Abstract
BACKGROUND:
The purpose of this study is to evaluate the utility of vasoactive-inotropic score (VIS) in predicting outcomes after left ventricular assist device (LVAD) implantation and explore possible mechanisms of post-operative hemodynamic instability.
METHODS:
Retrospective review was performed in 418 consecutive patients with LVAD implantation. VIS was calculated as dopamine + dobutamine + 10 × milrinone + 100 × epinephrine + 100 × norepinephrine (all μg/kg/min) + 10000 × vasopressin (U/kg/min) after initial stabilization in the operating room and upon arrival at the intensive care unit. The primary outcome was in-hospital mortality. The secondary outcomes were a composite of in-hospital mortality, delayed right ventricular assist device (RVAD) implantation, and continuous renal replacement therapy. The pre-operative biomarkers of inflammation, oxidative stress, endotoxemia and gut-derived metabolite trimethylamine-N-oxide (TMAO) were measured in a subset of 61 patients.
RESULTS:
Median VIS was 20.0 (interquartile range 13.3−27.9). VIS was an independent predictor of in-hospital mortality (odds ratio [OR] 1.06, 95% confidence interval [CI] 1.03−1.09, p < 0.001) and composite outcome (OR 1.03, 95% CI 1.01−1.06, p = 0.008). In-hospital mortality increased for each VIS quartile (0% vs 3.9% vs 7.6% vs 12.3%, p = 0.002). VIS was superior to other established LVAD risk models as a predictor of in-hospital mortality (area under the curve 0.73, 95% CI 0.64−0.82). The optimal cut-off point for VIS as a predictor of in-hospital mortality was 20. Pre-operative hemoglobin level was the only independent predictor of VIS ≥ 20 (p = 0.003). Patients with a high VIS were more likely to have elevated TMAO pre-operatively (53.6% vs 25.8%, p = 0.03).
CONCLUSIONS:
A high post-operative VIS is associated with adverse in-hospital outcomes and is a better predictor of in-hospital mortality compared with existing LVAD risk models. Whether early hemodynamic stabilization using RVAD may benefit patients with a high VIS remains to be investigated.
Keywords: ventricular assist device, inotropes, vasopressors, in-hospital mortality, trimethylamine-N-oxide
The use of durable continuous flow left ventricular assist devices (LVADs) has been shown to improve the survival and quality of life in patients with advanced heart failure (HF).1 However, in-hospital mortality at the time of LVAD implantation still remains as high as 6.0% in the modern era.2 Post-operative complications such as the need for right ventricular assist device (RVAD) and continuous renal replacement therapy (RRT) are reported to be 6%−9%3,4 and 14%,5 respectively. The discriminatory ability of LVAD risk scores, such as HeartMate II Risk Score (HMRS) and Right Ventricular Failure Risk Score (RVFRS), for the prediction of post-operative outcomes has been limited.6,7 In addition, the pathophysiological basis for early unfavorable outcomes following LVAD surgery is poorly understood. Inflammation, oxidative stress, endotoxemia and gut-derived metabolites, such as trimethylamine-N-oxide (TMAO), have been associated with HF progression and outcomes, and as such might predispose to untoward post-LVAD complications.8–15 In addition, cardiac surgery provokes a vigorous inflammatory and oxidative response that together may culminate in severe peripheral vasodilatation and myocardial dysfunction.
Vasoactive-inotropic score (VIS) has been extensively studied in pediatric patients undergoing cardiopulmonary bypass (CPB) to measure illness severity and as a surrogate marker for hemodynamic cardiovascular derangement at the time of intensive care unit admission. The initial inotrope score was developed by Wernovsky et al16 and then modified as VIS by Gaies et al17 to include additional vasoactive medications used in modern clinical practice. Prior studies in infants and adults undergoing cardiac surgery showed that an elevated VIS was associated with in-hospital mortality and adverse events, including cardiac arrest, RRT, and neurological injury.17–20 However, the utility of VIS in patients with LVAD implantation has never been studied.
Our main objectives were to (1) investigate whether VIS is an independent predictor of mortality, RVAD implantation, and RRT at index hospitalization among adult patients with HF undergoing LVAD implantation; (2) determine the optimal cut-off point of VIS as a predictor of outcomes; and (3) identify pre-operative and intraoperative predictors of a high VIS. In addition, as an exploratory aim, we investigated the association of pre-operative inflammation, endotoxemia, oxidative stress, and TMAO with the development of post-operative hemodynamic instability in patients with advanced HF undergoing LVAD surgery.
Methods
Study population
This study was approved by the Columbia University Irving Medical Center Institutional Review Board with a waiver of consent. A total of 469 adult (age ≥ 18) patients received LVAD implantation from April 2004 to December 2015 at Columbia University Irving Medical Center. Of those, 418 (89.1%) patients were included in this study after excluding 19 (4.1%) patients who received concomitant RVAD at the time of initial LVAD implantation and 32 (6.8%) patients with limited chart availability.
Calculation of VIS and hemodynamic data
VIS was calculated using the following formula14,15: dopamine + dobutamine + milrinone (× 10) + epinephrine (× 100) + norepinephrine (× 100) (all μg/kg/min) + vasopressin (× 10000) (U/kg/min). VIS and hemodynamic data (Fick cardiac index, systemic vascular resistance [SVR], mean arterial pressure [MAP], central venous pressure [CVP], and mean pulmonary artery pressure [PAP]) were acquired after initial stabilization in the operating room and upon arrival to the intensive care unit.
Data collection
Patient data were obtained from electronic medical records by coauthors (JH and AP). Pre-operative laboratory values and hemodynamics were collected within 24−48 hours prior to LVAD implantation. Pre-operative inotrope support was defined as the use of milrinone, dobutamine, and dopamine immediately prior to LVAD implantation. Pre-operative vasopressor support was defined as the use of norepinephrine, epinephrine, and vasopressin. The primary outcome was all-cause in-hospital mortality. The secondary outcome was a composite of in-hospital mortality, delayed RVAD implantation, or a need for RRT during index hospitalization. The existing risk models for patients with LVAD implantation, such as HMRS and RVFRS, were calculated to assess their relationship with in-hospital mortality as well as with VIS.
Measurements of plasma and serum biomarkers
As an exploratory analysis, in a cohort of 61 patients who underwent LVAD implantation from February 2016 to December 2017, we prospectively obtained measures of biomarkers of inflammation, oxidative stress, endotoxemia, and TMAO at a single time point pre-operatively (median 3, interquartile range [IQR] 1−8; days before surgery). The biomarkers of endotoxemia (lipopolysaccharide [LPS] and soluble CD14 [sCD14]), inflammation (C-reactive protein [CRP], interleukin-6 [IL-6], tumor necrosis factor-α [TNF-α], endothelin-1 [ET-1], and adiponectin), and oxidative stress (isoprostane) were measured in plasma or serum. Serum CRP was measured using a high sensitivity (0.3 mg/liter) particle enhanced turbidimetric assay on the automated analyzer, Integra 400 plus (Roche Diagnostics, Indianapolis, IN). Serum IL-6, TNF-α, sCD14, and ET-1 were assessed using a high-sensitivity enzyme-linked immunoassay (ELISA) kit (RD Systems, Minneapolis, MN). Serum adiponectin was measured using a Millipore Radioimmunoassay (RIA) kit (cat no. HADP-61HK, Billerica, MA). Plasma LPS was measured using a Limulus Amebocyte Lysate Chromogenic Endotoxin Quantitation Kit (Pierce Thermo Scientific, Rockford, IL). Plasma isoprostane (8, 12-iso-iPF2a-VI) was measured in butylated hydroxytoluene-treated human plasma samples using liquid chromatography-tandem mass spectrometry (LC-MS)/tandem mass spectrometry (MS) on Acquity UPLC-Xevo TQS (Waters. Milford, MA). TMAO was measured in human plasma samples using ultraperformance LC-MS after protein precipitation using deuterated (D9)-TMAO as the internal standard. LC-MS analysis was performed on a platform comprising Eksigent UPLC 100 integrated to an API 4,000 mass spectrometer, which was controlled by Analyst 1.6 (AB Sciex, Foster City, CA).
Statistical analysis
Stata 14 software (Stata Corp, College Station, TX) was used in statistical analyses. Statistical significance was determined based on a pre-determined two-sided α of 0.05. Logistic regression models were derived for a range of VIS cut-off points and Youden’s index was used to identify the optimal cut-off point of VIS for in-hospital mortality. The entire cohort was dichotomized into high vs low VIS groups based on the optimal cut-off point. The categorical variables are summarized with frequencies and percentages and were compared across VIS groups using chi-squared or Fisher’s exact tests as appropriate. The continuous variables are summarized as mean ± standard deviation (among all patients) or mean ± standard error (among VIS groups) for normally distributed data and as median (IQR) for non-normally distributed data. The differences in continuous variables between VIS groups were compared with either two-sample t-tests or Mann-Whitney-Wilcoxon tests, as appropriate. A series of univariate logistic models from pre-and intraoperative characteristics listed in Table 1 were used to determine whether VIS was an independent predictor of in-hospital mortality as the primary outcome and composite in-hospital mortality, delayed RVAD implantation, and RRT as secondary outcomes. The pre-operative variables at threshold of p < 0.1 and potential confounders including age, body mass index, and gender were then entered into a multivariate model. The final model was selected using Mallow’s CP as a criterion. To identify pre-and intraoperative predictors of high VIS, a series of univariate logistic models regressed high VIS on pre-and intraoperative predictors of high VIS. The variables at a threshold of p < 0.1 and clinically relevant variables were then entered into a multivariate model. The final model was selected using Mallow’s CP as a criterion. In a subset of patients with biomarker values, the biomarkers were dichotomized using a median split and were compared across high and low VIS groups using chi-squared tests. For TMAO, we further utilized a linear regression model to additionally assess the relationship between log TMAO values and continuous VIS.
Table 1.
Pre-Operative and Intraoperative Baseline Characteristics
| All patients(n = 418) | VIS ≥ 20(n = 210) | VIS < 20(n = 208) | p-value | |
|---|---|---|---|---|
|
| ||||
| Demographic data | ||||
| Age (years) | 57.8 ± 13.1 | 59.0 ± 0.88 | 56.6 ± 0.93 | 0.06 |
| BMI (kg/m2) | 26.3 ± 5.4 | 25.4 ± 0.35 | 27.2 ± 0.39 | <0.001 |
| Male, n (%) | 343 (82.1) | 172 (81.9) | 171 (82.2) | 0.94 |
| Ischemic etiology, n (%) | 169 (40.4) | 86 (41.0) | 83 (39.9) | 0.06 |
| LVAD indication | 0.86 | |||
| BTT, n (%) | 265 (63.4) | 134 (63.8) | 131 (63.0) | |
| DT, n (%) | 153 (36.6) | 76 (36.2) | 77 (37.0) | |
| Prior sternotomy (%) | 132 (32.0) | 79 (37.8) | 53 (26.0) | 0.010 |
| Pre-operative inotrope support | 350 (83.7) | 172 (81.9) | 178 (85.6) | 0.31 |
| Pre-operative vasopressor, n (%) | 34 (8.4) | 18 (8.8) | 16 (7.9) | 0.73 |
| Pre-operative ventilator, n (%) | 17 (4.2) | 12 (5.9) | 5 (2.5) | 0.08 |
| Pre-operative IABP, n (%) | 119 (28.5) | 69 (33.0) | 50 (24.0) | 0.042 |
| Pre-operative mechanical | ||||
| Circulatory support, n (%) | 41 (9.8) | 29 (13.8) | 12 (5.8) | 0.006 |
| ECMO | 7 (1.7) | 5 (2.4) | 2 (1.0) | 0.25 |
| Impella | 6 (1.4) | 5 (2.4) | 1 (0.5) | 0.10 |
| CentriMag VAD | 32 (7.7) | 23 (11.0) | 9 (4.3) | 0.011 |
| INTERMACS profile | 0.004 | |||
| 1 | 32 (7.7) | 22 (10.5) | 10 (4.8) | |
| 2 | 129 (30.9) | 62 (29.5) | 67 (32.2) | |
| 3 | 58 (13.9) | 27 (8.1) | 41 (19.7) | |
| 4 | 10 (2.4) | 4 (1.9) | 6 (2.9) | |
| Hemodynamic data | ||||
| CVP (mm Hg) | 10.8 ± 5.3 | 10.7 ± 0.4 | 11.0 ± 0.4 | 0.58 |
| Mean PAP (mm Hg) | 35.0 ± 9.8 | 34.3 ± 0.7 | 35.7 ± 0.7 | 0.17 |
| PCWP (mm Hg) | 23.7 ± 7.9 | 24.0 ± 0.6 | 23.4 ± 0.6 | 0.52 |
| CO (liter/min) | 3.48 ± 1.11 | 3.48 ± 0.1 | 3.46 ± 0.1 | 0.11 |
| PVR (Wood units) | 4.02 ± 2.64 | 3.98 ± 0.2 | 4.05 ± 0.2 | 0.81 |
| Laboratory values | ||||
| Hemoglobin (g/dl) | 11.4 ± 2.1 | 10.9 ± 0.1 | 11.9 ± 0.1 | <0.001 |
| Creatinine (mg/dl) | 1.4 (1.1–1.7) | 1.4 (1.1–1.7) | 1.4 (1.1–1.7) | 0.58 |
| Albumin (g/dl) | 3.7 (3.2–4.0) | 3.6 (3.1–3.9) | 3.7 (3.4–4.1) | 0.007 |
| AST (IU/liter) | 24 (18–34) | 25 (19–40) | 22 (18–31) | 0.032 |
| Total bilirubin (mg/dl) | 1.0 (0.7–1.7) | 1.1 (0.7–1.9) | 1.0 (0.7–1.5) | 0.11 |
| Medications | ||||
| ACE inhibitor use, n (%) | 116 (31.0) | 51 (27.4) | 65 (34.6) | 0.14 |
| Beta blocker use, n (%) | 285 (75.8) | 139 (74.7) | 146 (76.8) | 0.63 |
| Amiodarone use, n (%) | 190 (50.8) | 111 (59.7) | 79 (42.0) | 0.001 |
| Intraoperative characteristics | ||||
| CPB time (min) | 87 (63–119) | 97 (67–130) | 79 (59–104) | <0.001 |
| Intraoperative blood products | ||||
| PRBC use, n (%) | 187 (45.4) | 115 (55.6) | 72 (35.1) | <0.001 |
| FFP use, n (%) | 293 (71.1) | 157 (75.9) | 136 (66.3) | 0.033 |
| Platelets use, n (%) | 335 (81.3) | 173 (83.6) | 162 (79.0) | 0.236 |
| Concomitant valve procedures | 171 (40.9) | 101 (48.1) | 70 (33.7) | 0.003 |
| Tricuspid valve | 75 (17.9) | 49 (23.3) | 26 (12.5) | 0.004 |
| Mitral valve | 77 (18.4) | 48 (22.9) | 29 (13.9) | 0.019 |
| Aortic valve | 60 (14.3) | 25 (11.9) | 35 (16.8) | 0.15 |
ACE, angiotensin-converting enzyme; AST, aspartate aminotransferase; BMI, body mass index; BSA, body surface area; BTT, bridge-to-transplantation; CO, cardiac output; CPB, cardiopulmonary bypass; CVP, central venous pressure; DT, destination therapy; ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; IABP, intra-aortic balloon pump; LVAD, left ventricular assist device; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PRBC, packed red blood cells; PVR, peripheral vascular resistance; VAD, ventricular assist device; VIS, vasoactive-inotropic score.
Results
VIS as a predictor of clinical outcomes
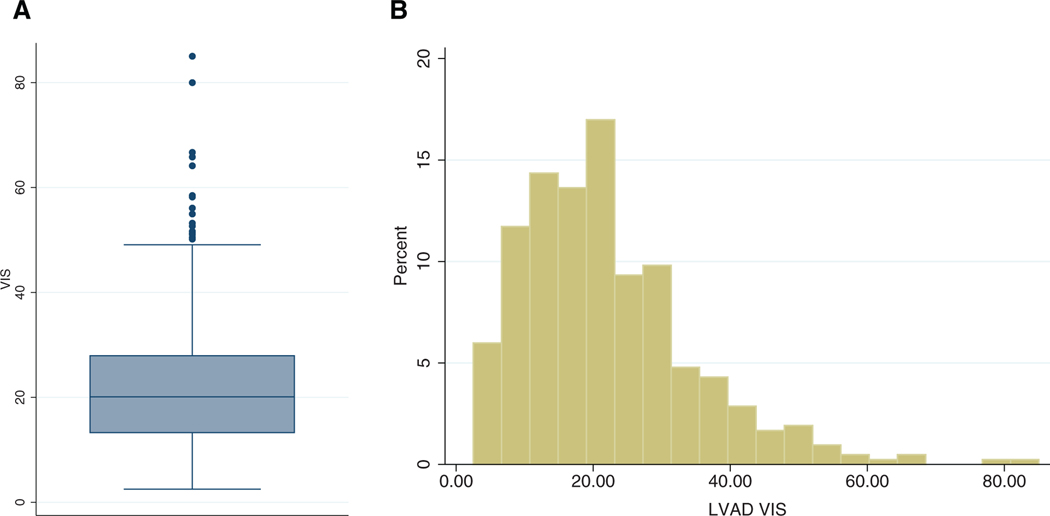
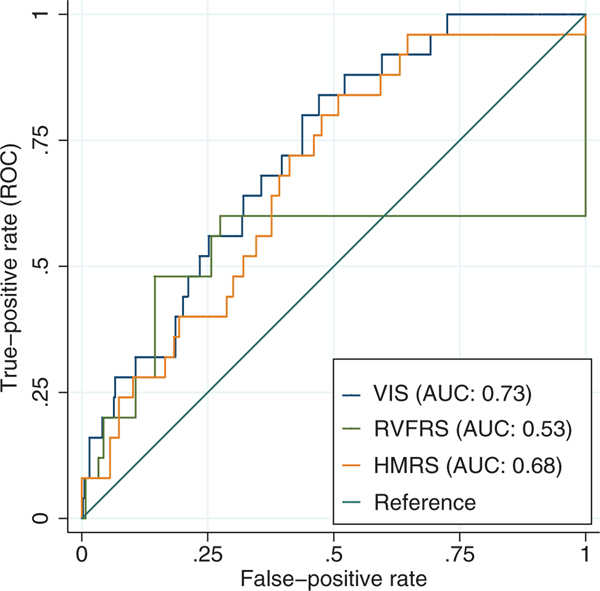
Pre- and intraoperative characteristics of all patients are outlined in Table 1. In the entire cohort, the mean age was 57.8 1 13.1 years, 82.1% were male, 36.6% were undergoing destination therapy, and 40.4% had ischemic etiology. A total of 83.7% of patients were on inotrope support and 8.4% patients were on vasopressors pre-operatively. The median VIS was 20.0 (IQR 13.3−27.9). The maximum VIS score was 85.0, and the overall distribution is shown in the boxplot and histogram in Figure 1. VIS was a significant predictor for in-hospital mortality with an OR of 1.06 per each unit increase in VIS (95% CI 1.03−1.09, p < 0.001) after adjusting for destination therapy, pre-operative vasopressor use, and pre-operative hemoglobin and creatinine levels (Table 2). Receiver operating curve (ROC) analyses (Figure 2) showed that VIS as a predictor of in-hospital mortality had an area under the curve (AUC) of 0.73 (95% CI 0.64−0.82) compared with other risk models for LVAD, such as HMRS (AUC 0.68, 95% CI 0.58−0.78) and RVFRS (AUC 0.53, 95% CI 0.35−0.70). VIS was also a significant predictor for the composite outcome of in-hospital mortality, delayed RVAD implantation, and the need for RRT therapy during index hospitalization with an OR of 1.03 for each unit increase in VIS (95% CI 1.01 −1.06, p = 0.008) after adjusting for pre-operative hemoglobin, albumin, and creatinine levels (Table 2).
Figure 1.
Distribution of vasoactive-inotropic score (VIS). (A) Boxplot of VIS. (B) Histogram of VIS. LVAD, left ventricular assist device.
Table 2.
Final Model for All Outcomes
| Final model for in-hospital mortality (Mallow’s CP 4.629) | |||
|---|---|---|---|
|
| |||
| Variable | OR | 95% CI | p-value |
|
| |||
| Destination therapy | 4.52 | 1.79–12.53 | 0.002 |
| Pre-operative vasopressor use | 3.37 | 0.91–11.07 | 0.053 |
| Pre-operative hemoglobin | 0.72 | 0.55–0.92 | 0.013 |
| Pre-operative creatinine | 1.81 | 1.10–3.21 | 0.026 |
| Vasoactive-inotropic score | 1.06 | 1.03–1.09 | <0.001 |
| Final model for composite outcome (Mallow’s CP 4.937) | |||
|
| |||
| Variable | OR | 95% CI | p-value |
|
| |||
| Pre-operative hemoglobin | 0.81 | 0.66–0.97 | 0.030 |
| Pre-operative albumin | 0.42 | 0.21–0.83 | 0.014 |
| Pre-operative creatinine | 2.27 | 1.45–3.71 | 0.001 |
| Vasoactive-inotropic score | 1.03 | 1.01–1.06 | 0.008 |
CI, confidence interval; OR, odds ratio.
Figure 2.
Receiver operating curve (ROC) for comparison of vasoactive-inotropic score (VIS) and left ventricular assist device (LVAD) risk models (HeartMate II Risk Score [HMRS] and Right Ventricular Failure Risk Score [RVFRS]) in the prediction of in-hospital mortality. AUC, area under the curve.
Optimal VIS cut-off point for prediction of clinical outcomes
An initial ROC analysis found VIS ≥ 20 as the optimal threshold for creating a dichotomous VIS variable. Based on Youden’s index, VIS ≥ 20 as a predictor of in-hospital mortality yielded a sensitivity of 0.84 and a specificity of 0.53. The results were nearly identical for the composite outcome with a cut-off point of 19.7 with a sensitivity of 0.72 and a specificity of 0.52.
Clinical characteristics based on a cut-off point of VIS ≥20
There were 210 (51.5%) patients with a high VIS (≥20) and 208 (48.5%) with a low VIS (<20). The patients with a high VIS had a lower body mass index and were more likely to have prior sternotomy, be on mechanical circulatory support, be on amiodarone, and have an INTERMACS 1 profile than patients with a low VIS (Table 1). In addition, the patients with a high VIS had lower hemoglobin and albumin and higher aspartate aminotransferase levels than those with a low VIS. The majority of patients (n = 329, 78.7%) received HeartMate II (Abbott Laboratories, Chicago, IL). A total of 44 (10.5%) patients received HeartWare (Medtronic, Minneapolis, MN) and 45 (10.8%) had other continuous flow devices. There was no association between device selection and VIS levels (p = 0.24). The patients with a high VIS were more likely to have required longer CPB time (97 minutes [IQR 67−130] vs 79 minutes [IQR 59−104], p < 0.001), concomitant valve procedures (48.1% vs 33.7%, p = 0.003), as well as a greater use of packed red blood cells (55.6% vs 35.1%, p < 0.001) and fresh frozen plasma (75.9% vs 66.3%, p = 0.033) intraoperatively.
Pre-and intraoperative predictors of VIS ≥20 after LVAD implantation
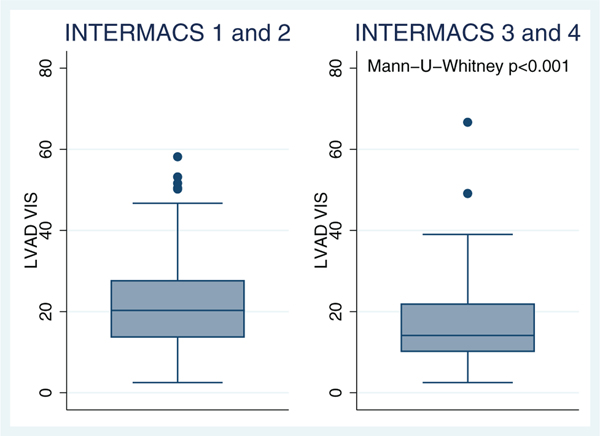
The multivariate logistic regression models showed that pre-operative hemoglobin level (OR 0.82, 95% CI 0.72−0.93, p = 0.003) was associated with increased post-operative VIS ≥ 20 (Table 3). Pre-operative thyroid function test, use of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, or amiodarone were not predictors of VIS ≥ 20. There was no correlation between existing pre-operative LVAD risk models, such as HMRS (R2 < 0.001, p = 0.62) and RVFRS (Kruskal−Wallis p = 0.58), with post-operative VIS ≥ 20. However, a high-risk pre-operative INTERMACS profile ≤2 was associated with a high VIS (Mann-U-Whitney p < 0.001) (Figure 3).
Table 3.
Predictors of Vasoactive-Inotropic Score (VIS) ≥ 20
| Predictors | OR | SE | (95% CI) | p-value |
|---|---|---|---|---|
|
| ||||
| Age | 1.017 | 0.010 | (0.997–1.038) | 0.089 |
| BMI | 0.958 | 0.022 | (0.916–1.002) | 0.063 |
| Pre-operative hemoglobin | 0.817 | 0.055 | (0.716–0.934) | 0.003 |
| Pre-operative SBP | 1.002 | 0.010 | (0.983–1.022) | 0.820 |
| Pre-operative albumin | 0.853 | 0.231 | (0.502–1.450) | 0.557 |
| Pre-operative MCS | 1.816 | 0.955 | (0.648–5.089) | 0.257 |
| Re-sternotomy | 1.252 | 0.355 | (0.718–2.182) | 0.429 |
| Number of valve procedures | 1.356 | 0.245 | (0.952–1.930) | 0.092 |
| Amiodarone use | 1.548 | 0.380 | (0.957–2.505) | 0.075 |
| ACE inhibitor use | 0.715 | 0.161 | (0.460–1.111) | 0.135 |
| Beta blocker use | 0.891 | 0.215 | (0.556–1.429) | 0.633 |
ACE, angiotensin-converting enzyme; BMI, body mass index; CI confidence interval; MCS, mechanical circulatory support; OR, odds ratio; SBP, systolic blood pressure; SE, standard error.
Figure 3.
Vasoactive-inotropic score (VIS) values across INTERMACS profiles. LVAD, left ventricular assist device.
Early post-operative clinical outcomes according to VIS ≥20 cut-off point
Table 4 shows early post-operative outcomes during index hospitalization. Twenty-five (6.0%) patients died. The most common causes of death were multi-organ failure (n = 10, 40.0%) followed by stroke (n = 5, 20.0%), bleeding (n = 2, 8.0%), and right HF (n = 2, 8.0%). A high VIS was associated with increased in-hospital mortality (10.0% vs 1.9%, p < 0.001). In-hospital mortality increased for each VIS quartile (0% [VIS 2.5−13.0] vs 3.9% [VIS 13.2−19.7] vs 7.6% [VIS 19.8−27.9] vs 12.3% [VIS 27.9−85.0], p = 0.002). A total of 37 (8.9%) patients needed post-operative RRT, and 12.9% of patients with a high VIS required RRT compared with 4.8% of patients with a low VIS (p = 0.004). There were 19 (4.5%) patients requiring delayed RVAD implantation without any significant difference among the VIS groups (p = 0.44). The patients with a high VIS spent longer periods of time in intensive care units than those with a low VIS (8 days [IQR 6−14] vs 7 days [IQR 5−11], p = 0.013) and a higher incidence of ventricular arrhythmias (31.8% vs 22.6%, p = 0.04).
Table 4.
Post-Operative Morbidity and Mortality during Index Hospitalization for Left Ventricular Assist Device (LVAD) Implantation
| All patients(N = 418) | VIS ≥ 20(n = 210) | VIS < 20(n = 208) | p-value | |
|---|---|---|---|---|
|
| ||||
| In-hospital mortality, n (%) | 25 (6.0) | 21 (10.0) | 4 (1.9) | <0.001 |
| Composite outcomea, n (%) | 54 (12.9) | 37 (17.6) | 17 (8.2) | 0.004 |
| Renal failure requiring RRT | 37 (8.9) | 27 (12.9) | 10 (4.8) | 0.004 |
| Delayed RVAD implantation, n (%) | 19 (4.6) | 12 (5.8) | 7 (3.3) | 0.44 |
| ICU stay (days) | 7 (5−12) | 8 (6−14) | 7 (5−11) | 0.013 |
| Post-operative complications, n (%) | ||||
| Take back for bleeding | 72 (18.3) | 43 (22.1) | 29 (14.7) | 0.06 |
| VF/VT | 107 (27.2) | 62 (31.8) | 45 (22.6) | 0.04 |
| Atrial fibrillation | 120 (28.7) | 55 (26.2) | 65 (31.3) | 0.43 |
| Sepsis | 33 (8.5) | 20 (10.5) | 13 (6.6) | 0.45 |
| Cerebral vascular accident b | 26 (6.3) | 14 (6.7) | 12 (5.8) | 0.70 |
ICU, intensive care unit; RRT, renal replacement therapy; RVAD, right ventricular assist device; VF, ventricular fibrillation; VIS, vasoactive-inotropic score; VT, ventricular tachycardia.
In-hospital mortality, delayed RVAD implantation, or a need for RRT during index hospitalization
Cerebral vascular accident was defined as new acute neurological deficit lasting >24 hours associated with acute infarction or hemorrhage on imaging
Post-operative hemodynamic parameters based on VIS ≥20 cut-off point
Patients with a high VIS had lower SVR (median 1,380 dynes sec cm—5, [IQR: 1,034−1,831] vs 1,562 [IQR: 1,199−1,963] dynes sec cm—5, p = 0.03), lower MAP (median 77 mmHg [IQR: 71−84] vs 81 [IQR: 75−87] mmHg, p < 0.001) and lower mean PAP (median 22 mmHg [IQR: 18−26] vs 25 [IQR: 20−29] mmHg, p = 0.03) compared to those with a low VIS. There was no difference in VIS and Fick cardiac index (median 3.75 liter/min/m2 [IQR 3.03−5.02] vs 3.51 liter/min/ m2 [IQR 2.87−4.51], p = 0.17) and CVP (median 9.5 mm Hg [IQR 7−13] vs 10 mm Hg [IQR 8−13], p = 0.58).
Biomarkers and VIS
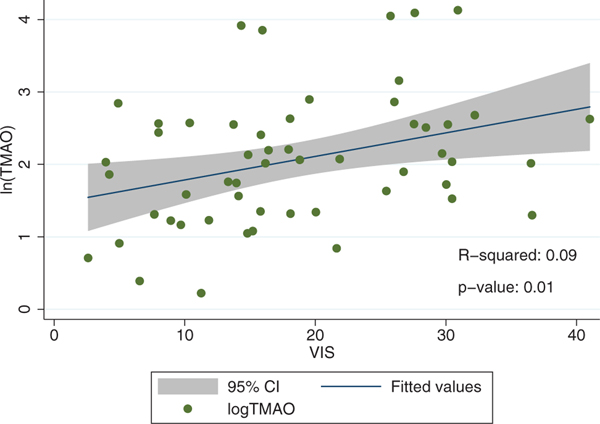
Overall, the baseline characteristics and the distribution of VIS between patients with biomarkers measured (2016−2017) and those in whom biomarkers were not measured (2004−2015) were similar (refer to Supplementary Material 1 available online at www.jhltonline.org and Figure S1). The patients with high post-operative VIS ≥ 20 were more likely to have elevated TMAO pre-operatively (53.6% vs 25.8%, p = 0.03). The relationship between VIS and log transformation of TMAO is shown in Figure 4 (R2 = 0.09). There was no significant association between VIS and pre-operative biomarkers of inflammation (IL-6, TNF-α, CRP, adiponectin, and ET-1), oxidative stress (isoprostane), and endotoxemia (sCD14 and LPS).
Figure 4.
Scatter plot of Log transformation of trimethyl-amine-N-oxide (TMAO) versus vasoactive-inotropic score (VIS). CI, confidence interval
Discussion
The purpose of this study was to evaluate the utility of post-operative VIS (a combined marker of inotrope and vasopressor requirement) to predict adverse outcomes when calculated after initial stabilization in the OR and upon arrival at the intensive care unit and to explore possible mechanisms for post-operative hemodynamic instability.
The findings show that VIS is an independent predictor and composite outcome of in-hospital mortality, delayed RVAD implantation and RRT, and the optimal cut-off point of VIS as a predictor of outcome is 20. Additionally, VIS is a better discriminator of in-hospital mortality compared with established LVAD risk models. Furthermore, pre-operative hemoglobin level is an independent predictor of elevated VIS and higher pre-operative plasma levels of TMAO are associated with a high VIS, while other biomarkers of inflammation, oxidative stress, and endotoxemia are not.
Prior studies in patients with LVAD implantation focused on identifying pre-operative predictors of short-term adverse events to allow better patient selection.21–26 However, few studies have tried to risk stratify patients immediately after LVAD surgery. Tecson et al27 recently reported that post-operative vasoplegia (defined as the “occurrence of normal cardiac index but with the need for intravenous vasopressors within 48 hours following surgery for >24 hours to maintain a MAP > 70 mm Hg”) was associated with poor post-operative outcomes, including mortality. Our study expands on this initial observation, by providing a reliable prediction tool that (1) integrates information on both vasopressor and inotrope requirement into one score that summarizes the severity of cardiovascular derangement, and (2) is calculated after initial stabilization in the OR and upon arrival at the intensive care unit. For this purpose and for the first time in the adult LVAD population, we are adopting VIS—a surrogate marker of overall cardiovascular derangement that has been associated with poor post-operative outcomes in pediatric and adult patients undergoing cardiac surgery.
Initial studies of VIS in pediatric patients used 24- or 48-hour maximum doses of drugs to calculate VIS.17–20 The results from our cohort show that VIS at a single time point still remains a significant predictor of in-hospital mortality and composite outcome of in-hospital mortality, delayed RVAD implantation, and RRT. The optimal cut-off point of VIS as a predictor of poor outcome in our cohort was 20. In a recent article by Yamazaki et al20 that utilized VIS as a predictor of morbidity and mortality in patients who underwent adult cardiac surgery, the optimal cut-off point was 5.5. This discrepancy in cut-off values is likely due to the differences in baseline characteristics and surgical procedures between studies, because the majority of the patients in their cohort had elective valve surgery, few had a left ventricular ejection fraction < 30%, and none underwent LVAD surgery for the treatment of endstage HF.21 Thus, VIS appears to maintain a prognostic role throughout a wide range of surgical procedures, although cut-off points may vary depending on patients’ characteristics and the type of intervention.
A high VIS captures the complex interplay between right ventricular dysfunction and vasoplegia, which one may define as “vasoplegia with relative RVF” that frequently follows LVAD implantation. Our results indicate that patients with a VIS ≥ 20 have a lower SVR [median 1,380 dynes sec cm−5 (IQR 1,034−1,831) vs 1,562 dynes sec cm−5 (IQR 1,199−1,963), p = 0.03], a lower MAP [median 77 mm Hg (IQR 71−84) vs 81 mm Hg (IQR 75−87), p < 0.001], and increased RRT requirement (12.9% vs 4.8%, p = 0.004) than those with a VIS < 20. Importantly, these data suggest that exposure to high, “toxic” doses of vasopressors to maintain MAP in a setting of marginal cardiac output may further limit perfusion of critical end-organs, particularly the kidneys, eventually causing acute kidney injury and ultimately patients’ demise.28 In addition, a greater incidence of ventricular arrhythmias in high VIS population (31.8% vs 22.6%, p = 0.04) may be driven by arrhythmogenic properties of inotropes and vasopressors. LVAD recipients with a high VIS deserve closer attention. In our opinion, these patients may benefit from early consideration of RVAD therapy, either surgical or percutaneous, to maintain a higher cardiac output and minimize vasopressor requirement before the kidneys and other organs sustain meaningful and eventually irreversible damage. Late interventions are often futile as demonstrated in our prior published experience.29 Our results indicate that VIS is a better predictor of in-hospital mortality than existing pre-operative risk models, such as HMRS or RVFRS, in patients with LVAD implantation.22–25 Notably, a high-risk INTERMACS profile (≤2) was a predictor of VIS ≥ 20, while the above LVAD risk models were not.
In our study, a lower hemoglobin level is associated with increased VIS. Our result is consistent with a prior study that showed anemia is associated with post-operative vasoplegia in patients with LVAD implantation.30 Pre-operative anemia is likely multifactorial. Determinants may include an underlying inflammatory state (anemia of chronic disease), poor nutrition and recurrent phlebotomies causing iron deficiency anemia, as well as hemolysis in patients on pre-operative mechanical circulatory support. While prior reports have identified the use of ACE inhibitors or beta-blockers as risk factors for post-bypass vasodilatory state and adverse events,31–33 our model does not show any association in the present cohort. Byrne et al 34 similarly reported that ACE inhibitors were not predictors of post-heart transplant vasoplegia. The long-term compensatory activation of the renin-angiotensin system in patients with HF (i.e., ACE escape) may explain this lack of association between ACE inhibitors and vasoplegia in this patient population.35
Our understanding of the pathophysiology that contributes to post-operative vasoplegia is incomplete. Cardiopulmonary bypass causes inflammation and smooth muscle relaxation via the release of nitric oxide.36 The pro-inflammatory milieu of advanced HF may also contribute to this hemodynamic response during Cardiopulmonary bypass. We investigated the association between VIS and biomarkers of inflammation, oxidative stress, and endotoxemia as well as the gut-derived metabolite TMAO in a subset of our study cohort. We report that a pre-operative value of TMAO is associated with a high VIS. Prior studies have shown that high TMAO levels are predictors of increased mortality in patients with chronic and acute HF,9–11 supporting the so-called gut-inflammatory hypothesis for the progression of this disease.12–14 One could speculate that TMAO may promote post-operative vasodilation through activation of nuclear factor-kB in endothelial cells and smooth muscle cells,15 although TMAO is a relatively novel biomarker and its precise role in the pathophysiology of vasoplegia is yet to be determined.
This investigation has limitations. First, this is a single-center retrospective study that lacks an external validation cohort. Future prospective multi-institutional studies are necessary to fully evaluate the utility of VIS in patients with LVAD implantation. Second, VIS is measured at a single time point rather than the precise definition of maximum doses within the first 24 hours. However, we believe that a single value offers a simple and rapid calculation of VIS, which helps to identify high-risk patients with LVAD implantation immediately after the surgery.
In conclusion, a high VIS after initial stabilization in the operating room and upon arrival at the intensive care unit is associated with adverse clinical outcomes and is a better predictor of in-hospital mortality compared with existing LVAD risk models. A lower hemoglobin level is an independent predictor of post-operative cardiovascular derangement. Based on an exploratory analysis, elevated preoperative TMAO levels are also associated with a high VIS. Whether early correction of hemodynamic compromise through mechanical right ventricular support therapy may prevent continued end-organ damage and improve clinical outcomes remains to be established.
Supplementary Material
Acknowledgments
This study was supported by the Lisa and Mark Schwartz Program to Reverse Heart Failure and the Susan and Lowell McAdam Program at the New York Presbyterian Hospital/Columbia University Irving Medical Center.
Footnotes
Supplementary data
Supplementary data associated with this article can be found in the online version at www.jhltonline.org/.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2019.05.007.
Disclosure statement
Y.N. received consulting fees from Abbott Laboratories/St Jude Medical. The remaining authors have no conflicts of interest to disclose.
References
- 1.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. [DOI] [PubMed] [Google Scholar]
- 2.Lampropulos JF, Kim N, Wang Y, et al. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open Heart 2014;1:e000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316–24. [DOI] [PubMed] [Google Scholar]
- 4.Aissaoui N, Morshuis M, Schoenbrodt M, et al. Temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J Thorac Cardiovasc Surg 2013;146:186–91. [DOI] [PubMed] [Google Scholar]
- 5.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007;357:885–96. [DOI] [PubMed] [Google Scholar]
- 6.Khayata M, Al-Kindi S, Panhwar MS, et al. Preoperative MEDL XI is not associated with mortality after LVAD. J Card Fail 2018;24:S106. [Google Scholar]
- 7.Kanwar MK, Lohmueller LC, Kormos RL, et al. Low accuracy of the HeartMate Risk Score for Predicting Mortality Using the INTERMACS Registry Data. ASAIO J 2017;63:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung RK, Wu YK. Circulating microbial RNA and health. Sci Rep 2015;5:16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N-oxide and prognosis in acute heart failure. Heart 2016;102: 841–8. [DOI] [PubMed] [Google Scholar]
- 10.Tang WH, Wang Z, Shrestha K, et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail 2015;21:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trøseid M, Ueland T, Hov JR, et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015;277:717–26. [DOI] [PubMed] [Google Scholar]
- 12.Nagatomo Y, Tang WH. Intersections Between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 2015;21:973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang WH, Wang Z, Fan Y, et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014;64:1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiattarella GG, Sannino A, Toscano E, et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–56. [DOI] [PubMed] [Google Scholar]
- 15.Seldin MM, Meng Y, Qi H, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kB. J Am Heart Assoc 2016;5. 10.1161/JAHA.115.002767 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 1995;92:2226–35. [DOI] [PubMed] [Google Scholar]
- 17.Gaies MG, Jeffries HE, Niebler RA, et al. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: an analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr Crit Care Med 2014;15:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234–8. [DOI] [PubMed] [Google Scholar]
- 19.Sanil Y, Aggarwal S. Vasoactive-inotropic score after pediatric heart transplant: a marker of adverse outcome. Pediatr Transplant 2013;17:567–72. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki Y, Oba K, Matsui Y, Morimoto Y. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018;32:167–73. [DOI] [PubMed] [Google Scholar]
- 21.Cotts WG, Mcgee EC, Myers SL, et al. Predictors of hospital length of stay after implantation of a left ventricular assist device: an analysis of the INTERMACS registry. J Heart Lung Transplant 2014;33:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabashnikov A, Mohite PN, Zych B, et al. Outcomes and predictors of early mortality after continuous-flow left ventricular assist device implantation as a bridge to transplantation. ASAIO J 2014;60:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews JC, Pagani FD, Haft JW, et al. Model for end-stage liver disease score predicts left ventricular assist device operative transfusion requirements, morbidity, and mortality. Circulation 2010;121:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teuteberg JJ, Ewald GA, Adamson RM, et al. Risk assessment for continuous flow left ventricular assist devices: does the destination therapy risk score work? An analysis of over 1,000 patients. J Am Coll Cardiol 2012;60:44–51. [DOI] [PubMed] [Google Scholar]
- 25.Cowger J, Sundareswaran K, Rogers JG, et al. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol 2013;61:313–21. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JC, Koelling TM, Pagani FD, Aaronson KD. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tecson KM, Lima B, Lee AY, et al. Determinants and outcomes of vasoplegia following left ventricular assist device implantation. J Am Heart Assoc 2018;7. 10.1161/JAHA.117.008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rona G Catecholamine cardiotoxicity. J Mol Cell Cardiol 1985;17:291–306. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka D, Takayama H, Garan RA, et al. Contemporary outcome of unplanned right ventricular assist device for severe right heart failure after continuous-flow left ventricular assist device insertion. Interact Cardiovasc Thorac Surg 2017;24:828–34. [DOI] [PubMed] [Google Scholar]
- 30.Argenziano M, Choudhri AF, Oz MC, et al. A prospective randomized trial of arginine vasopressin in the treatment of vasodilatory shock after left ventricular assist device placement. Circulation 1997;96 (Suppl 9):II–286. [PubMed] [Google Scholar]
- 31.Bandeali SJ, Kayani WT, Lee VV, et al. Outcomes of preoperative angiotensin-converting enzyme inhibitor therapy in patients undergoing isolated coronary artery bypass grafting. Am J Cardiol 2012;110:919–23. [DOI] [PubMed] [Google Scholar]
- 32.Miceli A, Capoun R, Fino C, et al. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 2009;54:1778–84. [DOI] [PubMed] [Google Scholar]
- 33.Levin MA, Lin HM, Castillo JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 2009;120:1664–71. [DOI] [PubMed] [Google Scholar]
- 34.Byrne JG, Leacche M, Paul S, et al. Risk factors and outcomes for ‘vasoplegia syndrome’ following cardiac transplantation. Eur J Cardiothorac Surg 2004;25:327–32. [DOI] [PubMed] [Google Scholar]
- 35.Ennezat PV, Berlowitz M, Sonnenblick EH, Le jemtel TH. Therapeutic implications of escape from angiotensin-converting enzyme inhibition in patients with chronic heart failure. Curr Cardiol Rep 2000;2: 258–62. [DOI] [PubMed] [Google Scholar]
- 36.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 2001;345:588–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.