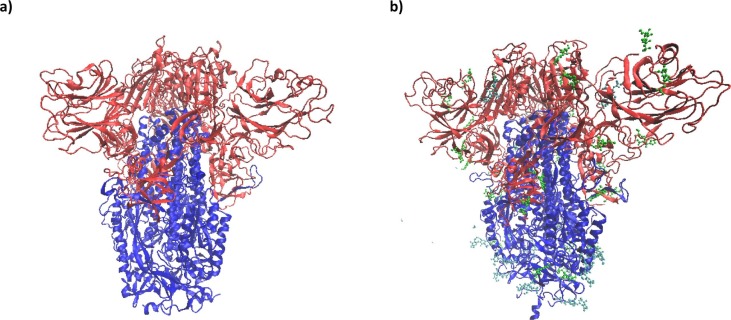
Graphical abstract
Abstract
A lot of effort has been made in developing vaccine and therapeutic agents against the SARS-CoV-2, concentrating on the Spike protein that binds angiotensin-converting enzyme 2 on human cells. Nowadays, some researches study the role of the N-linked glycans as potential targets for vaccines and new agents. Due to the flexibility and diversity of the N-linked glycans, in this work, we focus on the N-Acetylglucosamine moiety, which is the precursor of nearly all eukaryotic glycans. We performed molecular dynamics simulations to study the effects of the N-Acetylglucosamine on the stability of the spike glycoprotein in SARS-CoV-2. After a 100 ns of simulation on the spike proteins without and with the N-Acetylglucosamine molecules, we found that the presence of N-Acetylglucosamine increases the local stability in their vicinity; even though their effect on the full structure is negligible. Thus; it can be inferred that the N-Acetylglucosamine moieties can potentially affect the interaction of the S protein with the ACE2 receptor. We also found that the S1 domain is more flexible than the S2 domain. We propose which of the experimentally observed glycans found on the spike may be more functional than the others. Detailed understanding of glycans is key for the development of new therapeutic strategies.
1. Introduction
The SARS-CoV-2 genome encodes various nonstructural proteins [1], [2], [3], [4] needed for replication and transcription, structural proteins [5], [6], [7], [8], [9], [10], [11] needed for virus assembly and entering the host cell and accessory proteins [12], [13], [14], [15], [16] which play important roles in pathogenesis. To date, a large amount of experimental [17], [18], [19], [20], [21], [22], [23] and theoretical studies [24], [25], [26], [27], [28] have been conducted elucidating the structure of the SARS-CoV-2. There are also a wide variety of studies showing that the spike [29], [30], [31], [32], [33], [34], the main protease [35], [36], [37], [38], the RNA-dependent RNA-Polymerase [39], [40] and some other non-structural proteins such as protein 1 [41], protein 15 [42] and protein 16 [43] can be potential targets in the development of therapeutic drugs and the design of vaccines to fight this virus [44], [45], [46], [47].
Among the proteins in SARS-CoV-2, the S protein, assumed to be the most important one in binding to a receptor and sneaking into the host cell, is the natural target of antibodies. For this reason, the three-dimensional shape and dynamics of this protein are extremely important to understand [48]. The SARSCoV-2 S glycoprotein exists as a homotrimer (I will call each monomer as Chain A, Chain B, Chain C) composed of two sub-units: The S1 subunit that binds to the host cell receptor, and the S2 subunit that takes part in the fusion of the viral and the cellular membranes. These two domains of the S glycoprotein are shown in Fig. 1 . Walls et al. [48] determined the cryo-EM structures of the SARSCoV-2 S glycoprotein in two distinct conformations and stored the data as 6VXX.pdb (closed SARS-CoV-2 S) and 6VYB.pdb (SARS-CoV-2 S with one SB open) in RCSB PDB. Some comparative studies have also been conducted among the spikes of other coronaviruses [49], [50], [51], [52], [53] and the coronavirus spikes with other viral proteins [54], [55].
Fig. 1.
S1 (red) ve S2 (blue) domains of a) S-without-GlcNAC b) S-with-GlcNAC.
Various related coronavirus S glycoproteins are extensively patched with heterogeneous N-linked glycans (NAGs) hanging from the trimer surface that are important for proper folding [56], viral entry and to create a shield against the human immune system on the protein surface [57], [58], [59], [60], [61]. Extensive NAG molecules have also been characterized at the interface of SARS-CoV-2 S (Fig. 2 ) [62], [63], [64]. Wang et al. [65] summarized the importance of the NAG molecules on the S protein in viral entry and antibody production. Yang et al. [66] demonstrated that N-glycans have a major role in viral entry developing Spike-mutant pseudovirus without extended N-glycans. In another study, to investigate the roles of these Glycans on viral entry, [67] carried out various N-glycosylation site mutations and found that N61Q and N801Q mutations impaired the viral entry. An MD study conducted by Woods et al. [64] revealed the importance of the S protein glycans in shielding the immune recognition. Amaro et al. [68] studied the role of glycans on the SARS-CoV-2 S protein in the process of infection. Namely, before the S protein, which has a glycan coat on its surface, interacts with the angiotensin- converting enzyme 2 (ACE2) on a host cell, it changes its shape to reveal the receptor-binding domain (RBD). These authors carried out the all-atom molecular dynamics simulations (MD) of the S protein buried in the viral membrane, and showed that two NAG molecules at positions N165 and N234 have an important structural role in stabilizing the RBD of S1 “up” conformation, which could help promote infection. They also experimentally observed that S protein’s binding to ACE2 was reduced by mutations made to not have glycans at N165 and N234. Another MD study by the same group [69] revealed the gating role of the certain glycan, N343, in spike opening, with the participation of D405, R408 and D427 amino acids with the help of the weighted ensemble MD simulations and some experimental techniques. Their work provided a better understanding of the spike RBD activation mechanism. Ghorbani et al. [70] further analyzed the dynamics of the individual glycans and microdomains of glycans with MD simulations and network analysis for both RBD-up (open state) and RBD-down (closed state). They found that the two NAG molecules in the open state at positions N165 and N234 show a high betweenness centrality due to their interactions with neighboring glycans; complementing and confirming [68]. Cao et al. [71] formed a variety of S trimer-antibody complexes based on fully glycosylated S protein models and emphasized the importance of glycans in S-antibody binding with MD simulations. Sikora et al. [72] performed MD simulations of the system including a viral membrane patch with fully glycosylated and palmitoylated S proteins to identify possible antibody binding sites; since the glycans that coat the outer surface of the spike can also interfere with the binding of antibodies. Bernardi et al. [73] computationally developed the fully glycosylated models for the fusion of the extracellular domain of ACE2 with the Fc domain of human immunoglobulin. They stated in their therapeutic design study that glycosylation affects protein structure, and so potentially the interaction between S protein and ACE2. Then, they expanded their model [74] by exploring the role of glycosylation in enhancing binding to ACE2 with the steered MD simulations. Lokhande et al. [75] suggested that carbohydrate binding agents, with their antiviral potential against coronaviruses and some enveloped viruses, could be used to target the NAG molecules of the S proteins. They studied the stability and binding of “Lectins” and “Pradimicin-A” with the S glycans by docking and MD simulations. The importance of the S protein glycosylation was also shown by the recent article written by Huang et al. [76]. They found that the Mono-GlcNAc-decorated spike vaccine was highly effective against the Covid-19 variants. In addition to the studies stating that the role of glycans in binding ACE2 is important, Nguyen et al. [77] interestingly stated that the RBD-ACE2 contact did not change in the presence or absence of different glycoforms, proposing that the interaction of RBD and ACE2 is robust. Mehdipour and Hummer [78] performed MD simulations by modeling the fully glycosylated ACE2 receptor bound to the RBD of the S protein. As a result, they found that glycosylation of the ACE2 receptor greatly contributes to virus binding. Rivetingly, N90 and N322 glycans have opposite effects on binding of spike protein. The N90 glycan may intervene in the binding of the spike protein as it partially covers the binding interface of the spike RBD and so may protect against the entry of the virus into the cell, while the N322 glycan tightly interacts with the RBD of the spike and strengthens the complex.
Fig. 2.
“QuickSurf” representation of SARS-CoV-2 S Protein a) without GlcNAc b) with GlcNAc at the interface*. (* GlcNAc moieties are represented by Blue CPK which we call as ANX and Red CPK which we call as NAN).
Experiments also observed that each glycosylation site of S protein contained a large number of glycans in different compositions and abundances [63], [79], [80], [81]. This large number of glycoforms can be reduced by selecting the compound with the highest abundance for each glycosylation site based on the biosynthetic pathway information of NAGs [82]. But, still there are so many combinations to model the S protein with glycans. All eukaryotic NAGs begin with the covalent attachment of N-acetylglucosamine (GlcNAc) to the Nitrogen atom of an Asparagine (Asn) (Asn-GlcNAc) [83]. Because of such glycan diversity, in this study, we concentrated on the Asn-GlcNAc moiety. Although the role of the GlcNAc on the S protein has not been well studied, it is known that it has important roles in cell signaling [84], [85], [86], [87], [88], [89]. Also, Lee [90] found that the Asn-linked GlcNAc of the calcitonin receptor N130 is an important peptide affinity enhancer for the amylin receptor 2 (AMY2) extracellular domain and an important role of Asn-linked GlcNAc in AMY2 function.
16 out of 22 SARS-CoV-2 S Asn-GlcNAc molecules per monomer (in total 48) were observed in cryo-EM [48] (Table 1, Fig. 2b); however, computer simulation of closed form (6VXX.pdb) spike protein with Asn-GlcNAc molecules is not easy for the reason that the existing force fields are not defined for such mixed molecules. So, I introduced the Asn-GlcNAc to the system as a pseudo-amino acid. Details are given in the “Material and Methods” section.
Table 1.
Positions of GlcNAc molecules in SARS-CoV-2 S.
| GlcNAc molecules | Corresponding residues | Given names |
|---|---|---|
| (in Ref.[48]) | (in this simulation) | |
| N61VT | N80VT | ANX |
| N122AT | N141AT | ANX |
| N165CT | N184CT | ANX |
| N234IT | N253IT | NAN |
| N282GT | N301GT | ANX |
| N331IT | N350IT | ANX |
| N343AT | N362AT | ANX |
| N603TS | N622TS | ANX |
| N616CT | N635CT | ANX |
| N657NS | N676NS | ANX |
| N709NS | N728NS | ANX |
| N717FT | N736FT | NAN |
| N801FS | N820FS | NAN |
| N1074FT | N1093FT | ANX |
| N1098GT | N1117GT | NAN |
| N1134NT | N1153NT | NAN |
In experiments, in modeling and simulations, and in therapeutic drug design studies, the interaction of glycosylated S protein with various receptors, membranes, surfaces or other viral proteins have been investigated. However, the effects of the GlcNAC molecules, which are the starting part of N-linked Glycans, in the stability of the spike protein have not been studied. In this work, we explore these effects and show that GlcNAC molecules contribute to the local stability of the spike protein. In this context, 100 ns MD simulations of closed SARS-CoV-2 S without GlcNAc (S-without-GlcNAc) and with GlcNAc (S-with-GlcNAc) have been performed to better understand the role of the GlcNAc molecule on the stability of the S protein.
2. Material and Methods
2.1. Setup of the system
The structure of the SARS-CoV-2 S glycoprotein (in the closed state) was taken from the Protein Data Bank with the 6VXX code [48]. The missing residues were added using the Swiss Pdb-Viewer program [91] in order to complete the structure before running the MD simulations. Since terminal residues were not present in the PDB coordinates, each -mer of the S protein was capped with an acetyl group (ACE) at the N-terminus and an amino group (NH2) at the C-terminus. Since Gromos 54a7 force field already has parameters for ACE and NH2, the coordinates for the capping groups were obtained via ArgusLab and added to our input coordinate file. But it should also be noted that; if, instead of adding capping groups, the amino group (–NH2) were chosen as the starting terminus type for ALA and the carboxyl group (–COOH) as the end-terminus type for SER, the partial charge distributions on the ALA and SER residues would have changed, even if the total charge of the system would not have changed [Table SI-1]. However, since the N- and C- termini of each -mer of the S protein are located far from the RBD [Figure SI-1], which is known to be essential in binding to ACE2, it is not expected to have much effect on the stability of the system.
The difficulty, as mentioned above, is that the GlcNAc molecule, and so the GlcNAc-linked amino acid, is not a known entity in any force field of GROMACS [92]. For this reason, the Asn-GlcNAc molecules were introduced as pseudo amino acids into the Gromos 54a7 united-atom force field [93]. The S protein resolved by Walls et al. [48], GlcNAc is linked to the Asparagine (Asn) in two configurations: One GlcNAc bound to Asn (Fig. 3 a) which I called as ANX; and two GlcNAcs bound to Asn which I called as NAN (Fig. 3b). The geometries of the ANX and NAN were created by ArgusLab [94] and then their geometry optimizations were carried out by molecular mechanics calculation. The partial charges of geometry-optimized structures were obtained by single point calculations at the B3LYP-D3/6-31G**++ level of theory, in vacuum, using the Jaguar 10.7 program package [95]. The topologies of ANX and NAN were obtained ArgusLab and ProDrug [96] and validated also by single point calculations at the B3LYP-D3/6-31G**++ level of theory. The description of atom types (including united-atom aliphatic CH, CH2, and CH3 groups) and the corresponding van der Waals interaction parameters are directly taken from the GROMOS 54A7 force field. The bonded parameters (bonds, angles, dihedrals and impropers) were directly taken from GROMOS 54A7 force field. The charges of C5 (in ANX) and C33 (in NAN) atoms corresponding to CB (neutral) are −0.37120 e and −0.34932 e, respectively, while C6 (in ANX) and C34 (in NAN) corresponding to CG (0.29000 e) atoms have charges of 0.91083 e and 0.93261 e, respectively. The charges of O7 (in ANX) and O35 (in NAN) atoms corresponding to OD1 (-0.45000 e) are −0.42449 e and −0.46066 e, respectively. The charge of the O22 atom that binds C5 (-0.05570 e) and C16 (-0.19349 e) in NAN is −0.03884 e. The charges of N8 (in ANX) and N36 (in NAN) atoms corresponding to ND2 (-0.72000 e) atoms are −0.58124 e and −0.45603 e, respectively. While O and N atoms are always negatively charged, C atoms can be either negative or positive. The bonded and non-bonded interactions described for CB, CG, OD1 and ND2 of ASN in Gromos 54a7 force field were also used for the corresponding atoms of ANX (C5, C6, O7 and N8) and NAN (C33, C34, O35 and N36). The bonds formed by the ND2 atom of ASN and the C atom of GlcNac (C9 for ANX and C19 for NAN) were represented by gb_21 which is defined for CHn - N, NT, NL, NZ, NE; and the C16 atom of the middle ring and the C5 atoms of the left ring with O22 were represented by gb_20 which is defined for CHn - OA (sugar). Then the parameters of new pseudo-amino acids, ANX and NAN, were added to the aminoacids.rtp file of Gromacs 54a7 force field. All of the parameters are provided in Table SI-2. Thus, we obtained the complete model of the S glycoprotein of SARS-CoV-2 (S-with-GlcNAc). Thus, we obtained the complete model of the S glycoprotein of SARS-CoV-2 (S-with-GlcNAc). Besides, in order to understand what effects the GlcNAc molecule has on the S protein, we also created a system which is devoid of the GlcNAc molecules (S-without-GlcNAc) for comparison.
Fig. 3.
Structures of a) ANX and b) NAN.
2.2. Molecular dynamics (MD) simulations
S-without-GlcNAC and S-with-GlcNAC proteins were put in separate rhombic dodecahedron box filled with a SPC [97] type water molecules. Minimum image convention is set with the S-trimers in the center of the boxes and at a distance 1.0 nm to the edges of the boxes. We added 15Na+ ions (5 Na+ ions per -mer) to neutralize the charge of the systems. Having obtained electro-neutral S-trimers in boxes of water, a two-stage minimization was carried out with the steepest-descent algorithm for S-with-GlcNAC, each with a maximum of 50,000 steps and a force of < 10.0 kJ/(mol.nm); first with define = -dflexible option (with 50,000 steps and < 10.0 kJ/mol.nm force) and then run without it (rigid water) and only the latter minimization was carried out for S-without-GlcNAC. Following minimizations, each structure was equilibrated in two steps as 100 ps NVT and 100 ps NPT respectively with position constraints for the protein, to stabilize the temperature at 300 K and the pressure at 1 bar. Initial velocities were generated from a Maxwell–Boltzmann distribution at 300 K at the NVT. Production runs of MD simulations were carried out for 100 ns in the absence of position constraints. For this data collection part for the trajectory analysis, the temperature was kept constant with a coupling time constant of 0.1 ps using a velocity rescaling thermostat [98] with two coupling groups (Protein and non-Protein groups). The pressure was kept constant with a coupling time constant of 2.0 ps using a Berendsen barostat [99] with a coupling time constant of 2.0 ps. To examine the time evolution of the S trimers (S-without-GlcNAC and S-with-GlcNAC separately), Newton's equation of motion was integrated numerically using the Leap-frog algorithm with a 2 fs time step. The linear constraint solver (LINCS) algorithm [100] was applied to bonds involving hydrogen atoms. Short-range electrostatic and van der Waals interactions were cut off at 1.4 nm using the Verlet scheme [101] and long-range electrostatic interactions were calculated using the particle mesh Ewald (PME) [102] algorithm. Long range dispersion corrections for both the energy and the pressure were applied to crank into the truncation of van der Waals terms. Periodic boundary conditions were applied in the ×,y and z directions. All the calculations were carried out using the GROMACS 2019 [92] code and the GROMOS 54a7 force field [93] including the ANX and NAN residues. The reason why we chose Gromos54a7 force field in this study is that potential type of Gromos force fields includes parameters covering proteins, nucleotides, sugars etc. and also there are various comparative and consistent MD simulations with different force fields, studies on sugar parameterization with gromacs54a7 force field in the literature [103], [104], [105], [106], [107]. The snapshots were obtained using the visual molecular dynamics (VMD) software [108].
Note that we have also performed two more 100 ns production MD simulations for each protein with the above given properties; a separate 100 ns production MD simulation was performed after a longer equilibration run (1000 ps NVT and then 1000 ps NPT). We also extended the simulation of S-with-GlcNAc for another 50 ns (i.e. 150 ns in total) to be sure about the stabilization of the system.
3. Results and discussion
The coronavirus spike protein has a coating of NAG molecules in various locations and forms; thus, because of their heterogeneity and flexibility, the simulation of the S protein with NAG molecules is difficult to model. For these reasons, the GlcNAc moieties, which are the branching sugar residues, in NAGs were taken into account; and we modified the Gromos 54a7 force field including ANX and NAN residues to get the S-with-GlcNAc. Then, to understand if the GlcNAc molecules lead the changes in the dynamics of the S protein, we analyzed the MD trajectories both in the presence (S-with-GlcNAC) and absence of GlcNAcs (S-without-GlcNAc).
Structural stability along the simulations was analyzed using the backbone root mean square deviation (RMSD) relative to the energy-minimized configuration of the starting structures in three different ways. First, the RMSD of the S-without-GlcNAC and S-with-GlcNAC were compared. The RMSD values for both of our systems range from 0.5 nm to 0.6 nm. This result is consistent with the findings of [9] for the corresponding temperature (30 °C), where the RMSD values were found to lie between 0.6 nm and 0.7 nm. In another MD study [109] it was also found that the RBD region of spike displays a RMSD of 0.5 nm. In another MD study by Sahihi and Faraudo [110], the average RMSD values without glycans of S protein were found to be about 4.96 ± 0.19 Å for down conformations (corresponds to our S-without-GlcNAc system)”. Both of our systems reach stability after the first 20 ns of the simulation (Fig. 4 a). We also looked at the sidechain RMSD of S-with-GlcNAc (Figure SI-2). Second, since the S protein is made up of two domains, S1 and S2, we also analyzed the RMSDs of these parts without and with GlcNAc separately. The results are as follows.
Fig. 4.
Comparison of the root mean square deviation (RMSD) of the a) S-without-GlcNAC (blue) and S-with-GlcNAC (red) b) S1 domain without (purple) and with GlcNAc (pink) c) S2 domain without (cyan) and with GlcNAc (green) d) vicinity of the GlcNAc-linked residues (red) and the corresponding ASN residues (blue).
RMSD values of the S1 region is larger than that of the S2 region for both without-GlcNAc (Figure SI-3a; 0.63 nm for S1 region and 0.43 nm for S2 region) and with-GlcNAc (Figure SI-3b; 0.71 nm for S1 region and 0.39 nm for S2 region) structures. Thus, it might be concluded that the S2 region is slightly more stable than the S1 region which is consistent with the findings of [9].
For the S1 region (Fig. 4b), after 20 ns simulation, the difference in the RMSD values between S1-without-GlcNAc and S1-with-GlcNAc ranges from 0.04 nm to 0.09 nm and S1-with-GlcNAc (on average 0.71 nm) has larger RMSD values than S1-without-GlcNAc (on average 0.63 nm). Consistent with our result, another MD study by [111] presented that the RBD region of Spike showed a variation up to 0.75 nm.
In the S2 region (Fig. 4c), after 20 ns simulation, the difference in the RMSD values between S2-without-GlcNAc and S2-with-GlcNAc ranges from 0.02 nm to 0.07 nm; and these differences are less than that of the S1 region. Moreover, unlike the S1 region (Fig. 4c), S2-wihout-GlcNAc (on average 0.43 nm) has larger RMSD values than S2-with-GlcNAc (on average 0.39 nm). It can be inferred that the presence of the GlcNAc molecule makes the S2-domain more stable.
Third, since the GlcNAc molecules are known to have important roles in the cell signaling, we examined the vicinity of the Asn-GlcNAc residues and the corresponding Asn residues. Namely, we calculated the RMSD values between the GlcNAc-linked amino acids (ANX and NAN; Table 1 ) and the RMSD values between the corresponding Asn residues (that is Asn aminoacids without GlcNAc). Striking differences between them are observed; that is to say the RMSD value of GlcNAc-linked residues, which is less than or equal to 0.9 nm, appears to be more stable than the one without GlcNAc which is 2.7 nm on average (Fig. 4d). It can be inferred that the GlcNAcs make the Asn residues more stable. Yang et al. [66] have also experimentally shown that loss of N-glycan can trigger the Spike protein to lose its intactness.
To ensure the reproducibility of our data, we carried out three more 100 ns MD production runs (referred to as run A, run B and run C). For Run C, we used longer equilibration runs. The results (Figure SI-4a) indicate that RMSDs of new MD trajectories show almost the same tendency as our results. Although fluctuation is higher in run C up to 65 ns compared to the others, the average RMSD is around 0.6 for all runs after this point (Figure SI-4a). We also extended the simulation of S-with-GlcNAc to 150 ns (50 ns more) to be sure about the stabilization of the system (Figure SI-4b).
Cα root mean square fluctuations (RMSF) of every residue were calculated in reference to initial minimized structure as a function of time for each chain of S-without-GlcNAC and S-with-GlcNAC, separately (Figure SI-5). Most residues of each chain in the S-without-GlcNAC and S-with-GlcNAC fluctuated with a magnitude of<0.2 nm, and the fluctuations exceeded 0.25 nm corresponds to the turn structure. When the RMSF values of ANX, NAN and corresponding Asn residues are examined (Fig. 5 and Table SI-3), one observes that 253NAN and 350ANX residues in Chain A, 184ANX and 1117NAN in Chain B, and 184ANX and 736NAN residues in Chain C show high RMSF than the corresponding Asn residues.
Fig. 5.
The root mean square fluctuations (RMSF) of ANX, NAN and corresponding Asn residues.
Ghorbani et al. [70], as some of their results were mentioned above, also found low BC for 234A (253NAN in my model; see Table 1) and 165B (184ANX in my model) in the close state; this supports the high RMSF values we obtained for the same ANX and NAN residues. Because there is an inverse relationship between RMSF and BC observed by Penkler et al. [112]. According to our findings, 728ANX in chain A, 635ANX in Chain B and 1093ANX in Chain C in closed state show the least fluctuation compared to the other GlcNAcs (Table SI-3), so their effects on the shielding of spike protein are considered to be significant.
As a measure of compactness, that is to get an idea of the overall dimensions of the structures, the radius of gyration (Rg) was calculated throughout the simulations in three different ways. As in the case of RMSD results, Rg calculation of S-without-GlcNAC and S-with-GlcNAC (Fig. 6 a) showed that the two structures exhibited nearly similar fluctuations. Gyration values of the S1 region is greater than the S2 region for both without and with GlcNAc structures (Figure SI-6a and Figure SI-6b); 4.5 nm for S1 and 3.5 nm for S2 for both. S1 and S2 regions in S-with-GlcNAC (Figure SI-6c) are larger than S1 and S2 regions in S-without-GlcNAC (Figure SI-6d), as expected. However, if one zooms into the vicinity of the GlcNAc-linked residues, one observes that the structure without GlcNAc is floppier; compact-uncompact, than the structure with GlcNAc (Fig. 6b). It should be noted that in this study, counter-ions were added so that the total charge of the system is zero. So, if these simulations were carried out at high ion concentration or high ionic strength, or if the chemical entities that would change the concentration used in the experiments were used, the compactness could change.
Fig. 6.
Comparison of radius of gyration (Rg) plot of a) S-without-GlcNAc (blue) and S-with-GlcNAc (red) b) vicinity of the GlcNAc-linked residues (red) and the corresponding Asn residues (blue).
Moreover, solvent accessible surface area (SASA) as a function of time was analyzed. SASA plot of S-without-GlcNAC and S-with-GlcNAC showed no considerable difference (Fig. 7 a); that is, there is no remarkable shielding by glycans (consistent with [64]). SASA of S1 is greater than S2 for both structures (Figures SI-7a and SI-7b). The presence of GlcNAc did not cause much fluctuation in the SASA of the S1 region (Fig. 7b), but reduced the fluctuation in S2 region (Fig. 7c). SASA of GlcNAc-linked amino acids (Fig. 7d) are almost twice more than the SASA of the Asn residues without GlcNAc.
Fig. 7.
Comparison of solvent accessible surface area (SASA) of the a) S-without-GlcNAc (blue) and S-with-GlcNAc (red) b) S1 domain without (purple) and with (pink) GlcNAc c) S2 domain without (cyan) and with (green) GlcNAc d) vicinity of the GlcNAc molecules (red) and the corresponding Asn residues (blue).
We calculated the solvent accessibility of the individual Asn-GlcNAc residues and the corresponding Asn residues. As it is seen from the Table 2 and Fig. 7d, for all three-chains, residues ANX and NAN have almost 2 times more solvent-accessible surface areas than the corresponding Asn residues without GlcNAc. Also, NAN has a larger solvent-accessible surface area compared to ANX.
Table 2.
SASA of the individual glycan residues and corresponding ASN residues.
| Residue # | A-without-GlcNAc |
A-with-GlcNAc |
B-without-GlcNAc |
B-with-GlcNAc | C-without-GlcNAc |
C-with- GlcNAc |
|---|---|---|---|---|---|---|
| 80 141 184 253 301 350 362 622 635 676 728 736 820 1093 1117 1153 |
2.758 2.684 2.726 2.761 2.779 2.748 2.761 2.719 2.734 2.774 2.778 2.731 2.772 2.782 2.707 2.739 |
5.151 5.036 5.095 7.497 5.119 5.018 5.137 5.104 5.146 5.053 4.497 7.397 7.392 5.093 7.371 7.402 |
2.758 2.748 2.756 2.736 2.754 2.745 2.757 2.745 2.756 2.763 2.754 2.744 2.754 2.748 2.687 2.744 |
5.169 5.009 5.021 7.533 5.137 4.975 5.097 5.152 5.160 5.121 4.945 7.470 7.178 4.903 7.127 7.423 |
2.739 2.753 2.742 2.767 2.729 2.733 2.771 2.734 2.749 2.747 2.756 2.768 2.744 2.776 2.732 2.763 |
5.137 5.113 5.060 7.450 5.083 5.092 5.102 5.075 5.133 5.096 5.133 6.675 7.511 5.139 6.632 7.442 |
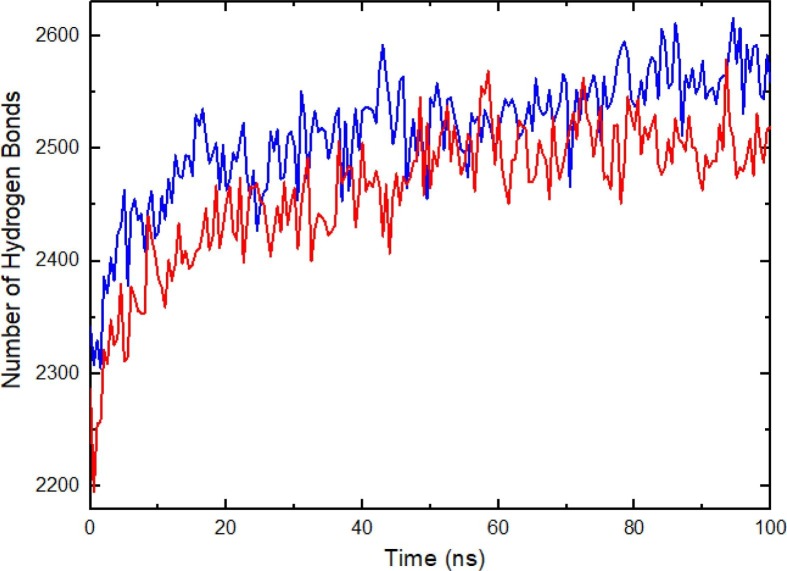
In the case of hydrogen bonds (Fig. 8 ) the cut-off distance and the cut-off angle criteria were set to 0.35 nm and 30° respectively. This analysis shows that the presence of the GlcNAc leads to a decrease in the hydrogen bonds network established within the structure. This further supports the DSSP results (Fig. 9 a,9b and Table 3 ). Namely; in the S-with-GlcNAC structure, the β-Sheet content decreases from 27 % to 24 %, while the coil content increases from 28 % to 30 %. Also, our results show similar secondary structure propensities with [111]; namely, secondary structure consists of “A-Helix + B-Sheet + B-Bridge + Turn”. The analysis of secondary structure as function of time was made using Dictionary of Secondary Structure of Proteins (DSSP) program by Kabsch and Sander [113]. There are no significant differences among the number of hydrogen bonds between the regions without and with GlcNAc structures (Figure SI-8a, Figure SI-8b, Figure SI-8c). In addition, Small-Angle X-ray Scattering (SAXS), which is another means of revealing the structure of the system, analysis was performed. In SI-9, we have depicted the SAXS structure factors for C-alpha atoms of S-with-NAG based on Cromer’s method. It also confirms that the structure reaches a dynamic equilibrium state after 100 ns simulation. The horizontal axis refers to the momentum transfer, while the vertical axis shows the intensity. The appearance of a distinct maximum is interpreted as the compactness of the structure.
Fig. 8.
Intra protein hydrogen bond pattern of S-without-GlcNAC (blue) and S-with-GlcNAC (red).
Fig. 9.
Secondary Structure content of the a) S-without-GlcNAC b) S-with-GlcNAC.
Table 3.
Percentage of Secondary Structure Content (Number).
| Secondary Structure | S-without-GlcNAc | S-with-GlcNAc |
|---|---|---|
| Structure Coil β- Sheet β- Bridge Bend Turn α- Helix 5- Helix 3-Helix |
56 % (381714) 28 % (186996) 27 % (180854) 2 % (12956) 15 % (98469) 10 % (64764) 18 % (123140) 0.0 (4 3 8) 1 % (8346) |
53 % (359283) 30 % (203426) 24 % (163319) 2 % (13641) 14 % (94905) 9 % (58685) 18 % (123638) 0.0 (1 8 0) 1 % (8521) |
Although our results regarding the stability of GlcNAc molecules on the S protein are consistent with other experimental/simulation studies in the literature as discussed, it should be noted that the results obtained in biomolecular simulations are always limited to the force fields used, hence further simulations with other force fields would be valuable.
4. Conclusions
The spike glycoprotein is the trimer structure decorated with the NAG molecules, located at the outermost part of the SARS-CoV-2, which enters the respiratory system by interacting with the human receptor ACE2. Some experiments and theoretical simulations show the importance of the NAG molecule on the stability of the different molecules. At the molecular level, however, the effect of NAG molecules on the SARS-CoV 2 structure is still not clear. Due to the structural diversity of NAGs, it is difficult to decide which type of glycan is linked to the Asn. However, it is very well known that the attachment of different glycans to Asn starts with the GlcNAc molecule. Therefore; in this study, Asn-GlcNAc molecules were introduced into the already-existing Gromos 54a7 force field as pseudo amino acids. 100 ns MD simulations of closed SARS-CoV-2 S (6VXX.pdb) were performed without and with GlcNAc to better understand the structures in atomic details and the importance of GlcNAc molecules on the stability of the SARS-CoV-2 S glycoprotein. This comparative study revealed that even though there are no striking differences between the stability of the full structures in the absence or presence of the GlcNAc molecules, GlcNAcs increase the local stability; that is to say the vicinity of GlcNAc molecules are much more stable than the corresponding Asn residues. If the presence or absence of GlcNAc moiety changes the protein’s stability and so its structure, it can be inferred that it can potentially affect the interaction of the S protein with the ACE2 receptor. Moreover, the S1 domain is more flexible than the S2 domain. Supporting the findings of Ghorbani et al. [70] and providing the additional findings, we found that 253NAN and 350ANX in Chain A, 184ANX and 1117NAN in Chain B, and 184ANX and 736NAN in Chain C are very fluctuated. Overall, these results can enlighten the nature of the SARS-CoV-2 S glycoprotein providing an information at the atomic level.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The numerical calculations reported in this paper were fully/partially performed at TUBITAK ULAKBIM, High Performance and Grid Computing Center (TRUBA resources). I would like to thank Dr. Süreyya Özcan Kabasakal, Dr. Ayşe B. Tekinay, Dr. İpek Güler and Dr. Jocelyne Vreede for useful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.comptc.2023.114049.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Yoshimoto F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2 or N-COV19), the Cause of COVID-19. Protein J. 2020;39(3):198–216. doi: 10.1007/s10930-020-09901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohaim M.A., El Naggar R.F., Clayton E., Munir M. Structural and Functional Insights Into Non-Structural Proteins of Coronaviruses. Microb Pathog. 2021;150 doi: 10.1016/j.micpath.2020.104641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benvenuto D., Angeletti S., Giovanetti M., Bianchi M., Pascarella S., Cauda R., et al. Evolutionary Analysis of SARS-CoV-2: How Mutation of Non-Structural Protein 6 (NSP6) Could Affect Viral Autophagy. J Infect. 2020;81(1):e24–e27. doi: 10.1016/j.jinf.2020.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R.N. Kirchdoerfer, A.B. Ward Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors, Nat. Commun., 10 (1) (2019), p. 2342. [DOI] [PMC free article] [PubMed]
- 5.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh C.-L., et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369(6510):1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi Y.K., et al. Structure, Dynamics, Receptor Binding, and Antibody Binding of the Fully Glycosylated Full-Length SARS-CoV-2 Spike Protein in a Viral Membrane. J. Chem. Theory Comput. 2021;17(4):2479–2487. doi: 10.1021/acs.jctc.0c01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arantes P.R., Saha A., Palermo G. Fighting COVID-19 Using Molecular Dynamics Simulations. ACS Cent. Sci. 2020;6(10):1654–1656. doi: 10.1021/acscentsci.0c01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rath S.L., Kumar K. Investigation of the Effect of Temperature on the Structure of SARS-CoV-2 Spike Protein by Molecular Dynamics Simulations. Front. Mol. Biosci. 2020;7(583523):1–13. doi: 10.3389/fmolb.2020.583523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong E., Huang X., Pearce R., Zhang Y., He Y. Computational design of SARS-CoV-2 spike glycoproteins to increase immunogenicity by T cell epitope engineering. Comput. Struct. Biotechnol. J. 2021;19:518–529. doi: 10.1016/j.csbj.2020.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 12.Velazquez-Salinas L., Zarate S., Eberl S., Gladue D.P., Novella I., Borca M.V. Positive Selection of ORF1ab, ORF3a, and ORF8 Genes Drives the Early Evolutionary Trends of SARS-CoV-2 During the 2020 COVID-19 Pandemic. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.550674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D.X., Fung T.S., Chong K.-K.-L., Shukla A., Hilgenfeld R. Accessory proteins of SARS-CoV and other coronaviruses. Antiviral Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson C.A., Pekosz A., Lee C.A., Diamond M.S., Fremont D.H. Structure and intracellular targeting of the SARS-coronavirus Orf7a accessory protein. Structure. 2005;13:75–85. doi: 10.1016/j.str.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang J., Han N., Chen Z., Peng Y., Li L., Zhou H., et al. Compositional Diversity and Evolutionary Pattern of Coronavirus Accessory Proteins. Brief Bioinform. 2021;22(2):1267–1278. doi: 10.1093/bib/bbaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redondo N, Zald́ıvar-Ló pez S, Garrido JJ and Montoya M (2021), SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns., Front. Immunol. 12:708264. [DOI] [PMC free article] [PubMed]
- 17.Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogando N.S., et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020;101(9):925–940. doi: 10.1099/jgv.0.001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee A., et al. Isolation, sequence, infectivity, and replication kinetics of severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2020;26:2054–2063. doi: 10.3201/eid2609.201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Zhong L.i. Genomics functional analysis and drug screening of SARS-CoV-2. Genes & Diseases. 2020;7(4):542–550. doi: 10.1016/j.gendis.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee A.K., Blanco M.R., Bruce E.A., Honson D.D., Chen L.M., Chow A., et al. SARS-CoV-2 disrupts splicing, translation, and protein trafficking to suppress host defenses. Cell. 2020;S0092–8674:31310–31316. doi: 10.1016/j.cell.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D. C. Malaspina and J. Faraudo, “Computer Simulations of the interaction between SARS-CoV-2 spike glycoprotein and different surfaces”, Biointerphases, 2020, 15(5), 051008 (1-10). [DOI] [PubMed]
- 25.A. Ali and R. Vijayan, “Dynamics of the ACE2–SARS‑CoV‑2/SARS‑CoV spike protein interface reveal unique mechanisms”, Sci. Rep., 2020, 10, 14214 (1-12). [DOI] [PMC free article] [PubMed]
- 26.Suárez D., Díaz N. SARS-CoV-2 Main Protease: A Molecular Dynamics Study. J. Chem. Inf. Model. 2020;60(12):5815–5831. doi: 10.1021/acs.jcim.0c00575. [DOI] [PubMed] [Google Scholar]
- 27.Selvaraj C., et al. Microsecond MD Simulation and Multiple-Conformation Virtual Screening to Identify Potential Anti-COVID-19 Inhibitors Against SARS-CoV-2 Main Protease. Front. Chem. 2021;8(595273):1–15. doi: 10.3389/fchem.2020.595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.J. A. Jaimes, N. M. André, J. S: Chappie, J. K. Millet, G. R. Whittaker, “Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically-sensitive activation loop”, J. Mol. Biol.2020, 432, 3309–3325. [DOI] [PMC free article] [PubMed]
- 29.Samrat S.K., Tharappel A.M., ZhongLi H.L. Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development. 2020;228 doi: 10.1016/j.virusres.2020.198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Struck A.-W., Axmann M., Pfefferle S., Drosten C., Meyer B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 2012;94:288–296. doi: 10.1016/j.antiviral.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh R. Identification of potential plant bioactive as SARS-CoV-2 Spike protein and human ACE2 fusion inhibitors. Computers in Biology and Medicine. 2021;136 doi: 10.1016/j.compbiomed.2021.104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhardwaj V.K., et al. Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors. Journal of Biomolecular Structure and Dynamics. 2021;39:3449–3458. doi: 10.1080/07391102.2020.1766572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhardwaj V.K., Singh R., Das P., R., Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs. Computers in Biology and Medicine. 2021;128 doi: 10.1016/j.compbiomed.2020.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R., et al. Benchmarking the ability of novel compounds to inhibit SARS-CoV-2 main protease using steered molecular dynamics simulations. Computers in Biology and Medicine. 2022;146 doi: 10.1016/j.compbiomed.2022.105572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chauhan M., et al. Theafavin 3-gallate inhibits the main protease (Mpro) of SARS-CoV-2 and reduces its count in vitro. Scientifc Reports. 2022;12:13146. doi: 10.1038/s41598-022-17558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh R., Bhardwaj V.K., Purohit R. Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach. Computers in Biology and Medicine. 2021;139 doi: 10.1016/j.compbiomed.2021.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.V. K.Bhardwaj et al. “Bioactive Molecules of Tea as Potential Inhibitors for RNA-Dependent RNA Polymerase of SARS-CoV-2”, Front. Med., 2021, 8, 684020, Sec. Precision Medicine. [DOI] [PMC free article] [PubMed]
- 41.R. Singh et al., “A computational approach for rational discovery of inhibitors for non-structural protein 1 of SARS-CoV-2”, Computers in Biology and Medicine 135 (2021) 104555. [DOI] [PMC free article] [PubMed]
- 42.Sharma J., et al. An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R. Singh et al., “In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors”, Journal of Traditional and Complementary Medicine 12 (2022) 35e43. [DOI] [PMC free article] [PubMed]
- 44.Mei-Yue Wang, Rong Zhao, Li-Juan Gao, Xue-Fei Gao, De-Ping Wang and Ji-Min Cao, SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development, Front. Cell. Infect. Microbiol., 2020, Sec. Virus and Host. [DOI] [PMC free article] [PubMed]
- 45.Yadav R., et al. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells. 2021;10:821. doi: 10.3390/cells10040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav R., Imran M., Dhamija P., Suchal K., Handu S. Virtual screening and dynamics of potential inhibitors targeting RNA binding domain of nucleocapsid phosphoprotein from SARS-CoV-2. J. Biomol. Struct. Dyn. 2020;1–16 doi: 10.1080/07391102.2020.1778536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walls et all., “Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein”, Cell, 2020, 180, 1–12. [DOI] [PMC free article] [PubMed]
- 49.Hatmal M.M., et al. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and Their Interactions with ACE2. Cells. 2020;9(2638):1–37. doi: 10.3390/cells9122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan M., et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coutard B., et al. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabaan A.A., et al. SARS-CoV-2, SARS-CoV, and MERS-CoV: A comparative overview. Infez. Med. 2020;28:174–184. [PubMed] [Google Scholar]
- 54.Watanabe Y., et al. “Vulnerabilities in coronavirus glycan shields despite extensive glycosylation” Nat. Commun. 2020;11:2688. doi: 10.1038/s41467-020-16567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Illanes-Álvarez F., et al. Similarities and differences between HIV and SARS-CoV-2. Int. J Med. Sci. 2021;18(3):846–851. doi: 10.7150/ijms.50133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossen J.W.A., et al. The Viral Spike Protein Is Not Involved in the Polarized Sorting of Coronaviruses in Epithelial Cells. J. Virol. 1998;72:497–503. doi: 10.1128/jvi.72.1.497-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walls A.C., et al. Glycan shield and epitope masking of a coronavirus spike protein observed by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2016;23:899–905. doi: 10.1038/nsmb.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong X., et al. Glycan shield and fusion activation of a deltacoronavirus spike glycoprotein fine-tuned for enteric infections. J. Virol. 2018;92:e01628–e1717. doi: 10.1128/JVI.01628-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y., et al. Two Mutations Were Critical for Bat-to-Human Transmission of Middle East Respiratory Syndrome Coronavirus. J. Virol. 2015;89:9119–9123. doi: 10.1128/JVI.01279-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pol-Fachin L., Verli H. Assessment of Glycoproteins Dynamics from Computer Simulations. Mini Rev. Org. Chem. 2011;8(3):229–238. [Google Scholar]
- 61.Huang X., et al. Glycosylation affects both the three-dimensional structure and antibody binding properties of the HIV-1IIIB GP120 Peptide RP135. Biochemistry. 1997;36:10846–10856. doi: 10.1021/bi9703655. [DOI] [PubMed] [Google Scholar]
- 62.Zhao P., et al. Virus-receptor interactions of glycosylated SARS-CoV-2 spike and human ACE2 receptor. Cell Host Microb. 2020;28(4):586–601. doi: 10.1016/j.chom.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant O.C., Montgomery D., Ito K., Woods R.J. Analysis of the SARS-CoV-2 spike protein glycan shield reveals implications for immune recognition. Sci. Rep. 2020;10(1):14991. doi: 10.1038/s41598-020-71748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao X., Chen H., Wang H. Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production. Front. Mol. Biosci. 2021;8(629873):1–10. doi: 10.3389/fmolb.2021.629873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Q., Hughes T.A., Kelkar A., Yu X., Cheng K., Park S., Huang W.C., Lovell J.F., Neelamegham S. Inhibition of SARS-CoV-2 viral entry upon blocking N- and O-glycan elaboration. eLife. 2020;9:e61552. doi: 10.7554/eLife.61552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Q., Kelkar A., Srıram A., Hombu R., Hughes T.A., Neelamegham S. “Role for N-glycans and calnexin-calreticulin chaperones in SARS-CoV-2 Spike maturation and viral infectivity”, Science. Advances. 2022;8 doi: 10.1126/sciadv.abq8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casalino L., et al. Beyond Shielding: The Roles of Glycans in SARS-CoV-2 Spike Protein. CS Cent. Sci. 2020;6(10):1722–1734. doi: 10.1021/acscentsci.0c01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sztain T., et al. A glycan gate controls opening of the SARS-CoV-2 spike protein. Nat. Chem. 2021;13:963–968. doi: 10.1038/s41557-021-00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghorbani M., Brooks B.R., Klauda J.B. Exploring dynamics and network analysis of spike glycoprotein of SARS-COV-2. Biophys. J. 2021;120:2902–2913. doi: 10.1016/j.bpj.2021.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Y., et al. Dynamic Interactions of Fully Glycosylated SARS-CoV-2 Spike Protein with Various Antibodies. J. Chem. Theory. Comput. 2021;17:6559–6569. doi: 10.1021/acs.jctc.1c00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sikora M., et al. Computational epitope map of SARS-CoV-2 spike protein. PLOS Comput. Biol. 2021;17(e1008790):1–16. doi: 10.1371/journal.pcbi.1008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernardi A., et al. Development and simulation of fully glycosylated molecular models of ACE2-Fc fusion proteins and their interaction with the SARS-CoV-2 spike protein binding domain. Plos One. 2020;15(e0237295):1–12. doi: 10.1371/journal.pone.0237295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y., et al. SARS-CoV-2 spike binding to ACE2 is stronger and longer ranged due to glycan interaction. Biophys. J. 2022;121:79–90. doi: 10.1016/j.bpj.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lokhande K.B., et al. Sensing the interactions between carbohydrate-binding agents and N-linked glycans of SARS-CoV-2 spike glycoprotein using molecular docking and simulation studies. J. Biomol. Struct. Dyn. 2020;1–19 doi: 10.1080/07391102.2020.1851303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang H.-Y., et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 2022;14(eabm0899):1–13. doi: 10.1126/scitranslmed.abm0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen K., Chakraborty S., Mansbach R.A., Korber B., Gnanakaran S. Exploring the Role of Glycans in the Interaction of SARS-CoV-2 RBD and Human Receptor ACE2. Viruses. 2021;13(927):1–18. doi: 10.3390/v13050927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehdipour A.R., Hummer G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. PNAS. 2021;118 (19), e2100425118:1–8. doi: 10.1073/pnas.2100425118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gong Y., Qin S., Dai L., Tian Z. The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct. Target. Ther. 2021;6(396):1–24. doi: 10.1038/s41392-021-00809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shajahan A., Pepi L.E., Rouhani D.S., Heiss C., Azadi P. Glycosylation of SARS-CoV-2: structural and functional insights. Anal. Bioanal. Chem. 2021;413:7179–7193. doi: 10.1007/s00216-021-03499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian Y., Parsons L.M., Jankowska E., Cipollo J.F. Site-Specific Glycosylation Patterns of the SARS-CoV-2 Spike Protein Derived From Recombinant Protein and Viral WA1 and D614G Strains. Front. Chem. 2021;9(767448):1–13. doi: 10.3389/fchem.2021.767448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Woo H., et al. Developing a Fully Glycosylated Full-Length SARS-CoV-2 Spike Protein Model in a Viral Membrane. J. Phys. Chem. B. 2020;124:7128–7137. doi: 10.1021/acs.jpcb.0c04553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.A. Varki et al., Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, Third Edition, ISBN is 9781621821328 / 1621821323.
- 84.Dennis J.W., Nabi I.R., Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139(7):1229–1241. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lau K.S., Khan S., Dennis J.W. Genome-scale identification of UDP-GlcNAc-dependent pathways. Proteomics. 2008;8(16):3294–3302. doi: 10.1002/pmic.200800208. [DOI] [PubMed] [Google Scholar]
- 86.T. Muramatsu, “Knockout Mice and Glycoproteins”, Editor: Hans Kamerling, Comprehensive Glycoscience, Elsevier, 2007, 121-147, ISBN 9780444519672.
- 87.J. B. Konopka, “N-Acetylglucosamine Functions in Cell Signaling”, Scientifica (Cairo). Vol: 2012; 2012, 489208 (1-15). [DOI] [PMC free article] [PubMed]
- 88.Moussian B. The role of GlcNAc in formation and function of extracellular matrices. Comp Biochem Physiol B Biochem Mol Biol. 2008;149(2):215–226. doi: 10.1016/j.cbpb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 89.Grigorian A., et al. Control of T cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J. Biol. Chem. 2007;282(27):20027–20035. doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- 90.Lee S. Asn-linked N–acetylglucosamine of the amylin receptor 2 extracellular domain enhances peptide ligand affinity. FEBS Open Bio. 2021;11:195–206. doi: 10.1002/2211-5463.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guex N., Peitsch M.C., Schwede T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis. 2009;30:S162–S173. doi: 10.1002/elps.200900140. [DOI] [PubMed] [Google Scholar]
- 92.M.J. Abraham, D. van der Spoel, E. Lindahl, B. Hess, and the GROMACS development team, GROMACS User Manual version 2019, http://www.gromacs.org.
- 93.Schmid N., et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur Biophys J. 2011;40:843–856. doi: 10.1007/s00249-011-0700-9. [DOI] [PubMed] [Google Scholar]
- 94.M. A. Thompson, “Molecular Docking Using ArgusLab, an Efficient Shape-Based Search Algorithm and the a Score Scoring Function”, ACS Meeting, Philadelphia, 2004.
- 95.Bochevarov A.D., et al. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013;113(18):2110–2142. [Google Scholar]
- 96.Schüttelkopf A.W., van Aalten D.M.F. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 97.Smith P., Vangunsteren W. The viscosity of SPC and SPC/E water at 277-K and 300-K. Chem. Phys. Lett. 1993;215(4):315–318. [Google Scholar]
- 98.Bussi G., Donadio D., Parrinello M.J. Canonical sampling through velocity rescaling. Chem. Phys. 2007;126:014101–014107. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 99.Berendsen H.J.C., Postma J.P.M., van Gunsteren W.F., DiNola A., Haak J.R. Molecular-Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 100.Hess B., Bekker H., Berendsen H., Fraaije J. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 1997;18(12):1463–1472. [Google Scholar]
- 101.Verlet L. Computer 'experiments' on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules. Phys. Rev. 1967;159:98–103. [Google Scholar]
- 102.Darden T., York D., Pedersen L. Particle mesh Ewald – an n log(n) method for Ewald sums in large systems. J. Chem. Phys. 1993;98(12):10089–10092. [Google Scholar]
- 103.F. Samsudin, A. Boags, T.J. Piggot, and S. Khalid, “Braun’s Lipoprotein Facilitates OmpA Interaction with the Escherichia coli Cell Wall”, Biophysical J.,2017, 113, 1496–1504. [DOI] [PMC free article] [PubMed]
- 104.Simončič M., Lukšič M. Mechanistic differences in the effects of sucrose and sucralose on the phase stability of lysozyme solutions. J Mol Liq. 2021 Mar;15(326) doi: 10.1016/j.molliq.2020.115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.R. D. Lins, P.H. Hunenberger, “A New GROMOS Force Field for Hexopyranose-Based Carbohydrates”, J Comput Chem. 2005 Oct;26(13):1400-12. [DOI] [PubMed]
- 106.Dhaliwal A., Khondker A., Alsop R., Rheinstädter M.C. Glucose Can Protect Membranes against Dehydration Damage by Inducing a Glassy Membrane State at Low Hydrations. Membranes (Basel) 2019 Jan;9(1):15. doi: 10.3390/membranes9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leekumjorn S., Sum A.K. Molecular Dynamics Study on the Stabilization of Dehydrated Lipid Bilayers with Glucose and Trehalose. J. Phys. Chem. B. 2008;112:10732–10740. doi: 10.1021/jp8025489. [DOI] [PubMed] [Google Scholar]
- 108.Humphrey W., Dalke A., Schulten K. VMD. visual molecular dynamics. J. Mol. Graphics Modell. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 109.Ahamad S., Kanipakam H., Gupta D. Insights into the structural and dynamical changes of spike glycoproteinmutations associated with SARS-CoV-2 host receptor binding. Journal of Biomolecular Structure And Dynamics. 2022;40(1):263–275. doi: 10.1080/07391102.2020.1811774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sahihi M., Faraudo J. Molecular Dynamics Simulations of Adsorption of SARS-CoV-2 Spike Protein on Polystyrene Surface. J. Chem. Inf. Model. 2022;62:3814–3824. doi: 10.1021/acs.jcim.2c00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D. F. de Lima Neto, et all. “Molecular dynamics simulations of the SARS-CoV-2 Spike protein and variants of concern. structural evidence for convergent adaptive evolution”, 2022, Journal of Biomolecular Structure and Dynamics. [DOI] [PubMed]
- 112.Penkler D.L., Atilgan C., Tastan Bishop Ö. Allosteric modulation of human Hsp90α conformational dynamics. J. Chem. Inf. Model. 2018;58:383–404. doi: 10.1021/acs.jcim.7b00630. [DOI] [PubMed] [Google Scholar]
- 113.Kabsch W., Sander C. Dictionary of protein secondary structure – pattern-recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.