BACKGROUND
The Government of India (GoI) has launched the fifth phase of the National AIDS and STD Control Programme (NACP) to anchor the national acquired immunodeficiency syndrome (AIDS) and sexually transmitted infection (STI) response in the country. Being fully funded by the GoI, NACP Phase-V will anchor the country’s response to achieve the end of the AIDS epidemic as a public health threat by 2030. This is consistent with the GoI commitment to the agenda of Sustainable Development Goals which calls for, inter alia, ending AIDS as a public health threat by 2030.[1,2]
The human immunodeficiency virus (HIV)/AIDS epidemic in India, highly diverse in terms of levels, trends, and drivers, is the second largest epidemic in the world with around 2.4 million people living with HIV/AIDS (PLHIV). The GoI is responding to this highly diverse epidemic through NACP since 1992. While the program has been phenomenally successful in preventing new HIV infections and AIDS-related mortality through four phases of NACP and the overall response is on track, there are specific implementation domains that require focus and prioritization to accelerate the progress towards the attainment of the 2030 goal.[3-6] Elimination of vertical transmission of HIV and syphilis (EVTHS) is one such area.
HIV and syphilis are two of the perinatally acquired infections identified for elimination globally. World Health Organization (WHO) has been releasing global guidance on the process and impact criteria for EVTHS since 2014. The guidance document has specific processes and impact targets for each vertical infection. Countries should have evidence of achievement and maintenance of process indicator targets for at least two years and impact indicators for at least one year at the national level before submission for validation. As of November 2021, 15 countries have been validated for the elimination of mother-to-child transmission (EMTCT) of HIV or syphilis.[7]
EVOLUTION AND PROGRESS ON EVTHS UNDER NACP
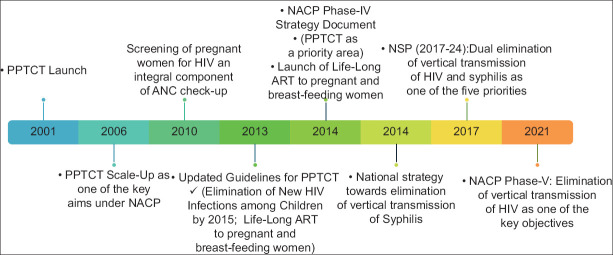
Interventions for the prevention of vertical transmission of HIV were launched under the second phase of the NACP of the GoI in the year 2002.[8,9] Since then, the EVTHS has remained one of the key objectives of the program [Figure 1]. Initially, the aim was to attain the elimination of vertical transmission by 2015, which was subsequently shifted to 2020. For HIV, the four-pronged strategy was adopted focusing on primary prevention of HIV, prevention of unintended pregnancies, prevention of vertical transmission, and care, support, and treatment of women living with HIV (WLHIV) and her children. The strategies for syphilis elimination called for sustained high-level commitment and advocacy, increasing access to, and improving the quality of reproductive, maternal, newborn, child health plus adolescents (RMNCH + A) and other relevant services including screening of pregnant women and treating syphilis-positive women, their partners, and new-born infants, and establishing/strengthening of surveillance, monitoring, and evaluation systems.[10-16]
Figure 1.
Elimination of vertical transmission and NACP
The progress on EVTHS has been significant but still, a lot needs to be done to attain the WHO’s prescribed targets for EVTHS [Table 1]. While registration of pregnant women for ante-natal care (ANC) services is hovering in the range of 92–97%, the progress on HIV testing/treatment aspects had never exceeded 82% with syphilis testing coverage among pregnant women being reported much lower than HIV under the program. As the new HIV infections declined significantly, the pediatric case rate declined below the target level of <50 per 100,000 live births, but the mother-to-child transmission (MTCT) rate continues to be high (24%) given the progress on testing and treatment coverage. Almost 8% of the estimated new HIV infections in India in 2021 were attributed to MTCT.[4,5,17,18]
Table 1.
Impact and process indicators and targets for elimination of vertical transmission of HIV and syphilis
| Infection | Indicator Type | Indicator | Target | Progress (2021) |
|---|---|---|---|---|
| HIV | Impact | HIV MTCT rate, and | <5% (breastfeeding populations) OR <2% (non-breastfeeding populations) | 24.2% |
| Case rate of new pediatric HIV infections due to MTCT | <50 per 100,000 live births | 24 | ||
| Process | ANC-1 coverage (at least one visit) | ≥95% | >=95% | |
| Coverage of HIV testing among pregnant women | ≥95% | 83% | ||
| ART coverage of pregnant women living with HIV | ≥95% | 64% | ||
| Syphilis | Impact | Case rate of congenital syphilis (CS) | ≤50 per 100,000 live births | - |
| Process | ANC-1 coverage (at least one visit) | ≥95% | >=95% | |
| Coverage of syphilis testing among pregnant women | ≥95% | 46% | ||
| Adequate treatment coverage of syphilis-seropositive pregnant women | ≥95% | 47% |
Recognizing the need to augment and accelerate progress, NACP Phase-V included the attainment of the EVTHS as one of the five high-level goals to be attained by 2025. Being ahead of the curve, NACP Phase-V has reflected the elimination of vertical transmission of HIV in terms of viral load suppression.[17] Having a priority reflected in high-level goals with higher benchmarks in vision and strategy documents underlines the country’s commitment to making progress and is a powerful motivator. The immediate next step would be to prepare strategic pathways to guide actions towards the attainment of EVTHS targets.
PATHWAY TO EVTHS
It has been almost two decades since the introduction of interventions for the prevention of vertical transmission under NACP. Overall, the HIV surveillance system under NACP has been indicative of a very low prevalence of HIV and syphilis among pregnant women in India.[19] While the disease burden for congenital syphilis is not available under NACP, almost 5000 new HIV infections may be attributed to vertical transmission annually.[5] In a low prevalence and low fertility yet high population settings of India, the challenges are vast and diverse. With EVTHS stated as one of the five high-level goals to be attained under NACP Phase-V, there is an urgent need to integrate, scale, expand, and accelerate the intervention’s mechanism and approach across the prevention-testing-treatment cascade in an integrated and holistic manner.
Disease eliminations are long-term and resource-intensive goals requiring sustained political commitments.[20] Attainment of EVTHS is not going to be different. The GoI has already demonstrated its commitment to the cause by including EVTHS as one of the five top-level goals under phase-V of NACP. As a next step, a dedicated campaign under the political leadership at the highest level, will not only catalyze the transition of EVTHS from policy to implementation but will also provide the required visibility gearing up the entire system, thus getting out of the “Business as Usual” approach. India has seen the direct and significant impact of charismatic leadership during the COVID-19 pandemic.[21] EVTHS needs to be distinguished for all other components of the NACP at all levels and political leadership will provide the required ecosystems to attain the same.
Equally important will be the shared strategic and operational framework for both HIV and syphilis in the context of EVTHS, across health systems, including the private sector. Till now, both had been largely targeted in isolation, in policy designing and implementation.[11,13,22] HIV testing is systematically linked with ANC clinics through dedicated integrated counselling and testing centres (ICTC) under NACP. Syphilis testing is mainly managed in the dermatology and venereology department, which is usually a very distinct setting from that of ANC clinics. Obstetricians may not necessarily refer a pregnant woman to the dermatology and venereology department, thus leading to a gap between HIV and syphilis testing. Similarly, ICTC under NACP may not be offering testing services for syphilis while doing so for HIV. As NACP structures for STI/ reproductive tract infection (RTI) management remains largely located at district-level facilities, the capacity of the National AIDS Control Organization (NACO) to drive the strategies for syphilis testing and treatment at sub-district level facilities is constrained. Given the fact that both have similarity in mode of transmission and prevention approaches, and also testing opportunities for both is common during pregnancy, having one strategic and implementation framework for both HIV and syphilis, across the NACP and National Health Mission (NHM), will boost the country’s progress enhancing efficiencies.[23]
ANC registration, HIV/syphilis testing for the diagnosis of infected pregnant women, and then suitable treatment for those found infected as well as follow-up of infants for screening and treatment services are fundamental building blocks of EVTHS. While progress on ANC registration is already at-par with the stated targets, there is a significant gap between ANC registration and testing revealing lost opportunity and systematic issues. Responding to the gaps will require not only ownership and integration of testing in the ANC care package by the frontline workers under NHM but will also require equal engagement from the private health care providers. The use of recent technology like point-of-care dual test kits for HIV and syphilis may help to achieve this universalization, but at the same time, the inclusion of offering of these services in routine program monitoring and conditional cash transfer program of Janani Suraksha Yojana may further boost the uptake of testing services.[24]
EVTHS calls for responding to every avenue of vertical transmission. Current guidelines usually ask for testing only once during the pregnancy which might miss the incident cases. HIV transmission during the breastfeeding period is a well-established route of transmission for HIV and accordingly, re-testing has been recommended.[25,26] In India, where almost three-fourths of 2 years old children continue to be breastfeeding, this becomes significant.[27] Given the high rate of vertical transmission, the national program may incorporate guidance on re-testing for HIV and syphilis during the pregnancy and breastfeeding period. A modeling exercise may be undertaken to assess the impact of such policies.
Detection of infected pregnant women or breastfeeding women should be followed by immediate initiation of treatment and retention (lifelong or until the completion of the course) as per the guidelines. And, then there are aspects of family planning services and follow-up testing services. Gaps exist in these aspects which can be addressed with due focus and unified service delivery approaches.[4] Re-engineering the operational flow of pregnant women or breastfeeding women bringing antiretroviral therapy (ART) centers in an anchoring role not only for treatment services but also for family planning and follow-up testing services for exposed babies may help to streamline the processes improving service uptake.
Shared and harmonized beneficiary-centric information-technology (IT) enabled strategic information across the complementary system of program monitoring, surveillance, and research will be vital to track the impact of the service delivery elements of the pathway. In general, strategic information to inform the policymakers and program managers on vertical transmission of HIV is much more robust than syphilis across the globe and India is not an exception.[17,28] Implementation of the integrated and enhanced surveillance and epidemiology framework for HIV, syphilis, and related co-morbidities under NACP will enrich the epidemiological evidence.[29] IT-enabled program monitoring for interventions across the pregnancy and breastfeeding period and also follow-up for testing and treatment services for exposed children will not only bridge the current evidence gap but will also provide crucial longitudinal information including that of treatment and retention informing both the program management and disease burden estimations.
CONCLUSION
By including the EVTHS as one of the five high-level goals, NACP has brought the issue to the forefront of the national health response. Remarkable progress in recent years, particularly for HIV screening and testing of pregnant women, has demonstrated that EVTHS is not merely aspirational but achievable. Political leadership to the cause of EVTHS will accelerate the progress immensely by driving administrative, service delivery, and community support. Shared strategic, and implementation framework anchoring integrated response across health systems and supported by expanded testing and re-engineered operational flows with harmonized IT-enabled strategic information for beneficiary-centric services to pregnant and breastfeeding HIV/syphilis mothers and their babies would be vital to take the country on the path of elimination.
REFERENCES
- 1.Government of India. Press release. 2022. Union Cabinet approves continuation of National AIDS and STD Control Programme (NACP, Phase-V) from 1st April 2021 to 31st March 2026. Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1808185 .
- 2.David M. Sustainable development goals (SDGs)-challenges for India. Indian J Public Health Res Dev. 2018;9 doi:10.5958/0976-5506.2018.00172.9. [Google Scholar]
- 3.Kumar P, Sahu D, Rajan S, Mendu VV, Das C, Kumar A, et al. District-level HIV estimates using the spectrum model in five states of India, 2017. Medicine. 2021;100:e26578. doi: 10.1097/MD.0000000000026578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National AIDS Control Organization. Sankalak: Status of National AIDS Response. Second edition, 2020. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2020. [Google Scholar]
- 5.National AIDS Control Organization &ICMR-National Institute of Medical Statistics. India HIV Estimates 2021: Fact Sheet. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2022. [Google Scholar]
- 6.Kumar P, Das C, Kumar A, Sahu D, Rai SK, Godbole S, et al. Diversity in HIV epidemic transitions in India:An application of HIV epidemiological metrices and benchmarks. PloS One. 2022;17:e0270886. doi: 10.1371/journal.pone.0270886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Global guidance on criteria and processes for validation: Elimination of Mother-to-child Transmission of HIV, Syphilis and Hepatitis B Virus. World Health Organization; 2021. [Google Scholar]
- 8.Kadri AM, Kumar P. Institutionalization of the NACP and way ahead. Indian J Community Med. 2012;37:83–8. doi: 10.4103/0970-0218.96088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National AIDS Control Organization. Elimination of Vertical Transmission of HIV &Syphilis. [Last accessed on 2022 Nov 19]. http://naco.gov.in/elimination-vertical-transmission-hiv-syphilis#:~:text=The%20Prevention%20of%20Parent%20to,five%20high%20HIV%20prevalence%20states.
- 10.National AIDS Control Organisation. National AIDS Control Programme Phase III [2006-2010] New Delhi: National AIDS Control Organization, Ministry of Health &Family Welfare, Government of India; 2006. Strategy and Implementation Plan. [Google Scholar]
- 11.National AIDS Control Organisation. New Delhi: National AIDS Control Organization, Ministry of Health &Family Welfare, Government of India; 2013. Updated Guidelines for Prevention of Parent to Child Transmission (PPTCT) of HIV using Multi-Drug Anti-retroviral Regimen in India. [Google Scholar]
- 12.National AIDS Control Organisation. National AIDS Control Programme Phase-IV [2012-2017] Strategy Document. New Delhi: National AIDS Control Organization, Ministry of Health &Family Welfare, Government of India; 2014. [Google Scholar]
- 13.National AIDS Control Organisation. New Delhi: National AIDS Control Organization, Ministry of Health &Family Welfare, Government of India 2015; The National Strategy &Operational Guidelines Towards Elimination of Congenital Syphilis. [Google Scholar]
- 14.Srinivas V, Turlapati PL, Bhola AK, Singh AK, Rajan S, Gupta RS, et al. Towards elimination of parent-to-child transmission of Syphilis in India:A rapid situation review to inform national strategy. WHO South East Asia J Public Health. 2015;4:197–203. doi: 10.4103/2224-3151.206690. [DOI] [PubMed] [Google Scholar]
- 15.National AIDS Control Organisation. National Strategic Plan for HIV/AIDS and STI, 2017 –2024. National AIDS Control Organization, Ministry of Health &Family Welfare, Government of India; 2017. [Google Scholar]
- 16.Ministry of Health & Family Welfare, Government of India. Priority Areas for HIV Programme (April 2021-March 2024) Available from: https://main.mohfw.gov.in/sites/default/files/Program%20priority%20Areas%20for%20EOI.pdf .
- 17.National AIDS Control Organization. Strategy Document:National AIDS and STD Control Programme Phase-V (2021-26) New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2022. [Google Scholar]
- 18.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global AIDS Monitoring 2022. Geneva: UNAIDS; 2022. [Google Scholar]
- 19.National AIDS Control Organization. ANC HSS 2019: Technical Report. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2020. [Google Scholar]
- 20.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(Suppl 2):22. [PMC free article] [PubMed] [Google Scholar]
- 21.Jahagirdar S, Chatterjee A, Behera S, Mohapatra A. Response to the COVID-19 pandemic in India:Case studies on leadership in crisis situations. Int J Health Allied Sci. 2020;9:81–4. [Google Scholar]
- 22.National AIDS Control Organisation. National AIDS Control Organization, Ministry of Health & Family Welfare, Government of India; 2011. Operational Guidelines For Programme Managers and Service Providers For Strengthening STI/RTI Services. [Google Scholar]
- 23.Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022-2030. Geneva: World Health Organization; 2022. Licence CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 24.Sen S, Chatterjee S, Khan PK, Mohanty SK. Unintended effects of Janani Suraksha Yojana on maternal care in India. SSM Popul Health. 2020;11:100619. doi: 10.1016/j.ssmph.2020.100619. doi:10.1016/j.ssmph. 2020.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Cock KM, Fowler MG, Mercier E, De Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries:Translating research into policy and practice. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Guidance on Global Scale-Up of the Prevention of Mother-to-Child Transmission of HIV. Geneva, Switzerland: The Interagency Task Team (IATT) on Prevention of HIV Infection in Pregnant Women, Mothers and their Children; 2007. [Google Scholar]
- 27.International Institute for Population Sciences (IIPS) and ICF. 2017. National Family Health Survey (NFHS-4) India. Mumbai: IIPS; 2015-16. [Google Scholar]
- 28.Taylor M, Newman L, Ishikawa N, Laverty M, Hayashi C, Ghidinelli M, et al. Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT):Process, progress, and program integration. PLoS Med. 2017;14:e1002329. doi: 10.1371/journal.pmed.1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National AIDS Control Organization. Integrated and Enhanced Surveillance and Epidemiology of HIV, STI and related Co-morbidities Under the National AIDS and STD Control Programme: Strategic Framework. New Delhi: NACO, Ministry of Health and Family Welfare, Government of India; 2022. [Google Scholar]