Summary
Drug-drug interaction of the antiviral sofosbuvir and the antiarrhythmics amiodarone has been reported to cause fatal heartbeat slowing. Sofosbuvir and its analog, MNI-1, were reported to potentiate the inhibition of cardiomyocyte calcium handling by amiodarone, which functions as a multi-channel antagonist, and implicate its inhibitory effect on L-type Cav channels, but the molecular mechanism has remained unclear. Here we present systematic cryo-EM structural analysis of Cav1.1 and Cav1.3 treated with amiodarone or sofosbuvir alone, or sofosbuvir/MNI-1 combined with amiodarone. Whereas amiodarone alone occupies the dihydropyridine binding site, sofosbuvir is not found in the channel when applied on its own. In the presence of amiodarone, sofosbuvir/MNI-1 is anchored in the central cavity of the pore domain through specific interaction with amiodarone and directly obstructs the ion permeation path. Our study reveals the molecular basis for the physical, pharmacodynamic interaction of two drugs on the scaffold of Cav channels.
Keywords: Sofosbuvir, Amiodarone, Drug safety, Drug-drug interactions, Severe bradycardia, L-type Cav channels, Cryo-EM, Cav1.1, Cav1.3, Voltage-gated calcium channels
Introduction
Sofosbuvir (or Sovaldi, short as SOF, Figure 1A), approved by the US Food and Drug Administration in 2013, has been an effective drug for the treatment of hepatitis C (Afdhal et al., 2014; Jacobson et al., 2013; Lawitz et al., 2013; Mangia et al., 2020). In 2015, severe slowing of heartbeat rate, including one fatal, was reported in patients following coadministration of SOF and amiodarone (AMIO, Figure 1A), a class III antiarrhythmic agent for the treatment of ventricular arrhythmias and atrial fibrillation (Back and Burger, 2015). SOF is a nucleotide analog inhibitor targeting Nonstructural protein 5B (NS5B) RNA polymerase of hepatitis C virus (HCV), and AMIO is a multi-channel blocker that can act on Ca2+, Na+, and K+ channels (Appleby et al., 2015; Kodama et al., 1997; Roy et al., 2000; Stedman, 2014). Ensuing investigations showed that SOF or its analog, MNI-1 (MRL, Merck & Co., Rahway, NJ, USA, Figure 1A), potentiated calcium-handling effects by AMIO in human cardiomyocytes, and increased AMIO’s inhibition of L-type Ca2+ channels (LTCC) (Lagrutta et al., 2017; Lagrutta et al., 2016; Millard et al., 2016). However, the underlying molecular mechanism was not clear.
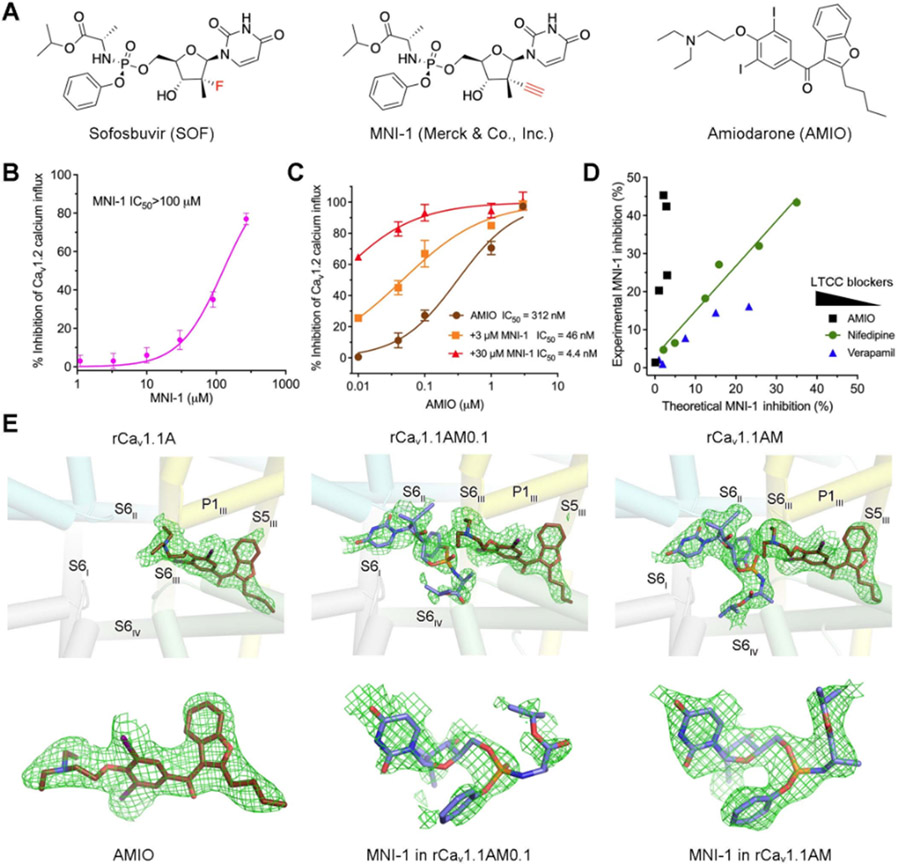
Figure 1 ∣. Cryo-EM analysis of rabbit Cav1.1 in the presence of AMIO and MNI-1.
(A) Chemical structures of sofosbuvir (SOF), MNI-1, and amiodarone (AMIO). (B) MNI-1 exhibits incomplete inhibition of Cav1.2 when applied at high concentrations. IC50 of MNI-1 was measured by Cav1.2 calcium influx assay. Values were normalized to the average response by 0.1 μM isradipine (as 100% inhibition). IC50 curves of all the figures were generated by Graphpad Prism 9. Points represent mean ± SEM obtained from at least three independent experiments performed in quadruplicate. (C) Synergistic effect of AMIO on MNI-1. IC50 values were calculated using MNI-1 as baseline. (D) Modeling of pharmacodynamic effects of LTCC blockers on MNI-1. This figure was plotted from a batch of IC50 experiment measuring LTCC blockers supplemented with 3 μM MNI-1 (AMIO), or 90 μM MNI-1 (nifedipine and verapamil). Please refer to STAR★Methods for detailed explanation of the modeling. The concentrations of LTCC blockers decrease from left to right. (E) EM densities of the bound ligands. Top: The ligand densities in the pore domain (PD) of rabbit Cav1.1 in the presence of the indicated chemicals. rCav1.1A: rabbit Cav1.1 implemented with 0.1 mM AMIO; rCav1.1AM0.1: with 0.1 mM AMIO and 0.1 mM MNI-1; rCav1.1AM: with 0.1 mM AMIO and 1 mM MNI-1. The domain coloured PD is shown as semi-transparent cylindrical cartoon. Bottom: Enlarged views of the EM densities for the indicated ligands. All the densities, shown as green meshes, are contoured at 4 σ. All structure figures are prepared in PyMol (DeLano, 2002). See also Figures S1, S2.
LTCCs, also known as the dihydropyridine receptors (DHPR), refer to the Cav1 subfamily of the voltage-gated Ca2+ channels (Carbone and Lux, 1984; Dolphin, 2006; Ertel et al., 2000; Kohlhardt and Fleckenstein, 1977; Nowycky et al., 1985). LTCCs are heteromeric channels consisting of a core α1 subunit, an extracellular α2δ subunit, an intracellular β subunit, and in some complexes a transmembrane γ subunit. Classification of Cav channels is based on the α1 subunit, which comprises four repeats each containing six transmembrane segments S1-S6 (Ertel et al., 2000). The S1-S4 segments in each repeat constitute the voltage-sensing domain (VSD), which undergoes conformational changes in response to fluctuations of the membrane potential. And the S5 and S6 segments of the four repeats enclose the central pore domain (PD) that is responsible for selective Ca2+ conductance (Tanabe et al., 1987; Wu et al., 2015).
Among the four Cav1 members, Cav1.1 is specialized for excitation-contraction coupling (ECC) of skeletal muscles; Cav1.4 predominantly functions in retina, playing a critical role for neurotransmitter release; and Cav1.2 and Cav1.3 exhibit a more diverse tissue distribution in heart, brain, and endocrine cells. Dysfunction of Cav1.2 and Cav1.3 has been characterized in various arrythmias. Cav1.2 controls ECC of cardiomyocytes, and Cav1.3, the primary subtype in the sinoatrial node and the atrioventricular node, is required for the pacemaker activity (Zamponi et al., 2015). Consistently, DDI of SOF/MNI-1 and AMIO interferes with calcium handling in cardiomyocyte models and Cav1.2 and Cav1.3 channel activity (Lagrutta et al., 2017; Lagrutta et al., 2016; Millard et al., 2016).
To dissect the molecular basis for the DDI of SOF/MNI-1 and AMIO on Cav1.2 and Cav1.3 channels, we set out to solve the structures of LTCCs supplemented with different drug combinations. For a long time, the multi-subunit Cav1.1 complex isolated from the skeletal muscle of rabbits, designated rCav1.1, has been a prototype for structural analysis of LTCC modulation by various anti-hypertension agents, such as DHP drugs and pore blockers (Gao and Yan, 2021; Wu et al., 2016; Zhao et al., 2019). Because of the high sequence similarity among the LTCC members, we first collected cryo-EM data for rCav1.1 treated with AMIO, or AMIO combined with MNI-1 (short as AM). To study the DDI on the physiologically more relevant human LTCCs, we managed to obtain the human ternary Cav1.3 complex, consisting of α1, α2δ-1, and β3 subunits, through recombinant expression in HEK293 cells (Yao et al., 2022). Cryo-EM data were collected for Cav1.3 separately incubated with AMIO, SOF, and AMIO together with SOF (short as AS).
Our systematic structural analyses demonstrate that AMIO alone inserts into the pore fenestration enclosed by repeat III and IV of LTCCs. Alone, neither SOF nor MNI-1 can bind to the channels; in the presence of AMIO however, either SOF or MNI-1 is able to act as a pore blocker that directly cuts off the ion permeation path in the central cavity of the channel. Our study reveals direct physical interaction of two small molecule blockbuster drugs within the scaffold of a protein, leading to clinically meaningful drug-drug interaction.
Results
Synergistic DDI of AMIO and MNI-1
Expanding on previously published data (Lagrutta et al., 2017; Millard et al., 2016), we used a HEK293 cell line that stably overexpresses human Cav1.2 channels for functional investigations of DDI. The pharmacodynamic interactions of MNI-1 with AMIO, or with two other LTCC blockers, nifedipine and verapamil, were separately explored. MNI-1 alone inhibited Cav1.2 with an IC50 value of more than 100 μM (Figure 1B). The dramatically enhanced inhibition by co-applied MNI-1 and AMIO indicates a strong synergistic effect between these two drugs (Figure 1C). In contrast, MNI-1 shows no synergistic interaction with nifedipine or verapamil (Figures S1A,B).
We modelled the effect of different Cav inhibitors tested on MNI-1 (Figure 1D; Figure S1C). In general, for two compounds that inhibit Ca2+ influx independently, the individual inhibition by one compound would match the total effect subtracted from the portion of blockage by the other compound, as seen in the modelling of no interaction between nifedipine and MNI-1 (Figure 1D; Figure S1C, middle). For AMIO, the experimental MNI-1 inhibition in the combination assays is consistently higher than predicted values, in accordance with the synergistic DDI of these two drugs (Figure 1D; Figure S1C, left). In contrast, the inhibition of Cav1.2 by MNI-1 was modelled to be diminished by verapamil at high concentrations, suggesting a competition between the two drugs for pore blocking (Figure 1D; Figure S1C, right).
Inspired by the synergistic DDI of MNI-1 and AMIO, we sought to employ the cryo-EM technology to examine the potential association of AMIO and MNI-1 with LTCCs.
Structural analysis of rCav1.1 bound to AMIO and MNI-1
Purification of rCav1.1 was performed following our reported protocol (Gao and Yan, 2021; Wu et al., 2016). For structural analysis of rCav1.1 in the presence of AMIO alone or in combination with MNI-1, AMIO was supplemented at a final concentration of 0.1 mM, and a titration of MNI-1 at 0.1 mM and 1 mM was used. After incubation at 4 °C for 30 min, cryo-grids were prepared for the three samples, referred to as rCav1.1A for rCav1.1 with AMIO only, rCav1.1AM0.1 for AMIO plus 0.1 mM MNI-1, and rCav1.1AM for AMIO plus 1 mM MNI-1. Following standard image acquisition and processing protocols, 3D EM reconstructions for rCav1.1A, rCav1.1AM0.1, and rCav1.1AM were obtained at 2.8 Å, 2.8 Å, and 3.0 Å, respectively (Table 1; Figure S2).
Table 1 ∣.
Statistics for data collection and structural refinement of rCav1.1 structures. See also Figure S2.
| rCav1.1A (EMD-27904) (PDB 8E56) |
rCav1.1AM0.1 (EMD-27905) (PDB 8E57) |
rCav1.1AM (EMD-27906) (PDB 8E58) |
|
|---|---|---|---|
| Data collection and processing | |||
| Magnification | 105,000 | 105,000 | 105,000 |
| Voltage (kV) | 300 | 300 | 300 |
| Electron dose (e-/Å2) | 50 | 50 | 50 |
| Defocus range (μm) | −2.1~−1.9 | −2.1~−1.9 | −2.1~−1.9 |
| Pixel size (Å) | 1.114 | 1.114 | 1.114 |
| Symmetry | C1 | C1 | C1 |
| Initial particle images (no.) | 831,227 | 688,386 | 1,014,749 |
| Final particle images (no.) | 400,307 | 190,835 | 83,355 |
| Map resolution (Å) | 2.8 | 2.8 | 3.0 |
| FSC threshold | 0.143 | 0.143 | 0.143 |
| Refinement | |||
| Initial model used (PDB code) | 5GJV | 5GJV | 5GJV |
| Model resolution (Å) | 2.9 | 3.0 | 3.1 |
| FSC threshold | 0.5 | 0.5 | 0.5 |
| Map sharpening B factor (Å2) | −67 | −57 | −65 |
| Model composition | |||
| Non-hydrogen atoms | 18,461 | 18,449 | 18,449 |
| Protein residues | 2247 | 2247 | 2247 |
| Ligands | 28 | 28 | 28 |
| B factors (Å2) | |||
| Protein | 68.25 | 73.00 | 82.9 |
| Ligand | 127.27 | 102.15 | 99.8 |
| R.m.s deviations | |||
| Bond lengths (Å) | 0.003 | 0.003 | 0.005 |
| Bond angles (°) | 0.555 | 0.518 | 0.620 |
| Validation | |||
| MolProbity score | 1.78 | 1.61 | 1.78 |
| Clashscore | 7.60 | 5.90 | 7.37 |
| Poor rotamers (%) | 1.06 | 0.66 | 0.91 |
| Ramachandran plot | |||
| Favored (%) | 95.07 | 95.84 | 94.48 |
| Allowed (%) | 4.93 | 4.16 | 5.43 |
| Disallowed (%) | 0.00 | 0.00 | 0.09 |
In all three maps, the density for AMIO was well resolved (Figure 1E). When applied at 0.1 mM, MNI-1 could not be reliably built into the discontinuous densities that are adjacent to AMIO (Figure 1E, middle). At 1 mM, a stretch of density that can be well fitted by MNI-1 is unambiguously resolved in the central cavity of the PD (Figure 1E, right). The MNI-1 density in rCav1.1AM serves as a reference to confirm that the weaker and noisier density in the cavity of rCav1.1AM0.1 should belong to MNI-1. We next focus on rCav1.1A and rCav1.1AM for structural analysis.
Coordination of AMIO by rCav1.1
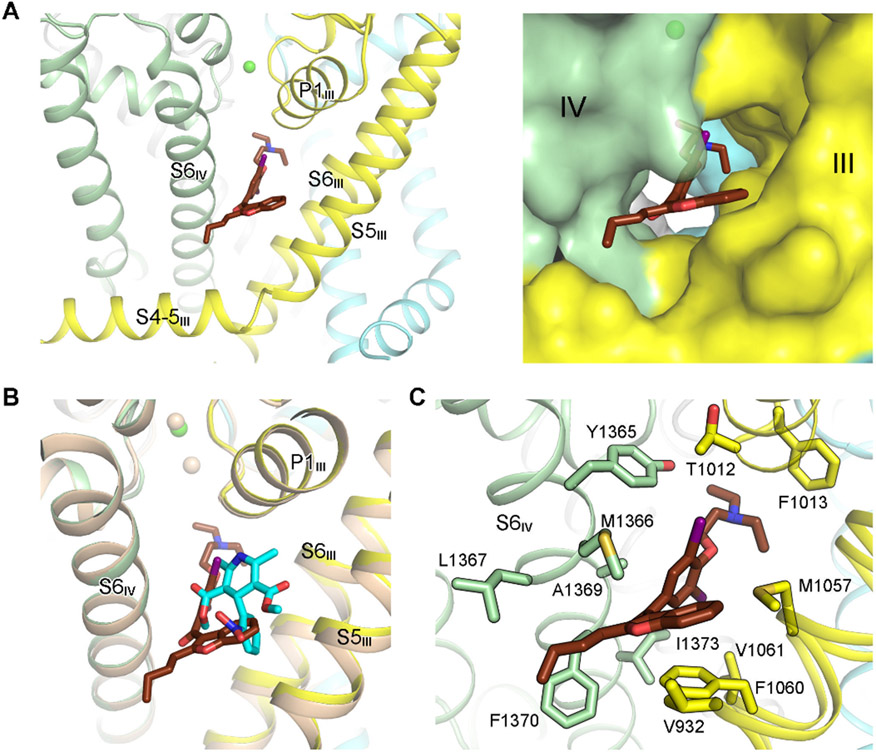
AMIO occupies the same site as for DHP compounds, i.e., the III-IV fenestration enclosed by S5III, S6III, and S6IV (Gao and Yan, 2021; Zhao et al., 2019) (Figure 2A). This observation immediately affords a molecular interpretation for the reported antagonism of the binding of a DHP drug nitrendipine to cardiac LTCC by AMIO (Lubic et al., 1994; Nokin et al., 1986).
Figure 2 ∣. Coordination of AMIO in rCav1.1.
(A) AMIO inserts into the fenestration on the interface of repeats III and IV (the III-IV fenestration). The PD of rCav1.1A is shown as ribbon cartoon (left) or semi-transparent surface (right) to highlight the binding pocket for AMIO. (B) AMIO occupies the same binding site as the DHP compounds. Structures of the α1 subunit of rCav1.1A and nifedipine (cyan sticks)-bound rCav1.1 (colored wheat, PDB code: 6JP5) can be superimposed with RMSD of 0.57 Å over 970 aligned Cα atoms. (C) AMIO is coordinated exclusively through van der Waals contacts. AMIO and the surrounding residues are shown as sticks. See also Figure S2.
Although the overall structures of AMIO- and nifedipine-bound rCav1.1 are nearly identical, with the root-mean-square deviation (RMSD) of 0.57 Å over 970 superimposed Cα atoms in the α1 subunit (Figure 2B), the coordination modes for the two classes of compounds deviate substantially. Unlike DHP compounds, whose LTCC-specificity is defined by several polar interactions (Gao and Yan, 2021; Zhao et al., 2019), AMIO is surrounded entirely by hydrophobic residues (Figure 2C). The fenestration-encompassing residues on S6III and S6IV are the major contributors for AMIO binding. In addition, Thr1012 and Phe1013 on the first pore helix of repeat III (P1III) are in the vicinity of the bound drug, and S5III engages one residue, Val932, for AMIO coordination.
Direct interaction of AMIO and MNI-1 within rCav1.1
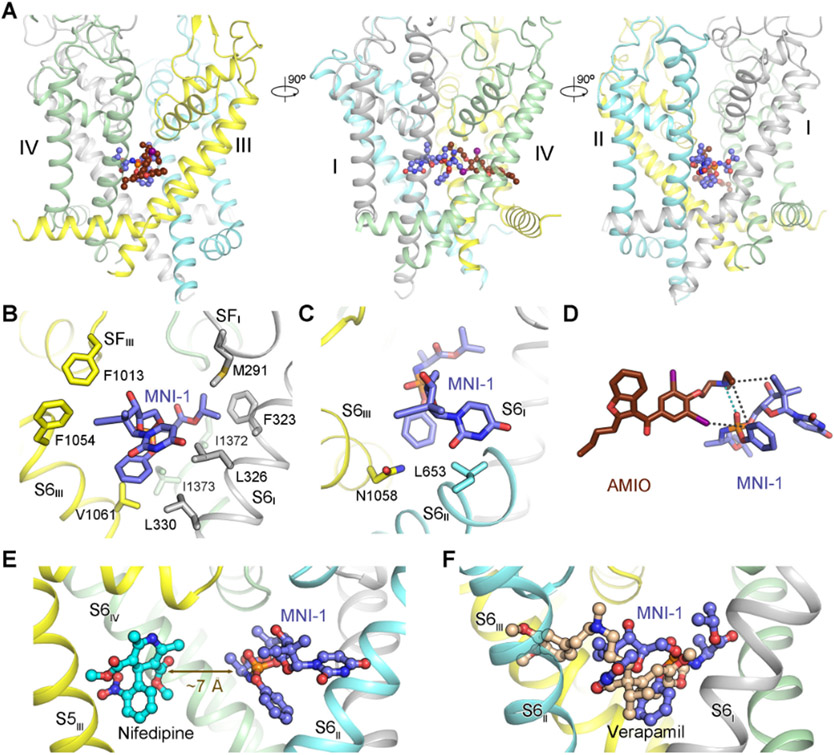
Accommodation of MNI-1 within the central cavity of the PD in the presence of AMIO is consistent with the mode of action (MOA) of a pore blocker (Figure 3A). MNI-1, which lies right below the selectivity filter (SF), stretches from S6II to S6IV, traversing the central cavity and contacting all four S6 helices. Scrutiny of MNI-1 coordination affords clues to its unfavored binding to the PD when AMIO is not present.
Figure 3 ∣. Direct drug-drug interaction (DDI) of AMIO and MNI-1 on rCav1.1.
(A) In the presence of AMIO, MNI-1 is accommodated in the central cavity of the PD. Three perpendicular side views of the PD are shown. AMIO and MNI-1 are shown as brown and purple ball-and-sticks, respectively. (B) The amphiphilic MNI-1 molecule is placed in a highly hydrophobic environment in rCav1.1. The MNI-1 coordinating residues, which are all hydrophobic, are shown as sticks. Repeat II and AMIO are omitted for visual clarity. (C) Less favored interactions between MNI-1 and rCav1.1 pore residues. Leu653 and Asn1058 are adjacent to polar and hydrophobic groups of MNI-1, respectively. (D) Direct interaction of AMIO and MNI-1. The polar interaction between the tertiary amine group in AMIO and the phosphate group in MNI-1 is indicated by cyan, dashed lines. The hydrophobic contacts between the two compounds are indicated by black, dashed lines. (E) Nifedipine does not interact with MNI-1 in rCav1.1. When the structure of nifedipine-bound rCav1.1 (PDB code: 6JP5) is overlaid with that of rCav1.1AM, the shortest distance between nifedipine and MNI-1 is ~ 7 Å, beyond the range for direction interactions. As the two structures are nearly identical, the protein scaffold of nifedipine-bound rCav1.1 is not shown. S6III and AMIO in rCav1.1AM are also omitted for clarity. (F) Overlapped binding poses of verapamil and MNI-1 explain their competition for binding to LTCCs. Verapamil-bound rCav1.1 structure (PDB code: 6JPA) is used for comparison with rCav1.1AM, but only the ligand is shown. See also Figure S3.
MNI-1 has a good aqueous solubility of ~ 3 mM. However, the water-soluble molecule is situated in a highly hydrophobic environment in Cav proteins (Figure 3B). The incongruent chemical properties of the ligand and the binding pocket may explain the low potency of MNI-1 on LTCC in the absence of AMIO (Figure 1B). While some of the hydrophobic residues, mainly on the S6 segments in repeats I, III, and IV, contribute to the binding of the hydrophobic groups of the compound (Figure 3B), Leu653 on S6II and Asn1058 on S6III are unfavorably positioned adjacent to the polar and hydrophobic groups of MNI-1, respectively (Figure 3C).
The structure shows that AMIO facilitates the accommodation of MNI-1 into the PD of LTCC by anchoring MNI-1 through direct physical association. In addition to the hydrophobic contacts, there is a polar interaction between the phosphate group of MNI-1 and the tertiary amine of AMIO (Figure 3D). This specific electrostatic coordination may play a critical role for stabilizing MNI-1 in the PD (Figure 3A).
We next examined the molecular basis for the lack of interactions between MNI-1 and nifedipine or verapamil (Figure 1D). For this, we compared the structures of rCav1.1 bound to nifedipine or verapamil (PDB codes: 6JP5 and 6JPA) with that of rCav1.1AM. Nifedipine, which is smaller than AMIO (Figure 2B), is distanced from MNI-1 by ~ 7 Å, beyond the range for direct interactions (Figure 3E). Structural comparison also provides an immediate explanation for the competition between verapamil and MNI-1, as their binding poses overlay in the central cavity (Figure 3F). Furthermore, verapamil, with its dominant binding pose revealed in the structure , does not form specific interactions with AMIO (Figure S3), consistent with the lack of synergetic effect between these two drugs (Lagrutta et al., 2016).
Interaction of AMIO and SOF in human Cav1.3
We then attempted to study the DDI on the physiologically more relevant human LTCCs. Our previously published functional data on HCV prodrugs interacting with AMIO demonstrated similar effects on hCav1.2 or hCav1.3 channels (Lagrutta et al., 2017; Millard et al., 2016), and it is likely that hCav1.3 plays a relevant role in the clinically reported DDI between SOF and AMIO, resulting in the severe bradycardia (Millard et al., 2016). Our recent studies on human Cav2.2 showed that selection of the β subunit was critical for improving recombinant expression of the channel complex in HEK293F cells (Gao et al., 2021). Indeed, in the presence of β3, which was used for the expression of the Cav2.2 channel complex, Cav1.3, together with α2δ-1, can be obtained to sufficient quantity for cryo-EM analysis. However, the expression level of Cav1.2 complex remained low. Based on these considerations and the high sequence similarity among the Cav1 subunits (Figure S4A), we used recombinant hCav1.3 complex for structural analysis.
SOF differs from MNI-1 only in the substituent on the tetrahydrofuran ring. The fluorine in SOF is replaced by the alkyne group in MNI-1 (Figure 1A). We have solved the following cryo-EM structures: hCav1.3A for hCav1.3 with 0.14 mM AMIO at 3.1 Å, hCav1.3S for hCav1.3 supplemented with 1.4 mM SOF at 3.3 Å, and hCav1.3AS for hCav1.3 with 0.14 mM AMIO and 1.4 mM SOF at 3.3 Å (Table 2; Figures S4, S5). Even in the presence of 1.4 mM SOF, there is no corresponding density in the central cavity of hCav1.3S (Figure S5A, left). Ligand densities are observed in the other two 3D EM reconstructions.
Table 2.
Statistics for data collection and structural refinement of hCav1.3 structures. See also Figure S4.
| hCav1.3A (EMD-27907) (PDB 8E59) |
hCav1.3S (EMD-27908) (PDB 8E5A) |
hCav1.3AS (EMD-27909) (PDB 8E5B) |
|
|---|---|---|---|
| Data collection and processing | |||
| Magnification | 105,000 | 105,000 | 105,000 |
| Voltage (kV) | 300 | 300 | 300 |
| Electron dose (e-/Å2) | 50 | 50 | 50 |
| Defocus range (μm) | −2.1~−1.9 | −2.1~−1.9 | −2.1~−1.9 |
| Pixel size (Å) | 1.114 | 1.114 | 1.114 |
| Symmetry | C1 | C1 | C1 |
| Initial particle images (no.) | 1,351,225 | 1,297,831 | 1,288,216 |
| Final particle images (no.) | 86,836 | 73,092 | 69,718 |
| Map resolution (Å) | 3.1 | 3.3 | 3.3 |
| FSC threshold | 0.143 | 0.143 | 0.143 |
| Refinement | |||
| Initial model used (PDB code) | 5GJV, 7MIY | hCav1.3A | hCav1.3A |
| Model resolution (Å) | 3.2 | 3.4 | 3.5 |
| FSC threshold | 0.5 | 0.5 | 0.5 |
| Map sharpening B factor (Å2) | −74 | −96 | −76 |
| Model composition | |||
| Non-hydrogen atoms | 20311 | 20259 | 20312 |
| Protein residues | 2502 | 2502 | 2502 |
| Ligands | 19 | 17 | 18 |
| B factors (Å2) | |||
| Protein | 56.14 | 65.35 | 50.29 |
| Ligand | 83.29 | 102.35 | 71.99 |
| R.m.s deviations | |||
| Bond lengths (Å) | 0.002 | 0.003 | 0.002 |
| Bond angles (°) | 0.485 | 0.661 | 0.515 |
| Validation | |||
| MolProbity score | 1.72 | 2.08 | 1.73 |
| Clashscore | 6.70 | 10.00 | 7.18 |
| Poor rotamers (%) | 0.54 | 1.86 | 0.36 |
| Ramachandran plot | |||
| Favored (%) | 94.86 | 94.82 | 95.15 |
| Allowed (%) | 5.10 | 4.98 | 4.81 |
| Disallowed (%) | 0.04 | 0.20 | 0.04 |
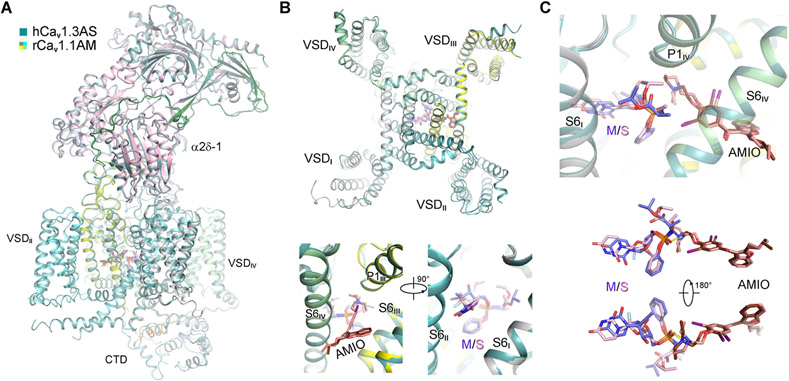
The three overall structures of hCav1.3 are nearly identical to that of rCav1.1. When hCav1.3AS is superimposed with rCav1.1AM, the RMSD for 1782 Cα atoms in α1 and α2δ-1 is 0.66 Å (Figure 4A). Substitution of the alkyne group with fluorine on tetrahydrofuran does not alter the binding mode with LTCCs. Coordination for these two compounds in rCav1.1AM and hCav1.3AS is nearly identical, including the interaction between the tertiary amine and the phosphate groups of the two drugs (Figures 4B,C).
Figure 4 ∣. AMIO and SOF interact within human Cav1.3 (hCav1.3).
(A) The overall structure of hCav1.3 bound to AMIO and SOF (hCav1.3AS) is identical to that of rCav1.1AM. The two structures can be superimposed with an RMSD of 0.66 Å for 1782 Cα atoms in the α1 and α2δ-1 subunits. rCav1.1AM is domain colored and hCav1.3AS is colored dark green. (B) SOF and MNI-1 share identical binding pose in the presence of AMIO. Top: A cytosolic view of superimposed α1 subunits from hCav1.3AS and rCav1.1AM. Bottom: Two opposite side views of superimposed α1 subunits from the two structures. (C) Direct interaction of SOF and AMIO within hCav1.3AS. SOF (pink) and MNI-1 (light purple), with similar chemical structures, interact with AMIO in the same way within the PD of LTCCs. See also Figures S4, S5.
The structures of rCav1.1 and hCav1.3 bound to AMIO and SOF/MNI-1 thus collectively demonstrate, to our best knowledge, an unprecedented mechanism of pharmacodynamic DDI through direct and specific physical interaction of the involved drugs, and reveal the molecular basis for the potentiation of AMIO action on LTCCs by SOF/MNI-1.
Discussion
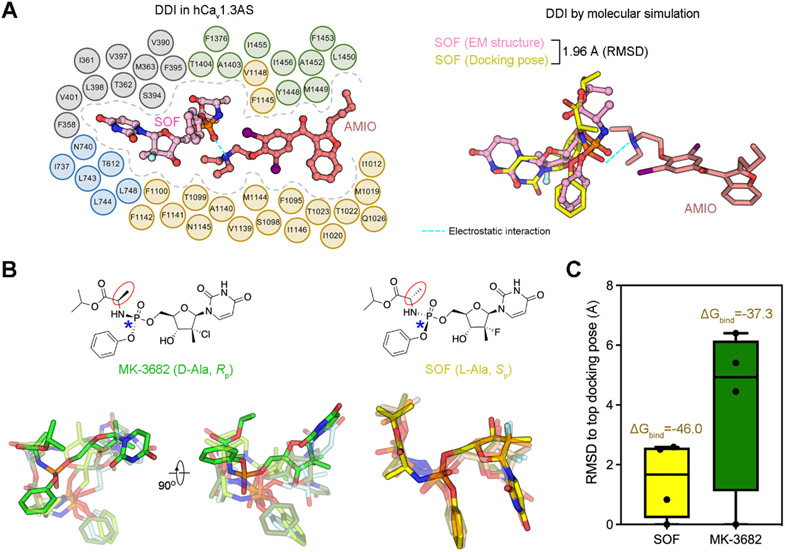
The stereochemistry of prodrug component of the HCV inhibitors determines their cardiac DDI with AMIO (Lagrutta et al., 2017). For example, no bradycardia is observed when MK-3682, which has opposite chirality at the amino acyl and phosphoryl groups compared to SOF, is combined with AMIO (Regan et al., 2016). To probe the molecular basis for the stereochemistry effect, we performed molecular docking simulation using hCav1.3AS as the template. Top ranking poses were selected for further comparison. Docking models of SOF align well with the experimental structure, with the RMSD of the top pose and the experimental one smaller than 2.0 Å (Figure 5A) (Hartshorn et al., 2007; Su et al., 2019). Moreover, the high ranking poses of SOF can be superimposed, with the RMSD values to the top one all less than 2.5 Å (Figures 5B,C).
Figure 5 ∣. Chiral specificity of SOF for interaction with AMIO.
(A) Molecular docking simulation of the interaction between SOF and AMIO. Left: Schematic illustration of the association of AMIO and SOF with hCav1.3AS. Residues within 4 Å from either drug are shown and domain colored. Right: Molecular docking simulation is consistent with the cryo-EM structure. Interactions were simulated using hCav1.3AS (without SOF) as the template. Shown in this figure are representative docking poses generated by Schrödinger software. The overall RMSD of the top ranking docking pose of SOF (yellow sticks) and the experimental one (pink ball-and-sticks) is < 2 Å, indicating high consistency (Hartshorn et al., 2007; Su et al., 2019). Importantly, the electrostatic interaction between the phosphate of SOF and the tertiary amine of AMIO, indicated by cyan dashes, is preserved in the simulation. (B) MK-3682, which has opposite chirality at the amino acyl and phosphoryl groups compared to SOF, displays variable binding poses in the molecular docking simulation. The stereochemical properties are labeled in parentheses below the chemical structures. L/D-Ala and S/RP indicate the chirality of the amino acyl group (red cycles) and the phosphoryl group (blue asterisks), respectively. Four docking models are shown for MK-3682 (left and middle) and SOF (right). For visuality, the top and other docking poses are shown as opaque and transparent sticks, respectively. Compared to those of SOF, which are relatively well aligned, the docking poses of MK-3682 vary substantially. (C) Less favored binding of MK-3682 to LTCC in the presence of AMIO. MK-3682 shows more diverse docking poses, manifested by the larger and broader RMSD values of the top docking poses. The average binding free energy ΔGbind (kcal/mol) of the top four docking poses for SOF (yellow) and MK-3682 (green), shown on the top of RMSD columns, was calculated with Prime MM-GBSA.
When MK-3682 was docked to AMIO-bound Cav1.3, the pyrimidinedione ring and other groups displayed rather flexible binding modes, resulting in a broader range of RMSD values of the four docking poses, all above 4 Å (Figure 5C). We used the molecular mechanics generalized Born surface area (MM/GBSA) method (Wang et al., 2019) to calculate the predicted free energies (ΔGbind) for the binding of MK-3682 or SOF with hCav1.3. The average ΔGbind values for the four docking poses of MK-3682 and SOF are −37.3 kcal/mol and −46.0 kcal/mol, respectively (Figure 5C). The larger RMSD and ΔGbind values consistently suggest a less favored binding of MK-3682 with LTCCs, thereby affording additional evidence for the chiral specificity of the interaction between SOF/MNI-1 and AMIO.
DDIs are a cause for adverse drug reactions, mostly through pharmacodynamics, pharmacokinetics, and pharmaceutical incompatibility (Niu et al., 2019; Palleria et al., 2013). Direct physical interactions enabled by one of the drug targets has been rarely described. Our systematic structural analysis shows a direct physical, pharmacodynamic interaction of an antiviral and an antiarrhythmic reagent on the scaffold of a protein, which underlies the clinically relevant, potentially life-threatening DDI of the two drugs.
Limitation of the study:
The present study is heavily structure-oriented. Mutational analysis was hindered by serious rundown of Cav1.2 and Cav1.3 mutants during electrophysiological recordings. Although the improved densities for MNI-1 and SOF in accordance with their increasing concentrations validate their binding poses (Figure 1E), these structures cannot reveal the dynamics or kinetics of the interactions between the drugs or with LTCCs. It is also unclear how SOF/MNI-1 gets access to the central cavity of LTCCs.
STAR★Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for reagents may be directed and will be fulfilled by Nieng Yan (nyan@princeton.edu), the lead contact.
Materials availability
Plasmids generated in this study will be made available on request, but we may require a payment and/or a completed Materials Transfer Agreement if there is potential for commercial application.
Data and code availability
Atomic coordinates of rCav1.1A, rCav1.1AM0.1, rCav1.1AM, hCav1.3A, hCav1.3S, and hCav1.3AS have been deposited in the Protein Data Bank (http://www.rcsb.org) and are publicly available as of the date of publication under the accession codes 8E56, 8E57, 8E58, 8E59, 8E5A, and 8E5B, respectively. The corresponding EM maps of rCav1.1A, rCav1.1AM0.1, rCav1.1AM, hCav1.3A, hCav1.3S, and hCav1.3AS have been deposited in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/pdbe/emdb/), under the accession codes EMD-27904, EMD-27905, EMD-27906, EMD-27907, EMD-27908, and EMD-27909, respectively. These accession numbers are also listed in the key resources table. All other data is available from the corresponding authors upon reasonable request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli BL21(DE3) | Novagen | Cat# 69387-1 |
| E. coli HST08 (Stellar™) | TaKaRa | Cat# 636766 |
| Biological samples | ||
| Rabbit muscle tissue | Pel-Freez | Cat# 41225 -2 |
| Chemicals, peptides, and recombinant proteins | ||
| Amiodarone | MedChemExpress | Cat# HY-14188 |
| MNI-1 | Merck & Co., Inc., Kenilworth, NJ, USA | N/A |
| Sofosbuvir | MedChemExpress | Cat# HY-15005 |
| Isradipine | Carbosynth Ltd | Cat# 12-35-21-00 |
| n-Dodecyl-β-D-Maltoside (DDM) | Anatrace | Cat# D310S |
| Glyco-diosgenin (GDN) | Anatrace | Cat# GDN101 |
| Cholesteryl hemisuccinate Tris salt (CHS) | Anatrace | Cat# CH210 |
| Aprotinin | VWR Life Science | Cat# 97062-754 |
| Pepstatin | VWR Life Science | Cat# 97064-248 |
| anti-Flag M2 affinity gel | MilliporeSigma | Cat# A2220 |
| Flag peptide | GenScript | Cat# RP10586-1 |
| Glutathione Sepharose® 4B | MilliporeSigma | Cat# GE17-0756-05 |
| Dulbecco’s modified Eagle Glutamax’s medium | Invitrogen | Cat# 10566-016 |
| Freestyle 293 medium | Thermo Fisher Scientific | Cat# 12338018 |
| Fetal bovine serum (influx assay) | Invitrogen | Cat# 10082-139 |
| Fetal Bovine Serum (protein expression) | Thermo Fisher Scientific | Cat# 10437028 |
| LB medium | Sigma | Cat# L3522 |
| Penicillin-streptomycin (100 U/mL) | Invitrogen | Cat# 15140-148 |
| Geneticin (G418) | Invitrogen | Cat# 10131-035 |
| Zeocin | Invitrogen | Cat# R250-01 |
| Hygromycin B | Invitrogen | Cat# 10687-010 |
| Polyethylenimine (PEI) | Polysciences | Cat# 24765-1 |
| Codex ACTOne® dye | Codex Biosolution Inc. | Cat#: CB-80599-301 |
| Superose 6™ Increase 10/300 GL | GE Healthcare | Cat# 29-0915-96 |
| Deposited data | ||
| Coordinates of rCav1.1A | This paper | PDB: 8E56 |
| Coordinates of rCav1.1AM0.1 | This paper | PDB: 8E57 |
| Coordinates of rCav1.1AM | This paper | PDB: 8E58 |
| Coordinates of hCav1.3A | This paper | PDB: 8E59 |
| Coordinates of hCav1.3S | This paper | PDB: 8E5A |
| Coordinates of hCav1.3AS | This paper | PDB: 8E5B |
| Cryo-EM map of rCav1.1A | This paper | EMDB: EMD-27904 |
| Cryo-EM map of rCav1.1AM0.1 | This paper | EMDB: EMD-27905 |
| Cryo-EM map of rCav1.1AM | This paper | EMDB: EMD-27906 |
| Cryo-EM map of hCav1.3A | This paper | EMDB: EMD-27907 |
| Cryo-EM map of hCav1.3S | This paper | EMDB: EMD-27908 |
| Cryo-EM map of hCav1.3AS | This paper | EMDB: EMD-27909 |
| Experimental models: Cell lines | ||
| HEK293F cells | Thermo Fisher Scientific | Cat# R79007 |
| HEK293 cells stably overexpressing human Cav1.2 channel complex and Kir2.3 | (Xia et al., 2004) | N/A |
| Recombinant DNA | ||
| Codon-optimized human Cav1.3-α1 cloned into a modified pCAG vector with tandem twin-strep and Flag tags at the amino (N) terminus | This paper | N/A |
| Codon-optimized human α2δ-1 subunit cloned into a modified pCAG vector | This paper | N/A |
| Codon-optimized human β3 subunit cloned into a modified pCAG vector with N-terminal Flag tag and C-terminal His10 tag | This paper | N/A |
| Software and algorithms | ||
| PyMOL | The PyMOL Molecular Graphics System, Schrodiner | https://pymol.org/2/ |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| MotionCor2 | (Zheng et al., 2017) | https://emcore.ucsf.edu/ucsf-motioncor2 |
| Coot | (Emsley et al., 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| UCSF Chimera | (Pettersen et al., 2004) | http://www.cgl.ucsf.edu/chimera |
| UCSF ChimeraX | (Goddard et al., 2018) | https://www.rbvi.ucsf.edu/chimerax/ |
| PHENIX | (Adams et al., 2010) | http://www.phenix-online.org/ |
| RELION 3.0 | (Zivanov et al., 2018) | https://www3.mrc-lmb.cam.ac.uk/relion/ |
| SerialEM | (Mastronarde, 2005) | http://bio3d.colorado.edu/SerialEM/ |
| Schrödinger Suite 2018-1 | Schrödinger, Inc. | https://www.schrodinger.com/ |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture
All E. coli cells were cultured in LB medium (Sigma) at 37 °C. The BL21(DE3) strain was used to express β1 subunit, and HST08 strain (Stellar™, TaKaRa) was used to amplify plasmids.
HEK293F suspension cells (Thermo Fisher Scientific, R79007) were cultured in Freestyle 293 medium (Thermo Fisher Scientific) at 37 °C supplied with 5% CO2 under 80% humidity.
HEK293 cells stably overexpressing human Cav1.2 channel complex and Kir2.3 inward rectifier K channel (Xia et al., 2004) were cultured in Dulbecco’s modified Eagle Glutamax’s medium (Invitrogen) with 10% fetal bovine serum (Invitrogen), 100 U/mL penicillin-streptomycin (Invitrogen), Geneticin (G418) (Invitrogen), Zeocin (Invitrogen), and Hygromycin B (Invitrogen) at 37 °C under a humidified atmosphere of 5% CO2.
METHOD DETAILS
Cav1.2 calcium influx assay
HEK293 cells overexpressing human Cav1.2 channel and Kir2.3 channel were plated on 96-well plates at a density of 60,000 cells/well (Xia et al., 2004). Cells were maintained in culture (37 °C, 5% CO2) overnight before use. On experiment day, cells were incubated with Codex ACTOne® dye (Codex Biosolution Inc.) formulated in PPB buffer containing 25 mM potassium (127 mM NaCl, 25 mM KCl, 0.005 mM CaCl2, 1.7 mM MgCl2, 10 mM HEPES 7.2) for 30 minute at room temperature, then test compounds were added for another 30-minute incubation at room temperature with a final volume of 100 μL. FDSS/μCell imaging platform simultaneously collected Ca2+ signals from 96-well plates, at a sampling rate of 16 Hz for 20 s as baseline, then a trigger buffer (119 mM NaCl, 25 mM KCl, 4 mM CaCl2, 1.7 mM MgCl2, 10 mM HEPES 7.2) was added using the dispenser of the FDSS/μCell instrument to generate Ca2+ transient for 40 s. The peak amplitude within the latter 40 s minus the average amplitude of the first 20 s is the final Ca2+ response of each well. Average response from wells treated with 0.1 μM Isradipine (reference Cav blocker, Carbosynth Ltd) was set as 100% inhibition (Rmax); and average response from wells treated with 0.1% DMSO was set as 0% inhibition (Rmin). Relative response of each well was calculated as follows:
Modelling of the Ca2+ influx data is based on the reported equation that inhibition of two independent blockers is Total%=A%+B%-A%*B%, where A% and B% are the percent inhibition of two blocker alone (Jarvis and Thompson, 2013). This equation can be presented as Total%-A% = B%(1-A%). In this study, we defined “% total inhibition - % inhibition by Cav blockers alone” as the experimental MNI-1 inhibition with the Cav blocker as baseline (Y axis), and the calculation by two drugs alone “% Inhibition by MNI-1 alone * (1 - % inhibition by Cav blockers alone)” as the theoretical MNI-1 inhibition (X axis). If there is no interaction between tested compounds (nifedipine and MNI-1), a close to 1:1 linear fitting is predicted. Also shown in Figure 1D are deviations from this 1:1 linearity, expected if there is competition between two drugs at high concentrations as the case for co-applying verapamil and MNI-1. AMIO and MIN-1 show a synergistic interaction as the experimental values are much higher than the theoretical ones.
Transient expression of human Cav1.3 in HEK293F cells
Codon-optimized cDNAs of CACNA1D for Cav1.3-α1 (2,161 residues, Uniprot Q01668-1), CACNA2D1 for α2δ-1 (1,103 residues, Uniprot P54289-1), and CACNB3 for β3 (484 residues, Uniprot P54284-1) were synthesized (Wuxi Qinglan Biotech. Inc). Full-length Cav1.3-α1, α2δ-1, and β3 were subcloned into the pCAG vector, with tandem twin-strep and Flag tags at the amino (N) terminus for α1 subunits, and N-terminal Flag tag and C-terminal His tag for β3 subunit. When HEK293F cell density reached 1.5-2.0 * 106 cells/mL, a mixture of the expressing plasmids, including 0.75 mg α1, 0.6 mg α2δ-1 and 0.5 mg β3, together with 3 mg polyethylenimine (Polysciences) was added to the cell culture for transient expression of the human Cav1.3 complex.
Protein preparation
The endogenous rCav1.1 complex was purified following identical protocol as reported (Gao and Yan, 2021). For hCav1.3, seven liters of suspension HEK293F cells were harvested approximately 72 hours after transfection by centrifugation at 3,600 g for 10 min and resuspended in the lysis buffer containing 25 mM HEPES (pH 7.4), 150 mM NaCl, and the protease inhibitor cocktail containing 2.6 μg/mL aprotinin (VWR Life Science) and 1.4 μg/mL pepstatin (VWR Life Science). After sonication on ice, the suspension was supplemented with glyco-diosgenin (GDN, Anatrace) to a final concentration of 1% (w/v), n-dodecyl-β-D-maltopyranoside (DDM, Anatrace) to 0.2% (w/v), and cholesteryl hemisuccinate Tris salt (CHS, Anatrace) to 0.04% (w/v). After incubation at 4 °C overnight, the mixture was centrifuged at 35,000g for 30 min, and the supernatant was applied to anti-Flag M2 affinity resin (Sigma). The resin was rinsed with wash buffer (buffer A) containing 25 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM CaCl2, and 0.01% GDN. Eluted with buffer A plus 0.2 mg/mL Flag peptide (synthesized by GenScript), the eluent was concentrated using a 100 kDa cut-off Amicon (Millipore) and further purified through size-exclusion chromatography (SEC, Superose 6 10/300 GL, GE Healthcare) pre-equilibrated in buffer A. The peak fractions were pooled and concentrated.
To prepare Cav-drug complexes, concentrated proteins (rCav1.1 at ~5 mg/mL, and hCav1.3 at ~15 mg/mL) were incubated with drugs at 4 °C for 30 min before making cryo-grids.
rCav1.1A: Amiodarone (MedChemExpress) was added to rCav1.1 proteins at a final concentration of 100 μM.
rCav1.1AM0.1: Amiodarone and MNI-1 (provided by Merck & Co., Inc., Rahway, NJ, USA) were added together to rCav1.1 proteins both at 100 μM.
rCav1.1AM: rCav1.1 proteins in the presence of 100 μM amiodarone and 1 mM MNI-1.
hCav1.3A: hCav1.3 proteins in the presence of 140 μM amiodarone.
hCav1.3S: hCav1.3 proteins in the presence of 1.4 mM sofosbuvir (MedChemExpress).
hCav1.3AS: hCav1.3 proteins in the presence of 140 μM amiodarone and 1.4 mM sofosbuvir.
Cryo-sample preparation and data collection
Aliquots of 3.5 μL concentrated samples were loaded onto glow-discharged holey carbon grids (Quantifoil Au 300 mesh, R1.2/1.3 and Quantifoil Cu 300 mesh, R1.2/1.3), which were blotted for 6 s and plunge-frozen in liquid ethane cooled by liquid nitrogen using a Vitrobot MarK IV (Thermo Fisher) at 8 °C with 100% humidity. Grids were transferred to a Titan Krios electron microscope (Thermo Fisher) operating at 300 kV and equipped with a Gatan Gif Quantum energy filter (slit width 20 eV) and spherical aberration (Cs) image corrector. Micrographs were recorded using a K2 Summit counting camera (Gatan Company) in super-resolution mode with a nominal magnification of 105,000x, resulting in a calibrated pixel size of 0.557 Å. Each stack of 32 frames was exposed for 5.6 s, with an exposure time of 0.175 s per frame. The total dose for each stack was ~ 50 e−/Å2. SerialEM was used for fully automated data collection (Mastronarde, 2005). All 32 frames in each stack were aligned, summed, and dose weighted using MotionCorr2 (Zheng et al., 2017) and 2-fold binned to a pixel size of 1.114 Å/pixel. The defocus values were set from −1.9 to −2.1 μm and estimated by Gctf (Zhang, 2016).
Image processing
A total of 1,677 (rCav1.1A), 1,794 (rCav1.1AM0.1), 2,013 (rCav1.1AM), 2,302 (hCav1.3A), 1,770 (hCav1.3S) and 2,032 (hCav1.3AS) cryo-EM micrographs were collected, and 831,227 (rCav1.1A), 688,386 (rCav1.1AM0.1), 1,014,749 (rCav1.1AM), 1,351,225 (hCav1.3A), 1,297,831 (hCav1.3S) and 1,288,216 (hCav1.3AS) particles were auto-picked by RELION-3.0 (Zivanov et al., 2018). Particle picking was performed using the selected 2D class of rabbit Cav1.1 as reference (Gao and Yan, 2021). All subsequent 2D and 3D classifications and refinements were performed using RELION-3.0.
Reference-free 2D classification were performed to remove ice spots, contaminants, and aggregates, yielding 777,536 (rCav1.1A), 581,605 (rCav1.1AM0.1), 911,176 (rCav1.1AM), 1,276,190 (hCav1.3A), 1,151,864 (hCav1.3S) and 1,121,237 (hCav1.3AS) particles. The particles were processed with a global search with K=1 to determine the initial orientation alignment parameters using bin2 particles. A published EM map of rabbit Cav1.1 (EMD-22426) low-pass filtered to 20 Å was used as an initial reference (Gao and Yan, 2021). The output of the 35–40 iterations was further applied to local angular search 3D classification with four classes. A total of 400,307 (rCav1.1A), 260,673 (rCav1.1AM0.1), 381,524 (rCav1.1AM), 516,088 (hCav1.3A), 407,272 (hCav1.3S) and 470,281 (hCav1.3AS) particles were selected by combining the good classes of the local angular search 3D classification. The particles were then re-extracted using a box size of 280 and pixel size of 1.114 Å. These particles yielded reconstructions at 3.0 Å (rCav1.1A), 3.1 Å (rCav1.1AM0.1), 3.6 Å (rCav1.1AM), 3.5 Å (hCav1.3A), 3.6 Å (hCav1.3S) and 4.0 Å (hCav1.3AS) after 3D auto-refinement with an adapted mask. Skip align 3D classification using bin1 particles and Bayesian polishing resulted in final reconstructions at 2.8 Å (rCav1.1A), 2.8 Å (rCav1.1AM0.1), 3.0 Å (rCav1.1AM), 3.1 Å (hCav1.3A), 3.3 Å (hCav1.3S) and 3.3 Å (hCav1.3AS) from 400,307 (rCav1.1A), 190,835 (rCav1.1AM0.1), 83,355 (rCav1.1AM), 86,836 (hCav1.3A), 73,092 (hCav1.3S) and 69,718 (hCav1.3AS) particles.
All 2D classification, 3D classification, and 3D auto-refinement were performed with RELION-3.0. Resolutions were estimated with the gold-standard Fourier shell correlation 0.143 criterion with high-resolution noise substitution (Chen et al., 2013; Rosenthal and Henderson, 2003).
Model building and refinement
The previously reported rCav1.1 structure (PDB: 5GJV) was used as starting model that was docked into the maps for rCav1.1-A/AM0.1/AM in Chimera (Pettersen et al., 2004), respectively. Model building of Cav1.3 was based on the reported structures of both Cav1.1 and Cav2.2. The starting model of Cav1.3 α1 subunit was built in SWISS-MODEL (Waterhouse et al., 2018) based on the structure of rCav1.1 (PDB: 5GJV), and those of α2δ-1 and β3 were based on the structure of Cav2.2 (PDB: 7MIY). The starting model of Cav1.3 was then manually docked into the 3.1 Å hCav1.3A 3D map in Chimera and manually adjusted in COOT (Emsley et al., 2010). For model building of the hCav1.3S/AS, the coordinates for α1, α2δ-1 and β3 from Cav1.3A were docked into the 3.3 Å Cav1.3S/AS map separately and manually adjusted in COOT.
Additional lipids and ligands were manually built to fit into the corresponding densities in COOT. Models were manually adjusted in COOT, followed by refinement against the corresponding maps by the phenix.real_space_refine program in PHENIX (Adams et al., 2010) with secondary structure and geometry restraints. Statistics of 3D reconstructions and model refinement can be found in Table 1 and 2. All structure figures were prepared in PyMol (DeLano, 2002), UCSF Chimera (Pettersen et al., 2004) and ChimeraX (Goddard et al., 2018).
Molecular docking simulation
After removing SOF from hCav1.3AS structure, SOF and MK-3682 were separately docked using Schrödinger Suite 2018-1 (Schrödinger, Inc.) in the presence of AMIO. The initial 3D conformations of ligands were generated from their 2D structures using the LigPrep program (Sastry et al., 2013) with the OPLS3 force field (Harder et al., 2016). The protein structure was processed by Protein Preparation Wizard using the coordinates of hCav1.3AS as input. Molecular docking simulations were performed using extra-precision docking (Glide XP) within the Glide program.
QUANTIFICATION AND STATISTICAL ANALYSIS
The local resolution map was calculated using RELION-3.0 (Zivanov et al., 2018). Resolutions were estimated with the gold-standard Fourier shell correlation 0.143 criterion (Rosenthal and Henderson, 2003) with high-resolution noise substitution. Statistical analysis of Cav1.2 calcium influx assays was performed using Prism 9 (GraphPad Software, San Diego, CA, USA). Data represent mean ± SEM obtained from three independent experiments performed in quadruplicate.
Supplementary Material
Figure S3 ∣ Verapamil does not have synergistic interaction with AMIO, Related to Figure 3. When the structures of verapamil-bound rCav1.1 (PDB:6JPA) and rCav1.1AM are superimposed, the protein backbones remain nearly identical. For visual clarity, only the protein scaffold of rCav1.1AM is shown in two identical extracellular views in the top row. MNI-1 is omitted in the right panels. Compared to the multiple contacts between MNI-1 and AMIO (left), there lack specific interactions between verapamil and AMIO (right). Moreover, the distance between the dimethoxybenzene group of verapamil in its present binding pose and the iodine of AMIO is less than 2 Å, representing a potential steric clash. The polar interaction, hydrophobic contacts and steric clashes are indicated by cyan, black and red dashes, respectively.
Figure S2 ∣ Cryo-EM analysis of rCav1.1 supplemented with AMIO and MNI-1, Related to Figures 1, 2 and Table 1. (A) Representative electron micrograph (left) and 30 classes of 2D class averages (right) for the rCav1.1A complex. Green circles indicate particles in distinct orientations. The box size for the 2D averages is 310 Å. (B) Gold-standard Fourier Shell Correlation (FSC) curves for the 3D reconstructions. A: rCav1.1 with 0.1 mM AMIO (black line); AM0.1: rCav1.1 with 0.1 mM AMIO and 0.1 mM MNI-1 (orange line); AM: rCav1.1 with 0.1 mM AMIO and 1 mM MNI-1 (red line). The graph was prepared in Graphpad Prism 9. (C) Workflow for cryo-EM data processing. Details can be found in STAR★Methods. (D) Heat maps for local resolutions of rCav1.1A, rCav1.1AM0.1 and rCav1.1AM. Orange squares indicate the AMIO binding site. The resolution maps were calculated in RELION-3.0 and prepared in Chimera. (E) Cryo-EM map for rCav1.1A. Two opposite side views of the EM map were prepared in ChimeraX. The extracellular α2δ-1 subunit is colored light pink for α2 and green for δ. The transmembrane α1 subunit is domain colored, and the γ1 subunit is colored bright orange. Sugars and AMIO are colored grey and brown, respectively. (F) Overall structure of rCav1.1A. The structure is with the same color scheme as in panel E. Two Ca2+ ions, one in the von Willebrand factor type A (VWA) domain of α2 and the other in the selectivity filter of α1, are shown as green spheres.
Figure S1 ∣ Amiodarone, but not nifedipine or verapamil, has synergistic effect with MNI-1 on Cav1.2, Related to Figure 1. (A) Total Cav1.2 inhibition by amiodarone (AMIO, left), nifedipine (middle), or verapamil (right) alone and in combination with MNI-1. Values were normalized to the average response by 0.1 μM Isradipine (as 100% inhibition). aIC50 (apparent IC50) curves were generated by calculating total inhibitory effects. Points represent mean ± SEM obtained from three independent experiments performed in quadruplicate. (B) IC50 curves using MNI-1 as baseline. The IC50 values of nifedipine and verapamil remained similar with or without MNI-1. (C) Modeling of pharmacodynamic effects of LTCC blockers on MNI-1. Each panel was plotted from three independent experiments for the LTCC blockers co-applied with two concentrations of MNI-1 (top and bottom). The numbers near each data point indicate the concentrations of AMIO (μM), nifedipine (nM) and verapamil (μM). Details can be found in STAR★Methods.
Figure S4 ∣ Cryo-EM analysis of hCav1.3 treated with AMIO, SOF, or both, Related to Figure 4 and Table 2. (A) AMIO and MNI-1-binding residues are highly conserved in LTCCs. Shown here is the sequence alignment of the segments in LTCCs involved in AMIO and MNI-1 binding. MNI-1 and AMIO binding residues are shaded green and cyan, respectively. The residues interacting with both ligands are shaded magenta. The secondary structural elements are shown above the sequences, and the residue numbers refer to those in rCav1.1. (B) Representative electron micrograph (left) and 25 classes of 2D class averages (right) for the hCav1.3A complex. Green circles indicate particles in distinct orientations. The box size for the 2D averages is 310 Å. Scale bar, 50 nm. (C) The gold-standard FSC curves for the 3D reconstructions. A: hCav1.3 with 0.14 mM AMIO (black line); S: hCav1.3 with 1.4 mM SOF (brown line); AS: hCav1.3 with 0.14 mM AMIO and 1.4 mM SOF (purple line). (D) Cryo-EM data processing workflow for hCav1.3 structures. Details can be found in STAR★Methods. (E) Heat maps for local resolutions of the hCav1.3A, hCav1.3S and hCav1.3AS complex. The local resolution maps were calculated in RELION-3.0 and prepared in Chimera.
Figure S5 ∣ EM densities for the bound drugs in hCav1.3, Related to Figure 4. (A) SOF density is resolved in hCav1.3 only in the presence of AMIO. The density for SOF, colored pink in the hCav1.3AS map (right), is missing in the map of hCav1.3S (left). The densities, shown as semi-transparent surface, were contoured at level of 0.02 in ChimeraX. (B) EM densities for AMIO and SOF in hCav1.3AS. The densities for the two drugs, shown as blue mesh, are contoured at 4 σ in PyMol.
Acknowledgements
We thank the cryo-EM facility at Princeton Imaging and Analysis Center operated by the Princeton Institute of Materials at Princeton University, which is supported in part by the Princeton Center for Complex Materials, a National Science Foundation Materials Research Science and Engineering Center (Grant No. DMR-2011750). The work was supported by grant from NIH (5R01GM130762 to N.Y. and R01DK125404 to Y.Y.).
Footnotes
Declaration of interests
Jixin Wang and Armando Lagrutta are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and potentially own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA.
References:
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. 10.1107/s0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P, et al. (2014). Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370, 1889–1898. 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- Appleby TC, Perry JK, Murakami E, Barauskas O, Feng J, Cho A, Fox D 3rd, Wetmore DR, McGrath ME, Ray AS, et al. (2015). Viral replication. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 347, 771–775. 10.1126/science.1259210. [DOI] [PubMed] [Google Scholar]
- Back DJ, and Burger DM (2015). Interaction between amiodarone and sofosbuvir-based treatment for hepatitis C virus infection: potential mechanisms and lessons to be learned. Gastroenterology 149, 1315–1317. 10.1053/j.gastro.2015.09.031. [DOI] [PubMed] [Google Scholar]
- Carbone E, and Lux HD (1984). A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature 310, 501–502. 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Chen S, McMullan G, Faruqi AR, Murshudov GN, Short JM, Scheres SH, and Henderson R (2013). High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35. 10.1016/j.ultramic.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL (2002). The PyMOL Molecular Graphics System. http://www.pymol.org. [Google Scholar]
- Dolphin AC (2006). A short history of voltage-gated calcium channels. Br J Pharmacol 147 Suppl 1, S56–62. 10.1038/sj.bjp.0706442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010). Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. 10.1107/s0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, et al. (2000). Nomenclature of voltage-gated calcium channels. Neuron 25, 533–535. 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Gao S, and Yan N (2021). Structural Basis of the Modulation of the Voltage-Gated Calcium Ion Channel Ca(v)1.1 by Dihydropyridine Compounds**. Angewandte Chemie-International Edition 60, 3131–3137. 10.1002/anie.202011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Yao X, and Yan N (2021). Structure of human Cav2.2 channel blocked by the painkiller ziconotide. Nature 596, 143–147. 10.1038/s41586-021-03699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, and Ferrin TE (2018). UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25. 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL, et al. (2016). OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J Chem Theory Comput 12, 281–296. 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- Hartshorn MJ, Verdonk ML, Chessari G, Brewerton SC, Mooij WT, Mortenson PN, and Murray CW (2007). Diverse, high-quality test set for the validation of protein-ligand docking performance. J Med Chem 50, 726–741. 10.1021/jm061277y. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, et al. (2013). Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 368, 1867–1877. 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- Jarvis GE, and Thompson AJ (2013). A golden approach to ion channel inhibition. Trends Pharmacol Sci 34, 481–488. 10.1016/j.tips.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama I, Kamiya K, and Toyama J (1997). Cellular electropharmacology of amiodarone. Cardiovasc Res 35, 13–29. 10.1016/s0008-6363(97)00114-4. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M, and Fleckenstein A (1977). Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn-Schmiedeberg's archives of pharmacology 298, 267–272. [DOI] [PubMed] [Google Scholar]
- Lagrutta A, Regan CP, Zeng H, Imredy JP, Koeplinger K, Morissette P, Liu L, Wollenberg G, Brynczka C, Lebrón J, et al. (2017). Cardiac drug-drug interaction between HCV-NS5B pronucleotide inhibitors and amiodarone is determined by their specific diastereochemistry. Sci Rep 7, 44820. 10.1038/srep44820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrutta A, Zeng H, Imredy J, Balasubramanian B, Dech S, Lis E, Wang J, Zhai J, DeGeorge J, and Sannajust F (2016). Interaction between amiodarone and hepatitis-C virus nucleotide inhibitors in human induced pluripotent stem cell-derived cardiomyocytes and HEK-293 Cav1.2 over-expressing cells. Toxicol Appl Pharmacol 308, 66–76. 10.1016/j.taap.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. (2013). Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368, 1878–1887. 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- Lubic SP, Nguyen KP, Dave B, and Giacomini JC (1994). Antiarrhythmic agent amiodarone possesses calcium channel blocker properties. J Cardiovasc Pharmacol 24, 707–714. 10.1097/00005344-199424050-00004. [DOI] [PubMed] [Google Scholar]
- Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, Ouzan D, Papatheodoridis G, Ramji A, Borgia SM, et al. (2020). Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int 40, 1841–1852. 10.1111/liv.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN (2005). Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51. 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Millard DC, Strock CJ, Carlson CB, Aoyama N, Juhasz K, Goetze TA, Stoelzle-Feix S, Becker N, Fertig N, January CT, et al. (2016). Identification of Drug-Drug Interactions In Vitro: A Case Study Evaluating the Effects of Sofosbuvir and Amiodarone on hiPSC-Derived Cardiomyocytes. Toxicol Sci 154, 174–182. 10.1093/toxsci/kfw153. [DOI] [PubMed] [Google Scholar]
- Niu J, Straubinger RM, and Mager DE (2019). Pharmacodynamic Drug-Drug Interactions. Clin Pharmacol Ther 105, 1395–1406. 10.1002/cpt.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokin P, Clinet M, Swillens S, Delisee C, Meysmans L, and Chatelain P (1986). Allosteric modulation of [3H]nitrendipine binding to cardiac and cerebral cortex membranes by amiodarone. J Cardiovasc Pharmacol 8, 1051–1057. 10.1097/00005344-198609000-00025. [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, and Tsien RW (1985). Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316, 440–443. 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Palleria C, Di Paolo A, Giofrè C, Caglioti C, Leuzzi G, Siniscalchi A, De Sarro G, and Gallelli L (2013). Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci 18, 601–610. [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004). UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Regan CP, Morissette P, Regan HK, Travis JJ, Gerenser P, Wen J, Fitzgerald K, Gruver S, DeGeorge JJ, and Sannajust FJ (2016). Assessment of the clinical cardiac drug-drug interaction associated with the combination of hepatitis C virus nucleotide inhibitors and amiodarone in guinea pigs and rhesus monkeys. Hepatology 64, 1430–1441. 10.1002/hep.28752. [DOI] [PubMed] [Google Scholar]
- Rosenthal PB, and Henderson R (2003). Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J Mol Biol 333, 721–745. DOI 10.1016/j.jmb.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, Kus T, Lambert J, Dubuc M, Gagne P, et al. (2000). Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 342, 913–920. 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- Sastry GM, Adzhigirey M, Day T, Annabhimoju R, and Sherman W (2013). Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27, 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Stedman C (2014). Sofosbuvir, a NS5B polymerase inhibitor in the treatment of hepatitis C: a review of its clinical potential. Therap Adv Gastroenterol 7, 131–140. 10.1177/1756283X13515825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M, Yang Q, Du Y, Feng G, Liu Z, Li Y, and Wang R (2019). Comparative Assessment of Scoring Functions: The CASF-2016 Update. J Chem Inf Model 59, 895–913. 10.1021/acs.jcim.8b00545. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, and Numa S (1987). Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328, 313–318. 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Wang E, Sun H, Wang J, Wang Z, Liu H, Zhang JZH, and Hou T (2019). End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem Rev 119, 9478–9508. 10.1021/acs.chemrev.9b00055. [DOI] [PubMed] [Google Scholar]
- Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, et al. (2018). SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46, W296–w303. 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yan Z, Li Z, Qian X, Lu S, Dong M, Zhou Q, and Yan N (2016). Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 A resolution. Nature 537, 191–196. 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M, and Yan N (2015). Structure of the voltage-gated calcium channel Cav1.1 complex. Science 350, aad2395. 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- Xia M, Imredy JP, Koblan KS, Bennett P, and Connolly TM (2004). State-dependent inhibition of L-type calcium channels: cell-based assay in high-throughput format. Anal Biochem 327, 74–81. 10.1016/j.ab.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Yao X, Gao S, and Yan N (2022). Structural basis for pore blockade of human voltage-gated calcium channel Ca(v)1.3 by motion sickness drug cinnarizine. Cell Res. 10.1038/s41422-022-00663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Striessnig J, Koschak A, and Dolphin AC (2015). The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol Rev 67, 821–870. 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K (2016). Gctf: Real-time CTF determination and correction. J Struct Biol 193, 1–12. 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Huang G, Wu J, Wu Q, Gao S, Yan Z, Lei J, and Yan N (2019). Molecular Basis for Ligand Modulation of a Mammalian Voltage-Gated Ca(2+) Channel. Cell 177, 1495–1506 e1412. 10.1016/j.cell.2019.04.043. [DOI] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332. 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJ, Lindahl E, and Scheres SH (2018). New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7. 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3 ∣ Verapamil does not have synergistic interaction with AMIO, Related to Figure 3. When the structures of verapamil-bound rCav1.1 (PDB:6JPA) and rCav1.1AM are superimposed, the protein backbones remain nearly identical. For visual clarity, only the protein scaffold of rCav1.1AM is shown in two identical extracellular views in the top row. MNI-1 is omitted in the right panels. Compared to the multiple contacts between MNI-1 and AMIO (left), there lack specific interactions between verapamil and AMIO (right). Moreover, the distance between the dimethoxybenzene group of verapamil in its present binding pose and the iodine of AMIO is less than 2 Å, representing a potential steric clash. The polar interaction, hydrophobic contacts and steric clashes are indicated by cyan, black and red dashes, respectively.
Figure S2 ∣ Cryo-EM analysis of rCav1.1 supplemented with AMIO and MNI-1, Related to Figures 1, 2 and Table 1. (A) Representative electron micrograph (left) and 30 classes of 2D class averages (right) for the rCav1.1A complex. Green circles indicate particles in distinct orientations. The box size for the 2D averages is 310 Å. (B) Gold-standard Fourier Shell Correlation (FSC) curves for the 3D reconstructions. A: rCav1.1 with 0.1 mM AMIO (black line); AM0.1: rCav1.1 with 0.1 mM AMIO and 0.1 mM MNI-1 (orange line); AM: rCav1.1 with 0.1 mM AMIO and 1 mM MNI-1 (red line). The graph was prepared in Graphpad Prism 9. (C) Workflow for cryo-EM data processing. Details can be found in STAR★Methods. (D) Heat maps for local resolutions of rCav1.1A, rCav1.1AM0.1 and rCav1.1AM. Orange squares indicate the AMIO binding site. The resolution maps were calculated in RELION-3.0 and prepared in Chimera. (E) Cryo-EM map for rCav1.1A. Two opposite side views of the EM map were prepared in ChimeraX. The extracellular α2δ-1 subunit is colored light pink for α2 and green for δ. The transmembrane α1 subunit is domain colored, and the γ1 subunit is colored bright orange. Sugars and AMIO are colored grey and brown, respectively. (F) Overall structure of rCav1.1A. The structure is with the same color scheme as in panel E. Two Ca2+ ions, one in the von Willebrand factor type A (VWA) domain of α2 and the other in the selectivity filter of α1, are shown as green spheres.
Figure S1 ∣ Amiodarone, but not nifedipine or verapamil, has synergistic effect with MNI-1 on Cav1.2, Related to Figure 1. (A) Total Cav1.2 inhibition by amiodarone (AMIO, left), nifedipine (middle), or verapamil (right) alone and in combination with MNI-1. Values were normalized to the average response by 0.1 μM Isradipine (as 100% inhibition). aIC50 (apparent IC50) curves were generated by calculating total inhibitory effects. Points represent mean ± SEM obtained from three independent experiments performed in quadruplicate. (B) IC50 curves using MNI-1 as baseline. The IC50 values of nifedipine and verapamil remained similar with or without MNI-1. (C) Modeling of pharmacodynamic effects of LTCC blockers on MNI-1. Each panel was plotted from three independent experiments for the LTCC blockers co-applied with two concentrations of MNI-1 (top and bottom). The numbers near each data point indicate the concentrations of AMIO (μM), nifedipine (nM) and verapamil (μM). Details can be found in STAR★Methods.
Figure S4 ∣ Cryo-EM analysis of hCav1.3 treated with AMIO, SOF, or both, Related to Figure 4 and Table 2. (A) AMIO and MNI-1-binding residues are highly conserved in LTCCs. Shown here is the sequence alignment of the segments in LTCCs involved in AMIO and MNI-1 binding. MNI-1 and AMIO binding residues are shaded green and cyan, respectively. The residues interacting with both ligands are shaded magenta. The secondary structural elements are shown above the sequences, and the residue numbers refer to those in rCav1.1. (B) Representative electron micrograph (left) and 25 classes of 2D class averages (right) for the hCav1.3A complex. Green circles indicate particles in distinct orientations. The box size for the 2D averages is 310 Å. Scale bar, 50 nm. (C) The gold-standard FSC curves for the 3D reconstructions. A: hCav1.3 with 0.14 mM AMIO (black line); S: hCav1.3 with 1.4 mM SOF (brown line); AS: hCav1.3 with 0.14 mM AMIO and 1.4 mM SOF (purple line). (D) Cryo-EM data processing workflow for hCav1.3 structures. Details can be found in STAR★Methods. (E) Heat maps for local resolutions of the hCav1.3A, hCav1.3S and hCav1.3AS complex. The local resolution maps were calculated in RELION-3.0 and prepared in Chimera.
Figure S5 ∣ EM densities for the bound drugs in hCav1.3, Related to Figure 4. (A) SOF density is resolved in hCav1.3 only in the presence of AMIO. The density for SOF, colored pink in the hCav1.3AS map (right), is missing in the map of hCav1.3S (left). The densities, shown as semi-transparent surface, were contoured at level of 0.02 in ChimeraX. (B) EM densities for AMIO and SOF in hCav1.3AS. The densities for the two drugs, shown as blue mesh, are contoured at 4 σ in PyMol.
Data Availability Statement
Atomic coordinates of rCav1.1A, rCav1.1AM0.1, rCav1.1AM, hCav1.3A, hCav1.3S, and hCav1.3AS have been deposited in the Protein Data Bank (http://www.rcsb.org) and are publicly available as of the date of publication under the accession codes 8E56, 8E57, 8E58, 8E59, 8E5A, and 8E5B, respectively. The corresponding EM maps of rCav1.1A, rCav1.1AM0.1, rCav1.1AM, hCav1.3A, hCav1.3S, and hCav1.3AS have been deposited in the Electron Microscopy Data Bank (https://www.ebi.ac.uk/pdbe/emdb/), under the accession codes EMD-27904, EMD-27905, EMD-27906, EMD-27907, EMD-27908, and EMD-27909, respectively. These accession numbers are also listed in the key resources table. All other data is available from the corresponding authors upon reasonable request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli BL21(DE3) | Novagen | Cat# 69387-1 |
| E. coli HST08 (Stellar™) | TaKaRa | Cat# 636766 |
| Biological samples | ||
| Rabbit muscle tissue | Pel-Freez | Cat# 41225 -2 |
| Chemicals, peptides, and recombinant proteins | ||
| Amiodarone | MedChemExpress | Cat# HY-14188 |
| MNI-1 | Merck & Co., Inc., Kenilworth, NJ, USA | N/A |
| Sofosbuvir | MedChemExpress | Cat# HY-15005 |
| Isradipine | Carbosynth Ltd | Cat# 12-35-21-00 |
| n-Dodecyl-β-D-Maltoside (DDM) | Anatrace | Cat# D310S |
| Glyco-diosgenin (GDN) | Anatrace | Cat# GDN101 |
| Cholesteryl hemisuccinate Tris salt (CHS) | Anatrace | Cat# CH210 |
| Aprotinin | VWR Life Science | Cat# 97062-754 |
| Pepstatin | VWR Life Science | Cat# 97064-248 |
| anti-Flag M2 affinity gel | MilliporeSigma | Cat# A2220 |
| Flag peptide | GenScript | Cat# RP10586-1 |
| Glutathione Sepharose® 4B | MilliporeSigma | Cat# GE17-0756-05 |
| Dulbecco’s modified Eagle Glutamax’s medium | Invitrogen | Cat# 10566-016 |
| Freestyle 293 medium | Thermo Fisher Scientific | Cat# 12338018 |
| Fetal bovine serum (influx assay) | Invitrogen | Cat# 10082-139 |
| Fetal Bovine Serum (protein expression) | Thermo Fisher Scientific | Cat# 10437028 |
| LB medium | Sigma | Cat# L3522 |
| Penicillin-streptomycin (100 U/mL) | Invitrogen | Cat# 15140-148 |
| Geneticin (G418) | Invitrogen | Cat# 10131-035 |
| Zeocin | Invitrogen | Cat# R250-01 |
| Hygromycin B | Invitrogen | Cat# 10687-010 |
| Polyethylenimine (PEI) | Polysciences | Cat# 24765-1 |
| Codex ACTOne® dye | Codex Biosolution Inc. | Cat#: CB-80599-301 |
| Superose 6™ Increase 10/300 GL | GE Healthcare | Cat# 29-0915-96 |
| Deposited data | ||
| Coordinates of rCav1.1A | This paper | PDB: 8E56 |
| Coordinates of rCav1.1AM0.1 | This paper | PDB: 8E57 |
| Coordinates of rCav1.1AM | This paper | PDB: 8E58 |
| Coordinates of hCav1.3A | This paper | PDB: 8E59 |
| Coordinates of hCav1.3S | This paper | PDB: 8E5A |
| Coordinates of hCav1.3AS | This paper | PDB: 8E5B |
| Cryo-EM map of rCav1.1A | This paper | EMDB: EMD-27904 |
| Cryo-EM map of rCav1.1AM0.1 | This paper | EMDB: EMD-27905 |
| Cryo-EM map of rCav1.1AM | This paper | EMDB: EMD-27906 |
| Cryo-EM map of hCav1.3A | This paper | EMDB: EMD-27907 |
| Cryo-EM map of hCav1.3S | This paper | EMDB: EMD-27908 |
| Cryo-EM map of hCav1.3AS | This paper | EMDB: EMD-27909 |
| Experimental models: Cell lines | ||
| HEK293F cells | Thermo Fisher Scientific | Cat# R79007 |
| HEK293 cells stably overexpressing human Cav1.2 channel complex and Kir2.3 | (Xia et al., 2004) | N/A |
| Recombinant DNA | ||
| Codon-optimized human Cav1.3-α1 cloned into a modified pCAG vector with tandem twin-strep and Flag tags at the amino (N) terminus | This paper | N/A |
| Codon-optimized human α2δ-1 subunit cloned into a modified pCAG vector | This paper | N/A |
| Codon-optimized human β3 subunit cloned into a modified pCAG vector with N-terminal Flag tag and C-terminal His10 tag | This paper | N/A |
| Software and algorithms | ||
| PyMOL | The PyMOL Molecular Graphics System, Schrodiner | https://pymol.org/2/ |
| Prism 9 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| MotionCor2 | (Zheng et al., 2017) | https://emcore.ucsf.edu/ucsf-motioncor2 |
| Coot | (Emsley et al., 2010) | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| UCSF Chimera | (Pettersen et al., 2004) | http://www.cgl.ucsf.edu/chimera |
| UCSF ChimeraX | (Goddard et al., 2018) | https://www.rbvi.ucsf.edu/chimerax/ |
| PHENIX | (Adams et al., 2010) | http://www.phenix-online.org/ |
| RELION 3.0 | (Zivanov et al., 2018) | https://www3.mrc-lmb.cam.ac.uk/relion/ |
| SerialEM | (Mastronarde, 2005) | http://bio3d.colorado.edu/SerialEM/ |
| Schrödinger Suite 2018-1 | Schrödinger, Inc. | https://www.schrodinger.com/ |