+ T-cell endpoint).
Conclusions
HVTN 702 and RV144 had distinct immunogenicity profiles. However, both identified significant correlations (univariate or interaction) for IgG V1V2 and polyfunctional CD4+ T cells with HIV-1 acquisition.
Clinical Trials Registration . NCT02968849.
Keywords: HIV-1 vaccine, HVTN 702, RV144, vaccine efficacy trial, T-cell immunogenicity, T-cell polyfunctionality, binding antibodies, correlates of risk, intracellular cytokine staining, vaccine-induced immune response
The immunogenicity profile of HVTN 702 differed from that of RV144. Both identified significant correlations (univariate or interaction) for IgG to V1V2 and polyfunctional CD4+ T cells with HIV-1 acquisition.
An efficacious preventive human immunodeficiency virus-1 (HIV-1) vaccine is important for inducing long-lived immunity and providing a practical, cost-effective method to fight the global epidemic, particularly for regions in sub-Saharan Africa with disproportionately high HIV-1 incidence despite decades of prevention efforts. Eight HIV-1 vaccine candidates have been studied in efficacy trials [1–8]; only the regimen tested in RV144 showed significant HIV-1 acquisition reduction. Estimated vaccine efficacy of the replication-defective canarypox vaccine (ALVAC) plus recombinant glycoprotein 120 (gp120) protein (AIDSVAX) vaccine at month 12 was 60% (95% confidence interval [CI], 22%–80%) [9]; however, efficacy waned to 31.2% (95% CI, 1.1%–52.1%; P = .04) by month 42 [5].
Given these results, the RV144 regimen was tested in South Africa in the HIV Vaccine Trials Network (HVTN) 097 phase 1b trial, which showed significantly higher cellular and humoral vaccine-induced responses among participants in South Africa than among participants in Thailand [10]. Shortly after the announcement of RV144, the Pox Protein Public–Private Partnership (P5) was formed to develop an RV144-analogous vaccine regimen that would aspirationally improve upon the 30% overall efficacy and overcome the large strain diversity between the focal A/E epidemic in Thailand versus the extremely diverse subtype C epidemic in South Africa [11]. The resultant regimen incorporated regionally adapted HIV-1 subtype C sub-Saharan African strains [7], MF59 adjuvant instead of alum, and a month 12 boost. Safety of this regimen was demonstrated in the HVTN 100 phase 1–2a trial [12], with humoral and cellular immune responses meeting the P5’s predefined criteria for studying the regimen’s preventive efficacy. HVTN 702, a phase 2b–3 efficacy trial, next evaluated the subtype C regimen in South African HIV-uninfected at-risk adults. However, the immunogenicity signal from HVTN 100 [12–15] did not translate to efficacy, and vaccinations in HVTN 702 were halted after prespecified vaccine efficacy futility criteria were met in January 2020. Participants were unblinded in February 2020. The estimated HIV hazard ratio (vaccine: placebo) for the first 24 months of follow-up was 1.02 (95% CI, .81–1.30, P = .84) [7].
Here we evaluated and compared HVTN 702 immune responses to those in RV144, HVTN 097, and HVTN 100 and assessed whether the RV144 correlates of HIV-1 risk would also correlate with HIV-1 risk in HVTN 702.
METHODS
Ethics
For the HVTN 702 trial (NCT02968849) [7], written informed consent was obtained from all participants and all procedures were conducted in accordance with the ethical standards of the Helsinki Declaration. The research ethics committees of the University of the Witwatersrand, University of Cape Town, University of KwaZulu-Natal, Sefako Makgatho University, and the South African Medical Research Council approved the trial.
Study Participants
HVTN 702 enrolled 5404 HIV-uninfected adults (3786 assigned female at birth, 1618 assigned male at birth) at 14 South African sites between 26 October 2016 and 21 June 2019. Participants were randomized to vaccine or placebo within each sex assigned at birth. The vaccine regimen was an ALVAC-HIV vector and an MF59-adjuvanted bivalent subtype C gp120. ALVAC-HIV (vCP2438; Sanofi Pasteur) expresses the subtype C ZM96.C HIV-1 envelope (Env) glycoprotein, along with subtype B LAI gp41 transmembrane sequence, gag, and protease. Bivalent subtype C gp120 (GSK) consists of 100 μg of TV1.C gp120 and 100 μg of 1086.C gp120. Participants received ALVAC-HIV or placebo at months 0 and 1, followed by 4 injections of ALVAC-HIV plus bivalent MF59-adjuvanted subtype C gp120 or placebo at months 3, 6, 12, and 18.
As few male participants acquired HIV-1 in the study [7], we restricted our analyses to female participants. Months 6.5 (2 weeks after fourth vaccination) and 12.5 (2 weeks after fifth vaccination) immune responses among vaccine recipients were used to profile immunogenicity, and were evaluated as predictors of HIV-1 acquisition through month 24, based on follow-up data collected through 18 February 2020. Using a case-control design frequency matched on age, we measured cellular and humoral immune responses in per-protocol females: 60 vaccine cases who acquired HIV-1 between month 6.5 and 24, 60 vaccine noncases who remained HIV-1 negative until month 24, 5 placebo cases, and 5 placebo noncases (Supplementary Text, Supplementary Figure 1).
Immune response data at month 6.5 from per-protocol female and male vaccine noncases from HVTN 100 (n = 184), HVTN 097 (n = 73), and RV144 (n = 201) were compared to HVTN 702 female per-protocol noncases (n = 60). Those who received the first 4 planned immunizations within specified visit windows were considered per protocol. HVTN 100 assessed the same regimen as HVTN 702, without the month 18 boost, enrolling 252 low-risk male and female participants in South Africa in 2015 with randomization to vaccine (n = 210) or placebo (n = 42) [13]. HVTN 097 assessed an RV144-related regimen (see below), enrolling 100 low-risk male and female participants in South Africa in 2013 with randomization to vaccine (n = 80) or placebo (n = 20) [10]. RV144 enrolled 16 402 males and females from the general population in Thailand between 2003 and 2005, with randomization to vaccine (n = 8197) or placebo (n = 8198). Like the heterologous prime-boost combination HVTN 100 and HVTN 702 vaccine regimens, the RV144 and HVTN 097 vaccine regimens consisted of 4 injections at months 0, 1, 3, and 6 of ALVAC-HIV (vCP1521), a canarypox vector expressing clade E Env, clade B gag, and clade B protease with 2 booster injections of alum-adjuvanted AIDSVAX B/E (a bivalent gp120) at months 3 and 6. For HVTN 097 and HVTN 100, immune response data are available on all per-protocol vaccine recipients (n = 73 and 184, respectively) whereas data are available on a subset of n = 201 RV144 vaccine noncases, selected for contemporaneous assaying with HVTN 100 specimens in 2016 [16] to inform whether to proceed with HVTN 702.
Laboratory Methods
All assays were performed blinded in HVTN laboratories utilizing validated methods [16–18]. CD4+ T-cell responses were measured by intracellular cytokine staining [19] and analyzed by flow cytometry (Supplementary Text). Serum HIV-1–specific immunoglobulin G (IgG), IgG3, and IgA binding antibody responses were measured by an HIV-1 binding antibody multiplex assay [4, 16] (Supplementary Text).
Immune Response End Points for Correlates of Risk Assessment
Three primary immune responses were selected based on previous RV144 immune correlates studies [16, 20–24]: (1) the COMPASS Env-specific CD4+ T-cell polyfunctionality score to ZM96 [23], defined as the estimated proportion of antigen-specific cell subsets detected, weighted by degree of functionality using the same 6 markers as the RV144 correlates analysis: CD40L, interferon-γ (IFN-γ), interleukin 2 (IL-2), tumor necrosis factor-α (TNF-α), IL-4, and IL-17a; (2) IgG binding antibody responses to AE.A244 V1V2; and (3) IgG3 binding antibody responses to C.1086 V1V2. Secondary immune responses were CD4+ polyfunctionality score to 1086 and to TV1, CD4+ T cells expressing IFN-γ and/or IL-2 and/or CD40L in response to ZM96, IgA binding antibody score, IgG binding antibody responses to RV144 vaccine-matched antigen (A244 gp120), and IgG binding antibody responses to the consensus antigen (A1.con.env03140CF).
Statistical Methods
Immunogenicity Characterization and Comparison
Given differences in the HVTN 702 versus HVTN 100, HVTN 097, and RV144 study populations (Table 1), we compared immunogenicity under a hypothetical scenario where baseline participant characteristics in the other trials (age, body mass index, South African region, and education level) follow the covariate distribution of the female per-protocol HVTN 702 cohort who were eligible for case-control sampling (Supplementary Text). Targeted minimum loss estimation was applied, with superlearning employed to model the mean (and 95% CI) immune response conditional on baseline covariates [25]. If the estimated response rate exceeded 90%, the Wilson score method was used to calculate the 95% CI as targeted minimum loss estimation can be unstable near the boundary. The Holm method controlled the family-wise error rate at 0.05 across each set of binary and continuous endpoints.
Table 1.
Demographic Characteristics of the Per-Protocol Vaccine Recipient Cohorts from HVTN 097, HVTN 100, RV144 Subset, and the Female Per-Protocol Noncases in the HVTN 702 Case-Control Set for Immunogenicity Analyses
| Characteristic | HVTN 097 (n = 73) | HVTN 100 (n = 184) | RV144 (n = 201) | HVTN 702 (n = 60) |
|---|---|---|---|---|
| Planned treatment | ||||
| Placebo | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Vaccine | 73 (100) | 184 (100) | 201 (100) | 60 (100) |
| Age in years at randomization | ||||
| Mean (SD) | 23 (4) | 24 (5) | … | 24 (4) |
| Range | 18–35 | 18–40 | … | 18–35 |
| Age category at randomization | ||||
| ≤20 | 27 (37) | 44 (24) | 56 (28) | 10 (17) |
| 21–25 | 17 (23) | 78 (42) | 97 (48) | 35 (58) |
| ≥26 | 29 (40) | 62 (34) | 48 (24) | 15 (25) |
| Sex assigned at birth | ||||
| Female | 33 (45) | 73 (40) | 79 (39) | 60 (100) |
| Male | 40 (55) | 111 (60) | 122 (61) | 0 (0) |
| BMI | ||||
| Mean (SD) | 23 (5) | 24 (5) | … | 27 (7) |
| Range | 17–37 | 16–39 | … | 17–47 |
| Region of enrollment | ||||
| Thailand | 0 (0) | 0 (0) | 201 (100) | 0 (0) |
| Central South Africa | 50 (68) | 100 (54) | 0 (0) | 34 (57) |
| KwaZulu-Natal South Africa | 0 (0) | 59 (32) | 0 (0) | 19 (32) |
| WestEast Cape South Africa | 23 (32) | 25 (14) | 0 (0) | 7 (12) |
| Education | ||||
| High school | 0 (0) | 135 (73) | 0 (0) | 55 (92) |
| Primary school | 0 (0) | 6 (3) | 0 (0) | 0 (0) |
| Tertiary college university | 0 (0) | 43 (23) | 0 (0) | 5 (8) |
| Prefer not to answer | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Not available | 73 (100) | … | 201 (0) | … |
Data are No. (%) except where indicated.
Abbreviation: BMI, body mass index.
Correlates of Risk Assessment
To evaluate immune responses among HVTN 702 female vaccine recipients as predictors of HIV-1 acquisition within the first 24 months since enrollment, univariate and multivariate Cox proportional hazard models were used. Each model accounted for the case-control sampling design and adjusted for age (≤ 25, >25 years) and a categorical baseline HIV risk score for women to control for potential confounding [7]. Univariate Cox models were fit for each individual categorical and continuous immune response variable at each time point. Four prespecified multivariate models were fit that included all categorical or continuous primary immune response variables at either month 6.5 or 12.5. At each time point, a separate multiplicity adjustment was applied across each set of endpoints. The Holm method controlled the family-wise error rate at 0.05 across the set of 3 primary variables and across the set of 6 secondary variables, with separate multiplicity adjustment for continuous and categorical variables. Q values for the set of exploratory variables were calculated using the Benjamini-Hochberg method [26], with Q < 0.2 considered statistically significant. Interactions among Month 6.5 immune responses were considered for their association with HIV-1 acquisition where prespecified criteria were met (Supplementary Text). Separate models were fit, each containing 1 interaction term and associated main effects, adjusting for age and HIV risk score, with Holm P value adjustment across the multiple models.
RESULTS
Different Immune Response Profiles Elicited in HVTN 702 Versus RV144
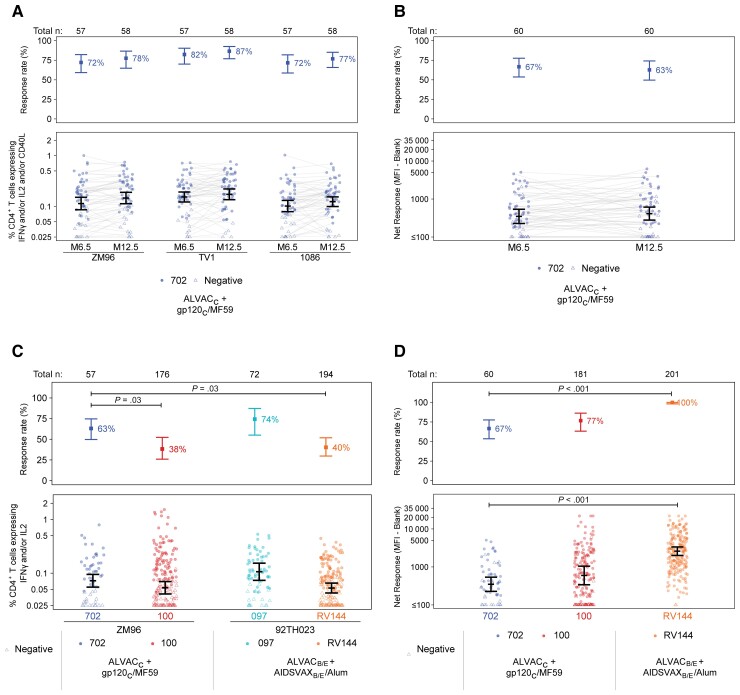
The HVTN 702 vaccine regimen induced CD4+ T cells expressing IFN-γ and/or IL-2 and/or CD40L in response to vaccine-matched HIV-1 envelope peptide pools in 72%–87% of vaccine recipient noncases, with similar responses at months 6.5 and 12.5 (Figure 1A). CD8+ T-cell response rates were low across HIV-1 peptide pools and time points: ≤12% (data not shown). IgG binding antibody response rates to 1086.C V1V2 were also similar across time points (67% and 63%, respectively; Figure 1B).
Figure 1.
Characterization of HVTN 702 cellular and humoral immune responses among per-protocol vaccinated noncases and comparison with HVTN 100, HVTN 097, and RV144 per-protocol vaccinated noncases. A, Response rates and magnitudes of CD4+ T cells expressing IFN-γ and/or IL-2 and/or CD40L among HVTN 702 vaccinated noncases, measured by intracellular cytokine staining at months 6.5 and 12.5. B, Month 6.5 and 12.5 IgG binding antibody responses to 1086.C V1V2, HVTN 702. C, Response rates and magnitudes of CD4+ T cells expressing IFN-γ and/or IL-2 among HVTN 702 vaccinated noncases compared to those in HVTN 100, HVTN 097, and RV144 at month 6.5, measured by intracellular cytokine staining. D, Month 6.5 IgG binding antibody responses to 1086.C V1V2 in HVTN 702 compared to HVTN 100 and RV144 (HVTN 097 data not available). Positive response rates and 95% CIs in the top panels and mean magnitudes and 95% CIs in the bottom panels are estimated by targeted maximum likelihood estimation. All Holm-adjusted P values < .05 for HVTN 702 contrasts with earlier trials are displayed. Abbreviations: CI, confidence interval; HVTN, HIV Vaccine Trials Network; IFN-γ, interferon-γ; IgG, immunoglobulin G; IL-2, interleukin 2; MFI, mean fluorescence intensity.
At month 6.5, the response rate of CD4+ T cells expressing IFN-γ and/or IL-2 to ZM96 was significantly higher in HVTN 702 than in HVTN 100 to ZM96 (63% vs 38%, P = .03) and was also significantly higher than that in RV144 to the analogous vaccine-matched envelope, 92TH023 (63% vs 40%, P = .03; Figure 1C). In contrast, the CD4+ T-cell response rate in HVTN 097 to 92TH023 (74%) was similar to HVTN 702. No significant differences were seen in these magnitudes across trials (all P > .23). Although the HVTN 702 IgG binding antibody response rate to 1086.C V1V2 was significantly lower than in RV144 (67% vs 100%, P < .001), it was similar to HVTN 100 (77%) (Figure 1D) and would have met the prespecified criteria for proceeding to efficacy testing. IgG 1086.C V1V2 magnitudes in HVTN 702 were also significantly lower than those in RV144 (P < .001) but similar to those in HVTN 100. Notably, only 3% of HVTN 702 (13% of HVTN 100) vaccine recipients had 1086.C V1V2 magnitudes in the upper tertile of RV144 (Supplementary Figure 2).
Cross-trial immunogenicity comparison across the subset of female per-protocol vaccine recipients in each trial (Supplementary Figure 3) yielded results highly comparable to those shown in Figure 1C and 1D that included both male and female participants.
No Significant Associations of Primary or Secondary Immune Response Variables With HIV-1 Acquisition in Univariate Correlate of Risk Analyses
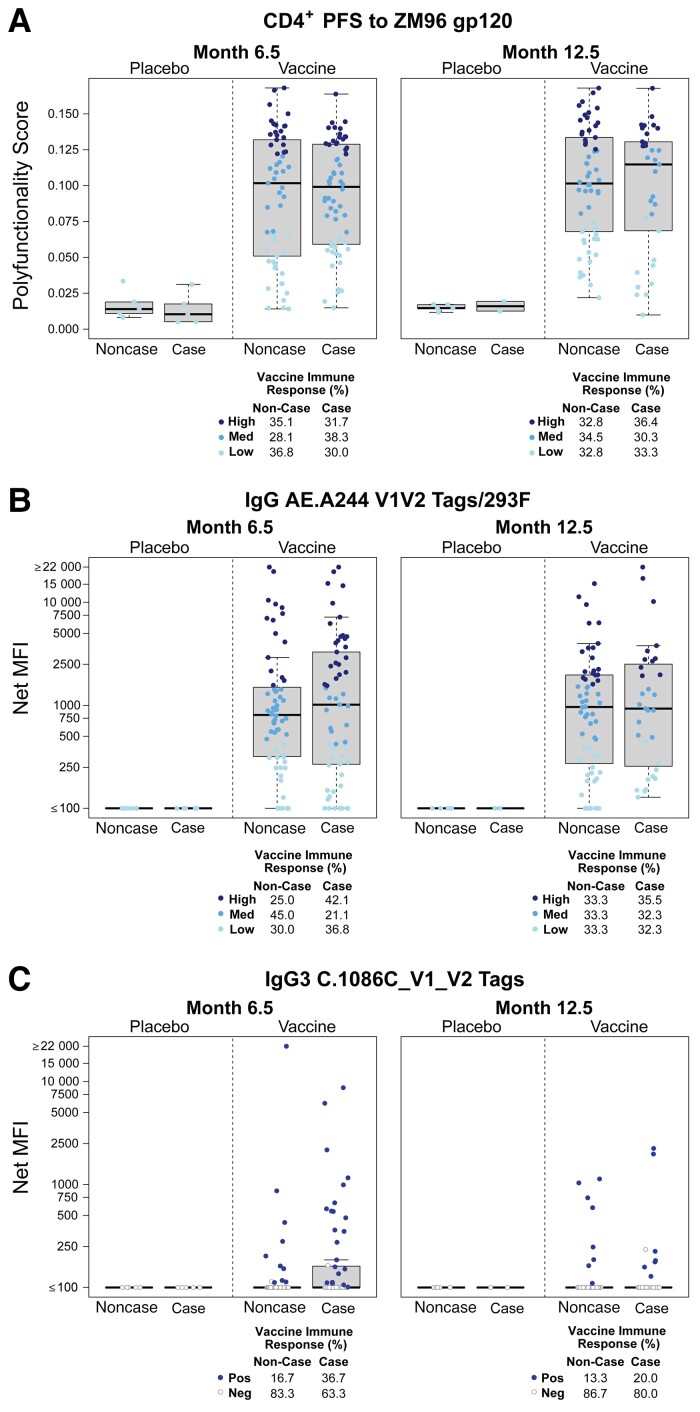
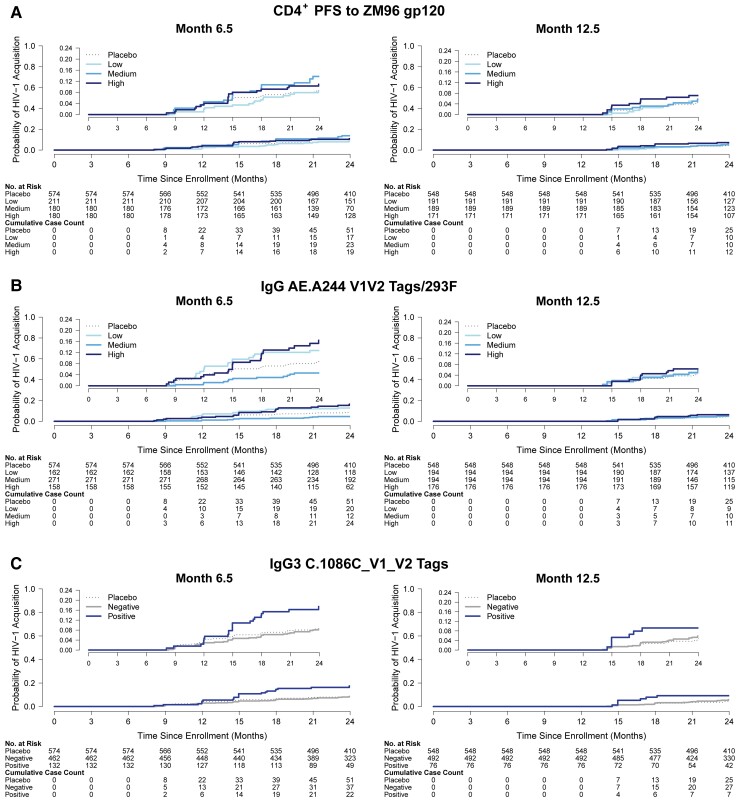
Given the lack of overall vaccine efficacy, we performed a limited correlates analysis on down-selected antibody and cellular immune measurements. For antibody measurements, we selected the protein boost clade C V1V2 sequence, which correlated with decreased HIV-1 risk in RV144 [21]. There was no significant association between month 6.5 or 12.5 Env ZM96 CD4+ polyfunctionality and HIV-1 acquisition, regardless of whether the quantitative immune response variable or the categorical variable high-versus-low response indicator and medium-versus-low response indicator was used (Table 2, Supplementary Table 1, Figure 2, and Figure 3). The CD4+ polyfunctionality profile is characterized by subsets that include IFN-γ, IL-2, IL-4, CD40L, and TNF-α (Supplementary Figure 4). Similarly, there was no significant association between month 6.5 or 12.5 IgG binding antibody responses to A244 V1V2 and HIV-1 acquisition when considered as a quantitative variable or a categorical high-versus-low response indicator and medium-versus-low response indicator (Table 2, Supplementary Table 1, Figure 2, and Figure 3). There was also no significant association between month 6.5 or 12.5 IgG3 binding antibody responses to 1086 V1V2 and HIV-1 acquisition when considered as a quantitative variable or a binary positive-versus-negative response indicator (Table 2, Supplementary Table 1, Figure 2, and Figure 3).
Table 2.
Results for Month 6.5 Primary and Secondary Immune Responses of Per-Protocol Vaccine Recipients
| Response Type | Variable | HR Scale | HR (95% CI) | Adjusted P | |
|---|---|---|---|---|---|
| Univariate results | |||||
| Primary variables | Cytokine-secreting T-cell | CD4+ PFS ZM96 | Per 1-SD | 1.16 (.84–1.61) | .79 |
| Binding antibody | IgG A244 V1V2 | Per 1-SD | .92 (.58–1.45) | .79 | |
| Binding antibody | IgG3 1086 V1V2 | Per 1-SD | 1.28 (.83–1.98) | .79 | |
| Cytokine-secreting T-cell | CD4+ PFS ZM96 | High vs low | 1.67 (.65–4.26) | .19 | |
| Cytokine-secreting T-cell | CD4+ PFS ZM96 | Medium vs low | 2.62 (.94–7.33) | ||
| Binding antibody | IgG A244 V1V2 | High vs low | 1.45 (.54–3.89) | .12 | |
| Binding antibody | IgG A244 V1V2 | Medium vs low | .38 (.13–1.12) | ||
| Binding antibody | IgG3 1086 V1V2 | Pos vs neg | 2.77 (1.04–7.38) | .12 | |
| Secondary variables | Cytokine-secreting T-cell | CD4+ PFS 1086 | Per 1-SD | 1.23 (.88–1.72) | 1.00 |
| Cytokine-secreting T-cell | CD4+ PFS TV1 | Per 1-SD | 1.20 (.83–1.73) | 1.00 | |
| Cytokine-secreting T-cell | CD4+ IFN-γ+/IL-2+/CD40L+ ZM96 | Per 1-SD | 1.39 (1.00–1.94) | .32 | |
| Binding antibody | IgG A1.con.env03 140 CF | Per 1-SD | 1.15 (.74–1.77) | 1.00 | |
| Binding antibody | IgG A244 gp120 | Per 1-SD | 1.08 (.72–1.63) | 1.00 | |
| Binding antibody | IgA score | Per 1-SD | 1.01 (.64–1.58) | 1.00 | |
| Cytokine-secreting T-cell | CD4+ PFS 1086 | High vs low | 1.32 (.52–3.37) | 1.00 | |
| Cytokine-secreting T-cell | CD4+ PFS 1086 | Medium vs low | 1.22 (.47–3.21) | ||
| Cytokine-secreting T-cell | CD4+ PFS TV1 | High vs low | 1.87 (.67–5.21) | 1.00 | |
| Cytokine-secreting T-cell | CD4+ PFS TV1 | Medium vs low | 1.29 (.50–3.32) | ||
| Cytokine-secreting T-cell | CD4+ IFN-γ+/IL-2+/CD40L+ ZM96 | High vs low | 2.01 (.75–5.37) | 1.00 | |
| Cytokine-secreting T-cell | CD4+ IFN-γ+/IL-2+/CD40L+ ZM96 | Medium vs low | 1.11 (.40–3.02) | ||
| Binding antibody | IgG A1.con.env03 140 CF | High vs low | 1.48 (.57–3.82) | 1.00 | |
| Binding antibody | IgG A1.con.env03 140 CF | Medium vs low | 1.10 (.57–3.93) | ||
| Binding antibody | IgG A244 gp120 | High vs low | 1.55 (.61–3.95) | 1.00 | |
| Binding antibody | IgG A244 gp120 | Medium vs low | 1.50 (.40–3.01) | ||
| Binding antibody | IgA score | High vs low | 1.45 (.53–3.97) | 1.00 | |
| Binding antibody | IgA score | Medium vs low | 1.04 (.40–2.70) | ||
| Multivariate resultsa | |||||
| Model 1, primary variables, magnitude | Cytokine-secreting T-cell | CD4+ PFS ZM96 | Per 1-SD | 1.04 (.71–1.53) | .84 |
| Binding antibody | IgG A244 V1V2 | Per 1-SD | .76 (.42–1.36) | .71 | |
| Binding antibody | IgG3 1086 V1V2 | Per 1-SD | 1.46 (.78–2.73) | .70 | |
| Model 2, primary variables, categorical | Cytokine-secreting T-cell | CD4+ PFS ZM96 | High vs low | 1.30 (.42–4.07) | .88 |
| Cytokine-secreting T-cell | CD4+ PFS ZM96 | Medium vs low | 1.33 (.38–4.61) | ||
| Binding antibody | IgG A244 | High vs low | 1.20 (.32–4.53) | .25 | |
| Binding antibody | IgG A244 | Medium vs low | .35 (.11–1.16) | ||
| Binding antibody | IgG3 1086 V1V2 | Pos vs neg | 1.88 (.52–6.77) | .66 | |
Univariate and multivariate Cox proportional hazards regression results are shown for the primary and secondary immune response variables, adjusted for the baseline covariates age (≤ 25, >25 years) and a previously derived categorical HIV risk score for women.
Abbreviations: CI, confidence interval; HR, hazard ratio; Neg, negative; Pos, positive.
Multivariate models 1 and 2 differ with respect to the immune response variables included: model 1 includes quantitative CD4+ polyfunctionality scores to ZM96, IgG binding antibody magnitude to A244, and IgG3 binding antibody magnitude to 1086 V1V2; model 2 replaces quantitative magnitude and polyfunctionality score variables with categorical low/medium/high or binary positive/negative response indicators.
Figure 2.
Distribution of primary immune response variables. Boxplots show the primary immune response variable distributions by HIV-1 acquisition status and treatment group: (A) CD4+ polyfunctionality score to ZM96; (B) IgG binding antibody response to A244 V1V2; and (C) IgG3 binding antibody response to 1086 V1V2. A, Month 6.5 polyfunctionality score categories were, high, ≥0.121; med, 0.067 to <0.121; low, <0.067. Month 12.5 polyfunctionality score categories were high, ≥0.125; med, 0.080 to <0.125; low, <0.080. B, The positive response rates were 88% at month 6.5 and 90.1% at month 12.5. Month 6.5 binding antibody categories were high, ≥1498.83 MFI; med, 421.08 to <1498.83 MFI; low, <421.08 MFI. Month 12.5 binding antibody categories were, high, ≥1603.5 MFI; med, 468.25 to <1603.5 MFI; low, <468.25 MFI. The mid-line of the box denotes the median and the ends of the box denote the 25th and 75th percentiles. The whiskers that extend from the top and bottom of the box extend to the most extreme data points that are no more than 1.5 times the interquartile range or if no value meets this criterion, to the data extremes. Abbreviations: HIV-1, human immunodeficiency virus 1; IgG, immunoglobulin G; Med, medium; MFI, mean fluorescence intensity; Neg, negative; PFS, polyfunctionality score; Pos, positive.
Figure 3.
HIV-1 acquisition incidence by vaccine recipient immune response subgroup. Plots show the cumulative incidence of HIV-1 acquisition among per-protocol vaccine recipients by primary categorical immune response variables at month 6.5 or month 12.5: (A) CD4+ T-cell polyfunctionality score to ZM96; (B) IgG binding antibody response to A244 V1V2; and (C) IgG3 binding antibody response to 1086 V1V2. A, Month 6.5 polyfunctionality score categories were high, ≥0.121; med, 0.067 to <0.121; low, <0.067. Month 12.5 polyfunctionality score categories were high, ≥0.125; med, 0.080 to <0.125; low, <0.080. B, The positive response rates were 88% at month 6.5 and 90.1% at month 12.5. Month 6.5 binding antibody categories were high, ≥1498.83 MFI; med, 421.08 to <1498.83 MFI; low, <421.08 MFI. Month 12.5 binding antibody categories were high, ≥1603.5 MFI; med, 468.25 to <1603.5 MFI; low, <468.25 MFI. Abbreviations: HIV-1, human immunodeficiency virus 1; IgG, immunoglobulin G; PFS, polyfunctionality score.
No significant associations with HIV-1 acquisition were observed with any secondary immune response variable at either time point, whether considered quantitatively or categorically (Table 1 and Table 2; Supplementary Figures 5–10). Pairwise correlations between primary and secondary endpoints are in Supplementary Figure 11.
No Significant Associations of Primary Immune Response Variables With HIV-1 Acquisition in Multivariate Analyses
When the Env ZM96 CD4+ polyfunctionality score, the IgG binding antibody A244 V1V2 responses, and the IgG3 binding antibody 1086 V1V2 responses were included in a multivariate Cox model, no significant associations with HIV-1 acquisition were observed at either month 6.5 or 12.5, whether continuous or categorical variables were considered (Table 1 and Table 2).
Interactions Between Month 6.5 IgG A244 V1V2 Binding Antibody and CD4+ T-Cell Responses Correlate With Risk of HIV-1 Acquisition
The association between CD4+ T-cell responses and HIV-1 risk qualitatively depended on the level of IgG A244 V1V2-directed binding antibody response (multiplicity-adjusted interaction P’s ≤ .03; Supplementary Figures 12–15). Among vaccinees with highest tertile IgG A244 V1V2 responses, vaccine-matched CD4+ T-cell endpoints (polyfunctional scores in response to Env-ZM96 and to 1086, triple-functional cells expressing IFN-γ, IL2, and CD40L in response to Env-ZM96) were associated with a 51%–60% lower acquisition risk (estimated hazard ratios = 0.40 to 0.49 per 1-SD increase in the respective CD4+ T-cell endpoint; Table 3). Conversely, among those with lowest tertile IgG A244 V1V2 binding antibody responses, CD4+ T-cell responses were associated with 2.2- to 3.6-fold higher risk of HIV-1 acquisition (Table 3). No significant interactions were observed between any primary or secondary endpoint and IgA score.
Table 3.
Results for Month 6.5 Immune Response Interaction Analyses of Per-Protocol Vaccine Recipients
| Variable, Continuous | IgG A244 V1V2, Categorical | HR Scale for Continuous Variable | HR (95% CI) | Unadjusted P | Adjusted P |
|---|---|---|---|---|---|
| CD4+ PFS ZM96 | Low | Per 1-SD | 2.20 (1.20–4.04) | ||
| Medium | Per 1-SD | 0.84 (.41–1.74) | <.0001 | .0017 | |
| High | Per 1-SD | 0.40 (.26–.63) | |||
| CD4+ PFS 1086 | Low | Per 1-SD | 3.59 (1.99–6.46) | ||
| Medium | Per 1-SD | 1.06 (.55–2.04) | <.0001 | .0002 | |
| High | Per 1-SD | 0.42 (.23–.76) | |||
| CD4+ PFS TV1 | Low | Per 1-SD | 2.30 (1.36–3.89) | ||
| Medium | Per 1-SD | 0.83 (.41–1.71) | .0016 | .033 | |
| High | Per 1-SD | 0.52 (.24–1.12) | |||
| CD4+ IFNg+/IL2+/CD40L+ ZM96 | Low | Per 1-SD | 3.20 (1.84–5.56) | ||
| Medium | Per 1-SD | 1.10 (.64–1.90) | <.0001 | .0001 | |
| High | Per 1-SD | 0.49 (.28–0.85) |
Cox proportional hazards regression results are shown for the models with significant interactions. Each model included 1 interaction term and associated main effects, adjusted for the baseline covariates age (≤25, >25 years) and a previously derived categorical HIV risk score for women.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Exploratory Immune Response Variables Show Little to No Evidence of a Significant Association With HIV-1 Acquisition in Univariate Analyses
Additional measurements including antigen-specific antibody responses were evaluated in exploratory analyses (Supplementary Text). Among the 206 exploratory immune responses assessed at month 6.5 and/or 12.5, one (IgG3 binding antibodies to gp70-TV1.GSKvacV1V2/293F, a subtype C vaccine protein-matched V1V2 antigen) was nominally significantly associated with HIV-1 acquisition among vaccine recipients in a univariate model when considered as a binary indicator (hazard ratio [HR] = 5.71; 95% CI, 1.97–16.54; Q = 0.13) at month 6.5. However, the number of positive responders was low among both cases (n = 9) and controls (n = 3), and this endpoint would not have passed the more stringent multiplicity correction applied to the primary/secondary correlates analysis. Moreover, the continuous version of the variable at month 6.5 was not significantly associated with HIV-1 acquisition (HR = 1.25; 95% CI, .80–1.93; Q = 0.82) and there were too few positive responders at month 12.5 for assessment (2 among the 34 cases, 4 among the 59 noncases; Supplementary Figure 16).
DISCUSSION
Through concerted effort and a significant body of work, immune correlates of HIV-1 acquisition risk in the RV144 trial have been established. However, it has remained an open question whether these correlates are generalizable to other populations and/or vaccine regimens. The HVTN 702 trial provided a unique opportunity to investigate this question in the context of a South African population vaccinated with a similar ALVAC/gp120 regimen. Several possibilities might explain why we did not identify a univariate correlation between any of the prespecified individual primary or secondary ALVAC/gp120 vaccine-induced T-cell or binding antibody immune responses and HIV-1 acquisition at either month 6.5 or 12.5. For instance, the IgG 1086 V1V2 response data are consistent with an explanation that the V1V2-directed binding antibody responses induced in HVTN 702 could have associated with HIV-1 acquisition but were of insufficient magnitude for this association to be detected. In addition, the V1V2 loop region among subtype C isolates has continued diversifying over the last decade, especially compared to the homogeneity in this region during RV144. This strain variation may also be a factor in the inability to identify associations in these nonneutralizing immune responses with HIV-1 acquisition. On the other hand, the CD4 polyfunctionality score and Env gp120-directed IgG binding antibody responses were also correlates of HIV-1 risk in RV144 and, despite high levels of these responses in HVTN 702, vaccine efficacy was not evident and, when considered univariately, these responses did not correlate with risk. Multivariate analyses of both RV144 and HVTN 505 supported that interactive combinations of antibody and T-cell responses impact HIV-1 risk [27, 28]. Our interaction results in HVTN 702 suggest that high levels of both IgG V1V2-directed binding antibodies and polyfuntional CD4+ T-cell responses may indicate protection from HIV-1 acquisition. This result, however, is partnered with evidence of a significant adverse association of polyfunctional CD4+ T-cell responses with HIV-1 acquisition when IgG V1V2 antibody responses are low.
The RV144 correlates of risk also may not directly translate to the HVTN 702 trial due to differences in vaccine regimen (eg, inserts, adjuvant, booster schedule), populations (eg, HIV incidence, HIV exposure, HLA background), and/or circulating viruses [29]. Different immune response types or specificities may be needed to prevent acquisition of the subtype C viruses that circulated in the HVTN 702 trial. In RV144, vaccine efficacy was greater against viruses with a vaccine-matched (vs mismatched) K169 residue [30], which was less frequent in circulating subtype C viruses than in RV144 placebo recipient CRF01_AE viruses [31]. A planned study of the HVTN 702 viral sequences may inform whether the vaccine applied immune pressure on viral genotypes. Genetic evolution in V1V2 has continued and the HVTN 702 vaccine regimen was less well matched, both overall and for the V2 region, to circulating strains in South Africa than the RV144 vaccine regimen to strains in Thailand [14]. It has also been reported that the A244 vaccine immunogen in the RV144 trial was a unique HIV-1 envelope immunogen in exposure of the V2 loop [32]. Additional study is needed to determine whether the V2-specific antibodies elicited here were to the alpha helical or beta sheet conformation; the former has been associated with decreased simian immunodeficiency virus risk [33]. In a preclinical alum-adjuvanted vaccine model, V2 antibodies correlated with reduced risk of acquisition [34, 35] (as in RV144 [16]). However, when the same preclinical vaccine was adjuvanted with MF59 [36], there were immunogenicity differences and no protection against virus acquisition [37], supporting that vaccine adjuvant significantly shapes the quality of the immune response and may impact vaccine efficacy.
The RV144 and HVTN 702 study populations also differed in the rate of HIV-1 incidence in women in the placebo group (0.3% in RV144 vs 4.2% in HVTN 702) [7], indicating that HIV-1 exposure may have been higher in the HVTN 702 trial. Immune correlates can be abrogated by frequent, heterogeneous, and/or high-pathogen-load exposure [38]. Vaccine efficacy in RV144 was higher in participants considered to be at low/moderate risk (vs high risk) of HIV-1 acquisition [9], whereas protection was not observed in any subgroup defined by baseline covariates in HVTN 702, including participants considered to be at low risk [7].
A limitation of this study was the lack of assessment of other immune biomarkers such as transcriptional signatures, mucosal responses, antibody Fc effector functions, host genetics, and genital inflammatory markers. Future work could examine whether any of these biomarkers associated with HIV-1 acquisition risk in HVTN 702. Additional limitations include the lack of data on HIV-1 exposures, the exclusion of individuals assigned male at birth due to low case counts, and the inability to assess the effect of booster doses [39] due to the limited case accrual after boost receipt.
Despite these limitations, the current study contributes to the HIV-1 vaccine field by demonstrating that the T-cell and binding antibody immune correlates of risk identified in RV144 were not significantly associated with HIV-1 acquisition in HVTN 702 and remain helpful benchmarks. Furthermore, our study raises the hypothesis that moderate to high levels of both Env V1V2-directed responses and high polyfunctional CD4+ T cells are needed for protection against HIV-1. These results expand the scientific knowledge from this valuable HIV-1 vaccine efficacy trial and, in concert with future studies including sequence analysis of breakthrough viruses, will further guide HIV-1 vaccine development.
Supplementary Data
Supplementary material is available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author
Supplementary Material
Contributor Information
Zoe Moodie, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
One Dintwe, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Cape Town HVTN Immunology Laboratory, Hutchinson Centre Research Institute of South Africa, Cape Town, South Africa.
Sheetal Sawant, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA.
Doug Grove, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Yunda Huang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Holly Janes, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Jack Heptinstall, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA.
Faatima Laher Omar, Cape Town HVTN Immunology Laboratory, Hutchinson Centre Research Institute of South Africa, Cape Town, South Africa.
Kristen Cohen, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Stephen C De Rosa, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Lu Zhang, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA.
Nicole L Yates, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA.
Marcella Sarzotti-Kelsoe, Department of Surgery, Duke University, Durham, North Carolina, USA; Department of Immunology, Duke University, Durham, North Carolina, USA.
Kelly E Seaton, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA.
Fatima Laher, Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Linda Gail Bekker, Desmond Tutu HIV Centre, University of Cape Town, Cape Town, South Africa.
Mookho Malahleha, Setshaba Research Centre, Soshanguve, South Africa; Synergy Biomed Research Institute, East London, South Africa.
Craig Innes, The Aurum Institute, Klerksdorp, South Africa.
Sheetal Kassim, Desmond Tutu HIV Centre, University of Cape Town, Cape Town, South Africa.
Nivashnee Naicker, Centre for the AIDS Programme of Research in South Africa, Durban, South Africa.
Vaneshree Govender, South African Medical Research Council, Durban, South Africa.
Modulakgotla Sebe, Aurum Institute, Tembisa, South Africa.
Nishanta Singh, South African Medical Research Council, Durban, South Africa.
Philip Kotze, Qhakaza Mbokodo Research Centre, Ladysmith, South Africa.
Erica Lazarus, Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Maphoshane Nchabeleng, Mecru Clinical Research Unit, Sefako Makgatho Health Sciences University, Pretoria, South Africa.
Amy M Ward, Department of Medicine, University of Cape Town, Cape Town, South Africa; Wellcome Centre for Infectious Diseases Research in Africa, Institute of Infectious Disease and Molecular Medicine, University of Cape Town, Cape Town, South Africa.
William Brumskine, Aurum Institute, Johannesburg, South Africa.
Thozama Dubula, Nelson Mandela Academic Clinical Research Unit and Department of Internal Medicine and Pharmacology, Walter Sisulu University, Mthatha, South Africa.
April K Randhawa, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Nicole Grunenberg, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
John Hural, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Jia Jin Kee, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
David Benkeser, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Yutong Jin, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Lindsay N Carpp, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Mary Allen, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Patricia D’Souza, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
James Tartaglia, Sanofi-Pasteur, Swiftwater, Pennsylvania, USA.
Carlos A DiazGranados, Sanofi-Pasteur, Swiftwater, Pennsylvania, USA.
Marguerite Koutsoukos, GSK, Wavre, Belgium.
Peter B Gilbert, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
James G Kublin, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lawrence Corey, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA.
Erica Andersen-Nissen, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Cape Town HVTN Immunology Laboratory, Hutchinson Centre Research Institute of South Africa, Cape Town, South Africa.
Glenda E Gray, Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa; South African Medical Research Council, Durban, South Africa.
Georgia D Tomaras, Center for Human Systems Immunology, Duke University, Durham, North Carolina, USA; Department of Surgery, Duke University, Durham, North Carolina, USA; Department of Immunology, Duke University, Durham, North Carolina, USA; Department of Molecular Genetics and Microbiology, Duke University, Durham, North Carolina, USA.
M Juliana McElrath, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Medicine, University of Washington, Seattle, Washington, USA.
Notes
Acknowledgments. We are indebted to the participants of the Uhambo/HVTN 702 clinical trial. We thank the clinical and protocol teams; members of the trial-site community and the HVTN core staff; staff members of the Hutchinson Centre Research Institute of South Africa, including Stephany Wilcox, Saleha Omarjee, Shamiska Rohith, Boitumelo Mosito, and Asiphe Besethi and Mahlodi Montlha, Brownwill Herringer, Sarah Everett, Chadwin Rushin, and Nicolette Schuller in the Cape Town HVTN Immunology Laboratory; the Statistical Center for HIV/AIDS Research and Prevention; the HVTN Laboratory Center teams including Kelvin Chiong, Yong Lin, Judith T. Lucas, Tara McNair, Mike Archibald, David Beaumont, Kristy Long, Xiaoying Shen; the Vaccine Research Program of the Division of AIDS of the NIH and the NIAID; the Pharmaceutical Affairs Branch; P5 partners, including Sanofi Pasteur, GSK, the Bill and Melinda Gates Foundation, the NIAID, the NIH, the US Military HIV Research Program, and the South African Medical Research Council; Nina Russell, Margaret Johnston, Susan Barnett, Lut Van Damme, Silvija Staprans, and Pervin Anklesaria of the Bill and Melinda Gates Foundation for their counsel; and Mary Marovich and Michael Pensiero of NIAID.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). GlaxoSmithKline Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors are solely responsible for final content and interpretation.
Financial support. This work was supported by the NIH, National Institute of Allergy and Infectious Diseases (NIAID) (grant numbers UM1 AI068614 to the HIV Vaccine Trials Network [HVTN], UM1 AI068635 to the HVTN Statistical Data and Management Center, Fred Hutchinson Cancer Research Center [FHCRC], and UM1 AI068618 to HVTN Laboratory Center, FHCRC); with additional support by the Center for AIDS Research, Duke University (NIH grant number AI P30 AI064518) and the Bill and Melinda Gates Foundation (grant number OPP1146996). Support was provided to Novartis Vaccines and Diagnostics (now part of the GlaxoSmithKline Biologicals SA) by NIAID (grant numbers HHSN272201300033C/HHSN272201600012C) for the selection and process development of the 2 gp120 envelope proteins TV1.C and 1086.C; and by the Bill and Melinda Gates Foundation Global Health (grant number OPP1017604) and NIAID for the manufacture and release of the gp120 clinical-grade material.
Potential conflicts of interest. M. K. is an employee of the GSK group of companies and holds shares in the GSK group of companies. Z. M., L. N. C., and P. B. G. received a contract from Sanofi Pasteur within the previous 3 years to conduct statistical analysis work (outside the scope of the current work; related to the CYD-TDV dengue vaccine) and submit the results for publication. P. B. G. is also on the Bill and Melinda Gates Foundation Advisory Boards on Vaccine Development (unpaid). C. A. D. G. was an employee of Sanofi Pasteur at the time of study execution. T. D. received funding from Fred Hutchinson Cancer Center to attend the HVTN regional and full group meetings. M. A. is employed by NIAID, the study sponsor. The coauthors are current recipients of NIAID funding, and the publication is a result of activities funded by NIAID. MA was not involved in the process of funding these awards, not in their administration or scientific aspects, and, in accordance with NIAID policies, is deferred from decisions regarding funding of coauthors for a requisite period. G. D. T. received consulting fees from Janssen, Axon Consulting, and Gilead within the past 36 months; received payment for lectures, presentations, speaker bureaus, manuscript writing or educational events from the UNC Scientific Advisory Board, the NIH Board of Scientific Counselors, and Johns Hopkins within the last 36 months; and received support from her institution for attending meetings/travel to scientific network meetings. N. G. declares that Sanofi Pasteur provided the study product for the trial (ALVAC-HIV), declares that GSK provided study product for the trial (bivalent gp120/adjuvant); and is a CoVPN member representative on Protocol Team and Protocol Safety Review Team for Sanofi’s phase 3 COVID-19 vaccine program with funding provided by the NIH via a grant paid to her institution. Y. H. received contracts through her institution from the World Health Organization within the previous 3 years to conduct statistical analysis work (outside the scope of the current work; related to the SARS-CoV-2 vaccine). S. C. D. R. was awarded contracts to his institution from Janssen and Battelle within the past 36 months; and was awarded grants to his institution from the Gates Medical Research Institute and the Paul G. Allen Family Foundation within the past 36 months. M. J. M. received funding from DAIDS for the HVTN Laboratory Center as Principal Investigator within the past 36 months. N. L. Y. received support from the HVTN in the form of registration payment for online conferences (paid directly to the conference). M. N. received support paid to their institution within the last 36 months from DAIDS for attending meetings/travel. J. H. and L. Z. received support from NIH NIAID to support attendance and travel to HVTN Full Group Meeting (paid directly to conference). E. L. received support from the NIH DAIDS HIV Vaccine Trials Unit in the form of salaries, equipment, supplies, and related costs to conduct the HVTN 702 clinical trial protocol at Soweto-Kliptown site. S. K. received support from the HVTN for travel and accommodation related to scientific meetings. K. E. S. received support from NIH NIAID in the form of registration payment for online conferences (paid directly to conference). J. T. is an employee of Sanofi and has performance shares awarded by the company. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Flynn NM, Forthal DN, Harro CD, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654–65. [DOI] [PubMed] [Google Scholar]
- 2. Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006; 194:1661–71. [DOI] [PubMed] [Google Scholar]
- 3. Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the step study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372:1881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 2011; 11:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 6. Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013; 369:2083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gray GE, Bekker LG, Laher F, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N Engl J Med 2021; 384:1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institutes of Health . News release. HIV vaccine candidate does not sufficiently protect women against HIV infection. 31 August 2021. https://www.nih.gov/news-events/news-releases/hiv-vaccine-candidate-does-not-sufficiently-protect-women-against-hiv-infection. Accessed 15 January 2022.
- 9. Robb ML, Rerks-Ngarm S, Nitayaphan S, et al. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 2012; 12:531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray GE, Huang Y, Grunenberg N, et al. Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa. Sci Transl Med 2019; 11:eaax1880. 10.1126/scitranslmed.aax1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell ND, Marovich MA. Pox-Protein Public Private Partnership program and upcoming HIV vaccine efficacy trials. Curr Opin HIV AIDS 2016; 11:614–9. [DOI] [PubMed] [Google Scholar]
- 12. Bekker LG, Moodie Z, Grunenberg N, et al. Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial. Lancet HIV 2018; 5:e366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laher F, Moodie Z, Cohen KW, et al. Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines. PLoS Med 2020; 17:e1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen X, Laher F, Moodie Z, et al. HIV-1 vaccine sequences impact V1V2 antibody responses: a comparison of two poxvirus prime gp120 boost vaccine regimens. Sci Rep 2020; 10:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao LP, Fiore-Gartland A, Carpp LN, et al. Landscapes of binding antibody and T-cell responses to pox-protein HIV vaccines in Thais and South Africans. PLoS One 2020; 15:e0226803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 2007; 323:39–54. 10.1016/j.jim.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 2008; 82:12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosseinipour MC, Innes C, Naidoo S, et al. Phase 1 human immunodeficiency virus (HIV) vaccine trial to evaluate the safety and immunogenicity of HIV subtype C DNA and MF59-adjuvanted subtype C envelope protein. Clin Infect Dis 2021; 72:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tomaras GD, Ferrari G, Shen X, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 2013; 110:9019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 2014; 9:e87572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yates NL, Liao HX, Fong Y, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. 10.1126/scitranslmed.3007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin L, Finak G, Ushey K, et al. COMPASS Identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 2015; 33:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zolla-Pazner S, Edlefsen PT, Rolland M, et al. Vaccine-induced human antibodies specific for the third variable region of HIV-1 gp120 impose immune pressure on infecting viruses. EBioMedicine 2014; 1:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benkeser D, Carone M, Laan MJV, Gilbert PB. Doubly robust nonparametric inference on the average treatment effect. Biometrika 2017; 104:863–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57:289–300. [Google Scholar]
- 27. Tomaras GD, Haynes BF. Advancing toward HIV-1 vaccine efficacy through the intersections of immune correlates. Vaccines (Basel) 2014; 2:15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fong Y, Shen X, Ashley VC, et al. Modification of the association between T-cell immune responses and human immunodeficiency virus type 1 infection risk by vaccine-induced antibody responses in the HVTN 505 trial. J Infect Dis 2018; 217:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zolla-Pazner S, Michael NL, Kim JH. A tale of four studies: HIV vaccine immunogenicity and efficacy in clinical trials. Lancet HIV 2021; 8:e449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rolland M, Edlefsen PT, Larsen BB, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 2012; 490:417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rademeyer C, Korber B, Seaman MS, et al. Features of recently transmitted HIV-1 clade C viruses that impact antibody recognition: implications for active and passive immunization. PLoS Pathog 2016; 12:e1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen X, Duffy R, Howington R, et al. Vaccine-induced linear epitope-specific antibodies to simian immunodeficiency virus SIVmac239 envelope are distinct from those induced to the human immunodeficiency virus type 1 envelope in nonhuman primates. J Virol 2015; 89:8643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silva de Castro I, Gorini G, Mason R, et al. Anti-V2 antibodies virus vulnerability revealed by envelope V1 deletion in HIV vaccine candidates. iScience 2021; 24:102047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pegu P, Vaccari M, Gordon S, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol 2013; 87:1708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaccari M, Fourati S, Gordon SN, et al. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14+ monocytes is associated with a decreased risk of SIVmac251 acquisition. Nat Med 2018; 24:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaccari M, Gordon SN, Fourati S, et al. Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med 2016; 22:762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tomalka JA, Pelletier AN, Fourati S, et al. The transcription factor CREB1 is a mechanistic driver of immunogenicity and reduced HIV-1 acquisition following ALVAC vaccination. Nat Immunol 2021; 22:1294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edlefsen PT. Leaky vaccines protect highly exposed recipients at a lower rate: implications for vaccine efficacy estimation and sieve analysis. Comput Math Method M 2014; 2014:813789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pitisuttithum P, Nitayaphan S, Chariyalertsak S, et al. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV 2020; 7:e238–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.