Abstract
Delivery of proteins to the vacuole of the yeast Saccharomyces cerevisiae provides an excellent model system in which to study vacuole and lysosome biogenesis and membrane traffic. This organelle receives proteins from a number of different routes, including proteins sorted away from the secretory pathway at the Golgi apparatus and endocytic traffic arising from the plasma membrane. Genetic analysis has revealed at least 60 genes involved in vacuolar protein sorting, numerous components of a novel cytoplasm-to-vacuole transport pathway, and a large number of proteins required for autophagy. Cell biological and biochemical studies have provided important molecular insights into the various protein delivery pathways to the yeast vacuole. This review describes the various pathways to the vacuole and illustrates how they are related to one another in the vacuolar network of S. cerevisiae.
Subcellular compartmentalization is an important feature of eukaryotic cells, preventing inappropriate meetings of certain intracellular components as well as facilitating efficient ordered reactions. To maintain this compartmentalization, cells have evolved mechanisms to ensure that specific proteins are delivered to specific organelles.
The vacuole of Saccharomyces cerevisiae is central to much of the physiology of this organism. Among the many roles of this organelle are pH and osmoregulation, protein degradation, and storage of amino acids, small ions, and polyphosphates. To carry out all of its functions, it is essential that the vacuole contain its full complement of proteins. The functions of the vacuole in sporulation, protein turnover, osmoregulation, pH homeostasis, and ion transport have been well documented and reviewed recently (81). The focus of this review is the biogenesis of the vacuole in S. cerevisiae and the processes involved in the formation and transmission of this organelle. In particular, the transport pathways responsible for the delivery of proteins to the vacuole will be discussed.
Over the past two decades, genetic screens have identified many genes as being involved in vacuolar biogenesis in yeast. These have included screens to identify genes involved in the delivery of various marker proteins to the vacuole. In the yeast S. cerevisiae proteins get to the vacuole by a number of different pathways, including (i) sorting of vacuolar proteins away from those to be delivered to the cell surface following transit through the early stages of the secretory pathway; (ii) endocytosis of material from the plasma membrane; (iii) cytoplasm-to-vacuole targeting pathways, which do not pass through the early stages of the secretory pathway; and (iv) inheritance of vacuolar material by daughter cells during cell division.
These routes are obviously different from one another, but, interestingly, genetic screens designed to identify components involved in each of them have shown that although there are genes unique to each pathway, there is a significant overlap of the genetic requirements for the various processes. This demonstrates that although these processes each define independent pathways for protein delivery to the vacuole, they are all intimately related.
One of the features of vacuolar protein sorting in S. cerevisiae that makes it an attractive model system for studying protein trafficking is the array of convenient marker proteins whose delivery to the vacuole can be monitored easily. Vacuolar proteases tend to be synthesized as inactive zymogens whose activation requires proteolytic cleavage to remove a propeptide (84). Processing occurs in the vacuole and requires an intact copy of the structural gene for proteinase A (PrA), PEP4, to initiate a vacuolar protease activation cascade (103, 108). The PEP4-dependent cleavage of many vacuolar proteins results in the formation of a lower-molecular-weight form of the protein, which can be distinguished from its precursor form through an increased mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Other proteins, such as endocytosed proteins, are completely degraded upon delivery to the vacuole. Such detectable proteolytic events provide convenient means to monitor the kinetics of vacuolar delivery. This, combined with the more classical tools of cell biology such as immunofluorescence microscopy and the ease with which S. cerevisiae can be manipulated genetically, makes the study of protein trafficking to the yeast vacuole a very attractive system.
SORTING OF PROTEINS FROM THE LATE GOLGI TO THE VACUOLE
Many vacuolar proteins such as the soluble vacuolar hydrolases carboxypeptidase Y (CPY), PrA, and proteinase B (PrB), as well as membrane proteins such as the 100-kDa subunit of the vacuolar ATPase (Vph1p), alkaline phosphatase (ALP or Pho8p), and dipeptidyl aminopeptidase B (Dap2p), travel through the early stages of the secretory pathway (66, 84, 123). Once these proteins reach the last compartment of the Golgi apparatus, they are sorted away from proteins destined for delivery to the cell surface (54). Soluble hydrolases require a sorting signal to mediate their vacuolar delivery from the Golgi apparatus (see below), and in the absence of such information, these proteins are delivered to the cell surface. In contrast to this, integral membrane proteins lacking information to localize them to a specific intracellular organelle are not delivered to the cell surface. Instead, these proteins are transported to the vacuole (130, 187).
Receptor-Mediated Sorting of Soluble Vacuolar Hydrolases
The posttranslational modifications received by CPY as it transits from the endoplasmic reticulum (ER) to the vacuole have been well characterized and have allowed its use as a marker protein to monitor protein trafficking to the vacuole (78, 159). CPY is synthesized as an inactive precursor, prepro-CPY, which is translocated into the lumen of the ER, where its signal sequence is removed by signal peptidase and it undergoes N-linked glycosylation to become the 67-kDa, ER (or p1) form of the protein. By means of vesicular trafficking, in a SEC-dependent manner, CPY is transported to and subsequently through the Golgi apparatus, where it receives further oligosaccharide modifications to produce the 69-kDa Golgi-modified (or p2) form of the zymogen. The last definable subcompartment of the Golgi apparatus (54) is that which contains the three processing proteinases involved in the maturation of the mating pheromone α-factor (Kex2p, Kex1p, and Ste13p) (15) before it is secreted from the cell. It is in this compartment that p2 CPY is diverted away from the secretory pathway through a receptor-mediated process that leads to its delivery to the vacuole, where it is cleaved by vacuolar proteases into its active, mature, 61-kDa form (mCPY).
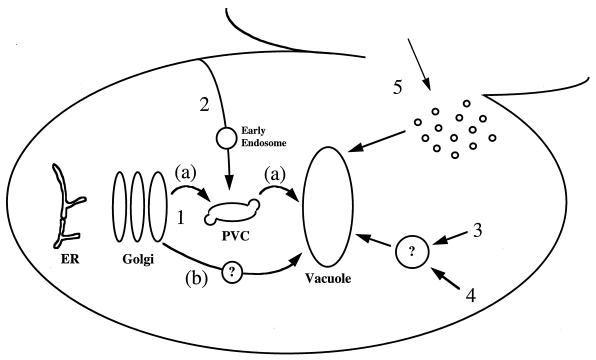
The sequence QRPL (residues 24 to 27) in the propeptide region of pro-CPY forms the core of the targeting signal required to divert p2 CPY away from the secretory pathway, into the vacuolar biogenesis pathway. Alteration of this signal results in the entry of the protein into Golgi-derived secretory vesicles and its subsequent secretion from the cell (78, 169, 170). Overproduction of CPY also results in its secretion from the cell, an observation that first suggested the involvement of a saturable receptor in CPY sorting (160). The receptor has been identified as being encoded by the VPS10 gene (98). Cells lacking Vps10p missort more than 90% of their CPY, and Vps10p can be cross-linked to pro-CPY but not to a sorting-defective form of pro-CPY. The current model of the pathway taken by CPY from the late Golgi to the vacuole, depicted in Fig. 1, is based largely on the delivery of proteins to the lysosome of mammalian cells by the mannose-6-phosphate receptor (90) and is as follows (19, 29): (i) p2 CPY binds to Vps10p in the late Golgi; (ii) the receptor-ligand complex travels to an endosomal or prevacuolar compartment (PVC) in Golgi-derived transport vesicles; (iii) and CPY dissociates from its receptor in the PVC and is transported to the vacuole, while Vps10p is recycled back to the Golgi to bind more CPY. This model accounts for the observation that Vps10p is synthesized at a 20-fold-lower rate than is CPY yet it binds its ligand with a 1:1 stoichiometry in vitro (29). In addition, Vps10p carries a tyrosine-based localization signal that is essential both for its steady-state localization to the trans-Golgi and its function as the CPY receptor (19, 29). Aromatic-residue-containing motifs are essential for the Golgi localization of membrane proteins, which continually cycle between the Golgi and the PVC (reviewed in reference 113), and it is evident that Vps10p also follows this itinerary (see below). As well as secreting CPY, vps10Δ cells secrete 50% of their newly synthesized PrA (29), suggesting that despite the lack of similarity between CPY and PrA, Vps10p can recognize both proteins as being destined for vacuolar delivery (29, 185). In addition, Vps10p seems to be responsible for targeting misfolded proteins that exit the ER to the vacuole (70). Therefore, while it is clear that the QRPL sequence is responsible for mediating interactions between pro-CPY and Vps10p, the receptor does seem to recognize a broad range of signals, whose exact nature remains unclear. While it has been found that the 54-residue propeptide of PrA can direct the normally secreted protein invertase to the vacuole in a VPS10-dependent manner, it has also been found that the propeptide is not essential for vacuolar targeting of PrA, suggesting that the protease can also reach the vacuole through a Vps10p-independent route (185). In addition, homologs of Vps10p have been identified within the S. cerevisiae genome, and there is evidence to suggest that at least one of these (VTH2) can act as a functional receptor for both CPY and PrA. These data suggest that S. cerevisiae contains a family of receptors that participate to various degrees in the sorting of soluble hydrolases to the vacuole (29, 185).
FIG. 1.
Protein transport pathways to the yeast vacuole. Depicted are six pathways used by proteins to reach the vacuole in S. cerevisiae: 1, sorting of vacuolar proteins in the late Golgi (a) through a PVC and (b) via an alternative route; 2, endocytosis of proteins from the cell surface; 3, biosynthetic cytoplasm to vacuolar targeting; 4, autophagy (degradative cytoplasm to vacuolar targeting); 5, vacuolar inheritance from mother to daughter cells, using homotypic fusion to fuse vacuolar vesicles.
VPS10 is identical to PEP1, and PEP1 is one of the group of genes identified in several genetic screens that were performed to identify functions required for vacuolar biogenesis. The pep mutants were isolated as being defective in CPY enzymatic activity (80). The vps mutants were isolated as a collection of mutants that fail to properly sort CPY or a CPY-invertase fusion protein to the vacuole and secrete the soluble hydrolase as a result of some block in the vacuolar biogenesis pathway (5, 137). Complementation analysis revealed extensive overlap between pep mutants and the vps mutants (136). Whereas the vast majority of PEP genes are required for the transport of proteins to the vacuole (reflected by the observation that most pep mutants secrete CPY), the PEP4 gene encodes PrA, a protease required for the activation of numerous vacuolar hydrolases (1, 188).
Trafficking Steps between the trans-Golgi Network and the Vacuole
The model outlined in Fig. 1 can be broken down into a number of different steps, and a variety of assays have been used to gain insight into the steps at which the various VPS/PEP genes function. This endeavor has been greatly enhanced by the cloning and molecular characterization of many VPS/PEP genes. This approach has revealed that many of the gene products involved in vacuolar protein sorting are homologous to those involved in protein trafficking in many other systems and has also allowed the isolation of conditional alleles. The use of conditional alleles that produce rapidly inactivatable proteins has proved particularly useful in determining the primary function(s) of these gene products.
An important stage in the transport of proteins to the vacuole is their segregation from secreted proteins and packaging into vesicles distinct from those bound for the cell surface. It is clear that this occurs at the level of the late Golgi, the yeast equivalent of the mammalian trans-Golgi network (TGN) (54), and that vacuolar proteins are not first delivered to the cell surface and then transported to the vacuole by endocytosis. Evidence that vacuolar delivery of CPY and the vacuolar membrane protein ALP occurs through an intracellular route comes from studies with mutants known to block the fusion of Golgi-derived secretory vesicles with the plasma membrane and mutants defective in early stages of endocytosis (112, 159). Both CPY and ALP reach the vacuole in these late-acting sec and end mutants, indicating that they do not require transit through the plasma membrane to reach their final destination. Recent studies have revealed that proteins can be sorted into one of at least five routes from the TGN: two to the cell surface (62), one that goes back to earlier Golgi subcompartments (61), and two that carry proteins to the vacuole (32, 117). This complexity demands a high level of regulation at the molecular level, and the factors involved in the formation of vacuolar-bound vesicles from the late Golgi are beginning to be uncovered.
Vesicle Formation at the trans-Golgi Network
Three VPS genes have been implicated in the budding of vesicles carrying vacuolar cargo from the Golgi apparatus. The observation that these genes are required for the formation of Golgi-derived vesicles that contain vacuolar cargo but not for the formation of those destined for the cell surface implies that they might somehow be involved in creating the specificity of these vesicles. VPS1 encodes a homolog of the mammalian protein dynamin (reviewed in reference 26), which is required for endocytosis and appears to be involved in the pinching off of vesicles from the plasma membrane (reviewed in reference 177). Yeast cells lacking Vps1p are not defective in endocytosis but instead secrete the Golgi-modified form of CPY and transport vacuolar membrane proteins to the plasma membrane (112). Such observations and the homology of Vps1p to dynamin suggest a role for it in the formation of vesicles that divert vacuolar proteins away from the secretory pathway in wild-type cells. Clathrin has also been implicated in the formation of these vesicles, and, indeed, a sudden loss of clathrin function also results in the mislocalization of vacuolar proteins to the cell surface (146). This shared phenotype is consistent with models in which Vps1p and clathrin act together to form vesicles carrying vacuolar cargo, and an absence of their function results in this cargo spilling over into secretory vesicles that transport them to the cell surface (26).
It has been proposed that Vps15p and Vps34p act together as part of a regulatory complex that functions at the late Golgi to drive vesicle formation (reviewed in reference 158). Vps15p exhibits homology to the Ser/Thr family of protein kinases and has been demonstrated to have kinase activity both in vitro and in vivo. The protein is myristylated at its N terminus and is thought to reside on the cytosolic face of Golgi membranes. Under restrictive conditions, vps15-ts mutants accumulate p2 CPY in membrane fractions enriched for Golgi markers. Vps15p is necessary for the membrane association of Vps34p. Vps34p is homologous to the 110-kDa catalytic subunit of mammalian phosphoinositide 3-kinase (PI3-kinase) (157). In mammalian cells, this subunit acts as part of a complex to phosphorylate phosphatidylinositol and other phosphorylated phosphoinositides in response to activated tyrosine kinases. Vps34p has also been demonstrated to have kinase activity, and, interestingly, vps15 mutant strains are defective in Vps34p PI3-kinase activity. These data have led to the model that Vps15p is responsible for recruiting Vps34p onto the membrane of the TGN and activating its lipid kinase activity, which, in turn, leads to the formation of vesicles carrying vacuolar cargo. This model predicts that vps15 and vps34 mutants will divert vacuolar traffic (such as Vps10p) to the cell surface as is the case in vps1 mutants, a prediction that remains to be tested. Vps15p and Vps34p are found in a hetero-oligomeric complex along with a number of as yet unidentified proteins. Identification of these proteins should aid our understanding of the link between kinase activity and membrane-trafficking events.
Vacuolar Delivery via a Prevacuolar/Endosomal Compartment
Subcellular fractionation studies have revealed that CPY transits another membrane-bound compartment on its journey from the late Golgi to the vacuole (172). This helped to form the model that Golgi-derived vesicles carrying pro-CPY fuse with a PVC in a manner analogous to the sorting of lysosomal proteins in mammalian cells (reviewed in references 72 and 158).
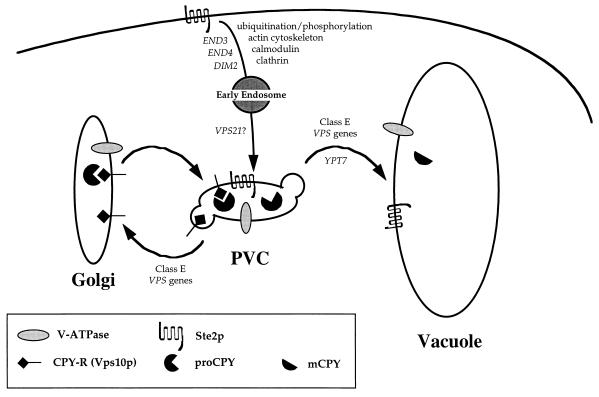
A large collection of vps mutants were divided into six classes (A to F) on the basis of a number of criteria including vacuolar morphology (6, 123). vps mutants exhibit one of a number of vacuolar morphologies, ranging from cells with near-wild-type vacuoles to those with highly fragmented vacuolar membranes. In many cases, it appears that mutants that fall into the same class are blocked in the same stage of vacuolar protein sorting (81, 158). For example, mutants from any of the 13 class E vps groups accumulate an exaggerated form of the PVC through which CPY transits en route to the vacuole. In addition to CPY, this class E compartment accumulates endocytosed proteins, such as the mating pheromone receptor Ste3p, as well as vacuolar membrane proteins, such as Vph1p, indicating that it represents a point of overlap between the endocytic pathway and the vacuolar biogenesis pathway (118) (see Fig. 3).
FIG. 3.
Endocytosis of proteins from the plasma membrane to the vacuole. Proteins such as the mating pheromone receptor Ste2p are endocytosed to the vacuole from the plasma membrane, passing through at least two membrane-bound compartments en route. The requirements for this process are indicated here. The requirement of class E VPS genes for endocytosis pinpoints the PVC as a convergence point for the biosynthetic and endocytic pathways.
Studies with vps27, vps28, and vps4 mutants indicate that these class E vps mutants are unaffected in anterograde traffic between the Golgi and the PVC and are competent for endocytosis as far as the PVC (4, 118, 127). An exaggerated form of this PVC can be seen as a multilamellar structure by electron microscopy (4, 127). The accumulation of this compartment seems to result from a block in membrane traffic out of the PVC both on to the vacuole and back to the Golgi apparatus. Such a block causes the entrapment of vacuolar proteins and recycling late-Golgi membrane proteins, as well as endocytosed proteins whose normal cellular itinerary includes transit through the PVC (16, 118).
Studies with the class E vps gene VPS27 provided evidence for the receptor-recycling model of CPY sorting outlined above (29, 118). As described above, VPS10 encodes a receptor that carries CPY from the late Golgi to the PVC (Fig. 1). By using a reversible, temperature-sensitive allele of VPS27, it has been shown that Vps10p, which accumulates in the class E compartment upon loss of VPS27 function, returns to the Golgi apparatus with a concomitant restoration of ability to properly sort CPY, upon restoration of VPS27 function, while Vph1p travels on to the vacuole.
Whereas class E Vps proteins appear to control traffic out of the PVC, class D Vps proteins act before vacuolar proteins gain entry into the PVC. VPS15 and VPS34 (described above) are examples of class D VPS genes that appear to act at the level of vesicle formation from the late Golgi, and thus a loss of function of these genes blocks traffic between the late Golgi and the PVC. Other class D mutations also block traffic between the Golgi and the PVC, but whereas vps15 and vps34 mutations appear to prevent the formation of Golgi-derived transport vesicles, these other class D mutations prevent the fusion of such vesicles with their acceptor membrane, the PVC.
SNARE Components of the VPS-Dependent Carboxypeptidase Y Pathway
Many lines of investigation suggest that the molecular mechanisms underlying vesicular docking and fusion are conserved across both species and organelles. For example, members of the highly conserved syntaxin-, Rab-, and Sec1p-like protein families are required for most, if not all, vesicle-mediated transport steps identified to date. Second, a single protein, NSF (NEM sensitive fusion protein) (Sec18p in yeast), seems to be required for the docking and/or fusion of transport vesicles derived from different organelles with their appropriate target membranes in many different species. The SNARE (soluble NSF receptors) hypothesis was proposed as a model to explain the specificity of vesicle targeting (44, 135, 156). It states that vesicles are identified by the possession of unique members of the v-SNARE family, which interact specifically with particular t-SNAREs on the appropriate target membrane. The complex of proteins formed when a vesicle docks generates a binding site for soluble NSF attachment proteins, which then recruit NSF to facilitate membrane fusion through an ATP hydrolysis-requiring step. The specificity of vesicular targeting is proposed to be inherent in the complementary molecular interactions between unique SNARE complex components, including Rab- and Sec1p-like proteins, which then recruit general membrane fusion factors that function at multiple points throughout the cell. Some aspects of the SNARE hypothesis, such as the role of SNARE proteins in determining the specificity of targeting, have recently been called into question (see below for examples of this); however, they are an integral part of the docking/fusion machinery.
The molecular mechanisms mediating transport between the trans-Golgi and the PVC are predicted to be similar to those involved in other vesicle-mediated transport steps. Among the proteins encoded by the class D genes are a syntaxin homolog (Pep12p/Vps6p), a Rab GTPase (Vps21p), and a Sec1p family member (Vps45p). Consistent with its homology to Sec1p, which is required for the fusion of Golgi-derived secretory vesicles with the plasma membrane, loss of Vps45p function results in the rapid accumulation of transport vesicles (∼60 nm in diameter) within the cytoplasm (30, 119). Immunofluorescence studies indicate that vacuolar proteins, such as Vph1p, do not reach the vacuole in vps45 mutants because they are trapped within these vesicles (117). The model that Vps45p is required for the fusion of Golgi-derived transport vesicles with the PVC is also supported by data from pulse-chase analyses of both soluble and integral membrane vacuolar proteins. These accumulate in their Golgi-modified, precursor forms in vps45 mutants, indicating that they do not reach a proteolytically active compartment (30, 117, 119). Other class D mutants also accumulate transport vesicles and Golgi-modified pro-CPY, and genetic and biochemical data suggest the existence of a complex containing the SNARE complex components Vps45p, Pep12p, Vps21p, and Sec18p, as well as Vac1p/Vps19p, which is believed to function in Golgi to PVC transport (18).
Pep12p is the presumed t-SNARE of the PVC and is required for the sorting of CPY. Although it has been shown that this syntaxin homolog fractionates away from Golgi and vacuolar markers (7), its positive assignment to the PVC remains to be assessed. Such studies are hindered by the lack of marker proteins for this compartment. Since the PVC is proposed to be the point at which the endocytic and Vps pathways intersect (118), experiments aimed at colocalizing Pep12p and other proposed markers of endosomes (such as Ypt51p [154; see below]) with endocytosed proteins in transit to the vacuole might resolve this issue.
The member of the v-SNARE family thought to direct Golgi-derived transport vesicles to the PVC is encoded by VTI1 (45). While many vti1 mutants secrete CPY, VTI1 is different from the other VPS genes in that it is essential for yeast cell viability. By isolating alleles of VTI1 that are temperature sensitive for either CPY secretion or viability, the essential function of the gene has been separated from its role in the Vps pathway. In addition to its interaction with Pep12p, Vti1p binds the t-SNARE Sed5p, which functions in traffic between the ER and the cis-Golgi. SED5, first isolated as a suppressor of the erd2 defect (60), was found to be a multicopy suppressor of a conditionally lethal vti1 mutation, an allele that blocks ER-to-Golgi traffic. This involvement of a single v-SNARE in distinct membrane-trafficking steps through interaction with two different t-SNAREs demands a modification of the original SNARE hypothesis. Besides interacting with Vti1p, Sed5p also recognizes the v-SNAREs Sec22p/Sly2p, Bet1p/Sly12p, and Bos1p, which are required for ER-to-cis-Golgi traffic, and the v-SNARE Sft1p, which is involved in retrograde traffic from later Golgi compartments back to the cis-Golgi. These data suggest that a t-SNARE defines a target organelle and can interact with v-SNAREs of all incoming vesicles rather than being restricted to interaction with one specific v-SNARE.
Transport from the Prevacuolar Compartment to the Vacuole
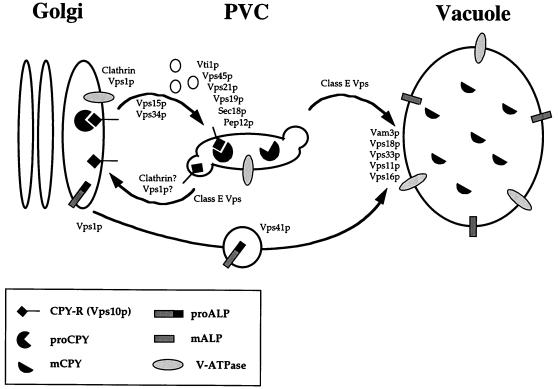
The mechanism of protein transport from the PVC on to the vacuole has remained something of an enigma. The fragmentation of vacuoles in class B and C vps mutants (6, 123) makes it tempting to speculate that the genes represented by these classes are required for the final trafficking step into the vacuole. Indeed, cells lacking the vacuolar t-SNARE Vam3p display a class B vps phenotype (34, 174) and are defective for traffic into the vacuole not only from the CPY pathway but also from the alternative pathway taken by ALP and Vam3p itself (see below). In addition, Vam3p is required for homotypic vacuole fusion (see below), as well as the Cvt pathway taken by aminopeptidase I (API) (see below). The four known class C VPS gene products (Vps11p, Vps16p, Vps18p, and Vps33p) have recently been localized to the vacuolar membrane and have been shown to interact with each other both physically and genetically (126). In this same study, a conditional allele of VPS18 was used to demonstrate an interaction between the class C VPS genes and VAM3 (126). Characterization of a vps18-ts mutant revealed that, like Vam3p, Vps18p functions in multiple protein transport pathways to the vacuole. Interestingly, rapid loss of Vps18p function leads to the accumulation of small vesicles, autophagosomes, and other membrane compartments. Taken together, these data suggest that the class C Vps proteins act as part of a complex to facilitate the delivery of multiple transport intermediates to the vacuole (Fig. 2).
FIG. 2.
Sorting of proteins from the late Golgi to the vacuole. Vacuolar proteins are sorted in vesicles bound for the vacuole at the level of the TGN. The soluble hydrolase, CPY, binds to its membrane receptor (Vps10p) in this compartment, and the receptor-ligand complex is delivered to the PVC. In the PVC, CPY dissociates from its receptor and travels on to the vacuole while Vps10p returns to the Golgi to bind more ligand. A second pathway from the TGN to the vacuole does not pass through this compartment and is taken by the vacuolar membrane proteins ALP and Vam3p. Interestingly, the two pathways converge at, or before, the vacuolar membrane.
The presence of SNARE complex homologs encoded by the class B and C VPS genes (e.g., Vps33p, Ypt7p, and Vam3p) makes it tempting to suggest that protein traffic from the PVC to the vacuole is vesicle mediated, and, as mentioned above, vps18-ts mutants do accumulate vesicles at the restrictive temperature (126). It has been proposed that in mammalian cells, endosomes mature with time and then fuse directly with lysosomes to deliver their contents there rather than utilizing a vesicle-mediated step (46). The involvement of SNARE complex proteins in PVC-to-vacuole trafficking does not argue against a similar maturation model since these proteins, as well as Sec18p (yeast NSF), have also been implicated in the homotypic fusion of vacuolar membranes (see below). Due to the apparent role of class C VPS genes in multiple protein transport pathways to the vacuole, vps18-ts mutants accumulate an array of intermediates upon exposure to restrictive conditions, and it therefore remains unclear whether the PVC-to-vacuole step is vesicle mediated. Among the intermediates that accumulate are 40- to 50-nm vesicles, but the identity of the cargo they carry awaits further analysis, perhaps by immunoelectron microscopy.
Trafficking of Alkaline Phosphatase to the Vacuole
Unlike most other vacuolar proteins that travel from the TGN to the vacuole that have been studied to date, the integral membrane protein ALP (encoded by PHO8) does not travel through the PVC defined by mutations in the class E genes VPS27 and VPS4 or in the Golgi-derived transport vesicles defined by vps45 mutations that require Pep12p for their targeting (32, 117). ALP is consistently delivered to the vacuole in cells that are blocked in the Vps pathway at these stages through a route that does not involve its transport to the cell surface and subsequent endocytosis (32, 117). The observation that ALP is not carried from the trans-Golgi by vesicles defined by vps45 mutations implies that there is a second class of vesicles that carry vacuolar cargo out of this organelle. The diversion of ALP, as well as proteins that utilize the Vps pathway, to the plasma membrane in vps1 mutant cells (112) provides evidence that Vps1p is involved in the formation of both classes of vesicles from the trans-Golgi, but it seems likely that clathrin is involved in the formation only of those bound for the PVC, since ALP is not significantly mislocalized to the cell surface of chc1-ts cells under restrictive conditions (147). Such a dual role for Vps1p is not surprising, since it is clear that its mammalian homolog, dynamin, participates both in rapid endocytosis from the plasma membrane and in the formation of caveolae (2).
The 33-residue N-terminal cytosolic tail of ALP is both necessary and sufficient for the diversion of membrane proteins from the Vps-dependent CPY pathway into the alternative route taken by ALP to the vacuole (32, 117). Although the precise signal required to mediate entry into this pathway remains to be defined, it is clear that ablation of this information from ALP results in trafficking of the protein to the PVC (and subsequently to the vacuole) in VPS45-controlled vesicles. Consistent with the identification of an ALP pathway targeting signal is the observation that this pathway is saturable. Overproduction of ALP leads to a portion that is now transported to the vacuole in a Pep12p-dependent manner (32).
Mutations in a number of class B genes, such as VAM3, which encodes a vacuole-localized syntaxin homolog (174) and also travels to the vacuole independently of the PVC (117), not only block the trafficking of ALP to the vacuole but also prevent transport of proteins by the Vps-dependent pathway. As well as being defective in both transport pathways from the TGN to the vacuole, vam3-ts cells are blocked in the cytoplasm-to-vacuole targeting pathway taken by API (see below) at the restrictive temperature, demonstrating that Vam3p acts at a convergence point for these three pathways (34). Similarly, YPT7 is required for the delivery of ALP to the vacuole and also for traffic from the late endosome/PVC to the vacuole (139, 186), in addition to its role in homotypic vacuolar fusion (see below).
Another class B VPS gene, VPS41, has been identified as being required for the vacuolar delivery of ALP but not carboxypeptidase S (CPS), which travels through the PVC via VPS45 vesicles (32). vps41 mutants accumulate structures that resemble mammalian multivesicular bodies (71), and these cells exhibit a block in the processing of ALP but process CPS and CPY with near-wild-type kinetics. In wild-type cells, newly synthesized ALP is found in a low-speed pellet fraction (containing vacuolar membranes) following a 30-min chase period and subcellular fractionation. In contrast, following exposure of vps41-ts mutant cells to restrictive conditions, ALP fractionates in the high-speed pellet, probably reflecting its entrapment in some transport intermediate defined by this mutation (32). The interpretation that the vps41-ts mutation selectively blocks the ALP pathway without affecting the CPY pathway is based on the assumption that the observed maturation of CPY and CPS reflects vacuolar delivery of these proteins. Unless confirmed by immunolocalization data, this assumption may be misleading, since it is clear that processing by vacuolar proteases does not always occur in the vacuole; for example, vps27 mutants (both null and temperature sensitive) process proteins in a PEP4-dependent manner in the PVC (118).
A genetic screen for factors specifically involved in transport of ALP to the vacuole was performed with an ALP-Ste13p fusion protein and identified Ap16p and Ap15p of the AP-3 adaptor protein complex (31). Adaptor proteins are believed to provide membrane binding sites for vesicle coat components such as clathrin and also interact with trafficking-membrane proteins to recruit specific cargo into transport vesicles (132). Recently, a new adaptor complex associated with the TGN and endosomes in mammalian cells has been described (36, 37, 150, 151), and a yeast equivalent of this complex has been identified by genetic screens aimed at suppressing the growth defects associated with endocytosis-deficient casein kinase I mutants (115). AP-3 is not found in association with clathrin, nor does it show genetic interaction with clathrin, and thus AP-3 most probably associates with other as yet unidentified coat proteins (115, 150). Interestingly, AP-3 has been shown to be involved in the trafficking of ALP, since disruption of genes encoding subunits of AP-3 result in the accumulation of pro-ALP (31). Although it is clear that the AP-3 complex functions along the alternative pathway, its precise site of action is unclear. While it is possible that AP-3 proteins function at the level of the TGN to sort proteins such as ALP into the alternative pathway, it is equally likely that the AP-3 complex functions at some intermediate compartment along the pathway. Nevertheless, these observations are provocative, since they imply that the alternative pathway followed by ALP defines a novel sorting pathway from the TGN that is present in all eukaryotes.
ENDOCYTOSIS OF PROTEINS FROM THE PLASMA MEMBRANE TO THE VACUOLE
Endocytosis is the uptake of extracellular and plasma membrane material from the cell surface into the cell. Endocytosis has been well characterized at the morphological level in mammalian cells, where both fluid-phase endocytic markers, such as horseradish peroxidase, and membrane-bound endocytic markers, such as colloidal gold-labeled transferrin receptors, can be internalized and visualized by electron microscopy. The best-characterized endocytic mechanism to date is clathrin-dependent endocytosis (141), in which markers are initially seen in invaginations of the plasma membrane, known as coated pits, coated on the cytoplasmic side of the plasma membrane with polymerized clathrin. In many cases, it has been shown that receptors are concentrated into these pits via an interaction between their cytosolic tails and clathrin adaptor proteins, which in turn associate with clathrin triskelions (116). After longer incubation times, endocytic markers label early endosomes and subsequently multivesicular late endosomes. It is through late endosomes that the mannose-6-phosphate receptor cycles as it targets proteins from the TGN to the lysosome, and these organelles seem to be the point at which the endocytic and lysosomal biogenesis pathways intersect (90, 101). After prolonged internalization, the marker proteins are delivered to lysosomes, the mammalian equivalent of the yeast vacuole. Endocytosis of extracellular fluid by S. cerevisiae has been demonstrated by using lucifer yellow (128) and the lipophilic dye FM4-64 (171), and receptor-mediated endocytosis can be monitored by using the mating pheromone receptors Ste2p (24, 77) and Ste3p (35).
Endocytosis of Mating-Pheromone Receptors
Yeast pheromone receptors function in cell-to-cell communication prior to mating. Each of the two haploid yeast cell types, a and α, secretes unique peptide pheromones and expresses a cell surface receptor for the pheromone secreted by the other cell type (64). Binding of pheromone to its receptor activates an intracellular signal transduction pathway that leads to the physiological alterations that facilitate mating. Following binding, the pheromone-receptor complex is internalized by endocytosis and degraded in the vacuole in a PEP4-dependent manner (35, 138). Riezman and colleagues used radiolabeled α-factor to monitor the uptake of the receptor-ligand complex from the cell surface to the vacuole and showed that the pheromone passes through at least two internal membrane-bound compartments with distinct densities, termed early and late endosomes, before being delivered to the vacuole and degraded by resident proteases (152, 153).
Ligand-Dependent versus Constitutive Endocytosis
Two distinct modes of endocytosis operate on both Ste3p and Ste2p (35): a constitutive (ligand-independent) mechanism and a ligand-dependent mechanism. In both cases, receptors follow the same intracellular itinerary from the cell surface to the vacuole, where they are degraded. The two modes are likely to be distinct mechanistically, since they can be dissected genetically: for example, a version of Ste3p lacking 105 of the 185 residues that make up the C-terminal cytosolic tail does not undergo constitutive endocytosis but is still susceptible to the ligand-induced mechanism (35). The failure of the truncated receptors to undergo constitutive endocytosis suggests that the constitutive pathway is not due to bulk endocytosis of the plasma membrane but, instead, requires some signal lacking in these truncated mutant receptors. Furthermore, not all plasma membrane proteins in yeast turn over as rapidly as the pheromone receptors do (e.g., the plasma membrane ATPase Pma1p has a half-life of >10 h [11], compared with 20 min and ∼2 h for Ste3p and Ste2p, respectively). Constitutive endocytosis of pheromone receptors may be necessary to remove receptor from the cell surface, since cells switch mating type in the wild as often as every cell division under some conditions (9). Along the same lines, endocytosis may aid in the process of cell orientation in response to a gradient of mating pheromone by clearing the receptor from regions of the cell not oriented toward the partner (74).
Ubiquitination as a Signal for Endocytosis
Both Ste2p and Ste3p become phosphorylated and ubiquitinated in response to ligand binding, prior to internalization (65, 134). The signal SINNDAKSS within the cytosolic tail of Ste2p has been identified as being sufficient for ligand-dependent receptor internalization (133). Although this signal is sufficient for the internalization of a truncated version of the receptor, it appears that the cytosolic tail of Ste2p contains other, perhaps redundant, internalization information, since this signal is not essential for internalization of the full-length receptor. The lysine residue contained within this signal is one of the eight lysines in the cytosolic tail that is modified by ubiquitin-conjugating enzymes in response to ligand binding (65). Abolition of this ubiquitination, either through mutation of the lysine to an arginine residue or by using ubc mutants, prevents internalization of Ste2p (reviewed in reference 129). In mammalian cells, surface receptors, including the epidermal growth factor receptor and the platelet-derived growth factor receptor, become ubiquitinated in response to ligand binding. These receptors are also internalized and are subsequently degraded in the lysosome in response to ligand binding, suggesting a role for ubiquitination in endocytosis in animal cells (49, 104). More direct evidence for this comes from studies with CHO cells carrying a temperature-sensitive form of the ubiquitin-activating enzyme. These cells are unable to internalize growth hormone receptor under restrictive conditions (161).
The role of phosphorylation in the internalization of the mating-pheromone receptors is poorly understood. Mutational analysis had demonstrated that phosphorylation of one of the three serine residues within the SINNDAKSS sequence is necessary for the internalization function of this sequence (65). It has been proposed that phosphorylation acts as a signal to mediate ubiquitination, as observed for certain cytosolic proteins (129).
The a-factor transporter, Ste6p, is constitutively endocytosed from the cell surface to the vacuole. Ste6p does not follow this itinerary in mutants lacking ubiquitin-conjugating enzymes (ubc mutants) but instead accumulates at the cell surface. Similarly, ubiquitinated forms of the protein accumulate at the cell surface in mutants blocked in early stages of endocytosis (see below) (14, 41, 88, 89). Such a role for ubiquitination in the endocytosis of Ste6p, as well as in the internalization of various permeases (47, 48, 94), implies that ubiquitination signals mediate constitutive as well as ligand-induced endocytosis in yeast cells. Consistent with this is the finding that Ste3p requires ubiquitination of the receptor for constitutive uptake as well as ligand-induced endocytosis (134).
Although ubiquitination is a well-characterized signal for the degradation of cytosolic protein by the proteasome (25), turnover of ubiquitinated pheromone receptors is not mediated by the proteasome but, rather, requires active vacuolar hydrolases (65, 129). It may be that the type of ubiquitination that proteins receive determines whether they are targeted for degradation by the proteasome or the vacuole. Multiubiquitination of substrates is generally required for recognition by the proteasome, but it seems that a monoubiquitinated species is the predominant form of modified Ste2p induced by ligand binding. These data suggest that a single ubiquitin moiety attached to a single lysine residue may lead to preferential recognition by the endocytic machinery instead of the proteasome (65). While the mechanism by which ubiquitin induces receptor internalization is unknown, it is clear that ubiquitination does not promote internalization by modulating the pheromone-stimulated signal transduction pathway (65). Models in which ubiquitin is recognized directly by the endocytic machinery or promotes the migration of receptors into domains of the plasma membrane that actively endocytose have been proposed. Another possibility is that ubiquitination distinguishes proteins to be degraded after endocytosis from those to be recycled to the cell surface after internalization (173).
Identification of Endocytic Machinery
The first genes identified as being required for the internalization of α-factor were END3 and END4. end3 and end4 mutants were identified as being deficient in pheromone uptake as well as in fluid-phase endocytosis (122). END4 is allelic to SLA2 (69), which was isolated as being synthetically lethal with the actin binding protein, Abp1p, and encodes a protein that localizes to cortical actin patches and exhibits homology to the mammalian focal adhesion protein talin (69). END3 is also required for the proper functioning of the actin cytoskeleton (10) and encodes a protein displaying similarity to domain I of eps15, a protein believed to facilitate interactions between clathrin and the epidermal growth factor receptor through the adaptor protein AP2 in mammalian cells (12, 13). Within this region (the EH domain, for eps15 homology), End3p contains a putative Ca2+ binding site, but no requirement for calcium in endocytosis has been shown. Interestingly, calmodulin is required for the internalization step of endocytosis (92), but the calcium binding function of the protein does not seem to be involved since calmodulin mutants that have lost their high-affinity calcium binding sites are not defective in endocytosis.
The endocytic tracer FM4-64 travels from the plasma membrane to the vacuole via punctate endocytic intermediates in a time-, temperature-, and energy-dependent manner (171). This molecule has been used in a fluorescence-activated cell sorter-based screen to isolate mutants defective in its uptake. The idea behind this screen was to isolate mutants defective in both clathrin-independent and clathrin-dependent endocytosis (see below), and although it is still possible that such mutants can be isolated by this approach, the two mutants reported to date, dim1 and dim2, also affect α-factor internalization (184), which occurs through the clathrin-dependent mechanism.
Like End3p, Dim2p/Pan1p contains an EH domain and localizes to cortical actin patches (10, 165). End3p and Pan1p interact genetically and physically both in vitro and in vivo (164). Since the localization of Pan1p is affected by end3 mutations, it has been proposed that End3p is important for the localization of Pan1p to the cortical actin skeleton (see below) (164), and perhaps the End3p/Pan1p-containing complex is involved in the formation of clathrin-coated pits at the cell surface through interactions with clathrin adaptor proteins (155) via EH domains. DIM1 is allelic to SHE4 and MYO4; mutants with mutations in MYO4 were isolated as having cytoskeletal defects (55), and those with mutations in SHE4 were isolated as having defects in mating-type switching (75). Both dim1 and dim2 mutants were shown to accumulate aberrant membranous structures that can be labeled with cationized ferritin as a marker of the endocytic pathway, suggesting that they represent intermediates in the internalization of plasma membrane material (184).
Other studies also suggest that the actin cytoskeleton plays a role in the internalization step of endocytosis in yeast (91). The act1-1 mutation leads to the production of an altered form of actin, and cells harboring this temperature-sensitive mutation are subjected to a rapid block in the internalization of α-factor by receptor-mediated endocytosis upon exposure to restrictive conditions. Also, an extension of the end mutant screen identified three genes that affect actin organization and are required for the internalization step in the endocytosis of Ste2p (107), and the yeast homolog of the actin-bundling protein fimbrin (Sac6p) is also required for the same stage in endocytosis (91).
Involvement of the Cytoskeleton in Endocytosis
Actin filaments assemble into two different structures in S. cerevisiae: actin cables, which extend through the cytoplasm, and cortical patches, which are found at the cell surface. While it has been proposed that cortical patches provide osmotic support during the insertion of new cell wall material, the physiological roles of both actin cables and the patches remain unclear. The small actin binding protein cofilin stimulates actin filament disassembly and thus stimulates a cycle of filament assembly and disassembly (96). Cofilin mutants are defective in the uptake of lucifer yellow suggesting that endocytosis requires the rapid turnover of actin cables induced by cofilin (96). Morphological evidence for the involvement of the actin cytoskeleton in endocytosis comes from immunoelectron microscopy experiments that demonstrate that actin in the cortical patches is associated with fingerlike invaginations of the plasma membrane (105). It has been suggested that organized bundles of actin filaments may be required to drive the scission of vesicles from invaginations of the plasma membrane (91) and that actin cables may be involved in producing the plasma membrane invaginations from which endocytic vesicles form (105). A role for actin in the scission of vesicles from the plasma membrane is consistent with observations following the treatment of mammalian cells with the drug cytochalasin D (52), when cells accumulate elongated clathrin-coated pits.
While not all mutations that affect cortical actin patches abolish endocytosis (e.g., the myo2-66 mutants endocytose α-factor with the same kinetics as do wild-type cells [97] despite having extensively delocalized cortical actin [79]), it remains possible that these structures play a role in the uptake of molecules from the cell surface. It has been suggested that mutations that affect the structure of cortical actin patches abolish endocytosis whereas those that affect the localization of these structures during the cell cycle do not (107). Unlike Myo2p, the type I yeast myosins Myo3p and Myo5p are involved in endocytosis. These molecules possess an N-terminal actin/ATP-dependent motor domain and a phospholipid binding, acidic C-terminal tail and have been postulated to be involved in vesicle movement (reviewed in reference 129).
Clathrin-Dependent and Clathrin-Independent Endocytosis
A role for clathrin in endocytosis in yeast has been identified in both constitutive and ligand-induced endocytosis by using strains expressing a temperature-sensitive clathrin heavy chain (163). In mammalian cells, endocytosis appears to operate through both a clathrin-dependent mechanism and a clathrin-independent mechanism (95); interestingly, yeast cells lacking clathrin heavy chain are capable of endocytosis at 30 to 50% of wild-type levels. Whether this residual uptake occurs through a second clathrin-independent pathway or because other elements of clathrin coats are still capable of limited vesiculation in the absence of clathrin heavy chain remains unclear.
Intersection of the Endocytic and VPS Pathways
Endocytosis mutants that disrupt actin organization are not required for the later stages of endocytosis, that is, the transport of endocytosed proteins from endosomal compartments to the vacuole (91). In a manner similar to the endocytic and lysosomal pathways in mammalian cells, the endocytic and vacuolar biogenesis pathways intersect at a post-Golgi PVC (118). A number of genes have been implicated as being required for trafficking of proteins out of this compartment and on to the vacuole. Class E vps mutants (118, 123) accumulate an exaggerated form of the PVC that contains endocytosed proteins as well as proteins en route to the vacuole via the biosynthetic pathway (118). Indeed, it is common for class E VPS genes to be identified in screens for endocytosis mutants: ren1 is allelic to vps2 and was isolated as being required for the endocytosis of Ste3p (35), and end13 is allelic to vps4 (106), confirming that transport from the PVC to the vacuole is part of the endocytic pathway. It is through the same compartment that Vps10p cycles in its role as the CPY receptor (29), and it is from here that late-Golgi membrane proteins are retrieved to achieve steady-state localization in the TGN (16) (Fig. 3).
Involvement of Rab Proteins in Endocytosis
Members of the small GTPase or Rab family of proteins have been implicated as being key regulators of endocytosis in mammalian cells at postinternalization stages (193). Rab7 is associated with late endosomes, and Rab5 is associated with early endosomes (21). The yeast homolog of Rab7, Ypt7p, is believed to control traffic between the PVC and the vacuole (139, 186), whereas homologs of Rab5, i.e., Ypt51p, Ypt52p, and Ypt53p, are proposed to be involved in an earlier transport step, such as between early endosomes and the PVC (154). Consistent with this model is the observation that the degradation of α-factor is more severely inhibited in ypt51 mutants than in ypt7 mutants (154), suggesting that in these cells the pheromone is trapped inside a proteolytically inactive compartment. In addition, the pheromone that accumulates in ypt7 cells fractionates to the same position in a density gradient as the kinetic intermediate defined by Singer et al. (152) as late endosomes, while the pheromone that accumulates in ypt51 mutants is found in fractions enriched for early endosomes (139). YPT51 is allelic to VPS21, a class D VPS gene, believed to function in the fusion of Golgi-derived transport vesicles with the PVC (18). The involvement of a member of the Rab family of GTPases in more than one transport step has two-well studied precedents: Rab1 facilitates transport between the ER and the Golgi apparatus and also seems to be required for transport between Golgi stacks (120), and Rab5 is involved in endosome-to-endosome fusion as well as in transport from the plasma membrane to early endosomes (17, 51). Similarly, in yeast, the small GTPase Ypt1p is required for ER–to–cis-Golgi transport as well as for cis- to medial-Golgi transport (76). However, conditional alleles of both VPS21/YPT51 and YPT7 are needed before we can convincingly assess the primary function of these Rab proteins in endocytic and biosynthetic vacuolar membrane traffic.
Most mutants defective in endocytosis isolated to date fall into two categories: those that block internalization of material from the plasma membrane and those that inhibit transport from the PVC to the vacuole. The latter category also shows defects in vacuolar protein sorting, pointing to an overlap between the endocytic and biosynthetic pathways. This model (Fig. 3) reconciles data from studies with class E vps mutants (discussed above), but the isolation of mutants such as vps34 as being defective in endocytosis (106) is perhaps harder to reconcile, since VPS34 has been implicated in the generation of Golgi-derived transport vesicles (see above).
CYTOPLASM-TO-VACUOLE TARGETING
Biosynthetic Pathway
Like many vacuolar proteins, the soluble vacuolar hydrolase API is synthesized as an inactive precursor (61 kDa) and converted to its active form (50 kDa) following a PEP4-dependent cleavage event (87). However, unlike most vacuolar proteins that have been characterized to date, API does not travel along the early stages of the secretory pathway to reach the vacuole. The delivery of both CPY and ALP to the vacuole is blocked in cells bearing mutations that block early stages in the secretory pathway (159), either by preventing traffic out of the ER or by blocking traffic through the Golgi apparatus (although not in sec mutants, which are specifically blocked in traffic from the Golgi apparatus to the cell surface). In contrast, the processing of API shows essentially the same kinetics in cells bearing temperature-sensitive alleles of sec23, sec12, sec18, or sec7 at the restrictive temperature as it does in wild-type cells, indicating that the delivery of API to the vacuole does not require trafficking out of the ER or through the Golgi apparatus (87). Further evidence in support of a model in which API reaches the vacuole through a route distinct from that taken by CPY and ALP comes from the time taken by these proteins to reach their final destination following synthesis. Both ALP and CPY are processed in a PEP4-dependent manner with half-lives of less than 10 min, whereas API is similarly processed with a much longer half-life of 45 min (87).
CPY is delivered to the vacuole by a receptor-mediated process (see above), and overproduction of CPY causes saturation of the receptor, resulting in a spillover of CPY into secretory vesicles in the late Golgi (160). Consequently, overproduction results in secretion of CPY from the cell. In contrast, overproduction of API does not cause secretion of the protein, but the unprocessed form instead accumulates in the cytosol; indeed, even without overproduction, it is evident that the precursor form of API does not reside within a membrane-bound compartment, since it is sensitive to proteases under conditions where mature API and all forms of CPY are not (87).
The above observations led to the model that API is synthesized in the cytosol and then transported into some “post-Golgi” compartment, originally postulated to be the vacuole itself, directly from the cytosol through the Cvt (cytoplasm-to-vacuole targeting) pathway. Such a route also appears to be taken by the product of the AMS1 gene, α-mannosidase I (Ams1p) (93, 191). Ams1p is initially synthesized as a 107-kDa precursor and is processed in a PEP4-dependent manner into 73- and 31-kDa forms upon delivery to the vacuole (192). Although the AMS1 gene product has seven consensus sequences for N-linked glycosylation (Asn-X-Ser/Thr) the protein is not glycosylated (192). By constructing a prepro-CPY–Ams1p fusion protein, which is delivered to the vacuole by the secretory pathway, it has been shown that the glycosylation sites within Ams1p are competent substrates for N-linked glycosylation and that the lack of such carbohydrate modification on Ams1p is probably because the protein is not exposed to the ER lumen. Similarly, API has four consensus N-linked glycosylation sites, and although initial characterization of the protein indicated that it was glycosylated (102), more recent studies involving lectin binding and tunicamycin treatment of cells demonstrate that the protein does not carry carbohydrate modifications (20, 87).
Ams1p and API have other features in common that set them apart from other vacuolar hydrolases and lend credence to the Cvt model. While having no effect on the sorting of CPY, overproduction of Ams1p increases the half-life of processing of API (as does overproduction of API itself), suggesting that the two have a common import mechanism and perhaps use the same saturable component to facilitate their entry into the vacuole (87). The converse experiment is hard to perform due to the long half-life of processing of Ams1p (10 h in wild-type cells at 30°C [192]).
Like API, the delivery of newly synthesized Ams1p to the vacuole is not dependent on the function of SEC12 or SEC23, as demonstrated by the observation that processing occurs in cells bearing temperature-sensitive mutations in these genes at the restrictive temperature (192). The other sec mutants analyzed in this study (sec7 and sec18) did not process Ams1p, but this is probably due to death of these mutants during the long incubation times at the restrictive temperature required in these studies (again due to the long half-life of processing of the protein).
Consistent with the data that neither API nor Ams1p is glycosylated, neither possesses a standard signal sequence as found in CPY, PrA, and PrB, specifying translocation into the ER lumen. They do, however, contain information targeting them to the vacuole (87, 192). For Ams1p, it has been shown that the extreme C terminus of the protein is capable of diverting the normally secreted protein invertase to the vacuole. The propeptide of API, which is removed upon delivery of the protein to the vacuole, is required for the targeting of the protein to the vacuole, and a mutant form of the protein lacking this pro region accumulates in the cytosol (114). This propeptide lacks any similarity to a standard signal sequence, because it is not hydrophobic in nature, and secondary-structure analysis predicts that it is composed of two α-helices separated by a β-turn, with the first of the two helices being amphipathic (20). Mutations within this amphipathic helix that substitute a charged amino acid for a nonpolar residue prevent membrane association of pro-API, suggesting that the amphipathic nature of this helix is vital for vacuolar targeting. In contrast, the periodicity of the second helix does not seem to be as important, and it has been suggested that its role might be to present the first helix in the correct context to allow it to facilitate Cvt transport (114).
Recent studies indicate that the precursor API oligomerizes in the cytosol prior to membrane binding (83), and a form of the protein carrying a temperature-sensitive mutation in the targeting signal has been used to show that the membrane-bound precursor is imported in its oligomeric form (83). The API oligomer is a dodecamer of 732 kDa, and this large size does not fit with translocation through a protein channel but would be accommodated by a vesicle-mediated mechanism such as macroautophagy (see below). Similarly, the temperature profiles of the import reaction both in vivo and in an in vitro reconstitution suggest that import does not occur through a proteinaceous channel, since the Cvt pathway is not operational at temperatures at which translocation into the ER and mitochondria occurs normally (144).
To identify components of the machinery involved in the Cvt pathway, Harding et al. used a genetic screen relying on the accumulation of precursor API to identify mutants defective in the cytoplasm to vacuole targeting of API (59). The original cvt mutants fall into eight complementation groups, five of which do not have a significant effect on vacuolar protein sorting through the secretory pathway and, by this criterion, appear to be specific for the Cvt targeting pathway. The cvt screen also identified VPS39 and VPS41 as being involved in the targeting of API, and this observation prompted a systematic screening of vps mutants. The discovery that vps1, vps8, vps15, vps16, vps17, vps18, and vps26 mutants all show defects in API processing as well as in α-mannosidase targeting suggests that there is overlap between the machinery used by the vps pathway taken by CPY and the Cvt pathway.
Degradative Pathway: Starvation-Induced Autophagy
Autophagy was first identified as the transport of cytoplasmic proteins to the vacuole as part of a response to nutrient deprivation in which cells degrade large amounts of intracellular proteins (reviewed in reference 40). Morphological studies have demonstrated that autophagocytosis in mammalian cells is used for bulk, nonselective transport of cytosol and organelle fragments to the lysosome, and it appears that there is also a more selective form of autophagocytic uptake that depends on the presence of a peptide signal and a membrane receptor (33, 38). Microautophagy is the sequestration of small portions of cytosol (including large molecules such as glycogen and also ribosomes) by an invagination of the lysosome membrane, resulting in the formation of membrane-bound intralysosomal vesicles, whose contents are subsequently degraded by lysosomal hydrolases. Macroautophagy is defined as the sequestration of organelles for degradation by the lysosome. Electron microscopy suggests that early autophagosomes are formed from the ER and that these then mature into late autophagosomes and subsequently into autolysosomes.
Under conditions of nitrogen starvation, S. cerevisiae cells degrade nearly half of their total cellular protein content within 24 h, with approximately 80% of this degradation taking place in the vacuole (166). As in mammalian cells, the nonselective component of this process has been demonstrated by monitoring the vacuolar uptake of several cytosolic enzymes under these conditions (42). Autophagy can be monitored morphologically in yeast and is characterized by the appearance of autophagic vesicles (whose contents are indistinguishable from cytosol, again demonstrating the nonselective nature of this uptake) inside vacuoles of protease-deficient strains under starvation conditions (149, 162). Two genetic screens have isolated mutants that fail to accumulate autophagic vesicles during starvation. The apg mutants were isolated through their reduced ability to survive during starvation (168), and the aut mutants were isolated as being defective in the breakdown of the cytosolic enzyme fatty acid synthase, whose degradation during starvation is dependent on vacuolar proteases (167).
Overlap between the Biosynthetic and Degradative Pathways
Autophagy appears to be a slow, nonselective, unsaturable pathway taken by proteins destined for degradation, whereas the Cvt pathway defined by API targeting delivers specific resident proteins to the vacuole in a saturable manner with relatively rapid kinetics. Despite the obvious differences between the two processes, both pathways transport fully synthesized proteins from the cytoplasm into the vacuole, and complementation analysis between the autophagy mutants and the cvt mutants was performed to identify overlap between API targeting and autophagy (58, 145). There is a significant amount of phenotypic and genetic (by using precursor API accumulation in diploids as an indication of noncomplementation) overlap between the two sets of mutants. For example, most of the autophagy mutants are blocked in the maturation of API without affecting the targeting of PrA or CPY. Despite this extensive overlap, a few mutants in each group showed little or no phenotypic overlap, implying that the pathway is only partially shared. These data are consistent with the model that API uptake from the cytoplasm to the vacuole is a selective process (see below), as seen for RNase A in mammalian cells, which requires the presence of a consensus sequence motif, KFERQ, in the protein (39).
Nutrient-Induced Autophagy
Peroxisomes are important for the oxidation of fatty acids and are induced when yeast cells are grown on oleic acid (43). Immunoelectron microscopy studies demonstrate that peroxisomes are transported to the vacuole when cells are shifted to glucose, resulting in destruction of the whole organelle in the vacuole (22). Similarly, other proteins are delivered to the vacuole in response to glucose exposure. For example, plasma membrane proteins such as the galactose and maltose transporters are endocytosed to the vacuole (22, 125). The key regulatory enzyme of the gluconeogenic pathway, fructose-1,6-bisphosphatase (FBPase), is also rapidly degraded when cells grown on poor carbon sources are provided with glucose to prevent a futile cycle of glucose anabolism and catabolism. FBPase has been localized to vacuoles both by immunofluorescence and by subcellular fractionation experiments by Chiang and Schekman under conditions that induce its degradation (23). The same group also found that this degradation requires PEP4 but not subunits of the proteasome, another major degradative site of the cell. In direct contrast to this, Schork et al. found that FBPase degradation does not require PEP4, but instead requires components of the proteasome (143). However, the results of Schork et al. are difficult to reconcile with immunolocalization of FBPase to the vacuolar lumen of glucose-treated pep4Δ yeast cells. Although this controversy awaits resolution (142), it does appear that autophagy can account for at least part of the FBPase degradation observed upon the addition of glucose.
Following glucose replenishment of protease-deficient cells, FBPase displays a punctate staining of the vacuole by indirect immunofluorescence (22). Electron microscopy studies showed that FBPase can be found at sites on the vacuolar membrane where invaginations occur, as well as being associated with vesicles inside the vacuole of protease-deficient strains. These results indicate that the enzyme is taken up into the vacuole by some sort of autophagic mechanism. Mutants that fail to degrade FBPase in response to glucose replenishment were isolated (68). Under such conditions, FBPase is found in the cytosol of most of these vid mutants, but in some vid mutants, it is found in punctate structures within the cytosol, consistent with its sequestration in membrane-bound vesicles (68). Glucose-induced FBPase degradation occurs more slowly at 22°C, and under such conditions it is possible to purify the vesicles that accumulate in the vid mutants from wild-type cells (73). FBPase associates transiently with these 30- to 40-nm vesicles upon glucose addition, suggesting that they represent an intermediate in the FBPase degradation pathway. The FBPase associated with these cytoplasmic vesicles is in a form protected from proteases, consistent with the model that the protein enters these membrane-enclosed structures before being taken up by the vacuole. Fractionation experiments show that these vesicles are distinct from the vacuole, peroxisomes, mitochondria, ER, Golgi, endosomes, and COPI or COPII vesicles. In addition, morphological studies suggest that they are distinct from early or late endosomes, which are 100 to 400 nm in diameter (153), the COPI and COPII vesicles, which are 50 nm in diameter (8, 82), and the post-Golgi vesicles, which are approximately 100 nm (175). These vid vesicles are also a substantially different size from the vesicles associated with API import (86), an observation in agreement with the fact that despite the substantial overlap between the cvt mutants and the apg/aut mutants, there is no overlap between the cvt mutants and the vid mutants. cvt mutants are not blocked in glucose-stimulated FBPase degradation, and vid mutants do not accumulate pro-API, indicating a separation of the Cvt/starvation-induced autophagy and nutrient-stimulated autophagy pathways (reviewed in reference 85).
The identification of many VPS genes as being required for the Cvt pathway as well as for the convergence of the CPY pathway, the ALP pathway, and the Cvt pathway on Vam3p (34) indicates an overlap between these protein-trafficking pathways. Similarly, an overlap between endocytosis and autophagocytosis can be demonstrated in mammalian cells by the appearance of endocytosed material in autophagic vesicles (50). The exact convergence point(s) between these pathways remains unclear. Ultrastructural analysis of yeast cells reveals double-membrane-layered autophagosome-like structures that can be found with their outer membranes in continuity with the vacuolar membrane (3) (Fig. 4). Membrane fusion following uptake of autophagic bodies by the lysosome through macroautophagy could also explain the appearance of single-membrane-surrounded vesicles in the vacuolar lumen (149, 162); therefore, the mechanism by which these membrane enclosed bodies enter the vacuole awaits elucidation.
FIG. 4.
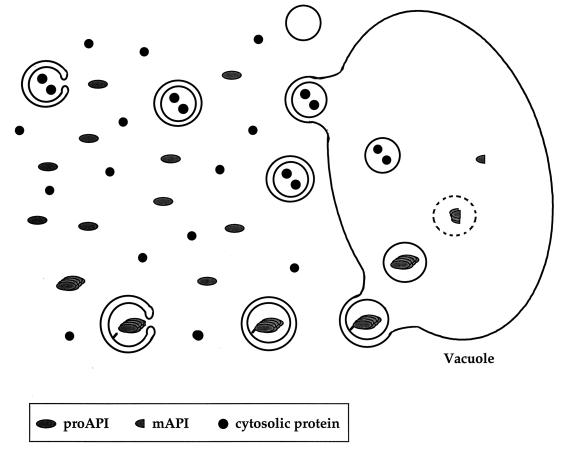
Cytoplasm-to-vacuole targeting. API reaches the vacuole through the Cvt pathway. There is extensive overlap between the Cvt and autophagic pathways to the vacuole (see the text for details). Oligomerized pro-API is sequestered into cytoplasmic vesicles through a saturable, perhaps receptor-mediated process. These vesicles are then taken up by the vacuole via an autophagic mechanism. Breakdown of these vesicles requires PrB, resulting in the release of API into the vacuole lumen and its subsequent maturation.
Models of Aminopeptidase I Import
Direct isolation of precursor API from subvacuolar vesicles indicates that API import involves a vesicular event (85, 144). The extensive overlap between the autophagic and Cvt pathways supports a model whereby API is taken up into a population of vesicles by macroautophagy and these vesicles subsequently fuse with the vacuole (Fig. 4). This model is supported by detection of double-membrane vesicles in the vacuole (3) and also by the observation that cvt1 and cvt17 mutants accumulate pro-API inside vesicles within the vacuole (58, 144). In addition, vps18-ts cells accumulate pro-API in a population of vesicles proposed to be an intermediate in the Cvt pathway (144). Vps18p/Pep3p is a class C Vps protein, localized to the vacuolar membrane and proposed to function in PVC-to-vacuole traffic (121, 131), and these data demonstrate another example of overlap between the Cvt and Vps pathways. The model outlined in Fig. 4 accommodates the large size of the oligomer imported into a membrane-bound compartment and explains the overlap between the two pathways. Since autophagy has been characterized as a slow process, targeting of API via this pathway might somehow concentrate the protein prior to targeting. It is possible that some sort of receptor-mediated macroautophagy increases the efficiency of the process, which would explain why only a small number of cvt mutants is not also defective in autophagy.
Molecular characterization of genes involved in cytoplasm-to-vacuole targeting is in its early stages (99, 140). The power of yeast genetics combined with the classical techniques of cell biology afforded through electron microscopy and biochemical studies should eventually heighten our understanding of the pathways described above.
VACUOLAR INHERITANCE
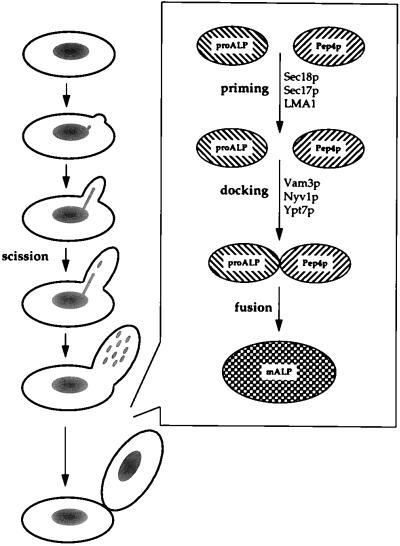
Cells do not synthesize organelles de novo but, instead, build upon material inherited from their mother cell. Cytological studies of mammalian cells have demonstrated vesiculation of nuclear, ER, and Golgi membranes at the beginning of mitosis and subsequent fusion of these vesicles to reconstitute these organelles after cytokinesis (reviewed in references 178 and 179). While not all organelles in S. cerevisiae follow this pattern of inheritance, yeast cells do inherit vacuoles from their mothers through a mechanism that involves the projection of vesicles and tubules, known as segregation structures, from the mother-cell vacuole into the growing daughter cell (124, 182) (Fig. 5). As with the inheritance of other organelles (reviewed in reference 179), vacuolar inheritance is coordinated by the cell cycle. Segregation structures are formed early in the cell cycle (67, 180), and then, sometime after cytokinesis, the vesicles deposited in the growing bud by these segregation structures use a homotypic fusion process to form one or a few larger vacuoles within the daughter cell (28) (Fig. 5).
FIG. 5.
Vacuolar inheritance. Mother cells donate approximately 50% of their vacuolar material to growing buds. Protrusions from the vacuole grow toward the emerging bud, and a scission event leads to the formation of vesicles, which travel into the bud. A homotypic fusion event between these vesicles (consisting of a priming event, docking, and, finally, fusion) forms the vacuole of the daughter cell.
Mutants defective in vacuolar inheritance were isolated initially by microscopic examination of cells that secrete CPY to identify those that lack segregation structures (180, 181). More recently, a fluorescence-activated cell sorter-based screen has been used to isolate cells that are defective in vacuolar inheritance (176). Most of these vac mutants secrete CPY, and many of the class C and D vps mutants lack segregation structures (6, 123), demonstrating that the sorting of proteins from late Golgi to vacuole and the process of vacuolar inheritance are intimately tied. It is apparent that the lack of vacuolar inheritance seen in vac mutants is not due to some gross perturbation of cell division, since many of the vac mutants are normal for the inheritance of other organelles such as the nucleus and mitochondria, an interpretation supported by the observation that the buds of many vac mutants grow to a normal size and have a normal complement of mitochondria and nuclear DNA but little or no vacuolar material (181). While it is clear that the inheritance of different organelles is individually controlled, it is likely that these processes are intimately related and indeed have common mechanisms; for example, the inheritance of many organelles requires actin (reviewed in references 63, 178, and 179).
Cell-Free Assays
A second approach to enhance the understanding of vacuolar inheritance at the molecular level has been undertaken by Wickner and coworkers with the development of cell-free assays for vacuolar inheritance (27, 28, 56). The formation of vacuole-derived tubules and vesicles can be observed in semi-intact cells by using the fluorescein derivative CDCFDA (28). In addition, a biochemical assay has been used to quantify the homotypic fusion of vacuoles isolated from strains carrying a gene disruption in either PEP4 or PHO8, by measuring ALP activity in vitro based on the maturation and activation of the unprocessed form of the vacuolar membrane protein ALP (encoded by PHO8) by the vacuolar protease PrA (Pep4p).
These assays have been used to demonstrate requirements for vacuolar inheritance, and it has been shown that the formation of segregation structures requires energy in the form of ATP and also the proteins encoded by VAC1 and VAC2 (28, 148). Such a link between a vacuolar inheritance phenotype in vivo and the requirement of the morphological cell-free assay for the proteins represented by these mutants is also seen in the biochemical assay. For example, cytosol from a vac5-1 mutant does not support the reaction with vacuoles isolated from wild-type cells, and vacuoles isolated from a vac1-1 mutant will not fuse in the reaction even in the presence of cytosol isolated from wild-type cells (110, 111).
Actin Cytoskeleton
vac15 mutations are allelic to mutations in MYO2 (67). Myo2p is an unconventional myosin (type V), which is required for polarized growth in S. cerevisiae. It has been proposed that Myo2p acts in the transport of small vesicles from the mother cell into a growing bud (53, 79). A mutation in MYO2 that is known to disrupt interactions between Myo2p and actin causes defects in vacuolar inheritance, and mutations in ACT1 that prevent the same interaction cause similar defects (67). These observations have been instrumental in the proposal of a model in which Myo2p serves as a motor for vacuole transport along actin filaments from the mother cell into the bud in a similar fashion to its proposed role in the movement of Golgi-derived secretory vesicles to the cell surface (53). Hill et al. undertook a systematic screening of various actin mutants to investigate the role of the actin cytoskeleton in vacuolar inheritance (67). As well as identifying the requirement of the myosin-actin interaction, they observed that filamentous actin is found in association with vacuolar membranes within the mother cell. Cortical actin is found at the site of bud emergence in unbudded cells, and a portion of the mother vacuole is also found there; it seems that this region of the vacuole travels into the bud as it emerges.
Machinery Involved
As mentioned above, there is significant overlap between the vac mutants and the vps mutants. Interestingly, vac2-1 mutants do not secrete soluble vacuolar hydrolases but are clearly unable to partition maternal vacuolar material into the emerging bud. Fluorescence microscopy studies indicate that vac2-1 cells are unable to initiate early steps in segregation structure formation, and it has been postulated that the mutation interferes with a cytoskeletally based transport system (148). However, it seems likely that the role of VAC2 in inheritance is also required at later stages, since vacuoles prepared from vac2-1 mutants are defective in the homotypic fusion assay (56).
Another VAC gene proposed to function early in the inheritance pathway is VAC8. VAC8 encodes a protein with homology to armadillo in Drosophila (and β-catenin in mammalian cells), which is found in cell adherence junctions. vac8 mutants show vacuolar inheritance defects similar to those of myo2 mutants. The buds receive very little vacuolar material from the mother, and vacuoles fail to migrate to the bud (14a). The removal of palmitoylation sites from the protein abolishes its function in vacuolar inheritance, and it has been proposed that the protein, which has been immunolocalized to the vacuolar membrane, may attach the vacuole to the bud site during cell division. Consistent with a role in the early stages of inheritance, anti-Vac8p antibodies do not inhibit the vacuolar fusion assay.
Mutations in VAC1 are allelic to vps19 mutations (183). VPS19 is a class D vps gene, which encodes a ring-finger-motif-containing protein that has been proposed to act as part of a Sec18p-dependent complex in Golgi-to-PVC traffic (18). Like other vac mutants that missort CPY, vac1 mutant cells show only a partial defect in vacuolar inheritance, in that they have a normal-size vacuole before they bud even though they inherit little vacuolar material from their mother (183). Whether the role of VAC1 in inheritance is related to its role in Golgi-to-vacuole transport or whether Vac1p is independently involved in protein sorting and vacuolar inheritance remains to be elucidated.
One subset of vac mutants seems to be defective in vacuole membrane scission (fusion of the internal faces of the vacuole membrane) (14a). Instead of a multilobed vacuole, these mutants contain a single, extremely large vacuole. Populations of these mutants contain cells in which the vacuole does extend into the bud, but even in the narrow confines of the bud neck there is a clear gap between opposing vacuolar membranes due to a lack of scission (it is worth noting that other mutants that have large vacuoles, such as class D vps mutants, do not form these “open figure eights”). Three mutants of this type have been identified to date: mutants with mutations in vac7-1, vac14-1, and fab1-2. Fab1p has significant homology to mammalian PI4 kinase, and since PI kinases have been implicated in other membrane fusion events (see above), it is tempting to draw analogies between vacuolar scission and bud formation. Indeed, since the scission event involved in the formation of clathrin-coated vesicles in mammalian cells seems to be mediated by dynamin (homologous to Vps1p), one might speculate that a dynamin homolog could be involved in vacuolar scission.
Dissection of Homotypic Vacuolar Fusion
By carefully studying the requirements of the biochemical assay for vacuolar fusion, the reaction was divided into four distinct stages (27). Stage I requires exposure of vacuoles to solutions of moderate ionic strength. It has been proposed that this somehow prepares the vacuoles for stage II, perhaps by removing some inhibitory molecule from the membrane. Stage II requires stage I vacuoles and cytosol. It seems likely that cytosolic factors bind to the vacuolar membrane during this stage, rendering them competent for fusion. Fusion is initiated in stage III and requires ATP, but it does not proceed until stage IV, when vacuoles must be present at or above a minimal concentration for the reaction to proceed. Stage III of the reaction is sensitive to inhibitors of the vacuolar ATPase as well as to proton ionophores, suggesting a requirement for an electrochemical gradient across the vacuolar membrane at this stage. Similarly, GTP-hydrolyzing proteins have been implicated at this stage through the inhibitory effect of GTPγS.
By using an in vitro assay for early endocytic events, it was demonstrated that rab5 is required for homotypic endosome fusion in mammalian cells (51); similarly, it has been demonstrated that Ypt7p functions in homotypic vacuolar fusion in S. cerevisiae. YPT7 was seen as being a prime candidate for the GTPase regulator of vacuolar fusion (whose existence was uncovered by the in vitro assay), since ypt7 cells have a class B vacuolar morphology phenotype in that they possess numerous small vacuoles (186), which may result from a failure of homotypic fusion. With antibodies to Ypt7p, a direct involvement for the small GTPase in the vacuole fusion reaction was demonstrated (57). In addition, purified Sec19p, the yeast homolog of the GDPase inhibitor protein Gdi1p, blocks homotypic fusion but not segregation structure formation, suggesting a role for Ypt7p in vacuolar inheritance (186).
Kinetic studies of the inhibitory affects of anti-Ypt7p antibodies on homotypic fusion suggest that the GTPase acts at the docking stage of the reaction (100), a stage that also requires the vacuolar SNARE proteins Vam3p and Nyv1p (109). The reaction has also been shown to require Sec18p and Sec17p (yeast homologs of the mammalian proteins NSF and α-SNAP [soluble NSF attachment protein]) and a low-molecular-weight heterodimer consisting of a thioredoxin subunit and an inhibitor of proteinase B (189) termed LMA1 (190) prior to the action of Ypt7p (99). According to the SNARE hypothesis, Sec18p is required after vesicles have docked with their acceptor membrane, directed by the binding of a v-SNARE with its cognate t-SNARE. In contrast to this, Sec18p and Sec17p are required in vacuolar homotypic fusion at a predocking stage (100), as determined both biochemically and microscopically. Therefore, it has been proposed that they are required to transiently modify the structure of the SNARE proteins involved in the fusion into a binding-competent state, which is stabilized by LMA1. Consistent with this theory is the finding that both partners must be exposed to Sec17p/Sec18p to render them competent for fusion.
OVERVIEW
Although we are beginning to gain some insight into the molecular mechanisms that underlie the biogenesis of the vacuole in S. cerevisiae by studying the delivery of its proteins through their various pathways, much obviously remains to be learned. Genetic screens that led to the isolation of mutants defective in these various pathways have greatly enhanced our knowledge, and the overlap of mutants identified by these screens has also uncovered much information. For example, the isolation of many class E vps mutants as being defective in endocytosis helped designate the PVC as the point of intersection between the endocytic and Vps pathways. The identification of the vacuolar syntaxin, Vam3p, as being necessary for the delivery of CPS, ALP, and API, which reach the vacuole through different routes, tells us that these pathways converge at or before the vacuole. Interestingly, VPS41 seems to be required for correct targeting of ALP and API but not CPS, perhaps indicating that the routes taken by the first two proteins converge before meeting up with that taken by CPS. However, such overlap is not always as immediately informative; for example, VPS34 was identified as being required for endocytosis, and it is not easy to see how this fits in with its proposed role in vesicle formation at the trans-Golgi. Such a block could arise from the lack of delivery of some component essential for endocytosis to the PVC or the vacuole, or VPS34 could function at more than one trafficking step. It should be possible to distinguish between such models through the use of conditional alleles, which allow the determination of which phenotypes arise as a direct effect of the loss of gene function.
ACKNOWLEDGMENTS
We thank Lois Weisman and Dan Klionsky for helpful discussions and for sharing data prior to publication. Members of the Stevens laboratory are also thanked for their input into this review.
Support from NIH (GM32448 and GM38006 to T.H.S.) is also acknowledged.
REFERENCES
- 1.Ammerer G, Hunter C P, Rothman J H, Saari G C, Valls L A, Stevens T H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986;6:2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artalejo C R, Lemmon M A, Schlessinger J, Palfrey H C. Specific role for the PH domain of dynamin-1 in the regulation of rapid endocytosis in adrenal chromaffin cells. EMBO J. 1997;16:1565–1574. doi: 10.1093/emboj/16.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba M, Takeshige K, Baba N, Ohsumi Y. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J Cell Biol. 1994;124:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babst M, Sato T K, Banta L M, Emr S D. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bankaitis V A, Johnson L M, Emr S D. Isolation of yeast mutants defective in protein targeting to the vacuole. Proc Natl Acad Sci USA. 1986;83:9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banta L M, Robinson J S, Klionsky D J, Emr S D. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol. 1988;107:1369–1383. doi: 10.1083/jcb.107.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becherer K A, Rieder S E, Emr S D, Jones E W. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bednarek S Y, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- 9.Bender A, Sprague G F., Jr Pheromones and pheromone receptors are the primary determinants of mating specificity in the yeast Saccharomyces cerevisiae. Genetics. 1989;121:463–476. doi: 10.1093/genetics/121.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetti H, Raths S, Crausaz F, Riezman H. The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol Biol Cell. 1994;5:1023–1037. doi: 10.1091/mbc.5.9.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benito B, Moreno E, Lagunas R. Half-life of the plasma membrane ATPase and its activating system in resting yeast cells. Biochim Biophys Acta. 1991;1063:265–268. doi: 10.1016/0005-2736(91)90381-h. [DOI] [PubMed] [Google Scholar]
- 12.Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of alpha-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- 13.Benmerah A, Gagnon J, Begue B, Megarbane B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131:1831–1838. doi: 10.1083/jcb.131.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkower C, Loayza D, Michaelis S. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. Mol Biol Cell. 1994;5:1185–1198. doi: 10.1091/mbc.5.11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Bonangelino C J, Catlett N L, Weisman L S. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant N J, Boyd A. Immunoisolation of Kex2p-containing organelles from yeast demonstrates colocalisation of three processing proteinases to a single Golgi compartment. J Cell Sci. 1993;106:815–822. doi: 10.1242/jcs.106.3.815. [DOI] [PubMed] [Google Scholar]
- 16.Bryant N J, Stevens T H. Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J Cell Biol. 1997;136:287–297. doi: 10.1083/jcb.136.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucci C, Parton R G, Mather I H, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 18.Burd C G, Peterson M, Cowles C R, Emr S D. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cereghino J L, Marcusson E G, Emr S D. The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol Biol Cell. 1995;6:1089–1102. doi: 10.1091/mbc.6.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y H, Smith J A. Molecular cloning and sequencing of genomic DNA encoding aminopeptidase I from Saccharomyces cerevisiae. J Biol Chem. 1989;264:6979–6983. [PubMed] [Google Scholar]
- 21.Chavrier P, Parton R G, Hauri H P, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 22.Chiang H-L, Schekman R, Hamamoto Selective uptake of cytosolic, peroxisomal, and plasma membrane proteins into the yeast lysosome for degradation. J Biol Chem. 1996;271:9934–9941. doi: 10.1074/jbc.271.17.9934. [DOI] [PubMed] [Google Scholar]
- 23.Chiang H L, Schekman R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature. 1991;350:313–318. doi: 10.1038/350313a0. [DOI] [PubMed] [Google Scholar]
- 24.Chvatchko Y, Howald I, Riezman H. Two yeast mutants defective in endocytosis are defective in pheromone response. Cell. 1986;46:355–364. doi: 10.1016/0092-8674(86)90656-2. [DOI] [PubMed] [Google Scholar]
- 25.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 26.Conibear E, Stevens T H. Vacuolar biogenesis in yeast: sorting out the sorting proteins. Cell. 1995;83:513–516. doi: 10.1016/0092-8674(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 27.Conradt B, Haas A, Wickner W. Determination of four biochemically distinct, sequential stages during vacuole inheritance in vitro. J Cell Biol. 1994;126:99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper A A, Stevens T H. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cowles C R, Emr S D, Horazdovsky B F. Mutations in the VPS45 gene, a SEC1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J Cell Sci. 1994;107:3449–3459. doi: 10.1242/jcs.107.12.3449. [DOI] [PubMed] [Google Scholar]
- 31.Cowles C R, Odorizzi G, Payne G S, Emr S D. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 32.Cowles C R, Snyder W B, Burd C G, Emr S D. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuervo A M, Dice J F. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 34.Darsow T, Rieder S E, Emr S D. A multi-specific syntaxin homologue, vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis N G, Horecka J L, Sprague G F., Jr cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell’Angelica E C, Ohno H, Ooi C E, Rabinovich E, Roche K W, Bonifacino J S. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dell’Angelica E C, Ooi C E, Bonifacino J S. Beta3A-adaptin, a subunit of the adaptor-like complex AP-3. J Biol Chem. 1997;272:15078–15084. doi: 10.1074/jbc.272.24.15078. [DOI] [PubMed] [Google Scholar]
- 38.Dice J F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 39.Dice J F. Selective degradation of cytosolic proteins by lysosomes. Ann N Y Acad Sci. 1992;31:58–64. doi: 10.1111/j.1749-6632.1992.tb27477.x. [DOI] [PubMed] [Google Scholar]
- 40.Dunn W J. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 41.Egner R, Kuchler K. The yeast multidrug transporter Pdr5 of the plasma membrane is ubiquitinated prior to endocytosis and degradation in the vacuole. FEBS Lett. 1996;378:177–181. doi: 10.1016/0014-5793(95)01450-0. [DOI] [PubMed] [Google Scholar]
- 42.Egner R, Thumm M, Straub M, Simeon A, Schuller H J, Wolf D H. Tracing intracellular proteolytic pathways. Proteolysis of fatty acid synthase and other cytoplasmic proteins in the yeast Saccharomyces cerevisiae. J Biol Chem. 1993;268:27269–27276. [PubMed] [Google Scholar]
- 43.Erdmann R, Veenhuis M, Mertens D, Kunau W H. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1989;86:5419–5423. doi: 10.1073/pnas.86.14.5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 45.Fischer von Mollard G, Nothwher S F, Stevens T H. The yeast v-SNARE Vti1p mediates two vesicles transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Futter C E, Pearse A, Hewlett L J, Hopkins C R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galan J M, Moreau V, Andre B, Volland C, Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J Biol Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 48.Galan J M, Volland C, Urban-Grimal D, Haguenauer-Tsapis R. The yeast plasma membrane uracil permease is stabilized against stress induced degradation by a point mutation in a cyclin-like “destruction box.”. EMBO J. 1994;13:3261–3271. doi: 10.1006/bbrc.1994.1767. [DOI] [PubMed] [Google Scholar]
- 49.Galcheva-Gargova Z, Theroux S J, Davis R J. The epidermal growth factor receptor is covalently linked to ubiquitin. Oncogene. 1995;11:2649–2655. [PubMed] [Google Scholar]
- 50.Gordon P B, Hoyvik H, Seglen P O. Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem J. 1992;283:361–369. doi: 10.1042/bj2830361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorvel J P, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham T R, Emr S D. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haarer B K, Petzold A, Lillie S H, Brown S S. Identification of MYO4, a second class V myosin gene in yeast. J Cell Sci. 1994;107:1055–1064. doi: 10.1242/jcs.107.4.1055. [DOI] [PubMed] [Google Scholar]
- 56.Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harding T M, Hefner-Gravink A, Thumm M, Klionsky D J. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole targeting pathway. J Biol Chem. 1996;271:17621–17624. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- 59.Harding T M, Morano K A, Scott S V, Klionsky D J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardwick K G, Pelham H R. SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris S L, Waters M G. Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. Cell. 1996;132:985–998. doi: 10.1083/jcb.132.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hermann G J, King E J, Shaw J M. The yeast gene, MDM20, is necessary for mitochondrial inheritance and organization of the actin cytoskeleton. J Cell Biol. 1997;137:141–153. doi: 10.1083/jcb.137.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herskowitz I. A regulatory hierarchy for cell specialization in yeast. Nature. 1989;342:749–757. doi: 10.1038/342749a0. [DOI] [PubMed] [Google Scholar]
- 65.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;271:10946–10952. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 66.Hill K J, Stevens T H. Vma22p is a novel endoplasmic reticulum-associated protein required for assembly of the yeast vacuolar H(+)-ATPase complex. J Biol Chem. 1995;270:22329–22336. doi: 10.1074/jbc.270.38.22329. [DOI] [PubMed] [Google Scholar]
- 67.Hill K L, Catlett N L, Weisman L S. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffman M, Chiang H-L. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics. 1996;143:1555–1566. doi: 10.1093/genetics/143.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holtzman D A, Yang S, Drubin D G. Synthetic-lethal interactions identify two novel genes, SLA1 and SLA2, that control membrane cytoskeleton assembly in Saccharomyces cerevisiae. J Cell Biol. 1993;122:635–644. doi: 10.1083/jcb.122.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong E, Davidson A R, Kaiser C A. A pathway for targeting soluble misfolded proteins to the yeast vacuole. J Cell Biol. 1996;135:623–633. doi: 10.1083/jcb.135.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hopkins C R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- 72.Horazdovsky B F, DeWald D B, Emr S D. Protein transport to the yeast vacuole. Curr Opin Cell Biol. 1995;7:544–551. doi: 10.1016/0955-0674(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 73.Huang P-H, Chiang H-L. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J Cell Biol. 1997;136:803–810. doi: 10.1083/jcb.136.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson C L, Hartwell L H. Courtship in S. cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell. 1990;63:1039–1051. doi: 10.1016/0092-8674(90)90507-b. [DOI] [PubMed] [Google Scholar]
- 75.Jansen R P, Dowzer C, Michaelis C, Galova M, Nasmyth K. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin myo4p and other cytoplasmic proteins. Cell. 1996;84:687–697. doi: 10.1016/s0092-8674(00)81047-8. [DOI] [PubMed] [Google Scholar]
- 76.Jedd G, Richardson C, Litt R, Segev N. The Ypt1 GTPase is essential for the first two steps of the yeast secretory pathway. J Cell Biol. 1995;131:583–590. doi: 10.1083/jcb.131.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenness D D, Spatrick P. Down regulation of the alpha-factor pheromone receptor in S. cerevisiae. Cell. 1986;46:345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- 78.Johnson L M, Bankaitis V A, Emr S D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987;48:875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- 79.Johnston G C, Prendergast J A, Singer R A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jones E W. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977;85:23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones E W, Webb G C, Hiller M A. Molecular biology of the yeast Saccharomyces cerevisiae. III. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 363–469. [Google Scholar]
- 82.Kaiser C A, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- 83.Kim J, Scott S V, Oda M N, Klionsky D J. Transport of a large oligomeric protein by the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1997;137:609–618. doi: 10.1083/jcb.137.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Klionsky D J, Herman P K, Emr S D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990;54:266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klionsky D J. Protein transport from the cytoplasm into the vacuole. J Membr Biol. 1997;157:105–115. doi: 10.1007/s002329900220. [DOI] [PubMed] [Google Scholar]
- 86.Klionsky, D. J. Personal communication.
- 87.Klionsky D J, Cueva R, Yaver D S. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolling R, Hollenberg C P. The ABC-transporter Ste6 accumulates in the plasma membrane in a ubiquitinated form in endocytosis mutants. Mol Biol Cell. 1994;5:1185–1198. doi: 10.1002/j.1460-2075.1994.tb06627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolling R, Losko S. The linker region of the ABC-transporter Ste6 mediates ubiquitination and fast turnover of the protein. EMBO J. 1997;16:2251–2261. doi: 10.1093/emboj/16.9.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 91.Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubler E, Schimmoller F, Riezman H. Calcium-independent calmodulin requirement for endocytosis in yeast. J Cell Sci. 1994;13:5539–5546. doi: 10.1002/j.1460-2075.1994.tb06891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuranda M J, Robbins P W. Cloning and heterologous expression of glycosidase genes from Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:2585–2589. doi: 10.1073/pnas.84.9.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai K, Bolognese C P, Swift S, McGraw P. Regulation of inositol transport in Saccharomyces cerevisiae involves inositol-induced changes in permease stability and endocytic degradation in the vacuole. J Biol Chem. 1995;270:2525–2534. doi: 10.1074/jbc.270.6.2525. [DOI] [PubMed] [Google Scholar]
- 95.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 96.Lappalainen P, Drubin D G. Cofilin promotes rapid actin filament turnover in vivo. Nature. 1997;388:78–82. doi: 10.1038/40418. [DOI] [PubMed] [Google Scholar]
- 97.Liu H P, Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989;57:233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- 98.Marcusson E G, Horazdovsky B F, Cereghino J L, Gharakhanian E, Emr S D. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 99.Matsuura A, Tsukada M, Wada Y, Ohsumi Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene. 1997;192:245–250. doi: 10.1016/s0378-1119(97)00084-x. [DOI] [PubMed] [Google Scholar]
- 100.Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 102.Metz G, Rohm K H. Yeast aminopeptidase I. Chemical composition and catalytic properties. Biochim Biophys Acta. 1976;429:933–949. doi: 10.1016/0005-2744(76)90338-7. [DOI] [PubMed] [Google Scholar]
- 103.Moehle C M, Dixon C K, Jones E W. Processing pathway for protease B of Saccharomyces cerevisiae. J Cell Biol. 1989;108:309–325. doi: 10.1083/jcb.108.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mori S, Heldin C H, Claesson-Welsh L. Ligand-induced polyubiquitination of the platelet-derived growth factor beta-receptor. J Biol Chem. 1992;267:6429–6434. [PubMed] [Google Scholar]
- 105.Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Munn A L, Riezman H. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Munn A L, Stevenson B J, Geli M I, Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nebes V L, Jones E W. Activation of the proteinase B precursor of the yeast Saccharomyces cerevisiae by autocatalysis and by an internal sequence. J Biol Chem. 1991;266:22851–22857. [PubMed] [Google Scholar]
- 109.Nichols B J, Ungermann C, Pelham H R, Wickner W T, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 110.Nicolson T, Conradt B, Wickner W. A truncated form of the Pho80 cyclin of Saccharomyces cerevisiae induces expression of a small cytosolic factor which inhibits vacuole inheritance. J Bacteriol. 1996;178:4047–4051. doi: 10.1128/jb.178.14.4047-4051.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nicolson T A, Weisman L S, Payne G S, Wickner W T. A truncated form of the Pho80 cyclin redirects the Pho85 kinase to disrupt vacuole inheritance in S. cerevisiae. J Cell Biol. 1995;130:835–845. doi: 10.1083/jcb.130.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nothwehr S F, Conibear E, Stevens T H. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nothwehr S F, Stevens T H. Sorting of membrane proteins in the yeast secretory pathway. J Biol Chem. 1994;269:10185–10188. [PubMed] [Google Scholar]
- 114.Oda M N, Scott S V, Hefner-Gravink A, Caffarelli A D, Klionsky D J. Identification of a cytoplasm to vacuole targeting determinant in aminopeptidase I. J Cell Biol. 1996;132:999–1010. doi: 10.1083/jcb.132.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panek H R, Stepp J D, Engle H M, Marks K M, Tan P K, Lemmon S K, Robinson L C. Suppressors of YCK-encoded yeast casein kinase 1 deficiency define the four subunits of a novel clathrin AP-like complex. EMBO J. 1997;16:4194–4204. doi: 10.1093/emboj/16.14.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pearse B M, Robinson M S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 117.Piper R C, Bryant N J, Stevens T H. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Piper R C, Cooper A A, Yang H, Stevens T H. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Piper R C, Whitters E A, Stevens T H. Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur J Cell Biol. 1994;65:305–318. [PubMed] [Google Scholar]
- 120.Plutner H, Cox A D, Pind S, Khosravi-Far R, Bourne J R, Schwaninger R, Der C J, Balch W E. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Preston R A, Manolson M F, Becherer K, Weidenhammer E, Kirkpatrick D, Wright R, Jones E W. Isolation and characterization of PEP3, a gene required for vacuolar biogenesis in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5801–5812. doi: 10.1128/mcb.11.12.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raymond C K, Howald-Stevenson I, Vater C A, Stevens T H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Raymond C K, O’Hara P J, Eichinger G, Rothman J H, Stevens T H. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990;111:877–892. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riballo E, Herweijer M, Wolf D H, Lagunas R. Catabolite inactivation of the yeast maltose transporter occurs in the vacuole after internalization by endocytosis. J Bacteriol. 1995;177:5622–5677. doi: 10.1128/jb.177.19.5622-5627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rieder S E, Emr S D. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2111–2118. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rieder S E, Banta L M, Kohrer K, McCaffery J M, Emr S D. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985;40:1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- 129.Riezman H, Munn A, Geli M I, Hicke L. Actin-, myosin- and ubiquitin-dependent endocytosis. Experientia. 1996;52:1033–1041. doi: 10.1007/BF01952099. [DOI] [PubMed] [Google Scholar]
- 130.Roberts C J, Nothwehr S F, Stevens T H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992;119:69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson J S, Graham T R, Emr S D. A putative zinc finger protein, Saccharomyces cerevisiae Vps18p, affects late Golgi functions required for vacuolar protein sorting and efficient alpha-factor prohormone maturation. Mol Cell Biol. 1991;11:5813–5824. doi: 10.1128/mcb.11.12.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robinson M S. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- 133.Rohrer J, Benedetti H, Zanolari B, Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled alpha-pheromone receptor in yeast. Mol Biol Cell. 1993;4:511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roth A F, Davis N G. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rothman J E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 136.Rothman J H, Howald I, Stevens T H. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989;8:2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rothman J H, Stevens T H. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- 138.Schandel K A, Jenness D D. Direct evidence for ligand-induced internalization of the yeast alpha-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schimmoller F, Riezman H, Wichmann H, Hengst L, Gallwitz D. Involvement of Ypt7p, a small GTPase, in traffic from late endosome to the vacuole in yeast. J Cell Sci. 1993;106:823–830. doi: 10.1242/jcs.106.3.823. [DOI] [PubMed] [Google Scholar]
- 140.Schlumpberger M, Schaeffeler E, Straub M, Bredschneider M, Wolf D H, Thumm M. AUT1, a gene essential for autophagocytosis in the yeast Saccharomyces cerevisiae. J Bacteriol. 1997;179:1068–1076. doi: 10.1128/jb.179.4.1068-1076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 142.Schork S M, Bee G, Thumm M, Wolf D H. Site of catabolite inactivation. FEBS Lett. 1994;369:283–284. doi: 10.1038/369283a0. [DOI] [PubMed] [Google Scholar]
- 143.Schork S M, Thumm M, Wolf D H. Catabolite inactivation of fructose-1,6-bisphosphatase of Saccharomyces cerevisiae. Degradation occurs via the ubiquitin pathway. J Biol Chem. 1995;270:26446–26450. doi: 10.1074/jbc.270.44.26446. [DOI] [PubMed] [Google Scholar]
- 144.Scott S V, Baba M, Ohsumi Y, Klionsky D J. Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism. J Cell Biol. 1997;138:37–44. doi: 10.1083/jcb.138.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scott S V, Hefner-Gravink A, Morano K A, Noda T, Ohsumi Y, Klionsky D J. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc Natl Acad Sci USA. 1996;93:12304–12308. doi: 10.1073/pnas.93.22.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seeger M, Payne G S. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992;11:2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seeger M, Payne G S. Selective and immediate effects of clathrin heavy chain mutations on Golgi membrane protein retention in Saccharomyces cerevisiae. J Cell Biol. 1992;118:531–540. doi: 10.1083/jcb.118.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shaw J M, Wickner W T. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. EMBO J. 1991;10:1741–1748. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simeon A, van der Klei I J, Veenhuis M, Wolf D H. Ubiquitin, a central component of selective cytoplasmic proteolysis, is linked to proteins residing at the locus of non-selective proteolysis, the vacuole. FEBS Lett. 1992;301:231–235. doi: 10.1016/0014-5793(92)81254-j. [DOI] [PubMed] [Google Scholar]
- 150.Simpson F, Bright N A, West M A, Newman L S, Darnell R B, Robinson M S. A novel adaptor-related protein complex. J Cell Biol. 1996;133:749–760. doi: 10.1083/jcb.133.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Simpson F, Peden A A, Christopoulou L, Robinson M S. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Singer B, Riezman H. Detection of an intermediate compartment involved in transport of alpha-factor from the plasma membrane to the vacuole in yeast. J Cell Biol. 1990;110:1911–1922. doi: 10.1083/jcb.110.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singer-Kruger B, Frank R, Crausaz F, Riezman H. Partial purification and characterization of early and late endosomes from yeast. Identification of four novel proteins. J Biol Chem. 1993;268:14376–14386. [PubMed] [Google Scholar]
- 154.Singer-Kruger B, Stenmark H, Dusterhoft A, Philippsen P, Yoo J S, Gallwitz D, Zerial M. Role of three rab5-like GTPases, Ypt51p, Ypt52p, and Ypt53p, in the endocytic and vacuolar protein sorting pathways of yeast. J Cell Biol. 1994;125:283–298. doi: 10.1083/jcb.125.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smythe E, Carter L L, Schmid S L. Cytosol- and clathrin-dependent stimulation of endocytosis in vitro by purified adaptors. J Cell Biol. 1992;119:1163–1171. doi: 10.1083/jcb.119.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sollner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 157.Stack J H, Emr S D. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J Biol Chem. 1994;269:31552–31562. [PubMed] [Google Scholar]
- 158.Stack J H, Horazdovsky B, Emr S D. Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- 159.Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- 160.Stevens T H, Rothman J H, Payne G S, Schekman R. Gene dosage-dependent secretion of yeast vacuolar carboxypeptidase Y. J Cell Biol. 1986;102:1551–1557. doi: 10.1083/jcb.102.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Strous G J, van Kerkhof P, Govers R, Ciechanover A, Schwartz A L. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO J. 1996;15:3806–3812. [PMC free article] [PubMed] [Google Scholar]
- 162.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tan P K, Davis N G, Sprague G F, Payne G S. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tang H-Y, Munn A, Cai M. EH domain proteins Pan1p and End3p are components of a complex that plays a dual role in organization of the cortical actin cytoskeleton and endocytosis in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4294–4304. doi: 10.1128/mcb.17.8.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tang H Y, Cai M. The EH-domain-containing protein Pan1 is required for normal organization of the actin cytoskeleton in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4897–4914. doi: 10.1128/mcb.16.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Teichert U, Mechler B, Muller H, Wolf D H. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J Biol Chem. 1989;264:16037–16045. [PubMed] [Google Scholar]
- 167.Thumm M, Egner R, Koch B, Schlumpberger M, Straub M, Veenhuis M, Wolf D H. Isolation of autophagocytosis mutants of Saccharomyces cerevisiae. FEBS Lett. 1994;349:275–280. doi: 10.1016/0014-5793(94)00672-5. [DOI] [PubMed] [Google Scholar]
- 168.Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- 169.Valls L A, Hunter C P, Rothman J H, Stevens T H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987;48:887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- 170.Valls L A, Winther J R, Stevens T H. Yeast carboxypeptidase Y vacuolar targeting signal is defined by four propeptide amino acids. J Cell Biol. 1990;111:361–368. doi: 10.1083/jcb.111.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vida T A, Emr S D. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vida T A, Huyer G, Emr S D. Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J Cell Biol. 1993;121:1245–1256. doi: 10.1083/jcb.121.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Volland C, Urban-Grimal D, Geraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- 174.Wada Y, Nakamura N, Ohsumi Y, Hirata A. Vam3p, a new member of syntaxin related protein, is required for vacuolar assembly in the yeast Saccharomyces cerevisiae. J Cell Sci. 1997;110:1229–1306. doi: 10.1242/jcs.110.11.1299. [DOI] [PubMed] [Google Scholar]
- 175.Walworth N C, Novick P J. Purification and characterization of constitutive secretory vesicles from yeast. J Cell Biol. 1987;105:163–174. doi: 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y X, Zhao H, Harding T M, Gomes de Mesquita D S, Woldringh C L, Klionsky D J, Munn A L, Weisman L S. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol Biol Cell. 1996;7:1375–1389. doi: 10.1091/mbc.7.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Warnock D E, Schmid S L. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 178.Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- 179.Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- 180.Weisman L S, Bacallao R, Wickner W. Multiple methods of visualizing the yeast vacuole permit evaluation of its morphology and inheritance during the cell cycle. J Cell Biol. 1987;105:1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weisman L S, Emr S D, Wickner W T. Mutants of Saccharomyces cerevisiae that block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad Sci USA. 1990;87:1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weisman L S, Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988;241:589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- 183.Weisman L S, Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J Biol Chem. 1992;67:618–623. [PubMed] [Google Scholar]
- 184.Wendland B, McCaffery J M, Xiao Q, Emr S D. A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15. J Cell Biol. 1996;135:1485–1500. doi: 10.1083/jcb.135.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Westphal V, Marcusson E G, Winther J R, Emr S D, van den Hazel H B. Multiple pathways for vacuolar sorting of yeast proteinase A. J Biol Chem. 1996;271:11865–11870. doi: 10.1074/jbc.271.20.11865. [DOI] [PubMed] [Google Scholar]
- 186.Wichmann H, Hengst L, Gallwitz D. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p) Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- 187.Wilcox C A, Redding K, Wright R, Fuller R S. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992;3:1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Woolford C A, Daniels L B, Park F J, Jones E W, Van Arsdell J N, Innis M A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986;6:2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu Z, Mayer A, Muller E, Wickner W. A heterodimer of thioredoxin and I(B)2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yoshihisa T, Anraku Y. Nucleotide sequence of AMS1, the structure gene of vacuolar alpha-mannosidase of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989;163:908–915. doi: 10.1016/0006-291x(89)92308-5. [DOI] [PubMed] [Google Scholar]
- 192.Yoshihisa T, Anraku Y. A novel pathway of import of alpha-mannosidase, a marker enzyme of vacuolar membrane, in Saccharomyces cerevisiae. J Biol Chem. 1990;265:22418–22425. [PubMed] [Google Scholar]
- 193.Zerial M, Stenmark H. Rab GTPases in vesicular transport. Curr Opin Cell Biol. 1993;5:613–620. doi: 10.1016/0955-0674(93)90130-i. [DOI] [PubMed] [Google Scholar]