Abstract
Background:
Considering the changing causative and resistance pattern of agents implicated in sexually transmitted infections (STIs), etiological diagnosis is imperative, especially in countries practicing syndromic management. This study was designed to identify etiological agents associated with cervicitis and to analyze their association with clinical and behavioral profile.
Materials and Methods:
Female STI clinic attendees presenting with cervico-vaginal discharge were examined for the presence of cervicitis. Endocervical swabs were collected for gram staining and real-time polymerase chain reaction was performed for various bacterial and viral STI agents in patients presenting with cervical discharge. A vaginal swab was also evaluated for bacterial vaginosis by Nugent's criteria.
Results:
Of 64 patients with vaginal discharge, 26.6% and 12.5% patients complained of genital itching and lower abdominal pain, respectively. Mean of 36.6 pus cells/hpf were observed, appreciably greater number in patients with Neisseria gonorrhoeae and Chlamydia trachomatis infections (P = 0.0063 and 0.0032, respectively). Pus cells were high (mean 68 pus cells/hpf) in patients with Ureaplasma urealyticum, though this may be attributed to coexisting N. gonorrhoeae. Agents isolated from endocervix were N. gonorrhoeae, 17 (26.6%), Trichomonas vaginalis, 4 (6.3%), HSV1 and C. trachomatis, 1 each (1.6%), HSV2, 9 (14.1%), U. urealyticum 5 (7.8%), Ureaplasma parvum 26 (40.6%), Mycoplasma genitalium (0%), and Mycoplasma hominis 11 (17.2%). Bacterial vaginosis was diagnosed in 14 (21.9%) patients. Multiple agents were isolated in 10 (two), 6 (three), 6 (four), and 1 (five) patients. Isolation of M. hominis and U. parvum was significantly associated with bacterial vaginosis (P = 0.04 and 0.003, respectively). Nonusage of condoms and mental stress predisposed to cervicitis.
Conclusion:
We concluded that there are changing etiological patterns of cervicitis. There is need to use tests that detect wider array of organisms, and can replace standard culture methods with molecular assays, to increase the ability to diagnose more number of organisms implicated in cervicitis.
Keywords: Cervicitis, chlamydia trachomatis, etiology, molecular tests, mycoplasma genitalium, neisseria gonorrhoeae, new agents, ureaplasma
Introduction
Cervicitis, “the ignored counterpart of urethritis in men,” has been associated with multiple complications in women. It is a clinical syndrome characterized by inflammation of the ectocervix due to infectious or noninfectious causes. The presentation can be acute or chronic. When patient presents with acute cervicitis, we suspect infectious etiology and in chronic cervicitis, both infectious and noninfectious etiologies are considered. The patient can have varied presentations involving a whole spectrum ranging from asymptomatic to mucoid or mucopurulent discharge. It is important to diagnose and treat patients of cervicitis since patients can develop complications, for example, salpingitis, endometritis, and pelvic inflammatory disease.[1]
Cervicitis is generally considered to result from infection due to sexually transmitted organisms Neisseria gonorrhoeae and Chlamydia trachomatis. Hence, the syndromic management kit for cervical discharge includes tablet azithromycin 1 g and tablet cefixime 400 mg stat.
However, there are ever-changing patterns of causative agents and their resistance patterns depending on the usage of antibiotics, this fact is especially relevant in countries practising syndromic management. To adequately treat the patient, it is imperative to identify the etiological agent. In this study, we aimed to identify etiological agents associated with cervicitis; and to analyze their association with the clinical and behavioral profile.
Materials and Methods
Study design
This retrospective cross-sectional study was conducted between October 1, 2019, and December 2020. The study was conducted at the Female Suraksha Clinic at a tertiary Reference Centre in North India.
Study participants and data collection
The case records of consecutive female sexually transmitted infections (STI) clinic attendees presenting with cervicovaginal discharge were examined for the presence of cervicitis till a minimum sample size of 50 was achieved as, according to the Guidelines for STI Surveillance, UN-AIDS/WHO Working Group on Global HIV/AIDS/STI Surveillance, a minimum sample size of 50 specimens from consecutive patients with the specified syndrome will provide adequate information for useful analyses.[2] Those with signs and symptoms of cervicitis were included in the study. Detailed sociodemographic and clinical history was taken by the STI counselor as per departmental predesigned pro forma. The presence of symptoms (discharge, dysuria, postcoital bleeding, dyspareunia, genital itching, pain abdomen, etc.), history of contraceptive use, detailed obstetric history, and behavioral history of the patient was also noted. Pregnant women, patients of cervical malignancy, and those on antibiotics within the past 2 weeks were excluded. The detailed history of any complaints in partners was taken, and partners were examined as well.
Clinical procedures and sample collection
All patients enrolled in the study received the standard clinical evaluation, which included physical and per speculum examination of the vagina and cervix. Note was made of any pathology of cervix and vagina such as cervicitis and cervical erosion. The amount, color, odor, character, and smell of discharge were noted.
After removing the cervical mucous secretions, two endocervical swabs were collected by the clinician. The first endocervical swab was used to prepare a smear for gram staining. The second swab was collected for performing real-time polymerase chain reaction (PCR) (nucleic acid tests) for various bacterial and viral STI agents. The swab was stored at −20°C till further processing. A vaginal swab was also collected and examined for bacterial vaginosis.
Treatment given
After sample collection, the patients were given syndromic management and if specific results were obtained after sample processing, the patients were called for follow-up and organism-based specific treatments were offered to the patient.
Laboratory analysis
The first endocervical swab smear was stained by Gram Stain. It was then examined for the presence of pus cells (number per high-power field), epithelial cells, bacterial, and fungal agents. The vaginal swab was also used to prepare a smear which was then evaluated for bacterial vaginosis by Nugent's criteria.
The second endocervical swab was used for DNA extraction using DNA isolation kit (Norgen Biotek Corp., Canada). The extracted DNA was then used to detect bacterial (N. gonorrhoeae, C. trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium), parasitic (Trichomonas vaginalis) and viral (Herpes Simplex Virus 1 and Herpes Simplex Virus 2) by mono-plex PCR assay using RealLine kits for each organism, supplied by Bioron Diagnostics GmbH. The Real-Time PCR assay was performed on Applied Biosystem 7500 Fast Dx Real-Time PCR Instrument supplied Thermo Fisher Scientific (Massachusetts, USA) as per kit instructions.
Definitions
Cervicitis-Two major diagnostic signs (either one or both) were considered to diagnose clinical cervicitis: (1) A purulent or mucopurulent endocervical exudate visible in the endocervical canal or by an endocervical swab specimen and (2) sustained endocervical bleeding easily induced by gentle passage of a cotton swab through the cervical os.[3]
Statistical analysis
All participant data included in the study were analyzed. The etiological agent implicated in cervicitis was identified as a sample being positive for the particular organism. Prevalence of each organism and also of coinfection was calculated and evaluated for risk of coinfections. Categorical variables were analyzed in number and percentage (%). Qualitative variables such as sociodemographic and behavioral data were associated using Chi-square test/Fisher's exact test. P < 0.05 was considered statistically significant. The data were entered into Microsoft Excel 2019 (developed by Microsoft Corporation) spreadsheet and analysis was performed using the Statistical Package for Social Sciences (SPSS)software, (IBM manufacturer, Chicago, USA).ver 21.0.
Observations and Results
Sociodemographic and clinical characteristics
A total of 64 patients with cervicovaginal discharge were included in the study. Table 1 shows the demographic profile of patients, clinical presentation, behavioral history, etc. The mean age of the patient was 33.4 years. Of 64 patients, 26.6% and 12.5% patients complained of genital itching and lower abdominal pain, respectively. Detailed behavioral history was taken, including condom usage, partners of 67.1% used condoms. Mental stress was present in 6.3% of patients.
Table 1.
Profile of study participants
| Item | Total n=64, n (%) |
|---|---|
| Mean age | 33.4 |
| Presenting complaints | |
| Vaginal discharge | 64 (100) |
| Itching | 17 (26.6) |
| Lower abdominal pain | 8 (12.5) |
| Genital ulcer | 4 (6.3) |
| Burning micturition | 1 (1.6) |
| Duration of symptoms | 10 days–20 years |
| Past history of STI | 3 (4.7) |
| Irregular menstrual history | 8 (12.5) |
| STI syndrome | - |
| Behavioral history | - |
| Contraceptive use | |
| Oral contraceptive | 3 (4.7) |
| IUCD | 6 (9.4) |
| Condom use | 43 |
| Emergency contraception | 3 (4.7) |
| Injectable contraceptives | 0 |
| Tubectomy | 3 (4.7) |
| Marital history | |
| Married | 54 (84.4) |
| Unmarried | 10 (15.6) |
| Abortion | 11 (17.2) |
| 1st trimester | 9 |
| 2nd trimester | 2 |
| 3rd trimester | 0 |
| Homosexual | 0 |
| Sex for money | 0 |
| Tobacco use | 1 (1.6) |
| Cigarette use | 1 (1.6) |
| Alcohol use | 1 (1.6) |
| Drug abuse | 1 (1.6) |
| History of travel | 2 (3.1) |
| Age of 1st sexual intercourse (years) | Mean age 21 |
| Mental stress | 4 (6.3) |
| Multiple partners | 1 (1.6) |
STI=Sexually transmitted infection, IUCD=Intrauterine contraceptive device
Risk factors for cervicitis
Various risk factors associated with cervicitis are detailed in Table 2. The risk factors assessed were age, menstrual irregularities, abortion, condom usage, and mental stress. On analysis, nonusage of condoms and mental stress were more frequently associated with cervicitis. Mean of 36.6 pus cells/hpf were observed, appreciably greater number in patients with N. gonorrhoeae and Trachomatous infections (P = 0.0063 and 0.0032, respectively). Pus cells were also high (mean 68 pus cells/hpf) in patients with U. urealyticum, though this may be attributed to coexisting N. gonorrhoeae.
Table 2.
Risk factors associated with cervicitis
| Factors | NG |
CT |
TV |
HSV 1 |
HSV 2 |
UU |
UP |
MH |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | Negative | |
| Total | 17 | 47 | 1 | 63 | 4 | 60 | 1 | 63 | 9 | 55 | 5 | 59 | 26 | 38 | 11 | 53 |
| Age (years) | 33.6 | 24 | 36 | 31 | 33 | 29 | 33.2 | 37.8 | ||||||||
| Menstrual history irregularities | 3 | 5 | 1 | 7 | 1 | 7 | 0 | 8 | 2 | 6 | 2 | 6 | 4 | 4 | 2 | 6 |
| P | 0.68 | 1 | 1 | 1 | 0.6 | 0.17 | 0.71 | 0.63 | ||||||||
| Pus cell count | 61.4 | 27.6 | 73 | 37.2 | 25 | 34.3 | 40 | 36.6 | 16 | 40 | 68.8 | 33.8 | 32.4 | 39.5 | 34.5 | 37.1 |
| P | 0.0063 | 0.0032 | 0.21 | 0.68 | 0.0213 | 0.1742 | 0.53 | 0.97 | ||||||||
| Abortion | 6 | 5 | 0 | 11 | 1 | 10 | 0 | 11 | 1 | 10 | 1 | 10 | 3 | 8 | 0 | 11 |
| P | 0.08 | 1 | 0.56 | 1 | 1 | 1 | 0.51 | 0.35 | ||||||||
| Condom | 4 | 10 | 0 | 14 | 2 | 12 | 0 | 14 | 2 | 12 | 0 | 14 | 0 | 14 | 1 | 13 |
| P | 1 | 1 | 0.3 | 1 | 1 | 0.58 | 0.0033 | 0.68 | ||||||||
| Mental stress | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 1 | 3 | 2 | 2 | 0 | 4 |
| P | 0.74 | 1 | 1 | 1 | 1 | 0.32 | 1 | 1 | ||||||||
| Age at 1st sex | 21.8 | 24 | 17.3 | 20.3 | 18.5 | 20.8 | 20 | |||||||||
NG=Neisseria gonorrhoeae, CT=Chlamydia trachomatis, UU=Ureaplasma urealyticum, UP=Ureaplasma parvum, MH=Mycoplasma hominis, TV=Trichomonas vaginalis
Etiological agents and coinfections
Table 3 shows various etiological agents isolated in the discharge. The most common agent isolated from endocervix was N. gonorrhoeae one out of every four patients. Other agents isolated were T. vaginalis, HSV1, C. trachomatis, HSV2, U. urealyticum, U. parvum, M. genitalium, and M. hominis. Bacterial vaginosis was diagnosed in around one-fifth of the patients. Multiple agents were isolated in 10 (two), 6 (three), 6 (four), and 1 (five) patients, as shown in Figure 1. Isolation of M. hominis and U. parvum was significantly associated with bacterial vaginosis (P = 0.04 and 0.003, respectively).
Table 3.
Etiological agents isolated
| Organism isolated | Frequency (%) |
|---|---|
| None | 9 (14.1) |
| CT | 1 (1.6) |
| NG | 17 (26.6) |
| TV | 4 (6.3) |
| Herpes simplex 1 | 1 (1.6) |
| Herpes simplex 2 | 9 (14.1) |
| UU | 5 (7.8) |
| UP | 26 (40.6) |
| Mycoplasma genitalium | 0 |
| MH | 11 (17.2) |
| Candida sp. | 17 (26.2) |
| Bacterial vaginosis | 14 (21.9) |
NG=Neisseria gonorrhoeae, CT=Chlamydia trachomatis, UU=Ureaplasma urealyticum, UP=Ureaplasma parvum, MH=Mycoplasma hominis, TV=Trichomonas vaginalis
Figure 1.
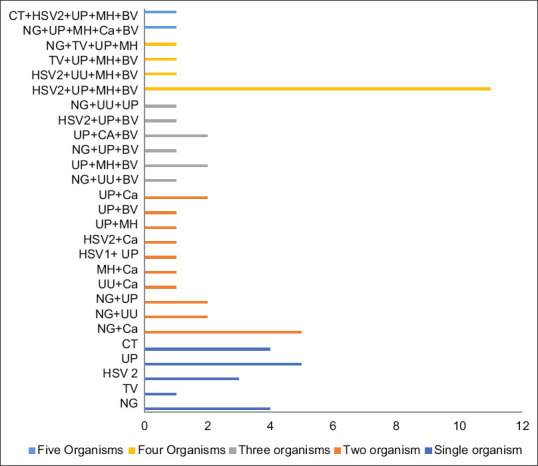
Frequency of multiple organisms implicated in cervicitis
Discussion
Sexually transmitted diseases play a significant role in impacting global health. There is increasing availability and application of newer molecular diagnostic tests for the detection of STI, including cervicitis. The list of causative agents implicated in cervicitis is increasing, including M. genitalium, herpes simplex virus, cytomegalovirus, bacterial vaginosis, and trichomonas. There is the rise in usage of broad-spectrum leading to the emergence of resistance that reinforced the need for targeted antibiotics for the management of cervicitis. A recent review by Lusk and Konecny[4] stated that as the understanding regarding the etiology of cervicitis, especially nonspecific cervicitis, will improve, better management will be possible. Hence, all patients who presented with presenting with cervicovaginal discharge were examined for the presence of cervicitis.
Demographic characteristics of patients complaining of cervico-vaginal discharge
Satterwhite et al.[5] found the incidence of cervicitis to be highest among the age group of 15–24 years. In our study, among the 64 cases with abnormal cervicovaginal discharge, the mean age group of patients was 33.4 years since they belong to the sexually active age group. However, this was not statistically significant.
Patients of cervicitis typically present with purulent or mucopurulent, intermenstrual or postcoital bleeding, dyspareunia, urethritis, and abdominal pain (pelvic inflammatory disease).[6] Of the 64 patients with cervicovaginal discharge, 26.6% and 12.5% of patients complained of genital itching and lower abdominal pain, respectively. There was no complaint of postcoital bleeding. These are comparable findings of the study conducted by Venugopal et al.[7] except that they saw postcoital bleeding in 13.6% of the cases.
Detailed behavioral history was taken, including condom usage, partners of 67.1% used condoms. In a series by Zapata et al.,[8] 29.5% used a barrier contraceptive. Patients of our series had more responsible behavior as far as condom usage was concerned.
In our series, mental stress was present in 6.3% of patients. Nansel et al.[9] in their study found that psychological stress was associated with increased incidence and prevalence of bacterial vaginosis independent of other risk factors. Chronic stress has been found to be associated with reduced immune functioning, which is due to chronic production of glucocorticoid hormones and catecholamines.[10]
Microscopic analysis of mucus taken from the endocervical canal was done. There is the presence of leukocytes throughout the genital tract, including the cervix.[11] More number of pus cells were seen in endocervical smears in patients with N. gonorrhoeae and C. trachomatis infections. Pus cells were high in patients with U. urealyticum, though this may be attributed to coexisting N. gonorrhoeae. Myzuik et al. and Randjelovic et al. in their study evaluated the gram smear findings along with the clinical findings. They stated that there are statistically significant data when >10 PMN/HPF were found in the presence of mucopurulent endocervical discharge, and abnormal cervical findings, for example, ectropion, and friability. They concluded that this did not result in high sensitivities, although the specificity was acceptable. Hence, according to these authors, for the diagnosis of C. trachomatis in resource-poor settings endocervical gram smears can be used or where advanced diagnostic facilities are not available.[12]
In our study, agents isolated from endocervix were N. gonorrhoeae, 26.6%, the most frequently found organism. There were very few C. trachomatis isolates and there was the absence of M. genitalium and HSV in one of every seven patients. Isolation of M. hominis and U. parvum was significantly associated with bacterial vaginosis (P = 0.04 and 0.003, respectively). In contrast, Nkwabong[13] in their study found among all women, genital tract mycoplasmas were the most encountered microorganisms (67.9%). This might reflect changing trends in causative organisms. Another aspect to be noted is that U. parvum and M. hominis are usually commensals; their high isolation, association with bacterial vaginosis and propensity to cause diseases in preterm infants and extragenital infections puts forth the need for further studies and regular monitoring of the agents and their association with cervicitis.
Multiple agents were isolated in 10 (two), 6 (three), 6 (four), and 1 (five) patients. Nkwabong found polymicrobial infections (≥2 g isolated), the association Mycoplasma and Chlamydia were usually encountered (40.9%), followed by mycoplasmas and Gardnerella vaginalis (13.6%). G. vaginalis, Staphylococcus aureus, and Streptococcus sp., when isolated, were found in association with other microorganisms. The finding of polymicrobial infection emphasizes the importance of laboratory tests and etiological diagnosis to identify various organisms responsible before starting empirical treatment. This will help in improving treatment outcomes and prevent resistance and relapse. This is in contradiction to the syndromic approach that is practiced in developing countries.[14]
Most of the published studies on cervicitis have used only a limited panel of organisms for identifying the etiological agents, leading to low etiological positivity in cases of cervicitis. Thus, in many cases etiology of cervicitis remains unknown or unclear.[15] In our study, etiological agents could be identified in 85.9% of cases, while Gaydos et al.[16] could identify an etiological agent in only 52.6% of the participants. Identification of a wider array of etiological agents, including viral and parasitic agents, increases the positivity of the etiological diagnosis of cervicitis. Furthermore, the use of molecular tests which have better performance characteristics (higher sensitivity and specificity) enhances the ability to diagnose more number of organisms implicated in cervicitis which are more often fastidious in nature and hence difficult to culture and isolate.
Some limitations of our study are we were not always able to verify the authenticity of data given by the women, especially regarding the number of sexual partners. A longer follow-up needs to be done to see the effect of treatment on clinical symptoms and the efficacy of treatment on these organisms.
Summary and recommendations
Very few C. trachomatis isolates, absence of M. genitalium and HSV in one of every 7 patient, highlights the changing etiological pattern of cervicitis
Although U. parvum and M. hominis are usually commensals, their high isolation, association with bacterial vaginosis and propensity to cause diseases in preterm infants and extragenital infections put forth the need for further studies and regular monitoring of the agents and their association with cervicitis
The use of tests to detect a wider array of organisms and replacing standard culture methods with molecular assays increases the ability to diagnose more number of organisms implicated in cervicitis
A larger follow-up study is needed to study the effect of treatment on clinical symptoms and the efficacy of treatment on these organisms.
Disclosure of interest statement
Apex Regional STD Centre is funded by the Safdarjung Hospital, New Delhi and National AIDS Control Organization, India. No pharmaceutical grants were received in the development of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ortiz-de la Tabla V, Gutiérrez F. Cervicitis: Aetiology, diagnosis and treatment. Enferm Infecc Microbiol Clin (Engl Ed) 2019;37:661–7. doi: 10.1016/j.eimc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS/WHO Working Group on Global HIV/AIDS/STI Surveillance. Geneva: WHO; 1999. [Last accessed on 2020 Oct 23]. Guidelines for Sexually Transmitted Infections Surveillance. Available from: http://www.who.int/emc . [Google Scholar]
- 3.Workowski A, Bolan GA. “Centers for Disease Control and Prevention. [Last accessed on 2021 Jul 07]. Sexually Transmitted Diseases Treatment Guidelines,” Available from: https://www.cdc.gov/std/tg2015/tg-2015-print.pdf .
- 4.Lusk MJ, Konecny P. Cervicitis: A review. Curr Opin Infect Dis. 2008;21:49–55. doi: 10.1097/QCO.0b013e3282f3d988. [DOI] [PubMed] [Google Scholar]
- 5.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187–93. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal U, Wills C. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Cervicitis. [PubMed] [Google Scholar]
- 7.Venugopal S, Gopalan K, Devi A, Kavitha A. Epidemiology and clinico-investigative study of organisms causing vaginal discharge. Indian J Sex Transm Dis AIDS. 2017;38:69–75. doi: 10.4103/2589-0557.203433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zapata LB, Pazol K, Curtis KM, Kane DJ, Jatlaoui TC, Folger SG, et al. Need for contraceptive services among women of reproductive age – 45 Jurisdictions, United States, 2017-2019. MMWR Morb Mortal Wkly Rep. 2021;70:910–5. doi: 10.15585/mmwr.mm7025a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194:381–6. doi: 10.1016/j.ajog.2005.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–8. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- 11.Stern JE, Givan AL, Gonzalez JL, Harper DM, White HD, Wira CR. Leukocytes in the cervix: A quantitative evaluation of cervicitis. Obstet Gynecol. 1998;91:987–92. doi: 10.1016/s0029-7844(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 12.Randjelovic I, Moghaddam A, Freiesleben de Blasio B, Moi H. The role of polymorphonuclear leukocyte counts from urethra, cervix, and vaginal wet mount in diagnosis of nongonococcal lower genital tract infection. Infect Dis Obstet Gynecol. 2018;2018:8236575.. doi: 10.1155/2018/8236575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nkwabong E. What are the most common sexually transmitted bacteria in women with cervico-vaginitis nowadays? Indian J Sex Transm Dis AIDS. 2020;41:39–42. doi: 10.4103/ijstd.IJSTD_143_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arzouni JP, Bouilloux JP, de Moüy D, Bicart-See A, Charbit C, Doeschler T, et al. Genital infections in women, in community practice. Comparison of two studies, 1987 and 2002. Med Mal Infect. 2004;34:92–6. doi: 10.1016/j.medmal.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Marrazzo JM. Mucopurulent cervicitis: No longer ignored, but still misunderstood. Infect Dis Clin North Am. 2005;19:333–49. doi: 10.1016/j.idc.2005.03.009. viii. [DOI] [PubMed] [Google Scholar]
- 16.Gaydos C, Maldeis NE, Hardick A, Hardick J, Quinn TC. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinics. Sex Transm Dis. 2009;36:598–606. doi: 10.1097/OLQ.0b013e3181b01948. [DOI] [PMC free article] [PubMed] [Google Scholar]


