Abstract
Aims/hypothesis
We aimed to evaluate associations of multiple recommended dietary patterns (i.e. the alternate Mediterranean diet [aMED], the Healthy Eating Index [HEI]-2015 and the healthful Plant-based Diet Index [hPDI]) with serum metabolite profile, and to examine dietary-pattern-associated metabolites in relation to incident diabetes.
Methods
We included 2842 adult participants free from diabetes, CVD and cancer during baseline recruitment of the Hispanic Community Health Study/Study of Latinos. Metabolomics profiling of fasting serum was performed using an untargeted approach. Dietary pattern scores were derived using information collected by two 24 h dietary recalls. Dietary-pattern-associated metabolites were identified using multivariable survey linear regressions and their associations with incident diabetes were assessed using multivariable survey Poisson regressions with adjustment for traditional risk factors.
Results
We identified eight metabolites (mannose, γ/β-tocopherol, N1-methylinosine, pyrraline and four amino acids) that were inversely associated with all dietary scores. These metabolites were detrimentally associated with various cardiometabolic risk traits, especially insulin resistance. A score comprised of these metabolites was associated with elevated risk of diabetes (RRper SD 1.54 [95% CI 1.29, 1.83]), and this detrimental association appeared to be attenuated or eliminated by having a higher score for aMED (pinteraction=0.0001), HEI-2015 (pinteraction=0.020) or hPDI (pinteraction=0.023). For example, RR (95% CI) of diabetes for each SD increment in the metabolite score was 1.99 (1.44, 2.37), 1.67 (1.17, 2.38) and 1.08 (0.86, 1.34) across the lowest to the highest tertile of aMED score, respectively.
Conclusions/interpretation
Various recommended dietary patterns were inversely related to a group of metabolites that were associated with elevated risk of diabetes. Adhering to a healthful eating pattern may attenuate or eliminate the detrimental association between metabolically unhealthy serum metabolites and risk of diabetes.
Keywords: Diabetes, Dietary Guidelines, Dietary Patterns, Metabolomics
Tweet:
A detrimental association between metabolically unhealthy serum metabolites (all were inversely associated with three recommended dietary patterns) and risk of diabetes appeared to be attenuated or eliminated by eating healthfully. (@EinsteinMed)
Introduction
The potential importance of dietary factors in the development of cardiometabolic diseases has been increasingly recognised. It is estimated that 45.4% of deaths in 2012 from CVD and diabetes among US adults aged ≥25 years were associated with suboptimal dietary habits [1]. Epidemiological findings from both prospective observational studies [2–5] and intervention studies [6, 7] support cardiometabolic benefits of high-quality diets. As such, the 2015–2020 Dietary Guidelines for Americans recommend multiple healthful eating patterns, including the Mediterranean-Style Eating Pattern, the Vegetarian Eating Pattern and the US-Style Eating Pattern, for reducing chronic disease risk and improving health status [8].
Various metabolic and molecular mechanisms that may underlie the potential cardiometabolic benefits of healthful dietary patterns have been proposed [4, 9, 10]. Metabolomics profiling of habitual diets may hold promise for providing novel mechanistic insights into the diet–disease association and discovering the potential therapeutic targets [11]. While previous studies have uncovered a wide range of metabolite biomarkers for various dietary intakes, these studies largely focused on individual foods, food groups or nutrients that often correlate and may interact with each other [12–17]. A few studies have identified circulating metabolites associated with one or more dietary patterns [18–23] and linked these metabolites to the development of CVD [22]. However, less is known about how the dietary-pattern-related metabolites may be associated with risk of diabetes, especially among US Hispanics/Latinos who have unique eating habits, poorer cardiometabolic features and higher diabetes burden as compared with other racial/ethnic groups in the country [24–26].
Using metabolomics data determined in a broad sample of US Hispanic/Latino adults [27, 28], we made the following assessments: (1) the associations of three recommended dietary patterns (i.e. the alternate Mediterranean diet [aMED], the Healthy Eating Index [HEI]-2015 and the healthful Plant-based Diet Index [hPDI]) with serum metabolite profile; (2) the relationship between the identified dietary-pattern-related metabolites and incident diabetes; and (3) the potential effect modification of healthful dietary patterns on the metabolite–diabetes association, since a healthful eating pattern may attenuate any detrimental association between diet-dependent metabolites and health risk [29]. Given the similar inverse associations between these dietary patterns and risk of chronic diseases [2–5], we hypothesised that these dietary patterns may share some mechanisms reflected by metabolite profiles that may underlie their potential benefits in terms of disease prevention.
Methods
Study design and population
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) is a prospective population-based study of 16,415 Hispanic/Latino adults aged 18–74 years who at recruitment were living in four metropolitan areas of the USA: Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA. Participants were recruited by using a two-stage probability sample design, as described previously [27, 28]. A comprehensive battery of interviews and clinical assessments with fasting blood draws were conducted by trained, certified and bilingual staff at in-person clinic visits from March 2008 to June 2011. The second visit period started in October 2014 and concluded in December 2017. The study was approved by the institutional review boards at all participating institutions, and all participants gave written informed consent.
Diet assessment and calculation of dietary scores
Information on dietary intake was collected by using two 24 h dietary recalls [24, 30]. The first recall was administered through in-person interviews conducted at the baseline visit and the second was performed primarily via telephone approximately 30 days after the first interview. Participants estimated portion sizes with the use of food models (in-person) or a food-amount booklet (telephone interviews). Data on foods and nutrients were collected and analysed using the multiple-pass methods of the Nutrition Data System for Research software (version 11) from the Nutrition Coordinating Center at University of Minnesota. For our current analysis, we used mainly the dietary data collected by the first recall to better capture the diet–metabolite associations, given the quick response of certain metabolites to dietary intakes [31]. We used the dietary data from the second recall for a small proportion (<3%) of participants for whom the dietary data from the first recall were missing, as described previously [31].
Components of the three dietary patterns and standards for scoring are presented in ESM Tables 1–3. Briefly, the aMED score was adapted by Fung et al [32] to reflect adherence to the Mediterranean diet, with each of its nine components being assigned a score of 0 or 1 point according to intake median or moderate drinking. The HEI-2015, which assesses the extent to which an individual’s diet aligns with the 2015–2020 Dietary Guidelines for Americans, includes 13 components and the score theoretically ranges from 0 to 100 [33]. While the aMED and HEI-2015 include both food and nutrient components, the hPDI comprises solely food groups that were coded based on intake quintiles [34]. Higher intakes of six ‘healthy plant foods’ (e.g. whole grains) were awarded points, while intakes of five animal foods and four ‘less healthy plant foods’ (e.g. sugar-sweetened beverages and fruit juices) were both reverse-coded, leading to a range of 15–75 for the total hPDI score.
Sample collection and metabolomics profiling
At both visits 1 and 2, participants were asked to fast for at least 8 h before the examination, consume only water and necessary medications, and to refrain from smoking or physical activity. Venous blood samples were collected, processed and frozen (at −70°C) on-site toward the beginning of the visit.
A total of 3972 participants randomly selected from the whole study population constituted the current subsample for metabolomics profiling. Based on discoveryHD4 platform at Metabolon (Durham, NC, USA), serum metabolite values were assessed by using an untargeted LC-MS-based metabolomic quantification protocol. More detailed experimental information on MS analysis, identification and classification of metabolites, and quality-control processes has been reported [35]. The platform captures information for a total of 1136 metabolites, including 782 metabolites with known structural identities and 354 unknown metabolites. As the identities for the 354 unknown metabolites are unclear, we considered only the metabolites with known structural identities for the current analysis. For a specific metabolite, its value varied among individual participants and for some participants this value was too low to be detected by the platform. When a metabolite could not be detected for 20% or more of the participants, it was excluded from our analysis to minimise the impact of missing data. These predefined criteria led to 624 known metabolites with an undetectable rate <20% being included in the present analysis. For these 624 metabolites, the median per cent undetectable value was 0.15%, and the missing values were imputed using half of the lowest values detected. A sensitivity analysis was performed to examine the diet–metabolite associations by excluding individuals with any metabolite values missing. Because of the skew distribution of the metabolite values, the values were transformed using a rank-based inverse normal transformation before all analyses to approximate a normal distribution [36].
Measurement of cardiometabolic traits
Using an automatic sphygmomanometer, three seated BP measures were obtained for each participant after a 5 min rest period, and their means were used to derive systolic BP (SBP) and diastolic BP (DBP) [37]. Centralised laboratory tests were performed to determine other cardiometabolic traits including plasma glucose, insulin, HbA1c, serum triacylglycerols and serum total cholesterol, HDL-cholesterol and LDL-cholesterol (all were fasting measures except for HbA1c) [38]. Non-HDL-cholesterol was computed by subtracting HDL-cholesterol from total cholesterol. For participants without self-reported diabetes or with fasting plasma glucose (FPG) ≤ 8.3 mmol/L (i.e. 150 mg/dl), measurements of 2 h plasma glucose were also performed following a standard OGTT (75 g glucose) [26]. HOMA-IR was derived using a common equation based on fasting glucose and insulin [39].
Assessment of diabetes
Participants were classified as having diabetes if they reported use of glucose-lowering medications or met one or more of the following ADA criteria: (1) FPG ≥ 7.0 mmol/L (i.e. 126 mg/dL); (2) 2 h OGTT plasma glucose ≥11.1 mmol/L (i.e. 200 mg/dL); and (3) HbA1c ≥ 48 mmol/mol (i.e. 6.5%). Based on these criteria, participants free of diabetes at visit 1 who were identified as having diabetes at visit 2 were deemed to be incident cases of diabetes [35].
Assessment of other covariates
Information on socioeconomic and demographic characteristics (e.g. Hispanic/Latino background), lifestyle factors, and medical and family histories was collected using structured questionnaires [28]. Physical activity was measured using the Global Physical Activity Questionnaire and data were summarised in metabolic equivalent-hours/day [40]. BMI was calculated as measured weight divided by measured height squared (kg/m2).
Statistical analysis
For the present analysis, we excluded participants with self-reported prevalent CVD or cancer (n=430), or self-reported prevalent diabetes with (n=509) or without (n=120) confirmation by the ADA criteria. We further excluded participants with missing dietary information (n=26) and those with implausible total energy intake (>25,104 or <2510.4 KJ/day [i.e. >6000 or <600 kcal/day] in men, or >16,736 or <1673.6 KJ/day [i.e. >4000 or <400 kcal/day] in women; n=45), leaving 2842 participants for further analyses.
To account for oversampling of specific population subgroups and/or non-response to the follow-up visit, all analyses incorporated HCHS/SOL complex study design and sampling weights, as described previously [28]. The three examined dietary pattern scores differed substantially in terms of both scale and variation. Thus, all dietary pattern scores were divided into quintiles and then examined for their associations with serum metabolites using survey linear regression models (quintile ranks of the dietary scores as predictors and inverse-normally transformed serum metabolites as response variables). Multivariable adjustment was made to account for age, sex, field centre, Hispanic/Latino background, education, annual household income, smoking status, drinking status (except for the analyses of aMED score), total energy intake, physical activity, BMI, use of antihypertensive drugs, use of lipid-lowering drugs and fasting time before blood sample collection. The yielded p values were corrected for false discovery rate (FDR) using the Benjamini–Hochberg procedure [41]. We performed a principal component (PC) analysis on metabolites to evaluate the variations in dietary pattern scores that could be explained by all metabolites included in this study and metabolites that were associated with all three dietary patterns. In addition, using doubly labelled water as an objective biomarker of total energy intake, we previously found in a substudy that HCHS/SOL participants with higher BMI or Dominican heritage underestimated their total energy intake [42]. Thus, we conducted a sensitivity analysis to assess the associations of dietary pattern with metabolites by excluding obese (BMI ≥30 kg/m2) or Dominican individuals.
For metabolites that were significantly associated with the dietary pattern scores, we calculated metabolite scores reflecting the direction of dietary pattern–metabolite associations. For example, metabolites positively associated with all dietary pattern scores were summed as one metabolite score (potentially beneficial), and those inversely associated with all dietary pattern scores were summed as another score (potentially detrimental). We used the same approach to sum unique metabolites that were inversely/positively associated with only one of the three dietary pattern scores. Thus, eight metabolite scores (four potentially beneficial and four potentially detrimental) were expected. We then evaluated cross-sectional relationships between the derived metabolite scores and various glycaemic traits, serum major lipids and BP by using partial Spearman correlation analysis after the multivariable adjustment as described above.
We next examined prospective associations of the derived metabolite scores with incident diabetes among 1966 participants with follow-up information for diabetes status at visit 2. We used multivariable survey Poisson regression models to estimate RR and 95% CI of incident diabetes according to tertiles of the metabolite scores, offsetting the lag time between the two study visits. To evaluate the potential effect modification of diet quality on the metabolite–diabetes association, we further examined the identified metabolite scores in relation to risk of diabetes within each tertile of the examined dietary pattern scores and tested the potential interactions. All statistical analyses were performed using R (version 3.3.2; R Foundation, Austria) and Stata (version 15.1; StataCorp, USA).
Results
Population characteristics
Age-adjusted baseline population characteristics according to quintiles of the three dietary pattern scores are reported in ESM Table 4. Individuals with a higher aMED, HEI-2015 or hPDI score were older, had higher levels of education and family income, and were less likely to be men or be current smokers. Differences in the dietary pattern scores across study field centres or Hispanic/Latino backgrounds were evident. Individual food or nutrient components of the dietary patterns distributed according to the dietary pattern scores largely as expected by design (ESM Table 5).
Dietary patterns and serum metabolites
After multivariable adjustment for demographic and socioeconomic factors, self-reported medication uses, lifestyle factors and BMI, 25.2% (157/624) of the assessed metabolites were significantly associated with at least one of the dietary scores (FDR-adjusted p<0.05). Of the 157 significant metabolites, 40 were associated with all dietary scores, while 50, 13 and 16 were specifically associated with aMED index, HEI-2015 and hPDI alone, respectively (Fig. 1a).
Fig. 1.
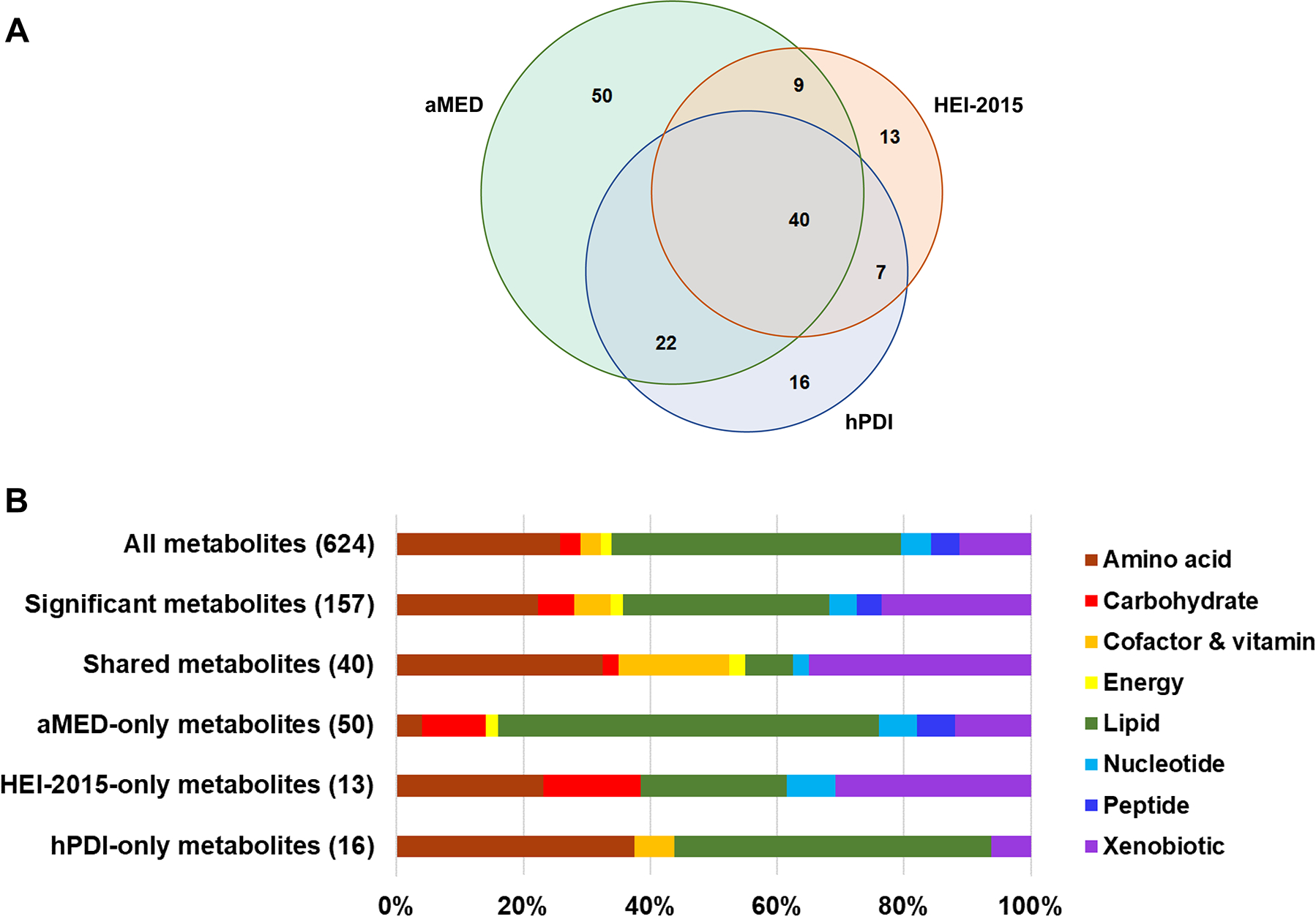
Serum metabolites associated with three dietary patterns (a) and the corresponding metabolism pathways (b). Dietary-pattern-related metabolites (FDR-adjusted p<0.05) were identified using survey linear regressions with adjustments for age, sex, study field centre, Hispanic/Latino background, education, annual household income, smoking status, drinking status (not for aMED-only metabolite score), total energy intake, physical activity, BMI, use of antihypertensive drugs, use of lipid-lowering drugs and fasting time before blood sample collection
The 157 significant metabolites with at least one dietary pattern association were largely lipids (32.5%), xenobiotics (23.6%) and amino acids (22.3%) (Fig. 1b and ESM Table 6). In contrast, the 40 metabolites that were associated with all dietary scores had substantially lower representation of lipids (7.5%), but relatively higher per cent amino acids (32.5%) and cofactor/vitamin metabolites (17.5%). For the metabolites associated with a single dietary score, lipids contributed to 60.0% (30/50) of aMED-only metabolites, 23.1% (3/13) of HEI-2015-only metabolites, and 50.0% (8/16) of hPDI-only metabolites. Of the 40 dietary-pattern-shared metabolites, 32 were positively and eight were inversely associated with all dietary scores.
As suggested by the results of PC analysis, the top five PCs based on all 624 metabolites explained a very small proportion of the variation in dietary scores (ranging from 1.1% for aMED to 5.5% for hPDI) (ESM Table 7). When using the top PCs based on the 40 metabolites that were associated with all three dietary patterns, there was a clear distinction between individuals with a higher dietary pattern score (top quintile) and those with a lower score (bottom quintile) (ESM Fig. 1). The top five PCs based on the 40 shared metabolites explained 16.8% (for hPDI score) to 19.4% (for aMED score) variation in the dietary pattern scores (ESM Table 7). In sensitivity analysis, the examined dietary pattern–metabolite associations among the study sample with imputed metabolites values were similar regardless of whether or not individuals with undetectable metabolites were included (EMS Fig. 2). The associations were also similar after excluding obese individuals or individuals with Dominican heritage who were more likely to underreport total energy intake (ESM Fig. 3).
Dietary-pattern metabolites and cardiometabolic risk traits
The score of 32 dietary-pattern-positive metabolites showed modest favourable correlations with various cardiometabolic traits (Fig. 2). Conversely, the score of eight dietary-pattern-inverse metabolites was detrimentally correlated with all glycaemic traits, lipid traits and BP (e.g. correlation with HOMA-IR, r=0.34). For other scores of metabolites specific for a single dietary pattern, the correlations with cardiometabolic traits appeared relatively weak and some were in unexpected directions (e.g. the score of hPDI-only-positive metabolites was associated with elevated HOMA-IR and serum non-HDL-cholesterol and triacylglycerols).
Fig. 2.
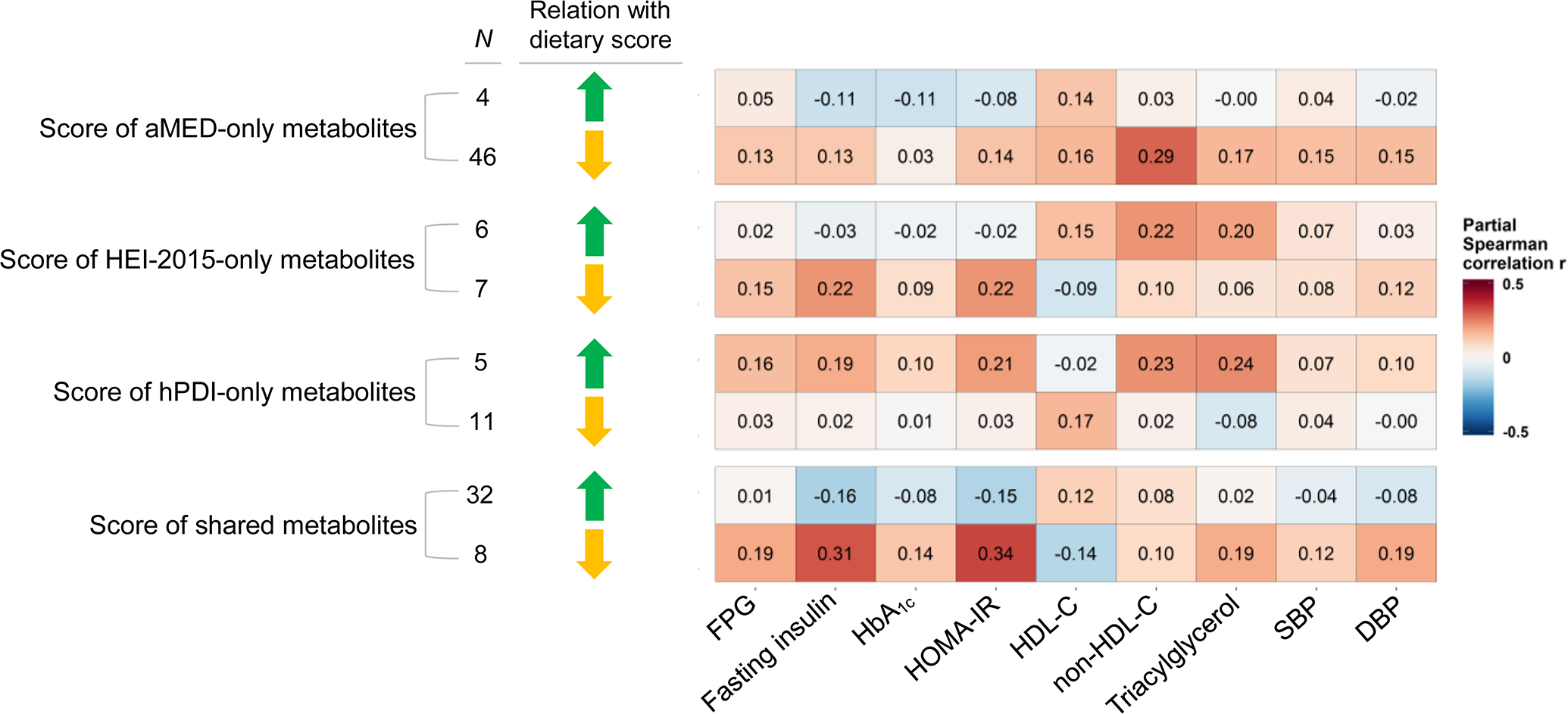
Cross-sectional association between scores of dietary-pattern-related metabolites and cardiometabolic traits. Metabolites indicated by green arrowhead were positvely associated with dietary pattern score(s), and metabolites indicated by yellow arrowhead were inversely associated with dietary pattern score(s). Results are partial Spearman correlations with adjustment for covariates listed in Fig. 1 legend. HDL-C, HDL-cholesterol
The 40 dietary-pattern-shared metabolites spanned distinct metabolic pathways (Fig. 3). The 32 dietary-pattern-positive metabolites were largely amino acids and plant xenobiotics, with most being modestly and favourably correlated with glycaemic traits, while a few were evidently correlated with an unfavourable lipid profile. The remaining eight dietary-pattern-inverse metabolites, which included mannose, γ/β-tocopherol, N1-methylinosine, pyrraline and four amino acids, were correlated with poorer glycaemic and lipid profiles and higher BP, especially so for mannose, γ/β-tocopherol, proline and N1-methylinosine.
Fig. 3.
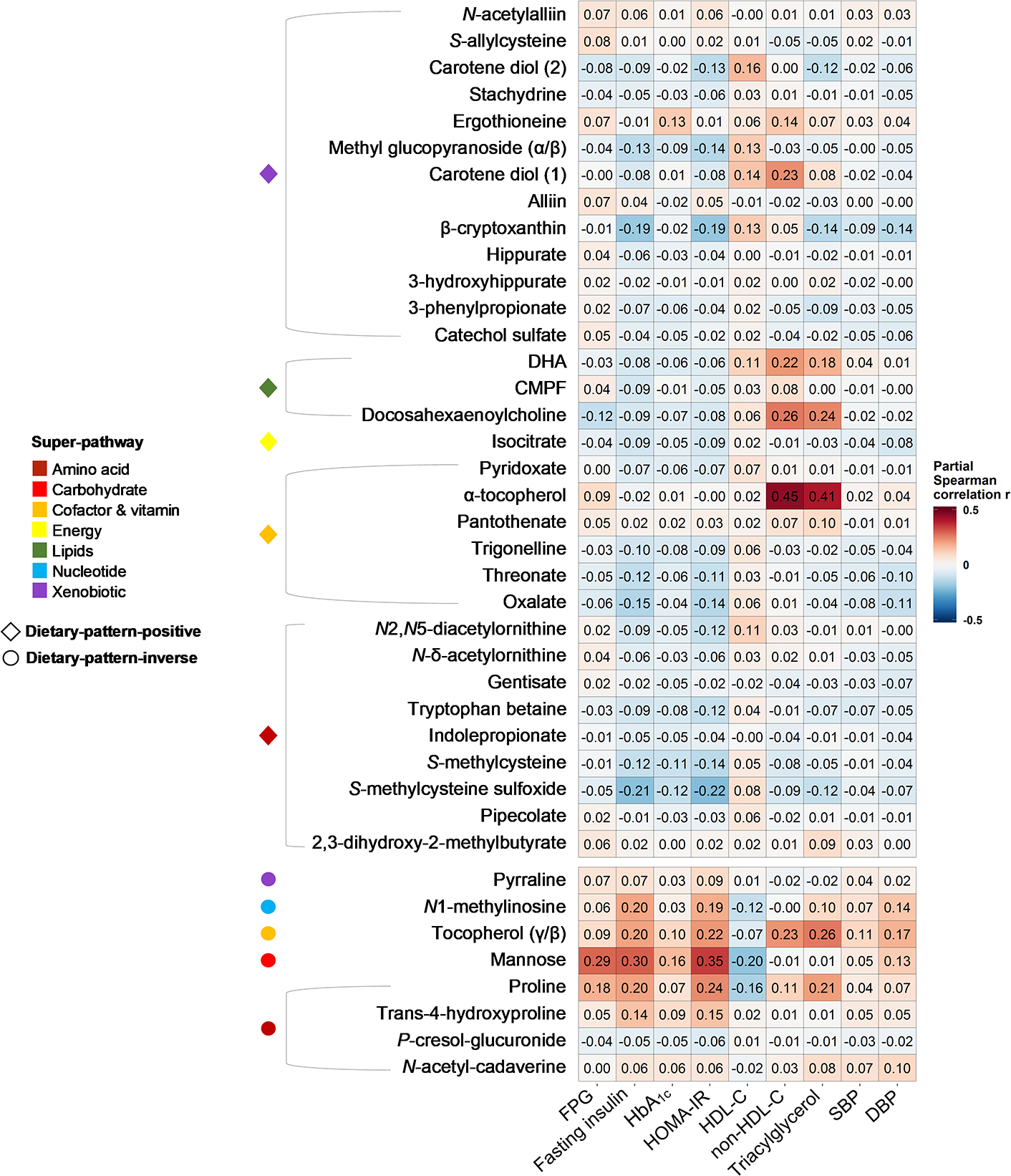
Cross-sectional association between 40 individual metabolites significantly associated with three dietary scores and cardiometabolic traits. Results are partial Spearman correlations with adjustment for age, sex, study field centre, Hispanic/Latino background, education, annual household income, smoking status, drinking status (not for aMED-only metabolite score), total energy intake, physical activity, BMI, use of antihypertensive drugs, use of lipid-lowering drugs and fasting time before blood sample collection. CMPF, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid; HDL-C, HDL-cholesterol
Dietary patterns, metabolites and incident diabetes
During an average 6 years of follow-up, 207 incident diabetes cases were identified. After multivariable adjustment, the score of 32 dietary-pattern-positive metabolites was inversely (non-significantly) associated with risk of diabetes (RRT3 vs T1 0.69 [95% CI 0.44, 1.09]; ptrend=0.11), while the score of eight dietary-pattern-inverse metabolites was strongly associated with elevated risk of diabetes (RRT3 vs T1 2.94 [95% CI 1.87, 4.63]; ptrend<0.0001) (Table 1). There was an unexpected positive association between the score of hPDI-only-positive metabolites and risk of diabetes (ptrend=0.021), while other scores of single-dietary-pattern metabolites were not associated with risk of diabetes. Given the unexpected correlations between dietary-pattern-positive metabolites and serum lipids (Figs 2 and 3), we repeated these analyses after excluding individuals with dislipidaemia at baseline. After that, only the score of eight dietary-pattern-positive metabolites remained associated with risk of diabetes (RRT3 vs T1 3.78 [95% CI 2.18, 6.56]; ptrend<0.0001) (ESM Table 8).
Table 1.
Multivariable-adjusted association between scores of dietary-pattern-related metabolites and risk of diabetes
| Metabolite score | No. of metabolites | Tertile for metabolite score | p trend | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Metabolites positively associated with dietary patterns | |||||
| aMED only | 4 | 1.00 (Referent) | 1.04 (0.70, 1.53) | 1.05 (0.67, 1.64) | 0.83 |
| HEI-2015 only | 6 | 1.00 (Referent) | 1.14 (0.68, 1.90) | 1.32 (0.77, 2.28) | 0.31 |
| hPDI only | 5 | 1.00 (Referent) | 0.83 (0.48, 1.44) | 1.63 (1.06, 2.51) | 0.021 |
| All dietary patterns | 32 | 1.00 (Referent) | 0.99 (0.65, 1.51) | 0.69 (0.44, 1.09) | 0.11 |
| Metabolites inversely associated with dietary patterns | |||||
| aMED only | 46 | 1.00 (Referent) | 0.95 (0.56, 1.62) | 1.16 (0.68, 1.99) | 0.59 |
| HEI-2015 only | 7 | 1.00 (Referent) | 1.09 (0.65, 1.82) | 0.97 (0.57, 1.66) | 0.84 |
| hPDI only | 11 | 1.00 (Referent) | 1.05 (0.65, 1.68) | 1.08 (0.66, 1.79) | 0.72 |
| All dietary pattern scores | 8 | 1.00 (Referent) | 1.77 (1.06, 2.96) | 2.94 (1.87, 4.63) | <0.0001 |
Data are presented as RRs (95% CIs) from survey Poisson regressions with adjustment for age, sex, study field centre, Hispanic/Latino background, education, annual household income, smoking status, drinking status (not for aMED-only metabolite score), total energy intake, physical activity, BMI, hypertension, dyslipidaemia and fasting time before blood sample collection
T, tertile
We then examined whether the healthful dietary patterns may modify the detrimental association between the score of eight dietary-pattern-inverse metabolites and risk of diabetes. There were significant interactions between this metabolite score and aMED (pinteraction=0.0001), HEI-2015 (pinteraction=0.020) and hPDI (pinteraction=0.023) scores on risk of diabetes (Fig. 4). The association of the metabolites score with risk of diabetes was apparently stronger among individuals in the first or second tertile of a dietary score than among individuals in the highest tertile of the corresponding dietary score, with the detrimental association being fully eliminated among individuals in the highest tertile of aMED score or HEI-2015 score. For example, RR (95% CI) of diabetes for each SD increment in the metabolite score was 1.54 (95% CI 1.29, 1.83) in the whole study sample, and was 1.99 (1.44, 2.37), 1.67 (1.17, 2.38) and 1.08 (0.86, 1.34) across the lowest to the highest tertile of aMED score, respectively.
Fig. 4.
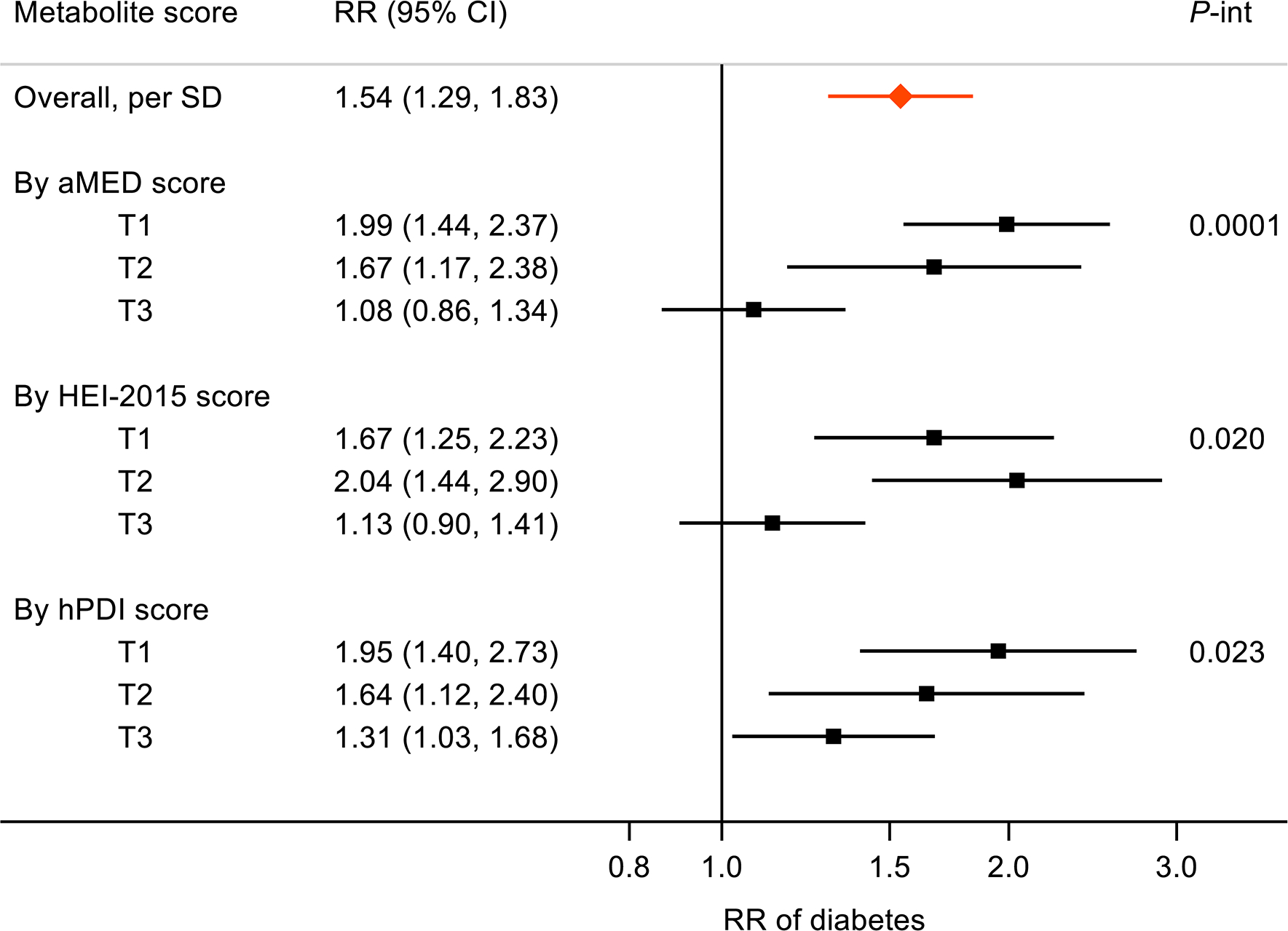
Association between score of eight metabolites (inversely associated with all dietary scores) and incident diabetes. Results are shown as RR (95% CI) from survey Poisson regressions with adjustment for age, sex, study field centre, Hispanic/Latino background, education, annual household income, smoking status, drinking status (not for aMED-only metabolite score), total energy intake, physical activity, BMI, hypertension, dyslipidaemia and fasting time before blood sample collection. T, tertile
Of the eight component metabolites inversely associated with all dietary scores, six were positively associated with risk of diabetes (RRper SD >1.10), and four (γ/β-tocopherol, mannose, proline, and N-acetyl-cadaverine) metabolite–diabetes associations were significant (RRper SD 1.20–1.76) (ESM Table 9). Mannose, pyrraline, N1-methylinosine showed interactions with one or more dietary scores on risk of diabetes. For example, mannose was not associated with risk of diabetes among individuals in the highest tertile of aMED score (RRper SD 1.00 [95% CI 0.62, 1.61]) but was associated with substantially elevated risk of diabetes among those with a lower aMED score (RRper SD 2.48 [95% CI 1.83, 3.37]) (pinteraction=0.0017).
Discussion
In a population-based study of US Hispanic/Latino adults, our analyses systematically assessed serum metabolite profiles associated with three recommended dietary patterns. While each dietary pattern was associated with a list of metabolites, 32 metabolites were positively associated and eight were inversely associated with all dietary patterns. A score of the eight dietary-pattern-inverse metabolites was associated with worse cardiometabolic traits (especially insulin resistance) and elevated risk of diabetes. The detrimental association between this metabolite score and risk of diabetes appeared to be modified by the degree of adherence to a healthful eating pattern, with a weaker or no association among individuals with a higher dietary pattern score. The score of 32 dietary-pattern-positive metabolites (or other scores of metabolites specific for a single dietary pattern) was not clearly associated with risk of diabetes.
A few previous studies have assessed metabolomics of hypothesis-driven dietary patterns [18–20, 22, 23]. McCullough et al [18] profiled serum metabolites of four hypothesis-driven dietary patterns (two of which, aMED and HEI-2015, were also included in our analysis) among 1367 US postmenopausal women. Their analysis highlighted 32 metabolites that distinguished high from low dietary pattern scores. Of these, several metabolites (e.g. γ/β-tocopherol, carotene diol-1 and docosahexaenoic acid [DHA]) that overlapped multiple dietary patterns were also associated with all dietary scores examined in our study. In another analysis of 1336 male smokers, Playdon et al [19] performed a metabolomics study of four healthful dietary patterns (aMED, HEI-2010, Healthy Diet Indicator, and Baltic Sea Diet) and found a suite of metabolites that were positively associated with multiple dietary patterns, especially those in the lysolipid or plant xenobiotic metabolism pathways. Using data from multi-cohorts of US and Spanish populations, Li et al [22] identified a set of metabolites associated with the Mediterranean diet, and a score of these metabolites was inversely associated with risk of major CVD [22].
The three dietary patterns examined in our study are comparable with regards to their emphases on intakes of minimally processed, nutritionally rich plant foods including whole grains, fruit, vegetables, nuts and legumes. Such similarities may, at least in part, explain the higher proportion of xenobiotic and cofactor/vitamin metabolites among the 40 shared metabolites as compared with composition of the 624 assessed metabolites. On the other hand, the three dietary pattern indices consist of distinctive components and apply different scoring criteria with regards to intake of animal food. For example, while aMED index emphasises lower consumption of red/processed meat, hPDI assigns equal weights for red/processed meat and fish, and red/processed meat consumption is not a specific component of HEI-2015. Such differences may have led to some unique metabolite profiles that were associated with a specific dietary pattern only. Despite the differences, the three examined dietary patterns concordantly captured a variety of serum metabolites that were associated with key cardiometabolic risk traits and risk of diabetes in expected directions. Thus, these data support our hypothesis that there might be common metabolic pathways underlying the extensively documented associations between high-quality diets and lower risk of cardiometabolic diseases.
We identified 32 metabolites positively associated with all examined dietary patterns. Previous studies have identified and replicated a number of these dietary-pattern-positive metabolites as potential biomarkers for intake of fruit (β-cryptoxanthin and methyl glucopyranoside [α/β]), vegetables (S-methylcysteine sulfoxide), nuts (tryptophan betaine), fish (DHA and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid) and coffee (theophylline) [14]. Some of these dietary-pattern-positive metabolites identified in our study and/or other previous studies might be originally from diets, as they occur naturally in foods (e.g. β-cryptoxanthin in tangerines, red peppers and pumpkin [43], DHA in fish [44], tryptophan betaine in legumes and nuts [45], and theophylline in coffee beans [46]). In our study, the 32 dietary-pattern-positive metabolites were modestly correlated with favourable glycaemic traits while a few were also correlated with an unfavourable lipid profile. A score comprised of these metabolites was not significantly associated with risk of diabetes. These observations suggest that the dietary-pattern-positive metabolites might be largely biomarkers of healthful eating patterns rather than metabolites that biologically underlie the association between high-quality diets and lower risk of diabetes.
We identified eight metabolites that were inversely related to all examined dietary patterns. Some of these metabolites also showed inverse associations with healthful dietary patterns in previous studies (e.g. mannose and the Mediterranean diet [47], and γ/β-tocopherol and three dietary scores including the aMED [18]). A score comprised of these eight metabolites was associated with elevated risk of diabetes, with the association being largely limited to individuals with a relatively lower dietary score. Similar differences according to dietary scores were observed for several component metabolites (especially mannose) and risk of diabetes. A higher level of circulating mannose has been consistently associated with insulin resistance [48] and elevated risk of diabetes in our study and several other population studies [49–51] and suggested to be a novel diabetes risk factor beyond blood glucose [49, 50]. Mannose is the main monosaccharide involved in protein glycosylation, a process predominantly occurring in the liver [52]. Abnormal glycosylation could lead to hepatic and whole-body insulin resistance [53]. Such insulin resistance might be attenuated by adherence to a healthful eating pattern, as evidence from both observational [54] and intervention studies [55] has demonstrated that the Mediterranean diet may improve insulin sensitivity among individuals with non-alcoholic fatty liver disease. Besides mannose, several other dietary-pattern-inverse metabolites (e.g. γ/β-tocopherol) were also detrimentally associated with risk of diabetes. Collectively, our findings suggest that a healthful eating pattern might lower risk of diabetes by decreasing the circulating concentrations and/or attenuating the adverse health impact of metabolically unhealthy metabolites. In line with our findings, a Mediterranean dietary intervention has been found to mitigate the deleterious association between plasma ceramides (a diet-dependent metabolite considered to be a potential novel cardiovascular biomarker [56]) and risk of major CVD [29]. However, it is also possible that the alterations in metabolite levels might be a consequence of improved cardiometabolic health due to healthy diet, since the causal role of these metabolites in human cardiometabolic health remains unknown.
Strengths of our study include the population-based design, the longitudinal data on diabetes status, and the representative sample of US Hispanic/Latino population covering the adult lifespan rather than a specific age or sex group. Additional strengths include the availability of the various clinical measures and other covariates collected using standard procedures, and a broad and unbiased spectrum of serum metabolite profiling.
Several limitations of our study need to be acknowledged. Dietary information was collected via self-report, so measurement errors are inevitable due to misreporting. First, our findings were derived from an observational study which cannot make causal inference. Although the dietary-pattern-associated metabolites could originate from nutrients or food components, the possibility of these metabolites being biomarkers of eating behaviours or other related lifestyle factors remains. Second, as compared with data derived by other dietary assessment instruments, such as food frequency questionnaires, dietary information estimated by 24 h recalls may poorly capture rarely consumed foods or be more reflective of recent intakes and thus may be more strongly associated with serum metabolites. In addition, misreporting of diet merits consideration, as specific individuals (e.g. obese and Dominican individuals in our study [42]) may underreport total energy intake, although the dietary pattern and metabolite associations were similar after excluding these individuals. Third, our analyses were based on a sample of US Hispanics/Latinos, among which some participants may be following their traditional diet characterised by a higher content of plant foods (e.g. grain products, vegetables and legumes) than found in the mainstream US diet [24]. These populations also have varying genetic compositions, poorer cardiometabolic features and higher diabetes burden as compared with other US racial/ethnic groups [25, 26]. As such, the identified metabolite markers of the dietary patterns may reflect not only participants’ habitual dietary intakes and the resultant biological consequences but also host genetic influences and the ability to digest, absorb and metabolise food components. Thus, it is uncertain to what extent our findings are ethnically specific and population-level generalisation of our findings should be made with caution. Fourth, there is evidence that metabolomic biomarkers of dietary patterns may vary by the time spent fasting before collection of the blood samples [57]. However, most (99.6%) of the participants in the current study fasted for at least 8 h and we controlled for fasting time throughout the analyses. Finally, as for other studies of dietary patterns and metabolites [18–20], our study was based on an untargeted metabolomic method that was unable to quantify the absolute values of individual metabolites. To make a direct comparison of metabolite levels across studies and to establish biomarkers linking diet and diseases that could be clinically applied, future studies using targeted metabolomics are still required.
In summary, this study of US Hispanics/Latinos identified that various serum metabolites spanning distinct biological pathwayswere associated with three recommended healthful eating patterns. A group of metabolites that were inversely associated with all dietary patterns were associated with worse cardiometabolic traits and elevated risk of diabetes. Our findings further highlighted that such a detrimental association between serum metabolites and risk of diabetes may be attenuated or eliminated by adhering to a healthful eating pattern. Additional studies are needed to confirm our findings and to verify whether these dietary-pattern-inverse metabolites could be therapeutic targets in dietary interventions that reduce risk of metabolic disorders.
Supplementary Material
Research in context.
What is already known about this subject?
Dietary factors are associated with altered metabolite profiles and risk of diabetes
What is the key question?
What are the associations between dietary-pattern-related metabolites and risk of diabetes?
What are the new findings?
Various recommended dietary patterns were inversely related to a group of metabolites that were associated with elevated risk of diabetes
Adhering to a healthful eating pattern may attenuate or eliminate the detrimental association between metabolically unhealthy serum metabolites and risk of diabetes
How might this impact on clinical practice in the foreseeable future?
The panel of dietary-pattern-inverse metabolites may have the potential to be therapeutic targets in dietary interventions that reduce risk of metabolic disorders
Acknowledgements
The authors thank the staff and participants of HCHS/SOL for their important contributions. A complete list of HCHS/SOL staff and investigators can be found in the reference [58] or at http://sites.cscc.unc.edu/hchs/.
Funding
The HCHS/SOL is a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (HHSN268201300001I / N01-HC-65233), University of Miami (HHSN268201300004I / N01-HC-65234), Albert Einstein College of Medicine (HHSN268201300002I / N01-HC-65235), University of Illinois at Chicago (HHSN268201300003I), Northwestern University (N01-HC-65236) and San Diego State University (HHSN268201300005I / N01-HC-65237). The following institutes/centres/offices have contributed to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities; National Institute on Deafness and Other Communication Disorders; National Institute of Dental and Craniofacial Research; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); National Institute of Neurological Disorders and Stroke; and NIH Institution-Office of Dietary Supplements.
This work is supported by the NHLBI R01HL060712 and NIDDK R01DK119268. Other funding sources for this study include: UM1 HG008898 from the National Human Genome Research Institute; K01HL129892, R01HL060712, R01HL140976 and R01HL136266 from the NHLBI; and R01DK112940, R01DK120870, P30DK046200 and the New York Regional Center for Diabetes Translation Research (P30 DK111022) from the NIDDK.
Abbreviations
- aMED
Alternate Mediterranean diet
- DBP
Diastolic BP
- DHA
Docosahexaenoic acid
- FDR
False discovery rate
- FPG
Fasting plasma glucose
- HCHS/SOL
Hispanic Community Health Study/Study of Latinos
- HEI
Healthy Eating Index
- hPDI
Healthful Plant-based Diet Index
- PC
Principal component
- SBP
Systolic BP
Data availability statement
The datasets analysed during the current study are available from the corresponding author based upon reasonable request in addition to a Data and Materials Distribution Agreement to protect the confidentiality and privacy of the participants and their families. Alternatively, de-identified data are publicly available at BioLINCC (https://biolincc.nhlbi.nih.gov/home/) and dbGaP (https://www.ncbi.nlm.nih.gov/gap/) for the subset of the study cohort that authorised general use of their data at the time of informed consent.
References
- 1.Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D (2017) Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA;317:912–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jannasch F, Kroger J, Schulze MB (2017) Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J Nutr;147:1174–1182 [DOI] [PubMed] [Google Scholar]
- 3.Qian F, Liu G, Hu FB, Bhupathiraju SN, Sun Q (2019) Association Between Plant-Based Dietary Patterns and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis. JAMA Intern Med;179:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satija A, Hu FB (2018) Plant-based diets and cardiovascular health. Trends Cardiovasc Med;28:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zampelas A, Magriplis E (2019) Dietary patterns and risk of cardiovascular diseases: a review of the evidence. Proc Nutr Soc;79:68–75 [DOI] [PubMed] [Google Scholar]
- 6.Estruch R, Ros E, Salas-Salvado J et al. (2018) Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med;378:e34. [DOI] [PubMed] [Google Scholar]
- 7.Salas-Salvado J, Bullo M, Babio N et al. (2011) Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care;34:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietary Guidelines Advisory Committee (2015). Dietary guidelines for Americans 2015–2020: Government Printing Office, Washington. [Google Scholar]
- 9.Tosti V, Bertozzi B, Fontana L (2018) Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J Gerontol A Biol Sci Med Sci;73:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salas-Salvado J, Becerra-Tomas N, Papandreou C, Bullo M (2019) Dietary Patterns Emphasizing the Consumption of Plant Foods in the Management of Type 2 Diabetes: A Narrative Review. Adv Nutr;10:S320–S331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guertin KA, Moore SC, Sampson JN et al. (2014) Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr;100:208–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanhineva K, Lankinen MA, Pedret A et al. (2015) Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr;145:7–17 [DOI] [PubMed] [Google Scholar]
- 13.Playdon MC, Sampson JN, Cross AJ et al. (2016) Comparing metabolite profiles of habitual diet in serum and urine. Am J Clin Nutr;104:776–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Gapstur SM, Carter BD et al. (2018) Untargeted Metabolomics Identifies Novel Potential Biomarkers of Habitual Food Intake in a Cross-Sectional Study of Postmenopausal Women. J Nutr;148:932–943 [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Yu B, Alexander D, Steffen LM, Boerwinkle E (2014) Human metabolome associates with dietary intake habits among African Americans in the atherosclerosis risk in communities study. Am J Epidemiol;179:1424–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmands WM, Ferrari P, Rothwell JA et al. (2015) Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am J Clin Nutr;102:905–913 [DOI] [PubMed] [Google Scholar]
- 17.Rebholz CM, Zheng Z, Grams ME et al. (2019) Serum metabolites associated with dietary protein intake: results from the Modification of Diet in Renal Disease (MDRD) randomized clinical trial. Am J Clin Nutr;109:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough ML, Maliniak ML, Stevens VL, Carter BD, Hodge RA, Wang Y (2019) Metabolomic markers of healthy dietary patterns in US postmenopausal women. Am J Clin Nutr;109:1439–1451 [DOI] [PubMed] [Google Scholar]
- 19.Playdon MC, Moore SC, Derkach A et al. (2017) Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr;105:450–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebholz CM, Lichtenstein AH, Zheng Z, Appel LJ, Coresh J (2018) Serum untargeted metabolomic profile of the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr;108:243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Perez I, Posma JM, Gibson R et al. (2017) Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol;5:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Guasch-Ferre M, Chung W et al. (2020) The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur Heart J;41:2645–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong TYN, Koulman A, Griffin JL, Wareham NJ, Forouhi NG, Imamura F (2020) A Combination of Metabolites Predicts Adherence to the Mediterranean Diet Pattern and Its Associations with Insulin Sensitivity and Lipid Homeostasis in the General Population: The Fenland Study, United Kingdom. J Nutr;150:568–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siega-Riz AM, Sotres-Alvarez D, Ayala GX et al. (2014) Food-group and nutrient-density intakes by Hispanic and Latino backgrounds in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr;99:1487–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menke A, Casagrande S, Geiss L, Cowie CC (2015) Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 26.Schneiderman N, Llabre M, Cowie CC et al. (2014) Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Diabetes Care;37:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavange LM, Kalsbeek WD, Sorlie PD et al. (2010) Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S et al. (2010) Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang DD, Toledo E, Hruby A et al. (2017) Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation;135:2028–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Jung M, Mossavar-Rahmani Y et al. (2016) Macronutrient Intake, Diagnosis Status, and Glycemic Control Among US Hispanics/Latinos With Diabetes. J Clin Endocrinol Metab;101:1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mei Z, Chen GC, Wang Z et al. (2021) Dietary factors, gut microbiota, and serum trimethylamine-N-oxide associated with cardiovascular disease in the Hispanic Community Health Study/Study of Latinos. Am J Clin Nutr;113:1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD (2006) Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr;136:466–472 [DOI] [PubMed] [Google Scholar]
- 33.Krebs-Smith SM, Pannucci TE, Subar AF et al. (2018) Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet;118:1591–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satija A, Bhupathiraju SN, Rimm EB et al. (2016) Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med;13:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen GC, Chai JC, Yu B et al. (2020) Serum sphingolipids and incident diabetes in a US population with high diabetes burden: the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Am J Clin Nutr;112:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Razquin C, Toledo E, Clish CB et al. (2018) Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care;41:2617–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorlie PD, Allison MA, Aviles-Santa ML et al. (2014) Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens;27:793–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi Q, Strizich G, Merchant G et al. (2015) Objectively Measured Sedentary Time and Cardiometabolic Biomarkers in US Hispanic/Latino Adults: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Circulation;132:1560–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia;28:412–419 [DOI] [PubMed] [Google Scholar]
- 40.Arredondo EM, Sotres-Alvarez D, Stoutenberg M et al. (2016) Physical Activity Levels in U.S. Latino/Hispanic Adults: Results From the Hispanic Community Health Study/Study of Latinos. Am J Prev Med;50:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. The annals of statistics;29:1165–1188 [Google Scholar]
- 42.Mossavar-Rahmani Y, Shaw PA, Wong WW et al. (2015) Applying Recovery Biomarkers to Calibrate Self-Report Measures of Energy and Protein in the Hispanic Community Health Study/Study of Latinos. Am J Epidemiol;181:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burri BJ, La Frano MR, Zhu C (2016) Absorption, metabolism, and functions of beta-cryptoxanthin. Nutr Rev;74:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strobel C, Jahreis G, Kuhnt K (2012) Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Souza RJ, Shanmuganathan M, Lamri A et al. (2020) Maternal Diet and the Serum Metabolome in Pregnancy: Robust Dietary Biomarkers Generalizable to a Multiethnic Birth Cohort. Curr Dev Nutr;4:nzaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guertin KA, Loftfield E, Boca SM et al. (2015) Serum biomarkers of habitual coffee consumption may provide insight into the mechanism underlying the association between coffee consumption and colorectal cancer. Am J Clin Nutr;101:1000–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macias S, Kirma J, Yilmaz A et al. (2019) Application of (1)H-NMR Metabolomics for the Discovery of Blood Plasma Biomarkers of a Mediterranean Diet. Metabolites;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S, Zhang C, Kilicarslan M et al. (2016) Integrated Network Analysis Reveals an Association between Plasma Mannose Levels and Insulin Resistance. Cell Metab;24:172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mardinoglu A, Stancakova A, Lotta LA et al. (2017) Plasma Mannose Levels Are Associated with Incident Type 2 Diabetes and Cardiovascular Disease. Cell Metab;26:281–283 [DOI] [PubMed] [Google Scholar]
- 50.Yu D, Moore SC, Matthews CE et al. (2016) Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics;12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menni C, Fauman E, Erte I et al. (2013) Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes;62:4270–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ichikawa M, Scott DA, Losfeld ME, Freeze HH (2014) The metabolic origins of mannose in glycoproteins. J Biol Chem;289:6751–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caro JF, Cecchin F, Sinha MK (1984) Is glycosylation in the liver needed for insulin binding, processing, and action? Evidence for heterogeneity. J Biol Chem;259:12810–12816 [PubMed] [Google Scholar]
- 54.Baratta F, Pastori D, Polimeni L et al. (2017) Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am J Gastroenterol;112:1832–1839 [DOI] [PubMed] [Google Scholar]
- 55.Ryan MC, Itsiopoulos C, Thodis T et al. (2013) The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol;59:138–143 [DOI] [PubMed] [Google Scholar]
- 56.Summers SA (2018) Could Ceramides Become the New Cholesterol? Cell Metab;27:276–280 [DOI] [PubMed] [Google Scholar]
- 57.Sedlmeier A, Kluttig A, Giegling I et al. (2018) The human metabolic profile reflects macro- and micronutrient intake distinctly according to fasting time. Sci Rep;8:12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lavange LM, Kalsbeek WD, Sorlie PD, et al. (2010) Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol; 20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author based upon reasonable request in addition to a Data and Materials Distribution Agreement to protect the confidentiality and privacy of the participants and their families. Alternatively, de-identified data are publicly available at BioLINCC (https://biolincc.nhlbi.nih.gov/home/) and dbGaP (https://www.ncbi.nlm.nih.gov/gap/) for the subset of the study cohort that authorised general use of their data at the time of informed consent.


