Abstract
Diabetic retinopathy (DR) is the most common microangiopathic complication of diabetes mellitus, representing a major cause of visual impairment in developed countries. Proliferative DR (PDR) represents the last stage of this extremely complex retinal disease, characterized by the development of neovascularization induced by the abnormal production and release of vascular endothelial growth factor (VEGF). The term VEGF includes different isoforms; VEGF-A represents one of the most important pathogenic factors of DR. Anti-VEGF intravitreal therapies radically changed the outcome of DR, due to combined anti-angiogenic and anti-edematous activities. Nowadays, several anti-VEGF molecules exist, characterized by different pharmacological features and duration. With respect to PDR, although anti-VEGF treatments represented a fundamental step forward in the management of this dramatic complication, a big debate is present in the literature regarding the role of anti-VEGF as substitute of panretinal photocoagulation or if these two approaches may be used in combination. In the present review, we provided an update on VEGF isoforms and their role in DR pathogenesis, on current anti-VEGF molecules and emerging new drugs, and on the current management strategies of PDR. There is an overall agreement regarding the relative advantage provided by anti-VEGF, especially looking at the management of PDR patients requiring vitrectomy, with respect to laser. Based on the current data, laser approaches might be avoided when a perfectly planned anti-VEGF therapeutic strategy can be adopted. Conversely, laser treatment may have a role for those patients unable to guarantee enough compliance to anti-VEGF injections.
Key messages
VEGF increased production, stimulated by retinal hypoperfusion and ischaemia, is a major pathogenic factor of neovascular complication onset in diabetic retinopathy and of DR stages progression.
Nowadays, several anti-VEGF molecules are available in clinical practice and other molecules are currently under investigation. Each anti-VEGF molecule is characterized by different targets and may interact with multiple biochemical pathways within the eye.
All the data agreed in considering anti-VEGF molecules as a first line choice for the management of diabetic retinopathy. Laser treatments may have a role in selected advanced cases and for those patients unable to guarantee enough compliance to intravitreal treatments schemes.
Keywords: Diabetic retinopathy, NPDR, PDR, neovascularization, VEGF, anti-VEGF, intravitreal injection, panretinal photocoagulation
Introduction
Vascular endothelial growth factor (VEGF) is one of the most important mediators involved in the pathogenesis of diabetic retinopathy (DR). The abnormal production and release of VEGF induces vascular endothelial cell proliferation and migration and increased vascular permeability [1]. VEGF is involved in the pathogenesis of DR-related complications, such as diabetic macular edoema (DME); furthermore, it represents a fundamental mediator for the development of retinal neovascularization leading to the possible onset of vitreous haemorrhage and tractional retinal detachment [2]. The term VEGF includes different isoforms, namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F and PGF (placental growth factor), the first one representing one of the most important pathogenic factors of DR. The introduction of anti-VEGF intravitreal therapies radically changed the course of DR and patients’ outcome, having a remarkable impact on the incidence of legal blindness. Nowadays, several anti-VEGF molecules exist, acting on different metabolic pathways and VEGF isoforms. The usage of anti-VEGF as first line treatment provided undoubted benefits for the management of DME; on the other side, the treatment of the proliferative form is quite controversial. Indeed, a big debate is still present in the literature regarding the role of laser approaches, administered through different strategies (prompt, deferred, etc.) in the anti-VEGF era.
In the present review, we provided an overall description of VEGF-targeting drugs and the acting mechanisms against the development of retinal neovascularization in DR.
Methods
We used keywords to explore all English language human subject articles in the MEDLINE library. The keywords included the following: diabetic retinopathy, exudation, diabetic macular edoema, vascular endothelial growth factor, VEGF, anti-VEGF, intravitreal injections, neovascularization, ischaemia. All the references were carefully examined by two expert researchers (FB, AA), who collated and arranged all the relevant information, bearing in mind this review’s main theme as expressed in the manuscript title.
Vascular endothelial growth factor: biochemical properties and mechanisms of action
VEGF, also known as vascular permeability factor, was firstly described as an endothelial cell-specific mitogen [3]. This group of molecules belong to the cystine-knot superfamily of hormones and extracellular signalling molecules, covering several functions in vertebrates [4]. VEGF is a dimeric glycoprotein of ∼40 kDa and is fundamental in promoting growth metabolic cascades and angiogenesis during the development of the vertebrate retina [5,6]. VEGF family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PGF molecules. These mediators originate from the alternative splicing of a common source molecule and are further characterized by different isoforms [7]. Alternative splicing of the human VEGF-A gene provides at least six different isoforms, namely 121, 145, 165, 183, 189, and 206 [8]. VEGF-A121 and VEGF-A165 represent the most expressed forms in mammalians. With respect to VEGF-B, at least two isoforms are known, namely 167 and 186 [9]. On the other side, although largely studied in mouse models, less is known about the VEGF-C and VEGF-D isoforms [10–12]. VEGF-E is a molecule of ∼20 kDa, identified in the genome of Orf virus, a parapoxvirus that affects occasionally humans, generating lesions with angiogenesis [13], whereas VEGF-F is a toxin identified in the snake venom, having several similarities with the other VEGF isoforms [14].
All VEGF isoforms bind to different types of VEGF tyrosine kinase trans-membrane receptors; VEGFR-1/Flt-1 (fms-like tyrosine kinase) and VEGFR-2/KDR/Flk-1 (kinase insert domain containing receptor/fetal liver kinase) are mainly associated with angiogenesis [15], whereas Flt-3/Flk-2 and VEGFR-3/Flt-4 are involved in haematopoiesis and lymphangiogenesis [16].
VEGF-A isoforms exert the most powerful angiogenetic activity, also increasing vascular permeability, resulting in leakage of proteins and other molecules towards the blood vessels [17]. Furthermore, previous findings support the involvement of VEGF-A in neurotrophic and neuroprotective activities [18,19]. The angiogenic role of VEGF-B is quite unclear; this isoform seems to act more as a regulator of cells homeostasis and survival than as a active pro-angiogenic factor [20]. Conversely, VEGF-C has been found to play a major role in the lymphangiogenesis [21], although this isoform can also stimulate the growth of blood vessels [22]. Similarly, VEGF-D showed both angiogenic and lymphangiogenic activities [23,24].
VEGF is considered as the major factor involved in the angiogenesis of the human retina [25], although many other molecules and mediators are involved in angiogenic physiologic processes, turning out to be dysregulated in retinal vascular diseases [26,27]. However, because of the central role of VEGF-A, with respect to the other isoforms, many times researchers and clinicians refer to this subtype as simply VEGF [27].
Overall, different stimuli governed the production and release of VEGF. Its production is up-regulated by different isoforms of hypoxia inducible factor-1 (HIF-1), which are released to promote physiological angiogenesis, whereas other HIF isoforms act more as regulatory mediators of VEGF expression [28]. Moreover, VEGF levels can also be increased by insulin-like growth factor 1 (IGF-1), playing an important role in retinal angiogenesis [29]. In the human retina, VEGF is mainly produced by retinal pigmented epithelium (RPE) cells [30], astrocytes [31], Müller cells [32], endothelium and ganglion cells [33]. In retinal diseases, VEGF release is upregulated by increased hypoxia and oxidative stress [34,35]. Furthermore, the dysregulation of many other mediators is involved in the promotion of VEGF release, including erythropoietin (EPO) [36], angiopoietins 1 and 2 (Ang-1 and Ang-2), Tie2 receptor [15], platelet derived growth factor (PDGF) [37] and advanced glycation end-products [38]. In addition, VEGF isoforms may synergically act to further reinforce the cascades of events leading to VEGF metabolism alterations [39]. On the other hand, VEGF abnormal release is also influenced by the downregulation of inhibitory mediators. For example, neovascularization and exudation processes are enhanced by the alterations of the VEGF/pigment epithelium derived factor (PEDF) ratio. PEDF is more released by the peripheral RPE cells than macular RPE, acting as a major inhibitor of angiogenesis [40,41]. From this point of view, the peripheral ischaemia characterizing several retinal vascular diseases, including DR, might be the basis for VEGF/PEDF ratio dysregulation, thus providing a possible basis to explain why neovascularization and macular edoema mainly occur in the macular region, compared with retinal periphery.
Vascular endothelial growth factor in diabetic retinopathy
DR is the most common microangiopathic complication of diabetes mellitus [2], affecting more than 100 million people worldwide and representing a major cause of visual impairment and legal blindness in developed countries [42]. DR is classically classified as non-proliferative (NPDR) and proliferative (PDR) (Figure 1). NPDR, further categorized in different stages, is characterized by several alterations including intraretinal haemorrhages, hard and soft exudates, cotton-wool spots, microaneurysms, venous calibre abnormalities, intraretinal microvascular anomalies and both macular and peripheral capillary nonperfusion; the onset of neovascularization characterize the progression towards PDR [2]. Most blindness cases are secondary to posterior PDR complications, such as vitreous haemorrhage and retinal detachment, whereas just 5% of blindness cases can be related to the onset of neovascular glaucoma [43]. The pathogenesis of DR is mainly characterized by the combined presence of neurovascular unit impairment, breakdown of the blood retinal barrier (BRB), inflammation, capillary non-perfusion/ischaemia, and neoangiogenesis [44]. All these anomalies lead to the increased production of angiogenic and inflammatory mediators. In this scenario, VEGF is undoubtedly a major pathogenic element characterizing DR and its complication. In DR, VEGF production is induced in response to ischaemic or hypoxic stimuli, causing several alterations at different levels. Indeed, VEGF increases the phosphorylation of tight junctions’ proteins, thus increasing retinal capillary permeability [45]. VEGF-related hyperpermeability is also caused by the induced modifications of several intercellular molecules, such as occludin, catenins and cadherins [46,47], by the increased transcytosis [48] and by the activated NOS-mediated mechanisms [49]. All these VEGF-related phenomena, mainly governed by VEGF-A isoforms, in combination with an extremely complex cascade of other events and mediators, lead to the onset and progression of a frequent DR-related complication, namely DME [50]. Furthermore, although few studies have been conducted, there are even growing evidences suggesting major roles of VEGF-B and PGF in DR pathogenesis. VEGF-B can promote neovascular phenomena and BRB breakdown towards non-inflammatory mechanisms, promoting potent survival stimuli on vascular and nonvascular cells [51]. Similarly, PGF acts as a powerful pro-angiogenic mediator, showing its serum and ocular concentrations strictly correlated with DR severity and risk of progression [51]. Hence, there is a consensus regarding the role of VEGF inhibition as a mandatory strategy for the management of DR [52].
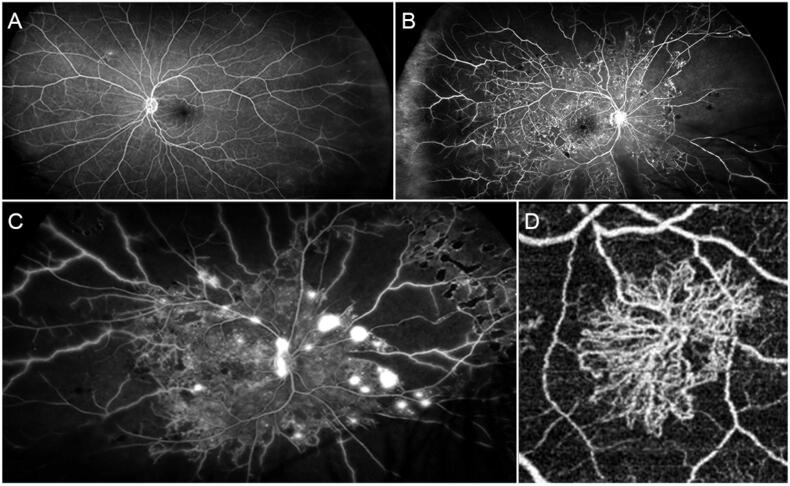
Figure 1.
Angiographic findings in different stages of diabetic retinopathy. (A) Mild form of NPDR characterised by preserved posterior pole and minor capillary non-perfusion changes detected in the extreme periphery. (B) Advanced form of NPDR, showing extensive peripheral capillary non-perfusion and central involvement with several microaneurysms and macular edoema. (C) A case of PDR with extensive peripheral capillary non-perfusion and neovascularizations detected both at the level of the optic nerve head and the retinal periphery. (D) Optical coherence tomography angiography reconstruction of a PDR-related neovascularization.
With respect to the neovascular complication, the increased production and release of VEGF activates endothelial cell proliferation and migration, thus promoting neoangiogenesis [53]. Indeed, VEGF molecules activate two tyrosine kinase receptors, namely VEGFR-1 and VEGFR-2, stimulating endothelial proliferation, migration, and survival, thus promoting the progression to PDR [54]. Also in this case, VEGF acts as a major cause of DR-related neovascular process, although being just a part of a more complex pathogenic pathway. Indeed, it was demonstrated that angiopoietin system, over than regulating vascular integrity, it can promote and enhance the effect of VEGF neovascular stimulus [55]. Furthermore, neuropilin-1 (NRP1) was found to act as an isoform-specific receptor for VEGF165 and as a co-receptor of VEGF receptor 2 [56]. In addition, another system implicated on DR pathogenesis and on the enhancement of the neovascular stimulus provided by VEGF is the renin/angiotensin system. Indeed, the severity of DR, expressed both as the rate of progression of NPDR or the progression to PDR, were found strictly related to the activity of the renin/angiotensin system [57].
Anti-vascular endothelial growth factor molecules
Based on all the above-described data, the main therapeutic target of anti-VEGF molecules regards the VEGF-A isoforms. It is worth of notice that endogenous anti-VEGF mechanisms already exist, although resulting impaired in retinal diseases such as DR. We have already described the role of VEGF/PEDF ratio dysregulation causing a reduced modulation of VEGF activity [40,41]. The physiologic anti-VEGF mechanisms represent a topic not deeply explored yet. Scant information came from animal models, reporting, for example, the anti-VEGF activity of VEGF165b isoform [58]. More in detail, VEGF165b seems to inhibit angiogenic stimuli induced by VEGF upregulation and hypoxia, also interfering with the migration and the proliferation of endothelial cells [58]. However, this topic would benefit from further dedicated studies to better define the endogenous anti-VEGF mechanisms occurring in animals and in human retina.
With respect to anti-VEGF drugs, the available molecules currently include: Bevacizumab (Avastin®, Hoffmann-La Roche), Pegaptanib (Macugen, Eyetech/Pfizer), Ranibizumab (Lucentis®, Novartis Pharmaceuticals Canada Inc.), Aflibercept (Eylea®, BAYER Pharma AG, Germany), Conbercept (Chengdu Kanghong Biotech Company, China), Brolucizumab (Beovu®, Novartis Pharmaceuticals Canada Inc.), Abicipar-pegol (Allergan, Inc., Irvine, CA) and Faricimab (Hoffmann-La Roche) (Figure 2).
Figure 2.
Main anti-VEGF molecules and respective VEGF isoforms targets.
Bevacizumab
Bevacizumab (Avastin®, Hoffmann-La Roche) is a fully humanized immunoglobulin G1 (IgG1) molecule of 148 kDa binding VEGF-A isoforms. This antibody was originally developed for cancer therapy [59]; its intravitreal use for retinal diseases is still considered as “off-label” treatment [60]. The mechanisms of action are quite simple; bevacizumab works as a pure anti-VEGF antibody, and its main effect is to block the neovascular stimulus and VEGF-induced increased vascular permeability [61]. Furthermore, bevacizumab can interact with HIF-1, thus interfering with its stimulating effect on VEGF production [62]. Although several studies described bevacizumab as an efficient and cost-effective treatment for retinal diseases [63–65], its usage is partially limited by its “off-label” classification. In 2007, the Diabetic Retinopathy Clinical Research Network (DRCR.net) reported the positive results regarding the employment of bevacizumab in DME on the basis of a phase II clinical trial [66]. Furthermore, the bevacizumab or laser therapy (BOLT) study reported the superiority of bevacizumab in the management of DME, with respect to laser approach [67,68]. Because of some doubts regarding the comparable profile of efficacy and safety of bevacizumab, the current recommendation of the European Society of Retina Specialists is to consider bevacizumab as a second choice, with respect to other anti-VEGF molecules [69].
Pegaptanib
Pegaptanib (Macugen, Eyetech/Pfizer) was the first drug to obtain FDA approval for the treatment of retinal diseases through the intravitreal administration. This molecule is a pegylated‐aptamer binding preferentially the heparin-binding domain of VEGF165 isoform [70]. Although pegaptanib was found efficient in inhibiting the neovascularization process [71], its molecular features strongly limit its ability to block VEGF-related pathways, thus making it a poorly considered therapeutic choice.
Ranibizumab
Ranibizumab (Lucentis®, Novartis Pharmaceuticals Canada Inc.) is a recombinant humanized immunoglobulin G1κ isotype monoclonal antibody fragment (Fab) of 48 kDa binding different VEGF-A isoforms and interfering with the interaction with VEGF receptors 1 and 2. The lack of fragment crystallizable (Fc) domain and the small molecule size might allowed to expand its affinity for more isoforms of VEGF-A (VEGF165, VEGF121, and VEGF110), increasing the penetration of the molecule within the retina and choroid [72,73]. Ranibizumab is characterized by only one binding site for VEGF; for this reason, two molecules of ranibizumab bind to one VEGF dimer [74]. This peculiar configuration allows the ranibizumab/VEGF-A complex having higher stability energy than bevacizumab [75] and greater molecular affinity to VEGF than bevacizumab and aflibercept [76]. Several clinical trials demonstrated high safety and efficacy of ranibizumab in the management of DME, exploring the usage of ranibizumab in different modalities and concentrations, as well as alone or in combination with laser, including READ-2 [77], RESOLVE [78], RESTORE [79], RISE and RIDE [80], LUCIDATE [81], REVEAL [82], RELIGHT [83], RETAIN [84] and READ-3 [85]. DRCR network conducted several multicenter clinical trials analyse similarities and differences of ranibizumab, with respect to other approaches, including other anti-VEGF molecules, corticosteroids, or laser, in the management of DR: Protocol S (ranibizumab vs. laser in PDR) [86,87], Protocol T (ranibizumab vs. aflibercept vs. bevacizumab in DME) [88] and Protocol I (fluocinolone acetonide vs. ranibizumab plus deferred laser in DME) [89] studies. Other meaningful clinical trials involving ranibizumab were: TREX-DME (ranibizumab “treat and extend” regimen with or without laser in DME) [90–92], ROTATE (ranibizumab in persistent DME after bevacizumab treatment) [93], RELATION (ranibizumab plus laser vs. laser alone in DR) [94] and REFINE (ranibizumab vs. laser in DME) [95] studies.
Aflibercept
Aflibercept (Eylea®, BAYER Pharma AG, Germany) is a dimeric glycoprotein of 115 kDa, also known as VEGF Trap. This is obtained from the fusion of the first three Ig domains of VEGFR-1 and the Fc region of human IgG1 [96]. These biochemical properties provide high affinity for VEGF-A isoforms and PGF, as well as relative affinity for VEGF-B. Another version of the molecule, differing from aflibercept only for its excipients and the higher osmolarity, and displaying an almost identical biochemical profile, is Ziv-aflibercept (Zaltrap; Sanofi-Aventis and Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) [97]. Although this molecule has been associated with promising effects in macular diseases, Ziv-aflibercept usage is still off-label [98]. The main trials dedicated on the assessment of the efficacy and safety of aflibercept in DR were VIVID-VISTA (aflibercept vs. laser in DME) [99,100], ENDURANCE (extension of VIVID-VISTA studies) [101], APOLLON (real-word data on aflibercept in DME) [102] and PANORAMA (aflibercept in NPDR) [103] studies. Furthermore, the DRCR network investigated the effect of aflibercept on DR progression, complications onset and outcome: Protocol V (aflibercept vs. laser vs. observation in DME) [102] and Protocol W (aflibercept vs sham in preventing vision-threatening complications in NPDR without DME) [103] studies.
Conbercept
Conbercept (Chengdu Kanghong Biotech Company, China) is a molecule belonging to VEGF Trap family. It represents a full human DNA sequence of 143 kDa, obtained from the fusion of extracellular domain 2 of VEGFR-1 and extracellular domains 3 and 4 of VEGFR-2 with the Fc portion of human IgG1 [104,105]. The molecular characteristics of Conbercept are quite similar to aflibercept’s; this molecule differs for the presence of a portion dedicated to VEGFR-2, which was developed to potentially increase the efficacy and stability of Conbercept and to produce relative affinity for VEGF-C [104,105]. It turned out to be superior to laser treatment in DME, as assessed by SAILING study [106]. Further real-life reports described the efficacy and safety of Conbercept for DME management [107–110].
Brolucizumab
Brolucizumab (Beovu®, Novartis Pharmaceuticals Canada Inc.) is a novel single-chain antibody fragment of 26 kDa, characterized by the absence of the Fc portion and developed to reduce molecule size and to improve the affinity for VEGF-A isoforms, compared with the other molecules [111,112]. Brolucizumab has been recently approved for the treatment of neovascular age-related macular degeneration, showing non-inferiority and higher penetrance within the retina and the choroid compared with the other anti-VEGF molecules [113,114]. With respect to DR, the ongoing KITE and KESTREL clinical trials reported preliminary positive results regarding the employment of brolucizumab in DME, compared with aflibercept [115], thus suggesting it will be approved for DR management soon.
Abicipar-pegol
Abicipar-pegol (Allergan, Inc., Irvine, CA, USA) belongs to the family of designed ankyrin repeat proteins (DARPins) molecules, a class of proteins that can mimic antibodies showing high affinity for VEGF target [116]. In more details, abicipar-pegol is a recombinant protein of 34 kDa coupled to a polyethylene glycol fraction binding all VEGF-A isoforms [117]. Its affinity for VEGF-A turned out to be comparable to aflibercept’s but remarkably greater than bevacizumab’s and ranibizumab’s [118]. A phase II clinical trial was completed in 2015 about the employment of abicipar-pegol in DME (ClinicalTrials.gov ID: NCT02186119). However, further studies are warranted to support its role in DME management.
Faricimab
Faricimab (Hoffmann-La Roche) is a novel molecule belonging to the DARPin family. This 150 kDa molecule can simultaneously and independently bind and neutralize both VEGF-A and Ang-2, the latter mechanism interfering with the Ang-1/Tie2 pathway [119]. The Ang-1/Tie2 pathway is a major pathogenic factor in the development of neovascularization and exudation. From this point of view, the multitargets profile of faricimab offers interesting new perspectives for the management of exudative retinal diseases. The phase 2 BOULEVARD trial provided prosing data regarding the employment of faricimab in DME, showing its superiority in terms of visual gain, compared with ranibizumab [120]. Furthermore, two Phase 3 clinical trials, RHINE and YOSEMITE (NCT03622593 and NCT03622580, respectively) are currently ongoing to compare the efficacy of faricimab to aflibercept [121]. However, also in this case, further studies are needed to draw more definite conclusions about the role of faricimab in DME management.
Emerging anti-VEGF molecules
In this section, we would just mention two new anti-VEGF molecules currently under investigation. KSI-301 (KODIAK sciences, Palo Alto, CA), is a new generation antibody biopolymer conjugate, under study in a phase 1 b trial (NCT03790852) and in a DAZZLE phase 2 trial (NCT04049266), obtained from the combination of humanized anti-VEGF monoclonal antibody and a phosphorylcholine-based polymer, specifically designed to increase the duration of anti-VEGF activity. OPT-302 is a VEGF-C/D inhibitor, currently under investigation for exudative age-related macular degeneration in a Phase 2 b clinical trial (NCT03345082). In addition, a Phase 3 clinical trial (NCT03610646) is currently ongoing comparing aflibercept to intravitreal MYL-1701P, a recombinant fusion protein that is an aflibercept biosimilar [121].
All the above-mentioned clinical trials are extensively reported in Table 1.
Table 1.
Main clinical trials dedicated on the assessment of anti-VEGF molecules in diabetic retinopathy. The order of appearance follows the year of publication.
| N | Title | Acronym | Design | Number of Patients | Max duration | Drug | Year of publication | Main conclusions | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Two-year outcomes of the ranibizumab for edoema of the mAcula in diabetes (READ-2) study | READ-2 | Prospective, randomized, interventional, multicenter clinical trial | 126 | 24 months | Ranibizumab | 2010 | Ranibizumab is effective in DME management with or without laser | 77 |
| 2 | Safety and efficacy of ranibizumab in diabetic macular edoema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study | RESOLVE | Randomized, controlled, double-masked, multicenter phase II study | 151 | 12 months | Ranibizumab | 2010 | Ranibizumab is effective in DME management | 78 |
| 3 | The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edoema | RESTORE | Randomized, double-masked, multicenter, laser-controlled phase III study | 345 | 12 months | Ranibizumab | 2011 | Ranibizumab with or without laser is superior to laser monotherapy for DME management | 79 |
| 4 | Ranibizumab for diabetic macular edoema: results from 2 phase III randomized trials: RISE and RIDE | RISE and RIDE | Two parallel, methodologically identical, phase III, multicenter, double-masked, sham injection-controlled, randomized studies | 377 (RISE) and 382 (RIDE) | 24 months | Ranibizumab | 2012 | Ranibizumab is effective in DME management | 80 |
| 5 | Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous haemorrhage from proliferative diabetic retinopathy. | Protocol N | Phase 3, double-masked, randomized, multicenter clinical trial | 261 | 4 months | Ranibizumab | 2013 | Ranibizumab is useful in vitrectomy setting of PDR | 122 |
| 6 | A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edoema (the LUCIDATE study). | LUCIDATE | Prospective, randomized, single-masked clinical trial | 33 | 12 months | Ranibizumab | 2014 | Ranibizumab is effective in DME management | 81 |
| 7 | The REVEAL Study: Ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edoema. | REVEAL | Randomized, double-masked, multicenter, laser-controlled, phase III study | 396 | 12 months | Ranibizumab | 2015 | Ranibizumab with or without laser is superior to laser monotherapy for DME management | 82 |
| 8 | Ranibizumab 0.5 mg for diabetic macular edoema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT Study. | RELIGHT | Phase IIIb, prospective, open-label, multicenter, single-arm study | 109 | 18 months | Ranibizumab | 2015 | Ranibizumab is effective in DME management | 83 |
| 9 | Panretinal photocoagulation vs intravitreous Ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial | Protocol S | Randomized, multicenter, clinical trial | 394 | Up to five years | Ranibizumab | 2015-2018 | Ranibizumab not inferior to PRP for PDR management | 86,87 |
| 10 | Intravitreal Aflibercept for diabetic macular edoema: 100-week results from the VISTA and VIVID studies. | VISTA and VIVID | Two similarly designed, double-masked, randomized, phase 3 trials, VISTA(DME) and VIVID(DME) | 872 | 24 months | Aflibercept | 2015 | Aflibercept is effective in DME management | 99,100 |
| 11 | A study of Abicipar Pegol in patients with diabetic macular edoema (NCT02186119). | N/A | Randomized, multicenter study | 151 | 12 months | Abicipar pegol | 2015 | Abicipar pegol non-inferior to ranibizumab for DME management | N/A |
| 12 | Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. | RETAIN | Single-masked, randomized study | 372 | 24 months | Ranibizumab | 2016 | Ranibizumab is effective in DME management | 84 |
| 13 | Twenty-four-month outcomes of the Ranibizumab for edoema of the macula in diabetes – protocol 3 with high dose (READ-3) Study | READ-3 | Randomized, controlled, double-masked (to the dose), interventional, multicenter clinical trial | 152 | 24 months | Ranibizumab | 2016 | Ranibizumab is effective in DME management | 85 |
| 14 | Aflibercept, bevacizumab, or ranibizumab for diabetic macular edoema | Protocol T | Randomized, multicenter, clinical trial | 660 | 24 months | Aflibercept, bevacizumab, and ranibizumab | 2016 | Aflibercept, bevacizumab, and ranibizumab non-inferiority study | 88 |
| 15 | Ranibizumab 0.3 mg for persistent diabetic macular edoema after recent, frequent, and chronic Bevacizumab: The ROTATE Trial. | ROTATE | Open-label, prospective study | 30 | 12 months | Ranibizumab and bevacizumab | 2016 | Ranibizumab demonstrated improved outcome in patients with persistent DME following bevacizumab | 93 |
| 16 | Outcomes with as-needed Aflibercept and macular laser following the Phase III VISTA DME Trial: ENDURANCE 12-month extension study. | ENDURANCE | Phase IV, multicenter, open-label extension study (12-month extension of VISTA and VIVID) | 60 | 36 months | Aflibercept | 2017 | Aflibercept with or without laser is superior to laser monotherapy for DME management | 101 |
| 17 | Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomized, controlled, phase 2 b, non-inferiority trial. | CLARITY | Phase 2 b, single-blind, non-inferiority trial | 232 | 12 months | Aflibercept | 2017 | Aflibercept is more effective than PRP for PDR management | 123 |
| 18 | Visual acuity outcomes in diabetic macular edoema with Fluocinolone Acetonide 0.2 μg/day versus Ranibizumab plus deferred laser (DRCR Protocol I). | Protocol I | Randomized, multicenter study | 188 | 36 months | Ranibizumab and Fluocinolone Acetonide | 2018 | Fluocinolone Acetonide was comparable to ranibizumab plus deferred laser for DME management | 89 |
| 19 | The RELATION study: efficacy and safety of ranibizumab combined with laser photocoagulation treatment versus laser monotherapy in NPDR and PDR patients with diabetic macular oedema. | RELATION | Double-masked, multicentre phase IIIb study | 128 | 12 months | Ranibizumab | 2018 | Ranibizumab with or without laser is superior to laser monotherapy for DME management | 94 |
| 20 | Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS Study). | PROTEUS | Prospective, randomized, multicenter, open-label, phase II/III study | 87 | 12 months | Ranibizumab | 2018 | Ranibizumab plus PRP superior to PRP monotherapy for DME management | 124 |
| 21 | Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS Study). | PROTEUS | Prospective, randomized, multicenter, open-label, phase II/III study | 87 | 12 months | Ranibizumab | 2018 | Ranibizumab plus laser superior than laser alone for PDR management | 124 |
| 22 | Efficacy and safety of ranibizumab 0.5 mg in Chinese patients with visual impairment due to diabetic macular edoema: results from the 12-month REFINE study. | REFINE | Phase III, double-masked, multicenter, laser-controlled study | 384 | 12 months | Ranibizumab | 2019 | Ranibizumab with or without laser is superior to laser monotherapy for DME management | 95 |
| 23 | Effect of initial management with Aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edoema involving the centre of the macula and good visual acuity: a randomized clinical trial. | Protocol V | Randomized, multicenter study | 702 | 24 months | Aflibercept | 2019 | Aflibercept is more effective than laser for DME management | 102 |
| 24 | Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-A with Faricimab in diabetic macular edoema: BOULEVARD Phase 2 randomized trial. | BOULEVARD | Prospective, randomized, active comparator-controlled, double-masked, multicenter, phase 2 study | 229 | 6 months | Faricimab | 2019 | Faricimab was statistically superior than ranibizumab for DME management | 120 |
| 25 | Real-world outcomes following 12 months of intravitreal aflibercept monotherapy in patients with diabetic macular edoema in France: results from the APOLLON study. | APOLLON | Prospective, observational cohort study | 147 | 12 months | Aflibercept | 2020 | Aflibercept is effective in DME management | 125 |
| 26 | A randomized, double-masked, multicenter, Phase III Study assessing the efficacy and safety of Brolucizumab versus Aflibercept in patients with visual impairment due to diabetic macular edoema (KITE). | KITE and KESTREL | Ongoing prospective, randomized, phase III clinical studies | 361 (KITE) and 561 (KESTREL) | 24 months | Brolucizumab and aflibercept | 2020 | Brolucizumab non-inferior to aflibercept for DME management | 115 |
| 27 | Long-term outcomes of treat-and-extend ranibizumab with and without navigated laser for diabetic macular oedema: TREX-DME 3-year results | TREX-DME | Multicenter, prospective, randomized clinical trial | 116 | 36 months | Ranibizumab | 2021 | Ranibizumab is effective in DME management | 90–92 |
| 28 | Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. | PANORAMA | Double-masked, multicenter, randomized clinical trial | 402 | 24 months | Aflibercept | 2021 | Aflibercept is effective in DME management | 126 |
| 29 | Effect of intravitreous anti-vascular endothelial growth factor vs sham treatment for prevention of vision-threatening complications of diabetic retinopathy: the protocol W randomized clinical trial. | Protocol W | Randomized, multicenter study | 328 | 24 months | Aflibercept | 2021 | Aflibercept is associated with lower progression to PDR than sham | 103 |
| 30 | Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomized controlled trial and open-label extension study. | SAILING | Multicentre, randomized, double-masked, double-sham, parallel controlled, phase III trial (Sailing Study), followed by a 12-month open-label extension study | 251/157 | 12 + 12 months | Conbercept | 2021 | Conbercept is more effective than laser for DME management | 106 |
| 31 | Effect of Aflibercept on diabetic retinopathy severity and visual function in the RECOVERY Study for proliferative diabetic retinopathy. | RECOVERY | Prospective, longitudinal, multicenter clinical trial | 40 | 12 months | Aflibercept | 2021 | Aflibercept is associated with improvement of Diabetic Retinopathy Severity Scale for PDR eyes | 127 |
| 32 | A multi centre, randomized, double-masked, active-controlled, comparative clinical study to evaluate the efficacy and safety of MYL-1701P and Eylea® in subjects with diabetic macular edoema | N/A | Multi Centre, randomized, double-masked, active-controlled, comparative clinical study | 355 | 12 months | MYL-1701P and aflibercept | N/A | Ongoing | 121 |
| 33 | A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of Faricimab (RO6867461) in patients with diabetic macular edoema (RHINE) | RHINE | Phase III, multicenter, randomized, double-masked, active comparator-controlled study | 951 | 24 months | Faricimab and aflibercept | N/A | Ongoing | 121 |
| 34 | A Phase III, multicenter, randomized, double-masked, active comparator-controlled study to evaluate the efficacy and safety of Faricimab (RO6867461) in patients with diabetic macular edoema (YOSEMITE) | YOSEMITE | Phase III, multicenter, randomized, double-masked, active comparator-controlled study | 940 | 24 months | Faricimab and aflibercept | N/A | Ongoing | 121 |
The role of anti-VEGF in DR-related retinal neovascularization
PDR is an extremely complex and vision-threating stage of DR. The management of this complicated form of DR has been mainly based on the employment of panretinal photocoagulation. The irreversible annihilation of peripheral ischaemic retina is associated with decreased production of VEGF and stabilization of the central retina, although having a remarkable impact on the visual field. Most of the above-described studies tried to assess the effect of anti-VEGF treatments on peripheral ischaemia and regression of the neovascularizations. The main question regards the possible replacement of panretinal photocoagulation with anti-VEGF injections alone. A Cochrane meta-analysis performed in 2014 reported low level of evidence regarding safety and efficacy of anti-VEGF in PDR, although the employment of intravitreal injections was associated with moderate reduction of the risk of intraocular bleeding [128]. A comparable low level of evidence was reported by another meta-analysis, although highlighting the higher morpho-functional outcome and benefits on vitrectomy setting obtained from anti-VEGF injections administered as adjuncts to panretinal photocoagulation [129]. The PROTEUS study assessed the role of adjunctive ranibizumab injections on panretinal photocoagulation vs laser treatment alone, reporting significant improvements of the visual acuity and fluorescein angiography features in PDR eyes undergoing combined approach [124]. DRCR Protocol S was a clinical trial specifically designed to compare panretinal photocoagulation to ranibizumab injections in PDR [86,87]. Overall, this study showed ranibizumab being non inferior to panretinal photocoagulation for PDR management. Eyes treated with laser showed higher risk of PDR progression, DME onset, and higher need of vitrectomy, compared with eyes treated by ranibizumab injections. Furthermore, Protocol S highlighted the importance of the starting PDR severity, associated with the presence of further complications, such as epiretinal membrane, neovascularization of the disc with neovascularization elsewhere, and vitreous haemorrhage on determining the achievable outcome [130]. The same attempt to compare panretinal photocoagulation with anti-VEGF injections was assessed by the CLARITY study, a phase 2 b, single-blind, noninferiority trial comparing aflibercept to panretinal photocoagulation in newly diagnosed or previously laser-treated active PDR [123]. Also in this case, the employment of anti-VEGF injections was associated with better outcome, compared with only laser-treated eyes, especially looking at neovascularization regression, visual acuity values and the need of vitrectomy. DRCR Protocol N was a phase 3, double-masked, randomized, multicenter clinical trial comparing intravitreal ranibizumab with intravitreal saline injections on vitrectomy rates for vitreous haemorrhage from PDR [122]. Because of the low overall rate of vitrectomies, the study could not detect differences in vitrectomy rates between groups. However, it showed how ranibizumab group showed better clinical outcome than eyes treated by saline injections. The non-inferiority of ranibizumab, compared with panretinal photocoagulation to manage PDR was furtherly confirmed by the five-year report of the DRCR network [87], which also reported relatively higher probability to develop DME and to have worse visual field in laser-treated group. Moreover, the recent RECOVERY study showed a statistically significant impact of anti-VEGF injections (in this case of aflibercept) on the improvement in DR severity progression registered after one year of follow-up in PDR eyes without DME [127]. Further recent meta-analysis studies provided even more support on the fact that, although not reaching enough level of statistical evidence, the use of anti-VEGF in PDR is associated with better visual outcome and less incidence of PDR-related complications, if compared with panretinal photocoagulation alone [131,132].
Overall considering all these findings, either anti-VEGF therapy or panretinal photocoagulation are feasible treatments for PDR patients. If panretinal photocoagulation was often criticized as inducing extensive loss of the visual field, the 5-year report of DRCR Protocol S highlighted a crucial point. Indeed, Maguire and colleagues [133] showed that, if the deterioration of the visual field was higher in the PDR group treated by laser during the first year, if compared with the eyes treated by anti-VEGF injections, the long-term visual field outcome was statistically similar between both groups at the end of the 5-year follow-up. These findings, showing similar long-term effect of laser and peripheral ischaemia on the visual field, further reinforced the role either of anti-VEGF and panretinal photocoagulation as effective treatments for PDR. As concluded by DRCR network, the therapeutic strategy for PDR should consider treatment recommendations, the relative advantages of each treatment approach and patients’ compliance with follow-up planning [134]. The other side of the medal is to always bear in mind the possible, although rare, risk of “anti-VEGF crunch syndrome”. This infrequent complication occurring in PDR is characterized by the progressive worsening of the fibrovascular tractional retinal detachment following anti-VEGF injections. This condition is still poorly defined, because of the very low incidence and the presence of many possible confounding factors, including panretinal photocoagulation. It might be determined by the fibrovascular regression induced by anti-VEGF injections, causing increased fibrosis leading to the worsening of the tractional component of the retinal detachment. As expected, the surgical approach and visual outcome are worse than PDR eyes not complicated by this occurrence [135]. The main interventional clinical trials specifically focussed on PDR are reported in Table 2.
Table 2.
Interventional clinical trials specifically focussed on proliferative diabetic retinopathy.
| N | Study ID | Title | Drug | Main Aim | Phase | Start Date | End Date | Status |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT00131144 | A randomized, controlled study on the efficacy and safety of octreotide acetate in microspheres in the therapy of patients with moderately severe or severe non-proliferate diabetic retinopathy (NPDR) or low risk proliferative diabetic retinopathy (PDR) | Octreotide acetate | To evaluate the efficacy of Octreotide acetate in PDR. | Phase III | 1999 | 2005 | Completed |
| 2 | NCT00130845 | A randomized, controlled study on the efficacy and safety of octreotide acetate in microspheres in the therapy of patients with moderately severe or severe non-proliferate diabetic retinopathy (NPDR) or low risk proliferative diabetic retinopathy (PDR) | Octreotide acetate | To evaluate the efficacy of Octreotide acetate in PDR. | Phase III | 2000 | 2005 | Completed |
| 3 | NCT00170742 | A randomized, open label, controlled study on the efficacy and safety of octreotide i.m. in patients with proliferative diabetic retinopathy (PDR) after start of laser coagulation | Octreotide, 30 mg i.m. | To evaluate monthly octreotide i.m. in comparison to no additional treatment in PDR after panretinal photocoagulation. | Phase III | 2003 | 2006 | Terminated |
| 4 | NCT00600262 | Efficacy of intravitreal bevacizumab for severe nonproliferative and proliferative diabetic retinopathy. | Bevacizumab | To evaluate the 3-month efficacy of a single dose of intravitreal bevacizumab on the progression of severe NPDR and PDR. | Phase II/III | 2005 | 2006 | Completed |
| 5 | NCT00443521 | Triamcinolone as adjunctive treatment to laser panretinal photocoagulation for proliferative diabetic retinopathy | Triamcinolone Acetonide | To evaluate the efficacy of adjuctive Triamcinolone Acetonide in PDR. | Phase II | 2005 | 2006 | Completed |
| 6 | NCT00248131 | An open-label extension study to evaluate the long-term safety and tolerability of octreotide acetate in microspheres in the therapy of patients with moderately severe or severe non-proliferative diabetic retinopathy (NPDR) or low risk proliferative diabetic retinopathy (PDR) | Octreotide acetate | To evaluate the efficacy of Octreotide acetate in PDR. | Phase III | 2005 | 2006 | Terminated |
| 7 | NCT00248157 | An open-label extension study to evaluate the long-term safety and tolerability of octreotide acetate in microspheres in the therapy of patients with moderately severe or severe non-proliferative diabetic retinopathy (NPDR) or low risk proliferative diabetic retinopathy (PDR) | Octreotide acetate | To evaluate the efficacy of Octreotide acetate in PDR. | Phase III | 2005 | 2006 | Terminated |
| 8 | NCT00423059 | Histologic changes of fibrovascular membrane associated with proliferative diabetic retinopathy after intravitreal Bevacizumab (Avastin®). | Bevacizumab | To evaluate the effect of the intravitreal bevacizumab on the fibrovascular membrane associated with PDR by objective histologic evaluation in eyes underwent vitrectomy. | N/A | 2006 | 2007 | Completed |
| 9 | NCT00446381 | Effect of Macugen (Pegaptanib) on surgical outcomes and growth factors including vascular endothelial growth factor (VEGF) levels in patients with proliferative diabetic retinopathy (PDR) and clinically significant diabetic macular edoema (CSDME). | Pegaptanib sodium | To quantify the reduction of intravitreal VEGF 165 levels in patients following intravitreal Macugen injection pre-operatively. | N/A | 2006 | 2008 | Completed |
| 10 | NCT00656435 | Bevacizumab pre-treatment and long acting gas infusion on the vitreous clear-up after diabetic vitrectomy | Bevacizumab | To evaluate the efficacy of bevacizumab before vitrectomy in PDR. | Phase III | 2006 | 2008 | Completed |
| 11 | NCT01041690 | Bevacizumab (Avastin) as an adjunct to vitrectomy in the management of severe proliferative diabetic retinopathy: a prospective case series. | Bevacizumab | To evaluate the role of preoperative intravitreal bevacizumab as an adjunct to vitrectomy in the management of PDR. | N/A | 2007 | 2008 | Completed |
| 12 | NCT00596505 | Intravitreal Bevacizumab (Avastin) pre-treatment for reducing intraoperative and postoperative preretinal haemorrhage in primary diabetic vitrectomy with silicone oil infusion. | Bevacizumab | To evaluate the effect of pre-operative becacizumab in PDR eyes undergoing vitrectomy. | N/A | 2007 | 2007 | Completed |
| 13 | NCT00548197 | Preoperative injection of Bevacizumab prior to vitreoretinal surgery in diabetic tractional retinal detachment. | Bevacizumab | To evaluate the preoperative injection of bevacizumab on PDR eyes undergoing vitrectomy. | Phase I | 2007 | 2008 | Completed |
| 14 | NCT00445003 | Intravitreal Ranibizumab or triamcinolone acetonide as adjunctive treatment to panretinal photocoagulation for proliferative diabetic retinopathy | Ranibizumab, Triamcinolone Acetonide | To evaluate the efficacy of intravitreal triamcinolone or intravitreal ranibizumab in preventing loss of vision caused by panretinal photocoagulation treatment in PDR. | Phase III | 2007 | 2010 | Completed |
| 15 | NCT00668785 | Intravitreal Ranibizumab to treat macular edoema after panretinal photocoagulation (Phase II) | Ranibizumab | To evaluate the efficacy of ranibizumab in PDR. | Phase II | 2007 | 2012 | Terminated |
| 16 | NCT00606138 | Investigation of Ranibizumab for the treatment of persistent diabetic neovascularization as assessed by super wide-field angiography (Optos). | Ranibizumab | To evaluate the efficacy of ranibizumab versus additional panretinal photocoagulation on diabetic neovascularization that is persistent despite previous treatment with panretinal photocoagulation. | Phase I/II | 2008 | 2010 | Completed |
| 17 | NCT00545870 | A randomized, double-masked study with intraocular Bevacizumab compared with intravitreal Ranibizumab in patients with persistent diabetic macular edoema or persistent active neovascularisation following lasercoagulation | Bevacizumab, ranibizumab | To evaluate safety and efficacy of ranibizumab vs bevacizumab following panretinal photocoagulation in PDR. | Phase III | 2008 | 2013 | Completed |
| 18 | NCT00511875 | Evaluation of effect of doxycycline verses placebo on diabetic retinopathy progression and retinal function in patients with severe non-proliferative or mild or moderate (non-high-risk) proliferative diabetic retinopathy. | Doxycycline monohydrate | To evaluate the effect of doxycycline monohydrate in DR and PDR. | Phase II | 2008 | 2012 | Completed |
| 19 | NCT00745498 | Efficacy study of pre- and intra-operative intravitreal Bevacizumab injection on postoperative vitreous haemorrhage after diabetic vitrectomy. | Bevacizumab | To evaluate the effect of pre- and intra-operative bevacizumab injection on PDR eyes undergoing vitrectomy. | N/A | 2008 | 2010 | Completed |
| 20 | NCT01102946 | Panretinal photocoagulation versus panretinal photocoagulation plus intravitreous Ranibizumab for high risk proliferative diabetic retinopathy. | Ranibizumab | To evaluate safety and efficacy of ranibizumab + panretinal photocoagulation in the regression of retinal neovascularization in PDR. | Phase II | 2009 | 2011 | Completed |
| 21 | NCT00907114 | Efficacy and safety of topic Ketorolac to treat centre point thickness secondary to panphotocoagulation in proliferative diabetic retinopathy. | Ketorolac tromethamine | To evaluate the efficacy and safety of topic ketorolac in treatment for centre point thickness secondary to panphotocoagulation in PDR. | Phase II | 2009 | 2015 | Completed |
| 22 | NCT01270542 | Effect of pre-operative adjunctive anti-VEGF on growth factors in severe proliferative diabetic retinopathy requiring surgical management | Bevacizumab | To evaluate the efficacy of bevacizumab before vitrectomy in PDR. | N/A | 2009 | 2012 | Completed |
| 23 | NCT01280929 | Prospective, randomized, multicenter, open label, Phase II Study to access efficacy and safety of Lucentis® monotherapy (Ranibizumab 0.5 mg intravitreal injections) compared with Lucentis® Plus panretinal photocoagulation (PRP) and PRP (monotherapy) in the treatment of patients with high risk proliferative. diabetic retinopathy | Ranibizumab | To evaluate safety and to compare the efficacy of ranibizumab + panretinal photocoagulation vs panretinal photocoagulation alone in the regression of retinal neovascularization in PDR. | Phase II | 2010 | 2013 | Completed |
| 24 | NCT01281098 | Prospective, randomized, open label, Phase II Study to assess efficacy and safety of Macugen® (Pegaptanib 0.3 mg intravitreal injections) plus panretinal photocoagulation (PRP) and PRP (monotherapy) in the treatment of patients with high risk proliferative diabetic retinopathy (PDR). | Pegaptanib sodium | To evaluate the safety and determine the efficacy of panretinal photocoagulation monotherapy or combination therapy (pegaptanib 0.3 mg plus panretinal photocoagulation) in PDR. | Phase II | 2010 | 2013 | Completed |
| 25 | NCT00996437 | An evaluation of intravitreal Ranibizumab for vitreous haemorrhage due to proliferative diabetic retinopathy. | Ranibizumab | To evaluate the efficacy of intravitreal ranibizumab in vitrectomy setting in PDR. | Phase II | 2010 | 2013 | Completed |
| 26 | NCT01213888 | Trientine Hydrochloride for the prevention of macular edoema associated with pan-retinal photocoagulation for severe non-proliferative and proliferative diabetic retinopathy | Trientine Hydrochloride | To evaluate the efficacy of Trientine Hydrochloride in PDR. | N/A | 2010 | 2013 | Terminated |
| 27 | NCT01487070 | A single-centre trial of intravitreous injections of Macugen (Pegaptanib Sodium) given at least 7 days before vitrectomy secondary to tractional retinal detachment in proliferative diabetic retinopathy | Pegaptanib sodium | To evaluate the safety and efficacy of intravitreal injections of pegaptanib sodium in PDR undergoing vitrectomy. | Phase I | 2011 | 2011 | Completed |
| 28 | NCT01746563 | Intravitreal Ranibizumab combined with panretinal photocoagulation in patients with treatment-naive proliferative diabetic retinopathy | Ranibizumab | To evaluate the efficacy of ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone in PDR. | Phase I | 2011 | 2012 | Completed |
| 29 | NCT01594281 | Multicenter randomized open-label three-arms controlled 12 months clinical proof of concept study to evaluate efficacy and safety of Ranibizumab alone or in combination with laser photocoagulation vs. laser photocoagulation alone in proliferative diabetic retinopathy. | Ranibizumab | To evaluate safety and to compare the efficacy of ranibizumab with or without panretinal photocoagulation vs panretinal photocoagulation alone in the regression of retinal neovascularization in PDR. | Phase II | 2012 | 2017 | Completed |
| 30 | NCT01589029 | A Pilot Study on the effects of ILARIS® on patients with proliferative diabetic retinopathy (PDRP). | Canakimumab | To evaluate the efficacy and safety of Canakinumab (ILARIS®) in PDR. | Phase I | 2012 | 2014 | Terminated (primary endpoint not met) |
| 31 | NCT01535495 | Propranolol for diabetic retinopathy. | Propanolol | To evaluate oral beta antagonist propranolol efficacy in inducing regression of retinal neovascularization in PDR. | Phase I | 2012 | 2015 | Completed |
| 32 | NCT02816073 | Single-session pattern scanning laser pan-retinal photocoagulation in proliferative diabetic retinopathy - a randomized study | Laser | To evaluate the safety and efficacy of single-session panretinal photocoagulation using Pattern Scan Laser (PASCAL) in PDR. | N/A | 2012 | 2016 | Completed |
| 33 | NCT01489189 | Prompt panretinal photocoagulation versus intravitreal Ranibizumab with deferred panretinal photocoagulation for proliferative diabetic retinopathy. | Ranibizumab | To evaluate the effect of prompt panretinal photocoagulation vs intravitreal ranibizumab with deferred panretinal photocoagulation in PDR. | Phase III | 2012 | 2018 | Completed |
| 34 | NCT01589718 | A Phase III randomized 1:1, masked, study of the safety, tolerability, and efficacy of intravitreal pre-op 0.3 mg Pegaptanib Sodium versus Sham, for adjuvant management of TRD and Vit Hem associated with PDR. | Pegaptanib sodium | To evaluate preoperative the efficacy of pegaptanib sodium in improving vitreous haemorrhage prior to vitrectomy in PDR. | Phase III | 2012 | 2014 | Withdrawn (No patients were enrolled) |
| 35 | NCT01854593 | Prospective randomized controlled study of intravitreal injection of 0.16 mg Bevacizumab one day before surgery for proliferative diabetic retinopathy. | Bevacizumab | To evaluate the efficacy of bevacizumab before vitrectomy in PDR. | Phase IV | 2012 | 2014 | Completed |
| 36 | NCT01627977 | A descriptive study to evaluate the effectiveness of the dye compound of the combination of Lutein, Zeaxanthin and brilliant blue in Chromovitrectomy | Dye of Lutein, Zeaxanthin and Brilliant Blue | To evaluate the efficacy of Lutein, Zeaxanthin and Brilliant Blue in vitrectomy setting in PDR. | Phase III | 2012 | 2014 | Completed |
| 37 | NCT01552408 | A Phase I/II, randomized, study for diabetic macular edoema using 0.3 mg Ranibizumab combined with targeted PRP monthly for 4 months, then PRN vs. 0.3 mg Ranibizumab 4 months monotherapy, then as needed(DME-AntiVEgf) DAVE | Ranibizumab | To evaluate the efficacy of ranibizumab combined with panretinal photocoagulation in PDR. | Phase I/II | 2012 | 2017 | Completed |
| 38 | NCT01769183 | Topical Squalamine in the treatment of retinal neovascularization from proliferative diabetic retinopathy. | Squalamine Lactate ophthalmic solution 0.2% | To determine the efficacy of topical Squalamine Lactate Ophthalmic Solution 0.2% in the treatment of retinal neovascularization in PDR. | Phase II | 2013 | 2014 | Completed |
| 39 | NCT01908816 | An open-label extended clinical protocol of Ranibizumab to evaluate safety and efficacy in rare VEGF driven ocular diseases. | Ranibizumab | To evaluate the safety and the efficacy of ranibizumab in DR. | Phase III | 2013 | 2016 | Completed |
| 40 | NCT01813773 | Treatment with intravitreal Aflibercept injection for proliferative diabetic retinopathy, The A.C.T Study. | Aflibercept | To evaluate the safety of intravitreal aflibercept injection in the treatment of PDR. | Phase II/III | 2013 | 2016 | Completed |
| 41 | NCT01805297 | Intravitreal Aflibercept injection as a surgical adjuvant in severe proliferative diabetic retinopathy | Aflibercept | To evaluate the effocacy of aflibercept in vitrectomy setting in PDR. | Phase II | 2013 | 2015 | Completed |
| 42 | NCT01869933 | An open label Phase I placebo controlled, dose escalation study assessing the ocular and systemic safety and tolerability of OC-10X | OC-10X | To evaluate safety and tolerability of OC-10X in PDR. | Phase I | 2013 | 2013 | Completed |
| 43 | NCT01941329 | Prospective, randomized, multicentre, open-label, Phase II/III Study to assess efficacy and safety of Ranibizumab 0.5 mg intravitreal injections plus panretinal photocoagulation (PRP) versus PRP in monotherapy in the treatment of subjects with high risk proliferative diabetic retinopathy. (PROTEUS) | Ranibizumab | To evaluate safety and to compare the efficacy of ranibizumab + panretinal photocoagulation vs panretinal photocoagulation alone in the regression of retinal neovascularization in PDR. | Phase II/III | 2014 | 2017 | Completed |
| 44 | NCT02735369 | A Phase II, randomized, placebo-controlled, study assessing efficacy and safety of OC-10X ophthalmic suspension in the treatment of proliferative diabetic retinopathy | 2% OC-10X | To evaluate the efficacy and safety of topical OC-10X Ophthalmic Suspension in PDR. | Phase II | 2014 | 2016 | Terminated |
| 45 | NCT03904056 | ETDRS panretinal photocoagulation (PRP) combined with intravitreal Ranibizumab (IVR) versus retinal photocoagulation targeted to ischaemic retina combined with IVR for the treatment of proliferative diabetic retinopathy. | Laser, ranibizumab | To compare panretinal photocoagulation combined with intravitreal injection of ranibizumab and retinal photocoagulation targeted to ischaemic retina combined with intravitreal injections of ranibizumab in PDR. | N/A | 2014 | 2017 | Completed |
| 46 | NCT02857491 | Comparison of intravitreal injection of Ranibizumab versus Sham Injection before vitrectomy in patients with proliferative diabetic retinopathy: a single-centre, prospective double-blinded randomized controlled trial. | Ranibizumab | To evaluate the effect of pre-operative injection of ranibizumab on peri-operative hemorrahge related complications in PDR. | N/A | 2014 | 2017 | Completed |
| 47 | NCT02475109 | A Phase 1 open-label, single-centre study to evaluate the safety and tolerability of topical ocular PAN-90806 in patients with proliferative diabetic retinopathy (PDR) | PAN-90806 Ophthalmic Solution | To evaluate the safety and tolerability of topical ocular PAN-90806 in PDR. | Phase I | 2015 | 2016 | Completed |
| 48 | NCT02447185 | 25-Gauge vitrectomy with Ranibizumab or Triamcinolone Acetonide on proliferative diabetic retinopathy in China: a randomized, Single Blind Trial. | Ranibizumab, Triamcinolone Acetonide | To evaluate the efficacy of intravitreal triamcinolone or intravitreal ranibizumab in vitrectomy setting in PDR. | Phase III | 2015 | 2021 | Recruiting |
| 49 | NCT02590094 | Comparison of interval variation and dosage in preoperative Bevacizumab and Ziv-Aflibercept administration in proliferative diabetic retinopathy undergoing vitrectomy | Bevacizumab, ziv-aflibercept | Bevacizumab versus ziv-aflibercept in PDR. | N/A | 2015 | 2023 | Recruiting |
| 50 | NCT02634333 | Intravitreous anti-vascular endothelial growth factor treatment for prevention of vision threatening diabetic retinopathy in eyes at high risk. | Aflibercept | To evaluate the efficacy and safety of intravitreous aflibercept injections versus sham injections (observation) for prevention of PDR. | Phase III | 2016 | 2022 | Active, not recruiting |
| 51 | NCT02816710 | Different Conbercept injection methods in treatment of severe proliferative diabetic retinopathy. | Conbercept | To evaluate the efficacy of pre-, intra- or post-operative conbercept in PDR eyes undergoing vitrectomy. | Phase IV | 2016 | 2017 | Completed |
| 52 | NCT02863354 | Intravitreal Aflibercept for retinal non-perfusion in proliferative diabetic retinopathy. | Aflibercept | To evaluate the safety and tolerability of 2 mg intravitreal aflibercept injections in PDR. | Phase II | 2016 | 2019 | Completed |
| 53 | NCT02705274 | Panretinal photocoagulation versus intravitreal Bevacizumab for proliferative diabetic retinopathy. | Bevacizumab | To evaluate the effect of bevacizumab and panretinal photocoagulation administered on the basis of DRCR Protocol S data in PDR. | Phase II/III | 2016 | 2017 | Completed |
| 54 | NCT02753400 | A multicenter, randomized, double-masked, placebo-controlled, pilot study to evaluate effects of Emixustat Hydrochloride on aqueous humour biomarkers associated with proliferative diabetic retinopathy. | Emixustat hydrochloride | To evaluate the effects of emixustat in PDR. | Phase II | 2016 | 2017 | Completed |
| 55 | NCT02858076 | Intravitreous anti-VEGF vs. prompt vitrectomy for vitreous haemorrhage from proliferative diabetic retinopathy. | Aflibercept | To evaluate the efficacy of aflibercept vs. laser in PDR. | Phase II | 2016 | 2020 | Completed |
| 56 | NCT03426540 | Safety and efficacy of intravitreal Conbercept injection after vitrectomy for the treatment of early proliferative diabetic retinopathy. | Conbercept | To evaluate safety and efficacy of intravitreal conbercept injection after vitrectomy in PDR. | Phase I | 2017 | 2019 | Completed |
| 57 | NCT03113006 | The individually-marked panretinal laser photocoagulation for proliferative diabetic retinopathy study: IMPETUS 2018 – TREAT. | Laser | To evaluate laser treatment protocol in PDR. | N/A | 2017 | 2019 | Completed |
| 58 | NCT02151695 | Phase 2 Study of Safety and Efficacy of Aflibercept in Proliferative Diabetic Retinopathy. | Aflibercept | To evaluate the efficacy and the safety of aflibercept intravitreal injections compared to panretinal photocoagulation in PDR. | Phase II | 2018 | 2020 | Completed |
| 59 | NCT03633266 | Feasibility study of anti-VEGF instead of intraoperative PRP in proliferative diabetic retinopathy. | Anti-VEGF | To evaluate the efficacy and safety of vitreoretinal surgery combined with anti-VEGF therapy in PDR. | N/A | 2018 | 2022 | Not yet recruiting |
| 60 | NCT02911311 | Conbercept vs. panretinal photocoagulation for the management of proliferative diabetic retinopathy. | Conbercept | To evaluate the efficacy and safety between intravitreal injection of conbercept and panretinal photocoagulation in PDR. | N/A | 2019 | 2022 | Enrolling by invitation |
| 61 | NCT04278417 | A 96-week, two-arm, randomized, single-masked, multi-centre, Phase III Study assessing the efficacy and safety of Brolucizumab 6 mg compared to panretinal photocoagulation laser in patients with proliferative diabetic retinopathy | Brolucizumab | To evaluate the efficacy and safety of brolucizumab compared to panretinal photocoagulation in PDR. | Phase III | 2020 | 2024 | Recruiting |
| 62 | NCT04800679 | Combination therapy for PDR. | Bevacizumab | To evaluate the efficacy of combination laser and bevacizumab therapy in PDR. | Phase II | 2020 | 2021 | Recruiting |
| 63 | NCT04464694 | Prospective, single-blind, randomized, controlled, multi-centre study to evaluate the benefit of Ranibizumab as an adjunctive therapy to vitrectomy for patients with proliferative diabetic retinopathy combined with diabetic macular oedema. | Ranibizumab | To evaluate ranibizumab's benefit on prevention of early postoperative vitreous haemorrhage in PDR. | Phase IV | 2020 | 2022 | Not yet recruiting |
| 64 | NCT04424290 | A first-in human trial to study safety and tolerability of single rising intravitreal dOses (open label, non-randomized, uncontrolled) and in addition the early biological response of multiple intravitreal dosing (single-masked, randomized, Sham-controlled) of BI 764524 in panretinaL photocoagulation (PRP) treated proliferative diabetic retinopathy (PDR) patients with diabetic macular ischaemia (DMI) - the HORNBILL Study | BI 764524 | To evaluate the efficacy of BI 764524 in PDR | Phase I/II | 2020 | 2022 | Recruiting |
| 65 | NCT04674254 | Macular perfusion changes in proliferative diabetic retinopathy following anti-VEGF therapy versus targeted and pan-retinal photocoagulation using optical coherence tomography angiography. | Bevacizumab | To evaluate the effect of bevacizumab, targeted retinal photocoagulation and standard retinal panphotocoagulation on macular perfusion in PDR. | Phase IV | 2021 | 2023 | Recruiting |
| 66 | NCT04661358 | A randomized clinical trial evaluating fenofibrate for prevention of diabetic retinopathy worsening. | Fenofibrate 160 mg | To evaluate the effect of fenofibrate compared with placebo for prevention of DR worsening. | Phase III | 2021 | 2027 | Recruiting |
| 67 | NCT04692688 | Randomized, placebo-controlled, double-masked study of the safety and efficacy of orally administered APX3330 in subjects with moderately severe to severe non-proliferative diabetic retinopathy and mild proliferative diabetic retinopathy | APX3330 | To evaluate the efficacy of APX3330 in PDR. | Phase II | 2021 | 2022 | Recruiting |
Source: https://clinicaltrials.gov/. The order of appearance follows the start date.
Final remarks and conclusions
Neovascular complication may have a remarkable negative impact on patients’ management and visual outcome in DR and is characterized by increased production of VEGF as a major causative factor. In the present review, we provided an overall description of the use of anti-VEGF molecules for the management of PDR, focussing on the current drugs, the molecules under investigation and the possible targets ruling the neovascular process and the development of further complications. It is unquestionable that the introduction of anti-VEGF therapy radically changed the management and prognosis of all the stages of DR, including PDR. Before the anti-VEGF era, the only therapeutic approach was represented by laser, performed on retinal periphery, through panretinal photocoagulation, or at the posterior pole, through focal/grid treatments. Laser approaches turned out to be useful in managing DME and neovascular progression, although the irreversible demolitive effect on retinal structures had a negative impact on the morpho-functional status of diabetic eyes. As highlighted by the present review, a big debate is nowadays present in the literature regarding the comparison between anti-VEGF and laser approaches, and the combined use of both treatments. Most of the current data do not provide enough level of evidence to draw definite conclusions. Through the many studies conducted, the DRCR network offered a strong contribution in the clinical and therapeutic management of DR, highlighting the importance of promptly and frequently repeated intravitreal injections. The overall feel is that laser approach might be avoided when a perfectly planned anti-VEGF therapeutic strategy can be adopted. However, we must bear in mind that the real-life situation is quite different from clinical trials settings. Probably, there is no absolute winning therapeutic choice for managing DR, but the choice of the treatments and of the follow-up timeline must be planned based on personalized strategies designed on patients’ characteristics. The clinical status, including glycemic control and presence of comorbidities, patient’s self-sufficiency, compliance with the frequency of follow-up visits and treatments represent key aspects ruling the overall clinical and ophthalmologic management of the DR patient. The future development of longer duration anti-VEGF treatments and of even more optimized molecules will have a remarkable impact on the feasibility and sustainability of DR patients care for the hospitals and the public health systems, and probably will modify the current indications to laser approaches. According to the EURETINA guidelines [67], anti-VEGF molecules represent the treatment of choice for most of DR patients, because of the high efficacy and safety profiles, and the feasible management. Possible contra-indications regard those patients characterized by high cardiovascular risks, where other approaches, such as corticosteroids [135–138], should be preferred. Laser approaches still maintain a role for diabetic eyes characterized by extremely severe form of DR and for those patients not guaranteeing adequate compliance to intravitreal treatments strategies.
Author contributions
FB and AA performed the revision of the current literature and wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s). Francesco Bandello is consultant for: Alcon (Fort Worth, Texas, USA), Alimera Sciences (Alpharetta, Georgia, USA), Allergan Inc (Irvine, California, USA), Farmila-Thea (Clermont-Ferrand, France), Bayer Shering-Pharma (Berlin, Germany), Bausch And Lomb (Rochester, New York, USA), Genentech (San Francisco, California, USA), Hoffmann-La-Roche (Basel, Switzerland), NovagaliPharma (Évry, France), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA). Alessandro Arrigo and Emanuela Aragona have no disclosures to declare.
Data availability statement
Data are available after a formal request to the corresponding author.
References
- 1.Aiello LP, Wong JS.. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000;77:S113–S9. [DOI] [PubMed] [Google Scholar]
- 2.Antonetti DA, Klein R, Gardner TW.. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Houck K, Jakeman L, et al. . Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13(1):18–32. [DOI] [PubMed] [Google Scholar]
- 4.Vitt UA, Hsu SY, Hsueh AJW.. Evolution and classification of cystine knot-containing hormones and related extracellular signaling molecules. Mol Endocrinol. 2001;15(5):681–694. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Carver-Moore K, Chen H, et al. . Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. [DOI] [PubMed] [Google Scholar]
- 6.Breier G. Functions of the VEGF/VEGF receptor system in the vascular system. Semin Thromb Hemost. 2000;26(5):553–559. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Shibuya M.. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci. 2005;109(3):227–241. [DOI] [PubMed] [Google Scholar]
- 8.Robinson CJ, Stringer SE.. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853–865. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson B, Pajusola K, von Euler G, et al. . Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J Biol Chem. 1996;271(32):19310–19317. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZG, Puri TS, Quigg RJ.. Characterization of novel VEGF (vascular endothelial growth factor)-C splicing isoforms from mouse. Biochem J. 2010;428(3):347–354. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin ME, Roufail S, Halford MM, et al. . Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J Biol Chem. 2001;276(47):44307–44314. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa S, Oku A, Sawano A, et al. . A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J Biol Chem. 1998;273(47):31273–31282. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki Y, Matsunaga Y, Tokunaga Y, et al. . Snake venom vascular endothelial growth factors (VEGF-Fs) exclusively vary their structures and functions among species. J Biol Chem. 2009;284(15):9885–9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancopoulos GD, Davis S, Gale NW, et al. . Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. [DOI] [PubMed] [Google Scholar]
- 16.Jussila L, Alitalo K.. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82(3):673–700. [DOI] [PubMed] [Google Scholar]
- 17.Dvorak HF, Brown LF, Detmar M, et al. . Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 18.Sondell M, Sundler F, Kanje M.. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. [DOI] [PubMed] [Google Scholar]
- 19.Storkebaum E, Lambrechts D, Carmeliet P.. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays. 2004;26(9):943–954. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Lee C, Tang Z, et al. . VEGF-B: a survival, or an angiogenic factor? Cell Adh Migr. 2009;3(4):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeltsch M, Kaipainen A, Joukov V, et al. . Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276(5317):1423–1425. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Linden P, Farnebo J, et al. . Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95(24):14389–14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marconcini L, Marchio S, Morbidelli L, et al. . c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci U S A. 1999;96(17):9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stacker SA, Caesar C, Baldwin ME, et al. . VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7(2):186–191. [DOI] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Aiello LP, Rosenfeld PJ.. Anti-vascular endothelial growth factor agents in the treatment of retinal disease: from bench to bedside. Ophthalmology. 2016;123(10S):S78–S88. [DOI] [PubMed] [Google Scholar]
- 26.Cabral T, Mello LGM, Lima LH, et al. . Retinal and choroidal angiogenesis: a review of new targets. Int J Retina Vitreous. 2017;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penn JS, Madan A, Caldwell RB, et al. . Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27(4):331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang GL, Semenza GL.. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–1237. [DOI] [PubMed] [Google Scholar]
- 29.Grant MB, Mames RN, Fitzgerald C, et al. . Insulin-like growth factor I as an angiogenic agent. In vivo and in vitro studies. Ann NY Acad Sci. 1993;692:230–242. [DOI] [PubMed] [Google Scholar]
- 30.Miller JW, Adamis AP, Aiello LP.. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev. 1997;13(1):37–50. [DOI] [PubMed] [Google Scholar]
- 31.Stone J, Itin A, Alon T, et al. . Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichenbach A, Bringmann A.. Glia of the human retina. Glia. 2020;68(4):768–796. [DOI] [PubMed] [Google Scholar]
- 33.Ida H, Tobe T, Nambu H, et al. . RPE cells modulate subretinal neovascularization, but do not cause regression in mice with sustained expression of VEGF. Invest Ophthalmol Vis Sci. 2003;44(12):5430–5437. [DOI] [PubMed] [Google Scholar]
- 34.Caldwell RB, Bartoli M, Behzadian MA, et al. . Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–524. [DOI] [PubMed] [Google Scholar]
- 35.Shankar A, Mitchell P, Rochtchina E, et al. . Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the blue mountains eye study. Am J Epidemiol. 2006;165(4):375–382. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe D, Suzuma K, Matsui S, et al. . Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353(8):782–792. [DOI] [PubMed] [Google Scholar]
- 37.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15(4):215–228. [DOI] [PubMed] [Google Scholar]
- 38.Puddu A, Sanguineti R, Durante A, et al. . Vascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularization. Mol Vis. 2012;18:2509–2517. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B, Ma A, Cai J, et al. . VEGF-A regulates the expression of VEGF-C in human retinal pigment epithelial cells. Br J Ophthalmol. 2006;90(8):1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SX, Wang JJ, Gao G, et al. . Pigment epithelium-derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF-VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol. 2006;37(1):1–12. [DOI] [PubMed] [Google Scholar]
- 41.Kociok N, Joussen AM.. Varied expression of functionally important genes of RPE and choroid in the macula and in the periphery of normal human eyes. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):101–113. [DOI] [PubMed] [Google Scholar]
- 42.Leasher JL, Bourne RR, Flaxman SR, on behalf of the Vision Loss Expert Group of the Global Burden of Disease Study, et al.. Vision loss expert group of the global burden of disease study. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. [DOI] [PubMed] [Google Scholar]
- 43.Havens SJ, Gulati V.. Neovascular glaucoma. Dev Ophthalmol. 2016;55:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stitt AW, Curtis TM, Chen M, et al. . The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016;51:156–186. [DOI] [PubMed] [Google Scholar]
- 45.Antonetti DA, Barber AJ, Hollinger LA, et al. . Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274(33):23463–23467. [DOI] [PubMed] [Google Scholar]
- 46.Behzadian MA, Windsor LJ, Ghaly N, et al. . VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. Faseb J. 2003;17(6):752–754. [DOI] [PubMed] [Google Scholar]
- 47.Gavard J, Gutkind JS.. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. [DOI] [PubMed] [Google Scholar]
- 48.Feng Y, Venema VJ, Venema RC, et al. . VEGF-induced permeability increase is mediated by caveolae. Invest Ophthalmol Vis Sci. 1999;40(1):157–167. [PubMed] [Google Scholar]
- 49.Hofman P, Blaauwgeers HG, Tolentino MJ, et al. . VEGF-A induced hyperpermeability of blood-retinal barrier endothelium in vivo is predominantly associated with pinocytotic vesicular transport and not with formation of fenestrations. Vascular endothelial growth factor-A. Curr Eye Res. 2000;21(2):637–645. [PubMed] [Google Scholar]
- 50.Daruich A, Matet A, Moulin A, et al. . Mechanisms of macular edema: beyond the surface. Prog Retin Eye Res. 2018;63:20–68. [DOI] [PubMed] [Google Scholar]
- 51.Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, et al. . Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102–115. [DOI] [PubMed] [Google Scholar]
- 52.Singh RP, Elman MJ, Singh SK, et al. . Advances in the treatment of diabetic retinopathy. J Diabetes Complications. 2019;33(12):107417. [DOI] [PubMed] [Google Scholar]
- 53.Witmer AN, Vrensen GF, Van Noorden CJ, et al. . Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. [DOI] [PubMed] [Google Scholar]
- 54.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39(5):469–478. [DOI] [PubMed] [Google Scholar]
- 55.Takagi H, Koyama S, Seike H, et al. . Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(1):393–402. [DOI] [PubMed] [Google Scholar]
- 56.Oh H, Takagi H, Otani A, et al. . Selective induction of neuropilin-1 by vascular endothelial growth factor (VEGF): a mechanism contributing to VEGF-induced angiogenesis. Proc Natl Acad Sci USA. 2002;99(1):383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaturvedi N, Sjolie AK, Stephenson JM, et al. . Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID study group. EURODIAB controlled trial of lisinopril in Insulin-Dependent diabetes mellitus. Lancet. 1998;351(9095):28–31. [DOI] [PubMed] [Google Scholar]
- 58.Rennel E, Guan WE, Schüler H, et al. . S. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98(7):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JC, Haworth L, Sherry RM, et al. . A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenfeld PJ, Moshfeghi AA, Puliafito CA.. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 61.Kreisl TN, Kim L, Moore K, et al. . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spasic M, Chow F, Tu C, et al. . Molecular characteristics and pathways of avastin for the treatment of glioblastoma multiforme. Neurosurg Clin N Am. 2012;23(3):417–427. [DOI] [PubMed] [Google Scholar]
- 63.Haritoglou C, Kook D, Neubauer A, et al. . Intravitreal bevacizumab (avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26(9):999–1005. [DOI] [PubMed] [Google Scholar]
- 64.Stefanini FR, Arevalo JF, Maia M.. Bevacizumab for the management of diabetic macular edema. World J Diabetes. 2013;4(2):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choovuthayakorn J, Tantraworasin A, Phinyo P, et al. . Factors associated with 1-year visual response following intravitreal bevacizumab treatment for diabetic macular edema: a retrospective single center study. Int J Retina Vitreous. 2021;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scott IU, Edwards AR, Beck RW, Diabetic Retinopathy Clinical Research Network, et al.. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114(10):1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michaelides M, Kaines A, Hamilton RD, et al. . A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086.e2. [DOI] [PubMed] [Google Scholar]
- 68.Rajendram R, Fraser-Bell S, Kaines A, et al. . A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130(8):972–979. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. . A. Guidelines for the management of diabetic macular edema by the european society of retina specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, Canny MD, De Erkenez A, et al. . A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc Natl Acad Sci USA. 2005;102(52):18902–18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. . VEGF inhibition study in ocular neovascularization clinical trial group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805–2816. [DOI] [PubMed] [Google Scholar]
- 72.Ferrara N, Damico L, Shams N, et al. . Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26(8):859–870. [DOI] [PubMed] [Google Scholar]
- 73.Lowe J, Araujo J, Yang J, et al. . Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res. 2007;85(4):425–430. [DOI] [PubMed] [Google Scholar]
- 74.Vaidyanathan U, Moshirfar M.. Ranibizumab. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [Google Scholar]
- 75.Platania CB, Di Paola L, Leggio GM, et al. . Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: a computational approach. Front Pharmacol. 2015;6:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J, Wang X, Fuh G, et al. . Comparison of binding characteristics and in vitro activities of three inhibitors of vascular endothelial growth factor A. Mol Pharm. 2014;11(10):3421–3430. [DOI] [PubMed] [Google Scholar]
- 77.Nguyen QD, Shah SM, Khwaja AA, et al. . READ-2 study group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. [DOI] [PubMed] [Google Scholar]
- 78.Massin P, Bandello F, Garweg JG, et al. . Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. . Weichselberger A; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen QD, Brown DM, Marcus DM, RISE and RIDE Research Group, et al.. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. [DOI] [PubMed] [Google Scholar]
- 81.Comyn O, Sivaprasad S, Peto T, et al. . A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am J Ophthalmol. 2014;157(5):960–970. [DOI] [PubMed] [Google Scholar]
- 82.Ishibashi T, Li X, Koh A, REVEAL Study Group, et al.. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402–1415. [DOI] [PubMed] [Google Scholar]
- 83.Pearce I, Banerjee S, Burton BJ, RELIGHT Study Group, et al.. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-Month, multicenter, phase IIIB RELIGHT study. Ophthalmology. 2015;122(9):1811–1819. [DOI] [PubMed] [Google Scholar]
- 84.Prünte C, Fajnkuchen F, Mahmood S, RETAIN Study Group, et al.. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100(6):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sepah YJ, Sadiq MA, Boyer D, READ-3 Study Group, et al.. Twenty-four-Month outcomes of the ranibizumab for edema of the macula in Diabetes – Protocol 3 with high dose (READ-3) study. Ophthalmology. 2016;123(12):2581–2587. [DOI] [PubMed] [Google Scholar]
- 86.Gross JG, Glassman AR, Jampol LM, Writing Committee for the Diabetic Retinopathy Clinical Research Network, et al.. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gross JG, Glassman AR, Liu D, Diabetic Retinopathy Clinical Research Network, et al.. Diabetic retinopathy clinical research network. Five-year outcomes of panretinal photocoagulation vs. intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wells JA, Glassman AR, Ayala AR, Diabetic Retinopathy Clinical Research Network, et al.. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singer MA, Miller DM, Gross JG, et al. . Visual acuity outcomes in diabetic macular edema with fluocinolone acetonide 0.2 μg/day versus ranibizumab plus deferred laser (DRCR protocol I). Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):698–706. [DOI] [PubMed] [Google Scholar]
- 90.Payne JF, Wykoff CC, Clark WL, TREX-DME Study Group, et al.. Randomized trial of treat and extend ranibizumab with and without navigated laser for diabetic macular edema: TREX-DME 1 year outcomes. Ophthalmology. 2017;124(1):74–81. [DOI] [PubMed] [Google Scholar]
- 91.Payne JF, Wykoff CC, Clark WL, TREX-DME Study Group, et al.. Randomized trial of treat and extend ranibizumab with and without navigated laser versus monthly dosing for diabetic macular edema: TREX-DME 2-Year outcomes. Am J Ophthalmol. 2019;202:91–99. [DOI] [PubMed] [Google Scholar]
- 92.Payne JF, Wykoff CC, Clark WL, TREX-DME Study Group, et al.. Long-term outcomes of treat-and-extend ranibizumab with and without navigated laser for diabetic macular oedema: TREX-DME 3-year results. Br J Ophthalmol. 2021;105(2):253–257. [DOI] [PubMed] [Google Scholar]
- 93.Fechter C, Frazier H, Marcus WB, et al. . Ranibizumab 0.3 mg for persistent diabetic macular edema after recent, frequent, and chronic bevacizumab: the ROTATE trial. Ophthalmic Surg Lasers Imag Retina. 2016;47(11):1–18. [DOI] [PubMed] [Google Scholar]
- 94.Lang GE, Liakopoulos S, Vögeler J, et al. . The RELATION study: efficacy and safety of ranibizumab combined with laser photocoagulation treatment versus laser monotherapy in NPDR and PDR patients with diabetic macular oedema. Acta Ophthalmol. 2018;96(3):e377–e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li X, Dai H, Li X, REFINE study group, et al.. Efficacy and safety of ranibizumab 0.5 mg in chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefes Arch Clin Exp Ophthalmol. 2019;257(3):529–541. [DOI] [PubMed] [Google Scholar]
- 96.Holash J, Davis S, Papadopoulos N, et al. . VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng YD, Yang H, Chen GQ, et al. . Molecularly targeted drugs for metastatic colorectal cancer. Drug Des Devel Ther. 2013;7:1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mansour AM, Al-Ghadban SI, Yunis MH, et al. . Ziv-aflibercept in macular disease. Br J Ophthalmol. 2015;99(8):1055–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. . Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. [DOI] [PubMed] [Google Scholar]
- 100.Brown DM, Schmidt-Erfurth U, Do DV, et al. . Intravitreal aflibercept for diabetic macular edema: 100-Week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044–2052. [DOI] [PubMed] [Google Scholar]
- 101.Wykoff CC, Le RT, Khurana RN, ENDURANCE Study Group, et al.. Outcomes with as-needed aflibercept and macular laser following the phase III VISTA DME trial: ENDURANCE 12-month extension study. Am J Ophthalmol. 2017;173:56–63. [DOI] [PubMed] [Google Scholar]
- 102.Baker CW, Glassman AR, Beaulieu WT, DRCR Retina Network, et al.. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maturi RK, Glassman AR, Josic K, DRCR Retina Network, et al.. Effect of intravitreous anti-Vascular endothelial growth factor vs sham treatment for prevention of Vision-Threatening complications of diabetic retinopathy: the protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang M, Zhang J, Yan M, et al. . Recombinant anti-vascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis. 2008;14:37–49. [PMC free article] [PubMed] [Google Scholar]
- 105.Suto K, Yamazaki Y, Morita T, et al. . Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005;280(3):2126–2131. [DOI] [PubMed] [Google Scholar]
- 106.Liu K, Wang H, He W, et al. . Intravitreal conbercept for diabetic macular oedema: 2-year results from a randomised controlled trial and open-label extension study. Br J Ophthalmol. 2021;:2020–318690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li F, Zhang L, Wang Y, et al. . One-year outcome of conbercept therapy for diabetic macular edema. Curr Eye Res. 2018;43(2):218–223. [DOI] [PubMed] [Google Scholar]
- 108.Cai S, Yang Q, Li X, et al. . The efficacy and safety of aflibercept and conbercept in diabetic macular edema. Drug Des Devel Ther. 2018;12:3471–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y, Rong A, Bi Y, et al. . Intravitreal conbercept injection with and without grid laser photocoagulation in the treatment of diffuse diabetic macular edema in real-life clinical practice. J Ophthalmol. 2016;2016:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H, Guo J, Tao S, et al. . One-Year effectiveness study of intravitreously administered conbercept® monotherapy in diabetic macular degeneration: a systematic review and meta-analysis. Diabetes Ther. 2020;11(5):1103–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yannuzzi NA, Freund KB.. Brolucizumab: evidence to date in the treatment of neovascular age-related macular degeneration. Clin Ophthalmol. 2019;13:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharma A, Kumar N, Kuppermann BD, et al. . Brolucizimab-leading an era of structural revolution for long-term VEGF suppression. Eye. 2020;34(4):611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nguyen QD, Das A, Do DV, et al. . Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020;127(7):963–976. [DOI] [PubMed] [Google Scholar]
- 114.Tadayoni R, Sararols L, Weissgerber G, et al. . Brolucizumab: a newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2021;244(2):93–101. [DOI] [PubMed] [Google Scholar]
- 115.Garweg JG. A randomized, Double-Masked, multicenter, phase III study assessing the efficacy and safety of brolucizumab versus aflibercept in patients with visual impairment due to diabetic macular edema (KITE). Klin Monbl Augenheilkd. 2020;237(4):450–453. [DOI] [PubMed] [Google Scholar]
- 116.Stumpp MT, Binz HK, Amstutz P.. DARPins: a new generation of protein therapeutics. Drug Discov Today. 2008;13(15–16):695–701. [DOI] [PubMed] [Google Scholar]
- 117.Souied EH, Devin F, Mauget-Faÿsse M, MP0112 Study Group, et al.. Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2014;158(4):724–732.e2. [DOI] [PubMed] [Google Scholar]
- 118.Rodrigues GA, Mason M, Christie LA, et al. . Functional characterization of Abicipar-Pegol, an anti-VEGF DARPin therapeutic that potently inhibits angiogenesis and vascular permeability. Invest Ophthalmol Vis Sci. 2018;59(15):5836–5846. [DOI] [PubMed] [Google Scholar]
- 119.Nicolò M, Ferro Desideri L, Vagge A, et al. . Faricimab: an investigational agent targeting the tie-2/angiopoietin pathway and VEGF-A for the treatment of retinal diseases. Expert Opin Investig Drugs. 2021;30(3):193–200. [DOI] [PubMed] [Google Scholar]
- 120.Sahni J, Patel SS, Dugel PU, et al. . Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth Factor-A with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology. 2019;126(8):1155–1170. [DOI] [PubMed] [Google Scholar]
- 121.Striglia E, Caccioppo A, Castellino N, et al. . Emerging drugs for the treatment of diabetic retinopathy. Expert Opin Emerg Drugs. 2020;25(3):261–271. [DOI] [PubMed] [Google Scholar]
- 122.Diabetic Retinopathy Clinical Research Network* Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131(3):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sivaprasad S, Prevost AT, Vasconcelos JC, CLARITY Study Group, et al.. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193–2203. [DOI] [PubMed] [Google Scholar]
- 124.Figueira J, Fletcher E, Massin P, EVICR.net Study Group., et al.. Ranibizumab plus panretinal photocoagulation versus panretinal photocoagulation alone for high-risk proliferative diabetic retinopathy (PROTEUS study). Ophthalmology. 2018;25(5):691–700. [DOI] [PubMed] [Google Scholar]
- 125.Korobelnik JF, Daien V, Faure C, et al. . Real-world outcomes following 12 months of intravitreal aflibercept monotherapy in patients with diabetic macular edema in France: results from the APOLLON study. Graefes Arch Clin Exp Ophthalmol. 2020;258(3):521–528. [DOI] [PubMed] [Google Scholar]
- 126.Brown DM, Wykoff CC, Boyer D, et al. . Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 2021;:e212809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alagorie AR, Velaga S, Nittala MG, et al. . Effect of aflibercept on diabetic retinopathy severity and visual function in the RECOVERY study for proliferative diabetic retinopathy. Ophthalmol Retina. 2021;5(5):409–419. [DOI] [PubMed] [Google Scholar]
- 128.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. . Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2014;2014(11):CD008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simunovic MP, Maberley DA.. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: a systematic review and meta-analysis. Retina. 2015;35(10):1931–1942. [DOI] [PubMed] [Google Scholar]
- 130.Bressler SB, Beaulieu WT, Glassman AR, et al. . Diabetic retinopathy clinical research network. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology. 2017;124(4):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao S, Lin Z, Shen X.. Anti-Vascular endothelial growth factor therapy as an alternative or adjunct to pan-retinal photocoagulation in treating proliferative diabetic retinopathy: meta-analysis of randomized trials. Front Pharmacol. 2020;11:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fallico M, Maugeri A, Lotery A, et al. . Intravitreal anti-vascular endothelial growth factors, panretinal photocoagulation and combined treatment for proliferative diabetic retinopathy: a systematic review and network meta-analysis. Acta Ophthalmol. 2021;99(6):e795–e805. [DOI] [PubMed] [Google Scholar]
- 133.Maguire MG, Liu D, Glassman AR, DRCR Retina Network, et al.. Visual field changes over 5 years in patients treated with panretinal photocoagulation or ranibizumab for proliferative diabetic retinopathy. JAMA Ophthalmol. 2020;138(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun JK, Glassman AR, Beaulieu WT, et al. . Diabetic retinopathy clinical research network. Rationale and application of the protocol S anti-vascular endothelial growth factor algorithm for proliferative diabetic retinopathy. Ophthalmology. 2019;126(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tan Y, Fukutomi A, Sun MT, et al. . Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: a review. Surv Ophthalmol. 2021;66(6):926–932. [DOI] [PubMed] [Google Scholar]
- 136.Boyer DS, Yoon YH, Belfort R, Jr, Ozurdex MEAD Study Group., et al.. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. [DOI] [PubMed] [Google Scholar]
- 137.Campochiaro PA, Brown DM, Pearson A, et al. . Kane FE; FAME study group. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635.e2. [DOI] [PubMed] [Google Scholar]
- 138.Campochiaro PA, Brown DM, Pearson A, FAME Study Group, et al.. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available after a formal request to the corresponding author.